Abstract
In an increasingly complex information society, demands for cognitive functioning are growing steadily. In recent years, numerous strategies to augment brain function have been proposed. Evidence for their efficacy (or lack thereof) and side effects has prompted discussions about ethical, societal, and medical implications. In the public debate, cognitive enhancement is often seen as a monolithic phenomenon. On a closer look, however, cognitive enhancement turns out to be a multifaceted concept: There is not one cognitive enhancer that augments brain function per se, but a great variety of interventions that can be clustered into biochemical, physical, and behavioral enhancement strategies. These cognitive enhancers differ in their mode of action, the cognitive domain they target, the time scale they work on, their availability and side effects, and how they differentially affect different groups of subjects. Here we disentangle the dimensions of cognitive enhancement, review prominent examples of cognitive enhancers that differ across these dimensions, and thereby provide a framework for both theoretical discussions and empirical research.
Keywords: Neuroenhancement, brain hacking, neuroethics, cognition, memory, working memory, attention, creativity
1. Introduction
An increasingly complex world exerts increasing demands on cognitive functions—functions that evolved for a fundamentally different environment. Daily life in an information society and a postindustrial economy require cognitive skills that have to be acquired through slow, effortful, and expensive processes of education and training. Likewise, these skills can become obsolete as the world changes ever faster or be lost by the processes of aging. People also vary in their mental abilities, allowing them to acquire certain skills more quickly or slower, which may have significant effects on life outcomes. Strategies to improve the acquisition and maintenance of cognitive skills are thus increasingly important on both an individual and societal level. These challenges of our times have fostered the exploration of strategies to enhance human brain function. While people have since time immemorial sought to improve their performance, the present era is unique in that not only the challenges are growing rapidly but so are technologies that promise to meet them. Just like the hacking culture in the realm of computer software and hardware, an increasing number of individuals experiment with strategies to creatively overcome the natural limitations of human cognitive capacity—in other words, to hack brain function. This development has led to both enthusiasm and dread, as observers have sharply differing intuitions about the feasibility, utility, risks, and eventual impact of enhancement technologies on the world.
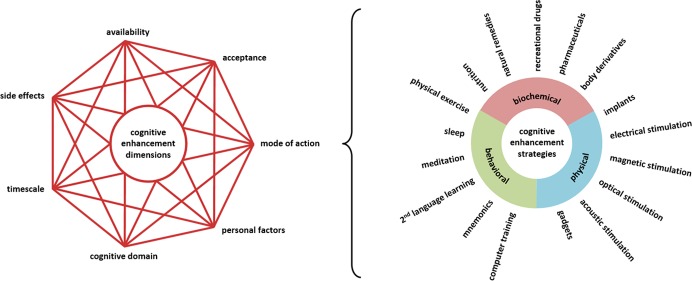
One reason for the often polarized debates has been the lack of hard evidence. Without empirical findings, it is easy to maintain any position, as well as regard opponents as having unfounded views. A further essential source of disagreement and theoretical confusion is a tendency to view enhancement as a unitary phenomenon to be judged as a whole, rather than as a broad set of techniques with important differences and diverging implications. Only on the basis of a clear picture on how a particular enhancement strategy might affect specific cognitive processes in specific populations, along with side effects and costs to be expected, an informed theoretical debate can evolve and a promising empirical research designs to test the strategy can be proposed. In the following, we aim to elucidate seven essential dimension of cognitive enhancement, namely, (a) its mode of action, (b) the cognitive domain targeted, (c) personal factors, (d) its time scale, (e) side effects, (f) availability, and (g) social acceptance (see Figure 1). Further, we will review empirical data of prominent examples of cognitive enhancers that differ across these dimensions and thereby illustrate some of their nuanced implications. The aim of our Review is to sketch a general framework that will foster both theoretical discussions and empirical research.
Figure 1.
Cognitive enhancement interventions differ across several interdependent dimensions.
2. Mode of Action
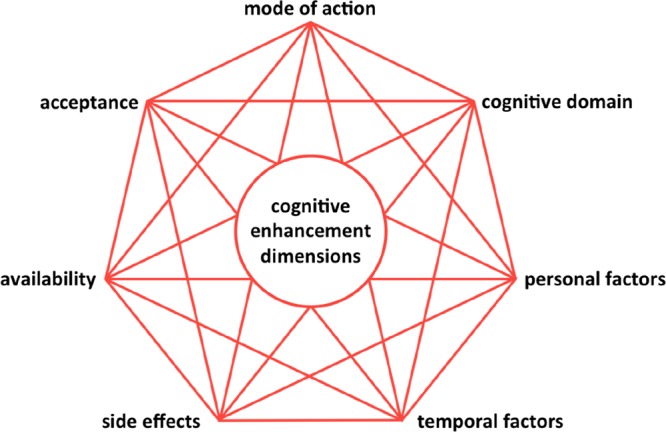
A widely cited definition characterizes enhancement as interventions in humans that aim to improve mental functioning beyond what is necessary to sustain or restore good health.1 While the current bioethical debate on cognitive enhancement is strongly focused on pharmacological ways of enhancement, improving mental capabilities also by nonpharmacological means has to be considered as cognitive enhancement proper according to the given characterization. We have reviewed elsewhere the efficacy of a number of nonpharmacological enhancers.2,3 To systematize the vast variety of different approaches of cognitive enhancement, we suggest clustering enhancement strategies into three major areas according to their main mode of action. Even though boundaries are not strict, most cognitive enhancement strategies can be considered to work as either biochemical, physical, or behavioral interventions (Figure 2). In the following, we will give an overview on the different cognitive enhancement strategies within these clusters.
Figure 2.
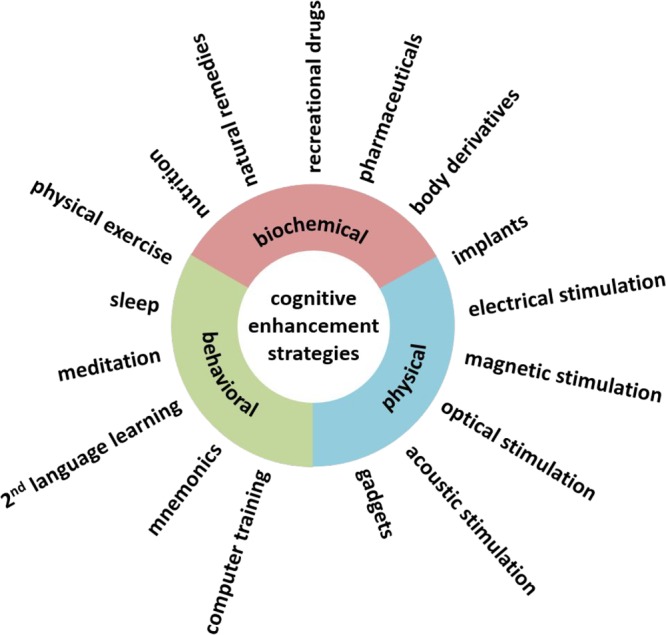
Cognitive enhancement interventions different in their mode of actions.
2.1. Biochemical Strategies
The prototypical cognitive enhancers addressed in the public debate are biochemical agents. However, biochemical interventions are not restricted to pharmaceutical “smart drugs”. Also application of ordinary substances such as oxygen has been shown to increase, e.g., memory processes4,5 and neural activation in memory-related brain regions.6
Biochemical enhancers with the longest tradition in human history are strategies to make use of certain nutritional components. Most widely used are probably glucose7 and caffeine,8,9 which both have demonstrated cognition-enhancing effects in numerous studies. In addition to coffee, other beverages from caffeine-bearing plants such as guarana have shown to enhance cognition.10 While the noncaffeine components in caffeine-bearing plants might exert independent effects on cognition,11 it has been doubted that industrially designed drinks contain cognitive enhancing components that go beyond caffeine, glucose, or guarana extract.12 Further nutritional components with some evidence for cognitive enhancing effects are flavonoids, e.g., in cocoa,13,14 curry powder (most likely due to the curcumin that it contains,15,16 folic acid,17 or omega-3 fatty acids.18 Besides specific dietary supplements, also the absence of food might enhance cognition: some evidence has been reported that fasting and general caloric restriction might improve memory in elderly individuals.19,20
Also certain traditional natural remedies have been discussed as cognitive enhancers: besides herbs that also grow in Western regions such as salvia,21 particularly traditional Chinese and Indian herbal medicines such as Bacopa monnieri have been ascribed with cognitive enhancing effects.22,23 However, with ginseng and ginkgo biloba, the most prominent examples of such traditional Asian herbal remedies so far have failed to consistently show positive effects on cognitive functions in healthy individuals.24,25
A further biochemical intervention with a long history concerns drugs that are being used recreationally and that have demonstrated the potential to enhance certain cognitive functions. For example, nicotine improves attention and memory,26−28 and even alcohol, despite impairing many cognitive functions, might enhance others such as creative processes29,30 or, retroactively, memory.31
Pharmaceuticals are in particular by the public regarded as prototypical cognitive enhancers: synthetic stimulants such as amphetamine, methylphenidate, or modafinil, or antidementia drugs such as acetylcholinesterase inhibitors and memantine are at the core of public debate on cognitive enhancement. However, evidence for their efficacy for augmenting brain function and cognition in healthy subjects is often markedly lower than assumed in theoretical discussions.32−37 Importantly, the lack of an objective effect on cognition can be accompanied by a considerable placebo effect: for example, users who believed to have received mixed-amphetamine salts subjectively rated themselves as performing better and even show minor objective performance increases, independent of actual medication state.38
While pharmacological enhancers are typically designed to affect or mimic certain neurotransmitters, also neural signaling molecules themselves such as adrenaline,39 GABA,40 glucocorticoids,41 ovarian hormones,42 and different neuropeptides43−45 have been suggested as cognitive enhancers.
A further biochemical strategy for cognitive enhancement consists of genetic modifications, which have been demonstrated to augment several learning and memory processes in animal models.46−51 Although progress has also been made in elucidating the genetic basis of cognitive traits in humans,52 genetic modifications in humans still have to be considered as future strategies rather than currently available enhancement options.
