Abstract
Objective.
We aimed to study the longitudinal associations between genetic risk, change in diet quality, and change in visceral adipose tissue (ΔVAT), abdominal subcutaneous adipose tissue (ΔSAT), and pericardial adipose tissue (ΔPAT).
Methods.
We analyzed 1,677 Framingham Heart Study participants who had ectopic fat depots measured using computed tomography. We quantified diet quality using a Mediterranean-style diet score (MDS) and genetic risk by depot-specific genetic risk scores (GRSs).
Results.
Per standard deviation improvement in MDS, there was 50 cm3 (95% CI: 14, 86; p=0.007) less fat accumulation in VAT; 52 cm3 (95% CI: 12, 92; p=0.01) less fat accumulation in SAT; and 1.3 cm3 (95% CI: 0.1, 2.4; p=0.04) less fat accumulation in PAT. No association was observed between GRSs and ΔVAT or ΔSAT. One standard deviation increase in the PAT GRS was associated with 1.2 cm3 (95% CI: 0.1, 2.3; p=0.03) increase in ΔPAT. In participants with higher PAT GRS, those with ΔMDS ≥0 had a favorable change in PAT compared to the counterparts with ΔMDS <0 (p=0.008).
Conclusions.
Longitudinal improvements in diet quality are associated with less ectopic fat accumulation. Our study suggests that diet quality may play a critical role in improving ectopic adiposity profiles.
Introduction
Visceral adipose tissue (VAT) has been associated with metabolic risk factors and hypothesized to have systemic effects on cardiovascular disease (1). Local ectopic fat depots, such as pericardial adipose tissue (PAT) that surrounds the heart, may also contribute to cardiovascular disease (2). For example, PAT has been associated with coronary artery calcium independent of VAT (3). Preventing excess fat accumulation in these depots therefore may be important to cardiometabolic health.
Adopting a healthy diet is recommended for the prevention of cardiometabolic diseases. Prior observational studies have shown that high quality diet, assessed using scores such as the Mediterranean-style diet score (MDS), was associated with reduced risk of incident diabetes and cardiovascular disease (4, 5, 6). Recent studies also showed that improved dietary scores were associated with reduced body weight (7) and less fat accumulation in the liver (8). However, the longitudinal associations between change in diet quality and change in VAT and PAT are not well studied.
In two large meta-analyses of genome-wide association studies (GWAS), 97 and 49 genetic loci were associated with body mass index (BMI) and waist to hip ratio (WHR), respectively, suggesting that genetic factors play an important role in determining the interindividual variation in adiposity (9, 10). In addition, one recent study identified several genetic loci that might affect fat distribution in VAT and PAT (11). However, these studies exclusively analyzed cross-sectional associations. Whether these genetic loci regulate changes in VAT and PAT is not well studied. Furthermore, the extent to which these genetic loci modify the associations between diet and VAT and PAT remains unclear.
To fill these knowledge gaps, we analyzed longitudinal data in the Framingham Heart Study (FHS) to examine the associations between change in diet quality and change in VAT, abdominal subcutaneous adipose tissue (SAT), and PAT. We also aimed to study the effect of previously identified genetic loci on change in VAT, SAT, and PAT and to test potential gene-diet interactions for ectopic fat accumulation in these depots.
Methods
Study sample.
We included participants from the FHS Offspring and Third Generation cohorts, which have been described elsewhere (12, 13). Briefly, 2,869 participants attended both the seventh examination (1998–2001) and eighth examination (2005–2008) in the Offspring cohort and 3,411 participants attended both the first examination (2002–2005) and the second examination (2008–2011) in the Third Generation cohort. After we excluded participants without computed tomography measures at the baseline examination (the seventh examination in the Offspring cohort and first examination in the Third Generation cohort) or follow-up examination (the eighth examination in the Offspring cohort and second examination in the Third Generation cohort), 2,001 participants remained with abdominal fat (VAT and SAT) measured at both baseline and follow-up. After additional exclusion of participants with missing dietary data and important covariates, 1,677 individuals (694 from the Offspring cohort and 983 from the Third Generation cohort) remained in the analysis for diet and abdominal fat change, 1,637 remained in the analysis for diet and PAT change, 1,583 remained in the gene-diet interaction analysis for abdominal fat change, and 1,544 remained in the gene-diet interaction analysis for PAT change. The FHS protocols and procedures were approved by the Institutional Review Board for Human Research at Boston University Medical Center. All participants provided written informed consent.
Diet quality scores.
We mailed a previously validated, self-administered, 126-item food frequency questionnaire (FFQ) to participants to measure consumption of dietary components for the year prior to the examinations (14). The FFQ was administered at both baseline and follow-up examinations. The collected data were used for analysis if less than 13 food items were missing and the total energy intakes were between 600 and 4200 kcal/day for men or between 600 and 4000 kcal/day for women.
