Abstract
Obesity has been recognized to increase the risk of such diseases as cardiovascular diseases, diabetes, and cancer. It indicates that obesity can impact genome stability. Oxidative stress and inflammation, commonly occurring in obesity, can induce DNA damage and inhibit DNA repair mechanisms. Accumulation of DNA damage can lead to an enhanced mutation rate and can alter gene expression resulting in disturbances in cell metabolism. Obesity-associated DNA damage can promote cancer growth by favoring cancer cell proliferation and migration, and resistance to apoptosis. Estimation of the DNA damage and/or disturbances in DNA repair could be potentially useful in the risk assessment and prevention of obesity-associated metabolic disorders as well as cancers. DNA damage in people with obesity appears to be reversible and both weight loss and improvement of dietary habits and diet composition can affect genome stability.
Keywords: DNA damage, obesity, inflammation, oxidative stress, ROS, cancer
1. Introduction
The rising prevalence of obesity has become a major health problem in adults, as well as among children and adolescents. Obesity is a complex chronic disease, characterized by a significant increase in body fat tissue mass, and it is associated with disturbances in lipid and glucose metabolism, chronic inflammation and oxidative stress, and an increased risk of several diseases, most notably cardiovascular diseases, diabetes, and cancers, and with a decrease in life expectancy [1,2,3]. In people with obesity accumulation of DNA damage has been reported and suggested to be involved in the development of obesity-related disease [4,5,6]. DNA lesions have an impact on DNA replication, leading to mutations and thus may create a hazard for cell metabolism and cell survival [7]. Body weight loss has been found to result in a reduction in the level of DNA damage [8].
The aim of this paper is to underline obesity as DNA damaging factor and to present the relationship between obesity, DNA damage and development of metabolic disorders, and cancer.
2. Inflammation and Reactive Oxygen Species (ROS)-Induced DNA Damage
Inflammation is activated to protect the body against these harmful stimuli [9]. Chronic inflammation has been linked to aging and numerous chronic diseases such as cardiovascular diseases, autoimmune diseases, and cancer [10,11]. Proinflammatory signal recruits and activates neutrophils and macrophages and in turn, endogenous oxygen and nitrogen species are created. Moreover, reactive oxygen species (ROS) are also formed in cells during mitochondrial oxidative metabolism, apoptosis or the enzymatic reaction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, superoxide dismutase (SOD), myeloperoxidase (MPO) and nitric oxide synthase (NOS) [12].
Despite the presence of the specific defense system against radicals, constant ROS production and low antioxidant activity can lead to the loss of balance between the formation of ROS and the operation of a protective system, resulted in the development of oxidative stress. The increased ROS production and oxidative stress may induce endogenous DNA damage, transcription interruption and induce cell-cycle arrest [13,14]. Lipid peroxidation processes are also induced by ROS and lead to the formation of DNA reactive lesions [15].
Among ROS, free radicals such as superoxide or hydroxyl radical are the most hazardous. The superoxide (O2 •−) is generated during aerobic respiration and hydrogen peroxide (H2O2) during dismutation of superoxide by superoxide dismutase. In addition, several oxidases can also produce hydrogen peroxide [16]. Hydroxyl radical (HO•) can be formed as a result of both Fenton reaction and Weber-Weiss reaction [17]. Activated macrophages and neutrophils involved in inflammation generate oxidants as peroxynitrite (ONOO−) and nitrosoperoxycarbonate (ONOOCO2−), hypohalous acids (HOCl, HOBr), and nitrosating agent (N2O3) [18]. Furthermore, ROS can participate in lipid peroxidation and generated products such as etheno-, propano-, and malondialdehyde interact with DNA, form DNA adducts and damage DNA structure [14,19]. ROS attack can lead to base lesions such as oxidation, alkylation, methylation, nitration, deamination and single or double-strand DNA breaks, or cross-links in DNA structure. The frequently occurred DNA lesions caused by ROS are 8-hydroxyguanine, 7,8-dihydro-8-oxoguanine (commonly termed 8-oxoguanine: 8-OHdG), thymine glycol, Fapy Ade (4,6-diamino-5-formamidopyrimidine) and Fapy Gua (2,6-diamino-4-hydroxy-5-formamidopyrimidine) [20]. The occurrence of DNA lesions can induce mutations during DNA replication, as 8-OHdG can cause a change in GC to TA (transversion), resulting in mutagenesis and cancer initiation [20,21].
3. DNA Damage Repair
The DNA repair system exists to overcome DNA damage and maintain the integrity of the DNA structure. In general, DNA damage repair process involves the recognition of DNA damage by specific sensors, generation, and amplification of the DNA damage signal, transduction of this signal to the cytoplasm and activation of specific effectors [21]. Inter-individual variations in the activity of enzymes that participate in DNA repair pathways have been described [22,23]. Therefore, some differences in the efficiency of DNA repair and observed levels of endogenous DNA damage can be expected [24,25].
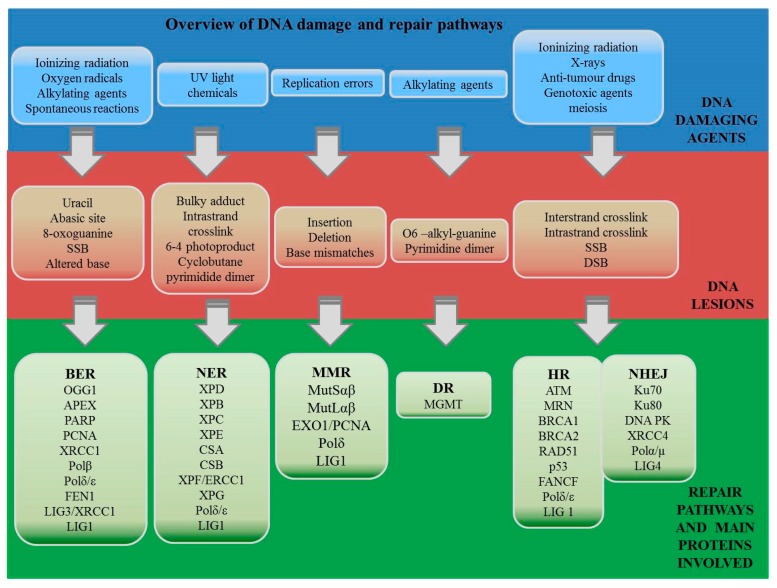
Among several known DNA repair mechanisms, direct repair occurs during the replication, while indirect repair takes place after the DNA synthesis [26]. The indirect repair strategy includes base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), the non-homologous end-joining (NHEJ) and homologous recombination (HR) pathways. Explanation of the pathways involved in DNA repair by three scientists was recognized by award them the Nobel Prize in chemistry in 2015 [27]. Tomas Lindahl described the model of BER, which is involved in modified bases repair [28]. Paul Modrich discovered a distinct pathway that detects and removes bases that are misincorporated during DNA replication. Finally, Aziz Sancar proposed the mechanism for removal of DNA adducts NER [29]. Overview of DNA damaging agents, induced DNA lesions, and their repair pathways is presented in Figure 1.
Figure 1.
Overview of DNA damaging agents, induced DNA lesions, and their repair pathways (BER—base excision repair, NER—nucleotide excision repair, MMR—mismatch repair, DR—direct repair, NHEJ—non-homologous end-joining; and HR—homologous recombination). Shortcuts are explained in the abbreviation section.
4. Obesity and DNA Damage
In people with obesity, a broad range of DNA lesions such as double strand breaks (DSB), single strand breaks (SSB) or oxidized bases and about 2-times higher DNA damage in lymphocytes than in normal weight subjects have been observed and a correlation between body–mass index (BMI) and DNA damage was also found [5,30,31]. A significant difference in levels of DNA damage measured by H2AX phosphorylation was also observed in children with overweight and obesity compared to lean controls [32]. Lymphocytes from people with obesity had more mitomycin C-induced DNA damage compared to cells from normal weight subjects [33]. However, available data regarding the relationship between obesity and levels of oxidized bases in DNA such as 8-oxodG and 8-OHdG are inconsistent [34,35,36,37].
There is well accepted that in obesity chronic energy overload results in enhanced ROS production and inflammation [38]. Available data indicate that ROS source can differ depending on the stage of obesity [39]. In the early stages of obesity increased adipocyte uptake of glucose and fatty acids activates NOX4, the major NADPH oxidase isoform in adipocytes, and induces ROS production. NOX4 silencing was reported to decrease ROS generation and inhibition of monocyte chemoattractant protein-1 [40]. Excessive accumulation of fat in adipocytes promotes proinflammatory adipokines production. Proinflammatory cytokines induce invasion of the target tissue by immune cells and development of chronic inflammation [41]. Accumulation of T-lymphocytes and macrophages in adipose tissue during obesity development promote ROS production by NOX2, the NADPH oxidase expressed in inflammatory cells. In addition, adipocytes and smooth muscle cells exposed to high FFA (free fatty acid) or high glucose concentrations showed increased mitochondrial fission and increased mitochondrial ROS production [42,43,44]. Characteristic for obesity excessive accumulation of triglycerides in adipocytes results in enhanced mitochondrial β-oxidation of FFA and increased mitochondrial ROS generation.
