Abstract
Prenatal substance exposure is a growing public health concern worldwide. Although the opioid crisis remains one of the most prevalent addiction problems in our society, abuse of cocaine, methamphetamines, and other illicit drugs, particularly amongst pregnant women, are nonetheless significant and widespread. Evidence demonstrates prenatal drug exposure can affect fetal brain development and thus can have long-lasting impact on neurobehavioral and cognitive performance later in life. In this review, we highlight research examining the most prevalent drugs of abuse and their effects on brain development with a focus on endoplasmic reticulum stress and oxidative stress signaling pathways. A thorough exploration of drug-induced cellular stress mechanisms during prenatal brain development may provide insight into therapeutic interventions to combat effects of prenatal drug exposure.
1. Introduction
A large number of scientific studies have indicated prenatal substance use poses harmful health risks for the developing fetus. For example, abuse of cocaine during pregnancy exposes approximately 45,000 infants per year to cocaine in the United States alone, resulting in a wide range of neurodevelopmental impairments (Chasnoff et al., 1998). Confounding variables like prenatal health, frequency and intensity of cocaine use, and polydrug usage have led to contradicting reports regarding the degree and/or presence of these impairments (Ackerman et al., 2010; Frank et al., 1998; Thompson et al., 2009) yet studies that have corrected for these variables confirm that high levels of in utero cocaine exposure consistently lead to neurological and neurobehavioral defects (Bandstra et al., 2004; Chiriboga et al., 1999, 2007; Delaney-Black et al., 1996; Mirochnick et al., 1995; Morrow et al., 2004; Tronick et al., 1996). Methamphetamine (METH) usage amongst pregnant women admitted into federally-funded substance treatment facilities in the United States has increased from 8 to 24%, based on the Treatment Episode Dataset from 1994 to 2006 (Terplan et al., 2009). Notably, more than 40% of pregnant METH users reported consistent METH use throughout all three trimesters (Della Grotta et al., 2010). Similarly, tobacco smoking during pregnancy continues to be an important public health concern as more than half of women who are regular smokers continue to smoke throughout their pregnancies (Ebrahim et al., 2000). Approximately 10% of pregnant women in the United States smoke according to the Pregnancy Risk Assessment and Monitoring System (PRAMS) data, and results in more than half a million infants exposed to maternal smoking each year (Tong et al., 2013).
The opioid epidemic is a continuing crisis in the United States; since 2014, opioid abuse has led seven states (Massachusetts, Virginia, Alaska, Arizona, Florida, Maryland, and Pennsylvania) to declare some form of public health emergency over the opioid crisis. This epidemic first emerged in the United States in the 1970s when neonatal abstinence syndrome (NAS) was first identified. NAS, also referred to as neonatal opioid withdrawal syndrome (NOWs), is characterized by withdrawal symptoms in newborns prenatally exposed to opioids such as heroin or methadone and these symptoms were identified in more than half of prenatally-exposed newborns. Common symptoms of NAS include irritability, sleep disturbances, high-pitched crying, tremors, feeding problems, projectile vomiting, diarrhea, sweating, and seizures. (Chasnoff and Gardner, 2015; Finnegan et al., 1975; McQueen and Murphy-Oikonen, 2016).
The endoplasmic reticulum (ER), important for processing and folding of newly synthesized proteins, can trigger the ER stress response in the presence of various pathophysiological insults, including hypoxia, redox imbalance, and a variety of drugs and chemicals (Chen et al., 2014; Harding et al., 2003; Kitamura, 2013; Walter and Ron, 2011). Disruption in ER protein-folding homeostasis induces ER stress which subsequently activates a set of signaling pathways termed the unfolded protein response (UPR), made up of the three signaling branches IRE1, PERK, and ATF6. UPR aims to promote cell survival by reducing protein misfolding and re-establishing protein folding function in the ER via upregulation of molecular chaperones and antioxidant proteins (Bravo et al., 2013; Walter and Ron, 2011). UPR can also promote apoptosis following prolonged and unresolved ER stress (Lin et al., 2007). When ER stress in neural tissue is initiated at high intensity or for prolonged periods, it can lead to aberrant neuronal differentiation and impaired dendritic outgrowth (Kawada et al., 2014; Li et al., 2013). Oxidative stress is another hallmark of cellular stress and is defined as an excess of reactive oxygen species (ROS) relative to antioxidant defenses (Betteridge, 2000). Research suggests that oxidative and ER stress are closely linked, given that protein folding in the ER requires a tightly controlled redox environment and excess ROS generation can severely affect ER homeostasis, either directly or indirectly (reviewed in Mahotra and Kaufman, 2007). Crosstalk between ER stress and oxidative stress is commonly seen in neurological disorders. For example, cocaine and METH can induce neurotoxicity via ER stress and oxidative stress pathways as revealed by studies in which both acute and chronic cocaine and/or METH exposure generated reactive oxygen species and altered CNS activities in various brain subregions (Bashkatova et al., 2005; Dietrich et al., 2005; Macedo et al., 2005; Pubill et al., 2005; Yamamoto and Zhu, 1998). Similar results of imbalanced ROS and dysregulated ER stress were observed in studies examining neurotoxicity of opioids and nicotine (Cai et al., 2016; Sil et al., 2018; Wong et al., 2016). Therefore, induction of ER and oxidative stress as a result of in utero substance exposure can be detrimental to brain development and impair neurological functions of affected infants. Despite extensive evidence from in vitro studies that suggest pivotal roles of ER and oxidative stress in the onset of neurodevelopmental impairments as a result of in utero exposure to various drugs of abuse, results from animal models and human studies have not conclusively defined mechanisms that underlie these impairments. The degree of involvement of cellular stress, including UPR, ER stress, and oxidative stress, in substance-mediated neurodevelopmental deficits remain largely unknown but has gathered great interest in the drug addiction field. In this review, we summarize in vitro, animal and human studies describing various drugs of abuse that are attributed to neurodevelopmental impairments, and also discuss underlying molecular and cellular mechanisms, particularly drug-induced ER and oxidative stress responses, that may potentially affect brain development of infants prenatally exposed to drugs of abuse (Fig. 1).
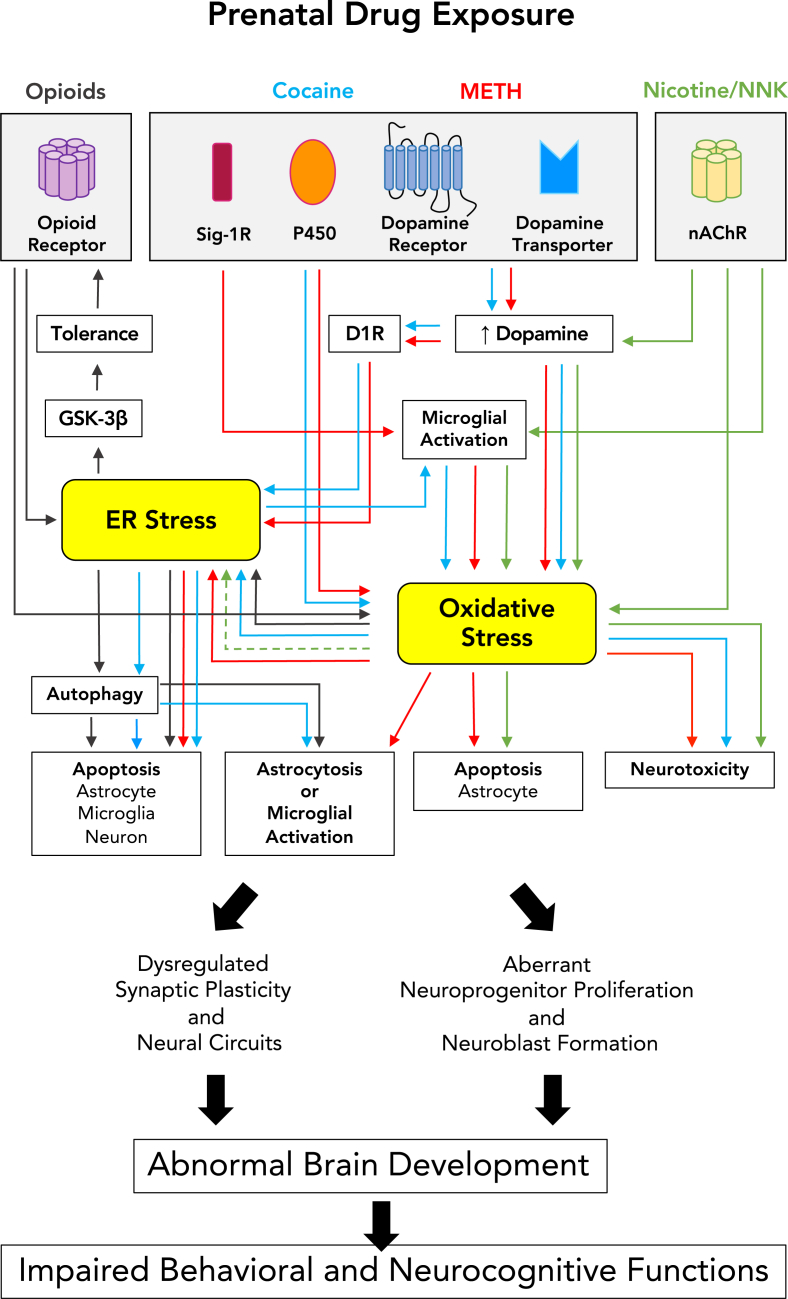
Fig. 1.
Proposed cellular stress mechanisms by which prenatal drug exposure can adversely affect brain development and neurobehavioral outcomes. Psychostimulants (cocaine and METH) can elevate dopamine levels and subsequently trigger ER and oxidative stress signaling pathways or, alternatively, cocaine and METH can activate Sig-1R or cytochrome P450 and trigger ER and oxidative stress in that manner. Similarly, nicotine/NNK can induce oxidative ER stress via regulations of dopamine releases and microglial activities. Opioids can cause apoptosis and astrocytosis through ER stress-autophagy axis pathway. Cellular responses to prenatal drug exposure, including cytotoxicity, astrocytosis, microglial activation, altered neurogenesis, and synaptic plasticity, can ultimately lead to behavioral consequences of abnormal brain development. Drug-mediated pathways are depicted in designated colors. Solid arrows indicate the pathway confirmed with in vivo or in vitro neural models whereas dashed arrows indicate studies conducted in non-neural systems. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2. Psychostimulants
2.1. Cocaine
2.1.1. Effects of prenatal cocaine exposure on brain development and neurobehavioral performance
Previous studies have indicated cocaine impacts neurobehavioral development via impaired human brain growth in utero (Behnke et al., 2006; Rando et al., 2013; Salzwedel et al., 2016; Singer et al., 2008, 2015). MRI studies have shown that prenatal cocaine exposure causes brain structural and functional abnormalities, particularly in neocortex, caudate nucleus, thalamus, cerebellum, and corpus callosum (Avants et al., 2007; Bellini et al., 2000; Dow-Edwards et al., 2006; Grewen et al., 2014; Liu et al., 2013; Salzwedel et al., 2016; Zakiniaeiz et al., 2017). Notably, cocaine-induced neocortical developmental defects were shown to impair cognition, intellectual ability and functional connectivity (Alessandri et al., 1998; Salzwedel et al., 2015; Singer et al., 2002, 2008, 2015), language development (Bandstra et al., 2004; Lewis et al., 2004; Morrow et al., 2004), and motor development (Arendt et al., 1999; Miller-Loncar et al., 2005; Thyssen Van Beveren et al., 2000). Likewise, morphological changes in the caudate nucleus, particularly those affecting its dopaminergic neuronal input, may lead to attention deficits in later years (Gabriel and Taylor, 1998; Heffelfinger et al., 1997; Karmel and Gardner, 1996). Finally, interruption of thalamic development was thought to cause learning disabilities in children prenatally exposed to cocaine (Gkioka et al., 2016; Salzwedel et al., 2016). Overall, these neuroimaging and neurobehavioral studies indicate a substantial link between in utero cocaine exposure and long-term central nervous system (CNS) morphological changes and their associated behavioral outcomes. Importantly, Richardson et al. (2013) and Rando et al. (2013) have linked prenatal cocaine abuse with increased susceptibility for substance abuse in adolescence though mechanisms remain unknown. The continued study of cocaine-induced neural impairments is therefore crucial to better understand neurodevelopmental deficits and addictive behavior resulting from in utero cocaine exposure.
2.1.2. Cocaine-induced ER and oxidative stress in the developing brain
Cocaine exerts its effects on prenatal brain development via distinct pathophysiological pathways, one of which is cocaine's direct pharmacological effect on CNS development (Benveniste et al., 2010). Monoamine transmitter (dopamine, serotonin, and noradrenaline) receptors appear during early stages of mammalian CNS development (Levitt et al., 1997), around the time monoamine axons reach the caudate nucleus and cerebral wall (Levitt and Moore, 1979; Lidov et al., 1980; Lidov and Molliver, 1982; Schlumpf et al., 1980; Verney et al., 1982, 1993; Zecevic and Verney, 1995). Because cocaine was shown to block presynaptic reuptake of monoamine neurotransmitters (Gawin and Ellinwood, 1988; Wise, 1984), brain areas containing high levels of monoamine receptors, including the caudate nucleus and cortex, are more susceptible to effects of in utero cocaine exposure (Levitt et al., 1997; Malanga and Kosofsky, 1999; Mayes, 1999). This in turn can impact neurotransmitter-mediated signals in those areas in early brain development (Levitt et al., 1997).
