Significance
Mechanochemistry, the influence of molecular-scale mechanical forces on chemical processes, can occur on actively translating ribosomes through the force-generating actions of motor proteins and the cotranslational folding of domains. Such forces are transmitted to the ribosome’s catalytic core and alter rates of protein synthesis, representing a form of mechanical allosteric communication. These changes in translation–elongation kinetics are biologically important because they can influence protein structure, function, and localization within a cell. Many fundamental questions are unresolved concerning the properties of protein domains that determine mechanical force generation; the effect of translation speed on this force; and exactly how, at the molecular level, force is generated. In this study we answer these questions using molecular simulations and statistical mechanical modeling.
Keywords: cotranslational folding, mechanical force, coarse-grained simulations, translation speed
Abstract
The concomitant folding of a nascent protein domain with its synthesis can generate mechanical forces that act on the ribosome and alter translation speed. Such changes in speed can affect the structure and function of the newly synthesized protein as well as cellular phenotype. The domain properties that govern force generation have yet to be identified and understood, and the influence of translation speed is unknown because all reported measurements have been carried out on arrested ribosomes. Here, using coarse-grained molecular simulations and statistical mechanical modeling of protein synthesis, we demonstrate that force generation is determined by a domain’s stability and topology, as well as translation speed. The statistical mechanical models we create predict how force profiles depend on these properties. These results indicate that force measurements on arrested ribosomes will not always accurately reflect what happens in a cell, especially for slow-folding domains, and suggest the possibility that certain domain properties may be enriched or depleted across the structural proteome of organisms through evolutionary selection pressures to modulate protein synthesis speed and posttranslational protein behavior.
Mechanical forces acting on a nascent polypeptide chain can alter the speed of protein synthesis by the ribosome (1–7). Such changes in translation speed can influence the timing and efficiency of a variety of cotranslational processes affecting the fold (8, 9), function (10, 11), and intracellular localization of the newly synthesized protein (12, 13). One source of mechanical force is the cotranslational folding of individual nascent chain domains as they emerge from the ribosome exit tunnel (1). The process of folding is presumed to generate a pulling force on residues remaining in the exit tunnel (Fig. 1 A and B). That pulling force is transmitted 10 nm back to the catalytic core of the ribosome in a form of mechanical allosteric communication, altering the energy barrier to peptide bond formation (7), thereby influencing translation speed. The factors governing cotranslational force generation have not yet been identified, but they are important to understand as they can modulate translation speed, which can alter protein structure and affect the evolutionary selection pressures shaping the sequence and properties of both mRNA and proteins.
Fig. 1.
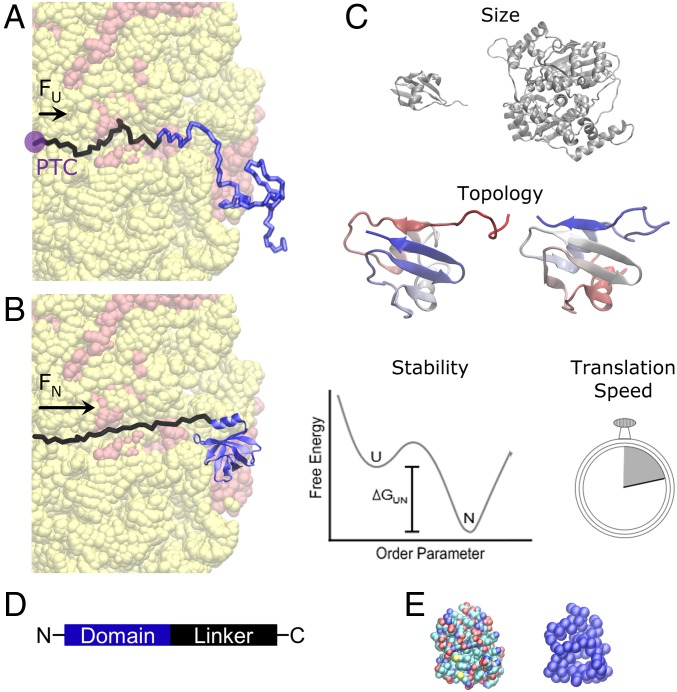
Cotranslational folding and the factors that might govern mechanical force generation. Cotranslational folding of a nascent protein domain (blue, in A and B) on the ribosome (red and yellow) has been implicated in generating a pulling force that is transmitted 10 nm through the exit tunnel to the peptidyl-transferase center. The domain’s unfolded (A) and folded state (B) each generate a pulling force that is transmitted 10 nm to the PTC of the ribosome and are denoted FU and FN, respectively. The difference between these two forces is the force generated by the act of domain folding (Eq. 6). Four factors may influence the extent of force generation: domain size, topology, stability, and translation speed (C). Differences in domain size are illustrated by a large and small domain, in gray. Differences in topology are illustrated by the rewiring of unstructured loops between secondary structural elements. (Note well, the colors of the domains shown under topology are from N terminus to C terminus, red to blue.) Native state stability is illustrated as a free energy difference, ∆GUN, between the unfolded (U) and folded (N) states in the free-energy profile as a function of a hypothetical order parameter. Translation speed is illustrated as a stopwatch, to metaphorically time how quickly ribosomes synthesize a protein. (D) Each domain in this study has an unstructured linker attached to its C terminus. (E) Illustration of an all-atom (Left) and coarse-grained representation (Right) of a protein domain.
Goldman et al. (1) estimate 12 pN of force is generated when the 106-residue-long Top7 protein folds on a translationally arrested ribosome. This force was estimated through a combination of experiments in which Top7’s midpoint folding force was measured off the ribosome, and the force required to relieve stalling by the arrest peptide SecM was measured in the absence of a folding domain (1). The relative strength of pulling forces generated by different proteins has also been measured using an expression assay comparing the amount of full-length protein produced to the amount of truncated protein produced due to translation stalling by SecM (3). If the pulling force is large enough at a given nascent chain length, it will overcome SecM-induced stalling and produce a full-length protein. Recent papers describe how forces measured in coarse-grained simulations can be used to generate estimates of the fraction of full-length protein (14, 15). Taken together, these studies demonstrate that different proteins produce different forces upon folding that can in turn alter translation speed.
Here we seek to identify what domain properties determine the magnitude of this pulling force and to test whether the forces measured in the artificial situation of translationally arrested ribosomes are the same as those that arise during continuous synthesis (Fig. 1C). To do this, we simulate the synthesis of five different Escherichia coli proteins by the ribosome using coarse-grained models. We find that three factors, the domain’s stability, topology, and translation rate, are the primary determinants of force generation and identify the conditions under which the forces measured on arrested ribosomes do not correspond to those measured on continually translating ribosomes. We also create a nonequilibrium statistical mechanical model that describes how the pulling force changes with codon translation speed, domain stability, and nascent chain length.
Results
Force Is Generated by Folding.
To examine whether folding generates a force on the catalytic core of the ribosome and to determine its range, we ran translationally arrested ribosome-nascent chain simulations at different nascent chain lengths for five different E. coli proteins: Protein thiS [Protein Data Bank (PDB) ID 1F0Z] (16), Pseudouridine synthase D (PDB ID 2IST) (17), Signal transduction protein PmrD (PDB ID 2JSO) (18), Protein yrbA (PDB ID 1NY8) (19), and Protein ybcJ (PDB ID 1P9K) (20). These proteins belong to the α/β structural class, and the simulated domains were assigned native-state stabilities typical of those found in experiment (Fig. 2) (21). An unstructured linker was attached to the C terminii of the domains to mimic domain folding in the context of a multidomain protein (Fig. 1D). We define linker residues as those nascent chain residues located after the C-terminal residue of the domain because they covalently link the folding domain to the P-site tRNA on the ribosome. The linker length is the number of residues composing the linker. The linker lengths used in these simulations were chosen such that they bracketed the length at which the midpoint of folding occurred. It was recently found that the unfolded state of a nascent chain can generate an entropic pulling force on the ribosome (7), and attractive interactions between the nascent chain and outer ribosome surface also have the potential to give rise to forces (22). To isolate the force arising from domain folding we ran additional simulations in which the domains always remain unfolded (SI Appendix) and simulated these folding-incompetent proteins on arrested ribosomes at the same linker lengths. The average pulling force at each linker length was then calculated as the average force at the C terminus of the nascent chain, which is located at the P site of the ribosome, minus the average force arising from the folding-incompetent protein (Eq. 6 in Methods).
Fig. 2.
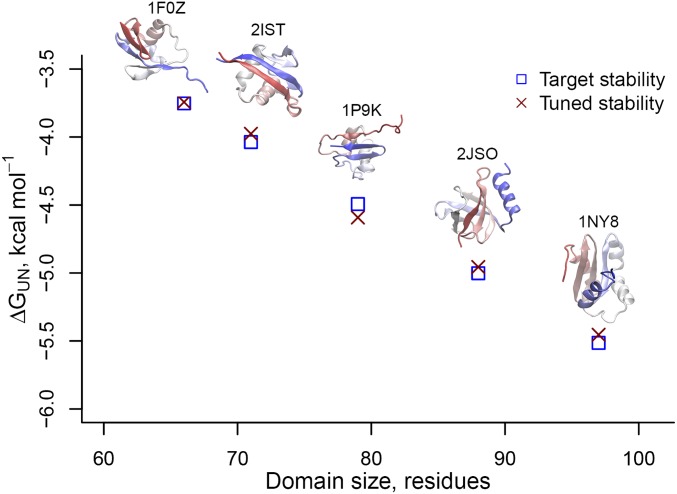
Setting a realistic energy scale for the domain’s coarse-grained force field. The free-energy difference between the native and unfolded state, , as a function of domain size, in units of residues, at 310 K, where is the gas constant; is the temperature; and and are the probabilities of being folded and unfolded, respectively. The target stabilities from the PREFUR algorithm (21) are shown as blue squares, and the free energy of each domain in the tuned simulation force field are shown as red crosses. The error bars (95% confidence intervals about the mean calculated from block averaging) for the calculated free energies are smaller than the size of the symbols. Stabilities are for five different proteins—1F0Z, 2IST, 1P9K, 2JSO, and 1NY8, as labeled. Their corresponding crystal structures are displayed in secondary structure format.
