Abstract
Background:
Alcohol use disorder (AUD) and its associated consequences remain significant public health concerns. Given that AUD represents a spectrum of severity, treatment options represent a continuum of care, ranging from single-session brief interventions to more intensive, prolonged, and specialized treatment modalities.
Objective:
This qualitative literature review seeks to describe the best practices for AUD by placing a particular emphasis on identifying those practices which have received the most empirical support.
Method:
This review summarizes psychological and pharmacological intervention options for AUD treatment, with a focus on the relapse prevention phase of recovery. Psychological and pharmacological treatments are summarized in terms of the empirical evidence favoring each approach and the level of AUD severity for which they are most indicated.
Scientific Significance:
One of the broad assertions from this review is that while AUD is highly prevalent, seeking treatment for AUD is not. There are a myriad of behavioral and pharmacological treatments that have shown compelling evidence of efficacy for the treatment of AUD. In the behavioral treatment literature, Cognitive Behavioral Therapy (CBT) has received the most consistent support. Opioid antagonism (via naltrexone) has been the most widely studied pharmacotherapy and has produced moderate effect sizes. While none of the treatments reviewed herein represents a so called “silver bullet” for AUD, they each have the potential to significantly improve the odds of recovery. Precision medicine, or the identification of best treatment matches for individual patients, looms as an important overarching goal for the field; although specific matches are not yet sufficiently reliable in their empirical evidence to warrant clinical dissemination.
Keywords: Alcohol use disorder, evidence-based treatment, psychosocial treatment, AUD treatment, psychopharmacology
Introduction
Alcohol use disorder (AUD) is a chronic, relapsing condition, characterized by continued use despite harmful medical, psychological, and social consequences. AUD and its associated consequences remain significant public health problems, as alcohol misuse was deemed the fifth largest risk factor for premature death and disability in 2010. Further, 3.3 million deaths (5.9% of all deaths) and 5.1% of the burden of disease and injury worldwide were attributable to alcohol consumption in 2012. More recently, it has been estimated that alcohol use and misuse contributed to over two hundred diseases and injury-related health conditions, including liver cirrhosis, cancers, and injuries (1).
Recent findings from the National Epidemiological Survey on Alcohol and Related Conditions III (NESARC-III) conducted between 2012–2013 by the National Institute on Alcohol Abuse and Alcoholism (2) indicated that the prevalence of DSM-5 12-month and lifetime AUD in the general population of adults in the United States was 13.9% and 29.1% respectively. Stratifying by severity, 12-month prevalence of AUD was estimated to be 7.3% for mild AUD, 3.2% for moderate AUD, and 3.4% for severe AUD. For lifetime AUD, these rates are 8.6%, 6.6%, and 13.9%, respectively, which represents a marked increase in disease severity rates from that of 12-month AUD. Mean age of AUD onset was 26.2 years. Age of onset seems to decrease with severity, with the age of onset approximating 30.1 years for mild AUD and 23.9 years for severe AUD (2), potentially reflecting subtypes of AUD with an early-onset of AUD indicating more chronic symptoms and a biological predisposition to AUD.
Results from NESARC-III compared to previous epidemiological studies of AUD show a marked increase in prevalence of AUD since 2002. Previous NESARC data (3), compared to the latest NESARC results (2), suggest that both the 12-month and lifetime prevalence of DSM-IV AUD substantially increased over the past decade, from 8.5% to 12.7% and from 30.3% to 43.6%, respectively. These increases may be attributable to significant increases in high-risk drinking from 2002–2013 (2). It is important to note a newly documented cohort effect, in that the gender gap between the prevalence of AUD between men and women appears to be narrowing (4, 5). Possible explanations for this effect include the notion that drinking norms may have become more liberal among women, coupled with increased educational and occupational opportunities (6), perhaps leading to increased alcohol use among women (7). What is clear is that ongoing monitoring of such cohort effects is warranted.
Overall, epidemiological studies estimate that about 44.6 million adults in the United States suffer from AUD in a given year, and 93.4 million will suffer from AUD in their lifetime (2) Further, it is estimated that the economic burden of AUD treatment is $250 billion nationally (8). These figures highlight the gravity of AUD as an important public health and economic concern, and call for an effort to develop and implement effective treatments to address AUD in the US.
Treatment
Although the recent increases in high-risk drinking and alcohol use disorder prevalence convey a significant public health concern, treatment rates for AUD remain extremely low. Among those with 12-month and lifetime diagnoses of AUD, only 7.7% and 19.8%, respectively, sought treatment (2). Of those who did seek treatment, the most commonly accessed treatment modalities included 12-step groups, health care practitioners, and outpatient and inpatient rehabilitation facilities (2). Common barriers to seeking treatment include fears of stigmatization and beliefs that AUD treatment is ineffective (9–11). Further, attitudinal barriers, including the belief that an individual should be strong enough to handle the problem without treatment, are highly prevalent in individuals with alcohol problems (12). It has been estimated that there is an average lag of approximately 8 years between the age of onset and the age at first treatment (13). Additionally, the COMBINE study, a multisite combined medication and psychotherapy trial conducted in the United States, reported that the gap between AUD onset and treatment-seeking in their sample was about 14 years, though it is unclear if the COMBINE participants engaged in previous treatment (14).
With this consideration, a recent study from our group conducted a comparison of the treatment-seeking COMBINE Study sample and a sample of non-treatment seeking participants enrolled in behavioral pharmacology studies for AUD. There were significant differences in sociodemographic, personality, psychological, and alcohol use pattern measures, and these inherent inconsistencies also predicted differential clinical outcomes. Specifically, COMBINE Study treatment-seekers tended to be older, reported more AUD symptoms, had a longer duration of AUD symptoms, were more likely to have a family history of AUD, and consumed more alcohol than non-treatment seekers with a current AUD diagnosis enrolled in behavioral pharmacology trials. COMBINE treatment seekers also tended to be female, less ethnically diverse (15, 16), have higher numbers of negative social consequences, and have higher rates of drug use and psychiatric severity (17).
In addition to differences by treatment seeking status, there is also a robust body of literature on natural recovery from AUD, which is defined as successful recovery without formal treatment. Epidemiologic data suggest that natural recovery is not only the most commonly reported method to mitigating AUD, but also that for a large subset of those suffering from AUD it is feasible to recover without formal treatment. Two Canadian surveys showed that more than 75% of those who recovered from an alcohol problem for a year or more, did so without formal treatment, and more than 50% maintained recovery for over 5 years (18). Additional findings on naturalistic recovery uphold the notion that social support is fundamental in recovery. Specifically, individuals with low social support were able to reach recovery unassisted, as long as their alcohol-related issues are mild in nature. Further, amongst young adults who drink heavily, a pattern of “aging out” is commonly seen (19, 20). In such cases, drinking begins in adolescence, increases and peaks during young adulthood (early twenties), and then decreases as the individual matures out of heavy drinking. Conversely, those with severe alcohol problems require greater amounts of social support in order to reach their recovery goals (21). It is important for clinicians to recognize that natural recovery is part of the landscape of AUD, particularly for individuals in the mild range of the disorder.
The focus of this qualitative review is to summarize best practices in the treatment of AUD, including psychosocial and pharmacological interventions with a focus on identifying those treatments receiving the most empirical support. This review will address evidence-based treatment for AUD relapse prevention, as opposed to the stabilization and medically supervised withdrawal phase of care or non-formal treatment interventions, such as natural recovery. This manuscript intends to provide a broad overview of key concepts in AUD treatment and to summarize evidence-based treatments, including pharmacotherapy and psychotherapy modalities. The inclusion of both treatment modalities in this review is purposeful since most reviews tend to focus on a single approach (i.e., pharmacotherapy or psychotherapy only).
Psychosocial Treatment
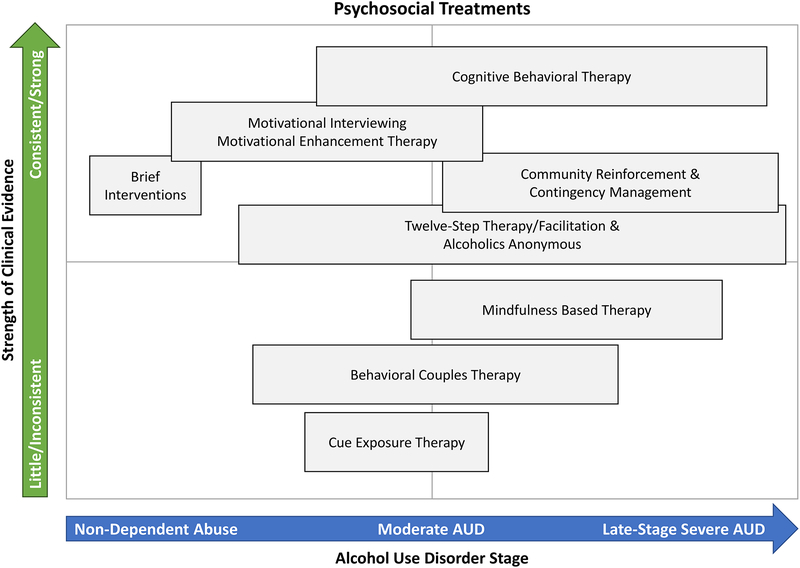
Numerous psychosocial treatments for AUD exist, although they vary in the degree to which they have received empirical support. Next we provide a brief overview of psychosocial treatments for AUD that are considered evidence-based, indicating that they have been empirically supported through randomized controlled trials (RCTs). These treatment modalities are listed in no particular order. Psychosocial treatments are summarized in Figure 1, which encompasses a summary of our subjective appraisal of the strength of the empirical scientific evidence for each treatment as well as our recommendation for their utilization relative to AUD severity.