We used a Mediterranean-style diet score (MDS) To assess overall diet quality. MDS was created based on the total consumption of the nine components (Table S1): vegetables, fruits, whole grains, legumes, nuts, fish, ratio of monounsaturated fatty acids (MUFA) to saturated fatty acids (SFA), red meat, and alcohol (15). We categorized intake of each dietary component (except for red meat and alcohol) into mutually exclusive, sex- and examination-specific quartiles, and assigned scores of 0, 1, 2, and 3 to the lowest to highest quartile categories. For red meat, we reversed the order of the scores so that the lowest to highest quartile categories were assigned scores of 3, 2, 1, and 0. For alcohol, we assigned a score of 1 if consumption was ≥10 grams/day and ≤25 grams/day for men or ≥5 grams/day and ≤15 grams/day for women and 0 for all other consumptions. We calculated the MDS by summing scores for all nine individual components. The MDS ranged from 0 (representing low diet quality) to 25 (representing high diet quality). As part of the sensitivity analysis, we also constructed the AHEI, which is comprised of 11 components (Table S1) (5). The AHEI, comprehensively ranging from 0 to 110, was developed based the 2010 Dietary Guideline for Americans (5).
Adipose tissue measurements.
The protocols for adiposity measurements in FHS have been described elsewhere (16). We used abdominal and chest multidetector computed tomography (CT, General Electric Health Care) to measure VAT, SAT, and PAT. We used an 8-slice scanner at baseline (2002–2005) and a 64-slice scanner at follow-up (2008–2011). We translated Hounsfield units (HU) to volume (cm3) for each fat depot using a dedicated offline workstation (Aquarius 3D Workstation). The readers manually outlined the abdominal muscular wall separating the visceral from the subcutaneous fat depots (16). We calculated the VAT to SAT ratio (VSR) to represent the tendency of fat storage in VAT relative to SAT. The readers also outlined the pericardial sac to estimate PAT volume (17). A previous study of FHS participants determined that reproducibility was excellent for VAT, SAT, and PAT volume measurements with an intra-observer correlation of ≥ 0.97 and inter-observer correlation of ≥ 0.95 (18).
Genetic variants selection and genotype.
To date, several single nucleotide polymorphisms (SNPs) associated with ectopic fat depots have been identified through a recent meta-analysis of GWAS (11). We analyzed all reported SNPs from this study (11), including two SNPs for VAT, three SNPs for SAT, three SNPs for VSR, and four SNPs for PAT (Table S2). Because effect sizes were not reported for these SNPs in the GWAS (11), we calculated unweighted, depot-specific genetic risk scores (GRSs) by summing the number of risk alleles at each locus for each trait. The ectopic fat depots of interest in the present study are directly correlated with general adiposity (1, 3). Therefore, we created two GRSs using SNPs identified in large consortia of GWAS for two easily accessible measures of body fat, 93 SNPs for BMI (Table S3) and 47 SNPs for waist to hip ratio (WHR) (Table S4) (9, 10). SNPs significant in GWAS but had imputation quality R-squared <0.3 in FHS were excluded (four for BMI GRS and two for WHR GRS). GRSs for BMI and WHR were weighted by multiplying the number of risk alleles at each locus with corresponding effect sizes reported in the meta-analysis of GWAS. Genotyping was performed with Affymetrix 550K array (replicated concordance >99% and overall call rate >95%). We used MACH with 1000G phase 1 version 3 (2012) to impute all variants (19).
Covariate assessments.
We assessed all covariates using standard protocols in the Framingham research clinic site (20). First, BMI was ascertained by dividing weight by height squared (kg/m2). We defined current smokers as participants who reported that they had smoked at least one cigarette daily in the previous year. We generated a physical activity score based on participants’ responses to a survey on the intensity of and time spent performing certain activities (21).
Statistical analysis.
Participants’ baseline characteristics across the quartiles of change in MDS (ΔMDS) between baseline and follow-up were presented as means and standard deviations (SDs) or proportions with counts. In our primary analysis, we examined the relations between ΔMDS and VAT volume change (ΔVAT), SAT volume change (ΔSAT), VSR change (ΔVSR) and PAT volume change (ΔPAT) between baseline and follow-up. The initial model adjusted for baseline measures of the outcome of interest (i.e. baseline VAT for ΔVAT as the outcome), baseline values of MDS, age, sex, baseline energy intake, baseline physical activity score, and baseline smoking status. We additionally adjusted for baseline BMI and changes in energy intake, physical activity score, and smoking status in a separate model. We used random effect linear mixed models to account for family structure in our study sample. The adjusted means and 95% confidence intervals (95%CIs) were estimated for all outcomes according to quartile categories for ΔMDS, as well as per SD increase in ΔMDS. We also tested linear association using continuous ΔMDS as an independent variable.
