Abstract
Identity disturbance is a core feature of borderline personality disorder (BPD). Autobiographical memory is a process of reflective thinking through which we form links between elements of life and self. It can be considered as an indirect index of identity integration. The present study was aimed to investigate the differences in brain activity patterns between BPD patients with identity diffusion and healthy controls using fMRI. We enrolled 24 BPD patients and 24 healthy controls. Identity integration in patients and controls was assessed with the Identity Disturbance Questionnaire (IDQ) score and was significantly different (p = 0.001). We analysed hemodynamic response in the regions of interest during presentation of resolved and unresolved life events. With reference to the condition “resolved”, increased cerebral activity in right anterior cingulate cortex (ACC), right medial prefrontal cortex (MPFC), right dorsolateral prefrontal cortex (DLPFC), and bilateral insula was registered in BPD patients compared with controls. In the condition “unresolved”, increased brain activity was observed in patients in bilateral ACC, bilateral DLPFC, and right temporo-parietal junction. Hyperactivity in ACC and DLPFC in BPD patients with both conditions (resolved and unresolved contexts) may be due to an inefficient attempt to reconstruct a coherent narrative of life events (resolved or not).
Keywords: Neuroscience, Psychiatry
1. Introduction
Borderline personality disorder (BPD) is a severe mental disturbance characterized by a pervasive pattern of instability of interpersonal relationships, affects, identity, and marked impulsivity that begins in early adulthood and is expressed in a variety of contexts (American Psychiatric Association, 2013). One of the most important and relatively understudied psychopathological feature of BPD is identity disturbance or diffusion. Stable and consistent identity implies a meaningful integration of the personal past, present and future in a coherent narration of the individual's life (MacIntyre, 1981; Carr, 1986). In patients with BPD a consistent sense of self and others is lacking and the process of identity formation is impaired (Yeomans et al., 2002; Jørgensen, 2006). The deficit of identity integration reveals itself in the fragmentation of the narrative self, dealing with a shifting view of oneself, with rapidly changing roles and relationships and an underlying feeling of inner emptiness (Fuchs, 2007). Moreover, research supports that individuals with BPD have difficulty with self-other differentiation, defining the psychological boundaries between oneself and another (Beeney et al., 2016) and show poorly differentiated self-referential cognitive and emotional processes also at the neurobiological level (Scherpiet et al., 2015). In the last decades Functional Magnetic Resonance Imaging (fMRI) studies, designed to identify brain areas involved in identity construction and integration, have received a growing interest (Damasio, 1998; Northoff et al., 2006; Lieberman, 2007; D'Argembeau et al., 2012; Wagner et al., 2012). For example, Beeney and colleagues (Beeney et al., 2016) performed an investigation on self-other representations in BPD patients during several social tasks in fMRI. They found that individuals with BPD had scarce temporal consistency in self and other representations and reported poor differentiation between self and others. In particular, BPD patients showed a hyperactivation in the medial prefrontal cortex, temporal parietal junction, several regions of the frontal pole, the precuneus and middle temporal gyrus, thus all crucial areas for social cognition.
However, to our knowledge, only one study investigated the relationship between personality functioning in terms of identity integration and brain structures activity in a sample of BPD patients (Doering et al., 2012). Authors found that a significantly decreased level of deactivation in the anterior and posterior cortical midline structures was able to predict low levels of personality functioning and identity integration.
The concept of personal identity is connected to a recall of autobiographical memories, a process of reflective thinking through which we form links between disparate elements of our life and the self (Habermas and Bluck, 2000; Wilkinson-Ryan and Westen, 2000; Startup et al., 2001; Jørgensen et al., 2012; Bech et al., 2015). In this view, autobiographical memory can be considered an indirect index of the level of identity integration and coherence (McLean and Pratt, 2006; Raffard et al., 2010; Lilgendahl and McAdams, 2011).
The brain network underpinnings of autobiographical memories have been extensively investigated in healthy subjects with neuroimaging studies using tasks consisting of recall of specific life events (Gilboa, 2004; Svoboda et al., 2006; Cabeza and St Jacques, 2007; McDermott et al., 2009; Spreng et al., 2009; Kim, 2012; Martinelli et al., 2013; D'Argembeau et al., 2014). Reviews of studies of autobiographical memory (Gilboa, 2004; Svoboda et al., 2006; Cabeza and St Jacques, 2007; Conway et al., 2003; Maguire, 2001) have shown a crucial role of posterior cingulate cortex (PCC), left medial prefrontal cortex (lMPFC), as well as hippocampus and surrounding regions. Several studies found a relationship between memories of life facts and activity pattern changes in the prefrontal cortex (PFC) (a region in the dorsal extent of the inferior frontal gyrus and a region of the right frontal polar cortex), lateral and medial posterior parietal cortex (PPC) (inferior parietal lobule complex) (McDermott et al., 1999; Shannon and Buckner, 2004; Wagner et al., 2005), temporoparietal junction (TPJ), and cerebellum (Svoboda et al., 2006.; Frith and Frith, 1999; Martin, 2001; O'Neill et al., 2015). With regard to BPD patients, only few studies have been conducted to examine the cerebral areas involved in autobiographical memory recall (Beblo et al., 2006; Schnell et al., 2007; Driessen et al., 2009). In particular, Beblo and colleagues (Beblo et al., 2006) evaluated the differences in brain activity between BPD patients and healthy controls during fMRI sessions. The researchers administered to patients and controls an Autobiographical Interview, from which significant life events (two resolved and two unresolved) were extrapolated. Authors defined unresolved life events as experiences of negative emotional valence still evoking serious emotional reactions. On the contrary, although resolved life event were defined as being also negative experiences, they were subjectively perceived as overcome and not leading to emotional arousal during recall (Beblo et al., 2006). Authors found that comparison of unresolved and resolved life events memories revealed different activations of neural networks in BPD patients compared to healthy subjects. In particular, they registered an increased activity of brain areas associated with the recall of unresolved life events compared to resolved life events in patients with BPD, but not in controls. The fMRI follow-up study performed by the same group aimed to investigate changes of neural activation patterns in response to the recall of unresolved life events compared with resolved life events over one year in the subgroup of BPD patients. Authors observed a substantial decrease in the right, relative to the left hemispheric activation of the brain areas (temporo-frontal neural activation patterns) that may be considered to be involved in the processing of autobiographically relevant, traumatic, or at least highly adverse and anxiety-related stimuli (Driessen et al., 2009). In another study (Schnell et al., 2007), authors evaluated the affective response of BPD patients that were exposed to visual stimuli: pictures from the Thematic Apperception Test (TAT) as a cue of aversive autobiographical memories and neutral pictures as reference condition. The main finding was the lack of differential activations between TAT and neutral stimuli in some brain areas (orbitofrontal, cingulate, and frontal areas) in BPD patients compared with controls. In addition, BPD patients showed increased activity in temporal areas and PCC, which indicates a deficit of selective attention involved in autobiographical memory retrieval and a general tendency towards self-referential information processing.
