Global phosphoproteomics on human testicular tissue was performed by using highly efficient titanium dioxide (TiO2) method coupled to LC-MS/MS. Mass spectrometry data were used to predict the most prominent kinases, and phosphoregulation was revealed as highly active during spermatogenesis. Protein kinases are good targets for pharmacological intervention in male fertility and testicular cancer progression. In fact, CDK12 and PAK4 were found to be potentially involved in the formation of sperm structures and cancer cell response to apoptosis, respectively.
Keywords: Phosphoproteome, Kinases*, Target identification, Phosphorylation, Developmental biology*, Human testis, Male reproduction, Spermatogenesis, TiOx enrichment
Graphical Abstract
Highlights
Identification of phosphoproteins and the most active kinases in the human testis.
Phosphoregulation by protein kinases is highly active during spermatogenesis.
CDK12 might be determinant for correct human sperm development and function.
PAK4 is a potential target for the treatment of testicular cancer progression.
Abstract
Spermatogenesis is a complex cell differentiation process that includes marked genetic, cellular, functional and structural changes. It requires tight regulation, because disturbances in any of the spermatogenic processes would lead to fertility deficiencies as well as disorders in offspring. To increase our knowledge of signal transduction during sperm development, we carried out a large-scale identification of the phosphorylation events that occur in the human male gonad. Metal oxide affinity chromatography using TiO2 combined with LC-MS/MS was conducted to profile the phosphoproteome of adult human testes with full spermatogenesis. A total of 8187 phosphopeptides derived from 2661 proteins were identified, resulting in the most complete report of human testicular phosphoproteins to date. Phosphorylation events were enriched in proteins functionally related to spermatogenesis, as well as to highly active processes in the male gonad, such as transcriptional and translational regulation, cytoskeleton organization, DNA packaging, cell cycle and apoptosis. Moreover, 174 phosphorylated kinases were identified. The most active human protein kinases in the testis were predicted both by the number of phosphopeptide spectra identified and the phosphorylation status of the kinase activation loop. The potential function of cyclin-dependent kinase 12 (CDK12) and p21-activated kinase 4 (PAK4) has been explored by in silico, protein-protein interaction analysis, immunodetection in testicular tissue, and a functional assay in a human embryonal carcinoma cell line. The colocalization of CDK12 with Golgi markers suggests a potential crucial role of this protein kinase during sperm formation. PAK4 has been found expressed in human spermatogonia, and a role in embryonal carcinoma cell response to apoptosis has been observed. Together, our protein discovery analysis confirms that phosphoregulation by protein kinases is highly active in sperm differentiation and opens a window to detailed characterization and validation of potential targets for the development of drugs modulating male fertility and tumor behavior.
Spermatogenesis is a complex cell differentiation process that takes place in the testis, within the seminiferous tubules (1). It includes tightly coordinated genetic, cellular, functional and structural changes to give rise to the highly specialized male gamete, the sperm cell (1–8). Spermatogenesis is divided in three main processes - mitosis, meiosis, and spermiogenesis - and includes different germ cell types that are mechanically and nutritionally supported by the somatic Sertoli cells (1, 9). Spermatogonia are in the basal part of the germinal epithelium, and experience successive mitotic divisions to undergo either self-renewal or differentiation to spermatocytes. Thereafter, spermatocytes go through two consecutive meiotic divisions, which results in the generation of haploid round spermatids. During the last step of spermatogenesis, spermatids elongate, most of the cytoplasm is lost, chromatin is extensively remodeled, and specialized structures for fertilization are formed, such as the flagellum and the acrosome (1, 3–5, 10, 11). At the end of the process, the spermatozoa are released to the lumen of the tubule, to continue the maturation in the epididymis (1, 12–16).
The process of spermatogenesis is very dynamic and disturbances in any of the steps would lead to fertility deficiencies. Therefore, it requires tight regulation at different levels. Although hormonal regulation of spermatogenesis by the hypothalamic-pituitary-testicular axis is well understood (17, 18, 27, 28, 19–26), other layers of regulation, such as signal transduction through phosphorylation, remain less well explored. Protein phosphorylation is well known to be involved in the regulation of cell cycle, cell growth, cell differentiation and cell death in many biological systems (29, 30). Also, a role for protein phosphorylation in the regulation of testis-specific events, such as the maintenance of the Sertoli cell blood testis barrier and basal ectoplasmic specializations, has been observed (31–33).
By adding phosphate groups to substrate proteins, protein kinases are key regulators of cell functions, and therefore, good targets for the modulation of spermatogenesis and the identification of potential causes of male infertility. During the past decades, many studies have been focused on specific kinase families, such as the mitogen-activated protein kinases (MAPKs), which are critical for sperm development (32, 34, 35). Other known kinases with roles in spermatogenesis are the cell cycle regulators POLO-like kinases (PLKs) (36, 37), the androgen receptor p21-activated kinase 6 (PAK6) (38–40), and the members of the testis-specific serine/threonine-protein kinase (TSSK) family, which have a role during the last stage of spermatogenesis called spermiogenesis (41). However, a large-scale identification of the phosphorylation events that occur in the human testis has not been conducted so far. The use of high-throughput techniques would provide an in-depth picture of the molecular regulation of spermatogenesis and identify additional kinases that might also be essential in the process. Phosphopeptide enrichment combined with MS has been used recently for the systematic analysis of the mouse testis phosphoproteome profile (37). However, because of biological and genetic differences that exist between rodent and primate spermatogenesis, the development of such a study in human testis is warranted.
In the present study we performed global phosphoproteomics on human testicular tissue with full spermatogenesis, to identify the most relevant signaling pathways taking place during the development of the male gamete. To this end, metal oxide affinity chromatography, using a highly efficient titanium dioxide (TiO2) method coupled to MS (42–44), was carried out to profile the phosphoproteome of human testes with histologically complete spermatogenesis. To further investigate phosphoregulation, MS data was used to predict the most active kinases in the human testis. Our study contributes to the understanding of the regulation of human spermatogenesis that is orchestrated by protein phosphorylation. In addition, the identification of the most active kinases in the human testis opens the window to a detailed characterization and validation of potential good targets for the development of drugs modulating male germ cell development and testicular cancer progression.
EXPERIMENTAL PROCEDURES
Biological Material
The human testis samples used in this study were donated after informed consent by patients undergoing bilateral orchidectomy as part of prostate cancer treatment at the AmsterdamUMC in Amsterdam (59–85 years old). Morphological analyses of the testes after eosin-hematoxylin staining showed normal full spermatogenesis in all cases, indicating a similar function to testis tissue of younger men in terms of generating testicular sperm. None of these men had previously received chemotherapy or radiotherapy. According to Dutch law and confirmed by the Dutch Central Committee on Research involving Human Subjects (CCMO), this spare tissue can be used for research purposes without further ethical approval, because no additional intervention is required to obtain the tissue. The testes from all patients were cut into small pieces, and either subsequently snap frozen at −196 °C for later analyses by MS or fixed for immunodetection.
Mouse testis samples were also included in this study for the validation of immunohistochemical analyses. Paraffin blocks of mouse testis from fertile adult C57BL/6 males were used from previous experiments that were approved by the animal ethical committee of the University of Utrecht. Testis tissues were fixed in diluted Bouin, before embedding in paraffin for immunohistochemical analyses.
The NCCIT cell line used for functional analysis represents an undifferentiated pluripotent cell type from embryonal carcinoma and was maintained in DMEM/F121 (Lonza, Basel, Switzerland), containing 10% FBS with penicillin and streptomycin (10.000 U/ml), at 37 °C under 5% CO2. Cells were cultured as described elsewhere (45).
Testis Tissue Lysis and Digestion
Human testicular tissue from three individuals was lysed in lysis buffer containing 9 m Urea, 20 mm HEPES pH 8.0, 1 mm Na3VO4 (orthovanadate), 2.5 mm Na4P2O7 (pyrophosphate), and 1 mm Na2C3H7PO6 (β-Glycerophosphate), by vortexing and sonication. After lysis, protein concentration was determined using the BCA method (ThermoPierce, Rockford, IL). Tissue lysates were reduced in 4.5 mm DTT for 30 min at 55 °C, cooled to room temperature, and alkylated in 11 mm iodoacetamide for 15 min in the dark. Subsequently, tissue lysates were diluted in 20 mm HEPES pH 8.0 to reduce the urea concentration to 2 m, and digested overnight with trypsin (Promega, Madison, WI) with an enzyme/protein ratio of 1:50 (w/w).
