Abstract
Alcohol liver disease (ALD) is one of the major chronic liver diseases worldwide, ranging from fatty liver, alcoholic hepatitis, cirrhosis, and potentially, hepatocellular carcinoma. Epidemiological studies suggest a potential link between ALD and impaired circadian rhythms, but the role of hepatic circadian proteins in the pathogenesis of ALD remains unknown. Here we show that the circadian clock protein BMAL1 in hepatocytes is both necessary and sufficient to protect mice from ALD. Ethanol diet-fed mice with liver-specific knockout (Bmal1-LKO) or depletion of Bmal1 develop more severe liver steatosis and injury as well as a simultaneous suppression of both de novo lipogenesis and fatty acid oxidation, which can be rescued by the supplementation of synthetic PPARα ligands. Restoring de novo lipogenesis in the liver of Bmal1-LKO mice by constitutively active AKT not only elevates hepatic fatty acid oxidation but also alleviates ethanol-induced fatty liver and liver injury. Furthermore, hepatic over-expression of lipogenic transcription factor ChREBP, but not SREBP-1c, in the liver of Bmal1-LKO mice also increases fatty acid oxidation and partially reduces ethanol-induced fatty liver and liver injury.
Conclusion:
we identified a protective role of BMAL1 in hepatocytes against ALD. The protective action of BMAL1 during alcohol consumption depends on its ability to couple ChREBP-induced de novo lipogenesis with PPARα-mediated fatty oxidation.
Alcohol over-consumption is the primary cause of liver-related mortality in western countries. In the United States, more than 50% of the population consumes alcohol.(1,2) As the primary site of alcohol metabolism, the liver is a major target organ of alcohol-induced injury. Three in 10 adults are heavy alcohol drinkers with a high risk for alcoholic liver disease (ALD).(3) ALD covers a spectrum of disease states that range from simple liver steatosis (fatty liver), steatohepatitis (combined with inflammation), to fibrosis and/or cirrhosis.(3) Both genetics and environmental factors contribute to the progression of ALD. Particularly, mice with circadian disruption have been reported to have a higher permeability of intestinal epithelial barrier, a risk factor of alcoholic tissue damage.(4) Human studies also indicated that night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking.(5) Although these observations point to a possible role of impairment of circadian rhythms in the pathogenesis of ALD, what and how circadian proteins are involved in ALD remain largely unknown.
The circadian clock functions in the majority of cell types in the human body. In hepatocytes, BMAL1 is one of the essential components of the circadian clock that drives the cyclic expression of a variety of metabolic genes involved in lipid, glucose, and cholesterol metabolism.(6) We previously discovered that BMAL1 is also essential for maintaining insulin responsiveness in the liver. A Bmal1 deficiency severely blunts insulin-stimulated AKT phosphorylation and reduces insulin-stimulated de novo lipogenesis.(7) Upon chronic HFD feeding, hepatic Bmal1 deficiency results in severe insulin resistance and liver steatosis,(8) indicating that impairment of BMAL1 expression or function may contribute to chronic liver metabolic diseases.
Alcohol exposure has been shown to impair lipid homeostasis in hepatocytes and cause liver injury during the development of ALD.(3) It has been reported that ethanol feeding increases SREBP-1c-driven de novo lipogenesis while inhibiting the PPARα activity in the liver.(9–11) Administration of a synthetic PPARα agonist reverses alcoholic fatty liver and improves liver function in mice.(9) The inhibitory effect of ethanol on the PPARα transcriptional activity was observed in cultured hepatocytes, suggesting that ethanol and its metabolites could impact the DNA-binding activity of PPARα in the liver.(10) Currently, it remains unclear how ethanol feeding alters the hepatic PPARα activity.
Given the pivotal role of PPARα in liver lipid homeostasis, extensive research has been done on how the PPARα activity is modulated in response to hormonal and nutritional signals. Unexpectedly, the PPARα activity is severely down-regulated in hepatocytes lacking FASN, indicating an intricate link between de novo lipogenesis and the biogenesis of endogenous PPARα ligands and the subsequent PPARα activation in the liver.(12) Follow-up studies show de novo lipogenesis is a major source of phosphatidyl-choline in hepatocytes that serves as endogenous ligands for PPARα.(13) However, how the connection between de novo lipogenesis and PPARα activation is regulated during ethanol feeding has not been tested. In this study, we explored the role of a key circadian protein BMAL1 in alcoholic liver disease using both hepatocyte-specific gain and loss of function models. Our findings suggest that in hepatocytes, BMAL1 is critical for reducing liver steatosis and hepatocyte apoptosis during chronic alcohol feeding. Moreover, BMAL1 is required for maintaining the PPARα activity in hepatocytes partially via its ability to promote ChREBP-induced de novo lipogenesis.
Materials and Methods
ANIMAL EXPERIMENTS
All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee at the University of Michigan. All animal care and use were in accordance with guidelines of the University of Michigan Institutional Animal Care and Use Committee. C57BL/6 mice were maintained on 12 hour/12 hour light/dark cycles with ad libitum access to food and water. Bmal1Flox/Flox mice were generously provided by Dr. Jiandie Lin at the University of Michigan. Bmal1 liver specific knockout (Bmal1-LKO) mice were generated by crossing Bmal1Flox/Flox mice with Albumin-Cre mice. Ethanol diet feeding and binge was performed with the protocol described previously.(14) For fenofibrate treatment, the mice were daily gavaged in the last 8 days of ethanol diet feeding (20mg/kg body weight). For liver-specific knockdown or overexpression, AdshLacZ vs. Ad-shBmal1, Ad-GFP vs. Ad-Flag-Bmal1, Ad-Akt2-CA or Ad-Flag-Chrebp adenoviruses were delivered via tail vein injection at a dose of 1×1012 plaque-forming units on the first day of ethanol diet feeding.
ELECTRON MICROSCOPY
Mouse liver was excised, minced into small pieces and fixed in a solution of 2% paraformaldehyde and 2% glutaraldehyde in PBS for 2 hours at room temperature. After washing in PBS, the tissue samples were post fixed in osmium tetroxide for 45 minutes at room temperature. Dehydration of the samples was accomplished by transferring the samples through a series of graded ethanol and then 100% propylene oxide. The tissue was then infiltrated by transferring the samples into increasing concentrations of Epon to propylene oxide solutions at 1:3, 1:1, 3:1, and then 100% Epon and finally embedded. Sections were made with a Leica EM UC7 ultra-microtome (Leica), stained for 15 minutes with 7% (saturated) aqueous uranyl acetate, washed, stained with lead citrate, and examined with a JEOL JEM 1400 plus transmission electron microscope (JEOL USA).
STATISTICS
Statistical analysis was performed using Prism version 6.0 (GraphPad Software, San Diego, CA). Statistical significance was determined either by unpaired two-tailed Student’s t-test for comparison between two groups or by one-way ANOVA with Tukey’s or Dunnett’s post-hoc test for multiple group comparison. All results were given as the mean ± SEM. Results were considered statistically significant with p value < 0.05.
Other detailed methods, including adenoviral production, serum ALT, liver histology, Nile Red staining, MPO staining, sucrose gradient sedimentation, TUNEL staining, liver triglycerides assay, primary mouse hepatocytes isolation and culture, cDNA synthesis and RT-qPCR, are available in the Supporting Information.
