Abstract
Major depression disorder, also known as depression, with a significant and persistent low mood as the main clinical features, is the main type of mood disorders. L-menthol (LM), the main active ingredient of mint, has been considered as safe and healthy natural ingredient by the Food and Drug Administration in the USA. In this study, LM (40 mg/kg, i.g.) produced antidepressant-like effect in the forced swimming test (FST) in mice. The sub-effective dose (5 mg/kg, i.g.) of LM combined with the sub-effective dose of fluoxetine (5 mg/kg, i.p.) or reboxetine (2.5 mg/kg, i.p.) could significantly shorten the immobility time in the FST. Pretreatment with ondansetron (a highly selective 5-HT3 receptor antagonist, 8 mg/kg, i.p.), bicuculline [a competitive γ-aminobutyric acid (GABA) antagonist, 4 mg/kg, i.p.] and haloperidol (a non-selective D2 receptor antagonist, 0.2 mg/kg, i.p.) significantly reversed the antidepressant-like effect of LM (40 mg/kg, i.g.). In contrast, prazosin (a α1-adrenoceptor antagonist, 1 mg/kg, i.p.) and N-methyl-d-aspartic acid (an agonist at the glutamate site, 75 mg/kg, i.p.) did not eliminate the antidepressant-like effect of LM. All of these above indicated that LM is able to induce an antidepressant-like effect mediated by the modification of 5-HTergic, GABAergic and DAergic systems in the FST. LM might be used as combination therapy in depressed patients and is a potential antidepressant.
Keywords: L-menthol, Antidepressant-like, Forced swimming test, Monoamine neurotransmitter
Introduction
Major depression disorder (MDD), also known as depression, with a significant and persistent low mood as the main clinical features, is the main type of mood disorders. MDD is a serious threat to human health due to its high prevalence, high recurrence rate, high disability, and high suicide rate. Depression brings pain to the patient and brings a great financial burden to society (Lepine and Briley 2011). On a global scale, the losses caused by depression have ranked second in all diseases. By 2030, the disease burden of depression will rank first (Ng et al. 2014). Therefore, scientific research related to the prevention and treatment of depression has become an important topic of concern. So far, the pathogenesis of depression is not entirely clear, the dominant point of view is the brain monoamine neurotransmitter metabolic disorders theory (Marecos et al. 2014). Drug therapy is the most common and important treatment for emotional disorders, especially MDD. The clinical commonly used antidepressants were usually divided into tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (NARIs) according to their chemical structure and mechanism of action (Sultana et al. 2014). Unfortunately, the efficacy of these drugs is unsatisfactory and often produces multiple unwanted side effects. So it is urgent to find a safe and effective antidepressant.
Mint, the dried aerial part of the Lamiaceae plant Mentha haplocalyx Briq., is cool-natured, acrid flavour, and enters the meridians of lungs and liver in the theory of Traditional Chinese Medicine (TCM). In the Tang Dynasty (Chinese), mint as a drug with dispersing stagnated liver “qi” which is defined as the motive force of life activity in TCM, was recorded in the “Xin Xiu Ben Cao”. The Chinese medicine theory displays that the pathogenesis of depression is the stagnation of liver qi. Treatment of depression should be based on disperse and rectify the depressed liver qi. L-menthol (LM) is the main active ingredient of the mint, and its content is up to 87% in the essential oil of mint (Lin et al. 2002). The previous study showed that LM could promote mouse activity, which might be associated with dopamine (Henderson et al. 2017). Therefore, this study would initially determine the antidepressant-like effect of LM and the mechanisms of action in a mice experimental model.
Methods and materials
Animal and agents
Male ICR mice, weighing 18–22 g, were provided by Changchun Yisi Experimental Animal Technology Co., Ltd. with certificate of quality no. SCXK 2016-0003 (Changchun, China). The mice were fed a standard diet in a controlled environment under a normal circadian rhythm at 25 ± 1 °C and 55 ± 5% humidity. All experiments were carried out in accordance with the Guide for Animal Experimentation of Jilin Agricultural University.
LM (purity 99.2%) was purchased from Macklin (Shanghai, China); PBS was from Hyclone (Beijing, China). Fluoxetine (FLU, an antidepressant drug belonging to 5-HT reuptake inhibitor), reboxetine (an antidepressant drug belonging to NE reuptake inhibitors) and tianeptine (an antidepressant drug belonging to TCAs), ondansetron (a highly selective 5-HT3 receptor antagonist), prazosin (α1-adrenoceptor antagonist), haloperidol (a non-selective D2 receptor antagonist), bicuculline (a competitive GABA antagonist) and N-methyl-d-aspartic acid (NMDA, an agonist at the glutamate site) were from Sigma (St. Louis, MO, USA). The detecting ELISA kits of 5-HT, DA, GABA, Glu and NE were from R&D Systems, Ltd. (Minneapolis, USA). All other reagents used in this study were of analytical grade.
The spontaneous locomotor activity test (SLT)
In order to exclude the possibility that the alteration in the immobility time in the forced swimming test (FST) was due to interference of the locomotor activity, spontaneous locomotor activity of each mouse was observed in a ZZ-6 mouse autonomic activity test instrument (Shanghai benefits of the medical equipment Development Co., Ltd., Shanghai, China). The total voluntary activity times of each mouse were measured within 20 min. The experimental video analysis system was placed in a sound attenuated testing room (Kamei et al. 1994; Tozzi et al. 2018; Yamagata et al. 2018).