Phosphopeptide Enrichment by Titanium Dioxide (TiO2)
For phosphopeptide enrichment a titanium dioxide-based workflow that was previously benchmarked for its high reproducibility was applied (43). For each human testis tissue sample, 500 μg of tryptic lysate digests were acidified by adding TFA to a final concentration of 1% and incubated on ice for 15 min. Samples were desalted with 30 mg OASISTM HLB cartridges (Waters Corporation, Milford, MA), previously activated with 100% ACN and equilibrated with 0.1% TFA. Briefly, peptides were loaded in the cartridge, washed with 0.1% TFA, and eluted with 0.1% TFA/80% ACN solution. Subsequently, desalted peptides were diluted 1:1 with lactic acid solution (0.3 g/ml lactic acid, 0.07% TFA, 53% ACN). For TiO2 capture, 2.5 mg of TiO2 beads (GL sciences, Eindhoven, The Netherlands; 10 μm) were packed in a 200-μl pipette tip fitted with a 16G-needle punch of C8 EMPORETM SPE material at the narrow end. Tips containing the TiO2 bed were washed with 200 μl of 0.1% TFA/80% ACN and equilibrated with 200 μl of lactic acid solution. Desalted peptides were loaded in the tips in 5 cycles of 200 μl of peptide mixture and centrifuged at 1500 × g, for 4 min. The TiO2 bed with bound phosphopeptides was then washed, first with 200 μl lactic acid solution, and then with 200 μl 0.1% TFA/80% ACN. All steps were performed by centrifugation at 1500 × g, for 4 min. Phosphopeptides were eluted in two steps with 50 μl 0.5% piperidine (Thermo Fisher Scientific, Bremen, Germany) and 50 μl 5% piperidine, respectively, and subsequently quenched in 100 μl 20% H3PO4. Phosphopeptides were desalted using 200-μl pipette tips fitted with a 16G-needle punch of SDB-XC EMPORETM SPE material at the narrow end, which was previously washed with 20 μl 0.1% TFA/80% ACN and equilibrated with 20 μl 0.1% TFA. Phosphopeptides mixtures were loaded and centrifuged for 3 min at 1000 × g,. SDB-XC beds were then washed with 20 μl 0.1% TFA, and desalted phosphopeptides were eluted with 20 μl 0.1% TFA/80% ACN. Phosphopeptides were dried in a vacuum centrifuge and dissolved in 20 μl 0.5% TFA/4% ACN.
Nano LC-MS/MS
The two tryptic peptide extracts from each human testis biological replicate, corresponding to the global and the phosphopeptide-enriched fractions, were separated using an Ultimate 3000 nanoLC-MS/MS system (Dionex LC-Packings, Amsterdam, The Netherlands) harboring a 20-cm fused silica column (75 μm inner diameter) custom packed with 1.9-μm ReproSil-Pur C18-AQ silica particles (120 Å pore diameter; Dr. Maisch GmbH, Ammerbuch-Entringen, Germany). First, peptides were trapped on a 10-mm trap column (100-μm inner diameter), packed with 5-μm ReproSil-Pur C18-AQ silica particles (120-Å pore diameter) at 6 μl/min and 2% buffer B (buffer A: 0.5% acetic acid; buffer B: 80% acetonitrile, 0.5% acetic acid), and separated at 300 nl/min in a 10–40% buffer B gradient in 60 min (90 min inject-to-inject). Eluting peptides were ionized at a potential of + 2 kV and nanosprayed into a Q Exactive mass spectrometer (Thermo Fisher Scientific). Intact masses were measured in the Orbitrap at a resolution of 70,000 (at m,/z, 200), using an automatic gain control (AGC) target value of 3 × 106 charges. The top 10 peptide signals (excluding charge state 1+) were submitted for MS/MS in the Higher-Energy Collisional Dissociation cell, using a 1.6-amu isolation width and 25% normalized collision energy. MS/MS spectra were acquired in the Orbitrap with a resolution of 17,500 (at m,/z, 200), using an AGC target value of 2 × 105 charges and an underfill ratio of 0.1%. Dynamic exclusion was applied with a repeat count of 1 and an exclusion time of 30 s.
Protein Identification from MS/MS Data
MS/MS spectra obtained after nanoLC-MS/MS were searched against a Uniprot human reference proteome FASTA file (release September 2015, canonical and isoforms, 42122 entries) supplemented with a common contaminants FASTA file (245 entries) using MaxQuant 1.5.2.8 software (46). Enzyme specificity was set to trypsin, and up to two missed cleavages were allowed. Cysteine carboxamidomethylation was set as fixed modification, and serine/threonine/tyrosine phosphorylation, methionine oxidation, and N-terminal acetylation as variable modifications. Default MaxQuant settings were used, that is peptide precursor ions were searched with a maximum mass deviation of 4.5 ppm, and fragment ions with a maximum mass deviation of 20 ppm. Peptide and protein identifications and site localizations were filtered at a FDR of 1% using the decoy database strategy. Phosphosite localizations were considered unambiguous when the localization probability was > 0.75. The minimal peptide length was 7 amino acids, the minimum Andromeda score for modified peptides was 40, and the minimum delta score was 6. Proteins that could not be differentiated based on MS/MS spectra alone were grouped into protein groups (default MaxQuant settings). Peptide identifications were propagated across samples using the match-between-runs (MBR) option checked. Lysate searches were performed with the label-free quantification option selected.
Label-free Phosphopeptide Quantification
Phosphopeptides were quantified by counting MS/MS spectra (spectral counts) (48) or by their extracted ion intensities (“Intensity” in MaxQuant). For each sample the phosphopeptide intensities were normalized on the median log10 intensity of all identified peptides in the sample using the MaxQuant evidence file (“normalized intensity”). For protein expression analysis of the corresponding lysates, the spectral counts were normalized on the sum of the counts in each sample.
Kinase Ranking
To identify the most active kinases in the human testis, an analysis pipeline was implemented in R using custom tables with data extracted from web resources, including currently recognized protein kinases from KinBase (http://kinase.com) (49), and the official gene symbols from HGNC (http://www.genenames.org) (50). An algorithm provided on the Phomics website (http://phomics.jensenlab.org) (51) was used to identify kinase activation loop peptides.
Phosphopeptide data from the MaxQuant “modificationSpecificPeptides” table were combined with spectral count data calculated above. Table rows with phosphopeptide data linked to multiple UniProt-derived gene symbols were deconvoluted into separate rows with a single gene symbol that was mapped to the official HGNC-approved gene symbol. Redundant rows linking the same phosphopeptide to different UniProt gene symbols but the same HGNC-mapped symbol were removed. For kinome (phosphokinase) analysis, the table was then filtered for rows with phosphopeptides derived from protein kinases (as defined in KinBase). For activation loop analysis, the latter table was further filtered for peptides residing in the activation segment of the kinase, as determined with the help of the Phomics website.
The kinome- and activation loop-centric tables were reduced to the data for phosphopeptides detected in all three testis tissue replicates, and spectral counts from replicates were averaged. To consider multiple phosphorylations of the same peptide, a modified metric was calculated by multiplying mean spectral counts by the number of phosphomodifications. For each analysis, after aggregation of peptide data to the protein level, a bar graph of (modified) mean spectral counts for the top 20 phosphokinases was plotted. Stacked bars represent the cumulated value for a given kinase, with bar segments representing contributions by individual phosphopeptides.
Bioinformatic Analyses of Human Testis Proteome and Phosphoproteome Data
The lists of phosphopeptides and phosphoproteins identified for each biological replicate were compared and visualized using Venny 2.1 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). The total list of proteins identified in human testis from the three individuals (phosphorylated and nonphosphorylated proteins; 3 replicates) was combined with a previous protein report from one human testis donor published by others (52), to complete the catalogue of human testicular proteins. GO and pathway enrichment analyses were performed in DAVID v6.8 (53, 54). After Bonferroni correction for multiple comparisons, p, values <0.001 were considered significant. Protein-protein interaction networks were identified using the STRING 10.0 database (http://string-db.org/). Only those interactions with high confidence interaction score (score ≥ 0.7 according to STRING 10.0 indications) were included in the analysis. Proteins directly interacting with the targets of interest (that is CDK12 and PAK4), were classified according to their biological function using information available at the Uniprot Knowledgebase (UniProtKB/Swiss-Prot) website (http://www.uniprot.org).