Results
HEPATOCYTE-SPECIFIC DELETION OF BMAL1 SENSITIZES MICE TO ETHANOL FEEDING-INDUCED LIVER STEATOSIS AND LIVER INJURY
To study the role of hepatic molecular circadian clock in the pathogenesis of ALD, we adopted the chronic-plus-binge feeding described by the Gao group to create a moderate ALD in mice.(14) Wildtype (WT) mice were fed a 5% ethanol diet for 10 days before binged with 5g ethanol per kg body weight at Zeitgeber time 3, and dissected 9 hours later. Ethanol feeding significantly increased liver triglycerides accumulation (Supporting Fig. S1A,B), ALT levels and Cyp2e1 expression level (Supporting Fig. S1C,D) along with enhanced expression of pro-inflammatory cytokines, ER stress and pro-apoptotic markers (Supporting Fig. S1E–G), as well as increased neutrophil recruitment (Supporting Fig. S1H), indicating that this chronic-plus-binge ethanol regimen induces mild to moderate liver steatosis and injury in WT mice.
We also observed enhanced acetylation of p65, a direct target of SIRT1,(15) in the liver of ethanol-fed mouse livers, indicative of potent suppression of the SIRT1 activity by ethanol feeding. However, ethanol feeding showed no effect on the abundance of SIRT1 (Supporting Fig. S2A,B). Given the important role of SIRT1 in regulating hepatic circadian clock,(16–19) we hypothesize that ethanol feeding could impact the circadian clock in the liver through SIRT1 inhibition. We found the diurnal rhythms of Dbp and Per1, two classical clock genes, were lost in the ethanol-fed liver (Supporting Fig. S2C). At the protein level, ethanol feeding led to an increase in both BMAL1 and CLOCK protein, but a reduction of AKT-PS473 in the liver (Supporting Fig. S2A,B). We previously showed that SIRT1 protects the BMAL1-CLOCK protein complex formation in the presence of palmitate in hepatocytes.(19) To test whether ethanol could impact the BMAL1-CLOCK interaction, we performed anti-FLAG immunoprecipitation in ethanol-treated primary mouse hepatocytes transduced with Ad-Flag-Bmal1 vs. Ad-GFP. We detected a strong interaction between FLAG-tagged BMAL1 and the endogenous CLOCK in vehicle-treated cells. However, the BMAL1-CLOCK interaction was weakened by ethanol treatment (Supporting Fig. S2D). To test whether ethanol feeding impairs the BMAL1-CLOCK complex in mouse liver, we performed sucrose gradient sedimentation using nuclear extracts isolated from both control diet-fed and ethanol diet-fed liver tissues. Consistent with in vitro findings, ethanol feeding reduced the number of fractions in which BMAL1 and CLOCK protein co-migrated (Supporting Fig. S2E). In summary, ethanol diet induces selective impairment of the hepatic circadian clock activity, in addition to liver steatosis and liver injury.
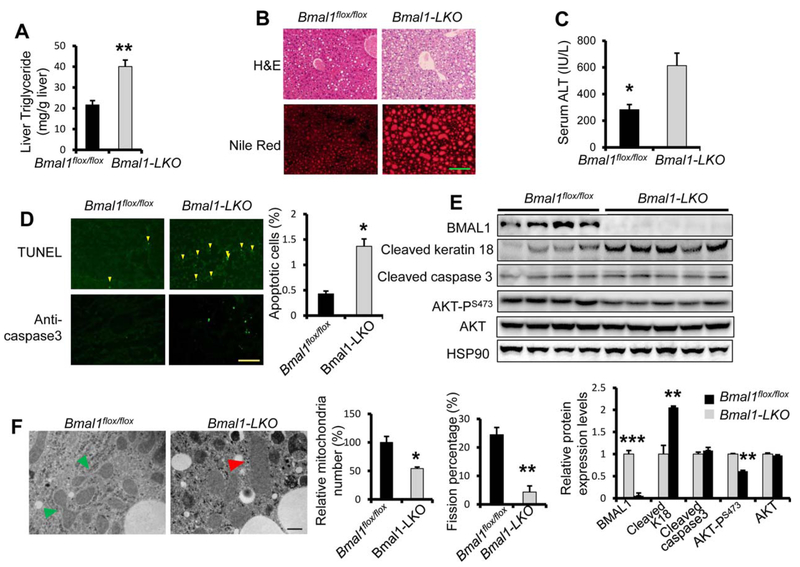
To evaluate the role of an intact molecular clock in hepatocytes during ALD, we subjected Bmal1flox/fox and Bmal1 liver-specific knockout (Bmal1-LKO) to the ethanol diet/binge model. Compared with Bmal1flox/fox control, Bmal1-LKO mice quickly lost weight on ethanol diet (Supporting Fig. SF3A), accumulated more lipids in the liver (Fig. 1A,B), developed more pronounced liver injury (Fig. 1C), and hepatocyte apoptosis by TUNEL staining and keratin 18 cleavage (Fig. 1D,E). However, increased liver injury in Bmal1-LKO liver was independent of gene expression of Cyp2e1 and markers of oxidative stress, ER stress, inflammation, and apoptosis (Supporting Fig. S3B–F). Neutrophil infiltration was comparable between Bmal1flox/fox and Bmal1-LKO mice (Supporting Fig. S3G).
FIG. 1.
Hepatocyte-specific Bmal1 knockout mice are sensitive to ethanol feeding-induced liver steatosis and liver injury. Eight-week-old Bmal1Flox/Flox littermates and Bmal1Flox/Flox Alb-Cre(+) (Bmal1-LKO) mice were fed 5% ethanol diet for 10 days before binge feeding with ethanol (5 g/kg body weight) at Zeitgeber Time (ZT)3 and dissected 9 hours later (n 5 4 for Bmal1Flox/Flox and n = 5 for Bmal1-LKO, both male and females). (A) Hepatic triglyceride levels. (B) H&E staining and Nile red staining. (C) ALT assay to assess liver injury. (D) TUNEL staining and immune-fluorescence against cleaved caspase3 to assess apoptosis. (E) Western blotting analysis of hepatic apoptotic markers. (F) Transmission electron microscopy (magnification 40,000X). Mitochondria undergoing fission are indicated with green arrowheads and swollen mitochondrial with red arrowheads. *p < 0.05, **p < 0.01 by two-tailed Student’s t test. Scale bar = 100 μM for B and D; Scale bar = 400 nM for F.
Mitochondrial dysfunction has been implicated in the pathogenesis of ALD.(20) The liver of mice with hepatocyte deficiency in Bmal1 showed a defect in mitochondrial fission following a 40-wk high-fat diet.(8) To test whether Bmal1-LKO mice also exhibited mitochondrial defects, we evaluated the mitochondrial morphology in the liver of Bmal1flox/fox vs. Bmal1-LKO mice with electron microscopy after ethanol feeding. In the Bmal1flox/fox liver, mitochondria displayed normal shape and active fission. However, we observed swollen mitochondria without fission in the liver of Bmal1-LKO after ethanol diet (Fig. 1F). In summary, Bmal1 deficiency in hepatocytes results in augmented liver steatosis and liver injury with mitochondrial impairment following ethanol diet independently of ER stress, oxidative stress, and induction of pro-apoptotic genes.