Although the role of medial PFC in processing self-relevant information and other social stimuli has been extensively studied in healthy subjects (D'Argembeau et al., 2014; Benoit et al., 2010; Araujo et al., 2015), additional studies demonstrated abnormal neural activation in BPD patients in regions that contribute to self-processing, such as dorsolateral prefrontal cortex (DLPFC) (D'Argembeau et al., 2014; Ruocco et al., 2013), a brain area involved in cognitive control across self-referential and non-self-referential processes (Lemogne et al., 2010).
Our study was aimed to investigate the differences in brain functioning between BPD patients and healthy controls during a task of autobiographical memory using fMRI. In particular, we evaluated brain functioning in patients with identity diffusion, a core psychopathological factor of BPD that had not been considered in previous investigations. Based on recent literature data (Beeney et al., 2016; Damasio, 1998; D'Argembeau et al., 2014; O'Neill et al., 2015; Beblo et al., 2006; Schnell et al., 2007; Araujo et al., 2015; Saxe and Wexler, 2005; Minzenberg et al., 2007; Craig, 2009; Singer et al., 2009; O'Neill and Frodl, 2012) about neural correlates involved in patients with BPD during autobiographical memories and self-referential processes we hypothesised that BPD patients with a deficit in identity integration may present a higher brain activation in medial prefrontal cortex, dorsolateral prefrontal cortex, insula, anterior cingulate cortex, posterior cingulate cortex, and temporal parietal junction during the recall of significant life events, both resolved and unresolved, in comparison with controls.
2. Materials & methods
2.1. Materials
The initial sample consisted of 24 consecutive outpatients who received a diagnosis of BPD based on DSM-5 criteria (American Psychiatric Association, 2013) and a group of 24 healthy subjects matched for gender, age, and education (number of years completed at school and university referred by patients and confirmed by school and academic certificates). All participants were aged between 18 and 60 years. Subjects included in the study had all right handed dominance with range for right handedness Laterality Index (LI) 48 ≤ LI < 100 (Oldfield, 1971). Both groups included males and females. Healthy subjects were recruited among general population and were tested with the Structured Clinical Interview for DSM.IV Axis I and II Disorders (First et al. 1997a, 1997b) to control for psychiatric disorders. Neurological diseases were also excluded. All BPD patients of our sample had to meet the criterion identity disturbance as one of the five diagnostic criteria required for the diagnosis. Patients attended the Center for Personality Disorders of the Department of Neuroscience at the University of Turin, Italy. The psychiatric diagnosis was made by an expert clinician and was confirmed with the Structured Clinical Interview for DSM.IV Axis I and II Disorders (First et al. 1997a, 1997b). The following were considered exclusion criteria: diagnoses of dementia, delirium, and other cognitive disorders; neurological diseases; schizophrenia and other psychotic disorders; bipolar disorders; concurrent major depressive episode; post-traumatic stress disorder, and substance abuse in the last two months. All subjects were free from psychiatric/neurological medications since 3 weeks, and from psychotherapy since 3 months. Female patients of childbearing age were excluded if they were not using adequate birth control methods (according to the judgment of clinicians). Each subject voluntarily participated in the study, after providing written informed consent. We observed the Declaration of Helsinki guidelines. Approval was obtained from the ethics committee of the University Hospital “Città della Salute e della Scienza –Ospedale dell’Ordine Mauriziano” of Turin. The trial was registered in the Australian New Zealand Clinical Trials Registry (ANZCTR) and allocated the code: ACTRN 12616000149460. The registration date was 8.02.2016.
2.2. Instrumentation
Both groups (patients and controls) were tested with the Autobiographical Interview (AI) (Witzel, 1985) and Identity Disturbance Questionnaire (IDQ) (Wilkinson-Ryan and Westen, 2000) to evaluate the degree of identity integration.
The Autobiographical Interview (AI) was performed on all subjects a week before fMRI by an expert clinician (psychiatrist) (P.B.). The interview covered the whole lifespan (childhood, youth, early adulthood, adulthood) and included social relationships, significant others, school, and employment. It allowed to obtain 2 unresolved life events and 2 resolved life events. Both, subject and clinicians selected the significant life events. Subject listed for each life event 4 keywords, that were used to trigger active recall during fMRI. Clinician and subject together ensured that the keywords triggered recall of the specific life-event. Moreover, the clinician, at the end of the interview, prepared a brief summary (words' number ranged from 25 to 27) for each event. Four brief summaries and 16 keywords were obtained for each subject from AI in order to present the unresolved versus resolved life events during fMRI. We considered as resolved event a life experience that the subject conceived as totally elaborated and concluded, while we considered as unresolved event a life experience that still produced its effects on the present and that the subject conceived as not completely integrated in his life.
We chose to present to subjects both life event summary and keywords related to the event in order to be sure that subjects during fMRI were actually recalling their specific life event.
In addition, before performing fMRI all subjects identified 4 neutral stories (words' number ranged from 25 to 27), each one of them associated to 4 keywords with a neutral meaning, among a pre-established set of neutral brief stories (21). Particular attention was payed to be sure that neutral stories did not include social content and interpersonal interactions. A preliminary set of neutral stories (44) was previously administered to 130 subjects, who indicated which stories and keywords they considered as neutral. Only stories that were found neutral by > 80% of subjects were used in the task as control conditions (21 stories).
