Abstract
The statistics from Europe and the USA have proven a high risk for skin diseases associated with plant contact. Therefore, plant-induced dermatitis is of increasing attention in dermatology. The focus of this paper was to present the current knowledge on aspects of contact allergy related to Asteraceae (Compositae) species. The Asteraceae family is one of the largest in the world with members across all continents. The PubMed/Medline databases have been searched. The Asteraceae representatives consist of diverse secondary metabolites, which exhibit various advantageous effects in humans. In particular, sesquiterpene lactones (SLs) may cause sensitization resulting in skin irritation and inflammation. In this study, we tried to reveal the allergenic potential of several Asteraceae species. The Asteraceae-related allergy symptoms involve eczema, hay fever, asthma, or even anaphylaxis. Furthermore, the evidence of severe cross-reactivity with food and pollen allergens (PFS) in patients sensitive to Asteraceae allergens have been announced. Further identification and characterization of secondary metabolites and possible allergens in Asteraceae are necessary for the better understanding of Asteraceae-related immune response. The Asteraceae allergy screening panel (the SL mix and the Compositae mix of five plant species) is a promising tool to improve allergy diagnostics and therapy.
Keywords: Allergic contact dermatitis, Irritant contact dermatitis, Allergy, Environment, Asteraceae, Sesquiterpene lactones
Introduction
The statistics from across the world have proven a high risk for skin diseases associated with plant contact (Crosby 2004; Fonacier et al. 2015). Therefore, plant-induced allergies are of increasing attention in medicine (Paulsen et al. 2017; Rozas-Muñoz et al. 2012). A number of different occupations are at risk of plant toxins and allergens exposure. The highest risk is associated with outdoor workers, e.g., farmers, gardeners, forestry and nursery workers, florists, cooks, housekeepers, and grocery store workers (Poljački et al. 2005; Spiewak 2001). In addition to professional activity, skin exposure to plant-related substances can occur during common outdoor or indoor activity (de Jong et al. 1998). Moreover, the increasing popularity of plant extracts in cosmetics (tonics, soaps, shampoos, creams) and massage or aromatherapy fragrance oils raises the chance of contact with hazardous substances (Thomson and Wilkinson 2000).
Plants, including Asteraceae species may imply contact or systemic allergy (Rozas-Muñoz et al. 2012). The Asteraceae species have been identified to produce numerous secondary metabolites, such as polyphenols, flavonoids, diterpenoids, and sesquiterpene lactones (SLs). These metabolites are dissolved or suspended in the latex sap or placed in specific trichomes found on plant organs, i.e., leaf, stem, flowers, seeds, and fruits (Salapovic et al. 2013; Jachuła et al. 2018b). The main group of chemicals relevant to cause allergies and systemic contact dermatitis are sesquiterpene lactones, i.e., lactones with α-methylene group on the γ-lactone ring (Menz and Winkelmann 1987; Nemery and Demedts 1989; Paulsen 2017; Paulsen et al. 2001). In Asteraceae, about 3000 compounds that belong to diverse classes of sesquiterpenoids: guaianolides, eudesmanolides, germacranolides, and pseudoguaianolides have been recognized (Paulsen et al. 2017; Salapovic et al. 2013; Zidorn 2008).
Almost 50% of SLs are potential contact allergens (Menz and Winkelmann 1987). These metabolites are present both in fresh and dried plants in various proportions from 0.01 to 8% per dry weight (Gordon 1999; Neerman 2003). It is also supposed that individuals with contact dermatitis to Asteraceae SLs can react to many other non-Asteraceae SLs-containing plants (Green and Ferguson 1994; Fuchs et al. 2011).
The main objective of this article was to review the evidence for the types of allergic reactions after contact with representatives of the Asteraceae family, which is one of the largest group of flowering plants distributed worldwide. The emphasis was put on the potential allergens from the common species used as popular food, ornamental plants, medicinal plants, and weeds. In particular, the common clinical symptoms of contact and systemic contact dermatitis caused by diverse bioactive molecules present in Asteraceae have been described.
Methods
The PubMed/Medline databases were searched, from inception to February 2018, using various combination of the following keywords: Asteraceae, Compositae, the names of plant species, sesquiterpene lactones, SLs, and contact dermatitis and related terms: irritant contact dermatitis, allergic contact dermatitis, and systemic contact dermatitis. Each reference retrieved was screened independently by two reviewers (MDP and ŁP), following predefined criteria to determine eligibility for the review.
Contact and systemic contact (allergic) dermatitis
Contact dermatitis is an inflammatory skin condition accounting for 70–90% of all occupational skin diseases (Adisesh et al. 2013). Contact dermatitis is induced by exposure to an external irritant or allergen and therefore, two types of contact dermatitis: irritant and allergic are distinguished (Rashid and Shim 2016). Approximately 80% of contact dermatitis are irritant contact dermatitis (ICD), which is a non-immunologic response to the direct damage of the skin, by chemical or physical agents (Fonacier and Sher 2014; Pigatto 2015; Tan et al. 2014). The clinical appearances differ between the acute and chronic ICD. The acute ICD includes macules and papules, erythematous, erythemoto-edematous or erytemato-squamous plaques. In the chronic ICD, dry skin, erytemato-squamous dermatitis, hyperkeratosis, and disappearance of fingerprints are found (Nosbaum et al. 2009). The rate of reactions and the severity of changes in skin depend on (i) nature and concentration of the responsible factor; (ii) duration, area, and frequency of contact with an agent; (iii) environment; (iv) skin type; and condition (Slodownik et al. 2008). The mechanism of skin irritation starts with skin damage and is followed by the release of numerous proinflammatory cytokines and chemokines (de Jongh et al. 2007). The primary source of ICD mediators are keratinocytes; however, new insight is given to the mast cells, macrophages, dendritic cells, and natural killers cells (Norman et al. 2008; Vocanson et al. 2005). The cytokines secreted in the ICD are IL-1, IL-6, IL-8, and TNF-α (Nosbaum et al. 2009; Vocanson et al. 2007).
Allergic contact dermatitis (ACD) compromises 20% of cases of contact dermatitis and includes two phases: (i) sensitization— maturation of potential to develop a cutaneous allergic reaction to allergen and (ii) elicitation—skin inflammation developed as a result of repeated exposure to the allergen in a sensitized person (Fonacier and Sher 2014). ACD is a type IV delayed hypersensitivity reaction to an external allergen with the circulating memory T cells involved as the main players. T cells home into the skin during r-exposure to an allergen and activate immunologic reaction causing skin inflammation, usually within 48 h (Burkemper 2015). The activated T cells produce cytokines, e.g., IL-2, IL-17, and INF-α, which further activate and damage skin cells (McFadden et al. 2013). The cellular apoptosis induces inflammation, recruitment, and mobilization of new cells in the skin resulting in eczema (Cavani 2008; Vocanson et al. 2007). The clinical symptoms and signs of ACD consists of erythema, edema, and oozing in the acute phase, while the chronic phase is characterized by lichenified, fissured, and pigmented skin. The location of clinical signs in the ACD is usually limited to the site of contact; however, in contrary to the ICD skin lesions might spread locally or at a distance (Asano et al. 2009; Nicolas et al. 2008). Summary and differential diagnosis between ICD and ACD are presented in Table 1.
Table 1.
Summary and differential diagnosis between ICD and ACD
| Criteria | Irritant contact dermatitis | Allergic contact dermatitis |
|---|---|---|
| Risk group | Anyone, especially people with repeated exposure | Previously sensitized, people genetically predisposed |
| Mechanism | Non-immunologic response to the direct damage of the skin | Immunologic, type IV delayed hypersensitivity reaction |
| Concentration of factor or allergen | Usually high, positive correlation between power of the agent’s concentration and sin lesion | Might be low, required threshold concentration |
| Symptoms | Burning, prickling, stinging | Pruritus, erythema, edema |
| Skin lesions’ area | Limited to the place of irritation | Site of contact, lesions might spread locally or at a distance |
| Onset of lesions | Appear rapidly, within minutes to hours | Appear within 24–72 h, possible late onset at 7 days after exposure |
| Diagnostic methods | None | Patch test |
Systemic contact (allergic) dermatitis (SCD) is an inflammatory skin disease and occurs in sensitized person after oral, inhalation, intravesical, intravenous, or transcutaneous exposure to the haptens (Nicolas et al. 2008; Veien 2011). Systemic reactions are induced by both humoral and cell-mediated mechanism including T cells and cytokine secretion (Paulsen 2017). Clinical symptoms include local allergic manifestations; however, in a person exposed to allergen, systematically noncutaneous symptoms might develop such as fever, chest pain, and urticarial (Andrews and Scheinman 2011).
