Abstract
A 12‐year‐old, neutered female, domestic medium hair cat was evaluated for a nonhealing, oral mucosal ulceration. The cat had a history of idiopathic hypercalcemia that had been treated with a bisphosphonate for 41 months. Oral examination identified exposed maxillary bone adjacent to a previous extraction site. Histopathology of the exposed bone and associated mucosa was most consistent with medication‐related osteonecrosis of the jaw. Treatment involved both medical and surgical interventions. Oral mucosal healing occurred after 6 months of treatment.
Keywords: alendronate, aminobisphosphonate, bisphosphonate‐related osteonecrosis of the jaw, hypercalcemia, osteomyelitis, pamidronate
Abbreviations
- AAOMS
American Association of Oral and Maxillofacial Surgeons
- IRIS
International Renal Interest Society
- MRONJ
medication‐related osteonecrosis of the jaw
- RI
reference interval
1. INTRODUCTION
Although medication‐related osteonecrosis of the jaw (MRONJ) is a well‐known adverse effect of bisphosphonate treatment in people, this is the first case report of MRONJ in a cat. Evaluation for potential risk factors before implementing bisphosphonate treatment is indicated, and serial oral examinations during treatment are imperative. Further research is needed to determine the frequency of and risk factors for MRONJ in small animal patients treated with bisphosphonates.
2. CASE REPORT
A 12‐year‐old, neutered female, domestic medium hair cat weighing 4.2 kg was referred to a tertiary veterinary hospital for evaluation of oral ulcerations and associated mucosal inflammation. The cat had a long‐term history of constipation managed by acacia fiber supplementation (1 teaspoon in food PO q12h), cisapride (1.25 mg/kg PO q12h), and once weekly SC fluid administration. The cat was diagnosed with idiopathic hypercalcemia at another tertiary veterinary hospital 4 years previously after a comprehensive diagnostic evaluation that included serial measurement of total and ionized calcium concentrations, parathyroid hormone and parathyroid hormone‐related protein concentrations, thoracic radiographs, and abdominal ultrasound examination. Total calcium concentration ranged from 12.3 to >15.3 mg/dL (reference interval [RI], 8.2‐10.8 mg/dL), ionized calcium concentration from 1.56‐1.83 mmol/L (RI, 1.13‐1.38 mmol/L) and 1.90 mmol/L (RI, 1.16‐1.34 mmol/L). Initial hypercalcemia treatment included more frequent SC fluid administration (150 mL 0.9%NaCl q24‐48h) and prednisolone (1.4 mg/kg PO q12‐24h). After no improvement in hypercalcemia with this regimen, the bisphosphonate pamidronate (1 mg/kg into 0.9% NaCl IV over 2 hours) was prescribed. Over the next 23 months, pamidronate was administered every 3‐26 weeks based on serial serum calcium concentrations and relevant clinical signs, including decreased appetite and weight loss. Furosemide administered PO (0.8 mg/kg PO q24h) was implemented approximately 15 months after pamidronate was started for attempted additional calciuresis. During the course of treatment, the pamidronate dose was gradually increased (up to 2.2 mg/kg in 0.9% NaCl IV over 2 hours) based on failure to restore normal total serum calcium concentrations. The time interval between pamidronate injections also decreased, with administration ultimately required every 4 weeks to control total serum hypercalcemia. The cat received cyproheptadine (0.5 mg/kg PO q12h) and ondansetron (0.5 mg/kg PO q12‐24h) as needed for hyporexia. After 18 pamidronate injections over approximately 23 months (121.2 mg, cumulative dose; 28.9 mg/kg), the cat was changed to alendronate because of recurrence of hypercalcemia (2.5 mg/kg PO once weekly, gradually increased to 2.5 mg/kg 3 times weekly and then 5 mg/kg once weekly because of difficulty in administration and occasional vomiting). During the 4 years of hypercalcemia treatment, the cat developed chronic kidney disease, fluctuating between International Renal Interest Society (IRIS) stage 1 and 2 (serum creatinine concentration, 1.3‐2.6 mg/dL; RI, 0.8‐2.4 mg/dL; urine specific gravity, 1.026 with trace proteinuria).