The IDQ (Wilkinson-Ryan and Westen, 2000) is a clinician rated scale including 40 items rated on a 1–5 scale (1 = not true at all; 5 = very true). In the scale a mixture of items that require some inference and items that describe relatively manifest aspects of the subject's life is included. The item set assesses different manifestations of identity disturbance, such as contradictory beliefs and behaviours, changes of values, painful inner incoherence and inconsistency, and confusion over sexual orientation. Wilkinson-Ryan and Westen identified four identity disturbance factors: role absorption (in which patients tend to define themselves in terms of a single role or cause), painful incoherence (a subjective sense of lack of coherence), inconsistency (an objective incoherence in thought, feeling, and behavior), and lack of commitment (e.g., to jobs or values). Each item was associated with one of these four factors. The score of each factor was obtained with the mean of the related items scores. In this study we used the mean score of the four factors (range values:1–5). Higher scores indicated a worse identity integration.
2.3. Methodology
In order to investigate the brain functioning in patients with identity diffusion, we applied a modified version of Beblo et al. (2006) as autobiographical memory recall task (Fig. 2). Specifically, we used an experimental design, in which participants were instructed to recall their life events (resolved and unresolved) and we added the presentation of the summary for each life event to prepare the recall. Before the fMRI session the experimental task was explained in detail to each participant using a computerized training with a set of different events from those utilized during the experimental task. Participants were instructed to approach trials, in order to remember content of each life event, resolved and unresolved. Neutral sentences and neutral keywords were presented to all subjects as control conditions during fMRI. The order of the trial visualization was pseudorandomized using the visual stimuli system E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) trough specific eyeglasses (Philips Resonance Technology, Inc.). The fMRI event related paradigm consisted of 24 trials (8 resolved, 8 unresolved, and 8 neutral) was administered. Each of them consisted of: 1) summary, presentation of the brief summary of the event for 15 s; 2) fixation cross for 5/6 s; 3) keyword for 5 s; 4) fixation cross for 6/7 s; 5) response screen for 4s, in which all participants were asked to identify the emotion they have felt among “positive”, “neutral”, and “negative” by clicking a button with the first three fingers of their right hand. This phase was used to control that the task was performed in a correct manner.
Fig. 2.
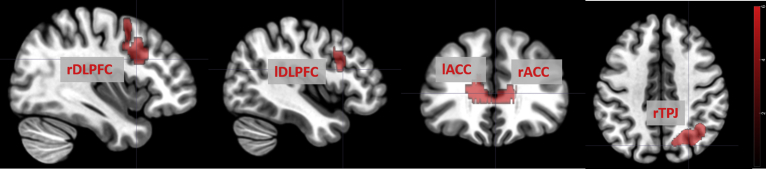
Significant neural activation in group analysis: patient group vs control group for the contrast unresolved keyword condition vs neutral keyword condition. Brain areas activated are labelled. Abbreviations: ACC: anterior cingulate cortex, DLPFC: dorsolateral prefrontal cortex, TPJ: temporal parietal junction, r: right, l: left. The colour bar indicates t-values.
Each trial started with the presentation of the summary for 15s on the screen, followed by a fixation cross for 5–7s, keyword for 6s, a fixation mark for 5–7s, and response screen for 4s.
Images were acquired using a 3.0 T MRI Scanner (Philips Ingenia) with a 32 channel array head coil, equipped with Philips specific eyeglasses (Resonance Tecnology, Inc.). Experimental session were performed at the Center of Brain Imaging 3T-NIT, at the Hospital Città della Salute e della Scienza in Turin, Italy.
The functional images were collected using an Echo-Planar Image sequence (EPI) (TR/TE = 3000/30 ms, 32 slices, matrix size = 92×96, slice gap = 0.5 mm, field of view (FOV) = 224 × 224 mm2, flip angle = 90°, slices aligned on the AC-PC line during functional run, consisting of 415 volumes). The first four volumes of run were discarded to allow the equilibration of T1 saturation effects. In the fMRI runs, structural images of the whole brain were acquired using a T1-weighted sequence (TR 8.1 ms, TI 900 ms, TE 3.7 ms, voxel size 1 × 1 × 1 mm³).
2.4. Analysis
Functional and structural images were analyzed using Statistical Parametric Mapping 8 (SPM8, Wellcome Department of Cognitive Neurology, London, UK) (Friston et al., 2007) implemented in Matlab (Mathworks, Cherborn, MA, USA). All functional images were spatially realigned to the first volume, co-registered to the mean image, and segmented in gray matter, white matter, and cerebrospinal fluid tissues in anatomical scans, normalized to the MNI (Montreal Neurological Institute) space, and smoothed with an 8 mm full-width half-maximum Gaussian Kernel (FWHM), with an additional 6 mm smoothing at the first level. In order to remove low-frequency drifts, high-pass temporal filtering with a cut-off of 128 s was applied. This methodological approach is broadly used in literature, for example in the study published by Fresco et al. (2017).
After pre-processing - for each participant - the General Linear Model (GLM) for statistical analysis was applied, convolving a stick function with a hemodynamic response function (HRF) to regressors of interest. For each of them, at the first level we computed and modelled three regressors: resolved keyword, unresolved keyword, neutral keyword. In addition, six parametric regressors of no interest to the design matrix were included in order to correct residual effects of head motion. All fMRI data underwent rigorous quality control check to exclude motion artefacts (threshold: > 2 mm translation and 2-degree rotation).
