Abstract
How neural representations of low-level visual information are accessed by higher-order processes to inform decisions and give rise to conscious experience is a longstanding question. Research on perceptual decision making has revealed a late event-related EEG potential (the Centro-Parietal Positivity, CPP) to be a correlate of the accumulation of sensory evidence. We tested how this evidence accumulation signal relates to externally presented (physical) and internally experienced (subjective) sensory evidence. Our results show that the known relationship between the physical strength of the external evidence and the evidence accumulation signal (reflected in the CPP amplitude) is mediated by the level of subjective experience of stimulus strength. This shows that the CPP closely tracks the subjective perceptual evidence, over and above the physically presented evidence. We conclude that a remarkably close relationship exists between the evidence accumulation process (i.e. CPP) and subjective perceptual experience, suggesting that neural decision processes and components of conscious experience are tightly linked.
Introduction
A central question in the study of decision making is how lower-level sensory information is accessed by higher-order processes to inform decisions and form conscious percepts. Research on perceptual decision making in human participants has established a late EEG potential characterized by a centro-parietal positivity (called CPP) as a neural correlate of sensory evidence accumulation1–3. The CPP is commensurate with the P300 family of event-related potentials (ERP)4 (for review, see5). In line with sequential information sampling models of decision making that assume integration of noisy evidence over time6, the CPP buildup rate increases proportionally with the strength of the exogenously presented sensory evidence to then peak at the time when a decision has been reached1. The CPP thus shows the defining “build-to-threshold” features of a decision variable7 and because it is neither specific to any particular sensory modality nor feature1, nor to any motor requirements2,8,9, it is believed to reflect evidence accumulation at an intermediate, abstract level of processing.
The process of evidence accumulation from early sensory stimulus representations depends not only on the strength of externally presented sensory evidence (stimulus intensity), but also on ‘internal’ sources of variability such as neural noise, in particular when the stimulus is weak. This can explain misperceptions (such as false alarms) in terms of erroneous evidence accumulation in sequential sampling models. Accordingly, it has been shown that the CPP is present not only for correct but also erroneous decisions1,8,10. Hence, the CPP can reflect not only external evidence but also an internal decision quantity. In line with this, a growing number of studies have focused on subjective aspects of the decision process such as decision confidence11–13. Interestingly, an evoked potential of similar latency and topography (Late Positivity, LP) has been shown to scale with perceptual awareness14, hence to be associated with subjectively reported (experienced) evidence.
Here, we wanted to directly investigate how the neural correlate of evidence accumulation, reflected in the CPP, relates to the externally presented (physical) and subjectively experienced evidence. We therefore examined the CPP as to its co-variation with physical versus subjectively experienced stimulus intensity. We manipulated the amount of available sensory evidence and asked our participants to explicitly rate the strength of their subjective experience, here defined as the clarity of the percept (using a four point scale, the Perceptual Awareness Scale (PAS)15). Our data show that the CPP amplitude more closely tracks the subjective reports of stimulus clarity than objective stimulus intensity, suggesting that a close relationship exists between the neural correlates of evidence accumulation and conscious awareness.
Results
While recording EEG, we presented difficult-to-detect visual stimuli that were either brighter or darker than the background at three intensity levels (i.e. contrast-from-background), collected discrimination performance and then asked the participants to rate the clarity of their perception on the PAS scale (Fig. 1). This allowed us to investigate the relationship of the CPP to both the amount of external sensory evidence and the level of subjective clarity of the percept (the internally experienced evidence).
Figure 1.
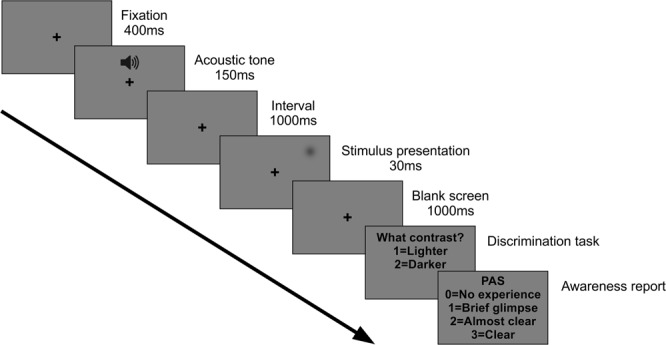
Single trial structure: Following an acoustic alerting tone, a brief visual stimulus was presented always at the same position in the upper right visual field. Stimuli could be either brighter or darker than the background and were presented at 3 different individually adjusted stimulus intensities (low, intermediate and high). After 1000 ms, participants were asked to report the brightness of the stimulus relative to the background (Discrimination task) and then rate the clarity of their perception on the Perceptual Awareness Scale (PAS) (Awareness report).
Behavioral responses to visual stimuli
To manipulate the amount of sensory evidence, we varied stimulus intensity (low, intermediate and high) by selecting 3 different contrast levels (i.e. stimulus levels corresponding to 25%, 50% and 75% detection rate, individually determined prior to the experiment; see experimental procedures). Participants were asked to indicate the brightness of the stimulus relative to the background (“lighter” or “darker”, prompted by a first question screen) for assessing discrimination accuracy, and to then rate the clarity of their percept (“no experience”, “brief glimpse”, “almost clear” or “clear”, prompted by the second question screen) (Fig. 1).
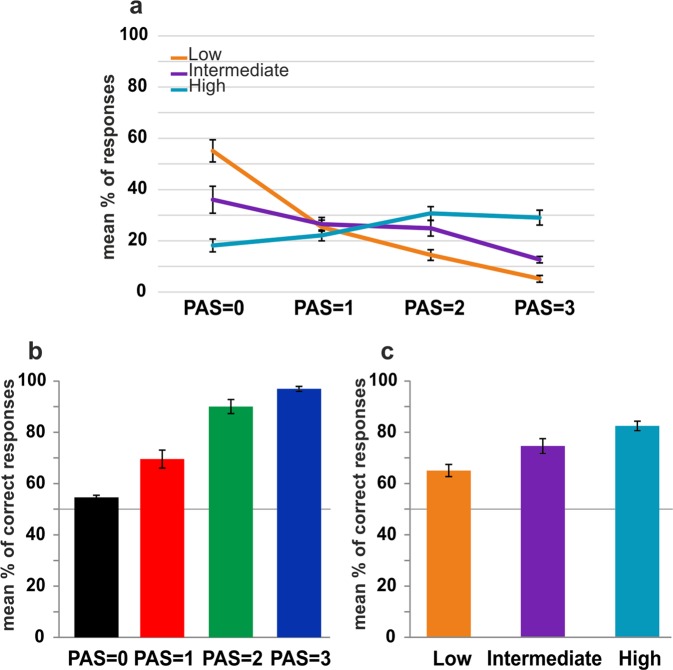
Figure 2a illustrates the distribution of subjective perceptual awareness ratings across the three intensity levels. As expected, for low intensity stimuli (orange line), participants indicated most often “no experience” (PAS = 0), followed by a “brief glimpse” (PAS = 1), “almost clear” experience (PAS = 2), and very few “clear” experience (PAS = 3). For intermediate intensity stimuli (purple line), the percentage of responses was more equally distributed across awareness rating levels. For high intensity stimuli (cyan line), participants indicated least often having “no experience” (PAS = 0), followed by more frequent “brief glimpses” (PAS = 1), “almost clear” (PAS = 2) and “clear” experiences (PAS = 3). Finally, catch trials were rated in 88.1% of trials as PAS = 0 (“no experience”).
