Abstract
The locus coeruleus (LC) has been studied in major depressive disorder (MDD) and bipolar disorder (BD). A major problem of immunocytochemical studies in the human LC is interference with the staining of the immunocytochemical end-product by the omnipresent natural brown pigment neuromelanin. Here, we used a multispectral method to untangle the two colors: blue immunocytochemical staining and brown neuromelanin. We found significantly increased tyrosine hydroxylase (TH) in the LC of MDD patients—thus validating the method—but not in BD patients, and we did not find significant changes in the receptor tyrosine-protein kinase ErbB4 in the LC in MDD or BD patients. We observed clear co-localization of ErbB4, TH, and neuromelanin in the LC neurons. The different stress-related molecular changes in the LC may contribute to the different clinical symptoms in MDD and BD.
Keywords: Major depressive disorder, Bipolar disorder, Tyrosine hydroxylase, ErbB4, Locus coeruleus
Introduction
Major depressive disorder (MDD) and bipolar disorder (BD) are the major subtypes of mood disorder. MDD is also known as major depression, or unipolar depression, the term ‘unipolar’ referring to the presence of one pole, or one extreme of depressed mood. BD, on the other hand, also known as manic depression, is a mental illness characterized by severely high (hypomania and mania) and low (depression) moods, thus ‘bipolar’, along with changes in sleep, energy, thinking, and behavior [1]. MDD and BD affect > 17% of the world’s population and impose heavy medical and social burdens on the individual, family, and society [2, 3]. Functional changes in emotion- and stress-related neuronal-networks are considered to be central in the pathogenesis of mood disorders. There are clear individual differences in the type of stress system involved and the level of vulnerability to environmental stress [4]. The locus coeruleus (LC) and the hypothalamo-pituitary-adrenal (HPA) axis are important hubs in these networks that interact closely [4–8].
The LC, strongly pigmented by melanin, is located in the brainstem and is the main source of norepinephrine (NE) in the brain. Tyrosine hydroxylase (TH) is the rate-limiting enzyme for NE production [9] and is regarded as a good biosynthetic index of LC activity [10]. The LC receives corticotropin-releasing hormone projections from the hypothalamus, while NE stimulates HPA activity [5]. In rats, chronic stress has been found to increase LC-TH expression [11, 12] and LC electrostimulation causes increased plasma adrenocorticotropic hormone levels, indicating corticotropin-releasing hormone activation [13]. In addition, increased TH levels have been found in the rostral, middle, and caudal parts of the LC in MDD patients [6], together with elevated NE in the cerebrospinal fluid (CSF) and increased plasma cortisol, indicating activation of both the LC and the HPA axis. Moreover, elevated CSF NE and plasma cortisol have been reported in patients with melancholic depression [5]. Furthermore, higher LC activity has been found in patients with suicidal depression, especially in those with violent suicidal behaviors [14, 15]. Only a few studies have addressed LC activity in BD patients. Lower LC TH-immunoreactivity has been reported in suicidal BD patients compared with normal controls [10], but no comparison has been made between BD patients who did not commit suicide and controls. Therefore, it remains unclear whether BD and MDD patients show different changes in LC activity.
Recently, the role of ErbB4 in the LC in schizophrenia and BD has drawn much attention [16–18]. ErbB4, a receptor for tyrosine kinase and a member of the epidermal growth factor receptor subfamily, is essential for neuronal development, including synapse formation, neuronal differentiation, axon navigation, and myelination [19]. ErbB4 is phosphorylated after binding to its ligand neuregulin (NRG)-1, followed by dimerization with ErbB2, leading to the phosphorylation and activation of signaling pathways such as the Akt and extracellular-regulated kinase (ERK) signaling pathways [2, 20]. ErbB4-mRNA is widely expressed in NE neurons in the LC of rats, suggesting a possible role of ErbB4 in the regulation of the LC-NE system [21]. In addition, a single-nucleotide polymorphism study in Dublin has shown that a polymorphism of ErbB4 is significantly associated with schizophrenia [22]. Higher ErbB4-mRNA levels have been found in the dorsolateral prefrontal cortex of schizophrenia patients than in controls [23]. Since BD and schizophrenia share a similar genetic background and similar clinical symptoms [24, 25], ErbB4-NRG1 expression has been proposed to play a role in BD. In rats, down-regulation of ErbB4-NRG1 in the hippocampus and prefrontal cortex is related to decreased glutamate decarboxylase-67 and GABA levels in the same regions [2]. A recent animal study has shown that ErbB4 signaling plays a critical role in regulating LC-NE neuronal function, and that dysfunction of the NE system may contribute to the pathogenesis of mania-associated disorders [26].
Therefore, it is important to quantify the changes of TH and ErbB4 in the LC of MDD and BD patients. However, in immunocytochemical studies of the human LC, there is an inherent problem: the immunocytochemical staining products are interfered with by the natural brown pigment neuromelanin which is present in the majority of large LC neurons. This pigment can even be useful as a marker when the number of immunoreactive LC neurons is counted, if quantification of the immunocytochemical product per cell is not essential [14, 15]. An alternative method is to extract proteins from LC tissue punches and to determine their levels [27], but this causes a loss of the LC morphological characteristics and makes impossible double labeling of the protein in LC neurons. It should also be noted that fluorescence is inapplicable to the human LC for quantitative immunocytochemical research, due to the strong autofluorescence of human brain tissue.
Therefore, in the present study we used a multispectral method to untangle the two colors: the genuine spectrum of the blue immunocytochemical staining was obtained in a brain area without neuromelanin, while the genuine spectrum of brown neuromelanin was based upon a ‘blank’ LC slide, in which the primary antibody was replaced by buffer in the staining procedure. As a first essential step for further functional studies on the neuromelanin-containing LC in different mood disorders, we used the multispectral analysis of TH and ErbB4 expression to compare BD and MDD patients with their matched controls. In addition, we studied the co-localization of TH and ErbB4 in neuromelanin-containing neurons of the human LC.