MICE WITH ACUTE DEPLETION OF Bmal1 IN HEPATOCYTES ARE MORE SUSCEPTIBLE TO ETHANOL-INDUCED FATTY LIVER AND LIVER INJURY
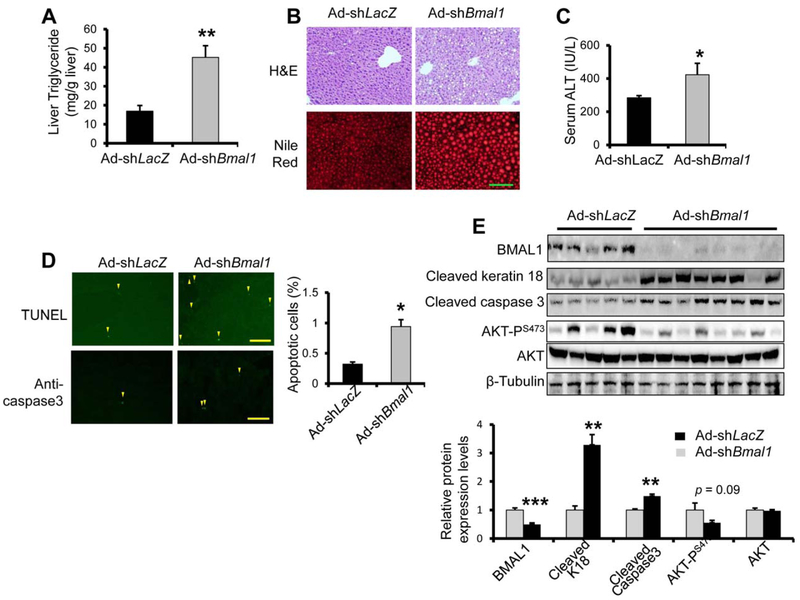
To test how acute depletion of hepatic Bmal1 in adult mice responds to ethanol feeding, we knocked down hepatic Bmal1 in adult WT mice by administrating AdshBmal1 via tail vein prior to ethanol feeding. Compared with Ad-shLacZ control, Ad-shBmal1-injected mice showed negative gain in body weight (Supporting Fig. S4A) but greater liver lipid accumulation (Fig. 2A,B) by liver triglycerides assay and Nile Red staining. Liver H&E staining also revealed increased lipid droplets in the liver of Ad-shBmal1 mice (Fig. 2B). Serum ALT assay detected greater liver injury in the mice of Ad-shBmal1 (Fig. 2C), which was further supported by a significant increase in hepatocyte apoptosis detected by TUNEL staining, caspase 3 staining, and immuno-blotting for apoptotic markers including cleaved keratin 18 and caspase 3 (Fig. 2D,E). Interestingly, acute Bmal1 knockdown did not result in altered expression of Cyp2e1, markers of inflammation, ER stress, and apoptosis (Supporting Fig. S4B–F). Altogether, our data suggest that acute hepatic clock deficiency by depleting Bmal1 also sensitizes mice to ethanol-induced fatty liver and liver injury.
FIG. 2.
Adult-onset hepatocyte Bmal1 depletion sensitizes mice to ethanol-induced fatty liver and liver injury. Eight-week-old mice were injected with either adenovirus expressing shRNA targeting Bmal1 (Ad-shBmal1, n =8) or Ad-shLacZ control (n =5) at MOI 1×1012 pfu, fed 5% ethanol diet for 10 days and then binged with ethanol (5 g/kg body weight) at ZT 3 of the eleventh day and were dissected 9 hours later. Liver samples were subjected to triglyceride assay (A), H&E staining and Nile Red staining (B) to assess hepatic lipid accumulation, ALT assay to assess liver injury (C), TUNEL staining and caspase3 immuno-staining to assess apoptosis (D), and Western analysis of apoptotic markers (E). *p < 0.05, **p < 0.01 by two-tailed Student’s t test. Scale bar = 100 uM.
MACROPHAGE BMAL1 IS DISPENSABLE IN PROTECTING MICE FROM ALD
A significant body of work has established the critical role of macrophage activation in the pathogenesis of ALD.(21,22) The circadian clock is also present in macrophages and plays an important role in regulating inflammatory cytokine production.(23) We hypothesized that deletion of Bmal1 in macrophages may also impact the severity of ALD in mice. We therefore generated macrophage-specific deletion of Bmal1 (Bmal1-MKO) with Lysosome-Cre and confirmed that the BMAL1 protein was largely absent in liver residential macrophages Kupffer cells isolated from Bmal1-MKO livers (Supporting Fig. S5A). Following ethanol feeding protocol, both Bmal1flox/flox and Bmal1-MKO displayed similar body weight, liver TAG and lipid droplet formation by H&E staining and Oil-Red-O staining (Supporting Fig. S5B–D). We also detected comparable levels of serum ALT between Bmal1flox/flox and Bmal1-MKO mice (Supporting Fig. S5E). EM showed intact mitochondrial morphology and mitochondrial fission in the liver of both Bmal1flox/flox and Bmal1-MKO mice (Supporting Fig. S5F). Taken together, BMAL1 in macrophages is likely to be dispensable during the pathogenesis of ALD.
LIVER-SPECIFIC Bmal1 OVER-EXPRESSION PROTECTS MICE FROM ALCOHOL FEEDING INDUCED FATTY LIVER AND LIVER INJURY
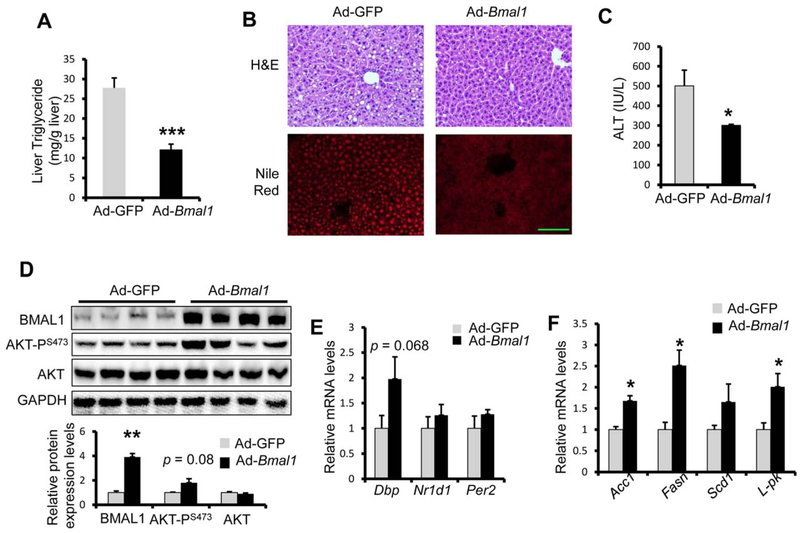
So far, we have demonstrated that lack of BMAL1 expression is detrimental to hepatocytes when challenged by ethanol feeding. It remains unclear whether over-expression of BMAL1 is sufficient to protect mice from ALDs. Female WT mice were injected with either Ad-GFP or Ad-Bmal1 prior to alcohol feeding/binge treatment. We first verified BMAL1 over-expression in the liver by immunoblotting with anti-FLAG. Over the course of alcohol feeding, mice injected with Ad-GFP showed similar body weight as mice with Ad-Bmal1 (Supporting Fig. S6A). However, Ad-Bmal1 injected mice showed approximately 50% reduction in liver TG content, which was further supported by H&E staining and Nile-Red staining (Fig. 3A,B). Moreover, Ad-Bmal1 expressing mice showed reduced serum ALT, suggesting BMAL1 over-expression protects mice against ethanol-induced liver injury (Fig. 3C). Moreover, BMAL1 over-expression led to enhanced AKTPS473 phosphorylation in the liver of alcohol-fed female mice (Fig. 3D). To determine whether this protection against ethanol-induced liver injury is gender-specific, we also assessed liver steatosis and injury in ethanol-fed male WT mice after injection of Ad-Bmal1. As shown in Supporting Fig. S6, BMAL1 overexpression indeed effectively reduced serum ALT, but to a lesser degree liver steatosis in male WT mice after ethanol feeding. Together these data support that ectopic expression of BMAL1 in the liver is sufficient to protect mice from alcohol feeding-induced fatty liver and liver injury.