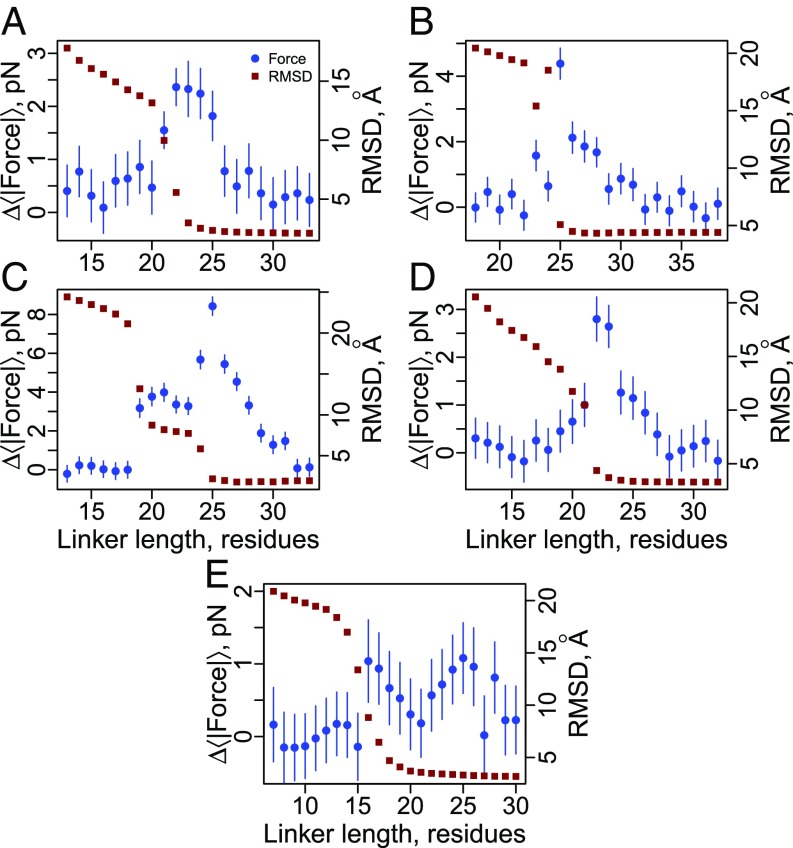
For all five proteins we observe that the average pulling force due to folding starts out at zero at short linker lengths, reaches a maximum near the midpoint of the folding transition of the domain, and decreases to zero again after the nascent chain elongates by just a few more residues (Fig. 3). For example, the pulling force for protein 1F0Z is at or near 0 pN below a linker length of 20 residues (blue circles, Fig. 3A), increases to a maximum of 2.5 pN at 22 residues, and then decreases back to 0 pN as the linker length increases to 29 residues. The force maximum at a linker length of 22 residues corresponds to the midpoint of folding as indicated by the inflection point in the average rmsd of the simulation structures from the crystal structure versus linker length profile (red squares, Fig. 3A). For these five proteins, we find the maximum folding force ranges between 1.0 and 8.4 pN at linker lengths between 16 and 26 residues and that the linker length locations of maximum force occur at or just after the greatest decrease in the rmsd from the crystal structure, i.e., the midpoint of folding.
Fig. 3.
Cotranslational folding generates piconewtons of force at the P site on translationally arrested ribosomes. Blue circles correspond to the average pulling force at the C-terminal nascent chain residue (Eq. 6) as a function of linker length for the five different proteins shown in Fig. 2: (A) 1F0Z, (B) 2IST, (C) 1P9K, (D) 2JSO, and (E) 1NY8. Red squares correspond to the average rmsd difference between domain structures sampled in the simulations and the folded crystal structure. Large rmsd values indicate the domain is unfolded; low values indicate it is folded. Note that 1NY8 folds noncooperatively, and the rmsd fails to report on the docking of the C terminus beginning at a length of 25 residues, which is seen when using the fraction of native contacts per secondary structural element as an order parameter (SI Appendix, Fig. S1E). All error bars represent 95% confidence intervals about the mean calculated from block averaging. Error bars on rmsd data points are smaller than the symbols.
Noncooperative Folding Leads to Skewed or Bimodal Force Profiles.
Three of the proteins exhibit a single maximum in their force profile (1F0Z, 2IST, and 2JSO in Fig. 3 A, B, and D), whereas protein 1P9K exhibits a shoulder (blue circles, Fig. 3C), and 1NY8 exhibits two maxima (blue circles, Fig. 3E). For the three single-peak proteins we observe they exhibit a cooperative, all-or-none folding transition in their rmsd profiles (i.e., a single inflection point; Fig. 3 A, B, and D), indicating that they do not populate any cotranslational folding intermediates, whereas 1P9K clearly exhibits noncooperative folding as indicated by two inflection points in its rmsd profile (red squares, Fig. 3C). Protein 1NY8 also exhibits two force peaks but has only one visible inflection point in its rmsd profile (red squares, Fig. 3E). As we show below, projecting the simulation trajectories of 1NY8 along other order parameters reveals it folds noncooperatively.
To determine the origin of the shape of these simulated pulling force profiles we analyzed for each protein the fraction of native contacts of each secondary structural element during synthesis. Consistent with the rmsd results, we find that there is cooperative formation of tertiary structure for proteins 1F0Z, 2IST, and 2JSO (i.e., all of the structural elements transition to the native fold at the same linker length), and partially folded intermediates are populated by 1P9K and 1NY8 (SI Appendix, Fig. S1). With this analysis we are able to identify that the folding intermediate of 1P9K consists of tertiary interactions between the three most N-terminal secondary structural elements (two α helices and a β strand), whereas the second transition consists of the docking of the most C-terminal β strand to form the folded state (SI Appendix, Fig. S1C). The partially folded intermediate of 1NY8 is composed of the six most N-terminal secondary structural elements (three α helices and three β strands in SI Appendix, Fig. S1E), and the second transition, at a linker length of 25 residues, involves the docking of the last C-terminal α helix to form the folded state. We emphasize that although the rmsd metric does not reflect this second transition (Fig. 3E), it is revealed using the fraction of native contacts formed by each secondary structural element as an order parameter (SI Appendix, Fig. S1E). In each case, these subdomain folding events correlate with an increase in force, indicating that partially folded intermediates can form and give rise to bimodal and asymmetric force profiles.
These results demonstrate that for these models, folding generates pulling forces on the piconewton scale 16 to 26 residues after synthesis of the most C-terminal structured residue in the domain and that formation of folding intermediates can also generate a force.
As a technical aside, because we are reporting the magnitude of the force (Eq. 6), it is possible that we are observing a compressive (pushing) rather than tensile (pulling) force. To confirm it is a tensile force, we calculated the average force vector at the C terminus of protein 2JSO at linker length 22, which yields a force vector of [33.2, −4.4, −13.2] pN. The angle formed between this vector and the long exit tunnel axis (which lies along the positive x axis) is 22°, demonstrating that this is a pulling force that is almost parallel with the tunnel and points toward the exit tunnel opening.
Protein folding in vitro is influenced by the stability of the domain, its topology, and its size (23, 24). In vivo, the speed of protein synthesis can also affect the cotranslational folding process (8, 10, 25–28). Therefore, we next sought to understand if these factors also influence force generation during cotranslational folding.
Domain Stability Influences Force Generation.
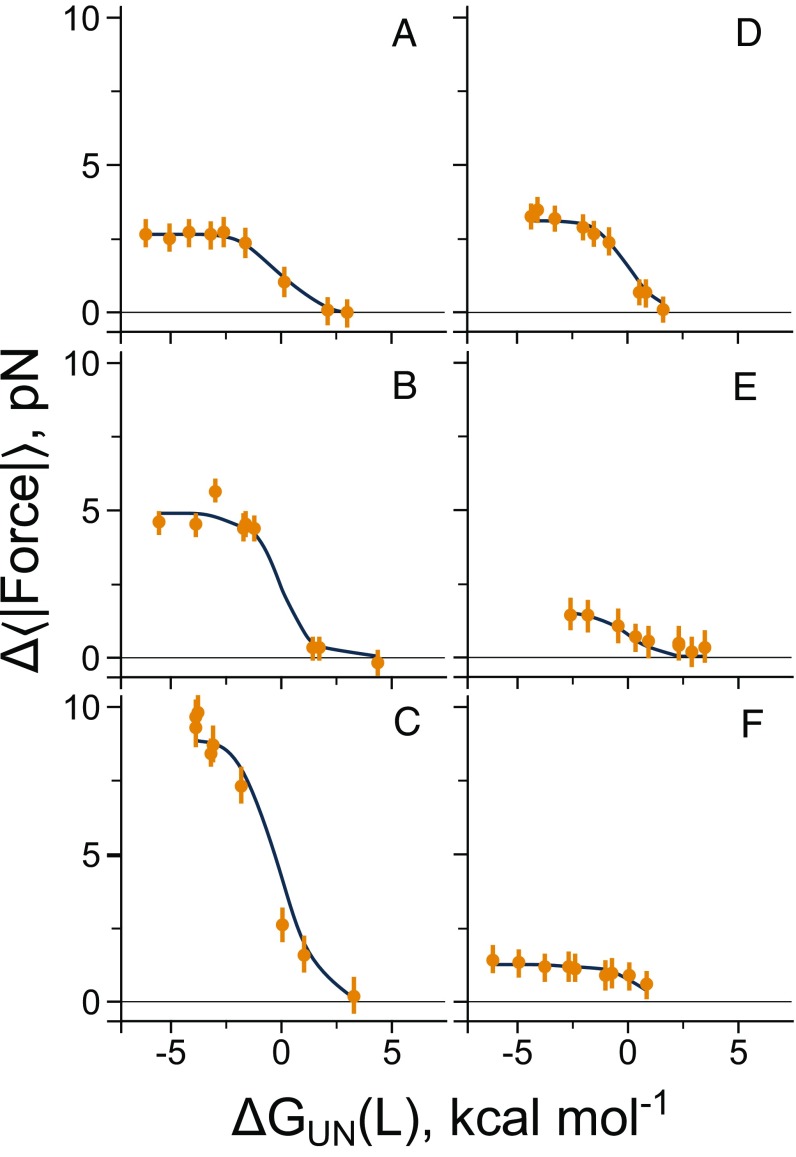
To study the influence of native state stability we altered the strength of the nonbonded interactions between residues that form contacts in the native state. Greater strength (i.e., a larger Lennard–Jones well depth; Eq. 3) results in a more stable folded state. This is analogous to experiments in which protein stability is modulated by changing solvent quality using denaturants and osmolytes. For each protein we created at least eight different native stabilities for the protein off the ribosome, ranging between −1 and −8 kcal/mol at 310 K. We then ran arrested ribosome simulations for each stability at the linker length that produced the largest force in the previous simulations (Fig. 3). Protein 1NY8 had a force profile with two maxima that are statistically indistinguishable; therefore, we ran simulations at both these linker lengths. We find that for all proteins the pulling force increases with increasing native-state stability, until it plateaus, where further increases in stability do not result in larger pulling forces at that linker length (orange circles, Fig. 4).