Figure 2.
Behavioral results. (a) PAS rating variability for each level of external sensory evidence. Error bars represent standard errors. Mean percentage of correct discrimination responses as a function of (b) PAS ratings and (c) stimulus intensity. Error bars represent standard errors and the solid line (50%) chance level.
Sorting trials according to the clarity of subjective experience (i.e. PAS = 0, PAS = 1, PAS = 2, PAS = 3) revealed that as the clarity of the percept increased, accuracy also increased (Fig. 2b), as expected [repeated-measures ANOVA (degrees of freedom corrected using Greenhouse-Geisser estimates of sphericity): F(1.688,16.882) = 113.2, p < 0.01; linear trend F(1,10) = 1000.7, p < 0.01]. Similarly, sorting trials according to the strength of the presented evidence (i.e. low, intermediate, high stimulus intensity) showed that as objective sensory information increased, accuracy also increased (Fig. 2c) [repeated-measures ANOVA: F(2,20) = 35.5, p < 0.01; linear trend F(1,10) = 89.9, p < 0.01].
Overall, these results thus show that both factors of interest (visual awareness and stimulus intensity level), i.e. the internally experienced and externally presented sensory evidence, co-vary, but also show considerable trial-by-trial variability.
Next, we examined to what extent the CPP amplitude is varying with each measure, when the alternative measure is accounted for. In addition, we ran a mediation analysis to test whether the known relationship between stimulus intensity and CPP1–3,16 is mediated by subjective experience.
Event-related potentials
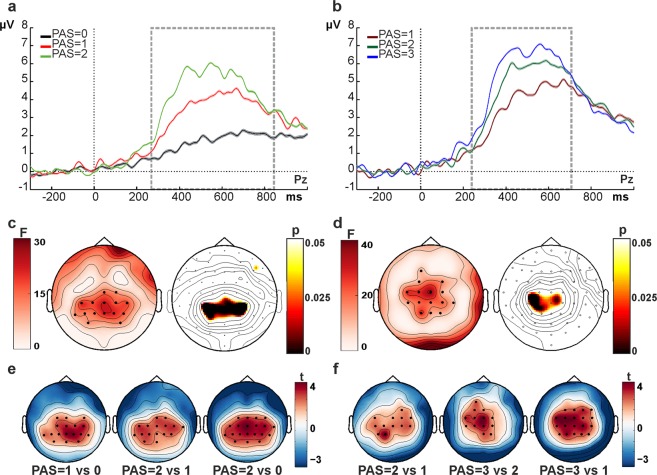
We first plotted the CPP as a function of external physical evidence (stimulus intensity), not taking into account internally experienced evidence (PAS ratings) (Fig. 3). As expected, the CPP scaled with stimulus intensity. However, note the large variability around the mean per stimulus intensity (Fig. 3, shaded areas). To test whether this variability is explained by the variability in PAS ratings observed for each stimulus intensity level (see Fig. 2a), we tested whether the CPP amplitude varies with subjective awareness ratings when physical stimulus properties were held constant. Conversely, we also tested whether this potential varies with physical stimulus properties when subjective ratings were held constant. That is, we compared ERP amplitudes evoked by different levels of subjective or objective evidence while controlling for the contribution of the alternative variable. To numerically equate the value of the alternative variable across all levels of comparison, we used random trial sub-sampling (see Methods).
Figure 3.
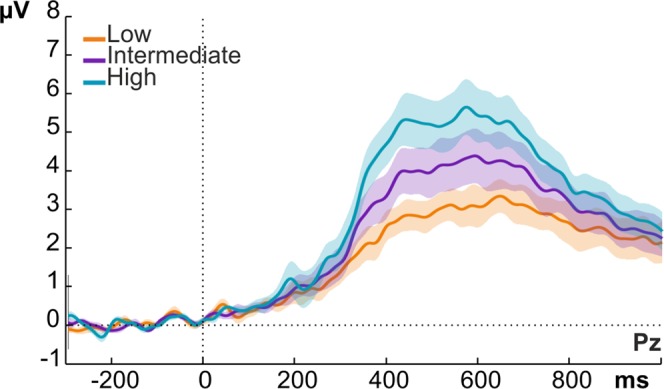
Grand average ERP waveforms over electrode Pz showing the late evoked potentials as a function of stimulus intensity, regardless of perceptual rating. Shaded areas represent standard errors at each time point.
Centro-parietal positivity co-varies with subjective clarity when stimulus contrast is equated across levels of comparisons
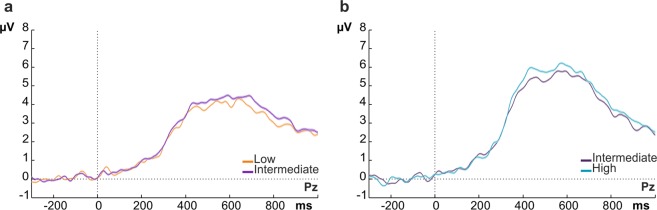
We compared CPP amplitude between awareness ratings for which we could equate stimulus intensities with a sufficient number of trials using trial subsampling (see “Statistical Analysis” section for details). PAS = 0, PAS = 1 and PAS = 2 were compared for stimuli presented at low and intermediate stimulus intensities (Fig. 4a) and PAS = 1, PAS = 2 and PAS = 3 for stimuli presented at intermediate and high stimulus intensities (Fig. 4b). The results were identical for both comparisons (cf. Fig. 4a vs. b). A late positive deflection was observed over central electrodes, peaking around 400–600 ms, which scaled with subjective awareness ratings (Fig. 4a,b), increasing in amplitude with stronger subjective experience. For statistical testing, we ran a non-parametric cluster-based permutation test17,18 (see Methods). This revealed a positive cluster over centro-parietal electrodes between roughly 200–800 ms after stimulus onset (highlighted by the dashed rectangle in Fig. 4a,b), independently of the awareness levels compared (see Fig. 4c,d, left maps for the results of the initial, random sub-sample, pcluster < 0.01). We corroborated this result by repeating the analysis in another 500 runs, randomly selecting a different subset of trials on each iteration (and always equating stimulus intensity across awareness ratings), revealing this effect to be highly consistent across sub-samples (see Fig. 4c,d, right maps for the topography of averaged p-values across the total of 500 runs). Additional, pairwise post-hoc comparisons between each awareness level, performed through cluster-based permutation t-tests averaged over the significant time window identified in the main analysis, showed that the CPP differed in amplitude across all awareness levels (Fig. 4e,f). Overall, these results show that the CPP is strongly related to subjective clarity of the percept, with higher amplitudes corresponding to higher clarity of perceptual experience. This effect was consistent across subjects (see Supplementary Fig. 1a for single subject data).