Cocaine is also suggested to affect prenatal brain development via induction of ER stress pathways. Gene profiling studies revealed that cocaine upregulated ER stress genes (BiP, CHOP, GADD34, XBP1, ATF6 and ATF4) in the striatum, medial prefrontal cortex (mPFC) and nucleus accumbens (NAcc) (Pavlovsky et al., 2013). Specifically, acute and repeated cocaine administration induced ER stress in the rat dorsal striatum via glutamate and dopamine receptor activation, as shown by increased immunoreactivity for ER stress proteins BiP and caspase-12 following acute or repeated cocaine treatment and attenuated immunoreactivity of these ER stress proteins by NMDA antagonist MK801 and dopamine receptor D1 antagonist SCH23390 (Ahn et al., 2007). Relatedly, repeated cocaine treatment in rat dorsal striatum caused an increase in extracellular glutamate levels that was mitigated by either selective mGluR1 or mGluR5 antagonists (Shin et al., 2007). Mechanistically, cocaine induced ER stress via glutamate receptor-mediated intracellular Ca2+ concentrations. This could be mediated by signaling crosstalk of group I mGluR-activated PLC/IP3 pathway, NMDA receptor-mediated Ca2+ influx, and group I mGluR-activated DAG/PKC pathway (Choe et al., 2011). Together, these studies suggest that cocaine induced ER stress via glutamate and dopamine receptor activation.
In addition to monoamine neurotransmitter pathways, cocaine was shown to mediate ER stress through alternative pathways. Cocaine can bind to ER chaperone protein sigma 1 receptor (Sig-1R) and promote its dissociation from the IP3 receptors on the ER and translocation to the proximity of the plasma membrane. This in turn enhances calcium efflux from ER to cytosol where Ca2+ might influence cytoskeletal protein expression or reorganization. This suggests cocaine may mediate cytoskeletal protein dynamics of dendritic spines and participate in the malfunction of axon growth and guidance via Sig-1R-mediated ER stress pathways (Hayashi and Su, 2007; Ka et al., 2016; Kourrich et al., 2013; Su and Hayashi, 2001; Tsai et al., 2009, 2012, 2015a, 2015b). Cocaine may also mediate astrogliosis and monocyte migration in the CNS via interactions with Sig-1Rs (Yang et al., 2016; Yao et al., 2010).
Cocaine-induced ER stress resulted in inhibition of neural progenitor proliferation, premature neuronal differentiation, and microglial cell death in vitro (Costa et al., 2013; Kindberg et al., 2014; Lee et al., 2008, 2009, 2011, 2017b). Specifically, Lee et al. (2008) demonstrated that cocaine induced ROS-mediated ER stress via N-oxidative metabolism of cocaine in cultured neural progenitors, which subsequently downregulated Cyclin A2 and inhibited cell proliferation via activation of the ER stress pathway PERK-eIF2α-ATF4. Pretreatment of neural progenitor cells with blockers of N-oxidative metabolism of cocaine (i.e. cytochrome P450 inhibitors SKF-525A or cimetidine) mitigated cocaine-induced ROS formation, confirming that N-oxidative metabolism of cocaine was involved in ROS generation. ROS generation also appears to be the source of cocaine-induced ER stress, as both SKF-525A and cimetidine also completely inhibited cocaine-induced ATF4 upregulation and cyclin A downregulation. The same group recently used 3D human pluripotent stem cell (hPSC)-derived cerebral organoids to identify a specific human cytochrome P450 isoform, CYP3A5, responsible for cocaine-induced ROS-mediated ER stress and subsequent defects in proliferation, neuronal differentiation, and organoid size in the developing human neocortex (Lee et al., 2017a, 2017b). Notably, CYP3A5 is polymorphically expressed in the general population, where about 60% of African-Americans but only 33% of Caucasians express active CYP3A5 while the rest have single-nucleotide polymorphisms in CYP3A5*3 and CYP3A5*6 alleles that cause a truncated form of this protein resulting in the absence of CYP3A5 activity. The polymorphic CYP3A5 expression study together with the recent study using the human stem cell model suggest that polymorphically expressing CYP3A5 genes might influence responses to oxidative metabolism of cocaine and cause variability in cocaine-induced developmental defects (Kuehl et al., 2001; Lee et al., 2017b). A deeper understanding of the link between genetic polymorphisms of human cytochrome P450 enzymes and cocaine-induced ER stress in the developing human brain is crucial to successfully translate research findings into clinical treatment.
The role of neuroinflammation in the onset and progression of cocaine-induced neurodevelopmental disorders is not clear. Nevertheless, a significant decrease in dopamine neurons accompanying an increase in activated microglia was observed amongst cocaine abusers (Little et al., 2009). Microglia act as immune cells in the brain and are involved in many aspects of development, synaptic plasticity, and neural circuitry maintenance through phagocytosis of neural precursors, newborn neurons, and synaptic elements (Brown and Neher, 2014; Cunningham et al., 2013; Paolicelli et al., 2011; Wake et al., 2013). Disturbance of microglial activity during critical development periods may lead to defective synapses and neural circuits and higher susceptibility to neurodevelopmental disorders such as autism spectrum disorder (Derecki et al., 2012; Paolicelli and Ferretti, 2017; Salter and Stevens, 2017). Notably, ROS-mediated ER stress has been implicated in cocaine-mediated induction of microglial activation. Cocaine-induced upregulation of immune response protein TLR2 was followed by microglia activation through the ATF4-TLR2 ROS-ER stress axis in vitro and in a rodent model (Liao et al., 2016). In addition, cocaine administration was shown to interrupt WNT/β-catenin signaling in the prefrontal cortex, dorsal striatum, amygdala, and nucleus accumbens of rodents (Cuesta et al., 2017; Dias et al., 2015), and alter gene expression of WNT/cadherin network in cerebral cortex of prenatally-exposed mice (Novikova et al., 2005). Microglia activation generated pro-inflammatory factors, including ROS, which interrupted neural progenitor proliferation and neuroblast formation via disruption of WNT/β-catenin signaling (L'Episcopo et al., 2012; Marchetti and Pluchino, 2013), a pathway previously linked to regulation of cell-cycle exit of neural progenitors, cortical layer formation, and cortical size determination (Chenn and Walsh 2002, 2003; Lee et al., 2015). Together, these findings suggest that exacerbated microglia activity may contribute to neurodevelopmental deficits of prenatal cocaine exposure by interrupting WNT/β-catenin signaling. Further studies on the underlying mechanisms of cocaine-induced microglial dysregulation may prove beneficial in the identification of therapeutic targets of prenatal cocaine abuse.
Cocaine was also able to induce astrocytosis and microglial activation through crosstalk between ER stress and autophagy pathways. Pharmacological and genetic approaches revealed that cocaine-induced astrocyte and microglia activation required sequential activation of ER stress and autophagy pathways, as demonstrated by cocaine-induced increase of autophagy markers BECN1, ATG5, MAP1LC3B-II, and SQSTM1 and their involvement in PERK (also known as EIF2AK3, eukaryotic translation initiation factor 2-a kinase 3)- and IRE1 (also known as ERN1, endoplasmic reticulum to nucleus signaling 1)-mediated ER stress pathways. Reducing ROS generation mitigated cocaine-induced PERK and IRE1 activation, suggesting that ROS acts as a molecular link between cocaine exposure and ER stress pathways (Guo et al., 2015; Periyasamy et al., 2016). In addition, cocaine may exert its neurotoxicity via the nitric oxide-dependent autophagic process (Guha et al., 2016). These results suggest potential oxidative- and ER stress-mediated autophagy mechanisms for cocaine-induced neurodevelopmental deficits.
Overall, cocaine-induced ER and oxidative stress seem to mediate several distinct pathophysiological pathways, including neuroinflammation via microglial activation, cytoskeletal dynamics via Sig-1R, and impaired cell proliferation and differentiation via N-oxidative metabolism of cocaine. Further research is necessary to continue to elucidate a link between the aforementioned pathways and how they may lead to prenatal neurodevelopmental defects as well as discovering potential new pathways by which cocaine may exert its impact.
2.2. Methamphetamine
2.2.1. Effects of prenatal methamphetamine exposure on brain development and neurobehavioral performance
MRI scans of adolescents prenatally exposed to METH showed significant changes in brain structure and cognitive performance, including reduced volumes of the caudate, thalamus, putamen, globus pallidus, and hippocampus, changes in white matter connections, and correlated deficits in attention and verbal memory (Chang et al., 2004, 2016; Warton et al., 2018a, 2018b). A similar study found that prenatal METH exposure led to cognitive deficits in adolescents as a result of striatal volume reductions (Sowell et al., 2010). Interestingly, Roos et al. (2014) reported enlargement of putamen in METH-exposed pre-adolescent children which, when taken together with previously mentioned studies (Chang et al., 2004; Sowell et al., 2010), suggests a potential neuropathology pattern of aberrant brain growth followed by slowed growth and impaired brain development. This atypical pattern has been previously reported in children suffering from autism spectrum disorder (Amaral et al., 2008; Courchesne, 2004). Thus, further longitudinal studies are necessary to precisely elucidate mechanisms of brain volume changes, especially in dopamine-rich brain regions, and their associated neurodevelopmental impacts in children with prenatal METH exposure.
2.2.2. Methamphetamine -induced ER and oxidative stress in the developing brain
Similar to cocaine, METH exerts its reinforcing effects mainly via an interaction with the mesolimbic dopamine reward system by blocking dopamine reuptake (Ashok et al., 2017; Volkow et al., 2012). However, METH can also stimulate release of cytoplasmic dopamine from presynaptic neurons into extracellular space (Espana and Jones, 2013; Siciliano et al., 2015) which is supported by studies showing METH causes greater dopamine release than cocaine in basal ganglia (Zhang et al., 2001). In addition to having different biochemical mechanisms of action, METH and cocaine yield different neurotoxic effects on the brain. METH was reported to cause extensive neurotoxicity on dopamine terminals in the striatum (Ares-Santos et al., 2014; Ellison et al., 1978; Wagner et al., 1980) that can produce long-lasting striatal dopamine depletion and destruction of dopaminergic terminals in abusers. Moreover, METH can also induce neuronal apoptosis in the striatum (Deng and Cadet, 2000; Deng et al., 2001; Zhu et al., 2006). In contrast, cocaine administration did not elicit neurotoxic effects as severe as METH (Kleven et al., 1988; Ryan et al., 1988). In vitro studies with fetal rat primary neuronal culture indicated that METH was neurotoxic to cultured mesencephalic dopamine cells as evidenced by impairments in dopamine reuptake and reduction in tyrosine hydroxylase (TH) and neuron-specific enolase immunostaining. Contrarily, cocaine neither impaired neuronal function nor altered dopamine cell survival in these cultures (Bennett et al., 1993a, 1993b). These findings indicate that various illicit stimulants can perturb dopaminergic function via different biochemical mechanisms and with varying neurotoxic potencies and suggests that METH mechanisms other than reuptake inhibition may be responsible for METH-induced neuronal damage.
Increase in dopamine levels following METH administration has been linked to enhanced oxidative stress (Cadet and Brannock, 1998; Jablonski et al., 2016). Augmented dopamine levels can lead to generation of free radical species including superoxide and hydrogen peroxide, derived from auto-oxidation of dopamine or enzymatic oxidation by monoamine oxidase (Yamamoto and Raudensky, 2008). METH-induced oxidative stress has been shown to cause striatal neuronal apoptosis via ER stress pathways, as shown by increased activity of proteases calpain and caspase-12 and upregulation of ER stress proteins BiP/GRP78, CHOP/GADD153, or Trib3 in rodent brains following METH injections (Jayanthi et al., 2004; Xu et al., 2017). Several METH-responsive ER genes have been identified (Jayanthi et al., 2009; Beauvais et al., 2011) and the upregulation of these genes were blocked by D1 and D2 receptor antagonist SCH23390 (Beauvais et al., 2011). METH and cocaine both activate ER stress in the striatum yet they differ in their capacity to induce cell death (Ahn et al., 2007; Jayanthi et al., 2004; Shin et al., 2007). METH generates greater neurotoxicity to striatal neurons compared to cocaine (Deng and Cadet, 2000; Deng et al., 2001; Kleven et al., 1988; Ryan et al., 1988; Zhu et al., 2006) yet the mechanism with which METH- or cocaine-induced ER stress promotes apoptosis or cytoprotection in striatal neurons remains unknown. One potential explanation is that excess dopamine released after METH administration might activate apoptotic machinery in striatal neurons once ER stress cannot be remedied. In contrast, cocaine releases smaller quantities of dopamine which might activate survival mechanisms that would prevent apoptosis in ER-stressed cells.
