Abstract
Background
Trigeminal‐mediated headshaking results from low‐threshold firing of the trigeminal nerve resulting in apparent facial pain. Magnesium may have neuroprotective effects on nerve firing that potentially dampen signs of neuropathic pain. This hypothesis has not been investigated in horses with trigeminal‐mediated headshaking.
Objective
To investigate head‐shaking behavior in affected horses after IV magnesium sulfate infusion.
Animals
Six geldings with trigeminal‐mediated headshaking.
Methods
Prospective randomized crossover study. Horses were controlled for diet and infused IV with 5% dextrose solution (DS; control solution at 2 mL/kg body weight [BW]) and MgSO4 50% solution (MSS at 40 mg/kg BW). Head‐shaking behavior was recorded at times T0 (baseline, before infusion) and T15, T30, T60, and T120 minutes post‐infusion. Venous blood variables such as pH, HCO3 −, standard base excess (SBE), Na+, Cl−, K+, Ca2+, Mg2+, total magnesium (tMg), glucose, and lactate were measured; strong ion difference (SID) and anion gap (AG) were calculated for each time point.
Results
Blood variables including pH, Na+, Cl−, K+, SID, AG, lactate, Ca2+, tMg, and Mg2+ had significant changes with MSS as compared to DS treatment. Glucose, SBE, and HCO3 − did not have significant changes. A 29% reduction in head‐shaking rate occurred after MSS treatment but no change occurred after DS treatment.
Conclusions and Clinical Importance
Administration of MSS IV increased plasma total and ionized magnesium concentrations and significantly decreased head‐shaking behavior in horses with trigeminal‐mediated headshaking.
Keywords: equine, trigeminal, head shakers, magnesium
Abbreviations
- AG
anion gap
- BW
body weight
- DS
dextrose solution group
- MSS
MgSO4 solution
- tMg
total magnesium
- SBE
standard base excess
- SID
strong ion difference
1. INTRODUCTION
Trigeminal‐mediated headshaking in horses is a disorder associated with a decreased threshold of firing of the trigeminal nerve leading to apparent episodic intractable facial pain, compromised performance and quality of life, and ultimately leading to euthanasia of severely affected horses thus representing a major welfare concern.1, 2 This disorder is manifested as violent, mostly vertical, head shakes, and other common signs including apparent pruritus, tingling, and presumed burning or electric‐like sensation in the horse's face, similar to that described for neuropathic pain in people.1, 2, 3, 4, 5, 6, 7, 8 Horses may snort, rub their nose on their limbs, or attempt to strike at their face with their thoracic limbs in attempts to get relief.3 Studies on somatosensory nerve conduction of the trigeminal nerve indicated at least a 10‐fold lower threshold to trigger nerve conduction of the maxillary branch of the trigeminal nerve in affected horses compared to control horses.1, 2 Results from such studies described a functional rather than anatomical abnormality, hence the name trigeminal‐mediated headshaking.1, 9, 10 This disorder has been reported in young, mature horses of several breeds, with a predominance of cases in geldings (72%).3, 5, 6 Head‐shaking behavior appears to have a seasonal predominance in 60% of horses, with peaks during the spring and summer months.3 Various treatments have been attempted, including nose nets, ultraviolet protective masks, nutritional supplements, drug treatment, nerve blocks, neurectomy or nerve compression, and electrical neuromodulation of the infraorbital nerve, but results have been inconsistent.11, 12, 13, 14, 15, 16, 17
Anecdotally, magnesium supplementation leads to a decrease in head‐shaking signs in 40% of affected horses.9, 18 Magnesium is the second most abundant intracellular cation, and it participates in >300 biochemical reactions including ATPase‐dependent ion pumps.19, 20 Intracellular and extracellular fluid magnesium concentrations play a role in the transmission of nerve impulses and might result in neuroprotective effects.21, 22, 23, 24 Intravenous infusion of MgSO4 has many uses in horses, including treatment for hypomagnesemia, and decreasing intraoperative and postoperative pain. It also has been used in humans as a treatment for neuropathic pain with variable results.4, 19, 23, 25, 26, 27 Horses affected by trigeminal‐mediated headshaking do not show signs of hypomagnesemia, although 50%‐60% of affected horses may have ionized magnesium (Mg2+) concentrations below the reference range.28 The objective of our study was to investigate the effect of an IV infusion of magnesium on head‐shaking behavior in horses with trigeminal‐mediated headshaking. Infusion of MgSO4 would be expected to temporarily increase blood magnesium concentrations.25, 27 We hypothesized that the infusion of MgSO4 would increase total (tMg) and ionized (Mg2+) magnesium concentrations in the blood and decrease head‐shaking behavior.
2. MATERIALS AND METHODS
2.1. Animals
Six geldings with naturally occurring head shaking donated to the Center for Equine Health were included in the study. Inclusion criteria consisted of fulfillment of the diagnosis of trigeminal‐mediated headshaking by exclusion of all other known causes of head shaking as described elsewhere.9 Horses included were Quarter Horses (n = 4) and Thoroughbreds (n = 2), aged 5‐13 years, and weighing between 472 and 580 kg. At the time of donation, horses had received all recommended vaccinations and deworming as per the Center for Equine Health protocols. These horses were known to be seasonal head shakers and had been donated because they were refractory to various treatments including antihistamines for possible allergies. These horses displayed head‐shaking behavior at the time of the study. The horses underwent thorough physical and neurologic examinations performed by a board‐certified large animal internist and neurologist, followed by a thorough diagnostic investigation. Diagnostic evaluation included oral, ophthalmic, and otoscopic examinations, CBC, serum biochemical profile, skull radiographs, and upper airway endoscopy including evaluation of the guttural pouches. Furthermore, none of the horses had apparent cervical or nuchal pain based on palpation, extension, and flexion of the neck that could have contributed to head‐shaking behavior. Computed tomography of the head to eliminate other causes was not performed. Based on historical seasonal head shaking, observed head‐shaking behavior by the authors during spring and summer, and lack of abnormal findings on various diagnostic modalities, the authors believed that all of these features supported a clinical diagnosis of trigeminal‐mediated headshaking. Furthermore, it is unlikely that these horses would have had abnormalities manifested in an intermittent fashion during spring and summer months. Horses were housed in 5 × 12 ft covered stalls that contained automatic watering devices (water provided free choice) and raised metal bins for hay. Horses were fed twice daily at 7:00 am and 5:00 pm. Stalls were cleaned and bedded with shavings daily.
