Abstract
Background
There is an association between Chiari malformations, syringomyelia (CMSM) and tethered cord syndrome (TCS) in people, suggesting Cavalier King Charles Spaniels (CKCS) with CMSM could also have TCS. Currently there are no data on the position of the caudal spinal cord structures in CKCS.
Objective
To describe and compare location of spinal cord termination in CKCS with weight‐matched controls and to examine the relationship between SM and spinal cord termination.
Animals
Thirty‐nine CKCS and 33 controls with thoracolumbar MRIs; 34 of 39 CKCS also had cervical MRIs.
Methods
Blinded retrospective study. Spinal cord and dural sac termination were determined from T2‐weighted sagittal and transverse images and half‐Fourier acquisition single‐shot turbo spin echo sequences. Intra‐observer reliability was determined using kappa analysis. Presence of SM was compared with location of spinal cord and dural sac termination.
Results
Intra‐observer reliability was moderate for identifying spinal cord termination (Kappa = 0.6) and good for dural sac termination (Kappa = 0.8). The spinal cord terminated at lumbar vertebra 6 (L6) in 1, 7 (L7) in 22, and sacrum in 16 CKCS versus 9 at L6, 23 at L7, 1 at sacrum in controls. Spinal cord (P < .001) and dural sac (P = .002) termination were significantly more caudal in CKCS compared to controls. The presence of thoracolumbar SM was associated with more caudal dural sac termination in CKCS (P = .03).
Conclusions and Clinical Importance
The relationship between TL SM and possible spinal cord tethering because of a more caudal dural sac termination should be investigated.
Keywords: conus medullaris, dural sac, filum terminale, syringomyelia
Abbreviations
- Cd
caudal vertebra
- CKCS
Cavalier King Charles Spaniel
- CM
Chiari‐like malformation
- CM1
Chiari‐type 1 malformation
- CMSM
Chiari malformations, syringomyelia
- CSF
cerebrospinal fluid
- HASTE
half‐Fourier acquisition single‐shot turbo spin echo
- L2
second lumbar vertebra
- L6
sixth lumbar vertebra
- L7
seventh lumbar vertebra
- LS
lumbosacral
- NC
North Carolina
- S1
first sacral vertebra
- SF
San Francisco
- SM
syringomyelia
- T1
first thoracic vertebra
- TCS
tethered cord syndrome
- TL
thoracolumbar
- VA
Virginia
1. INTRODUCTION
Chiari‐like malformation (CM) is a skull anomaly in Cavalier King Charles Spaniels (CKCS) that results in crowding of the caudal fossa and is ubiquitously present in the breed. An estimated 70% of CKCS also develop syringomyelia (SM).1, 2 Syringomyelia was first described in the cervical spinal cord and postulated to result from altered cerebrospinal fluid (CSF) flow dynamics at the craniocervical junction.3, 4, 5, 6, 7 Symptomatic dogs show signs of neuropathic pain and paresthesias, which often manifest as phantom scratch, focused on the head and neck region.1, 8, 9, 10, 11, 12, 13, 14 However, in many dogs, SM also occurs in the thoracic and lumbar spinal cord and although thoracolumbar (TL) pain is frequently noted we also observed that many CKCS have lumbosacral (LS) pain.1, 7, 8, 15, 16 This is consistent with the findings in Chiari‐type 1 malformation (CM1), the equivalent human condition.17, 18, 19, 20, 21 Headaches, neck pain, and paresthesia of the upper extremities dominate the clinical presentation in these patients, but lower back pain also occurs.18
The normal spinal cord tapers after the lumbar intumescence to form the conus medullaris, ending in the filum terminale, a structure containing pia mater, glial, and ependymal cells. This ultimately fuses with the dura mater and attaches to the caudal vertebrae (Cd), thus anchoring the spinal cord.22 Alterations in this anchoring can increase tension on the caudal spinal cord with clinical consequences, so‐called tethered cord syndrome (TCS) in people.23, 24 There is an association among a subset of CM1 patients, as well as those suffering from SM, with TCS.19, 25, 26, 27, 28, 29, 30 To date, the relationship between Chiari malformations, syringomyelia (CMSM) and TCS has not been explored in dogs; although, a recent study using a 3‐dimensional computer model suggested tethering as a potential mechanism for SM development within the canine spinal cord.31
Tethered cord syndrome occurs more commonly in animals with coexisting anomalies such as spina bifida although also as a unique condition.31, 32, 33, 34, 35, 36, 37, 38 Diagnosis in people relies on a combination of clinical signs, MRI findings, such as caudal displacement of the conus medullaris and thickened filum terminale, and surgical findings.23, 24, 39 In dogs, interpretation of imaging is difficult because there are limited MRI or CT data on the location of the conus medullaris and filum terminale.22, 37, 38 The purpose of this retrospective study was to generate objective data on the location of spinal cord and dural sac termination in small breed dogs in order to determine whether additional study of this region is merited. The primary aim of this study was to determine and compare the location of the spinal cord and dural sac termination in CKCS and other weight‐matched breeds using MRI. The second aim was to investigate whether the presence of SM, cervical or TL, in CKCS was associated with the site of spinal cord and dural sac termination. We hypothesized that the spinal cord and dural sac terminate more caudally in CKCS compared to other breeds and that the presence of TL SM is associated with the location of spinal cord and dural sac termination.