Sig-1R chaperones at the ER-mitochondrion interface were upregulated in rodent brains following METH self-administration and have been implicated in METH's adverse effects on the brain (Hayashi et al., 2010; Robson et al., 2012; Stefanski et al., 2004). METH interacts with Sig-1Rs at physiologically relevant levels and blockage of Sig-1Rs can mitigate METH-induced neurotoxicity, striatal dopamine depletion, striatal dopamine transporter downregulation, locomotor stimulation, memory deficiencies, and hyperthermia, though the neuroprotective mechanisms remain unknown (Matsumoto et al., 2008; Nguyen et al., 2005; Robson et al., 2013a; Seminerio et al., 2013). Moreover, pretreatment with a Sig-1R antagonist can attenuate METH-induced microglial activation and upregulation of proinflammatory cytokines in the striatum (Robson et al., 2013b). Previous studies have indicated that microglia activation leads to generation of reactive species that help orchestrate METH-induced neurotoxicity (LaVoie et al., 2004; Thomas and Kuhn, 2005; Thomas et al., 2004). Thus, further exploration of Sig-1R antagonists may shed light on better pharmacological protection against METH-induced neurotoxicity and neurobehavioral changes.
METH exposure has also been associated with altered astrocytic functions. Oxidative stress coupled with reactive astrogliosis in the striatum is an indication of METH toxicity (Granado et al., 2011a, 2011b; Zhu et al., 2005). During CNS development, astrocytes play a role in neuronal differentiation, migration, synaptogenesis, myelination, and activity-dependent synaptic plasticity (Chung et al., 2015; Fellin, 2009; Haydon, 2001; Ishibashi et al., 2006; Molofsky et al., 2012). Impairments in astrocyte function can therefore negatively impact neuronal development and precise formation of neural circuitry necessary for proper CNS function (Khakh and Sofroniew, 2015; Pekny et al., 2016; Sofroniew, 2014; Sofroniew and Vinters, 2010; Stipursky et al., 2012). In addition, cytochrome P450 2E1 (CYP2E1) which is expressed and catalytically active in the human brain (Farin and Omiecinski, 1993; Howard et al., 2003; Ledesma et al., 2014; Montoliu et al., 1995; Tindberg and Ingelman-Sundberg, 1996; Upadhya et al., 2000), can be induced by METH in cultured human astrocytes (Shah et al., 2013). The authors demonstrated that METH can induce ROS-mediated apoptosis of astrocytes via a NADPH oxidase-coupled CYP2E1-dependent pathway, suggesting that CYP2E1 effects on astrocytes may contribute in part to METH neurotoxicity on the developing brain. Genetic polymorphisms in CYP2E1 might also alter enzymatic activity and alter effects of METH-induced oxidative stress in astrocytes (Miksys and Tyndale, 2004). A clearer understanding of inter-individual variability of CYP2E1 in response to METH could lead to identification of factors contributing to astrocytic dysfunction in the striatum against METH.
2.3. Tobacco
2.3.1. Effects of prenatal tobacco exposure on brain development and neurobehavioral performance
Tobacco smoke contains numerous chemicals including nicotine, hydrogen cyanide, carbon monoxide, nitrosamines, polycyclic aromatic hydrocarbons, benzene, etc, many of which cause harmful effects to the central nervous, cardiovascular, and pulmonary systems. In this section, we review key findings focusing specifically on effects of nicotine and tobacco-specific nitrosamines.
As shown by neuroimaging studies, prenatal tobacco exposure leads to abnormalities in brain morphology and function, including cortical gray matter volume reduction, coherent anterior corona radiata fibers and thinning of the superior frontal, superior parietal, and precentral cortical areas in children (Chang et al., 2016; El Marroun et al., 2014, 2016; Rivkin et al., 2008). Notably, these prenatally-exposed children exhibited more emotional problems compared to healthy controls, likely due to thinning of the superior frontal and precentral cortices (El Marroun et al., 2014). Another set of studies assessed adolescents whose mothers smoked during pregnancy and identified similar cortical thinning, particularly in orbitofrontal, middle frontal, and parahippocampal cortices and interestingly, females were more severely affected than males (Toro et al., 2008). The orbitofrontal cortex plays a significant role in antisocial behavior, impulsivity, and cue-mediated drug intake (Blair, 2004; Everitt et al., 2007; Rolls 2000, 2004; Seguin, 2004). Prenatal tobacco exposure can hinder development of the orbitofrontal cortex and in turn lead to alterations in social behavior and increase susceptibility to drug use in adolescence (Lotfipour et al., 2009; Toro et al., 2008). Prenatal tobacco exposure also caused a reduction in pallidum and amygdala volumes (Haghighi et al., 2013; Liu et al., 2013) and in the size of the corpus callosum, which is the fiber tract connecting the two cerebral hemispheres (Paus et al., 2008). Interestingly, only females exhibited smaller corpus callosum, particularly in its posterior part. Posterior corpus callosum contains interhemispheric fibers forming ventral and dorsal visual streams (Hofer and Frahm, 2006; Park et al., 2008) and tobacco-induced reduction in posterior corpus callosum impaired visual attention (Espy et al., 2011; Jacobsen et al., 2007). Taken together, these findings suggest prenatal tobacco exposure can negatively affect cortical development and expands our knowledge of the causal relationship between brain structure and behavior; however, further research is necessary to elucidate precise mechanisms underlying these structural impairments.
2.3.2. Tobacco-induced ER and oxidative stress in the developing brain
Nicotine, the primary psychoactive component of tobacco, binds to nicotinic acetylcholine receptors (nAChRs) expressed in neural progenitors during early embryonic cortical development (Atluri et al., 2001). Cholinergic neurons innervate the cerebral cortex during a critical period of neuronal differentiation and synaptogenesis, and acetylcholine, as a neuromodulator, can modulate the growth, differentiation, and plasticity of developing cortical neurons (Hohmann and Berger-Sweeney, 1998; Lauder and Schambra, 1999; Picciotto et al., 2012). Sustained exposure to nicotine can hinder progenitor self-renewal and promote premature neuronal differentiation via activation of α4β2 nicotinic acetylcholine receptors (α4β2-nAChRs), as shown in vitro with primary neural progenitors derived from fetal rodent neocortex (Takarada et al., 2012). In animal models, over-stimulation of nAChRs by nicotine during gestation seems to alter nAChR expression and disrupt cortical development, specifically targeting neural progenitor division and differentiation, synaptic development, and synaptic activity, ultimately resulting in cognitive and executive control impairments in children with prenatal tobacco exposure (Aoyama et al., 2016; Bassey et al., 2018; Bryden et al., 2016; Muhammad et al., 2012; Mychasiuk et al., 2013; Parameshwaran et al., 2013; Slikker et al., 2005; Slotkin, 2004; Slotkin et al., 2005). Therefore, prenatal nicotine exposure disturbs trophic effects of acetylcholine on cell proliferation, neuronal differentiation, and synaptic development that are required for proper assembly of the brain.
Nicotine can induce oxidative stress in multiple brain regions, including prefrontal cortex, striatum, hippocampus, and cerebellum (Bhagwat et al., 1998; Budzynska et al., 2013; Das et al., 2009; Qiao et al., 2005), and increased oxidative stress may underlie some of the adverse effects on brain development caused by prenatal nicotine exposure. Stimulation of nAChRs by nicotine resulted in dopamine release in prefrontal cortex and striatum (Benowitz, 2009; D'Souza and Markou, 2011), and this excess dopamine may lead to generation of free radicals. Furthermore, Das et al. (2009) showed that nicotine inhibited mitochondrial electron transport chain complexes and produced nitric oxide, subsequently repressing mitochondrial oxidative stress scavenger systems in the brain and causing lipid peroxidation and protein oxidation in the mitochondria of several brain regions. Notably, increased nitric oxide induced by nicotine was shown to inhibit proliferation of mouse neural stem cells through upregulation of histone deacetylase 1 (HDAC1) (Lee et al., 2014), a protein involved in epigenetic regulation of neurogenesis in the developing brain (Montgomery et al., 2009). Furthermore, in vitro studies have demonstrated that nicotine inhibited proliferation of neural progenitors through induction of oxidative stress (Qiao et al., 2005). These results suggest that nicotine-induced oxidative stress and nitric oxide production might elicit neurotoxicant actions in the developing brain.
As of yet, no evidence links prenatal nicotine exposure to ER stress in the developing brain; however, oxidative stress induced by prenatal nicotine exposure might indirectly lead to ER stress activation and subsequently contribute to the observed neurodevelopmental deficits. For example, Repo et al. (2014) showed that nicotine inhibited proliferation of human trophoblast cells via ROS-mediated ER stress responses. Moreover, prenatal nicotine exposure in rodents impaired placental function through activation of ER stress pathway (Wong et al., 2015).
Astrocytes contain high amount of ROS scavenger molecules which protect astroglial cells against deleterious effects of ROS and promote neuronal survival (Desagher et al., 1996; Dringen et al., 1999; Fernandez-Fernandez et al., 2012; Ferrero-Gutierrez et al., 2008). Astrocytes participate in the defense of surrounding neurons by providing prosurvival trophic factors and antioxidant molecules such as glial cell-line derived neurotrophic factor (GDNF) and glutathione precursors (Hamdi et al., 2016; Lin et al., 2006; Masmoudi-Kouki et al., 2011; Sandhu et al., 2009). Hamdi et al. (2016) recently demonstrated that cultured astrocytes isolated from newborn rats prenatally exposed to tobacco were more vulnerable to oxidative insult than astrocytes isolated from control animals, indicating that despite their high antioxidant capacity, astrocytes derived from prenatally-exposed animals could not survive under increased oxidative stress, and the loss of antioxidative ability of astrocytes following tobacco exposure may partially explain the brain's susceptibility to additional oxidative stress and its progression towards developmental dysfunction.
4-Methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific procarcinogen, can activate microglia in the mouse brain and cause neuronal damage (Ghosh et al., 2009). In contrast to nicotine-induced oxidative stress occurring in microsomes and mitochondria of rat brain, NNK-induced oxidative damage occurred predominantly in the brain microsomes (Bhagwat et al., 1998). Notably, expression of microsomal CYP2E1, involved in the metabolic activation of NNK in humans (Abdel-Rahman et al., 2000; Smith et al., 1992; Yamazaki et al., 1992), was greatly increased in brains of monkeys and rats following nicotine exposure (Anandatheerthavarada et al., 1993; Joshi and Tyndale 2006a, 2006b). These findings indicated that nicotine-induced CYP2E1 upregulation in the brain may influence onset or progression of tobacco-mediated neurodevelopmental defects. Taken together, the understanding of the interface between oxidative stress, ER stress, and microglial inflammation is important to develop therapeutic strategies to prevent neurodevelopment abnormalities in utero after exposure to tobacco.
2.4. Opioids
2.4.1. Effects of prenatal opiate exposure on brain development and neurobehavioral performance
Assessments of prenatal opioid exposure in children and modeling prenatal opioid exposure in animals have provided insights on the effects of NOWs on neurobehavioral functioning of neonates. In animal studies, prenatal opioid exposure is correlated with lower birth weights, reduced brain mass, increased depressive-like behavior, craniofacial anomalies, and higher mortality rate (Byrnes and Vassoler, 2018; Devarapalli et al., 2016; Hung et al., 2013; Hutchings et al., 1996; Slamberova et al., 2005; Wu et al., 2014). In humans, prenatal opioid exposure can lead to various adverse health problems in infants, longer hospital stays, and a higher rate of admission to intensive care units (Kivisto et al., 2015; Tolia et al., 2018).
In animal models, prenatal exposure to opioid is associated with abnormal CNS function including dysregulated cholinergic activity, delayed neural tube defects, decreased myelination, impaired memory performance, impaired fear memory extinction and hippocampal CA1 synaptic plasticity, enhanced activity of blood-brain barrier transport system of opiate peptides, delayed development of monoamine oxidase isoforms, decreased brain volume, and altered neurochemistry (Banks et al., 1996; Nasiraei-Moghadam et al., 2005; Robinson et al., 1996; Sanchez et al., 2008; Tan et al., 2015; Tsang et al., 1986; Zagon and McLaughlin, 1977).
Human neuroimaging studies examining early cerebral connective tract development showed that methadone-exposed infants demonstrated higher mean diffusivity in the superior longitudinal fasciculus regionally (Walhovd et al., 2012) and reduced fractional anisotropy and altered microstructure in major white matter tracts (Monnelly et al., 2018). Moreover, information processing deficits were observed in young boys as a result of in utero exposure to methadone (Guo et al., 1994). Prenatal opioid exposure (methadone, buprenorphine, or heroin) may also be associated with brain volume reduction and subsequent cognitive and behavioral deficits (Walhovd et al., 2007; Yuan et al., 2014). Studies have documented that prenatal opioid exposure, specifically methadone and buprenorphine, impairs cognitive development in early childhood (Bauman and Levine, 1986; Konijnenberg and Melinder, 2011; Soepatmi, 1994; van Baar, 1990; van Baar and de Graaff, 1994). Additionally, a longitudinal study suggesting prenatal opioid exposure reduced IQ scores, with male subjects demonstrating poorer cognitive functions than female subjects (Nygaard et al., 2015).
