Abstract
Background:
A single measure that distills complex body mass index (BMI) trajectories into one value could facilitate otherwise complicated analyses. This study creates and assesses the validity of such a measure: average excess BMI.
Methods:
We use data from Waves I-IV of the National Longitudinal Study of Adolescent to Adult Health (n=17 669). We calculate average excess BMI by integrating to find the area above a healthy BMI trajectory and below each subject-specific trajectory and divide this value by total study time. To assess validity and utility, we (1) evaluate relationships between average excess BMI from adolescence to adulthood and adult chronic conditions, (2) compare associations and fit to models using subject-specific BMI trajectory parameter estimates as predictors, and (3) compare associations to models using BMI trajectory parameter estimates as outcomes.
Results:
Average excess BMI from adolescence to adulthood is associated with increased odds of hypertension (OR = 1.56; 95% CI: 1.47, 1.67), hyperlipidemia (OR = 1.36; 95% CI: 1.26, 1.47), and diabetes (OR = 1.57; 95% CI: 1.47, 1.67). The odds associated with average excess BMI are higher than the odds associated with the BMI intercept, linear, or quadratic slope. Correlations between observed and predicted health outcomes are slightly lower for some models using average excess BMI as the focal predictor compared to those using BMI intercept, linear, and quadratic slope. When using trajectory parameters as outcomes, some covariates associate with the intercept, linear, and quadratic slope in contradicting directions.
Conclusions:
This study supports the utility of average excess BMI as an outcome. The higher an individual’s average excess BMI from adolescence to adulthood, the greater their odds of chronic conditions. Future studies investigating longitudinal BMI as an outcome should consider using average excess BMI, whereas studies that conceptualize longitudinal BMI as the predictor should continue using traditional latent growth methods.
INTRODUCTION
Evaluations of body mass index (BMI) over time provide insights into the development of obesity and comorbidities above cross-sectional anthropometrics. Beyond weight at a single point, rapidly growing BMI trajectories (1–3) and more time spent overweight (4, 5) increase risk for disease. Current methods for measuring BMI longitudinally pose challenges when we want to consider the entire trajectory as an outcome, as trajectories are jointly defined by multiple parameters. A single, longitudinal BMI measure would overcome this challenge.
BMI trajectories are commonly assessed via latent growth modeling (LGM). This defines trajectories by multiple parameters: starting BMI (intercept), growth (linear slope), and curvature (quadratic slope). Yet, parameters are separate pieces of information, and analyses using BMI trajectories as an outcome are complicated by having multiple dependent variables instead of one. Healthy BMI trajectories are those that: 1) moderately increase over adolescence (small, positive, linear slope); and 2) taper in adulthood (small, negative, quadratic slope) (6). However, a single factor might associate with trajectory parameters in different directions, making it unclear if the factor provides overall risk or protection. For example, Fuemmeler and colleagues found disengaged parenting was associated with a lower linear BMI slope across adolescence but a higher quadratic slope (less “leveling off’) compared with authoritative parenting (7). This invites the question: what parenting promotes healthier trajectories? We cannot obtain a comprehensive picture when considering trajectory pieces individually, fueling the need to capture trajectories in a single value. The present study develops and assesses the validity of such a measure: average excess BMI.
Average excess BMI is an average deviance above a healthy BMI over a specified period. This is an innovative way of operationalizing trajectories that joins multiple parameters from LGM into one. Average excess BMI is a methodological advancement for evaluating BMI longitudinally, as it: 1) is more parsimonious than considering trajectory parameter estimates independently; and 2) captures the continuous, developmental nature of BMI.
Other methods for operationalizing longitudinal BMI exist, including “obese-years” or “cumulative exposure to excess adiposity,” but with limitations (4, 5). Unlike LGM, these variables assume BMI at a measurement occasion carries forward to the next occasion rather than recognizing changes occur between measurements. This ignores the developmental nature of BMI and becomes problematic as timing between measurements widens.
Another alternative for operationalizing trajectories is growth mixture modeling. This assumes different distributions of parameters govern growth in subgroups, producing finite latent classes with characteristics such as “normal,” “becoming obese,” and “persistently obese” (8). However, with mixture modeling, the researcher cannot discern whether true population heterogeneity is being modeled, and classification is not robust to minor sampling changes. Experts caution this technique can result in obfuscating significant or identifying spurious relationships (9).