2. MATERIALS AND METHODS
2.1. Study cohort
A search was conducted using the AnimalScan and North Carolina State University's imaging databases for CKCS and size‐matched control breeds. To find size‐matched control dogs, the search was conducted for dogs with appropriate TL MRIs (imaged from thoracic vertebra 1 through sacrum) that weighed between 5 and 14 kg based on the normal weight range for CKCS. Within the cohort of CKCS with TL images, a subset with cervical images was also identified to investigate the relationship between SM and spinal cord termination. Weight‐matched controls were used based on the knowledge that the location of spinal cord termination is influenced by body size in dogs.22 The databases included dogs that were imaged in North Carolina (NC), Virginia (VA), and San Francisco (SF) between the years of 2004 and 2017. The cases were initially filtered by using report keywords to exclude cases with diagnoses that might impact the position of the spinal cord (ie, neoplasia, fractures, and Intervertebral Disc Disease). The selected cases were anonymized by assignment of a study identity (ID) number, and to ensure blinding of the reviewer, the cervical studies were separated from the associated TL images and given individual study IDs. Dogs' age and weight were recorded. Images were examined for sufficient image quality and appropriate image sequences (T2‐weighted sagittal images from the first thoracic vertebra [T1] to the sacrum, T2‐weighted transverse images of the LS junction, and TL sagittal half‐Fourier acquisition single‐shot turbo spin echo [HASTE] sequences; T2‐weighted sagittal images were required for cervical study analysis). Additional cases were excluded based on the criteria listed under image evaluation. The reviewer was blinded from breed identification until after statistical analyses were completed. All dogs were imaged with a 1.5 Tesla unit (NC, VA ‐ before 2015, SF ‐ Symphony, Siemens Medical Solutions USA Inc., Malvern, Pennsylvania; VA ‐ after 2015, Signa Excite, GE Healthcare Headquarters, Chicago, Illinois).
2.2. Image evaluation
All images were evaluated by 1 of the authors (C.R.S). Observations of 15 randomly selected dogs were repeated once at least 60 days after initial observations were made to establish intra‐observer consistency. Images were analyzed by eUnity software (Version 6.3.0.1.4, Client Outlook Inc, Waterloo, Ontario). The following were criteria for exclusion: cases with lesions caudal to the second lumbar vertebra (L2) that completely interrupted the HASTE signal or cases with lesions cranial to L2 that interrupted the HASTE signal spanning more than 1 vertebral level. The TL images were evaluated to determine the location of spinal cord and dural sac termination, presence (T2‐hyperintensity on T2‐sagittal images greater than 2 mm in height) and location (thoracic, lumbar spinal cord) of SM. The cervical images (only in CKCS) were analyzed for the presence of SM.
Termination of the spinal cord was determined using T2‐weighted sagittal and transverse images viewed simultaneously using reference lines. T2‐weighted sagittal images were used to locate the point at which the spinal cord was no longer tapering, representing the termination of the spinal cord, and confirmed using transverse views (Figure 1A). Consecutive transverse images were used to identify the slice at which the spinal cord signal disappeared within the vertebral column (Figure 1B). This was recorded according to the vertebral level at which it occurred (eg, L6, L7, S). Dural sac termination was identified using HASTE and T2‐weighted sagittal sequences in a 2 × 1 series view as the end of the brightest HASTE signal, which represents the CSF within the dural sac. Then, the corresponding vertebral location was identified on T2‐weighted sagittal images by the triangulation tool (Figure 1C). If the location of spinal cord or dural sac termination occurred over a disc space, the more cranial vertebra was recorded (eg, L7/S1 disc space was recorded as L7). The presence and location (cervical, thoracic, or lumbar) of SM (in the subset of CKCS with both cervical and TL MRI studies) was determined using T2‐weighted sagittal views of the TL, and cervical spinal cord and was defined as linear hyperintensity greater than 2 mm in height.
Figure 1.
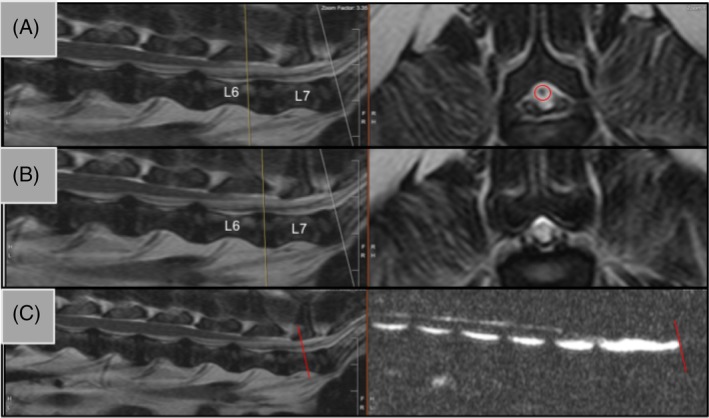
Identification of A‐B, spinal cord termination and C, dural sac termination. Panels A and B show representative T2‐sagittal views (left) and transverse views (right). The yellow line is a reference line showing the corresponding location of the transverse slice. In panel A, the hypointense signal representing the spinal cord is still present and is seen within the red circle at L6. Panel B shows the next transverse slice where the spinal cord signal has disappeared at L6/L7 disc space. Dural sac termination is shown in C with a T2‐weighted sagittal view and a half‐Fourier acquisition single‐shot turbo spin echo (HASTE) sequence side‐by‐side. The red line indicates HASTE termination and the triangulation tool was used to identify the same location on the T2‐weighted sagittal view
2.3. Statistical analysis
All analyses were carried out by JMP software (JMP Pro 12.2.0, SAS Version 9.4, Cary, North Carolina). Dogs were assigned to breed groups by the author who searched the database (I.R.) after all measurements had been recorded. Initially, dogs were assigned to 1 of 2 groups, either CKCS or control breed and the 2 breed groups were compared. In the next analysis, dogs were assigned to 1 of 3 groups (CKCS, brachycephalic control breed, and non‐brachycephalic control breed) and comparisons were made between each breed group. The identity of these groups remained masked until statistical analysis had been completed. Summary statistics for spinal cord termination, dural sac termination, and presence and location of SM were generated for each group. Normality was assessed for age, weight, and maximum SM height by a Shapiro‐Wilk test. Normally distributed data were reported using mean and SD and non‐normal data were described using median and range values. The age and weight of each group of dogs were compared by Wilcoxon rank sum tests. In addition, the relationship between weight and location of spinal cord and dural sac termination was examined in the whole cohort using linear regression to determine whether weight should be a covariate in the subsequent analyses. Similarly, in CKCS alone, the impact of age on the presence of SM was determined using linear regression.