FIG. 3.
Liver-specific Bmal1 over-expression protects mice from alcohol feeding-induced fatty liver and liver injury. Eight-week-old mice were injected with adenovirus expressing either Bmal1 (Ad-Bmal1, n = 8) or GFP control (Ad-GFP, n = 5), and then fed 5% ethanol diet for 10 days and binged with 5 g ethanol per kg body weight at ZT3 of the eleventh day and were dissected 9 hours later. (A,B) Bmal1 overexpression lowers ethanol-induced liver toxicity. Hepatic triglycerides assay (A), H&E staining and Nile red staining (B) were utilized to assess hepatic lipid accumulation. Liver injury was assessed with ALT assay (C). (D-F) Bmal1 overexpression activates hepatic lipogenesis. AKT phosphorylation was assessed with western blotting (D). Circadian genes (E) and lipogenic genes (F) were assessed with RT-qPCR. *p < 0.05, ***p < 0.001 by two-tailed Student’s t test. Scale bar = 100 uM.
What is the underlying mechanism for the protective role of BMAL1? Given the role of BMAL1 as a key regulator of the circadian clock, we first examined the expression of BMAL1 targets in the liver of Ad-Bmal1-injected female mice following alcohol feeding. To our surprise, we only detected modest induction of Dbp, Nr1d1, and Per2 in the liver (Fig. 3E). In contrast, we observed robust induction of lipogenic genes (Fasn, Acc1, and Scd1) and the glycolytic gene Lpk (Fig. 3F). Moreover, the expression of genes in ER stress, apoptosis, and oxidative stress were comparable between Ad-GFP and Ad-Bmal1 group (Supporting Fig. S7). These data suggest that BMAL1 overexpression may protect mice against ALD in part via its ability to regulate hepatic lipid metabolism, particularly de novo lipogenic pathway.
HEPATOCYTE BMAL1 PROTECTS ALD VIA PROMOTING PPARα-MEDIATED β-OXIDATION
We further investigated how BMAL1 could impact hepatic lipid metabolism during the pathogenesis of ALD. Previously, we discovered that hepatocyte BMAL1 is required for post-prandial de novo lipogenesis,(7) while others reported that BMAL1 regulates the PPARα expression and fatty acid oxidation in hepatocytes.(24,25) Dysregulated hepatic lipid metabolism is one of major pathological feature of ALD, which is characterized by increased de novo lipogenesis and suppressed fatty acid oxidation. We therefore hypothesize that BMAL1 may protect hepatocytes against ethanol toxicity by maintaining lipid homeostasis in the liver.
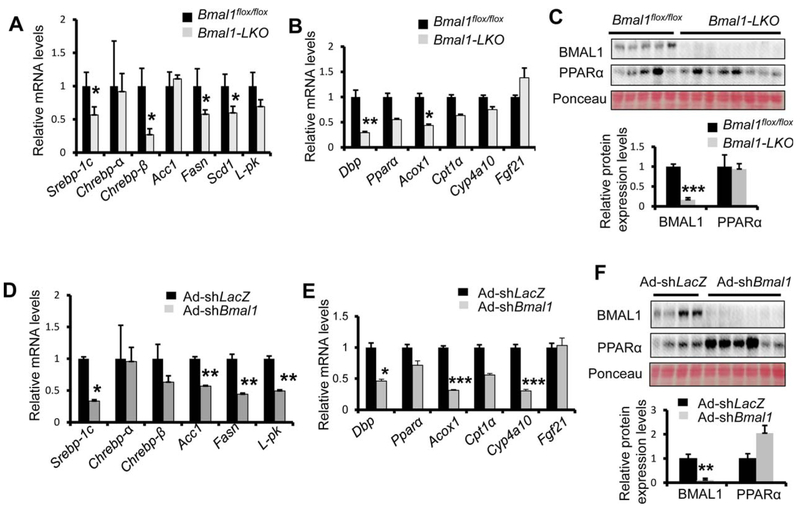
We first measured the mRNA expression of lipid metabolism in the mouse liver with various levels of Bmal1 expression following ethanol feeding. Compared with Bmal1flox/flox mice, we observed reduced expression of genes in de novo lipogenesis and fatty acid oxidation in Bmal1-LKO liver (Fig. 4A,B). A similar down-regulation of de novo lipogenesis and fatty acid oxidation genes in Ad-shBmal1-injected liver was also observed vs. Ad-shLacZ mice (Fig. 4D,E), suggesting that Bmal1 deficiency leads to a suppression of both de novo lipogenesis and fatty acid oxidation pathways.
FIG. 4.
Hepatic Bmal1 deficiency results in decreased gene expression of both de novo lipogenesis and β-oxidation in the liver of ethanol-fed mice. (A-C) Hepatocyte-specific deficiency of Bmal1 results in decreased PPARα activity in BLKO mice liver after ethanol treatment. Mice were treated as described in Fig. 1. Relative mRNA levels of lipogenic genes (A) and PPARα target genes (B), and protein levels of PPARα (C) in the livers of Bmal1-LKO mice (n=5), were compared to those of Bmal1Flox/Flox mice (n=4). (D-F) Adult-onset Bmal1 depletion in the liver decreases the PPARα activity after ethanol treatment. Relative mRNA levels of lipogenic genes (D) and PPARα target genes (E), and protein levels of PPARα (F) in the livers of Ad-shBmal1-injected mice (n=8), were compared with those of Ad-shLacZ control (n=5). *p < 0.05, ***p < 0.001 by two-tailed Student’s t test.