The forced swimming test (FST)
After 60 min of a single-dose drug treatment, the mice were placed in a glassware (20 cm high, 14 cm in diameter), containing 15 cm of fresh water at 25 °C. After an initial 2 min period of vigorous activity, each animal assumed a typical immobile posture. A mouse was immobile when it remained floating in the water without struggling, making only minimum movements of its limbs necessary to keep its head above water. The total duration of immobility was recorded during the next 4 min of a total 6 min test. The changes in immobility duration were studied after administering drugs in separate groups of mice. Each mouse was used only once (Porsolt et al. 1977; Hiraoka et al. 2017).
Drug treatment
LM and other medicines were dissolved in physiological saline (0.9% NaCl aq) immediately before use. The administration operators were blind to the drugs including the vehicles, the tested materials, antidepressant drugs and receptor antagonists and agonists. The observers were also blind to the drug treatment. According to the existing experimental protocol (Lin et al. 2017; Palucha-Poniewiera et al. 2017; Bian et al. 2018), specific tests were arranged as follows.
The effect of LM on locomotor activity in the SLT
After 30 min of a single-dose LM (1 mg/kg, 5 mg/kg, 20 mg/kg, 40 mg/kg and 80 mg/kg, i.g., respectively) administration, mice were placed in the autonomous instrument. The times of autonomous activities were evaluated over a 20-min period. Each animal was used only once.
The antidepressant-like effect of LM in the FST
The mice of blank control group, LM groups (1 mg/kg, 5 mg/kg, 20 mg/kg, 40 mg/kg and 80 mg/kg, i.g., respectively) were administrated and the immobility period was recorded after 60 min of a single-dose administration. The mice of FLU group were administered with a single 20 mg/kg of FLU by intraperitoneal (i.p.) injection 60 min prior to the FST.
The antidepressant-like effect of co-administration of LM (5 mg/kg) and antidepressant drugs in the FST
Positive drug groups (sub-effective doses): the mice were administered with a single-dose of FLU (5 mg/kg, i.p.), tianeptine (15 mg/kg, i.p.) and reboxetine (2.5 mg/kg, i.p.) 60 min prior to the FST, respectively. Positive drug + LM groups (sub-effective doses): the mice were administered with a single-dose of FLU (5 mg/kg, i.p.), tianeptine (15 mg/kg, i.p.) and reboxetine (2.5 mg/kg, i.p.) immediately after the administration of a single-dose of LM (5 mg/kg, i.g.) 60 min prior to the FST. And all mice were performed the FST after 60 min.
The roles of different central nervous system (CNS) functions in the antidepressant-like effect of LM (40 mg/kg) in the FST
Ondansetron group: the mice were administered with a single-dose of ondansetron (8 mg/kg, i.p.) 60 min prior to the FST. LM + ondansetron group: the mice were administered a single-dose of ondansetron (8 mg/kg, i.p.) 30 min prior to a single-dose of LM (40 mg/kg, i.g.) treatment. And all mice were performed the FST after 60 min.
Prazosin group: the mice were administered with a single-dose of prazosin (1.0 mg/kg, i.p.) 60 min prior to the FST. LM + prazosin group: the mice were administered with a single-dose of prazosin (1.0 mg/kg, i.p.) 30 min prior to a single-dose of LM (40 mg/kg, i.g.) treatment. And all mice were performed the FST after 60 min.
Bicuculline group: the mice were administered with a single-dose of bicuculline (4.0 mg/kg, i.p.) 60 min prior to the FST. LM + bicuculline group: the mice were administered with a single-dose of bicuculline (4 mg/kg, i.p.) 30 min prior to a single-dose of LM (40 mg/kg, i.g.) treatment. And all mice were performed the FST after 60 min.
Haloperidol group: the mice were administered with a single-dose of haloperidol (0.2 mg/kg, i.p.) 60 min prior to the FST. LM + haloperidol group: the mice were administered with a single-dose of haloperidol (0.2 mg/kg, i.p.) 30 min prior to a single-dose of LM (40 mg/kg, i.g.) treatment. And all mice were performed the FST after 60 min.
NMDA group: the mice were administered with a single-dose of NMDA (75 mg/kg, i.p.) 60 min prior to the FST. LM + NMDA: the mice were administered with a single-dose of NMDA (75 mg/kg, i.p.) 30 min prior to a single-dose of LM (40 mg/kg, i.g.) treatment. And all mice were performed the FST after 60 min.
Biochemical measurements
After the FST, all mice were sacrificed by cervical dislocation, and the brains were harvested for biochemical analysis. The mice brain tissues were washed with ice-cold physiological saline, weighed the weight, added 9 times of cold PBS (pH 7.4) and homogenized, shaken for 10 s and centrifuged at 12,000 rmp for 5 min at 4 °C. The supernatants were collected for the detection of 5-HT, DA, NE, GABA, Glu contents by ELISA kits.
Statistical analysis
All experimental data were expressed as mean ± SEM. The data were analyzed using the t test and one-way analysis of variance (ANOVAs), followed by Tukey’s post-hoc multiple comparison test. A value of p < 0.05 was considered statistically significant for analysis.