Based on the soundness of a measure that joins multiple LGM parameters into one—and its advancements over previous longitudinal BMI measures—we use data from the National Longitudinal Study of Adolescent to Adult Health (Add Health) to evaluate average excess BMI’s utility. To validate the measure as an outcome, we must demonstrate it behaves as we expect when predicting later chronic conditions; i.e., average excess BMI from adolescence to young adulthood should be positively associated with disease in adulthood, similarly to the trajectory intercept, linear, and quadratic slope. Thus, this study contains two aims: 1) Create a single measure for BMI trajectories that captures average excess BMI over time; and 2) Evaluate the validity and utility of this measure relative to traditional LGM metrics.
SUBJECTS AND METHODS
Data Source
This study uses data from Add Health (P01-HD31921), a longitudinal study of a nationally representative sample of 20 745 adolescents in grades 7–12 during 1994–95 (10). We use data from Waves I (ages 13–21), II (13–21), III (18–28), and IV (24–31). Informed consent was obtained in the original study. The University of North Carolina IRB granted exemption from human subjects’ research approval for the present study.
Sample.
For analyses, we restrict the sample to respondents aged 13–31 with valid Wave I sampling weights and BMI measured at Wave II, III, or IV. We drop data for time points when the respondent is pregnant and for respondents who were underweight the entire study, as being underweight poses health risks not examined. This results in 17 669 respondents—over 80% power to detect a small standardized effect (d = 0.02) at a significant level (two-sided p = 0.05).
Measures
Body mass index.
Study staff measured height and weight in Waves II-IV, and we calculate BMI as kilogram (kg)/meter (m)2. The focal predictors in Aim 2 include: 1) average excess BMI; and 2) BMI trajectory parameter estimates (i.e., intercept, linear/quadratic slope). We calculate these variables for each respondent based on individual BMI growth parameter estimates (in Analytic Approach).
Health outcomes.
Health outcomes are based on the joint classification of biomarkers, self-report physician diagnosis, and medication use at Wave IV. Study staff took three measures of blood pressure at 30-second intervals, and the mean of the second and third classified blood pressure. We classify participants as having hypertension if they met the 2017 American Heart Association Task Force hypertension guidelines (11)—systolic blood pressure ≥130 mm HG or diastolic pressure ≥ 80 mm HG—or if they reported a diagnosis of hypertension. Staff obtained whole blood spots via finger prick, analyzed for glucose and HbA1c. We classify participants as having diabetes if their HbA1c ≥ 6.5%, or fasting glucose ≥126 mg/dL, or non-fasting glucose ≥200 mg/dL, or they reported anti-diabetic medication use in the past month or history of diabetes (except during pregnancy). We classify participants as having hyperlipidemia if they reported a history of hyperlipidemia or antihyperlipidemic medication in the past month.
Covariates.
Based on a literature review, Aim 2 covariates include sex (male or female), age at Wave IV (in years), race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic, other), household structure (two biological parents, two parents with at least one non-biological, single parent, other), parent education (< high school, high school, some college, college graduate), parent employment (employed or unemployed), child birth weight (in ounces), if a child was exclusively breastfed for 6+ months, if the respondent was US-born, pubertal status of respondent at Wave I (scale, 1–5), and if a biological parent was obese (12–16).
Analytic Approach
Aim 1: Create average excess BMI.
The first step in calculating average excess BMI is to model BMI over time (by age) and obtain subject-specific trajectory parameter estimates using LGM (Supplemental File 1). With Mplus v.7.31® statistical software, we estimate a linear, quadratic, and piecewise LGM stratified by sex, and we compare Bayesian Information Criteria (BIC) between models to determine best-fit. We estimate models with full information maximum likelihood to accommodate planned outcome missingness imposed by study design and unplanned missingness due to item non-response (17). Analyses adjust estimates to account for clustering and include sampling weights.