Furthermore, chronic inflammation associated with obesity is strongly involved in the formation of DNA lesion [38]. Activated macrophages secrete cytokines such as TNFα, and IL-6 which can induce DNA damage in non-targeted tissue distant from the site of inflammation [45,46]. Released cytokines can travel to different regions of the body and activate resident macrophages to produce proinflammatory molecules such as COX2, NOS, superoxide, ROS, and NO [47,48]. The release of these molecules can lead to oxidative DNA damage in cells. Also, macrophages that must absorb apoptotic cells can move to another region of the body and then release factors inducing DNA damage [49]. The translocation of DNA damaging factors via macrophages can deliver a high amount of damage-inducing signals from distant sites and can also be specific to regions where the macrophages are likely to travel (e.g., gut, spleen, skin, lymph nodes). Thus, obesity-associated oxidative stress and inflammation can induce DNA damage in different tissues.
Published intervention weight loss trails in obese in which DNA damage assessment was performed are limited. A significant decrease in levels of DNA damage was found after a low caloric diet-induced weight loss [8,50]. Improvement of genomic stability, characterized by a reduction of oxidative damage in saliva, was observed also after bariatric surgery associated weight loss in patients with morbid obesity [51].
4.1. Obesity and DNA Damage Repair
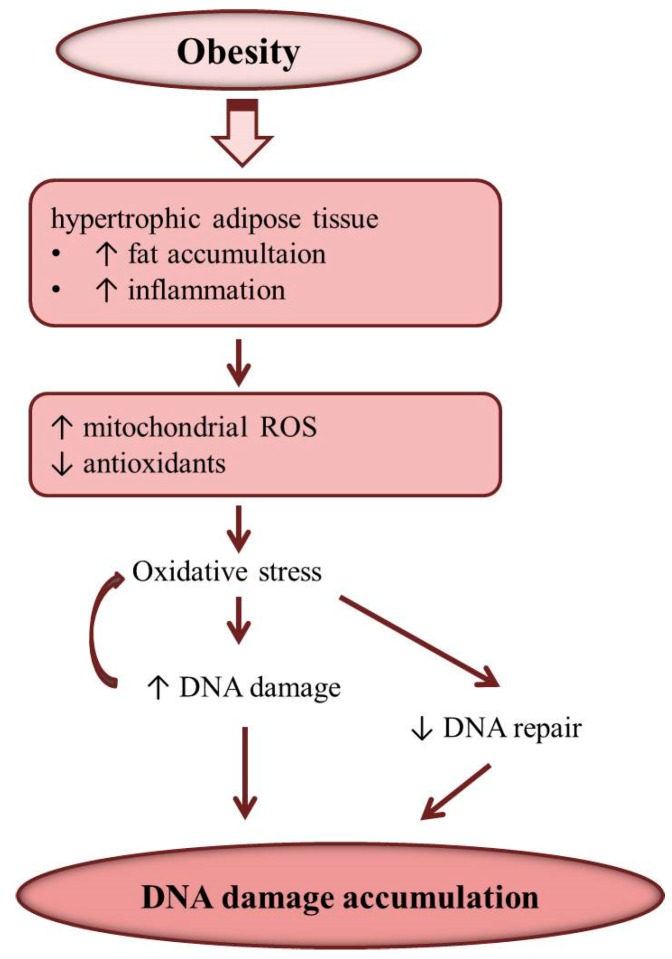
Disturbances in DNA damage response pathway related to enhanced increased body weight were reported [52]. An inverse association between BMI and nucleotide excision repair (NER) capacity was found in young females [53]. Presence of obesity was also recognized to alter the repair of DSBs induced by genotoxic agents [34]. Obesity-associated enhanced ROS production can modulate the DNA damage response through the impact on the expressions of genes involved in DNA repair (Figure 2) [54,55]. Inhibition of DNA repair enzymes provoked by the oxidative stress has been reported [56,57]. In obese alter expression of genes related to response to stress and toxic agents were also recognized [58].
Figure 2.
Obesity and DNA damage. Obesity is associated with inflammation and oxidative stress which induces DNA damage and inhibits DNA damage repair resulting in the accumulation of DNA damage in adipocyte and other tissues.
It can be hypothesized that epigenetic mechanisms might be involved in the regulation of genes encoding DNA repair proteins. Unbalanced and high-fat diet commonly observed in overweight and obese subjects can alter methyl group availability and disturb epigenetic regulation of DNA repair genes. Enhanced dietary fat consumption was reported to significantly alter DNA methylation and gene expression [59,60]. Recently high-fat diet was also found to suppress DNA damage repair by increasing lysine homocysteination in proteins involved in DNA damage repair [61]. Low intakes of vitamins C and E, as well as vitamins B and zinc, were reported to be associated with enhanced DNA damage [62,63]. In obese women, daily intakes of vitamins C and E were recognized to significantly affect DNA damage in lymphocytes indicating that in obese adequate vitamins C and E consumption could reduce levels of basal DNA damage probably, by the antioxidant effect of this vitamins [5]. However, in obese mice, an active antioxidant, EGCG (epigallocatechin-3-gallate) was shown to enhance methylation of MGMT (O6-Methylguanine-DNA methyltransferase) and MLH1 (MutL homolog 1) genes involved in direct and mismatch DNA repair, respectively [64]. Reduction of MLH1 gene expression was also observed and methylation rate of MLH1 was related to DNA damage in mice on high-fat diet supporting the role of epigenetic mechanisms in the regulation of DNA damage response [65].
4.2. Obesity and Mitochondrial DNA Damage
DNA lesions occur in both nuclear and mitochondrial DNA (mtDNA). Because of the absence of nucleotide excision repair mechanism in mitochondria, mtDNA is more susceptible to damage caused by reactive species than nuclear DNA. Photodimers and bulky adducts arising as a result of oxidative stress related to inflammation and environmental factors are not efficiently removed from mtDNA [66,67]. Despite mtDNA encodes only 1% of the mitochondrial proteins, mitochondrial diseases are associated with a high number of lesions in mtDNA [68]. In addition, enhanced degradation of mtDNA and decreased mtDNA copy number are related also to diabetes, cancer or neurodegenerative diseases [69,70,71]. The accumulation of oxidative mtDNA lesions may result in rearrangements or point mutations which can be maternally inherited [72].
In obesity, mitochondrial dysfunction leads to failure in fatty acid (FA) oxidation and disturbances in glucose homeostasis [43,73,74]. Elevated urinary excretion of mtDNA observed in morbidly obese patients was found significantly reduced after bariatric surgery associated weight loss [75]. The animal study showed that in mice fed high-fat diet mtDNA damage increased and was associated with mitochondrial dysfunction [76]. Additionally, oxidized mtDNA was found to induce synthesis of proinflammatory cytokines such as IL-6, TNF-α, pro-IL-1β through the activation of toll-like receptor 9 (TLR-9) [77,78]. Therefore obesity-associated inflammation could be in part both a cause and a consequence of the accumulation of mtDNA lesions.
4.3. Effect of Parental Obesity-Related DNA Damage on Offspring
The obesity-associated DNA damage may at least in part be responsive for disturbances in reproductive capacity of obese subjects and their offspring’s health [79]. Adiposity was recognized to cause sperm DNA fragmentation, affect DNA methylation and cause aberrancies in chromatin in male germ cells [80,81]. In patients with obesity, high DNA fragmentation index (DFI) in sperm and reduced fertility was recognized [82,83]. DNA damage in germ cells may be a result of increased ROS production characteristic for obesity [59,84,85]. DNA damage was present in the daughter cells after subsequent cell division indicating ineffective DNA damage response [86]. Therefore, it is suspected that the appearance of DNA lesions in germ cells can be transmitted to the genome of future generations [87]. Maternal obesity may cause de novo mutations in the embryo, change the methylation status of the genes in embryo and through miRNA affects the expression of embryonic proteins [88,89,90,91].