2.2. Physical Strategies
The current most widely discussed physical strategies for cognitive enhancement include a number of brain stimulation technologies. Whereas the cognition enhancing effects of invasive methods such as deep brain stimulation53,54 are restricted to subjects with pathological conditions, several forms of allegedly noninvasive stimulation strategies are increasingly used on healthy subjects, among them electrical stimulation methods such transcranial direct current stimulation (tDCS55), transcranial alternating current stimulation (tACS56), transcranial random noise stimulation (tRNS57), transcranial pulsed current stimulation (tPCS58,59), transcutaneous vagus nerve stimulation (tVNS60), or median nerve stimulation (MNS61). Details of the stimulation procedures appear to be crucial: commercial do-it-yourself electrical brain stimulators might impair rather than enhance cognition,62 and systematic reviews have shed doubt on a clear and simple enhancing effect of electrical brain stimulation on different cognitive domains also under controlled laboratory conditions.63,64 Recent studies have even questioned if some of the most commonly used setups for electrical brain stimulation have neurophysiologically meaningful effects at all.65−68 On this background, the development of noninvasive deep brain stimulation via temporally interfering electric fields might provide a more systematic and targeted mechanism compared to the currently used approaches.68
Besides electrical stimulation methods, also for transcranial magnetic stimulation (TMS69), optical stimulation with lasers,70 and several forms of acoustic stimulation, such as transcranial focused ultrasound stimulation,71 binaural beats,72,73 or auditory stimulation of the EEG theta rhythm74 or sleep EEG slow oscillations,75 a potential for cognitive enhancement has been reported.
Physical enhancement methods that target brain processes more indirectly include whole body vibrations,76 stochastic resonance motor control improvement,77,78 and several forms of neurofeedback,79 with, e.g., EEG neurofeedback in the upper alpha band enhancing memory,80 working memory,81 and visuospatial skills.82 Besides classical neurofeedback training that involves unspecific but active effort of the subject, also neurofeedback interventions that automatically feedback low energy currents in response to EEG activity have been developed, thereby allowing the subject to receive the training procedure passively.83 Recently, the use of fMRI neurofeedback, utilizing multivariate pattern analysis, has shown the potential to increase sustained attention84 or visuospatial memory.85
Finally, humans have always deployed physical tools to assist cognitive functioning. In current developments that converge minds and machines, these tools become more closely integrated with the person.86 Crowdfunding or biohacking communities have developed numerous novel technical devices to increase cognitive functions transiently with, e.g., wearable electronic memory aids or augmented reality gadgets,87,88 or more permanently as in the case of cognition enhancing or extending bodily implants.88,89 Neural implants or prosthetics have progressed; in controlled laboratory settings, implants could facilitate human memory.90 In addition, Brain–Computer Interfaces connect the central nervous system with computers through wearable or implanted electrodes and may afford a range of applications that enhance cognitive functions or joint outputs of minds coupled with machines.91,92
2.3. Behavioral Strategies
Although not commonly recognized as such by the public, cognitive enhancers with the most wide use and longest history are probably behavioral strategies: a rapidly growing body of evidence shows that everyday activities such as sleep93 or physical exercise94−96 improve cognitive functioning. Also well-established cultural activities such as musical training,97,98 dancing,99 or learning a second language100 have been demonstrated to enhance cognition beyond the specifically trained skills.
In addition to these natural and cultural standard activities, several behavioral strategies have been developed to enhance certain brain functions intentionally. Two strategies that reach back to ancient times are mnemonic techniques to enhance learning and memory101,102 and meditation training to enhance attention processes and mindfulness.103,104 In contrast, commercial video games105,106 and customized computer trainings107 represent historically very recent developments that are targeted to enhance specific cognitive capacities and skills. In contrast to several years of enthusiasm and widespread commercial application, however, more recent controlled studies and meta-analyses have shed some doubt on the efficacy of computerized brain training programs,108 particularly criticizing claims of “far transfer” of training gains to cognitive domains considerably different from the specifically trained skills.109,110
3. Cognitive Domain
The human mind is not a monolithic entity, but consists of a broad variety of cognitive functions. Not surprisingly, no single cognitive enhancer augments every cognitive function. Instead, most cognitive enhancers have specific profiles regarding their efficacy for different cognitive domains. Memory is, e.g., strongly enhanced by mnemonic strategies, but not by meditation; attention, in turn, is strongly enhanced by meditation training, but not training in mnemonic strategies.101,103,104 Sleep, in contrast, enhances both cognitive capacities.111,112 Some computerized cognitive trainings have been found to enhance memory, processing speed and visuospatial skills, but not executive functions or attention.107 It is currently highly debated in how far specific training strategies exert transfer effects also to nontrained cognitive domains.113
Different cognitive tasks require different optimal levels of receptor activation, thus requiring different doses of pharmacological enhancers targeting the respective neurotransmitter system depending on the cognitive domain targeted.114 Of note, effects of pharmacological enhancement on different cognitive domains might even differ depending on the cognitive test battery used, illustrating the fragility of the respective effects.115
Some interventions might even enhance one but impair another cognitive domain: Intranasal application of oxytocin has been shown to enhance social cognition and cognitive flexibility but impairs long-term memory.116,117 Methylphenidate improves the ability to resist distraction but impairs cognitive flexibility.118 Computerized working memory training has been reported to enhance working memory, reasoning, and arithmetic abilities; however, it might deteriorate creativity.119 Also for amphetamines and modafinil, potential impairments on creativity are discussed besides their enhancing effects on other domains.120,34 In contrast, alcohol might enhance creative processes while impairing most other cognitive functions.29
The costs and benefits of a single cognitive enhancer might even change through slight changes in the application process: for example, electrical stimulation of posterior brain regions was found to facilitate numerical learning, whereas automaticity for the learned material was impaired. In contrast, stimulation on frontal brain regions impaired the learning process, whereas automaticity for the learned material was enhanced.121 Brain stimulation has thus been suggested to be a zero-sum game, with costs in some cognitive functions always being paid for gains in others.122 This implies that enhancement may have to be tuned to the task at hand, in order to focus on the currently most important cognitive demands.
4. Personal Factors
The efficacy of cognitive enhancers does not only differ for different cognitive domains, but also for different users. An important factor in this regard are the cognitive skills of the individual prior to the enhancement intervention. Many pharmaceuticals, including amphetamine,123 modafinil, and methylphenidate,124 work mainly in individuals with low baseline performance. In some cases, even impairments in individuals with higher performance at baseline have been reported, e.g., in the case of amphetamine,125 methylphenidate,124 nicotine,27 or acute choline supplementation.126 The phenomenon of enhanced cognition in individuals with low baseline performance and impairments in those with high baseline performance can be explained by the classical inverted U-model,127,128 where performance is optimal at intermediate levels of the targeted neurochemicals and impaired at levels that are either too low or too high.129−131 For some drugs such as methylphenidate, enhancement dependency on the baseline might even differ between cognitive functions, with performance in specific tasks being improved in low, while impaired in high, performers,124 but showing the opposite pattern for other tasks.132
The baseline-dependency of cognitive enhancement is not restricted to pharmaceuticals: also in the case of video games,133 cognitive training,134 or brain stimulation,135,136 individuals starting at a lower baseline performance benefit more than those with an already high performance at baseline. In contrast, sleep appears to improve memory particularly in subjects with a higher baseline performance of memory,137 working memory138 or intelligence.139 Also mnemonic training appears to work particularly well in individuals with a higher cognitive baseline performance.140 This has been interpreted in terms of an amplification model, in which high baseline performance and cognitive enhancement interventions show synergistic effects.141
Cognitive enhancers can also affect individuals differently depending on basic biological, psychological, or social factors. For example, effects of training interventions on selective attention can depend on the genotype of the trainee;142 effects of methylphenidate on creativity can depend on personality characteristics;143 the cognition enhancing effects of sleep144 or video games145 are modulated by gender. In turn, such modulations of enhancement effects might reduce existing differences in cognitive profiles, as seen, e.g., in action video game training, that have the potential to eliminate gender differences in spatial attention and decrease the gender disparity in mental rotation ability.146 Also the hormonal status of subjects affects how strongly they profit, e.g., from sleep144−148 or brain stimulation.149 Caffeine enhances working memory particularly in extraverted individuals,150 and memory enhancement through sleep151 or mnemonic training140 has been reported to depend on the age of subjects. Health status affects how much users benefit from different kinds of cognitive enhancers, including pharmaceuticals,3 mnemonics,152 or sleep.153−156 Finally, also socioenvironmental factors such as social resources, parental occupation, or family composition can modulate cognitive enhancement interventions, e.g., with cognitive training programs.157
5. Time Scale
Interventions for cognitive enhancement differ in the specific time scale they require to achieve their aims. The prototypical “smart pill” discussed in popular accounts of cognitive enhancement needs practically no preparation time, exerts its effects within seconds or minutes, and lasts for several hours. While this is close to reality in the case of some pharmacological enhancers, the temporal pattern of most other enhancement strategies differs strongly from these time scales. In particular, the time needed for application and the duration of their effects markedly varies between enhancement interventions.
Most pharmacological enhancers can be applied quickly and without further preparation; however, some drugs such as acetylcholinesterase inhibitors or memantine are thought to require longer periods of intake to be effective.33 Also some nutritional enhancers such as glucose and caffeine exert their effects rather quickly, whereas other nutritional supplements have to be taken over extended periods to show an impact on cognition.158,18 Obviously, behavioral strategies like sleep, exercise, video games or mnemonic training need hours or weeks to robustly enhance cognition. Some effects of meditation might even take years of training.159 For brain stimulation methods, both immediate effects of acute stimulation, but also more delayed effects after repeated stimulation haven been observed.55,69 Technological gadgets or implants need some preparation to be installed and accommodated to, however then exert their cognition augmenting effects on demand.
Enhancing effects of most quickly acting pharmacological or nutritional cognitive enhancers also wear off rather rapidly. In contrast to such transient effects, interventions such as brain stimulation,160,161,57 sleep,162 mnemonic strategies163 or genetic modifications46 have the potential for long-term up to chronic enhancement. However, in the latter case, the reversibility of the effects (and side effects) of an enhancement intervention might be a further aspect to be considered.
Interventions can also differ regarding the time point of application relative to the situation when enhanced cognitive performance is needed. For example, application of stress hormones such as cortisol or adrenaline before or after memory encoding enhances memory, whereas application before retrieval impairs memory;41 benzodiazepines impair memory when given before and enhance memory when given after encoding;164 in contrast, caffeine before learning enhances memory under certain conditions but might impair memory when consumed afterward.165,9 Mnemonic strategies on the other hand work solely when taught/applied before/during encoding, but can hardly be applied afterward.