We examined the associations between GRSs (unweighted depot-specific GRSs) and baseline and change in ectopic fat depots of interest. For example, we examined the cross-sectional association between the VAT-specific GRS and baseline VAT as well as the longitudinal association between the VAT-specific GRS and ΔVAT. In the cross-sectional analysis, we adjusted for sex, age, and smoking status in the base model and additionally adjusted for physical activity score, energy intake, MDS, BMI, and GRSs for BMI and WHR in a multivariable model. In the longitudinal analysis, we adjusted for sex, age, smoking status, and baseline outcome (i.e. for ΔVAT, we adjusted for baseline VAT) in the base model and additionally adjusted for baseline BMI, GRSs for BMI and WHR, physical activity score, energy intake, MDS, and change in MDS, energy intake, smoking status, and physical activity in a multivariable model. To test the potential gene-diet interaction, we included the product term of ΔMDS and GRS in the longitudinal analysis (e.g., ΔMDS × VAT-specific GRS in the longitudinal analysis for ΔVAT). Secondarily, we also tested the abovementioned associations and interaction using BMI GRS and WHR GRS to replace depot-specific GRSs. Baseline BMI was not adjusted for in the analysis using BMI GRS.
Given the established sex dimorphism on abdominal fat distribution, we performed an interaction analysis by testing the significance of the product of ΔMDS and sex for change in ectopic depots. We also conducted sex-specific analyses for the associations between ΔMDS and change in ectopic depots. We conducted all statistical analyses using R statistical analysis software (version 3.3.3; https://www.Rproject.org). We considered a two-tailed p <0.05 value as statistically significant given the exploratory nature of the present study.
Results
Participant characteristics.
Baseline characteristics for the 1,677 participants are shown in Table 1. Median follow-up was six years. There were no noticeable differences in age, sex, smoking status, physical activity levels, and BMI across the quartile categories of ΔMDS. We observed a large interindividual variation for ΔMDS (mean ± SD: 0.3 ± 4.0; range: −15 – 16; Figure S1). In addition, the distribution of ΔMDS was largely similar in men and women (Figure S2). Overall, in participants with improved diet quality (i.e. increased ΔMDS), intake of all MDS components improved (Table S5).
Table 1.
Baseline participant characteristics according to quartiles of MDS change
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Median MDS change | −4 | −1 | 1 | 5 |
| Range MDS change | (−15, −3) | (−2, 0) | (1, 2) | (3, 16) |
| N | 390 | 489 | 313 | 485 |
| Age, years | 51±10 | 52±10 | 51±9 | 51±10 |
| Female, % (n) | 48 (189) | 48 (237) | 51 (161) | 52 (250) |
| Current smoker, % (n) | 9 (37) | 10 (47) | 7 (21) | 9 (45) |
| Physical activity score | 37±7 | 37±7 | 38±7 | 37±7 |
| BMI, kg/m2 | 27.4±4.8 | 27.6±4.9 | 27.6±5.1 | 27.5±5.1 |
| VAT volume, cm3 | 1735±927 | 1804±961 | 1711±973 | 1744±1029 |
| SAT volume, cm3 | 2894±1389 | 2863±1364 | 2955±1456 | 2777±1270 |
| VSR | 0.66±0.37 | 0.69±0.37 | 0.62±0.34 | 0.67±0.39 |
| PAT volume, cm3 | 106±37 | 110±43 | 110±41 | 108±41 |
| Energy intake, kcal | 2053±624 | 1989±638 | 1893±631 | 1876±595 |
| Baseline MDS | 15±4 | 13±4 | 12±4 | 10±4 |
Values are mean ± standard deviation or percent (count)
BMI=Body Mass Index; VAT=Visceral Adipose Tissue; SAT= Abdominal Subcutaneous Adipose Tissue; VSR=VAT to
SAT Ratio; PAT=Pericardial Adipose Tissue; MDS=Mediterranean-style diet score
Change in MDS and ΔVAT.
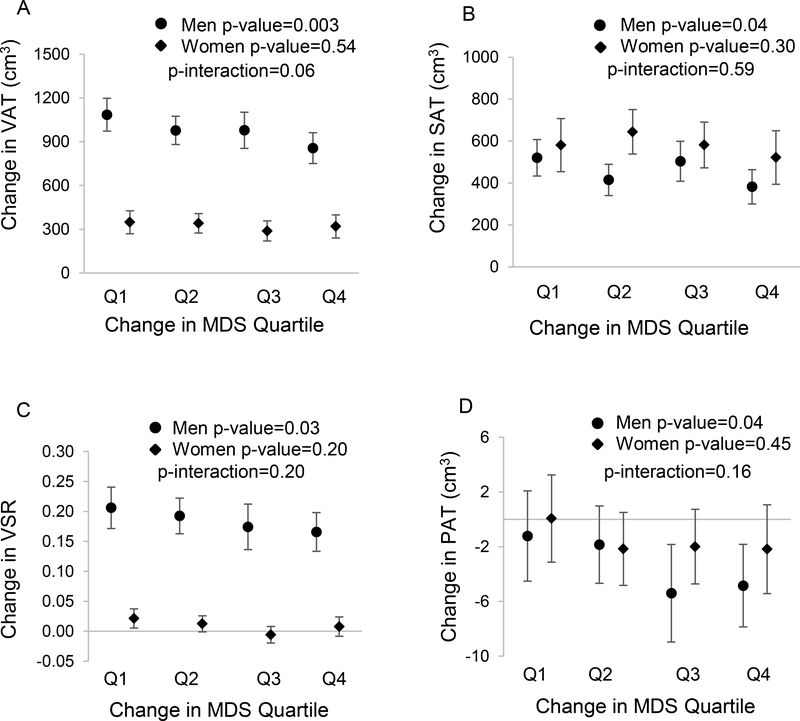
Improved diet quality was associated with less fat accumulation in VAT (Table 2). After adjustment for sex, age, baseline VAT volume, baseline MDS, and baseline and change in energy intake, physical activity level, and smoking status, as well as baseline BMI, ΔVAT was 715 cm3 (95% CI: 646, 784), 658 cm3 (95% CI: 599, 716), 631 cm3 (95% CI: 557, 704), and 595 cm3 (95% CI: 533, 657) from the lowest quartile of ΔMDS (i.e. largest decline in diet quality score) to the highest quartile of ΔMDS (i.e. largest improvement in diet quality score). For each SD increase in ΔMDS, there was 50 cm3 (95% CI: 14, 86) less fat accumulation in VAT (p-value=0.007). As shown in Figure 1A, the inverse association between ΔMDS and ΔVAT was significant in men (p-value=0.003) but not in women (p-value=0.54). However, the interaction between sex and ΔMDS for ΔVAT was not statistically significant (p-interaction=0.06).