Figure 1.
Summary of available psychological treatments for AUD with the y-axis indicating the strength of the evidence in favor of a particular treatment (as perceived in this qualitative review) and the x-axis indicating the recommended placement of that treatment across the continium of AUD severity. This figure summarizes the authors’ subjective evaluation of the scientific literature, which has not been subjected to quantitative analyses (e.g., effect size estimations).
(1) Brief interventions (BI) entail a succinct screening for heavy alcohol use, negotiation of a behavioral change plan, and encouragement of follow-up or engagement with resources. BI is typically delivered in a single session by a health care professional, with no additional benefit from sessions greater than 60 minutes in duration (22). Research samples have typically been opportunistic and meta-analyses have consistently shown a benefit in the reduction of alcohol use (23–25). In non-treatment seeking heavy drinkers and in individuals with mild AUD, BI can be a powerful tool to reduce use and should be considered as a routine part of any interview occurring with individuals with an alcohol or substance use disorder. However, BI have been found to be ineffective in individuals with moderate-to-severe AUD (26), and therefore should not replace long-term, addiction centered treatment plans for those seeking treatment or for individuals with recurrent, moderate-to-severe AUD (25, 27).
(2) Motivational interviewing (MI) or motivational enhancement therapy (MET) seeks to enhance the motivation and commitment to change. The therapist helps the patient recognize and grapple with ambivalence between the factors maintaining alcohol use and the problems that are consequent to his/her alcohol use. Typically delivered in 1–4 sessions, or in conjunction with other therapies (28), MET is designed to meet patients at their respective stages along the continuum of change in a collaborative, empathetic, and non-confrontational manner (29). Several meta-analyses have supported the efficacy of MI and MET, particularly in emergency departments (30) and primary care (31) settings. There is also evidence that a combination of MET and Cognitive Behavioral Therapy may be effective for reducing alcohol consumption and depression among individuals with comorbid AUD and major depression (28).
(3) Cognitive behavioral therapies (CBT) typically begin with a functional analysis of alcohol use, which elucidates the individual’s cycles of thoughts, feelings, and behaviors underlying excessive drinking. The therapist provides psychoeducation about AUDand teaches skills to cope with cravings and mood fluctuations. Alongside the patient, the therapist helps to identify and plan for triggers of alcohol-related thoughts and urges, and works to develop a relapse prevention plan (32). Numerous studies, reviews, and meta-analyses corroborate the efficacy of CBT for treating AUD (33–35); although there are larger effect sizes for other substances of abuse compared to alcohol (34, 36). CBT for AUD may also be more effective for women than men (37). While those findings warrant replication, CBT remains one of the most widely studied and empirically supported treatments for AUD.
(4) Behavioral couples therapy (BCT) presupposes a reciprocal relationship between substance use and relationship distress. Thus, BCT treats substance use within the dyad of the substance user and his/her partner. Interventions typically include psychoeducation, communication skills, behavioral activation to increase positive shared activities, negotiation of sobriety contracts, and relapse prevention with the goals of improving relationship functioning, mutual coping, and decreasing use (38). BCT has been shown superior to individual treatment in reducing negative consequences of substances use, increasing relationship satisfaction, and improving treatment retention for married and cohabitating individuals with AUD (38, 39). Notably, BCT has been associated with reduced risk of domestic violence (38). As reviewed recently by McCrady and colleagues (2016) (40), new frontiers in BCT include adaptions for non-traditional couples and development of flexible technology-based models to promote dissemination.
(5) Reinforcement-based treatments are predicated in operant conditioning theory and assume that alcohol use is inherently rewarding. Therefore, these approaches attempt to reduce the relative reinforcing value of alcohol and increase reinforcement from other non-drinking activities. The community reinforcement approach (CRA) aims to produce changes in both the individual’s lifestyle and embeddedness within his/her social environment, thereby increasing healthy, pleasurable, drug-free behaviors that are also reinforcing. For instance, the social/recreational counseling and activity sampling modules focuses on helping the individual to initiate or renew engagement in hobbies and social activities that were replaced by alcohol consumption (41). Evidence for CRA is generally favorable for AUD and can be paired with contingency management (CM) (42). CM programs provide incentives based upon the demonstration of a planned reduction or cessation behavior (43). These approaches are shown to be efficacious in a number of settings, across diverse populations, and with different substances (43, 44). Within alcohol users, CM has been found to be efficacious in reducing drinking in non-treatment seeking heavy drinkers (45) and in individuals with AUD with co-occurring mental illness (46). Limitations of this approach include the high cost and concern that treatment effects progressively degrade once reinforcements are no longer in place (43).
(6) Classical conditioning therapies include Cue-exposure therapy (CET) and aversion therapy. CET involves repeated exposure to alcohol-related stimuli, without the reinforcement of alcohol consumption, to produce a decrease in alcohol craving and an increase in self-efficacy for coping with urges and high-risk situations (47). CET has received some empirical support from a number of studies, including a comparison to CBT (48, 49), in conjunction with pharmacotherapies (50), and in fMRI work (51). Despite the theory-driven approach of CET, data have been mixed. One study did not show that CET yielded additional benefits beyond CBT alone (52). Two meta-analyses have respectively found the overall effect size of CET to be small (d < 0.10) (47); and that CET has little to no impact on drinking outcomes (53). Novel approaches for the delivery of CET are currently under investigation, specifically via smartphone applications (54). Aversion therapy involves repeated pairings of alcohol with an unpleasant stimulus, such as an electric shock or chemical emetic, to target unconscious memory associations involving alcohol craving. While there is initial evidence that aversion therapy results in improved abstinence rates (55), it has not been widely studied, potentially due to ethical concerns regarding this approach.
(7) Twelve-step therapies (TST) include Alcoholics Anonymous (AA) and 12-step facilitation (TSF) which are rooted in the AA tradition. TSF is an individual therapy delivered in 12 weekly sessions that facilitate involvement in AA through understanding, acceptance, and engagement (56). TST represent a widely available, free resource for individuals with alcohol problems which espouses the goal of long-term, complete abstinence. Thus, according to the TST program, psychological wellbeing, ability to cope, and adaptation to a sober lifestyle are foundational elements of recovery. Experimental research assessing efficacy of AA has evolved over the years (57). An early review of AA found that these programs elicited small reductions in alcohol use consistent with other behavioral treatments (58). Subsequent meta-analyses noted that AA evaluation studies exhibit significant heterogeneity in outcomes, were likely underpowered to detect effects, and were confounded by participant selection bias (59, 60). More recent meta-analyses including controlled studies have found either mixed results or inconclusive findings regarding the effectiveness of AA in reducing alcohol consumption (57, 61). There is some evidence, however, that increased AA attendance among adolescents with substance use disorders strengthens the benefits of existing community outpatient treatments (62). Strengths of TST include the length of treatment, cost, ease, social support, and self-regulated exposure to common therapeutic components (63). Given the accessibility of TST and the evidence for its efficacy, we encourage clinicians to support their patients in seeking a TST group that is a good fit for them.
(8) Mindfulness-based therapies are based in Buddhist traditions regarding meditation and mindfulness practice. Promoted concepts include awareness of the “here and now” and nonjudgmental stances towards one’s current state, emotional and otherwise. In AUD treatment mindfulness is used to cope with urges, such as cravings, and to prevent relapse. Specifically, Mindfulness-Based Relapse Prevention (MBRP) (64) aims to increase awareness of triggers, habitual behavioral patterns, and reactions, and the automatic responses to these discomforts in order to modify response (65). MBRP has shown initial efficacy at reducing substance use and craving (66, 67). A meta-analysis of 42 studies published in 2017 found that MBRP had significant effects on the reduction of craving, reducing substance misuse, and lessening stress levels (68). Based on the literature thus far, we encourage clinicians to consider mindfulness-based interventions in their practice with individuals with AUD.
(9) Technology-based interventions are an emerging treatment approach which utilize web- and telephone-based technology. Technology based interventions largely translate evidence-based, in-person treatments, such as CBT or MET, into web-based formats. Initial evidence indicates that technology-based interventions have promise to treat individuals with a lower severity of alcohol problems (69, 70) and for individuals who may not seek face-to-face treatment (71). However, efficacy testing for these novel approaches has been variable and many available studies pertain only to college-student populations (72–74). Web-based interventions have also been used as an add-on support to in-person treatment. A recent RCT found that the addition of a web-based intervention focused on skills for achieving and maintaining abstinence resulted in an increase in abstinence compared to treatment-as usual (75). This effect was more pronounced in individuals with a positive breath alcohol at study entry (75). A recent NIAAA study compared a computer based delivery platform, Take Control, to traditional therapist-delivered interventions (76). Take Control is a seven module intervention rooted in principles of psychoeducation, skill building, goal setting, and AUD treatment education. Devine and colleagues (2016) found that Take Control was comparable to therapist-delivered intervention on participant retention and medication adherence, indicating that such technology-based interventions may be a viable, cost-effective alternative to traditional face-to-face interventions. Such technology based interventions have a host of potential benefits, including reduction of therapist bias in RCTs, decreased cost (76), and the ability to reach historically underserved populations, including women, younger individuals, and other at risk populations. Thus, we recommend that clinicians become familiar with the latest developments in evidence-based applications of psychological principles to AUD treatment through mobile technology and computerized interventions. These resources, much like TST, represent highly accessible options to patients with AUD, and as such, deserve careful consideration in order to broaden the scope and accessibility of care.