We use the best-fitting LGM to generate empirical Bayes (EB) estimates for each respondent. EB estimates are subject-specific estimates of trajectory parameters (18). We use EB estimates to generate each respondent’s model-implied trajectory, which allows us to calculate average excess BMI from adolescence to adulthood. Using MATLAB®, we integrate to find the area above the upper-limit of a healthy BMI trajectory (6) and below each respondent’s model-implied trajectory from 13 to 31 years. Because overweight is associated with various diseases (19–21), we use the CDC overweight thresholds for the upper-limit of a healthy BMI (i.e., sex/age-specific 85th percentile for individuals below 20 years and a BMI of 25 kg/m2 for 20+ years). We refer to the integrated area as excess BMI, and it depends on the magnitude of deviance from a healthy BMI and time spent at an unhealthy BMI. Due to the latter dependence, excess BMI is a function of the length of follow-up. To provide estimates comparable across studies of different durations, we divide excess BMI by total study time (18 years) to generate a measure that equates to the average excess BMI from adolescence to adulthood. We provide code for this integration in Supplemental File 2. Figure 1 illustrates respondents’ model-implied BMI trajectories and excess BMI, representing a no, low, moderate, and high, average excess BMI.
Figure 1. Excess body mass index for four respondents.

Dashed lines indicate sex and age specific healthy body mass index ranges for males as established by the Centers for Disease Control and Prevention, where the top lines indicate the overweight threshold (sex/age-specific 85th percentile for individuals below 20 years and a BMI of 25 kg/m2 for 20+ years) and the bottom lines indicate the underweight threshold (sex/age-specific 5th percentile for individuals below 20 years and a BMI of 18 kg/m2 for 20+ years). Solid lines indicate respondents’ model-implied body mass index trajectories. Diagonal lines represent each respondent’s excess BMI including no (a), low (b), moderate (c), and high (d), excess BMI.
Aim 2: Validity and utility of average excess BMI.
Aim 2 assesses the relationship between BMI trajectories and health outcomes to validate average excess BMI. We conduct 50 imputations to impute values for all health outcomes and covariates using multiple imputation with chained equations (22). We use Stata 14.2® MI and SVY command suite to create and analyze imputed survey data.
In our first logistic regressions, we evaluate the relationship between average excess BMI from adolescence to adulthood and three chronic conditions in adulthood (hypertension, hyperlipidemia, and diabetes), controlling for covariates listed above.
| (Model Set 1) |
To evaluate the predictive validity of average excess BMI relative to traditional BMI trajectory parameter estimates, we conduct a second set of regressions predicting chronic conditions from EB estimates of trajectory parameters (i.e., intercept, linear, and quadratic slope), controlling for covariates.
| (Model Set 2) |
To aid model comparisons, we standardize all focal predictors to a scale with a mean of zero and standard deviation of one. To assess model fit, we obtain estimates of correlations between observed and predicted health outcomes.
To evaluate the utility of using average excess BMI as an outcome, we compare associations between models using BMI trajectory parameter estimates as outcomes. We find evidence for the utility of a consolidated trajectory measure if the same covariate associates with trajectory parameter estimates in contradicting directions.
| (Model Set 3) |
Code availability.
Annotated code is available for generating average excess BMI. This includes Mplus® code for estimating a LGM and obtaining EB estimates (Supplemental File 1), and MatLab® code to calculate average excess BMI (Supplemental File 2).
RESULTS
The final sample is 17 669. Of the original 20 744 respondents, 117 were not in the desired age range, 1 826 did not have information on sampling weights, 1 133 had no BMI measurements, 27 were underweight at all waves, and 2 had no information on sex. Table 1 provides descriptive statistics.
Table 1.
Descriptive statistics for respondents from the National Longitudinal Study of Adolescent to Adult Health included in the analytic sample.
| Variable | Mean (SE) or % |
|---|---|
| Average excess BMI | 2.8 (0.077) |
| Body mass index (kg/m2) | |
| Wave II | 23.2 (0.105) |
| Wave III | 26.5 (0.113) |
| Wave IV | 28.9 (0.130) |
| Biological sex | |
| Male | 51% |
| Female | 49% |
| Parental obesity | |
| Obese | 24% |
| Not obese | 76% |
| Parent education | |
| < High school | 12% |
| High school | 28% |
| Some college | 30% |
| ≥ College | 30% |
| Parent employment | |
| Employed | 70% |
| Unemployed | 30% |
| Race | |
| White | 66% |
| Black | 16% |
| Hispanic | 12% |
| Other | 6.7% |
| Breastfed as infant, exclusive for 6 months | |
| Breastfed | 20% |
| Not breastfed | 80% |
| Birthweight, in ounces | 118.8 (0.331) |
| Family structure | |
| Two biological parent | 55% |
| Two parents, at least one non-biological | 17% |
| Single parent | 24% |
| Other | 4.6% |
| US born | |
| US born | 94% |
| Not US born | 6.1% |
| Pubertal status | 3.2 (0.016) |
| N | 17,669 |
Estimates based on analytic sample of 17,669 respondents across 50 multiply imputed datasets. All estimates account for survey clustering and weighting.