Four months after starting PO alendronate treatment, the cat was evaluated for intermittent ptyalism and suspected gingival ulceration. On oral examination, 2 fistulous draining tracts were observed in the buccal gingiva of the right maxillary canine tooth (104). This tooth as well as a maxillary premolar (208) and mandibular molar (309) were extracted because of radiographic evidence of advanced periodontal disease. Histopathology of the extracted right canine tooth and associated soft tissue was consistent with chronic active hyperplastic gingivitis and necrotizing alveolar osteomyelitis. Cefovecin (Convenia, Zoetis, Parsippany, New Jersey) recently had been administered (7.6 mg/kg SC), and buprenorphine (0.015 mg/kg transmucosally q12h) was prescribed. The extraction and biopsy sites healed without complication.
The cat continued to have episodic ptyalism for the next 14 months. It was reevaluated for a mucosal ulceration over the alveolus of the previously extracted canine tooth (104) that had not responded to cefovecin (8.8 mg/kg SC). A CBC was within RI. A serum biochemistry panel showed mild hypermagnesemia (2.8 mEq/L; RI, 1.5‐2.5 mEq/L), mild hypertriglyceridemia (164 mg/dL; RI, 25‐160 mg/dL, and hyperamylasemia (1627 U/L; RI, 100‐1200 U/L). The cat remained in IRIS stage 2 (serum creatinine concentration, 2.0 mg/dL; RI, 0.6‐2.4 mg/dL). The cat was feline leukemia virus antigen, feline immunodeficiency virus antibody, and heartworm antibody negative. Oral examination under anesthesia disclosed an approximately 3‐mm area of exposed maxillary bone at the alveolus of the previously extracted canine tooth (Figure 1). The gingiva around both mandibular canine teeth (304, 404) and a mandibular premolar (308) was inflamed and lifted away from the underlying bone. Dental radiographs showed decreased trabecular bone density and sclerotic appearance of the rostral mandible (Figure 2); the previous extraction site also had decreased trabecular bone density with irregular alveolar bone margins. A doxycycline polymer filling (Doxirobe, Zoetis) was placed in periodontal pockets of the right mandibular canine tooth (404). The exposed maxillary bone and associated mucosa were excised and submitted for histopathology. No tissue was submitted for bacterial culture. The biopsy site was debrided and closed with a mucosal flap. The cat recovered uneventfully.
Figure 1.
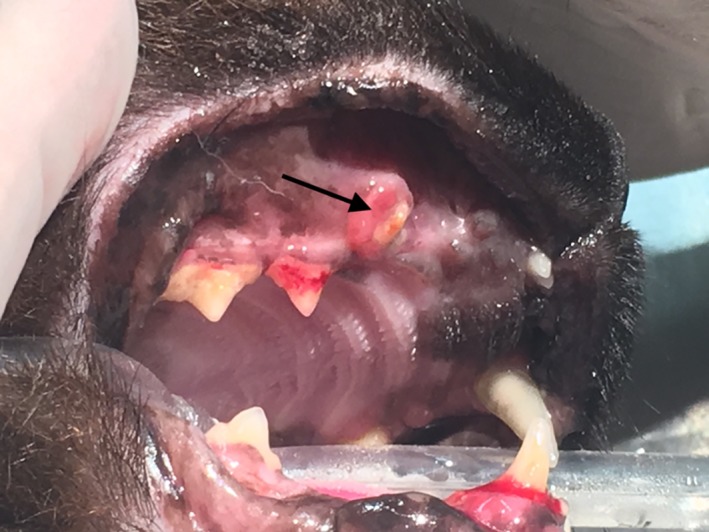
Exposed maxillary bone (black arrow) at the site of previously extracted tooth 104
Figure 2.