Summary of Psychosocial Treatments:
In summary, the psychosocial treatment modalities described above show, at least, some degree of empirical support for the treatment of AUD. However, no single treatment has surpassed all others at treating this complex disorder (77). Psychosocial treatments may be optimally effective when combined with another psychosocial modality and/or with pharmacotherapy in the form of medication assisted treatment. Several studies have examined the effectiveness of several psychosocial treatments. For example, Project MATCH (78, 79) compared the efficacy of CBT, TSF, and MET. This study enrolled outpatients and aftercare patients recently discharged from private, public, and Department of Veterans Affairs inpatient alcohol treatment facilities. Patients’ alcohol use was measured at one and three years post treatment. At one year follow-up, TSF appeared to slightly outperform the other two modalities, but this difference dissipated at the three year follow-up. The overall abstinence rate across conditions was approximately 30% at three year follow-up, although patients in aftercare had higher rates of abstinence compared to the outpatients, 35% vs. 20% respectively (79). Of potential moderators of response, only pretreatment anger level and severity of alcohol dependence predicted positive treatment outcome to MET and TSF, respectively (78). The COMBINE study analyzed the role of drinking goals on drinking outcomes. Individuals with the goal of complete abstinence had the highest abstinence rates, whereas individuals with the goal of controlled drinking had the fewest drinks per drinking day (80). Additional research is needed to identify the clinical characteristics and moderators that will make treatments more efficacious for subgroups of individuals with AUD.
Pharmacological Treatment
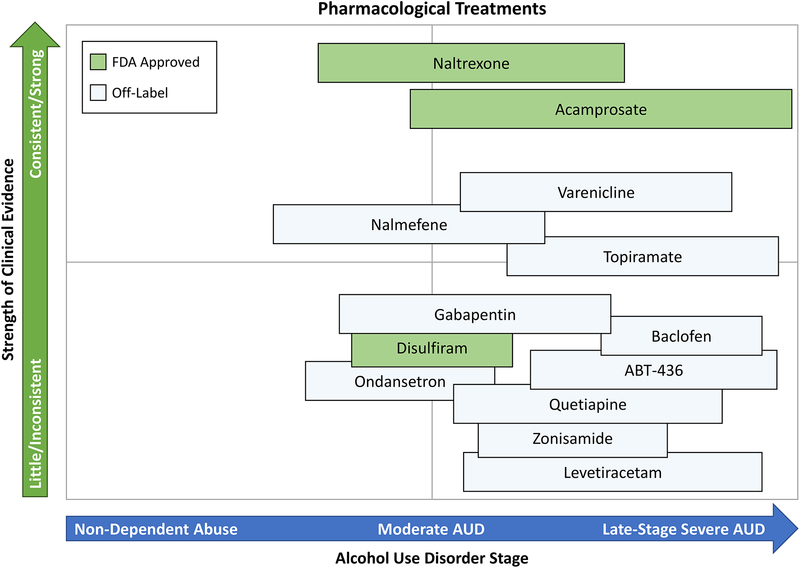
Pharmacotherapy for AUD is used less often than psychosocial interventions. Aside from medically supervised withdrawal treatment, when pharmacological agents are often used to manage alcohol withdrawal symptoms, few community programs combine pharmacotherapy and psychosocial interventions to treat AUD. The use of pharmacotherapy to treat AUD has been limited, in part due to lack of awareness of medications, limited marketing efforts, shortage of physicians trained in addiction treatment, patient refusal to take medication, and perceptions of lack of efficacy. This section of the review will discuss medications for the relapse prevention phase of AUD treatment, divided into FDA-approved medications and off-label pharmacotherapies (Figure 2). These pharmacotherapies are listed in no particular order and are further summarized in Figure 2, which provides a visual representation of our subjective appraisal of the strength of the empirical scientific evidence for each treatment as well as our recommendation for their utilization relative to AUD severity.
Figure 2.
Summary of available pharmacological treatments for AUD with the y-axis indicating the strength of the evidence in favor of a particular treatment (as perceived in this qualitative review) and the x-axis indicating the recommended placement of that treatment across the continium of AUD severity. Pharmacotherapies are divided into FDA-approved and off-label treatments. This figure summarizes the authors’ subjective evaluation of the scientific literature, which has not been subjected to quantitative analyses (e.g., effect size estimations).
Food and Drug Administration (FDA) Approved Medications
The only pharmacotherapies currently approved by the FDA for the treatment of AUD are disulfiram (Antabuse), acamprosate, oral naltrexone, and extended-release injectable naltrexone (Vivitrol) (81–83).
(1) Naltrexone is the most studied of the FDA approved medications. Naltrexone is an opioid antagonist, with the highest affinity for the mu-opioid receptor. The neurobiological basis for the use of naltrexone stems from the neurocircuitry through which alcohol exerts its effects (84, 85). Alcohol increases release of endogenous opioids in the mesolimbic dopamine system contributing to the pleasurable effects of alcohol (84, 85), thus an opioid antagonist is proposed to block these reinforcing effects. Oral naltrexone was approved by the FDA in 1994 after initial trials suggested that naltrexone resulted in significantly fewer drinking days and lower rates of relapse, defined as drinking 5 or more drinks on an occasion for men or four or more drinks on an occasion for women (23% relapse rate for naltrexone versus 54% relapse rate for placebo) following three months of treatment (86, 87). These initial results have been largely supported by more recent trials of naltrexone that have generally demonstrated naltrexone reduces the subjective pleasurable effects of alcohol (88, 89), craving for alcohol (90, 91), drinks per drinking day (92), rates of relapse (93, 94), and time to first relapse (95, 96). However, the support for naltrexone is not uniform. A few trials, including a large multisite trial, have reported no significant outcome differences between naltrexone and placebo treated patients (97–99). Moreover, the effect sizes of previous findings are often modest even when they reach statistical significance. Extended-release injectable naltrexone was developed to address poor medication adherence with oral naltrexone (100). A multi-site trial of long-acting naltrexone identified significant reductions in the number of heavy drinking days over a period of 6 months compared to placebo (101); however this effect was only significant for men (101). Improvements in drinking outcomes were greater in individuals who were abstinent for at least 4 days prior to randomization (101, 102). In sum, studies of naltrexone suggest only a moderate effect on the reduction of alcohol use.
(2) Acamprosate was approved by the FDA in 2004. While the specific mechanisms of action for acamprosate are still under investigation, recent studies suggest that acamprosate interacts with the glutamatergic system, acting as a partial co-agonist at the N-Methyl-D-aspartic acid (NMDA) receptor (103). Additionally, acamprosate results in the release of taurine, which is an inhibitory neuromodulator. Acamprosate’s actions on NMDA receptors and taurine may attenuate neuronal hyperexcitability, which occurs during acute alcohol withdrawal and abstinence. Thus, acamprosate is proposed to be more effective in achieving and maintaining abstinence, as opposed to preventing relapse if drinking occurs (104). This effect was supported in a recent review demonstrating reduced risk of returning to any drinking, as well as fewer drinking days (105). Acamprosate is thought to have neuroprotective effects (106, 107), which may be particularly important in AUD treatment considering neuronal changes that may occur following chronic alcohol abuse (108, 109).
(3) Disulfiram was the first medication approved for the treatment of AUD in 1951. Disulfiram is an aldehyde dehydrogenase inhibitor that exerts its clinical effect by blocking the metabolism of alcohol, which produces an aversive unpleasant response after alcohol intake that results in severe nausea and vomiting (110). Disulfiram has demonstrated mixed clinical efficacy, largely due to poor adherence (111, 112); however a recent meta-analysis reported a significant effect of disulfiram in open-label trials (113). Supervised administration of Disulfiram has been suggested to still have a role in AUD treatment if an individual is having difficulty attaining sobriety (114); however, compliance and medical management issues, including the risk for severe medical complications when the medication is combined with alcohol, limit the widespread utilization of disulfiram in clinical practice for AUD.
(4) Comparing Naltrexone and Acamprosate. There have been four double-blind, placebo controlled trials comparing naltrexone and acamprosate (115). The first trial, a single-center study, was conducted in Germany and randomized patients to receive naltrexone, acamprosate, a combination of naltrexone and acamprosate, or placebo for 12 weeks (95). Results from this study indicated that naltrexone, acamprosate, and their combination were more effective than placebo. Moreover, this study found that the combination of medications was more effective than acamprosate alone, but not naltrexone alone. In the second trial, the COMBINE Study, patients were randomized to naltrexone, acamprosate, or placebo in combination with a behavioral intervention (combine behavioral intervention; CBI) or medication management (MM), using a multi-factorial research design (116, 117). Results from the COMBINE Study found that patients receiving medical management (MM) with naltrexone, CBI, or both fared better on drinking outcomes, whereas acamprosate showed no evidence of efficacy, with or without CBI. No combination of medications produced better efficacy than naltrexone or CBI alone in the presence of medical management (118). The third study was a multi-center trial conducted in Australia (115); patients were randomized to receive naltrexone, acamprosate, or placebo for 12 weeks. This study did not find an effect of naltrexone or acamprosate compared to placebo. However, this study did find that naltrexone appeared to be an effective treatment in patients with no depression. The fourth trial, called the PREDICT study, was conducted in Germany and was designed to match the protocol of the COMBINE study (14). The PREDICT study did not find significant differences between naltrexone or acamprosate and placebo.