Although there remain inconsistencies amongst studies, perhaps partly due to limited sample sizes, study populations, varied analytical approaches and/or experimental designs, these results strongly suggest that opioid-exposed infants are more likely to develop cognitive and behavioral difficulties. Collectively, these studies raise concerns on the potential effects of prenatal opioid exposure on cognitive development and performance. Further investigation of opioids’ effects on prenatal brain development are warranted.
2.4.2. Opioid-induced ER and oxidative stress in the developing brain
Although strong evidence suggests prenatal opioid exposure leads to various neurobehavioral defects later in life, the underlying neurobiological mechanisms remain unelucidated. Prenatal opioid exposure may lead to depressive-like behavior due to imbalanced oxidative stress. For example, rat pups exposed to high doses of buprenorphine (1 mg/kg/day) during gestation exhibited elevated oxidative stress as shown by increased lipid peroxidation levels and reduced glutathione (GSH) levels and glutathione activity. High doses of buprenorphine also impaired expression and activation of key neurotrophin signaling factors, including reduced plasma BDNF and serotonin levels, reduced ERK and TrkB phosphorylation, and impaired protein kinase A activity (Hung et al., 2013). These results were supported by a separate study that noted reduced BDNF expression and impaired neurogenesis in prenatal buprenorphine-exposed rat pups (Wu et al., 2014). These studies collectively propose a potential mechanism underlying in utero opioid exposure and development of depressive-like behavior.
Prenatal exposure to buprenorphine may also affect myelin formation during brain development. Oligodendrocytes are myelinating cells found in the CNS and oligodendrocyte-regulated myelination provides valuable support to axonal function, synaptic plasticity, and CNS injury repair that are critical for proper CNS development and function (Bradl and Lassmann, 2010; Fields, 2005; Miron et al., 2011; Sanchez et al., 2008; Simons and Nave, 2015). Sanchez et al. (2008) examined effects of buprenorphine on myelination and found that high doses of buprenorphine (1 mg/kg/day) during gestation reduced expression of myelin basic protein splicing isoforms during CNS development. Although prenatal exposure to buprenorphine increased the caliber of myelinated axons, thinner myelin sheaths were observed in these axons.
Prenatal heroin and morphine exposure were both found to induce apoptosis and impair learning and memory (Nasiraei-Moghadam et al., 2005, 2010; Wang and Han, 2009). For example, hippocampi from mice exposed to heroin (10 mg/kg/day) during gestation exhibited elevated protein and mRNA levels of apoptotic markers caspase-3 and Bax, and reduced levels of anti-apoptosis marker Bcl-2. Caspase-3 was significantly activated in the dentate gyrus (DG) and CA1 of the hippocampus in postnatal animals. Prenatal heroin exposure may consequently impair short-term spatial memory later in life, as shown by decreased dendritic arborization in cortical layers II and III and poor performance in location preference tasks (Lu et al., 2012).
Similarly, prenatal morphine exposure led to neuronal loss and memory impairments in rat embryos; they were smaller in size, displayed neural tube deficits between E9.5 and E13.5 (Nasiraei-Moghadam et al., 2005), and exhibited increased apoptosis throughout the CNS (Nasiraei-Moghadam et al., 2010). Specifically, Ghafari and Golalipour (2014) reported that prenatal exposure to morphine reduced pyramidal neuron density in mouse hippocampus which offers an explanation for postnatal learning and memory task deficits. Prenatal morphine exposure may also impair memory performance via long-term depression (LTD) and cytoskeletal network rearrangements, as suggested by reports of impaired synaptic plasticity in DG, reduced BDNF levels, and reduced expression of postsynaptic marker PSD95 and neuronal nitric oxide synthase (nNOS) in postnatal rat pups exposed to morphine prenatally (Niu et al., 2009; Yang et al., 2006) Prenatal morphine exposure may also affect brain development by lowering activity of brain cyclin-dependent kinase 5 (Cdk5) in embryos. Interestingly, prenatal cocaine exposure exhibited the opposite effect on Cdk5 activity, and co-administration of cocaine and morphine tended to obliterate their individual effects on Cdk5 activity (Bhat et al., 2006). These data suggested prenatal exposure to heroin or morphine during embryonic neurogenesis may disturb apoptotic pathways and pose long-term impacts on postnatal learning and memory defects.
Recent studies have shed light on ER stress-integrated mechanisms of opioid-mediated impairments on brain development and cognitive functions. Sil et al. (2018) revealed morphine exposure induced astrocytosis and subsequent inflammatory responses via ER stress-autophagy axis. Morphine exposure not only resulted in upregulation of the ER chaperone BiP, also led to induction of autophagy and autophagasome accumulation as indicated by elevated autophagy markers BECN1, LC3-II, and p62. Morphine-impaired ER-autophagy axis further induced proinflammatory cytokine production in a mu opioid receptor (MOR)-dependent fashion. The study also showed morphine induced profound astrocytosis in the cerebellum of rhesus macaques. Similarly, morphine exerted detrimental synaptic regulations via sequential induction of ROS, ER stress, and autophagy in the hippocampus. Morphine-mediated MOR activation induced NADPH oxidase-mediated ROS generation, activated ER stress (pPERK, IRE1α, and ATF6) and autophagy (Beclin1, ATG5/ATG7, and LC3-II), and eventually led to synaptic density alterations that can contribute to cognitive disabilities (Cai et al., 2016). These findings suggested ER stress crosstalk plays a pivotal role in morphine-mediated neuroplasticity.
Emerging studies also suggest that ER and oxidative stress regulate morphine tolerance (Abdel-Zaher et al., 2010; Dobashi et al., 2010; Lauro et al., 2016; Motaghinejad et al., 2015). Repeated morphine exposure leads to development of morphine tolerance (Roeckel et al., 2016) which might partially be controlled by ER and oxidative stress signaling pathways, including ER stress-mediated glycogen synthase kinase 3beta (GSK-3β) activation (Dobashi et al., 2010). Phosphoproteomic analyses of morphine-tolerant rat spinal cords revealed potential underlying mechanisms that contribute to the development of morphine tolerance, including ER stress, mitochondrial dysfunction, cytoskeleton reorganization, receptor trafficking, and biomolecular metabolism (Liaw et al., 2014).
Reports have also indicated morphine-induced apoptosis in microglia, neurons, and astrocytes (Deb and Das, 2011; Hu et al., 2002). Other opioids, including oxycodone, tramadol, and tapentadol, were reported to induce oxidative stress and ER stress in various brain regions (Awadalla and Salah-Eldin, 2016; Fan et al., 2015; Faria et al., 2016; Zhuo et al., 2012). On the contrary, morphine reduced astrocyte apoptosis upon cytotoxic insults (Kim et al., 2001; Lee et al., 2004). A recent study demonstrated morphine exerted a protective effect in response to glutamate-stimulated apoptosis of spinal cord astrocytes and did so via reducing calcium overloading and suppressing ER stress sensors (Zhang et al., 2016). Interestingly, the opioid antagonist naloxone was also found to cause apoptosis and induce multiple ER chaperones (BiP, ERp29 and PDI) and ER stress sensors (ATF-6, IRE1, and PERK) in vitro (Seo et al., 2014).
3. Concluding remarks
The continued rise of drug abuse-related deaths necessitates improved therapies to treat drug addictions in combination with revised laws and policies to control distribution of addictive substances. Prenatal drug exposure can cause neurodevelopmental deficits and lead to neurobehavioral changes, although each substance can induce ER and oxidative stress via different pathways and no addiction scenario is the same. Thus, when it comes to addiction therapies, “one treatment fits all” simply does not work; many studies are instead supporting the concept of personalized medicine to revolutionize the treatment plan for drug addiction.
While methadone and buprenorphine are commonly used detoxification drugs to wean off opioid addiction, clinical studies have indicated that patients often develop drug dependency after extended treatment periods. This poses additional challenges for opioid abuse treatments and necessitates the development of alternative strategies to cope with the ongoing opioid crisis. In addition to improving postnatal care for substance-exposed newborns, more actions should be taken to mitigate adverse outcomes of infants born to mothers with opioid and other substance use disorders during pregnancy. For instance, enriched environments and increasing exercises are both proven to improve cognitive functions of prenatally-exposed children (Ahmadalipour et al., 2018). Treatments with platelet derived growth factor (PDGF) ameliorated morphine-induced ROS productions and restored spine densities (Cai et al., 2016). Furthermore, dextromethorphan, an active ingredient found in cough syrup, may be repurposed as a mitigator of adverse opioid effects during gestation and lactation periods since co-administration of dextromethophen with morphine were found to significantly attenuate morphine-induced neurologic effects (Tao et al., 2011; Yang et al., 2006).
Recently, the National Institute on Drug Abuse embarked on a 10-year longitudinal study of adolescent brain cognitive development (ABCD Study) to observe factors that affect brain growth. The multi-institute collaborative research project is expected to bring forth valuable information (Volkow et al., 2018). Animal studies suggested that many alterations of neuronal activity occurred in infants during the first two weeks after birth, a period which was shown to be critical for proper brain development and which should be a primary focus for any interventions aimed at reducing brain damage cause by prenatal drug exposure. For long-term therapeutic strategies, the ABCD Study should serve as a research model with which to implement systematic evaluation and longitudinal monitoring on cognitive function and neurobehavioral outcomes of children with prenatal substance exposure. Since details of ER and oxidative stress mechanisms affecting brain development is scarce, further studies on the cellular pathways contributing to drug-induced behavioral and neurocognitive impairments would be greatly beneficial to effectively develop new therapeutic strategies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2018.100145.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abdel-Rahman S.Z., Salama S.A., Au W.W., Hamada F.A. Role of polymorphic CYP2E1 and CYP2D6 genes in NNK-induced chromosome aberrations in cultured human lymphocytes. Pharmacogenetics. 2000;10:239–249. doi: 10.1097/00008571-200004000-00005. [DOI] [PubMed] [Google Scholar]
- Abdel-Zaher A.O., Abdel-Rahman M.S., FM E.L. Blockade of nitric oxide overproduction and oxidative stress by Nigella sativa oil attenuates morphine-induced tolerance and dependence in mice. Neurochem. Res. 2010;35:1557–1565. doi: 10.1007/s11064-010-0215-2. [DOI] [PubMed] [Google Scholar]
- Ackerman J.P., Riggins T., Black M.M. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadalipour A., Ghodrati-Jaldbakhan S., Samaei S.A., Rashidy-Pour A. Deleterious effects of prenatal exposure to morphine on the spatial learning and hippocampal BDNF and long-term potentiation in juvenile rats: beneficial influences of postnatal treadmill exercise and enriched environment. Neurobiol. Learn. Mem. 2018;147:54–64. doi: 10.1016/j.nlm.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Ahn S.M., Kim S.W., Choe E.S. Cocaine increases immunoglobulin heavy chain binding protein and caspase-12 expression in the rat dorsal striatum. Psychopharmacology (Berlin) 2007;195:407–414. doi: 10.1007/s00213-007-0922-9. [DOI] [PubMed] [Google Scholar]
- Alessandri S.M., Bendersky M., Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev. Psychol. 1998;34:565–573. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Anandatheerthavarada H.K., Williams J.F., Wecker L. Differential effect of chronic nicotine administration on brain cytochrome P4501A1/2 and P4502E1. Biochem. Biophys. Res. Commun. 1993;194:312–318. doi: 10.1006/bbrc.1993.1821. [DOI] [PubMed] [Google Scholar]