Figure 4.
CPP scales with the strength of subjective evidence. (a,b) Grand average ERP waveforms over electrode Pz, obtained for each visual awareness rating (a: PAS = 0 vs. 1 vs. 2; b: PAS = 1 vs. 2 vs. 3) for trials with equated stimulus intensity levels. Each waveform represents the average ERP over 500 random trial draws; shaded areas represent standard errors at each time point (note that the standard errors are very small and therefore almost invisible). Time windows of significant differences are highlighted by the dashed rectangle. (c,d) Cluster analysis results for awareness-related signals (based on ANOVAs across awareness levels). The maps on the left show the topographic distribution of F-values, while the black dots represent significant electrodes (initial draw out of 500 subsamples). The maps on the right show the topography of the p-values averaged over all 500 random draws in the time windows where the most consistent effects were found. (e,f) Pairwise post-hoc comparisons for awareness-related signals in the significant time window (dashed rectangle in a,b). Each map shows the t-value distribution with black dots indicating significant electrodes.
Centro-parietal positivity shows no relation to stimulus contrast when subjective awareness ratings are equated across levels of comparisons
Next, we compared CPP amplitude across stimulus intensity levels for which we could equate the value of awareness ratings with a sufficient number of trials through random trial subsampling. Low versus intermediate stimulus intensity levels were compared after equating PAS values 0, 1 and 2 across stimulus categories (Fig. 5a) and intermediate versus high stimulus intensity levels were contrasted after equating PAS values 1, 2 and 3 across stimulus categories (Fig. 5b). The results reveal very small variations of the CPP with stimulus intensity for both comparisons (see Fig. 5a,b: compare low vs. intermediate intensity waveform in a; and intermediate vs. high intensity waveform in b), with the differences being an order of magnitude smaller than those observed for the awareness effects (cf. Fig. 4a,b). Running a non-parametric cluster-based permutation t-test per stimulus intensity comparison did not reveal any significant cluster of electrodes (all pcluster > 0.5 for low vs intermediate comparisons, all pcluster > 0.3 for intermediate vs high comparisons). The absence of any effect was not driven by trial selection as confirmed by repeating the trial selection 500 times (random draws). Hence, the CPP was not modulated by the different stimulus intensities when accounting for the subjective experience ratings. The lack of effect was evident across subjects (see Supplementary Fig. 1b for single subject data).
Figure 5.
CPP amplitude and stimulus intensity: ERP amplitudes over electrode Pz per each of the stimulus intensity comparisons (a: low vs. intermediate; b: intermediate vs. high) for which awareness ratings could be equated. No differences were found across stimulus intensities with equated PAS ratings (compare low vs. intermediate waveform in (a) and intermediate vs. high waveform in (b)). Note that the amplitude-differences between a and b are due to the need for including different PAS ratings in the respective averages (PAS 0, 1 and 2 for waveforms in (a), PAS 1, 2 and 3 for waveforms in (b)). Each waveform represents the average ERP over 500 trial selections (random draws), shaded areas represent standard errors at each time point.
Centro-parietal positivity: accuracy and catch trials
Given the relatively stronger co-variation of the CPP with subjectively experienced than with physically presented sensory evidence, we expected an uncoupling of this potential from performance accuracy. This was confirmed in two separate analyses. First, we compared the CPP across PAS ratings on correct response trials only (PAS ratings from 0 to 3 included), and then repeated this analysis on incorrect response trials (only including PAS = 0 and 1 ratings to have a sufficient number of numerically equated trials between the two categories, see Behavioral results).
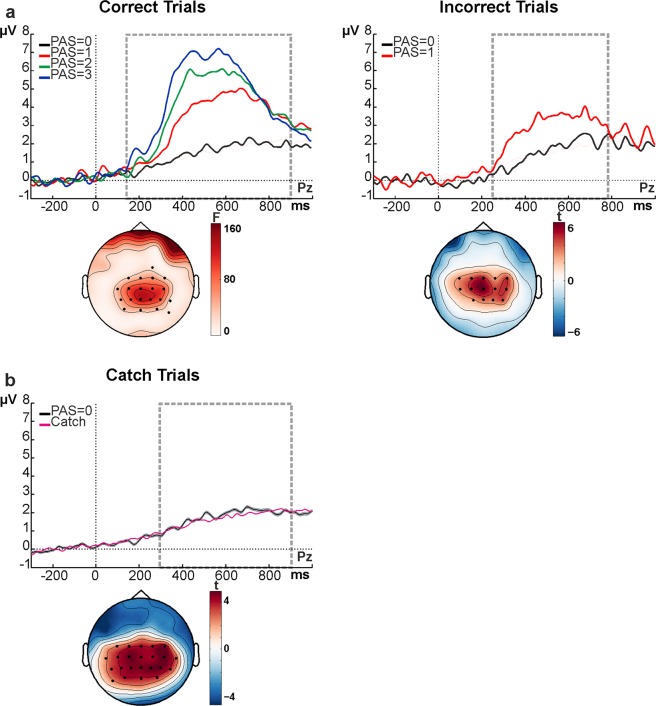
For correct trials, we confirmed the existence of an awareness rating effect. The higher the visual awareness rating, the higher the amplitude of the CPP (Fig. 6a, left panel). As above, the cluster-based permutation test on ERPs across subjective rating (PAS), performed on the whole epoch (0 to 900 ms), revealed a significant positive centro-parietal electrode cluster (pcluster < 0.01, from 196 to 900 ms, Fig. 6a, left panel, map). The cluster was also present in each pairwise post-hoc comparison between PAS ratings when performed through cluster-based permutation t-tests on the mean amplitude of the significant time window (p values ranging from 0.046 to 0.002).
Figure 6.