Both the COMBINE and PREDICT studies explored a precision medicine approach. Specifically, the COMBINE Study examined whether a single nucleotide polymorphism (SNP) in the mu opioid receptor (OPRM1) gene, the Asn40Asp SNP predicted clinical response to naltrexone, an opioid antagonist. Results indicated that if treated with MM alone and naltrexone, 87.1% of Asp40 carriers had a good clinical outcome, compared with only 54.8% of individuals with the Asn40/Asn40 genotype, while, if treated with placebo, 48.6% of Asp40 carriers and 54.0% of individuals with the Asn40/Asn40 genotype had a good clinical outcome (119). However, a more recent RCT of naltrexone prospectively genotyped participants for the OPRM1 Asn40Asp SNP and did not find evidence for a genotype × treatment interaction (120), indicating that the Asn40Asp polymorphism may not be an effective biomarker for naltrexone efficacy. The PREDICT study examined if reward and relief drinking phenotypes moderated treatment response (121). This sub-group analysis found a significant interaction between the reward subgroup and naltrexone; such that in the reward drinkers, naltrexone predicted an 83% decreased likelihood of any heavy drinking, compared to placebo. Further, the PREDICT study investigated if patients’ pre-treatment neural response to alcohol cues moderated medication efficacy (122). They reported an interaction between pre-treatment brain activation to alcohol cues and medication on time to relapse, such that in individuals with high reactivity to alcohol cues, the risk of relapse was lower in patients assigned to naltrexone compared to those assigned to acamprosate. In sum, these results show the promise of a precision medicine approach to improving treatment response.
Promising Off-Label Medications
Numerous promising pharmacotherapies have been examined as possible treatments for AUD. In fact, in 2007 the National Institute on Alcohol Abuse and Alcoholism (NIAAA) established the Clinical Investigations Group (NCIG) as a formalized effort to support Phase II clinical trials of promising medications. To date, NCIG has completed 6 Phase II multi-site, placebo-controlled, randomized trials. Next, we review several pharmacotherapies recently tested off-label for the treatment of AUD.
(1) Nalmefene is a mu- and delta-opioid antagonist and a partial kappa-opioid receptor agonist that is approved for the treatment of AUD in Europe. Notably, nalmefene is the only pharmacological treatment approved for the reduction of alcohol consumption, rather than abstinence. Initial results have supported the efficacy for nalmefene to prevent relapse to heavy drinking in comparison to placebo (123, 124), although some studies have been null (125). A recent meta-analysis of 7 placebo-controlled studies found that nalmefene is effective at reducing heavy drinking days and total alcohol consumption (126).
(2) Varenicline is a partial α4β2 nicotinic agonist and full α7 agonist that is currently FDA approved for the treatment of nicotine dependence. Varenicline was examined as part of the NCIG initiative (127), and found to significantly reduce weekly percent heavy drinking days, drinks per day, drinks per drinking day, as well as alcohol craving. Varenicline was also well tolerated suggesting that it may serve as a promising option for AUD treatment. Moderator analyses indicated that varenicline was more efficacious in reducing drinking in smokers who also reduced their cigarette smoking (128). Recent studies investigating varencline’s efficacy in heavy drinking smokers have supported this finding (129, 130). New findings indicate that varenicline’s may be more effective as an AUD treatment in men, as varenicline combined with medication management decreased heavy drinking only in men, but improved smoking abstinence in both men and women (131). This medication may be especially relevant to smokers, given epidemiological estimates that 20–25% of current smokers are heavy drinkers (132).
(3) Anti-convulsants are another class of medications that has been examined for the treatment of AUD. Gabapentin is thought to modulate GABA activity by indirectly interacting with voltage-gated calcium channels (133). A recent 12-week clinical trial revealed that gabapentin significantly improved rates of heavy drinking and abstinence, and was well tolerated with no serious adverse events (134). Additional single-site RCTs have demonstrated initial efficacy for gabapentin to reduce drinking outcomes (135, 136), and in combination with naltrexone produced superior effects compared to naltrexone alone (137). A Cochrane Review found gabapentin to significantly reduce rates of heavy drinking, but reported no effects on attenuating alcohol craving (138). It should be noted that gabapentin does carry the potential for misuse and abuse, particularly in individuals with opioid use disorders (139); therefore careful consideration should be given before recommending gabapentin to individuals with co-morbid substance use disorder.
Another promising anti-convulsant medication is topiramate. While the precise mechanisms of action remain unclear, topiramate is thought to reduce neuronal excitability through inhibition at glutamate AMPA/kainate receptors and L-type calcium channels. This could conceivably attenuate behavioral symptoms of protracted withdrawal from alcohol. Topiramate facilitates brain GABA function and may even increase GABA levels. Both of these effects (i.e., glutamate blockade, GABA facilitation) can in turn reduce or inhibit mesolimbic DA activity. It has been suggested that topiramate may indirectly influence midbrain dopaminergic activity, thereby reducing craving (140). A trial found that topiramate reduced drinking and alcohol craving over a 12-week treatment period (140). A larger, multi-site trial of topiramate similarly found a reduction in the percentage of heavy drinking days and improved self-report drinking outcomes during the 14-week treatment period (141). A more recent trial found topiramate to reduce drinks per day, percent days drinking, and percent days heavy drinking (142). Side effects of topiramate include cognitive impairment, such as slight reductions in verbal fluency and working memory (142). A recent review concluded that topiramate demonstrates clinical efficacy in various drinking outcomes, however longer-term trials of topiramate are warranted to further establish the optimal treatment dose, duration, and tolerability (143).
Lastly, zonisamide, an FDA approved adjunct treatment for partial seizures, is a new generation of anticonvulsant with several molecular actions in the brain, including enhancing GABA function. Zonisamide has shown initial efficacy in reducing number of heavy drinking day and drinks per week (144), as well as reducing urge to drink (145). A recent trial examining the efficacy of three anticonvulsants found that zonisamide and topiramate reduced the number of drinks per drinking day, number of drinking days, and number of heavy drinking days, compared to placebo (142). However, both zonisamide and topiramate produce cognitive side effects, which should be monitored during treatment.
(4) Baclofen is a GABAB agonist that is currently FDA approved for muscle spasticity. While pre-clinical trials have shown reduced alcohol intake, clinical trials have produced mixed results (146–148). A recent meta-analysis found that while baclofen is associated with higher rates of abstinence than placebo, it did not increase number of days abstinent, or decrease heavy drinking, craving, anxiety, or depression (149).
(5) Ondansetron is a 5-HT3 antagonist that is FDA approved to treat nausea and vomiting. Ondansetron has demonstrated effectiveness, relative to placebo, in the reduction of drinking among individuals with early onset AUD (150). Although the mechanism of action is unclear, it has been speculated that ondansetron might address the serotonergic dysfunction thought to characterize early onset AUD (150, 151) and that it might reduce craving for alcohol through the influence of 5-HT3 projections to mesolimbic dopaminergic connections in the midbrain (150, 151).
(6) ABT-436 is a novel arginine vasopressin V1B receptor antagonist that has shown some efficacy in treating AUD. Vasopressin receptors have a regulatory role during alcohol consumption, as well in stress and anxiety (152). Animal studies have suggested that blocking V1B receptors results in decreased alcohol consumption (153, 154). A recent multisite clinical trial found that ABT-436 increased the percent days abstinent compared with placebo (155). The trial also found that patients receiving ABT-436 had a lower percentage of heavy drinking days compared to placebo; however, this difference was not statistically significant. Further, a moderator analysis found that individuals with higher baseline levels of stress had a lower percentage of heavy drinking days on ABT-436 compared to placebo (133). ABT-436 appears to exert some selective effects on specific drinking outcomes and for a subset of individuals.
Off-Label Medications with Poor Efficacy
In addition to the promising medications reviewed above, a series of pharmacotherapies have been tested with results suggesting poor efficacy, despite initial promising findings.
(1) Quetiapine, an atypical antipsychotic, is often used in treating psychiatric disorders, such as schizophrenia, bipolar disorder, and major depression. Quetiapine blocks multiple dopamine and serotonin receptors. Quetiapine, showed initial efficacy in heavy drinkers (156–158). However, an examination of Quetiapine as part of the NCIG trials found no benefit of quetiapine on percent of heavy drinking days, percent days abstinent, drinks per day, or percent heavy drinking days (159). However, quetiapine improved sleep and decreased depressive symptoms in comparison to placebo. Another study examined quetiapine’s efficacy in reducing alcohol consumption in individuals with bipolar disorder and comorbid AUD, and did not find an effect of quetiapine in reducing drinks per day or on other alcohol measures (160).
(2) Levetiracetam is a FDA-approved anticonvulsant, which has shown poor efficacy as a treatment for AUD despite early promising results. Levetiracetam initially showed promise in limiting harmful drinking in both animal models (161) and reducing alcohol withdrawal symptoms in a clinical sample (162). When examined in NCIG, Levetiracetam was no different than placebo on primary and secondary drinking outcome measures (163). Levetiracetam was compared to other anticonvulsants (topiramate and zonisamide) and found to be less efficacious for AUD than the others (142).