Immunodetection of the Kinases CDK12 and PAK4 in Human and Mouse Testis Tissue
Two of the active kinases identified in the testis phosphoproteome were selected to be further studied by immunodetection procedures. Sections from fixed and embedded human and mouse testicular tissues were cut at 5 μm and mounted on superfrost plus slides (Menzel, Braunschweig, Germany), dried overnight at 37 °C, and stored at 4 °C until further use.
For immunohistochemical analyses, the testis sections were deparaffinated and treated with 0.3% hydrogen peroxide in PBS for 10 min, to inhibit endogenous peroxidase. Nonspecific adhesion sites were blocked in 5% BSA supplemented with 0.5% acetylated BSA and 5% goat serum for 1 h at room temperature. For CDK12 detection, testis sections were incubated with 2 μg/ml anti-CDK12 (orb317586; Biorbyt, San Francisco, CA) overnight at 4 °C. For PAK4 detection, sections were incubated with 4 μg/ml anti-PAK4 (sc-390507; Santa Cruz Biotechnology Inc, Santa Cruz, CA) overnight at 4 °C. Isotype controls containing the same concentrations of IgG were also included. Signal was visualized on sections by incubation with Powervision poly horseradish peroxidase conjugated anti-mouse/rabbit/rat antibody (Immuno Vision Technologies, Burlingame, CA) for 1 h at room temperature. Subsequently, 3,3′-diaminobenzidine (DAB) was used as a substrate, and hematoxylin as counterstain. Slides were examined using an Olympus BX41 bright field microscope (Olympus America Inc, Center Valley, PA).
For immunofluorescence analyses, deparaffinized slides were incubated with Trueblack (Biotium, Fremont, CA) autofluorescence quencher and superblock (AAA999, ScyTek Laboratories, Logan, UT) to block nonspecific staining. Slides were incubated with anti-CDK12 as described above in combination with 4 μg/ml anti-GM130 (sc-55590; Santa Cruz Biotechnology Inc) or 2 μg/ml VASA/DDX4 (sc-517247; Santa Cruz Biotechnology Inc) and DAPI as counterstain. Signal was visualized with donkey-anti-rabbit AF488 (for CDK12) or goat-anti-mouse AF555 (for GM130 and VASA/DDX4). Slides were examined using a Leica DM 5000B (Leica Microsystems, Amsterdam, The Netherlands).
MTS Cell Proliferation Assay After Apoptosis Induction on PAK4 siRNA NCCIT Transfected Cells
To assess the effect of PAK4 down-regulation on cell proliferation, NCCIT cells, an undifferentiated cell line from human embryonic carcinoma, were seeded in a 6-well plate at 2 × 105 cells/well and transfected the day after at 50% confluency. ON-TARGET Plus Human PAK4 siRNA (SMART pool, l-003615–00, Dharmacon, GE life sciences, Lafayette, CO) was used for transfection. As negative control, cells were transfected with ON-TARGET Plus Nontargeting siRNA #1 (d-001810–01-05). NCCIT cells were incubated in 2 ml transfection medium containing 10 nm siRNA diluted in DharmaFECT4 Transfection Reagent (Dharmacon) at 37 °C under 5% CO2 for 48 h, following manufacturer's instructions. The efficiency of the PAK4 siRNA transfection was determined by quantitative PCR. Briefly, cells were harvested in QIAzol Lysis Reagent (Qiagen GmbH, Hilden, Germany), and mRNA was isolated following manufacturer's indications and measured using the NanoDrop 2000c Spectophotometer (Thermo Fisher Scientific). Subsequently, the mRNA quality was monitored with the Agilent 2200 TapeStation System (Agilent Technologies, Santa Clara, CA). RT-PCR was performed with 0.5 μg of RNA and the iSCRIPTTM cDNA Synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCR analysis was conducted in a CFX96 thermocycler (Bio-Rad) with iQTM SYBR® Green Supermix (Bio-Rad), and the RT2 qPCR Primer Assay for Human PAK4 (Qiagen). Two analytical replicates were made. Expression levels of HNRNPK, NONO and NUDT21 were used as endogenous controls. Relative quantification with multiple reference gene normalization, inter-run calibration and error propagation was calculated in qbase+ (Biogazelle, Zwijnaarde, Belgium) according to Hellemans et al., (55).
Equal numbers of transfected NCCIT cells (either with PAK4 siRNA or nontargeting siRNA) were seeded in culture medium in a 96-wells plate. For the induction of apoptosis, medium was replaced the day after by either fresh regular culture medium (control, n, = 3), fresh medium containing 0.1% FBS (serum starvation, n, = 3), or fresh medium containing 100 mm Paclitaxel (cell death inducer by mitotic arrest; Sanbio, Uden, The Netherlands; n, = 3). Cells were then incubated for 48 h at 37 °C under 5% CO2. To detect the levels of cell proliferation after the different treatments, the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) was performed following the manufacturer's indications.
Experimental Design and Statistical Rationale
Human testicular tissue from three individuals with morphologically normal spermatogenesis was used for global phosphopeptide enrichment by the TiO2 method. This approach is known to have high reproducibility of phosphopeptide identification within three biological replicates, and no bias toward phosphorylated amino acid, sequence context, peptide length or hydrophobicity (43). Any potential source of technical variance associated to this procedure was discarded after additional quality analysis of the MS data obtained in this study. To reduce the impact of the biological variability of the testis samples on our results, only those phosphopeptides identified in all three donors were used for kinase ranking. The functional involvement of the identified proteins was predicted by enrichment analysis on GO and Reactome terms, in which the significance was calculated by an EASE Score corresponding to a one-tail Fisher Exact p, value. Bonferroni correction for multiple comparisons was applied and p, values <0.001 were considered significant. Further, protein-protein interactions were predicted using high confidence interaction score (score ≥0.7).
The characterization of the expression of two of the identified kinases, CDK12 and PAK4, in the human testis was conducted by immunohistochemical and immunofluorescence staining on testicular tissue from 3 men and 2 adult mice. Sections stained with isotype IgG antibodies served as controls.
The involvement of PAK4 in cell apoptosis response was explored in three biological replicates of NCCIT cells transiently transfected with PAK4 siRNA, for each of the tested conditions. Nontargeting siRNA was taken along as a control. Normal distribution was assumed, and data were statistically analyzed using a multiple t, test in Graphpad Prism 7 (GraphPad Software, La Jolla, CA). The results were corrected for multiple comparisons using the Holm-Sidak method. p, values <0.05 were considered significant.
RESULTS
Composition of the Human Testis Phosphoproteome and Proteome
The global phosphopeptide enrichment conducted in this study using the TiO2 method combined with LC-MS/MS analysis resulted in the identification of 8187 different phosphopeptides in the human testis with full spermatogenesis (Fig. 1A,, supplemental Table S1), corresponding to 2661 individual phosphoproteins (Fig. 1B,; supplemental Table S1). Of note, the percentage of identified phosphopeptides among the obtained hits in terms of intensity ranged from 90.45 to 92.11% (supplemental Fig. S1), indicating highly efficient phosphopeptide enrichment. The phosphosite localization analysis in MaxQuant assigned around 89.7% of the phosphosites to serines, 9.8% to threonines, and 0.5% to tyrosines (supplemental Fig. S1, supplemental Table S1). High quantitative correlations for the identified phosphoproteins were observed between the three donor samples included in this analysis, with Pearson correlation coefficients ranging from r, = 0.954 to r, = 0.968 and linear regression values ranging from r2 = 0.9093 to r2 = 0.9370 (supplemental Fig. S1). The overlap of the MS identification between the three donor samples was 47.4% for the phosphopeptide identification (3878 phosphopeptides), and 64.5% in terms of individual phosphoproteins (1716 phosphoproteins) (Fig. 1A,–1B,). Phosphopeptides belonging to the 3-replicate overlap showed higher spectral counts than those that were not identified in all three samples (supplemental Table S1).
Fig. 1.
