Abstract
Background.
The contemporary limb outcomes and costs of stent-based vs non-stent based strategies in endovascular revascularization of femoropopliteal (FP) peripheral artery disease (PAD) are not well understood.
Methods and Results.
We present data from the ongoing United States multicenter Excellence in Peripheral Artery Disease Registry between 2006–2016 to compare stent vs non-stent treatment outcomes and associated costs in FP interventions. A total of 2910 FP interventions were performed in 2162 patients (mean age, 66 years), comprising 1339 stent based (superficial femoral artery, 93%) in 1007 patients and 1571 non-stent interventions (superficial femoral artery, 85%) in 1155 patients. A growing trend for non-stent based interventions and a declining trend in repeat revascularization rate at 1 year were observed across years of registry enrollment. Stent implantation was the prevailing strategy in treating longer FP lesions (mean length, 152 mm vs 105 mm; P<.001) and chronic total occlusions (65% vs 40%; P<.001), while stent implantation was employed less frequently when treating in-stent restenotic lesions (14% vs 20%; P<.001). Stent and non-stent interventions had similar 1-year limb outcomes in all-cause death, target-limb revascularization, target-vessel revascularization, and major or minor amputation. The average procedure costs for the stent group were significantly higher than the non-stent group ($6215 vs $4790; P<.001).
Conclusion.
There is a growing trend for non-stent FP artery interventions, with a significant decline in 1-year target-limb revascularization rates over time. One-year limb outcomes in stent-based compared to non-stent interventions are similar; however, at a significantly higher procedural cost.
Keywords: chronic total occlusions, superficial femoral artery
Endovascular intervention is a prevailing strategy over surgical revascularization for treatment of patients with symptomatic peripheral artery disease (PAD).1 Along with an exponential growth in the number of lower-extremity (LE) interventions over the last decade in the United States, numerous new interventional devices have been approved for specific use in the femoropopliteal (FP) segment.2 Recognition of the dynamic nature, anatomic specifications, and treatment challenges of the superficial femoral (SFA) and popliteal arteries has fueled the development of dedicated new stents, including drug-eluting stent (DES) options, novel atherectomy technologies, and drug-coated balloon (DCB) options. Despite a proliferation of novel devices, the optimal treatment approach to these lesions remains elusive. Knowledge of how and when contemporary stent and non-stent modalities are applied, together with clinical outcomes and comparative costs, is crucial to guide contemporary clinical decision-making and for developing cost-effective paradigms for the treatment of patients with symptomatic PAD.
This study compares the contemporary use of stent vs non-stent strategies in endovascular revascularization of FP-PAD and its associated limb outcomes and costs using ongoing multicenter data from the Excellence in Peripheral Artery Disease (XLPAD) registry.
Methods
We analyzed data on FP interventions included in the XLPAD registry (NCT01904851). This ongoing multicenter registry includes demographic, medication, procedure, and 1-year clinical outcomes data from 23 United States hospital sites. Both retrospective and prospective data collection are permitted as per the decision of each participating site institutional review board that has provided approval to the study. Currently, 85% of the data are retrospectively collected and 15% are prospectively collected. All patient data are entered into a secure online data collection REDCap portal hosted and managed at the University of Texas Southwestern Medical Center, Dallas, Texas.3 The registry requires submission of all clinically indicated procedure angiograms and Duplex ultrasounds (DUS) that are independently adjudicated by the Angiographic and Ultrasound Core Laboratory at Veterans Affairs North Texas Health Care System in Dallas, Texas. Periodic data audits and query resolution are performed and both remote and on-site monitoring of sites is conducted. Access to registry information and data-entry portal is provided through www.XLPAD.org.
Use of a stent in FP intervention designates the procedure as stent based, while absence of stent use classifies it as a non-stent procedure. All commercially available bare-metal stent (BMS) options, DES options, conventional (plain-old) balloon angioplasty (PTA), scoring or cutting balloons, atherectomy, and DCB options are included in the registry. Descriptions of XLPAD registry variables have been previously described.4
Lesion-crossing strategy, guidewire type(s), support catheter(s), debulking, balloon, DCB and stent device(s), anticoagulation regimen, and anti-platelet therapy treatments were at the discretion of the operator. We also compared technical success, procedural success, and patient outcomes between stent and non-stent groups. Technical success is defined as placement of a guidewire in the distal true lumen, past the occluded infrainguinal artery segment (in chronic total occlusion [CTO] lesions), confirmed by either angiography or intravascular ultrasound (IVUS). Procedural success is defined as successful revascularization of the lesion with ≤30% angiographic residual diameter stenosis. Patient outcome measures include periprocedural complications and limb outcomes. Periprocedural complication is defined as the occurrence of a flow-limiting dissection, arterial perforation, access-site hematoma, retroperitoneal hematoma, distal embolization, major bleed requiring blood transfusion, or emergency surgery. Clinical outcomes include all-cause mortality, clinically driven endovascular target-vessel revascularization (TVR), and target-limb revascularization comprising surgical and endovascular revascularization, and major or minor amputations of the target limb. The primary outcome measure of this analysis is 12-month target-limb revascularization.
Statistical analysis.
Descriptive statistics of continuous variables are presented as mean ± standard deviation, and categorical variables as frequencies and percentages. T-statistics and Chi-square statistics were conducted to test group differences in continuous and categorical variables, respectively. Time to event analyses used Cox proportional hazard model and cumulative incidence function (CIF) plots. This analysis examines presence of competing risk for events. To address competing risks, we used Cox models with sub-distributional hazard functions introduced by Fine and Gray.5 These hazard functions were estimated using two groups of patients: (1) those who have not experienced the specific adverse event, adverse limb events (TVR and amputation); and (2) those who have experienced a competing risk event, death, previously. This strategy differs from Kaplan-Meier patient group analysis, which only considers patients who are free from both adverse limb events and death at each month.
