Significance
The causes and consequences of animal migration have received substantial research attention, yet the mechanisms underlying this global phenomenon remain largely untested in marine systems. By combining 10 years of satellite tracking data on blue whales with simultaneous remotely-sensed oceanographic measurements in the North Pacific, we demonstrate that both long-term memory and resource tracking play key roles in the long-distance migrations of marine megafauna. These findings have important implications for long-lived species across systems and taxa, as long-range migrants conditioned by historical environmental processes may struggle in response to rapid environmental change. Finally, our study reveals that ecological theory of animal migrations is conserved across marine and terrestrial systems.
Keywords: marine megafauna, migration, movement ecology, resource wave, spatial memory
Abstract
In terrestrial systems, the green wave hypothesis posits that migrating animals can enhance foraging opportunities by tracking phenological variation in high-quality forage across space (i.e., “resource waves”). To track resource waves, animals may rely on proximate cues and/or memory of long-term average phenologies. Although there is growing evidence of resource tracking in terrestrial migrants, such drivers remain unevaluated in migratory marine megafauna. Here we present a test of the green wave hypothesis in a marine system. We compare 10 years of blue whale movement data with the timing of the spring phytoplankton bloom resulting in increased prey availability in the California Current Ecosystem, allowing us to investigate resource tracking both contemporaneously (response to proximate cues) and based on climatological conditions (memory) during migrations. Blue whales closely tracked the long-term average phenology of the spring bloom, but did not track contemporaneous green-up. In addition, blue whale foraging locations were characterized by low long-term habitat variability and high long-term productivity compared with contemporaneous measurements. Results indicate that memory of long-term average conditions may have a previously underappreciated role in driving migratory movements of long-lived species in marine systems, and suggest that these animals may struggle to respond to rapid deviations from historical mean environmental conditions. Results further highlight that an ecological theory of migration is conserved across marine and terrestrial systems. Understanding the drivers of animal migration is critical for assessing how environmental changes will affect highly mobile fauna at a global scale.
Spatiotemporal variation in resources is understood to be a major driver of migration across terrestrial and marine taxa (1), but which mechanisms underlie the when and where of migratory movements remains a key question in ecology. There is growing recognition, however, that the distribution of resources across space and time can influence the timing and pace of migration (2, 3). Both during migration and within seasonal ranges, mobile consumers that move to exploit heterogeneity in resources across space and time benefit from enhanced resource gain (4–6). Resource tracking should be favored in environments with resource waves, or ephemeral pulses of resources that propagate across spatiotemporal gradients (7). There are numerous examples of both primary and secondary consumers exploiting resource waves (7), from migratory geese tracking a narrow phenological window of high-quality forage (8) to grizzly bears seeking out salmon spawning events across streams (9, 10).
Although resource waves may not be the primary driver of migration, when a resource wave propagates across space, the timing and pace of migration may be influenced by the progression of the resource wave (11). The green wave hypothesis formalizes this notion, suggesting that migratory herbivores should match their movements with ephemeral peaks in high-quality forage that progress across the landscape, a behavior termed “surfing the green wave” (8). The green wave hypothesis originated to explain the migratory movements of barnacle geese (12), and has recently been applied to ungulate migration (13). Animal movements may respond to resource waves that are contemporaneous, as conceptualized by the green wave hypothesis, or to resource waves that are climatological—namely the progression of a shifting mosaic of predictably high-quality resource patches averaged over longer (e.g., decadal) timescales. Although there is a growing body of work to suggest that many terrestrial migrants track resource waves (11, 14, 15) and derive an energetic benefit as a result (4, 6), it remains unclear if and how resource tracking interacts with spatial memory to shape migration patterns.
Recently, the role of memory in shaping animal movements, and particularly migration patterns, has gained increased attention as a mechanism for enhancing fitness beyond tracking proximate resources (16, 17). Memory allows migrants to make movement decisions using information beyond their immediate perceptual ranges (16), and allows forecasting of future conditions based on long-term averages of past conditions (17). Indeed, migratory birds and ungulates have been shown to track climatological resource waves, having developed expectations of resource availability via long-term memory (17, 18). Spatial memory can also improve efficiency of movement, for example by facilitating navigation to high-quality forage patches (19–21), landscape features (19), or stopover sites (22). As a result of these potential benefits, memory is expected to play an important role in environments that exhibit predictable temporal dynamics of resources (23).
Pinpointing the mechanisms underlying migration is important given ongoing global reductions in animal migration due to human activities (24), especially for emblematic and endangered species (25). While the drivers of terrestrial migration patterns have been relatively well explored (2, 4, 26–29), those in marine systems are less well understood (30, 31). Investigations into marine megafauna migrations are difficult because despite recent technological advances, large-scale tracking efforts are still extremely costly, and data on resource distributions in highly dynamic ocean habitats are difficult to attain (32). Thus, while there has been suggestion that marine predators track seasonal cycles of primary production and water temperature (33), no empirical investigation of resource tracking among long-distance marine migrants exists. Rather, most studies link the phenology of marine migrants with the phenology of resources in a single foraging location (34–36). As such, the role of memory in the movement patterns of marine megafauna and the drivers of long-distance movements are both considered key knowledge gaps (30). We address these questions using a 10-y dataset on ocean productivity and migratory movements of blue whales (Balaenoptera musculus).