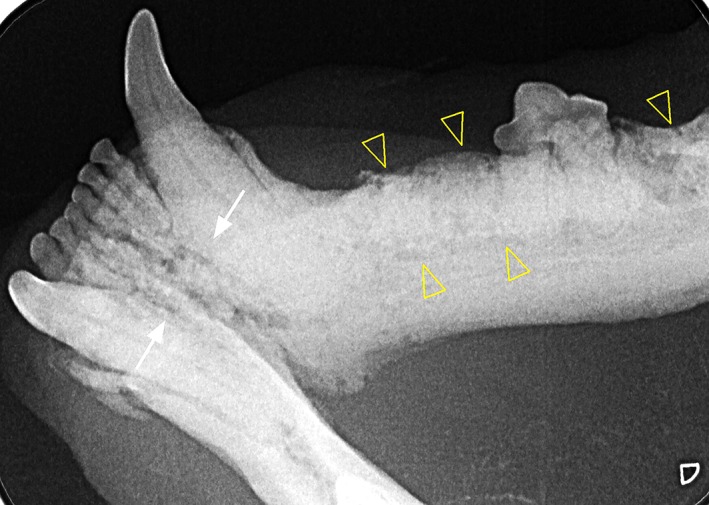
Radiographically decreased trabecular density (open yellow arrow heads) and sclerotic appearance of the rostral mandible (solid white arrows)
Histological evaluation of the maxillary bone and adjacent soft tissue showed hyperplastic and variably ulcerated mucosa with clusters of mixed bacteria. The submucosa had marked edema, mild hemorrhage, and congested granulation tissue with foamy macrophages, neutrophils, and reactive fibroblasts; a section of submucosa also contained degenerative granulation tissue and fibrosis. Adjacent to the fibrotic tissue were variably scalloped bony trabeculae and many macrophages and neutrophils. Alveolar bony lacunae were often empty or contained pale osteocytes, consistent with osteonecrosis (Figure 3). The overall histopathological diagnosis was marked chronic gingivitis and osteomyelitis with ulceration and edema, soft tissue hemorrhage and granulation tissue, and bony lysis and osteonecrosis. Given the cat's history of chronic bisphosphonate treatment, these histological findings were believed to be most consistent with MRONJ and concurrent osteomyelitis. The cat was treated with clindamycin (11 mg/kg PO q12h for 14 days), and PO alendronate was discontinued. On reevaluation, exposure of bone associated with the left mandibular canine tooth (304) secondary to gingival sloughing was observed. Over the next 14 weeks, this alveolar bone exposure progressed (Figure 4) despite various treatments, including saline oral rinse, dental water additives (cat::essential healthymouth; HealthyMouth LLC, Malibu, California; Breathalyzer, imRex Inc, Burlington, Ontario, Canada), a topical cleansing gel (Maxi/Guard Oral Cleansing Gel, Addison Biological Laboratory, Fayette, Missouri) and antibiotic treatment (amoxicillin, 12.5 mg/kg PO q12h for 14 days).
Figure 3.
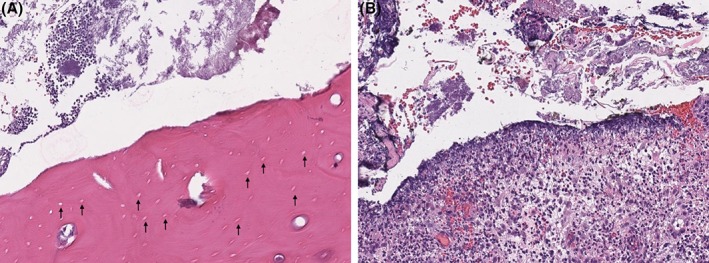
Histopathology of exposed maxillary bone and adjacent soft tissue. A, Mandibular bone with black arrows noting absence of osteocytes within numerous lacunae, consistent with osteonecrosis. B, Marked suppurative to histiocytic ulcerative gingivitis with mixed bacteria
Figure 4.
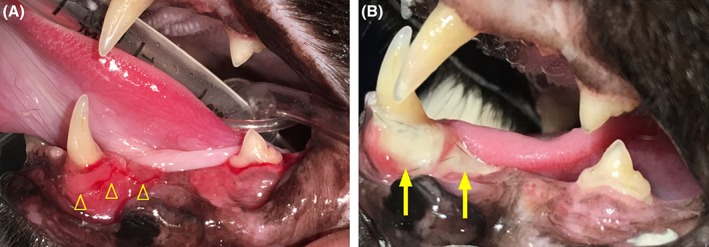
A, Initial gingival inflammation adjacent to left mandibular canine tooth (yellow open arrowheads). B, Exposed mandibular bone adjacent to left mandibular canine tooth (yellow solid arrows) that developed secondary to mucosal sloughing approximately 6 weeks later
Surgical debridement of the necrotic alveolar bone was suggested with caution given the unpredictable mucosal healing and unknown outcome of MRONJ in cats. Dental radiographs obtained under general anesthesia showed persistence of the decreased trabecular bone density of the rostral mandible and subjective worsening of the associated bone sclerosis. Both mandibular canine teeth (304, 404) were surgically extracted, and the nonvital bone near the coronal portion of each tooth was debrided using a round diamond bur. The sites were irrigated with sterile saline and closed with mucogingival flaps. The cat recovered uneventfully and was treated with amoxicillin (12.5 mg/kg PO q12h).