Fig. 4.
A statistical mechanical model describes how the pulling force changes with native-state stability at a fixed linker length. Pulling force (Eq. 6) versus native state stability for each of the five domains is plotted at a fixed linker length. Proteins (A) 1F0Z, (B) 2IST, (C) 1P9K, (D) 2JSO, (E) 1NY8, and (F) 1NY8 at linker lengths of 23, 25, 25, 22, 16, and 25 residues, respectively. Results from the arrested-ribosome simulations are shown as circles, whereas the fit to the statistical mechanical model (Eq. 1) is shown as a solid line. Error bars represent 95% confidence intervals about the mean calculated from block averaging.
To understand how the pulling force at a fixed linker length changes with native state stability we created a statistical mechanical model in which each domain can exist in one of two states—the native or unfolded state—and each state generates a characteristic pulling force that is independent of the native-state stability. With these assumptions it can be shown that the pulling force is (SI Appendix)
| [1] |
where and are the characteristic pulling forces of the native and unfolded states, respectively, at linker length ; is the probability the domain is in the folded state at linker length ; is the free energy difference between the native and unfolded states at linker length ; and where is the Boltzmann constant and is the temperature. Using as a fitting parameter, we see this equation reasonably describes how the pulling force changes with stability at a given linker length (solid lines, Fig. 4). [We also provide a generalization of Eq. 1 that describes the folding force when an arbitrary number of intermediates are populated (SI Appendix, Eq. S10).] In the limit of an unstable native state, i.e., the folded population goes to zero, , and therefore, . At the midpoint of stability, , and hence, is half its maximal value. Finally, when the folded state is very stable, i.e., , , and the force reaches an effectively constant value of .
Thus, a domain’s native state stability influences the magnitude of the pulling force at a fixed nascent chain length. Greater stability leads to greater pulling forces up to a point, beyond which further increases in stability negligibly alter the native state population, resulting in no further change in the force at that fixed linker length.
Domain Topology Influences Force Generation.
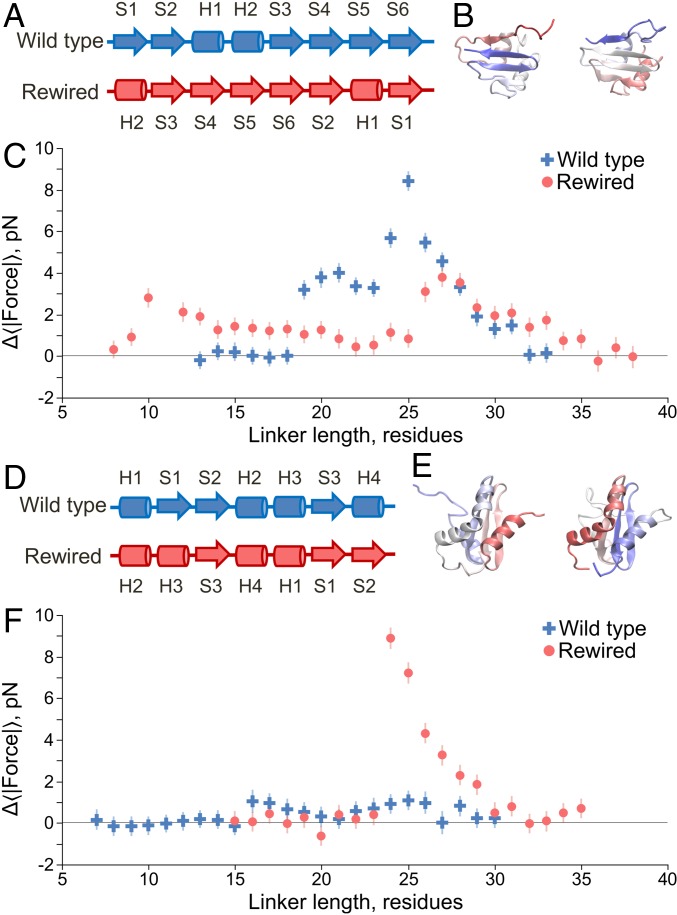
Next, we examined whether domain topology affects force generation. To isolate this effect, we need to control for domain size and stability. Therefore, we took the approach of rewiring the domains, i.e., reconnecting the loops between the secondary structural elements of the domain (29), and adjusted the strength of the nonbonded interactions in the simulation force field so as to maintain isostability between the original and rewired forms (SI Appendix). Domain size is maintained with this procedure as the total number of residues composing the domain stayed constant before and after rewiring; the ordering of the secondary structural elements is the only change. There are many possible ways to rewire the loops, and simulations on the ribosome are computationally expensive. Therefore, we first focused on rewiring one domain based on the hypothesis described below.
Protein 1P9K exhibits the largest folding force (Fig. 3C) and is the only domain in our set of proteins to have a β hairpin (i.e., two antiparallel β strands that are connected by a loop and form tertiary structure) at the C terminus of the domain (Fig. 5 A and B). This β-hairpin segment is the last portion of the domain to emerge from the exit tunnel during synthesis. We analyzed 1P9K’s cotranslational folding pathways and found that as the β hairpin folds (blue circles, SI Appendix, Fig. S2A), the more N-terminal β strand flips back toward the ribosome surface (black squares, SI Appendix, Fig. S2B) along with the rest of the domain to form contacts with the more C-terminal β strand. This process pushes the domain against the ribosome surface, as evidenced by the increased number of contacts between the domain and ribosome (red squares, SI Appendix, Fig. S2A), which we hypothesized caused this large pulling force. To test this hypothesis we rewired the loops to move the C-terminal β hairpin to the center of the domain (Fig. 5A), while maintaining the same folded-state stability off the ribosome. The average rmsd between the secondary structural elements in the original and rewired domain is 1.6 Å at 310 K; thus, the overall shape of the protein is not perturbed by rewiring. We find that moving the β hairpin in this way causes the maximum force to decrease from 8.5 to 3.8 pN (Fig. 5C), thus supporting our hypothesis. As a further test of this hypothesis we took protein 1NY8, which has a maximum force of 1.1 pN and no C-terminal β hairpin, and rewired its loops to create a C-terminal hairpin (Fig. 5 D and E) while maintaining its stability. We find the maximum force increases to 8.9 pN (Fig. 5F), again supporting our hypothesis.
Fig. 5.
Topology influences the pulling force, with C-terminal β hairpins giving rise to large forces. (A) Linear secondary structure representation, from N to C terminus, of the domain 1P9K. Arrows indicate β strands, and cylinders indicate α helices. The original wild-type structure is shown at the top, whereas the rewired mutant is shown below. The rewired mutant maintains the same secondary structure labels (H1, S1, etc.) as the wild type to illustrate how their relative positioning has changed along the primary structure due to rewiring. (B) Crystal structures of the wild-type (Left) and rewired (Right) domains, with the N terminii in red and the C terminii in blue. (C) Pulling force (Eq. 6) versus linker length for the wild-type (blue crosses) and rewired mutant (red circles) domains at 310 K. Moving the C-terminal β hairpin (formed by strands S5–S6) to the middle of the domain generates a lower maximum force in the rewired mutant. (D) Same as A except for protein 1NY8, which in the wild type has no C-terminal β hairpin but in the rewired mutant has one formed by strands S1–S2. (E) Same as B except for protein 1NY8. (F) Same as C but for protein 1NY8; wild-type results are blue crosses, and mutant results are red circles. Error bars represent 95% confidence intervals about the mean calculated from block averaging.
These results demonstrate that topology can influence the pulling force and that the presence of C-terminal β hairpins can generate relatively large forces.
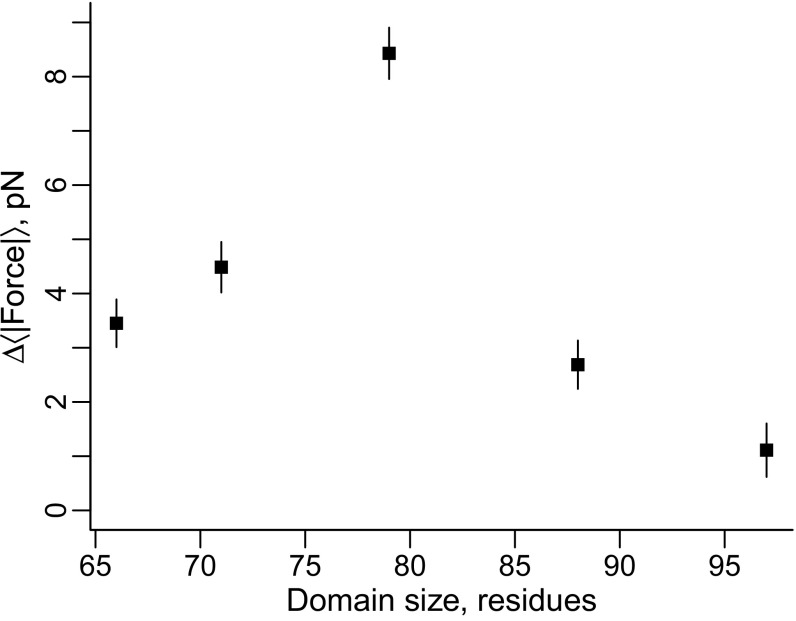
Domain Size Has No Apparent Effect.