Late evoked potential scales with awareness regardless of accuracy. (a) ERP waveforms over electrode Pz, obtained for each visual awareness level in correct trials (left panel) and for visual awareness levels PAS = 0 and PAS = 1 in incorrect trials (right panel). The map illustrates the topographical distribution of F- and t-values for the significant time window (highlighted by the dashed rectangle). Black dots represent significant positive electrode clusters. (b) Evoked potentials in catch trials versus PAS = 0 trials (obtained after 500 random draws from low and intermediate stimulus intensity trials) over electrode Pz. Shaded areas around PAS = 0 waveforms represent standard errors at each time point. Map: t-value distributions from the comparison between catch trial data in the window after expected stimulus onset (marked by 0) versus its baseline “pre-stimulus” interval. Black dots represent significant positive electrode clusters.
Importantly, the awareness rating effect was also observed for incorrect trials, suggesting a dissociation of the effect from task accuracy (Fig. 6a, right panel). The cluster-based permutation t-test (performed on the whole epoch, 0 to 900 ms) again revealed a positive cluster over centro-parietal areas (pcluster < 0.01, from 248 to 788 ms, Fig. 6a, right panel, map).
As a variant of testing for a link of the CPP to an ‘internal’ decision quantity, we analysed catch trials to examine whether this late positivity can also occur in the absence of any external sensory evidence. The corresponding cluster-based permutation t-test performed on the whole epoch (0 to 900 ms), with the pre-stimulus period (−300 to 0 ms) as a reference interval, again revealed a positive cluster over centro-parietal areas (pcluster < 0.01, starting at 248 ms until the end of the epoch, Fig. 6b, map). Therefore, even if no veridical sensory information is available, the CPP is still present, and its amplitude matches the amplitude of stimulus-present trials with PAS reports of zero (“no experience”) (Fig. 6b). Please note that the high number of PAS 0 reports in catch trials prevented a comparison across PAS levels.
Mediation analysis
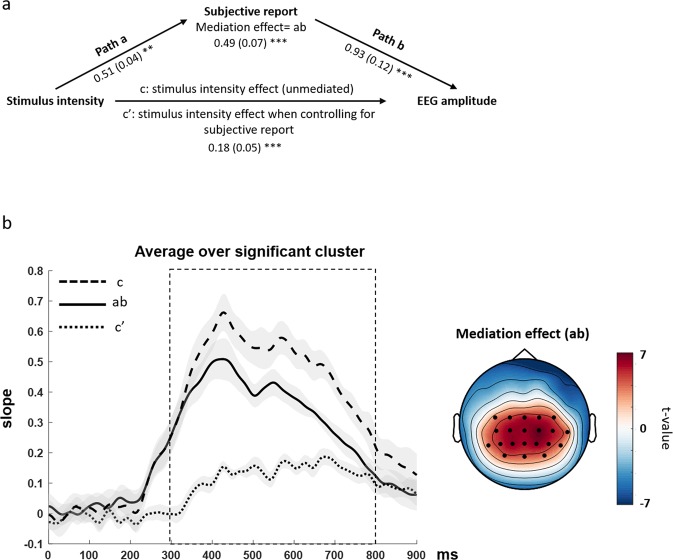
To further investigate the relationship between CPP amplitude, stimulus strength and subjective experience, we ran a mediation analysis linking the three variables, without any prior trial selection. A mediation analysis allows for estimation of the extent to which a proposed mediator variable accounts for the relationship between a predictor and an outcome variable19,20. Here, based on the abundant literature linking the amount of available evidence to the CPP amplitude e.g.1,3,12,16, we considered stimulus intensity (from catch trials to High, 4 levels) as predictor of the EEG amplitude (outcome variable), while subjective experience measured by PAS scale ratings (0–3, 4 levels) was included as a proposed mediator (see Fig. 7a). The choice of the awareness rating as the mediator is also in line with our behavioural results (Fig. 2a), indicating that the awareness rating is predicted by the stimulus strength, thus acknowledging that subjective perception is mostly driven by external evidence. Indeed, in the mediation analysis, variations in levels of the predictor must significantly account for variations in the proposed mediator. Please note that by the use of the mediation analysis, we aimed to investigate the strength of the stimulus intensity-CPP amplitude relationship whilst controlling for subjective experience, without implying that the subjective evidence causally influences the CPP.
Figure 7.
Mediation hypothesis and results. (a) The mediation model included the independent variable (stimulus intensity) as predictor of the outcome variable (ERP amplitude). Subjective reports were introduced as a proposed mediator and tested as to whether they accounted for the relationship between predictor and outcome. (b) The mediation effect (ab) time course averaged across electrodes within the significant cluster is shown on the left, together with the total effect of stimulus intensity (c) and the direct effect (c′) when controlling for subjective reports. The rectangle represents the significant time window of mediation. On the right, the topography of the mediation effect is shown with the significant positive electrode cluster superimposed (black dots).
Therefore, in our model (Fig. 7a), path a represents the relationship between stimulus intensity and PAS rating and path b the relationship between PAS rating and EEG amplitude when controlling for stimulus intensity. Path c represents the total stimulus intensity effect (unmediated) on EEG amplitude and path c’ represents the direct stimulus intensity–EEG amplitude effect when controlling for PAS ratings. The product of the path a and path b coefficients (ab) represents the mediation effect. Testing for a significant mediation effect involves testing if the predictor-outcome relationship (stimulus intensity – EEG amplitude) is significantly reduced by including the mediator (PAS ratings) in the model (a*b = c-c′ > 0). To test this, ab coefficients calculated for each channel and each time point from 0 to 900 ms after stimulus presentation were tested against 0 by means of a cluster based permutation t-test. We found a significant positive cluster (pcluster = 0.007; also note path a p < 0.05), spanning from 300 to 800 ms and including several centro-parietal electrodes. Figure 7b shows ab values over time averaged across electrodes within the significant cluster (on the left) and its corresponding topography (on the right). Importantly, the time window and the topography of the mediation effect exactly mirrored the CPP component. This result indicates that the inclusion of the mediator “subjective report” in the model significantly decreased the predictive power of stimulus strength on CPP (compare dashed lines in Fig. 7b). In summary, the mediation analysis confirmed and extended our initial results, showing that when the subjective experience is accounted for, the stimulus strength does not significantly modulate the CPP amplitude anymore.
Discussion
Our study sheds light on how subjective and objective evidence strength relate to the known EEG evidence accumulation signal (CPP): when the variation of the CPP amplitude with subjective stimulus clarity is accounted for, there is little residual variation as a function of physical stimulus intensity. In addition, this relationship with subjective evidence strength is observed for error as well as correct trials. Hence, the magnitude of the evidence accumulation signal is strongly linked to the subjective experience of stimulus clarity, irrespective of performance accuracy.