(3) Selective-serotonin reuptake inhibitors (SSRIs) are FDA-approved medications for the treatment of mood and anxiety disorders. The efficacy of SSRIs in reducing drinking has been modest (164, 165), although it has been suggested that they may work better in certain subgroups, including alcoholic type (Type A, low-risk/severity subtype characterized by later onset of problem drinking, less severe dependence, and better treatment response vs. Type B, high-risk/severity subtype characterized by familial risk factors, early onset of alcohol problems, and poor treatment prognosis) (166, 167) and in those with genetic polymorphisms in the SLC6A4 gene (168). SSRIs reduced depressive symptoms in depressed individuals with AUD to the same degree as depressed individuals without an AUD (169), indicating that these medications can be used in combination with more efficacious AUD treatments to address comorbid presentations.
Summary of Pharmacological Treatments:
In summary, RCTs have provided sufficient evidence of the efficacy, albeit modest, for acamprosate, oral naltrexone, and Vivitrol for AUD treatment (97, 170–172). Of the NCIG trials conducted to date, Varenicline appears to be the most promising off-label medication in development for AUD (127). In addition, there are a number of opportunities for research in the field of pharmacotherapies for AUD, including the need to identify psychosocial predictors of medication compliance and efficacy (97), expand our knowledge of dosing issues (173), improve the dissemination of research findings to clinicians in the field (174), examine the combined effects of psychosocial and pharmacotherapy treatments (175), and study the role of genetic factors as predictors of response to pharmacotherapies (176). Perhaps most importantly, there is a strong recommendation that novel targets and novel compounds be screened for efficacy for AUD treatment (177, 178), given the recognition that repurposing psychiatric medications for AUD treatment has been met with limited success. Thus we anticipate that future AUD clinical trials will branch towards novel compounds and targets that can more effectively mitigate the neurotoxic effects of alcohol and ameliorate alcohol-induced neuroadaptations.
Conclusions
Alcohol use disorder has multifaceted etiology, maintenance, and relapse processes. Research reviewed in this article underscores the complex nature of AUD and its treatment. One of the broad assertions from the work reviewed herein is that while AUD is highly prevalent, with rates of 13.9% and 29.1% of adults in the US meeting criteria for 12-month and lifetime diagnoses of AUD, seeking treatment for AUD is not, as only 7.7% and 19.8% of individuals with current and lifetime diagnoses sought treatment (2). There is substantial naturalistic recovery and brief intervention may be able to catalyze recovery in mild cases, yet the vast majority of individuals with moderate-to-severe AUD will require specialized treatment to overcome this disorder. To that end, identifying treatments that are evidence-based is a rather challenging task. To mitigate this concern, NIAAA has launched a new initiative called NIAAA Alcohol Treatment Navigator (https://alcoholtreatment.niaaa.nih.gov/), with the overarching goal of helping patients and families identify evidence-based care for AUD. We encourage providers to become familiar with the resources in the NIAAA Alcohol Treatment Navigator and to consider using it in their practice. Furthermore, future research should focus on the identification of barriers to effectively implement these evidence-based treatments in the community. This article sought to review the literature on evidence-based care for AUD at the level of psychosocial and pharmacological interventions. There are a myriad of behavioral and pharmacological treatments that have shown compelling evidence of efficacy for the treatment of AUD. While none of those treatments represents a so called “silver bullet” for AUD, they each have the potential to significantly improve the odds of AUD recovery. While beyond the scope of this review, there are significant barriers to the utilization (or “uptake”) of treatments in real-world treatment settings which also require careful consideration.
Precision medicine, or identification of best treatment matches for individual patients, looms as an important overarching goal for the field, but specific matches are not yet reliable in their research evidence to warrant clinical dissemination. Nonetheless, there is a strong recognition among providers and patients alike that “more is better” and that giving patients access to multiple evidence-based resources for behavioral and pharmacological treatment of AUD is likely to improve each individual’s chances of sustained recovery from AUD. As the field of AUD treatment moves towards the development of novel pharmacotherapies and the adaptation of behavioral therapies to the high-technology environment we live in, we are reminded that decades of research have resulted in the currently-available treatments that are evidence-based. As such, each individual patient is entitled to receive those treatments as he/she embarks on his/her recovery from AUD.
Acknowledgments
Funding Sources: Supported by NIH grants DA041226, AA026006, and AA023669 (LR); and training grants T32 DA007272 (AV and AL) and T32DA024635 (RG and EG).
Footnotes
Financial Disclosures: None of the authors have any conflicts of interest or financial disclosures.
References
- 1.Organization WH, Unit WHOMoSA. Global status report on alcohol and health, 2014: World Health Organization, 2014. [Google Scholar]
- 2.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry 2015; 72 (8), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend 2004; 74 (3), 223–234. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017; 74 (9), 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend 2008; 93 (1–2), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Archives of general psychiatry 2009; 66 (7), 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyes KM, Li G, Hasin DS. Birth cohort effects and gender differences in alcohol epidemiology: a review and synthesis. Alcoholism, clinical and experimental research 2011; 35 (12), 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. American journal of preventive medicine 2015; 49 (5), e73–e79. [DOI] [PubMed] [Google Scholar]
- 9.Keyes KM, Hatzenbuehler ML, McLaughlin KA, Link B, Olfson M, Grant BF, Hasin D. Stigma and treatment for alcohol disorders in the United States. Am J Epidemiol 2010; 172 (12), 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoast RA, Wilford BB, Hayashi SW. Encouraging physicians to screen for and intervene in substance use disorders: obstacles and strategies for change. Journal of addictive diseases 2008; 27 (3), 77–97. [DOI] [PubMed] [Google Scholar]
- 11.Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 2007; 86 (2–3), 214–221. [DOI] [PubMed] [Google Scholar]
- 12.Schuler MS, Puttaiah S, Mojtabai R, Crum RM. Perceived Barriers to Treatment for Alcohol Problems: A Latent Class Analysis. Psychiatric services (Washington, D.C.) 2015; 66 (11), 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry 2007; 64 (7), 830–842. [DOI] [PubMed] [Google Scholar]
- 14.Mann K, Lemenager T, Hoffmann S, Reinhard I, Hermann D, Batra A, Berner M, Wodarz N, Heinz A, Smolka MN, Zimmermann US, Wellek S, Kiefer F, Anton RF. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addiction biology 2013; 18 (6), 937–946. [DOI] [PubMed] [Google Scholar]
- 15.Ray LA, Bujarski S, Yardley MM, Roche DJO, Hartwell EE. Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse 2017; 43 (6), 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohn MC, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L. Differences Between Treatment-Seeking and Nontreatment-Seeking Alcohol-Dependent Research Participants: An Exploratory Analysis. Alcoholism, clinical and experimental research 2017; 41 (2), 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisner C, Matzger H. A prospective study of the factors influencing entry to alcohol and drug treatment. The journal of behavioral health services & research 2002; 29 (2), 126–137. [DOI] [PubMed] [Google Scholar]
- 18.Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: prevalence in two population surveys. American journal of public health 1996; 86 (7), 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulia N, Karriker-Jaffe KJ, Witbrodt J, Bond J, Williams E, Zemore SE. Racial/ethnic differences in 30-year trajectories of heavy drinking in a nationally representative U.S. sample. Drug Alcohol Depend 2017; 170, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlamangla A, Zhou K, Reuben D, Greendale G, Moore A. Longitudinal trajectories of heavy drinking in adults in the United States of America. Addiction (Abingdon, England) 2006; 101 (1), 91–99. [DOI] [PubMed] [Google Scholar]
- 21.Bischof G, Rumpf HJ, Hapke U, Meyer C, John U. Types of natural recovery from alcohol dependence: a cluster analytic approach. Addiction (Abingdon, England) 2003; 98 (12), 1737–1746. [DOI] [PubMed] [Google Scholar]
- 22.Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, Heather N, Saunders J, Burnand B. Effectiveness of brief alcohol interventions in primary care populations. The Cochrane database of systematic reviews 2007; (2), Cd004148. [DOI] [PubMed] [Google Scholar]
- 23.Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Archives of internal medicine 2005; 165 (9), 986–995. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio G, Degutis LC. Preventive care in the emergency department: screening and brief intervention for alcohol problems in the emergency department: a systematic review. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 2002; 9 (6), 627–638. [DOI] [PubMed] [Google Scholar]