Intra‐observer reliability for the location of spinal cord and dural sac termination was determined by Kappa analysis. Kappa values between 0.81 and 1.0 indicate very good agreement, 0.61‐0.80 indicate good agreement, 0.41‐0.6 indicate moderate agreement, 0.21‐0.40 indicate fair agreement, and ≤0.2 indicate poor agreement.40 The relationship between breed group and location of spinal cord and dural sac termination was then examined by contingency tables and chi‐square tests. Fisher's exact tests were used when there were <5 observations in a category.
Associations among SM presence and spinal cord and dural sac termination were then explored in CKCS only using the subset with both cervical and TL MRI studies. Chi‐square tests and contingency tables were used to analyze the presence of SM (cervical or TL) and spinal cord and dural sac termination sites. Linear regression was used to determine whether age was significantly associated with SM height in CKCS. Holm‐Bonferroni method was used to correct for multiple comparisons. A P value of <.05 was considered statistically significant. The identity of the breed groups was revealed once statistical analysis was complete.
3. RESULTS
One hundred‐six cases were identified and anonymized and, of these, 34 were subsequently excluded based on the predetermined exclusion criteria. A total of 39 CKCS and 33 weight‐matched controls were analyzed. The weight‐matched controls consisted of brachycephalic (n = 20) and non‐brachycephalic breeds (n = 13). Control breeds included Beagles (n = 3), Jack Russell Terriers (n = 4), Poodle (n = 1), Schnauzers (n = 5), Cocker Spaniels (n = 2), Lhaso Apsos (n = 8), Pekingese (n = 1), and Shih Tzus (n = 9). The ages of dogs for the whole cohort ranged from 1.5 to 15 years with a median of 6 years and there were an equal number of females and males (n = 36 each). The weights of dogs ranged from 4.8 to 14 kg with a median of 8 kg. Weights were not significantly different among CKCS and all control dogs grouped together (P adj = .09, Table 1). In addition, CKCS weights were not significantly different from brachycephalic (P adj = .06) and non‐brachycephalic controls (P adj = .96). Within the weight range of dogs selected for this study, weight did not significantly impact spinal cord (P = .34) or dural sac (P = .87) termination and therefore was not considered a covariate. Cohort characteristics are provided in Table 1.
Table 1.
Cohort characteristics for Cavalier King Charles Spaniel (CKCS) and control dogs and CKCS with and without syringomyelia (SM). Weight reported in kilograms (kg)
| CKCS (n = 39) | Control (n = 33) | P adj | |
|---|---|---|---|
| Age (median, range) | 6, 1.5‐11 | 7, 2‐15 | .63 |
| Sex (F, M) | 17, 22 | 19, 17 | 1.0 |
| Weight (median, range) | 9, 5.45‐14 | 7.6, 4.82‐13.6 | .09 |
| CKCS with SM (n = 16) | CKCS without SM (n = 18) | P adj | |
|---|---|---|---|
| Age (median, range) | 7, 2‐11 | 5, 1.5‐10 | .24 |
| Sex (F, M) | 10, 6 | 5, 13 | .19 |
| Weight (median, range) | 8, 6.5‐13 | 9.5, 6‐14 | .11 |
Intra‐observer reliability was moderate for locating spinal cord termination (Kappa = 0.6) and good for dural sac termination (Kappa = 0.8). The spinal cord terminated at sixth lumbar vertebra (L6) in 10 dogs, seventh lumbar vertebra (L7) in 45 dogs, and the sacrum in 17 dogs for the total cohort. The dural sac terminated at L7 in 24 dogs, the sacrum in 41 dogs, and Cd in 7 dogs. When separated according to breed groups, differences became clear (Figure 2). Spinal cord termination was significantly more caudal in CKCS compared to all controls (Figure 3A, P adj = .0001). Notably, the spinal cord terminated at the level of the sacrum in 16 (41%) CKCS but in only 1 (3%) of the control dogs. When separated according to skull shape, the spinal cord terminated more caudally in CKCS compared to both brachycephalic (P adj = .002) and non‐brachycephalic (P adj = .002) dogs. However, the 2 control groups were not significantly different with respect to spinal cord termination (Figure 3B, P adj = .82).
Figure 2.