Results
Effect of LM on the locomotor activity
The results of the SLT are shown in Fig. 1. After a single-dose of LM (1 mg/kg, 5 mg/kg, 20 mg/kg, 40 mg/kg and 80 mg/kg, i.g., respectively) administration, there were no significant changes in the number of activities and standing times of mice in the LM treatments compared with those in the blank control group. It showed the LM treatment did not affect the spontaneous activity of mice. Therefore, the false positive effect in the behavioral despair tests could be ruled out.
Fig. 1.
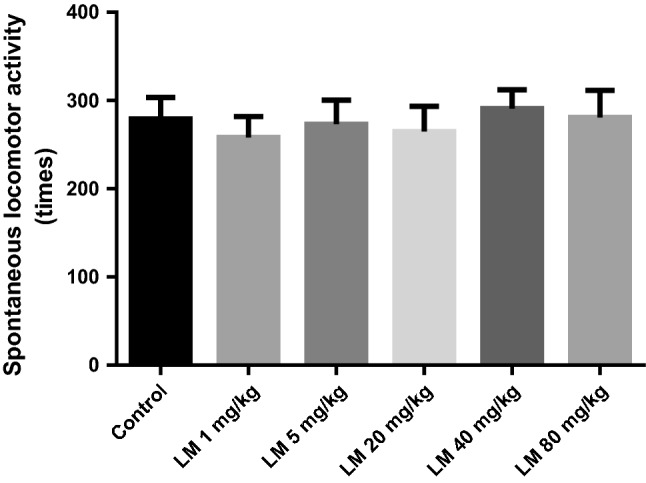
Effects of LM on spontaneous locomotor activity. The values are represented as the mean ± SEM (n = 10). Data were analyzed by t test and one-way ANOVAs
Effects of LM on immobility time in the FST
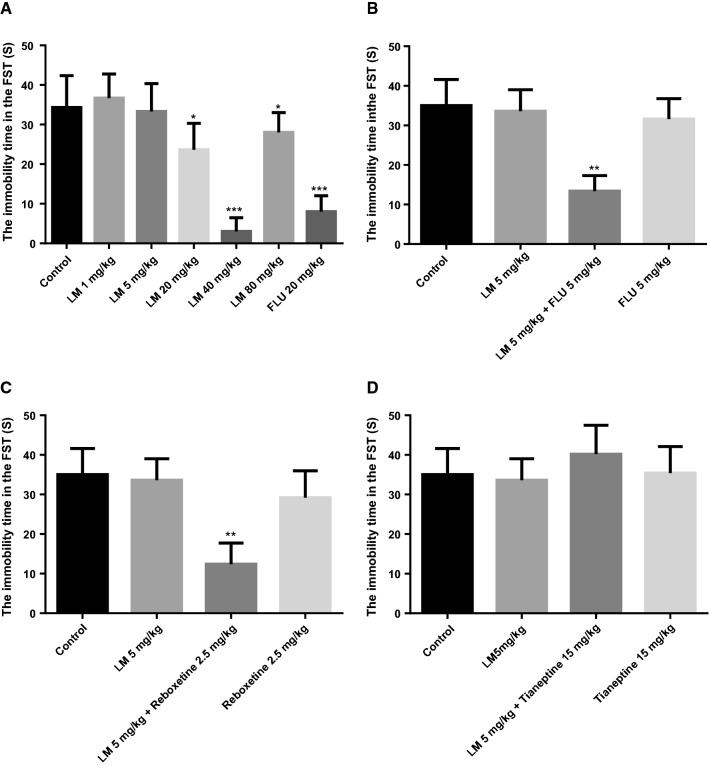
The change in immobility time of mice in the FST is a common indicator of the success of animal behavioral despair model and has been used for rapid screening and evaluation of antidepressants. As revealed in Fig. 2a, compared with the blank control group, a single-dose administration of LM (20 mg/kg, 40 mg/kg and 80 mg/kg, i.g., respectively) significantly reduced the immobility time (F (1, 18) = 38.585, p < 0.01 or F (1, 18) = 1.347, p < 0.05); and the 40 mg/kg of LM treatment exhibited the strongest activity; but 1 mg/kg and 5 mg/kg of LM treatment did not display effect on immobility time. Therefore, we selected 5 mg/kg and 40 mg/kg as the sub-effective dose and the effective dose, respectively.
Fig. 2.
a Effects of different doses of LM on the immobility time in the FST, b the antidepressant-like effect of the co-administration of sub-effective dose of LM (5 mg/kg, i.g.) with fluoxetine (5 mg/kg, i.p.) in the FST, c the antidepressant-like effect of the co-administration of sub-effective dose of LM (5 mg/kg, i.g.) with reboxetine (2.5 mg/kg, i.p.) in the FST, d the antidepressant-like effect of the co-administration of sub-effective dose of LM (5 mg/kg, i.g.) with tianeptine (15 mg/kg, i.p.) in the FST. The values were represented as the mean ± SEM (n = 10). Data were analyzed by t test and one-way ANOVAs, followed by Tukey’s post hoc test. ***p < 0.001; **p < 0.01; *p < 0.05 compared with the blank control group
The antidepressant-like effect of co-administration of the sub-effective doses of LM (5 mg/kg) and antidepressant drugs in the FST
The results of co-administration in the FST were shown in Fig. 2b–d. As revealed in Fig. 2b, in the FST, compared with that of the blank control group, the sub-effective dose of FLU (5 mg/kg, i.p.) or the sub-effective dose of LM (5 mg/kg, i.g.) could not reduce the immobility time, while the co-administration of the sub-effective dose of FLU (5 mg/kg, i.p.) and LM (5 mg/kg, i.g.) led to a significant reduction in the immobility time (F (1, 18) = 39.673, p < 0.01).