The largest animal to ever exist, blue whales are listed as endangered under both the US Endangered Species Act and the International Union for Conservation of Nature Red List (37). In the eastern North Pacific, blue whales perform seasonal latitudinal migrations between winter/breeding grounds in the Gulf of California or the Costa Rica Dome and productive foraging grounds at higher latitudes in the California Current and Gulf of Alaska (38–41) (SI Appendix, Fig. S1). In the spring, blue whales depart the breeding grounds and travel north, spending the summer months feeding progressively northward along the North American west coast from Baja California to as far north as British Columbia (38). In the California Current, seasonal upwelling in the spring and summer drives a phytoplankton bloom (here, “green-up”) that occurs progressively later at more northern latitudes (42, 43). Though the onset and duration of the upwelling season are characterized by significant interannual variability (42, 43), the northward migration of blue whales occurs during the same season as the bloom (39, 44). Blue whales are specialist feeders on krill (45–47), but because krill availability is difficult to estimate, previous studies on blue whale foraging ecology have used time-lagged chlorophyll-a concentration as a proxy (35, 48–52). Blue whales demonstrate temporal synchrony with their prey (34, 36), and simulations have shown that temporal heterogeneity in ocean productivity leads to the emergence of their observed migratory behavior (53). However, despite blue whales garnering significant conservation and research attention, the relative roles of proximate cues and memory in shaping their migratory behavior are unknown. Elucidating these drivers is key to understanding blue whales’ behavioral plasticity to changing environmental conditions.
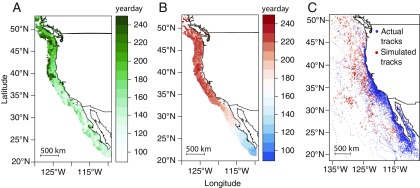
We investigated the role of resource tracking, based on both contemporaneous and climatological resource waves, in shaping the phenology of blue whales’ northward migrations in the California Current. We consider here contemporaneous resource tracking to indicate a response to proximate cues, and tracking of long-term average conditions to indicate a memory-based mechanism (17). Using daily telemetry data for 60 blue whales tagged between 1999 and 2008, we compared the phenology of migratory movements with the phenology of peak resource availability, as measured by time-lagged chlorophyll-a (34, 35, 49, 54) (Fig. 1A). We calculated the mean climatology of chlorophyll-a over a decade to reflect long-term average conditions. We also compared blue whale locations with the presence of 15 to 17 °C sea surface temperatures (SSTs; Fig. 1B), which are hypothesized to influence blue whale presence directly via thermal preferences of the whales themselves (48–50) or indirectly via thermal associations of krill (55). We considered in this study two sets of hypotheses and predictions: (i) If contemporaneous resource tracking dominates migration timing, blue whales should adjust the timing of their movements in response to the proximate availability of resources; and (ii) if memory dominates migration timing, migration phenology should track the climatological green-up (i.e., in interannually predictable resource hotspots). We tested these hypotheses using a three-pronged approach to (i) evaluate the influence of environmental resources on blue whale migration phenology using telemetry-derived movement data, (ii) compare empirical results with those of simulated random migration tracks (Fig. 1C), and (iii) evaluate the abundance and long-term predictability in resource availability at blue whale foraging locations.
Fig. 1.
(A and B) Average timing of (A) peak chlorophyll-a concentration and (B) 15 to 17 °C sea surface temperature along the western coast of North America between 1998 and 2010. (C) Observed blue whale tracks (blue points) and simulated random migrants (red points) used in the analysis.
Results
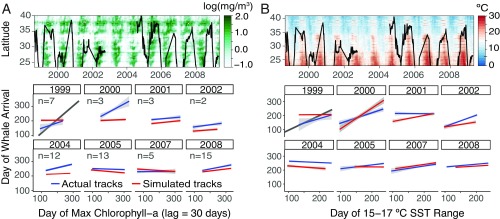
Regression between dates of whale use and contemporaneous dates of peak chlorophyll-a concentration, with individuals nested as a random effect, showed no significant relationship when tracks were pooled across all years (linear regression P = 0.23). The only year with significant contemporaneous chlorophyll-a tracking was 2004 (P < 0.001) (Fig. 2A). There was a weak but significant correlation between whale use and the contemporaneous timing of 15 to 17 °C SSTs (P < 0.001), but this effect was also present in simulated random tracks (P < 0.01; Fig. 2B).
Fig. 2.
(Top) Annual blue whale latitudinal movements averaged over the population (black line) overlaid on Hovmoller plots of (A) chlorophyll-a and (B) SST. (Bottom) Annual linear regressions of whale movement phenology with contemporaneous (A) chlorophyll-a and (B) SST (observed tracks in blue; simulated tracks in red). Gray lines in the 1999 regressions indicate a 1:1 relationship.
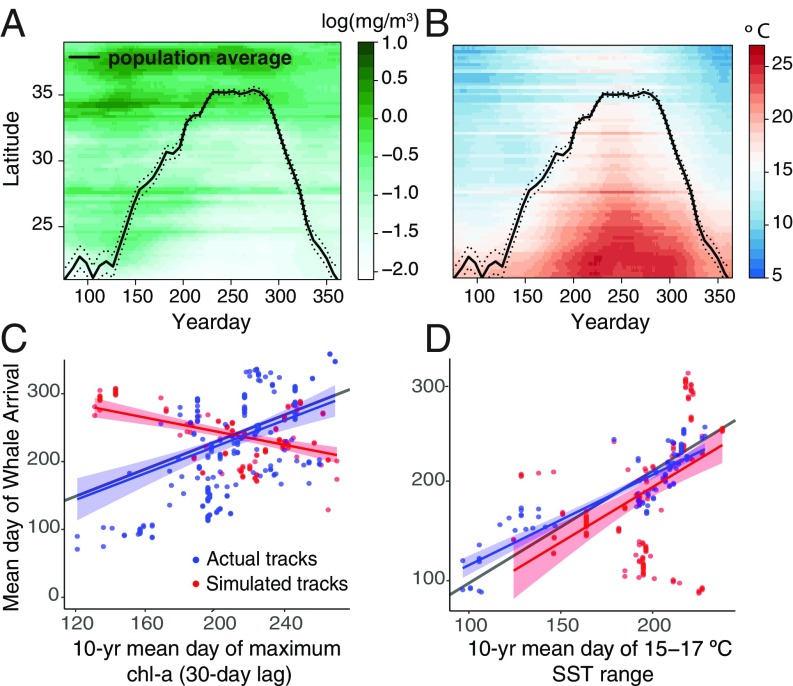
In contrast, blue whale migratory movements were significantly correlated with the climatological (10-y average) timing of peak chlorophyll-a (linear regression P < 0.001; Fig. 3C). Based on linear regression slopes, the climatological timing of peak chlorophyll-a bloom matched the movements of blue whales more than threefold better than those of simulated random migrants. Blue whales’ migration phenology was also significantly correlated with the climatological timing of 15 to 17 °C SSTs (P < 0.001), but the same was true for random migrants, suggesting this relationship is correlative but not causative (Fig. 3D).