5. DNA Damage and Obesity-Related Metabolic Disorders
Obesity is well recognized to be involved in the development of diabetes and atherosclerosis-related diseases and to increase the mortality rate, particularly deaths from cardiovascular diseases [92]. In mice and humans, mutations of genes related to the DNA repair result in phenotypic changes similar to those observed in obesity-associated metabolic and cardiovascular abnormalities [93]. The p53 protein, the transcriptional factor mediating the DNA damage response and involved in preserving genomic stability, has been shown to affect obesity-associated diseases [94]. DNA damage-induced p53 activation was found to be involved in aging-related diseases, since mice with a p53 mutant allele associated with p53 activation developed premature aging and mice overexpressing naturally occurring truncated p53 isoform have a short lifespan [95,96]. A higher mutation amount in the p53 gene was found in cancers [97]. In breast cancer enhanced BMI was related to p53 mutation [98]. Activation of p53 signaling in vessels, heart, and the visceral adipose tissue of obese was found to contribute to diabetes progression and atherosclerosis [99]. DNA damage in obese adipocytes can induce p53 pathway involved in altered metabolism of adipocytes. In consequences of adipocyte dysfunction, tissue inflammation and insulin resistance appear [100]. Under a high-calorie diet, the p53/p21-signaling pathway was found to be involved in adipocyte differentiation, hypertrophy, induction of inflammation, and development of systemic insulin resistance, which commonly occurs in obese patients. In mice overexpression of Δ40p53, p53 isoform alters the balance between the full-length and short isoforms and hyperactivates p53 resulting in increased p21 expression and developed of hypoinsulinemia, glucose intolerance, and diabetes [101]. Accumulation of DNA damage in pancreatic β-cell as well as in adipocytes results in cell senescence, which contributes to the development of disturbances in glucose metabolism and systemic insulin resistance [100,102]. Insulin resistance is an important underlying mechanism accelerating development of obesity-associated comorbidities [103]. The relationship between type 2 diabetes and DNA double-strand breaks was recognized. In people with obesity and diabetes, BMI was positively correlated with oxidative DNA damage measured by serum 8-OHdG [104]. Observations from in vitro studies indicate that hyperglycemia can cause DNA damage and mutation [105,106]. Hyperglycemia increases production of AGEs (advanced glycation end products) which promote DNA breaks and 8-OHdG accumulation [107,108]. High glucose levels may induce DNA damage in cells through AKT (Protein Kinase B) phosphorylation and tuberin phosphorylation [109,110]. The AKT pathway is involved in cell growth and DNA repair. AKT activation leads to inhibition of protein recruitment to DNA damage site and therefore disturbs homologous repair [111]. ROS-related AKT induction was associated with low expression of OGG1, a protein involved in repair of oxidative DNA lesion and resulted in the accumulation of DNA damage [109,112,113]. Also, XPD (Xeroderma Pigmentosum group D) gene involved in DNA repair by NER was down-regulated as a result of long-term exposure to high glucose concentration. Insulin was found to affect XPD gene expression via p70S6 kinase signaling pathway critical for cell-cycle progression and via RAS—a regulator of DNA damage checkpoint [114,115].
There is no doubt that obesity is associated with oxidative stress causing DNA damage. Repair of oxidized, saturated, and ring-fragmented bases via the BER pathway are known to be critical for maintaining genomic stability. On the other hand, an important role of DNA repair proteins in modulating mitochondrial energetics and whole-body energy balance was shown [116]. Products of such genes as OGG1, NTH1, NEIL1, and NEIL2 participate in the initiation of repair of oxidative DNA lesions. NEIL1 is an enzyme that initiates BER of ring-fragmented purines and some saturated pyrimidines [117,118]. The neil1 knockout mice developed symptoms consistent with metabolic syndrome: severe obesity, fatty liver, dyslipidemia, and insulin resistance [119]. OGG1, a critical enzyme of the BER repair pathway, participates in the repair of the most common oxidative DNA lesion as 8-oxo-7,8-dihydroguanine (8-oxoG) and OGG1 expression is induced in response to a high-fat diet [120]. Mice lacking OGG1 (Ogg1−/−) developed features of metabolic syndrome, including increased adiposity, fatty liver, elevated triglycerides, and impaired glucose tolerance [116].
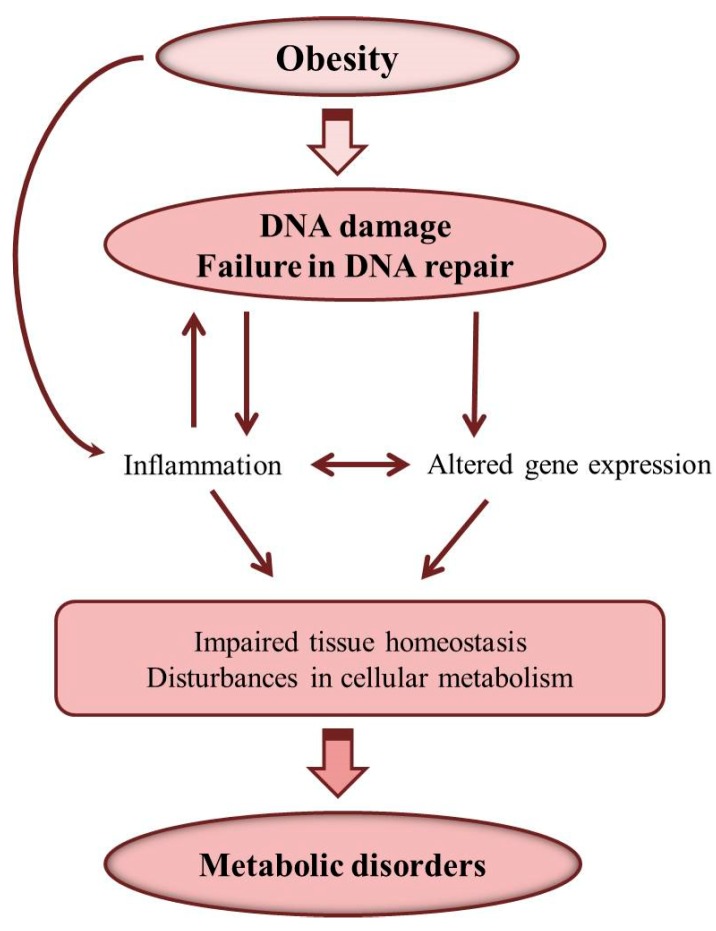
Therefore, obesity can induce DNA damage and disturbances in DNA repair resulting in cellular accumulation of DNA damage, which causes inflammation and alterations in gene expression and disturbances in cellular metabolism (Figure 3). As a consequence of these alterations, metabolic disorders can develop, and reduction of DNA damage may be important for the prevention and treatment of obesity-related metabolic diseases [5,121].
Figure 3.
Obesity-induced DNA damage and development of metabolic disorders.
6. DNA Damage and Development of Obesity-Related Cancer
Obesity-induced DNA damage and dysregulation of the DNA repair pathways can lead to increased mutation rate and transformation of healthy tissues to cancer [25,122,123,124,125].
The International Agency for Research on Cancer has identified several cancers associated with overweight and obesity including postmenopausal breast cancer, endometrial cancer, renal cell carcinoma, esophageal adenocarcinoma, pancreatic, colorectal, and liver cancers [126,127,128,129,130]. About 55% of all cancers diagnosed in women and 24% of those diagnosed in men are associated with overweight and obesity [131]. Between 2005–2014 cancers associated with overweight and obesity, excluding colorectal cancer, increased 7%. Colorectal cancer incidence decreased by 23%, but this is due in large part to the screening. The pooled analysis of 42 prospective and 14 retrospective studies have shown that each increase in BMI by 5 kg/m2 was significantly associated with an 18% higher risk of colorectal cancer [132]. The meta-analysis of 82 studies on breast cancer (including 213,075 breast cancer survivors with 23,182 deaths) has shown that relative risks of mortality are 1.75 for pre-menopausal and 1.34 for postmenopausal breast cancer for obese women. Each 5 kg/m2 BMI increase before and after 1 year of cancer diagnosis increases risks by 18% and 29% for breast cancer mortality, respectively. In this case, obesity was associated with poorer breast cancer survival regardless of BMI ascertainment period [126]. Cancers associated with overweight and obesity, excluding colorectal cancer, increased among adults younger than 75 years. Moreover, there is an increase in the frequency of cancers associated with overweight and obesity (by 7%) in comparison to non-obesity cancers (13% drop).