As revealed in Fig. 2c, the results showed that LM (5 mg/kg, i.g.) or reboxetine (2.5 mg/kg, i.p.) administered alone exhibited no significant effect on the immobility time, while the co-administration of sub-effective doses of reboxetine (2.5 mg/kg, i.p.) and LM (5 mg/kg, i.g.) could significantly reduce immobility time compared with the blank control group (F (1, 18) = 35.568, p < 0.01) in the FST.
Figure 2d revealed that the co-administration of sub-effective doses of LM (5 mg/kg, i.g.) and tianeptine (15 mg/kg, i.p.) could not reduce the immobility time compared with that of the blank control group in the FST. The results showed there was no difference for LM treatment, tianeptine administration, co-administration of tianeptine and LM, and the blank control.
The roles of different CNS functions in the antidepressant-like effect of LM (40 mg/kg) in the FST
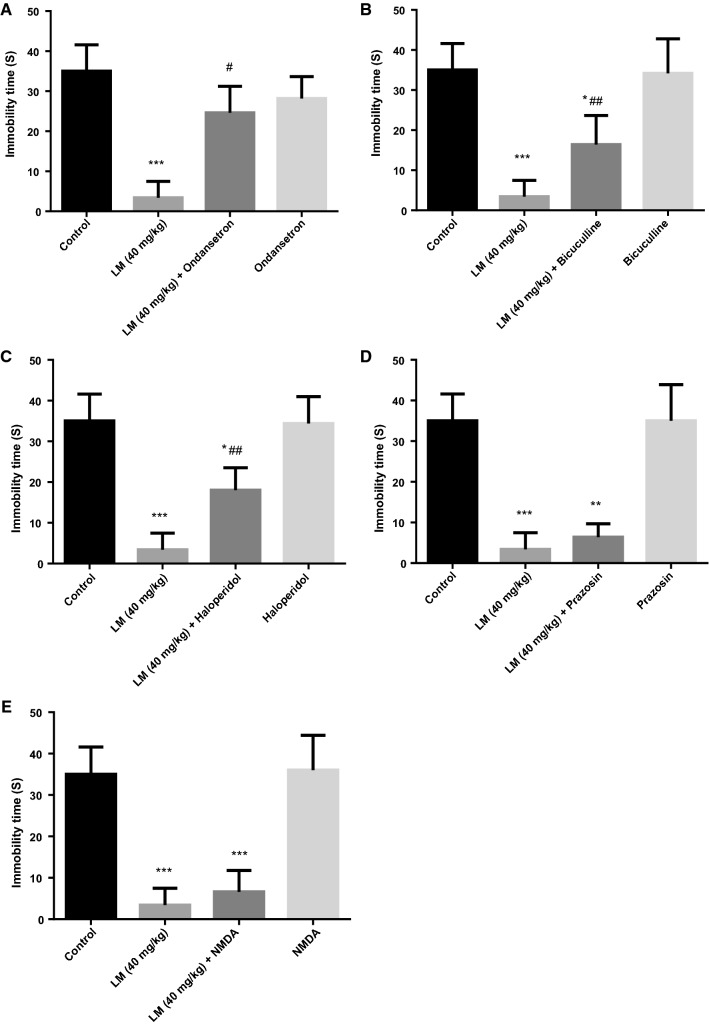
As shown in Fig. 3a–c, compared with the LM (40 mg/kg, i.g.) administered alone, LM (40 mg/kg, i.g.) + ondansetron or LM (40 mg/kg, i.g.) + bicuculline or LM (40 mg/kg, i.g.) + haloperidol could significantly increase the immobility time (F (1, 18) = 38.019, p < 0.01; F (1, 18) = 15.115, p < 0.01; F (1, 18) = 23.721, p < 0.01), which indicated that the ondansetron or bicuculline or haloperidol partly eliminated the antidepressant-like effect of LM.
Fig. 3.
a Effects of pre-treatment with ondansetron (8 mg/kg, i.p.) on the antidepressant-like effect induced by LM (40 mg/kg, i.g.) in the FST, b effects of pre-treatment with bicuculline (4 mg/kg, i.p.) on the antidepressant-like effect induced by LM (40 mg/kg, i.g.) in the FST, c effects of pre-treatment with haloperidol (0.2 mg/kg, i.p.) on the antidepressant-like effect induced by LM (40 mg/kg, i.g.) in the FST, d effects of pre-treatment with prazosin (1 mg/kg, i.p.) on the antidepressant-like effect induced by LM (40 mg/kg, i.g.) in the FST, e effects of pre-treatment with NMDA (75 mg/kg, i.p) on the antidepressant-like effect induced by LM (40 mg/kg, i.g) in the FST; The values were represented as the mean ± SEM (n = 10 in each group). Data were analyzed by t test and one way ANOVAs, followed by Tukey’s post hoc test. ***p < 0.001; **p < 0.01; *p < 0.05 compared with the blank control group; ##p < 0.01; #p < 0.05 compared with LM group
As shown in Fig. 3d, e, LM (40 mg/kg, i.g.) + prazosin or LM (40 mg/kg, i.g.) + NMDA compared with the blank group exhibited a significant difference, but there was no difference compared with LM (40 mg/kg, i.g.), which indicated that prazosin and NMDA did not reduce the antidepressant-like effect of LM.