Table 2.
Associations between change in MDS and change in ectopic fat depots (adjusted mean and 95% confidence interval)
| Quartile ΔMDS |
||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Per SD Increase in ΔMDS | ||
| Median ΔMDS | −4 | −1 | 1 | 5 | P-value | |
| Change in VAT volume, cm3 | ||||||
| Model 1 | 713 (645, 781) | 655 (596, 714) | 632 (559, 706) | 598 (537, 658) | −47 (−82, −13) | 0.007 |
| Model 2 | 715 (646, 784) | 658 (599, 716) | 631 (557, 704) | 595 (533, 657) | −50 (−86, −14) | 0.007 |
| Change in SAT volume, cm3 | ||||||
| Model 1 | 546 (471, 621) | 529 (464, 594) | 544 (462, 625) | 462 (395, 529) | −37 (−75, 1) | 0.06 |
| Model 2 | 563 (486, 639) | 535 (470, 600) | 545 (464, 626) | 442 (373, 510) | −52 (−92, −12) | 0.01 |
| Change in VSR | ||||||
| Model 1 | 0.11 (0.09, 0.13) | 0.10 (0.09, 0.12) | 0.09 (0.07, 0.11) | 0.08 (0.07, 0.10) | −0.01 (−0.02, 0.00) | 0.008 |
| Model 2 | 0.11 (0.09, 0.13) | 0.10 (0.08, 0.12) | 0.09 (0.07, 0.11) | 0.09 (0.07, 0.11) | −0.01 (−0.02, 0.00) | 0.04 |
| Change in PAT volume, cm3 | ||||||
| Model 1 | –0.7 (−3.0, 1.5) | −1.9 (−3.8, 0.0) | −4.1 (−6.5, −1.7) | −3.1 (−5.1, −1.1) | −1.2 (−2.3, 0.0) | 0.046 |
| Model 2 | 0.8 (−4.1, 5.7) | −0.4 (−5.1, 4.3) | −2.7 (−7.5, 2.1) | −1.8 (−6.5, 2.9) | −1.3 (−2.4, −0.1) | 0.04 |
Model 1 adjusted for sex, age, baseline adipose tissue (e.g., baseline VAT for ΔVAT or baseline VSR for ΔVSR), baseline MDS, baseline energy intake, baseline physical activity, and baseline smoking status; Model 2 adjusted for model 1 covariates plus baseline BMI, change in energy intake, physical activity, and smoking status. In model 2, we also adjusted for baseline SAT in analysis for ΔVAT and baseline VAT in analysis for ΔSAT. BMI=Body Mass Index; VAT=Visceral Adipose Tissue; SAT= Abdominal Subcutaneous Adipose Tissue; VSR=VAT to SAT Ratio; PAT=Pericardial Adipose Tissue; MDS=Mediterranean-style diet score; SD=Standard Deviation
Figure 1.
Associations of change in MDS and change in each ectopic fat depot volume by sex. Figures 1A, 1B, 1C, and 1D represent change in VAT, SAT, VSR, and PAT, respectively. Changes in MDS were categorized into quartiles, Q1 representing a tendency towards a worse diet and Q4 representing a tendency towards a healthier diet. We used a model adjusting for age, baseline ectopic fat volume (i.e. VAT for ΔVAT analysis), MDS, and BMI, and baseline and change in smoking status, physical activity, and energy intake. P-values were calculated from linear association tests using continuous MDS. P-interactions were p-values from interaction analysis between sex and ΔMDS.BMI=Body Mass Index; VAT=Visceral Adipose Tissue; SAT=Abdominal Subcutaneous Adipose Tissue; VSR=Visceral to Subcutaneous Adipose Tissue Ratio; PAT=Pericardial Adipose Tissue; MDS=Mediterranean-style diet score.
Change in MDS and ΔSAT.