Polymer theory models and experimental observation demonstrate that an entropic pulling force can be generated when an inert sphere is attached to the end of an unbranched polymer that is, itself, covalently attached to a surface (30, 31). This pulling force increases as the radius of the bead increases until its size becomes comparable to the polymer’s contour length, after which this force approaches a constant value (30). We examined whether a similar size-dependent force might arise due to the size of the folded domain (the sphere) attached to the linker (the polymer) that is attached in turn to the ribosome (the surface). To do this we controlled for stability effects by tuning all five domains to a native state stability of −4.5 kcal/mol off the ribosome and determined the maximum pulling force generated during cotranslational folding, again on arrested ribosomes. No correlation between force and domain size is found when domain size is defined as the number of residues in the domain (Fig. 6) nor by its radius of gyration (SI Appendix, Fig. S3), suggesting domain size has no effect. We emphasize that this result does not definitively rule out an effect from domain size. We have seen that domain topology can have significant effects on the magnitude of the pulling force. Because we did not control for differences in topology between domains, any contribution from domain size has the potential to be obscured. Additionally, the polymer model (30) predicts that for the domain sizes used in this study, there would only be a 0.25 pN force difference between the smallest and largest domain, a force that again could be obscured by topological differences.
Fig. 6.
The size of the domain has no apparent effect on the magnitude of the force. Pulling force versus domain size for the five different domains. No statistically significant trend is observed. Error bars represent 95% confidence intervals about the mean calculated from block averaging.
Translation Speed Influences Force Generation.
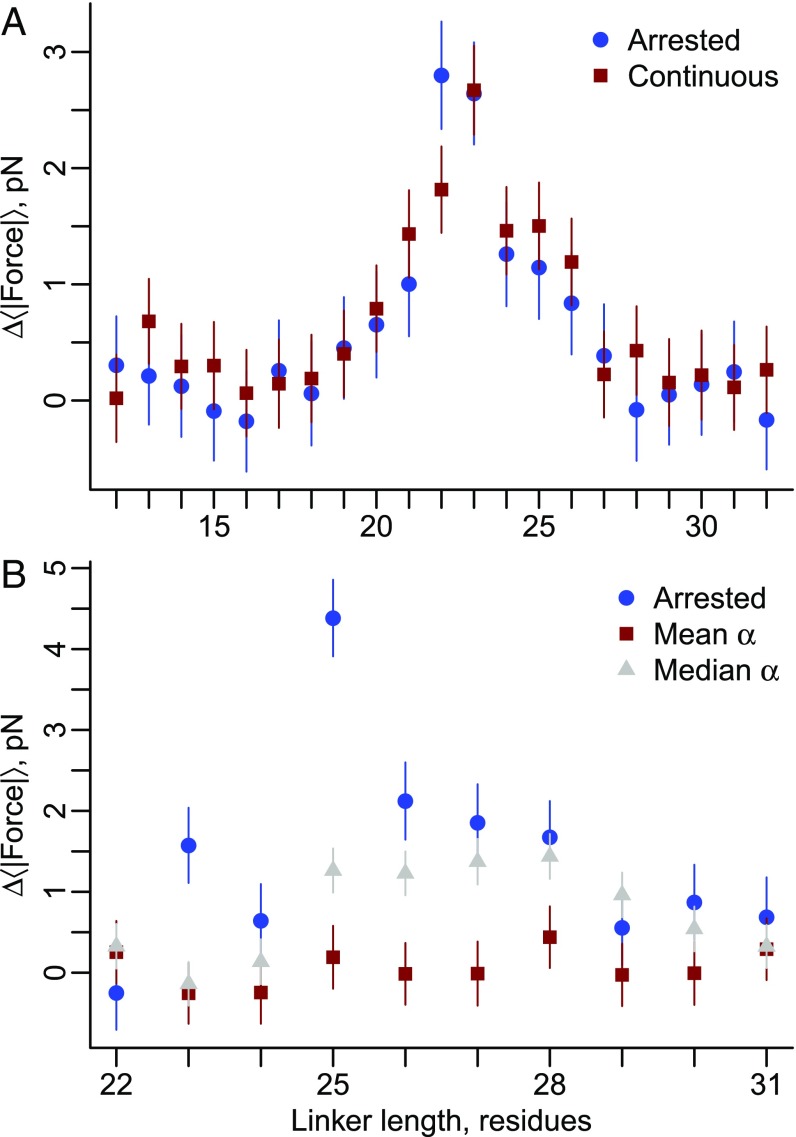
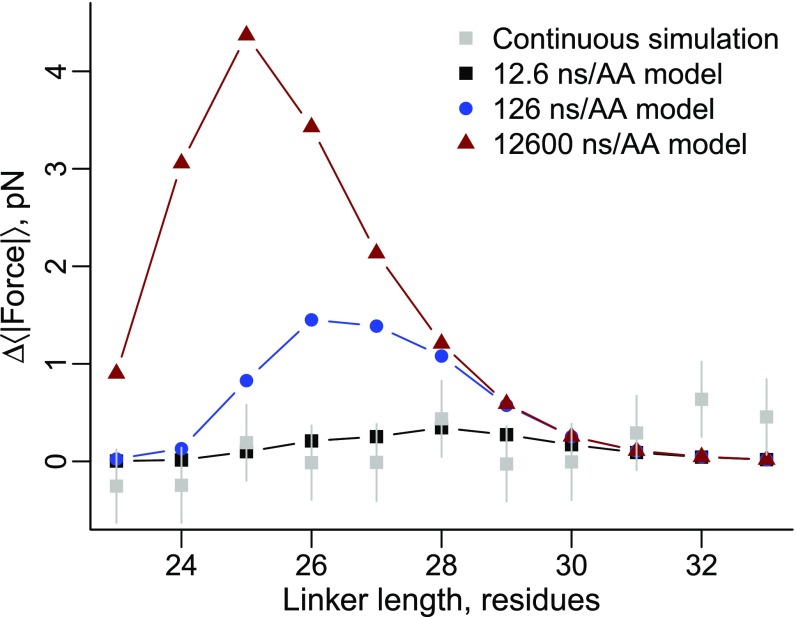
The results we have reported thus far are based on arrested ribosome simulations in which the cotranslational folding process is at equilibrium at each nascent chain length. Continuous synthesis, being irreversible, is a nonequilibrium process, and therefore, folding, and hence mechanical forces, have the potential to differ between arrested and continuous translation (25, 32). To test if the folding force differs between these two situations we ran continuous synthesis simulations of proteins 2JSO and 2IST (Fig. 7). We emphasize that although our coarse-grained simulations exhibit accelerated dynamics (33), we maintain a realistic ratio of time scales between domain folding and amino acid addition (Eq. 4), which allows us to estimate that 50 ms of experimental time corresponds to 12.6 ns of simulation time based on the average value of α from SI Appendix, Table S1 (see also Eq. 5). For 2JSO, the force profile obtained from continuous synthesis simulations is consistent with the force profile from the arrested ribosome-nascent chain simulations (Fig. 7A). Protein 2IST’s force profile, however, generated no force during continuous synthesis (red squares, Fig. 7B). When continuous translation was further slowed down by using the median value of α, the pulling force remained lower than that found on the arrested ribosome (gray triangles, Fig. 7B). Thus, translation speed can decrease or abolish the pulling force generated by proteins on arrested ribosomes.
Fig. 7.
Pulling forces generated on arrested ribosomes can differ from those undergoing active translation. (A) Pulling force versus linker length for protein 2JSO on arrested ribosomes (blue circles) and undergoing continuous synthesis (red squares). (B) Same as A except for protein 2IST and two translation rates, the mean α (red squares) and the median α (gray triangles). The force profiles arising from arrested and continuous synthesis are in good agreement for 2JSO, whereas for 2IST the pulling force decreases during continuous synthesis, and the extent of change depends on the exact translation rates. Error bars represent 95% confidence intervals about the mean calculated from block averaging (arrested simulations) or bootstrapping (continuous simulations). Using a Wilcoxon signed-rank test to compare the force profiles in this folding region, P < 0.05 between the arrested and median α, and P < 0.001 between the mean α and median α and between the arrested and mean α.
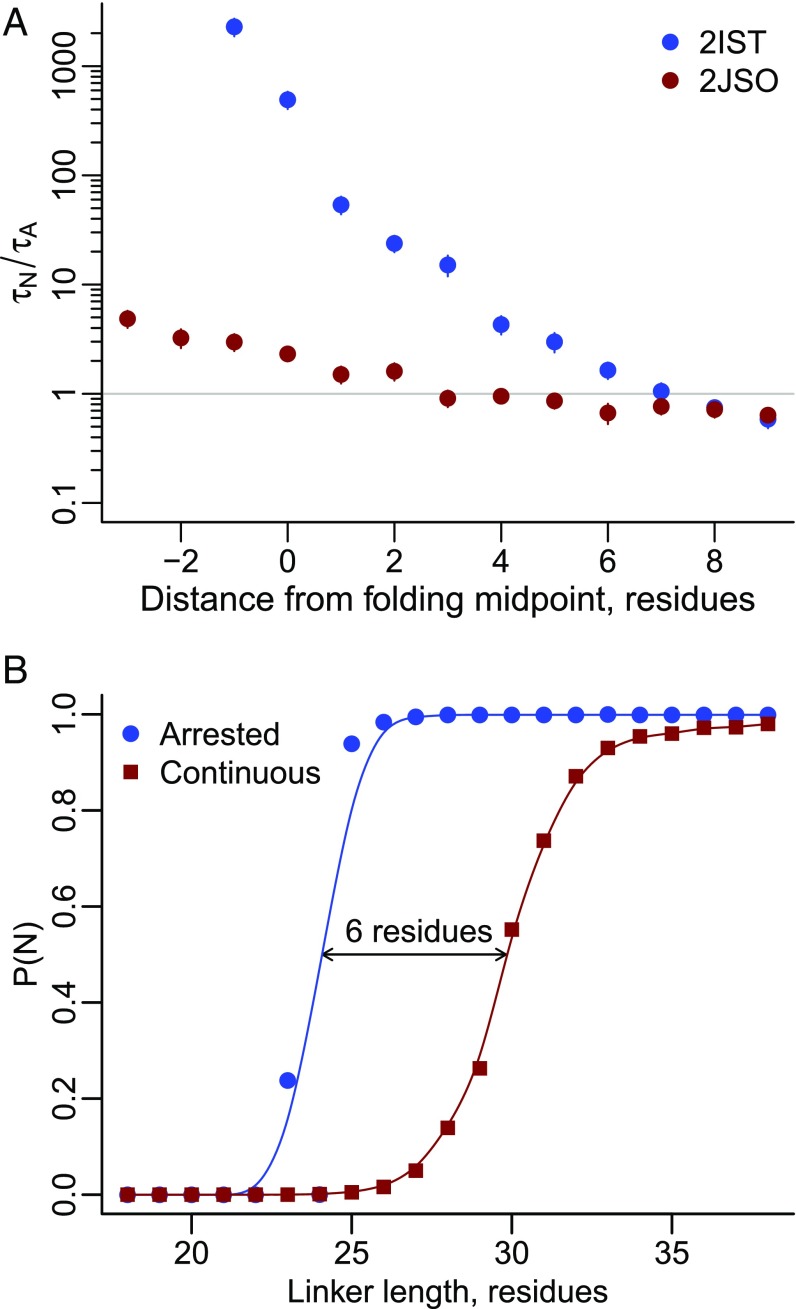
During continuous translation the cotranslational folding of a domain occurs at longer nascent chain lengths when the mean folding time of the domain () is longer than the time scale at which amino acids are added to the elongating chain () by the ribosome (25). We hypothesized that such a delay in folding was causing 2IST’s pulling force to decrease during continuous synthesis. To test this, we calculated the mean folding time of the 2IST and 2JSO proteins at a range of linker lengths that bracket the midpoint of folding in the arrested ribosome simulations. We find that although 2IST folds two times faster than 2JSO off the ribosome, on the ribosome, 2JSO’s ratio of is less than 1 at a majority of linker lengths (i.e., folding is faster than amino acid addition), whereas 2IST’s ratio is greater than 1 up to seven residues beyond the midpoint length of folding (Fig. 8A). This has the effect of shifting 2IST’s midpoint of folding to six residues later during continuous synthesis compared with on an arrested ribosome (Fig. 8B). This delay in folding means that when 2IST finally folds during continuous synthesis, it is no longer pushing against the ribosome surface and hence cannot generate a pulling force. This interpretation is supported from the simulations: at a linker length of 24 residues the folded state of 2IST is in contact with the ribosome surface 100% of the time, whereas at linker length 30 the folded state is only in contact 40% of the time.