Effects of LM on the levels of 5-HT, GABA, DA, NE and Glu in mice exposed to the FST
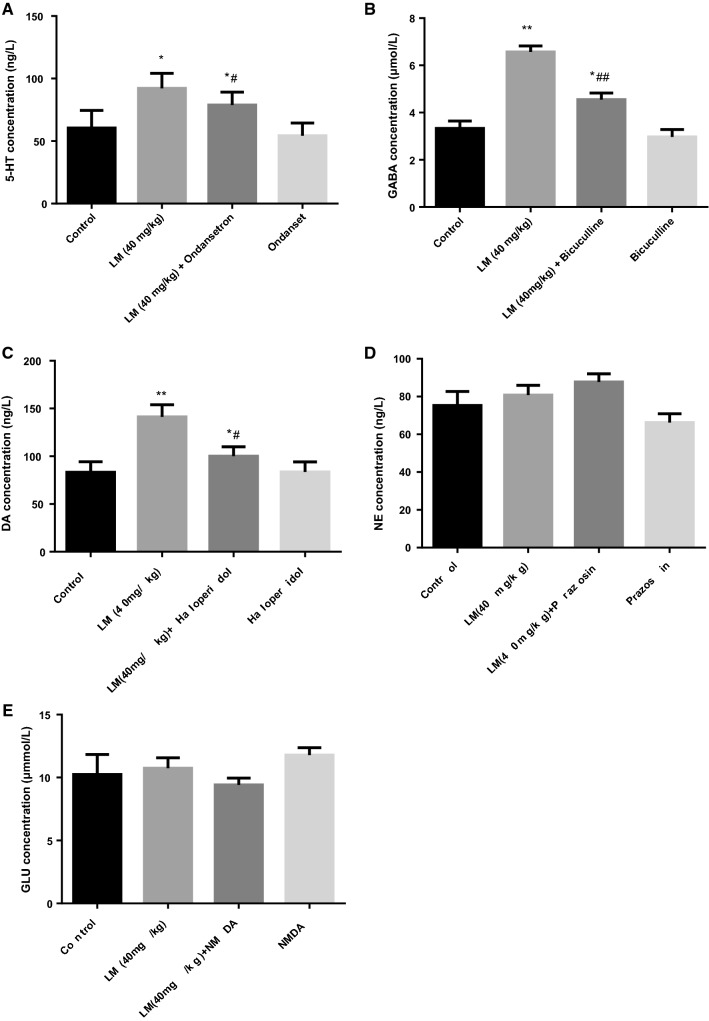
As revealed in Fig. 4a–c, compared with the blank control group, the levels of 5-HT, GABA and DA were evidently increased in the LM (40 mg/kg) group (F (1, 18) = 5.929, p < 0.05; F (1, 18) = 131.183; p < 0.01; F (1, 18) = 23.663, p < 0.01) and co-treatment group (F (1, 18) = 2.196, p < 0.05; F (1, 18) = 16.950; p < 0.01; F (1, 18) = 2.572, p < 0.05). Meanwhile, compared with the ondansetron group, bicuculline group and haloperidol group, the levels of 5-HT, GABA and DA evidently increased in the co-treatment group (F (1, 18) = 5.704, p < 0.05; F (1, 18) = 28.064; p < 0.01; F (1, 18) = 2.592, p < 0.05). However, as revealed in Fig. 4d, e, compared with the blank control group, LM (40 mg/kg) + prazosin group or LM (40 mg/kg) + NMDA group showed no difference; and there was no difference between the co-treatment groups and the prazosin group or NMDA group.
Fig. 4.
Effects of LM, ondansetron, bicuculline, haloperidol, prazosin, NMDA and co-treatments on the 5-HT (a), GABA (b), DA (c), NE (d) and Glu (e) levels in the brains of mice exposed to the FST. The values were represented as the mean ± SEM (n = 10 in each group). Data were analyzed by t test and one-way ANOVAs, followed by Tukey’s post hoc test., *p < 0.05; **p < 0.01 compared with control group; #p < 0.05; ##p < 0.01 compared with ondansetron (a), bicuculline (b) and haloperidol (c)
Discussion
The FST is one of the most widely used behavioral despair models to assess the antidepressant activity of drugs (Mezadri et al. 2011; Pesarico et al. 2014). To exclude the possibility that the alteration in the immobility time in the FST was due to interference of the locomotor activity, the mice were subjected to the locomotor activity test (Bian et al. 2018). In this study, it was indicated that LM exhibited antidepressant-like effects. The previous studies showed that the co-administration with antidepressant drugs might produce a better effect or faster onset speed than mono-therapy (Tardito et al. 2012; Zhang et al. 2014). This study indicated that the combination of LM and reboxetine, as well as LM and FLU showed synergistic effects, while LM and tianeptine did not display this performance in combination. As we known, FLU works as 5-HT reuptake inhibitor, and reboxetine acts as NE reuptake inhibitor, while tianeptine (an antidepressant drug belonging to TCAs) act as an atypical agonist of the μ-opioid receptor with clinically negligible effects on the δ- and κ-opioid receptors (Gassaway et al. 2014); obviously, these three antidepressant drugs have different mechanisms of action. Based on the results of this study, it can be speculated that the mechanism of action of LM is closer to those of FLU and reboxetine.