Trends of limb outcomes and device use patterns were depicted using annual rates. Annual rates of outcomes and medical device use were calculated as numbers of events and device use divided by a total number of interventions, respectively. One-year rates of all-cause death, repeat endovascular or surgical revascularization, and major and minor amputation were plotted against annual rates of device use, BMS, DES, DCB, and atherectomy, and PTA use was plotted separately, with annual rates of any use of PTA and PTA use without other adjunctive devices (PTA only). Non-linear regression was used to test a linear trend of each limb outcome and its breaking points (SAS Proc NLIN). When a linear trend was rejected by a fit diagnostic test, piecewise regressions were then conducted to examine a change in a trend for each limb outcome. Following graphic analysis results, breaking points (years of change) in trends of limb outcomes were selected and tested. Years of 2008, 2009, 2010, 2012, and 2014 were tested as breaking points for all limb outcomes. Year 2008 was selected for 1-year repeat endovascular or surgical revascularization and mortality outcomes, year 2012 for 1-year major amputation, and year 2013 for 1-year minor amputation.
Costs of procedure were calculated based on a methodology using micro-costs calculated by procedural information collected in the XLPAD registry and prices of devices and expenditures obtained from the Dallas VA Medical Center, and are consistent with non-VA hospital costs.6 All costs were converted to 2015 constant dollars. To calculate an estimated benefit of stent use, this study utilized an opportunity-cost concept.7 The opportunity costs of using stents were captured as difference in estimated numbers of TVR, target-limb surgical revascularization, and major and minor amputation at 1 year, separately, between the stent and non-stent groups. We then calculated net costs (costs — benefits) for each adverse event following a cost-benefit analysis, as previously published by our group.7 Opportunity costs for use of stents compared to no use of stents were calculated as a difference in estimated numbers of all clinical adverse events at 12 months times the average cost of each intervention. Estimated numbers of clinical adverse event per procedure were calculated by a logistic model per stent use as follows: Y (clinical adverse event = yes) = b0 + b1⋆group (stent vs no-stent group) + b2⋆age + b3⋆gender + b4⋆diabetes + b5⋆smoking status + b6⋆hypertension + b7⋆hypercholester- olemia + b8*lesion length + error, where b values are estimates. Mean and bootstrap 95% confidence intervals (CIs) of net cost per group were estimated. Bootstrap method with 1000 resampling technique was used to construct 95% CIs. All statistical tests were 2-sided and P-value <.05 was set as the criterion for statistical significance; analyses were conducted using SAS version 9.4 (SAS Institute).
Results
This analysis from the XLPAD registry reports on 2910 interventions performed in 2162 patients between 2006 and 2016. Baseline characteristics of patients are depicted in Table 1A. Over 51% patients had diabetes mellitus and 25% are women. Over one-half of the interventions (55%) were performed in claudicants (Rutherford category [RC] 2–3) and one-third (27%) were performed in critical limb ischemia (CLI) patients (RC 4–6). Approximately 18% had unreported RC. Mean ankle-brachial index (ABI) was 0.75 ± 0.23. Patients who underwent stent-based infrainguinal interventions had a significantly lower ABI at baseline compared with the non-stent group.
Table 1A.
Patient characteristics in stent and non-stent treatment groups.
| Demographic and Clinical Characteristics | All (n = 2162) | Stent Based (n = 1007) | Non-Stent Based (n = 1155) | P-Value |
|---|---|---|---|---|
| Age (years) | 66.2 ± 10.2 | 64.6 ± 9.6 | 67.3 ± 10.4 | <.001 |
| Female | 532 (24.6%) | 208 (20.7%) | 324 (28.1%) | <.001 |
| Caucasians | 1435 (66.4%) | 675 (67.0%) | 884 (62.6%) | .61 |
| African-Americans | 460 (21.3%) | 214 (21.3%) | 246 (21.3%) | |
| Hispanics | 187 (8.7%) | 84 (8.3%) | 103 (8.9%) | |
| Other | 33 (1.5%) | 17 (1.7%) | 16 (1.4%) | |
| Unknown race | 47 (2.2%) | 17 (1.7%) | 30 (2.6%) | |
| Hypertension | 1925 (89.0%) | 903 (89.7%) | 1022 (88.5%) | .38 |
| Diabetes mellitus | 1102 (51.0%) | 512 (50.8%) | 590 (51.1%) | .91 |
| Hyperlipidemia | 1761 (81.5%) | 817 (81.1%) | 944 (81.7%) | .72 |
| Chronic kidney disease | 323 (14.9%) | 140 (13.9%) | 183 (15.8%) | .21 |
| Coronary artery disease | 1307 (60.5%) | 594 (59.0%) | 713 (61.7%) | .19 |
| Heart failure | 338 (15.6%) | 175 (17.4%) | 163 (14.1%) | .04 |
| Prior non-fatal MI | 487 (22.5%) | 254 (25.2%) | 233 (21.1%) | <.01 |
| Prior stroke | 170 (7.9%) | 72 (7.2%) | 98 (8.5%) | .25 |
| Rutherford class I | 3 (0.1%) | 2 (0.2%) | 1 (0.1%) | .22 |
| Rutherford class II-III | 1197 (55.4%) | 584 (58.0%) | 613 (53.1%) | |
| Rutherford class IV-VI | 583 (27.0%) | 261 (12.1%) | 322 (27.9%) | |
| Rutherford class unknown | 379 (17.5%) | 160 (15.9%) | 219 (19.0%) | |
| Ankle-brachial index | 0.75 ± 0.23 | 0.72 ± 0.19 | 0.77 ± 0.23 | <.001 |
| Toe-brachial index | 0.51 ± 0.28 | 0.47 ± 0.23 | 0.53 ± 0.28 | .34 |
| Medications | ||||
| Aspirin | 1180 (54.6%) | 625 (62.1%) | 555 (41.6%) | <.001 |
| Dual-antiplatelet therapy | 646 (29.9%) | 328 (32.6%) | 318 (27.5%) | .01 |
| Clopidogrel | 623 (28.9%) | 302 (30.0%) | 321 (27.8%) | .26 |
| Anticoagulation therapy | 120 (5.6%) | 56 (5.6%) | 64 (5.5%) | .98 |
| Warfarin | 74 (3.4%) | 34 (3.4%) | 40 (3.5%) | .91 |
| Cilastazol | 59 (2.7%) | 37 (3.7%) | 22 (1.9%) | .01 |
| Lipid-lowering therapy | 1139 (52.7%) | 596 (59.2%) | 543 (47.0%) | <.001 |
| Statin therapy | 1090 (50.4%) | 580 (57.6%) | 510 (44.2%) | <.001 |
| ACEI/ARB | 862 (39.9%) | 475 (47.2%) | 387 (33.5%) | <.001 |
| Beta-blockers | 857 (39.6%) | 472 (46.6%) | 385 (33.3%) | <.001 |
Data presented as mean ± standard deviation or number (%).