Fig. 8.
A delay in cotranslational folding due to continuous synthesis causes 2IST’s pulling force to vanish. (A) The ratio of folding time to amino acid addition time versus linker length for proteins 2IST (blue circles) and 2JSO (red circles) as calculated from temperature quenching simulations on arrested ribosomes. Although 2IST folds faster in bulk solution, its folding rate slows at short linker lengths on the ribosome. Error bars represent 95% confidence intervals about the mean, calculated from bootstrapping. (B) Probability of folding versus linker length for protein 2IST on arrested ribosomes (blue circles) and undergoing continuous synthesis (red squares). The longer folding time for 2IST causes the protein to fold six residues later during continuous synthesis than on an arrested ribosome.
Thus, translation speed can influence force generation when the time scale of folding is longer than the amino acid addition time scale, which shifts the folding process from a quasi-equilibrium to a nonequilibrium regime (32). As little as a six-residue delay in folding can diminish or abolish force generation.
Statistical Mechanical Model Explains Trends in Force Profiles.
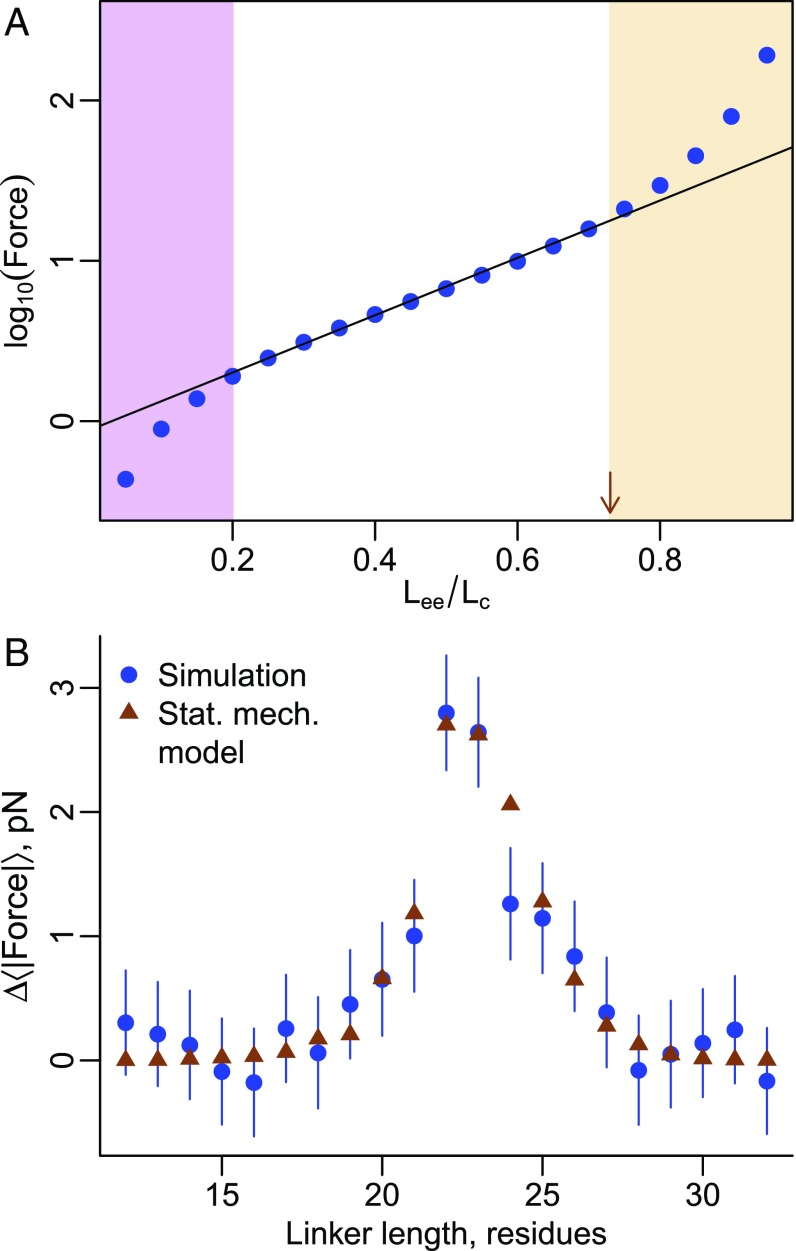
We demonstrated that a statistical mechanical model can describe how the pulling force changes with native-state stability at a given nascent chain length (Fig. 4). If we understand force generation during synthesis, we should be able to create a model that predicts the pulling force at all linker lengths () during arrested or continuous synthesis. To create this model (see SI Appendix for derivation) we calculate the intrinsic difference in force between the native (N) and unfolded () states and multiply it by the probability the domain is folded at a given length, , and by the conditional probability that given the domain is folded and touching the ribosome (), it does not have slack in the linker, . Based on a polymer physics model of a worm-like chain that exhibits a crossover to a high-force regime in its force versus extension curve (brown arrow, Fig. 9A) (34), we define slack as being present if the linker’s end-to-end distance, , is less than 73% of its contour length, (Fig. 9A). When is used as a fitting parameter, the product is consistent with the raw values calculated directly from the simulations. is related to the folding [] and unfolding [] rates of the domain and the codon translation rates [] at a given length by the function (35). The resulting equation is
| [2] |
We note that in the limit of infinitely slow translation [, i.e., an arrested ribosome], , where is the free energy as a function of linker length, and we get back that the force is a function of the native-state free energy at each length.
Fig. 9.
A statistical mechanical model describes how the pulling force changes with linker length on arrested ribosomes. (A) A force versus extension curve for a worm-like chain polymer with a persistence length of 9 Å and contour length (Lc) of 84 Å and confined to an inert cylinder with a 15-Å diameter at 310 K (34). The abscissa plots the end-to-end extension of the polymer (Lee) divided by its contour length. Low, intermediate, and high force regimes are present, with the line of best fit to the intermediate regime demarcating it from the high force regime at Lee/LC = 0.73 (red arrow). As noted in Methods, this value was used as a threshold. (B) The statistical mechanical model (Eq. 2) accurately describes the force versus linker length results from the simulations of protein 2JSO. Error bars represent 95% confidence intervals about the mean calculated from block averaging.
We first tested the accuracy of this equation by comparing its predicted force profile to that from the arrested ribosome simulations of protein 2JSO. Setting and plugging in , , , and , which were determined from the simulations (SI Appendix), we find that Eq. 2 reasonably describes 2JSO’s force profile (Fig. 9B).
Next, we tested how this equation performs in describing the continuous synthesis force profile from 2IST. Using the same codon translation rates as in the simulation, we find Eq. 2 correctly predicts 2IST will not produce a force at these rates (black squares, Fig. 10) and will produce a force when translation is very slow compared with folding (red triangles, Fig. 10). We illustrate in Fig. 10 (blue circles) that Eq. 2 can also predict force profiles at intermediate translation speeds.
Fig. 10.
A statistical mechanical model describes the influence of translation speed on force. We calculated folding probabilities for protein 2IST at a range of linker lengths for various amino acid addition rates and used the statistical mechanical model Eq. 2, to estimate the pulling force at each length and speed. The black squares represent the rate used in the continuous synthesis simulations, whereas the blue circles and red triangles represent one and three orders of magnitude faster, respectively. Error bars represent 95% confidence intervals about the mean, calculated from bootstrapping.
Thus, Eq. 2 is a nonequilibrium statistical mechanical model that can describe cotranslational folding force generation on both arrested and continuously translating ribosomes.