Depression is a complex brain disease. Epidemiological studies show that the risk of suffering from depression about 40–50% is caused by genetic causes (Ikeda et al. 2002; Belmaker and Agam 2008). At present, people have determined the psychological, biological, social environment and other factors involved in the pathogenesis of depression, and these factors do not work alone. So far, the pathogenesis of depression is not very clear; the researchers put forward a variety of hypothesis since 1965. Some studies have found that NE, 5-HT, DA and other monoamine neuron function is insufficient induced the occurrence of depression (Mayberg 2003; Rajkowska 2003). In addition, the researchers also found that some other neurotransmitters, such as acetylcholine and Glu, are closely related to the occurrence of depression (Walker et al. 2013). Monoamine hypothesis is gradually recognized as one of the most important hypotheses about the pathogenesis of depression. The monoamine hypothesis indicates that depression is due to a low level of monoamine neurotransmitters in the brain.
5-HT3 is a ligand-gated ion channel receptor. Studies have shown that 5-HT3 closely related to the pathogenesis of many diseases, such as mood disorders, cognitive dysfunction, bowel syndrome, addiction and so on (Walstab et al. 2010; Guo et al. 2018). The mice who knocked out 5-HT3 genes showed anxiolytic behavior, 5-HT3 antagonists have also shown a series of anxiolytic effects (Thompson and Lummis 2005). Expression of 5-HT3 in the hippocampus has been shown to be negatively correlated with the incidence of depression in women, that is to say, the expression of 5-HT3 is involved in the regulation of mood (Krzywkowski et al. 2008). Ondansetron is a highly selective 5-HT3 receptor antagonist, ondansetron can also bind to 5-HT1 or 5-HT2 receptor, but the affinity with the 5-HT3 receptor is 250–500 times that with other receptors. In this study, ondansetron could partially block the antidepressant-like activity of LM, and partly curb the increase of 5-HT level in the brain caused by LM, which indicated that the antidepressant-like activity of LM might participate in the serotonergic system.
GABA is the most important inhibitory neurotransmitter in the CNS of mammals; approximately 50% of neurotransmitters in the CNS are GABA (Salat et al. 2013). In the brain, GABA acts primarily as an inhibitory neurotransmitter (Tóth et al. 2018). Central GABA levels and GABA receptor dysfunction are associated with epilepsy, depression and mental illness (Frisardi et al. 2011; Hao et al. 2018). Studies of depression in animal models have shown that GABA is significantly reduced in experimental animals in the chronic stress depression model, forced swimming, and other animal models; and supplement action of GABA can alleviate depression-like behavior (Sherman and Petty 1980; Borsini et al. 1988; Gronli et al. 2007; Machado-Vieira et al. 2009). In this study, bicuculline (a competitive GABA antagonist) partially blocked the antidepressant-like activity of LM, and partly prevented the increase of GABA level in the brain caused by LM, indicating that the antidepressant-like activity of LM might participate in the GABA system.
The disturbance of DA circuitry is well acknowledged in the pathophysiology of mood disorders; and it is emphasized for the implication and role of dopaminergic neurotransmission in the pathophysiology of depression (Brocco et al. 2006). Pharmacologic silencing of the dopaminergic system through a chemical blockade of its receptors or depletion of DA content of dopaminergic neurons can mimic depressive-like behavior in animal models (Ellrich 2005; Dunlop and Nemeroff 2007; Gershon et al. 2007). Pharmacologic experiments suggest that the DA D2-like receptors play a key role in the response to biological antidepressant treatments. Several reports have shown that chronic antidepressant treatment increases D2-like binding activity in the nucleus accumbens of rats (Frye et al. 2007; Amiri et al. 2016). The haloperidol acts as a D2 antagonist with high affinity for the receptors. In this study, haloperidol (a classic non-selective D2 receptor antagonist) partially inhibited the antidepressant-like activity of LM, and partly restrained the increase of DA level in the brain caused by LM, indicating that LM might play an antidepressant-like role through the dopaminergic pathway.
Biogenic amines NE and Glu also play important roles in the pathophysiology of MDD. However, the results of this study showed that the LM did not take part in the noradrenergic system or glutamatergic system of the mice in the FST.
As mention above, based on the synergistic effects of LM and reboxetine, as well as LM and FLU, the changes in brain neurotransmitters caused by LM, including NE, GABA and DA, and the cases that the antidepressant-like effects of LM partially inhibited by ondansetron (a highly selective 5-HT3 receptor antagonist), bicuculline (a competitive GABA antagonist), and haloperidol (a classic non-selective D2 receptor antagonist), the mechanism of antidepressant-like effects of LM may be related to the 5-HTergic, GABAergic and DAergic systems. The further mechanisms of action of LM on the regulating monoamine neurotransmitters in the brain will be designed and studied in our subsequent experiments.