Fig. 3.
(A and B) Climatology of blue whale migrations (black lines ± 1 SE) along the US west coast and Baja California between 1998 and 2010 in relation to climatological (A) chlorophyll-a concentration and (B) SST. (C and D) Relationship between phenology of tracks and long-term climatologies of (C) chlorophyll-a; observed tracks (blue; linear regression mean 0.14 ± 0.03; P < 0.001) and random tracks (red; mean −0.04 ± 0.02; P = 0.015), and (D) SST; observed tracks (mean 0.26 ± 0.04; P < 0.001) and random tracks (mean = 0.24 ± 0.03; P < 0.001). Shading indicates 95% confidence interval around fitted values. Gray lines indicate a 1:1 relationship. Results of all tracks pooled and averaged are shown.
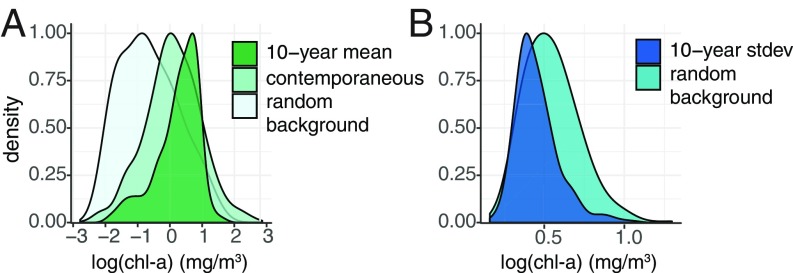
Analysis of 2,373 foraging locations identified by a state-space model (Movement Data) revealed that these areas were characterized by significantly higher long-term average chlorophyll-a concentrations compared with that of contemporaneous chlorophyll-a concentrations experienced by the whales, and both of these distributions were significantly greater than climatological background distributions available in the environment (10-y average x̅ = 0.24 ± 0.67 mg/m3; contemporaneous x̅ = 0.08 ± 0.87 mg/m3; background x̅ = −0.64 ± 0.65 mg/m3; 10-y-contemporaneous, background-contemporaneous, and background-10-y Bhattacharyya similarity coefficients = 0.68, 0.26, and 0.11, respectively; Kolmogorov–Smirnov test and Welch’s t test P < 0.001 for all; Fig. 4A). All chlorophyll-a concentration values reported are on a log scale. In addition, foraging locations had significantly lower interannual variability in productivity compared with background distributions (10-y temporal SD x̅ = 0.44 ± 0.13 mg/m3; background temporal SD x̅ = 0.55 ± 0.17 mg/m3; Bhattacharyya similarity coefficient = 0.30; Kolmogorov–Smirnov test and Welch’s t test P < 0.001; Fig. 4B).
Fig. 4.
(A) Distributions of 10-y average and contemporaneous chlorophyll-a concentrations for n = 2,373 area-restricted search locations, and 10-y average chlorophyll-a concentrations from 10,000 randomly generated locations in the study area. (B) Distributions of 10-y temporal SDs in chlorophyll-a concentrations for area-restricted search locations and randomized locations.
Discussion
Understanding the mechanisms driving the movement behaviors of animals can inform our expectations of how animals will respond to changing resource phenology and distributions as predicted under climate change. Across both marine and terrestrial systems, animals should benefit from increased resource gain by matching their movements in space and time to the availability of resources (4, 7). Thus, the movement of an animal should be fined-tuned to its resource landscape, and different resource landscapes should favor different movement mechanisms, such as random search, memory, and taxis (i.e., gradient tracking) (23). Animals likely use a mixture of different movement mechanisms to interact with their environment. Here, we demonstrate that resource tracking in highly dynamic environments can be enhanced by long-term memory of highly productive and relatively stable foraging sites. Memory is hypothesized to be favored in organisms that are long-lived, which can have extended periods of learning, and in resource landscapes that are heterogeneous and predictable (16). Our findings are consistent with these predictions and, in addition, provide a test of the green wave hypothesis in a marine system.
Our results demonstrate that blue whales track the long-term average phenology of the spring/summer phytoplankton bloom as they forage progressively farther north along the west coast of North America, signifying that memory plays an important role in the movement decisions of these long-lived animals. In other words, we find that blue whales surf climatological resource waves, using memory to track shifting hotspots of predictable and high-quality resources. Long-term memory has been shown to be a strong driver of migration patterns across taxa (17, 22), and in some cases a stronger driver than proximate cues. For example, the migration direction of zebra during long-distance migrations in southern Africa was predicted significantly better by memory (modeled as tracking of past average conditions) than by tracking of contemporaneous resource waves (17). Similarly, several species of long-distance avian migrants have been shown to track decadal averages of vegetation conditions (18). In addition, many migratory megafauna display extreme individual-level fidelity to their interannual migration routes (56–58). Our study indicates that an interplay between both long-term memory and resource tracking shapes the long-distance migrations of marine megafauna.