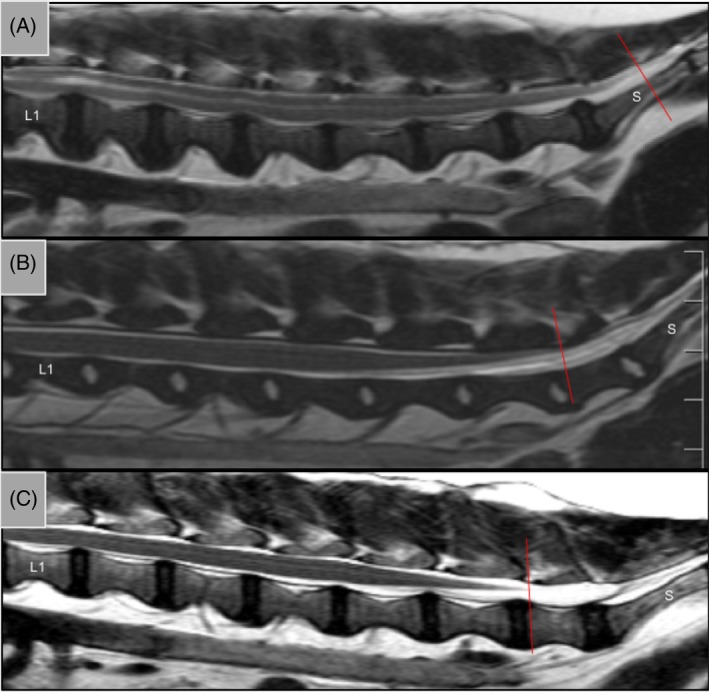
Representative T2 sagittal MRIs from lumbar vertebrae 1 (L1) to the sacrum (S) in A, Cavalier King Charles Spaniel; B, brachycephalic control; and C, non‐brachycephalic control. Red line indicates spinal cord termination
Figure 3.
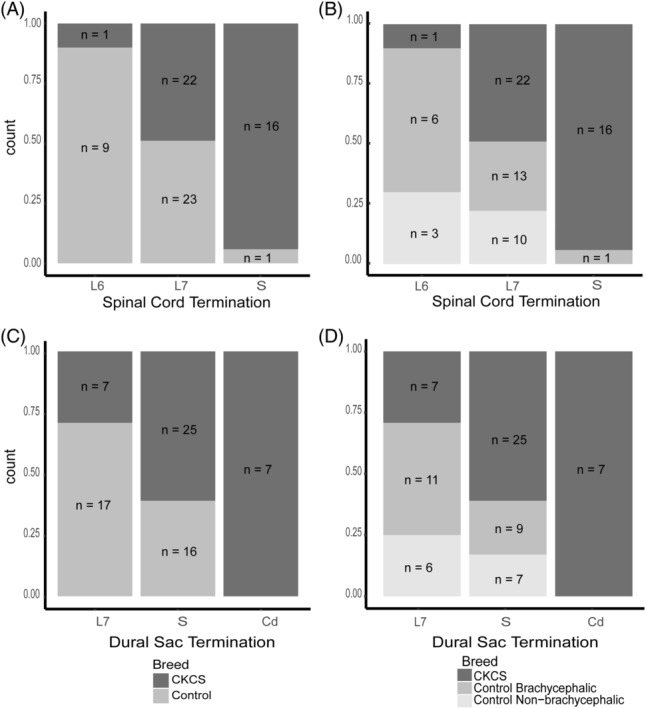
Spinal cord and dural sac termination in Cavalier King Charles Spaniel (CKCS) and size‐matched controls. Each stacked bar shows the relative count for each breed terminating at every vertebral level. A, SC termination in CKCS and size‐matched controls. B, SC termination in CKCS, brachycephalic controls, and non‐brachycephalic controls. C, Dural sac termination in CKCS and size‐matched controls. D, Dural sac termination in CKCS, brachycephalic controls, and non‐brachycephalic controls. L6, lumbar vertebrae 6; L7, lumbar vertebrae 7; S, sacrum
Representative MRIs of dural sac termination using HASTE sequences can be seen in Figure 4. Dural sac termination was significantly more caudal in CKCS compared to all controls (Figure 3C, P adj = .002). Seven (18%) CKCS had a dural sac that terminated at the level of the Cd whereas the dural sac did not extend beyond the sacrum in any of the controls. Additionally, when control breeds were differentiated by skull shape, dural sac termination was significantly more caudal in CKCS compared to brachycephalic control dogs (P adj = .02) and non‐brachycephalic control dogs (P adj = .03), whereas control breeds were not significantly different from one another (P adj = .73).
Figure 4.
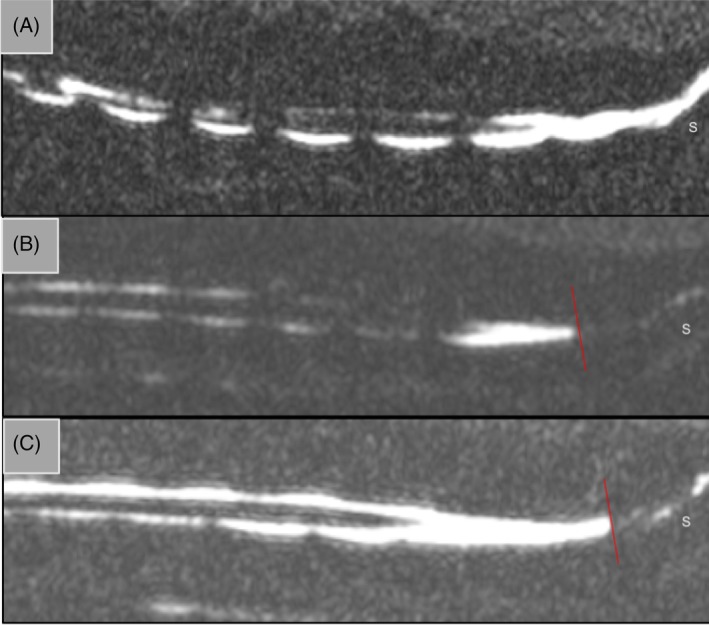
Representative sagittal HASTE images showing termination of the dural sac in A, Cavalier King Charles Spaniel; B, brachycephalic control; and C, non‐brachycephalic control. The red line indicates dural sac termination in B and C, however, the dural sac extends beyond the sacrum in A. S, sacrum
There were 34 CKCS with MRIs of the cervical and TL spine available for evaluation of the relationship between SM and termination of the spinal cord and dural sac. Sixteen CKCS had SM and 18 did not (Table 1). Of the 16 with SM, 3 dogs had SM in the cervical spinal cord alone, 2 dogs had SM in the TL spinal cord alone, and 11 dogs had SM in both regions. Age was not significantly associated with the presence of SM in the cervical (P = .33) or TL spinal cord (P = .38). The location of spinal cord termination was not different based on the presence of either cervical (P adj = .28) or TL SM (P adj = .15) or the combination of both cervical and TL SM (P adj = .21). Dural sac termination was not associated with the presence of cervical SM (P adj = 1.0) or the combination of cervical and TL SM (P adj = .33; Table 2). However, the presence of SM in the TL spinal cord (P adj = .03) was significantly associated with a more caudal location of dural sac termination. Notably, the 2 dogs with TL SM only had dural sac termination sites in the Cd. By comparison, the dogs with cervical SM only had more cranial dural sac termination sites (Table 2).