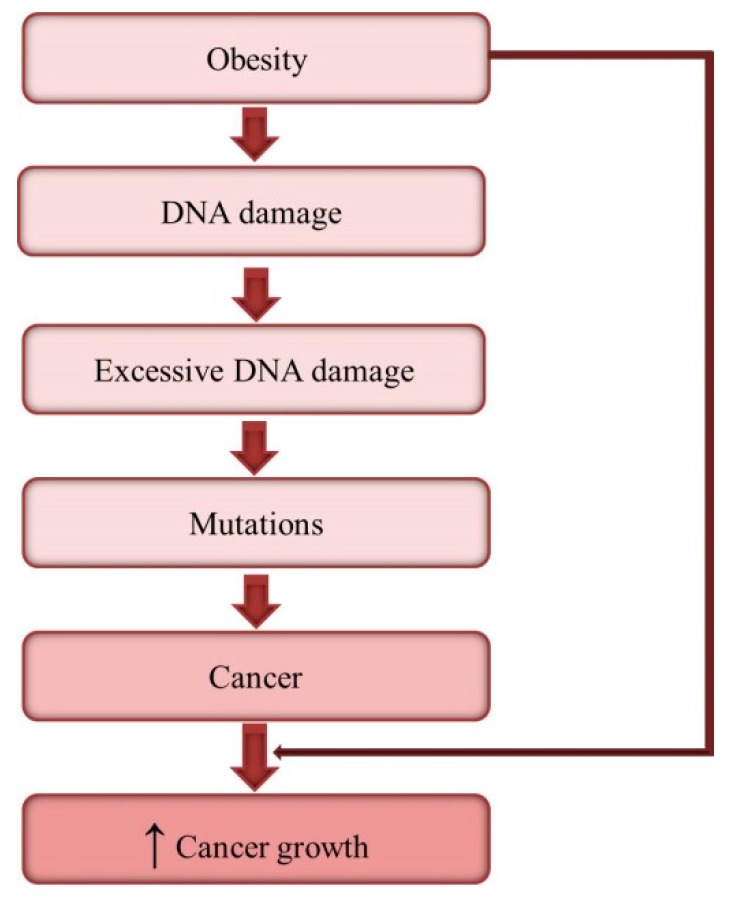
Obesity-associated DNA damage cannot only enhance cancer risk but also promote cancer growth (Figure 4). DNA damage induced chronic inflammation, insulin resistance and alter gene expression can favor cancer cell proliferation and migration, resistance to apoptosis as well as tumor angiogenesis [133,134,135,136,137]. In addition, associated with obesity reduced secretion of adiponectin and increased secretion of leptin by adipose tissue can promote cancer development in obese. Adiponectin possesses anti-inflammatory and anti-angiogenic properties and can inhibit cancer growth [138]. Some tumor cells express adiponectin receptors, thus adiponectin by binding and activating these receptors can downstream signaling pathways in cancer cells and adiponectin deficiency excludes such action [139,140]. Leptin is a mitogenic, anti-apoptotic, pro-angiogenic, and proinflammatory factor [141]. Therefore, high leptin favors cancer growth and the relationship between circulating leptin concentrations and colorectal cancer risk has been demonstrated [142]. Obesity-associated abnormalities in the secretion of adipokines and cytokines lead to the activation of oncogenic intracellular molecular networks such as NF-κB, JAK2/STAT3 or PI3K/AKT pathways [143,144]. NF-κB signaling plays important role in modulating cancer cell response to DNA damage [145,146]. Hyperinsulinemia, commonly observed in obese, can reduce PI3K/AKT and affect p53 function. Gain-of-function p53 mutations enhance activation of AKT and, in turn, a modified response of cancer cells to insulin, leading to increased proliferation and migration [147].
Figure 4.
Obesity-induced DNA damage and cancer development.
The described above effect of DNA damage on the development of disturbances in glucose metabolism and hyperglycemia, commonly observed in obese, may promote tumor growth by providing cancer cells with energy and allowing them to maintain a rapid rate of cell division [148,149,150]. The high rate of glucose metabolism has been also reported to be associated with both activation of oncogenes and loss of tumor suppressors [150,151,152].
The accumulation of adipose tissue is a significant source of estrogens after the menopause. Enhance levels of estrogens can increase cell proliferation in the breast and uterus and increase the risk of cancer. ROS generation during estrogen metabolism may also promote oxidative DNA base damage [153]. Quinone and semiquinone metabolites of endogenous estrogens undergo redox cycling in breast epithelial cells, resulting in superoxide radical anion and H2O2 production [154,155]. In addition, 2,3-quinone and 3,4-quinone have the potential to initiate the cancer process by forming DNA adducts [156,157]. Estrogen signaling has been also recognized as a factor regulating DDR (DNA damage response) proteins such as ATM, ATR, p53, BRCA1, and BRCA2, as well as directly interacting with the DNA repair machinery [158,159]. Any disruption of DNA repair pathways may support cancer cell proliferation. Obesity-induced alterations in expression of proteins involve in DNA repair, such as PARP1, γH2AX, ATM, FANCD2, PTEN, BRCA1, and p53 were found to affect carcinogenesis and disease outcomes [160,161,162].
7. Conclusions
Obesity, caused mainly by chronic energy overload resulting from consumption of high-energy meals and reduced physical activity, and associated with oxidative stress and inflammation, has been recognized as a key factor inducing DNA damage and inhibiting DNA damage repair mechanisms, favoring accumulation of DNA damage, and leading to enhanced mutation rate and altering gene expression. Cellular response to DNA damage can result in irreversible cell-cycle arrest, activation of several proteins which can induce adipocyte differentiation and hypertrophy, inflammation, disturbances in cell metabolism, impair glucose metabolism, and promote the development of systemic insulin resistance. Accumulation of mutagenic DNA lesions is related to cancer development. In addition, obesity-associated metabolic disturbances and excessive DNA damage can promote cancer growth by favoring cancer cell proliferation and migration, and resistance to apoptosis. Estimation of the DNA damage and/or disturbances in DNA repair could be potentially useful in the early risk assessment and prevention of obesity-associated metabolic disorders as well as cancers since DNA damage in obesity appears to be reversible and both weight loss and improvement of dietary habits and diet composition can affect genome stability.
Acknowledgments
This work was supported by the National Science Centre (grant number N404042/32/0945) and carried out with the use of CePT infrastructure financed by the European Union—the European Regional Development Fund within the Operational Program (Innovative economy for 2007–2013).
Abbreviations
| 8-OHdG | 8-OH deoxyguanosine |
| AGEs | advanced glycation end products |
| APE | apurinic endonuclease |
| ATM | ataxia telangiectasia–mutated protein |
| BER | base excision repair |
| BLM | bloom syndrome protein |
| BMI | body mass index |
| COX-2 | cyclooxygenase 2 |
| CSA | cockayne syndrome protein A |
| CSB | cockayne syndrome protein B |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DDR | DNA damage response |
| DFI | DNA fragmentation index |
| DNA-PK | DNA-dependent kinase |
| DR | direct repair |
| DSB | double-strand breaks |
| ELISA | enzyme-linked immunosorbent assay |
| ENDS | electronic nicotine delivery devices |
| EtBr | ethidium bromide |
| EXO1 | human exonuclease 1 |
| FANCF | fanconi anemia complementation group |
| Fapy Ade | 4,6-diamino-5-formamidopyrimidine |
| Fapy Gua | 2,6-diamino-4-hydroxy-5-formamidopyrimidine |
| FEN1 | flap endonuclease 1 |
| FFA | free fatty acids |
| FISH | fluorescence in situ hybridization |
| GC-MS | gas chromatography-mass spectrometry |
| GGR | global genome repair |
| H2O2 | hydrogen peroxide |
| HPLC | high performance liquid chromatography |
| HR | homologous recombination |
| IL-6 | interleukin 6 |
| LIG | DNA ligase |
| MGMT | O6-methylguanine DNA methyltransferase |
| MN | micronucleus assay |
| MMR | mismatch repair |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NER | nucleotide excision repair |
| NF-κB | nuclear factor κB |
| NHEJ | non-homologous end-joining |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NOX4 | NADPH oxidase 4 |
| OGG1 | 8-Oxoguanine glycosylase |
| PAH | polycyclic aromatic hydrocarbons |
| PARP | poly ADP-ribose polymerase 1 |
| PCR | polymerase chain reaction |
| PCNA | proliferating cell nuclear antigen |
| PhIP | 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine |
| PTEN | phosphatase and tensin homolog |
| ROS | reactive oxygen species |
| RPA | replication protein A |
| SCGE | Single cell gel electrophoresis |
| SSB | single-strand breaks |
| ssDNA | single-strand DNA |
| SOD | superoxide dismutase |
| TCR | transcription-coupled repair |
| TFIIH | transcription factor complex IIH |
| TNFα | tumor necrosis factor |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling UV |
| UV | ultraviolet |
| WHO | World Health Organization |
| WRN | Werner syndrome protein |
| XP | xeroderma pigmentosium |
| XRCC | X-ray repair cross-complementing group |
Author Contributions
M.W. designed this work, collected the data, and wrote the manuscript. G.N. collected the data and co-wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Leung Y.M., Pollack L.M., Colditz G.A., Chang S.H. Life years lost and lifetime health care expenditures associated with diabetes in the U.S., National Health Interview Survey, 1997–2000. Diabetes Care. 2015;38:460–468. doi: 10.2337/dc14-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ligibel J.A., Alfano C.M., Courneya K.S., Demark-Wahnefried W., Burger R.A., Chlebowski R.T., Fabian C.J., Gucalp A., Hershman D.L., Hudson M.M., et al. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 2014;32:3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer P.E., Hill J.A. Obesity, diabetes, and cardiovascular diseases: A compendium. Circ. Res. 2016;118:1703–1705. doi: 10.1161/CIRCRESAHA.116.308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerda C., Sanchez C., Climent B., Vazquez A., Iradi A., El Amrani F., Bediaga A., Saez G.T. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv. Exp. Med. Biol. 2014;824:5–17. doi: 10.1007/978-3-319-07320-0_2. [DOI] [PubMed] [Google Scholar]