Skin severe reactions caused by bioactive chemicals from the representatives of the Asteraceae family have been described worldwide (in North America, Europe, Asia, Australia); however, patient sensitivity varied between geographical regions or seasons and is associated with both sex and age (Gordon 1999; Thomson and Wilkinson 2000). In Europe, Asteraceae-related allergy is among the top ten contact sensitivities, in most cases noted in Central and South Europe (Alexander et al. 2013; Paulsen and Andersen 2016). The allergic reactions after contact with Asteraceae SLs differ between countries, ranging from 0.1% to 2.7%, with a mean prevalence of 1.5% (Paulsen 2017).
Some authors even indicate that the detection for Asteraceae-related skin dermatitis is insufficient due to the low awareness of the problem among patients and their doctors (Spiewak 2001). Typical routes of accidental exposure are skin or eye contact or ingestion (Gordon 1999; Jovanović and Poljacki 2003; Neerman 2003). Positive reactions to Asteraceae allergens (SLs, flavonoids, proteins) may be caused not only by plant allergy, but also by cross-reactivity with, for instance, fragrance terpenes (Paulsen 2017; Paulsen and Andersen 2016).
Diverse reactions have been documented after transcutaneous absorption of toxins from Asteraceae (Paulsen and Andersen 2016; Zidorn 2008). Generalized eczema (20–30%), eczema of exposed body surfaces, i.e., hands and face (24%), facial eczema (11–28%), eczema of V of the neck, and forearms are found among clinical manifestations of Asteraceae-derivative symptoms (Jovanović and Poljacki 2003). In addition, areas protected from the sunlight exposure such as retroauricular regions (Wilkinson triangle), eyelids, and nasolabial folds are also at risk, allowing its differentiation from a true photo-related dermatitis (Gordon 1999). Among Asteraceae-sensitive individuals, 78.8% exhibit different contact skin inflammations, e.g., to nickel in 33.3% of patients or photosensitivity in 22–75% of persons (Jovanović and Poljacki 2003). Asteraceae allergy screening panel developed by the North American Contact Dermatitis Group comprises of two standard Asteraceae allergens responsible for contact skin inflammation (1) sesquiterpene lactones mix (SL mix; mix of three common SLs (alantolactone, dehydrocostus lactone, and costunolide) and (2) Compositae mix (CM) comprises the biological substances from five Asteraceae species, i.e., Arnica montana, Matriacaria recutica, Tanacetum parthenium, Tanacetum vulgare, and Achillea millefolium (Alexander et al. 2013). Both the SL mix and the Compositae mix are considered as efficient screening for Asteraceae-related allergy (Green and Ferguson 1994; Paulsen et al. 2001). Potential allergens derivative from Asteraceae species are displayed in Table 2 and structures of several common SLs compounds are shown in Fig. 1.
Table 2.
Species of the Asteraceae (Compositae) family with potential allergens
| Name | Distribution | Allergens |
|---|---|---|
| Edible plants | ||
| Lettuce, Lactuca sativa L. | Cultivated worldwide | SLs—guaianolides, lactucin, lactucopicrin, 8-deoxylactucopicrin |
| Endivie, Cichorium endivia L. | Mediterranean region | SLs—lactucopicrin, kaempferol malonyl glucoside |
| Chicory, Cichorium intybus var. sativum L. | Europe and North America | SLs—grosheimin, guaianolide ixerisoside D, glycosides |
| Globe artichoke, Cynara scolymus L. | Mediterranean region of Europe and North Africa | SLs, flavonoids, hydroxycinnamic acids, tyrosols, and lignans |
| Sunflower, Helianthus annuus L. | Central America, cultivated in moderate climate zones and semi-arid regions | SLs—niveusin B and argophyllin A and B, diterpene acids, grandifloric acid, ciliaric acid and 17-hydroxy-ent-isokaur-15(16)-en-19-oic acid, albumins |
| Ornamental plants | ||
| Chrysanths, Chrysanthemum sp. | Native to Asia and northeastern Europe, known and cultivated worldwide | SLs—guaianolides cumambrin A, dihydrocumambrin A, pyrethrum |
| Dahlia, Dahlia sp. | Cultivated in Europe, Asia | SLs—causesin |
| Zinnia, Zinnia sp. | Cultivated in Europe, Asia | SLs—zinagrandinolides A-C (1–3), delta-elemanolide 4 |
| Herbs | ||
| Dandelion, Taraxacum officinale L. | Native to Europe and Asia; naturalized worldwide | Taraxinic acid-1ƒ-O-b--glucopyranoside |
| Marigold, Calendula officinalis L. | Southern Europe; naturalized in temperate regions | Triterpenoids, flavonoids, coumarins, quinones, volatile oil, carotenoids and amino acids |
| Wild chamomile, Matricaria chamomilla L.; Chamomilla recutita; Dog fennel, Anthemis cotula L. | Common in Europe, North Africa, and Asia | SLs, a-peroxyachifolid, herniarin, nobilin, bisabolol coumarin, anthecotulide flavonoids |
| Echinacea, Echinacea purpurea (L.) Moench.) | Native to North America, used worldwide | SLs cimifugaside, caryophyllene |
| Tansy, Tanacetum vulgare L. | Native to Europe and Asia; naturalized in North America | SLs tatridin A, |
| Feverfew, Tanacetum parthenium L. | Common in Europe, North America, Canada | SLs— germacranolides (e.g., parthenolide), eudesmanolides guaianolides, artecanin, artemorin, balchanin, canin, costunolide |
| Mugwort, Artemisia vulgaris L. | Native to Europe, Asia, Northern Africa; naturalized in North America | SLs, psilostachyin, psilostachyin-C, artemisin |
| Yarrow, Achillea millefolium L. | Common in Europe, Asia, and North America | SLs—a-peroxyachifolid, flavonoids |
| Great burdock, Arctium lappa L. | Common in Europe, Asia, and North America | SLs, actiopicrin |
| Arnica, Arnica montana L. | Central Europe | SLs—xanthalongin, helenalin |
| Ragweed, Ambrosia artemisifolia L. | Native to North America and Canada; naturalized in Europe | SLs—psilostachyin, psilostachyin B and psilostachyin C; pseudoguaianolides cumanin, peruvin and dihydrocumanin |
| Santa Maria feverfew, Parthenium hysterophorus L. | Native to tropical regions of America; invasive in India, Australia, and Africa | SLs—parthenin |
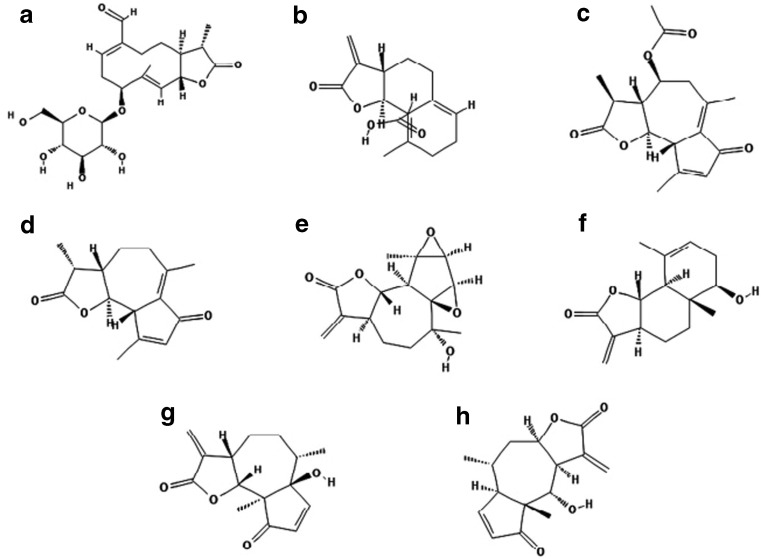
Fig. 1.