Table 2.
The location of spinal cord and dural sac termination sites in Cavalier King Charles Spaniel (CKCS) with cervical and thoracolumbar (TL) syringomyelia (SM), cervical SM only, TL SM only, and those without SM. The presence or absence of SM at each vertebral location is displayed as the number, n, of dogs in each category
| Cervical and thoracolumbar SM (n = 11) | Cervical SM only (n = 3) | TL SM only (n = 2) | No SM (n = 18) | |
|---|---|---|---|---|
| Termination sites | Spinal cord termination | |||
| L6 | 0 | 0 | 0 | 2 |
| L7 | 4 | 2 | 1 | 11 |
| S | 7 | 1 | 1 | 5 |
| Termination sites | Dural sac termination | |||
| L7 | 0 | 2 | 0 | 4 |
| S | 8 | 1 | 0 | 13 |
| Cd | 3 | 0 | 2 | 1 |
4. DISCUSSION
In this retrospective morphometric study, we blindly evaluated the location of spinal cord and dural sac termination in CKCS compared with weight‐matched small breed dogs. The results showed that the spinal cord and dural sac ended in a more caudal location in CKCS compared to other breeds. We then demonstrated a relationship between the location of dural sac termination and the presence of TL SM in CKCS leading to speculation that more caudal dural sac termination might be associated with spinal cord tethering and that spinal cord tethering could be associated with TL SM in CKCS.
The termination of the spinal cord is complex, and ultimately achieves anchoring of the spinal cord and meninges to the vertebral column. Spinal cord elongation during development is completed before the vertebral column finishes growing, and as a result, the segments at the terminal end of the spinal cord lie rostral to their respective vertebrae. Because of the differences in body size among breeds, the spinal cord terminates around L6 in larger dog breeds compared with L7 in smaller breeds.41 However, there are limited data on different breeds or on the MRI definition of the termination of the spinal cord.22, 38 In this study, we chose dogs of approximately the same body size (based on weight) before comparing breeds to address this influence on the location of the terminal structures associated with the spinal cord. The conus medullaris is continued by a narrow strand known as the filum terminale that consists of pia mater, glial cells, and ependymal cells. It is impossible to distinguish caudal conus medullaris from filum terminale on MRI, and so for the purposes of this study, we defined a specific MRI appearance to represent the termination of the spinal cord and performed all of our observations with breed identity masked. The intra‐observer kappa statistic shows that these observations were made in a moderately consistent fashion. The dural sac, containing CSF, extends approximately 2 cm beyond the conus medullaris after which it fuses with the filum terminale to form the caudal ligament or the spinal dura mater filament which, in turn, goes on to attach to the sacral or Cd.22, 37, 41 The presence of CSF is highlighted on HASTE images and therefore we chose to identify the termination of the dural sac as another method of evaluating the structures associated with the termination and tethering of the spinal cord. Kappa analysis suggested that these observations were made in a consistent fashion and were in better agreement than the spinal cord termination observations. Overall, we chose to make observations on the termination of both the spinal cord and the dural sac to increase our ability to define this critical but often overlooked region.
Tethered cord syndrome in people could result from a wide variety of different anomalies, including spina bifida and spinal cord dysraphism, and these conditions are readily visible on MRI.23 However, TCS can occur in isolation without other anomalies and is diagnosed based on clinical signs and imaging finding of a low conus medullaris on MRI, presence of a thickened filum terminale (the structure could be fibrous or include fatty tissue), as well as surgical findings.23 We designed our study to capture the location of the conus medullaris and, because of the ease of identifying the end of the dural sac on HASTE images, we also included this variable. Review of images at the time of study design revealed challenges in clearly identifying the filum terminale for measurement of its thickness and so this variable was not assessed. This is comparable to the challenges of image analysis of the region in people, with filum terminale measurements possible in only 12% of children and 9% of adults in one study.27 We compared CKCS with weight‐matched controls because of the importance of body size on location of spinal cord termination in dogs and, in support of our hypothesis, found that the spinal cord and dural sac terminated in a more caudal location in CKCS than the other breeds.41 Dogs can be classified according to their nose length among other variables such as brachycephalic, mesaticephalic or dolichocephalic, and there are numerous differences in skull morphology among dogs in these different groups.22 Cavalier King Charles Spaniels are classified as a brachycephalic breed and this classification is considered important in morphometric analyses of their skull and brain.42, 43 While initial veterinary research on CMSM focused on skull and brain morphology, the importance of the craniocervical junction morphology became clearer over time, leading some authors to rename the disorder craniocervical junctional disorder.44 The possibility that there is also a mismatch between the vertebral column and spinal cord length is now being investigated by us and others.31 In light of this, given there may be differences in vertebral column and spinal cord relationships between breeds with different skull shapes, we subdivided control dogs into brachycephalic and mesaticephalic breeds. When comparing CKCS to these smaller subcategories of control dogs, we found that both spinal cord and dural sac termination were significantly different in CKCS compared to both brachycephalic and non‐brachycephalic controls, whereas the control breeds were not different from one another.