The cat underwent a third surgical procedure 10 weeks after the initial surgery because of persistence of mandibular bone exposure. Under general anesthesia, a bur was used to debride the exposed, necrotic bone adjacent to the extraction sites of both mandibular canine teeth. The affected sites were irrigated with sterile saline and closed with a mucogingival flap. Immediately after this procedure, the cat received 10 hyperbaric oxygen treatments (1.75‐2.0 atmospheres absolute, 60 minutes each) over 6 days as well as 5 cold laser treatments focused on the rostral mandible (class 4 laser, 3 watts over 2 minutes each). Three weeks later, the mucogingival flaps were adequately healed. Subsequent oral evaluations over 13 months have documented no evidence of devitalized oral mucosa or bone exposure.
After the MRONJ diagnosis, alendronate was discontinued and the cat's hypercalcemia was managed with prednisolone (1.1 mg/kg PO q24h, decreased to 0.55 mg/kg PO q24h) and SC fluid treatment for attempted calciuresis, although fluid treatment eventually was discontinued because of progressive difficulty of administration. Acacia fiber supplementation, originally used in management of the cat's constipation, was continued for its possible benefit in lowering serum calcium concentration. Except for a single increased serum ionized calcium concentration (1.42 mmol/L; RI, 1.20‐1.32 mmol/L), the cat remained normocalcemic for approximately 14 months after discontinuation of bisphosphonate treatment. Since recurrence of hypercalcemia, the cat has been treated with SC fluid treatment (100 mL 0.9% NaCl SC q24‐48h), prednisolone (0.55 mg/kg PO q12h), and high fiber prescription diets, which all are currently accepted treatments for idiopathic hypercalcemia in cats.1
3. DISCUSSION
Bisphosphonates are a class of antiresorptive drugs used to treat numerous bone diseases in people, including osteoporosis, multiple myeloma, metastatic bone lesions, and hypercalcemia of malignancy, among others.2 The phosphate and hydroxyl groups of bisphosphonates allow preferential binding to hydroxyapatite crystals within the bone matrix. Accumulation of bisphosphonates within the bone matrix results in osteoclast apoptosis, decreased bone resorption, and subsequent accumulation of necrotic bone.3 Osteonecrosis of the jaw bones is a serious adverse effect of bisphosphonates that was first described in people in 2003.4 The American Association of Oral and Maxillofacial Surgeons (AAOMS) has since preferred the term “medication‐related osteonecrosis of the jaw” (MRONJ) to include other antiresorptive and antiangiogenic drugs that also have resulted in mandibular or maxillary necrosis or both.5 The reported prevalence of MRONJ varies widely, but overall is considered rare; incidence among osteoporosis patients ranges from 0.001% to 7%, and incidence among cancer patients ranges from approximately 0.7% to 15%.6, 7, 8, 9, 10
The pathophysiology of MRONJ has not been definitively determined but is thought to be multifactorial.5 The long‐term inhibition of osteoclastic activity by bisphosphonates likely contributes to the characteristic osteonecrosis of MRONJ.8 Their suppression of bone remodeling is most pronounced in areas of high bone turnover, which could explain why MRONJ occurs primarily within the alveolar bone of the mandible and maxilla.5 Local oral risk factors, namely inflammation and infection, have been strongly implicated in the development of MRONJ. Introduction of bacteria can occur from either physiological microdamage within the oral cavity or from disruption of the oral mucosa from injury or dental procedure.8, 11
A number of risk factors for the development of MRONJ have been established in people. Patients with MRONJ are more likely to have received IV bisphosphonates, with >80% having received either zoledronate, pamidronate, or a combination of them.10, 12, 13, 14 Longer duration of bisphosphonate treatment is also a key risk factor for MRONJ development in people.5, 6, 7, 10, 15 The highest risk of MRONJ occurs after 3 or more years of treatment with zoledronate6 and beyond 4 years of treatment for PO bisphosphonates.5, 7 As expected, underlying primary disease also is a documented MRONJ risk factor because patients with malignancies require higher cumulative bisphosphonate doses.8, 10
Approximately 52%‐72% of patients who develop MRONJ have had ≥1 tooth extractions during bisphosphonate treatment,6, 9, 10, 12, 13, 15 and patients who undergo a tooth extraction when on IV bisphosphonate treatment have a 33‐fold higher risk of MRONJ development.9 Furthermore, oral inflammation also is a well‐recognized risk factor, with periodontal disease having been diagnosed in up to 84% of MRONJ patients.10, 12, 13, 15 Evaluation of concurrent corticosteroid administration, which can delay wound healing by antiangiogenic or immunosuppressive effects, has produced conflicting results.6, 15, 16