Tracking of climatological resource waves suggests that blue whales time their northward migrations to exploit expected resource availability in interannually predictable productivity hotspots. Even in years with the largest sample sizes of tagged individuals (e.g., 2005, 2008), we did not detect a significant signal for tracking of proximate resource waves (Fig. 2). Thus, we did not find support for the green wave hypothesis for tracking of contemporaneous resource waves among blue whales. Because the timing of upwelling-driven productivity in the California Current Ecosystem has significant interannual variability and habitat patches are highly dynamic (42, 43), blue whales may instead maximize their resource gain by targeting predictable foraging areas, a strategy that should theoretically favor memory (23). This conclusion is supported by evidence that during migration, foraging areas selected by blue whales were characterized by low year-to-year variability and high long-term productivity compared with contemporaneous measurements as well as with habitats available in their environment (Fig. 4). Similarly, a recent study found that over an 11-y period, blue whales consistently arrived in Monterey Bay during periods when prey availability was more predictable, with low interannual variance, relative to time periods of higher but more variable prey density (36). Interestingly, similar responses to the tradeoff between selecting habitats with consistent versus potentially higher but more variable resource availability have been observed in migratory ungulates; specifically, saiga antelope (Saiga tatarica) selected habitats in their spring range that had lower forage abundance but also more consistent year-to-year productivity than other available habitats (59). In coastal marine systems, persistent productivity hotspots are often geographically fixed at capes and headlands along the coast, which generate increased upwelling and primary productivity (60) and form upwelling shadows favorable for krill aggregations (61, 62). Indeed, one blue whale for which tag data were available in successive years arrived at the same foraging area near Cape Mendocino within the same week 1 y later, suggesting it timed its arrival to an expected increase in food availability (39).
Clarifying the mechanistic drivers underlying movements of wide-ranging species is a multifaceted and challenging endeavor given the noisiness of environmental gradients inherent to natural systems (11, 63). While we demonstrate that memory of climatological resource waves may lead whales to historically productive foraging areas, blue whales likely also fine-tune their movements at finer spatial scales in response to local, proximate conditions to locate individual prey patches. At subbasin scales, baleen whales are hypothesized to use a combination of social and sensory information to locate prey (64); however, substantial knowledge gaps remain. In addition, exogenous cues or endogenous factors, such as body condition, may play a role in other aspects of migratory behavior, such as whether and when migration is first initiated.
Testing the roles of memory and resource tracking in driving the timing and pace of migration is necessary for assessing how migratory species will respond to a world with changing resource distributions and phenology. As a K-selected, highly migratory species, blue whales are under threat from human activity and are a species of conservation concern (65–67). Climate change is likely to affect blue whales in the eastern North Pacific in ways that are not fully understood. Reliance on expectations developed by past average conditions may be detrimental as novel ecosystem states emerge. Climate variability underlies changes in prey abundance and distribution (68), as well as regime shifts to ecologically similar prey (69). Certain top predators, such as the sympatrically foraging humpback whale (Megaptera novaeangliae), can prey switch from krill to forage fish to buffer against ecosystem variability (70), but blue whales are specialists that must find dense patches of krill to achieve sufficient foraging efficiency (45–47). Warming oceans may affect the vertical distribution of krill by deepening the thermocline and increasing stratification of the water column (71), and krill populations may shift poleward or even decline over time in response to warming temperatures and predicted changes in coastal upwelling systems (72–75). Further, blue whale habitat is predicted to decrease significantly with current climate change projections (76). Whatever patterns and processes arise over the next century, it is likely that behavioral plasticity will be required for blue whales to continue to thrive in this ecosystem.
To further elucidate the drivers of animal migration, it is important to assess the interplay between resource tracking and memory across a wide range of species, in a diverse range of resource landscapes. The drivers of resource phenology vary across systems (e.g., marine versus terrestrial) and differ depending on the target resource. For example, in terrestrial systems, the emergence of highly nutritious plant growth can be influenced by static features, such as elevation (5), and less predictable weather events, such as rainfall (2). Likewise, the resource landscape of marine consumers is influenced by a suite of factors that shape primary production (e.g., winds, ocean currents, and mixing and stratification of the water column) (77) and prey distribution (e.g., intrinsic factors such as growth rates or physical features such as fronts and eddies that aggregate prey) (78). Despite these differences across systems, our study suggests that ecological theory of migration, and in particular the importance of resource tracking, is conserved across marine and terrestrial systems.
Methods
Movement Data.
We used daily telemetry data regularized with a Bayesian state-space model from previously published studies of blue whales in which 104 Argos-linked satellite tags were deployed between 1994 and 2008 (38, 39, 48). These data are published in the Movebank Data Repository (79). We examined tracks with at least 14-d duration, totaling 10,495 locations for 60 individuals (mean tag duration 134 ± 90 d; tag years 1999 to 2002, 2004 to 2005, and 2007 to 2008; SI Appendix, Fig. S2). We focused analyses on spring/summer northward movements from the southern tip of Baja California to British Columbia, coinciding with the northward progression of the spring/summer phytoplankton bloom (42, 43). We used changes in net squared displacement from the first location of each track to delineate the start and end of each northward migration annually (11, 80).
Following Thorup et al. (18) and Aikens et al. (11), we compared observed migration tracks with simulated random migration tracks to test a null hypothesis in which any observed relationships could simply be by-products of latitudinal migratory behavior decoupled from the environment. For each empirical whale track, 40 random migration tracks were simulated from correlated random walks based on empirical step length and turn angle distributions (Fig. 1C; see ref. 48 for details). A flag value was assigned to each simulated track indicating its similarity to the empirical track based on distance and net angular displacement from the true track (48, 81). To ensure random tracks simulated a realistic latitudinal migration, only random tracks in the upper 90th percentile of flag values were used for comparison.