2.2. Experimental design
The study had a randomized, controlled crossover experimental design, in which each horse served as its own control. The horses were randomized to 1 of the 2 treatment groups, with a washout period of >1 week between treatments. The entire study took place over 5 weeks in the early summer to avoid seasonal and environmental variability. All horses received both treatments. An IV catheter was placed in the left jugular vein using aseptic technique. Horses received sterile fluids IV: the Dextrose Solution (DS) group received 5% dextrose solution at 2 mL/kg body weight (BW), and the Magnesium Sulfate Solution (MSS) group received 50% MgSO4 solution at 40 mg/kg BW diluted in 5% dextrose solution. The infusion time was 5‐10 minutes, and after infusion the catheter was removed.
2.3. Sample collection
Heparinized blood samples were collected by venipuncture from the right jugular vein at T0 (baseline, before infusion) and T5, T15, T30, T60, and T120 minutes post‐infusion and placed on ice immediately.
2.4. Blood analysis
Venous blood pH, standard base excess (SBE), HCO3 −, Na+, Cl−, K+, Ca2+, lactate and glucose concentrations were determined using an ABL815 FLEX (Radiometer America Inc, Brea, California). Total magnesium (tMg) and ionized magnesium (Mg2+) concentrations were determined using a NOVA 8 (NOVA Biomedical, Waltham, Massachusetts). Strong ion difference (SID) was calculated using the Stewart equation as follows: [(Na+ + K+ + Ca2+ + Mg2+) − (Cl−)].26 Anion gap (AG) was calculated using the equation as follows: [(Na+ + K+) − (Cl− + HCO3 −)].26
2.5. Behavioral analysis
Horses were placed in individual round pens and evaluated by 3 independent, trained evaluators for head‐shaking behavior while at a walk (1 minute), trot (1‐3 minutes), canter (1 minute), and walk again (1 minute) and at 5 time points, T0 (before infusion), T15, T30, T60, and T120 minutes post‐infusion. This protocol was selected because light exercise in this group of horses resulted in exacerbation of signs as seen in a previous pilot study. After each time point, horses were returned immediately to their stalls. Evaluators were unaware of which treatment each horse received on any particular day. The head‐shaking behavior (including head shakes [vertical head shakes]/minute, head‐tossing [nonvertical head movements]/minute, nose rubbing/minute, dropped head/minute, and snorting/minute) was recorded for each horse during each level of exercise at each time point by the 3 evaluators. A median of each head‐shaking behavior per minute for each horse at each time point during each level of exercise was determined. Variability among evaluators was evaluated. The study was approved by our institutional animal care and use committee.
2.6. Statistical analysis
Data were analyzed using Stata Statistical Software, Release 14 (StataCorp LP 2015, College Station, Texas). For all blood variables, the results were presented as mean and SD in tabular form and indicated if within or outside the reference range. A multilevel mixed‐effects analysis of variance model also was used to evaluate the main and interactive effects of treatment groups (DS or MSS) and time (T0, T5, T15, T30, T60, and T120 minutes post‐infusion), with individual horses as random effect, on blood variables (pH, K+, Na+, Cl−, SBE, HCO3 −, Ca2+, glucose, lactate, tMg, Mg2+) and calculated values of SID and AG. A P‐value <.05 was considered significant. The statistical model utilized in this study included standard error of the mean for the calculation and representation of the data for all figures presented here.
For head‐shaking behavior, a multilevel mixed‐effects Poisson regression model was used to examine the main and interactive fixed effects of treatment groups (DS or MSS), time (T0, T15, T30, T60, and T120 minutes post‐infusion), gait and breed, with individual horses as the random effect, on head shaking. Each gait (first walk, trot, canter, second walk) was evaluated separately. Within each gait, a model was created to evaluate the possible interaction between treatment and time. If the interaction was significant, 2 additional sets of analyses (effect of treatment at individual times and effect of time within individual treatments) were performed. If the interaction was not significant, a main effects‐only model was fit. Results are presented as incidence rate ratios (IRR), P‐values, and 95% confidence intervals (CI). A P‐value <.05 was considered significant. A plot with the regression line was used to display longitudinal data for individual horses.
3. RESULTS
3.1. Blood results
After the infusion of MSS, Mg2+ peaked at 5 minutes post‐administration and steadily and rapidly decreased over the next time points, returning nearly to baseline by 120 minutes post‐infusion (Figure 1). Ionized magnesium significantly increased after MSS treatment when compared to DS (+0.20 mmol/L, P < .001, Figure 1). Ionized and tMg measurements indicated that significant changes occurred in MSS treatment (P < .001, Figures 1 and 2). Blood variables including pH, Na+, Cl−, K+, lactate, SID, AG, and Ca2+ were significantly different after MSS compared to DS treatment (P < .01, Figure 3). Glucose, SBE, and HCO3 − did not have significant changes.
Figure 1.
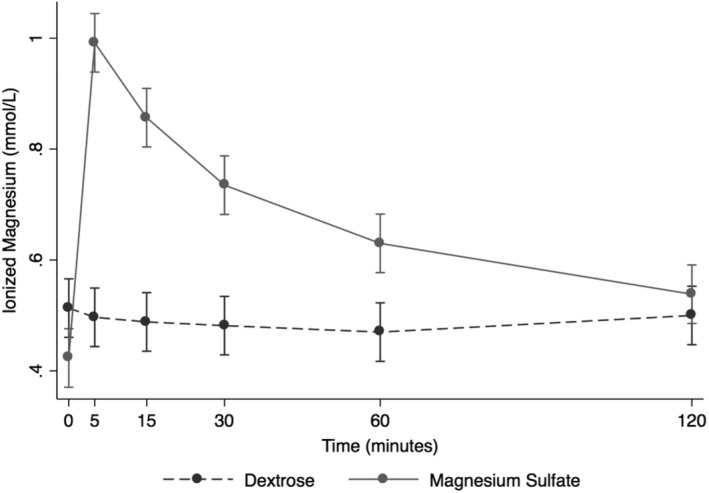
Overall effect of treatment on the blood ionized magnesium (Mg2+) over time. The mean and SEM venous blood Mg2+ for all 6 horses. Black = DS 5% solution (2 mL/kg BW); gray = MgSO4 50% solution (40 mg/kg BW). BW, body weight; DS, dextrose solution group; MSS, MgSO4 solution group
Figure 2.