Our results are complementary to a recent study by Kang et al.21, in which participants were asked to report the timing of their commitment to a perceptual decision. By demonstrating that the subjective report corresponds to the time of decision termination, Kang et al. provided evidence that the neurophysiological mechanisms mediating the decision completion might be also responsible for the piercing of conscious awareness. However, while Kang et al. focused on the timing of the decision process (its termination), we asked our participants to report the clarity of their percept, demonstrating that subjective reports closely match the absolute magnitude reached by the evidence accumulation signal (i.e. the CPP). Interestingly, this suggests that decision processes and mechanisms of conscious perception may be more tightly linked than commonly thought (see also21). Moreover, and in further analogy to Kang et al.21, our study suggests a remarkable degree of reliance that can be placed on observers’ subjective impressions to gain access to core internal decision quantities. Intriguingly, a parallel line of research on the neural correlates of consciousness22 has also identified a late potential of similar topography and latency that tracks conscious reports23–28, further substantiating a link between the CPP and conscious perception.
With weak sensory stimuli as employed in the present study, the measure of the clarity of percepts will represent an ‘internal’ subjective quantity, which likely equates to another such quantity: the meta-cognitive measure of confidence in the decision. If participants perceive the stimulus more clearly, they are also likely to be more certain about their decision. A small number of single-unit recordings have demonstrated that neural evidence accumulation signals in parietal neurons index choice confidence, besides representing the choice itself (e.g.11). Similar results have been reported in human studies for parietal and frontal sources of activity12,29–32, and for the decision-related P300 component33, (but see24 for a possible dissociation of response confidence from awareness). It is unclear whether the awareness rating scale used here relates to the confidence of having correctly detected the stimulus (its presence), or alternatively the confidence in having made the correct choice in the discrimination component of the task (darker/lighter than the background). Given the specific instructions provided to the participants (‘rate the clarity regardless of accuracy confidence’), we tend to favor the former scenario. Precisely how the CPP is related to different types of confidence report (see34) is worthy of further study. Irrespective of this, our findings represent a clear demonstration of the link between the CPP and subjective perceptual experience, by relying on the explicit report of subjective awareness, as opposed to indirect behavioral metrics such as post-decisional choice wagering and reaction times11,12. Overall, our findings demonstrate that the CPP reflects an evidence accumulation process, the output of which seems to relate not only to the decision itself but also to the associated visual awareness levels.
It is of interest to consider the differences in experimental design between our and previous works. In evidence accumulation experiments1–3,9, (see also35,36), sensory stimuli are classically shown continuously or sequentially (e.g. moving random dot sequences) until a decision is reached, i.e. until all of the evidence needed for a confident report is ‘accumulated’ and high awareness levels have presumably been reached. In contrast, we used brief static stimuli at peri-threshold levels to capture a range of subjective awareness levels across a range of sensory evidence values. This design allowed us to examine the relationship between both experienced stimulus clarity and physical stimulus properties and the CPP. Our results are in line with previous studies revealing a buildup of the CPP even during erroneous decisions1,8,10 and earlier neuroimaging findings of stronger neural activity for false alarms compared to misses37 found even at low levels of sensory processing, i.e. in V1–V338. All of these findings follow from the core concept that random variability in noisy sensory signals impacts on perceptual experience and decisions (see the phenomenon of “choice probability”39). Moreover, our data show that the CPP was also present in catch trials, when no visual evidence was physically displayed. The signal recorded during catch trials resembles the shallow evidence accumulation signal observed in monkey parietal areas when no evidence was displayed40, and replicates previous findings in humans showing a low amplitude late potential in catch trials or when subliminal stimuli were presented26,41. This CPP buildup in catch trials also follows naturally from the evidence accumulation account of the CPP, given that the CPP builds positively for evidence that favors either decision alternative (see also1). By extension, the CPP is expected not to average to zero even in the absence of evidence as long as there is an attempt to read out sensory information. In the present work, the CPP therefore likely reflects an attempt to access evidence for report (regardless of the presence of the actual physical stimulus) that however fails in most catch trials (around 90%) to reach the threshold for categorizing the percept as a brief glimpse or more (PAS ≥ 1). Interestingly, previous research suggests that a small fraction of catch trials can even be associated with readout of visual information, as indicated by the finding of object perception in pure noise stimuli42,43.
Conclusion
Our results reveal that subjective perceptual experiences closely match the magnitude of the neural signature of sensory evidence accumulation (the CPP) during decision formation. Hence, when probed on clarity of perception, participants may directly ‘read’ the magnitude reached by the decision variable, showing a remarkably tight link between the neural correlates of evidence accumulation and conscious perception.
Materials and Methods
Participants
14 participants (7 females, 2 left-handed, mean age ± standard deviation: 23.79 ± 3.17) were recruited. All reported normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. They all gave written informed consent to participate in the study, which was approved by the College of Science and Engineering Ethics Committee of the University of Glasgow and conducted in accordance with the 2013 Declaration of Helsinki. Data from three participants were excluded from the analysis because of a low number of trials ( < 20) in one or more conditions after artifact removal. The final sample was thus composed of 11 participants (6 females, 1 left-handed, mean age ± standard deviation: 23.6 ± 3.32). Note that this dataset has been used in44 to address another question unrelated to the CPP.
Experimental Procedure
The experiment comprised two sessions performed over two consecutive days. The first session served for calibrating stimulus intensity by threshold assessment (see Determination of Stimulus Intensity) and to familiarize participants with the behavioral task. During the second session, after a threshold re-assessment, participants were prepared for EEG recordings. They then performed a forced choice discrimination task, while EEG was continuously recorded (see “EEG Experiment”).
Stimuli
The stimuli were black or white circular patches with a Gaussian envelope (size = 1.3°), presented on a gray background in the upper right visual field (5° of vertical and 10° of horizontal eccentricity from the fixation cross). The luminance of the stimuli was individually adjusted to obtain six contrast levels (three lighter and three darker than the background) by means of a threshold assessment procedure (see next paragraph for further details). The contrast of the stimuli presented varied from 0.025 to 0.116% of the maximal contrast of the black/white patch.