At the second level, in order to investigate the neural correlates involved during recall of resolved, unresolved life events compared to neutral conditions, we used SPM8 (Welcome Department of Imaging Neuroscience, London, UK) software. We have performed a full-factorial design with the ‘group’ being the independent between-subject factor and the factor life-event being measured at three levels (resolved keyword, unresolved keyword, neutral keyword). Linear contrasts were calculated for the comparisons of life-event between conditions (resolved keyword, unresolved keyword, neutral keyword): i) resolved keyword condition vs neutral keyword condition, and ii) unresolved keyword condition vs neutral keyword condition. We applied the accepted procedure defined by Poldrack et al. (2008) in line with this we initially looked at ROIs and after a whole-brain data in order to reduce type-I error rates (Bosco et al., 2017). In fact, in the light of our hypothesis on the role of brain networks in the process of autobiographical memories, we defined multiple a priori ROIs, and used small volume corrections in predefined regions. In particular, we used small volume correction with a sphere of 10 mm radius centered on coordinates from previous neuroimaging studies and meta-analyses (Spreng et al., 2009; Kim, 2012) to detect neural correlates involved during resolved and unresolved conditions, in the following regions: left posterior cingulate cortex (lPCC, x = -8, y = -52, z = 4) (Kim, 2012), right posterior cingulate cortex (rPCC x = 8, y = -52, z = 14) (Kim, 2012), left medial prefrontal cortex (lMPFC, x = -38, y = 44, z = 26) (Kim, 2012), right medial prefrontal cortex (rMPFC, x = 36, y = 40, z = 23) (Kim, 2012) right anterior insula (rAI, x = 40, y = 24, z = 6) (Kim, 2012), left anterior insula (lAI, x = -46, y = 20, z = -4) (Spreng et al., 2009), right dorsolateral prefrontal cortex (rDLPFC, x = 23, y = 46, z = 39) (Spreng et al., 2009), right anterior cingulate cortex (rACC, x = -3, y = 47, z = -1 (Spreng et al., 2009), left anterior cingulate cortex (lACC, x = -5, y = 33, z = 22 (Spreng et al., 2009), left dorsolateral prefrontal cortex (lDLPFC, x = -46, y = 24, z = 21 (Spreng et al., 2009), right temporal parietal junction (rTPJ, x = 49, y = -59, z = 27) (Spreng et al., 2009), left temporal parietal junction (lTPJ, x = -47, y = -61, z = 26) (Spreng et al., 2009). All these ROIs were applied for both contrasts: 1. life event resolved condition vs neutral condition; 2. life event unresolved condition vs neutral condition (threshold level was corrected for number of ROIs: Pcorrected = 0.05/12 = 0.004). Results obtained by the SVC-based analyses were considered significant for a threshold of p < 0.004 peak level (FWE-corrected). At the second level, other brain activations by the whole brain without SVC-based analyses were not highlighted. All ROIs Talairach coordinates (Spreng et al., 2009; Kim, 2012) were converted to MNI using http://www.sdmproject.com. Finally, we calculated the correlation of neural activity with IDQ scores for the contrast life event unresolved condition vs life event neutral condition to examine potential relationships between the internal brain activation and external behaviour. Spearman's Rank non-parametric (i.e. Spearman ρ) correlations were computed. In particular, we extracted the first-level contrast estimates from the primary results and correlated these scores with IDQ scores. These parameters were extracted using the toolbox REX (http://web.mit.edu/swg/software.htm).
3. Results
In the group of BPD patients the mean age ± SD was 37.17 ± 13.23, fifteen (62.5%) were females and nine were males. In the group of controls, the mean age ± SD was 36.36 ± 12.85, thirteen (54.16%) were females and eleven were males. T-test and chi-square test did not register differences in age (t = 0.730; p = 0.398), gender distribution (χ2 = 0.343; p = 0.558), level of education (t = 0.983; p = 0.327), and handedness (t = -1.5; p = 0.190) between BPD patients and healthy controls. As expected, BPD patients showed a significantly lower degree of identity integration (higher IDQ score) than controls. IDQ score registered in healthy subjects group was: mean ± SD = 1.34 ± 0.52, while in BPD patients group it was: mean ± SD = 3.04 ± 1.05 (t = 6.83; df = 43; P = 0.001) (Table 1).
Table 1.
Demographic and clinical characteristics of BPD patients and healthy controls. Demographic variables and IDQ score were compared with t-test and chi-square test.
| BPD Mean ± SD |
Healthy controls Mean ± SD |
t/χ2 | P | |
|---|---|---|---|---|
| Demographic variables | ||||
| Age | 37.17 ± 13.23 | 36.36 ± 12.84 | 0.730 | 0.398 |
| Female gender N (%) | 15 (62.5%) | 13 (54.16) | 0.343 | 0.558 |
| Level of education | 13.30 ± 3.19 | 13.00 ± 2.56 | 0.983 | 0.327 |
| Clinical variables | ||||
| IDQ total score | 3.04 ± 1.05 | 1.34 ± 0.52 | 6.83 | 0.001 |
BPD = Borderline Personality Disorder; SD = Standard Deviation; IDQ = Identity Disturbance Questionnaire.
After visual inspection of the anatomical scans three subjects were excluded from further analyses due to neurological abnormalities: 1 patient and 2 controls. The final sample consisted of 23 BPD patients (15 females and 8 males) and 22 healthy controls (14 females and 8 males).
Full factorial within-group analysis was performed.
We report the following results about t-contrasts of keyword analyzed for each group:
- the contrast unresolved vs resolved conditions for group of BPD patients did not show significant differences in brain activity. Instead, for group of healthy subjects we found a significant increased activity in bilateral temporo-parietal junction (rTPJ: x = 42; y = -57; z = 33), (lTPJ: x = - 41; y = -52; z = 35), in right orbitofrontal cortex (rOFC: x = -47; y = 44; z = -10), in right dorsolateral prefrontal cortex (rDLPFC: x = 54; y = 19; z = 17).
- the contrast resolved vs neutral condition for the group BPD patients showed significant increased activity in the right temporoparietal junction (rTPJ: x = 51; y = -45; z = 49) and in the right insula (x = 49; y = 16; z = -4). In healthy participants no significant differences in brain activity were found.
Full factorial between-group analysis, BPD patients versus controls, showed significant differences in brain activations in the following t-contrasts (Table 2): 1) resolved keyword condition vs neutral keyword condition, and 2) unresolved keyword condition vs neutral keyword condition.
Table 2.