- 5.Wlodarczyk M., Jablonowska-Lietz B., Olejarz W., Nowicka G. Anthropometric and dietary factors as predictors of DNA damage in obese women. Nutrients. 2018;10:578. doi: 10.3390/nu10050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaki M., Basha W., El-Bassyouni H.T., El-Toukhy S., Hussein T. Evaluation of DNA damage profile in obese women and its association to risk of metabolic syndrome, polycystic ovary syndrome and recurrent preeclampsia. Genes Dis. 2018;5:367–373. doi: 10.1016/j.gendis.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irigaray P., Belpomme D. Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis. 2010;31:135–148. doi: 10.1093/carcin/bgp252. [DOI] [PubMed] [Google Scholar]
- 8.Heilbronn L.K., de Jonge L., Frisard M.I., DeLany J.P., Larson-Meyer D.E., Rood J., Nguyen T., Martin C.K., Volaufova J., Most M.M., et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou-Raya S., Abou-Raya A., Naim A., Abuelkheir H. Chronic inflammatory autoimmune disorders and atherosclerosis. Ann. N. Y. Acad. Sci. 2007;1107:56–67. doi: 10.1196/annals.1381.007. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Candales A., Burgos P.M.H., Hernandez-Suarez D.F., Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J. Nat. Sci. 2017;3:e341. [PMC free article] [PubMed] [Google Scholar]
- 12.Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bont R., van Larebeke N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y., Cui Y., Niedernhofer L.J., Wang Y. Occurrence, biological consequences, and human health relevance of oxidative stress-induced DNA damage. Chem. Res. Toxicol. 2016;29:2008–2039. doi: 10.1021/acs.chemrestox.6b00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luczaj W., Skrzydlewska E. DNA damage caused by lipid peroxidation products. Cell Mol. Biol. Lett. 2003;8:391–413. [PubMed] [Google Scholar]
- 16.Jena N.R. DNA damage by reactive species: Mechanisms, mutation and repair. J. Biosci. 2012;37:503–517. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- 17.Kehrer J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C., Mackey M.M., Diaz A.A., Cox D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- 19.Dedon P.C., Tannenbaum S.R. Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Kamiya H. Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: Approaches using synthetic oligonucleotides and nucleotides: Survey and summary. Nucleic Acids Res. 2003;31:517–531. doi: 10.1093/nar/gkg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sova H., Jukkola-Vuorinen A., Puistola U., Kauppila S., Karihtala P. 8-Hydroxydeoxyguanosine: A new potential independent prognostic factor in breast cancer. Br. J. Cancer. 2010;102:1018–1023. doi: 10.1038/sj.bjc.6605565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGowan C.H., Russell P. The DNA damage response: Sensing and signaling. Curr. Opin. Cell Biol. 2004;16:629–633. doi: 10.1016/j.ceb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Allione A., Guarrera S., Russo A., Ricceri F., Purohit R., Pagnani A., Rosa F., Polidoro S., Voglino F., Matullo G. Inter-individual variation in nucleotide excision repair pathway is modulated by non-synonymous polymorphisms in ERCC4 and MBD4 genes. Mutat. Res. 2013;751–752:49–54. doi: 10.1016/j.mrfmmm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Nagel Z.D., Chaim I.A., Samson L.D. Inter-individual variation in DNA repair capacity: A need for multi-pathway functional assays to promote translational DNA repair research. DNA Repair. 2014;19:199–213. doi: 10.1016/j.dnarep.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabzinski J., Mucha B., Cuchra M., Markiewicz L., Przybylowska K., Dziki A., Dziki L., Majsterek I. Efficiency of Base Excision Repair of Oxidative DNA Damage and Its Impact on the Risk of Colorectal Cancer in the Polish Population. Oxid Med. Cell Longev. 2016;2016:3125989. doi: 10.1155/2016/3125989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slyskova J., Lorenzo Y., Karlsen A., Carlsen M.H., Novosadova V., Blomhoff R., Vodicka P., Collins A.R. Both genetic and dietary factors underlie individual differences in DNA damage levels and DNA repair capacity. DNA Repair. 2014;16:66–73. doi: 10.1016/j.dnarep.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27:589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleaver J.E. Profile of Tomas Lindahl, Paul Modrich, and Aziz Sancar, 2015 Nobel Laureates in Chemistry. Proc. Natl. Acad. Sci. USA. 2016;113:242–245. doi: 10.1073/pnas.1521829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindahl T., Karran P., Wood R.D. DNA excision repair pathways. Curr. Opin. Genet. Dev. 1997;7:158–169. doi: 10.1016/S0959-437X(97)80124-4. [DOI] [PubMed] [Google Scholar]
- 30.Sancar A. Excision repair in mammalian cells. J. Biol. Chem. 1995;270:15915–15918. doi: 10.1074/jbc.270.27.15915. [DOI] [PubMed] [Google Scholar]
- 31.Bukhari S.A., Rajoka M.I., Ibrahim Z., Jalal F., Rana S.M., Nagra S.A. Oxidative stress elevated DNA damage and homocysteine level in normal pregnant women in a segment of Pakistani population. Mol. Biol. Rep. 2011;38:2703–2710. doi: 10.1007/s11033-010-0413-7. [DOI] [PubMed] [Google Scholar]
- 32.Tomasello B., Malfa G., Galvano F., Reins M. DNA damage in normal-weight obese syndrome measured by Comet assay. Mediterr. J. Nutr. Metab. 2011;2:99–104. doi: 10.1007/s12349-010-0035-6. [DOI] [Google Scholar]
- 33.Scarpato R., Verola C., Fabiani B., Bianchi V., Saggese G., Federico G. Nuclear damage in peripheral lymphocytes of obese and overweight Italian children as evaluated by the gamma-H2AX focus assay and micronucleus test. FASEB J. 2011;25:685–693. doi: 10.1096/fj.10-168427. [DOI] [PubMed] [Google Scholar]
- 34.Azzara A., Pirillo C., Giovannini C., Federico G., Scarpato R. Different repair kinetic of DSBs induced by mitomycin C in peripheral lymphocytes of obese and normal weight adolescents. Mutat. Res. 2016;789:9–14. doi: 10.1016/j.mrfmmm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Donmez-Altuntas H., Sahin F., Bayram F., Bitgen N., Mert M., Guclu K., Hamurcu Z., Aribas S., Gundogan K., Diri H. Evaluation of chromosomal damage, cytostasis, cytotoxicity, oxidative DNA damage and their association with body-mass index in obese subjects. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;771:30–36. doi: 10.1016/j.mrgentox.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Karbownik-Lewinska M., Szosland J., Kokoszko-Bilska A., Stepniak J., Zasada K., Gesing A., Lewinski A. Direct contribution of obesity to oxidative damage to macromolecules. Neuro Endocrinol. Lett. 2012;33:453–461. [PubMed] [Google Scholar]
- 37.Kocael A., Erman H., Zengin K., Kocael P.C., Korkmaz G.G., Gelisgen R., Taskin M., Ersan Y., Uzun H. The effects on oxidative DNA damage of laparoscopic gastric band applications in morbidly obese patients. Can. J. Surg. 2014;57:183–187. doi: 10.1503/cjs.008113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setayesh T., Nersesyan A., Misik M., Ferk F., Langie S., Andrade V.M., Haslberger A., Knasmuller S. Impact of obesity and overweight on DNA stability: Few facts and many hypotheses. Mutat. Res. 2018;777:64–91. doi: 10.1016/j.mrrev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Iyer A., Fairlie D.P., Prins J.B., Hammock B.D., Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat. Rev. Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 40.Han C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016;40:272–279. doi: 10.4093/dmj.2016.40.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han C.Y., Umemoto T., Omer M., den Hartigh L.J., Chiba T., LeBoeuf R., Buller C.L., Sweet I.R., Pennathur S., Abel E.D., et al. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J. Biol. Chem. 2012;287:10379–10393. doi: 10.1074/jbc.M111.304998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao C.L., Zhu C., Zhao Y.P., Chen X.H., Ji C.B., Zhang C.M., Zhu J.G., Xia Z.K., Tong M.L., Guo X.R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell Endocrinol. 2010;320:25–33. doi: 10.1016/j.mce.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 44.Heo J.W., No M.H., Park D.H., Kang J.H., Seo D.Y., Han J., Neufer P.D., Kwak H.B. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J. Physiol. Pharmacol. 2017;21:567–577. doi: 10.4196/kjpp.2017.21.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fehsel K., Kolb-Bachofen V., Kolb H. Analysis of TNF alpha-induced DNA strand breaks at the single cell level. Am. J. Pathol. 1991;139:251–254. [PMC free article] [PubMed] [Google Scholar]
- 46.Arango Duque G., Descoteaux A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rastogi S., Boylan M., Wright E.G., Coates P.J. Interactions of apoptotic cells with macrophages in radiation-induced bystander signaling. Radiat. Res. 2013;179:135–145. doi: 10.1667/RR2969.1. [DOI] [PubMed] [Google Scholar]