Materials and Methods
Participants
Postmortem LC samples were obtained from the Netherlands Brain Bank (NBB), Amsterdam. All material was collected from donors from whom written informed consent for brain autopsy and the use of the material and clinical information for research purposes had been obtained by the NBB. Diagnosis of MDD or BD at any time during life was made by qualified psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III-R/DSM-IV criteria. The absence of neuropathological changes, both in the mood disorder groups and the control groups, was confirmed by systematic neuropathological investigation [28].
The LCs were dissected at autopsy and fixed in 0.1 mol/L phosphate-buffered 4% (w/v) formaldehyde (pH 7.2) for 1–2 months. Two separate control groups of 11 largely-overlapping patients (9 each) were set to match the 9 BD and 9 MDD patients (for P values of matching see Tables 1 and 2). The pH of the CSF was slightly lower in the BD group (mean, 6.34) than in the control group (mean, 6.63, P = 0.075). A lower CSF pH has been reported to be an endophenotype of BD [29] and thus was not considered to be a confounder. In addition, there was a significant difference in the month of death between the MDD group (mean, July ± 2 months) and its controls (mean, March ± 2 months, P = 0.01, Table 1), to which we paid special attention in the analysis (see Results and Discussion).
Table 1.
Clinico-neuropathological information of the bipolar disorder (BD) patients and matched controls.
| NBB | Group | Sex | Age (years) | PMD (h:min) | CTD | MOD | CSF pH | BW (g) | Braak stage | Storage time (days) | Suicide ideation | Medication in the last 3 months | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008-061 | BD | F | 62 | 05:00 | 07:00 | 7 | 6.11 | 1110 | I | 3179 | Yes | Li, antipsychotic, BZD | Cachexia by gall bladder carcinoma |
| 2004-040 | BD | F | 76 | 04:40 | 04:00 | 5 | 6.50 | 1103 | I | 4701 | No | BZD, antipsychotic | Liver failure, obstruction of the biliary tract, serious infection and dehydration |
| 2014-041 | BD | F | 79 | 08:00 | 16:45 | 7 | 6.31 | 990 | II | 988 | No | Antipsychotic | Renal insufficiency |
| 1997-058 | BD | F | 90 | 06:30 | 10:15 | 5 | / | 1143 | 7258 | No | MAOI | Respiratory insufficiency, possibly due to lung embolism. According to daughter: recurrent lung embolism and cardiac decompensation | |
| 1996-118 | BD | M | 55 | / | 00:40 | 1 | / | / | I | 7744 | Yes | BZD antipsychotic |
Intracranial trauma due to .22 cal. bullet wounds, self-inflicted. Death in minutes. Secondary to manic depression for years |
| 2014-070 | BD | M | 66 | 07:35 | 16:40 | 11 | 5.83 | 31320 | I | 865 | Yes | Li BZD antipsychotic |
Acute myeloid leukemia |
| 2002-014 | BD | M | 68 | / | 12:00 | 2 | 6.64 | 1414 | I | 5521 | No | / | Subdural hematoma |
| 2006-021 | BD | M | 70 | 06:23 | 13:07 | 3 | 6.53 | 1368 | III | 4032 | No | Li | Severe neck trauma and pneumonia |
| 2008-085 | BD | M | 71 | 06:25 | 22:50 | 10 | 6.49 | 1380 | I | 3087 | No | None | Metastasized pulmonary carcinoma |
| Median/phi ± sa | 70 | 06:25 | 12:04 ± 4:59 | 4.21 ± 2.51 | 6.49 | 1231.5 | I | 4032 | |||||
| 1998-061 | CNTR | F | 64 | 06:00 | 12:00 | 5 | 6.87 | 1122 | I | 6893 | No | BZD | Cachexia |
| 2004-015 | CNTR | F | 69 | 04:20 | 23:50 | 2 | 6.12 | 1183 | I | 4791 | No | None | Cardiac decompensation due to myocardial infarction, sepsis, and lung embolisms |
| 2001-096 | CNTR | F | 77 | 05:40 | 05:00 | 8 | 6.32 | 1111 | I | 5705 | No | None | Apnea. Starvation/dehydration |
| 2003-006 | CNTR | F | 91 | 05:20 | 02:40 | 1 | 6.51 | 1080 | III | 5187 | No | None | Consequences of a heart attack |
| 1998-006 | CNTR | M | 50 | 08:30 | 11:00 | 1 | 6.65 | 1401 | 0 | 7013 | No | / | Cardiac arrest |
| 2011-044 | CNTR | M | 51 | 07:45 | 16:30 | 5 | 7.05 | 1450 | 0 | 2145 | No | None | Suicide by refusing food and water |
| 2002-008 | CNTR | M | 62 | 09:35 | 10:00 | 1 | 6.58 | 1163 | 0 | 5552 | No | None | Metastasized adenocarcinoma of the head of the pancreas. Rupture of the ileum, bacterial peritonitis, and sepsis |
| 2005-073 | CNTR | M | 87 | 06:05 | 08:05 | 10 | 6.96 | 1468 | III | 4183 | No | None | / |
| 1995-062 | CNTR | M | 80 | 04:25 | 17:40 | 6 | 6.59 | 1429 | II | 8109 | No | / | / |
| Median/phi ± sa | 69 | 06:00 | 9:39±4:41 | 2.58±2.44 | 6.59 | 1183 | I | 5552 | |||||
| P | 1.0 | 0.91 | 0.96 | 0.67 | 0.80 | 0.08 | 0.63 | 0.51 | 0.20 |
BD bipolar disorder, Braak stage Braak stage of Alzheimer disease [42], BW brain weight, BZD benzodiazepine, CSF cerebrospinal fluid, CNTR control group, CTD clock time at death, F female, Li lithium, M male, MAOI monoamine oxidase inhibitor, MOD month of death, NBB Netherlands Brain Bank number, PMD postmortem delay, / no information.
aMean angle ± mean angular deviation in Mardia-Watson-Wheeler test.
Table 2.