- Aoyama Y., Toriumi K., Mouri A., Hattori T., Ueda E. Prenatal nicotine exposure impairs the proliferation of neuronal progenitors, leading to fewer glutamatergic neurons in the medial prefrontal cortex. Neuropsychopharmacology. 2016;41:578–589. doi: 10.1038/npp.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt R., Angelopoulos J., Salvator A., Singer L. Motor development of cocaine-exposed children at age two years. Pediatrics. 1999;103:86–92. doi: 10.1542/peds.103.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ares-Santos S., Granado N., Espadas I., Martinez-Murillo R., Moratalla R. Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology. 2014;39:1066–1080. doi: 10.1038/npp.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok A.H., Mizuno Y., Volkow N.D., Howes O.D. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:511–519. doi: 10.1001/jamapsychiatry.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri P., Fleck M.W., Shen Q., Mah S.J., Stadfelt D. Functional nicotinic acetylcholine receptor expression in stem and progenitor cells of the early embryonic mouse cerebral cortex. Dev. Biol. 2001;240:143–156. doi: 10.1006/dbio.2001.0453. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Hurt H., Giannetta J.M., Epstein C.L., Shera D.M. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr. Neurol. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Awadalla E.A., Salah-Eldin A.E. Molecular and histological changes in cerebral cortex and lung tissues under the effect of tramadol treatment. Biomed. Pharmacother. 2016;82:269–280. doi: 10.1016/j.biopha.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Bassey R.B., Gondré-Lewis M.C. Combined early life stressors: prenatal nicotine and maternal deprivation interact to influence affective and drug seeking behavioral phenotypes in rats. Behav. Brain Res. 2018;18 doi: 10.1016/j.bbr.2018.07.022. 10605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra E.S., Vogel A.L., Morrow C.E., Xue L., Anthony J.C. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst. Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A., Kastin A.J., Harrison L.M., Zadina J.E. Perinatal treatment of rats with opiates affects the development of the blood-brain barrier transport system PTS-1. Neurotoxicol. Teratol. 1996;18:711–715. doi: 10.1016/s0892-0362(96)00128-6. [DOI] [PubMed] [Google Scholar]
- Bashkatova V., Meunier J., Maurice T., Vanin A. Memory impairments and oxidative stress in the hippocampus of in-utero cocaine-exposed rats. Neuroreport. 2005;16:1217–1221. doi: 10.1097/00001756-200508010-00017. [DOI] [PubMed] [Google Scholar]
- Bauman P.S., Levine S.A. The development of children of drug addicts. Int. J. Addict. 1986;21:849–863. doi: 10.3109/10826088609027399. [DOI] [PubMed] [Google Scholar]
- Beauvais G., Atwell K., Jayanthi S., Ladenheim B., Cadet J.L. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke M., Eyler F.D., Warner T.D., Garvan C.W., Hou W., Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J. Pediatr. Psychol. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C., Massocco D., Serra G. Prenatal cocaine exposure and the expanding spectrum of brain malformations. Arch. Intern. Med. 2000;160:2393. doi: 10.1001/archinte.160.15.2393. [DOI] [PubMed] [Google Scholar]
- Bennett B.A., Hyde C.E., Pecora J.R., Clodfelter J.E. Differing neurotoxic potencies of methamphetamine, mazindol, and cocaine in mesencephalic cultures. J. Neurochem. 1993;60:1444–1452. doi: 10.1111/j.1471-4159.1993.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Bennett B.A., Hyde C.E., Pecora J.R., Clodfelter J.E. Long-term cocaine administration is not neurotoxic to cultured fetal mesencephalic dopamine neurons. Neurosci. Lett. 1993;153:210–214. doi: 10.1016/0304-3940(93)90324-e. [DOI] [PubMed] [Google Scholar]
- Benowitz N.L. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H., Fowler J.S., Rooney W.D., Scharf B.A., Backus W.W. Cocaine is pharmacologically active in the nonhuman primate fetal brain. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1582–1587. doi: 10.1073/pnas.0909585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge D.J. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Bhagwat S.V., Vijayasarathy C., Raza H., Mullick J., Avadhani N.G. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem. Pharmacol. 1998;56:831–839. doi: 10.1016/s0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Bhat R., Chari G., Rao R., Wirtshafter D. Prenatal cocaine and morphine alter brain cyclin-dependent kinase 5 (Cdk5) activity in rat pups. Neurotoxicol. Teratol. 2006;28:625–628. doi: 10.1016/j.ntt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Blair R.J. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Bradl M., Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Parra V., Gatica D., Rodriguez A.E., Torrealba N. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.C., Neher J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Bryden D.W., Burton A.C., Barnett B.R., Cohen V.J., Hearn T.N. Prenatal nicotine exposure impairs executive control signals in medial prefrontal cortex. Neuropsychopharmacology. 2016;41:716–725. doi: 10.1038/npp.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., Skalicka-Wozniak K., Michalak A. Effects of imperatorin on nicotine-induced anxiety- and memory-related responses and oxidative stress in mice. Physiol. Behav. 2013;122:46–55. doi: 10.1016/j.physbeh.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Byrnes E.M., Vassoler F.M. Modeling prenatal opioid exposure in animals: current findings and future directions. Front. Neuroendocrinol. 2018 Oct;51:1–13. doi: 10.1016/j.yfrne.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J.L., Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem. Int. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Cai Y., Yang L., Hu G., Chen X., Niu F. Regulation of morphine-induced synaptic alterations: role of oxidative stress, ER stress, and autophagy. J. Cell Biol. 2016;215:245–258. doi: 10.1083/jcb.201605065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Oishi K., Skranes J., Buchthal S., Cunningham E., Yamakawa R., Hayama S., Jiang C.S., Alicata D., Hernandez A., Cloak C., Wright T., Ernst T. Sex-specific alterations of white matter developmental trajectories in infants with prenatal exposure to methamphetamine and tobacco. JAMA Psychiatry. 2016;73:1217–1227. doi: 10.1001/jamapsychiatry.2016.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Smith L.M., LoPresti C., Yonekura M.L., Kuo J. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatr. Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chasnoff I.J., Anson A., Hatcher R., Stenson H., Iaukea K., Randolph L.A. Prenatal exposure to cocaine and other drugs. Outcome at four to six years. Ann. N. Y. Acad. Sci. 1998;846:314–328. [PubMed] [Google Scholar]
- Chasnoff I.J., Gardner S. Neonatal abstinence syndrome: a policy perspective. J. Perinatol. 2015;35:539–541. doi: 10.1038/jp.2015.53. [DOI] [PubMed] [Google Scholar]
- Chen S., Melchior W.B., Jr., Guo L. Endoplasmic reticulum stress in drug- and environmental toxicant-induced liver toxicity. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2014;32:83–104. doi: 10.1080/10590501.2014.881648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A., Walsh C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A., Walsh C.A. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cerebr. Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Chiriboga C.A., Brust J.C., Bateman D., Hauser W.A. Dose-response effect of fetal cocaine exposure on newborn neurologic function. Pediatrics. 1999;103:79–85. doi: 10.1542/peds.103.1.79. [DOI] [PubMed] [Google Scholar]
- Chiriboga C.A., Kuhn L., Wasserman G.A. Prenatal cocaine exposures and dose-related cocaine effects on infant tone and behavior. Neurotoxicol. Teratol. 2007;29:323–330. doi: 10.1016/j.ntt.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe E.S., Ahn S.M., Yang J.H., Go B.S., Wang J.Q. Linking cocaine to endoplasmic reticulum in striatal neurons: role of glutamate receptors. Basal Ganglia. 2011;1:59–63. doi: 10.1016/j.baga.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.S., Welsh C.A., Barres B.A., Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B.M., Yao H., Yang L., Buch S. Role of endoplasmic reticulum (ER) stress in cocaine-induced microglial cell death. J. Neuroimmune Pharmacol. 2013;8:705–714. doi: 10.1007/s11481-013-9438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Cuesta S., Severin M.J., Batuecas J., Rosso S.B., Pacchioni A.M. Wnt/beta-catenin pathway in the prefrontal cortex is required for cocaine-induced neuroadaptations. Addict. Biol. 2017;22:933–945. doi: 10.1111/adb.12377. [DOI] [PubMed] [Google Scholar]
- Cunningham C.L., Martinez-Cerdeno V., Noctor S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza M.S., Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict. Sci. Clin. Pract. 2011;6:4–16. [PMC free article] [PubMed] [Google Scholar]
- Das S., Gautam N., Dey S.K., Maiti T., Roy S. Oxidative stress in the brain of nicotine-induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl. Physiol. Nutr. Metabol. 2009;34:124–135. doi: 10.1139/H08-147. [DOI] [PubMed] [Google Scholar]
- Deb I., Das S. Thyroid hormones protect astrocytes from morphine-induced apoptosis by regulating nitric oxide and pERK 1/2 pathways. Neurochem. Int. 2011;58:861–871. doi: 10.1016/j.neuint.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V., Covington C., Ostrea E., Jr., Romero A., Baker D. Prenatal cocaine and neonatal outcome: evaluation of dose-response relationship. Pediatrics. 1996;98:735–740. [PubMed] [Google Scholar]
- Della Grotta S., LaGasse L.L., Arria A.M., Derauf C., Grant P. Patterns of methamphetamine use during pregnancy: results from the infant development, environment, and lifestyle (IDEAL) study. Matern. Child Health J. 2010;14:519–527. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Cadet J.L. Methamphetamine-induced apoptosis is attenuated in the striata of copper-zinc superoxide dismutase transgenic mice. Brain Res. Mol. Brain Res. 2000;83:121–124. doi: 10.1016/s0169-328x(00)00169-8. [DOI] [PubMed] [Google Scholar]
- Deng X., Wang Y., Chou J., Cadet J.L. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res. Mol. Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Derecki N.C., Cronk J.C., Lu Z., Xu E., Abbott S.B. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S., Glowinski J., Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J. Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarapalli M., Leonard M., Briyal S., Stefanov G., Puppala B.L. Prenatal oxycodone exposure alters CNS endothelin receptor expression in neonatal rats. Drug Res. (Stuttg) 2016;66:246–250. doi: 10.1055/s-0035-1569279. [DOI] [PubMed] [Google Scholar]
- Dias C., Dietz D., Mazei-Robison M., Sun H., Damez-Werno D. Dishevelled-2 regulates cocaine-induced structural plasticity and Rac1 activity in the nucleus accumbens. Neurosci. Lett. 2015;598:23–28. doi: 10.1016/j.neulet.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J.B., Mangeol A., Revel M.O., Burgun C., Aunis D., Zwiller J. Acute or repeated cocaine administration generates reactive oxygen species and induces antioxidant enzyme activity in dopaminergic rat brain structures. Neuropharmacology. 2005;48:965–974. doi: 10.1016/j.neuropharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Dobashi T., Tanabe S., Jin H., Mimura N., Yamamoto T. BiP, an endoplasmic reticulum chaperone, modulates the development of morphine antinociceptive tolerance. J. Cell Mol. Med. 2010;14:2816–2826. doi: 10.1111/j.1582-4934.2009.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D.L., Benveniste H., Behnke M., Bandstra E.S., Singer L.T. Neuroimaging of prenatal drug exposure. Neurotoxicol. Teratol. 2006;28:386–402. doi: 10.1016/j.ntt.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R., Kussmaul L., Gutterer J.M., Hirrlinger J., Hamprecht B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J. Neurochem. 1999;72:2523–2530. doi: 10.1046/j.1471-4159.1999.0722523.x. [DOI] [PubMed] [Google Scholar]
- Ebrahim S.H., Floyd R.L., Merritt R.K., 2nd, Decoufle P., Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987-1996. JAMA. 2000;283:361–366. doi: 10.1001/jama.283.3.361. [DOI] [PubMed] [Google Scholar]
- El Marroun H., Schmidt M.N., Franken I.H., Jaddoe V.W., Hofman A. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology. 2014;39:792–800. doi: 10.1038/npp.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H., Tiemeier H., Franken I.H., Jaddoe V.W., van der Lugt A. Prenatal cannabis and tobacco exposure in relation to brain morphology: a prospective neuroimaging study in young children. Biol. Psychiatry. 2016;79:971–979. doi: 10.1016/j.biopsych.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Ellison G., Eison M.S., Huberman H.S., Daniel F. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science. 1978;201:276–278. doi: 10.1126/science.26975. [DOI] [PubMed] [Google Scholar]
- Espana R.A., Jones S.R. Presynaptic dopamine modulation by stimulant self-administration. Front Biosci. (Schol. Ed.) 2013;5:261–276. doi: 10.2741/s371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy K.A., Fang H., Johnson C., Stopp C., Wiebe S.A. Prenatal tobacco exposure: developmental outcomes in the neonatal period. Dev. Psychol. 2011;47:153–156. doi: 10.1037/a0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Hutcheson D.M., Ersche K.D., Pelloux Y., Dalley J.W., Robbins T.W. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann. N. Y. Acad. Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fan R., Schrott L.M., Snelling S., Ndi J., Arnold T., Korneeva N.L. Chronic oxycodone induces integrated stress response in rat brain. BMC Neurosci. 2015;16:58. doi: 10.1186/s12868-015-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria J., Barbosa J., Queiros O., Moreira R., Carvalho F., Dinis-Oliveira R.J. Comparative study of the neurotoxicological effects of tramadol and tapentadol in SH-SY5Y cells. Toxicology. 2016;359–360:1–10. doi: 10.1016/j.tox.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Farin F.M., Omiecinski C.J. Regiospecific expression of cytochrome P-450s and microsomal epoxide hydrolase in human brain tissue. J. Toxicol. Environ. Health. 1993;40:317–335. doi: 10.1080/15287399309531797. [DOI] [PubMed] [Google Scholar]
- Fellin T. Communication between neurons and astrocytes: relevance to the modulation of synaptic and network activity. J. Neurochem. 2009;108:533–544. doi: 10.1111/j.1471-4159.2008.05830.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez S., Almeida A., Bolanos J.P. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 2012;443:3–11. doi: 10.1042/BJ20111943. [DOI] [PubMed] [Google Scholar]