Stent-based and non-stent procedures completed 1-year follow-up. P-values based on Chi-square statistics. ACEI/ARB = angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers; MI = myocardial infarction.
The SFA was the most frequent intervention target (89%) and was more frequently treated with a stent-based strategy (93% vs 85%; P<.001) (Table 1B). Concurrent iliac artery and below-the-knee (BTK) artery interventions were performed during 11% and <1% of FP procedures, respectively. Mean lesion length was 120.9 ± 92.8 mm and included nearly 51% CTO lesions. Use of atherectomy was significantly higher in the non-stent group (26% vs 51%; P<.001). Descriptions of treatment strategies in the stent and nonstent procedures are shown in Table 1B.
Table 1B.
Description of stent and non-stent based femoropopliteal procedures (completed 1-year follow-up).
| All (n = 2910) | Stent Based (n = 1339) | Non-Stent Based (n = 1571) | P-Value | |
|---|---|---|---|---|
| Target vessels | ||||
| Superficial femoral artery | 2582 (88.7%) | 1241 (92.7%) | 1341 (85.4%) | <.001 |
| Ostial | 376 (12.9%) | 185 (13.8%) | 191 (12.1%) | .18 |
| Proximal | 827 (28.4%) | 440 (32.9%) | 387 (24.6%) | <.001 |
| Mid | 858 (29.5%) | 418 (31.2%) | 440 (28.0%) | .06 |
| Distal | 734 (25.2%) | 338 (25.2%) | 396 (25.2%) | .98 |
| Popliteal | 336 (11.6%) | 99 (7.4%) | 237 (15.1%) | <.001 |
| Concurrent intervention | ||||
| Below the knee | 11 (0.4%) | 2 (0.2%) | 9 (0.6%) | .06 |
| Iliac artery | 319 (11.0%) | 148 (11.1%) | 171 (10.9%) | .88 |
| Common iliac artery | 155 (5.3%) | 63 (4.7%) | 92 (5.9%) | .17 |
| External iliac artery | 216 (7.4%) | 105 (7.8%) | 111 (7.1%) | .43 |
| Bilateral iliac artery | 103 (3.5%) | 39 (2.9%) | 64 (4.1%) | .09 |
| Below-the knee run-off vessels (n) | 2.27 ± 0.80 | 2.29 ± 0.80 | 2.26 ± 0.81 | .56 |
| Lesion description | ||||
| Mean lesion length (mm) | 120.9 ± 92.8 | 151.7 ± 94.8 | 105.0 ± 87.6 | <.001 |
| Lesions ≥100 mm in length | 1483 (51.0%) | 782 (58.4%) | 701 (44.6%) | <.001 |
| Lesions ≥200 mm in length | 629 (21.6%) | 347 (25.9%) | 282 (18.0%) | <.001 |
| In-stent restenosis | 498 (17.1%) | 188 (14.0%) | 310 (19.7%) | <.001 |
| Chronic total occlusion | 1487 (51.1%) | 863 (64.5%) | 624 (39.7%) | <.001 |
| Severe calcification | 1302 (44.7%) | 625 (46.7%) | 677 (43.1%) | .05 |
| Diffuse disease | 1787 (61.4%) | 849 (63.4%) | 938 (59.7%) | .04 |
| Treatment | ||||
| Chronic total occlusion crossing device | ||||
| Wire catheter | 1309 (40.1%) | 633 (48.7%) | 676 (34.4%) | <.001 |
| Crossing device | 474 (16.3%) | 271 (20.2%) | 203 (12.9%) | <.001 |
| Re-entry device use | 197 (6.8%) | 142 (10.6%) | 55 (3.5%) | <.001 |
| Plain old balloon angioplasty | 2520 (86.6%) | 1208 (90.2%) | 1312 (83.5%) | <.001 |
| Scoring-balloon angioplasty | 478 (16.4%) | 235 (17.6%) | 243 (15.5%) | .13 |
| Drug-coated balloon | 388 (13.3%) | 121 (9.0%) | 267 (17.0%) | <.001 |
| Atherectomy | 1215 (41.8%) | 343 (25.6%) | 872 (51.5%) | <.001 |
| Stent types | ||||
| Bare-metal stent | – | 836 (62.4%) | – | – |
| Drug-eluting stent | – | 233 (17.4%) | – | – |
| Vascular mimetic stent | – | 257 (19.2%) | – | – |
| Covered stent | – | 129 (9.6%) | – | – |
| Bail-out stenting | – | 110 (8.2%) | – | – |
| Stent length (mm) | 163.99 ± 120.97 | |||
| Number of stents | ||||
| 1 | 636 (47.5%) | |||
| 2 | 347 (25.9%) | |||
| 3 | 236 (17.6%) | |||
| 4 | 55 (4.1%) | |||
| 5 | 29 (2.2%) | |||
| Missing | 36 (2.7%) | |||
| Balloon length (mm) | 149.7 ± 111.4 | 164.5 ± 114.3 | 143.1 ± 109.5 | <.001 |
| Number of balloons | ||||
| 1 | 1441 (49.5%) | 708 (52.9%) | 733 (46.7%) | |
| 2 | 762 (27.0%) | 333 (24.9%) | 429 (27.3%) | |
| 3 | 412 (12.7%) | 201 (15.0%) | 211 (13.4%) | |
| No balloon or unknown | 295 (10.1%) | 198 (12.6%) | 97 (7.24%) | |