Clinico-pathological information of major depressive disorder (MDD) patients and matched controls.
| NBB | Group | Sex | Age (years) | PMD (h:min) |
CTD | MOD | CSF pH | BW (g) | Braak stage | Storage time (days) | Suicide ideation | Medication in last 3 months | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999-115 | MDD | F | 57 | 05:30 | 20:45 | 9 | 6.28 | 1325 | 0 | 6405 | No | BZD | Euthanasia |
| 2012-097 | MDD | F | 73 | 05:45 | 15:30 | 9 | 6.70 | 1205 | III | 1656 | No | None | Euthanasia |
| 2005-065 | MDD | F | 93 | 04:25 | 16:55 | 9 | 7.30 | 1203 | I | 4213 | Yes | SSRI | Unknown |
| 2011-051 | MDD | F | 100 | 05:50 | 10:50 | 6 | 6.40 | 990 | II | 2114 | No | / | Natural death |
| 2014-012 | MDD | M | 68 | 08:55 | 05:45 | 3 | 6.82 | 1510 | 0 | 1110 | Yes | SSRI | Sudden death, possibly related to panlobular emphysema, pneumonia, or cardiac arrest |
| 2006-026 | MDD | M | 70 | 07:15 | 08:00 | 3 | 6.50 | 1380 | I | 4032 | No | Antipsychotic | Respiratory insufficiency (and cardiac symptomology) |
| 2002-051 | MDD | M | 81 | 06:00 | 15:30 | 6 | 6.50 | 1280 | III | 5401 | No | Antipsychotic | Renal insufficiency |
| 2011-058 | MDD | M | 83 | 10:40 | 05:00 | 7 | 6,5 | II | 2084 | No | None | Acute heart failure | |
| 2007-060 | MDD | M | 93 | 06:00 | 21:10 | 9 | 6.37 | 1369 | I | 3483 | No | BZD | Cachexia in combination with CVA or failure of renal functions |
| Median/phi ± sa | 81 | 06:00 | 14:17 ± 4:56 | 7.37 ± 2.05 | 6.5 | 1302.5 | I | 3483 | |||||
| 1998-061 | CNTR | F | 64 | 06:00 | 12:00 | 5 | 6.87 | 1122 | I | 6893 | No | None | Cachexia |
| 2004-015 | CNTR | F | 69 | 04:20 | 23:50 | 2 | 6.12 | 1183 | I | 4791 | No | / | Cardiac decompensation due to myocardial infarction, sepsis, and lung embolisms |
| 2011-072 | CNTR | F | 76 | 07:15 | 05:00 | 9 | 6.87 | 1072 | II | 2022 | No | BZD | Hepatic failure and colon carcinoma |
| 2003-006 | CNTR | F | 91 | 05:20 | 02:40 | 1 | 6.51 | 1080 | III | 5187 | No | None | Consequences of a heart attack |
| 2011-044 | CNTR | M | 51 | 07:45 | 16:30 | 5 | 7.05 | 1450 | 0 | 2145 | No | None | Suicide by refusing food and water |
| 2002-008 | CNTR | M | 62 | 09:35 | 10:00 | 1 | 6.58 | 1163 | 0 | 5552 | No | / | Metastasized adenocarcinoma of the head of the pancreas. Rupture of the ileum, bacterial peritonitis, and sepsis |
| 1995-062 | CNTR | M | 80 | 04:25 | 17:40 | 6 | 6.59 | 1429 | II | 8109 | – | / | / |
| 2001-021 | CNTR | M | 82 | 07:40 | 02:50 | 2 | 6.07 | 1318 | I | 5886 | No | None | Heart attack |
| 2005-073 | CNTR | M | 87 | 06:05 | 08:05 | 10 | 6.96 | 1468 | III | 4183 | No | None | / |
| Median / phi ± sa | 76 | 06:05 | 6:12±4:52 | 2.50±2.39 | 6.59 | 1183 | I | 5187 | |||||
| P | 1.00 | 0.34 | 0.81 | 0.96 | 0.01 | 0.86 | 0.71 | 1.0 | 0.10 |
Braak stage Braak stage of Alzheimer disease [42], BW brain weight, BZD benzodiazepine, CSF cerebrospinal fluid, CTD clock time at death, CNTR control, CVA cerebrovascular accident, F female, M male, MDD major depressive disorder, MOD month of death, NBB Netherlands Brain Bank number, PMD postmortem delay, SSRI selective serotonin reuptake inhibitor; / no information.
aMean angle ± mean angular deviation in Mardia-Watson-Wheeler test.
Brainstem containing the LC was dehydrated in graded ethanols, embedded in paraffin, and serially cut at 6 μm in the rostro-caudal axis on a Leitz microtome (Leica Biosystems, Nussloch, Germany) and stored at room temperature (RT). Every 50th section was mounted as an unstained paraffin section to locate the mid-level of the LC by the presence of the largest number of brown-pigmented neurons. The sections around the mid-level of the LC were stained for either TH or ErbB4. Subsequently, adjacent sections were used for double-staining for TH and ErbB4.
Patients were characterized as belonging to the suicidal group if they experienced suicidal ideation (n =2), had attempted suicide (n =2), or had committed suicide (n =1). There were 3 BD and 2 MDD suicidal patients. In addition, there were 2 legal euthanasia MDD patients and 1 control who died by voluntary starvation (Tables 1 and 2), for whom extra analyses were performed.