- 25.Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction (Abingdon, England) 2002; 97 (3), 279–292. [DOI] [PubMed] [Google Scholar]
- 26.Substance A, Mental HSAU, General OotS. Facing addiction in America: The Surgeon General’s report on alcohol, drugs, and health. 2016. [PubMed]
- 27.Saitz R Alcohol screening and brief intervention in primary care: Absence of evidence for efficacy in people with dependence or very heavy drinking. Drug and alcohol review 2010; 29 (6), 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riper H, Andersson G, Hunter SB, de Wit J, Berking M, Cuijpers P. Treatment of comorbid alcohol use disorders and depression with cognitive-behavioural therapy and motivational interviewing: a meta-analysis. Addiction (Abingdon, England) 2014; 109 (3), 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller WR, Rollnick S. Motivational interviewing: Helping people change: Guilford press, 2012. [Google Scholar]
- 30.Schmidt CS, Schulte B, Seo HN, Kuhn S, O’Donnell A, Kriston L, Verthein U, Reimer J. Meta-analysis on the effectiveness of alcohol screening with brief interventions for patients in emergency care settings. Addiction (Abingdon, England) 2016; 111 (5), 783–794. [DOI] [PubMed] [Google Scholar]
- 31.Jonas DE, Garbutt JC, Amick HR, Brown JM, Brownley KA, Council CL, Viera AJ, Wilkins TM, Schwartz CJ, Richmond EM, Yeatts J, Evans TS, Wood SD, Harris RP. Behavioral counseling after screening for alcohol misuse in primary care: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Annals of internal medicine 2012; 157 (9), 645–654. [DOI] [PubMed] [Google Scholar]
- 32.Monti PM. Treating alcohol dependence: A coping skills training guide: Guilford Press, 2002. [Google Scholar]
- 33.Carroll KM, Kiluk BD. Cognitive behavioral interventions for alcohol and drug use disorders: Through the stage model and back again. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 2017; 31 (8), 847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magill M, Ray LA. Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. J Stud Alcohol Drugs 2009; 70 (4), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. The Psychiatric clinics of North America 2010; 33 (3), 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 2008; 165 (2), 179–187. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognitive therapy and research 2012; 36 (5), 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Farrell TJ, Fals-Stewart W. Behavioral couples therapy for alcoholism and drug abuse. Journal of substance abuse treatment 2000; 18 (1), 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers MB, Vedel E, Emmelkamp PM. Behavioral couples therapy (BCT) for alcohol and drug use disorders: a meta-analysis. Clinical psychology review 2008; 28 (6), 952–962. [DOI] [PubMed] [Google Scholar]
- 40.McCrady BS, Wilson AD, Munoz RE, Fink BC, Fokas K, Borders A. Alcohol-Focused Behavioral Couple Therapy. Family process 2016; 55 (3), 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyers RJ, Roozen HG, Smith JE. The community reinforcement approach: an update of the evidence. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism 2011; 33 (4), 380–388. [PMC free article] [PubMed] [Google Scholar]
- 42.Roozen HG, Boulogne JJ, van Tulder MW, van den Brink W, De Jong CA, Kerkhof AJ. A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction. Drug Alcohol Depend 2004; 74 (1), 1–13. [DOI] [PubMed] [Google Scholar]
- 43.Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Preventive medicine 2016; 92, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzsimons H, Tuten M, Borsuk C, Lookatch S, Hanks L. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015; 152, 62–67. [DOI] [PubMed] [Google Scholar]
- 45.Dougherty DM, Lake SL, Hill-Kapturczak N, Liang YY, Karns TE, Mullen J, Roache JD. Using Contingency Management Procedures to Reduce At-Risk Drinking in Heavy Drinkers. Alcoholism-Clinical and Experimental Research 2015; 39 (4), 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonell MG, Leickly E, McPherson S, Skalisky J, Srebnik D, Angelo F, Vilardaga R, Nepom JR, Roll JM, Ries RK. A Randomized Controlled Trial of Ethyl Glucuronide-Based Contingency Management for Outpatients With Co-Occurring Alcohol Use Disorders and Serious Mental Illness. American Journal of Psychiatry 2017; 174 (4), 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction (Abingdon, England) 2002; 97 (2), 155–167. [DOI] [PubMed] [Google Scholar]
- 48.Loeber S, Croissant B, Heinz A, Mann K, Flor H. Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. The British journal of clinical psychology 2006; 45 (Pt 4), 515–529. [DOI] [PubMed] [Google Scholar]
- 49.Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction (Abingdon, England) 2001; 96 (8), 1161–1174. [DOI] [PubMed] [Google Scholar]
- 50.Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcoholism, clinical and experimental research 2001; 25 (11), 1634–1647. [PubMed] [Google Scholar]
- 51.Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry 2011; 69 (11), 1060–1066. [DOI] [PubMed] [Google Scholar]
- 52.Kavanagh DJ, Sitharthan G, Young RM, Sitharthan T, Saunders JB, Shockley N, Giannopoulos V. Addition of cue exposure to cognitive-behaviour therapy for alcohol misuse: a randomized trial with dysphoric drinkers. Addiction (Abingdon, England) 2006; 101 (8), 1106–1116. [DOI] [PubMed] [Google Scholar]
- 53.Mellentin AI, Skot L, Nielsen B, Schippers GM, Nielsen AS, Stenager E, Juhl C. Cue exposure therapy for the treatment of alcohol use disorders: A meta-analytic review. Clinical psychology review 2017; 57, 195–207. [DOI] [PubMed] [Google Scholar]
- 54.Mellentin AI, Stenager E, Nielsen B, Nielsen AS, Yu F. A Smarter Pathway for Delivering Cue Exposure Therapy? The Design and Development of a Smartphone App Targeting Alcohol Use Disorder. JMIR mHealth and uHealth 2017; 5 (1), e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JW, Frawley PJ, Polissar L. 6-Month and 12-Month Abstinence Rates in Inpatient Alcoholics Treated with Aversion Therapy Compared with Matched Inpatients from a Treatment Registry. Alcoholism-Clinical and Experimental Research 1991; 15 (5), 862–870. [DOI] [PubMed] [Google Scholar]
- 56.Walitzer KS, Dermen KH, Barrick C. Facilitating involvement in Alcoholics Anonymous during out-patient treatment: a randomized clinical trial. Addiction (Abingdon, England) 2009; 104 (3), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferri M, Amato L, Davoli M. Alcoholics Anonymous and other 12-step programmes for alcohol dependence. The Cochrane database of systematic reviews 2006; (3), Cd005032. [DOI] [PubMed] [Google Scholar]
- 58.Emrick CD, Tonigan JS, Montgomery H, Little L. Alcoholics Anonymous: What is currently known? 1993.
- 59.Kownacki RJ, Shadish WR. Does Alcoholics Anonymous work? The results from a meta-analysis of controlled experiments. Substance use & misuse 1999; 34 (13), 1897–1916. [DOI] [PubMed] [Google Scholar]
- 60.Tonigan JS, Toscova R, Miller WR. Meta-analysis of the literature on Alcoholics Anonymous: sample and study characteristics moderate findings. Journal of studies on alcohol 1996; 57 (1), 65–72. [DOI] [PubMed] [Google Scholar]
- 61.Kaskutas LA. Alcoholics anonymous effectiveness: faith meets science. Journal of addictive diseases 2009; 28 (2), 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly JF, Dow SJ, Yeterian JD, Kahler CW. Can 12-step group participation strengthen and extend the benefits of adolescent addiction treatment? A prospective analysis. Drug Alcohol Depend 2010; 110 (1–2), 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly JF, Magill M, Stout RL. How do people recover from alcohol dependence? A systematic review of the research on mechanisms of behavior change in Alcoholics Anonymous. Addiction Research & Theory 2009; 17 (3), 236–259. [Google Scholar]
- 64.Witkiewitz K, Marlatt GA, Walker D. Mindfulness-based relapse prevention for alcohol and substance use disorders. Journal of cognitive psychotherapy 2005; 19 (3), 211. [Google Scholar]
- 65.Bowen S, Chawla N, Marlatt G. Mindfulness-based relapse prevention for addictive behaviors A clinician guide New York. London, The Guilford press, 2011. [Google Scholar]
- 66.Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, Carroll HA, Harrop E, Collins SE, Lustyk MK, Larimer ME. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry 2014; 71 (5), 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Witkiewitz K, Bowen S, Douglas H, Hsu SH. Mindfulness-based relapse prevention for substance craving. Addictive behaviors 2013; 38 (2), 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Howard MO, Garland EL, McGovern P, Lazar M. Mindfulness treatment for substance misuse: A systematic review and meta-analysis. Journal of substance abuse treatment 2017; 75, 62–96. [DOI] [PubMed] [Google Scholar]
- 69.Bewick BM, Trusler K, Barkham M, Hill AJ, Cahill J, Mulhern B. The effectiveness of web-based interventions designed to decrease alcohol consumption--a systematic review. Preventive medicine 2008; 47 (1), 17–26. [DOI] [PubMed] [Google Scholar]
- 70.Rosa C, Campbell AN, Miele GM, Brunner M, Winstanley EL. Using e-technologies in clinical trials. Contemp Clin Trials 2015; 45 (Pt A), 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Litvin EB, Abrantes AM, Brown RA. Computer and mobile technology-based interventions for substance use disorders: an organizing framework. Addictive behaviors 2013; 38 (3), 1747–1756. [DOI] [PubMed] [Google Scholar]