Putative foraging locations were identified as area-restricted search (ARS) locations from the state-space model (38). The state-space model estimated one location per day and produced a continuous “behavioral mode” value from 1 to 2 for each location, with 1 representing idealized transiting behavior and 2 representing idealized ARS behavior (38, 82). Following previous studies (38, 82), we conservatively used values >1.75 to classify ARS behavior. ARS locations were time-matched based on date to contemporaneous and 10-y mean chlorophyll-a concentrations to examine the distribution of environmental conditions in putative foraging areas. In addition, the 10-y SD of chlorophyll-a concentration, representing the interannual variability, was extracted for each ARS location. Finally, we sampled the long-term mean and SD chlorophyll values at 10,000 randomly generated locations over the same time period within the study area to compare the distribution of variables at foraging locations with those available in the environment.
Tagging was approved by the Oregon State University Institutional Animal Care and Use Committee (permit nos. 2284, 2715, and 3158; to B.R.M.) and conducted under National Marine Fisheries Service research permits (nos. 841, 369-1440, and 369-1757; to B.R.M.). Tagging in Mexican waters was conducted under permits issued by the Secretarìa de Medio Ambiente y Recursos Naturales (nos. DOO 028319 and SGPA/DGVS 0576).
Environmental Data.
Because higher trophic levels are difficult to quantify over large spatial extents and timescales, satellite-based estimates of primary production have been used to examine spatiotemporal variability in food availability (83), including for secondary consumers (18, 54). We used chlorophyll-a concentration quantifying phytoplankton biomass (GlobColor merged product; 25-km spatial resolution; daily temporal resolution) to proxy food availability, as chlorophyll concentration is commonly related to zooplankton abundance and predator foraging behavior in marine systems (54, 84, 85), and in particular is a significant predictor of blue whale presence and foraging behavior (34, 44, 48–50, 52). Because secondary production lags primary production, and blue whale presence has been shown to lag chlorophyll peaks by 0 to 3 mo (34, 35, 49), we tested the relationship between blue whale presence and peak chlorophyll at lag times of 2 wk and 1, 2, and 3 mo, in addition to testing peaks in contemporaneous chlorophyll-a. We found that a 1-mo lag relative to peak chlorophyll-a best predicted blue whale use over the study area, as measured by linear regression slope (Statistical Analyses). To reflect long-term average conditions, we calculated the mean climatology (i.e., long-term average for each Julian day) from 1998 to 2010. The period of 1998 to 2010 was chosen because it encompasses the study period, and because chlorophyll data at the requisite extent and resolution are not available before September 1997.
Sea surface temperature data were obtained from AVHRR Pathfinder version 5.3 L3-Collated (25-km spatial resolution; daily temporal resolution). The majority of blue whale tracks stayed within a “goldilocks zone” of 15 to 17 °C throughout the annual migration cycle (Fig. 2B and SI Appendix, Fig. S3). The average SST experienced by blue whales during the northward migrations was 16.33 ± 2.00 °C, which is only slightly higher than the mean temperature they experience over the full year (15.69 ± 2.40 °C), consistent with other observations of the species (50, 86). We therefore examined blue whale use in relation to the median date that a location’s SST was within this goldilocks zone.
Because the seasonal green-up in the California Current is a nearshore phenomenon driven by interactions between alongshore winds, coastal topography, and subsurface nutrient conditions (42), we examined environmental layers within 200 km of the coast, where the majority of blue whale locations occurred (Fig. 1). Testing a narrower band (within 50 km of the coast) had negligible impacts on results.
Statistical Analyses.
Studies of resource tracking typically compare the date an animal uses a given location with the date of optimal resource availability at the same location (9–11, 18, 87). We used linear mixed-effects regression to evaluate the relationships between the date of blue whale use and the date of peak productivity (lagged by 1 mo), while controlling for SST, for (i) each year (to reflect current conditions), and (ii) the mean climatology. Individuals were nested as a random effect. We repeated analyses using tracks from the simulated random migrants. We tested for similarity between the distributions of contemporaneous, long-term mean, and long-term SD chlorophyll values at foraging locations and those available in the environment using the Bhattacharyya similarity coefficient, which ranges from 0 (no overlap in distributions) to 1 (perfect overlap), and tested for significance using Kolmogorov–Smirnov tests and Welch t tests. All spatial and statistical analyses were conducted in R 3.4.1 (88).
Supplementary Material
Acknowledgments
We thank the many people who assisted with tagging and tag development from the Marine Mammal Institute, as well as the various crews of the R/V Pacific Storm. We thank our organizations for supporting our time in writing this manuscript. We are grateful to Alexandre Zerbini and two anonymous reviewers for providing valuable comments that strengthened this manuscript. E.O.A. was supported by the Wyoming NASA Space Grant Consortium (NASA Grant NNX15AI08H). J.A.G. and M.S.S. were supported in part by a Terman Fellowship from Stanford University. Support for blue whale satellite tagging and data acquisition includes the National Geographic Society, Tagging of Pacific Pelagics program of the Census of Marine Life, Alfred P. Sloan Foundation, Moore Foundation, Packard Foundation, Office of Naval Research (Grants 9610608, 0010085, and 0310862), NASA (Grant NNX11AP71G), and private donors to Oregon State University’s Marine Mammal Institute endowment.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Movebank Data Repository (https://doi.org/10.5441/001/1.5ph88fk2).
See Commentary on page 5217.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819031116/-/DCSupplemental.
