Abstract
Purpose
Although Fuchs corneal dystrophy (FCD) is considered an eye disease, a small number of studies have identified genes related to both FCD and hearing loss. Whether FCD is related to hearing loss is unknown.
Method
This is a case–control study comparing pure-tone audiometry hearing thresholds in 180 patients with FCD from a hospital-based ophthalmology clinic with 2,575 population-based controls from a nationally representative survey, the National Health and Nutrition Examination Survey (from cycles 2005-06 and 2009-10). Generalized estimating equations were used to compare mean better-hearing ear thresholds in the 2 groups adjusted for age, sex, race, and noise exposure.
Results
Patients with FCD had higher hearing thresholds (worse hearing) in lower frequencies (mean difference at 0.5 kHz = 3.49 dB HL) and lower hearing thresholds (better hearing) in higher frequencies (difference at 4 kHz = −4.25 dB HL) compared with population-based controls.
Conclusion
In the first study to use objectively measured hearing, FCD was associated with poorer low-frequency and better high-frequency audiometric thresholds than population controls. Further studies are needed to characterize this relationship.
Fuchs' corneal dystrophy (FCD) is a genetic disorder of the corneal endothelium resulting in a reduced density of endothelial cells and thickening of Descemet's membrane, which may lead to corneal transplantation. FCD is the most common corneal dystrophy, affecting about 4% of the population over 40 years of age. FCD is more common in women and tends to present in the sixth decade (Gipson, 2013; Musch, Niziol, Stein, Kamyar, & Sugar, 2011). FCD is inherited in an autosomal dominant pattern, although some cases present as sporadic (Cross, Maumenee, & Cantolino, 1971; Eghrari & Gottsch, 2010).
Recent genetic literature shows that genes associated with hearing loss are also related to FCD, suggesting a genetic link for hearing loss as a possible phenotype of FCD (Iliff, Riazuddin, & Gottsch, 2012; Riazuddin et al., 2012). A 2011 study from the Netherlands linked FCD with the subjective impact of hearing loss using the Hearing Handicap Inventory for the Elderly-Screening; authors reported a significant association of perceived hearing loss disability with FCD after adjusting for age, noise exposure, and diabetes (odds ratio = 1.97, 95% CI [1.04, 3.75]; Stehouwer, Bijlsma, & Van der Lelij, 2011). Although some congenital corneal dystrophies have been linked with sensorineural hearing loss (SNHL), no study has used objective hearing measures to assess hearing in patients with FCD (Desir et al., 2007; Grillet et al., 2009; Riazuddin et al., 2012). This pilot project represents the first study using objective audiometric data to quantify and describe the potential association of FCD with hearing impairment. The aim of this study is to characterize the objective audiometric measurements associated with FCD compared with population averages in a case–control study design.
Method
Participants
FCD cases were recruited at the Johns Hopkins Wilmer Eye Institute Cornea Clinic, where all patients with FCD were offered onsite audiogram (from January 1, 2013, to December 31, 2015). Patients with a history of hearing aid use were allowed to submit a recent (< 1 year) audiogram performed in another clinic in lieu of onsite audiogram. Population-based controls (matched on demographic characteristics) were obtained using the combined 2005-06 (AUX D and AUQ D Data Files) and 2009-10 (AUX F and AUQ F Data Files) cycles of the National Health and Nutrition Examination Survey (NHANES), a nationally representative cross-sectional survey assessing health in the noninstitutionalized, civilian population.
Two hundred four patients with FCD were recruited, 180 of whom were included in this analysis. Six were excluded for cerumen impaction; and two, for recent chemotherapy. In addition, two left the clinic without completing an audiogram, one provided unreliable test results, four hearing aid users failed to submit outside audiograms, six failed to complete questionnaire data, and three were excluded because of older age (> 84 years) that exceeded the range of NHANES controls. Six patients submitted recent audiograms performed at outside locations by a licensed audiologist.
There were 40,857 records provided in the initial data set of potential NHANES control participants. Of these, 32,775 were excluded because of missing or incomplete audiometric measures. Of the remaining participants, 3,227 were excluded for failing to match to FCD cases (2,614 did not match in race, and 2,713 did not match in age). The remaining 2,575 participants were analyzed in the control cohort. A subset of controls was frequency-matched (2:1) to patients with FCD on age (by deciles in patients with FCD), race, and sex.