| Intravascular ultrasound | 187 (6.4%) | 105 (7.8%) | 82 (5.2%) | .01 |
| Embolic protection device | 751 (25.8%) | 236 (17.6%) | 515 (32.8%) | <.001 |
| Distal filter device | 648 (22.3%) | 180 (13.4%) | 468 (29.8%) | <.001 |
| Embolic protection balloon | 19 (0.7%) | 12 (0.4%) | 7 (0.5%) | .13 |
| Aspiration thrombectomy | 136 (4.7%) | 69 (5.2%) | 67 (4.3%) | .26 |
| Thrombolytic therapy | 235 (8.1%) | 144 (10.8%) | 91 (5.8%) | <.001 |
| Glycoprotein IIb/IIIa | 11 (0.4%) | 7 (0.5%) | 4 (0.3%) | .24 |
| Unfractionated heparin | 2330 (80.1%) | 1117 (83.4%) | 1213 (77.2%) | <.001 |
| Bivalirudin | 273 (9.4%) | 121 (9.0%) | 152 (9.7%) | .56 |
| Medication post intervention | ||||
| Aspirin | 2224 (76.4%) | 1045 (78.0%) | 1179 (75.1%) | .06 |
| Dual-antiplatelet therapy | 706 (24.3%) | 431 (32.2%) | 275 (17.5%) | <.001 |
| Clopidogrel | 861 (29.6%) | 505 (37.7%) | 356 (22.7%) | <.001 |
| Anticoagulation therapy | 51 (1.8%) | 24 (1.8%) | 27 (1.7%) | .88 |
| Warfarin | 33 (1.1%) | 15 (1.1%) | 18 (1.2%) | .95 |
| Cilastazol | 28 (10.0%) | 13 (10.0%) | 15 (0.9%) | .96 |
| Lipid-lowering therapy | 1889 (64.9%) | 899 (67.1%) | 990 (63.0%) | .02 |
| ACEI/ARB | 1282 (44.1%) | 626 (46.8%) | 656 (41.8%) | <.01 |
| Beta-blockers | 1349 (46.3%) | 649 (48.5%) | 700 (44.6%) | .04 |
Data presented as mean ± standard deviation or number (%).
Stent-based and non-stent procedures completed 1-year follow-up. P-values based on Chi-square statistics. ACEI/ARB = angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers.
Use of guideline-directed medical therapy post intervention was suboptimal across both groups; antiplatelet, lipid-lowering, angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEI/ARB), and beta-blocker therapies were significantly under-utilized in the non-stent group compared with the stent group. Baseline and postintervention medical therapies are depicted in Table 1A and Table 1B, respectively.
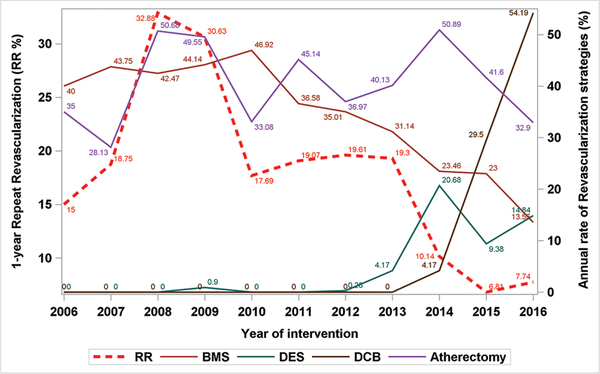
Stenting was more prevalent in long, CTO, heavily calcified, and diffuse lesions (Table 1B), but less frequent in restenotic lesions (14% vs 20%; P<.001). Although a stent-based treatment strategy was employed in 46% of interventions overall, Figure 1 shows a declining trend in stent use, and at the same time a rising trend in a non-stent approach during the registry data collection period. It also depicts an increased use of DES since 2013, DCB since 2014, and an overall upward trend for atherectomy use since 2008, with 50% peak and 28% lowest use in enrolled procedures. Piecewise regression results indicate that annual repeat revascularization (endovascular and surgical) rates increased until 2008 (slope = 8.94; P=.21), but decreased significantly in later years (slope = −2.98; P<.01) (Supplemental Figure S1; supplemental figures available at www.invasivecardiology.com). Trends for 1-year all-cause death, major amputation, and minor amputation were also tracked (Supplemental Figures 2–4).
FIGURE 1.
A time trend of 1-year repeat revascularization [RR] and treatment modalities from XLPAD registry between 2006 and 2016. BMS = bare-metal stent; DCB = drug-coated balloon; DES = drug-eluting stent.
Technical and procedure success, periprocedural complications, and 12-month mortality and limb outcomes in the stent and non-stent groups are shown in Table 2. Overall, the stent-based group has significantly higher rates of both technical and procedural success than the non-stent group. The residual dissection rate was significantly higher in the stent group than in the non-stent group (3.6% vs 2.3%; P=.04). While not statistically significant, rates of retroperitoneal hematoma, distal embolization, and acute renal failure in the stent group were numerically higher. There were no significant group differences in other complications.
Table 2.