Composition of the human testis phosphoproteome and proteome. A,, Venn diagram showing the number of phosphopeptides (based on spectral counting) identified by TiO2-based phosphoproteomics in the three human testicular tissue samples from donors with full spermatogenesis included in this study. B,, Total number of phosphoproteins identified from the testicular phosphopeptide list and the overlap within biological replicates. C,, Compilation of proteins identified in the human testis by LC-MS/MS approaches. The total list of proteins identified in the present study was combined with the previous report from Liu et al., (52). D,, Proportion of phosphoproteins identified within the total human testicular proteome (present study and Liu et al.,, 2013(52)).
MS/MS analysis of the total tissue lysates resulted in the identification of 3576 different proteins (supplemental Table S2). The combination of these proteins with the complete list of phosphoproteins resulted in a total of 5158 different proteins identified in at least one of the analyzed human testis tissue samples (Fig. 1C,). When this list of human testicular proteins was compared with the data reported previously by Liu and colleagues, who identified a total of 6985 different proteins using human testicular tissue from one individual with normal spermatogenesis (52), 1105 proteins were found exclusively detected in the present study (Fig. 1C,). The compiled list of testicular proteins (representing the combination of our study and that of Liu et al.,, 2013 (52)) resulted in a total of 8090 gene products identified so far in the human testis with full spermatogenesis by using MS techniques (Fig. 1C,). From these, 32.9% were found to be phosphorylated in this study (Fig. 1D,).
Functional Annotations of the Human Testis Phosphoproteome
The testis phosphoproteome was subjected to enrichment analyses of GO and Reactome terms with DAVID v6.8. By doing this, the phosphorylation events taking place in human testicular tissue were found to be functionally involved mainly in chromosome organization and DNA packaging, cell cycle, RNA splicing, and cellular response to stress (Fig. 2A,, supplemental Table S3). Interestingly, the process of spermatogenesis was also overrepresented. Specifically, 81 of the phosphoproteins identified in this study have been linked to sperm production and development (Bonferroni-corrected p, value < 0.0001; Fig. 2A,, supplemental Table S3). Further, the top 5 enriched Reactome pathways in this data set revealed the involvement of phosphorylated proteins in signaling by NGF and EGFR, the cell cycle, processing of capped intron containing pre-mRNA, and apoptosis (Table I).
Fig. 2.
Functional involvement of testicular phosphoproteins and kinase ranking. A,, Abundance of the most overrepresented biological processes in the human testis phosphoproteome, based on GO terms. Bonferroni corrected p, values < 0.001. The GO annotations are sorted by p, value (highest significance on the top), and by the number of gene products found in the human testis for each GO category. B,, Protein kinase ranking based on spectral counting of the phosphopeptides identified in all the three biological replicates. Stacked bars represent the cumulated value for a given kinase, with bar segments representing contributions by individual phosphopeptides from the same kinase. C,, Protein kinase ranking based on spectral counting of phosphopeptides belonging to the kinase activation loop. Only the phosphopeptides identified in all three biological replicates were used for kinase ranking. Stacked bars represent the cumulated value for a given kinase, with bar segments representing contributions by individual phosphopeptides from the same kinase.
Table I. Over-representation of Reactome terms in the human testis phosphoproteome data set. Analysis performed with DAVID v6.8. Reactome term: overrepresented pathway in the human phosphoproteome data set and the corresponding code; Gene count: number of genes corresponding to human phosphoproteins identified in the human testis that belong to the specific reactome category; Bonferroni p value: one-tail Fisher Exact p value after the application of the Bonferroni correction for multiple comparisons.
| Reactome term | Gene count | Bonferroni p, value |
|---|---|---|
| REACT_578:Apoptosis | 44 | 8.30E-05 |
| REACT_125:Processing of Capped Intron-Containing Pre-mRNA | 41 | 1.50E-05 |
| REACT_152:Cell Cycle, Mitotic | 85 | 1.10E-05 |
| REACT_9417:Signaling by EGFR | 28 | 9.70E-09 |
| REACT_11061:Signalling by NGF | 69 | 2.00E-11 |
According to GO cellular component enrichment analysis, testicular phosphoproteins seem to be located in all cell compartments (Table II). Remarkably, the spliceosome is overrepresented in the data set (Table II), which agrees with the enrichment of mRNA-related processes shown in Fig. 2A,.
Table II. Gene Ontology Cellular Component terms enrichment analysis of the human testis phosphoproteome data set. Analysis made with DAVID v6.8. GO Cellular component term: over-represented GO cellular component annotations in the human phosphoproteome data set; Gene count: number of genes corresponding to human phosphoproteins identified in the human testis that belong to GO Cellular component category; Bonferroni p value: one-tail Fisher Exact p value after the application of the Bonferroni correction for multiple comparisons.
| GO Cellular Component Term | Gene Count | Bonferroni p value |
|---|---|---|
| Nuclear lumen | 387 | 9.30E-43 |
| Cytosol | 349 | 4.20E-36 |
| Nucleoplasm | 244 | 1.60E-27 |
| Microtubule cytoskeleton | 160 | 1.70E-19 |
| Chromosome | 141 | 4.30E-19 |
| Cell junction | 134 | 3.30E-11 |
| Ribonucleoprotein complex | 123 | 1.20E-07 |
| Spliceosome | 42 | 1.00E-04 |
Prediction of the Most Active Human Kinases in the Testis Tissue
Among the phosphopeptides identified in the human testis, a subset was derived from protein kinase sequences. Specifically, phosphopeptides corresponding to 174 different kinases were identified in the human testis phosphoproteome data set, which covers the 32% of the human kinome. Kinases are the enzymes responsible for phosphorylation, and their activation depends also on their own phosphorylation state. In addition, the number of observed phosphopeptide MS/MS spectra shows for a certain kinase in the tissue is correlated to the activity of that kinase in a pathway (56). Taking this into account, MS/MS data from the subset of phosphopeptides identified in the three biological replicates (47.3% of the identified phosphopeptides; Fig. 1A,) were used for kinase ranking (supplemental Table S4). The top 20 most prominent phosphorylated kinases, according to the number of counted spectra, are shown in Fig. 2B,. Interestingly, most of those kinases were also found to be phosphorylated in the activation loop (Fig. 2C,, supplemental Table S5), which indicates the active state of these proteins in the human testis.
The human testicular kinase ranking conducted in this study identified some groups of protein kinases belonging to the same family, such as the MAPKs which are known to be critically involved in the regulation of spermatogenesis. Additional families for which multiple active kinases were identified in the human testis included cyclin-dependent kinases (CDKs), serine/threonine-protein kinases D (PRKDs), and p21-activated kinases (PAKs), among others (Fig. 2B,–2C,). Interestingly, based on information available at the Uniprot Knowledgebase (UniProtKB/Swiss-Prot) website, most of these predicted active kinases are functionally related to overrepresented GO annotations found in the total human testis phosphoproteome (Fig. 2A,). For instance, CDK12 is involved in mRNA splicing, and PAK4 in the regulation of apoptosis and cell cycle. Of note, neither of these two kinases has been studied in spermatogenesis so far. Regardless, both CDK12 and PAK4 have been reported to be highly expressed in testis and have been linked to other spermatogenesis-related proteins, such as Cyclin K and PAK6, respectively (information extracted from the UniProtKB Knowledgebase, http://www.uniprot.org; and The Human Protein Atlas, https://www.proteinatlas.org/). Therefore, the potential role of CDK12 and PAK4 in the human testis has been further explored in this study.
CDK12 Protein-Protein Interactions and Expression in the Testis
CDK12 was identified as one of the most prominent active kinases in the human testis. Specifically, 11 CDK12 phosphopeptides were identified, and one of them belongs to the kinase active loop (Fig. 2B,–2C,, supplemental Table S4, S5).
STRING analysis including the total human testicular proteome (phosphorylated and nonphosphorylated proteins identified in the present study and combined with the data set published previously by Liu and colleagues (52), Fig. 1C,) revealed high-confidence interactions of CDK12 with 23 human testicular proteins (Fig. 3A,). CDK12-interacting proteins were divided in three functional groups according to information available at the Uniprot Knowledgebase: (1) cell cycle regulation, (2) mRNA splicing, and (3) regulation of transcription (Fig. 3A,).
Fig. 3.