Discussion
Tensile forces acting on the nascent protein can alter the speed of translation elongation in a form of mechanical allosteric communication that links what happens outside the exit tunnel to the ribosome’s catalytic core (1–7). It has been found that protein folding on translationally arrested ribosomes can generate a pulling force that is transmitted through the nascent protein backbone to the peptidyl transferase center (PTC), which can alter peptide bond formation (1, 4, 7). Specifically, a previous study used quantum mechanics/molecular mechanics simulations to show that pulling forces applied to the PTC can decrease the free energy of the transition state barrier to peptide bond formation (7). The results from this coarse-grained study quantify this form of allosteric communication within the ribosome–nascent chain complex, identify the factors influencing the strength of this allosteric signal, and present statistical mechanical models to predict these forces. This tensile-force feedback mechanism may potentially be a common occurrence during protein synthesis because it is estimated that one-third of the E. coli proteome cotranslationally folds (25), and these results lay the ground work for future research exploring that possibility. The domain properties and series of molecular events that give rise to this force and the influence of continuous translation were previously unknown. Understanding the impact of these factors provides insight into this source of mechanochemistry on the ribosome and how it could influence translation speed and opens up avenues to understanding the evolutionary pressures that have shaped properties of domains.
In this study we examined four different factors that we hypothesized had the potential to influence mechanical force generation on the ribosome. Utilizing a coarse-grained simulation model and statistical mechanics, we demonstrated that a domain’s stability and topology, as well as translation speed, determine cotranslational folding force generation on the ribosome. A number of theoretical and experimental studies have demonstrated that the pulling force a polymer experiences as it is extruded through a pore is proportional to the change in free energy of the polymer during translocation (36–38). This is broadly consistent with our finding that the free energy of folding influences the pulling force.
Topology is another factor that governs the pulling force. We found that inserting and removing C-terminal β hairpins increased and decreased the pulling force, respectively. This is because to form a C-terminal β hairpin, the rest of the domain (if it is already folded) must flip back toward the ribosome surface and press against it. This topological effect suggests the possibility that the presence of C-terminal β hairpins in domains may have been naturally selected for in domain structures to modulate translation speed. Our study of five α/β proteins is not comprehensive, and therefore, other topological features may also have systematic effects on force. For example, the presence of C-terminal α hairpins might have a similar effect because a similar series of structural transitions might occur (SI Appendix, Fig. S2).
Translation speed was found to have no effect on the pulling force when a domain’s folding time was equal to or less than the codon translation time but diminished the force when folding was slower than the elongation time. It was previously shown that slow-folding domains fold at longer nascent chain lengths during continuous synthesis compared with the folding of those same domains on arrested ribosomes and that for 20% of cytosolic E. coli proteins, translational kinetics can switch their domains from folding cotranslationally to posttranslationally (25). We found that protein 2IST’s slow folding time caused it to fold at longer nascent chain lengths and that when it folded, it was no longer near the ribosome surface to press against it and generate a pulling force. This suggests two things: that for experiments, some of the force measurements on arrested ribosomes may not be relevant to continuous synthesis, especially if the domains fold slower than the codon translation times [i.e., slower than about 200 ms (39)], and that forces may generally tend to be lower during continuous synthesis. For example, 2IST’s force completely disappears during continuous synthesis simulations.
Due to limitations in experimental spatial resolution, the structural basis for force generation is unknown, although a number of hypotheses have been stated in the literature (1, 2, 35). Our simulations provide insight into the structural changes that occur concomitant with force generation, and our statistical mechanical modeling reveals the essential features. For example, if all we consider is the folding status of the domain during synthesis, then we cannot accurately model the force profiles seen in the simulations (blue circles, SI Appendix, Fig. S4). If we then speculate that we need to know not just whether the domain is folded but also when it is touching and pressing against the surface of the ribosome to generate a force, we still cannot accurately describe the force profile (orange diamonds, SI Appendix, Fig. S4). The reason for this is that there are many structures where the domain is folded and touching the surface but the linker has slack in it. It is only when we require that the domain be folded, be pressing against the surface, and have little slack in the linker that we see good agreement between the predicted force profile and the measured force profile (black squares, SI Appendix, Fig. S4). Thus, at the molecular level, forces are generated just as a domain emerges from the exit tunnel, begins to fold, and pushes against the ribosome surface, and there is little slack in the linker. All three of these structural features of the domain and linker are essential components of force generation.
We observed a maximum pulling force of between 1 and 9 pN. The only experimentally estimated pulling force was slightly higher at 12 pN for the protein Top7 (1). Several features of the Top7 protein help to explain why its force is larger. The Top7 domain is an engineered domain that is highly stable for its small size—the free energy of folding is −13.2 kcal/mol, which is more than twice the free energy of folding of our most stable domain (40). Additionally, the Top7 protein contains a β hairpin at its C terminus (40), just like our two highest force-producing constructs. Thus, a larger force should be expected for Top7 compared with the proteins we studied.
Two unexpected results from this study are the lack of any effect from domain size and the differing effect of the ribosome on folding rates. Larger domains were predicted to exhibit a greater pulling force due to the larger increase in entropy they can achieve by moving away from the ribosome surface. We observe no correlation, however, between the maximum force and domain size when we control for differences in domain stability. We emphasize that this result does not definitively rule out an effect from domain size. We have seen that domain topology can have significant effects on the magnitude of the pulling force. Because we did not control for topology in this comparison (because it is not possible to isotropically change a domain’s size), any contribution from domain size has the potential to be obscured by topological effects, especially in light of the force differences predicted by the polymer theory model (30). Less germane to the purpose of this study, we observed an example of a change in ordering of fast- and slow-folding proteins when they fold cotranslationally. Specifically, we observe that off the ribosome protein 2IST folds 2.2-fold faster than protein 2JSO. However, on the ribosome, protein 2JSO can fold 210-fold faster than protein 2IST (compare points at an abscissa value of 0 in Fig. 8A). This indicates differences in native state topologies can play an important role in folding kinetics on the ribosome.
A key technical point we wish to convey is the challenge of acquiring equilibrium results from the arrested ribosome simulations even for these relatively small domains. If the simulations are not at equilibrium, the calculated force can be inaccurately very high or low. A key test for equilibrium sampling in the replica exchange (REX) simulations used here is observing a constant number of transitions from the high to low temperature replicas in different portions of the simulation. For example, for protein 1F0Z at a linker length of 24 residues, we observe a constant number of transitions in the various deciles of the replica exchange simulation (SI Appendix, Fig. S5B). If there was nonequilibrium sampling, this quantity would exhibit a systematic drift with time. In our view, to help ensure accurate results, such a demonstration of equilibration should be required of any REX simulations from which forces or other thermodynamic averages are calculated.
We have used simulations and created statistical mechanical models to identify trends in the pulling force arising from various factors. Our results concerning the influence of domain stability, topology, and translation rate could be tested experimentally using expression assays comparing the amount of full-length protein produced to the amount of truncated protein produced due to translation stalling by SecM. To test stability, domains could be expressed in solvents of varying solvent quality (41), or point mutations could be introduced (42). Translation speed effects might be tested by introducing synonymous codon mutations in the mRNA (43), and altering the topology using circular permutation is a common technique in protein engineering (29, 44).
In summary, we have identified the factors determining mechanical force generation on the ribosome due to cotranslational protein folding, created statistical mechanical models that describe how force profiles depend on these factors, and described the series of molecular events giving rise to this force. In future studies it would be interesting to probe proteome-wide structural domain properties and transcriptome-wide codon usage bias to look for signatures of evolutionary selection acting on these properties and the biological benefit of this form of translation speed modulation through mechanochemistry.
Methods
Ribosome and Nascent Chain Models.
Five cytosolic E. coli proteins were randomly selected from the PDB with the requirements that they be of the α/β structural class, bind no ligands, have no posttranslational modifications, and are between 50 and 100 residues in length. This resulted in four single-domain proteins (PDB IDs 1F0Z, 1P9K, 1NY8, and 2JSO), whereas the fifth was a two-domain protein, but only the N-terminal domain was studied here (PDB ID 2IST, residues 2–72).
Coarse-grained structure-based models were created for each domain as previously described (45), where each amino acid is represented as a single interaction site centered on its α carbon and assigned a ±1 or 0 e− charge based on the overall charge of the amino acid at pH 7. Counter ion screening is represented implicitly using Debye–Hückel theory with a screening length of 10 Å. The starting structure for coarse-graining the 50S subunit of the E. coli ribosome was PDB ID 3UOS (46). Ribosomal proteins were coarse-grained in the same manner as the nascent proteins. Ribosomal RNA was represented as three or four interactions sites per nucleotide with a −1 charge assigned to the phosphate center, and for efficiency, the bulk of the ribosome is fixed in space and interacts with the nascent chain only via excluded volume and electrostatic interactions. Residues 42–59 of the ribosomal L24 protein were not fixed and allowed to fluctuate because these residues sit at the end of the exit tunnel and can affect cotranslational folding. The ribosome was trimmed in an umbrella shape, with the PTC at the base of the narrow section (SI Appendix, Fig. S6). Ribosomal beads within 10 Å of the walls of the exit tunnel and a large portion of the outer ribosomal surface surrounding the exit tunnel were included in the final structure, resulting in a coarse-grained ribosome structure consisting of 6,612 interaction sites.
All simulations were performed using Chemistry at Harvard Macromolecuar Mechanics (CHARMM) version c35b5 (47), which expresses the potential energy of a system as
| [3] |
where, from left to right, the summations are over bond, bond angle, dihedral, electrostatic, and van der Waals energy terms. Parameters for each energy term in Eq. 3 were taken from ref. 45. Note that the parameters for the first four terms are fully transferable between proteins, whereas the last term is structure-based and hence not transferable. For all simulations the system was propagated in time using Langevin dynamics with a friction coefficient of 0.05 ps−1 and an integration time step of 0.015 ps. To tune the native-state stability of each domain to the value predicted by the PREFUR method (21), in Eq. 3 was multiplied by a number η. A range of η values were tested for which the thermodynamic properties of each domain were calculated using REX simulations (48). At least 50,000 exchanges were attempted in these simulations that had at least eight temperature windows, with the first 10,000 exchanges discarded to allow for equilibration. Free energies of stability were calculated using the weighted histogram analysis method (WHAM) (49), and η was adjusted until the native-state stability in the model matched the desired stability.
Arrested Ribosome Simulations.