Determination of Stimulus Intensity
Participants sat in a dimly lit testing room in front of a CRT monitor (resolution 1280 × 1024, refresh rate of 100 Hz, viewing distance of 57 cm), with their head stabilized by a chin rest. The aim of the first session was to individually determine 3 levels of stimulus intensity (low, intermediate and high) that corresponded to 25%, 50% and 75% of correct detection rate (by manipulating contrast from the medium gray background in both directions, leading to three lighter and three darker patches from background). The thresholds were measured using the method of constant stimuli45. At the beginning of threshold assessment, ten evenly spaced contrast values ranging from 0.025 to 0.116% of the maximal contrast of the black/white patches were presented in a randomized order, in the upper periphery of the right visual field (see Stimuli for details). This first phase included two blocks: on each block, all contrast values were tested seven times together with 14 stimulus-absent trials (catch trials), resulting in a total number of 308 trials per participant. On each trial, the stimulus appeared after a 1000 ms interval following a brief (150 ms) warning tone (1000 Hz). Participants were asked to keep their eyes on a central fixation cross and to press the spacebar on a keyboard whenever they saw a stimulus. At the end of the two blocks, sigmoid functions were fit to the data from both the light and dark stimulus trials separately and contrast values yielding detection thresholds of 25%, 35%, 50%, 65% and 75% were extracted for each participant. These contrast levels were tested again in two blocks, including 10 trials for each contrast and stimulus type (light and dark stimuli) and 14 catch trials, resulting in a total of 228 trials per participant.
On the second day of testing and prior to EEG recording, a short threshold re-assessment was performed, to verify that participants’ performance was comparable to that obtained in the first session. The previously identified contrast values (5 for light and 5 for dark patches) and contrast levels corresponding to 0% and 100% detection accuracy were each presented seven times together with 14 catch trials, for a total of 182 trials. If contrast values resulting in detection thresholds of about 25%, 50% and 75% were confirmed, they were selected for the behavioral task during the EEG recording. Otherwise, sigmoid functions were again fit to the data and new contrast levels were extracted and tested with the same procedure. The threshold assessment procedure had to be repeated for 4 participants.
EEG Experiment
During EEG recordings, participants performed a two-alternative forced choice discrimination task. Each trial (Fig. 1) started with a central black fixation cross, followed after 400 ms by a 1000-Hz warning tone (150 ms). After a 1000 ms interval, a light or a dark gray Gaussian patch (the luminance values of which had been determined by threshold assessment) was presented for 30 ms (3 frames) in the upper periphery of the right visual field. A 1000-ms blank screen was then followed by a response prompt asking the participants to judge the brightness of the stimulus relative to the gray background, pressing a button for “lighter” and another button for “darker”. The participants were asked to guess when they did not perceive any stimulus. After the button press, another response prompt asked participants to rate the clarity of their perception on the four-point Perceptual Awareness Scale (PAS15). The four PAS categories are: 0) “no experience” of the stimulus, 1) a “brief glimpse”, 2) an “almost clear experience” and 3) a “clear experience”. Responses were given by pressing four different buttons on the keyboard. The experimental session was divided into ten blocks. Each block was composed of 80 trials: 10 trials for each individually adjusted stimulus contrast (25%, 50% and 75% of detection threshold) and stimulus type (light and dark), together with 20 catch trials, thus yielding a total of 800 trials. The order of the trials was fully randomized. Both the threshold assessment and the actual behavioral task were programmed and run in MATLAB (MathWorks Inc.), using the Psychophysics Toolbox extension46,47.
EEG recording and Event-Related Potential (ERP) Analysis
EEG was continuously recorded with a BrainAmp system (Brain Products GmbH, Munich, Germany – BrainVision Recorder) using a Fast’n Easy cap with 61 Ag/AgCl pellet pin electrodes (EasyCap GmbH, Herrsching, Germany) placed according to the 10–05 International System. An additional electrode was positioned below the left eye to record eye movements (after being referenced to Fp1), whereas horizontal eye movements were detected by referencing AF7 to AF8 off-line. Two extra electrodes served as ground (TP9) and on-line reference (AFz). All scalp channels were re-referenced off-line to the average of all electrodes. Electrode impedances were kept below 10 kΩ. The digitization rate was 1000 Hz with a low cut-off of 0.01 Hz and a high cut-off of 100 Hz.
The continuous EEG signal was pre-processed off-line using Brain Vision Analyzer 2.0 (BrainProducts). Data were filtered with a second order high-frequency cutoff of 85 Hz and a second order low-frequency cutoff of 0.1 Hz. A band rejection filter with a bandwidth of 2 Hz was then used to remove 50 Hz interference. Independent component analysis (ICA48) was applied to remove eye blinks and muscle artifacts. The EEG data were then cut into epochs of 1300 ms starting 300 ms before stimulus-onset and baseline corrected using the 300 ms pre-stimulus period. All segments were visually inspected and removed if still contaminated by residual eye movements, blinks, strong muscle activity or excessively noisy EEG. On average, ~5% of the trials were discarded. Finally, data were down-sampled to 250 Hz before averaging.
Analysis of the Event-Related Potentials (ERPs) was performed using the Fieldtrip toolbox18; (see http://www.fieldtriptoolbox.org/). Averaging was carried out separately according to visual awareness (subjective rating) and stimulus intensity (detection threshold). To evaluate the impact of visual awareness on the late positivity, we randomly selected trials so that each PAS ERP average would include an equal number of trials with stimuli presented at the different intensities, in order to control for the stimulus intensity factor. In a second analysis, we focused on the impact of physical stimulus properties, i.e. different intensities. In this case, trials were randomly selected so that ERPs evoked by each stimulus intensity would include an equal number of trials receiving the different perceptual ratings on the PAS, to control for the visual awareness factor.
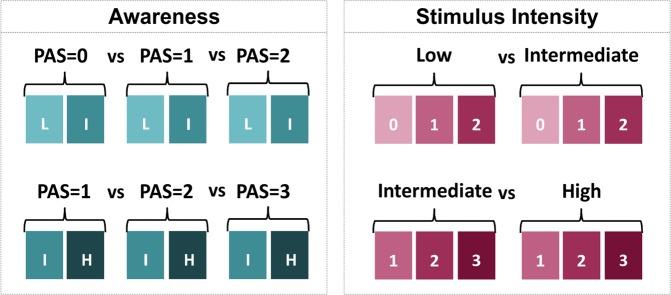
Because of a low number of trials for the combination of low stimulus intensity (25% detection threshold) with rating 3, and high stimulus intensity (75% detection thresholds) with rating 0, comparisons between perceptual ratings 0, 1 and 2 included trials with stimuli presented at low and intermediate intensity (25% and 50% detection thresholds), while comparisons between perceptual ratings 1, 2 and 3 included trials with stimuli presented at intermediate and high intensity (50% and 75% detection thresholds). For the same reason, the comparison between low and intermediate intensity levels only included ratings 0, 1 and 2 and the comparison between intermediate and high intensity levels only included ratings 1, 2 and 3 (Fig. 8).
Figure 8.