We also observed an inverse association between ΔMDS and ΔSAT (Table 2). After adjusting for multiple covariates, the ΔSAT was highest, 563 cm3 (95% CI: 486, 639), in those with the greatest decline in diet quality (i.e. the lowest quartile of ΔMDS). The longitudinal ΔSAT gradually reduced in other quartiles of ΔMDS: 535 cm3 (95% CI: 470, 600), 545 cm3 (95% CI: 464, 626), and 442 cm3 (95% CI: 373, 510) from the second to the fourth quartiles of ΔMDS. Fat accumulation in SAT was 52 cm3 (95% CI: 12, 92; p-value=0.01) less for one SD increase in ΔMDS (i.e. diet quality improved). We found no significant interaction between sex and ΔMDS for ΔSAT (p-interaction=0.59; Figure 1B). Nevertheless, in sex-specific analyses, the inverse association was significant in men (p-value=0.04) but not in women (p-value=0.30).
Change in MDS and ΔVSR.
The mean VSR increased by 0.10 (95% CI: 0.09, 0.11; p-value<0.001) from baseline to follow-up, indicating that fat was more likely to accumulate in VAT relative to SAT over time. However, as shown in Table 2, ΔVSR tended to be smaller in participants whose diet quality improved (i.e. ΔMDS increased). For one SD increase in ΔMDS, ΔVSR reduced by 0.01 (95% CI: 0, 0.02), p-value=0.04. No significant sex-ΔMDS interaction was detected for ΔVSR (p-interaction=0.20). As shown in Figure 1C, ΔVSR was reduced along with increases in MDS in both men and women; however, the association was significant in men (p-value=0.03) but not in women (p-value=0.20).
Change in MDS and ΔPAT.
As shown in Table 2, PAT decreased more in participants who had an increased ΔMDS (i.e. diet quality improved) compared with those with a decreased ΔMDS (i.e. diet quality declined). PAT decreased an additional 1.3 cm3 (95% CI: 0.1, 2.4; p-value=0.04) for each SD increase in ΔMDS after adjustment for multiple covariates. Similar to abdominal adipose tissues, we observed no significant interaction between sex and ΔMDS for ΔPAT (p-interaction=0.16); however, a significant association between ΔMDS and ΔPAT was observed in men (p-value=0.04) but not women (p-value=0.45; Figure 1D).
Sensitivity analysis using AHEI.
When ΔAHEI was analyzed, we observed similar associations (Table S6) to those for MDS. In the overall study sample, increased ΔAHEI was associated with less fat accumulation in VAT (p-value=0.02), SAT (p-value=0.002), and PAT (p-value<0.001) after adjustment for multiple covariates. In men, increased ΔAHEI was associated with less fat accumulation in VAT (p-value=0.008), smaller increases in VSR (p-value=0.04), and less fat accumulation in PAT (p-value=0.006). Whereas in women, increased ΔAHEI was associated with less fat accumulation in SAT and PAT only (p-value=0.006 and 0.008, respectively).
Genetic risk factors and change in ectopic fat depots.
GRSs were generally associated with baseline fat depots but not with change over time (Table 3). We observed that, in models adjusting for baseline BMI and other covariates, the VAT GRS was associated with baseline VAT (p-value=0.01), the VSR GRS was associated with baseline VSR (p-value=0.03), and the PAT GRS was associated with baseline PAT (p-value<0.001). The SAT GRS was associated with baseline SAT in the base model without adjustment for baseline BMI (p-value=0.03). However, adjustment for baseline BMI attenuated the association between the SAT GRS and baseline SAT to nonsignificant (p-value=0.38). We only observed that the PAT GRS was associated with ΔPAT (p-value=0.03) insofar that PAT increased by 1.2 cm3 (95%CI: 0.1, 1.6) for one SD increase in the PAT GRS. No significant associations were observed between the VAT GRS and ΔVAT, the SAT GRS and ΔSAT, or the VSR GRS and ΔVSR (Table 3).
Table 3.