Immunocytochemical Staining for TH
The specificity of the monoclonal mouse-anti-TH (Millipore MAB318) has been extensively confirmed by Western blotting and immunohistochemistry [30, 31]. One mid-level LC section per patient was used for TH staining. In brief, the sections were deparaffinized and rehydrated using xylene and decreasing grades of ethanol. After rinsing in distilled water (1 × 5 min), the sections were placed in 0.05 mol/L Tris-HCl (pH 9) and microwave-treated for 10 min at maximum power (800 W) for antigen retrieval. After cooling to RT, the sections were rinsed in Tris-buffered saline (TBS) (pH 7.6) and incubated overnight at 4°C with monoclonal mouse anti-TH diluted 1:8000 in supermix (0.25% gelatin and 0.5 mL Triton X-100 in 100 mL TBS, pH 7.6). On the second day, the sections were rinsed in TBS and incubated for 90 min with donkey anti-mouse IgG conjugated to alkaline phosphatase (cat. no. 715-056-150, Jackson ImmunoResearch, West Grove, PA) diluted 1:200 in supermix at RT. After washing in TBS and pre-incubation in buffer (in mmol/L: 100 NaCl, 5 MgCl2, 100 Tris-HCl pH 9.5), the LC was visualized with color solution containing 0.34 mg/mL nitro-blue tetrazolium chloride (NBT), 0.175 mg/mL 5-bromo-4-chloro-3’-indolyphosphate p-toluidine salt (BCIP), and 0.24 mg/mL Levamisole (L-9756, Sigma-Aldrich, St. Louis, MO) in Buffer 2. Color development took place in the dark at RT for 30 min. Reactions were terminated by rinses in distilled water (2 × 5 min) followed by methanol (1 × 5 min) to remove the brown discoloration, and in water (2 × 5 min) before coverslipping the sections.
Immunocytochemical Staining for ErbB4
The immunohistochemical protocol for ErbB4 was largely similar to that of the TH staining, except that after rehydration, sections were boiled in 0.01 mol/L citrate buffer (pH 6.0). After a 1-h wash in TBS and incubation with 5% milk to reduce the background, sections were incubated with a monoclonal rabbit anti-ErbB4 antibody (Abcam ab32375, lot number: GR3174336-3) diluted 1:50. The specificity of this antibody has been established by negative staining in LC-ErbB4-/- mice [26]. Detection was performed with anti-rabbit IgG conjugated to alkaline phosphatase (cat. no. 711-055-152, Jackson ImmunoResearch, 1:1 in glycerol). The time needed for color development was 90 min. Two sections per patient were used, and their average value was calculated.
Double-Staining for TH and ErbB4 in Human LC
For immunocytochemical studies of the co-localization of TH and ErbB4, the same antibodies were used as in single staining. Double staining for TH and ErbB4 was performed on sections around the mid-level of the LC for each sample. The sections were deparaffinized and rehydrated in graded ethanols, and then placed in a microwave oven in 0.05 mol/L Tris-HCl (pH 9) buffer for 10 min. Sections were then incubated overnight in a mixture of rabbit-anti-ErbB4 (dilution 1:15) and mouse-anti-TH (1:8000). The next day, the sections were rinsed in TBS for 3 × 10 min and subsequently incubated with anti-rabbit IgG conjugated to alkaline phosphatase (1:200) at RT for 1 h. ErbB4 was stained blue using a Fast Blue kit (cat. No. SK-5300, Vector Laboratories, Burlingame, CA) for 50 min. Then the sections were rinsed for 3 × 5 min and incubated with biotinylated anti-mouse (1:200) at RT for 1 h and with avidin-biotin complexes (ABC, 1:800) at RT for 1 h. TH was stained red using the 3-amino-9-ethylcarbazole (AEC) red kit (cat. No. SK-4200, Vector Laboratories) for 50 min.
Image Analysis
The experimental procedures are summarized in Fig. 1. The specificity and sensitivity of this multispectral method were based upon the specific immunocytochemical staining for each of the target proteins by thresholding in such a way that the mask exactly covered the specific immunocytochemical staining: (1) a stack of images of each section was collected at different wavelengths ranging from 520 to 700 nm in 20-nm steps on an Axioskop microscope (Zeiss, Oberkochen, Germany) equipped with a 20× (for TH) or a 10× (for ErbB4) objective and a Nuance FX multispectral camera (Perkin Elmer, Waltham, MA); (2) the pure spectrum of the NBT/BCIP staining (blue) was based on the ErbB4 staining of human hippocampus, while the pure spectrum of the brown neuromelanin was based on a ‘blank’ LC slide, in which the primary antibody was replaced by supermix in the staining process. This allowed untangling of these two colors [32] with Nuance software (Version 3.0.1.2, Cambridge Research Instrumentation; Woburn, MA; Fig. 2). When there was negligible or no bleed-through of neuromelanin into the blue spectrum, the Nuance software was used to analyze the blue spectrum. Because of the large inter-patient variance in background intensity, the mask threshold had to be set for each patient individually.
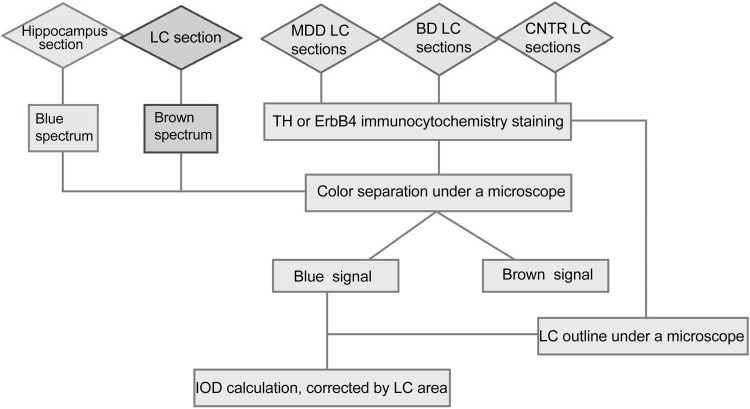
Fig. 1.
Flowchart of the multispectral method used to prevent the interference of neuromelanin with the immunocytochemical signal of tyrosine hydroxylase (TH) or ErbB4 in postmortem human locus coeruleus (LC) tissue. MDD, major depressive disorder; BD, bipolar disorder; CNTR, control group; IOD, integrated optical density.
Fig. 2.
Images of immunoreactivity (ir) signals of tyrosine hydroxylase (TH) in human locus coeruleus (LC) neurons. According to the different wavelengths of light, TH-ir (blue) was separated from the signal of neuromelanin (brown). Microscope images showed both the TH-ir and neuromelanin signals in the LC neurons (A). The brown signal (B) and blue signal (C) were separated from the original image (scale bar, 200 μm).