- 48.Speed N., Blair I.A. Cyclooxygenase- and lipoxygenase-mediated DNA damage. Cancer Metastasis Rev. 2011;30:437–447. doi: 10.1007/s10555-011-9298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faber T.J., Japink D., Leers M.P., Sosef M.N., von Meyenfeldt M.F., Nap M. Activated macrophages containing tumor marker in colon carcinoma: Immunohistochemical proof of a concept. Tumour Biol. 2012;33:435–441. doi: 10.1007/s13277-011-0269-z. [DOI] [PubMed] [Google Scholar]
- 50.O’Callaghan N.J., Clifton P.M., Noakes M., Fenech M. Weight loss in obese men is associated with increased telomere length and decreased abasic sites in rectal mucosa. Rejuvenation Res. 2009;12:169–176. doi: 10.1089/rej.2008.0819. [DOI] [PubMed] [Google Scholar]
- 51.Fejfer K., Buczko P., Niczyporuk M., Ladny J.R., Hady H.R., Knas M., Waszkiel D., Klimiuk A., Zalewska A., Maciejczyk M. Oxidative modification of biomolecules in the nonstimulated and stimulated saliva of patients with morbid obesity treated with bariatric surgery. Biomed. Res. Int. 2017;2017:4923769. doi: 10.1155/2017/4923769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Himbert C., Thompson H., Ulrich C.M. Effects of intentional weight loss on markers of oxidative stress, DNA repair and telomere length—A systematic review. Obes. Facts. 2017;10:648–665. doi: 10.1159/000479972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyson J., Caple F., Spiers A., Burtle B., Daly A.K., Williams E.A., Hesketh J.E., Mathers J.C. Inter-individual variation in nucleotide excision repair in young adults: Effects of age, adiposity, micronutrient supplementation and genotype. Br. J. Nutr. 2009;101:1316–1323. doi: 10.1017/S0007114508076265. [DOI] [PubMed] [Google Scholar]
- 54.Adcock I.M., Cosio B., Tsaprouni L., Barnes P.J., Ito K. Redox regulation of histone deacetylases and glucocorticoid-mediated inhibition of the inflammatory response. Antioxid. Redox Signal. 2005;7:144–152. doi: 10.1089/ars.2005.7.144. [DOI] [PubMed] [Google Scholar]
- 55.Kidane D., Chae W.J., Czochor J., Eckert K.A., Glazer P.M., Bothwell A.L., Sweasy J.B. Interplay between DNA repair and inflammation, and the link to cancer. Crit. Rev. Biochem. Mol. Biol. 2014;49:116–139. doi: 10.3109/10409238.2013.875514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R.H., Hotchkiss J.H. Potential genotoxicity of chronically elevated nitric oxide: A review. Mutat. Res. 1995;339:73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 57.McAdam E., Brem R., Karran P. Oxidative stress-induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy. Mol. Cancer Res. 2016;14:612–622. doi: 10.1158/1541-7786.MCR-16-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikodemova M., Yee J., Carney P.R., Bradfield C.A., Malecki K.M. Transcriptional differences between smokers and non-smokers and variance by obesity as a risk factor for human sensitivity to environmental exposures. Environ. Int. 2018;113:249–258. doi: 10.1016/j.envint.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zwamborn R.A., Slieker R.C., Mulder P.C., Zoetemelk I., Verschuren L., Suchiman H.E., Toet K.H., Droog S., Slagboom P.E., Kooistra T., et al. Prolonged high-fat diet induces gradual and fat depot-specific DNA methylation changes in adult mice. Sci. Rep. 2017;7:43261. doi: 10.1038/srep43261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keleher M.R., Zaidi R., Hicks L., Shah S., Xing X., Li D., Wang T., Cheverud J.M. A high-fat diet alters genome-wide DNA methylation and gene expression in SM/J mice. BMC Genom. 2018;19:888. doi: 10.1186/s12864-018-5327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang D., Zhao R., Qu Y.Y., Mei X.Y., Zhang X., Zhou Q., Li Y., Yang S.B., Zuo Z.G., Chen Y.M., et al. Colonic lysine homocysteinylation induced by high-fat diet suppresses DNA damage repair. Cell Rep. 2018;25:398–412. doi: 10.1016/j.celrep.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 62.Harreus U., Baumeister P., Zieger S., Matthias C. The influence of high doses of vitamin C and zinc on oxidative DNA damage. Anticancer Res. 2005;25:3197–3201. [PubMed] [Google Scholar]
- 63.Huang H.Y., Helzlsouer K.J., Appel L.J. The effects of vitamin C and vitamin E on oxidative DNA damage: Results from a randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2000;9:647–652. [PubMed] [Google Scholar]
- 64.Remely M., Ferk F., Sterneder S., Setayesh T., Roth S., Kepcija T., Noorizadeh R., Rebhan I., Greunz M., Beckmann J., et al. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxid Med. Cell Longev. 2017;2017:3079148. doi: 10.1155/2017/3079148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Remely M., Ferk F., Sterneder S., Setayesh T., Kepcija T., Roth S., Noorizadeh R., Greunz M., Rebhan I., Wagner K.H., et al. Vitamin E modifies high-fat diet-induced increase of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Nutrients. 2017;9:607. doi: 10.3390/nu9060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Houten B., Hunter S.E., Meyer J.N. Mitochondrial DNA damage induced autophagy, cell death, and disease. Front. Biosci. 2016;21:42–54. doi: 10.2741/4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein A., Sia E.A. Mitochondrial DNA repair and damage tolerance. Front. Biosci. 2017;22:920–943. doi: 10.2741/4525. [DOI] [PubMed] [Google Scholar]
- 68.Taanman J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta. 1999;1410:103–123. doi: 10.1016/S0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 69.Wang J., Xiong S., Xie C., Markesbery W.R., Lovell M.A. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J. Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 70.Nishikawa M., Oshitani N., Matsumoto T., Nishigami T., Arakawa T., Inoue M. Accumulation of mitochondrial DNA mutation with colorectal carcinogenesis in ulcerative colitis. Br. J. Cancer. 2005;93:331–337. doi: 10.1038/sj.bjc.6602664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bensch K.G., Mott J.L., Chang S.W., Hansen P.A., Moxley M.A., Chambers K.T., de Graaf W., Zassenhaus H.P., Corbett J.A. Selective mtDNA mutation accumulation results in beta-cell apoptosis and diabetes development. Am. J. Physiol. Endocrinol. Metab. 2009;296:E672–E680. doi: 10.1152/ajpendo.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chinnery P.F., Hudson G. Mitochondrial genetics. Br. Med. Bull. 2013;106:135–159. doi: 10.1093/bmb/ldt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutherland L.N., Capozzi L.C., Turchinsky N.J., Bell R.C., Wright D.C. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: Potential mechanisms and the relationship to glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1076–E1083. doi: 10.1152/ajpendo.90408.2008. [DOI] [PubMed] [Google Scholar]
- 74.Turner N., Bruce C.R., Beale S.M., Hoehn K.L., So T., Rolph M.S., Cooney G.J. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–2092. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 75.Lee H., Oh S., Yang W., Park R., Kim H., Jeon J.S., Noh H., Han D.C., Cho K.W., Kim Y.J., et al. Bariatric surgery reduces elevated urinary mitochondrial DNA copy number in obese patients. J. Clin. Endocrinol. Metab. 2019 doi: 10.1210/jc.2018-01935. [DOI] [PubMed] [Google Scholar]
- 76.Yuzefovych L.V., Musiyenko S.I., Wilson G.L., Rachek L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE. 2013;8:e54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pazmandi K., Agod Z., Kumar B.V., Szabo A., Fekete T., Sogor V., Veres A., Boldogh I., Rajnavolgyi E., Lanyi A., et al. Oxidative modification enhances the immunostimulatory effects of extracellular mitochondrial DNA on plasmacytoid dendritic cells. Free Radic. Biol. Med. 2014;77:281–290. doi: 10.1016/j.freeradbiomed.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 78.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patro B., Liber A., Zalewski B., Poston L., Szajewska H., Koletzko B. Maternal and paternal body mass index and offspring obesity: A systematic review. Ann. Nutr. Metab. 2013;63:32–41. doi: 10.1159/000350313. [DOI] [PubMed] [Google Scholar]