Finally, some interventions can also influence the timing of cognitive performance itself: stimulants such as methylphenidate, modafinil, and caffeine might increase the time subjects take to perform a given task, with impairing effects under time pressure and potentially enhancing effects in the absence of temporal constraints.166
6. Side Effects
The pharmaceutical platitude that there is no effect without side effects holds true also for many nonpharmacological enhancement interventions. It appears obvious that cognitive enhancers differ in the severity and form of side effects: prima facie, deep brain stimulation or implants have higher risks for side effects than sleep or cognitive training. However, also more indirect enhancement strategies such as neurofeedback potentially bear the risk of side effects up to inducing epileptiform activity,167 and even gentle intervention such as meditation training might exert negative effects on specific cognitive domains: a negative relationship between mindfulness and implicit learning168,169 and an increased susceptibility to false memory formation after mindfulness meditation170 have been observed. Here, the intended training goal of nonjudgemental mindfulness opposes tasks where either a more critical or automatic mindset was needed. Further examples of side effects intrinsically associated with the enhancement goal are trade-offs between stability vs flexible updating of memory systems:129 memories can also become “too stable” due to a memory enhancement intervention, as observed, e.g., for the anti-obesity drug rimonabant.171
It has been suggested to differentiate enhancement strategies according to their level of invasiveness.172,173 However, while invasiveness has a more or less definite meaning in its original medical context, physically breaching the skin or entering the body deeply through an external orifice,174 it is difficult to determine the level of invasiveness in the context of cognitive enhancement. Both nutritional supplements and pharmaceuticals enter the body, and thus could be considered invasive in a narrow medical sense, as might be certain forms of physical exercise due to the risk of bruises or scratches as common, e.g., in martial arts or a hike through the woods. Brain stimulation that does not break the skin would, by contrast, be classified as noninvasive. This taxonomy can be disputed for good reasons.175 Besides known risks of these stimulation methods such as scalp burns from tDCS or seizures from TMS, the “known unknowns” have been suggested to pose potentially even greater risks: potential build-up effects across multiple sessions or in sensitive nontarget areas.176 Of note, only few neuroscientists use brain stimulation on themselves for cognitive enhancement.176 Given the still unclear risks and side effects of do-it-yourself brain stimulation use, it has been proposed to extend existing medical device legislation to cover also nonpharmacological and in particular physically acting cognitive enhancement devices.177,178 In contrast to strict medical definitions, the more intuitively assessed level of invasiveness of an intervention often seems to depend on familiarity and cultural traditions. This leads to the Western attitude according to which changing one’s diet or performing exercise appears less invasive than taking pharmaceuticals or applying brain stimulation, independent of their actual effects on health.
Related to the time scale dimension, side effects of short- vs long-term use of cognitive enhancers can be differentiated. For example, while side effects for the acute use of methylphenidate include increased heart rate, headache, anxiety, nervousness, dizziness, drowsiness, and insomnia, in the case of long-term use side effects such as abnormal prefrontal brain function and impaired plasticity have been reported.179,180 Also addiction is a well-known side effect for the long-term use of pharmacological enhancers, which is particularly detrimental to the aim of enhancement if combined with tolerance effects such that larger doses are needed to achieve the same effect (or prevent impairing withdrawal effects). Also behavioral addictions have been observed, e.g., physical exercise181 or the use of technological gadgets.182
A somewhat nonobvious negative effect of some cognitive enhancers is their illusional efficacy: users sometimes believe their performance to be enhanced by amphetamine in absence of any verified and objectively visible enhancing effects, even if administered in a double blind manner.183,184,38 This is particularly counterproductive in cases of already high-functioning individuals whose cognitive capabilities might be impaired rather than enhanced by amphetamine.125,184 Also for caffeine, under certain conditions higher subjectively perceived mental energy in the absence of objectively enhancing effects have been observed.185 The often subtle effects of enhancers can be hidden or amplified by placebo effects.
7. Availability
Cognitive enhancers differ in at least three aspects of availability: legal status, cost, and application time. In terms of legal regulation, different enhancement methods are regulated by sometimes drastically varying frameworks. Pharmaceuticals, for instance, are regulated by strict international control regimes that effectively prohibit nontherapeutic uses or by more lenient domestic drug laws. Brain-stimulation methods, by contrast, fall under medical device regulations, pertaining to basic safety standards in terms but not proscribing the uses to which they might be put.177,178 Behavioral strategies are usually not regulated at all. The regulatory landscape is thus vast and possibly incoherent (for a review, see ref (186)). Besides practical hurdles to the acquisition of illicit drugs for cognitive enhancement, the legal status appears to affect the motivation of users to decide which cognitive enhancers to take.166
A common ethical argument in the enhancement debate concerns distributive justice: also legally available enhancers come with cost barriers, restricting individuals of low socioeconomic status from access.187 A main factor in the costs of cognitive enhancers is their patentability, which is not restricted to pharmaceuticals.188 However, in particular, behavioral enhancement strategies are typically not subject to patentability or other cost-driving factors: sleep, exercise, meditation, or training in mnemonic strategies are largely free of cost and, thus, in contrast to pharmaceuticals or technological strategies, are available independent from the financial background of the user. On the other hand, these behavioral strategies require some time and effort: the 24/7 working manager as the cliché user of cognitive enhancement drugs might have the financial means to afford quickly taking his expensive smart pill between two meetings, but might be unable or unwilling to spend extended periods of time with sleep, meditation, or mnemonic training.
8. Social Acceptance
Largely independent from their specific enhancing effects on different cognitive capacities, social acceptance of cognitive enhancement interventions differs strongly depending on traditions, their perceived naturalness, and the perceived directness of their mode of action. Enhancement interventions with a tradition of thousands of years such as meditation and nutrition are typically much better accepted than many currently debated enhancement strategies such as brain stimulation and pharmaceuticals.189 Also more “natural” interventions such as sleep or exercise are seen in a more positive light compared to technological innovations.190 Moreover, in how far the mode of action is perceived as psychologically mediated or more biologically direct, affecting the brain indirectly through the senses or more directly through the cranium or metabolism, often plays a role for their social acceptance: if an enhancement intervention such as intense cognitive or physical training requires extended efforts or is seen as a quick and effortless shortcut to the same goal as in the case of smart pills or brain stimulation touches different intuitions about human virtues and is thus valuated differently. Even though views based on such purely intuitive aspects of tradition, naturalness, or directness often rely on cognitive biases rather than rational argument,191 a negative social perception for whatever reason might generate indirect psychological costs for users, which in turn might influence also rational evaluations of the respective enhancement intervention.192
Accordingly, one of the central points in the ethical controversy revolves around the question of whether enhancement strategies only relevantly differ with respect to their outcomes, i.e., their benefits and side effects,193 or also with respect to their mode of operation.194 Some argue that the relevant ethical distinction runs along the lines of enhancements that are active, in the sense of requiring participation, and those that work on persons more passively.195
Not surprisingly, different views on cognitive enhancement prevail in different (sub)cultures, with, e.g., a more positive view on enhancement interventions in Asia196 or in younger populations.197 Empirical studies on attitudes toward cognitive enhancement interventions found medical safety, coercion, and fairness the most common concerns, with nonusers displaying more concerns regarding medical safety and fairness than users.198 Sometimes readily available substances for cognitive enhancement such as caffeine, energy drinks, or herbal drugs are dubbed “soft enhancers”;199 however, considering that prohibition of substances is not only based on their potential harm, but also on historical circumstances, this differentiation between soft and hard enhancers appears questionable.
A further aspect that determines the social acceptance of cognitive enhancement is the aim of the given intervention. Taken by face value, the term cognitive enhancement denotes any action or intervention that improves cognitive capacities, independent from the specific aim of this improvement. The use of the term in the empirical, philosophical, and sociopolitical literature, however, varies with regard of the specific aim of enhancement interventions: people appear to be more tolerant toward enhancement of traits considered less fundamental to self-identity,200 and also more tolerant toward enhancement in individuals with cognitive impairments or low performance baselines compared to enhancement of normal or high achievers.201,202 At least four different aims can be identified, each leading to different research strategies and different ethical evaluations of existing or potential enhancement strategies.203 The least problematic concept of cognitive enhancement targets those everyday medical or psychological interventions that are meant to treat pathological deficiencies. Closely related are those cognitive enhancement interventions that aim to prevent or attenuate cognitive decline that is associated also with healthy aging.204 Slightly less accepted appear to be those enhancement strategies that aim to improve cognition in completely healthy individuals, but still clearly stay within the normal limits of cognition. The probably most widely used and ethically most controversial concept of cognitive enhancement aims at the augmentation of cognitive capacities beyond normal function, as is represented in the cliché of high-functioning students or managers trying to further improve their performance by taking smart pills.
Besides these differentiations between enhancement of impaired vs healthy cognition, another difference in the aims of cognitive enhancement touches the ultimate deed of the enhancement intervention: due to the central role of cognitive capacities in defining humans as a species, it is tempting to consider the improvement of these defining human capacities as a value in itself. However, most philosophical or religious approaches do not center on objective cognitive performance markers, but propose values only indirectly related to cognitive performance such as living a happier or more meaningful life in general. In this light, human enhancement in more general terms does not need to aim for individual cognitive or neural processes, but can also be achieved by sociopolitical reforms targeted at the population level.205,206
9. Conclusions
Cognitive enhancement clearly is a multidimensional endeavor. However, not every dimension is important for every theoretical or empirical research question. For example, many empirical researchers of cognitive enhancement are primarily interested in the understanding of the neurobiological and psychological mechanisms underlying cognitive functions.207 For this aim, the availability and social acceptance dimensions are largely irrelevant. In contrast, many theorists are interested in the social and ethical implications of cognitive enhancement,208 where these dimensions might be of prime importance. Also side effects and temporal factors might be of secondary importance to empirical researchers with an interest in the neural mechanisms of certain cognitive processes, whereas these would be highly relevant for users who ponder the question which cognitive enhancement strategy to choose for a certain aim. When comparing different cognitive enhancement strategies, different dimensions might thus be differently weighed or completely ignored, depending on the aim of the comparison.
Up until now, direct comparisons between cognitive enhancement strategies with radically different modes of actions have rarely been made (but see, e.g., ref (165)), and more comprehensive comparisons across dimensions might be difficult: practical issues of information availability from the different dimensions aside, interventions typically differ on different dimensions and are thus difficult to compare globally. In addition, multiple interactions between different enhancers exist, which further complicates the situation. Interactions have been reported, e.g., for glucose and caffeine,209 diet and exercise,210 exercise and working memory training,211 video games and sleep,212 video games and brain stimulation,213 exercise and brain stimulation,214 and brain stimulation and sleep.215,216 Also different dimensions discussed here can interact in multiple ways, as, e.g., computerized cognitive training can differentially enhance different cognitive processes depending on personal factors such as age;217 and social acceptance of different enhancement strategies depends on both the baseline performance of users and the cognitive domain targeted.200,201
Despite—or because of—these complexities, in our opinion, both theoretical discussions and empirical research would strongly benefit from a more differentiated approach. Specific research questions might require the emphasis on some dimensions of cognitive enhancement over others, and for some research questions some dimensions might be entirely irrelevant. Nevertheless, keeping in mind that cognitive enhancement is not a monolithic phenomenon will help to solve and avoid a number of confusions and disagreements that are still present in the public debate on cognitive enhancement.