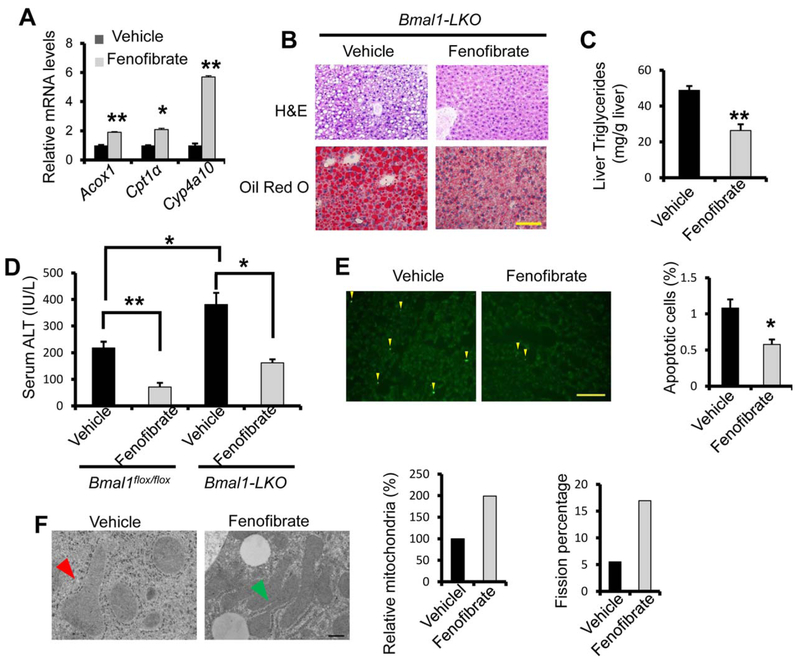
Previous studies demonstrated that Pparα knockout mice are more sensitive to ethanol-induced liver injury.(26) We suspected that suppression of the PPARα pathway contributes to the severity of ALD in Bmal1-LKO and hepatic Bmal1-depleted mice. Although we detected about 40% and 20% reduction of Pparα mRNA in the Bmal1-LKO and Bmal1-depleted liver respectively, the PPARα protein abundance in the liver was comparable between Bmal1flox/flox and Bmal1-LKO mice or between AdshLacZ and Ad-shBmal1 mice (Fig. 4C,F), suggesting that BMAL1 may regulate the biogenesis of endogenous ligands for PPARα without affecting its protein abundance. Thus, we speculated that supplementation of synthetic PPARα ligands could ameliorate ALD in Bmal1-LKO mice. As a synthetic PPARα agonist, fenofibrate has been clinically used to treat hyperlipidemia.(27,28) Of note, we firstly used ethanol-fed WT mice to confirm the efficacy of fenofibrate. At the dose of 20 mg/kg, daily treatment of fenofibrate was sufficient to reduce liver steatosis and serum ALT (Supporting Fig. S8), consistently with the previous report.(29) We therefore tested our hypothesis by treating Bmal1-LKO mice daily with either corn oil or fenofibrate during the course of ethanol feeding. As expected, fenofibrate stimulated the expression of PPARα target genes in the liver of Bmal1-LKO mice such as Acox1, Cpt1a, and Cyp4a10 (Fig. 5A). Fenofibrate treatment reduced lipid accumulation in the liver of Bmal1-LKO mice (Fig. 5B,C). Additionally, fenofibrate lowered serum ALT and reduced TUNEL-positive hepatocytes (Fig. 5D,E). Electron microscopy also showed higher density of mitochondria in active fission in the liver of fenofibrate-treated Bmal1-LKO mice (Fig. 5F). Taken together, we found that reactivation of PPARα signaling by fenofibrate could reverse liver steatosis, liver injury, and mitochondrial impairment in ethanol-fed Bmal1-LKO mice.
FIG. 5.
Fenofibrate administration rescues ethanol-induced liver injury in BLKO mice. Eight-week-old BLKO mice were fed 5% ethanol diet for 10 days. From the third day of ethanol diet feeding, mice were gavaged daily with fenofibrate (20 mg/ kg body weight) till the last day of ethanol diet feeding. On the eleventh day, mice were binged with 5g ethanol/kg body weight at ZT3, and were dissected 9 hours later (n = 5 for control and n = 7 for fenofibrate, both males and females). (A) PPARα activation by fenofibrate was confirmed by the induction of PPARα target genes with RT-qPCR. (B,C) Fenofibrate treatment reduces ethanol feeding-induced liver steatosis in Bmal1-LKO mice, assessed by H&E staining and Oil-Red-O staining (B) and liver triglycerides assay (C). (D-F) Fenofibrate treatment ameliorates ethanol-induced liver injury in Bmal1-LKO mice. Liver injury was assessed with ALT assay (D), TUNEL staining (E) and electron microscopy (magnification 40,000X) (F). Mitochondria undergoing fission were indicated with green arrowheads, and swollen mitochondria with red arrowheads. *p < 0.05, **p < 0.01 by two-tailed Student’s t test. Scale bar = 100 uM for B and E; Scale bar 5 400 nM for F.
CONSTITUTIVELY ACTIVE AKT RESTORES PPARα ACTIVITY AND REVERSES LIVER INJURY IN ETHANOL DIET-FED Bmal1-LKO MICE
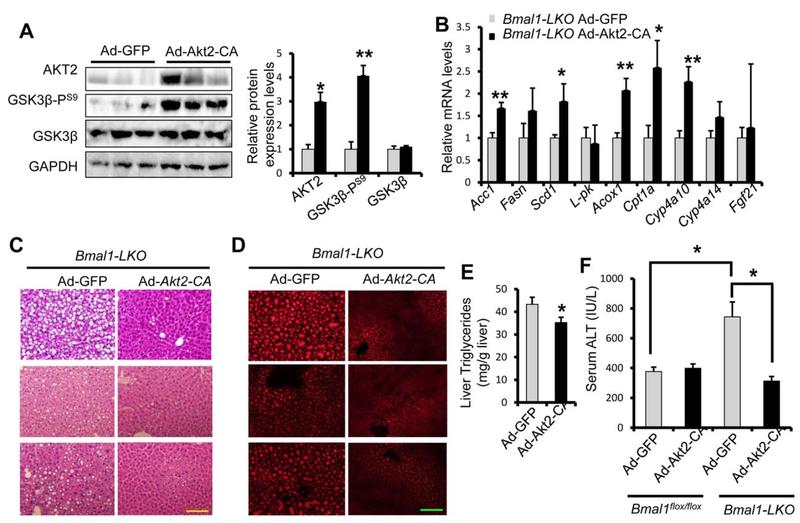
Hepatocyte PPARα signaling plays an essential role in maintaining lipid homeostasis in the liver. PPARα activity is dynamically regulated by nutritional status and various hormonal inputs. It is thought that PPARα activity elevates during fasting and becomes suppressed by feeding. Unexpectedly, hepatocytespecific Fasn knockout mice show reduced PPARα activity and liver steatosis, suggesting a positive crosstalk between hepatic de novo lipogenesis and PPARα-mediated fatty acid oxidation.(12) It has been proposed that hepatic lipogenesis may be responsible for de novo biogenesis of endogenous PPARα ligands.(12,13,30) We also demonstrated previously that restoring AKT activity by expressing constitutively active AKT2 (AKT2-CA) can rescue the defect of de novo lipogenesis in the Bmal1 knockout liver.(7) Since de novo lipogenesis and PPARα pathways were both repressed in the ethanolfed Bmal1-LKO mice, we investigated whether BMAL1-driven de novo lipogenesis pathway could impact the PPARα transcription activity. To test this hypothesis, we first determined whether restoring cellular AKT could induce PPARα target genes in hepatocytes with Bmal1 deficiency. In Bmal1-LKO hepatocytes, over-expression of AKT2-CA increased the expression of not only lipogenic genes but also a subset of β-oxidation genes (Supporting Fig. S9). To test how AKT2-CA overexpression in the liver affects PPARα target gene expression in Bmal1-LKO, we injected Bmal1-LKO mice with either Ad-GFP or Ad-Akt2-CA prior to ethanol feeding. Overexpression of AKT2-CA was confirmed by immunoblotting for total AKT2 and GSK3β-PS9, a direct phosphorylation target of AKT (Fig. 6A). In ethanol-fed Bmal1-LKO mouse livers, over-expression of AKT2-CA induced lipogenic genes, and more importantly, increased several PPARα targets (Fig. 6B). Notably, over-expression of AKT2-CA showed stronger effects on PPARα targets in vivo than in primary hepatocytes, possibly due to a longer duration of expression.