Procedure and Materials
This study was approved by the Johns Hopkins Institutional Review Board. Audiograms at the Johns Hopkins Wilmer Cornea Clinic were performed in a double-walled sound booth calibrated to American National Standards Institute S3.1-1991 for maximum permissible noise levels. After otoscopy, audiometric thresholds were obtained on a calibrated AD629 Interacoustics audiometer with E-A-RTONE 3A insert earphones using modified Hughson–Westlake procedures at 0.5, 1, 2, 4, and 8 kHz bilaterally (Carhart & Jerger, 1959). Likewise, objective air-conduction pure-tone audiometric data were collected in a soundproof mobile booth on a subset of NHANES participants. Notably, NHANES uses both supra-aural and insert earphones (in the case of collapsed canals). NHANES participants completed questionnaires (NHANES AUQ D and AUD F Data Files) to determine the history of personal and/or occupational noise exposure and history of firearm use. A survey replicating these questions was administered to the patients with FCD for comparison (Centers for Disease Control and Prevention, 2011). Using a conservative approach, an answer of “yes” to any of these three questions was coded as positive noise exposure in this study, regardless of reported use of hearing protection. Hearing data were summarized as a better-hearing ear pure-tone average (PTA) of 0.5, 1, 2, and 4 kHz to represent functional hearing at important speech frequencies. In addition, hearing was modeled as the individual frequencies (0.5–8 kHz) in the better-hearing ear.
Data Analysis
Linear regression was used to model mean differences in PTA comparing patients with FCD with NHANES controls, adjusting for age, sex, race, and noise exposure. Model assumptions were assessed using component residual plots and smoothed residual plots. Robust standard errors were used to address heteroscedasticity.
Generalized estimating equations were used to estimate mean hearing thresholds across all frequencies in the better-hearing ear, accounting for the correlation between multiple frequency measures in an individual. An independence working correlation structure was specified, but standard errors are consistent even when the working correlation structure is misspecified and the residuals are heteroskedastic. Models were adjusted for age, sex, race, noise exposure, and an interaction term between age and sex. In addition, interaction terms were included between each covariate and frequency to allow the estimated effect of FCD to vary by frequency. Adequacy of fit was assessed using smoothed residual plots.
Data were analyzed using R Version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results and Discussion
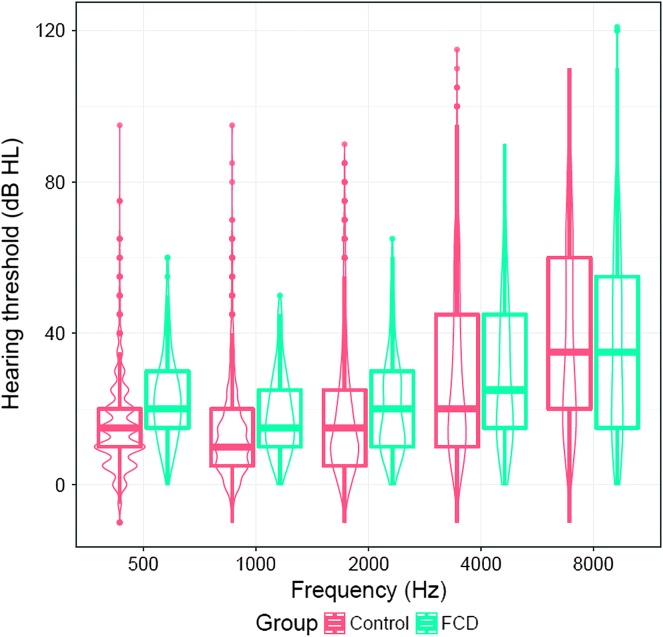
On average, patients with FCD were older (67 vs. 59 years of age) and more likely to be female (72% vs. 50%) and White (vs. Black) (91% vs. 73%) as compared with controls (see Table 1). In unadjusted comparisons, patients with FCD had higher thresholds (worse hearing) at lower frequencies (see Table 1 and Figure 1).
Table 1.