References
- 1.Avgar T, Street G, Fryxell JM. On the adaptive benefits of mammal migration. Can J Zool. 2014;92:481–490. [Google Scholar]
- 2.Holdo RM, Holt RD, Fryxell JM. Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti. Am Nat. 2009;173:431–445. doi: 10.1086/597229. [DOI] [PubMed] [Google Scholar]
- 3.Bauer S, Gienapp P, Madsen J. The relevance of environmental conditions for departure decision changes en route in migrating geese. Ecology. 2008;89:1953–1960. doi: 10.1890/07-1101.1. [DOI] [PubMed] [Google Scholar]
- 4.Middleton AD, et al. Green-wave surfing increases fat gain in a migratory ungulate. Oikos. 2018;20:741–749. [Google Scholar]
- 5.Albon SD, Langvatn R. Plant phenology and the benefits of migration in a temperate ungulate. Oikos. 1992;65:502–513. [Google Scholar]
- 6.Deacy WW, et al. Phenological tracking associated with increased salmon consumption by brown bears. Sci Rep. 2018;8:11008. doi: 10.1038/s41598-018-29425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong JB, Takimoto G, Schindler DE, Hayes MM, Kauffman MJ. Resource waves: Phenological diversity enhances foraging opportunities for mobile consumers. Ecology. 2016;97:1099–1112. doi: 10.1890/15-0554.1. [DOI] [PubMed] [Google Scholar]
- 8.van der Graaf SAJ, Stahl J, Klimkowska A, Bakker JP, Drent RH. Surfing on a green wave—How plant growth drives spring migration in the barnacle goose Branta leucopsis. Ardea. 2006;94:567–577. [Google Scholar]
- 9.Schindler DE, et al. Riding the crimson tide: Mobile terrestrial consumers track phenological variation in spawning of an anadromous fish. Biol Lett. 2013;9:20130048. doi: 10.1098/rsbl.2013.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deacy W, Leacock W, Armstrong JB, Stanford JA. Kodiak brown bears surf the salmon red wave: Direct evidence from GPS collared individuals. Ecology. 2016;97:1091–1098. doi: 10.1890/15-1060.1. [DOI] [PubMed] [Google Scholar]
- 11.Aikens EO, et al. The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecol Lett. 2017;20:741–750. doi: 10.1111/ele.12772. [DOI] [PubMed] [Google Scholar]
- 12.Drent RH, Ebbinge B, Weijand B. Balancing the energy budgets of arctic-breeding geese throughout the annual cycle: A progress report. Verh Ornithol Ges Bayern. 1980;23:239–264. [Google Scholar]
- 13.Bischof R, et al. A migratory northern ungulate in the pursuit of spring: Jumping or surfing the green wave? Am Nat. 2012;180:407–424. doi: 10.1086/667590. [DOI] [PubMed] [Google Scholar]
- 14.Merkle JA, et al. Large herbivores surf waves of green-up during spring. Proc Biol Sci. 2016;283:20160456. doi: 10.1098/rspb.2016.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesmer BR, et al. Is ungulate migration culturally transmitted? Evidence of social learning from translocated animals. Science. 2018;361:1023–1025. doi: 10.1126/science.aat0985. [DOI] [PubMed] [Google Scholar]
- 16.Fagan WF, et al. Spatial memory and animal movement. Ecol Lett. 2013;16:1316–1329. doi: 10.1111/ele.12165. [DOI] [PubMed] [Google Scholar]
- 17.Bracis C, Mueller T. Memory, not just perception, plays an important role in terrestrial mammalian migration. Proc Biol Sci. 2017;284:20170449. doi: 10.1098/rspb.2017.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorup K, et al. Resource tracking within and across continents in long-distance bird migrants. Sci Adv. 2017;3:e1601360. doi: 10.1126/sciadv.1601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polansky L, Kilian W, Wittemyer G. Elucidating the significance of spatial memory on movement decisions by African savannah elephants using state-space models. Proc Biol Sci. 2015;282:20143042. doi: 10.1098/rspb.2014.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merkle JA, Fortin D, Morales JM. A memory-based foraging tactic reveals an adaptive mechanism for restricted space use. Ecol Lett. 2014;17:924–931. doi: 10.1111/ele.12294. [DOI] [PubMed] [Google Scholar]
- 21.Bracis C, Gurarie E, Van Moorter B, Goodwin RA. Memory effects on movement behavior in animal foraging. PLoS One. 2015;10:e0136057. doi: 10.1371/journal.pone.0136057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mettke-Hofmann C, Gwinner E. Long-term memory for a life on the move. Proc Natl Acad Sci USA. 2003;100:5863–5866. doi: 10.1073/pnas.1037505100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller T, Fagan WF. Search and navigation in dynamic environments—From individual behaviors to population distributions. Oikos. 2008;117:654–664. [Google Scholar]
- 24.Tucker MA, et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science. 2018;359:466–469. doi: 10.1126/science.aam9712. [DOI] [PubMed] [Google Scholar]
- 25.Wilcove DS, Wikelski M. Going, going, gone: Is animal migration disappearing. PLoS Biol. 2008;6:e188. doi: 10.1371/journal.pbio.0060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fryxell JM, Sinclair AR. Causes and consequences of migration by large herbivores. Trends Ecol Evol. 1988;3:237–241. doi: 10.1016/0169-5347(88)90166-8. [DOI] [PubMed] [Google Scholar]
- 27.Teitelbaum CS, et al. How far to go? Determinants of migration distance in land mammals. Ecol Lett. 2015;18:545–552. doi: 10.1111/ele.12435. [DOI] [PubMed] [Google Scholar]
- 28.Martin J, et al. Common drivers of seasonal movements on the migration—Residency behavior continuum in a large herbivore. Sci Rep. 2018;8:7631. doi: 10.1038/s41598-018-25777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fryxell JM, Greever J, Sinclair A. Why are migratory ungulates so abundant? Am Nat. 1988;131:781–798. [Google Scholar]
- 30.Hays GC, et al. Key questions in marine megafauna movement ecology. Trends Ecol Evol. 2016;31:463–475. doi: 10.1016/j.tree.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Costa DP, Breed GA, Robinson PW. New insights into pelagic migrations: Implications for ecology and conservation. Annu Rev Ecol Evol Syst. 2012;43:73–96. [Google Scholar]
- 32.Hussey NE, et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science. 2015;348:1255642. doi: 10.1126/science.1255642. [DOI] [PubMed] [Google Scholar]
- 33.Block BA, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475:86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- 34.Croll DA, et al. From wind to whales: Trophic links in a coastal upwelling system. Mar Ecol Prog Ser. 2005;289:117–130. [Google Scholar]
- 35.Visser F, Hartman KL, Pierce GJ, Valavanis VD, Huisman J. Timing of migratory baleen whales at the Azores in relation to the North Atlantic spring bloom. Mar Ecol Prog Ser. 2011;440:267–279. [Google Scholar]
- 36.Fossette S, et al. Resource partitioning facilitates coexistence in sympatric cetaceans in the California Current. Ecol Evol. 2017;7:9085–9097. doi: 10.1002/ece3.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reilly SB, et al. 2008 Balaenoptera musculus. The IUCN Red List of Threatened Species. Available at www.iucnredlist.org/. Accessed October 1, 2018.