Author Contributions
All authors developed the concept of this paper and jointly wrote the manuscript.
This work was supported by the Volkswagen Foundation, Germany.
The authors declare no competing financial interest.
References
- Juengst E. T. (1998) What does enhancement mean? In Enhancing Human Traits: Ethical and Social Implications (Parens E., Ed.), pp 29–47, Georgetown University Press. [Google Scholar]
- Dresler M.; Sandberg A.; Ohla K.; Bublitz C.; Trenado C.; Mroczko-Wąsowicz A.; Kühn S.; Repantis D. (2013) Non-pharmacological cognitive enhancement. Neuropharmacology 64, 529–43. 10.1016/j.neuropharm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Dresler M., and Repantis D. (2015) Cognitive enhancement in humans. In Cognitive Enhancement: Pharmacologic, Environmental and Genetic Factors (Knafo S., and Venero C., Eds.), pp 273–306, Elsevier, Amsterdam. [Google Scholar]
- Moss M. C.; Scholey A. B. (1996) Oxygen administration enhances memory formation in healthy young adults. Psychopharmacology (Berl) 124, 255–60. 10.1007/BF02246665. [DOI] [PubMed] [Google Scholar]
- Scholey A. B.; Moss M. C.; Neave N.; Wesnes K. (1999) Cognitive performance, hyperoxia, and heart rate following oxygen administration in healthy young adults. Physiol. Behav. 67, 783–9. 10.1016/S0031-9384(99)00183-3. [DOI] [PubMed] [Google Scholar]
- Yu R.; Wang B.; Li S.; Wang J.; Zhou F.; Chu S.; He X.; Wen X.; Ni X.; Liu L.; Xie O.; Huang R. (2015) Cognitive enhancement of healthy young adults with hyperbaric oxygen: A preliminary resting-state fMRI study. Clin. Neurophysiol. 126, 2058–2067. 10.1016/j.clinph.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Smith M. A.; Riby L. M.; Eekelen J. A.; Foster J. K. (2011) Glucose enhancement of human memory: a comprehensive research review of the glucose memory facilitation effect. Neurosci. Biobehav. Rev. 35, 770–83. 10.1016/j.neubiorev.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Glade M. J. (2010) Caffeine-Not just a stimulant. Nutrition 26, 932–8. 10.1016/j.nut.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Nehlig A. (2010) Is caffeine a cognitive enhancer?. J. Alzheimer's Dis. 20, S85–94. 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- Haskell C. F.; Kennedy D. O.; Wesnes K. A.; Milne A. L.; Scholey A. B. (2007) A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guaraná in humans. J. Psychopharmacol. 21, 65–70. 10.1177/0269881106063815. [DOI] [PubMed] [Google Scholar]
- Haskell C. F.; Dodd F. L.; Wightman E. L.; Kennedy D. O. (2013) Behavioural effects of compounds co-consumed in dietary forms of caffeinated plants. Nutr. Res. Rev. 26, 49–70. 10.1017/S0954422413000036. [DOI] [PubMed] [Google Scholar]
- McLellan T. M.; Lieberman H. R. (2012) Do energy drinks contain active components other than caffeine?. Nutr. Rev. 70, 730–44. 10.1111/j.1753-4887.2012.00525.x. [DOI] [PubMed] [Google Scholar]
- Rendeiro C.; Guerreiro J. D.; Williams C. M.; Spencer J. P. (2012) Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proc. Nutr. Soc. 71, 246–62. 10.1017/S0029665112000146. [DOI] [PubMed] [Google Scholar]
- Socci V.; Tempesta D.; Desideri G.; De Gennaro L.; Ferrara M. (2017) Enhancing Human Cognition with Cocoa Flavonoids. Frontiers in Nutrition 4, 19. 10.3389/fnut.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. P.; Chiam P. C.; Lee T.; Chua H. C.; Lim L.; Kua E. H. (2006) Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 164, 898–906. 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- Cox K. H.; Pipingas A.; Scholey A. B. (2015) Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 29, 642–51. 10.1177/0269881114552744. [DOI] [PubMed] [Google Scholar]
- Enderami A.; Zarghami M.; Darvishi-Khezri H. (2018) The effects and potential mechanisms of folic acid on cognitive function: a comprehensive review. Neurol. Sci. 39, 1667. 10.1007/s10072-018-3473-4. [DOI] [PubMed] [Google Scholar]
- Luchtman D. W.; Song C. (2013) Cognitive enhancement by omega-3 fatty acids from child-hood to old age: findings from animal and clinical studies. Neuropharmacology 64, 550–565. 10.1016/j.neuropharm.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Witte A. V.; Fobker M.; Gellner R.; Knecht S.; Flöel A. (2009) Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. U. S. A. 106, 1255–60. 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S.; Choi I. Y.; Wei M.; Cheng C. W.; Sedrakyan S.; Navarrete G.; Dubeau L.; Yap L. P.; Park R.; Vinciguerra M.; Di Biase S.; Mirzaei H.; Mirisola M. G.; Childress P.; Ji L.; Groshen S.; Penna F.; Odetti P.; Perin L.; Conti P. S.; Ikeno Y.; Kennedy B. K.; Cohen P.; Morgan T. E.; Dorff T. B.; Longo V. D. (2015) A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 22, 86–99. 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tildesley N. T.; Kennedy D. O.; Perry E. K.; Ballard C. G.; Savelev S.; Wesnes K. A.; Scholey A. B. (2003) Salvia lavandulaefolia (Spanish sage) enhances memory in healthy young volunteers. Pharmacol., Biochem. Behav. 75, 669–74. 10.1016/S0091-3057(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Howes M. J.; Houghton P. J. (2003) Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol., Biochem. Behav. 75, 513–27. 10.1016/S0091-3057(03)00128-X. [DOI] [PubMed] [Google Scholar]
- Kongkeaw C.; Dilokthornsakul P.; Thanarangsarit P.; Limpeanchob N.; Norman Scholfield C. (2014) Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J. Ethnopharmacol. 151, 528–35. 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Geng J.; Dong J.; Ni H.; Lee M. S.; Wu T.; Jiang K.; Wang G.; Zhou A. L.; Malouf R. (2010) Ginseng for cognition. Cochrane Database Syst. Rev. 12, CD007769. 10.1002/14651858.CD007769.pub2. [DOI] [PubMed] [Google Scholar]
- Laws K. R.; Sweetnam H.; Kondel T. K. (2012) Is Ginkgo biloba a cognitive enhancer in healthy individuals? A meta-analysis. Hum. Psychopharmacol. 27, 527–33. 10.1002/hup.2259. [DOI] [PubMed] [Google Scholar]
- Warburton D. M. (1992) Nicotine as a cognitive enhancer. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 16, 181–91. 10.1016/0278-5846(92)90069-Q. [DOI] [PubMed] [Google Scholar]
- Niemegeers P.; Dumont G.; Quisenaerts C.; Morrens M.; Boonzaier J.; Fransen E.; de Bruijn E.; Hulstijn W.; Sabbe B. (2014) The effects of nicotine on cognition are dependent on baseline performance. Eur. Neuropsychopharmacol. 24, 1015–1023. 10.1016/j.euroneuro.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Valentine G.; Sofuoglu M. (2018) Cognitive Effects of Nicotine: Recent Progress. Curr. Neuropharmacol 16, 403–414. 10.2174/1570159X15666171103152136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz A. F.; Colflesh G. J.; Wiley J. (2012) Uncorking the muse: alcohol intoxication facilitates creative problem solving. Conscious Cogn 21, 487–93. 10.1016/j.concog.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Benedek M.; Panzierer L.; Jauk E.; Neubauer A. C. (2017) Creativity on tap? Effects of alcohol intoxication on creative cognition. Conscious Cogn 56, 128–134. 10.1016/j.concog.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle M.; Dumay N.; Roberts K.; McAndrew A.; Stevens T.; Lawn W.; Morgan C. J. A. (2017) Improved memory for information learnt before alcohol use in social drinkers tested in a naturalistic setting. Sci. Rep. 7, 6213. 10.1038/s41598-017-06305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repantis D.; Schlattmann P.; Laisney O.; Heuser I. (2010) Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacol. Res. 62, 187–206. 10.1016/j.phrs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Repantis D.; Laisney O.; Heuser I. (2010) Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: a systematic review. Pharmacol. Res. 61, 473–81. 10.1016/j.phrs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Battleday R. M.; Brem A. K. (2015) Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. Eur. Neuropsychopharmacol. 25, 1865–81. 10.1016/j.euroneuro.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Fond G.; Micoulaud-Franchi J. A.; Brunel L.; Macgregor A.; Miot S.; Lopez R.; et al. (2015) Innovative mechanisms of action for pharmaceutical cognitive enhancement: a systematic review. Psychiatry Res. 229, 12–20. 10.1016/j.psychres.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodríguez E.; Barciela Veras A.; Barbosa Rocha N.; Budde H.; Machado S. (2018) An Overview of the Clinical Uses, Pharmacology, and Safety of Modafinil. ACS Chem. Neurosci. 9, 151–158. 10.1021/acschemneuro.7b00374. [DOI] [PubMed] [Google Scholar]
- Schleim S.; Quednow B. B. (2018) How Realistic Are the Scientific Assumptions of the Neuroenhancement Debate? Assessing the Pharmacological Optimism and Neuroenhancement Prevalence Hypotheses. Front. Pharmacol. 9, 3. 10.3389/fphar.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey K. L.; Schiavon S.; Hendricks P. S.; Froelich M.; Lentowicz I.; Fargason R. (2017) Mixed-amphetamine salts expectancies among college students: Is stimulant induced cognitive enhancement a placebo effect?. Drug Alcohol Depend. 178, 302–309. 10.1016/j.drugalcdep.2017.05.024. [DOI] [PubMed] [Google Scholar]
- Cahill L.; Alkire M. T. (2003) Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol. Learn. Mem. 79, 194–8. 10.1016/S1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Steenbergen L.; Sellaro R.; Stock A. K.; Beste C.; Colzato L. S. (2015) γ-Aminobutyric acid (GABA) administration improves action selection processes: a randomised controlled trial. Sci. Rep. 5, 12770. 10.1038/srep12770. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Het S.; Ramlow G.; Wolf O. T. (2005) A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30, 771–84. 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Smith M. J.; Adams L. F.; Schmidt P. J.; Rubinow D. R.; Wassermann E. M. (2002) Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 51, 599–603. 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- Kunath N., and Dresler M. (2014) Ghrelin and memory. In Central Functions of the Ghrelin Receptor (Portelli J., and Smolders I., Eds.), pp 167–176, Springer, New York: 10.1007/978-1-4939-0823-3_10. [DOI] [Google Scholar]