Descriptive statistics of the analytic cohort.
| Characteristics | FCD (n = 180) | Control (n = 2,575) |
|---|---|---|
| Demographics | ||
| Age (years), M (SD) | 67 (8.9) | 59 (12.1) |
| Female sex, n (%) | 130 (72) | 1,275 (50) |
| Male sex, n (%) | 50 (28) | 1,300 (50) |
| White race, n (%) | 164 (91) | 1,883 (73) |
| Black race, n (%) | 16 (9) | 692 (27) |
| Audiometric measures | ||
| History of noise exposure, n (%) | 72 (40) | 1,238 (48) |
| Better-ear PTA, M (SD) | 23 (10.9) | 18.9 (13.2) |
| 0.5-kHz threshold, M [IQR] | 20 [15, 30] | 15 [10, 20] |
| 1 kHz, M [IQR] | 15 [10, 25] | 10 [5, 20] |
| 2 kHz, M [IQR] | 20 [10, 30] | 15 [5, 25] |
| 4 kHz, M [IQR] | 25 [15, 45] | 20 [10, 45] |
| 8 kHz, M [IQR] | 35 [15, 55] | 35 [20, 60] |
Note. FCD = Fuchs corneal dystrophy; PTA = pure-tone average (defined as four-frequency in the better-hearing ear); IQR = interquartile range.
Figure 1.
Unadjusted violin plot of the distribution of hearing thresholds in patients with Fuchs corneal dystrophy (FCD) and population-based controls. Boxplots represent an interquartile range with the middle line as the median number.
In a multivariable-adjusted analysis, the average difference in PTA between patients with FCD and controls was 0.19 dB HL (95% CI [−1.25, 1.62], p = .798; see Table 2). In generalized estimating equation models of the relationship between mean hearing thresholds at each of the five frequencies (0.5, 1, 2, 4, and 8 kHz), after full adjustment, the association between FCD and mean audiometric thresholds varied by frequency, with participants with FCD exhibiting higher thresholds (worse hearing) at low frequencies and lower thresholds (better hearing) at high frequencies. At 0.5 kHz, the difference comparing patients with FCD with controls was 3.49 dB HL (95% CI [1.83, 5.15]). By comparison, the adjusted difference comparing patients with FCD with controls was −4.25 dB HL (95% CI [−6.94, −1.68]) and −11.11 dB HL (95% CI [−14.23, −7.98]) at 4 and 8 kHz, respectively.
Table 2.
Differences in mean better-hearing–ear hearing thresholds between participants with Fuchs corneal dystrophy and population-based controls.
| Hearing | Estimate (dB HL) | Lower CI (dB HL) | Upper CI (dB HL) | p value: difference = 0 |
|---|---|---|---|---|
| PTA a | 0.19 | −1.25 | 1.62 | .798 |
| Individual frequencies | ||||
| 0.5 kHz | 3.49 | 1.83 | 5.15 | < .0001* |
| 1 kHz | 1.12 | −0.41 | 2.64 | .1530 |
| 2 kHz | −0.87 | −2.74 | 1.00 | .3628 |
| 4 kHz | −4.31 | −6.94 | −1.68 | .0013* |
| 8 kHz | −11.11 | −14.23 | −7.98 | < .0001* |
Note. Models were adjusted for age, sex, race, and noise exposure.
PTA was defined as a four-frequency (0.5, 1, 2, and 4 kHz) pure-tone average in the better-hearing ear.
p < .05.
Discussion
In a clinical cohort of 180 FCD cases and 2,575 population-based controls from a nationally representative U.S. survey, patients with FCD had worse hearing at lower frequencies and better hearing at higher frequencies. These findings represent the first frequency-specific characterization of objectively measured hearing loss among patients with FCD.
A previous 2011 case–control study found that 72 patients with FCD had 1.97 times the odds of significant subjective handicap from hearing impairment compared with 144 control patients without FCD after adjusting for age, noise exposure, and diabetes mellitus (Stehouwer et al., 2011). Notably, subjective hearing results do not always correlate well with audiometric data (Kamil, Genther, & Lin, 2015).
The biological mechanism by which FCD could potentially, differentially affect hearing at different frequencies is unknown; however, it is possible that similar mechanisms could underlie both FCD and hearing loss. FCD is a disease of ion transporters in corneal endothelial cells. Corneal endothelial cells contain multiple ion pumps regulating H+, Na+, K+, Cl−, and HCO3 − to maintain osmotic balance. In at least a subset of FCD, the Na+/K+ ATPase is downregulated, leading to endothelial cell edema and eventually death (Bonanno, 2012). Similarly, the cochlea contains a variety of ion channels. The stria vascularis generates the endocochlear potential through multiple ion channels through maintaining a high-potassium ion concentration in the endolymph (Wangemann, 2006). The main hair cell signal transduction channel is a nonspecific cation channel that allows passage of principally potassium ions from the endolymph leading to hair cell depolarization. Previous literature has implicated the stria vascularis' involvement in low-frequency hearing loss (Schuknecht & Gacek, 1993; Schuknecht et al., 1974).