Significantly activated brain regions.
| Contrasts of interest | MNI Coordinates |
Z-scores | T values | P values | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| BPD patients vs Healthy subjects | ||||||
| Life event resolved condition vs life event neutral condition | ||||||
| Right prefrontal cortex (rMPFC) | 11 | 51 | 9 | 4.09 | 4.28 | = .001 |
| Right anterior insula (rAI) | 48 | 25 | 4 | 4.10 | 4.32 | <.001 |
| Left anterior insula (lAI) | -43 | 21 | 7 | 4.05 | 4.24 | = .002 |
| Right dorsolateral prefrontal cortex (rDLPFC) | 35 | 28 | 31 | 4.07 | 4.27 | = .001 |
| Right anterior cingulate cortex (rACC) | 7 | 42 | 14 | 4.02 | 4.23 | = .002 |
| Life event unresolved condition vs life event neutral condition | ||||||
| Right anterior cingulate cortex (rACC) | 8 | 39 | 12 | 4.39 | 4.46 | <.001 |
| Left anterior cingulate cortex (lACC) | -10 | 29 | 12 | 3.89 | 3.81 | = .002 |
| Right dorsolateral prefrontal cortex (rDLPFC) | 41 | 17 | 30 | 4.01 | 4.15 | = .003 |
| Left dorsolateral prefrontal cortex (lDLPFC) | -37 | 19 | 26 | 4.47 | 4.67 | <001 |
| Right temporal parietal junction (rTPJ) | 42 | -56 | 34 | 3.96 | 3.86 | = .002 |
Peak activity coordinates are given in MNI space.
Significant neural activation in group analysis: patient group vs control group for the contrasts 1) life event resolved condition vs life event neutral condition, and 2) life event unresolved condition vs life event neutral condition.
All contrasts were analysed using a small volume correction (SVC) with a sphere of 10 mm radius Statistical threshold of p < .004 family-wise error corrected for multiple comparisons at the voxel level over small volumes of interest.
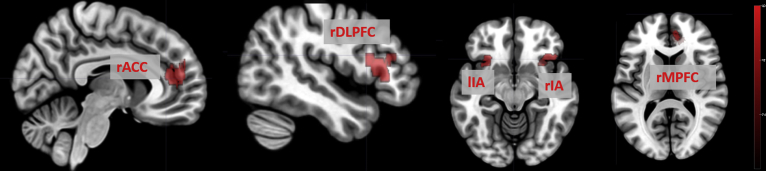
For the contrast resolved keyword condition vs neutral keyword condition, we observed a significantly increased activity of the right medial prefrontal cortex (rMPFC, x = 11, y = 51, z = 9), the bilateral anterior insula (rAI: x = 48; y = 25; z = 4), (lAI: x = -43; y = 21; z = 7) the right dorsolateral prefrontal cortex (rDLPFC: x = 35; y = 28; z = 31), and the right anterior cingulate cortex (rACC: x = 7; y = 42; z = 14) in BPD patients relative to healthy subjects (Fig. 1).
Fig. 1.
Significant neural activation in group analysis: patient group vs control group for the contrast resolved keyword condition vs neutral keyword condition. Brain areas activated are labelled. Abbreviations: ACC: anterior cingulate cortex, AI: anterior insula, DLPFC: dorsolateral prefrontal cortex, MPFC: medial prefrontal cortex, r: right, l: left. The color bar indicates t-values.
For the contrast unresolved keyword condition vs neutral keyword condition, we found a significantly increased activation in the bilateral anterior cingulate cortex (rACC: x = 8; y = 39; z = 12), (lACC: x = -10; y = 29; z = 12), the bilateral dorsolateral prefrontal cortex (rDLPFC: x = 41; y = 17; z = 30), (lDLPFC: x = -37; y = 19; z = 26), and the right temporal parietal junction (rTPJ: x = 42; y = -56; z = 34) in BPD patients compared with healthy controls (Fig. 1). In addition, we calculated the correlation of neural activity with IDQ scores for the contrast life event unresolved condition vs life event neutral condition in each group. In the patient group, we found that response in the rTPJ region was positive correlated with IDQ scores (ρ = 0.44.; p = 0.018). No other brain region showed a significant correlation with IDQ scores. For the control group, IDQ scores did not correlate with activation in any of the ROIs (Fig. 2).
4. Discussion
The present study was aimed to investigate by fMRI the differences of brain areas activation between BPD patients and healthy controls during a task of autobiographical memory (an indirect measure of identity integration) comparing resolved versus unresolved life events.
We hypothesized that BPD patients with identity diffusion, including lack of inner coherence and consistency, would exhibit different cerebral functioning during the recall of crucial life events in comparison with healthy subjects. The level of identity integration in patients and controls was assessed with the IDQ score, which revealed pronounced between-group differences.
Our findings showed significant differences in functioning of specific brain areas between BPD patients and healthy controls. In particular, the significant differences registered between the two groups concerned the recruitment of the following brain regions: insula, anterior cingulate cortex (ACC), medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), and temporo-parietal junction (TPJ).
With reference to the condition resolved, increased cerebral activity in right ACC, right MPFC, right DLPFC, and bilateral insula was registered in BPD patients compared with controls. On the other hand, when considering the condition unresolved increased brain activity was observed in bilateral ACC, bilateral DLPFC, and right TPJ in patients compared with healthy subjects.
Some areas of brain activations were the same in both conditions resolved and unresolved in BPD patients compared with healthy subjects. In particular, a greater activation of the ACC and the DLPFC was registered. Anterior cingulate cortex is a brain region implicated in attentional and emotional processing and is often described as a point of integration for visceral, attentional, and emotional information concerning self-regulation and adaptability (Devinsky et al., 1995; Bush et al., 2000). Dorsolateral prefrontal cortex is typically involved during memory search and controlled retrieval processes to reconstruct past events (Cabeza and St Jacques, 2007). This brain area may mediate the reconstruction of details of past episodes in order to mentally re-experience the situations in their original context (D'Argembeau et al., 2014). Some investigators suggested that ACC and DLPFC may play a central role in the creation of personal narratives, contributing to form a coherent and stable sense of self and identity (Schnell et al., 2007; Lemogne et al., 2010; Brass et al., 2005). Activations of these regions have been interpreted to reflect an excessive self-focus, negative affect, and attempt of cognitive control (Lemogne et al., 2010). Hyperactivity in ACC and DLPFC in BPD patients with both conditions, resolved and unresolved, may be due to an effortful but inefficient attempt to reconstruct a coherent narrative of significant life events, whether resolved or not. Therefore, we can infer that patients with BPD show a tendency to experience most of their life events as poorly integrated, in an emotional and cognitive perspective. Furthermore, an alternative explanation could be that BPD patients have the tendency to over-generalize autobiographical memories. These over-general memories may serve as a protective function in BPD patients by preventing some of the negative affect associated with the memories. This tendency may reflect in the increased brain activity in the DLPFC and ACC.