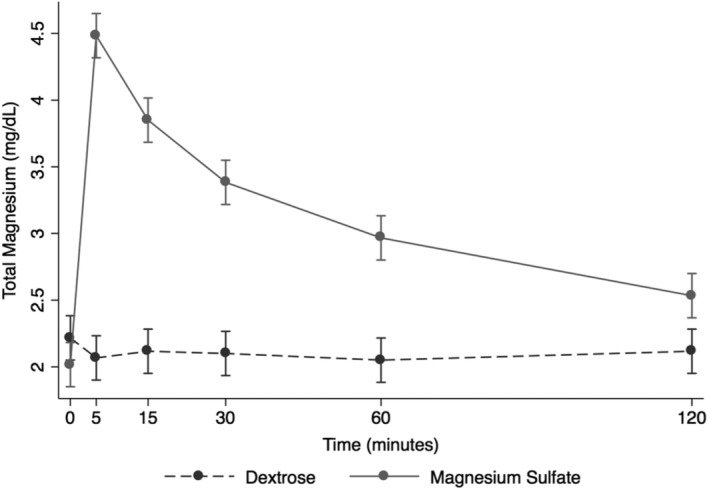
Overall effect of treatment on the blood total magnesium (tMg) over time. The mean and SEM venous blood tMg for all 6 horses. Black = DS 5% solution (2 mL/kg BW); gray = MgSO4 50% solution (40 mg/kg BW). BW, body weight; DS, dextrose solution group; MSS, MgSO4 solution group
Figure 3.
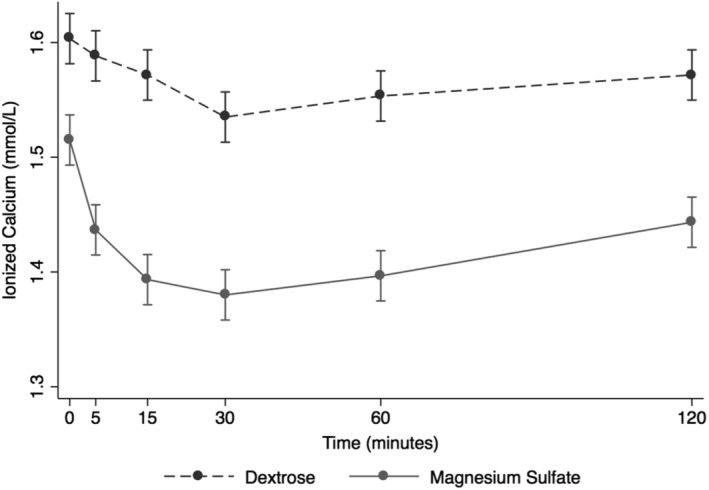
Overall effect of treatment on the blood ionized calcium (Ca2+) over time. The mean and SEM venous blood Ca2+ for all 6 horses. Black = DS 5% solution (2 mL/kg BW); gray = MgSO4 50% solution (40 mg/kg BW). BW, body weight; DS, dextrose solution group; MSS, MgSO4 solution group
At baseline, all blood variables measured were within reference range with a few exceptions (Tables 1 and 2). Standard base excess uniformly was above reference range (mean, 6.1 ± 1.2 mmol/L for DS; 6.5 ± 1.5 mmol/L for MSS; Table 1), and Cl− was above reference range at baseline (mean, 103 ± 1.7 mmol/L for DS; 103 ± 1.9 mmol/L for MSS; Table 1). Anion gap was below reference range at baseline (mean, 7.9 ± 1.8 mmol/L for DS; 4.4 ± 1.8 mmol/L for MSS; Table 2). Sodium was below reference range in MSS only (mean, 134 ± 3.4 mmol/L; Table 1). For MSS, SID was above reference range at baseline (mean, 37.7 ± 2.3 mmol/L; Table 2). For MSS, the baseline measurement for Mg2+ was below reference range (mean, 0.42 ± 0.05 mmol/L; Table 1). Overall, no changes in blood pH, HCO3 −, and SBE after DS were observed (Table 1).
Table 1.