In addition, there may also be a genetic link between FCD and hearing loss. However, molecular mechanisms accounting for these complex results are unlikely to be a single gene or protein mutation. Several genetic alleles responsible for both corneal dystrophy and SNHL have been reported. SLC4A11 encodes for an ion transporter that regulates sodium and hydroxide ion cell entry as well as sodium and borate co-transport (Desir et al., 2007; Grillet et al., 2009). Homozygous mutations in SLC4A11 can lead to Harboyan syndrome, a congenital endothelial dystrophy with the progressive SNHL, whereas heterozygous mutations in SLC4A11 have been linked to late-onset FCD (Vithana et al., 2006). SLC4A11 ion transporter defects interfere with the osmotic regulation of the endothelial cells, leading to endothelial cell death and corneal clouding. A mouse model of SLC4A11 mutant mice demonstrated impaired potassium transport through the fibrocytes underlying the stria vascularis, leading to reduced endocochlear potentials (Gröger et al., 2010). There are many other common ion channels to both the corneal endothelium and cochlea, including the chloride channel and calcium activated channel, ATP1A1 sodium/potassium ATP-ase, and KCNJ10 potassium channel (Bonanno, 2012; Hatou et al., 2013; Kim & Marcus, 2010; Wang, Zhang, Su, & Lin, 2014; Wangemann, 2006). Future studies are needed to elucidate how shared ion channels might contribute to common corneal and cochlear pathology.
Although no known mutations would lead to the variable hearing impact observed in participants with FCD in this study, both FCD and genetic SNHL are heterogeneous. Some, but not all, mutations that lead to FCD also cause hearing loss. It is possible that an as-yet-undiscovered mutation leads to FCD and hearing preservation in the lower audiometric frequencies. Because this study did not separate patients by genotype, these results only present an aggregate of hearing patterns in all patients with FCD, and therefore there are likely many pathophysiologic factors leading to this audiometric result.
Nonbiologic explanations for results are also possible, including unmeasured and residual confounding. Although patients with FCD and NHANES controls were measured using different sound booths and equipment, systematic measurement error in either study group is not expected given regular calibration of equipment. Calibration of Wilmer Eye Institute was also checked post–data collection to ensure there are no differential effects by frequency.
Our study is limited in that pure-tone thresholds were obtained via air conduction only so conductive component contributions, although unlikely, are unmeasured. Furthermore, patients with FCD represent a clinical convenience sample and may not be representative of the entire population with FCD. Moreover, the ecological design of this study does limit the results as different equipment was used. Future research should include cases and controls matched on potentially confounding variables and assessed on the same equipment to improve internal validity, as transducer type may impact results. The cross-sectional design does not allow us to determine the temporal sequence between the exposure and the outcome. Further longitudinal research is needed to confirm the results and elucidate the relationship between FCD and hearing loss. Future research should also include comprehensive audiometric assessment, including middle ear function, to rule out confounding from conductive components and more specifically describe the relationship.
Although the current research is limited and speculative in its nature as a first-in-kind study, it does have potential implications for clinical care following a more methodologically rigorous study. First, hearing loss may serve as a biomarker for FCD, and thus hearing testing should be encouraged among persons seeking ophthalmologic care. Second, hearing care could be an aspect of recommended care for patients with FCD. There is no known cure for FCD, but surgical treatment in the form of total or partial corneal replacement is possible. However, even with surgical options, vision may not be completely corrected. In the cases of persistent vision problems, hearing care, including amplification and appropriate counseling, should be recommended to treat any comorbid hearing loss to maximize the potential for sensory substitution measures (i.e., relying on hearing when vision is limited).
Conclusion
Compared with population-based controls, patients with FCD had poorer lower-frequency hearing and better high-frequency hearing. There is a plausible genetic basis for this relationship as well as numerous shared structures in the cornea and cochlea that could link FCD with hearing loss. The nature of this relationship should be further studied and replicated in methodologically rigorous future studies.
Acknowledgments
The work was supported by National Eye Institute Grant R01 EY016835 and Kwok Research Fund, both awarded to John D. Gottsch, and the Johns Hopkins University Cochlear Center for Hearing and Public Health. Dr. Deal was supported by National Institutes of Health/National Institute on Aging Grant K01AG054693. Dr. Reed was supported by National Institutes of Health Grant 5KL2TR001077-05.