Having established that CKCS do have a more caudal location of their spinal cord terminal structures than other similar sized breeds, the relationship between the presence of SM and these structures was explored in CKCS. Spinal cord termination was not significantly different in CKCS with and without SM in the cervical or TL spinal cord, and our data suggest that a more caudal spinal cord termination is breed‐wide in CKCS. However, we found that dural sac termination was significantly more caudally located in CKCS with TL SM. Although these results demonstrate an association only, the possibility that there is a causal relationship between TL SM and location of the termination of the dural sac deserves investigation. These findings corroborate a recent 3‐dimensional computer modeling study that suggested cord tethering might be involved in SM development in the canine spinal cord.31 There remains a need to investigate whether the caudal location of the spinal cord termination in CKCS simply represents the relative lengths of the spinal cord and vertebral column in this breed or is the result of tethering, and if so, whether tethering contributes to the development of SM.
The implications of these results are subject to certain limitations. The first issue encountered was identifying TL MRI studies in dogs of appropriate breeds without lesions that might alter the position of the terminal spinal cord and the HASTE signal. As a result, the number of dogs of different breeds was lower than the number of CKCS. There were also challenges associated with identifying spinal cord termination. First, using MRI we are unable to differentiate exactly where the conus medullaris ends and the filum terminale starts, and this is reflected by the Kappa analysis revealing moderate intra‐observer agreement. Furthermore, determining the end of the dural sac using HASTE sequences was problematic because although many MRI studies showed an abrupt ending of T2‐hyperintense signal, others had a trailing off of signal that weakened in intensity in the caudal direction. We therefore defined dural sac termination as the location where the brightest HASTE signal ended and this may have moved the location rostrally. Nonetheless, the breed identity was masked and the reviewer made observations in a consistent manner for all cases. Finally, an important imaging finding for the diagnosis of TCS in people is the presence of a thickened filum terminale. We did not attempt to establish a reference range of filum terminale thickness in this study because we could not reliably identify this structure in all dogs. This may be achievable in a prospective study that obtained thin transverse images through the sacral region, or in CT images. In future work, larger prospective studies across different breeds of dog, both with and without CMSM and incorporating dynamic views of the LS region would be of interest.38
Despite these weaknesses, we identified significant differences in the location of the termination of the spinal cord and CSF signal in CKCS compared to control dogs. These data raise the question of whether CKCS with CMSM mirror the full spectrum of abnormalities in people in whom TCS may occur alongside CM1 and SM. The conus medullaris was significantly more caudal in children with CM1 than in healthy patients, and 14% of patients with CM1 had clinical signs of TCS.27 Although contentious, studies have implicated a traction‐based mechanism to explain the coexistence of CM1 and TCS whereby the inelastic filum terminale displaces the caudal brainstem and cervical spinal cord causing descent of the cerebellar tonsils.17, 23, 27, 28, 45, 46, 47 Furthermore, transection of the tethered filum terminale has been shown to alleviate clinical signs and in some cases ascent of the cerebellar tonsils occurred.27, 28, 45, 48 By contrast, others have failed to demonstrate tonsillar ascent after transection of the filum terminale and suggest that the coexistence of these conditions is the result of a genetic basis for CM1 and TCS alone, with no causal relationship.25, 26, 49
To conclude, we found that CKCS have a caudally displaced spinal cord and dural sac when compared with a range of weight‐matched breeds. These findings suggest that CKCS could be predisposed to TCS and that spinal cord tethering could be involved in the development of TL SM. Future work is needed to determine whether there is spinal cord tethering in CKCS, potentially through dynamic studies, by consideration of the clinical signs and exploratory surgery.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank AnimalScan for allowing the use of their database to find cases and the American Cavalier King Charles Spaniel Club Charitable Trust for their continued support toward imaging Cavalier King Charles Spaniels. The authors thank Drs Gabriela Seiler and Erin Keenihan for their time and recommendations for MRI analysis. This work was presented at the 2018 ACVIM Forum, Seattle, Washington.