Changes in blood variables after IV infusion of DS and MSS. DS, 5% dextrose solution (2 mL/kg BW) and MSS, MgSO4 50% solution (40 mg/kg BW) in 5% dextrose
| Treatments | ||||
|---|---|---|---|---|
| DS | MSS | |||
| Blood variable | Mean | SD | Mean | SD |
| pH (ref 7.35 to 7.45) | ||||
| 0 | 7.375 | ±0.021 | 7.390 | ±0.017 |
| 5 | 7.402 | ±0.015 | 7.393 | ±0.014 |
| 15 | 7.388 | ±0.015 | 7.395 | ±0.007 |
| 30 | 7.403 | ±0.022 | 7.400 | ±0.023 |
| 60 | 7.425 | ±0.014 | 7.397 | ±0.013 |
| 120 | 7.413 | ±0.021 | 7.397 | ±0.006 |
| SBE mmol/L (ref −3.0 to +3.0) | ||||
| 0 | 6.1 | ±1.2 | 6.5 | ±1.5 |
| 5 | 5.7 | ±1.4 | 5.6 | ±1.1 |
| 15 | 6.0 | ±1.2 | 6.3 | ±0.8 |
| 30 | 6.5 | ±1.5 | 6.7 | ±1.3 |
| 60 | 7.1 | ±1.9 | 6.0 | ±1.2 |
| 120 | 6.5 | ±1.5 | 6.0 | ±1.2 |
| HCO3 − mmol/L (ref 25 to 32) | ||||
| 0 | 31.1 | ±1.3 | 31.4 | ±1.5 |
| 5 | 30.4 | ±1.4 | 30.4 | ±1.2 |
| 15 | 30.9 | ±1.1 | 31.1 | ±0.9 |
| 30 | 31.3 | ±1.5 | 31.5 | ±1.4 |
| 60 | 31.5 | ±2.1 | 30.7 | ±1.2 |
| 120 | 31.1 | ±1.6 | 30.7 | ±1.4 |
| Na+ mmol/L (ref 135 to 145) | ||||
| 0 | 138 | ±2.2 | 134 | ±3.4 |
| 5 | 134 | ±1.8 | 131 | ±2.3 |
| 15 | 135 | ±1.8 | 132 | ±2.4 |
| 30 | 136 | ±1.9 | 133 | ±2.2 |
| 60 | 136 | ±1.6 | 133 | ±1.9 |
| 120 | 136 | ±2.4 | 132 | ±1.5 |
| Cl− mmol/L (ref 94 to 102) | ||||
| 0 | 103 | ±1.7 | 103 | ±1.9 |
| 5 | 101 | ±1.6 | 101 | ±1.1 |
| 15 | 102 | ±1.8 | 101 | ±2.0 |
| 30 | 103 | ±2.2 | 101 | ±2.0 |
| 60 | 103 | ±2.4 | 101 | ±1.9 |
| 120 | 102 | ±2.7 | 101 | ±1.9 |
| K+ mmol/L (3.3 to 5.0) | ||||
| 0 | 4.4 | ±0.8 | 4.3 | ±1.0 |
| 5 | 4.9 | ±0.7 | 4.3 | ±0.5 |
| 15 | 4.8 | ±0.8 | 4.2 | ±0.5 |
| 30 | 5.0 | ±0.7 | 4.2 | ±0.3 |
| 60 | 4.8 | ±0.5 | 4.0 | ±0.5 |
| 120 | 4.3 | ±0.6 | 3.8 | ±0.4 |
| Glucose mg/dL (ref 77 to 110) | ||||
| 0 | 91 | ±8 | 96 | ±7 |
| 5 | 156 | ±15 | 152 | ±9 |
| 15 | 134 | ±16 | 133 | ±9 |
| 30 | 98 | ±16 | 103 | ±15 |
| 60 | 89 | ±4 | 89 | ±8 |
| 120 | 92 | ±6 | 89 | ±6 |
| Lactate mmol/L (ref < 2) | ||||
| 0 | 0.8 | ±0.1 | 0.9 | ±0.5 |
| 5 | 1.0 | ±0.2 | 0.8 | ±0.2 |
| 15 | 1.1 | ±0.3 | 0.7 | ±0.1 |
| 30 | 0.9 | ±0.2 | 0.8 | ±0.2 |
| 60 | 0.9 | ±0.3 | 0.6 | ±0.2 |
| 120 | 1.1 | ±0.6 | 0.7 | ±0.2 |
| Ca2+ mmol/L (ref 1.40 to 1.60) | ||||
| 0 | 1.60 | ±0.05 | 1.52 | ±0.05 |
| 5 | 1.59 | ±0.04 | 1.44 | ±0.05 |
| 15 | 1.57 | ±0.05 | 1.39 | ±0.05 |
| 30 | 1.54 | ±0.03 | 1.38 | ±0.06 |
| 60 | 1.55 | ±0.05 | 1.40 | ±0.05 |
| 120 | 1.57 | ±0.07 | 1.44 | ±0.03 |
| tMg mg/dL (ref 1.9 to 3.0) | ||||
| 0 | 2.2 | ±0.2 | 2.0 | ±0.2 |
| 5 | 2.1 | ±0.1 | 4.5 | ±0.5 |
| 15 | 2.1 | ±0.1 | 3.9 | ±0.3 |
| 30 | 2.1 | ±0.2 | 3.4 | ±0.3 |
| 60 | 2.1 | ±0.1 | 3.0 | ±0.2 |
| 120 | 2.1 | ±0.2 | 2.5 | ±0.3 |
| Mg2+ mmol/L (ref 0.47 to 0.70) | ||||
| 0 | 0.51 | ±0.09 | 0.42 | ±0.05 |
| 5 | 0.50 | ±0.09 | 0.99 | ±0.10 |
| 15 | 0.49 | ±0.10 | 0.86 | ±0.09 |
| 30 | 0.48 | ±0.09 | 0.74 | ±0.09 |
| 60 | 0.47 | ±0.08 | 0.63 | ±0.06 |
| 120 | 0.50 | ±0.07 | 0.54 | ±0.04 |
Numbers in bold for the following variables, Na+, Cl−, K+, glucose, Ca2+, tMg, and Mg2+, indicate values above or below the reference range. Note: For SBE, the baseline values for all horses were above the reference range. Bold indicates difference from baseline values.
Abbreviations: DS, dextrose solution group; MSS, MgSO4 solution group; SBE, standard base excess; tMg, total magnesium.
Table 2.
Calculated blood results
| Treatments | ||||
|---|---|---|---|---|
| DS | MSS | |||
| Blood variable | Mean | SD | Mean | SD |
| SID mmol/L (ref 38‐42) | ||||
| 0 | 41.1 | ±1.9 | 37.7 | ±2.3 |
| 5 | 39.8 | ±1.3 | 36.9 | ±1.9 |
| 15 | 40.2 | ±0.9 | 37.5 | ±2.4 |
| 30 | 40.2 | ±1.3 | 38.2 | ±2.3 |
| 60 | 40.3 | ±1.8 | 37.4 | ±1.2 |
| 120 | 40.2 | ±1.2 | 37.1 | ±2.2 |
| AG mmol/L (ref 9‐17) | ||||
| 0 | 7.9 | ±1.8 | 4.4 | ±1.8 |
| 5 | 7.4 | ±1.2 | 4.1 | ±2.2 |
| 15 | 6.9 | ±1.1 | 4.1 | ±2.6 |
| 30 | 6.9 | ±1.6 | 4.5 | ±2.1 |
| 60 | 6.7 | ±1.5 | 4.6 | ±1.7 |
| 120 | 6.9 | ±1.5 | 4.4 | ±1.3 |
Changes in calculated blood variables after IV infusion of DS and MSS. DS (5% dextrose solution at 2 mL/kg BW) and MSS (MgSO4 50% solution (40 mg/kg BW) in 5% dextrose. Numbers in bold indicates values above or below the reference range for that blood parameter.