Conclusion
The results of this study demonstrated that LM was able to induce an antidepressant-like effect in a mouse model of depressive behavior, and this effect might be partially mediated by dopaminergic, 5-HTergic and GABAergic. On the other hand, the combination of LM and reboxetine, as well as LM and FLU showed synergistic effects, and LM might be used as combination therapy in depressed patients. As a safe and healthy natural ingredient, LM might be a potential antidepressant-like drug and food additive.
Acknowledgements
The authors are grateful for the National key R&D program (Grant No. 2016YFC0500303), the Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201303111), and Jilin Province Science and Technology Development Program (Grant Nos. 20160307005YY, 20150307012YY).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Yan Zhao, Phone: +86 431 84533358, Email: zhaoyan@jlau.edu.cn.
Yugang Gao, Phone: +86 431 84533358, Email: gaoyugang_2006@163.com.
References
- Amiri S, Amini-Khoei H, Mohammadi-Asl A, Alijanpour S, Haj-Mirzaian A, Rahimi-Balaei M, Razmi A, Olson CO, Rastegar M, Mehdizadeh M, Zarrindast MR. Involvement of D1 and D2 dopamine receptors in the antidepressant-like effects of selegiline in maternal separation model of mouse. Physiol Behav. 2016;163:107–114. doi: 10.1016/j.physbeh.2016.04.052. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bian X, Liu X, Liu J, Zhao Y, Li H, Cai E, Li P, Gao Y. Study on antidepressant activity of chiisanoside in mice. Int Immunopharmacol. 2018;57:33–42. doi: 10.1016/j.intimp.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Borsini F, Mancinelli A, D’Aranno V, Evangelista S, Meli A. On the role of endogenous GABA in the forced swimming test in rats. Pharmacol Biochem Behav. 1988;29(2):275–279. doi: 10.1016/0091-3057(88)90156-6. [DOI] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Papp M, Millan MJ. Antidepressant-like properties of the anti-Parkinson agent, piribedil, in rodents: mediation by dopamine D2 receptors. Behav Pharmacol. 2006;17(7):559–572. doi: 10.1097/01.fbp.0000236267.41806.5b. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ellrich J. Dopamine D2-like receptor activation antagonizes long-term depression of orofacial sensorimotor processing in anesthetized mice. Brain Res. 2005;1035(1):94–99. doi: 10.1016/j.brainres.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Frisardi V, Panza F, Farooqui AA. Late-life depression and Alzheimer’s disease: the glutamatergic system inside of this mirror relationship. Brain Res Rev. 2011;67(1–2):344–355. doi: 10.1016/j.brainresrev.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Frye MA, Grunze H, Suppes T, McElroy SL, Keck PE, Jr, Walden J, Leverich GS, Altshuler LL, Nakelsky S, Hwang S, Mintz J, Post RM. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry. 2007;164(8):1242–1249. doi: 10.1176/appi.ajp.2007.06060981. [DOI] [PubMed] [Google Scholar]
- Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl Psychiatry. 2014;4(7):e411. doi: 10.1038/tp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biol Psychiatry. 2007;61(2):145–153. doi: 10.1016/j.biopsych.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Gronli J, Fiske E, Murison R, Bjorvatn B, Sorensen E, Ursin R, Portas CM. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res. 2007;181(1):42–51. doi: 10.1016/j.bbr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Guo D, Gan J, Tan T, Tian X, Wang G, Ng KT. Neonatal exposure of ketamine inhibited the induction of hippocampal long-term potentiation without impairing the spatial memory of adult rats. Cogn Neurodyn. 2018;12(4):377–383. doi: 10.1007/s11571-018-9474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Yang Z, Gong P, Lei J. Maintenance of postsynaptic neuronal excitability by a positive feedback loop of postsynaptic BDNF expression. Cogn Neurodyn. 2018;12(4):403–416. doi: 10.1007/s11571-018-9479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA. Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology. 2017;42(12):2285–2291. doi: 10.1038/npp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Motomura K, Yanagida S, Ohashi A, Ishisaka-Furuno N, Kanba S. Pattern of c-Fos expression induced by tail suspension test in the mouse brain. Heliyon. 2017;3(6):e00316. doi: 10.1016/j.heliyon.2017.e00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Kitajima T, Iwata N, Ozaki N. Molecular genetics of mood disorders. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22(5):137–143. [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Iwamoto Y, Funada M, Suzuki T, Misawa M, Nagase H, Kasuya Y. Effects of diabetes on spontaneous locomotor activity in mice. Neurosci Lett. 1994;178(1):69–72. doi: 10.1016/0304-3940(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Krzywkowski K, Davies PA, Feinberg-Zadek PL, Brauner-Osborne H, Jensen AA. High-frequency HTR3B variant associated with major depression dramatically augments the signaling of the human 5-HT3AB receptor. Proc Natl Acad Sci USA. 2008;105(2):722–727. doi: 10.1073/pnas.0708454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(Suppl 1):3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Tian J, Huang G, Li T, Li F. Analysis of menthol in three traditional Chinese medicinal herbs and their compound formulation by GC-MS. Biomed Chromatogr. 2002;16(3):229–233. doi: 10.1002/bmc.131. [DOI] [PubMed] [Google Scholar]