Medial prefrontal cortex, insula and temporo-parietal junction were found engaged in different test conditions. In particular, MPFC and insula presented a higher level of activity in BPD patients compared with controls during the recall of resolved life events and related keywords, while TPJ showed a more pronounced activation in BPD patients versus controls during the recall of unresolved life events and related keywords.
MPFC, insula and TPJ are brain areas that have received increasing attention for their role in self-referential processing (Beeney et al., 2016; Spreng et al., 2009; Kim, 2012; Martinelli et al., 2013; D'Argembeau et al., 2014; O'Neill et al., 2015; Benoit et al., 2010; Van der Meer et al., 2010).
Medial prefrontal cortex is involved in the processing self-relevant information, in the generation of personal significance (D'Argembeau et al., 2007), and in mentalizing self versus other (Kim, 2012). In BPD patients an abnormal neural activity was found in regions that contribute to self-processing, including MPFC (Beeney et al., 2016; Ruocco et al., 2013). The insula is recognized for its involvement in different aspects of the interoceptive awareness, especially in subjective feelings associated with internal states (Craig, 2009). Insula activation is most likely to occur in tasks with perceptual processes and salient emotional component (D'Argembeau et al., 2007; Ochsner et al., 2005; Gasquoine, 2014; Sterzer and Kleinschmidt, 2010). Reasoning about other people's beliefs or mental states is retained to be linked to TPJ, that is well-known for its association with the theory of mind and abilities of mentalization (D'Argembeau et al., 2007; Ochsner et al., 2005; Pfeifer and Peake, 2012). Greater recruitment of MPFC, insula, and TPJ was observed in the deficit of emotional self-awareness and in the impairment of theory of mind that were described in several mental disorders, such as BPD, schizophrenia, and autism (Pfeifer and Peake, 2012; Sharp et al., 2011; Lombardo et al., 2010). In our findings, bilateral insula, and MPFC were activated when patients faced resolved situations. A possible explanation is that resolved life events in BPD patients cannot be considered actually resolved and integrated in self-narratives. In fact, they retain an excessive emotional charge that produces a hyperactivity of the insula, the brain region responsible for processing of inner emotions (interoceptive awareness). Moreover, hyperactivity of MPFC can be associated with the tendency of these patients to over-interpret and over-attribute self and other mental states and the difficulty in processing self-significant information during emotionally-charged situations (D'Argembeau et al., 2014; Domsalla et al., 2014). TPJ was hyperactive during the recall of unresolved experiences. In order to propose a reason for this finding, we have to consider that unresolved events are characterized by a different and peculiar meaning in BPD patients compared with controls. In fact, this kind of events are strictly related to patients' negative experiences with dysfunctional interpersonal relationships. As problems in interpersonal relationships are commonly associated in BPD with the deficit of theory of mind and abilities of mentalization, the hyper-recruitment of TPJ may be interpreted as a compensatory activity. The role of TPJ in BPD patients with identity disturbance is particularly notable as it is the only brain area that showed a significant correlation with IDQ score in our sample.
It is not easy to compare our findings with those obtained in other studies, due to the important differences in methods and design of experimental tasks on brain functioning and identity disturbance in patients with BPD. To our knowledge, three studies about autobiographical memories in BPD in comparison with controls have been published (Beblo et al., 2006; Schnell et al., 2007; Driessen et al., 2009). In particular, in the two studies performed by Beblo and colleagues (Beblo et al., 2006) and Driessen and colleagues (Driessen et al., 2009) the unresolved experiences used as trigger in fMRI had all a negative/traumatic connotation; the retrieval cues were presented via auditory stimuli; brain activity was evaluated while listening to the keywords in comparison with resting state as reference condition. In addition, patients enrolled presented several psychiatric disturbances in comorbidity (including post-traumatic stress disorder), and medications and psychotherapies were admitted during the studies. As for the study performed by Schnell and colleagues (Schnell et al., 2007), evaluation was focused on affective response of BPD patients to aversive/negative visual stimuli derived from TAT. Nevertheless, some considerations can be made. Our results are in contrast with those from the studies of Beblo and colleagues (Beblo et al., 2006; Driessen et al., 2009), while are more consistent with the findings reported by Schnell and colleagues (Schnell et al., 2007). In the studies by Beblo et al. (Beblo et al., 2006; Driessen et al., 2009), results showed an increased activity of insula, the right orbitofrontal cortex, the right temporal lobe, amigdala, and the cerebellum vermis in BPD patients for the contrast unresolved life events versus resolved life events, but not for the contrast resolved versus neutral events. As already mentioned, methodological differences between the studies of Beblo et al. (2006) and our investigation may be a reason for the diverging findings. In contrast, findings exposed in our study are mainly concordant with those presented by Schell and colleagues (Driessen et al., 2009). They found that BPD patients displayed hyperactivity in the prefronto-cingulate brain areas for both autobiographical and neutral stimuli. This result is in line with our hypothesis that BPD patients show a tendency to experience most of their life events as poorly integrated and not completely resolved.
Some limitations must be considered while interpreting our results. The first limitation is that sample size is rather small. The second limitation is the exclusion of comorbid conditions. This criterion was applied in order to select a group with more homogeneous clinical characteristics and to avoid the effects of co-occurring psychiatric disorders. Given that psychiatric comorbidities are common in BPD, the study sample may present clinical features that are partially different from those typically found in clinical practice, thereby compromising generalisability of our findings. The third limitation consists of the lack of another control group including BPD patients without identity disturbance. The fourth limitation concerns the unstable sense of the self and the uncertain narrative identity of BPD patients, being prone to change of opinion with regard to resolved or unresolved significant life events during the week between administration of Autobiographical Interview and fMRI scans. In order to minimize the effect of this limitation, participants were asked to read the summaries of life events immediately before fMRI scans and to confirm that each event was correctly allocated among resolved or unresolved experiences. Moreover, the level of dissociation was not assessed in our sample with specific evaluation instruments and this variable may have an impact on identity and neural activity. Finally, even though we have corrected fMRI results for the number of ROIs to reduce the chance of a Type-I error, this can represent a limitation in methodological approach.