FIG. 6.
Restoring AKT activity rescues both de novo lipogenesis and PPARα pathway and reverses liver injury in Bmal1-LKO mice. Eight-week-old Bmal1-LKO mice were injected with Ad-Akt2-CA (n = 6) or Ad-GFP (n = 5), and then fed 5% ethanol diet for 10 days, binged with 5g ethanol/kg body weight at ZT3 on the eleventh day, and were dissected 9 hours later. (A) AKT2 activity was confirmed by western blotting with anti-AKT2 and anti-GSK3β phosphorylation. (B) Restoring AKT activity induces both de novo lipogenic genes and β-oxidation genes with RT-qPCR. (C-F) AKT activation reduces ethanol-induced liver steatosis and injury in Bmal1-LKO mice with H&E staining (C) and Nile Red staining (D), liver triglycerides assay (E) and serum ALT assay (F). *p <0.05, **p < 0.01 by two-tailed Student’s t test. Scale bar = 100 μM.
Next, we examined the impact of AKT2-CA over-expression on the severity of ALD in BLKO mice. H&E staining and Nile-Red staining showed reduced lipid accumulation in the liver of AKT2-CA injected mice (Fig. 6C,D) in addition to reduced liver TG (Fig. 6E). Restoring of AKT activity also reduced liver injury detected by lower ALT level (Fig. 6F), fewer TUNEL-positive cells and reduced caspase 3 cleavage (Supporting Fig. S10A,B). Interestingly, the protective effects of AKT2-CA overexpression were only observed in Bmal1-LKO liver but not in WT mice (Fig. 6F, Supporting Fig. S11). Taken together, restoring hepatocyte AKT is sufficient to reverse ALD in Bmal1-LKO mice. Furthermore, increasing AKT activity in Bmal1-LKO mice not only promotes de novo lipogenesis but also enhances the expression of β-oxidation genes. To our knowledge this is the first evidence showing that active AKT signaling pathway enhances both de novo lipogenesis and β-oxidation while protecting against ALD.
OVEREXPRESSION OF ChREBP IN THE LIVER PARTIALLY RESCUES LIVER INJURY IN ETHANOL-FED Bmal1-LKO MICE
Both ChREBP and SREBP-1c are two major lipogenic transcription factors in hepatic lipid biosynthesis.(31) SREBP-1c has been implicated as a direct downstream effector of AKT-mTORC1 signaling in insulin-induced de novo lipogenesis,(32) whereas ChREBP has been more associated with high carbohydrate feeding, in particular, fructose feeding.(33,34) A recent study showed loss of Chrebp may sensitize mice from binge-drinking-induced liver injury.(35) We observed that L-pk, the hallmark target of ChREBP, was reduced in the liver of both Bmal1-LKO and mice with liver specific depletion of Bmal1 but induced in Ad-Bmal1 liver (Fig. 3F, Fig. 4A,D), indicating a functional link between BMAL1 and ChREBP during the process of de novo lipogenesis. Furthermore, we evaluated the effects of ChREBP over-expression in primary hepatocyte isolated from Bmal1flox/flox and Bmal1-LKO mice on the expression of enzymes in lipogenesis and fatty acid oxidation. We also included SREBP-1c as a comparison. Consistent with their roles in driving de novo lipogenesis, both SREBP-1c and ChREBP overexpression significantly induced key genes of lipogenic program (Supporting Fig. S12A,C). However, only ChREBP was able to potently induce the expression of β-oxidation genes (Supporting Fig. S12B,D). Of note, compared with Bmal1flox/flox hepatocytes, Bmal1-LKO hepatocytes showed a more robust induction of β-oxidation genes in response to ChREBP overexpression. These data unveiled a diverging effect of ChREBP and SREBP-1c on β-oxidation in the liver.
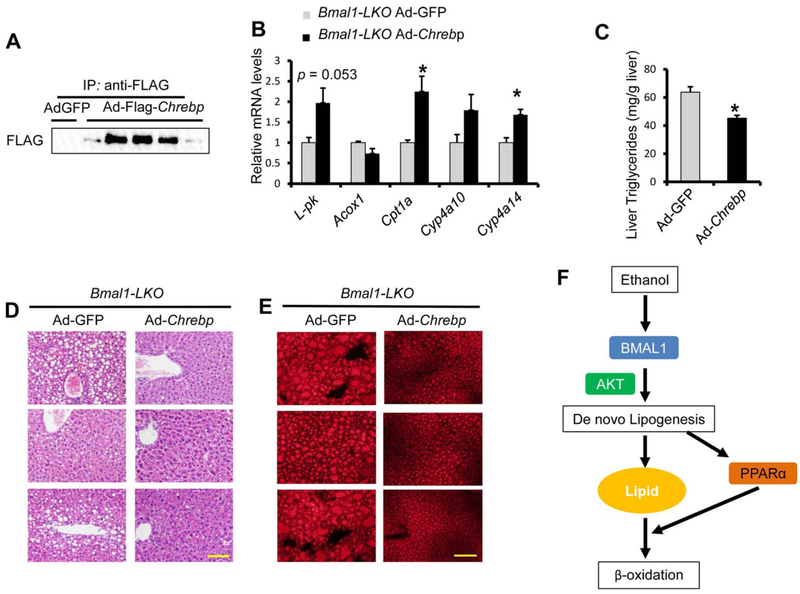
These observations prompted us to ask whether over-expression of ChREBP in liver could affect the degree of liver injury in ethanol-fed Bmal1-LKO mice. Over-expression of FLAG-ChREBP in the liver after Ad-Flag-Chrebp adenovirus injection was confirmed by immunoblotting with anti-FLAG (Fig. 7A). Compared with Ad-GFP injection, Ad-Flag-Chrebp injection induced hepatic expression of β-oxidation genes (Fig. 7B), reduced fat accumulation in the liver of Bmal1-LKO mice (Fig. 7C–E). We also detected significantly reduced apoptosis in the liver in addition to modestly decreased serum ALT (Supporting Fig. S13). Interestingly, the protective effect of ChREBP overexpression was only observed in the Bmal1-LKO liver but not in that of WT mice (Supporting Fig. S14).
FIG. 7.