Technical and procedural success, periprocedural complications, and 12-month major adverse events in stent and no-stent groups.
| Demographic and Clinical Characteristics | All (n = 2910) | Stent based (n = 1339) | Non-Stent Based (n = 1171) | P-Value |
|---|---|---|---|---|
| Technical success | 2763 (95.0%) | 1328 (99.1%) | 1435 (91.3%) | <.001 |
| Procedure success | 2706 (93.0%) | 1293 (96.6%) | 1413 (89.9%) | <.001 |
| Procedural complications | ||||
| Residual dissection | 84 (2.9%) | 48 (3.6%) | 36 (2.3%) | .04 |
| Access-site hematoma | 17 (0.6%) | 10 (0.8%) | 7 (0.5%) | .29 |
| Retroperitoneal hematoma | 9 (0.3%) | 7 (0.5%) | 2 (0.1%) | .06 |
| Bleeding diathesis | 3 (0.1%) | 3 (0.2%) | 0 (0.0%) | .60 |
| Distal embolization | 37 (1.3%) | 20 (1.5%) | 17 (1.1%) | .32 |
| Acute renal failure | 5 (0.2%) | 4 (0.3%) | 1 (0.1%) | .13 |
| Perforation | 15 (0.5%) | 7 (0.5%) | 8 (0.5%) | .96 |
| Emergency surgery | 4 (0.2%) | 1 (0.1%) | 3 (0.2%) | .37 |
| Death | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| Amputation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | - |
| 12-month adverse eventsa | ||||
| Death | 48 (2.2%) | 22 (2.2%) | 26 (2.3%) | .92 |
| Non-fatal myocardial infarction | 47 (1.6%) | 21 (1.6%) | 26 (1.7%) | .85 |
| Stroke | 8 (0.3%) | 2 (0.2%) | 6 (0.4%) | .23 |
| Target-limb revascularization | 417 (14.3%) | 201 (15.0%) | 216 (13.8%) | .33 |
| Endovascular | 361 (12.4%) | 169 (12.6%) | 192 (12.2%) | .74 |
| Surgical | 64 (2.2%) | 36 (2.7%) | 28 (1.8%) | .10 |
| Target-vessel revascularization | 360 (12.4%) | 169 (12.6%) | 191 (12.2%) | .70 |
| Target-limb amputation | 127 (4.4%) | 54 (4.0%) | 73 (4.7%) | .42 |
| Major | 54 (1.9%) | 21 (1.6%) | 33 (2.1%) | .29 |
| Minor | 82 (2.8%) | 37 (2.8%) | 45 (2.9%) | .87 |
Data presented as number (%).
Based on 2162 patients (2910 procedures) with completed 1-year follow-up. P-values based on Chi-square statistics.
At 1 year, all-cause mortality rates in the stent and non-stent groups were 2.2% and 2.3%, respectively (P=.92). The mean time to death from index procedure was 201.0 ± 149.6 days. The mean follow-up time for patients with complete 12-month follow-up and censored due to death was 358.4 ± 41.1 days. TVR rates were similar between stent and non-stent groups (12.6% vs 12.2%, respectively; P=.70). Overall, target-limb revascularization rates in stent and non-stent groups were similar (15.0% vs 13.4%, respectively; P=.23), while the surgical revascularization rate was higher in the stent group than the non-stent group (2.7% vs 1.8%, respectively; P=.097). Target-limb major and minor amputation rates were similar between stent and non-stent groups (4.0% vs 4.7%, respectively; P=.42).
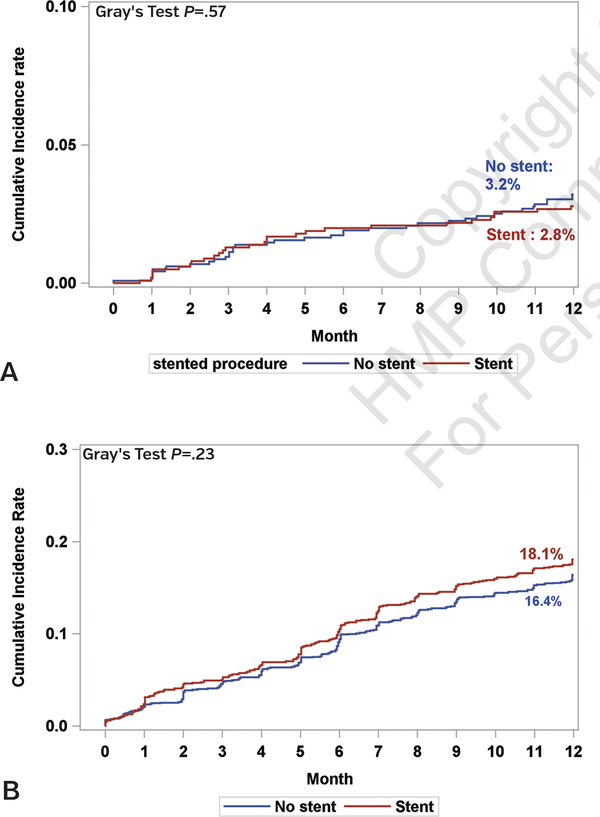
Fine and Gray’s competing risk CIF plots show that the stent group’s 1-year mortality risk did not significantly differ from that of the non-stent group (Gray’s test X2=0.32; P=.57) (Figure 2A). When considering the competing risk of death, the cumulative target-lesion revascularization rates at 1 year in the stent and non-stent groups were 18.1% and 16.4%, respectively (Gray’s test X2=1.46; P=.23) (Figure 2B). TVR rates at 1 year in the stent and non-stent groups were similar (Gray’s test X2=0.63; P=.43), but was numerically higher in the stent group (15.5%) compared with the non-stent group (14.5%). Cumulative major and minor amputation rates were similar between stent and non-stent groups (X2=1.49 and P=.22 for major amputations; X2=0.04 and P=.85 for minor amputations; data not shown).
FIGURE 2.
[A] 12-month mortality cumulative incidence function plots in the stent and no-stent groups. [B] 12-month cumulative incidence function plots for target-vessel revascularization in stent and no-stent groups.