The innovative contribution of the present study is the aim to evaluate whether BPD patients with identity disturbance differ from healthy subjects in brain functioning during a specifically designed autobiographical task. To our knowledge this is the first study that specifically investigated this issue. As we retain that identity disturbance is a core psychopathological feature in BPD the present study represents a further effort in the comprehension of the brain mechanism that are associated with the core psychopathology of BPD.
Declarations
Author contribution statement
Bozzatello Paola, Morese Rosalba, Valentini Maria Consuelo, Rocca Paola, Bosco Francesca, Bellino Silvio: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This study was supported by Ministero dell'Istruzione, dell'Università e della Ricerca-MIUR projects “Dipartimenti di Eccellenza 2018–2022” to the Department of Neuroscience “Rita Levi Montalcini”.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at theAustralian New Zealand Clinical Trials Registry (ANZCTR) under the registration number ACTRN12616000149460.
Acknowledgements
We would like to thank Dr. Thomas Beblo and colleagues, as our task is an adaptation of the task used in their investigations.
We would like to thank Dr. Marco Bosia for preparing the manuscript according to the journal rules.
We would like to thank Dr. Maria Uscinska for the revision of the English language.
References
- American Psychiatric Association . fifth ed. American Psychiatric Press; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Araujo H.F., Kaplan J., Damasio H., Damasio A. Neural correlates of different self domains. Brain Behav. 2015;21(12):5. doi: 10.1002/brb3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beblo T., Driessen M., Mertens M., Wingenfeld K., Piefke M., Rullkoetter N., Silva-Saavedra A., Mensebach C., Reddemann L., Rau H., Markowitsch H.J., Wulff H., Lange W., Berea C., Ollech I., Woermann F.G. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychol. Med. 2006;36(6):845–856. doi: 10.1017/S0033291706007227. [DOI] [PubMed] [Google Scholar]
- Bech M., Elklit A., Simonsen E. Autobiographical memory in borderline personality disorder-A systematic review. Personal Ment Health. 2015;9(2) doi: 10.1002/pmh.1294. 162–17. [DOI] [PubMed] [Google Scholar]
- Beeney J.R., Hallquist M.N., Ellison W.D., Levy K.N. Self-other disturbance in borderline personality disorder: neural, self-report, and performance-based evidence. Personal Disord. 2016;7(1):28–39. doi: 10.1037/per0000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit R.G., Gilbert S.J., Volle E., Burgess P.W. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50(3):1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Bosco F.M., Parola A., Valentini M.C., Morese R. Neural correlates underlying the comprehension of deceitful and ironic communicative intentions. Cortex. 2017 Sep;94:73–86. doi: 10.1016/j.cortex.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Brass M., Ullsperger M., Knoesche T.R., von Cramon D.Y., Phillips N.A. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J. Cogn. Neurosci. 2005;17(9):1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cognit. Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R., St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cognit. Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Carr D. University of Indiana Press; Bloomington: 1986. Time, Narrative, and History. [Google Scholar]
- Conway M.A., Pleydell-Pearce C.W., Whitecross S.E., Sharpe H. Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia. 2003;41(3):334–340. doi: 10.1016/s0028-3932(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. Investigating the biology of consciousness. Phil. Trans. Biol. Sci. 1998;353:1879–1882. doi: 10.1098/rstb.1998.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A., Ruby P., Collette F., Degueldre C., Balteau E., Luxen A. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J. Cogn. Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A., Cassol H., Phillips C., Balteau E., Salmon E., Van der Linden M. Brains creating stories of selves: the neural basis of autobiographical reasoning. Soc. Cognit. Affect Neurosci. 2014;9(5):646–652. doi: 10.1093/scan/nst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A., Jedidi H., Balteau E., Bahri M., Phillips C., Salmon E. Valuing one's self: medial prefrontal involvement in epistemic and emotive investments in self-views. Cerebr. Cortex. 2012;22(3):659–667. doi: 10.1093/cercor/bhr144. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Doering S., Enzi B., Faber C., Hinrichs J., Bahmer J., Northoff G. Personality functioning and the cortical midline structures--an exploratory FMRI study. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsalla M., Koppe G., Niedtfeld I., Vollstädt-Klein S., Schmahl C., Bohus M., Lis S. Cerebral processing of social rejection in patients with borderline personality disorder. Soc. Cognit. Affect Neurosci. 2014;9(11):1789–1797. doi: 10.1093/scan/nst176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen M., Wingenfeld K., Rullkoetter N., Mensebach C., Woermann F.G., Mertens M., Beblo T. One-year functional magnetic resonance imaging follow-up study of neural activation during the recall of unresolved negative life events in borderline personality disorder. Psychol. Med. 2009;39(3):507–516. doi: 10.1017/S0033291708003358. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L. American Psychiatric Press; Washington (DC): 1997. Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M. American Psychiatric Press; Washington (DC): 1997. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) [Google Scholar]
- Fresco D.M., Roy A.K., Adelsberg S., Seeley S., García-Lesy E., Liston C., Mennin D.S. Distinct functional connectivities predict clinical response with emotion regulation therapy. Front. Hum. Neurosci. 2017;3:11–86. doi: 10.3389/fnhum.2017.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J., Kiebel S.J., Nichols T.E., Penny W.D. Academic Press; 2007. Statistical Parametric Mapping: the Analysis of Functional Brain Images. [Google Scholar]
- Frith U., Frith C.D. Interacting minds—a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Fuchs T. Fragmented selves: temporality and identity in borderline personality disorder. Psychopathology. 2007;40(6):379–387. doi: 10.1159/000106468. [DOI] [PubMed] [Google Scholar]
- Gasquoine P.G. Contributions of the insula to cognition and emotion. Neuropsychol. Rev. 2014;24:77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory--one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42(10):1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Habermas T., Bluck S. Getting a life: the emergence of the life story in adolescence. Psychol. Bull. 2000;126(5):748–769. doi: 10.1037/0033-2909.126.5.748. [DOI] [PubMed] [Google Scholar]
- Jørgensen C.R. Disturbed sense of identity in borderline personality disorder. J. Personal. Disord. 2006;20(6):618–644. doi: 10.1521/pedi.2006.20.6.618. [DOI] [PubMed] [Google Scholar]