CDK12 protein-protein interactions and expression in the human testis. A,, Protein-protein interactions of CDK12 with human testicular proteins (left), predicted by STRING 10.0 database (confidence score > 0.7). The interacting proteins are distributed in functional groups according to data from the Uniprot Knowledgebase (UniProtKB/Swiss-Prot) (right). B,, A representative image of CDK12 immunohistochemistry in human testicular tissue with full spermatogenesis. Insets are a higher magnification of the CDK12 staining in specific round structures of human spermatocytes, indicated by arrows (left). The same concentration of IgG isotype was used as negative control (right). C,, Co-staining of CDK12 with the Golgi apparatus marker GM130 (left side) and the nuage marker DDX4 (right side). Note the co-localization of CDK12 surrounding Golgi structures, indicated by white arrowheads. Cells were counterstained with DAPI.
Immunohistochemical analysis of CDK12 revealed that it is expressed in distinct structures in late spermatocytes of the seminiferous tubules of the human testis (Fig. 3B,). Comparable staining was observed in mouse testicular tissue sections (supplemental Fig. S2), and not detected in the corresponding isotype IgG controls (Fig. 3B, and supplemental Fig. S2). Interestingly, using immunofluoresence microscopy, co-localization of CDK12 with the Golgi apparatus marker GM130 is observed (Fig. 3C,), whereas no overlap is detected with DDX4, a marker for nuage granules (a germ cell-specific organelle containing piRNAs and RNA-binding proteins; Fig. 3C,). Specifically, CDK12 seems to be localized to the surroundings of the Golgi apparatus (Fig. 3C,).
PAK4 Protein-Protein Interactions and Localization in the Testis
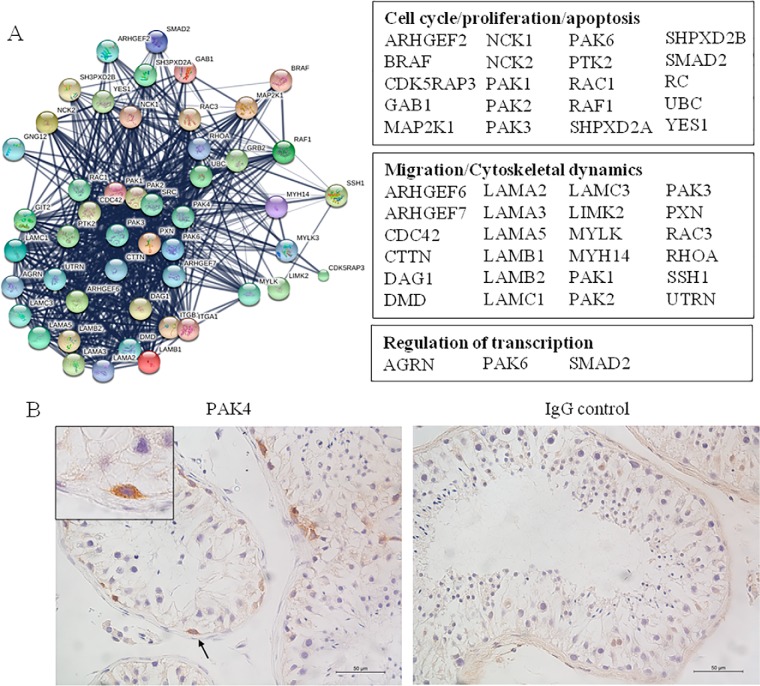
Three PAK4 phosphopeptides were identified by MS in the 3 donor samples (Fig. 2B,, supplemental Table S4), one of which represents the active loop of the kinase (Fig. 2C,, supplemental Table S5). STRING analysis revealed 48 high-confidence protein-protein interactions between PAK4 and other proteins identified in the human testis by MS approaches in this study as well as in Liu et al., (52) (Fig. 4A,). Predicted PAK4-interacting proteins were divided into three main groups, according to their corresponding biological function, as follows: (1) those involved in cell migration and cytoskeletal dynamics, (2) those involved in the regulation of transcription, and (3) those involved in the regulation of the cell cycle, proliferation and apoptosis (Fig. 4A,).
Fig. 4.
PAK4 protein-protein interactions and expression in the human testis. A,, Protein-protein interactions of PAK4 with human testicular proteins (left), predicted by STRING 10.0 database (confidence score > 0.7). The interacting proteins are categorized into functional groups according to data from the Uniprot Knowledgebase (UniProtKB/Swiss-Prot) (right). B, Representative image of PAK4 immunohistochemistry in human testicular tissue with full spermatogenesis. Insets are a higher magnification of the specific PAK4 staining in a subset of human spermatogonia, indicated by arrows (left). The same concentration of isotype IgG was used as negative control (right).
In seminiferous tubules, PAK4 expression was observed on a subset of human spermatogonia (Fig. 4B,). In mouse testis, PAK4 was detected also in Sertoli Cells, in addition to spermatogonia (supplemental Fig. S2). No staining in spermatogonia and Sertoli cells was observed in the corresponding isotype controls performed on human and mouse testis sections (Fig. 4B, and supplemental Fig. S2).
The Role of PAK4 in Embryonal Carcinoma Cells in Response to Apoptotic Stimuli
siRNA transfection targeting PAK4 in NCCIT cells resulted in a reduction of 77% of the PAK4 mRNA levels (Fig. 5A,). Transfected cells were incubated under either serum deprivation conditions (0.1% FBS medium) or 100 mm Paclitaxel, to induce cell stress and apoptosis. The competence of siPAK4-transfected NCCIT cells to respond to apoptotic stimuli was monitored by the measurement of the capacity of bioreduction of the MTS tetrazolium compound by metabolically active cells. Although the effect of transient PAK4 knock-down in NCCIT cells was reduced by the end of a 48-hour culture period (Fig. 5A,), the number of metabolically active (live) cells was significantly decreased (p, value <0.01; Fig. 5B,). Interestingly, major differences were observed after treatment with the apoptosis inducer Paclitaxel, with 60% less metabolically active cells in the transient knock-down cells compared with in control NCCIT cells (Fig. 5B,).
Fig. 5.

PAK4 role in embryonal carcinoma cell response to apoptotic stimuli. A,, Efficiency of transient knock-down of PAK4 at the mRNA level obtained after PAK4 siRNA transfection in NCCIT cells (siPAK4) compared with negative controls (siNT). PAK4 mRNA levels were determined right after transfection (Post-transfection), and after 48h in the presence of either regular culture medium (10% FBS medium), serum deprivation (0.1% FBS medium) or Paclitaxel in culture medium (100 mm Paclitaxel). B,, Determination of the response of NCCIT cells to apoptotic stimuli using the CellTiter 96® AQueous One Solution Cell Proliferation Assay. The OD490 values (after background subtraction) represent the proportion of metabolically active cells.
DISCUSSION
In the present study we show for the first time a comprehensive analysis of the phosphoproteome of the human testis with full spermatogenesis. The advances in MS-based approaches make phosphoproteomics the most powerful technique nowadays for the global analysis of signaling networks in defined biological systems (57). In the field of male reproduction, phosphoproteomics has been used to unravel the molecular mechanisms of sperm motility (58, 59). However, an in-depth study of the phosphoregulation in the human testis was not conducted so far. Through phosphopeptide enrichment using the TiO2 method combined with LC-MS/MS, we have identified 8187 phosphopeptides from 2661 proteins, resulting in the most complete report of the human testicular phosphoproteins to date. It is important to highlight that the global phosphopeptide enrichment approach conducted in this study has been proven to be highly reproducible and robust (43). Therefore, the limited overlap between the three donors described here for the identification of both the phosphopeptides and the individual proteins, exposes the intrinsic biological heterogeneity between different donors.
According to the results reported herein, phosphoproteins represent the 32.9% of the human testis proteome and are closely related to highly active processes in the male gonad, such as transcriptional and translational regulation, cytoskeleton organization, DNA packaging, cell cycle, and apoptosis. Of note, the term of spermatogenesis was also found overrepresented in the GO enrichment analysis conducted in the present study. This, together with the fact that the 32% of the human kinome was included in the human testicular phosphoproteome, confirms that phosphoregulation by protein kinases is highly active in cellular differentiation from spermatogonia to spermatozoa.