- Kunath N.; Müller N.; Tonon M.; Konrad B. N.; Kopczak A.; Pawlowski M.; Kühn S.; Repantis D.; Ohla K.; Müller D.; Fernandez G.; Tschoep M.; Steiger A.; Czisch M.; Dresler M. (2016) Ghrelin modulates encoding-related brain activity without enhancing memory formation in humans. NeuroImage 142, 465–473. 10.1016/j.neuroimage.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Asua D.; Bougamra G.; Calleja-Felipe M.; Morales M.; Knafo S. (2018) Peptides acting as cognitive enhancers. Neuroscience 370, 81–87. 10.1016/j.neuroscience.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Tang Y. P.; Shimizu E.; Dube G. R.; Rampon C.; Kerchner G. A.; Zhuo M.; Liu G.; Tsien J. Z. (1999) Genetic enhancement of learning and memory in mice. Nature 401, 63–9. 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Diana G.; Valentini G.; Travaglione S.; Falzano L.; Pieri M.; Zona C.; Meschini S.; Fabbri A.; Fiorentini C. (2007) Enhancement of learning and memory after activation of cerebral Rho GTPases. Proc. Natl. Acad. Sci. U. S. A. 104, 636–641. 10.1073/pnas.0610059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Viti S.; Martino A.; Musilli M.; Fiorentini C.; Diana G. (2010) The Rho GTPase activating CNF1 improves associative working memory for object-in-place. Behav. Brain Res. 212, 78–83. 10.1016/j.bbr.2010.03.049. [DOI] [PubMed] [Google Scholar]
- Dubal D. B.; Yokoyama J. S.; Zhu L.; Broestl L.; Worden K.; Wang D.; Sturm V. E.; Kim D.; Klein E.; Yu G. Q.; Ho K.; Eilertson K. E.; Yu L.; Kuro-o M.; De Jager P. L.; Coppola G.; Small G. W.; Bennett D. A.; Kramer J. H.; Abraham C. R.; Miller B. L.; Mucke L. (2014) Life extension factor klotho enhances cognition. Cell Rep. 7, 1065–1076. 10.1016/j.celrep.2014.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounallah-Saad H.; Sharma V.; Edry E.; Rosenblum K. (2014) Genetic or pharmacological reduction of PERK enhances cortical-dependent taste learning. J. Neurosci. 34, 14624–32. 10.1523/JNEUROSCI.2117-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez G.; Vidal R. L.; Mardones P.; Serrano F. G.; Ardiles A. O.; Wirth C.; Valdés P.; Thielen P.; Schneider B. L.; Kerr B.; Valdés J. L.; Palacios A. G.; Inestrosa N. C.; Glimcher L. H.; Hetz C. (2016) Regulation of Memory Formation by the Transcription Factor XBP1. Cell Rep. 14, 1382–1394. 10.1016/j.celrep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A.; de Quervain D. J. (2011) Genetics of human episodic memory: dealing with complexity. Trends Cognit. Sci. 15, 381–7. 10.1016/j.tics.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Suthana N.; Haneef Z.; Stern J.; Mukamel R.; Behnke E.; Knowlton B.; Fried I. (2012) Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 366, 502–10. 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman C. S.; Manns J. R.; Bijanki K. R.; Bass D. I.; Hamann S.; Drane D. L.; Fasano R. E.; Kovach C. K.; Gross R. E.; Willie J. T. (2018) Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proc. Natl. Acad. Sci. U. S. A. 115, 98–103. 10.1073/pnas.1714058114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman B. A.; Clark V. P.; Parasuraman R. (2014) Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. NeuroImage 85, 895–908. 10.1016/j.neuroimage.2013.07.083. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E.; Polizzotto N. R.; Godone M.; Giovannelli F.; Feurra M.; Matzen L.; Rossi A.; Rossi S. (2013) Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr. Biol. 23, 1449–53. 10.1016/j.cub.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Snowball A.; Tachtsidis I.; Popescu T.; Thompson J.; Delazer M.; Zamarian L.; et al. (2013) Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Curr. Biol. 23, 987–992. 10.1016/j.cub.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberzadeh S.; Bastani A.; Zoghi M. (2014) Anodal transcranial pulsed current stimulation: A novel technique to enhance corticospinal excitability. Clin. Neurophysiol. 125, 344–351. 10.1016/j.clinph.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Morales-Quezada L.; Cosmo C.; Carvalho S.; Leite J.; Castillo-Saavedra L.; Rozisky J. R.; Fregni F. (2015) Cognitive effects and autonomic responses to transcranial pulsed current stimulation. Exp. Brain Res. 233, 701. 10.1007/s00221-014-4147-y. [DOI] [PubMed] [Google Scholar]
- Colzato L. S.; Ritter S. M.; Steenbergen L. (2018) Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia 111, 72–76. 10.1016/j.neuropsychologia.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Carvalho S.; French M.; Thibaut A.; Lima W.; Simis M.; Leite J.; Fregni F. (2018) Median nerve stimulation induced motor learning in healthy adults: A study of timing of stimulation and type of learning. Eur. J. Neurosci 48, 1667. 10.1111/ejn.13990. [DOI] [PubMed] [Google Scholar]
- Steenbergen L.; Sellaro R.; Hommel B.; Lindenberger U.; Kühn S.; Colzato L. S. (2016) ″Unfocus″ on foc.us: commercial tDCS headset impairs working memory. Exp. Brain Res. 234, 637–43. 10.1007/s00221-015-4391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch E. R.; Santarnecchi E.; Antal A.; Born J.; Celnik P. A.; Classen J.; Gerloff C.; Hallett M.; Hummel F. C.; Nitsche M. A.; Pascual-Leone A.; Paulus W. J.; Reis J.; Robertson E. M.; Rothwell J. C.; Sandrini M.; Schambra H. M.; Wassermann E. M.; Ziemann U.; Cohen L. G. (2017) Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clin. Neurophysiol. 128, 589–603. 10.1016/j.clinph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Reteig L. C.; Talsma L. J.; van Schouwenburg M. R.; Slagter H. A. (2017) Transcranial electrical stimulation as a tool to enhance attention. J. Cogn Enhanc 1, 10–25. 10.1007/s41465-017-0010-y. [DOI] [Google Scholar]
- Lafon B.; Henin S.; Huang Y.; Friedman D.; Melloni L.; Thesen T.; Doyle W.; Buzsáki G.; Devinsky O.; Parra L. C.; Liu A. A. (2017) Low frequency transcranial electrical stimulation does not entrain sleep rhythms measured by human intracranial recordings. Nat. Commun. 8, 1199. 10.1038/s41467-017-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut A.; Zafonte R.; Morse L. R.; Fregni F. (2017) Understanding Negative Results in tDCS Research: The Importance of Neural Targeting and Cortical Engagement. Front. Neurosci. 11, 00707. 10.3389/fnins.2017.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin B. L.; Bhandari M.; Glen J. C.; Walsh V. (2018) The physiological effects of transcranial electrical stimulation do not apply to parameters commonly used in studies of cognitive neuromodulation. Neuropsychologia 10.1016/j.neuropsychologia.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Grossman N.; Bono D.; Dedic N.; Kodandaramaiah S. B.; Rudenko A.; Suk H. J.; Cassara A. M.; Neufeld E.; Kuster N.; Tsai L. H.; Pascual-Leone A.; Boyden E. S. (2017) Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 169, 1029. 10.1016/j.cell.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B.; Lisanby S. H. (2014) Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). NeuroImage 85, 961–70. 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F.; Barrett D. W. (2014) Augmentation of cognitive brain functions with transcranial lasers. Front. Syst. Neurosci. 8, 36. 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W.; Sato T. F.; Opitz A.; Mueller J.; Barbour A.; Williams A.; Tyler W. J. (2014) Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 17, 322–9. 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- Reedijk S. A.; Bolders A.; Hommel B. (2013) The impact of binaural beats on creativity. Front. Hum. Neurosci. 7, 786. 10.3389/fnhum.2013.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L. S.; Barone H.; Sellaro R.; Hommel B. (2017) More attentional focusing through binaural beats: evidence from the global-local task. Hematol. Cell Ther. 81, 271–277. 10.1007/s00426-015-0727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. M.; Clarke A.; Addante R. J.; Ranganath C. (2018) Entrainment enhances theta oscillations and improves episodic memory. Cogn Neurosci 9, 181. 10.1080/17588928.2018.1521386. [DOI] [PubMed] [Google Scholar]
- Ngo H. V.; Martinetz T.; Born J.; Mölle M. (2013) Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–53. 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Fuermaier A. B.; Tucha L.; Koerts J.; van Heuvelen M. J.; van der Zee E. A.; Lange K. W.; Tucha O. (2014) Good vibrations--effects of whole body vibration on attention in healthy individuals and individuals with ADHD. PLoS One 9, e90747 10.1371/journal.pone.0090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenado C.; Mikulić A.; Manjarrez E.; Mendez-Balbuena I.; Schulte-Mönting J.; Huethe F.; Hepp-Reymond M. C.; Kristeva R. (2014) Broad-band Gaussian noise is most effective in improving motor performance and is most pleasant. Front. Hum. Neurosci. 8, 22. 10.3389/fnhum.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenado C.; Mendez-Balbuena I.; Manjarrez E.; Huethe F.; Schulte Mönting J.; Feige B.; Hepp-Reymond M.; Kristeva R. (2014) Enhanced corticomuscular coherence by external stochastic noise. Front. Hum. Neurosci. 8, 325. 10.3389/fnhum.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruzelier J. H. (2014) EEG-neurofeedback for optimizing performance. I: a review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 44, 124–141. 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Escolano C.; Olivan B.; Lopez-del-Hoyo Y.; Garcia-Campayo J.; Minguez J. (2012) Double-blind single-session neurofeedback training in upper-alpha for cognitive enhancement of healthy subjects. Conf Proc. IEEE Eng. Med. Biol. Soc. 2012, 4643–7. 10.1109/EMBC.2012.6347002. [DOI] [PubMed] [Google Scholar]
- Escolano C.; Aguilar M.; Minguez J. (2011) EEG-based upper alpha neurofeedback training improves working memory performance. Conf Proc. IEEE Eng. Med. Biol. Soc. 2011, 2327–30. 10.1109/IEMBS.2011.6090651. [DOI] [PubMed] [Google Scholar]
- Zoefel B.; Huster R. J.; Herrmann C. S. (2011) Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage 54, 1427–31. 10.1016/j.neuroimage.2010.08.078. [DOI] [PubMed] [Google Scholar]
- Ochs L. (2006) The Low Energy Neurofeedback System (LENS): Theory, background, and introduction. J. Neurotherapy 10 (2–3), 5–39. 10.1300/J184v10n02_02. [DOI] [Google Scholar]