Abbreviations: AG, anion gap; DS, dextrose solution group; MSS, MgSO4 solution group; SID, strong ion difference.
The DS group experienced a decrease in Na+ at T5 (mean, 134 ± 1.8 mmol/L; Table 1); otherwise, it remained within the reference range. Serum chloride concentration was above reference range at baseline and was again above reference range at T30 and T60 (T30 mean, 103 ± 2.2 mmol/L; T60 mean, 103 ± 2.4 mmol/L; Table 1). Blood glucose concentration was above reference range at T5 and T15 before returning to reference range (T5 mean, 156 ± 15 mg/dL; T15 mean, 134 ± 16 mg/dL; Table 1). All other blood variables remained within reference range. Serum osmolality was estimated by equation using sodium and glucose ([Na+ mmol/L × 2] + [glucose mg/dL/18]). Baseline mean and SD osmolality was 277 ± 7.0 mOsm/kg (range, 265‐286; reference range, 270‐300 mOsm/kg). In the DS group, mean and SD osmolality was 277 ± 3.6 mOsm/kg (range, 268‐283 mOsm/kg), whereas in the MSS group, the osmolality was 270 ± 4.2 mOsm/kg (range, 264‐278 mOsm/kg).
Infusion of MSS caused a decrease in Na+ at all time points, compared with the DS group. Blood glucose concentration was above reference range at T5 and T15 before returning to reference range (T5 mean, 152 ± 9 mg/dL; T15 mean, 133 ± 9 mg/dL; Table 1). Serum ionized calcium decreased below reference range at T15 and T30 (T15 mean, 1.39 ± 0.05 mmol/L; T30 mean, 1.38 ± 0.06 mmol/L; Table 1). Total magnesium concentration increased above reference range at T5, T15, and T30 (T5 mean, 4.5 ± 0.5 mg/dL; T15 mean, 3.9 ± 0.3 mg/dL; T30 mean, 3.4 ± 0.3 mg/dL; Table 1). Ionized magnesium concentration was below reference range at baseline but increased above reference range at T5, T15, and T30 before returning to reference range (T5 mean, 0.99 ± 0.1 mmol/L; T15 mean, 0.86 ± 0.09 mmol/L; T30 mean, 0.74 ± 0.09 mmol/L; Table 1).
3.2. Behavior results
Head‐shaking behavior, which included head shakes/minute, head‐tossing/minute, nose rubbing/minute, dropped head/minute, and snorting/minute, varied widely among horses. For statistical analysis, we focused on head shakes/minute because it was the most important and consistent behavior of affected horses. The range of head shakes/minute among horses varied widely, because some horses were very severely affected (eg, baseline at trot 11 head shakes/minute and at canter 26 head shakes/minute), whereas other behaviors were not. Nonetheless, overall head‐shaking behavior was altered by the infusion of MgSO4. Longitudinal data for individual horses can be found in Figure 4.
Figure 4.

Head‐shaking behavior for 6 individual horses and overall change over time. A and B, At the trot. C and D, At the canter. Each horse is displayed with a different marker, and the overall change in head‐shaking behavior is represented by the regression line. Top graphs (A and C) = DS 5% solution (2 mL/kg BW); bottom graphs (B and D) = MgSO4 50% solution (40 mg/kg BW). BW, body weight; DS, dextrose solution group; MSS, MgSO4 solution group
A mixed‐effects Poisson regression model evaluated the effects of treatment and time while controlling for breed and gait on median head shakes/minute. An effect of MSS treatment was observed controlling for time, breed, and gait (first walk, trot, canter, second walk) with a mean of 0.71 times the rate of head shaking compared to DS group or a 29% decrease in median head shakes/minute (IRR, 0.710; CI, 0.61‐0.82; P < .001; Table 3). When restricted to canter, the MSS group experienced 0.49 times the rate of median head shakes/minute compared to DS or a 51% decrease in head shaking (IRR, 0.493; CI, 0.40‐0.61; P < .001; Table 3). There was an effect of trot and DS treatment, while controlling for time and breed, with a 10‐fold increase in median head shakes/minute (IRR, 10.250; CI, 5.66‐18.54; P < .001; Table 4). There was an effect of canter and DS treatment, while controlling for time and breed, with a 22‐fold increase in median head shakes/minute (IRR, 22.667; CI, 12.71‐40.41; P < .001; Table 4) as compared to first walk. There was an effect of trot and MSS treatment, while controlling for time and breed, with a 15‐fold increase in median head shakes/minute (IRR, 15.000; CI, 7.91‐28.45; P < .001; Table 4). There was an effect of canter and MSS treatment, while controlling for time and breed, with a 13‐fold increase in median head shakes/minute (IRR, 13.400; CI, 7.04‐25.48; P < .001; Table 4) as compared to first walk. There was an effect of time while controlling for treatment, breed, and gait on median head shakes/minute with T60 having 0.77 times the rate of head shaking as compared to T0 or a 24% decrease in head shaking (IRR, 0.765; CI, 0.60‐0.96; P = .02). There was an effect of time when restricted to DS treatment and controlling for gait and breed with a 37% increase in median head shakes/minute at T120 (IRR, 1.367; CI, 1.02‐1.83; P = .04; Table 5). There was an effect of time at T30 when restricted to MSS treatment and controlling for gait and breed with 0.64 times the rate of head shaking as compared to T0 or a 36% decrease in head shaking (IRR, 0.644; CI, 0.46‐0.90; P = .01; Table 5). There was an effect of time at T60 when restricted to MSS treatment and controlling for gait and breed with 0.47 times the rate of head shaking compared to T0 or a 53% decrease in head shaking (IRR, 0.471; CI, 0.33‐0.68; P < .001; Table 5). There was an effect of time at T120 when restricted to MSS treatment and controlling for gait and breed with 0.48 times the rate of head shaking as compared to T0 or a 52% decrease in head shaking (0.483 IRR; CI, 0.33‐0.69, P < .001, Table 5). Thoroughbreds had a numerically greater reduction in head shakes per minute in response to MSS treatment compared to Quarter Horses, but the limited number of animals of each breed in the study did not permit statistical comparison.
Table 3.