Bisphosphonates are used in veterinary practice for treatment of pain associated with skeletal neoplasia, medical management of hyperparathyroidism, and idiopathic hypercalcemia in cats.1, 17, 18, 19, 20, 21, 22, 23, 24, 25 Information regarding adverse effects of chronic bisphosphonate use is currently very limited for dogs and cats.17 Dogs have a bone remodeling rate similar to that of humans, and mandibular necrosis has occurred in some experimental canine models of MRONJ with months to years of bisphosphonate administration.16, 26, 27, 28 Among these dogs, only 1 exhibited bone exposure after 3 months of high‐dose zoledronate, which resolved quickly without intervention.27 In a prospective study evaluating alendronate treatment for odontoclastic resorptive lesions in cats, no cats had gross evidence of oral bone exposure or ulcerations after 11 months of treatment.22 Similarly, no adverse effects were reported in 3 other studies involving chronic alendronate treatment (ranging from 6 to 30 months) of idiopathic hypercalcemia in cats.23, 24, 25 However, a dog with appendicular osteosarcoma developed oral mucosal ulceration and exposed mandibular bone after 46 consecutive monthly IV injections of zoledronate; histopathological evaluation of the mandible showed osteonecrosis, bacterial osteomyelitis, and metastatic osteosarcoma.29 Most recently, a cat was diagnosed with bilateral patellar fractures and radiographically thickened cortices after 8 years of alendronate treatment.30
To our knowledge, ours is the first case report of osteonecrosis secondary to bisphosphonate treatment in a cat. In people, MRONJ is defined by the following: current or previous treatment with antiresorptive or antiangiogenic agents, exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for >8 weeks, and no history of radiation treatment to the jaws or obvious metastatic disease to the jaws.5 The cat of our study fulfilled these criteria and had histological evidence of osteonecrosis on maxillary bone biopsy. This cat also had several risk factors for MRONJ development, including long duration of bisphosphonate treatment (41 months), high cumulative dose of bisphosphonate (121.2 mg pamidronate and 2160 mg alendronate), and concurrent corticosteroid use. Because bisphosphonates are excreted by the kidneys,17 this cat's chronic kidney disease could have resulted in higher plasma concentrations of bisphosphonate. This cat also had multiple dental extractions and osteomyelitis while receiving alendronate approximately 15 months before development of maxillary bone exposure. Although this time to development seems prolonged, the duration between a potential inciting cause and onset of BRONJ in people is highly variable.10 Furthermore, the dog in the previous report similarly had a history of mandibular bacterial osteomyelitis approximately 13 months earlier.29
This cat required 3 surgical treatments and multiple courses of antibiotic treatment over 6 months before complete mucosal healing was achieved. Treatment guidelines for MRONJ in humans remain both controversial and unestablished.14 Described treatments include a conservative, nonsurgical approach with systemic antibiotic administration and oral antimicrobial rinses, minimally invasive surgery with bone curettage, soft tissue debridement and wound closure, and more radical surgical procedures, including segmental mandibular resection and partial maxillectomies.8 In this cat, mucosal healing improved after hyperbaric oxygen treatment and laser treatment. These treatment modalities show promise as supplemental treatments in people, but proof of beneficial effect has not yet been established. Ongoing research into these modalities of treatment is warranted.14, 31, 32, 33 Bisphosphonate treatment was discontinued in this cat, and its corticosteroid dosage was temporarily decreased in the event the higher dosage was contributing to delayed wound healing. Currently, no data confirms that discontinuation of bisphosphonate treatment improves MRONJ outcome,6, 8, 10 but the AAOMS recognizes a theoretical benefit for the discontinuation of bisphosphonates in high‐risk patients before oral surgery.5
Alendronate has been shown to accumulate in high concentrations within the trabecular bone of cats, and its plasma half‐life has been estimated for this species.22 However, the biological half‐life of bisphosphonates in cats currently is unknown. The biological half‐life of bisphosphonates in people is estimated to exceed 10 years, whereas the estimated half‐life in dogs is approximately 3 years.34 If the bisphosphonate half‐life in cats is similar to dogs or people, this patient could have had ongoing bisphosphonate release secondary to natural bone remodeling contributing to its 14 months of normocalcemia after discontinuation of bisphosphonate treatment.