- 38.Bailey H, et al. Behavioural estimation of blue whale movements in the northeast Pacific from state-space model analysis of satellite tracks. Endanger Species Res. 2009;10:93–106. [Google Scholar]
- 39.Irvine LM, et al. Spatial and temporal occurrence of blue whales off the U.S. West Coast, with implications for management. PLoS One. 2014;9:e102959. doi: 10.1371/journal.pone.0102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballance LT, Pitman RL, Fiedler PC. Oceanographic influences on seabirds and cetaceans of the eastern tropical Pacific: A review. Prog Oceanogr. 2006;69:360–390. [Google Scholar]
- 41.Mate BR, Lagerquist BA, Calambokidis J. Movements of North Pacific blue whales during the feeding season off southern California and their southern fall migration. Mar Mamm Sci. 1999;15:1246–1257. [Google Scholar]
- 42.Foukal NP, Thomas AC. Biogeography and phenology of satellite-measured phytoplankton seasonality in the California Current. Deep Sea Res Part I. 2014;92:11–25. [Google Scholar]
- 43.Bograd SJ, et al. Phenology of coastal upwelling in the California Current. Geophys Res Lett. 2009;36:L01602. [Google Scholar]
- 44.Burtenshaw JC, et al. Acoustic and satellite remote sensing of blue whale seasonality and habitat in the northeast Pacific. Deep Sea Res Part II. 2004;51:967–986. [Google Scholar]
- 45.Goldbogen JA, et al. Mechanics, hydrodynamics and energetics of blue whale lunge feeding: Efficiency dependence on krill density. J Exp Biol. 2011;214:131–146. doi: 10.1242/jeb.048157. [DOI] [PubMed] [Google Scholar]
- 46.Hazen EL, Friedlaender AS, Goldbogen JA. Blue whales (Balaenoptera musculus) optimize foraging efficiency by balancing oxygen use and energy gain as a function of prey density. Sci Adv. 2015;1:e1500469. doi: 10.1126/sciadv.1500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiedler PC, et al. Blue whale habitat and prey in the California Channel Islands. Deep Sea Res Part II. 1998;45:1781–1801. [Google Scholar]
- 48.Hazen EL, et al. WhaleWatch: A dynamic management tool for predicting blue whale density in the California Current. J Appl Ecol. 2017;54:1415–1428. [Google Scholar]
- 49.Prieto R, Tobeña M, Silva MA. Habitat preferences of baleen whales in a mid-latitude habitat. Deep Sea Res Part II. 2017;141:155–167. [Google Scholar]
- 50.Gill PC, et al. Blue whale habitat selection and within-season distribution in a regional upwelling system off southern Australia. Mar Ecol Prog Ser. 2011;421:243–263. [Google Scholar]
- 51.Croll DA, et al. An integrated approch to the foraging ecology of marine birds and mammals. Deep Sea Res Part II. 1998;45:1353–1371. [Google Scholar]
- 52.Baines M, Reichelt M, Griffin D. An autumn aggregation of fin (Balaenoptera physalus) and blue whales (B. musculus) in the Porcupine Seabight, southwest of Ireland. Deep Sea Res Part II. 2017;141:168–177. [Google Scholar]
- 53.Pirotta E, et al. A dynamic state model of migratory behavior and physiology to assess the consequences of environmental variation and anthropogenic disturbance on marine vertebrates. Am Nat. 2018;191:E40–E56. doi: 10.1086/695135. [DOI] [PubMed] [Google Scholar]
- 54.Suryan RM, Santora JA, Sydeman WJ. New approach for using remotely sensed chlorophyll a to identify seabird hotspots. Mar Ecol Prog Ser. 2012;451:213–225. [Google Scholar]
- 55.Saborowski R, Salomon M, Buchholz RF. The physiological response of Northern krill (Meganyctiphanes norvegica) to temperature gradients in the Kattegat. Hydrobiologia. 2000;426:157–160. [Google Scholar]
- 56.Horton TW, et al. Route fidelity during marine megafauna migration. Front Mar Sci. 2017;4:422. [Google Scholar]
- 57.Abrahms B, et al. Climate mediates the success of migration strategies in a marine predator. Ecol Lett. 2018;21:63–71. doi: 10.1111/ele.12871. [DOI] [PubMed] [Google Scholar]
- 58.Sawyer H, Kauffman MJ. Stopover ecology of a migratory ungulate. J Anim Ecol. 2011;80:1078–1087. doi: 10.1111/j.1365-2656.2011.01845.x. [DOI] [PubMed] [Google Scholar]
- 59.Singh NJ, Grachev IA, Bekenov AB, Milner-Gulland EJ. Saiga antelope calving site selection is increasingly driven by human disturbance. Biol Conserv. 2010;143:1770–1779. [Google Scholar]
- 60.Fiechter J, Edwards CA, Moore AM. Wind, circulation, and topographic effects on alongshore phytoplankton variability in the California Current. Geophys Res Lett. 2018;45:3238–3245. [Google Scholar]
- 61.Santora JA, Sydeman WJ, Schroeder ID, Wells BK, Field JC. Mesoscale structure and oceanographic determinants of krill hotspots in the California Current: Implications for trophic transfer and conservation. Prog Oceanogr. 2011;91:397–409. [Google Scholar]
- 62.Santora JA, Zeno R, Dorman JG, Sydeman WJ. Submarine canyons represent an essential habitat network for krill hotspots in a large marine ecosystem. Sci Rep. 2018;8:7579. doi: 10.1038/s41598-018-25742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guttal V, Couzin ID. Social interactions, information use, and the evolution of collective migration. Proc Natl Acad Sci USA. 2010;107:16172–16177. doi: 10.1073/pnas.1006874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres LG. A sense of scale: Foraging cetaceans’ use of scale-dependent multimodal sensory systems. Mar Mamm Sci. 2017;104:2511–2524. [Google Scholar]