- Lin M, Li H, Zhao Y, Cai E, Zhu H, Gao Y, Liu S, Yang H, Zhang L, Tang G, Wang R. Ergosteryl 2-naphthoate, an ergosterol derivative, exhibits antidepressant effects mediated by the modification of GABAergic and glutamatergic systems. Molecules. 2017;22(4):565. doi: 10.3390/molecules22040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist. 2009;15(5):525–539. doi: 10.1177/1073858409336093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marecos C, Ng J, Kurian MA. What is new for monoamine neurotransmitter disorders? J Inherit Metab Dis. 2014;37(4):619. doi: 10.1007/s10545-014-9697-4. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Positron emission tomography imaging in depression: a neural systems perspective. Neuroimaging Clin N Am. 2003;13(4):805–815. doi: 10.1016/S1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-de-Oliveira C. Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J Neurosci Methods. 2011;195(2):200–205. doi: 10.1016/j.jneumeth.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha-Poniewiera A, Podkowa K, Lenda T, Pilc A. The involvement of monoaminergic neurotransmission in the antidepressant-like action of scopolamine in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):155–161. doi: 10.1016/j.pnpbp.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Pesarico AP, Sampaio TB, Stangherlin EC, Mantovani AC, Zeni G, Nogueira CW. The antidepressant-like effect of 7-fluoro-1,3-diphenylisoquinoline-1-amine in the mouse forced swimming test is mediated by serotonergic and dopaminergic systems. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54(15):179–186. doi: 10.1016/j.pnpbp.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- Rajkowska G. Depression: what we can learn from postmortem studies. Neuroscientist. 2003;9(4):273–284. doi: 10.1177/1073858403252773. [DOI] [PubMed] [Google Scholar]
- Salat K, Kulig K, Gajda J, Wieckowski K, Filipek B, Malawska B. Evaluation of anxiolytic-like, anticonvulsant, antidepressant-like and antinociceptive properties of new 2-substituted 4-hydroxybutanamides with affinity for GABA transporters in mice. Pharmacol Biochem Behav. 2013;110:145–153. doi: 10.1016/j.pbb.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Sherman AD, Petty F. Neurochemical basis of the action of antidepressants on learned helplessness. Behav Neural Biol. 1980;30(2):119–134. doi: 10.1016/S0163-1047(80)91005-5. [DOI] [PubMed] [Google Scholar]
- Sultana J, Italiano D, Spina E, Cricelli C, Lapi F, Pecchioli S, Gambassi G, Trifiro G. Changes in the prescribing pattern of antidepressant drugs in elderly patients: an Italian, nationwide, population-based study. Eur J Clin Pharmacol. 2014;70(4):469–478. doi: 10.1007/s00228-013-1636-z. [DOI] [PubMed] [Google Scholar]
- Tardito D, Molteni R, Popoli M, Racagni G. Synergistic mechanisms involved in the antidepressant effects of agomelatine. Eur Neuropsychopharmacol. 2012;22(Suppl 3):S482–S486. doi: 10.1016/j.euroneuro.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2005;12(28):3615–3630. doi: 10.2174/138161206778522029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Höfner G, Wanner KT. Synthesis and biological evaluation of novel N-substituted nipecotic acid derivatives with an alkyne spacer as GABA uptake inhibitors. Bioorg Med Chem. 2018;26(12):3668–3687. doi: 10.1016/j.bmc.2018.05.049. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Peters JF, Cankaya MN. The informational entropy endowed in cortical oscillations. Cogn Neurodyn. 2018;12(5):501–507. doi: 10.1007/s11571-018-9491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AJ, Burnett SA, Hasebe K, McGillivray JA, Gray LJ, McGee SL, Walder K, Berk M, Tye SJ. Chronic adrenocorticotrophic hormone treatment alters tricyclic antidepressant efficacy and prefrontal monoamine tissue levels. Behav Brain Res. 2013;242:76–83. doi: 10.1016/j.bbr.2012.12.033. [DOI] [PubMed] [Google Scholar]
- Walstab J, Rappold G, Niesler B. 5-HT 3 receptors: role in disease and target of drugs ☆. Pharmacol Ther. 2010;128(1):146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Yamagata H, Uchida S, Matsuo K, Harada K, Kobayashi A, Nakashima M, Higuchi F, Watanuki T, Matsubara T, Watanabe Y. Altered plasma protein glycosylation in a mouse model of depression and in patients with major depression. J Affect Disord. 2018;233:79–85. doi: 10.1016/j.jad.2017.08.057. [DOI] [PubMed] [Google Scholar]
- Zhang LM, Zhao N, Guo WZ, Jin ZL, Qiu ZK, Chen HX, Xue R, Zhang YZ, Yang RF, Li YF. Antidepressant-like and anxiolytic-like effects of YL-IPA08, a potent ligand for the translocator protein (18 kDa) Neuropharmacology. 2014;81:116–125. doi: 10.1016/j.neuropharm.2013.09.016. [DOI] [PubMed] [Google Scholar]