Trial sampling and comparisons. Left panel: comparisons performed to investigate the visual awareness effect. Different rating scores were compared by including an equal number of trials for each stimulus intensity (low (L), intermediate (I) and high (H)). To compare PAS = 0 vs. PAS = 1 vs. PAS = 2, stimuli at low and intermediate intensities were included (left upper panel), whereas stimuli at intermediate and high intensities were included to compare PAS = 1 vs. PAS = 2 vs. PAS = 3 (left bottom panel). Right panel: comparisons performed to investigate the stimulus intensity effect. Different stimulus intensity levels were compared after equating the number of trials for each rating. Stimuli of low and intermediate intensities were compared including an equal number of trials rated with PAS 0, 1 and 2 (right upper panel), whereas intermediate and high intensities were compared including an equal number of trials with PAS ratings 1, 2 and 3 (right bottom panel).
The mean number of trials for each comparison was: 48.55 for rating 0 vs 1 vs 2 and 44.55 for rating 1 vs 2 vs 3; 72.82 for the low vs intermediate stimulus intensities and 66.82 for the intermediate and high intensities.
To investigate the effect of accuracy, correct trials were averaged together according to their awareness rating and regardless of stimulus intensity. For PAS = 0 the mean number of correct trials was 115, 101 for PAS = 1, 112.63 for PAS = 2 and 86.54 for PAS = 3 (the decrease in correct trials with increasing PAS depends on the proportion of given ratings, i.e. there were overall more trials for lower PAS ratings. See also Fig. 2a). For incorrect trials, we could only compare PAS = 0 and 1. The difference in trial numbers between the two ratings was overcome by equating the number of trials through a random selection, which resulted in a mean number of trials of 39.81.
Finally, for each participant, average waveforms were computed for the catch trials (mean number of trials after artifact rejection: 186.18).
Statistical Analysis
Behavioral data
To evaluate the effectiveness of the experimental manipulations, two separate repeated-measures analyses of variance (ANOVA) were carried out on discrimination accuracy for trials sorted according to either awareness rating (within-subject factor: PAS rating. 4 levels: PAS = 0, PAS = 1, PAS = 2 and PAS = 3) or stimulus intensity (within-subject factor: stimulus intensity. 3 levels: low, intermediate and high). We expected higher accuracy for higher ratings and intensity levels.
EEG data
To investigate the effect of different levels of visual awareness (PAS = 0 vs. 1 vs. 2; PAS = 1 vs. 2 vs. 3) and stimulus intensity (low vs. intermediate intensity; intermediate vs. high intensity) on EEG data, non-parametric cluster-based permutation analyses were used17,18. For every sample (channel x time point), conditions were compared by means of a repeated-measures ANOVA (for awareness rating) or by a paired-samples T-Test (for stimulus intensity), on a time window spanning from 0 to 900 ms after stimulus presentation. Those samples whose F- or t-value exceeded a critical value (p < 0.05) were selected and clustered according to spatial and temporal adjacency. Following this, within every cluster, F- or t-values were summed to calculate cluster-level statistics. These cluster-based statistics were evaluated through a non-parametric permutation analysis, with labels from different conditions randomly permuted over 500 iterations. For each permutation, cluster-based statistics were calculated and a reference distribution was built, from which the Monte Carlo p-value was estimated according to the proportion of the randomization null distribution exceeding the maximum cluster statistic. When ANOVAs on visual awareness comparisons were significant, post-hoc analyses were performed through non-parametric cluster-based permutation t-tests between each rating condition. The paired-samples T-Tests were run on the mean amplitude of the significant time window identified by the main ANOVA.
In order to ensure that the random selection of trials performed to equate the number of trials was not biasing the results, trial sampling was repeated 500 times for each comparison and statistical analyses performed for each random draw. The p values obtained after each draw and statistical analysis were averaged together for each comparison, to confirm the significant effects.
Furthermore, a cluster-based permutation t-test was performed on catch trials to test whether the amplitude of evoked activity significantly differed from a baseline period (−300 to 0 ms before stimulus onset) including all channels and time points from 0 to 900 ms after stimulus presentation.
To investigate the influence of discrimination accuracy on the visual awareness effect, ERP amplitudes evoked by different visual awareness ratings in correct trials only were compared by means of a cluster-based permutation ANOVA (500 permutations, time window from 0 to 900 ms after stimulus onset). Finally, incorrect trials receiving a rating of 0 or 1 were compared by means of a cluster-based permutation t-test (500 permutations, time window from 0 to 900 ms after stimulus onset).
Lastly, a hierarchical (two-level) mediation analysis was carried out using the Mediation Toolbox (http://wagerlab.colorado.edu/tools19,49). First, each path was estimated for each participant, channel and data point (from 0–900 ms after stimulus presentation) using linear regression. The single-trial stimulus contrast values (coded as 0,1,2,3) were entered into the model as the predictor (X) variable and single-trial EEG amplitudes were entered as the outcome variable (Y). Single-trial PAS ratings were tested as a possible mediator (M) of the X-Y relationship. To test for a systematic mediation effect (ab) (i.e. if ab slopes across participants are significantly different from zero), we ran a cluster-based permutation t-test against 0, including all channels and data points.
Supplementary information
Acknowledgements
This work was supported by a Wellcome Trust Award to Gregor Thut [Grant Number 098434]. We would like to thank the anonymous reviewers for their very insightful and constructive comments on a previous version of the manuscript.
Author Contributions
C.F.T., D.V., C.S.Y.B., R.C., S.S. and G.T. designed the research; C.F.T. and D.V. performed the research; C.F.T., D.V. and G.T. analyzed the data; C.F.T., D.V., C.S.Y.B., R.C., S.S. and G.T. wrote the paper.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chiara F. Tagliabue and Domenica Veniero contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41024-4.