Effect of MSS treatment and gait on IRR of median head shakes/minute across all times and breeds
| Treatment group: MSS | |||
|---|---|---|---|
| Behavior | IRR | P value | 95% CI |
| Head shakes | |||
| All gaits | 0.710 | <.001 | 0.61‐0.83 |
| First walk | 0.833 | .67 | 0.36‐1.93 |
| Trot | 1.219 | .10 | 0.96‐1.55 |
| Canter | 0.493 | <.001 | 0.40‐0.61 |
| Second walk | 0.499 | .06 | 0.24‐1.03 |
P < .05 was considered significant.
Abbreviations: CI, confidence intervals; IRR, incidence rate ratios; MSS, MgSO4 solution group.
Table 4.
Effect of gait (trot and canter) and treatment (DS and MSS) on IRR of median head shakes/minute across all times and breeds
| Treatment | IRR | P value | 95% CI |
|---|---|---|---|
| DS | |||
| Trot | 10.250 | <.001 | 5.66‐18.54 |
| Canter | 22.667 | <.001 | 12.71‐40.41 |
| MSS | |||
| Trot | 15.000 | <.001 | 7.91‐28.45 |
| Canter | 13.400 | <.001 | 7.05‐25.48 |
P < .05 was considered significant.
Abbreviations: CI, confidence intervals; DS, dextrose solution group; IRR, incidence rate ratios; MSS, MgSO4 solution group.
Table 5.
Effect of each treatment and time on IRR of median head shakes/minute as compared to T0
| Treatment group | ||||||
|---|---|---|---|---|---|---|
| DS | MSS | |||||
| Behavior | IRR | P value | 95% CI | IRR | P value | 95% CI |
| Head shakes, min | ||||||
| T15 | 0.936 | .69 | 0.68‐1.29 | 0.908 | .54 | 0.67‐1.23 |
| T30 | 1.038 | .81 | 0.76‐1.41 | 0.644 | .01 | 0.46‐0.90 |
| T60 | 1.088 | .59 | 0.80‐1.48 | 0.471 | <.001 | 0.33‐0.68 |
| T20 | 1.367 | .04 | 1.02‐1.83 | 0.483 | <.001 | 0.33‐0.69 |
P < .05 was considered significant.
Abbreviations: CI, confidence intervals; DS, dextrose solution group; IRR, incidence rate ratios; MSS, MgSO4 solution group.
There was a significant interaction between treatment and time while controlling for treatment, gait, and breed with MSS and T30 having 0.62 times the rate of head shaking compared to DS and T0 or a 38% decrease in head shaking (IRR, 0.620; CI, 0.39‐0.98; P = .04). There was a significant interaction between treatment and time while controlling for treatment, gait, and breed with MSS and T60 having 0.43 times the rate of head shaking compared to DS and T0 or a 57% decrease in head shaking (IRR, 0.432; CI, 0.27‐0.70; P = .001). There was a significant interaction between treatment and time while controlling for treatment, gait, and breed with MSS and T120 having 0.35 times the rate of head shaking compared to DS and T0 or a 65% decrease in head shaking (IRR, 0.353; CI, 0.22‐0.56; P < .001). There was a significant interaction between treatment and time while restricted to the trot, with MSS and T60 having 0.42 times the rate of head shaking compared to DS and T0 or a 58% decrease in head shaking (IRR 0.416; CI 0.19‐0.93; P = .03). There was a significant interaction between treatment and time while restricted to the trot, with MSS and T120 having 0.45 times the rate of head shaking compared to DS and T0 or a 55% decrease in head shaking (IRR, 0.451; CI 0.22‐0.95; P = .04). There was a significant interaction between treatment and time while restricted to the canter, with MSS and T60 having 0.40 times the rate of head shaking compared to DS and T0 or a 60% decrease in head shaking (IRR, 0.404; CI 0.21‐0.80; P = .009). There was a significant interaction between treatment and time while restricted to the canter, with MSS and T120 having 0.20 times the rate of head shaking compared to DS and T0 or an 80% decrease in head shaking (IRR, 0.200; CI, 0.09‐0.44; P < .001). There were no significant interactions between treatment and gait. There were no significant interactions between treatment and breed.
3.3. Agreement between evaluators
Spearman's correlation was used to assess interobserver variability among the 3 evaluators. There was a strong agreement between observer 1 and 2 (rs = 0.94; P < .0001), observer 1 and 3 (rs = 0.84; P < .0001), and observer 2 and 3 (rs = 0.88; P < .0001).
4. DISCUSSION
We evaluated the effects on head‐shaking behavior of IV administration of MSS. These effects were expected to be observed over a short time frame because of the blood and tissue distribution of the solutions after a single infusion. The MSS treatment induced a 29% decrease in median head shakes/minute when compared to DS across all gaits (first walk, trot, canter, second walk). As exercise intensity increased from walk to trot to canter, median head shakes/minute also increased, suggesting that exercise might have contributed to the triggering or exacerbation of the observed signs in horses affected with trigeminal‐mediated headshaking. Similar observations have been reported anecdotally, and our study results support this assumption.6, 18 The largest decrease in head shakes/minute was 51% for the canter in MSS. The faster the gait, the larger the decrease in median head shakes/minute after MSS treatment. Therefore, our study highlights the possible blunting effects of magnesium during faster gaits that are likely to exacerbate head‐shaking signs. An important finding is the effect of MSS treatment on decreasing median head shakes/minute starting at T30 and continuing to T120. This blunting effect might have persisted longer than our study period of 2 hours. Therefore, further evaluation to determine how long this blunting effect persists is warranted. Although there was a larger decrease in the rate of head shaking in Thoroughbreds compared to Quarter Horses in this study, the low number of horses per breed precluded any conclusion with respect to the effect of breed in the response to MSS treatment in head shaking.