Immunocytochemical signals were quantified by determining the optical density (OD) in an outline of the LC after thresholding: only signals > 2.5× (TH) or > 2× (ErbB4) background density were masked and measured. Staining was assessed for its OD, and then multiplied by the area of the mask to obtain the integrated optical density (IOD), which was subsequently corrected by the total area of the LC in the same section to calculate the corrected IOD. Images to outline total area were obtained using the Axioskop 9811 microscope (20× objective for TH, 10× for ErbB4) with a Sony 77 × c camera to tile multiple pictures into one large snapshot. Manual correction of artifacts was kept to the absolute minimum. For TH, image analysis was largely similar to that of ErbB4. However, background intensity was lower and more consistent, so all samples were analyzed with the same threshold (2.5× average-background).
For the analysis of TH-ErbB4 double staining, Nuance 3.0.1.2 software was used to build up spectral libraries of the chromogens [32]. In brief, using the 40× objective, a single LC TH-stained or LC ‘blank’ slide, or a hippocampal ErbB4-stained slide was scanned with the aim of defining the respective spectral curves, which were then used to distinguish the three chromogens (red, blue, and brown) in the double-stained sections for signal quantification. To define the specific signal during masking, the OD threshold was set at 4× the background for the red and blue chromogens. Subsequently, the co-localization of TH (red) neurons and ErbB4 (blue) was analyzed.
Statistics
All data were analyzed with IBM SPSS version 23 (IBM, Armonk, NY). Data were first checked for normality with the Shapiro-Wilk test and for equality of variance with Levene’s test. Since most data were normally distributed, differences in corrected IOD values between 2 groups were tested with Student’s t-test. In addition, analysis of covariance was applied to analyze the effect of CSF pH on TH- and ErBB4-immunoreactivity(ir). Pearson’s test was applied to analyze correlations. Since there was a large overlap between the two control groups, Bonferroni correction was applied after t-tests. All tests were two-tailed and P ≤ 0.025 was considered significant.
Results
TH-ir Was Increased in the LC of MDD but not BD Patients
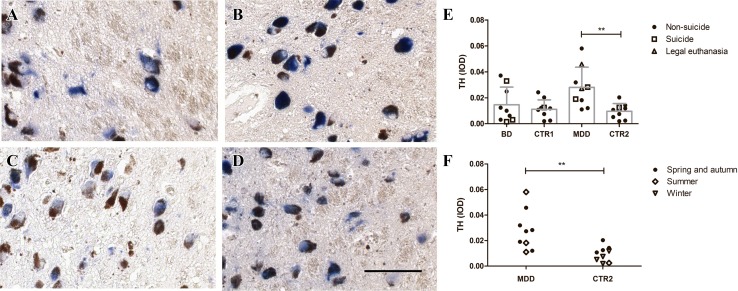
TH-ir was present in the cytoplasm and fibers of neurons containing neuromelanin, both in controls and mood disorder patients. The distribution and appearance of the TH-ir neurons in the mood disorder patients were similar to those of the control cases, but the intensity of the staining was higher in some of the MDD patients (Fig. 3A–D). The corrected IODs of LC TH-ir were significantly higher in the MDD group than in its controls (P = 0.008), while those in the BD group did not differ from their controls (P = 0.560). The TH-ir of the 3 BD patients who had attempted suicide or had suicidal ideation (mean 0.013, range 0.002–0.033) was within the range of the other BD patients who had not attempted suicide and had not had such ideation (n = 6, mean 0.016, range 0.003–0.037). In addition, the TH-ir of the 2 MDD patients who had attempted suicide or had suicidal ideation (mean 0.024, range 0.019–0.028) was within the range of the other MDD patients who had not attempted suicide and had not had ideation (n = 7, mean 0.029, range 0.011–0.058). Furthermore, when the 2 MDD patients who died of euthanasia were included in the suicide group, the TH-ir levels (n = 4, mean 0.030, range 0.019–0.045) did not differ from the other MDD patients (n = 5, mean 0.026, range 0.011–0.058; P = 0.599, Fig. 3E). In addition, the TH-ir level of the control (0.013) who died by refusing food and water was within the range of levels of the other controls (n = 10, mean 0.011, range 0.002–0.024, Fig. 3E). Moreover, there were no significant differences in TH-ir levels between the samples from participants who died during June–August and those who died in other months (for selection of these months see Discussion), either in the MDD group or its control group (Fig. 3F). Furthermore, there was no correlation between CSF pH and TH-ir, either in the BD group (r = 0.514, P = 0.238) or its control group (r = 0.457, P = 0.216). Lastly, with CSF pH as a covariant, there was no effect of BD group on the level of TH-ir (F(1,16) = 0.510, P = 0.510), although there was a trend for a lower CSF pH effect on TH-ir level (F(1,16) = 3.835, P = 0.074).
Fig. 3.
Tyrosine hydroxylase (TH) immunoreactivity (ir) in the locus coeruleus (LC) neurons of mood disorder patients. TH-ir (blue) was present in the cytoplasm of neurons containing neuromelanin (dark brown), both in controls (A and C) and in patients with major depressive disorder (MDD, B) or bipolar disorder (BD, D). TH-ir was significantly higher in the MDD group than in its control group, while there was no significant difference in TH-ir between the BD and control groups (E). Note that the TH-ir levels in patients who committed suicide were in the range of those who did not have suicidal ideation or attempts. In addition, the TH-ir levels of those who died in summer (Jul-Aug) showed no significant differences from those who died in the other seasons (F). The data are shown as mean + SD. IOD, integrated optical density; CTR1, control group for BD; CTR2, control group for MDD; **P < 0.01; scale bar, 200 μm.
