Abstract
Glucocorticoids are the leading cause of secondary osteoporosis. In the current study, the in vivo effects of Antarctic krill (Euphausia superba) oil (AKO) on dexamethasone-treated mice were investigated. Results showed that AKO significantly prevents bone loss, as evidenced by improved bone mineral density, biomechanical strength, and cancellous bone microstructure. Fluorescence double-labeling of femur showed that AKO induces new bone formation. Toluidine blue staining of marrow cavity indicated that AKO increases the number of trabecula, and decreases the generation of adipose cells. Runt-related transcription factor 2 (Runx2) and Peroxisome proliferator-activated receptor γ (PPARγ) are the switches for osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells, respectively. AKO significantly promoted the expression of Runx2 protein, and reduced PPARγ expression in bone tissue. Furthermore, AKO increased the mRNA expression of osteogenesis-related genes and decreased the expression of adipogenesis-related genes. In conclusion, AKO improved glucocorticoid-induced osteoporosis via promoting bone formation.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0463-5) contains supplementary material, which is available to authorized users.
Keywords: Antarctic krill oil, Dexamethasone, Bone formation, Runt-related transcription factor 2, Peroxisome proliferator-activated receptor γ
Introduction
Glucocorticoids (GCs) are widely applied clinically as immune-modulators for treating a variety of chronic diseases, such as rheumatoid arthritis, polymyalgia rheumatica and inflammatory bowel disease. However, long-term use of GCs leads to some adverse effects, including a high incidence of osteoporosis (Pasqualetti et al., 2015). GCs are the leading cause of secondary osteoporosis. They have been found to induce the destruction of bone microstructure, thereby accelerating bone loss and increasing the risk of fracture (Reid et al., 2009; van Staa et al., 2005).
GCs inhibit bone formation through their direct and indirect effects on osteoblasts, osteoclasts, and osteocytes (Weinstein et al., 2010). Loss of vitality and differentiation capacity of osteogenic cells is the principal cause of GC-induced bone degeneration. Osteoblasts are differentiated from bone marrow mesenchymal stem cells (BMSCs). GCs stimulate BMSCs differentiation into adipocytes instead of osteoblasts, resulting in the decreased osteogenesis (Li et al., 2013; Zhou et al., 2014).
Since the existing drugs for treating osteoporosis have adverse effects, it necessary to extract novel alternatives from natural products. Omega-3 polyunsaturated fatty acids (ω-3PUFAs) are a group of essential fatty acids, and they play important roles in the protection of bone metabolism (Bonnet and Ferrari, 2011). There are several biological pathways whereby ω-3 PUFAs regulate bone formation and resorption. First, ω-3 PUFAs could change the fatty acid composition of membrane phospholipids, specifically the biosynthesis of pro-inflammatory prostaglandin E2 which promotes bone resorption (Blackwell et al., 2010; Fetterman and Zdanowicz, 2009). In addition, the suppression of the production of other inflammatory cytokines, such as TNF-α, IL-1, and IL-6, helps ω-3 PUFAs to inhibit bone resorption and prevent bone loss (Appleton et al., 2011). Second, ω-3 PUFAs could promote bone formation by preventing the creation of products that inhibit osteoblastogenesis, such as lipid peroxide (Poulsen et al., 2007). Both ω-6 and ω-3 PUFAs can act on precursor cells of osteoblasts and adipocytes, but ω-3 PUFAs do not exhibit a strong adipogenic effect, thus, stimulating osteoblast differentiation (Casado-Diaz et al., 2013).
Antarctic krill (Euphausia superba) has an estimated biomass of 379 million tonnes, and it attracts more and more attention due to its high nutritional content (Atkinson et al., 2009). Two major ω-3PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoicacid (DHA), are abundant in the oil of Antarctic krill (Phleger et al., 2002). In the current study, oil was extracted from Antarctic krill, and its anti-osteoporotic activity was investigated on dexamethasone-treated mice for the first time.
Materials and methods
Materials and reagents
Tetracycline and calcein were purchased from Sigma-Aldrich (St. Louis, MO, USA). Moloney murine leukemia virus reverse transcriptase (M-MLV), random primers, dNTPs, and PageRuler prestained protein ladder were purchased from TaKaRa Bio Inc (Otsu, Shiga, Japan). FastStart Universal SYBR Green Master (Rox) came from Roche (Basel, Switzerland). Primers for bone sialoprotein (BSP), type I procollagen (Col1a), APETALA2 (ap2) and fatty acid binding protein 4 (FABP4) were synthesized by GENEWIZ (Suzhou, China). Primary rabbit anti-Runt-related transcription factor 2 (Runx2, #12556) and peroxisome proliferator-activated receptor-γ (PPARγ, #2345) were from Cell Signaling (Beverly, MA, USA). The goat anti-rabbit IgG-HRP secondary antibody was from proteintech (Catalog: SA00001-2, Chicago, IL, USA).
Preparation of Antarctic krill oil (AKO)
Freeze-dried Antarctic krill (purchased from China national fisheries CORP, Beijing, China) powder was extracted using 95% ethanol at 45 °C for 6 h (1:12, m/v). Then the supernatant was filtered and concentrated to obtain crude extract of AKO. 20% chitosan was added in AKO, and agitated at 40 °C for 60 min to remove free fatty acids. 10% β-cyclodextrin was added in AKO, and agitated at 40 °C for 30 min to remove cholesterol. Ceramic membrane filtration and molecular distillation technologies were applied to remove other impurities, and finally obtained high-quality AKO. It contained approximately 46.1% of phospholipids, 35.2% of triglycerides, 2.2% of cholesterol and 12.6% of free fatty acids. There were 26.72% of EPA and 18.31% of DHA in AKO.