- 72.Donoghue K, Patton R, Phillips T, Deluca P, Drummond C. The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: a systematic review and meta-analysis. Journal of medical Internet research 2014; 16 (6), e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White A, Kavanagh D, Stallman H, Klein B, Kay-Lambkin F, Proudfoot J, Drennan J, Connor J, Baker A, Hines E, Young R. Online alcohol interventions: a systematic review. Journal of medical Internet research 2010; 12 (5), e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott-Sheldon LA, Carey KB, Elliott JC, Garey L, Carey MP. Efficacy of alcohol interventions for first-year college students: a meta-analytic review of randomized controlled trials. Journal of consulting and clinical psychology 2014; 82 (2), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robins L, Newby J, Wilhelm K, Smith J, Fletcher T, Ma T, Finch A, Campbell L, Andrews G. Internet-delivered cognitive behaviour therapy for depression in people with diabetes: study protocol for a randomised controlled trial. BMJ Open Diabetes Res Care 2015; 3 (1), e000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devine EG, Ryan ML, Falk DE, Fertig JB, Litten RZ. An exploratory evaluation of Take Control: A novel computer-delivered behavioral platform for placebo-controlled pharmacotherapy trials for alcohol use disorder. Contemp Clin Trials 2016; 50, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcoholism, clinical and experimental research 2015; 39 (4), 579–584. [DOI] [PubMed] [Google Scholar]
- 78.Allen J, Anton RF, Babor TF, Carbonari J. Project MATCH secondary a priori hypotheses. Addiction (Abingdon, England) 1997; 92 (12), 1671. [PubMed] [Google Scholar]
- 79.GROUP PMR. Matching patients with alcohol disorders to treatments: Clinical implications from Project MATCH. Journal of Mental Health 1998; 7 (6), 589–602. [Google Scholar]
- 80.Bujarski S, O’Malley SS, Lunny K, Ray LA. The effects of drinking goal on treatment outcome for alcoholism. Journal of consulting and clinical psychology 2013; 81 (1), 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrakis IL. A rational approach to the pharmacotherapy of alcohol dependence. Journal of clinical psychopharmacology 2006; 26 (6), S3–S12. [DOI] [PubMed] [Google Scholar]
- 82.Pettinati HM, Rabinowitz AR. Choosing the right medication for the treatment of alcoholism. Curr Psychiatry Rep 2006; 8 (5), 383–388. [DOI] [PubMed] [Google Scholar]
- 83.Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. Journal of studies on alcohol and drugs. Supplement 2014; 75 Suppl 17, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 2012; 4 (116), 116ra116. [DOI] [PubMed] [Google Scholar]
- 85.Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience 2005; 8 (11), 1445–1449. [DOI] [PubMed] [Google Scholar]
- 86.O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Archives of general psychiatry 1992; 49 (11), 881–887. [DOI] [PubMed] [Google Scholar]
- 87.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of general psychiatry 1992; 49 (11), 876–880. [DOI] [PubMed] [Google Scholar]
- 88.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Archives of general psychiatry 2007; 64 (9), 1069–1077. [DOI] [PubMed] [Google Scholar]
- 89.Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcoholism, clinical and experimental research 2004; 28 (9), 1362–1370. [DOI] [PubMed] [Google Scholar]
- 90.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002; 160 (1), 19–29. [DOI] [PubMed] [Google Scholar]
- 91.Richardson K, Baillie A, Reid S, Morley K, Teesson M, Sannibale C, Weltman M, Haber P. Do acamprosate or naltrexone have an effect on daily drinking by reducing craving for alcohol? Addiction (Abingdon, England) 2008; 103 (6), 953–959. [DOI] [PubMed] [Google Scholar]
- 92.O’Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J, Romano DM, Wu R, Toll BA, Sher KJ, Gueorguieva R, Kranzler HR. Reduction of alcohol drinking in young adults by naltrexone: a double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. The Journal of clinical psychiatry 2015; 76 (2), e207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction (Abingdon, England) 2001; 96 (11), 1565–1573. [DOI] [PubMed] [Google Scholar]
- 94.Carmen B, Angeles M, Ana M, María AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction (Abingdon, England) 2004; 99 (7), 811–828. [DOI] [PubMed] [Google Scholar]
- 95.Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Archives of general psychiatry 2003; 60 (1), 92–99. [DOI] [PubMed] [Google Scholar]
- 96.Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramirez M, Mengual I, Gonzalvo B, Segura L, Trujols J, Casas M. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcoholism, clinical and experimental research 2002; 26 (9), 1381–1387. [DOI] [PubMed] [Google Scholar]
- 97.Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology 2000; 22 (5), 493–503. [DOI] [PubMed] [Google Scholar]
- 98.Krystal JH, Cramer J, Krol W, Kirk G, Rosenheck R. Naltrexone in the treatment of alcohol dependence. N Engl J Med 2003; 200113 (345), 24. [DOI] [PubMed] [Google Scholar]
- 99.Streeton C, Whelan G. Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcohol 2001; 36 (6), 544–552. [DOI] [PubMed] [Google Scholar]
- 100.Johnson BA. Naltrexone long-acting formulation in the treatment of alcohol dependence. Ther Clin Risk Manag 2007; 3 (5), 741–749. [PMC free article] [PubMed] [Google Scholar]
- 101.Garbutt JC. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial (vol 293, pg 1617, 2005). Jama-J Am Med Assoc 2005; 293 (16), 1978–1978. [DOI] [PubMed] [Google Scholar]
- 102.O’Malley SS, Garbutt JC, Gasfriend DR, Dong QM, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. Journal of Clinical Psychopharmacology 2007; 27 (5), 507–512. [DOI] [PubMed] [Google Scholar]
- 103.Mason BJ, Heyser CJ. Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS & neurological disorders drug targets 2010; 9 (1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosner S, Leucht S, Lehert P, Soyka M. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol 2008; 22 (1), 11–23. [DOI] [PubMed] [Google Scholar]
- 105.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama 2014; 311 (18), 1889–1900. [DOI] [PubMed] [Google Scholar]
- 106.Koob GF, Mason BJ, De Witte P, Littleton J, Siggins GR. Potential neuroprotective effects of acamprosate. Alcoholism, clinical and experimental research 2002; 26 (4), 586–592. [PubMed] [Google Scholar]
- 107.Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. Journal of addiction medicine 2007; 1 (3), 115–125. [DOI] [PubMed] [Google Scholar]
- 108.Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. European journal of pharmacology 1990; 176 (3), 289–296. [DOI] [PubMed] [Google Scholar]
- 109.Davidson M, Shanley B, Wilce P. Increased NMDA-induced excitability during ethanol withdrawal: a behavioural and histological study. Brain research 1995; 674 (1), 91–96. [DOI] [PubMed] [Google Scholar]
- 110.Vallari RC, Pietruszko R. Human aldehyde dehydrogenase: mechanism of inhibition of disulfiram. Science (New York, N.Y.) 1982; 216 (4546), 637–639. [DOI] [PubMed] [Google Scholar]
- 111.Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, James KE, Lacoursiere RB, Lee KK, Lowenstam I, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. Jama 1986; 256 (11), 1449–1455. [PubMed] [Google Scholar]
- 112.Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. Jama 1999; 281 (14), 1318–1325. [DOI] [PubMed] [Google Scholar]
- 113.Skinner MD, Lahmek P, Pham H, Aubin HJ. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PloS one 2014; 9 (2), e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fuller RK, Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction (Abingdon, England) 2004; 99 (1), 21–24. [DOI] [PubMed] [Google Scholar]
- 115.Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction (Abingdon, England) 2006; 101 (10), 1451–1462. [DOI] [PubMed] [Google Scholar]
- 116.Group TCSR. Testing combined pharmacotherapies and behavioral interventions for alcohol dependence (the COMBINE study): a pilot feasibility study. Alcoholism, clinical and experimental research 2003; 27 (7), 1123–1131. [DOI] [PubMed] [Google Scholar]
- 117.Group TCSR. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcoholism, clinical and experimental research 2003; 27 (7), 1107–1122. [DOI] [PubMed] [Google Scholar]
- 118.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama 2006; 295 (17), 2003–2017. [DOI] [PubMed] [Google Scholar]
- 119.Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of general psychiatry 2008; 65 (2), 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oslin DW, Leong SH, Lynch KG, Berrettini W, O’Brien CP, Gordon AJ, Rukstalis M. Naltrexone vs Placebo for the Treatment of Alcohol Dependence A Randomized Clinical Trial. Jama Psychiatry 2015; 72 (5), 430–437. [DOI] [PubMed] [Google Scholar]
- 121.Mann K, Roos CR, Hoffmann S, Nakovics H, Lemenager T, Heinz A, Witkiewitz K. Precision Medicine in Alcohol Dependence: A Controlled Trial Testing Pharmacotherapy Response Among Reward and Relief Drinking Phenotypes. Neuropsychopharmacology 2018; 43 (4), 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mann K, Vollstadt-Klein S, Reinhard I, Lemenager T, Fauth-Buhler M, Hermann D, Hoffmann S, Zimmermann US, Kiefer F, Heinz A, Smolka MN. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcoholism, clinical and experimental research 2014; 38 (11), 2754–2762. [DOI] [PubMed] [Google Scholar]
- 123.Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Archives of general psychiatry 1999; 56 (8), 719–724. [DOI] [PubMed] [Google Scholar]
- 124.Mann K, Bladstrom A, Torup L, Gual A, van den Brink W. Extending the treatment options in alcohol dependence: a randomized controlled study of as-needed nalmefene. Biol Psychiatry 2013; 73 (8), 706–713. [DOI] [PubMed] [Google Scholar]
- 125.Anton RF, Pettinati H, Zweben A, Kranzler HR, Johnson B, Bohn MJ, McCaul ME, Anthenelli R, Salloum I, Galloway G, Garbutt J, Swift R, Gastfriend D, Kallio A, Karhuvaara S. A multi-site dose ranging study of nalmefene in the treatment of alcohol dependence. J Clin Psychopharmacol 2004; 24 (4), 421–428. [DOI] [PubMed] [Google Scholar]
- 126.Mann K, Torup L, Sorensen P, Gual A, Swift R, Walker B, van den Brink W. Nalmefene for the management of alcohol dependence: review on its pharmacology, mechanism of action and meta-analysis on its clinical efficacy. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 2016; 26 (12), 1941–1949. [DOI] [PubMed] [Google Scholar]