Treatment with MSS supplied a large bolus of magnesium that should be 100% bioavailable. Potential adverse effects such as sweating, agitation, muscle fasciculations, arrhythmias, and collapse resulting from the infusion of MSS were not detected in any of these horses.29 However, the dosage of magnesium used in our study was below that previously associated with toxicity in horses (1500‐2000 mg/kg of PO MgSO4).29 Infusion of MSS corrected the lower concentrations of Mg2+ observed at baseline, which also was seen in a previous study of horses affected by trigeminal‐mediated headshaking.28 The decrease in head‐shaking behavior could be a result of Mg2+ inhibiting nerve transmission.30 Such inhibition can occur when Mg2+ acts as a blocking agent to N‐methyl‐D‐aspartate (NMDA) receptors, which have been shown to have an effect on trigeminal neurons at the trigeminal subnucleus caudalis (ie, the orofacial nociceptive processing center) in rats.30 The NMDA receptors are a subtype of glutamate receptors and, once activated, are permeable to calcium and other cations.30, 31, 32 Magnesium and zinc can bind to the NMDA receptor and prevent activation.31, 32 Magnesium also is involved in the regulation of neuroexcitation and can inhibit Ca2+‐dependent presynaptic excitation‐secretion coupling.22 Magnesium also participates in neuromuscular blockade by inhibiting calcium channels, which inhibits acetylcholine release and muscle excitability.23 Furthermore, magnesium has been utilized in anesthesia as an analgesic agent.23, 33 The exact mechanism of how magnesium acts as a pain reducer is not known but may involve inhibition of neurotransmission by blockade of receptors involved in cell excitation.22, 31, 32 Intravenous magnesium supplementation in our study was expected to have short‐acting effects on magnesium concentrations and head shaking as fluid distribution occurred in the body. However, magnesium concentrations increased and remained increased 120 minutes after infusion. The time required for normalization of plasma magnesium concentrations after infusion was not determined in our study.
Some preexisting differences in baseline blood variables were present in horses of both treatment groups. These differences might have reflected individual physiological variations. Significant changes also occurred in blood Ca2+, pH, K+, Na+, Cl−, SID, AG, and lactate after administration of treatment solutions. Blood Ca2+ decreased significantly in MSS compared to DS. In support of this finding, hypomagnesemia has been shown to be induced by hypercalcemia that occurs after administration of calcium gluconate solution IV.34 Higher plasma concentrations of Ca2+ activate release of neurotransmitters and signal the nerve to fire, whereas decreased plasma Ca2+ concentrations result in less calcium available for activation of the trigeminal nerve.35 It has been found that Ca2+ is involved in neuropathic pain of the trigeminal nerve, which also could be a possible mechanism by which magnesium treatment resulted in a decrease in head shaking (decrease in neuropathic pain) in this group of horses.36 Blood sodium concentrations decreased below reference range in MSS treatment group. This decrease in Na+ amounted to an average of 4 mmol/L and occurred concurrently with an increase in blood glucose concentration. Plasma osmolality was not measured in this study but calculated using an equation employing Na+ and glucose. Plasma osmolality in these horses remained within physiologic range after treatment, despite mild changes in Na+. Because the changes in blood sodium concentration were mild and transient with treatment, the biological relevance to clinical manifestations of head shaking were likely minimal. A previous study showed that metabolic alkalosis induced by administration of hypertonic sodium bicarbonate IV resulted in a decrease in head‐shaking behavior.28
The main limitations of our study included the small number of horses and lack of control groups (healthy control group [unaffected horses] and affected untreated horses [no IV solution]). A detailed diagnostic investigation was done in these horses to eliminate other causes of head shaking. Computed tomography would have been helpful to further eliminate other causes but was not performed. Local anesthesia of the infraorbital nerve was not performed because it is not specific, and any underlying cause located in areas innervated by this nerve would have been affected by local anesthesia. Nerve conduction velocity studies of the trigeminal nerve would have confirmed trigeminal‐mediated headshaking but would have required general anesthesia. Therefore, such studies were not performed in these horses. Furthermore, effects of riding, tack, and rider expertise were not evaluated in our study.
In conclusion, the administration of MSS IV increased tMg and Mg2+ and significantly decreased head‐shaking behavior in horses with trigeminal‐mediated headshaking. Our study supports anecdotal reports of the perceived benefits of magnesium supplementation in affected horses. Therefore, future studies should investigate practical PO supplementation of magnesium with high bioavailability for long‐term use. Doing so might help address a major welfare challenge in equine medicine and possibly avoid euthanasia. Further investigation is warranted to determine if a cutoff concentration of Mg2+ would be associated with the prevention of or decrease in head shaking in affected horses.
CONFLICTS OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC of the University of California, Davis.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
The authors thank Drs. Phil Kass, Jerome Baron, and Aki Tanaka for their assistance with statistical analysis and Dr. Gary Magdesian for scientific advice.