Structures of several common SL compounds found in plant tissue of the Asteraceae (Compositae) species presented according to their chemical classification. 1. Germacranolides—lactuside A (A), taraxinix acid (B); 2. Guaianolides—matricarin (C), achillin (D); 3. Eudesmanolides—artecanin (E), balchanin = santamarin (F); 4. Pseudoguaianolides—parthenin (G), helenalin (H)
Asteraceae family—characteristic and representatives
The Asteraceae family (Compositae) is one of the major group of flowering plants, comprising approximately 1000 genera and 23,000 species widespread worldwide (Heywood 1993; Tutin et al. 1980; Czarnecka and Denisow 2014). The Asteraceae species are distributed from the polar zone to the subtropical and tropical zones, except Antarctica (Stevens 2001). The Asteraceae species occur in various habitats and represent 8–20% of native floras (Tutin et al. 1980). The characteristic feature is flower reduction and organizing in the capitulum-type inflorescence. The members are mainly herbaceous species; however, trees and shrubs are also found. Numerous of these species are economically important and are cultivated as crops (Heywood 1993). The plants are grown as vegetables: lettuce (Lactuca sativa L.), endive (Cichorium endivia L.), cardoon (Cynara cardunulus L. var. sylvestris), globe artichoke (Cynara cardunculus L. var. scolymus), common salsify (Tragopogon porrifolius L.), black salsify (Scorzonera hispanica L.), the garden ornamentals useful in flowering arrangements (e.g., Ageratum, Aster, Dahlia, Tagetes—marigold, Zinnia); several species are popular as indoor plants (Chrysanthemum, Gerbera, Senecio) (Tutin et al. 1980). A large number of species are recognized as weeds, e.g., Cirsium sp., Carduus sp., coltsfoot (Tussilago farfafa L.), dandelion (Taraxacum officinale L.), yarrow (A. millefolium L.), tansy (T. vulgare L.), and are characteristic for areas with different levels of anthropopressure (Denisow 2011; Wrzesień et al. 2016a, b; Jachuła et al. 2018a, b). Some species are popular as medical herbs, i.e., arnica (A. montana L.), German chamomile (Chamomilla recutita (L.) Rauch.) or marigold (Calendula officinalis L.) (Reider et al. 2001). Invasive plant species are also common among Asteraceae species (Denisow et al. 2017). Some anemophilous Asteraceae species are responsible for severe pollinosis, e.g., common ragweed (Ambrosia artemisifolia L.), mugwort (Artemisia vulgaris L.), marsh elder (Iva xanthifolia Nutt.), or cocklebur (Xanthium strumarium L.) (D'Amato et al. 2007).
Edible Asteraceae species
Lettuce (L. sativa L.) is an important leaf vegetable with numerous varieties commonly cultivated worldwide and increasing production in European countries (Paulsen et al. 2001). Among SLs are the guaianolides, of which lactucin, lactucopicrin, and 8-deoxylactucopicrin are the most representative (Salapovic et al. 2013). However, a 9-kDa lipid transfer protein (Lac s 1) is considered the major allergen in lettuce (Hartz et al. 2007). Two Lac s 1 isoforms were recognized, with an amino-acid showing the high sequence identity to Pru p 3 from peach, apple allergen Mal d 3, and to the London plane tree pollen (LTPs pla a 3) (Franck et al. 2000). The allergic patients’ sera showed specific IgE binding to an nLac s protein from the lettuce extract (Hartz et al. 2007). Lettuce protein (Lac s 1) may result in cross-reactivity with other lipid transfer protein-containing foods (LTPs), e.g., from Rosaceae family (peach, apple, apricot); other plant sources (mugwort, peanut, hazelnut, chestnut, grapes, maize, beans, orange, onion, tomatoes, strawberry); pollen; or animal food (milk, fish, sea food, chicken) (Avila Castanon et al. 2002; Diaz et al. 2013; Díaz-Perales et al. 2000; Pastorello et al. 2000; Vila et al. 1998).
In central and northern Europe, the allergy to lettuce is not frequently noted (de Jongh et al. 2007). However, allergy symptoms of a lettuce allergy reaction can invoke after ingestion and can range from mild to severe (Diaz et al. 2013; Vila et al. 1998). In some regions, the species is included among major Asteraceae skin irritants (Alexander et al. 2013). In particular, the allergic response to lettuce is associated with birch pollinosis, and the symptoms are usually limited to the oropharyngeal system (Díaz-Perales et al. 2000). In the Mediterranean countries, the allergic reactions are independent on pollinosis, and the individuals manifest systemic reactions associated with nonspecific lipid transfer proteins (nsLTP) (San Miguel-Moncín et al. 2003). Considering common lettuce consumption, the contact allergy is relatively rarely reported; however, several occupational cases have been documented, therefore, the lettuce-related allergy may be underdiagnosed (Paulsen and Andersen 2016; Vila et al. 1998). In clinical practice, diverse symptoms associated with allergy to lettuce protein have been described, i.e., urticaria, gastrointestinal symptoms, OAS, and angioedema (Hartz et al. 2007).
Occupational contact dermatitis has been revealed in employees working with green business (greenhouse workers, gardeners, cookers) (Helander 1984; Krook 1977). Lettuce has been reported to encounter for lip and facial swelling. In isolated cases, an aggravation of pre-existing dermatitis has been recorded (Oliwiecki et al. 1991). Several patients have been described to have anaphylaxis that occurs in response to lettuce (Morita et al. 2007; Olive-Perez and Pineda 2003; San Miguel-Moncín et al. 2003).
Endivie (C. endivia L.), a bitter-leafed vegetable, is particularly common in the Mediterranean region. In this region, the endivie is responsible for 20–30% of skin allergies (Alexander et al. 2013). Occupational hand dermatitis has been reported in SL-sensitive patients (Rozas-Muñoz et al. 2012). The patients with severe chronic skin irritation to lettuce (L. sativa) can have cross-sensitivity to endivie (Krook 1977).
Chicory (Cichorium intybus var. sativum L., succory, coffee weed, cornflower, wild chicory) is a species common in the wild in Europe and North America (Heywood 1993; Tutin et al. 1980). It is of substantial economic, culinary, and medicinal potential. The plant is grown for its roots, which are known for the high concentration of inulin, the polysaccharide that is reported to have diverse advantages to the human body, i.e., enhance the immune system and stabilize blood sugars and lipids level (Figueira et al. 2003). IgE-mediated allergy with skin irritant reactions, facial erythema, dyspnea, chronic eczema, as well as severe bronchospastic reactions has been reported after contact with chicory root or leaves (Das et al. 2016). The skin reaction to chicory allergens may be delayed, and the symptoms may occur even 2 years after the first contact to cultivating chicory plants (Morita et al. 2007). The plant can cross-react with birch pollen, and in some individuals with birch pollen allergies, it causes the oral allergy syndrome (Cadot et al. 2003; Willi et al. 2009). In rare cases, the anaphylactic type I allergy to chicory was also reported (Morita et al. 2007; Olive-Perez and Pineda 2003; Willi et al. 2009).
Globe artichoke (Cynara scolymus L., syn.; C. cardunculus var. scolymus L.) is a perennial herb, native to the Mediterranean region of Europe and North Africa, used as a vegetable plant with edible head inflorescence (Heywood 1993). The plant is used in phytomedicine to enhance the kidneys and stimulate bile acid excretion and flow (Ben Salem et al. 2015). The development of occupational rhinitis and bronchial asthma has been reported in vegetable warehouse workers after sensitization to artichoke (Miralles et al. 2003).
Sunflower (Helianthus annuus L., common sunflower) is an annual plant, native to Central America (Heywood 1993). It is widely cultivated as an oilseed crop and livestock forage in semi-arid regions (Tutin et al. 1980). The protein allergens with high cross-reactivity (32, 24, 55, and 55 kDa albumins, LTPs Hel a 1, Hel a 2) have been found in sunflower pollen (de la Hoz et al. 1994; International Union of Immunological Societies Allergen Nomenclature). Pollen allergens differ from seed allergens Hel a 3 (Macias et al. 2014). In patients allergic to sunflower proteins, generalized urticaria, angioedema, oral allergy syndrome, and other symptoms were reported (Vandenplas et al. 1998). Although the sunflower seed dust can result in allergic symptoms with serious anaphylaxis, the incidents are very rare (Vandenplas et al. 1998). The safety of sunflower oil ingestion in patients with IgE-mediated hypersensitivity to sunflower seed was reported by Halsey et al. (1986).