- 80.Wahl S., Drong A., Lehne B., Loh M., Scott W.R., Kunze S., Tsai P.C., Ried J.S., Zhang W., Yang Y., et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campbell J.M., Lane M., Owens J.A., Bakos H.W. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: A systematic review and meta-analysis. Reprod. Biomed. Online. 2015;31:593–604. doi: 10.1016/j.rbmo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 82.Kort H.I., Massey J.B., Elsner C.W., Mitchell-Leef D., Shapiro D.B., Witt M.A., Roudebush W.E. Impact of body mass index values on sperm quantity and quality. J. Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 83.Evenson D.P., Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology. 2006;65:979–991. doi: 10.1016/j.theriogenology.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Abdelbaki S.A., Sabry J.H., Al-Adl A.M., Sabry H.H. The impact of coexisting sperm DNA fragmentation and seminal oxidative stress on the outcome of varicocelectomy in infertile patients: A prospective controlled study. Arab. J. Urol. 2017;15:131–139. doi: 10.1016/j.aju.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tunc O., Bakos H.W., Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 86.Lezaja A., Altmeyer M. Inherited DNA lesions determine G1 duration in the next cell cycle. Cell Cycle. 2018;17:24–32. doi: 10.1080/15384101.2017.1383578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soubry A. Epigenetic inheritance and evolution: A paternal perspective on dietary influences. Prog. Biophys. Mol. Biol. 2015;118:79–85. doi: 10.1016/j.pbiomolbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Hammoud S.S., Nix D.A., Hammoud A.O., Gibson M., Cairns B.R., Carrell D.T. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum. Reprod. 2011;26:2558–2569. doi: 10.1093/humrep/der192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McPherson N.O., Fullston T., Aitken R.J., Lane M. Paternal obesity, interventions, and mechanistic pathways to impaired health in offspring. Ann. Nutr. Metab. 2014;64:231–238. doi: 10.1159/000365026. [DOI] [PubMed] [Google Scholar]
- 90.Noblanc A., Damon-Soubeyrand C., Karrich B., Henry-Berger J., Cadet R., Saez F., Guiton R., Janny L., Pons-Rejraji H., Alvarez J.G., et al. DNA oxidative damage in mammalian spermatozoa: Where and why is the male nucleus affected? Free Radic. Biol. Med. 2013;65:719–723. doi: 10.1016/j.freeradbiomed.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 91.Palmer N.O., Fullston T., Mitchell M., Setchell B.P., Lane M. SIRT6 in mouse spermatogenesis is modulated by diet-induced obesity. Reprod. Fertil. Dev. 2011;23:929–939. doi: 10.1071/RD10326. [DOI] [PubMed] [Google Scholar]
- 92.Van Gaal L.F., Mertens I.L., de Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 93.Shimizu I., Yoshida Y., Suda M., Minamino T. DNA damage response and metabolic disease. Cell Metab. 2014;20:967–977. doi: 10.1016/j.cmet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 94.Vousden K.H., Lane D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 95.Maier B., Gluba W., Bernier B., Turner T., Mohammad K., Guise T., Sutherland A., Thorner M., Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tyner S.D., Venkatachalam S., Choi J., Jones S., Ghebranious N., Igelmann H., Lu X., Soron G., Cooper B., Brayton C., et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 97.Petitjean A., Mathe E., Kato S., Ishioka C., Tavtigian S.V., Hainaut P., Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Hum. Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 98.Ochs-Balcom H.M., Marian C., Nie J., Brasky T.M., Goerlitz D.S., Trevisan M., Edge S.B., Winston J., Berry D.L., Kallakury B.V., et al. Adiposity is associated with p53 gene mutations in breast cancer. Breast Cancer Res. Treat. 2015;153:635–645. doi: 10.1007/s10549-015-3570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minamino T., Orimo M., Shimizu I., Kunieda T., Yokoyama M., Ito T., Nojima A., Nabetani A., Oike Y., Matsubara H., et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 100.Vergoni B., Cornejo P.J., Gilleron J., Djedaini M., Ceppo F., Jacquel A., Bouget G., Ginet C., Gonzalez T., Maillet J., et al. DNA Damage and the Activation of the p53 Pathway Mediate Alterations in Metabolic and Secretory Functions of Adipocytes. Diabetes. 2016;65:3062–3074. doi: 10.2337/db16-0014. [DOI] [PubMed] [Google Scholar]
- 101.Hinault C., Kawamori D., Liew C.W., Maier B., Hu J., Keller S.R., Mirmira R.G., Scrable H., Kulkarni R.N. Delta40 Isoform of p53 controls beta-cell proliferation and glucose homeostasis in mice. Diabetes. 2011;60:1210–1222. doi: 10.2337/db09-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tavana O., Zhu C. Too many breaks (brakes): Pancreatic beta-cell senescence leads to diabetes. Cell Cycle. 2011;10:2471–2484. doi: 10.4161/cc.10.15.16741. [DOI] [PubMed] [Google Scholar]
- 103.Castro A.V., Kolka C.M., Kim S.P., Bergman R.N. Obesity, insulin resistance and comorbidities? Mechanisms of association. Arq. Bras. Endocrinol. Metabol. 2014;58:600–609. doi: 10.1590/0004-2730000003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Aubaidy H.A., Jelinek H.F. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur. J. Endocrinol. 2011;164:899–904. doi: 10.1530/EJE-11-0053. [DOI] [PubMed] [Google Scholar]
- 105.Lee S.C., Chan J.C. Evidence for DNA damage as a biological link between diabetes and cancer. Chin. Med. J. 2015;128:1543–1548. doi: 10.4103/0366-6999.157693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y., Zhou J., Wang T., Cai L. High level glucose increases mutagenesis in human lymphoblastoid cells. Int. J. Biol. Sci. 2007;3:375–379. doi: 10.7150/ijbs.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stopper H., Schinzel R., Sebekova K., Heidland A. Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett. 2003;190:151–156. doi: 10.1016/S0304-3835(02)00626-2. [DOI] [PubMed] [Google Scholar]
- 108.Fukami K., Yamagishi S., Kaifu K., Matsui T., Kaida Y., Ueda S., Takeuchi M., Asanuma K., Okuda S. Telmisartan inhibits AGE-induced podocyte damage and detachment. Microvasc. Res. 2013;88:79–83. doi: 10.1016/j.mvr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Simone S., Gorin Y., Velagapudi C., Abboud H.E., Habib S.L. Mechanism of oxidative DNA damage in diabetes: Tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626–2636. doi: 10.2337/db07-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Habib S.L., Liang S. Hyperactivation of Akt/mTOR and deficiency in tuberin increased the oxidative DNA damage in kidney cancer patients with diabetes. Oncotarget. 2014;5:2542–2550. doi: 10.18632/oncotarget.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu N., Lao Y., Zhang Y., Gillespie D.A. Akt: A double-edged sword in cell proliferation and genome stability. J. Oncol. 2012;2012:951724. doi: 10.1155/2012/951724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Habib S.L., Phan M.N., Patel S.K., Li D., Monks T.J., Lau S.S. Reduced constitutive 8-oxoguanine-DNA glycosylase expression and impaired induction following oxidative DNA damage in the tuberin deficient Eker rat. Carcinogenesis. 2003;24:573–582. doi: 10.1093/carcin/24.3.573. [DOI] [PubMed] [Google Scholar]
- 113.Habib S.L. Molecular mechanism of regulation of OGG1: Tuberin deficiency results in cytoplasmic redistribution of transcriptional factor NF-YA. J. Mol. Signal. 2009;4:8. doi: 10.1186/1750-2187-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piscitello D., Varshney D., Lilla S., Vizioli M.G., Reid C., Gorbunova V., Seluanov A., Gillespie D.A., Adams P.D. AKT overactivation can suppress DNA repair via p70S6 kinase-dependent downregulation of MRE11. Oncogene. 2018;37:427–438. doi: 10.1038/onc.2017.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Merkel P., Khoury N., Bertolotto C., Perfetti R. Insulin and glucose regulate the expression of the DNA repair enzyme XPD. Mol. Cell Endocrinol. 2003;201:75–85. doi: 10.1016/S0303-7207(02)00432-X. [DOI] [PubMed] [Google Scholar]
- 116.Komakula S.S.B., Tumova J., Kumaraswamy D., Burchat N., Vartanian V., Ye H., Dobrzyn A., Lloyd R.S., Sampath H. The DNA Repair Protein OGG1 Protects Against Obesity by Altering Mitochondrial Energetics in White Adipose Tissue. Sci. Rep. 2018;8:14886. doi: 10.1038/s41598-018-33151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dizdaroglu M. Substrate specificities and excision kinetics of DNA glycosylases involved in base-excision repair of oxidative DNA damage. Mutat. Res. 2003;531:109–126. doi: 10.1016/j.mrfmmm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Dou H., Mitra S., Hazra T.K. Repair of oxidized bases in DNA bubble structures by human DNA glycosylases NEIL1 and NEIL2. J. Biol. Chem. 2003;278:49679–49684. doi: 10.1074/jbc.M308658200. [DOI] [PubMed] [Google Scholar]