Sheldon SA, Aleman M, Costa LRR, Santoyo AC, Howey Q, Madigan JE. Intravenous infusion of magnesium sulfate and its effect on horses with trigeminal‐mediated headshaking. J Vet Intern Med. 2019;33:923–932. 10.1111/jvim.15410
Funding information Gift from anonymous private donors towards the Equine and Comparative Neurology Research Group
REFERENCES
- 1. Aleman M, Williams DC, Brosnan RJ, et al. Sensory nerve conduction and somatosensory evoked potentials of the trigeminal nerve in horses with idiopathic headshaking. J Vet Intern Med. 2013;27:1571‐1580. [DOI] [PubMed] [Google Scholar]
- 2. Aleman M, Rhodes D, Williams DC, et al. Sensory evoked potentials of the trigeminal nerve for the diagnosis of idiopathic headshaking in a horse. J Vet Intern Med. 2014;28:250‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madigan JE, Bell SA. Characterisation of headshaking syndrome–31 cases. Equine Vet J Suppl. 1998;27:28‐29. [DOI] [PubMed] [Google Scholar]
- 4. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bell AJ. Headshaking in a 10‐year‐old thoroughbred mare. Can Vet J. 2004;45:153‐155. [PMC free article] [PubMed] [Google Scholar]
- 6. Madigan JE, Bell SA. Owner survey of headshaking in horses. J Am Vet Med Assoc. 2001;219:334‐337. [DOI] [PubMed] [Google Scholar]
- 7. Mills DS, Cook S, Taylor K, et al. Analysis of the variations in clinical signs shown by 254 cases of equine headshaking. Vet Rec. 2002;150:236‐240. [DOI] [PubMed] [Google Scholar]
- 8. Li KW, Yu YP, Zhou C, et al. Calcium channel alpha2delta1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem. 2014;289:7025‐7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pickles K, Madigan J, Aleman M. Idiopathic headshaking: is it still idiopathic? Vet J. 2014;201:21‐30. [DOI] [PubMed] [Google Scholar]
- 10. Roberts VL, Fews D, McNamara JM, et al. Trigeminal nerve root demyelination not seen in six horses diagnosed with trigeminal‐mediated headshaking. Front Vet Sci. 2017;4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mills DS, Taylor K. Field study of the efficacy of three types of nose net for the treatment of headshaking in horses. Vet Rec. 2003;152:41‐44. [DOI] [PubMed] [Google Scholar]
- 12. Mills DS, Cook S, Jones B. Reported response to treatment among 245 cases of equine headshaking. Vet Rec. 2002;150:311‐313. [DOI] [PubMed] [Google Scholar]
- 13. Madigan JE, Kortz G, Murphy C, et al. Photic headshaking in the horse: 7 cases. Equine Vet J. 1995;27:306‐311. [DOI] [PubMed] [Google Scholar]
- 14. Talbot WA, Pinchbeck GL, Knottenbelt DC, et al. A randomised, blinded, crossover study to assess the efficacy of a feed supplement in alleviating the clinical signs of headshaking in 32 horses. Equine Vet J. 2013;45:293‐297. [DOI] [PubMed] [Google Scholar]
- 15. Roberts VL, McKane SA, Williams A, et al. Caudal compression of the infraorbital nerve: a novel surgical technique for treatment of idiopathic headshaking and assessment of its efficacy in 24 horses. Equine Vet J. 2009;41:165‐170. [DOI] [PubMed] [Google Scholar]
- 16. Roberts VL, Perkins JD, Skarlina E, et al. Caudal anaesthesia of the infraorbital nerve for diagnosis of idiopathic headshaking and caudal compression of the infraorbital nerve for its treatment, in 58 horses. Equine Vet J. 2013;45:107‐110. [DOI] [PubMed] [Google Scholar]
- 17. Roberts VL, Patel NK, Tremaine WH. Neuromodulation using percutaneous electrical nerve stimulation for the management of trigeminal‐mediated headshaking: a safe procedure resulting in medium‐term remission in five of seven horses. Equine Vet J. 2016;48:201‐204. [DOI] [PubMed] [Google Scholar]
- 18. Lane JG, Mair TS. Observations on headshaking in the horse. Equine Vet J. 1987;19:331‐336. [DOI] [PubMed] [Google Scholar]
- 19. Stewart AJ. Magnesium disorders in horses. Vet Clin North Am Equine Pract. 2011;27:149‐163. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka M, Shimizu S, Nishimura W, et al. Relief of neuropathic pain with intravenous magnesium. Masui. 1998;47:1109‐1113. [PubMed] [Google Scholar]
- 21. Bowen JM, Blackmon DM, Heavner JE. Effect of magnesium ions on neuromuscular transmission in the horse, steer, and dog. J Am Vet Med Assoc. 1970;157:164‐173. [PubMed] [Google Scholar]
- 22. Hoenderop JG, Bindels RJ. Epithelial Ca2+ and Mg2+ channels in health and disease. J Am Soc Nephrol. 2005;16:15‐26. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez‐Rubio L, Solis Garcia Del Pozo J, Nava E, et al. Interaction between magnesium sulfate and neuromuscular blockers during the perioperative period. A systematic review and meta‐analysis. J Clin Anesth. 2016;34:524‐534. [DOI] [PubMed] [Google Scholar]
- 24. Bowen JM, McMullan WC. Influence of induced hypermagnesemia and hypocalcemia on neuromuscular blocking property of oxytetracycline in the horse. Am J Vet Res. 1975;36:1025‐1028. [PubMed] [Google Scholar]
- 25. Stewart AJ. Treatment for hypomagnesemia In: Fielding CL, Magdesian KG, eds. Equine Fluid Therapy. Ames, IA: John Wiley & Sons, Inc; 2015:76‐87. [Google Scholar]
- 26. Stewart PA. Modern quantitative acid‐base chemistry. Can J Physiol Pharmacol. 1983;61:1444‐1461. [DOI] [PubMed] [Google Scholar]
- 27. Lopes MA, Walker BL, White NA, et al. Treatments to promote colonic hydration: enteral fluid therapy versus intravenous fluid therapy and magnesium sulphate. Equine Vet J. 2002;34:505‐509. [DOI] [PubMed] [Google Scholar]
- 28. Sheldon S, Aleman M, Costa L, et al. Alterations in metabolic status and headshaking behavior following intravenous administration of hypertonic solutions in horses with trigeminal‐mediated headshaking. Animals. 2018;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henninger RW, Horst J. Magnesium toxicosis in two horses. J Am Vet Med Assoc. 1997;211:82‐85. [PubMed] [Google Scholar]
- 30. Chen L, Mae Huang L‐Y. Protein kinase C reduces Mg2+ block of NMDA‐receptor channels as a mechanism of modulation. Nature. 1992;356:521‐523. [DOI] [PubMed] [Google Scholar]
- 31. Zhang L, Rzigalinski BA, Ellis EF, et al. Reduction of voltage‐dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921‐1923. [DOI] [PubMed] [Google Scholar]
- 32. Wang LY, MacDonald JF. Modulation by magnesium of the affinity of NMDA receptors for glycine in murine hippocampal neurones. J Physiol. 1995;486(Pt 1):83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhatia A, Kashyap L, Pawar DK, et al. Effect of intraoperative magnesium infusion on perioperative analgesia in open cholecystectomy. J Clin Anesth. 2004;16:262‐265. [DOI] [PubMed] [Google Scholar]
- 34. Toribio RE, Kohn CW, Rourke KM, et al. Effects of hypercalcemia on serum concentrations of magnesium, potassium, and phosphate and urinary excretion of electrolytes in horses. Am J Vet Res. 2007;68:543‐554. [DOI] [PubMed] [Google Scholar]
- 35. Sudhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4:a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li K‐W, Yu YP, Zhou C, et al. Calcium channel α(2)δ(1) proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem. 2014;289:7025‐7037. [DOI] [PMC free article] [PubMed] [Google Scholar]