- Ferrero-Gutierrez A., Perez-Gomez A., Novelli A., Fernandez-Sanchez M.T. Inhibition of protein phosphatases impairs the ability of astrocytes to detoxify hydrogen peroxide. Free Radic. Biol. Med. 2008;44:1806–1816. doi: 10.1016/j.freeradbiomed.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Fields R.D. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–531. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan L.P., Connaughton J.F., Jr., Kron R.E., Emich J.P. Neonatal abstinence syndrome: assessment and management. Addict. Dis. 1975;2:141–158. [PubMed] [Google Scholar]
- Frank D.A., Augustyn M., Zuckerman B.S. Neonatal neurobehavioral and neuroanatomic correlates of prenatal cocaine exposure. Problems of dose and confounding. Ann. N. Y. Acad. Sci. 1998;846:40–50. doi: 10.1111/j.1749-6632.1998.tb09725.x. [DOI] [PubMed] [Google Scholar]
- Gabriel M., Taylor C. Prenatal exposure to cocaine impairs neuronal coding of attention and discriminative learning. Ann. N. Y. Acad. Sci. 1998;846:194–212. [PubMed] [Google Scholar]
- Gawin F.H., Ellinwood E.H., Jr. Cocaine and other stimulants. Actions, abuse, and treatment. N. Engl. J. Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- Ghafari S., Golalipour M.J. Prenatal morphine exposure reduces pyramidal neurons in CA1, CA2 and CA3 subfields of mice hippocampus. Iran J. Basic Med. Sci. 2014;17:155–161. [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Mishra M.K., Das S., Kaushik D.K., Basu A. Tobacco carcinogen induces microglial activation and subsequent neuronal damage. J. Neurochem. 2009;110:1070–1081. doi: 10.1111/j.1471-4159.2009.06203.x. [DOI] [PubMed] [Google Scholar]
- Gkioka E., Korou L.M., Daskalopoulou A., Misitzi A., Batsidis E. Prenatal cocaine exposure and its impact on cognitive functions of offspring: a pathophysiological insight. Rev. Neurosci. 2016;27:523–534. doi: 10.1515/revneuro-2015-0064. [DOI] [PubMed] [Google Scholar]
- Granado N., Ares-Santos S., Oliva I., O'Shea E., Martin E.D. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol. Dis. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Granado N., Lastres-Becker I., Ares-Santos S., Oliva I., Martin E. Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia. 2011;59:1850–1863. doi: 10.1002/glia.21229. [DOI] [PubMed] [Google Scholar]
- Grewen K., Burchinal M., Vachet C., Gouttard S., Gilmore J.H. Prenatal cocaine effects on brain structure in early infancy. Neuroimage. 2014;101:114–123. doi: 10.1016/j.neuroimage.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha P., Harraz M.M., Snyder S.H. Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1417–1422. doi: 10.1073/pnas.1524860113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M.L., Liao K., Periyasamy P., Yang L., Cai Y. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy. 2015;11:995–1009. doi: 10.1080/15548627.2015.1052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Spencer J.W., Suess P.E., Hickey J.E., Better W.E., Herning R.I. Cognitive brain potential alterations in boys exposed to opiates: in utero and lifestyle comparisons. Addict. Behav. 1994;19:429–441. doi: 10.1016/0306-4603(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Haghighi A., Schwartz D.H., Abrahamowicz M., Leonard G.T., Perron M. Prenatal exposure to maternal cigarette smoking, amygdala volume, and fat intake in adolescence. JAMA Psychiatry. 2013;70:98–105. doi: 10.1001/archgenpsychiatry.2012.1101. [DOI] [PubMed] [Google Scholar]
- Hamdi Y., Madfai H., Belhareth R., Mokni M., Masmoudi-Kouki O., Amri M. Prenatal exposure to cigarette smoke enhances oxidative stress in astrocytes of neonatal rat. Toxicol. Mech. Methods. 2016;26:231–237. doi: 10.3109/15376516.2016.1156205. [DOI] [PubMed] [Google Scholar]
- Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Justinova Z., Hayashi E., Cormaci G., Mori T. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J. Pharmacol. Exp. Therapeut. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Haydon P.G. GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Heffelfinger A., Craft S., Shyken J. Visual attention in children with prenatal cocaine exposure. J. Int. Neuropsychol. Soc. 1997;3:237–245. [PubMed] [Google Scholar]
- Hofer S., Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hohmann C.F., Berger-Sweeney J. Cholinergic regulation of cortical development and plasticity. New twists to an old story. Perspect. Dev. Neurobiol. 1998;5:401–425. [PubMed] [Google Scholar]
- Howard L.A., Miksys S., Hoffmann E., Mash D., Tyndale R.F. Brain CYP2E1 is induced by nicotine and ethanol in rat and is higher in smokers and alcoholics. Br. J. Pharmacol. 2003;138:1376–1386. doi: 10.1038/sj.bjp.0705146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Sheng W.S., Lokensgard J.R., Peterson P.K. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Hung C.J., Wu C.C., Chen W.Y., Chang C.Y., Kuan Y.H. Depression-like effect of prenatal buprenorphine exposure in rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings D.E., Hamowy A.S., Williams E.M., Zmitrovich A.C. Prenatal administration of buprenorphine in the rat: effects on the rest-activity cycle at 22 and 30 days of age. Pharmacol. Biochem. Behav. 1996;55:607–613. doi: 10.1016/s0091-3057(96)00287-0. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Dakin K.A., Stevens B., Lee P.R., Kozlov S.V. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S.A., Williams M.T., Vorhees C.V. Mechanisms involved in the neurotoxic and cognitive effects of developmental methamphetamine exposure. Birth Defects Res. C Embryo Today. 2016;108:131–141. doi: 10.1002/bdrc.21130. [DOI] [PubMed] [Google Scholar]
- Jacobsen L.K., Slotkin T.A., Mencl W.E., Frost S.J., Pugh K.R. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Jayanthi S., Deng X., Noailles P.A., Ladenheim B., Cadet J.L. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S., McCoy M.T., Beauvais G., Ladenheim B., Gilmore K. Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M., Tyndale R.F. Induction and recovery time course of rat brain CYP2E1 after nicotine treatment. Drug Metab. Dispos. 2006;34:647–652. doi: 10.1124/dmd.105.008029. [DOI] [PubMed] [Google Scholar]
- Joshi M., Tyndale R.F. Regional and cellular distribution of CYP2E1 in monkey brain and its induction by chronic nicotine. Neuropharmacology. 2006;50:568–575. doi: 10.1016/j.neuropharm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ka M., Kook Y.H., Liao K., Buch S., Kim W.Y. Transactivation of TrkB by Sigma-1 receptor mediates cocaine-induced changes in dendritic spine density and morphology in hippocampal and cortical neurons. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmel B.Z., Gardner J.M. Prenatal cocaine exposure effects on arousal-modulated attention during the neonatal period. Dev. Psychobiol. 1996;29:463–480. doi: 10.1002/(SICI)1098-2302(199607)29:5<463::AID-DEV5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kawada K., Iekumo T., Saito R., Kaneko M., Mimori S. Aberrant neuronal differentiation and inhibition of dendrite outgrowth resulting from endoplasmic reticulum stress. J. Neurosci. Res. 2014;92:1122–1133. doi: 10.1002/jnr.23389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B.S., Sofroniew M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Cheong Y.P., So H.S., Lee K.M., Kim T.Y. Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase) Biochem. Pharmacol. 2001;61:779–786. doi: 10.1016/s0006-2952(01)00541-x. [DOI] [PubMed] [Google Scholar]
- Kindberg A.A., Bendriem R.M., Spivak C.E., Chen J., Handreck A. An in vitro model of human neocortical development using pluripotent stem cells: cocaine-induced cytoarchitectural alterations. Dis. Model Mech. 2014;7:1397–1405. doi: 10.1242/dmm.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M. The unfolded protein response triggered by environmental factors. Semin. Immunopathol. 2013;35:259–275. doi: 10.1007/s00281-013-0371-y. [DOI] [PubMed] [Google Scholar]
- Kivisto K., Tupola S., Kivitie-Kallio S. Prenatally buprenorphine-exposed children: health to 3 years of age. Eur. J. Pediatr. 2015;174:1525–1533. doi: 10.1007/s00431-015-2562-0. [DOI] [PubMed] [Google Scholar]
- Kleven M.S., Woolverton W.L., Seiden L.S. Lack of long-term monoamine depletions following repeated or continuous exposure to cocaine. Brain Res. Bull. 1988;21:233–237. doi: 10.1016/0361-9230(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Konijnenberg C., Melinder A. Prenatal exposure to methadone and buprenorphine: a review of the potential effects on cognitive development. Child Neuropsychol. 2011;17:495–519. doi: 10.1080/09297049.2011.553591. [DOI] [PubMed] [Google Scholar]
- Kourrich S., Hayashi T., Chuang J.Y., Tsai S.Y., Su T.P., Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl P., Zhang J., Lin Y., Lamba J., Assem M. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- L'Episcopo F., Tirolo C., Testa N., Caniglia S., Morale M.C. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson's disease involves cross talk between inflammatory and Wnt/beta-catenin signaling pathways: functional consequences for neuroprotection and repair. J. Neurosci. 2012;32:2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder J.M., Schambra U.B. Morphogenetic roles of acetylcholine. Environ. Health Perspect. 1999;107(Suppl. 1):65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro F., Giancotti L.A., Ilari S., Dagostino C., Gliozzi M. Inhibition of spinal oxidative stress by bergamot polyphenolic fraction attenuates the development of morphine induced tolerance and hyperalgesia in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie M.J., Card J.P., Hastings T.G. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp. Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ledesma J.C., Miquel M., Pascual M., Guerri C., Aragon C.M. Induction of brain cytochrome P450 2E1 boosts the locomotor-stimulating effects of ethanol in mice. Neuropharmacology. 2014;85:36–44. doi: 10.1016/j.neuropharm.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Lee C.T., Bendriem R.M., Kindberg A.A., Worden L.T., Williams M.P. Functional consequences of 17q21.31/WNT3-WNT9B amplification in hPSCs with respect to neural differentiation. Cell Rep. 2015;10:616–632. doi: 10.1016/j.celrep.2014.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.T., Bendriem R.M., Wu W.W., Shen R.F. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017;24:59. doi: 10.1186/s12929-017-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.T., Chen J., Hayashi T., Tsai S.Y., Sanchez J.F. A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Med. 2008;5:e117. doi: 10.1371/journal.pmed.0050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.T., Chen J., Kindberg A.A., Bendriem R.M., Spivak C.E. CYP3A5 mediates effects of cocaine on human neocorticogenesis: studies using an in vitro 3D self-organized hPSC model with a single cortex-like unit. Neuropsychopharmacology. 2017;42:774–784. doi: 10.1038/npp.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.T., Chen J., Worden L.T., Freed W.J. Cocaine causes deficits in radial migration and alters the distribution of glutamate and GABA neurons in the developing rat cerebral cortex. Synapse. 2011;65:21–34. doi: 10.1002/syn.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.T., Lehrmann E., Hayashi T., Amable R., Tsai S.Y. Gene expression profiling reveals distinct cocaine-responsive genes in human fetal CNS cell types. J. Addiction Med. 2009;3:218–226. doi: 10.1097/ADM.0b013e318199d863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Park J.R., Yang J., Kim E., Hong S.H. Nicotine inhibits the proliferation by upregulation of nitric oxide and increased HDAC1 in mouse neural stem cells. In Vitro Cell. Dev. Biol. Anim. 2014;50:731–739. doi: 10.1007/s11626-014-9763-0. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim M.S., Park C., Jung E.B., Choi D.H. Morphine prevents glutamate-induced death of primary rat neonatal astrocytes through modulation of intracellular redox. Immunopharmacol. Immunotoxicol. 2004;26:17–28. doi: 10.1081/iph-120029941. [DOI] [PubMed] [Google Scholar]
- Levitt P., Harvey J.A., Friedman E., Simansky K., Murphy E.H. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Levitt P., Moore R.Y. Development of the noradrenergic innervation of neocortex. Brain Res. 1979;162:243–259. doi: 10.1016/0006-8993(79)90287-7. [DOI] [PubMed] [Google Scholar]