After each protein domain was tuned to its target stability, an unstructured peptide linker between 1 and 40 residues in length was covalently attached to the C terminus of the domain (SI Appendix, Table S2) (50), and the C terminus of the resulting nascent chain was harmonically restrained with a force of 10 kcal/mol/Å2 at the P-site location found in the crystal structure. This restraint was used because it allowed for the fastest equilibration of the force. This value should not affect the value of the pulling force because the pulling force is the difference of the magnitudes of the wild-type and destabilized versions of the domain, removing the influence of the restraint. For each wild-type sequence, REX simulations were carried out consisting of between 8 and 14 temperature windows and 300,000 attempted replica exchanges. For the destabilized proteins, only 100,000 exchanges were attempted because no phase transition can occur. The first 10,000 exchanges were discarded to permit equilibration. Error bars were computed for REX simulations by breaking each simulation into blocks of 50 exchanges and using the average from each block to compute 95% confidence intervals. Fifty exchanges per block were used because it resulted in uncorrelated data (51).
Mapping Simulation Time to Experimental Time.
Coarse-graining and low-friction Langevin dynamics accelerate processes. To map these accelerated dynamics to real-world time scales we simulated the folding of 18 proteins with our coarse-grained model. By comparing the simulated folding time to the experimentally measured value we determined the proportionality constant between the simulation and experimental time scales. Specifically, coarse-grained models, as previously described, were built for six α-helical (SI Appendix, Table S3), six β-sheet (SI Appendix, Table S4), and seven α/β proteins (SI Appendix, Table S5) whose lengths vary from 58 to 155 residues and for which free energy changes upon folding (52, 53) and mean folding times (52, 54–58) have been experimentally measured. Three to six REX simulations with various η values were carried out for each protein and the free-energy of folding computed for each value of η using the WHAM equations. The fraction of native contacts ( was used to differentiate folded and unfolded conformations. The threshold separating the and populations at equilibrium, , was identified as the value of at which the cumulative probability of at the model’s melting temperature () equals 0.5. The data for each protein were then fit to the equation , and the η that results in matching the experimental stability, denoted η*, was calculated as , where and are the slope and y axis intercept, respectively. All values of were computed at the temperature at which was obtained (). The values calculated by this method range from 0.998 to 1.628, with an average value of 1.235 (SI Appendix, Tables S3–S5).
CG models were constructed for all 19 proteins at their respective values. The mean time of folding for each protein, denoted , was then determined from temperature quench simulations. Five-hundred independent trajectories were run, with a 1,000-K equilibration period of between 20 ns and 2 µs followed by a quench to 310 K lasting between 100 ns and 10 μs. The durations of these two phases of the simulation were chosen such that the length of the 1,000-K equilibration was 20% the duration of the 310-K quench. Kinetic thresholds, denoted , were defined for each protein to differentiate the unfolded and folded ensembles during the quench period. This parameter was calculated as the arithmetic mean of and the most probable value of observed at 310 K in the REX simulation at the value of closest to η* for a given protein. We required that the folded state remain folded for at least 150 ps before the protein was considered to have folded at the initial frame at which . The time-dependent survival probability of the unfolded state was calculated as , where is the number of trajectories that have populated the folded state based on the above kinetic definition of folding at least once by simulation time and is the number of statistically independent trajectories included in the analysis. Survival probability curves for all proteins except ABP1 SH3 (PDB ID 1JO8) were fit to the equation using Python, where is the sole fitting parameter and the mean folding time is calculated as . The survival probability distribution for ABP1 SH3 was found to be best fit by the sum of two exponential terms; the fit equation for ABP1 SH3 was modified to the form , where . Curve fitting revealed that , , , and ; the value of reported in SI Appendix, Table S5, for ABP1 SH3 was calculated as , where and . Pearson values for all fits were ≥0.98. Note that very few trajectories for dihydrofolate reductase (PDB ID 1RX4) folded during 10 µs of quench, and therefore could not be calculated.
We maintain the experimentally observed ratio of a protein’s folding time to the time scale of amino acid addition by requiring that
| [4] |
in which we have introduced the parameter , which reports the relative acceleration of protein folding observed in our simulations compared with experiment. The , , and other relevant parameters for each of the 18 proteins are reported in SI Appendix, Table S1. The mean value of over the 18 proteins in the dataset was found to be . The average codon translation rate in E. coli increases from 8 to 22 amino acids per second as E. coli’s growth rate increases (59). Therefore, we set s (corresponding to 20 AA/s), yielding
| [5] |
That is, 12.6 ns of simulation time equals 50 ms of experimental time. We emphasize that the acceleration of folding we observe, and therefore this mean in silico translation time, is dependent on the specifics of our coarse-grained model (i.e., the force field, frictional coefficient, temperature, model resolution, transition state barrier height, etc.) and is thus not transferrable to other coarse-grained models. On a side note, bacterial translation rates are faster than eukaryotic translation rates due to their faster growth rates. For example, Saccharomyces cerevisae and human cells have translation rates between 5 and 9 amino acids per second (60, 61).
Continuous Synthesis Simulations.
For the continuous synthesis simulations, 1,100 independent trajectories were run for the wild-type and destabilized versions of proteins 2JSO and 2IST starting from a nascent chain length of 40 residues and ending at 138 and 121 residues, respectively. The same unstructured linker as in the arrested ribosome simulations was added to the C terminus of the domain and synthesized starting from 89 residues, for 2JSO, and 72 residues, for 2IST, which allowed the domains to fully emerge from the exit tunnel. Residues were added every 12.6 ns on average, with dwell times stochastically sampled from an exponential distribution. Error bars on average properties calculated from these simulation represent 95% confidence intervals computed from bootstrapping 10,000 times.
Analyses.
The , , and components of the force (, , ) from the harmonic restraint applied to the C-terminal bead of the nascent chain was calculated for each frame using CHARMM. Because the force is the negative gradient of the potential energy, and the potential energy of the restraint () is calculated with the equation , where is the current location and is the position of the restraint, each force component is calculated with the equation and then converted to pN (33). Thus, a 10-pN force applied along the x axis would cause a displacement of 0.014 Å because 1 kcal mol−1 Å−2 is equivalent to 69.5 pN. The magnitude of the force was then calculated as The average magnitude of the force and the backbone rmsds at 310 K were then calculated for each stability and linker length using the WHAM equations (49). To isolate the effect of cotranslational folding, the quantity was computed as
| [6] |
where is the average magnitude of the force, averaged over all of the saved configurations from the simulations, and is the average magnitude of the force of the destabilized version of the protein at that same linker length and temperature. In this way, the force arising solely from the folding is measured.
Temperature Quench Simulations.
Temperature quench simulations were performed by simulating the system at 800 K for 120 ns, then instantaneously quenching to 310 K for 1,500 ns or until the domain was folded. The time required to fold was defined by the first occurrence where the rmsd dropped below the threshold and remained below that threshold for 105 time steps or 1.5 ns. For each system at each nascent chain length, 100 trajectories were simulated. Error bars represent 95% confidence intervals computed from bootstrapping 10,000 times.
Supplementary Material
Acknowledgments
We thank Patrick Dudas for helping create Figs. 4, 5, and 7, and Liam Jackson and the E.P.O. laboratory members for a critical reading of the manuscript. We acknowledge funding from NSF Grant MCB-1553291 and from NIH Grant R35-GM124818.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813003116/-/DCSupplemental.
References
- 1.Goldman DH, et al. Ribosome. Mechanical force releases nascent chain-mediated ribosome arrest in vitro and in vivo. Science. 2015;348:457–460. doi: 10.1126/science.1261909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson OB, et al. Cotranslational protein folding inside the ribosome exit tunnel. Cell Rep. 2015;12:1533–1540. doi: 10.1016/j.celrep.2015.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marino J, von Heijne G, Beckmann R. Small protein domains fold inside the ribosome exit tunnel. FEBS Lett. 2016;590:655–660. doi: 10.1002/1873-3468.12098. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson OB, Müller-Lucks A, Kramer G, Bukau B, von Heijne G. Trigger factor reduces the force exerted on the nascent chain by a cotranslationally folding protein. J Mol Biol. 2016;428:1356–1364. doi: 10.1016/j.jmb.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Butkus ME, Prundeanu LB, Oliver DB. Translocon “pulling” of nascent SecM controls the duration of its translational pause and secretion-responsive secA regulation. J Bacteriol. 2003;185:6719–6722. doi: 10.1128/JB.185.22.6719-6722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomkiewicz D, Nouwen N, Driessen AJ. Pushing, pulling and trapping–Modes of motor protein supported protein translocation. FEBS Lett. 2007;581:2820–2828. doi: 10.1016/j.febslet.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Fritch B, et al. Origins of the mechanochemical coupling of peptide bond formation to protein synthesis. J Am Chem Soc. 2018;140:5077–5087. doi: 10.1021/jacs.7b11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komar AA. A pause for thought along the co-translational folding pathway. Trends Biochem Sci. 2009;34:16–24. doi: 10.1016/j.tibs.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Nicola AV, Chen W, Helenius A. Co-translational folding of an alphavirus capsid protein in the cytosol of living cells. Nat Cell Biol. 1999;1:341–345. doi: 10.1038/14032. [DOI] [PubMed] [Google Scholar]
- 10.Zhang G, Hubalewska M, Ignatova Z. Transient ribosomal attenuation coordinates protein synthesis and co-translational folding. Nat Struct Mol Biol. 2009;16:274–280. doi: 10.1038/nsmb.1554. [DOI] [PubMed] [Google Scholar]
- 11.Chang HC, Kaiser CM, Hartl FU, Barral JM. De novo folding of GFP fusion proteins: High efficiency in eukaryotes but not in bacteria. J Mol Biol. 2005;353:397–409. doi: 10.1016/j.jmb.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Pechmann S, Willmund F, Frydman J. The ribosome as a hub for protein quality control. Mol Cell. 2013;49:411–421. doi: 10.1016/j.molcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter P, Johnson AE. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- 14.Tian P, et al. Folding pathway of an Ig domain is conserved on and off the ribosome. Proc Natl Acad Sci USA. 2018;115:E11284–E11293. doi: 10.1073/pnas.1810523115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niesen MJM, Müller-Lucks A, Hedman R, von Heijne G, Miller TF., 3rd Forces on nascent polypeptides during membrane insertion and translocation via the Sec translocon. Biophys J. 2018;115:1885–1894. doi: 10.1016/j.bpj.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Xi J, Begley TP, Nicholson LK. Solution structure of ThiS and implications for the evolutionary roots of ubiquitin. Nat Struct Biol. 2001;8:47–51. doi: 10.1038/83041. [DOI] [PubMed] [Google Scholar]
- 17.Foster PG, Finer-Moore JS, Stroud RM. 2006 Data from “Crystal structure of RluD from E. coli.” RCSB PDB. https://www.rcsb.org/structure/2IST.