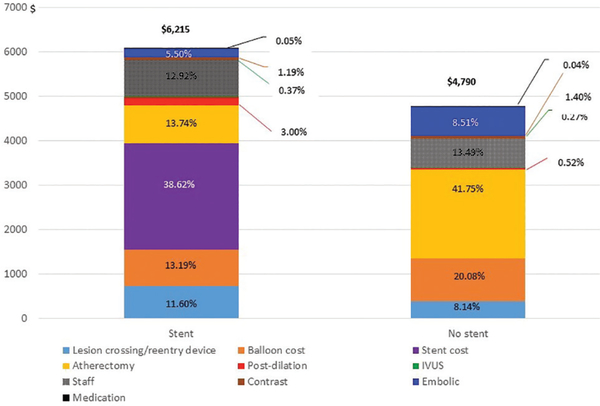
The average procedure costs in the stent group (mean cost, $6215 ± $3543) were significantly higher than the non-stent group (mean cost, $4790 ± $2909; P<.001). Itemized procedure cost includes stents, balloons, lesion crossing, re-entry device, atherectomy device, postdilation balloons, IVUS, staff, contrast volume, embolic protection, and medications. Figure 3 illustrates the average cost per each item of the total procedure costs. The device costs represent 83% of the total procedure costs for the stent group and 79% for the non-stent group.
FIGURE 3.
Percentages of itemized procedural cost by stent and no-stent groups.
Cost-effectiveness analysis was conducted and is shown in Table 3. The difference in average total procedure cost between stent and non-stent groups was $1425. The average benefit of stent use for 12-month major amputation was $22 (0.0003 × $71,980), while the results were negative for 12-month TVR, surgical revascularization, and minor amputation. Thus, a stent-based strategy for FP intervention was not cost-effective compared to a non-stent strategy.
Table 3.
Estimated costs and numbers of repeat revascularization procedures and amputations.
| Average Cost Difference | Target-Vessel Revascularizationc | Surgical Revascularization | Major Amputation | Minor Amputation | |
|---|---|---|---|---|---|
| Stent - No stent | $1425a | $5391f | $32,793g | $71,980h | $34,278h |
| Mean estimate (95% CI) | Mean estimate (95% CI) | Mean estimate (95% CI) | Mean estimate (95% CI) | ||
| Stent - No stent | Estimated number of events difference per procedureb | 0.015498 (−0.034988 to 0.11189) | 0.0002 (−0.0011 to 0.0159) | −0.0003 (−0.00347 to 0.0011) | 0.02 (−0.004 to 0.11384) |
| Benefits (costs, $)d | −$89.5 (−603.2 to 188.6) | −$6.6 (−521.4 to 36.1) | $21.6 (−72.0 to 249.8) | −$648.5 (−3902.2 to 137.1) | |
| Total costs (benefits, $)e | −$542.5 (−5098.8 to 611.6) | ||||
The average procedure cost was calculated as procedure cost divided by number of lesions treated within the procedure.
A logistic model calculated estimated reduction in numbers of repeat endovascular or surgical revascularization and amputation per procedure. Model: Y (clinical event = yes) = b0 + b1*group (stent vs no stent group) + b2*age + b3*gender + b4*diabetes + b5*current smoking status + b6*hypertension + b7*hypercholesterolemia + b8*lesion length + error, where b values are estimates. P(Y) group = stent) = exp (b0 + b1* group = stent) / 1 − exp (b0 + b1*group = stent) and P(Y|group = no stent) = b0 / 1 − exp (b0). Difference in P(Y) = P(Y) group = stent) − P(Y) group = nostent). Difference in estimated based on numbers of events per procedure between stent and non-stent groups that was calculated as a group difference in estimated numbers of repeated intervention, surgical revascularization, and major and minor amputation, respectively.
Target-vessel revascularization (TVR) cost was calculated using the average procedure cost per lesion treated.
Benefits of stent use compared to no use were calculated as difference in estimated numbers of needed intervention • average cost of procedure cost per lesion treated.
Total benefits were calculated as a sum of TVR, surgical revascularization, and major and minor amputation costs.
Average cost of procedure cost per lesion treated.
Cost figure is from a published article,6 and converted to 2015 constant dollars using Consumer Price Index (CPI).
Cost figure is an average cost of below-the-knee and above-the-knee amputation costs from the publication,22 and cost was converted to 2015 constant dollars using CPI.
Discussion
This report from the XLPAD registry provides important insights on contemporary trends, comparative outcomes, and costs associated with stent and non-stent strategies employed for endovascular treatment of FP-PAD. The key findings from this analysis are that most FP peripheral artery interventions are performed in patients with diabetes mellitus, for claudication, in the SFA distribution, and include a CTO. There is a clear trend for an increased adoption of a non-stent strategy. However, stenting was performed in nearly 46% of cases. It was more frequently used in more complex lesions with significantly higher technical and procedural success rates and an overall similar all-cause death, TVR and target-limb revascularization, and amputation rates at 12 months compared with a non-stent approach, albeit at a higher average procedural cost.
These results from the XLPAD registry reflect the growth in non-stent based procedures in the United States over the last decade.8 Forty-three hospitals participating in the BMC2 VIC registry between 2006–2013 have also reported a rise in non-stent treatments during peripheral vascular interventions.2 This included use of primarily balloon angioplasty, cutting and scoring balloons. No significant changes were found with stenting and atherectomy. In contrast, the fee-for-service Medicare claims data from 2011 to 2014 registered a 60% increase in the use of atherectomy, especially in the office-based catheterization laboratories.9 These data clearly indicate changing trends for stent and non-stent based LE peripheral artery interventions. Recognition of limitations associated with stenting, such as stent fracture, in-stent restenosis (ISR), and stent thrombosis (ST), introduction of DCBs in the United States, and a higher reimbursement for atherectomy procedures may have contributed to the growth of the non-stent leave-nothing behind approach.10 These observed trends make a comparison of patient outcomes and costs associated with the selection of a stent-based or non-stent based treatment for FP revascularization highly relevant.