- Lewis B.A., Singer L.T., Short E.J., Minnes S., Arendt R. Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicol. Teratol. 2004;26:617–627. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Li S., Yang L., Selzer M.E., Hu Y. Neuronal endoplasmic reticulum stress in axon injury and neurodegeneration. Ann. Neurol. 2013;74:768–777. doi: 10.1002/ana.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K., Guo M., Niu F., Yang L., Callen S.E., Buch S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J. Neuroinflammation. 2016;13:33. doi: 10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw W.J., Tsao C.M., Huang G.S., Wu C.C., Ho S.T. Phosphoproteomics and bioinformatics analyses of spinal cord proteins in rats with morphine tolerance. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidov H.G., Grzanna R., Molliver M.E. The serotonin innervation of the cerebral cortex in the rat--an immunohistochemical analysis. Neuroscience. 1980;5:207–227. doi: 10.1016/0306-4522(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Lidov H.G., Molliver M.E. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res. Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- Lin C.H., Cheng F.C., Lu Y.Z., Chu L.F., Wang C.H., Hsueh C.M. Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Exp. Neurol. 2006;201:225–233. doi: 10.1016/j.expneurol.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Lin J.H., Li H., Yasumura D., Cohen H.R., Zhang C. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little K.Y., Ramssen E., Welchko R., Volberg V., Roland C.J., Cassin B. Decreased brain dopamine cell numbers in human cocaine users. Psychiatr. Res. 2009;168:173–180. doi: 10.1016/j.psychres.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Liu J., Lester B.M., Neyzi N., Sheinkopf S.J., Gracia L. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. 2013;167:348–354. doi: 10.1001/jamapediatrics.2013.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S., Ferguson E., Leonard G., Perron M., Pike B. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch. Gen. Psychiatr. 2009;66:1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- Lu R., Liu X., Long H., Ma L. Effects of prenatal cocaine and heroin exposure on neuronal dendrite morphogenesis and spatial recognition memory in mice. Neurosci. Lett. 2012;522:128–133. doi: 10.1016/j.neulet.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Macedo D.S., de Vasconcelos S.M., dos Santos R.S., Aguiar L.M., Lima V.T. Cocaine alters catalase activity in prefrontal cortex and striatum of mice. Neurosci. Lett. 2005;387:53–56. doi: 10.1016/j.neulet.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Mahotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants Redox Signal. 2007;9:2277–2294. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Malanga C.J., 3rd, Kosofsky B.E. Mechanisms of action of drugs of abuse on the developing fetal brain. Clin. Perinatol. 1999;26:17–37. (v-vi) [PubMed] [Google Scholar]
- Marchetti B., Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol. Med. 2013;19:144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi-Kouki O., Douiri S., Hamdi Y., Kaddour H., Bahdoudi S. Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J. Neurochem. 2011;117:403–411. doi: 10.1111/j.1471-4159.2011.07185.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto R.R., Shaikh J., Wilson L.L., Vedam S., Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: evidence from in vivo and in vitro studies. Eur. Neuropsychopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes L.C. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev. Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- McQueen K., Murphy-Oikonen J. Neonatal abstinence syndrome. N. Engl. J. Med. 2016;375:2468–2479. doi: 10.1056/NEJMra1600879. [DOI] [PubMed] [Google Scholar]
- Miksys S., Tyndale R.F. The unique regulation of brain cytochrome P450 2 (CYP2) family enzymes by drugs and genetics. Drug Metab. Rev. 2004;36:313–333. doi: 10.1081/dmr-120034149. [DOI] [PubMed] [Google Scholar]
- Miller-Loncar C., Lester B.M., Seifer R., Lagasse L.L., Bauer C.R. Predictors of motor development in children prenatally exposed to cocaine. Neurotoxicol. Teratol. 2005;27:213–220. doi: 10.1016/j.ntt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Mirochnick M., Frank D.A., Cabral H., Turner A., Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. J. Pediatr. 1995;126:636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- Miron V.E., Kuhlmann T., Antel J.P. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim. Biophys. Acta. 2011;1812:184–193. doi: 10.1016/j.bbadis.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Molofsky A.V., Krencik R., Ullian E.M., Tsai H.H., Deneen B. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly V.J., Anblagan D., Quigley A., Cabez M.B., Cooper E.S. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin. 2018;18:9–14. doi: 10.1016/j.nicl.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R.L., Hsieh J., Barbosa A.C., Richardson J.A., Olson E.N. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoliu C., Sancho-Tello M., Azorin I., Burgal M., Valles S. Ethanol increases cytochrome P4502E1 and induces oxidative stress in astrocytes. J. Neurochem. 1995;65:2561–2570. doi: 10.1046/j.1471-4159.1995.65062561.x. [DOI] [PubMed] [Google Scholar]
- Morrow C.E., Vogel A.L., Anthony J.C., Ofir A.Y., Dausa A.T., Bandstra E.S. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J. Pediatr. Psychol. 2004;29:543–554. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaghinejad M., Karimian S.M., Motaghinejad O., Shabab B., Asadighaleni M., Fatima S. The effect of various morphine weaning regimens on the sequelae of opioid tolerance involving physical dependency, anxiety and hippocampus cell neurodegeneration in rats. Fundam. Clin. Pharmacol. 2015;29:299–309. doi: 10.1111/fcp.12121. [DOI] [PubMed] [Google Scholar]
- Muhammad A., Mychasiuk R., Nakahashi A., Hossain S.R., Gibb R., Kolb B. Prenatal nicotine exposure alters neuroanatomical organization of the developing brain. Synapse. 2012;66:950–954. doi: 10.1002/syn.21589. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Muhammad A., Gibb R., Kolb B. Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Res. 2013;1499:53–60. doi: 10.1016/j.brainres.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S., Kazeminezhad B., Dargahi L., Ahmadiani A. Maternal oral consumption of morphine increases Bax/Bcl-2 ratio and caspase 3 activity during early neural system development in rat embryos. J. Mol. Neurosci. 2010;41:156–164. doi: 10.1007/s12031-009-9312-6. [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S., Sahraei H., Bahadoran H., Sadooghi M., Salimi S.H. Effects of maternal oral morphine consumption on neural tube development in Wistar rats. Brain Res. Dev. Brain Res. 2005;159:12–17. doi: 10.1016/j.devbrainres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Nguyen E.C., McCracken K.A., Liu Y., Pouw B., Matsumoto R.R. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Niu L., Cao B., Zhu H., Mei B., Wang M. Impaired in vivo synaptic plasticity in dentate gyrus and spatial memory in juvenile rats induced by prenatal morphine exposure. Hippocampus. 2009;19:649–657. doi: 10.1002/hipo.20540. [DOI] [PubMed] [Google Scholar]
- Novikova S.I., He F., Bai J., Lidow M.S. Neuropathology of the cerebral cortex observed in a range of animal models of prenatal cocaine exposure may reflect alterations in genes involved in the Wnt and cadherin systems. Synapse. 2005;56:105–116. doi: 10.1002/syn.20134. [DOI] [PubMed] [Google Scholar]
- Nygaard E., Moe V., Slinning K., Walhovd K.B. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr. Res. 2015;78:330–335. doi: 10.1038/pr.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Paolicelli R.C., Ferretti M.T. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front. Synaptic Neurosci. 2017;9:9. doi: 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameshwaran K., Buabeid M.A., Bhattacharya S., Uthayathas S., Kariharan T. Long term alterations in synaptic physiology, expression of beta2 nicotinic receptors and ERK1/2 signaling in the hippocampus of rats with prenatal nicotine exposure. Neurobiol. Learn. Mem. 2013;106:102–111. doi: 10.1016/j.nlm.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Park H.J., Kim J.J., Lee S.K., Seok J.H., Chun J. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Nawazkhan I., Leonard G., Perron M., Pike G.B. Corpus callosum in adolescent offspring exposed prenatally to maternal cigarette smoking. Neuroimage. 2008;40:435–441. doi: 10.1016/j.neuroimage.2007.10.066. [DOI] [PubMed] [Google Scholar]
- Pavlovsky A.A., Boehning D., Li D., Zhang Y., Fan X., Green T.A. Psychological stress, cocaine and natural reward each induce endoplasmic reticulum stress genes in rat brain. Neuroscience. 2013;246:160–169. doi: 10.1016/j.neuroscience.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M., Pekna M., Messing A., Steinhauser C., Lee J.M. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- Periyasamy P., Guo M.L., Buch S. Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy. 2016;12:1310–1329. doi: 10.1080/15548627.2016.1183844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M.R., Higley M.J., Mineur Y.S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubill D., Chipana C., Camins A., Pallas M., Camarasa J., Escubedo E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol. Appl. Pharmacol. 2005;204:57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Qiao D., Seidler F.J., Slotkin T.A. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Rando K., Chaplin T.M., Potenza M.N., Mayes L., Sinha R. Prenatal cocaine exposure and gray matter volume in adolescent boys and girls: relationship to substance use initiation. Biol. Psychiatry. 2013;74:482–489. doi: 10.1016/j.biopsych.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repo J.K., Pesonen M., Mannelli C., Vahakangas K., Loikkanen J. Exposure to ethanol and nicotine induces stress responses in human placental BeWo cells. Toxicol. Lett. 2014;224:264–271. doi: 10.1016/j.toxlet.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Richardson G.A., Larkby C., Goldschmidt L., Day N.L. Adolescent initiation of drug use: effects of prenatal cocaine exposure. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin M.J., Davis P.E., Lemaster J.L., Cabral H.J., Warfield S.K. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.E., Mo Q., Maher J.R., Wallace M.J., Kunko P.M. Perinatal exposure to methadone affects central cholinergic activity in the weanling rat. Drug Alcohol Depend. 1996;41:119–126. doi: 10.1016/0376-8716(96)01238-0. [DOI] [PubMed] [Google Scholar]
- Robson M.J., Noorbakhsh B., Seminerio M.J., Matsumoto R.R. Sigma-1 receptors: potential targets for the treatment of substance abuse. Curr. Pharmaceut. Des. 2012;18:902–919. doi: 10.2174/138161212799436601. [DOI] [PubMed] [Google Scholar]
- Robson M.J., Seminerio M.J., McCurdy C.R., Coop A., Matsumoto R.R. Sigma Receptor antagonist attenuation of methamphetamine-induced neurotoxicity is correlated to body temperature modulation. Pharmacol. Rep. 2013;65:343–349. doi: 10.1016/s1734-1140(13)71009-0. [DOI] [PubMed] [Google Scholar]
- Robson M.J., Turner R.C., Naser Z.J., McCurdy C.R., Huber J.D., Matsumoto R.R. SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation. Exp. Neurol. 2013;247:134–142. doi: 10.1016/j.expneurol.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeckel L.A., Le Coz G.M., Gaveriaux-Ruff C., Simonin F. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience. 2016;338:160–182. doi: 10.1016/j.neuroscience.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cerebr. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Roos A., Jones G., Howells F.M., Stein D.J., Donald K.A. Structural brain changes in prenatal methamphetamine-exposed children. Metab. Brain Dis. 2014;29:341–349. doi: 10.1007/s11011-014-9500-0. [DOI] [PubMed] [Google Scholar]
- Ryan L.J., Martone M.E., Linder J.C., Groves P.M. Cocaine, in contrast to D-amphetamine, does not cause axonal terminal degeneration in neostriatum and agranular frontal cortex of Long-Evans rats. Life Sci. 1988;43:1403–1409. doi: 10.1016/0024-3205(88)90307-4. [DOI] [PubMed] [Google Scholar]
- Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- Salzwedel A.P., Grewen K.M., Vachet C., Gerig G., Lin W., Gao W. Prenatal drug exposure affects neonatal brain functional connectivity. J. Neurosci. 2015;35:5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel A.P., Grewen K.M., Goldman B.D., Gao W. Thalamocortical functional connectivity and behavioral disruptions in neonates with prenatal cocaine exposure. Neurotoxicol. Teratol. 2016;56:16–25. doi: 10.1016/j.ntt.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E.S., Bigbee J.W., Fobbs W., Robinson S.E., Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56:1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu J.K., Gardaneh M., Iwasiow R., Lanthier P., Gangaraju S. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 2009;33:405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Schlumpf M., Shoemaker W.J., Bloom F.E. Innervation of embryonic rat cerebral cortex by catecholamine-containing fibers. J. Comp. Neurol. 1980;192:361–376. doi: 10.1002/cne.901920210. [DOI] [PubMed] [Google Scholar]
- Seguin J.R. Neurocognitive elements of antisocial behavior: relevance of an orbitofrontal cortex account. Brain Cogn. 2004;55:185–197. doi: 10.1016/S0278-2626(03)00273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminerio M.J., Hansen R., Kaushal N., Zhang H.T., McCurdy C.R., Matsumoto R.R. The evaluation of AZ66, an optimized sigma receptor antagonist, against methamphetamine-induced dopaminergic neurotoxicity and memory impairment in mice. Int. J. Neuropsychopharmacol. 2013;16:1033–1044. doi: 10.1017/S1461145712000831. [DOI] [PubMed] [Google Scholar]
- Seo S., Kwon Y.S., Yu K., Kim S.W., Kwon O.Y. Naloxone induces endoplasmic reticulum stress in PC12 cells. Mol. Med. Rep. 2014;9:1395–1399. doi: 10.3892/mmr.2014.1935. [DOI] [PubMed] [Google Scholar]
- Shah A., Kumar S., Simon S.D., Singh D.P., Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E.H., Bian S., Shim Y.B., Rahman M.A., Chung K.T. Cocaine increases endoplasmic reticulum stress protein expression in striatal neurons. Neuroscience. 2007;145:621–630. doi: 10.1016/j.neuroscience.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Siciliano C.A., Calipari E.S., Ferris M.J., Jones S.R. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem. Neurosci. 2015;6:27–36. doi: 10.1021/cn5002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil S., Periyasamy P., Guo M.L., Callen S., Buch S. Morphine-mediated brain region-specific astrocytosis involves the ER stress-autophagy Axis. Mol. Neurobiol. 2018 Aug;55(8):6713–6733. doi: 10.1007/s12035-018-0878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Nave K.A. Oligodendrocytes: myelination and axonal support. Cold Spring Harb. Perspect Biol. 2015;8:a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer L.T., Arendt R., Minnes S., Farkas K., Salvator A. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer L.T., Minnes S., Min M.O., Lewis B.A., Short E.J. Prenatal cocaine exposure and child outcomes: a conference report based on a prospective study from Cleveland. Hum. Psychopharmacol. 2015;30:285–289. doi: 10.1002/hup.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer L.T., Nelson S., Short E., Min M.O., Lewis B. Prenatal cocaine exposure: drug and environmental effects at 9 years. J. Pediatr. 2008;153:105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R., Riley M.A., Vathy I. Cross-generational effect of prenatal morphine exposure on neurobehavioral development of rat pups. Physiol. Res. 2005;54:655–660. [PubMed] [Google Scholar]