- deBettencourt M. T.; Cohen J. D.; Lee R. F.; Norman K. A.; Turk-Browne N. B. (2015) Closed-loop training of attention with real-timebrain imaging. Nat. Neurosci. 18, 470–475. 10.1038/nn.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenfeld C.; Nellessen N.; Dogan I.; Kuhn H.; Müller C.; Papa F.; Ketteler S.; Goebel R.; Heinecke A.; Shah N. J.; Schulz J. B.; Reske M.; Reetz K. (2017) Cognitive Improvement and Brain Changes after Real-Time Functional MRI Neurofeedback Training in Healthy Elderly and Prodromal Alzheimer’s Disease. Front Neurol 8, 384. 10.3389/fneur.2017.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. (2015) Thinking in the Cloud: The Cognitive Incorporation of Cloud-Based Technology. Philosophy & Technology 28, 261–296. 10.1007/s13347-014-0153-z. [DOI] [Google Scholar]
- Brenninkmeijer J.; Zwart H. (2017) From ’Hard’ Neuro-Tools to ’Soft’ Neuro-Toys? Refocussing the Neuro-Enhancement Debate. Neuroethics 10, 337–348. 10.1007/s12152-016-9283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick K. (2014) The Cyborg Revolution. NanoEthics 8, 263–273. 10.1007/s11569-014-0212-z. [DOI] [Google Scholar]
- Warwick K. (2014) A tour of some brain/neuronal–computer interfaces. Brain-Computer-Interfaces in their ethical, social and cultural contexts. International Library of Ethics, Law and Technology 12, 131–145. 10.1007/978-94-017-8996-7_12. [DOI] [Google Scholar]
- Hampson R. E.; Song D.; Robinson B. S.; Fetterhoff D.; Dakos A. S.; Roeder B. M.; She X.; Wicks R. T.; Witcher M. R.; Couture D. E.; Laxton A. W.; Munger-Clary H.; Popli G.; Sollman M. J.; Whitlow C. T.; Marmarelis V. Z.; Berger T. W.; Deadwyler S. A. (2018) Developing a hippocampal neural prosthetic to facilitate human memory encoding and recall. J. Neural Eng. 15, 036014. 10.1088/1741-2552/aaaed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F.; Merkow M. B.; Jacobs J.; Kahana M. J.; Zaghloul K. A. (2015) Brain computer interface to enhance episodic memory in human participants. Front. Hum. Neurosci. 8, 1055. 10.3389/fnhum.2014.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert S.; Bublitz C.; Jox R.; Friedrich O. (2018) Doing things with thoughts. Philos. Technol. 10.1007/s13347-018-0308-4. [DOI] [Google Scholar]
- Feld G. B.; Diekelmann S. (2015) Sleep smart-optimizing sleep for declarative learning and memory. Front Psychol 6, 622. 10.3389/fpsyg.2015.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. K.; Labban J. D.; Gapin J. I.; Etnier J. L. (2012) The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 1453, 87–101. 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Roig M.; Nordbrandt S.; Geertsen S. S.; Nielsen J. B. (2013) The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci. Biobehav. Rev. 37, 1645–66. 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Hötting K.; Röder B. (2013) Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 37, 2243–57. 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Schlaug G.; Norton A.; Overy K.; Winner E. (2005) Effects of music training on the child’s brain and cognitive development. Ann. N. Y. Acad. Sci. 1060, 219–30. 10.1196/annals.1360.015. [DOI] [PubMed] [Google Scholar]
- Seinfeld S.; Figueroa H.; Ortiz-Gil J.; Sanchez-Vives M. V. (2013) Effects of music learning and piano practice on cognitive function, mood and quality of life in older adults. Front. Psychol. 4, 810. 10.3389/fpsyg.2013.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coubard O. A.; Duretz S.; Lefebvre V.; Lapalus P.; Ferrufino L. (2011) Practice of contemporary dance improves cognitive flexibility in aging. Front. Aging Neurosci. 3, 13. 10.3389/fnagi.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E.; Craik F. I.; Luk G. (2012) Bilingualism: consequences for mind and brain. Trends Cognit. Sci. 16, 240–50. 10.1016/j.tics.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen J. B., and Hunt R. R. (2010) Mnemonology, Psychology Press, New York. [Google Scholar]
- Dresler M.; Shirer W. R.; Konrad B. N.; Müller N. C. J.; Wagner I. C.; Fernández G.; Czisch M.; Greicius M. D. (2017) Mnemonic Training Reshapes Brain Networks to Support Superior Memory. Neuron 93, 1227–1235. 10.1016/j.neuron.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A.; Calati R.; Serretti A. (2011) Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev. 31, 449–64. 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Sedlmeier P.; Eberth J.; Schwarz M.; Zimmermann D.; Haarig F.; Jaeger S.; Kunze S. (2012) The psychological effects of meditation: a meta-analysis. Psychol Bull. 138, 1139–71. 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Green C. S.; Bavelier D. (2012) Learning, attentional control, and action video games. Curr. Biol. 22, R197–R206. 10.1016/j.cub.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S.; Gleich T.; Lorenz R. C.; Lindenberger U.; Gallinat J. (2014) Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol. Psychiatry 19, 265–271. 10.1038/mp.2013.120. [DOI] [PubMed] [Google Scholar]
- Lampit A.; Hallock H.; Valenzuela M. (2014) Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 11, e1001756 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons D. J.; Boot W. R.; Charness N.; Gathercole S. E.; Chabris C. F.; Hambrick D. Z.; Stine-Morrow E. A. (2016) Do ″Brain-Training″ Programs Work?. Psychol Sci. Public Interest 17, 103–186. 10.1177/1529100616661983. [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M.; Redick T. S.; Hulme C. (2016) Working Memory Training Does Not Improve Performance on Measures of Intelligence or Other Measures of ″Far Transfer″: Evidence From a Meta-Analytic Review. Perspect Psychol Sci. 11, 512–34. 10.1177/1745691616635612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanoski B.; Lyons K. M.; Pearce A. A. A.; Owen A. M. (2018) Targeted training: Converging evidence against the transferable benefits of online brain training on cognitive function. Neuropsychologia 117, 541–550. 10.1016/j.neuropsychologia.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Diekelmann S. (2014) Sleep for cognitive enhancement. Front. Syst. Neurosci. 8, 46. 10.3389/fnsys.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosekind M. R.; Smith R. M.; Miller D. L.; Co E. L.; Gregory K. B.; Webbon L. L.; Gander P. H.; Lebacqz J. V. (1995) Alertness management: strategic naps in operational settings. J. Sleep Res. 4 (S2), 62–66. 10.1111/j.1365-2869.1995.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Noack H.; Lövdén M.; Schmiedek F. (2014) On the validity and generality of transfer effects in cognitive training research. Hematol. Cell Ther. 78, 773–789. 10.1007/s00426-014-0564-6. [DOI] [PubMed] [Google Scholar]
- Mehta M. A.; Swainson R.; Ogilvie A. D.; Sahakian J.; Robbins T. W. (2001) Improved short-term spatial memory but impaired reversallearning following the dopamine D(2) agonist bromocriptine in humanvolunteers. Psychopharmacology (Berl) 159, 10–20. 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Lees J.; Michalopoulou P. G.; Lewis S. W.; Preston S.; Bamford C.; Collier T.; Kalpakidou A.; Wykes T.; Emsley R.; Pandina G.; Kapur S.; Drake R. J. (2017) Modafinil and cognitive enhancement in schizophrenia and healthy volunteers: the effects of test battery in a randomised controlled trial. Psychol. Med. 47, 2358–2368. 10.1017/S0033291717000885. [DOI] [PubMed] [Google Scholar]
- Hurlemann R.; Patin A.; Onur O. A.; Cohen M. X.; Baumgartner T.; Metzler S.; Dziobek I.; Gallinat J.; Wagner M.; Maier W.; Kendrick K. M. (2010) Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 30, 4999–5007. 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B.; Leonzino M.; Braida D.; Sala M. (2014) Learning about oxytocin: pharmacologic and behavioral issues. Biol. Psychiatry 76, 360–6. 10.1016/j.biopsych.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Fallon S. J.; van der Schaaf M. E.; Ter Huurne N.; Cools R. (2017) The Neurocognitive Cost of Enhancing Cognition with Methylphenidate: Improved Distractor Resistance but Impaired Updating. J. Cogn Neurosci 29, 652–663. 10.1162/jocn_a_01065. [DOI] [PubMed] [Google Scholar]
- Takeuchi H.; Taki Y.; Sassa Y.; Hashizume H.; Sekiguchi A.; Fukushima A.; Kawashima R. (2011) Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. PLoS One 6, e23175 10.1371/journal.pone.0023175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M. J.; Illes J.; Cook-Deegan R.; Gardner H.; Kandel E.; King P.; Parens E.; Sahakian B.; Wolpe P. R. (2004) Neurocognitive enhancement: what can we do and what should we do?. Nat. Rev. Neurosci. 5, 421–5. 10.1038/nrn1390. [DOI] [PubMed] [Google Scholar]
- Iuculano T.; Cohen Kadosh R. (2013) The mental cost of cognitive enhancement. J. Neurosci. 33, 4482–6. 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem A. K.; Fried P. J.; Horvath J. C.; Robertson E. M.; Pascual-Leone A. (2014) Is neuroenhancement by noninvasive brain stimulation a net zero-sum proposition?. NeuroImage 85, 1058–68. 10.1016/j.neuroimage.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva I.; Boland J.; Farah M. J. (2013) Objective and subjective cognitive enhancing effects of mixed amphetamine salts in healthy people. Neuropharmacology 64, 496–505. 10.1016/j.neuropharm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Finke K.; Dodds C. M.; Bublak P.; Regenthal R.; Baumann F.; Manly T.; Müller U. (2010) Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology (Berl) 210, 317–29. 10.1007/s00213-010-1823-x. [DOI] [PubMed] [Google Scholar]
- Chou H. H.; Talledo J. A.; Lamb S. N.; Thompson W. K.; Swerdlow N. R. (2013) Amphetamine effects on MATRICS Consensus Cognitive Battery performance in healthy adults. Psychopharmacology (Berl) 227, 165–76. 10.1007/s00213-012-2948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott V.; de la Salle S.; Choueiry J.; Impey D.; Smith D.; Smith M.; Beaudry E.; Saghir S.; Ilivitsky V.; Labelle A. (2015) Neurocognitive effects of acute choline supplementation in low, medium and high performer healthy volunteers. Pharmacol., Biochem. Behav. 131, 119–29. 10.1016/j.pbb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Yerkes R. M.; Dodson J. D. (1908) The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology 18, 459–482. 10.1002/cne.920180503. [DOI] [Google Scholar]
- Duffy E. (1957) The psychological significance of the concept of ″arousal″ or ″activation. Psychological Review 64, 265–275. 10.1037/h0048837. [DOI] [PubMed] [Google Scholar]