Hepatic overexpression of ChREBP restores the PPARα activity and partially ameliorates liver injury in ethanol-fed Bmal1-LKO mice. Eight-week-old Bmal1-LKO mice were injected with Ad-Flag-Chrebp (n = 5) or Ad-GFP (n = 3), and then fed 5% ethanol diet for 10 days and binged with ethanol 5g/kg body weight at ZT3, and were dissected 9 hours later. (A) Hepatic ChREBP overexpression by adeovirus was confirmed by immunoprecipitation with anti-FLAG beads and western blotting with anti-FLAG. (B) Hepatic ChREBP overexpression activates the expression of L-pk and PPARα target genes measured by RT-qPCR. (C-E) Hepatic ChREBP overexpression reduces ethanol feeding-induced liver steatosis in Bmal1-LKO mice with liver triglycerides assay (C) H&E staining (D) and Nile Red staining (E). (E,F) Hepatic ChREBP overexpression ameliorates ethanol-induced liver injury in Bmal1-LKO mice with serum ALT assay (E). (F) Model depicting that BMAL1 limits ethanol-induced lipotoxicity via increased β-oxidation by activating de novo lipogenesis to provide ligands for PPARα. *p < 0.05, by two-tailed Student’s t test. Scale bar = 100 μM
Meanwhile, we also evaluated whether over-expression of SREBP-1c in the liver could impact the degree of liver injury in ethanol-fed Bmal1-LKO mice. As shown in Supporting Fig. S15B–D, ectopic expression of SREBP-1c failed to reduce liver steatosis and liver injury after ethanol diet. Moreover, SREBP-1c overexpression reduced the expression of β-oxidation genes in ethanol-fed Bmal1-LKO mice (Supporting Fig. S15E). In summary, these data suggest that ChREBP overexpression in the Bmal1-LKO liver efficiently reverses alcoholic fatty liver and reduces liver injury. Our study also demonstrated a clear distinction between ChREBP and SREBP-1c in hepatic regulation of de novo lipogenesis and fatty acid oxidation during ethanol feeding.
To investigate the clinical relevance of the BMAL1-AKT-ChREBP axis in human ALD, we examined the abundance of BMAL1, AKT-PS473, AKT, and ChREBP in the liver samples from patients with alcoholic hepatitis vs. normal controls. Indeed, BMAL1 protein levels were severely decreased in the livers of patients with ALD compared with normal controls (Supporting Fig. S16). In the same liver samples, the levels of AKT-PS473 were greatly reduced. Moreover, ChREBP protein levels were reduced in the liver of ALD patients. Collectively, our data suggest that the impairment of the BMAL1-AKT-ChREBP axis could be involved in the development of human ALD.
Discussion
In the current study, we presented evidence supporting that the intact circadian clock in the liver is required to protect mice from ethanol feeding-induced liver steatosis and injury. Either chronic or acute loss of Bmal1 aggravates liver steatosis and injury after chronic ethanol-binge feeding. Impaired PPARα signaling may drive the severity of ALD in the Bmal1-deficient condition since PPARα agonist fenofibrate rescues liver injury in Bmal1-LKO mice. Further investigations reveal an intimate relationship between the BMAL1-AKT-ChREBP axis-mediated de novo lipogenesis and PPARα-induced fatty acid oxidation. In summary, our work demonstrated a pivotal role of the circadian clock protein BMAL1 in the liver in protecting against ALD via maintaining hepatic lipid homeostasis.
Recent studies suggest a potential crosstalk between alcohol metabolism and circadian rhythms could impact the progression and severity of ALD.(4,36) We observed severe liver steatosis and liver injury following ethanol feeding in two different mouse models with circadian clock deficiency in the liver, pointing to the protective role of an intact hepatic circadian clock against ALD. Interestingly, our study revealed that it is the circadian clock in hepatocytes rather than macrophages that protects the liver in response to ethanol feeding, highlighting the cell type-specific function of the circadian clock. Our data suggest that hepatic circadian deficiency or impairment could be a risk factor for the development of advanced ALD. It has been reported that high-fat diet greatly dampens the hepatic circadian clock even before the development of obesity.(37) Social factors such as jet-lag can also disturb the hepatic circadian clock.(16) Therefore, restoring or maintaining a normal circadian clock could be key to the prevention or treatment of ALD.
Our study also suggests that BMAL1 overexpression is also sufficient in protecting mice from alcoholic liver injury. Liver-specific expression of BMAL1 using adenovirus provides almost full protection against ALD in both female and male mice. This exciting finding suggests that elevating the BMAL1 abundance in hepatocytes might be an effective approach to treat ALD. In our study, we also found that BMAL1 protein is modestly increased in the liver of ethanol-fed WT mice although the clock activity is suppressed. It is well known that the Bmal1 mRNA oscillates in hepatocytes during a 24-hr cycle and multiple transcription factors are implicated in its mRNA oscillation. In contrast, the BMAL1 protein levels remain steady with modest changes throughout the circadian cycle.(38) It has been suggested that BMAL1 protein abundance and activity are subject to multiple post-translational modifications including O-linked glycosylation, acetylation, and phosphorylation.(38–41) We suspect that elevated BMAL1 protein in the ethanolfed WT mouse liver is a net result of altered multiple upstream signaling pathways. Given that BMAL1 overexpression is sufficient to protect mice from ALD, the modest induction of BMAL1 in the liver of ethanol-fed WT mice might be a compensatory mechanism to preserve the clock activity in hepatocytes.
Based on our findings, one of the major downstream pathways could be AKT-ChREBP-lipogenesis. During ethanol feeding, BMAL1 over-expression up-regulates liver AKT activity and ChREBP target gene expression, whereas Bmal1 deficiency reduces liver AKT activation and ChREBP target gene expression. Restoring AKT or ChREBP in Bmal1-LKO mice attenuates fatty liver and more importantly activates the β-oxidation pathway. How BMAL1 regulates the ChREBP transcriptional activity via AKT remains to be determined in future study. A recent study suggests AKT can activate ChREBP in brown adipose tissue upon cold exposure.(42) It is intriguing to speculate that a similar pathway is conserved in hepatocytes in the context of ethanol feeding.
Our findings have shed light on an under-appreciated function of hepatocyte de novo lipogenesis in promoting PPARα-dependent β-oxidation pathway. The Semenkovich group used hepatocyte-specific Fasn knockout mice as a model to concept that de novo lipogenesis in hepatocytes promotes PPARα and β-oxidation pathway by generating newly synthesized PPARα-specific ligands.(12,13) For alcoholic fatty liver disease, a canonical model is that lipogenesis over-activation leads to liver steatosis and lipotoxicity. However, a recent report showed that although ethanol feeding induced liver steatosis in mice, lipogenic gene expression was repressed.(43) Our findings support this concept in Bmal1-LKO mice by demonstrating hepatic BMAL1 acts as a central regulator that coordinates de novo lipogenesis and PPARα-dependent β-oxidation in hepatocytes when challenged with ethanol feeding. Given the fact that BMAL1, ChREBP, and SREBP-1c all can stimulate the transcriptional program of de novo lipogenesis, it will be interesting to use unbiased lipodomics to differentiate lipid end-products specific for each lipogenic factor after over-expression.
The Bailey group showed that chronic ethanol consumption disrupts the core circadian clock and diurnal rhythms of metabolic genes in the liver.(44) The Duffield group also showed the circadian clock impairment in the mouse liver with alcohol–induced steatosis.(45) In agreement with these two studies, our study showed disrupted diurnal rhythms of core clock genes and a potent suppression of SIRT1 activity in the ethanolfed mouse liver. Compared with the two studies, our study used a different chronic feeding/binge protocol. Therefore, there are noticeable differences in the type and pattern of clock genes oscillations affected by the duration of feeding, degree of liver steatosis, and severity of injury. It is likely that ethanol feeding impacts the expression of circadian genes in both clock-dependent and clock-independent manners.