Associations between genetic risk scores (GRSs) for ectopic fat and baseline and change in corresponding ectopic fat volumes in overall sample
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| Effect | SE | p-value | Effect | SE | p-value | |
| VAT GRS (per standard deviation increase) | ||||||
| Baseline VAT, cm3 | 41 | 21 | 0.05 | 35 | 14 | 0.01 |
| Change in VAT, cm3 | −12 | 17 | 0.48 | −12 | 17 | 0.49 |
| SAT GRS (per standard deviation increase) | ||||||
| Baseline SAT, cm3 | 74 | 34 | 0.03 | 16 | 18 | 0.38 |
| Change in SAT, cm3 | 6 | 18 | 0.73 | 5 | 18 | 0.80 |
| VSR GRS (per standard deviation increase) | ||||||
| Baseline VSR | 1.644 | 0.675 | 0.02 | 1.441 | 0.682 | 0.03 |
| Change in VSR | −0.445 | 0.472 | 0.35 | −0.282 | 0.472 | 0.55 |
| PAT GRS (per standard deviation increase) | ||||||
| Baseline PAT, cm3 | 3.0 | 0.9 | 0.002 | 3.5 | 0.8 | <0.001 |
| Change in PAT, cm3 | 1.1 | 0.5 | 0.05 | 1.2 | 0.6 | 0.03 |
For the analyses of association between GRSs and baseline ectopic fat volumes, Model 1 adjusted for sex, age, and smoking status and Model 2 additionally adjusted for BMI GRS, WHR GRS, and baseline smoking status, physical activity, energy intake, MDS, and BMI; For the analyses of GRSs and association between change in ectopic fat depot volumes, Model 1 adjusted for sex, age, smoking status, and baseline ectopic fat volume and Model 2 additionally adjusted for BMI GRS, WHR GRS, baseline BMI, and change in smoking status, physical activity, energy intake, and MDS. VAT=Visceral Adipose
Tissue; SAT= Abdominal Subcutaneous Adipose Tissue; VSR=VAT to SAT Ratio; PAT=Pericardial Adipose Tissue; GRS=Genetic Risk Score; MDS=Mediterranean-style Diet Score; BMI=Body Mass Index; WHR=Waist to Hip Ratio; SE=Standard Error
As shown in Table S7, the BMI GRS was associated with baseline VAT (p-value=0.01) and baseline SAT (p-value<0.001) and the WHR GRS was associated with baseline VAT (p-value<0.001) and baseline VSR (p-value=0.002). However, the associations between the BMI GRS and WHR GRS and change in fat depots were not significant (all p-value>0.05).
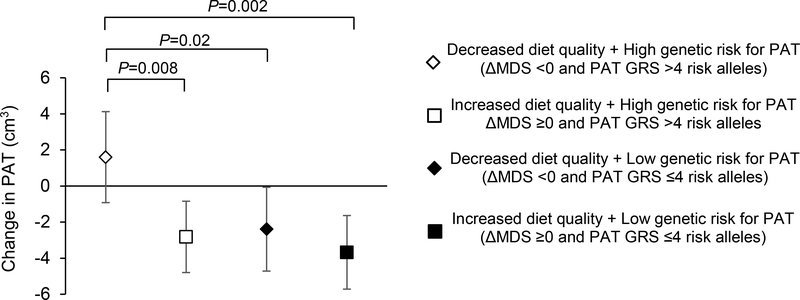
There was no significant interaction between the GRSs and ΔMDS in relation to ΔVAT, ΔSAT, ΔVSR, or ΔPAT in our study sample (all p-interaction >0.05). However, even among those with a high genetic risk for PAT (defined as PAT GRS above the median, i.e., four risk alleles), PAT volume decreased over time in participants with an increased or maintained MDS (ΔMDS ≥0), but not in those with a decreased MDS (Figure 2; p-value=0.008).
Figure 2.
Analysis of change in MDS (ΔMDS) and PAT genetic risk score (GRS) in relation to change in PAT. Participants were categorized into one of four mutual exclusive groups using medians of ΔMDS (0) and PAT GRS (4). ΔMDS >0 represents an improved diet quality and PAT GRS >4 reflects a higher genetic risk for increasing PAT. Model adjusted for baseline PAT, baseline MDS, age, sex, baseline BMI, and baseline and change in smoking status, physical activity, and energy intake. PAT=Pericardial Adipose Tissue; MDS=Mediterranean-style diet score.
Discussion
Our study found that longitudinal improvements in diet quality, quantified by an increased MDS, were associated with favorable changes in ectopic fat depots represented by less visceral and pericardial fat accumulation over approximately six years of follow-up. We also found that genetic factors identified through GWASs affect fat depots differently. The VAT GRS and SAT GRS were only associated with baseline volumes of VAT and SAT, respectively. Whereas, the PAT GRS was associated with both baseline and change in PAT. Nevertheless, we observed no interaction between GRSs and diet for abdominal or pericardial fat change. Overall, these findings suggest that the genetic influence (as reflected in the GRS) on adiposity has already manifested in adult age. Furthermore, the GRS, which is derived from cross-sectional phenotype data, is not an optimal predictor for further changes in ectopic fat depots among older adults. However, diet, a modifiable factor, may play a key role in the longitudinal change of these depots.
A few observational studies have studied the relation between long-term dietary intake and ectopic fat depots (22, 23). For instance, one study found that better adherence to the Mediterranean diet was associated with reduced VAT in 4,399 adults (22). Another study including 5,079 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) found that those with a higher diet quality score, developed using components of a Mediterranean-style diet, also had lower volumes of VAT and PAT (23). However, most of these studies employ a cross-sectional design, which cannot establish a temporal relationship.
Longitudinal studies aimed to directly examine the association between overall diet quality and ectopic fat depots are limited. Several studies have analyzed the longitudinal association between dietary intake and general weight gain. For example, one large cohort study showed that adopting a high quality diet, assessed using multiple diet scores, was associated with less weight gain over four-year increments (7). The present study is an extension of our prior work, which demonstrated that increased diet quality was associated with less fat accumulation in the liver over time (8). Thus, the present study enhances the literature by showing that improving overall diet quality may be associated with favorable adiposity changes in the abdomen and pericardial sac in free-living adults.