- 127.Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. Journal of addiction medicine 2013; 7 (4), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Falk DE, Castle IJ, Ryan M, Fertig J, Litten RZ. Moderators of Varenicline Treatment Effects in a Double-Blind, Placebo-Controlled Trial for Alcohol Dependence: An Exploratory Analysis. Journal of addiction medicine 2015; 9 (4), 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 2009; 66 (2), 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012; 223 (3), 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, Bold KW, Petrakis I, Muvvala S, Jatlow P, Gueorguieva R. Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking A Randomized Clinical Trial. Jama Psychiatry 2018; 75 (2), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Research and Health 2000; 24 (4), 201–208. [PMC free article] [PubMed] [Google Scholar]
- 133.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current opinion in pharmacology 2006; 6 (1), 108–113. [DOI] [PubMed] [Google Scholar]
- 134.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA internal medicine 2014; 174 (1), 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcoholism, clinical and experimental research 2008; 32 (8), 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. The Journal of clinical psychiatry 2007; 68 (11), 1691–1700. [DOI] [PubMed] [Google Scholar]
- 137.Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin combined with naltrexone for the treatment of alcohol dependence. American Journal of Psychiatry 2011; 168 (7), 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pani PP, Trogu E, Pacini M, Maremmani I. Anticonvulsants for alcohol dependence. Cochrane Database of Systematic Reviews 2010; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction (Abingdon, England) 2016; 111 (7), 1160–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet (London, England) 2003; 361 (9370), 1677–1685. [DOI] [PubMed] [Google Scholar]
- 141.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM, Adv TA. Topiramate for treating alcohol dependence - A randomized controlled trial. Jama-J Am Med Assoc 2007; 298 (14), 1641–1651. [DOI] [PubMed] [Google Scholar]
- 142.Knapp CM, Ciraulo DA, Sarid-Segal O, Richardson MA, Devine E, Streeter CC, Oscar-Berman M, Surprise C, Colaneri L, Putnam M, Waters M, Richambault C. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol 2015; 35 (1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olmsted CL, Kockler DR. Topiramate for alcohol dependence. The Annals of pharmacotherapy 2008; 42 (10), 1475–1480. [DOI] [PubMed] [Google Scholar]
- 144.Arias AJ, Feinn R, Oncken C, Covault J, Kranzler HR. Placebo-controlled trial of zonisamide for the treatment of alcohol dependence. J Clin Psychopharmacol 2010; 30 (3), 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarid-Segal O, Knapp CM, Burch W, Richardson MA, Bahtia S, DeQuattro K, Afshar M, Richambault C, Sickels L, Devine E, Ciraulo DA. The anticonvulsant zonisamide reduces ethanol self-administration by risky drinkers. Am J Drug Alcohol Abuse 2009; 35 (5), 316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol 2002; 37 (5), 504–508. [DOI] [PubMed] [Google Scholar]
- 147.Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcoholism, clinical and experimental research 2010; 34 (11), 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morley KC, Baillie A, Leung S, Addolorato G, Leggio L, Haber PS. Baclofen for the Treatment of Alcohol Dependence and Possible Role of Comorbid Anxiety. Alcohol Alcohol 2014; 49 (6), 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rose AK, Jones A. Baclofen: its effectiveness in reducing harmful drinking, craving, and negative mood. A meta-analysis. Addiction (Abingdon, England) 2018. [DOI] [PubMed] [Google Scholar]
- 150.Johnson BA, Ait-Daoud N, Prihoda TJ. Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypotheses to preliminary clinical evidence. Alcoholism, clinical and experimental research 2000; 24 (5), 737–742. [PubMed] [Google Scholar]
- 151.Johnson BA, Ait-Daoud N. Neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Psychopharmacology (Berl) 2000; 149 (4), 327–344. [DOI] [PubMed] [Google Scholar]
- 152.Caldwell HK, Wersinger SR, Young WS, 3rd. The role of the vasopressin 1b receptor in aggression and other social behaviours. Progress in brain research 2008; 170, 65–72. [DOI] [PubMed] [Google Scholar]
- 153.Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addiction biology 2012; 17 (1), 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcoholism, clinical and experimental research 2011; 35 (10), 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K, Mahoney E, Ciraulo DA, Sickles-Colaneri L, Ait-Daoud N, Johnson BA, Ransom J, Scott C, Koob GF, Litten RZ. A Phase 2, Double-Blind, Placebo-Controlled Randomized Trial Assessing the Efficacy of ABT-436, a Novel V1b Receptor Antagonist, for Alcohol Dependence. Neuropsychopharmacology 2017; 42 (5), 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martinotti G, Andreoli S, Di Nicola M, Di Giannantonio M, Sarchiapone M, Janiri L. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol 2008; 23 (5), 417–424. [DOI] [PubMed] [Google Scholar]
- 157.Monnelly EP, Ciraulo DA, Knapp C, LoCastro J, Sepulveda I. Quetiapine for treatment of alcohol dependence. Journal of Clinical Psychopharmacology 2004; 24 (5), 532–535. [DOI] [PubMed] [Google Scholar]
- 158.Sattar SP, Bhatia SC, Petty F. Potential benefits of quetiapine in the treatment of substance dependence disorders. J Psychiatry Neurosci 2004; 29 (6), 452–457. [PMC free article] [PubMed] [Google Scholar]
- 159.Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, Murtaugh C, Ciraulo D, Green AI, Johnson B, Pettinati H, Swift R, Afshar M, Brunette MF, Tiouririne NA, Kampman K, Stout R. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcoholism, clinical and experimental research 2012; 36 (3), 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown ES, Davila D, Nakamura A, Carmody TJ, Rush AJ, Lo A, Holmes T, Adinoff B, Caetano R, Swann AC, Sunderajan P, Bret ME. A randomized, double-blind, placebo-controlled trial of quetiapine in patients with bipolar disorder, mixed or depressed phase, and alcohol dependence. Alcoholism, clinical and experimental research 2014; 38 (7), 2113–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zalewska-Kaszubska J, Bajer B, Gorska D, Andrzejczak D, Dyr W, Bienkowski P. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol preferring rats chronically treated with lamotrigine. Physiology & behavior 2015; 139, 7–12. [DOI] [PubMed] [Google Scholar]
- 162.Muller CA, Schafer M, Schneider S, Heimann HM, Hinzpeter A, Volkmar K, Forg A, Heinz A, Hein J. Efficacy and safety of levetiracetam for outpatient alcohol detoxification. Pharmacopsychiatry 2010; 43 (5), 184–189. [DOI] [PubMed] [Google Scholar]
- 163.Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, Rickman WJ, Scott C, Ciraulo D, Green AI, Tiouririne NA, Johnson B, Pettinati H, Strain EC, Devine E, Brunette MF, Kampman K, D AT, Stout R. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcoholism, clinical and experimental research 2012; 36 (8), 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Litten RZ, Fertig J, Mattson M, Egli M. Development of medications for alcohol use disorders: recent advances and ongoing challenges. Expert opinion on emerging drugs 2005; 10 (2), 323–343. [DOI] [PubMed] [Google Scholar]
- 165.Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol 2008; 75 (1), 34–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcoholism, clinical and experimental research 1996; 20 (9), 1534–1541. [DOI] [PubMed] [Google Scholar]
- 167.Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism, clinical and experimental research 2000; 24 (7), 1041–1049. [PubMed] [Google Scholar]
- 168.Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, Pettinati H, Oncken C. A double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. J Clin Psychopharmacol 2011; 31 (1), 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry 2004; 56 (10), 785–792. [DOI] [PubMed] [Google Scholar]
- 170.Mann K Pharmacotherapy of alcohol dependence. CNS drugs 2004; 18 (8), 485–504. [DOI] [PubMed] [Google Scholar]
- 171.Myrick H, Brady KT, Malcolm R. New developments in the pharmacotherapy of alcohol dependence. The American journal on addictions 2001; 10 Suppl, 3–15. [DOI] [PubMed] [Google Scholar]
- 172.Schaffer A, Naranjo CA. Recommended drug treatment strategies for the alcoholic patient. Drugs 1998; 56 (4), 571–585. [DOI] [PubMed] [Google Scholar]
- 173.Mason BJ. Dosing issues in the pharmacotherapy of alcoholism. Alcoholism, clinical and experimental research 1996; 20 (7 Suppl), 10a–16a. [DOI] [PubMed] [Google Scholar]
- 174.Meza E, Kranzler HR. Closing the gap between alcoholism research and practice: the case for pharmacotherapy. Psychiatric services (Washington, D.C.) 1996; 47 (9), 917–920. [DOI] [PubMed] [Google Scholar]
- 175.McCaul ME, Petry NM. The role of psychosocial treatments in pharmacotherapy for alcoholism. The American journal on addictions 2003; 12 Suppl 1, S41–52. [DOI] [PubMed] [Google Scholar]
- 176.Kranzler HR, Smith RV, Schnoll R, Moustafa A, Greenstreet-Akman E. Precision medicine and pharmacogenetics: what does oncology have that addiction medicine does not? Addiction (Abingdon, England) 2017; 112 (12), 2086–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Litten RZ, Falk DE, Ryan ML, Fertig JB. Discovery, Development, and Adoption of Medications to Treat Alcohol Use Disorder: Goals for the Phases of Medications Development. Alcoholism, clinical and experimental research 2016; 40 (7), 1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addiction biology 2012; 17 (3), 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]