In summary, we have presented evidence that BMAL1, a key circadian protein, is both necessary and sufficient to protect mice against ALD. Our findings identified the BMAL1-AKT-ChREBP-lipogenesis axis as a major signaling route in the pathogenesis of ALD. Our study suggests that activation of BMAL1-dependent de novo lipogensis in the liver could offer therapeutics to treat human ALD.
Supplementary Material
Acknowledgement:
We thank Dr. Jiandie Lin (University of Michigan) for providing Bmal1flox/flox mice.
Supported by NIH R01(DK099593) and R21 (AA022720) to L.Y. and R01 (DK47918) to M.B.O.. Part of the work was also supported by pilot grants from Michigan Nutrition and Obesity Research Center to L.Y. and X.T. (P30 DK089503) and Michigan Diabetes Research Training Center to X.T. (P60DK020572).
Abbreviations:
- AKT2-CA
constitutively active AKT2
- ALD
Alcohol liver disease
- ALT
alanine aminotransferase
- EM
electron microscope
- HFD
high-fat diet
- LKO
liver-specific knockout
- MKO
macrophage-specific knockout
- TG
triglycerides
- WT
wildtype
Footnotes
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29878/suppinfo.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Massey VL, Arteel GE. Acute alcohol-induced liver injury. Front Physiol 2012;3:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Poppitt SD. Beverage Consumption: Are Alcoholic and Sugary Drinks Tipping the Balance towards Overweight and Obesity? Nutrients 2015;7:6700–6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12:231–242. [DOI] [PubMed] [Google Scholar]
- 4).Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, et al. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PloS One 2013;8:e67102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Swanson GR, Gorenz A, Shaikh M, Desai V, Kaminsky T, Van Den Berg J, et al. Night workers with circadian misalignment are susceptible to alcohol-induced intestinal hyperpermeability with social drinking. Am J Gastrointest Liver Physiol 2016;311: G192–G201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015;161:84–92. [DOI] [PubMed] [Google Scholar]
- 7).Zhang D, Tong X, Arthurs B, Guha A, Rui L, Kamath A, et al. Liver clock protein BMAL1 promotes de novo lipogenesis through insulin-mTORC2-AKT signaling. J Biol Chem 2014; 289:25925–25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell metab 2015;22: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor α (PPARα) agonist treatment reverses PPARα dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 2003;278:27997–28004. [DOI] [PubMed] [Google Scholar]
- 10).Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and dna binding activity of peroxisome proliferator-activated receptor α is inhibited by ethanol metabolism. a novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem 2001;276:68–75. [DOI] [PubMed] [Google Scholar]
- 11).You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 2002;277: 29342–29347. [DOI] [PubMed] [Google Scholar]
- 12).Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, et al. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 2005;1:309–322. [DOI] [PubMed] [Google Scholar]
- 13).Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, et al. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell 2009;138:476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 2013;8:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Yin H, Hu M, Zhang R, Shen Z, Flatow L, You M. Micro-RNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J Biol Chem 2012;287:9817–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008;134:317–328. [DOI] [PubMed] [Google Scholar]
- 17).Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, et al. The NAD1-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008;134:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD1 salvage pathway by CLOCK-SIRT1. Science 2009;324:654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Tong X, Zhang D, Arthurs B, Li P, Durudogan L, Gupta N, et al. Palmitate inhibits SIRT1-dependent BMAL1/CLOCK interaction and disrupts circadian gene oscillations in hepatocytes. PloS One 2015;10:e0130047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 2002;122:2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci 2012;343:244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 2016;64:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol 2013;13:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor α defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol 2006;20:1715–1727. [DOI] [PubMed] [Google Scholar]
- 25).Oishi K, Shirai H, Ishida N. CLOCK is involved in the circa-dian transactivation of peroxisome-proliferator-activated receptor α (PPARα) in mice. Biochem 2005;386:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, et al. Peroxisome proliferator-activated receptor α protects against alcohol-induced liver damage. HEPATOLOGY 2004;40:972–980. [DOI] [PubMed] [Google Scholar]
- 27).Forcheron F, Cachefo A, Thevenon S, Pinteur C, Beylot M. Mechanisms of the triglyceride-and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes 2002;51:3486–3491. [DOI] [PubMed] [Google Scholar]
- 28).Vega GL, Ma PT, Cater NB, Filipchuk N, Meguro S, Garcia-Garcia AB, et al. Effects of adding fenofibrate (200 mg/day) to simvastatin (10 mg/day) in patients with combined hyperlipidemia and metabolic syndrome. American J Cardiol 2003;91:956–960. [DOI] [PubMed] [Google Scholar]
- 29).Tsutsumi M, Takase S. Effect of fenofibrate on fatty liver in rats treated with alcohol. Alcohol Clin Exp Res 2001;25(6 Suppl): 75S–89S. [DOI] [PubMed] [Google Scholar]
- 30).Liu S, Alexander RK, Lee C-H. Lipid metabolites as metabolic messengers in inter-organ communication. Trends Endocrinol Metab 2014;25:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Zhang D, Yin L. Transcriptional Regulation of De novo Lipo-genesis in Liver In: Ntambi J, ed. Hepatic De novo Lipogenesis and Regulation of Metabolism. 1st ed. Switzerland: Springer; 2016;1–31. [Google Scholar]
- 32).Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 2011;14:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Zhang DQ, Tong X, VanDommelen K, Gupta N, Stamper K, Brady GF, et al. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J Clin Invest 2017;127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Abdul-Wahed A, Guilmeau S, Postic C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab 2017;26:324–341. [DOI] [PubMed] [Google Scholar]
- 35).Marmier S, Dentin R, Daujat-Chavanieu M, Guillou H, Bertrand-Michel J, Gerbal-Chaloin S, et al. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. HEPATOLOGY 2015;62:1086–1100. [DOI] [PubMed] [Google Scholar]
- 36).Udoh US, Valcin JA, Gamble KL, Bailey SM. The molecular circadian clock and alcohol-induced liver injury. Biomolecules 2015;5:2504–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007;6:414–421. [DOI] [PubMed] [Google Scholar]
- 38).Li MD, Ruan HB, Hughes ME, Lee JS, Singh JP, Jones SP, et al. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab 2013;17:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, et al. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007;450:1086–1090. [DOI] [PubMed] [Google Scholar]
- 40).Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3β-mediated phosphorylation. PloS One 2010;5:e8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Tamaru T, Hirayama J, Isojima Y, Nagai K, Norioka S, Takamatsu K, et al. CK2α phosphorylates BMAL1 to regulate the mammalian clock. Nat Struc Mol Biol 2009;16:446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Sanchez-Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Martinez Calejman C, Gujja S, et al. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab 2018;27:195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Yang Z, Tsuchiya H, Zhang Y, Lee S, Liu C, Huang Y, et al. REV-ERBα activates C/EBP homologous protein to control small heterodimer partner–mediated oscillation of alcoholic fatty liver. American J Pathol 2016;186:2909–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Filiano AN, Millender-Swain T, Johnson R Jr, Young ME, Gamble KL, Bailey SM. Chronic ethanol consumption disrupts the core molecular clock and diurnal rhythms of metabolic genes in the liver without affecting the suprachiasmatic nucleus. PloS One 2013;8:e71684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Zhou P, Ross RA, Pywell CM, Liangpunsakul S, Duffield GE. Disturbances in the murine hepatic circadian clock in alcohol-induced hepatic steatosis. Sci Rep 2014;4:3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.