Aim 1: Average excess BMI.
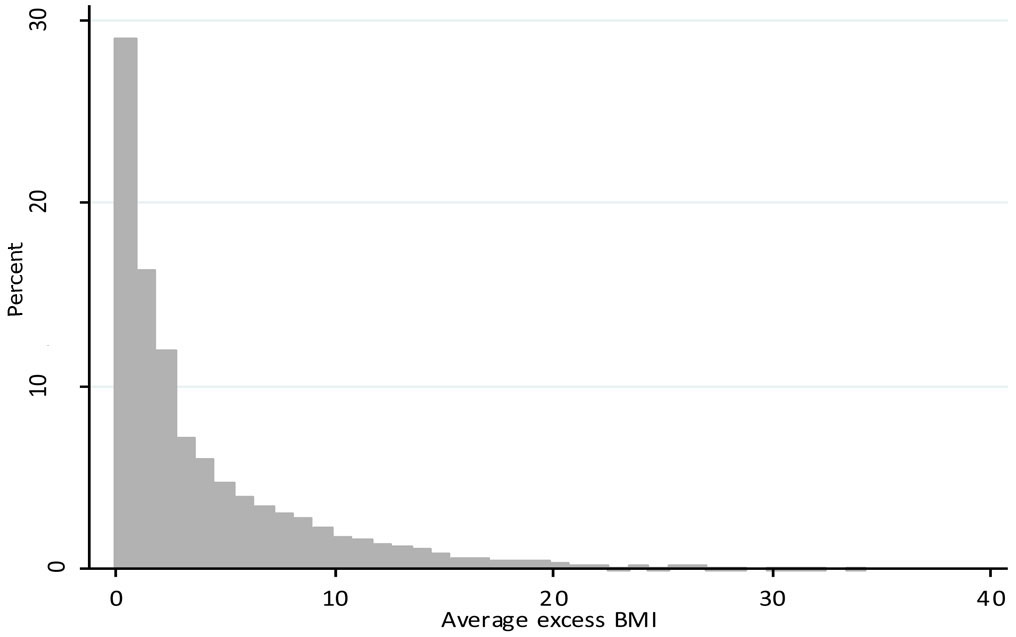
The quadratic LGM yielded superior fit (male BIC = 113 085; female BIC = 126 999) compared to the piecewise (male BIC = 113 248; female BIC = 127 095) and linear LGM (male BIC = 113 414 female BIC = 127 279). Therefore, we used the quadratic LGM to obtain individual model-implied BMI trajectories. The mean average excess BMI was 2.79 kg/m2 (s.d. = 4.23) (Figure 2).
Figure 2.
Histogram of average excess BMI.
Aim 2: Validity and utility of average excess BMI.
Average excess BMI from adolescence to adulthood is associated with increased odds of hypertension, hyperlipidemia, and diabetes in adulthood, and the odds associated with average excess BMI are higher than the odds associated with traditional BMI trajectory parameter estimates (Table 2). For every one unit standard deviation increase in average excess BMI, the odds of hypertension increase by a factor of 1.56 (95% CI: 1.47, 1.67), the odds of hyperlipidemia increase by a factor of 1.36 (95% CI: 1.26, 1.47), and the odds of diabetes increase by a factor of 1.57 (95% CI: 1.47, 1.67).
Table 2.
Odds ratios and corresponding 95% confidence intervals for associations between adolescent to adult body mass index trajectory variables and adult chronic conditions.