Ornamental Asteraceae species
Chrysanths (Chrysanthemum sp.) are native to Asia and northeastern Europe. In Japan, the chrysanthemum is an imperial and national flower (Tutin et al. 1980). In many European countries (Italy, Poland), the ornamental chrysanthemum is restricted to use mainly in cemetery arrangements (Heywood 1993). The first description of a severe skin irritation after contact with the Chrysanthemum plants was made by Howe JS in 1887. Currently, the Chrysanthemum species and varieties are considered to be a primary sensitizer and principle agent of contact occupational dermatitis in Western Europe (60%) (Alexander et al. 2013). The allergens in chrysanths (mainly SLs) are found in the flowers and leaves, as well as in the hairs (trichomes) developed on all plant parts (Salapovic et al. 2013). The trichomes easily become airborne and can contact nose and eyes mucosa (Menz and Winkelmann 1987). The symptoms and complaints due to the direct contact with the Chrysanthemum plant parts can vary from urticaria to allergic rhinoconjunctivitis and asthma (de Jong et al. 1998). The contact dermatitis often begins with fingerstrips and extend to the face and forearms and can occur minutes after contact (Alexander et al. 2013). Cross-sensitization allergy symptoms after contact with several Asteraceae members (e.g., Matricaria, Solidago) have also been reported (de Jong et al. 1998).
Dahlia (Dahlia sp.) is a perennial ornamental plant (Heywood 1993). The causes of dahlia dermatitis have been described in Asian countries (Nandakishore and Pasricha 1994). Sensitization occurs through direct and airborne skin contact. Allergic symptoms are noted mainly in face and hands (Alexander et al. 2013).
Asteraceae herbaceous plants
Dandelion (T. officinale L.) is a herbaceous perennial weed native to Europe and Asia; however, it is naturalized and found on all continents (Heywood 1993; Tutin et al. 1980). The plant is found in abundance in meadows, roadsides, and ruderal places. In several countries, it is recognized as a severe weed in agriculture and gardening; in others, as a beneficial apicultural plant (Denisow 2011). Dandelion contains many pharmacologically active compounds and is used as herbal medicine in Europe, North America, and China (Schutz et al. 2006). Among potential allergens, an 18-kDa Bet v 1 related-protein with high expression in roots and stems has been extracted from dandelion (Xu et al. 2000). The dandelion sensitivity is expected in patient allergic to the pollen of wind-pollinated Asteraceae (e.g., Ambrosia, Artemisia, Iva) as the cross-reactive epitopes have been shown in several Asteraceae members (Paulsen and Andersen 2016; Syhaieva 2006). For example, the ingestion of bee pollen recommended as food supplementation can result in acute allergic reactions (Cohen et al. 1979; Denisow and Denisow-Pietrzyk 2016; Helander 1984). Several studies described a seasonal cutaneous allergy after contact with dandelion (Cohen et al. 1979; Hausen and Schulz 1978; Ingber 2000; Jovanovic et al. 2004; Lovell and Rowan 1991; Poljacki et al. 2005; Thomson and Wilkinson 2000). In the Korean study, the sensitization to dandelion occurred in 8.5% of patients with respiratory allergy (Lee et al. 2007). In an atopic patient with hay fever, even an anaphylactic reaction has been observed after intake of mixed pollen with 15% of dandelion participation (Chivato et al. 1996).
Arnica (A. montana L.) is a herbaceous perennial plant widespread in the nutrient-poor siliceous meadows of Central Europe (Tutin et al. 1980). The plant extracts are used in alternative medicine and cosmetic products. The arnica-related allergy is not often and have been detected in approximately 1.13% patients; however, the contact allergy with skin irritation to arnica have been described (Reider et al. 2001; Rudzki and Grzywa 1977). Given that sensitization to arnica cannot be assessed by testing with the Compositae or sesquiterpene mix alone, the authors suggest that these allergies are more common and contribute considerably to the contact dermatitis, and presumably are recognized as general plant/Asteraceae allergy (Neerman 2003; Reider et al. 2001).
Marigold (C. officinalis L., pot marigold, ruddles, common marigold, garden marigold, English marigold, or Scottish marigold) is an herbaceous, very aromatic perennial known in folk medicine (Heywood 1993). The species is native to southern Europe; currently, it is naturalized in temperate regions (Tutin et al. 1980). Calendula extract contains triterpenoids, flavonoids, coumarins, quinones, volatile oil, carotenoids, and amino acids, with multiple medical activities, i.e., anti-inflammatory, cytotoxic, hepatoprotective, spasmolytic, and spasmogenic (Muley et al. 2009; Silva et al. 2007). Marigold extracts are common in diverse creams, which shows the protective action in humans with irritant contact dermatitis (ICD) (Fuchs et al. 2011; Fuchs et al. 2005). Although adverse reactions to marigolds are rare, approximately 2.0% of allergic patients reacted to allergens in marigold, contact skin allergies, and severe anaphylaxis have been noted (D'Amato et al. 2007; Wintzen et al. 2003).
Yarrow (A. millefolium L.) is an herbaceous perennial native to temperate areas in Europe, Asia, and North America (Heywood 1993). It is commonly found in grasslands, ruderal areas, and open forests and is also frequently cultivated as an ornamental plant. The plant is an ingredient in herbal teas. One patient had a flare-up of dermatitis after drinking tea made from A. millefolium (Wrangsö et al. 1990).
Chamomile is a common name of several plant species spread over Europe, North Africa, and Asia (Matricaria chamomilla L.—wild chamomile, German chamomile in Poland, Germany, France; Anthemis nobilis L.—common chamomile in England, Spanish, Germany; Anthemis arvensis—common chamomile in Asores, Iran, Denmark, Ukraine; and Anthemis cotula L.—dog’s fennel, May weed, stinking chamomile) (Heywood 1993; Tutin et al. 1980). Chamomile is considered as a medicinal plant containing diverse bio-active molecules, e.g., terpenoids, flavonoids, and volatile oils, contributing to its medicinal usage and is listed on the FDA’s GRAS, commonly recommended as a safe list (Srivastava et al. 2010). Chamomile plays an important role in phytomedicine and is known from its antispasmodic and sedative usefulness. Therapeutic effects of chamomile herbs or flowers have been established against hay fever, inflammation, muscle spasms, disorders in a menstrual cycle, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids (Zidorn 2008). However, in a low percentage of individuals, chamomile can be dangerous and initiate allergic reactions, including contact dermatitis reactions (Budzinski et al. 2000; Pereira et al. 1997; Rodríguez-Serna et al. 1998). The tests conducted by Budzinski et al. (2000) revealed that 3.1% of the patients develop an Asteraceae-related allergic reaction, and of these individuals, 56.5% demonstrated allergy to chamomile. As another example, in hay fever patients with an inflammation of meibomian glands, the chamomile employed as fluid extract exacerbates the inflammation syndromes (Subiza et al. 1990). It is presumable that reported allergic effects may result from contamination of common chamomile herb by A. cotula, the species very similar to the other chamomiles, difficult to distinguish, and known for its allergenic properties (Budzinski et al. 2000). The plant is even classified as poison (Toxic plants ASPCA 2014). The cases of severe anaphylactic reaction have been reported in a 38-year-old Caucasian man and in an 8-year-old boy, who ingested chamomile as a herbal tea (Andres et al. 2009; Subiza et al. 1989). The allergen protein, a homolog of Bet v 1 has been identified in chamomile (Reider et al. 2000). These high-weight molecules (23–50 kDa) may presumably induce the cross-reactivity with foods and pollen allergens. However, the subjects sensitive to mugwort seldom reveal allergenic reaction to chamomile. On the contrary, the patients sensitive to chamomile are usually allergic to mugwort (Barrett et al. 2010). Furthermore, the authors suggest that evidence of cross-reactivity with food and pollen allergens is highly probable in subjects sensitized to chamomile (de la Torre Morín et al. 2001; Reider et al. 2000). In particular, establishing general recognition of safety of chamomile products is needed before usage in children; pregnant women; or patients with allergy, kidney and liver diseases.
Echinacea (Echinacea purpurea (L.) Moench.)—purple coneflower) is a herbaceous, perennial plant, native to North America commonly used to enhance the immunology system and prevent against cold infections (Barrett et al. 2010; Stevens 2001). Adverse reactions to Echinacea have been documented in Australian patients (Mullins and Heddle 2002). The Echinacea-related symptoms included severe urticaria (hives), swelling, acute asthma attacks, and anaphylaxis.