Sparks CR, Robertson I, Olby NJ. Morphometric analysis of spinal cord termination in Cavalier King Charles Spaniels. J Vet Intern Med. 2019;33:717–725. 10.1111/jvim.15437
Funding information American Cavalier King Charles Spaniel Club Charitable Trust; American Kennel Club Canine Health Foundation, Grant/Award Number: MOU CHF 02‐162
REFERENCES
- 1. Hechler AC, Moore SA. Understanding and treating Chiari‐like malformation and syringomyelia in dogs. Top Companion Anim Med. 2018;33:1‐11. [DOI] [PubMed] [Google Scholar]
- 2. Parker JE, Knowler SP, Rusbridge C, Noorman E, Jeffery ND. Prevalence of asymptomatic syringomyelia in Cavalier King Charles Spaniels. Vet Rec. 2011;168:667‐667. [DOI] [PubMed] [Google Scholar]
- 3. Cerda‐Gonzalez S, Olby NJ, Broadstone R, McCullough S, Osborne JA. Characteristics of cerebrospinal fluid flow in Cavalier King Charles Spaniels analyzed using phase velocity cine magnetic resonance imaging. Vet Radiol Ultrasound. 2009;50:467‐476. [DOI] [PubMed] [Google Scholar]
- 4. Driver CJ, Volk HA, Rusbridge C, Van Ham LM. An Update on the pathogenesis of syringomyelia secondary to Chiari‐like malformations in dogs. Vet J. 2013;198:551‐559. [DOI] [PubMed] [Google Scholar]
- 5. Fenn J, Schmidt MJ, Simpson H, Driver CJ, Volk HA. Venous sinus volume in the caudal cranial fossa in Cavalier King Charles Spaniels with syringomyelia. Vet J. 2013;197:896‐897. [DOI] [PubMed] [Google Scholar]
- 6. Driver CJ, Rusbridge C, Cross HR, McGonnell I, Volk HA. Relationship of brain parenchyma within the caudal cranial fossa and ventricle size to syringomyelia in Cavalier King Charles Spaniels. J Small Anim Pract. 2010;51:382‐386. [DOI] [PubMed] [Google Scholar]
- 7. Rusbridge C, Greitz D, Iskandar BJ. Syringomyelia: current concepts in pathogenesis, diagnosis, and treatment. J Vet Intern Med. 2006;20:469‐479. [DOI] [PubMed] [Google Scholar]
- 8. Sparks CR, Cerda‐Gonzalez S, Griffith EH, Lascelles BDX, Olby NJ. Questionnaire‐based analysis of owner‐reported scratching and pain signs in Cavalier King Charles Spaniels screened for Chiari‐like malformation and syringomyelia. J Vet Intern Med. 2018;32:331‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rutherford L, Wessmann A, Rusbridge C, et al. Questionnaire‐based behaviour analysis of Cavalier King Charles Spaniels with neuropathic pain due to Chiari‐like malformation and syringomyelia. Vet J. 2012;194:294‐298. [DOI] [PubMed] [Google Scholar]
- 10. Cerda‐Gonzalez S, Olby NJ, Griffith EH. Longitudinal study of the relationship among craniocervical morphology, clinical progression, and syringomyelia in a cohort of Cavalier King Charles Spaniels. J Vet Intern Med. 2016;30:1090‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ives EJ, Doyle L, Holmes M, Williams TL, Vanhaesebrouck AE. Association between the findings on magnetic resonance imaging screening for syringomyelia in asymptomatic Cavalier King Charles Spaniels and observation of clinical signs consistent with syringomyelia in later life. Vet J. 2015;203:129‐130. [DOI] [PubMed] [Google Scholar]
- 12. Plessas IN, Rusbridge C, Driver CJ, et al. Long‐term outcome of Cavalier King Charles Spaniel dogs with clinical signs associated with Chiari‐like malformation and syringomyelia. Vet Rec. 2012;171:501. [DOI] [PubMed] [Google Scholar]
- 13. Nalborczyk ZR, McFadyen AK, Jovanovik J, et al. MRI characteristics for "phantom" scratching in canine syringomyelia. BMC Vet Res. 2017;13:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thøfner MS, Stougaard CL, Westrup U, et al. Prevalence and heritability of symptomatic syringomyelia in Cavalier King Charles Spaniels and long‐term outcome in symptomatic and asymptomatic littermates. J Vet Intern Med. 2015;29:243‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loderstedt S, Benigni L, Chandler K, et al. Distribution of syringomyelia along the entire spinal cord in clinically affected Cavalier King Charles Spaniels. Vet J. 2011;190:359‐363. [DOI] [PubMed] [Google Scholar]
- 16. Rusbridge C, Carruthers H, Dubé M‐, Holmes M, Jeffery ND. Syringomyelia in Cavalier King Charles Spaniels: the relationship between syrinx dimensions and pain. J Small Anim Pract 2007;48:432–436. [DOI] [PubMed] [Google Scholar]
- 17. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005‐1017. [DOI] [PubMed] [Google Scholar]
- 18. Fischbein R, Saling J, Marty P, et al. Patient‐reported Chiari malformation type I symptoms and diagnostic experiences: a report from the national Conquer Chiari Patient Registry database. Neurol Sci. 2015;36:1617‐1624. [DOI] [PubMed] [Google Scholar]
- 19. Massimi L, Novegno F, di Rocco C. Chiari type I malformation in children. Adv Tech Stand Neurosurg. 2011;37:143‐211. [DOI] [PubMed] [Google Scholar]
- 20. Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA. Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and Chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine. 2007;7:601‐609. [DOI] [PubMed] [Google Scholar]
- 21. Speer MC, Enterline DS, Mehltretter L, et al. Review article: Chiari type I malformation with or without syringomyelia: prevalence and genetics. J Genet Couns. 2003;12:297‐311. [DOI] [PubMed] [Google Scholar]
- 22. Evans HE. Miller's Anatomy of the Dog. Elsevier Saunders: St. Louis, MO; 2013. [Google Scholar]
- 23. Lew SM, Kothbauer KF. Tethered cord syndrome: an updated review. Pediatr Neurosurg. 2007;43:236‐248. [DOI] [PubMed] [Google Scholar]