References
- 1.O’Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci. 2012;15:1729–1735. doi: 10.1038/nn.3248. [DOI] [PubMed] [Google Scholar]
- 2.Kelly SP, O’Connell RG. Internal and external influences on the rate of sensory evidence accumulation in the human brain. J Neurosci. 2013;33:19434–19441. doi: 10.1523/JNEUROSCI.3355-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loughnane GM, et al. Target selection signals influence perceptual decisions by modulating the onset and rate of evidence accumulation. Curr Biol. 2016;26:496–502. doi: 10.1016/j.cub.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Sutton S, Braren M, Zubin J, John E. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 5.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Shadlen MN, Kiani R. Decision making as a window on cognition. Neuron. 2013;80:791–806. doi: 10.1016/j.neuron.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinemann NA, O’Connell RG, Kelly SP. Decisions are expedited through multiple neural adjustments spanning the sensorimotor hierarchy. Nat. Commun. 2018;9:3627. doi: 10.1038/s41467-018-06117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twomey DM, Kelly SP, O’Connell RG. Abstract and Effector-Selective Decision Signals Exhibit Qualitatively Distinct Dynamics before Delayed Perceptual Reports. J. Neurosci. 2016;36:7346–7352. doi: 10.1523/JNEUROSCI.4162-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afacan-Seref K, Steinemann NA, Blangero A, Kelly SP. Dynamic Interplay of Value and Sensory Information in High-Speed Decision Making. Curr Biol. 2018;28:795–802. doi: 10.1016/j.cub.2018.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324:759–764. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gherman S, Philiastides MG. Neural representations of confidence emerge from the process of decision formation during perceptual choices. Neuroimage. 2015;106:134–143. doi: 10.1016/j.neuroimage.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg R, et al. A common mechanism underlies changes of mind about decisions and confidence. Elife. 2016;5:e12192. doi: 10.7554/eLife.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagliabue CF, Mazzi C, Bagattini C, Savazzi S. Early Local Activity in Temporal Areas Reflects Graded Content of Visual Perception. Front Psychol. 2016;7:572. doi: 10.3389/fpsyg.2016.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsøy TZ, Overgaard M. Introspection and subliminal perception. Phenomenol Cogn Sci. 2004;3:1–23. doi: 10.1023/B:PHEN.0000041900.30172.e8. [DOI] [Google Scholar]
- 16.Twomey DM, Murphy PR, Kelly SP, O’Connell RG. The classic P300 encodes a build-to-threshold decision variable. Eur J Neurosci. 2015;42:1636–1643. doi: 10.1111/ejn.12936. [DOI] [PubMed] [Google Scholar]
- 17.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wager TD, et al. Brain mediators of cardiovascular responses to social threat, Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 21.Kang YHR, Petzschner FH, Wolpert DM, Shadlen MN. Piercing of Consciousness as a Threshold-Crossing Operation. Curr Biol. 2017;27:2285–2295. doi: 10.1016/j.cub.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch, C. The Quest for Consciousness: A Neuroscientific Approach. Denver: Roberts & Co. (2004).
- 23.Del Cul A, Baillet S, Dehaene S. Brain Dynamics Underlying the Nonlinear Threshold for Access to Consciousness. PLoS Biol. 2007;5:2408–2423. doi: 10.1371/journal.pbio.0050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salti M, Bar-Haim Y, Lamy D. The P3 component of the ERP reflects conscious perception, not confidence. Conscious Cogn. 2012;21:961–968. doi: 10.1016/j.concog.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 26.Koivisto M, Salminen-Vaparanta N, Grassini S, Revonsuo A. Subjective visual awareness emerges prior to P3. Eur J Neurosci. 2016;43:1601–1611. doi: 10.1111/ejn.13264. [DOI] [PubMed] [Google Scholar]
- 27.Pitts MA, Metzler S, Hillyard SA. Isolating neural correlates of conscious perception from neural correlates of reporting one’s perception. Front Psychol. 2014;5:1078. doi: 10.3389/fpsyg.2014.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitts MA, Padwal J, Fennelly D, Martínez A, Hillyard SA. Gamma band activity and the P3 reflect post-perceptual processes, not visual awareness. Neuroimage. 2014;101:337–350. doi: 10.1016/j.neuroimage.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy PR, Robertson IH, Harty S, O’Connell RG. Neural evidence accumulation persists after choice to inform metacognitive judgments. Elife. 2015;4:e11946. doi: 10.7554/eLife.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: a timing diagram. J Neurosci. 2006;26:8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratcliff R, Philiastides MG, Sajda P. Quality of evidence for perceptual decision making is indexed by trial-to-trial variability of the EEG. Proc Natl Acad Sci USA. 2009;106:6539–6544. doi: 10.1073/pnas.0812589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philiastides MG, Heekeren HR, Sajda P. Human Scalp Potentials Reflect a Mixture of Decision-Related Signals during Perceptual Choices. J Neurosci. 2014;34:16877–16889. doi: 10.1523/JNEUROSCI.3012-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eimer M, Mazza V. Electrophysiological correlates of change detection. Psychophysiology. 2005;42:328–342. doi: 10.1111/j.1469-8986.2005.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandberg K, Timmermans B, Overgaard M, Cleeremans A. Measuring consciousness: is one measure better than the other? Conscious Cogn. 2010;19:1069–1078. doi: 10.1016/j.concog.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 35.De Lange FP, van Gaal S, Lamme VAF, Dehaene S. How awareness changes the relative weights of evidence during human decision-making. PLoS Biol. 2011;9:e1001203. doi: 10.1371/journal.pbio.1001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melloni L, Schwiedrzik CM, Müller N, Rodriguez E, Singer W. Expectations change the signatures and timing of electrophysiological correlates of perceptual awareness. J Neurosci. 2011;31:1386–1396. doi: 10.1523/JNEUROSCI.4570-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Summerfield C, Egner T, Mangels J, Hirsch J. Mistaking a house for a face: neural correlates of misperception in healthy humans. Cereb Cortex. 2006;16:500–508. doi: 10.1093/cercor/bhi129. [DOI] [PubMed] [Google Scholar]
- 38.Ress D, Heeger DJ. Neuronal correlates of perception in early visual cortex. Nat Neurosci. 2003;6:414–20. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci. 1998;21:227–77. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 40.de Lafuente V, Jazayeri M, Shadlen MN. Representation of accumulating evidence for a decision in two parietal areas. J Neurosci. 2015;35:4306–4318. doi: 10.1523/JNEUROSCI.2451-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverstein BH, Snodgrass M, Shevrin H, Kushwaha R. P3b, consciousness, and complex unconscious processing. Cortex. 2015;73:216–227. doi: 10.1016/j.cortex.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Gosselin F, Schyns PG. Superstitious perceptions reveal properties of internal representations. Psychol Sci. 2003;14:505–509. doi: 10.1111/1467-9280.03452. [DOI] [PubMed] [Google Scholar]
- 43.Smith ML, Gosselin F, Schyns PG. Measuring internal representations from behavioral and brain data. Curr Biol. 2012;22:191–196. doi: 10.1016/j.cub.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 44.Benwell CSY, et al. Prestimulus EEG Power Predicts Conscious Awareness But Not Objective Visual Performance. eNeuro. 2017;4:1–17. doi: 10.1523/ENEURO.0182-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban FM. The method of constant stimuli and its generalizations. Psychol. Rev. 1910;17:229–259. doi: 10.1037/h0074515. [DOI] [Google Scholar]
- 46.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- 47.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- 48.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 49.Woo, C.-W., Roy, M., Buhle, J. T. & Wager, T. D. Distinct Brain Systems Mediate the Effects of Nociceptive Input and Self-Regulation on Pain. PLOS Biol. 13, e1002036. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.