Tansy (T. vulgare L., syn. C. vulgare (L.) Bernh.) is a perennial, herbaceous plant native to Europe and Asia, and is naturalized in North America and Canada. Yellow flower heads are flattened. Fresh tansy herb yields between 0.2% and 0.6% volatile oil of highly variable geographically dependent composition with high participation of monoterpene camphor (Keskitalo et al. 2001). The β-thujone, a compound reported to be highly toxic to brain, liver, and kidney tissues is also a well-known ingredient in tansy (Chiasson et al. 2001). The irritant contact dermatitis has been documented after prolonged exposure to tansy (Paulsen and Andersen 2016). Sesquiterpene lactones (SLs) present in Asteraceae species, e.g., pathenolides, are presumably responsible for severe cross-sensitivity between tansy and chrysanthemum (Paulsen et al. 2001). The allergy for tansy herb have been evidenced in 60.6–77.0% of individuals sensitive to Asteraceae (Paulsen 2017). Clinical symptoms cover the face, hands, and/or forearms, and usually occur after irritant contact with the herb in the wild or through the use of cosmetics (Salapovic et al. 2013; Zidorn 2008).
Feverfew (T. parthenium (L.) Sch. Bip., syn. C. parthenium (L.) Bernch.) is a perennial plant which grows in most of Europe, North America, and Canada (Tutin et al. 1980; Stevens 2001). It has been used in herbal remedies for centuries (Neerman 2003; Zidorn 2008). The skin irritation symptoms (in eyes, face, neck, and scalp) have been documented in a 45- and 25-year-old woman after usage of moisturizers containing feverfew extracts (Neerman 2003). The patch test with the NACDG revealed positive reaction of both patients to sesquiterpene lactone and to Compositae mix. It is thought that both of these eruptions are a result of contact dermatitis from the Asteraceae family (Killoran et al. 2007).
Mugwort (A. vulgaris L. felon herb, chrysanthemum weed, or St. John’s herb, common wormwood). This perennial herb is native to Europe, Asia, Northern Africa, and is naturalized to North America (Heywood 1993; Tutin et al. 1980). The genus Artemisia includes 57 species in Europe (Stevens 2001). Mugwort is present in urban, suburban, and rural areas. In tradition folk medicine, the herb is used to release abdominal and menstrual pain and rheumatic arthritis, as an antimalarial drug (Liu et al. 2006). Mugwort (Artemisia) and ragweed (Ambrosia) are indicated among the most involved in pollinosis among Asteraceae species (D'Amato et al. 2007). In the last decade, the pollen of Artemisia campestris have been also identified in airborne pollen in Europe (Grewling et al. 2015). As reported by Park (2005), 42.7% of subjects who experienced the allergic rhinitis and asthma develop positive reactions to mugwort on skin prick testing. Mugwort pollen is known to cross-react with some fruits (peach, apple) and vegetables foods belonging to the Brassicaceae family, such as cauliflower, cabbage, or broccoli (Sugita et al. 2016). The allergic irritation dermatitis revealed after contact with the herbal patch with mugwort ingredient has been reported in a 43-year-old atopic Korean man (Haw et al. 2010). However, the exact Artemisia species used for the herbal patch was not identified; therefore, the authors suggest the need for further studies to explain whether there are any differences in skin reaction according to divers Artemisia species.
Ragweed (A. artemisifolia L.) is an annual herb, native to North America and Canada (Heywood 1993). The species became naturalized in Europe, and currently, it is widespread as an invasive species (Stevens 2001). The sensitization rate against ragweed pollen is high among humans and is compared to that of grass pollen and is expected to increase due to plant migration across Europe (Rodinkova et al. 2018; Buters et al. 2015). The major allergen of ragweed is Amb a 1, a member of the pectatelyases that catalyzes the breakdown of pectin (the major plant cellular wall component). Ragweed cross-reacts with mugwort (A. vulgaris). Clinical symptoms of ragweed-related allergy involve allergic dermatitis, oral allergy syndromes, allergic rhinoconjunctivitis, and asthma (Buters et al. 2015; Möller et al. 2002). According to these authors, contact with vegetative parts (leaves) of ragweed may induce hands, underarms, and face eczema with papulo-vesicles or chronic hyperkeratotic eczema. Severe cross-reactivity symptoms between Asteraceae allergens and food allergens, e.g., celery-mugwort-spice syndromes, and mugwort-peach, mugwort-chamomile, mugwort-mustard, ragweed-melon-banana have been also reported (Popescu 2015). Pollen from other Asteraceae species recorded in the atmosphere (i.e., Iva, Xanthium) are also known to cause allergy (Sikoparija et al. 2017; Rysiak and Czarnecka 2018).
Conclusion
In conclusion, Asteraceae species are risk factors for a potential contact and systemic allergy. It is advisable to discriminate the Asteraceae species and use of Asteraceae extracts/herbal teas/cosmetics with caution in highly sensitive persons. The symptoms after contact with Asteraceae species vary widely and could be severe in atopic patients. The evidence of cross-reactivity of Asteraceae species with other plants and anaphylactic reactions have been reported. Moreover, cross-reactivity occurs between Asteraceae allergens and food allergens. Therefore, no allergenicity of Asteraceae products needs to be proven for each case. In diagnosis, it is recommended to use patch tests with either additional plant extracts or commercial Compositae mix adjusted to local conditions.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Łukasz Pietrzyk, Phone: +48 81 448 60 50, Email: lukasz.pietrzyk@wp.pl.
Bożena Denisow, Phone: +48 81 445 65 05, Email: bozena.denisow@up.lublin.pl.
References
- Adisesh A, Robinson E, Nicholson PJ, Sen D, Wilkinson M, Standards of Care Working Group Standards of care working group. U.K. standards of care for occupational contact dermatitis and occupational contact urticaria. Br J Dermatol. 2013;168:1167–1175. doi: 10.1111/bjd.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RJ, Patricia MD, Norris L, et al. Allergic contact dermatitis to plant extracts in cosmetics. Semin Cutan Med Surg. 2013;32:140–146. doi: 10.12788/j.sder.0019. [DOI] [PubMed] [Google Scholar]
- Andres C, Chen WC, Ollert M, Mempel M, Darsow U, Ring J. Anaphylactic reaction to chamomile tea. Allergol Int. 2009;58:135–136. doi: 10.2332/allergolint.C-08-63. [DOI] [PubMed] [Google Scholar]
- Andrews ID, Scheinman P. Systemic hypersensitivity reaction (without cutaneous manifestations) to an implantable cardioverter-defibrillator. Dermatitis. 2011;22:161–164. [PubMed] [Google Scholar]
- Asano Y, Makino T, Norisugi O, et al. Occupational cobalt induced systemic contact dermatitis. Eur J Dermatol. 2009;19:166–168. doi: 10.1684/ejd.2008.0581. [DOI] [PubMed] [Google Scholar]
- ASPCA (2014) Toxic plants ASPCA. http://www.aspca.org/pet-care/animal-poison-control/toxic-and-non-toxic-plants/chamomile. Accessed 10 May 2018
- Avila Castanon L, Perez Lopez J, del Rio Navarro BE, et al. Hypersensitivity detected by skin tests to food in allergic patients in the hospital Infantil de Mexico Federico Gomez. Rev Alerg Mex. 2002;49:74–79. [PubMed] [Google Scholar]