- Slikker W., Jr., Xu Z.A., Levin E.D., Slotkin T.A. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin T.A. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin T.A., Seidler F.J., Qiao D., Aldridge J.E., Tate C.A. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C: neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology. 2005;30:129–144. doi: 10.1038/sj.npp.1300544. [DOI] [PubMed] [Google Scholar]
- Smith T.J., Guo Z., Gonzalez F.J., Guengerich F.P., Stoner G.D., Yang C.S. Metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human lung and liver microsomes and cytochromes P-450 expressed in hepatoma cells. Cancer Res. 1992;52:1757–1763. [PubMed] [Google Scholar]
- Soepatmi S. Developmental outcomes of children of mothers dependent on heroin or heroin/methadone during pregnancy. Acta Paediatr. Suppl. 1994;404:36–39. doi: 10.1111/j.1651-2227.1994.tb13382.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Astrogliosis. Cold Spring Harb. Perspect Biol. 2014;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Leow A.D., Bookheimer S.Y., Smith L.M., O'Connor M.J. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J. Neurosci. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R., Justinova Z., Hayashi T., Takebayashi M., Goldberg S.R., Su T.P. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berlin) 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Stipursky J., Spohr T.C., Sousa V.O., Gomes F.C. Neuron-astroglial interactions in cell-fate commitment and maturation in the central nervous system. Neurochem. Res. 2012;37:2402–2418. doi: 10.1007/s11064-012-0798-x. [DOI] [PubMed] [Google Scholar]
- Su T.P., Hayashi T. Cocaine affects the dynamics of cytoskeletal proteins via sigma(1) receptors. Trends Pharmacol. Sci. 2001;22:456–458. doi: 10.1016/s0165-6147(00)01740-5. [DOI] [PubMed] [Google Scholar]
- Takarada T., Nakamichi N., Kitajima S., Fukumori R., Nakazato R. Promoted neuronal differentiation after activation of alpha4/beta2 nicotinic acetylcholine receptors in undifferentiated neural progenitors. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.W., Duan T.T., Zhou Q.X., Ding Z.Y., Jing L. Impaired contextual fear extinction and hippocampal synaptic plasticity in adult rats induced by prenatal morphine exposure. Addict. Biol. 2015;20:652–662. doi: 10.1111/adb.12158. [DOI] [PubMed] [Google Scholar]
- Tao P.L., Chen C.F., Huang E.Y. Dextromethorphan attenuated the higher vulnerability to inflammatory thermal hyperalgesia caused by prenatal morphine exposure in rat offspring. J. Biomed. Sci. 2011;18:64. doi: 10.1186/1423-0127-18-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terplan M., Smith E.J., Kozloski M.J., Pollack H.A. Methamphetamine use among pregnant women. Obstet. Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Thomas D.M., Kuhn D.M. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J. Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Thomas D.M., Walker P.D., Benjamins J.A., Geddes T.J., Kuhn D.M. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J. Pharmacol. Exp. Therapeut. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thompson B.L., Levitt P., Stanwood G.D. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat. Rev. Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyssen Van Beveren T., Little B.B., Spence M.J. Effects of prenatal cocaine exposure and postnatal environment on child development. Am. J. Hum. Biol. 2000;12:417–428. doi: 10.1002/(SICI)1520-6300(200005/06)12:3<417::AID-AJHB12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Tindberg N., Ingelman-Sundberg M. Expression, catalytic activity, and inducibility of cytochrome P450 2E1 (CYP2E1) in the rat central nervous system. J. Neurochem. 1996;67:2066–2073. doi: 10.1046/j.1471-4159.1996.67052066.x. [DOI] [PubMed] [Google Scholar]
- Tolia V.N., Murthy K., Bennett M.M., Miller E.S., Benjamin D.K. Antenatal methadone vs buprenorphine exposure and length of hospital stay in infants admitted to the intensive care unit with neonatal abstinence syndrome. J. Perinatol. 2018;38:75–79. doi: 10.1038/jp.2017.157. [DOI] [PubMed] [Google Scholar]
- Tong V.T., Dietz P.M., Morrow B., D'Angelo D.V., Farr S.L. Trends in smoking before, during, and after pregnancy--pregnancy risk assessment monitoring system, United States, 40 sites, 2000-2010. MMWR Surveill Summ. 2013;62:1–19. [PubMed] [Google Scholar]
- Toro R., Leonard G., Lerner J.V., Lerner R.M., Perron M. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33:1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- Tronick E.Z., Frank D.A., Cabral H., Mirochnick M., Zuckerman B. Late dose-response effects of prenatal cocaine exposure on newborn neurobehavioral performance. Pediatrics. 1996;98:76–83. [PMC free article] [PubMed] [Google Scholar]
- Tsai S.Y., Chuang J.Y., Tsai M.S., Wang X.F., Xi Z.X. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6562–E6570. doi: 10.1073/pnas.1518894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S.Y., Hayashi T., Harvey B.K., Wang Y., Wu W.W. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22468–22473. doi: 10.1073/pnas.0909089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S.Y., Pokrass M.J., Klauer N.R., Nohara H., Su T.P. Sigma-1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6742–6747. doi: 10.1073/pnas.1422001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S.Y., Rothman R.K., Su T.P. Insights into the Sigma-1 receptor chaperone's cellular functions: a microarray report. Synapse. 2012;66:42–51. doi: 10.1002/syn.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang D., Ho K.P., Wen H.L. Effect of maternal methadone administration on the development of multiple forms of monoamine oxidase in rat brain and liver. Brain Res. 1986;391:187–192. doi: 10.1016/0165-3806(86)90282-8. [DOI] [PubMed] [Google Scholar]
- Upadhya S.C., Tirumalai P.S., Boyd M.R., Mori T., Ravindranath V. Cytochrome P4502E (CYP2E) in brain: constitutive expression, induction by ethanol and localization by fluorescence in situ hybridization. Arch. Biochem. Biophys. 2000;373:23–34. doi: 10.1006/abbi.1999.1477. [DOI] [PubMed] [Google Scholar]
- van Baar A. Development of infants of drug dependent mothers. JCPP (J. Child Psychol. Psychiatry) 1990;31:911–920. doi: 10.1111/j.1469-7610.1990.tb00833.x. [DOI] [PubMed] [Google Scholar]
- van Baar A., de Graaff B.M. Cognitive development at preschool-age of infants of drug-dependent mothers. Dev. Med. Child Neurol. 1994;36:1063–1075. doi: 10.1111/j.1469-8749.1994.tb11809.x. [DOI] [PubMed] [Google Scholar]
- Verney C., Berger B., Adrien J., Vigny A., Gay M. Development of the dopaminergic innervation of the rat cerebral cortex. A light microscopic immunocytochemical study using anti-tyrosine hydroxylase antibodies. Brain Res. 1982;281:41–52. doi: 10.1016/0165-3806(82)90111-0. [DOI] [PubMed] [Google Scholar]
- Verney C., Milosevic A., Alvarez C., Berger B. Immunocytochemical evidence of well-developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. J. Comp. Neurol. 1993;336:331–344. doi: 10.1002/cne.903360303. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018 Aug;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Tomasi D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G.C., Ricaurte G.A., Seiden L.S., Schuster C.R., Miller R.J., Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wake H., Moorhouse A.J., Miyamoto A., Nabekura J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013;36:209–217. doi: 10.1016/j.tins.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Moe V., Slinning K., Due-Tonnessen P., Bjornerud A. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage. 2007;36:1331–1344. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Watts R., Amlien I., Woodward L.J. Neural tract development of infants born to methadone-maintained mothers. Pediatr. Neurol. 2012;47:1–6. doi: 10.1016/j.pediatrneurol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang Y., Han T.Z. Prenatal exposure to heroin in mice elicits memory deficits that can be attributed to neuronal apoptosis. Neuroscience. 2009;160:330–338. doi: 10.1016/j.neuroscience.2009.02.058. [DOI] [PubMed] [Google Scholar]
- Warton F.L., Meintjes E.M., Warton C.M.R., Molteno C.D., Lindinger N.M., Carter R.C., Zöllei L., Wintermark P., Jacobson J.L., van der Kouwe A., Jacobson S.W. Prenatal methamphetamine exposure is associated with reduced subcortical volumes in neonates. Neurotoxicol. Teratol. 2018;65:51–59. doi: 10.1016/j.ntt.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warton F.L., Taylor P.A., Warton C.M.R., Molteno C.D., Wintermark P., Lindinger N.M., Zöllei L., van der Kouwe A., Jacobson J.L., Jacobson S.W., Meintjes E.M. Prenatal methamphetamine exposure is associated with corticostriatal white matter changes in neonates. Metab. Brain Dis. 2018;33:507–522. doi: 10.1007/s11011-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A. Neural mechanisms of the reinforcing action of cocaine. NIDA Res. Monogr. 1984;50:15–33. [PubMed] [Google Scholar]
- Wong M.K., Holloway A.C., Hardy D.B. Nicotine directly induces endoplasmic reticulum stress response in rat placental trophoblast giant cells. Toxicol. Sci. 2016;151:23–34. doi: 10.1093/toxsci/kfw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.K., Nicholson C.J., Holloway A.C., Hardy D.B. Maternal nicotine exposure leads to impaired disulfide bond formation and augmented endoplasmic reticulum stress in the rat placenta. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.C., Hung C.J., Shen C.H., Chen W.Y., Chang C.Y. Prenatal buprenorphine exposure decreases neurogenesis in rats. Toxicol. Lett. 2014;225:92–101. doi: 10.1016/j.toxlet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Xu X., Huang E., Tai Y., Zhao X., Chen X. Nupr1 modulates methamphetamine-induced dopaminergic neuronal apoptosis and autophagy through CHOP-Trib3-Mediated endoplasmic reticulum stress signaling pathway. Front. Mol. Neurosci. 2017;10:203. doi: 10.3389/fnmol.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto B.K., Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J. Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto B.K., Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J. Pharmacol. Exp. Therapeut. 1998;287:107–114. [PubMed] [Google Scholar]
- Yamazaki H., Inui Y., Yun C.H., Guengerich F.P., Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992;13:1789–1794. doi: 10.1093/carcin/13.10.1789. [DOI] [PubMed] [Google Scholar]
- Yang L., Yao H., Chen X., Cai Y., Callen S., Buch S. Role of sigma receptor in cocaine-mediated induction of glial fibrillary acidic protein: implications for HAND. Mol. Neurobiol. 2016;53:1329–1342. doi: 10.1007/s12035-015-9094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.N., Liu C.A., Chung M.Y., Huang H.C., Yeh G.C. Alterations of postsynaptic density proteins in the hippocampus of rat offspring from the morphine-addicted mother: beneficial effect of dextromethorphan. Hippocampus. 2006;16:521–530. doi: 10.1002/hipo.20179. [DOI] [PubMed] [Google Scholar]
- Yao H., Yang Y., Kim K.J., Bethel-Brown C., Gong N. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Rubic M., Seah J., Rae C., Wright I.M. Do maternal opioids reduce neonatal regional brain volumes? A pilot study. J. Perinatol. 2014;34:909–913. doi: 10.1038/jp.2014.111. [DOI] [PubMed] [Google Scholar]
- Zakiniaeiz Y., Yip S.W., Balodis I.M., Lacadie C.M., Scheinost D., Constable R.T., Mayes L.C., Sinha R., Potenza M.N. Altered functional connectivity to stressful stimuli in prenatally cocaine-exposed adolescents. Drug Alcohol Depend. 2017;180:129–136. doi: 10.1016/j.drugalcdep.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon I.S., McLaughlin P.J. Effect of chronic maternal methadone exposure on perinatal development. Biol. Neonate. 1977;31:271–282. doi: 10.1159/000240975. [DOI] [PubMed] [Google Scholar]
- Zecevic N., Verney C. Development of the catecholamine neurons in human embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J. Comp. Neurol. 1995;351:509–535. doi: 10.1002/cne.903510404. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang C., Ren J., Guo X., Yun K. Morphine protects spinal cord astrocytes from glutamate-induced apoptosis via reducing endoplasmic reticulum stress. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Loonam T.M., Noailles P.A., Angulo J.A. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann. N. Y. Acad. Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Zhu J.P., Xu W., Angulo J.A. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res. 2005;1049:171–181. doi: 10.1016/j.brainres.2005.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.P., Xu W., Angulo J.A. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo H.Q., Huang L., Huang H.Q., Cai Z. Effects of chronic tramadol exposure on the zebrafish brain: a proteomic study. J. Proteomics. 2012;75:3351–3364. doi: 10.1016/j.jprot.2012.03.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.