Interestingly, the functional involvement of phosphoproteins in human sperm development is like rodent spermatogenesis, because comparable functional annotations were observed previously in the mouse testis phosphoproteome (37). However, the main protein kinases orchestrating this regulation seem to be different between mouse and man. Although POLO-like kinases (PLKs) were found as highly active in the mouse testis (37), no human PLKs were found in the top 20 after kinase ranking in this study. Instead, we found MAPK1 and MAPK3 (also known as ERK2 and ERK1, respectively) as two of the most active kinases in the human testis, indicated by the abundance of their phosphopeptides and the presence of phosphosites in their kinase activation loop. These data are consistent with several publications reporting that MAPK1 and -3 play critical roles in spermatogenesis. Specifically, they are known to be involved in the regulation of mitosis, meiosis, and the Sertoli-Sertoli and Sertoli-germ cell interface (32, 34, 35). In addition, MAPK1 and -3 regulate cell proliferation after activation by Serine/threonine-protein kinase D1 (PRKD1) (60), which was also identified in the human testis kinase ranking.
Other highly prominent kinases in the human testis are known to be involved in the regulation of mRNA splicing in different cell types, such as the pre-mRNA processing factor 4B (PRPF4B) and cyclin-dependent kinases CDK12 and CDK13 (61–64). Alternative splicing allows a single gene to encode different or multiple proteins (65) and this is particularly important for complex cell differentiation processes that require tight regulation, as is the case in spermatogenesis (66, 67). In fact, testis is a tissue with one of the highest levels of alternative splicing in the body, together with brain and liver (68), which is consistent with the overrepresentation of this pathway in the human testis phosphoproteomic profile. Further in silico, analysis of CDK12 protein-protein interactions have also suggested such a role for CDK12 in the male gonad, as well as potential involvements in regulation of transcription and the cell cycle. CDK12 is a cyclin-dependent kinase which requires the interaction with a cyclin-regulatory partner to become active, such as the cyclin K (61, 62, 69, 70). In fact, the CDK12/cyclin K complex was found by others to be required for the regulation of DNA damage response genes through phosphorylation of the C-terminal domain of RNA polymerase II in mammalian cells (61, 69). Interestingly, potential roles of cyclin K in spermatogenesis were also suggested by others. In particular, cyclin K is known to be expressed in rodent testicular tissue in a developmentally regulated manner and ends with a specific localization in primary spermatocytes of adult testes (71). Here we found CDK12 associated with specific structures from human mid-to-late pachytene spermatocytes at epithelial stages VII-XII, according to the stage classification proposed by Muciaccia and colleagues (72). However, transcriptional activity becomes dramatically reduced at post-meiotic steps, which could suggest additional roles for this kinase in the human testis. Therefore, because of its confined expression in the human testis, as well as its function in other cell types and tissues, two localizations could be envisioned for CDK12 in the male gonad: either (1) an association with the nuage, a germ-cell specific organelle that stores RNA and RNA binding proteins and plays crucial roles in spermatogenesis, such as the regulation of transposon elements (73–75), or (2) the association with pro-acrosomal vesicle-related structures, such as the Golgi apparatus. To determine the localization of CDK12 we conducted immunofluorescence analyses, which revealed the specific localization of CDK12 surrounding the Golgi apparatus marker GM130, whereas no co-localization was observed with the nuage component DDX4. In mammals, Golgi structures develop into the acrosome during the last stages of spermatogenesis, which is a unique organelle containing various hydrolytic enzymes which, when secreted, allow sperm penetration of the oocyte zona pellucida (76, 77). Further, according to results reported by others (78), CDK12 expression in the human testis seems to be limited to structures that might be identified as pro-acrosomal granules. Therefore, and although the potential involvement of CDK12 in the initiation of acrosome biogenesis demand deeper investigation, the results observed in this study, together with the high expression of CDK12 in testis compared with other tissues as indicated in The Human Protein Atlas, would suggest a determinant role for this kinase in sperm development and function. CDK12 might thus be a potential pharmacological candidate for the development of drugs that modulate male fertility.
Protein kinases are heavily pursued targets for drug development because of their capacity to modulate signaling pathways in many diseases. Especially attractive candidates are those kinases that regulate cell survival and cell death, many of which have been identified in this study as highly abundant in the human testis. One of these is the serine/threonine-protein kinase PAK4, a member of the group II of the PAK family (79). Although PAK4 role has never been studied in the testicular environment, the results of the present study suggest a potential role in the regulation of cytoskeletal dynamics and cell survival in sperm development. Also, PAK4 was found to be expressed uniquely in human spermatogonia, which suggests an involvement of this kinase in the proliferative stage of spermatogenesis. Therefore, PAK4 might be involved in the initiation of sperm development and that its ablation would induce alterations in this process. In addition, PAK4 is known to protect cells from apoptosis, preventing the activation of caspase through two different mechanisms: (1) through phosphorylation of the pro-apoptotic protein Bad, which results in the deactivation of cytochrome-3 release and the blocking of the caspase cascade (80); and (2) by inhibiting caspase-8 in a kinase-independent manner (81). In a similar fashion, PAK4 can also protect cancer cells from apoptosis, and thus, represents a potential therapeutic target for the treatment of malignancies. In fact, PAK4 is the only PAK family member that is considered oncogenic, and it has been found overexpressed in several tumor cell lines, such as those from breast and prostate (79, 82). Similarly, here we show that PAK4-deficient embryonal carcinoma cells are less capable to resist to apoptotic stimuli, leading to a decrease number of metabolic active cells. Collectively, we suggest that PAK4 inhibitors would provide an interesting pharmacological target for the treatment of testicular cancer.
Together, the comprehensive analysis of the human testicular phosphoproteome contributes to our molecular knowledge of human sperm development and allowed us to identify the main protein kinases involved in the phosphoregulation of spermatogenesis. Because human testis tissue from healthy fertile men for research purposes is scarce and difficult to obtain, the phosphoproteomic description of the human testis was conducted in testicular samples that, although showing morphological normal spermatogenesis, do not represent the population and age of healthy fertile men. Further studies are now required to identify age-derived changes as well as to relate the results described here with the fertilization capacity of sperm, which is also influenced by the genetic and epigenetic profile of the cells and post-testicular processes. Also, these results open a window to validate CDK12, PAK4, and additional predicted active kinases that have been identified in the human testis as potential candidates for pharmacological interventions in male fertility or testicular cancer.
DATA AVAILABILITY
The mass spectrometry data have been submitted to the ProteomeXchange Consortium (47) via the PRIDE partner repository, with the dataset identifier PXD010246 (https://www.ebi.ac.uk/pride/archive/projects/PXD010246).
Supplementary Material
Acknowledgments
We thank Dr Leendert H. J. Looijenga (Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands) for kindly providing the NCCIT embryonal carcinoma cell line.
Footnotes
* This work was supported by grants from EU-FP7-PEOPLE-2013-ITN 603568: “GROWSPERM” to A.M.M. van Pelt and B.J.H. Jansen.
 This article contains supplemental Figures and Tables. The authors declare no competing financial interest.
This article contains supplemental Figures and Tables. The authors declare no competing financial interest.
1 The abbreviations used are:
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- Fetal bovine serum
- FDR
- false discovery rate
- GO
- Gene Ontology
- IgG
- Immunoglobulin G
- MS/MS
- tandem mass spectrometry
- TiO2
- Titanium dioxide.