- Barrett B, Brown R, Rakel D, Mundt M, Bone K, Barlow S, Ewers T. Echinacea for treating the common cold: a randomized trial. Ann Intern Med. 2010;153:769–777. doi: 10.7326/0003-4819-153-12-201012210-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Salem M, Affes H, Ksouda K, Dhouibi R, Sahnoun Z, Hammami S, Zeghal KM. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum Nutr. 2015;70:441–453. doi: 10.1007/s11130-015-0503-8. [DOI] [PubMed] [Google Scholar]
- Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Burkemper NM. Contact dermatitis, patch testing and allergen avoidance. Mo Med. 2015;112:296–300. [PMC free article] [PubMed] [Google Scholar]
- Buters J, Alberternst B, Nawrath S, Wimmer M, Traidl-Hoffmann C, Starfinger U, Behrendt H, Schmidt-Weber C, Bergmann KC. Ambrosia artemisiifolia (ragweed) in Germany—current presence, allergological relevance and containment procedures. Allergo J Int. 2015;24:108–120. doi: 10.1007/s40629-015-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadot P, Kochuyt AM, van Ree R, Ceuppens JL. Oral allergy syndrome to chicory associated with birch pollen allergy. Int Arch Allergy Immunol. 2003;131:19–24. doi: 10.1159/000070430. [DOI] [PubMed] [Google Scholar]
- Cavani A. T regulatory cells in contact hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:294–298. doi: 10.1097/ACI.0b013e3283079ea4. [DOI] [PubMed] [Google Scholar]
- Chiasson H, Bélanger A, Bostanian N, Vincent C, Poliquin A. Acaricidal properties of Artemesia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. J Econ Entomol. 2001;94:167–171. doi: 10.1603/0022-0493-94.1.167. [DOI] [PubMed] [Google Scholar]
- Chivato T, Juan F, Montoro A, et al. Anaphylaxis induced by ingestion of a pollen compound. J Investig Allergol Clin Immunol. 1996;6:208–209. [PubMed] [Google Scholar]
- Cohen SH, Yunginger JW, Rosenberg N, Fink JN. Acute allergic reaction after composite pollen ingestion. J Allergy Clin Immunol. 1979;64:270–274. doi: 10.1016/0091-6749(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Crosby DG. The poisoned weed: plants toxic to skin. Oxford: England; 2004. [Google Scholar]
- Czarnecka B, Denisow B. Floral biology of Senecio macrophyllus M. Bieb. (Asteraceae), a rare central European steppe plant. Acta Soc Bot Pol. 2014;83(1):29–37. [Google Scholar]
- D'Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- Das S, Vasudeva N, Sharma S. Cichorium intybus: a concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Dev Ther. 2016;7:1–12. [Google Scholar]
- de Jong NW, Vermeulen AM, Gerth van Wijk R, et al. Occupational allergy caused by flowers. Allergy. 1998;53:204–209. doi: 10.1111/j.1398-9995.1998.tb03872.x. [DOI] [PubMed] [Google Scholar]
- de Jongh CM, Lutter R, Verberk MM, Kezic S. Differential cytokine expression in skin after single and repeated irritation by sodium lauryl sulphate. Exp Dermatol. 2007;16:1032–1040. doi: 10.1111/j.1600-0625.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- de la Hoz F, Melero JA, Gonzalez R, et al. Isolation and partial characterization of allergens from Helianthus annuus (sunflower) pollen. Allergy. 1994;49:848–854. doi: 10.1111/j.1398-9995.1994.tb00786.x. [DOI] [PubMed] [Google Scholar]
- de la Torre MF, Sánchez Machín I, García Robaina JC, et al. Clinical cross-reactivity between Artemisia vulgaris and Matricaria chamomilla (chamomile) J Investig Allergol Clin Immunol. 2001;11:118–122. [PubMed] [Google Scholar]
- Denisow B (2011) Pollen production of selected ruderal plant species in the Lublin area. Univ Plant Sciences in Lublin Press, 351: 86
- Denisow B, Denisow-Pietrzyk M. Biological and therapeutic properties of bee pollen: a review. J Sci Food Agric. 2016;96:4303–4309. doi: 10.1002/jsfa.7729. [DOI] [PubMed] [Google Scholar]
- Denisow B, Wrzesień M, Mamchur Z, Chuba M. Invasive flora within urban railway areas: a case study from Lublin (Poland) and Lviv (Ukraine) Acta Agrobot. 2017;70:1727. [Google Scholar]
- Diaz EH, De Las HM, Herranz JC, et al. Study of new lettuce (Lactuca sativa) allergens. J Allergy Clin Immunol. 2013;131:AB18. [Google Scholar]
- Díaz-Perales A, Lombardero M, Sánchez-Monge R, et al. Lipid-transfer protein as potential plant-panallergens: cross-reactivity among proteins of Artemisia pollen, Castanea, nut and Rosaceae fruits, with different IgE-binding capacities. Clin Exp Allergy. 2000;30:1403–1410. doi: 10.1046/j.1365-2222.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- Figueira GM, Park KJ, Brod FP, et al. Evaluation of desorption isotherms, drying rates and inulin concentration of chicory roots (Cichorium intybus L.) with and without enzymatic activation. J Food Eng. 2003;63:273–280. [Google Scholar]
- Fonacier LS, Sher JM. Allergic contact dermatitis. Ann Allergy Asthma Immunol. 2014;113:9–12. doi: 10.1016/j.anai.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Fonacier L, Bernstein DI, Pacheco K, Holness DL, Blessing-Moore J, Khan D, Lang D, Nicklas R, Oppenheimer J, Portnoy J, Randolph C, Schuller D, Spector S, Tilles S, Wallace D American Academy of Allergy, Asthma & Immunology American College of Allergy, Asthma & Immunology Joint Council of allergy, Asthma & Immunology. (2015) Contact dermatitis: a practice parameter-update 2015. J Allergy Clin Immunol Pract 3: S1–39 [DOI] [PubMed]
- Franck P, Kanny G, Dousset B, Nabet P, Moneret-Vautrin DA. Lettuce allergy. Allergy. 2000;55:201–202. doi: 10.1034/j.1398-9995.2000.00373.x. [DOI] [PubMed] [Google Scholar]
- Fuchs SM, Schliemann-Willers S, Fischer TW, Elsner P. Protective effects of different marigold (Calendula officinalis L.) and rosemary cream preparations against sodium-lauryl-sulfate-induced irritant contact dermatitis. Skin Pharmacol Physiol. 2005;18:195–200. doi: 10.1159/000085865. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Rauber-Luthy C, Kupferschmidt H, et al. Acute plant poisoning: analysis of clinical features and circumstances of exposure. Clin Toxicol. 2011;49:671–680. doi: 10.3109/15563650.2011.597034. [DOI] [PubMed] [Google Scholar]
- Gordon LA. Compositae dermatitis. Australas J Dermatol. 1999;40:123–130. doi: 10.1046/j.1440-0960.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151–153. doi: 10.1111/j.1600-0536.1994.tb01954.x. [DOI] [PubMed] [Google Scholar]
- Grewling Ł, Kasprzyk I, Borycka K, Chłopek K, Kostecki Ł, Majkowska-Wojciechowska B, Malkiewicz M, Myszkowska D, Nowak M, Piotrowska-Weryszko K, Puc M, Stawińska M, Balwierz Z, Szymańska A, Smith M, Sulborska A, Weryszko-Chmielewska E. Searching for a trace of Artemisia campestris pollen in the air. Acta Agrobot. 2015;68:399–404. [Google Scholar]
- Halsey AB, Martin ME, Ruff ME, Jacobs FO, Jacobs RL. Sunflower oil is not allergenic to sunflower seed-sensitive patients. J Allergy Clin Immunol. 1986;78:408–410. doi: 10.1016/0091-6749(86)90025-4. [DOI] [PubMed] [Google Scholar]
- Hartz C, San Miguel-Moncin MM, Cistero-Bahima A, et al. Molecular characterisation of lac s 1, the major allergen from lettuce (Lactuca sativa) Mol Immunol. 2007;44:2820–2830. doi: 10.1016/j.molimm.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Hausen BM, Schulz KH. Allergic contact dermatitis caused by dandelions (Taraxacum officinale Wiggers) Derm Beruf Umwelt. 1978;26:198. [PubMed] [Google Scholar]
- Haw S, Cho HR, Lee MH. Allergic contact dermatitis associated with mugwort (Artemisia vulgaris) Contact Dermatitis. 2010;62:61–63. doi: 10.1111/j.1600-0536.2009.01672.x. [DOI] [PubMed] [Google Scholar]
- Helander I. Contact dermatitis to lettuce. Contact Dermatitis. 1984;11:249. doi: 10.1111/j.1600-0536.1984.tb00993.x. [DOI] [PubMed] [Google Scholar]
- Heywood VH. Flowering plants of the world. New York: Oxford Univ Press; 1993. pp. 204–221. [Google Scholar]