- 18.Fu W, et al. First structure of the polymyxin resistance proteins. Biochem Biophys Res Commun. 2007;361:1033–1037. doi: 10.1016/j.bbrc.2007.07.144. [DOI] [PubMed] [Google Scholar]
- 19.Swapna GVT, et al. 2004 Data from “Solution structure of protein yrbA from Escherichia coli: Northeast Structural Genomics Consortium target ER115.” RCSB PDB. https://www.rcsb.org/structure/1NY8.
- 20.Volpon L, et al. The solution structure of YbcJ from Escherichia coli reveals a recently discovered alphaL motif involved in RNA binding. J Bacteriol. 2003;185:4204–4210. doi: 10.1128/JB.185.14.4204-4210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sancho D, Muñoz V. Integrated prediction of protein folding and unfolding rates from only size and structural class. Phys Chem Chem Phys. 2011;13:17030–17043. doi: 10.1039/c1cp20402e. [DOI] [PubMed] [Google Scholar]
- 22.Knight AM, et al. Electrostatic effect of the ribosomal surface on nascent polypeptide dynamics. ACS Chem Biol. 2013;8:1195–1204. doi: 10.1021/cb400030n. [DOI] [PubMed] [Google Scholar]
- 23.Plaxco KW, Simons KT, Ruczinski I, Baker D. Topology, stability, sequence, and length: Defining the determinants of two-state protein folding kinetics. Biochemistry. 2000;39:11177–11183. doi: 10.1021/bi000200n. [DOI] [PubMed] [Google Scholar]
- 24.Naganathan AN, Muñoz V. Scaling of folding times with protein size. J Am Chem Soc. 2005;127:480–481. doi: 10.1021/ja044449u. [DOI] [PubMed] [Google Scholar]
- 25.Ciryam P, Morimoto RI, Vendruscolo M, Dobson CM, O’Brien EP. In vivo translation rates can substantially delay the cotranslational folding of the Escherichia coli cytosolic proteome. Proc Natl Acad Sci USA. 2013;110:E132–E140. doi: 10.1073/pnas.1213624110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siller E, DeZwaan DC, Anderson JF, Freeman BC, Barral JM. Slowing bacterial translation speed enhances eukaryotic protein folding efficiency. J Mol Biol. 2010;396:1310–1318. doi: 10.1016/j.jmb.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 27.Nissley DA, O’Brien EP. Timing is everything: Unifying codon translation rates and nascent proteome behavior. J Am Chem Soc. 2014;136:17892–17898. doi: 10.1021/ja510082j. [DOI] [PubMed] [Google Scholar]
- 28.Sharma AK, O’Brien EP. Increasing protein production rates can decrease the rate at which functional protein is produced and their steady-state levels. J Phys Chem B. 2017;121:6775–6784. doi: 10.1021/acs.jpcb.7b01700. [DOI] [PubMed] [Google Scholar]
- 29.Graf R, Schachman HK. Random circular permutation of genes and expressed polypeptide chains: Application of the method to the catalytic chains of aspartate transcarbamoylase. Proc Natl Acad Sci USA. 1996;93:11591–11596. doi: 10.1073/pnas.93.21.11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segall DE, Nelson PC, Phillips R. Volume-exclusion effects in tethered-particle experiments: Bead size matters. Phys Rev Lett. 2006;96:088306. doi: 10.1103/PhysRevLett.96.088306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milstein JN, Chen YF, Meiners JC. Bead size effects on protein-mediated DNA looping in tethered-particle motion experiments. Biopolymers. 2011;95:144–150. doi: 10.1002/bip.21547. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien EP, Ciryam P, Vendruscolo M, Dobson CM. Understanding the influence of codon translation rates on cotranslational protein folding. Acc Chem Res. 2014;47:1536–1544. doi: 10.1021/ar5000117. [DOI] [PubMed] [Google Scholar]
- 33.Klimov DK, Thirumalai D. Viscosity dependence of the folding rates of proteins. Phys Rev Lett. 1997;79:317–320. [Google Scholar]
- 34.Wang J, Gao H. Stretching a stiff polymer in a tube. J Mater Sci. 2007;42:8838–8843. [Google Scholar]
- 35.Sharma AK, Bukau B, O’Brien EP. Physical origins of codon positions that strongly influence cotranslational folding: A framework for controlling nascent-protein folding. J Am Chem Soc. 2016;138:1180–1195. doi: 10.1021/jacs.5b08145. [DOI] [PubMed] [Google Scholar]
- 36.Ismail N, Hedman R, Schiller N, von Heijne G. A biphasic pulling force acts on transmembrane helices during translocon-mediated membrane integration. Nat Struct Mol Biol. 2012;19:1018–1022. doi: 10.1038/nsmb.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail N, Hedman R, Lindén M, von Heijne G. Charge-driven dynamics of nascent-chain movement through the SecYEG translocon. Nat Struct Mol Biol. 2015;22:145–149. doi: 10.1038/nsmb.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikoofard N, Khalilian H, Fazli H. Directed translocation of a flexible polymer through a cone-shaped nano-channel. J Chem Phys. 2013;139:074901. doi: 10.1063/1.4818419. [DOI] [PubMed] [Google Scholar]
- 39.Fluitt A, Pienaar E, Viljoen H. Ribosome kinetics and aa-tRNA competition determine rate and fidelity of peptide synthesis. Comput Biol Chem. 2007;31:335–346. doi: 10.1016/j.compbiolchem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhlman B, et al. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302:1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]
- 41.Samelson AJ, Jensen MK, Soto RA, Cate JH, Marqusee S. Quantitative determination of ribosome nascent chain stability. Proc Natl Acad Sci USA. 2016;113:13402–13407. doi: 10.1073/pnas.1610272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan L, Lv Q, Zhang Y. STRUM: Structure-based stability change prediction upon single-point mutation. Bioinformatics. 2016;32:2911–2919. doi: 10.1093/bioinformatics/btw361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komar AA, Lesnik T, Reiss C. Synonymous codon substitutions affect ribosome traffic and protein folding during in vitro translation. FEBS Lett. 1999;462:387–391. doi: 10.1016/s0014-5793(99)01566-5. [DOI] [PubMed] [Google Scholar]
- 44.Marsden AP, et al. Investigating the effect of chain connectivity on the folding of a beta-sheet protein on and off the ribosome. J Mol Biol. 2018;430:5207–5216. doi: 10.1016/j.jmb.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien EP, Christodoulou J, Vendruscolo M, Dobson CM. Trigger factor slows co-translational folding through kinetic trapping while sterically protecting the nascent chain from aberrant cytosolic interactions. J Am Chem Soc. 2012;134:10920–10932. doi: 10.1021/ja302305u. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J, Lancaster L, Trakhanov S, Noller HF. Crystal structure of release factor RF3 trapped in the GTP state on a rotated conformation of the ribosome. RNA. 2012;18:230–240. doi: 10.1261/rna.031187.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks BR, et al. CHARMM: The biomolecular simulation program. J Comput Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugita Y, Okamoto Y. Replica-exchange dynamics method for protein folding. Chem Phys Lett. 1999;314:141–151. [Google Scholar]
- 49.Kumar S, et al. The weighted histogram analysis method for free-energy calculations on biomolecules. J Comput Chem. 1992;13:1011–1021. [Google Scholar]
- 50.Trovato F, O’Brien EP. Fast protein translation can promote co- and posttranslational folding of misfolding-prone proteins. Biophys J. 2017;112:1807–1819. doi: 10.1016/j.bpj.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frenkel D, Smit B. Understanding Molecular Simulation, from Algorithms to Applications. Academic; San Diego: 2002. [Google Scholar]
- 52.De Sancho D, Doshi U, Muñoz V. Protein folding rates and stability: How much is there beyond size? J Am Chem Soc. 2009;131:2074–2075. doi: 10.1021/ja808843h. [DOI] [PubMed] [Google Scholar]
- 53.Kumar MDS, et al. ProTherm and ProNIT: Thermodynamic databases for proteins and protein-nucleic acid interactions. Nucleic Acids Res. 2006;34:D204–D206. doi: 10.1093/nar/gkj103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wittung-Stafshede P, Gray HB, Winkler JR. Rapid formation of a four-helix bundle. Cytochrome b562 folding triggered by electron transfer. J Am Chem Soc. 1997;119:9562–9563. doi: 10.1073/pnas.96.12.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reid KL, Rodriguez HM, Hillier BJ, Gregoret LM. Stability and folding properties of a model β-sheet protein, Escherichia coli CspA. Protein Sci. 1998;7:470–479. doi: 10.1002/pro.5560070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Nuland NAJ, et al. Slow cooperative folding of a small globular protein HPr. Biochemistry. 1998;37:622–637. doi: 10.1021/bi9717946. [DOI] [PubMed] [Google Scholar]
- 57.López-Hernández E, Serrano L. Structure of the transition state for folding of the 129 aa protein CheY resembles that of a smaller protein, CI-2. Fold Des. 1996;1:43–55. [PubMed] [Google Scholar]
- 58.Raschke TM, Marqusee S. The kinetic folding intermediate of ribonuclease H resembles the acid molten globule and partially unfolded molecules detected under native conditions. Nat Struct Biol. 1997;4:298–304. doi: 10.1038/nsb0497-298. [DOI] [PubMed] [Google Scholar]
- 59.Young R, Bremer H. Polypeptide-chain-elongation rate in Escherichia coli B/r as a function of growth rate. Biochem J. 1976;160:185–194. doi: 10.1042/bj1600185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonven B, Gulløv K. Peptide chain elongation rate and ribosomal activity in Saccharomyces cerevisiae as a function of the growth rate. Mol Gen Genet. 1979;170:225–230. doi: 10.1007/BF00337800. [DOI] [PubMed] [Google Scholar]
- 61.Boström K, et al. Pulse-chase studies of the synthesis and intracellular transport of apolipoprotein B-100 in Hep G2 cells. J Biol Chem. 1986;261:13800–13806. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.