Concerns regarding potential development and prevention of MRONJ in companion animals undergoing bisphosphonate treatment recently were reviewed.35 Our case report, in addition to the recent case report of mandibular osteonecrosis in a dog secondary to zoledronate treatment,29 confirms that MRONJ is a possible complication of long‐term bisphosphonate use in small animal patients. A substantial decrease in MRONJ frequency occurs in people when dental hygiene is optimized before bisphosphonate initiation,6, 36, 37 and full dental cleaning, dental radiographs, and complete healing of any extraction sites are strongly recommended before starting bisphosphonate treatment.5, 8 Similar recommendations should be considered in veterinary patients before starting bisphosphonate treatment to maximize oral health and decrease the risk of MRONJ development.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Cefovecin (Convenia, Zoetis, Parsippany, NJ) was used off‐label in this cat for treatment of possible periodontal infection.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Larson MJ, Oakes AB, Epperson E, Chew DJ. Medication‐related osteonecrosis of the jaw after long‐term bisphosphonate treatment in a cat. J Vet Intern Med. 2019;33:862–867. 10.1111/jvim.15409
REFERENCES
- 1. Galvão DB, Parker V, Schenck PA, Chew DJ. Update on feline ionized hypercalcemia. Vet Clin North Am Small Anim Pract. 2017;47:273‐292. [DOI] [PubMed] [Google Scholar]
- 2. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003;5:65‐74. [DOI] [PubMed] [Google Scholar]
- 4. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115‐1117. [DOI] [PubMed] [Google Scholar]
- 5. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication‐related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938‐1956. [DOI] [PubMed] [Google Scholar]
- 6. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active‐controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341‐1347. [DOI] [PubMed] [Google Scholar]
- 7. Lo JC, O'Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan AA, Morrison A, Kendler DL, et al. Case‐based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the international task force on ONJ. J Clin Densitom. 2017;20:8‐24. [DOI] [PubMed] [Google Scholar]
- 9. Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate‐related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356‐5362. [DOI] [PubMed] [Google Scholar]
- 10. Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landesberg R, Woo V, Cremers S, et al. Potential pathophysiological mechanisms of ONJ. Ann N Y Acad Sci. 2011;1218:62‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate‐induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567‐1575. [DOI] [PubMed] [Google Scholar]
- 13. Woo S, Hellstein JW, Kalmar JR. Annals of internal medicine systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753‐761. [DOI] [PubMed] [Google Scholar]
- 14. Fliefel R, Tröltzsch M, Kühnisch J, et al. Treatment strategies and outcomes of bisphosphonate‐related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44:568‐585. [DOI] [PubMed] [Google Scholar]
- 15. Barasch A, Cunha‐Cruz J, Curro FA, et al. Risk factors for osteonecrosis of the jaws: a case‐control study from the CONDOR dental PBRN. J Dent Res. 2011;90:439‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen MR, Chu TMG, Ruggiero SL. Absence of exposed bone following dental extraction in beagle dogs treated with 9 months of high‐dose zoledronic acid combined with dexamethasone. J Oral Maxillofac Surg. 2013;71:1017‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plumb DC. Plumb's Veterinary Drug Handboook. 8th ed Stockholm, Wis: PharmaVet Inc; 2015:33‐35 556–557, 1103‐1105. [Google Scholar]
- 18. Hostutler RA, Chew DJ, Jaeger JQ, et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. J Vet Intern Med. 2005;19:29‐33. [DOI] [PubMed] [Google Scholar]