- Ingber A. Seasonal allergic contact dermatitis from Taraxacum officinale (dandelion) in an Israeli florist. Contact Dermatitis. 2000;43:49. [PubMed] [Google Scholar]
- International Union of Immunological Societies Allergen Nomenclature IUIS official list. http://www.allergen.org. Accessed 16 May 2018
- Jachuła J, Denisow B, Wrzesień M. Validation of floral food resources for pollinators in agricultural landscape in SE Poland. J Sci Food Agric. 2018;98(7):2672–2680. doi: 10.1002/jsfa.8761. [DOI] [PubMed] [Google Scholar]
- Jachuła J, Konarska A, Denisow B. Micromorphological and histochemical attributes of flowers and floral reward in Linaria vulgaris (Plantaginaceae) Protoplasma. 2018;255(6):1763–1776. doi: 10.1007/s00709-018-1269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović M, Poljacki M. Compositae dermatitis. Med Pregl. 2003;56:43–49. doi: 10.2298/mpns0302043j. [DOI] [PubMed] [Google Scholar]
- Jovanovic M, Poljacki M, Mimica-Dukic N, Boza P, Vujanovic L, Duran V, Stojanovic S. Sesquiterpene lactone mix patch testing supplemented with dandelion extract in patients with allergic contact dermatitis, atopic dermatitis and non-allergic chronic inflammatory skin diseases. Contact Dermatitis. 2004;51:101–110. doi: 10.1111/j.0105-1873.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- Keskitalo M, Pehu E, Simon JE. Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem Syst Ecol. 2001;29:267–285. doi: 10.1016/s0305-1978(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Killoran CE, Crawford GH, Pedvis-Leftick A. Two cases of Compositae dermatitis exacerbated by moisturizer containing feverfew. Dermatitis. 2007;18:225–229. doi: 10.2310/6620.2007.06063. [DOI] [PubMed] [Google Scholar]
- Krook G. Occupational dermatitis from Lactuca sativa (lettuce) and Chicorium (endive). Simultaneous occurrence of immediate and delayed allergy as a cause of contact dermatitis. Contact Dermatitis. 1977;3:27–36. doi: 10.1111/j.1600-0536.1977.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Lee YW, Choi SY, Lee EK, Sohn JH, Park JW, Hong CS. Cross-allergenicity of pollens from the Compositae family: Artemisia vulgaris, Dendranthema grandiflorum, and Taraxacum officinale. Ann Allergy Asthma Immunol. 2007;99:526–533. doi: 10.1016/S1081-1206(10)60382-1. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao Y, Wang Y. Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biotechnol. 2006;72:11–20. doi: 10.1007/s00253-006-0452-0. [DOI] [PubMed] [Google Scholar]
- Lovell CR, Rowan M. Dandelion dermatitis. Contact Dermatitis. 1991;25:185–188. doi: 10.1111/j.1600-0536.1991.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Macias ML, Gomez F, Aranda A, Blanca-Lopez N, Mayorga C, Torres MJ, Canto G, Diaz-Perales A, Blanca M. Identification of Helianthus annuus allergens in subjects with allergy to sunflower. Clin Transl Allergy. 2014;4:P14. [Google Scholar]
- McFadden JP, Puangpet P, Basketter DA, et al. Why does allergic contact dermatitis exist? Br J Dermatol. 2013;168:692–699. doi: 10.1111/bjd.12145. [DOI] [PubMed] [Google Scholar]
- Menz J, Winkelmann RK. Sensitivity to wild vegetation. Contact Dermatitis. 1987;16:169–173. doi: 10.1111/j.1600-0536.1987.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Miralles JC, Garcia-Sells J, Bartolome B, et al. Occupational rhinitis and bronchial asthma due to artichoke (Cynara scolymus) Ann Allergy Asthma Immunol. 2003;91:92–95. doi: 10.1016/s1081-1206(10)62066-2. [DOI] [PubMed] [Google Scholar]
- Möller H, Spiren A, Svensson A, et al. Contact allergy to the Asteraceae plant Ambrosia artemisiifolia L (ragweed) in sesquiterpene lactone-sensitive patients in southern Sweden. Contact Dermatitis. 2002;47:157–160. doi: 10.1034/j.1600-0536.2002.470306.x. [DOI] [PubMed] [Google Scholar]
- Morita A, Inomata N, Kondou M, Shirai T, Ikezawa Z. Occupational contact urticaria syndrome caused handling lettuce and chicory: cross-reactivity between lettuce and chicory. J Allergy Clin Immunol. 2007;119:S24. [Google Scholar]
- Muley BP, Khadabadi SS, Banarase NB. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn (Asteraceae): a review. Trop J Pharm Res. 2009;8:455–465. [Google Scholar]
- Mullins RJ, Heddle R. Adverse reactions associated with Echinacea: the Australian experience. Ann Allergy Asthma Immunol. 2002;88:42–51. doi: 10.1016/S1081-1206(10)63591-0. [DOI] [PubMed] [Google Scholar]
- Nandakishore T, Pasricha JS. Pattern of cross-sensitivity between 4 Compositae plants, Parthenium hysterophorus, Xanthium strumarium, Helianthus annuus and Chrysanthemum coronarium, in Indian patients. Contact Dermatitis. 1994;30:162–167. doi: 10.1111/j.1600-0536.1994.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Neerman ME. Sesquiterpene lactones: a diverse class of compounds found in essential oils possessing antibacterial and antifungal properties. Int J Aromather. 2003;13:114–120. [Google Scholar]
- Nemery B, Demedts M. Occupational asthma in a chicory grower. Lancet. 1989;1:672–673. doi: 10.1016/s0140-6736(89)92177-6. [DOI] [PubMed] [Google Scholar]
- Nicolas JF, Testud F, Vocanson M. Sensibilisation versus tole ´rance dans l’ecze ´ma de contact. Ann Dermatol Venereol. 2008;135:733–736. doi: 10.1016/j.annder.2008.07.047. [DOI] [PubMed] [Google Scholar]
- Norman MU, Hwang J, Hulliger S, Bonder CS, Yamanouchi J, Santamaria P, Kubes P. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am J Pathol. 2008;172:1638–1649. doi: 10.2353/ajpath.2008.070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF. Allergic and irritant contact dermatitis. Eur J Dermatol. 2009;19:325–332. doi: 10.1684/ejd.2009.0686. [DOI] [PubMed] [Google Scholar]
- Olive-Perez A, Pineda F. Anaphylactic reaction to ‘Tudela’ lettuce hearts. Allergy. 2003;58:1205–1206. doi: 10.1046/j.0105-4538.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Oliwiecki S, Beck MH, Hausen BM. Compositae dermatitis aggravated by eating lettuce. Contact Dermatitis. 1991;24:318–319. doi: 10.1111/j.1600-0536.1991.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Park YM. Relationship between sensitization to outdoor aeroallergen and month of birth. Pediatr Allergy Respir Dis. 2005;15:257–262. [Google Scholar]
- Pastorello EA, Farioli L, Pravettoni V, Ispano M, Scibola E, Trambaioli C, Giuffrida MG, Ansaloni R, Godovac-Zimmermann J, Conti A, Fortunato D, Ortolani C. The maize major allergen, which is responsible for food-induced allergic reactions, is a lipid transfer protein. J Allergy Clin Immunol. 2000;106:744–751. doi: 10.1067/mai.2000.108712. [DOI] [PubMed] [Google Scholar]
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1–10. doi: 10.1111/cod.12671. [DOI] [PubMed] [Google Scholar]
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67–75. doi: 10.1111/cod.12458. [DOI] [PubMed] [Google Scholar]
- Paulsen E, Andersen KE, Hausen BM. Sensitization and cross-reaction patterns in Danish Compositae-allergic patients. Contact Dermatitis. 2001;45:197–204. doi: 10.1034/j.1600-0536.2001.450402.x. [DOI] [PubMed] [Google Scholar]
- Paulsen E, Hyldgaard MG, Andersen KE, et al (2017) Allergic sesquiterpene lactones from cushion bush (Leucophyta brownie Cass): new and old sensitizers in a shrub-turned-a-pot plant 76: 280-286 [DOI] [PubMed]
- Pereira F, Santos R, Pereira A. Contact dermatitis from chamomile tea. Contact Dermatitis. 1997;36:307. doi: 10.1111/j.1600-0536.1997.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Pigatto PD. Conctact dermatitis: some important topics. Eur Ann Allergy Clin Immunol. 2015;47:188–191. [PubMed] [Google Scholar]