| Focal predictor | Model 1 | Model 2 | |
|---|---|---|---|
| Hypertension |
Average excess BMI | 1.56 (1.46, 1.67) |
-- |
| Intercept | -- | 1.23 (1.05, 1.43) |
|
| Linear slope | -- | 1.30 (1.08, 1.56) |
|
| Quadratic slope | -- | 1.12 (1.03, 1.22) |
|
| Correlation between observed and predicted |
0.34 (p < 0.01) |
0.36 (p < 0.01) |
|
| Hyperlipidemia |
Average excess BMI | 1.36 (1.26, 1.47) |
-- |
| Intercept | -- | 1.28 (1.08, 1.51) |
|
| Linear slope | -- | 1.10 (0.92, 1.33) |
|
| Quadratic slope | -- | 1.11 (1.01, 1.21) |
|
| Correlation between observed and predicted |
0.11 (p < 0.01) |
0.11 (p < 0.01) |
|
| Diabetes |
Average excess BMI | 1.57 (1.47, 1.67) |
-- |
| Intercept | -- | 1.31 (1.08, 1.57) |
|
| Linear slope | -- | 1.31 (1.05, 1.63) |
|
| Quadratic slope | -- | 1.01 (0.91, 1.13) |
|
| Correlation between observed and predicted |
0.22 (p < 0.01) |
0.22 (p < 0.01) |
|
Estimates based on analytic sample of 17,669 respondents from 50 multiply imputed datasets. All focal predictors are standardized. Models 1 and 2 control for biological sex, racial identity, parent obesity status, parent employment, birthweight, family structure, whether breastfed for 6 months, parent education, age at Wave IV, pubertal status at Wave I, and whether the respondents was born in the U.S. All estimates account for survey clustering and weighting.
According to Model Set 2, the model whose focal predictors were BMI trajectory intercept, linear, and quadratic slope estimates had an insignificantly higher correlation between observed and predicted hypertension compared to the model whose predictor was average excess BMI (r = 0.36, p < 0.01 compared to r = 0.34, p < 0.01). Correlations between observed and predicted diabetes (r = 0.22; p < 0.01) and hyperlipidemia (r = 0.11; p < 0.01) were equivalent between models.
Per Model Set 3, being male associated with the trajectory parameter estimates in contradicting directions, whereby being male was associated with a lower BMI intercept (B = −0.14; p = 0.12), a higher linear slope (B = 0.022; p < 0.01), and greater leveling off into adulthood (B = −0.001; p < 0.01). Similar contradicting patterns existed among those with two biological parents and having a parent with a high school education—these later estimates did not achieve statistical significance.
DISCUSSION
The present study supports the utility of average excess BMI. The higher an individual’s average excess BMI from adolescence to adulthood, the greater the odds of obesity-related conditions in adulthood. Because this measure performs similarly well to the combination of BMI intercept, linear, and quadratic slope when predicting poor health, it is a valid longitudinal BMI outcome whose parsimony can simplify complex analyses.
Because average excess BMI condenses trajectories into a single value, it is useful for questions that hold overall BMI trajectories as the outcome. By joining three trajectory parameters into one measure, we can more easily explore what explains or buffers relationships between risk factors and longitudinal BMI, rather than assessing what mediators or moderators exist for each BMI trajectory parameter. This parsimonious analysis facilitates identifying intervention targets for improving longitudinal BMI, especially when potential targets associate with trajectory parameters in conflicting directions as in the present analyses.
Beyond intervention research, this measure could be translated to medical practice by providing patients with an interpretable BMI trajectory measure. While a person’s linear BMI slope is unintuitive, their average excess BMI is how overweight they have been over a period of time. The reference period could be changed to the last year, ten years, and lifetime, and these numbers could be another tool for physicians to discuss weight with patients in a way that captures both the degree and duration of overweight.
An additional advantage of average excess BMI compared to individual LGM parameters is its applicability across developmental stages. High values of linear slope are less of a health risk when such growth is considered healthy—e.g., early childhood—compared to periods when BMI is expected to remain constant—e.g., adulthood (6). However, average excess BMI is meaningful across the life course, as it captures deviations above a sex- and age-specific healthy range, and thus inherently accounts for appropriate increases in BMI. Because average excess BMI represents an individual’s expected excess BMI at any moment in time, this measure accommodates different time durations and is comparable across studies of different lengths. Moreover, this approach may be useful for measuring other outcomes over time, such as cumulative stressors or psychological symptoms.
Although the focus of this paper is to validate average excess BMI as an outcome, traditional LGM trajectory parameters are more useful as predictors. Because the intercept, linear, and quadratic slope together explain more variance in an outcome than average excess BMI alone, there is reason to include all trajectory parameters in predictive models. Another limitation of average excess BMI is the potential for non-equivalent trajectories to produce equivalent values of average excess BMI. For example, an individual who had excess weight in childhood with a healthy range in adulthood could have the same average excess BMI as one who was in a healthy range in childhood with excess weight in adulthood. Future studies could differentially weight periods to investigate if excess BMI is more problematic during specific life stages. However, the present data did not include early childhood, and future work should consider broader age ranges to better investigate how this measure operates during different periods.