- 19. Feldman EC, Nelson RW. Hypercalcemia and primary hyperparthyroidism in dogs In: Bonagura JD, ed. Kirk's Current Veterinary Therapy XIII. Philadelphia, PA: WB Saunders Co; 2000:345‐348. [Google Scholar]
- 20. Fan TM. The role of bisphosphonates in the management of patients that have cancer. Vet Clin North Am Small Anim Pract. 2007;37:1091‐1110. [DOI] [PubMed] [Google Scholar]
- 21. Spugnini EP, Vincenzi B, Caruso G, et al. Zoledronic acid for the treatment of appendicular osteosarcoma in a dog. J Small Anim Pract. 2009;50:44‐46. [DOI] [PubMed] [Google Scholar]
- 22. Mohn KL, Jacks TM, Schleim KD, et al. Alendronate binds to tooth root surfaces and inhibits progression of feline tooth resorption: a pilot proof‐of‐concept study. J Vet Dent. 2009;26:74‐81. [DOI] [PubMed] [Google Scholar]
- 23. Whitney JL, Barrs VR, Wilkinson MR, et al. Use of bisphosphonates to treat severe idiopathic hypercalcaemia in a young Ragdoll cat. J Feline Med Surg. 2011;13:129‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hardy BT, de Brito Galvao JF, Green TA, et al. Treatment of ionized hypercalcemia in 12 cats (2006‐2008) using PO‐administered alendronate. J Vet Intern Med. 2015;29:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pellaz U, Pellaz V, Schumacher M. Long‐term treatment of idiopathic hypercalcaemia in a cat with an oral bisphosophonate (alendronate). Kleintierpraxis. 2014;59:252‐259. [Google Scholar]
- 26. Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg. 2008;66:987‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allen MR, Kubek DJ, Burr DB, et al. Compromised osseous healing of dental extraction sites in zoledronic acid‐treated dogs. Osteoporos Int. 2011;22:693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burr DB, Allen MR. Mandibular necrosis in beagle dogs treated with bisphosphonates. Orthod Craniofac Res. 2009;12:221‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lundberg AP, Roady PJ, Somrak AJ, et al. Zoledronate‐associated osteonecrosis of the jaw in a dog with appendicular osteosarcoma. J Vet Intern Med. 2016;30:1235‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Council N, Dyce J, Drost WT, et al. Bilateral patellar fractures and increased cortical bone thickness associated with long‐term oral alendronate treatment in a cat. JFMS Open Rep. 2017;3:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freiberger JJ, Padilla‐Burgos R, McGraw T, et al. What is the role of hyperbaric oxygen in the management of bisphosphonate‐related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J Oral Maxillofac Surg. 2012;70:1573‐1583. [DOI] [PubMed] [Google Scholar]
- 32. Martins MA, Martins MD, Lascala CA, et al. Association of laser phototherapy with PRP improves healing of bisphosphonate‐related osteonecrosis of the jaws in cancer patients: a preliminary study. Oral Oncol. 2012;48:79‐84. [DOI] [PubMed] [Google Scholar]
- 33. Vescovi P, Manfredi M, Merigo E, et al. Surgical approach with Er: YAG laser on osteonecrosis of the jaws (ONJ) in patients under bisphosphonate therapy (BPT). Lasers Med Sci 2010;25:101–113. [DOI] [PubMed] [Google Scholar]
- 34. Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75‐85. [DOI] [PubMed] [Google Scholar]
- 35. Stepaniuk K. Bisphosphonate related osteonecrosis of the jaws: a review. J Vet Dent. 2011;28:277‐281. [DOI] [PubMed] [Google Scholar]
- 36. Bonacina R, Mariani U, Villa F, et al. Preventive strategies and clinical implications for bisphosphonate‐related osteonecrosis of the jaw: a review of 282 patients. J Can Dent Assoc. 2011;b147:77. [PubMed] [Google Scholar]
- 37. Vandone AM, Donadio M, Mozzati M, et al. Impact of dental care in the prevention of bisphosphonate‐associated osteonecrosis of the jaw: a single‐center clinical experience. Ann Oncol. 2012;23:193‐200. [DOI] [PubMed] [Google Scholar]