- 24. Kesler H, Dias MS, Kalapos P. Termination of the normal conus medullaris in children: a whole‐spine magnetic resonance imaging study. Neurosurg Focus. 2007;23:1‐5. [DOI] [PubMed] [Google Scholar]
- 25. Massimi L, Peraio S, Peppucci E, Tamburrini G, Di Rocco C. Section of the filum terminale: is it worthwhile in Chiari type I malformation? Neurol Sci. 2011;32(Suppl 3):S349‐S351. [DOI] [PubMed] [Google Scholar]
- 26. Valentini LG, Selvaggio G, Visintini S, Erbetta A, Scaioli V, Solero CL. Tethered cord: natural history, surgical outcome and risk for Chiari malformation 1 (CM1): a review of 110 detethering. Neurol Sci. 2011;32:353‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Milhorat TH, Bolognese PA, Nishikawa M, et al. Association of Chiari malformation type I and tethered cord syndrome: preliminary results of sectioning filum terminale. Surg Neurol. 2009;72:20‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Royo‐Salvador MB, Sole‐Llenas J, Domenech JM, Gonzalez‐Adrio R. Results of the section of the filum terminale in 20 patients with syringomyelia, scoliosis and Chiari malformation. Acta Neurochir. 2005;147:515‐523. discussion 523. [DOI] [PubMed] [Google Scholar]
- 29. Vidmer S, Sergio C, Veronica S, et al. The neurophysiological balance in Chiari type 1 malformation (CM1), tethered cord and related syndromes. Neurol Sci. 2011;32(Suppl 3):S311‐S316. [DOI] [PubMed] [Google Scholar]
- 30. Glenn C, Cheema AA, Safavi‐Abbasi S, et al. Spinal cord detethering in children with tethered cord syndrome and Chiari type 1 malformations. J Clin Neurosci. 2015;22:1749‐1752. [DOI] [PubMed] [Google Scholar]
- 31. Cirovic S, Lloyd R, Jovanovik J, Volk HA, Rusbridge C. Computer simulation of syringomyelia in dogs. BMC Vet Res. 2018;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ricci E, Cherubini GB, Jakovljevic S, Aprea F, Cantile C. MRI findings, surgical treatment and follow‐up of a myelomeningocele with tethered spinal cord syndrome in a cat. J Feline Med Surg. 2011;13:467‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zani DD, De Zani D, Morandi N, et al. Imaging diagnosis‐‐split cord malformation. Vet Radiol Ultrasound. 2010;51:57‐60. [DOI] [PubMed] [Google Scholar]
- 34. Shamir M, Johnston D, Rochkind S. Surgical treatment of tethered spinal cord syndrome in a dog with myelomeningocele. Vet Rec. 2001;148:755‐756. [DOI] [PubMed] [Google Scholar]
- 35. Plummer SB, Bunch SE, Khoo LH, Spaulding KA, Kornegay JN. Tethered spinal cord and an intradural lipoma associated with a meningocele in a Manx‐type cat. J Am Vet Med Assoc. 1993;203:1159‐1161. [PubMed] [Google Scholar]
- 36. Fingeroth JM, Johnson GC, Burt JK, Fenner WR, Cain LS. Neuroradiographic diagnosis and surgical repair of tethered cord syndrome in an English bulldog with spina bifida and myeloschisis. J Am Vet Med Assoc. 1989;194:1300‐1302. [PubMed] [Google Scholar]
- 37. De DS, Gregori T, Kenny PJ, et al. Tethered cord syndrome associated with a thickened filum terminale in a dog. J Vet Intern Med. 2015;29:405‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Decker S, Watts V, Neilson DM. Dynamic lumbosacral magnetic resonance imaging in a dog with tethered cord syndrome with a tight filum terminale. Front Vet Sci. 2017;4:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamada S, Won DJ, Siddiqi J, Yamada SM. Tethered cord syndrome: overview of diagnosis and treatment. Neurol Res. 2004;26:719‐721. [DOI] [PubMed] [Google Scholar]
- 40. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 41. Pasquini C, Pasquini S. Applied Clinical Dog Dissection Guide: A Regional Approach. Pilot Point, TX: Sudz Publishing; 2015. [Google Scholar]
- 42. Schmidt MJ, Kramer M, Ondreka N. Comparison of the relative occipital bone volume between Cavalier King Charles Spaniels with and without syringohydromyelia and French bulldogs. Vet Radiol Ultrasound. 2012;53:540‐544. [DOI] [PubMed] [Google Scholar]
- 43. Schmidt MJ, Neumann AC, Amort KH, Failing K, Kramer M. Cephalometric measurements and determination of general skull type of Cavalier King Charles Spaniels. Vet Radiol Ultrasound. 2011;52:436‐440. [DOI] [PubMed] [Google Scholar]
- 44. Marino D, Luoghin C, Dewey C, et al. Morphometric features of the craniocervical junction region in dogs with suspected Chiari‐like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am J Vet Res. 2012;73:105‐111. [DOI] [PubMed] [Google Scholar]
- 45. Royo‐Salvador MB. Syringomyelia, scoliosis and idiopathic Arnold‐Chiari malformations: a common etiology. Rev Neurol. 1996;24:937‐959. [PubMed] [Google Scholar]
- 46. GARCEAU GJ. The filum terminale syndrome (the cord‐traction syndrome). J Bone Joint Surg Am. 1953;35‐A:711‐716. [PubMed] [Google Scholar]
- 47. Wu L, Qiu Y, Wang B, Zhu ZZ, Ma WW. The left thoracic curve pattern: a strong predictor for neural axis abnormalities in patients with "idiopathic" Scoliosis. Spine. 2010;35:182‐185. [DOI] [PubMed] [Google Scholar]
- 48. Gluncic V, Turner M, Burrowes D, Frim D. Concurrent Chiari decompression and spinal cord untethering in children: feasibility in a small case series. Acta Neurochir. 2011;153:109‐114. discussion 114. [DOI] [PubMed] [Google Scholar]
- 49. Tubbs RS, Loukas M, Shoja MM, Oakes WJ. Observations at the craniocervical junction with simultaneous caudal traction of the spinal cord. Childs Nerv Syst. 2007;23:367‐369. [DOI] [PubMed] [Google Scholar]