- 65.Maxwell SM, et al. Cumulative human impacts on marine predators. Nat Commun. 2013;4:2688. doi: 10.1038/ncomms3688. [DOI] [PubMed] [Google Scholar]
- 66.Berman-Kowalewski M, et al. Association between blue whale (Balaenoptera musculus) mortality and ship strikes along the California coast. Aquat Mamm. 2010;36:59–66. [Google Scholar]
- 67.Redfern JV, et al. Assessing the risk of ships striking large whales in marine spatial planning. Conserv Biol. 2013;27:292–302. doi: 10.1111/cobi.12029. [DOI] [PubMed] [Google Scholar]
- 68.Williams R, et al. Evidence for density-dependent changes in body condition and pregnancy rate of North Atlantic fin whales over four decades of varying environmental conditions. ICES J Mar Sci. 2013;70:1273–1280. [Google Scholar]
- 69.Chavez FP, Ryan J, Lluch-Cota SE, Niquen C M. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science. 2003;299:217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- 70.Fleming AH, Clark CT, Calambokidis J, Barlow J. Humpback whale diets respond to variance in ocean climate and ecosystem conditions in the California Current. Glob Change Biol. 2016;22:1214–1224. doi: 10.1111/gcb.13171. [DOI] [PubMed] [Google Scholar]
- 71.Palacios DM. Long-term and seasonal trends in stratification in the California Current, 1950–1993. J Geophys Res. 2004;109:307–312. [Google Scholar]
- 72.Rykaczewski RR, et al. Poleward displacement of coastal upwelling‐favorable winds in the ocean’s eastern boundary currents through the 21st century. Geophys Res Lett. 2015;42:6424–6431. [Google Scholar]
- 73.Sydeman WJ, et al. Climate change. Climate change and wind intensification in coastal upwelling ecosystems. Science. 2014;345:77–80. doi: 10.1126/science.1251635. [DOI] [PubMed] [Google Scholar]
- 74.Roemmich D, McGowan J. Climatic warming and the decline of zooplankton in the California Current. Science. 1995;267:1324–1326. doi: 10.1126/science.267.5202.1324. [DOI] [PubMed] [Google Scholar]
- 75.Di Lorenzo E, Miller AJ, Schneider N, McWilliams JC. The warming of the California Current System: Dynamics and ecosystem implications. J Phys Oceanogr. 2005;35:336–362. [Google Scholar]
- 76.Hazen EL, et al. Predicted habitat shifts of Pacific top predators in a changing climate. Nat Clim Chang. 2012;3:234–238. [Google Scholar]
- 77.Jacox MG, Hazen EL, Bograd SJ. Optimal environmental conditions and anomalous ecosystem responses: Constraining bottom-up controls of phytoplankton biomass in the California Current System. Sci Rep. 2016;6:27612. doi: 10.1038/srep27612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodson CB, Litvin SY. Ocean fronts drive marine fishery production and biogeochemical cycling. Proc Natl Acad Sci USA. 2015;112:1710–1715. doi: 10.1073/pnas.1417143112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mate BR, Palacios DM, Irvine LM, Follett TM. 2019 doi: 10.5441/001/1.5ph88fk2. “Behavioural estimation of blue whale movements in the northeast Pacific from state-space model analysis of satellite tracks.” Movebank Data Repository. Available at . . Deposited February 8, 2019. [DOI]
- 80.Bunnefeld N, et al. A model-driven approach to quantify migration patterns: Individual, regional and yearly differences. J Anim Ecol. 2011;80:466–476. doi: 10.1111/j.1365-2656.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- 81.Willis-Norton E, et al. Climate change impacts on leatherback turtle pelagic habitat in the southeast Pacific. Deep Sea Res Part II. 2015;113:260–267. [Google Scholar]
- 82.Jonsen ID, Myers RA, James MC. Identifying leatherback turtle foraging behaviour from satellite telemetry using a switching state-space model. Mar Ecol Prog Ser. 2007;337:255–264. [Google Scholar]
- 83.Pettorelli N, et al. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol Evol. 2005;20:503–510. doi: 10.1016/j.tree.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Bailey H, et al. Identification of distinct movement patterns in Pacific leatherback turtle populations influenced by ocean conditions. Ecol Appl. 2012;22:735–747. doi: 10.1890/11-0633. [DOI] [PubMed] [Google Scholar]
- 85.Boustany AM, Matteson R, Castleton M, Farwell C, Block BA. Movements of Pacific bluefin tuna (Thunnus orientalis) in the eastern North Pacific revealed with archival tags. Prog Oceanogr. 2010;86:94–104. [Google Scholar]
- 86.Whitehead H, McGill B, Worm B. Diversity of deep-water cetaceans in relation to temperature: Implications for ocean warming. Ecol Lett. 2008;11:1198–1207. doi: 10.1111/j.1461-0248.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 87.Lok EK, et al. Spatiotemporal associations between Pacific herring spawn and surf scoter spring migration: Evaluating a “silver wave” hypothesis. Mar Ecol Prog Ser. 2012;457:139–150. [Google Scholar]
- 88.R Core Team 2018 R: A Language and Environment for Statistical Computing. Version 3.5.1. Available at https://www.R-project.org/. Accessed July 2, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.