This study supports the utility and validity of average excess BMI. Future studies should test this measure against other consolidated BMI trajectory measures, and research investigating mediators or moderators of the relationship between exposures and longitudinal BMI should consider using average excess BMI as an outcome.
Supplementary Material
ACKNOWLEDGEMENTS
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain Add Health data files is available on the website: http://www.cpc.unc.edu/addhealth. No direct support was received from grant P01-HD31921 for this analysis.
Funding source: This work was supported by the National Institute of Child Health and Human Development (T32-HD07376), and by the National Institute on Drug Abuse of the National Institutes of Health (K01 DA035153). The funding sources had no involvement in the study design, data collection, analysis, interpretation, or writing of the report.
Benjamin Sokol provided MATLAB® coding support, with the application of the user-contributed M Files, hatchfill2 © 2016 Takeshi Ikuma, and legendflex © 2015 Kelly Kearney.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Supplementary information is available at the International Journal of Obesity’s website.
REFERENCES
- 1.Boyer BP, Nelson JA, Holub SC. Childhood body mass Index trajectories predicting cardiovascular risk in adolescence. Journal of Adolescent Health 2015;56(6):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attard SM, Herring AH, Howard AG, Gordon-Larsen P. Longitudinal trajectories of BMI and cardiovascular disease risk: the national longitudinal study of adolescent health. Obesity 2013;21(11):2180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding M, Hu Y, Schwartz J, Koh WP, Yuan JM, Sesso HD, et al. Delineation of body mass index trajectory predicting lowest risk of mortality in U.S. men using generalized additive mixed model. Ann Epidemiol 2016;26(10):698–703.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. Jama 2013;310(3):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol 2012;176(2):99–107. [DOI] [PubMed] [Google Scholar]
- 6.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey 2002(246):1–190. [PubMed] [Google Scholar]
- 7.Fuemmeler BF, Yang C, Costanzo P, Hoyle RH, Siegler IC, Williams RB, et al. Parenting styles and body mass index trajectories from adolescence to adulthood. Health Psychology 2012;31(4):441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leiby BE. Growth curve mixture models. Shanghai Archives of Psychiatry 2012;24(6):355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: implications for overextraction of latent trajectory classes. Psychol Methods 2003;8(3):338–63. [DOI] [PubMed] [Google Scholar]
- 10.Harris K, Halpern C, Whitsel E, Hussey J, Tabor J, Entzel P, et al. The National Longitudinal Study of Adolescent to Adult Health: Research Desig. 2009. URL: http://wwwcpcuncedu/projects/addhealth/design. Accessed 2016;5. [Google Scholar]
- 11.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex: : 1979). 2017. [Google Scholar]
- 12.Anderson PM, Butcher KF, Levine PB. Maternal employment and overweight children. Journal of health economics 2003;22(3):477–504. [DOI] [PubMed] [Google Scholar]
- 13.Harris KM, Perreira KM, Lee D. Obesity in the transition to adulthood: predictions across race/ethnicity, immigrant generation, and sex. Archives of pediatrics & adolescent medicine 2009;163(11):1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons TJ, Power C, Logan S, Summerbelt C. Childhood predictors of adult obesity: a systematic review. International journal of obesity 1999;23. [PubMed]
- 15.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 2005;115(5):1367–77. [DOI] [PubMed] [Google Scholar]
- 16.De Leonibus C, Marcovecchio ML, Chiavaroli V, de Giorgis T, Chiarelli F, Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatr Obes 2014;9(4):292–9. [DOI] [PubMed] [Google Scholar]
- 17.Graham JW. Missing data analysis: Making it work in the real world. Annual review of psychology 2009;60:549–76. [DOI] [PubMed] [Google Scholar]
- 18.Muthén BO. Beyond SEM: General latent variable modeling. Behaviormetrika 2002;29(1):81–117. [Google Scholar]
- 19.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New England Journal of Medicine 2003;348(17):1625–38. [DOI] [PubMed] [Google Scholar]
- 20.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Jama 1999;282(16):1523–9. [DOI] [PubMed] [Google Scholar]
- 21.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The lancet 2014;384(9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science 2007;8(3):206–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.