REFERENCES
- 1. de Kretser D. M., Loveland K. L., Meinhardt A., Simorangkir D., and Wreford N. (1998) Spermatogenesis. Hum. Reprod. 13, 1–8 [DOI] [PubMed] [Google Scholar]
- 2. Sutovsky P., and Manandhar G. (2006) in The Sperm Cell: Production, Maturation, Fertilization, Regeneration, eds De Jonge CJ, Barratt CL, pp 1–30, Cambridge University Press, New York [Google Scholar]
- 3. Oliva R. (2006) Protamines and male infertility. Hum. Reprod. Update 12, 417–435 [DOI] [PubMed] [Google Scholar]
- 4. Kimmins S., and Sassone-Corsi P. (2005) Chromatin remodelling and epigenetic features of germ cells. Nature 434, 583–589 [DOI] [PubMed] [Google Scholar]
- 5. Oliva R., and Dixon G. H. (1991) Vertebrate protamine genes and the histone-to-protamine replacement reaction. Prog. Nucleic Acids Res. Mol. Biol. 40, 25–94 [DOI] [PubMed] [Google Scholar]
- 6. Davies D. V., and Mann T. (1947) Functional development of accessory glands and spermatogenesis. Nature 160, 295. [DOI] [PubMed] [Google Scholar]
- 7. Fawcett D. W., and Chemes H. E. (1979) Changes in distribution of nuclear pores during differentiation of the male germ cells. Tissue Cell 11, 147–162 [DOI] [PubMed] [Google Scholar]
- 8. Oliva R., and Castillo J. (2011) Proteomics and the genetics of sperm chromatin condensation. Asian J. Androl. 13, 24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber J. E., Russell L. D., Wong V., and Peterson R. N. (1983) Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli–Sertoli and Sertoli–germ-cell relationships. Am. J. Anat. 167, 163–179 [DOI] [PubMed] [Google Scholar]
- 10. Oliva R., and Castillo J. (2011) in Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction, eds Zini A, Agarwal A, pp 45–60, Springer, New York [Google Scholar]
- 11. Soler-Ventura A., Castillo J., de la Iglesia A., Jodar M., Barrachina F., Ballesca J. L., and Oliva R. (2018) Mammalian sperm protamine extraction and analysis: a step-by-step detailed protocol and brief review of protamine alterations. Protein Pept. Lett. 25, 424–433 [DOI] [PubMed] [Google Scholar]
- 12. Holstein A. F., Schulze W., and Davidoff M. (2003) Understanding spermatogenesis is a prerequisite for treatment. Reprod. Biol. Endocrinol. 1, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saez F., Frenette G., and Sullivan R. (2003) Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J. Androl. 24, 149–154 [DOI] [PubMed] [Google Scholar]
- 14. Dacheux J. L., and Dacheux F. (2014) New insights into epididymal function in relation to sperm maturation. Reproduction 147, R27–42 [DOI] [PubMed] [Google Scholar]
- 15. Sullivan R., and Mieusset R. (2016) The human epididymis: Its function in sperm maturation. Hum. Reprod. Update 22, 574–587 [DOI] [PubMed] [Google Scholar]
- 16. Castillo J., Jodar M., and Oliva R. (2018) The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Update 24, 535–555 [DOI] [PubMed] [Google Scholar]
- 17. Cheng C. Y., and Mruk D. D. (2010) A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 6, 380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tilbrook A. J., and Clarke I. J. (2001) Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol. Reprod. 64, 735–742 [DOI] [PubMed] [Google Scholar]
- 19. Alves M. G., Rato L., Carvalho R. A., Moreira P. I., Socorro S., and Oliveira P. F. (2013) Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 70, 777–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlatt S., and Ehmcke J. (2014) Regulation of spermatogenesis: An evolutionary biologist's perspective. Semin. Cell Dev. Biol. 29, 2–16 [DOI] [PubMed] [Google Scholar]
- 21. Dierich A., Sairam M. R., Monaco L., Fimia G. M., Gansmuller A., LeMeur M., and Sassone-Corsi P. (1998) Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. 95, 13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abel M. H., Wootton A. N., Wilkins V., Huhtaniemi I., Knight P. G., and Charlton H. M. (2000) The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction 1. Endocrinology 141, 1795–1803 [DOI] [PubMed] [Google Scholar]
- 23. De Gendt K., Swinnen J. V., Saunders P. T. K., Schoonjans L., Dewerchin M., Devos A., Tan K., Atanassova N., Claessens F., Lecureuil C., Heyns W., Carmeliet P., Guillou F., Sharpe R. M., and Verhoeven G. (2004) A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. 101, 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holdcraft R. W. (2003) Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131, 459–467 [DOI] [PubMed] [Google Scholar]
- 25. Chang C., Chen Y.-T., Yeh S.-D., Xu Q., Wang R.-S., Guillou F., Lardy H., and Yeh S. (2004) Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. 101, 6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang R. S., Yeh S., Tzeng C. R., and Chang C. (2009) Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 30, 119–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Escott G., da Rosa L., and Loss E. (2015) Mechanisms of hormonal regulation of sertoli cell development and proliferation: a key process for spermatogenesis. Curr. Mol. Pharmacol. 7, 96–108 [DOI] [PubMed] [Google Scholar]
- 28. Rudolph L. M., Bentley G. E., Calandra R. S., Paredes A. H., Tesone M., Wu T. J., and Micevych P. E. (2016) Peripheral and central mechanisms involved in the hormonal control of male and female reproduction. J. Neuroendocrinol. 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fisher D., Krasinska L., Coudreuse D., and Novak B. (2012) Phosphorylation network dynamics in the control of cell cycle transitions. J. Cell Sci. 125, 4703–4711 [DOI] [PubMed] [Google Scholar]
- 30. Venerando A., Cesaro L., and Pinna L. A. (2017) From phosphoproteins to phosphoproteomes: a historical account. FEBS J. 284, 1936–1951 [DOI] [PubMed] [Google Scholar]
- 31. Wan H. T., Mruk D. D., Tang E. I., Xiao X., Cheng Y. H., Wong E. W. P., Wong C. K. C., and Cheng C. Y. (2014) Role of non-receptor protein tyrosine kinases in spermatid transport during spermatogenesis. Semin. Cell Dev. Biol. 30, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong C. H., and Cheng C. Y. (2005) Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: A review of recent data. Dev. Biol. 286, 1–15 [DOI] [PubMed] [Google Scholar]
- 33. Zhang H., Yin Y., Wang G., Liu Z., Liu L., and Sun F. (2014) Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep. 4, 4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walker W. H. (2010) Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 365, 1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xia W., and Cheng C. Y. (2005) TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Dev. Biol. 280, 321–343 [DOI] [PubMed] [Google Scholar]
- 36. Jordan P. W., Karppinen J., and Handel M. A. (2012) Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes. J. Cell Sci. 125, 5061–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qi L., Liu Z., Wang J., Cui Y., Guo Y., Zhou T., Zhou Z., Guo X., Xue Y., and Sha J. (2014) Systematic analysis of the phosphoproteome and kinase-substrate networks in the mouse testis. Mol. Cell. Proteomics 13, 3626–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee S. R., Ramos S. M., Ko A., Masiello D., Swanson K. D., Lu M. L., and Balk S. P. (2002) AR and ER interaction with a p21-activated kinase (PAK6). Mol. Endocrinol. 16, 85–99 [DOI] [PubMed] [Google Scholar]
- 39. Liu X., Busby J., John C., Wei J., Yuan X., and Lu M. L. (2013) Direct Interaction between AR and PAK6 in Androgen-Stimulated PAK6 Activation. PLoS ONE 8, e77367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schrantz N., Da Silva Correia J., Fowler B., Ge Q., Sun Z., and Bokoch G. M. (2004) Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling. J. Biol. Chem. 279, 1922–1931 [DOI] [PubMed] [Google Scholar]
- 41. Kueng P., Nikolova Z., Djonov V., Hemphill A., Rohrbach V., Boehlen D., Zuercher G., Andres A. C., and Ziemiecki A. (1997) A novel family of serine/threonine kinases participating in spermiogenesis. J. Cell Biol. 139, 1851–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kyono Y., Sugiyama N., Imami K., Tomita M., and Ishihama Y. (2008) Successive and selective release of phosphorylated peptides captured by hydroxy acid-modified metal oxide chromatography. J. Proteome Res. 7, 4585–4593 [DOI] [PubMed] [Google Scholar]
- 43. Piersma S. R., Knol J. C., de Reus I., Labots M., Sampadi B. K., Pham T. V., Ishihama Y., Verheul H. M. W., and Jimenez C. R. (2015) Feasibility of label-free phosphoproteomics and application to base-line signaling of colorectal cancer cell lines. J. Proteomics 127, 247–258 [DOI] [PubMed] [Google Scholar]
