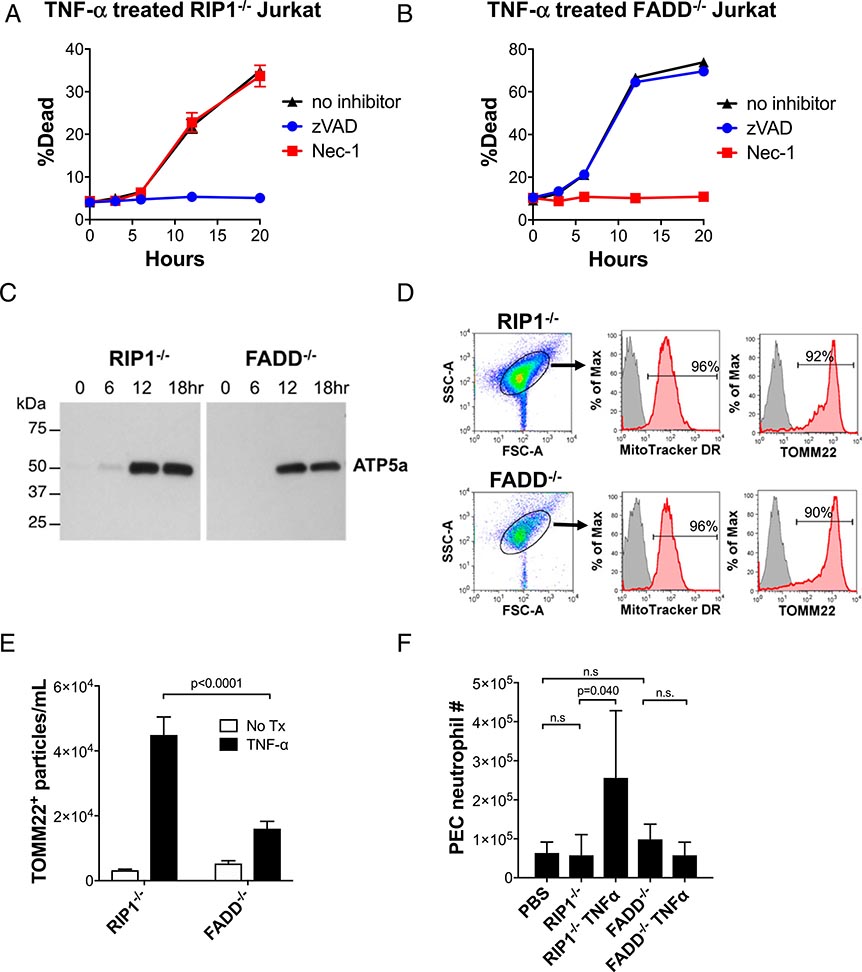
In Fig 6A and 6B, there was an inadvertent transposition of the graphs. The graph that appeared in panel (A) under the heading TNF-α treated RIP-1−/− Jurkat should have appeared in panel (B) under the heading TNF-α treated FADD−/− Jurkat and the graph that appeared in panel (B) under the heading TNF-α treated FADD−/− Jurkat should have appeared in panel (A) under the heading TNF-α treated RIP-1−/− Jurkat. A corrected version of Fig. 6 with the graphs under the correct headings is shown below. The figure legend was correct as published and is shown below for reference.
In addition, the first sentence of the Discussion section should start with “In this article…”
The figure and text have also been corrected in the online article.
FIGURE 6. Human T cells undergoing apoptosis and necroptosis release mitochondria, and apoptotic mitochondria recruit neutrophils.
To repeat our findings using human cells, RIP1−/− and FADD−/− Jurkat cell lines were used. (A and B) Treatment with TNF-α causes both cell lines to undergo cell death. Use of inhibitors of apoptosis (zVAD) and necroptosis (Nec-1) demonstrate that RIP1−/− Jurkat cells specifically undergo apoptosis and that FADD−/− Jurkat cells specifically undergo necroptosis in response to TNF-α treatment. (C and D) Mitochondria are released by apoptotic and necroptotic Jurkat cells. Following TNF-α treatment of the Jurkat cell lines, the cell-free SN microparticles were collected, and their protein lysates were examined by Western blot analysis for ATP5a, a mitochondria-specific protein. SN microparticles were then stained with a mitochondria-specific dye, MitoTracker DR, and with anti-TOMM22 and FACS analyzed. Red shading indicates microparticles stained with MitoTracker DR or TOMM2, indicative of mitochondria. Gray shading indicates unstained microparticles (negative control). Horizontal lines indicate percentage of microparticles staining positively for MitoTracker DR or TOMM2, respectively. (E) Quantification of TOMM22+ microparticles in the SN of TNF-α–treated Jurkat cell lines. (F) PEC neutrophil assay following i.p. mitochondria injection. C57BL/6 mice were injected with mitochondria (300 mg) purified from RIP1−/− or FADD−/− Jurkat cells that were untreated or treated with TNF-α. An equal volume of PBS (vehicle) was injected as a negative control. Eighteen hours after injection, PECs were harvested and analyzed for Ly6G+Ly6B.2+ neutrophils by FACS (n = 6 for all conditions). Comparisons were performed using a Mann–Whitney U test (E) or a Kruskal–Wallis with Dunn multiple comparison test (F). A p value < 0.05 was considered significant.