Stented lesions were significantly longer, diffusely diseased, or totally occluded and with heavy calcification. A non-stent based approach was used more frequently in CLI patients, and with a significantly greater involvement of the popliteal and BTK arteries. The higher mean ABI in the non-stent group is attributed to the significantly greater representation of older patients and those with chronic kidney disease with non-compressible ABIs. Both at baseline and post intervention, medical therapy including dual-antiplatelet agents are under-used in this cohort of patients with symptomatic PAD. This observation is consistent with prior reports of suboptimal medical treatment of PAD patients and presents an important opportunity to improve both cardiovascular and limb outcomes of patients with PAD.11 Such improvements in outcomes have been demonstrated with newer antithrombotic agents, such as vorapaxar and rivaroxaban.12,13 The effect of medical therapy on cardiovascular outcomes, patency, and limb salvage after peripheral vascular intervention is an important area for future research.
Despite higher technical and procedural success rates in the stent group, the higher residual dissection rate noted during independent adjudication of angiograms was attributed mainly to a higher prevalence of CTO lesions and significantly greater use of subintimal dissection and true lumen re-entry. In both study groups, use of combination treatments was high. Such combination treatments saw frequent use of atherectomy. Although the use of atherectomy has remained high, our registry indicates a decline in the last two reporting years. Most stents were BMS, with ~17% DES use. In the non-stent group, DCB use was 17%; however, the current trend suggests an expected increase over subsequent years. A recent study comparing combination treatments in de novo SFA lesions (mean length of 65.9 ± 46.8 mm and 56% CTOs) reported that treatment with paclitaxel DCB and stenting was superior to PTA and stenting or directional atherectomy (DA) in terms of angiographic diameter stenosis at 6 months and target-lesion revascularization at 24 months.14 These findings taken together may support broader DCB use in the FP distribution as a treatment before stenting. However, this approach needs to be rigorously tested and compared with conventional balloon predilation and DES use as well as other non-stent combination therapies.
Non-stent based combination treatments — especially involving atherectomy and DCB — may be encouraging in heavily calcified FP lesions. Vessel preparation with directional atherectomy (DA) followed by DCB showed favorable results compared with PTA + DCB in a recently published pilot study that was under-powered to detect differences in clinical outcomes.15 In this study, technical success was superior for DA + DCB (89.6% vs 64.2%; P<.01). Bail-out stenting rate was 3.7% for DCB and 2% for DA + DCB (P=.01). One-year primary outcome of angiographic percent diameter stenosis, clinically driven target-lesion revascularization, and freedom from major adverse events was similar in both groups. Stenting for a bail-out indication in our study constituted nearly 8% of cases in the stent group.
Introduction of DES and DCB technologies in the United States for the treatment of FP arteries has demonstrated sustainable results. The 2-year outcomes from the pivotal Zilver PTX randomized control trial, wherein 6-month, 1-year, and 2-year primary patencies were 95.1%, 82.7%, and 74.8%, respectively, were sustained through 5 years at 72.4%.16 These results represent >40% relative risk reduction for restenosis and target-lesion revascularization through 5 years for the overall DES compared with standard care, and for provisional DES compared with provisional BMS. In a more recent prospective, single-arm, multicenter trial of a new DES platform that enrolled 57 patients with FP de novo or restenotic lesions with a mean lesion length of 70.8 ± 28.1 mm and 46% CTO, Duplex-ultrasound derived primary patency at 12 months was 96%.17 An 85.3% Kaplan-Meier freedom from target-lesion revascularization at 3 years was recently reported.18 A network meta-analysis from 15 randomized controlled trials and 10 prospective, multicenter, single-arm trials evaluated target-lesion revascularization in 2912 patients with 3151 person-years of follow-up. It demonstrated that DCB provided better reduction compared with PTA (68% reduction; P<.001) and BMS (53% reduction; P=.04).19 BMS, polymer-covered metal stents (CMS), and DES reduced target-lesion revascularization by 33%, 48%, and 58%, respectively compared with PTA, with statistical significance achieved for CMS and DES. These results need to be considered in light of new evidence on stent platforms that are by design less prone to mechanical failure (for example, the Supera vascular mimetic stent by Abbott Vascular). Per-protocol analysis of the multicenter RAPID trial showed a 12-month primary patency estimate of 74.7% in the DCB + Supera group vs 62.0% in the Supera stent alone group (P=.27).20 The new, hybrid, heparin-bonded, nitinol-ring Tigris stent (Gore Medical) demonstrated a 86.1 ± 5.9% freedom from target-lesion revascularization when used for the treatment of lesions located in the popliteal artery.21 These data together demonstrate a continuous and ongoing refinement of stent and non-stent technologies and its applications for FP artery revascularization.
In light of these new developments, the ongoing XLPAD registry will continue to capture and report treatment trends and adjudicated outcomes in endovascular revascularization of PAD in the real-world setting. Capitalizing on the 10-year multicenter XLPAD registry data, herein we attempted to provide an evidence-based comparative assessment of clinical and cost-effectiveness of stent and non-stent based strategies for endovascular treatment of FP-PAD.
Study limitations.
Important limitations of this study include its observational nature, selection bias due to non-randomized treatment assignments, missing follow-up information, and missing durations of medical therapies and device-specific information. The XLPAD registry enrolls patients at hospitals performing both outpatient and inpatient peripheral artery interventions; however, data regarding patient disposition are not included in the registry. The registry executive committee is working to include this important information. Despite these limitations, the findings of this study provide important insights into contemporary endovascular management of FP-PAD.
Conclusion
A majority of FP peripheral arterial interventions are performed in patients with diabetes mellitus and include a CTO. Stents are used in more complex lesions and have significantly higher technical and procedural success, but similar overall clinical outcomes at 1-year. The average procedure cost of a stent-based approach is significantly higher than non-stent procedures.
Supplementary Material
Acknowledgment.