No Significant Changes in the LC ErbB4-ir in MDD or BD Patients
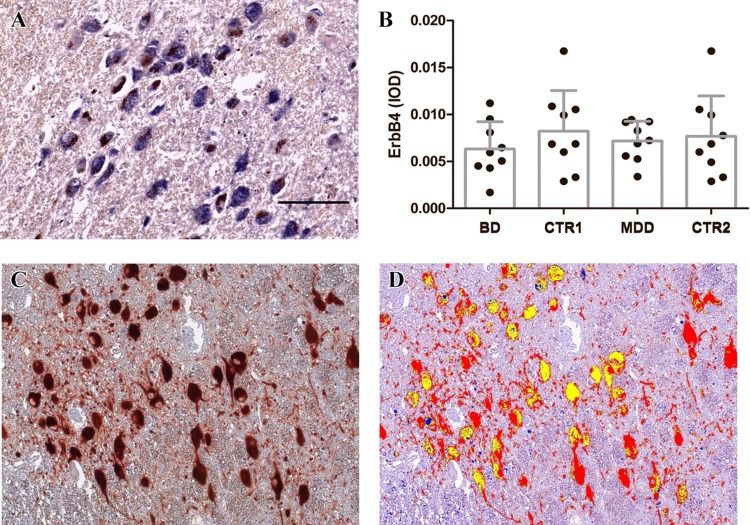
ErbB4-ir was present in the cytoplasm of the LC neurons containing neuromelanin, both in controls and mood disorder patients (Fig. 4A). The distribution and appearance of the ErbB4-ir neurons in mood disorder patients were similar to those of the controls.
Fig. 4.
ErbB4 immunoreactivity (ir) in the locus coeruleus (LC) neurons of mood disorder patients, and co-localization of tyrosine hydroxylase (TH)-ir and ErbB4-ir in the human LC. A ErbB4-ir (blue) was present in the cytoplasm in neurons containing neuromelanin (dark brown) both in controls and mood disorder patients. The distribution and appearance of the ErbB4-ir neurons in mood disorder patients were similar to those of controls. B There was no difference between the IODs of ErbB4-ir in MDD patients and their controls, or BD patients and their controls. C Original microscope image showing signals of TH-ir (red), ErbB4-ir (blue), and neuromelanin (brown). D After color separation, the TH-ir (red) and ErbB4-ir (blue) signals are presented in one image. Co-localization (yellow) was observed in a considerable number of LC neurons. The data are shown as mean + SD. IOD, integrated optical density; CTR1, control group for BD; CTR2, control group for MDD; scale bar, 200 μm.
There was no difference in the corrected IODs of LC ErbB4-ir between the MDD group and its controls (P = 0.761), or between the BD group and its controls (P = 0.289, Fig. 4B). There was no correlation between CSF pH and LC-ErbB4-ir, either in the BD group (r = 0.563, P = 0.188) or its control group (r = − 0.110, P = 0.779). In addition, there was no effect of BD (F(1,16) = 0.880, P = 0.367), or CSF pH (F(1,16) = 0.226, P = 0.643) on the level of ErbB4-ir. And the interaction between the BD group and CSF pH was not significant either (F(1,16) = 0.853, P = 0.374).
No correlation was found between LC-ErbB4-ir and LC-TH-ir in the pooled controls (n = 11, r = − 0.192, P = 0.572), the BD patients (n = 9, r = 0.116, P = 0.766), or the MDD patients (n = 9, r = − 0.217, P = 0.575).
Co-localization of TH and ErbB4 in the LC
ErbB4-ir and TH-ir showed clear co-localization in the LC of MDD and BD patients as well as controls (Fig. 4C and D).
Discussion
In the present study, we validated our method of quantitative immunocytochemical analysis of formalin-fixed paraffin-embedded postmortem human LC tissue by confirming that TH-ir was significantly elevated in the LC of MDD patients. This is in line with an earlier study [6], which was performed using an independent technique—protein extraction from LC punches. A novel finding was that there was no increase of TH-ir in BD patients. This indicates a different state of LC activity between these two subtypes of mood disorder, which may contribute to the differences in their clinical symptoms. It should, however, be noted that none of the BD patients in this study died in the manic stage. Thus, although it seems that the LC-TH-ir change is not a trait marker of BD, further study is warranted to determine whether LC-TH-ir changes in BD patients in the manic phase, if such material can be obtained. In addition, the method we used here allowed us to show that ErbB4-ir and TH-ir were clearly co-localized in the neuromelanin-containing neurons of the LC in MDD and BD patients as well as in controls.
The multispectral method we developed to untangle the two spectra of the immunocytochemical staining of a certain protein in the LC and the natural brown neuromelanin solved the inherent problem in immunocytochemical studies of the human LC, and may facilitate quantitative studies of specific proteins expressed in the LC. In addition, this method could be applied to immunocytochemical studies of other human brain areas that contain natural pigments, such as the substance nigra. Both structures are involved in several neurodegenerative disorders.
Baumann et al. (1999) found that the TH-ir level in the LC of suicidal depressed patients was higher than that in non-suicidal depressed patients [14]. Our limited data do not support this result, either in BD or MDD, since the data from suicidal depressed patients were in the same range as the depressed patients who did not commit suicide. However, it should be noted that the very small number of suicidal patients in our study does not allow a firm conclusion on this topic and calls for confirmation. It should also be noted that some studies found that people who die in summer (June to August) have a higher density of TH-expressing neurons in the midbrain than those who die in winter (November to January) [33]. Although we found that the average month of death in the MDD group was July while it was March in the control group, we did not find a significant difference in TH-ir levels between those who died during June to August and those who died in the other months, either in the MDD group or its control group (Fig. 3F). This deserves investigation of the biological rhythms of TH-ir in the LC.