- de Jongh R.; Bolt I.; Schermer M.; Olivier B. (2008) Botox for the brain: enhancement of cognition, mood and pro-social behavior and blunting of unwanted memories. Neurosci. Biobehav. Rev. 32, 760–76. 10.1016/j.neubiorev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hills T.; Hertwig R. (2011) Why aren’t we smarter already: Evolutionary trade-offs and cognitive enhancements. Current Directions in Psychological Science 20, 373–377. 10.1177/0963721411418300. [DOI] [Google Scholar]
- Wood S.; Sage J. R.; Shuman T.; Anagnostaras S. G. (2014) Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. 66, 193–221. 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaaf M. E.; Fallon S. J.; Ter Huurne N.; Buitelaar J.; Cools R. (2013) Working memory capacity predicts effects of methylphenidate on reversal learning. Neuropsychopharmacology 38, 2011–2018. 10.1038/npp.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock L. A.; McLaughlin A. C.; Allaire J. C. (2012) Individual differences in response to cognitive training: Using a multi-modal, attentionally demanding game-based intervention for older adults. Computers in Human Behavior 28, 1091–1096. 10.1016/j.chb.2012.01.012. [DOI] [Google Scholar]
- Jaeggi S. M.; Buschkuehl M.; Jonides J.; Perrig W. J. (2008) Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. U. S. A. 105 (19), 6829–6833. 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E.; Muller T.; Rossi S.; Sarkar A.; Polizzotto N. R.; Rossi A.; Cohen Kadosh R. (2016) Individual differences and specificity of prefrontal gamma frequency-tACS on fluid intelligence capabilities. Cortex 75, 33–43. 10.1016/j.cortex.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Habich A.; Klöppel S.; Abdulkadir A.; Scheller E.; Nissen C.; Peter J. (2017) Anodal tDCS Enhances Verbal Episodic Memory in Initially Low Performers. Front. Hum. Neurosci. 11, 542. 10.3389/fnhum.2017.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I.; Metzkow-Mészàros M.; Knapp S.; Born J. (2012) Sleep-dependent consolidation of procedural motor memories in children and adults: the pre-sleep level of performance matters. Dev Sci. 15, 506–15. 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- Fenn K. M.; Hambrick D. Z. (2012) Individual differences in working memory capacity predict sleep-dependent memory consolidation (2012). J. Exp. Psychol. Gen. 141, 404–410. 10.1037/a0025268. [DOI] [PubMed] [Google Scholar]
- Fenn K. M.; Hambrick D. Z. (2015) General intelligence predicts memory change across sleep. Psychon Bull. Rev. 22, 791–9. 10.3758/s13423-014-0731-1. [DOI] [PubMed] [Google Scholar]
- Langbaum J. B.; Rebok G. W.; Bandeen-Roche K.; Carlson M. C. (2009) Predicting memory training response patterns: results from ACTIVE. J. Gerontol.. Ser. B 64, 14–23. 10.1093/geronb/gbn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P.; Marcoen A. (1996) On the mechanisms of plasticity in young and older adults after instruction in the method of loci: evidence for an amplification model. Psychol Aging 11, 164–78. 10.1037/0882-7974.11.1.164. [DOI] [PubMed] [Google Scholar]
- Isbell E.; Stevens C.; Pakulak E.; Hampton Wray A.; Bell T. A.; Neville H. J. (2017) Neuroplasticity of selective attention: Research foundations and preliminary evidence for a gene by intervention interaction. Proc. Natl. Acad. Sci. U. S. A. 114, 9247–9254. 10.1073/pnas.1707241114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gvirts H. Z.; Mayseless N.; Segev A.; Lewis D. Y.; Feffer K.; Barnea Y.; Bloch Y.; Shamay-Tsoory S. G. (2017) Novelty-seeking trait predicts the effect of methylphenidate on creativity. J. Psychopharmacol. 31, 599–605. 10.1177/0269881116667703. [DOI] [PubMed] [Google Scholar]
- Genzel L.; Kiefer T.; Renner L.; Wehrle R.; Kluge M.; Grözinger M.; Steiger A.; Dresler M. (2012) Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology 37, 987–98. 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Okagaki L.; Frensch P. A. (1994) Effects of video game playing on measures of spatial performance: Gender effects in late adolescence. J. Appl. Dev Psychol 15, 33–58. 10.1016/0193-3973(94)90005-1. [DOI] [Google Scholar]
- Feng J.; Spence I.; Pratt J. (2007) Playing an action video game reduces gender differences in spatial cognition. Psychol Sci. 18, 850–5. 10.1111/j.1467-9280.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- Genzel L.; Bäurle A.; Potyka A.; Wehrle R.; Adamczyk M.; Friess E.; Steiger A.; Dresler M. (2015) Diminished nap effects on memory consolidation are seen under oral contraceptive use. Neuropsychobiology 70, 253–61. 10.1159/000369022. [DOI] [PubMed] [Google Scholar]
- Dresler M.; Genzel L.; Kluge M.; Schüssler P.; Weber F.; Rosenhagen M.; Steiger A. (2010) Off-line memory consolidation impairments in multiple sclerosis patients receiving high-dose corticosteroid treatment mirror consolidation impairments in depression. Psychoneuroendocrinology 35, 1194–202. 10.1016/j.psyneuen.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Krause B.; Cohen Kadosh R. (2014) Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 8, 25. 10.3389/fnsys.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie L. D.; Gökçen E. (2010) Caffeine enhances working memory for extraverts. Biol. Psychol. 85, 496–498. 10.1016/j.biopsycho.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Pace-Schott E. F.; Spencer R. M. (2014) Sleep-dependent memory consolidation in healthy aging and mild cognitive impairment. Curr. Top. Behav. Neurosci. 25, 307–30. 10.1007/7854_2014_300. [DOI] [PubMed] [Google Scholar]
- Yesavage J. A.; Sheikh J. I.; Friedman L.; Tanke E. (1990) Learning mnemonics: roles of aging and subtle cognitive impairment. Psychol Aging 5, 133–137. 10.1037/0882-7974.5.1.133. [DOI] [PubMed] [Google Scholar]
- Dresler M.; Kluge M.; Genzel L.; Schüssler P.; Steiger A. (2010) Impaired off-line memory consolidation in depression. Eur. Neuropsychopharmacol. 20, 553–61. 10.1016/j.euroneuro.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Dresler M.; Kluge M.; Pawlowski M.; Schüssler P.; Steiger A.; Genzel L. (2011) A double dissociation of memory impairments in major depression. J. Psychiatr. Res. 45, 1593–1599. 10.1016/j.jpsychires.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Genzel L.; Ali E.; Dresler M.; Steiger A.; Tesfaye M. (2011) Sleep-dependent memory consolidation of a new task is inhibited in psychiatric patients. J. Psychiatr. Res. 45, 555–60. 10.1016/j.jpsychires.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Genzel L.; Dresler M.; Cornu M.; Jäger E.; Konrad B.; Adamczyk M.; Friess E.; Steiger A.; Czisch M.; Goya-Maldonado R. (2015) Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol. Psychiatry 77, 177–86. 10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Segretin M. S.; Lipina S. J.; Hermida M. J.; Sheffield T. D.; Nelson J. M.; Espy K. A.; Colombo J. A. (2014) Predictors of cognitive enhancement after training in preschoolers from diverse socioeconomic backgrounds. Front. Psychol. 5, 205. 10.3389/fpsyg.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E.; Macpherson H.; Vitetta L.; Kirk J.; Sali A.; Pipingas A. (2012) Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: a randomised, placebo-controlled trial. Hum. Psychopharmacol. 27, 370–377. 10.1002/hup.2236. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis J. A.; Lutz A.; Schaefer H. S.; Levinson D. B.; Davidson R. J. (2007) Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Natl. Acad. Sci. U. S. A. 104, 11483–8. 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J.; Schambra H. M.; Cohen L. G.; Buch E. R.; Fritsch B.; Zarahn E.; Celnik P. A.; Krakauer J. W. (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. U. S. A. 106, 1590–5. 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R.; Soskic S.; Iuculano T.; Kanai R.; Walsh V. (2010) Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr. Biol. 20, 2016–2020. 10.1016/j.cub.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U.; Hallschmid M.; Rasch B.; Born J. (2006) Brief sleep after learning keeps emotional memories alive for years. Biol. Psychiatry 60, 788–790. 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Dresler M.; Shirer W. R.; Konrad B. N.; Müller N. C. J.; Wagner I. C.; Fernández G.; Czisch M.; Greicius M. D. (2017) Mnemonic Training Reshapes Brain Networks to Support Superior Memory. Neuron 93, 1227. 10.1016/j.neuron.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs J. V.; Ghoneim M. M.; Mewaldt S. P. (1984) Diazepam and memory: retrograde facilitation produced by interference reduction. Psychopharmacology (Berl) 84, 158–162. 10.1007/BF00427439. [DOI] [PubMed] [Google Scholar]
- Mednick S. C.; Cai D. J.; Kanady J.; Drummond S. P. (2008) Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav. Brain Res. 193, 79–86. 10.1016/j.bbr.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. G.; Gränsmark P.; Agricola A.; Schühle K.; Rommel T.; Sebastian A.; Balló H. E.; Gorbulev S.; Gerdes C.; Frank B.; Ruckes C.; Tüscher O.; Lieb K. (2017) Methylphenidate, modafinil, and caffeine for cognitive enhancement in chess: A double-blind, randomised controlled trial. Eur. Neuropsychopharmacol. 27, 248–260. 10.1016/j.euroneuro.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Hammond D. C.; Kirk L. (2007) Negative effects and the need for standards of practice in neurofeedback. Biofeedback 35, 139–145. [Google Scholar]
- Whitmarsh S.; Uddén J.; Barendregt H.; Petersson K. M. (2013) Mindfulness reduces habitual responding based on implicit knowledge: evidence from artificial grammar learning. Conscious Cogn 22, 833–845. 10.1016/j.concog.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Stillman C. M.; Feldman H.; Wambach C. G.; Howard J. H. Jr; Howard D. V. (2014) Dispositional mindfulness is associated with reduced implicit learning. Conscious Cogn 28, 141–150. 10.1016/j.concog.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. M.; Mickes L.; Stolarz-Fantino S.; Evrard M.; Fantino E. (2015) Increased false-memory susceptibility after mindfulness meditation. Psychol. Sci. 26, 1567. 10.1177/0956797615593705. [DOI] [PubMed] [Google Scholar]