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Texas Southwestern Medical Center.1 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Footnotes
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Banerjee reports honoraria from Medtronic, Gore, Astra Zeneca, Janssen; research grants (institutional) from Boston Scientific, Abbott Vascular, Merck; Board of Directors for the Cardiovascular Innovations Foundation; Dr Armstrong reports honoraria from Medtronic, Abbott Vascular, Boston Scientific Corporation, Cardiovascular Systems, Merck and Spectranetics. Dr Bajzer reports honoraria from Abbott Vascular. Dr Prasad reports honoraria from Osprey Medical, Abiomed, Gilead, and GE; research grants from Medtronic, Acist Medical. Dr Addo reports honoraria from Astra Zeneca. Dr Niazi reports grant support from Surmodics. Dr Shammas reports educational and research grants from Intact Vascular, Philips, Boston Scientific, and Bard. Dr Brilakis reports honoraria from Abbott Vascular, ACIST, Amgen, Asahi Intecc, CSI, Elsevier, GE Healthcare, Medicure, and Nitiloop; research support from Boston Scientific and Osprey; Board of Directors for Cardiovascular Innovations Foundation; Board of Trustees for the Society of Cardiovascular Angiography and Interventions. The remaining authors report no conflicts of interest regarding the content herein.
References
- 1.Wiseman JT, Fernandes-Taylor S, Saha S, et al. Endovascular versus open revascularization for peripheral arterial disease. Ann Surg. 2017;265:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas MP, Jung Park Y, Grey S, et al. Temporal trends in peripheral arterial interventions: observations from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2 PVI). Catheter Cardiovasc Interv. 2017;89:728–734. [DOI] [PubMed] [Google Scholar]
- 3.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee S, Sarode K, Mohammad A, et al. Femoropopliteal arterystent thrombosis: report from the Excellence in Peripheral Artery Disease registry. Circ Cardiovasc Interv. 2016;9:e002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin H, Rajan M, Tseng CL, Pogach L, Sinha A, Mph M. Cost of lower-limb amputation in U.S. veterans with diabetes using health services data in fiscal years 2004 and 2010. J Rehabil Res Dev. 2014;51:1325–1330. [DOI] [PubMed] [Google Scholar]
- 6.Jaff MR, Cahill KE, Yu AP, Birnbaum HG, Engelhart LM. Clinical outcomes and medical care costs among medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24:577–587. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Jeon-Slaughter H, Tsai S, et al. Comparative assessment of procedure cost and outcomes between guidewire and crossing device strategies to cross peripheral artery chronic total occlusions. JACC Cardiovasc Interv. 2016;9:2243–2252. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee S, Pershwitz G, Sarode K, et al. Stent and non-stent based outcomes of infrainguinal peripheral artery interventions from the multicenter XLPAD Registry. J Invasive Cardiol. 2015;27:14–18. [PubMed] [Google Scholar]
- 9.Mukherjee D, Hashemi H, Contos B. The disproportionate growth of office-based atherectomy. J Vasc Surg. 2017;65:495–500. [DOI] [PubMed] [Google Scholar]
- 10.Jones WS, Mi X, Qualls LG, et al. Trends in settings for peripheral vascular intervention and the effect of changes in the outpatient prospective payment system. J Am Coll Cardiol. 2015;65:920–927. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer AJ, Sedrakyan A, Connolly PH, Ellozy S, Schneider DB, Vascular Study Group of greater New York. Risk factors for suboptimal utilization of statins and antiplatelet therapy in patients undergoing revascularization for symptomatic peripheral arterial disease. Ann Vasc Surg. 2018;46:234–240. Epub 2017 Jun 8. [DOI] [PubMed] [Google Scholar]
- 12.Bonaca MP, Creager MA, Olin J, et al. Peripheral revascularization in patients with peripheral artery disease with vorapaxar: insights from the TRA 2 degrees P-TIMI 50 trial. JACC Cardiovasc Interv. 2016;9:2157–2164. [DOI] [PubMed] [Google Scholar]
- 13.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. Epub 2017 Nov 10. [DOI] [PubMed] [Google Scholar]
- 14.Ott I, Cassese S, Groha P, et al. Randomized comparison of paclitaxel-eluting balloon and stenting versus plain balloon plus stenting versus directional atherectomy for femoral artery disease (ISAR-STATH). Circulation. 2017;135:2218–2226. [DOI] [PubMed] [Google Scholar]
- 15.Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR Study. Circ Cardiovasc Interv. 2017;10:e004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133:1472–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller-Hulsbeck S, Keirse K, Zeller T, Schroe H, Diaz-Cartelle J. Twelve-month results from the MAJESTIC trial of the Eluvia paclitaxel-eluting stent for treatment of obstructive femoropopliteal disease. J Endovasc Ther. 2016;23:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller-Hulsbeck S, Keirse K, Zeller T, Schroe H, Diaz-Cartelle J. Longterm results from the MAJESTIC trial of the Eluvia paclitaxel-eluting stent for femoropopliteal treatment: 3-year follow-up. Cardiovasc Intervent Radiol. 2017;40:1832–1838. [DOI] [PubMed] [Google Scholar]
- 19.Jaff MR, Nelson T, Ferko N, Martinson M, Anderson LH, Hollmann S. Endovascular interventions for femoropopliteal peripheral artery disease: a network meta-analysis of current technologies. J Vasc Interv Radiol. 2017;28:1617–1627.e1. [DOI] [PubMed] [Google Scholar]
- 20.de Boer SW, van den Heuvel DAF, de Vries-Werson DAB, et al. Short-term results of the RAPID randomized trial of the Legflow pacli-taxel-eluting balloon with Supera stenting vs Supera stenting alone for the treatment of intermediate and long superficial femoral artery lesions. J Endovasc Ther. 2017;24:783–792. [DOI] [PubMed] [Google Scholar]
- 21.Parthipun A, Diamantopoulos A, Kitrou P, et al. Use of a new hybrid heparin-bonded nitinol ring stent in the popliteal artery: procedural and mid-term clinical and anatomical outcomes. Cardiovasc Intervent Radiol. 2015;38:846–854. [DOI] [PubMed] [Google Scholar]
- 22.Franklin H, Rajan M, Tseng C, Pogach L, Sinha A. Cost of lower-limb amputation in U.S. veterans with diabetes using health services data in fiscal years 2004 and 2010. J Rehabil Res Dev. 2014;51:1325–1330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.