- Jørgensen C.R., Berntsen D., Bech M., Kjølbye M., Bennedsen B.E., Ramsgaard S.B. Identity-related autobiographical memories and cultural life scripts in patients with Borderline Personality Disorder. Conscious. Cognit. 2012;21(2):788–798. doi: 10.1016/j.concog.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Kim H. A dual-subsystem model of the brain's default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage. 2012;61(4):966–977. doi: 10.1016/j.neuroimage.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Lemogne C., Mayberg H., Bergouignan L., Volle E., Delaveau P., Lehéricy S., Allilaire J.F., Fossati P. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. J. Affect. Disord. 2010;124(1-2):196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D. Social cognitive neuroscience: a review of core processes. Annu. Rev. Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lilgendahl J.P., McAdams D.P. Constructing stories of self-growth: how individual differences in patterns of autobiographical reasoning relate to well-being in midlife. J Pers. 2011;79(2):391–428. doi: 10.1111/j.1467-6494.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Sadek S.A., Pasco G., Wheelwright S.J., Suckling J. Atypical neural self-representation in autism. Brain. 2010;133(2):611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- MacIntyre A. University of Notre Dame Press; Notre Dame: 1981. After Virtue. [Google Scholar]
- Maguire E.A. Neuroimaging studies of autobiographical event memory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356(1413):1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. Functional neuroimaging of semantic memory. In: Cabeza A. Kingstone., editor. Handbook of Functional Neuroimaging of Cognition. The MIT Press; London, England: 2001. pp. 153–186. [Google Scholar]
- Martinelli P., Sperduti M., Piolino P. Neural substrates of the self-memory system: new insights from a meta-analysis. Hum. Brain Mapp. 2013;34(7):1515–1529. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott K.B., Buckner R.L., Petersen S.E., Kelley W.M., Sanders A.L. Set- and code-specific activation in frontal cortex: an fMRI study of encoding and retrieval of faces and words. J. Cogn. Neurosci. 1999;11(6):631–640. doi: 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- McDermott K.B., Szpunar K.K., Christ S.E. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47(11):2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- McLean K.C., Pratt M.W. Life's little (and big) lessons: identity statuses and meaning-making in the turning point narratives of emerging adults. Dev. Psychol. 2006;42(4):714–722. doi: 10.1037/0012-1649.42.4.714. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Fan J., New A.S., Tang C.Y., Siever L.J. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;15(155(3)):231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;15(31(1)):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- O'Neill A., Frodl T. Brain structure and function in borderline personality disorder. Brain Struct. Funct. 2012;217:767–782. doi: 10.1007/s00429-012-0379-4. [DOI] [PubMed] [Google Scholar]
- Ochsner J.S., Beer E.R., Robertson J.C., Cooper J.D., Gabrieli J.F., Kihsltrom M., D’Esposito K.N. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O'Neill A., D'Souza A., Samson A.C., Carballedo A., Kerskens C., Frodl T. Dysregulation between emotion and theory of mind networks in borderline personality disorder. Psychiatry Res. 2015;231(1):25–32. doi: 10.1016/j.pscychresns.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Peake S.J. Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Dev Cogn Neurosci. 2012;2(1):55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Fletcher P.C., Henson R.N., Worsley K.J., Brett M., Nichols T.E. Guidelines for reporting an fMRI study. Neuroimage. 2008 Apr 1;40(2):409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffard S., D'Argembeau A., Lardi C., Bayard S., Boulenger J.P., Van der Linden M. Narrative identity in schizophrenia. Conscious. Cognit. 2010;19(1):328–340. doi: 10.1016/j.concog.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Amirthavasagam S., Choi-Kain L.W., McMain S.F. Neural correlates of negative emotionality in borderline personality disorder: an activation-likelihood-estimation meta-analysis. Biol. Psychiatry. 2013;15(73(2)):153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Saxe R., Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43(10):1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Scherpiet S., Herwig U., Opialla S., Scheerer H., Habermeyer V., Jäncke L., Brühl A.B. Reduced neural differentiation between self-referential cognitive and emotional processes in women with borderline personality disorder. Psychiatry Res. 2015;30(233(3)):314–323. doi: 10.1016/j.pscychresns.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Schnell K., Dietrich T., Schnitker R., Daumann J., Herpertz S.C. Processing of autobiographical memory retrieval cues in borderline personality disorder. J. Affect. Disord. 2007;97(1–3):253–259. doi: 10.1016/j.jad.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Shannon B.J., Buckner R.L. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004;24(45):10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C., Pane H., Ha C., Venta A., Patel A.B., Sturek J., Fonagy P. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(6):563–573. doi: 10.1016/j.jaac.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cognit. Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Mar R.A., Kim A.S. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Startup M., Heard H., Swales M., Jones B., Williams J.M., Jones R.S. Autobiographical memory and parasuicide in borderline personality disorder. Br. J. Clin. Psychol. 2001;40(2):113–120. doi: 10.1348/014466501163535. [DOI] [PubMed] [Google Scholar]
- Sterzer P., Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct. Funct. 2010;214:611–622. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M.C., Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer M.A., Johnson A., Schmitzer-Torbert N.C., Redish A.D. Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron. 2010;67(1):25–32. doi: 10.1016/j.neuron.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Shannon B.J., Kahn I., Buckner R.L. Parietal lobe contributions to episodic memory retrieval. Trends Cognit. Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wagner D.D., Haxby J.V., Heatherton T.F. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:451–470. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson-Ryan T., Westen D. Identity disturbance in borderline personality disorder: an empirical investigation. Am. J. Psychiatry. 2000;157(4):528–541. doi: 10.1176/appi.ajp.157.4.528. [DOI] [PubMed] [Google Scholar]
- Witzel A. Das problemzentrierte interview. In: Juttemann G., editor. Qualitative Forschung in der Psychologie. Asanger; Weinheim: 1985. pp. 227–255. [Google Scholar]
- Yeomans F., Clarkin J.F., Kernberg O.F. Aronson; New York: 2002. A Primer of Transference Focused Psychotherapy for the Borderline Patient. [Google Scholar]