- Poljački M, Jovanovic M, Boza P, et al. Is Vojvodina a risk area for contact weed allergies? Med Pregl. 2005;58:123–126. doi: 10.2298/mpns0504123p. [DOI] [PubMed] [Google Scholar]
- Popescu FD. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015;5:31–50. doi: 10.5662/wjm.v5.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid RS, Shim TN (2016) Contact dermatitis. BMJ. 30: 353: i3299 [DOI] [PubMed]
- Reider N, Sepp N, Fritsch P, Weinlich G, Jensen-Jarolim E. Anaphylaxis to chamomile: clinical features and allergen cross reaction. Clin Exp Allergy. 2000;30:1436–1443. doi: 10.1046/j.1365-2222.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Reider N, Komericki P, Hausen BM, Fritsch P, Aberer W. The seamy side of natural medicines: contact sensitization to arnica (Arnica montana L.) and marigold (Calendula officinalis L.) Contact Dermatitis. 2001;45:269–272. doi: 10.1034/j.1600-0536.2001.450503.x. [DOI] [PubMed] [Google Scholar]
- Rodinkova V, Palamarchuk O, Toziuk O, Yermishev O. Modeling hay fever risk factors caused by pollen from Ambrosia spp. using pollen load mapping in Ukraine. Acta Agrobotanica. 2018;71(3):1742. [Google Scholar]
- Rodríguez-Serna M, Sánchez-Motilla JM, Ramón R, et al. Allergic and systemic contact dermatitis from Matricaria chamomilla tea. Contact Dermatitis. 1998;39:192–193. doi: 10.1111/j.1600-0536.1998.tb05892.x. [DOI] [PubMed] [Google Scholar]
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, Giménez-Arnau A. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456–477. doi: 10.1016/j.ad.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Rudzki E, Grzywa Z. Dermatitis from Arnica montana. Contact Dermatitis. 1977;3:281–282. doi: 10.1111/j.1600-0536.1977.tb03682.x. [DOI] [PubMed] [Google Scholar]
- Rysiak A, Czarnecka B. The urban heat island and the features of the flora in the Lublin City area, SE Poland. Acta Agrobot. 2018;71(2):1736. [Google Scholar]
- Salapovic H, Geier J, Reznicek G. Quantification of sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. 2013;81:807–818. doi: 10.3797/scipharm.1306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel-Moncín M, Krail M, Scheurer S, et al. Lettuce anaphylaxis: identification of a lipid transfer protein as the major allergen. Allergy. 2003;58:511–517. doi: 10.1034/j.1398-9995.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- Schutz K, Reinchold C, Schieber A. Taraxacum—a review on its phytochemical and pharmacological profile. J Ethnopharmacol. 2006;107:313–323. doi: 10.1016/j.jep.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Sikoparija B, Galán C, Smith M, EAS QC Working Group Pollen-monitoring: between analyst proficiency testing. Aerobiologia. 2017;33:191–199. [Google Scholar]
- Silva EJ, Gonçalves ES, Aguiar F, et al. Toxicological studies on hydroalcohol extract of Calendula officinalis L. Phytother Res. 2007;21:332–336. doi: 10.1002/ptr.2009. [DOI] [PubMed] [Google Scholar]
- Slodownik D, Lee A, Nixon R. Irritant contact dermatitis: a review. Australas J Dermatol. 2008;49:1–9. doi: 10.1111/j.1440-0960.2007.00409.x. [DOI] [PubMed] [Google Scholar]
- Spiewak R. Causes for occupational skin diseases in farmers other than pesticides. Przyczyny zawodowych chorób skory u rolników inne niż środki ochrony roślin. In: Tos-Luty S, editor. Ryzyko zdrowotne stosowania pestycydów - Problemy Teoretyczne i Praktyczne. Lublin: Institute of Agricultural Medicine; 2001. pp. 65–75. [Google Scholar]
- Srivastava A, Srivastava JK, Shankar E, et al. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895–901. doi: 10.3892/mmr.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens PF (2001) Angiosperm phylogeny website. http://www.mobot.org/MOBOT/research/APweb. Accessed 2 May 2018
- Subiza J, Subiza JL, Hinojosa M, et al. Anaphylactic reaction after the ingestion of chamomile tea: a study of cross reactivity with other composite pollens. J Allergy Clin Immunol. 1989;84:353–358. doi: 10.1016/0091-6749(89)90420-x. [DOI] [PubMed] [Google Scholar]
- Subiza J, Subiza JL, Alonso M, et al. Allergic conjunctivitis to chamomile tea. Ann Allergy. 1990;65:127–132. [PubMed] [Google Scholar]
- Sugita Y, Makino T, Mizawa M, et al. Mugwort-mustard allergy syndrome due to broccoli consumption. Case Rep Dermatol Med. 2016;84:13767. doi: 10.1155/2016/8413767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syhaieva IA (2006) Efficiency of specific immunotherapy in treatment of patients with seasonal allergic rhinitis. [Ukrainian] Lik Sprava (1-2): 51-53 [PubMed]
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116–124. doi: 10.1016/j.clindermatol.2013.05.033. [DOI] [PubMed] [Google Scholar]
- Thomson KF, Wilkinson SM. Allergic contact dermatitis to plant extracts in patients with cosmetic dermatitis. Br J Dermatol. 2000;142:84–88. doi: 10.1046/j.1365-2133.2000.03245.x. [DOI] [PubMed] [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, et al. Flora Europaea. Cambridge: Cambridge University Press; 1980. [Google Scholar]
- Vandenplas O, Vander Borght T, Delwiche JP. Occupational asthma caused by sunflower-seed dust. Allergy. 1998;53:907–908. doi: 10.1111/j.1398-9995.1998.tb04003.x. [DOI] [PubMed] [Google Scholar]
- Veien NK. Systemic contact dermatitis. Int J Dermatol. 2011;50:1445–1456. doi: 10.1111/j.1365-4632.2011.05104.x. [DOI] [PubMed] [Google Scholar]
- Vila L, Sánchez G, Sanz ML, et al. Study of a case of hypersensitivity to lettuce (Lactuca sativa) Clin Exp Allergy. 1998;28:1031–1035. doi: 10.1046/j.1365-2222.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Vocanson M, Hennino A, Chavagnac C, Saint-Mezard P, Dubois B, Kaiserlian D, Nicolas JF. Contribution of CD4+ and CD8+ T cells in contact hypersensitivity and allergic contact dermatitis. Expert Rev Clin Immunol. 2005;1:75–86. doi: 10.1586/1744666X.1.1.75. [DOI] [PubMed] [Google Scholar]
- Vocanson M, Hennino A, Poyet G, et al. Experimental models of contact dermatitis. Rev Fr Allergol. 2007;47:314–317. [Google Scholar]
- Willi R, Pfab F, Huss-Marp J, Buters JTM, Zilker T, Behrendt H, Ring J, Darsow U. Contact anaphylaxis and protein contact dermatitis in a cook handling chicory leaves. Contact Dermatitis. 2009;60:226–227. doi: 10.1111/j.1600-0536.2008.01461.x. [DOI] [PubMed] [Google Scholar]
- Wintzen M, Donker AS, van Zuuren EJ. Recalcitrant atopic dermatitis due to allergy to Compositae. Contact Dermatitis. 2003;48:87–88. doi: 10.1034/j.1600-0536.2003.480206.x. [DOI] [PubMed] [Google Scholar]
- Wrangsö K, Ros AM, Wahlberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148–154. doi: 10.1111/j.1600-0536.1990.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Wrzesień M, Denisow B, Mamchur Z, Chuba M, Resler I. Composition and structure of the flora in intra-urban railway areas. Acta Agrobot. 2016;69(3):1666. [Google Scholar]
- Wrzesień M, Jachuła J, Denisow B. Railway embankments—refuge areas for food flora and pollinators in agricultural landscape. J Apic Sci. 2016;60(1):97–110. [Google Scholar]
- Xu X-Y, Bewley JD, Greenwood JS. Cloning and characterization of an 18 kiloDalton protein in the roots of the perennial weed Taraxacum officinale Weber (dandelion) which has allergen- and pathogenesis-related protein properties. Plant Cell Environ. 2000;23:1227–1236. [Google Scholar]
- Zidorn C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Phytochemistry. 2008;69:2270–2296. doi: 10.1016/j.phytochem.2008.06.013. [DOI] [PubMed] [Google Scholar]