To the best of our knowledge, we are the first to study the possible presence of alterations in LC ErbB4 in MDD and BD patients. Although ErbB4 co-localized with TH in human LC neurons, which is in accordance with the findings in rats [21] and suggests a possible role of ErbB4 in LC activity, we found no correlation between TH-ir and ErbB4-ir. In addition, we did not find any significant change in ErbB4-ir in MDD or BD patients. Further, a change in the LC similar to that reported in the ErbB4-/- mouse that showed manic-like behavior [26] was not found in the LC-ErbB4 levels in human mood disorders. However, several limitations should be noted here in relation to this issue. First, the human LC is much larger than that of a mouse. The LCs of both mouse and human are topographically organized, i.e. different parts of the LC project to different brain regions, performing different functions. For instance, neurons in the dorsal part of the LC project to the neocortex and hippocampus, those in the ventral part project to the spinal cord, basal ganglia, thalamus, hypothalamus, and cerebellum [34, 35], those in the rostral part project to the forebrain, and those in the caudal part project to the spinal cord [35]. Since we used mid-level LC sections, the ErbB4 expression might not reflect that in the whole LC but may be present solely in a subarea that is more associated with BD symptoms. The presence of possible region-specific alterations in ErbB4-ir in the LC still needs further study. Second, ErbB4 is phosphorylated after binding to its ligand NRG1, followed by activation of signaling pathways [2, 20]. Previous studies have shown that chronic unpredictable mild stress in rats, a model for depression, elevates phosphorylated-ErbB4 and NRG1 in the prefrontal cortex and decreases phosphorylated-ErbB4 and NRG1 in the hippocampus [20]. This indicates that phosphorylated-ErbB4 in the LC in mood disorders deserves further study.
One of the inherent potential confounding factors in a postmortem study is medication use. Indeed, one animal study has shown that the selective serotonin reuptake inhibitors (SSRIs), which were used by some MDD patients in the present study, delay the TH-ir decrease in rat LC after chronic stress [36]. However, our study showed that the LC TH-ir levels were increased in MDD patients, and the levels in those who used SSRIs (n = 2, mean = 0.024, range 0.019–0.028) were in the range of those who did not (n = 7, mean = 0.029, range 0.011–0.058). In addition, animal studies have shown that benzodiazepines, for example olanzapine, may elevate LC TH-ir [37, 38]. However, we do not think that our main conclusion is confounded by these drugs since the TH-ir levels in BD patients who had used benzodiazepines (n = 4, mean = 0.011, range 0.002–0.033) did not differ from those of the other BD patients who had not used such drugs (n = 5, mean = 0.018, range 0.003–0.037, P = 0.286). In addition, the TH-ir levels of two MDD patients who had used benzodiazepines (mean = 0.030, range 0.027–0.032) did not differ from those who had not (n = 7, mean = 0.027, range 0.011–0.058). Lithium was used by many patients in our study (Table 1). However, it has been reported that lithium does not affect TH-mRNA levels in the ventral tegmental area in rats [39]. Moreover, anti-psychotic medication has been reported to decrease CSF-NE levels in BD patients in the manic stage, who then change to the depressive stage [40, 41]. In our study, the LC TH-ir in BD patients who used anti-psychotic drugs (n = 5, mean = 0.014, range 0.003–0.033) did not significantly differ from those who had not (n = 4, mean = 0.016, range 0.003–0.037, P = 0.850). Furthermore, the LC TH-ir of MDD patients who had used anti-psychotic drugs (n = 2, mean = 0.012, range 0.011–0.012) was below the range of those who had not (n = 7, mean = 0.033, range 0.018–0.058), so if they had an effect it would lead to an underestimation of the increase in TH-ir. For ErbB4, animal studies with drugs are not available.
Conclusions
The multispectral method allowed us to quantify the immunocytochemical changes in the neuromelanin-containing neurons of the LC. In addition, we established with this method the co-localization of TH and ErbB4 in these neurons in the human LC. The LC was found to be significantly activated in MDD but not BD patients, which may contribute to the difference in clinical symptoms between them. ErbB4 changes were not found in the human LC, but samples from patients who died in a manic state were not available.
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFC1306700) and the National Natural Science Foundation of China (91332102 and 31271130). We thank Mr. Bart Fisser, Hugo McGurran, Ling Shan, and Michiel Kooreman for technical support, Prof. Xiao-Ming Li for insightful comments, and Wilma Verweij for secretarial assistance.
References
- 1.Kocsis RN. Book Review: Diagnostic and Statistical Manual of Mental Disorders: fifth edition (DSM-5) Int J Offender Ther Comp Criminol. 2013;57:1546–1548. doi: 10.1177/0306624X13511040. [DOI] [Google Scholar]
- 2.Wang N, Zhang GF, Liu XY, Sun HL, Wang XM, Qiu LL, et al. Downregulation of neuregulin 1-ErbB4 signaling in parvalbumin interneurons in the rat brain may contribute to the antidepressant properties of ketamine. J Mol Neurosci. 2014;54:211–218. doi: 10.1007/s12031-014-0277-8. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Zhu Y, Zhu Y, Wu S, Liu H, Zhang W, et al. Molecular, functional, and structural imaging of major depressive disorder. Neurosci Bull. 2016;32:273–285. doi: 10.1007/s12264-016-0030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao AM, Ruhe HG, Gao SF, Swaab DF. Neurotransmitters and neuropeptides in depression. Handb Clin Neurol. 2012;106:107–136. doi: 10.1016/B978-0-444-52002-9.00008-5. [DOI] [PubMed] [Google Scholar]
- 5.Chandley MJ, Szebeni K, Szebeni A, Crawford J, Stockmeier CA, Turecki G, et al. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J Psychiatry Neurosci. 2013;38:276–284. doi: 10.1503/jpn.120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, et al. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–1286. doi: 10.1016/S0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 7.Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Sigitova E, Fisar Z, Hroudova J, Cikankova T, Raboch J. Biological hypotheses and biomarkers of bipolar disorder. Psychiatry Clin Neurosci. 2017;71:77–103. doi: 10.1111/pcn.12476. [DOI] [PubMed] [Google Scholar]