Funding Statement
The work was supported by National Eye Institute Grant R01 EY016835 and Kwok Research Fund, both awarded to John D. Gottsch, and the Johns Hopkins University Cochlear Center for Hearing and Public Health. Dr. Deal was supported by National Institutes of Health/National Institute on Aging Grant K01AG054693. Dr. Reed was supported by National Institutes of Health Grant 5KL2TR001077-05.
References
- Bonanno J. A. (2012). Molecular mechanisms underlying the corneal endothelial pump. Experimental Eye Research, 95(1), 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart R., & Jerger J. (1959). Preferred method for clinical determination of pure-tone thresholds. Journal of Speech and Hearing Disorders, 24, 330–345. [Google Scholar]
- Centers for Disease Control and Prevention. (2011). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Audiometry Questionnaire. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Retrieved from https://www.cdc.gov/nchs/nhanes/index.htm [Google Scholar]
- Cross H. E., Maumenee A. E., & Cantolino S. J. (1971). Inheritance of Fuchs' endothelial dystrophy. Archives of Ophthalmology, 85(3), 268–272. [DOI] [PubMed] [Google Scholar]
- Desir J., Moya G., Reish O., Van Regemorter N., Deconinck H., David K. L., … Abramowicz M. J. (2007). Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. Journal of Medical Genetics, 44(5), 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghrari A. O., & Gottsch J. D. (2010). Fuchs' corneal dystrophy. Expert Review of Ophthalmology, 5(2), 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson I. K. (2013). Age-related changes and diseases of the ocular surface and cornea. Investigative Ophthalmology & Visual Science, 54(14), ORSF48–ORSF53. [DOI] [PubMed] [Google Scholar]
- Grillet N., Schwander M., Hildebrand M. S., Sczaniecka A., Kolatkar A., Velasco J., … Müller U. (2009). Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans. The American Journal of Human Genetics, 85(3), 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger N., Fröhlich H., Maier H., Olbrich A., Kostin S., Braun T., & Boettger T. (2010). SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. Journal of Biological Chemistry, 285(19), 14467–14474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatou S., Yoshida S., Higa K., Miyashita H., Inagaki E., Okano H., … Shimmura S. (2013). Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/β-catenin signaling. Stem Cells and Development, 22(5), 828–839. [DOI] [PubMed] [Google Scholar]
- Iliff B. W., Riazuddin S. A., & Gottsch J. D. (2012). The genetics of Fuchs' corneal dystrophy. Expert Review of Ophthalmology, 7(4), 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil R. J., Genther D. J., & Lin F. R. (2015). Factors associated with the accuracy of subjective assessments of hearing impairment. Ear and Hearing, 36(1), 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. X., & Marcus D. C. (2010). Inward-rectifier chloride currents in Reissner's membrane epithelial cells. Biochemical and Biophysical Research Communications, 394(2), 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch D. C., Niziol L. M., Stein J. D., Kamyar R. M., & Sugar A. (2011). Prevalence of corneal dystrophies in the United States: Estimates from claims data. Investigative Ophthalmology & Visual Science, 52(9), 6959–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S. A., Parker D. S., McGlumphy E. J., Oh E. C., Iliff B. W., Schmedt T., … Gottsch J. D. (2012). Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. The American Journal of Human Genetics, 90(3), 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht H. F., & Gacek M. R. (1993). Cochlear pathology in presbycusis. Annals of Otology, Rhinology & Laryngology, 102(1, Pt. 2), 1–16. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. F., Watanuki K., Takahashi T., Belal A. A. Jr., Kimura R. S., Jones D. D., & Ota C. Y. (1974). Atrophy of the stria vascularis, a common cause for hearing loss. Laryngoscope, 84, 1777–1821. [DOI] [PubMed] [Google Scholar]
- Stehouwer M., Bijlsma W. R., & Van der Lelij A. (2011). Hearing disability in patients with Fuchs' endothelial corneal dystrophy: Unrecognized co-pathology? Clinical Ophthalmology (Auckland, NZ), 5, 1297–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithana E. N., Morgan P., Sundaresan P., Ebenezer N. D., Tan D. T., Mohamed M. D., … Aung T. (2006). Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2). Nature Genetics, 38(7), 755–757. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang C., Su X., & Lin D. (2014). Kcnj10 is a major type of K+ channel in mouse corneal epithelial cells and plays a role in initiating EGFR signaling. American Journal of Physiology-Cell Physiology, 307(8), C710–C717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. (2006). Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. The Journal of Physiology, 576(1), 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]