- 44. Sugiyama N., Masuda T., Shinoda K., Nakamura A., Tomita M., and Ishihama Y. (2007) Phosphopeptide enrichment by aliphatic hydroxy acid-modified metal oxide chromatography for nano-LC-MS/MS in proteomics applications. Mol. Cell. Proteomics 6, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 45. Eini R., Stoop H., Gillis A. J. M., Biermann K., Dorssers L. C. J., and Looijenga L. H. J. (2014) Role of SOX2 in the etiology of embryonal carcinoma, based on analysis of the NCCIT and NT2 cell lines. PLoS ONE 9, e83585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cox J., and Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 47. Vizcaíno J. A., Deutsch E. W., Wang R., Csordas A., Reisinger F., Ríos D., Dianes J. A., Sun Z., Farrah T., Bandeira N., Binz P. A., Xenarios I., Eisenacher M., Mayer G., Gatto L., Campos A., Chalkley R. J., Kraus H. J., Albar J. P., Martinez-Bartolomé S., Apweiler R., Omenn G. S., Martens L., Jones A. R., and Hermjakob H. (2014) ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu H., Sadygov R. G., and Yates J. R. (2004) A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193–4201 [DOI] [PubMed] [Google Scholar]
- 49. Manning G., Whyte D. B., Martinez R., Hunter T., and Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 50. Gray K. A., Yates B., Seal R. L., Wright M. W., and Bruford E. A. (2015) Genenames.org: The HGNC resources in 2015. Nucleic Acids Res. 43, D1079–D1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Munk S., Refsgaard J. C., Olsen J. V., and Jensen L. J. (2016) in Methods in Molecular Biology, pp 307–321, Springer, New York: [DOI] [PubMed] [Google Scholar]
- 52. Liu M., Hu Z., Qi L., Wang J., Zhou T., Guo Y., Zeng Y., Zheng B., Wu Y., Zhang P., Chen X., Tu W., Zhang T., Zhou Q., Jiang M., Guo X., Zhou Z., and Sha J. (2013) Scanning of novel cancer/testis proteins by human testis proteomic analysis. Proteomics 13, 1200–1210 [DOI] [PubMed] [Google Scholar]
- 53. Huang D. W., Sherman B. T., and Lempicki R. A. (2009) Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang D. W., Sherman B. T., and Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 55. Hellemans J., Mortier G., De Paepe A., Speleman F., and Vandesompele J. (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rikova K., Guo A., Zeng Q., Possemato A., Yu J., Haack H., Nardone J., Lee K., Reeves C., Li Y., Hu Y., Tan Z., Stokes M., Sullivan L., Mitchell J., Wetzel R., MacNeill J., Ren J. M., Yuan J., Bakalarski C. E., Villen J., Kornhauser J. M., Smith B., Li D., Zhou X., Gygi S. P., Gu T. L., Polakiewicz R. D., Rush J., and Comb M. J. (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 [DOI] [PubMed] [Google Scholar]
- 57. Macek B., Mann M., and Olsen J. V. (2009) Global and site-specific quantitative phosphoproteomics: principles and applications. Annu. Rev. Pharmacol. Toxicol. 49, 199–221 [DOI] [PubMed] [Google Scholar]
- 58. Amaral A., Castillo J., Ramalho-Santos J., and Oliva R. (2014) The combined human sperm proteome: Cellular pathways and implications for basic and clinical science. Hum. Reprod. Update 20, 40–62 [DOI] [PubMed] [Google Scholar]
- 59. Parte P. P., Rao P., Redij S., Lobo V., D'Souza S. J., Gajbhiye R., and Kulkarni V. (2012) Sperm phosphoproteome profiling by ultra performance liquid chromatography followed by data independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J. Proteomics 75, 5861–5871 [DOI] [PubMed] [Google Scholar]
- 60. Jadali A., and Ghazizadeh S. (2010) Protein kinase D is implicated in the reversible commitment to differentiation in primary cultures of mouse keratinocytes. J. Biol. Chem. 285, 23387–23397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen H.-H., Wang Y.-C., and Fann M.-J. (2006) Identification and characterization of the CDK12/Cyclin L1 complex involved in alternative splicing regulation. Mol. Cell. Biol. 26, 2736–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen H. H., Wong Y. H., Geneviere A. M., and Fann M. J. (2007) CDK13/CDC2L5 interacts with L-type cyclins and regulates alternative splicing. Biochem. Biophys. Res. Commun. 354, 735–740 [DOI] [PubMed] [Google Scholar]
- 63. Corkery D. P., Holly A. C., Lahsaee S., and Dellaire G. (2015) Connecting the speckles: Splicing kinases and their role in tumorigenesis and treatment response. Nucleus 6, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schneider M., Hsiao H. H., Will C. L., Giet R., Urlaub H., and Lührmann R. (2010) Human PRP4 kinase is required for stable tri-snRNP association during spliceosomal B complex formation. Nat. Struct. Mol. Biol. 17, 216–221 [DOI] [PubMed] [Google Scholar]
- 65. Andreadis A., Gallego M. E., and Nadal-Ginard B. (1987) Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu. Rev. Cell Biol. 3, 207–242 [DOI] [PubMed] [Google Scholar]
- 66. Huang X., Li J., Lu L., Xu M., Xiao J., Yin L., Zhu H., Zhou Z., and Sha J. (2005) Novel development-related alternative splices in human testis identified by cDNA microarrays. J. Androl. 26, 189–196 [DOI] [PubMed] [Google Scholar]
- 67. Venables J. P. (2002) Alternative splicing in the testes. Curr. Opin. Genet. Dev. 12, 615–619 [DOI] [PubMed] [Google Scholar]
- 68. Yeo G., Holste D., Kreiman G., and Burge C. B. (2004) Variation in alternative splicing across human tissues. Genome Biol 5, R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blazek D., Kohoutek J., Bartholomeeusen K., Johansen E., Hulinkova P., Luo Z., Cimermancic P., Ule J., and Peterlin B. M. (2011) The cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 25, 2158–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loyer P., Trembley J. H., Katona R., Kidd V. J., and Lahti J. M. (2005) Role of CDK/cyclin complexes in transcription and RNA splicing. Cell. Signal. 17, 1033–1051 [DOI] [PubMed] [Google Scholar]
- 71. Xiang X., Deng L., Zhang J., Zhang X., Lei T., Luan G., Yang C., Xiao Z. X., Li Q., and Li Q. (2014) A distinct expression pattern of cyclin K in mammalian testes suggests a functional role in spermatogenesis. PLoS ONE 9, e101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Muciaccia B., Boitani C., Berloco B. P., Nudo F., Spadetta G., Stefanini M., de Rooij D. G., and Vicini E. (2013) Novel stage classification of human spermatogenesis based on acrosome development1. Biol. Reprod. 89, 60. [DOI] [PubMed] [Google Scholar]
- 73. Yokota S. (2012) Nuage proteins: their localization in subcellular structures of spermatogenic cells as revealed by immunoelectron microscopy. Histochem. Cell Biol. 138, 1–11 [DOI] [PubMed] [Google Scholar]
- 74. Onohara Y., and Yokot S. (2012) in Meiosis - Molecular Mechanisms and Cytogenetic Diversity, InTech. [Google Scholar]
- 75. Soper S. F. C., van der Heijden G. W., Hardiman T. C., Goodheart M., Martin S. L., de Boer P., and Bortvin A. (2008) Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev. Cell 15, 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Berruti G., and Paiardi C. (2011) Acrosome biogenesis. Spermatogenesis 1, 95–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leuchtenberger C., and Schrader F. (1950) The chemical nature of the acrosome in the male germ cells. Proc. Natl. Acad. Sci. U.S.A. 36, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang H., Wan H., Li X., Liu W., Chen Q., Wang Y., Yang L., Tang H., Zhang X., Duan E., Zhao X., Gao F., and Li W. (2014) Atg7 is required for acrosome biogenesis during spermatogenesis in mice. Cell Res. 24, 852–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wells C. M., and Jones G. E. (2010) The emerging importance of group II PAKs. Biochem. J. 425, 465–473 [DOI] [PubMed] [Google Scholar]
- 80. Gnesutta N., Qu J., and Minden A. (2001) The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J. Biol. Chem. 276, 14414–14419 [DOI] [PubMed] [Google Scholar]
- 81. Gnesutta N., and Minden A. (2003) Death receptor-induced activation of initiator caspase 8 is antagonized by serine/threonine kinase PAK4. Mol. Cell. Biol. 23, 7838–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Callow M. G., Clairvoyant F., Zhu S., Schryver B., Whyte D. B., Bischoff J. R., Jallal B., and Smeal T. (2002) Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277, 550–558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry data have been submitted to the ProteomeXchange Consortium (47) via the PRIDE partner repository, with the dataset identifier PXD010246 (https://www.ebi.ac.uk/pride/archive/projects/PXD010246).