- 9.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 10.Wiste AK, Arango V, Ellis SP, Mann JJ, Underwood MD. Norepinephrine and serotonin imbalance in the locus coeruleus in bipolar disorder. Bipolar Disord. 2008;10:349–359. doi: 10.1111/j.1399-5618.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Kitayama I, Nomura J. Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci. 1998;62:2083–2092. doi: 10.1016/S0024-3205(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 12.Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M, Sabban EL. Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry. 1999;45:853–862. doi: 10.1016/S0006-3223(98)90360-2. [DOI] [PubMed] [Google Scholar]
- 13.Sved AF, Cano G, Passerin AM, Rabin BS. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiol Behav. 2002;77:737–742. doi: 10.1016/S0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- 14.Baumann B, Danos P, Diekmann S, Krell D, Bielau H, Geretsegger C, et al. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is reduced in depressed non-suicidal patients but normal in depressed suicide patients. Eur Arch Psychiatry Clin Neurosci. 1999;249:212–219. doi: 10.1007/s004060050089. [DOI] [PubMed] [Google Scholar]
- 15.Gos T, Krell D, Bielau H, Brisch R, Trubner K, Steiner J, et al. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is elevated in violent suicidal depressive patients. Eur Arch Psychiatry Clin Neurosci. 2008;258:513–520. doi: 10.1007/s00406-008-0825-8. [DOI] [PubMed] [Google Scholar]
- 16.Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan GH, Liu YY, Hu XL, Yin DM, Mei L, Xiong ZQ. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2011;15:258–266. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- 19.Woo RS, Lee JH, Yu HN, Song D, Baik TK. Expression of ErbB4 in the apoptotic neurons of Alzheimer’s disease brain. Anat Cell Biol. 2010;43:332–339. doi: 10.5115/acb.2010.43.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang R, Cai H, Zhang L, Liang D, Lv C, Guo Y, et al. Dysregulation of Neuregulin-1/ErbB signaling in the prefrontal cortex and hippocampus of rats exposed to chronic unpredictable mild stress. Physiol Behav. 2016;154:145–150. doi: 10.1016/j.physbeh.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 22.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 23.Joshi D, Fullerton JM, Weickert CS. Elevated ErbB4 mRNA is related to interneuron deficit in prefrontal cortex in schizophrenia. J Psychiatr Res. 2014;53:125–132. doi: 10.1016/j.jpsychires.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Chen J, Huang K, Ji W, Wang T, Li T, et al. Analysis of association between common SNPs in ErbB4 and bipolar affective disorder, major depressive disorder and schizophrenia in the Han Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:17–21. doi: 10.1016/j.pnpbp.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Goes FS, Rongione M, Chen YC, Karchin R, Elhaik E, Potash JB. Exonic DNA sequencing of ERBB4 in bipolar disorder. PLoS One. 2011;6:e20242. doi: 10.1371/journal.pone.0020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao SX, Zhang Y, Hu XY, Hong B, Sun P, He HY, et al. ErbB4 deletion in noradrenergic neurons in the locus coeruleus induces mania-like behavior via elevated catecholamines. Elife 2018, 7. [DOI] [PMC free article] [PubMed]
- 27.Zhu M, Klimek V, Haycock JW, Ordway GA. Quantitation of tyrosine hydroxylase protein in the locus coeruleus from postmortem human brain. J Neurosci Methods. 2000;99:37–44. doi: 10.1016/S0165-0270(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 28.van de Nes JA, Kamphorst W, Ravid R, Swaab DF. Comparison of beta-protein/A4 deposits and Alz-50-stained cytoskeletal changes in the hypothalamus and adjoining areas of Alzheimer’s disease patients: amorphic plaques and cytoskeletal changes occur independently. Acta Neuropathol. 1998;96:129–138. doi: 10.1007/s004010050872. [DOI] [PubMed] [Google Scholar]
- 29.Hagihara H, Catts VS, Katayama Y, Shoji H, Takagi T, Huang FL, et al. Decreased brain pH as a shared endophenotype of psychiatric disorders. Neuropsychopharmacology. 2018;43:459–468. doi: 10.1038/npp.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karolewicz B, Johnson L, Szebeni K, Stockmeier CA, Ordway GA. Glutamate signaling proteins and tyrosine hydroxylase in the locus coeruleus of alcoholics. J Psychiatr Res. 2008;42:348–355. doi: 10.1016/j.jpsychires.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snow DM, Carman HM, Smith JD, Booze RM, Welch MA, Mactutus CF. Cocaine-induced inhibition of process outgrowth in locus coeruleus neurons: role of gestational exposure period and offspring sex. Int J Dev Neurosci. 2004;22:297–308. doi: 10.1016/j.ijdevneu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 32.van der Loos CM. Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J Histochem Cytochem. 2008;56:313–328. doi: 10.1369/jhc.2007.950170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aumann TD, Raabus M, Tomas D, Prijanto A, Churilov L, Spitzer NC, et al. Differences in number of midbrain dopamine neurons associated with summer and winter photoperiods in humans. PLoS One. 2016;11:e0158847. doi: 10.1371/journal.pone.0158847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 35.Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73:273–283. doi: 10.1016/S0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Asakura M, Kanai S, Tanaka D, Hishinumai T, Nagashima H. Effect of concurrent treatment of SSRI on the tyrosine hydroxylase immunoreactivity in the rat locus coeruleus treated with chronic variable stress. Nihon Shinkei Seishin Yakurigaku Zasshi. 2004;24:21–27. [PubMed] [Google Scholar]
- 37.Ordway G, Szebeni K. Effect of repeated treatment with olanzapine or olanzapine plus fluoxetine on tyrosine hydroxylase in the rat locus coeruleus. Int J Neuropsychopharmacol. 2004;7:321–327. doi: 10.1017/S1461145704004468. [DOI] [PubMed] [Google Scholar]
- 38.Verma V, Rasmussen K, Dawe GS. Effects of short-term and chronic olanzapine treatment on immediate early gene protein and tyrosine hydroxylase immunoreactivity in the rat locus coeruleus and medial prefrontal cortex. Neuroscience. 2006;143:573–585. doi: 10.1016/j.neuroscience.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Ferrie L, Young AH, McQuade R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int J Neuropsychopharmacol. 2006;9:729–735. doi: 10.1017/S1461145705006243. [DOI] [PubMed] [Google Scholar]
- 40.Kurita M. Noradrenaline plays a critical role in the switch to a manic episode and treatment of a depressive episode. Neuropsychiatr Dis Treat. 2016;12:2373–2380. doi: 10.2147/NDT.S109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manji HK, Quiroz JA, Payne JL, Singh J, Lopes BP, Viegas JS, et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- 42.Braak H, Braak E. Neuropathological staging of Alzheimer’s disease-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]