Sex differences for abdominal adipose tissue distribution have been well documented (24). Men and women may also have differing responses to dietary intake with respect to fat mass. In the present study, we observed that the association between diet quality change and VAT change was only statistically significant in men. This observation coincides with the findings from a nutrition intervention study aimed to promote adherence to a Mediterranean-style diet (25). This study found that body fat significantly decreased in men but not in women after the 12-week nutrition counseling intervention. Nevertheless, future studies are needed to examine the potential sex-diet interaction in relation to abdominal fat depots, as well as the underlying mechanisms.
As expected, GRSs constructed based on known genetic risk factors identified in the published GWAS were cross-sectionally associated with abdominal and pericardial fat volume. In the longitudinal analyses, only the association between the PAT GRS and PAT volume change was significant. We acknowledge that a larger sample size may be required to detect the association between genetic factors and change in ectopic fat depots. Nevertheless, our observations may agree with one recent study which showed that the cross-sectional association between the BMI GRS and BMI was higher in middle adulthood, defined as 45 to 50 years in men and 45 to 65 years in women, compared with other ages (26). Additionally, the longitudinal analysis in this study showed a weak association between the BMI GRS and weight gain from 45 to 65 years in women and no association in men. As such, this study coupled with our findings suggest that known genetic risk identified through GWAS may be already established in middle-aged to older adults and environmental exposures, such as diet, may play a critical role to determine the change in adiposity in this age group.
One prior study showed that higher whole grain consumption was inversely associated with VAT and SAT, whereas, refined grain intake was positively associated with VAT and SAT (27). In addition, other cohort studies also showed that increased fruit and vegetable consumptions was associated with lower weight gain (28) and higher nut consumption was associated with lower risk of weight grain (29). Therefore, it is possible that change in ectopic fat depots may be attributed to multiple dietary components and mechanisms.
Our study used robust longitudinal data, including high resolution imaging, genotype, diet, and lifestyle factors, collected from free-living adults in the FHS. However, several limitations exist. We used an 8-slice CT scanner at baseline and 64-slice CT scanner at follow-up. The two devices might impact our measurements for ectopic fat volumes; however, such differences were likely nondifferential in our study participants. We constructed GRSs based on SNPs identified through published GWASs; however, these studies explain only a small proportion of the heritability of adiposity (9, 10, 11). It is possible that there are unknown genetic factors that regulate adiposity changes in middle-aged to older adults. The limited genetic information and small sample size may restrict our gene-diet interaction analyses. The change in MDS may reflect regression to the mean phenomenon that may bias the present observations. Furthermore, the self-report dietary assessment was subject to response bias. We did not assess diet quality between examinations, which might also affect our results. Although we used multivariable models adjusting for lifestyle risk factors, there may be additional confounders that are present in the analyses. Finally, our study sample included mostly participants of European ancestry, limiting its generalizability to diverse populations.
In conclusion, the present study demonstrates that, in middle-aged to older adults, known genetic risk factors may have limited power to account for longitudinal changes in ectopic fat depots. On the other hand, our data emphasize that improved diet quality may play an important role in preventing fat accumulation in the viscera and pericardium in middle-aged to older adults. These findings provide useful hypotheses into dietary interventions that may substantially improve cardiometabolic health despite genetic predisposition. Nevertheless, future studies with racially diverse populations are needed to investigate novel genetic variants that may affect ectopic adiposity changes in middle-aged to older adults, as well as to study mechanisms underlying the favorable association between diet quality and ectopic fat depots.
Supplementary Material
Figure S1. Distribution of ΔMDS (Change in Mediterranean-style diet score)
Figure S2. Sex-specific distribution of ΔMDS (Change in Mediterranean-style diet score).
Table S1. Components of the Mediterranean-style Diet Score (MDS) and Alternative Healthy Eating Index (AHEI)
Table S2. Genetic variants included in each ectopic fat GRS.
Table S3. Genetic variants included in the BMI GRS.
Table S4. Genetic variants included in the WHR GRS.
Table S5. Intakes of dietary components of the MDS according quartile categories of ΔMDS
Table S6. Associations between change in AHEI and change in ectopic fat depots.
Table S7. Associations between genetic risk scores for BMI and WHR and baseline and change in corresponding ectopic fat volumes in overall sample.
What is already known about this project?
Diet quality change has been associated with weight gain and liver fat accumulation
Genetic variants at several loci have been identified for visceral adipose tissue, abdominal subcutaneous adipose tissue, and pericardial adipose tissue
What does this study add?
The present study demonstrated that improving diet quality is associated with long-term reduction of abdominal and pericardial adipose tissue
The present study showed that increased or maintained diet quality may mitigate the predisposed genetic effect on fat accumulation in pericardial adipose tissue
Acknowledgements
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and its contract with Affymetrix for genotyping services (Contract No. N02-HL-6–4278). Several authors are employees of the National Heart, Lung and Blood Institute and their research is supported by its Division of Intramural Research (JM, RH, CL, DL). NMM is supported by the USDA (Agreement No. 58–1950-4–003).