- 119.Vartanian V., Lowell B., Minko I.G., Wood T.G., Ceci J.D., George S., Ballinger S.W., Corless C.L., McCullough A.K., Lloyd R.S. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc. Natl. Acad. Sci. USA. 2006;103:1864–1869. doi: 10.1073/pnas.0507444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sampath H., Vartanian V., Rollins M.R., Sakumi K., Nakabeppu Y., Lloyd R.S. 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS ONE. 2012;7:e51697. doi: 10.1371/journal.pone.0051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bankoglu E.E., Arnold C., Hering I., Hankir M., Seyfried F., Stopper H. Decreased Chromosomal Damage in Lymphocytes of Obese Patients After Bariatric Surgery. Sci. Rep. 2018;8:11195. doi: 10.1038/s41598-018-29581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gavande N.S., VanderVere-Carozza P.S., Hinshaw H.D., Jalal S.I., Sears C.R., Pawelczak K.S., Turchi J.J. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol. Ther. 2016;160:65–83. doi: 10.1016/j.pharmthera.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Turgeon M.O., Perry N.J.S., Poulogiannis G. DNA Damage, Repair, and Cancer Metabolism. Front. Oncol. 2018;8:15. doi: 10.3389/fonc.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lord C.J., Ashworth A. BRCAness revisited. Nat. Rev. Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 125.Chae Y.K., Anker J.F., Carneiro B.A., Chandra S., Kaplan J., Kalyan A., Santa-Maria C.A., Platanias L.C., Giles F.J. Genomic landscape of DNA repair genes in cancer. Oncotarget. 2016;7:23312–23321. doi: 10.18632/oncotarget.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chan D.S., Vieira A.R., Aune D., Bandera E.V., Greenwood D.C., McTiernan A., Rosenblatt D.N., Thune I., Vieira R., Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kocarnik J.M., Chan A.T., Slattery M.L., Potter J.D., Meyerhardt J., Phipps A., Nan H., Harrison T., Rohan T.E., Qi L., et al. Relationship of prediagnostic body mass index with survival after colorectal cancer: Stage-specific associations. Int. J. Cancer. 2016;139:1065–1072. doi: 10.1002/ijc.30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nimptsch K., Pischon T. Body fatness, related biomarkers and cancer risk: An epidemiological perspective. Horm. Mol. Biol. Clin. Investig. 2015;22:39–51. doi: 10.1515/hmbci-2014-0043. [DOI] [PubMed] [Google Scholar]
- 129.Pischon T., Nimptsch K. Obesity and Risk of Cancer: An Introductory Overview. Recent Results Cancer Res. 2016;208:1–15. doi: 10.1007/978-3-319-42542-9_1. [DOI] [PubMed] [Google Scholar]
- 130.Secord A.A., Hasselblad V., von Gruenigen V.E., Gehrig P.A., Modesitt S.C., Bae-Jump V., Havrilesky L.J. Body mass index and mortality in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2016;140:184–190. doi: 10.1016/j.ygyno.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.The L. The link between cancer and obesity. Lancet. 2017;390:1716. doi: 10.1016/S0140-6736(17)32659-4. [DOI] [PubMed] [Google Scholar]
- 132.Ning Y., Wang L., Giovannucci E.L. A quantitative analysis of body mass index and colorectal cancer: Findings from 56 observational studies. Obes. Rev. 2010;11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 133.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 134.Fernandez-Sanchez A., Madrigal-Santillan E., Bautista M., Esquivel-Soto J., Morales-Gonzalez A., Esquivel-Chirino C., Durante-Montiel I., Sanchez-Rivera G., Valadez-Vega C., Morales-Gonzalez J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jung U.J., Choi M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun B., Karin M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramos-Nino M.E. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013;2013:697521. doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Riondino S., Roselli M., Palmirotta R., Della-Morte D., Ferroni P., Guadagni F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014;20:5177–5190. doi: 10.3748/wjg.v20.i18.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Otani K., Ishihara S., Yamaguchi H., Murono K., Yasuda K., Nishikawa T., Tanaka T., Kiyomatsu T., Hata K., Kawai K., et al. Adiponectin and colorectal cancer. Surg. Today. 2017;47:151–158. doi: 10.1007/s00595-016-1334-4. [DOI] [PubMed] [Google Scholar]
- 140.Barb D., Pazaitou-Panayiotou K., Mantzoros C.S. Adiponectin: A link between obesity and cancer. Expert Opin. Investig. Drugs. 2006;15:917–931. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- 141.Stattin P., Lukanova A., Biessy C., Soderberg S., Palmqvist R., Kaaks R., Olsson T., Jellum E. Obesity and colon cancer: Does leptin provide a link? Int. J. Cancer. 2004;109:149–152. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 142.Stattin P., Palmqvist R., Soderberg S., Biessy C., Ardnor B., Hallmans G., Kaaks R., Olsson T. Plasma leptin and colorectal cancer risk: A prospective study in Northern Sweden. Oncol. Rep. 2003;10:2015–2021. doi: 10.3892/or.10.6.2015. [DOI] [PubMed] [Google Scholar]
- 143.Orecchioni S., Reggiani F., Talarico G., Bertolini F. Mechanisms of obesity in the development of breast cancer. Discov. Med. 2015;20:121–128. [PubMed] [Google Scholar]
- 144.Karin M., Greten F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 145.McCool K.W., Miyamoto S. DNA damage-dependent NF-kappaB activation: NEMO turns nuclear signaling inside out. Immunol. Rev. 2012;246:311–326. doi: 10.1111/j.1600-065X.2012.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang W., Mani A.M., Wu Z.H. DNA damage-induced nuclear factor-kappa B activation and its roles in cancer progression. J. Cancer Metastasis Treat. 2017;3:45–59. doi: 10.20517/2394-4722.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valentino E., Bellazzo A., di Minin G., Sicari D., Apollonio M., Scognamiglio G., di Bonito M., Botti G., del Sal G., Collavin L. Mutant p53 potentiates the oncogenic effects of insulin by inhibiting the tumor suppressor DAB2IP. Proc. Natl. Acad. Sci. USA. 2017;114:7623–7628. doi: 10.1073/pnas.1700996114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 149.Macheda M.L., Rogers S., Best J.D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 150.Koppenol W.H., Bounds P.L., Dang C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 151.Levine A.J., Puzio-Kuter A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 152.Rose D.P., Gracheck P.J., Vona-Davis L. The Interactions of Obesity, Inflammation and Insulin Resistance in Breast Cancer. Cancers. 2015;7:2147–2168. doi: 10.3390/cancers7040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marnett L.J. Chemistry and biology of DNA damage by malondialdehyde. IARC Sci. Publ. 1999;150:17–27. [PubMed] [Google Scholar]
- 154.Voulgaridou G.P., Anestopoulos I., Franco R., Panayiotidis M.I., Pappa A. DNA damage induced by endogenous aldehydes: Current state of knowledge. Mutat. Res. 2011;711:13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 155.Wen C., Wu L., Fu L., Wang B., Zhou H. Unifying mechanism in the initiation of breast cancer by metabolism of estrogen (Review) Mol. Med. Rep. 2017;16:1001–1006. doi: 10.3892/mmr.2017.6738. [DOI] [PubMed] [Google Scholar]
- 156.Fussell K.C., Udasin R.G., Smith P.J., Gallo M.A., Laskin J.D. Catechol metabolites of endogenous estrogens induce redox cycling and generate reactive oxygen species in breast epithelial cells. Carcinogenesis. 2011;32:1285–1293. doi: 10.1093/carcin/bgr109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yager J.D., Liehr J.G. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 158.Yasuda M.T., Sakakibara H., Shimoi K. Estrogen- and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes Environ. 2017;39:10. doi: 10.1186/s41021-016-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Caldon C.E. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front. Oncol. 2014;4:106. doi: 10.3389/fonc.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wysham W.Z., Mhawech-Fauceglia P., Li H., Hays L., Syriac S., Skrepnik T., Wright J., Pande N., Hoatlin M., Pejovic T. BRCAness profile of sporadic ovarian cancer predicts disease recurrence. PLoS ONE. 2012;7:e30042. doi: 10.1371/journal.pone.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mhawech-Fauceglia P., Wang D., Kim G., Sharifian M., Chen X., Liu Q., Lin Y.G., Liu S., Pejovic T. Expression of DNA repair proteins in endometrial cancer predicts disease outcome. Gynecol. Oncol. 2014;132:593–598. doi: 10.1016/j.ygyno.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 162.Godoy H., Mhawech-Fauceglia P., Beck A., Miller A., Lele S., Odunsi K. Expression of poly (adenosine diphosphate-ribose) polymerase and p53 in epithelial ovarian cancer and their role in prognosis and disease outcome. Int. J. Gynecol. Pathol. 2011;30:139–144. doi: 10.1097/PGP.0b013e3181fa5a64. [DOI] [PMC free article] [PubMed] [Google Scholar]