- Shiflett M. W.; Rankin A. Z.; Tomaszycki M. L.; DeVoogd T. J. (2004) Cannabinoid inhibition improves memory in food-storing birds, but with a cost. Proc. R. Soc. London, Ser. B 271, 2043–2048. 10.1098/rspb.2004.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A.; Bostrom N. (2006) Converging cognitive enhancements. Ann. N. Y. Acad. Sci. 1093, 201–227. 10.1196/annals.1382.015. [DOI] [PubMed] [Google Scholar]
- Sarewitz D., and Karas T. (2012) Policy implications of technologies for cognitive enhancement. In Neurotechnology: Premises, Potential and Problems (Giordano J., Ed.), pp 267–285, CRC Press, Boca Raton, FL. [Google Scholar]
- Northrop R. B. (2017) Non-Invasive Instrumentation and Measurement in Medical Diagnosis, CRC Press, Boca Raton, FL. [Google Scholar]
- Davis N. J.; van Koningsbruggen M. G. (2013) ″Non-invasive″ brain stimulation is not non-invasive. Front. Syst. Neurosci. 7, 76. 10.3389/fnsys.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y.; Hewitt M.; Paulus W. (2014) Neuroscientists Do Not Use Non-invasive Brain Stimulation on Themselves for Neural Enhancement. Brain Stimul 7, 618–9. 10.1016/j.brs.2014.01.061. [DOI] [PubMed] [Google Scholar]
- Maslen H.; Douglas T.; Cohen Kadosh R.; Levy N.; Savulescu J. (2014) The regulation of cognitive enhancement devices: extending the medical model. Journal of Law and the Biosciences 1, 68–93. 10.1093/jlb/lst003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler A. (2017) The Social Context of ″Do-It-Yourself″ Brain Stimulation: Neurohackers, Biohackers, and Lifehackers. Front. Hum. Neurosci. 11, 224. 10.3389/fnhum.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K. R.; Gao W. J. (2013) Methylphenidate and the juvenile brain: enhancement of attention at the expense of cortical plasticity?. Med. Hypotheses 81, 988–994. 10.1016/j.mehy.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban K. R.; Gao W. J. (2014) Performance enhancement at the cost of potential brain plasticity: neural ramifications of nootropic drugs in the healthy developing brain. Front. Syst. Neurosci. 8, 38. 10.3389/fnsys.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi E. (2013) Exercise addiction. Sports Med. 43, 111–9. 10.1007/s40279-012-0013-x. [DOI] [PubMed] [Google Scholar]
- Yung K.; Eickhoff E.; Davis D. L.; Klam W. P.; Doan A. P. (2015) Internet addiction disorder and problematic use of Google Glass in patient treated at a residential substance abuse treatment program. Addict Behav 41, 58–60. 10.1016/j.addbeh.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Ilieva I. P.; Farah M. J. (2013) Enhancement stimulants: perceived motivational and cognitive advantages. Front. Neurosci. 7, 198. 10.3389/fnins.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva I.; Boland J.; Farah M. J. (2013) Objective and subjective cognitive enhancing effects of mixed amphetamine salts in healthy people. Neuropharmacology 64, 496–505. 10.1016/j.neuropharm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Ullrich S.; de Vries Y. C.; Kühn S.; Repantis D.; Dresler M.; Ohla K. (2015) Feeling smart: Effects of caffeine and glucose on cognition, mood and self-judgment. Physiol. Behav. 151, 629–37. 10.1016/j.physbeh.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Bublitz C. (2016) Drugs, enhancements, and rights. Ten points for lawmakers to consider. In Jotterand/Dubljevic, Cognitive Enhancement: Ethical and Policy Implications in International Perspectives, Oxford University Press, New York. [Google Scholar]
- Farah M. J.; Haimm C.; Sankoorikal G.; Chatterjee A. (2009) When we enhance cognition with Adderall, do we sacrifice creativity? A preliminary study. Psychopharmacology 202, 541–7. 10.1007/s00213-008-1369-3. [DOI] [PubMed] [Google Scholar]
- Nordberg A. (2015) Patentability of methods of human enhancement. J. of Intellectual Property Law & Pract 10, 19–28. 10.1093/jiplp/jpu203. [DOI] [Google Scholar]
- Bergström L. S.; Lynöe N. (2008) Enhancing concentration, mood and memory in healthy individuals: an empirical study of attitudes among general practitioners and the general population. Scandinavian Journal of Public Health 36 (5), 532–537. 10.1177/1403494807087558. [DOI] [PubMed] [Google Scholar]
- Caviola L.; Faber N. S. (2015) Pills or push-ups? Effectiveness and public perception of pharmacological and non-pharmacological cognitive enhancement. Front. Psychol. 6, 1852. 10.3389/fpsyg.2015.01852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviola L.; Mannino A.; Savulescu J.; Faulmüller N. (2014) Cognitive biases can affect moral intuitions about cognitive enhancement. Front. Syst. Neurosci. 8, 195. 10.3389/fnsys.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulmüller N.; Maslen H.; de Sio F. S. (2013) The indirect psychological costs of cognitive enhancement. Am. J. Bioeth 13, 45–7. 10.1080/15265161.2013.794880. [DOI] [PubMed] [Google Scholar]
- Levy N.Cognitive Enhancement: Defending the Parity Principle. In Neuro-Interventions and the Law: Regulating Human Mental Capacity (Vincent N., Ed.), Oxford University Press, New York: (in press). [Google Scholar]
- Bublitz J. C.; Merkel R. (2014) Crimes against minds: on mental manipulations, harms and a human right to mental self-determination. Criminal Law and Philosophy 8, 51–77. 10.1007/s11572-012-9172-y. [DOI] [Google Scholar]
- Focquaert F.; Schermer M. (2015) Moral enhancement: Do means matter morally?. Neuroethics 8, 139–151. 10.1007/s12152-015-9230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macer D. (2012) Ethical consequences of the positive views of enhancement in Asia. Health Care Anal 20, 385–97. 10.1007/s10728-012-0230-3. [DOI] [PubMed] [Google Scholar]
- Singhammer J. (2012) Age and gender specific variations in attitudes to performance enhancing drugs and methods. Sport Science Review 21, 29–48. 10.2478/v10237-012-0017-3. [DOI] [Google Scholar]
- Schelle K. J.; Faulmüller N.; Caviola L.; Hewstone M. (2014) Attitudes toward pharmacological cognitive enhancement-a review. Front. Syst. Neurosci. 8, 53. 10.3389/fnsys.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakoni E.; Schaub M. P.; Maier L. J.; Glauser G. V.; Liechti M. E. (2015) The use of prescription drugs, recreational drugs, and ″soft enhancers″ for cognitive enhancement among Swiss secondary school students. PLoS One 10, e0141289 10.1371/journal.pone.0141289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis J.; Simmons J. P.; Goodwin G. P. (2008) Preferences for psychological enhancements: The reluctance to enhance fundamental traits. Journal of Consumer Research 35, 495–508. 10.1086/588746. [DOI] [Google Scholar]
- Sabini J.; Monterosso J. (2005) Judgments of the fairness of using performance enhancing drugs. Ethics and Behavior 15, 81–94. 10.1207/s15327019eb1501_6. [DOI] [PubMed] [Google Scholar]
- Medaglia J. D.; Yaden D. B.; Helion C.; Haslam M. (2019) Moral attitudes and willingness to enhance and repair cognition with brain stimulation. Brain Stimul. 12, 44. 10.1016/j.brs.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. L.; Strand N. K. (2015) Cognitive enhancement and beyond: recommendations from the Bioethics Commission. Trends Cognit. Sci. 19, 549–551. 10.1016/j.tics.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Pieramico V.; Esposito R.; Cesinaro S.; Frazzini V.; Sensi S. L. (2014) Effects of non-pharmacological or pharmacological interventions on cognition and brain plasticity of aging individuals. Front. Syst. Neurosci. 8, 153. 10.3389/fnsys.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke J.; Partridge B. (2013) Towards a smart population: a public health framework for cognitive enhancement. Neuroethics 6, 419–427. 10.1007/s12152-012-9167-3. [DOI] [Google Scholar]
- Schleim S. (2014) Whose well-being? Common conceptions and misconceptions in the enhancement debate. Front. Syst. Neurosci. 8, 148. 10.3389/fnsys.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J. L.; Roozendaal B. (2009) Drug enhancement of memory consolidation:historical perspective and neurobiological implications. Psychopharmacology 202, 3–14. 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Savulescu J., and Bostrom N. (2009) Human Enhancement, Oxford University Press, Oxford. [Google Scholar]
- Adan A.; Serra-Grabulosa J. M. (2010) Effects of caffeine and glucose, alone and combined, on cognitive performance. Hum. Psychopharmacol. 25, 310–7. 10.1002/hup.1115. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F.; Zhuang Y.; Feng J.; Ying Z.; Fan G. (2011) Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci 33, 383–390. 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. M.; Spiegler K. M.; Sauce B.; Wass C. D.; Sturzoiu T.; Matzel L. D. (2013) Voluntary aerobic exercise increases the cognitive enhancing effects of working memory training. Behav. Brain Res. 256, 626–35. 10.1016/j.bbr.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijamini F.; Pereira S. I.; Cini S. A.; Louzada F. M. (2014) After being challenged by a video game problem, sleep increases the chance to solve it. PLoS One 9, e84342 10.1371/journal.pone.0084342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi C. Y.; Duta M.; Brem A. K.; Huber S.; Nuerk H. C.; Cohen Kadosh R. (2016) Combining brain stimulation and video game to promote long-term transfer of learning and cognitive enhancement. Sci. Rep. 6, 22003. 10.1038/srep22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau D.; Wang C. H.; Tseng P.; Juan C. H. (2015) Blending transcranial direct current stimulations and physical exercise to maximize cognitive improvement. Front. Psychol. 6, 678. 10.3389/fpsyg.2015.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L.; Helgadottir H.; Molle M.; Born J. (2006) Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Antonenko D.; Diekelmann S.; Olsen C.; Born J.; Mölle M. (2013) Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur. J. Neurosci 37, 1142–1151. 10.1111/ejn.12118. [DOI] [PubMed] [Google Scholar]
- Cappelletti M.; Gessaroli E.; Hithersay R.; Mitolo M.; Didino D.; Kanai R.; Cohen Kadosh R.; Walsh V. (2013) Transfer of cognitive training across magnitude dimensions achieved with concurrent brain stimulation of the parietal lobe. J. Neurosci. 33, 14899–14907. 10.1523/JNEUROSCI.1692-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]