The authors thank Shih-Jen Hwang (Population Sciences Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD) for assistance in data preparation and Martin G. Larson (Framingham Heart Study and Department of Biostatistics, Boston University School of Public Health) for suggestions in statistical analysis.
Sources of Funding: This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and its contract with Affymetrix for genotyping services (Contract No. N02-HL-6–4278). Several authors are employees of the National Heart, Lung and Blood Institute and their research is supported by its Division of Intramural Research (JM, RH, CL, DL). NMM is supported by the USDA (Agreement No. 58–1950-4–003).
Footnotes
The authors do not have any competing interests to report.
Competing Interests: Null.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116: 39–48. [DOI] [PubMed] [Google Scholar]
- 2.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation 2011;124: e837–841. [DOI] [PubMed] [Google Scholar]
- 3.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008;117: 605–613. [DOI] [PubMed] [Google Scholar]
- 4.Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of Changes in Diet Quality with Total and Cause-Specific Mortality. The New England journal of medicine 2017;377: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition 2012;142: 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Changes in Diet Quality Scores and Risk of Cardiovascular Disease Among US Men and Women. Circulation 2015;132: 2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung TT, Pan A, Hou T, Chiuve SE, Tobias DK, Mozaffarian D, et al. Long-Term Change in Diet Quality Is Associated with Body Weight Change in Men and Women. The Journal of nutrition 2015;145: 1850–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PF, et al. Improved Diet Quality Associates With Reduction in Liver Fat-Particularly in Individuals With High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu AY, Deng X, Fisher VA, Drong A, Zhang Y, Feitosa MF, et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nature genetics 2017;49: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. American journal of epidemiology 2007;165: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. American journal of epidemiology 1979;110: 281–290. [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology 1992;135: 1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 15.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, et al. Visceral and subcutaneous fat quality and cardiometabolic risk. JACC Cardiovascular imaging 2013;6: 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain SH, Massaro JM, Hoffmann U, Rosito GA, Vasan RS, Raji A, et al. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes care 2009;32: 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. International journal of obesity 2007;31: 500–506. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Yang Q, Hwang SJ, Fox CS, Chu AY. Genetic risk score and risk of stage 3 chronic kidney disease. BMC nephrology 2017;18: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Savage PJ Sr., Levy D, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation 2009;120: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. American heart journal 1986;112: 820–825. [DOI] [PubMed] [Google Scholar]
- 22.Bertoli S, Leone A, Vignati L, Bedogni G, Martinez-Gonzalez MA, Bes-Rastrollo M, et al. Adherence to the Mediterranean diet is inversely associated with visceral abdominal tissue in Caucasian subjects. Clinical nutrition 2015;34: 1266–1272. [DOI] [PubMed] [Google Scholar]
- 23.Shah RV, Murthy VL, Allison MA, Ding J, Budoff M, Frazier-Wood AC, et al. Diet and adipose tissue distributions: The Multi-Ethnic Study of Atherosclerosis. Nutrition, metabolism, and cardiovascular diseases : NMCD 2016;26: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuente-Martin E, Argente-Arizon P, Ros P, Argente J, Chowen JA. Sex differences in adipose tissue: It is not only a question of quantity and distribution. Adipocyte 2013;2: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leblanc V, Hudon AM, Royer MM, Corneau L, Dodin S, Begin C, et al. Differences between men and women in dietary intakes and metabolic profile in response to a 12-week nutritional intervention promoting the Mediterranean diet. Journal of nutritional science 2015;4: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song M, Zheng Y, Qi L, Hu FB, Chan AT, Giovannucci EL. Longitudinal Analysis of Genetic Susceptibility and BMI Throughout Adult Life. Diabetes 2018;67: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKeown NM, Troy LM, Jacques PF, Hoffmann U, O’Donnell CJ, Fox CS. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: the Framingham Heart Study. The American journal of clinical nutrition 2010;92: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertoia ML, Mukamal KJ, Cahill LE, Hou T, Ludwig DS, Mozaffarian D, et al. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLoS Med 2015;12: e1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bes-Rastrollo M, Wedick NM, Martinez-Gonzalez MA, Li TY, Sampson L, Hu FB. Prospective study of nut consumption, long-term weight change, and obesity risk in women. The American journal of clinical nutrition 2009;89: 1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of ΔMDS (Change in Mediterranean-style diet score)
Figure S2. Sex-specific distribution of ΔMDS (Change in Mediterranean-style diet score).
Table S1. Components of the Mediterranean-style Diet Score (MDS) and Alternative Healthy Eating Index (AHEI)
Table S2. Genetic variants included in each ectopic fat GRS.
Table S3. Genetic variants included in the BMI GRS.
Table S4. Genetic variants included in the WHR GRS.
Table S5. Intakes of dietary components of the MDS according quartile categories of ΔMDS
Table S6. Associations between change in AHEI and change in ectopic fat depots.
Table S7. Associations between genetic risk scores for BMI and WHR and baseline and change in corresponding ectopic fat volumes in overall sample.