Animals and experimental design
This study was approved by the Ethical Committee of Experimental Animal Care at Ocean University of China (Certificate number: SYXK20120014). Ten-week-old female C57BL/6J mice (n = 40, 20 ± 2.0 g) were purchased from Vital River Laboratory Animal Center (Beijing, China; license ID: SCXK2012-0001). The animals were housed four per cage at 22–24 °C with a 12-h light/12-h dark cycle and given food and water ad libitum. Thirty mice were randomly divided into three groups (n = 10 per group): Model group, alendronate sodium (ALF) group as positive control and AKO group. They were subcutaneous injected with dexamethasone sodium phosphate (10 mg/kg body weight) three times a week to establish a glucocorticoid-induced osteoporosis model. The remaining 10 mice were subcutaneously injected with physiological saline, serving as a control group. The injection volume was 1 mL/100 g body weight. At the same time, the ALF and AKO groups were intragastrically treated with alfacalcidol (0.15 μg/kg body weight) and AKO (160 mg/kg body weight), respectively. The control and model groups were both intragastrically treated with physiological saline. Animals in each group were given intragastric administration of physiological saline or respective drugs (1 mL/100 g body weight) once a day. After 90 days of treatment, serum was collected to measure bone turnover indicators. Femur and tibia were isolated immediately after sacrifice, followed by an evaluation of bone mineral density (BMD), biomechanical properties, bone microstructure, and gene expression.
Measurement of serum biochemical indicators
The bone formation indicators including bone morphogenetic protein 2 (BMP2), carboxy-terminal propeptide of procollagen type Ι (PICP), tartrate resistant acid phosphatase (TRAP) and C-telopeptide of type 1 collagen (CTX-1) were measured in serum using corresponding commercial ELISA kits (R&D, Minneapolis, MN, USA).
BMD and biomechanical property testing
BMD of femur was determined using a GK99-UNIGAMMA X-RAY PLUS Bone Densitometer (L’ACN, Napoli, Italy). Biomechanical property of tibia, including ultimate load and stiffness, were tested using a YLS-16A bone strength tester (Yiyan CORP, Jinan, China).
Histological analysis
The distal femur was fixed in 10% neutral formaldehyde for 24 h and decalcified in 10% ethylene diamine tetraacetic acid (EDTA) for 4 weeks. The decalcified tissue was then embedded in paraffin after dehydration with ascending grades of ethanol. The embedded samples were serially sectioned (4 µm thickness) and stained with hematoxylin and eosin (H&E) or toluidine blue. Finally, the tissue sections were observed and photographed using BH-2 microscope (Olympus, Tokyo, Japan) equipped with matching camera software.
Fluorescence double-labeling
Mice were treated with tetracycline (20 mg/kg body weight) and calcein (20 mg/kg body weight) by intraperitoneal injection 13 and 3 days before sacrifice, respectively. The distal femur was collected and decalcified in 10% EDTA for 3 h. After dehydration in ascending grades of ethanol, tissue was embedded in paraffin and serially sectioned at 10 μm thickness. Tissue sections were dewaxed in xylene for 1 h, followed immediately by the addition of fluorescence quenching agent in a dropwise manner. Finally, tissue sections were analyzed and photographed under a Leica DM2500 fluorescence microscope (Scanco Medical AG, Bassersdorf, Switzerland).
Real time PCR
Total RNA from the proximal femur was extracted using TRIzol reagent, and 1 μg of RNA was reverse transcribed into cDNA using M-MLV. Real time PCR analysis was performed on a Multicolor Real-Time PCR Detection System (Bio-RAD, Hercules, CA, USA). Sequences of the primers used in this study are listed in Table S1. Each real time PCR reaction was assembled in a total volume of 25 μL containing 12.5 μL of FastStart Universal SYBR Green Master, 5 μL of cDNA template, 0.75 μL of forward primer (10 μM), 0.75 μL of reverse primer (10 μM), and 6 μL of sterile Milli-Q water. The reaction program was set as follows: denaturation at 95 °C for 10 min, 45 cycles of 15 s at 95 °C, 20 s at 60 °C, 30 s at 72 °C, and a final extension at 72 °C for 10 min. Standard curves were constructed from PCR reactions using fivefold serial dilutions of mixture samples. The relative mRNA expression was calculated as the ratio of signal intensity of the target genes to that of β-actin.
Western blot
The proximal femur was ground into powder using liquid nitrogen. Next, 0.1 g of the powder was lysed in Western and IP lysis buffer (Applygen, Beijing, China) and centrifuged to obtain liquid supernatant. The obtained proteins were separated on a 10% SDS-PAGE gel and then electro-transferred onto polyvinylidene fluoride membrane. The membrane was blocked with 5% bovine serum albumin and incubated with primary antibodies overnight at 4 °C. Subsequently, the membrane was incubated with horseradish peroxidase conjugated IgG secondary antibody at indoor temperature for 2 h. Protein bands were visualized using an ECL stain kit (Applygen, Beijing, China) and quantified with the Image J program.
Statistical analysis
All data were subject to one-way analysis of variance (ANOVA) using the SPSS statistical program (Version 17.0). Significant differences among treatments were compared by least-significance-difference (LSD) method multiple range test. The results were presented as mean ± standard deviation. Values of p < 0.05 were considered as significant.
Results and discussion
AKO improved bone turnover
In order to evaluate the changes of bone turnover, bone formation markers BMP2 and PICP, as well as resorption markers TRAP and CTX-1 were measured in serum. As shown in Table 1, the bone formation activity in the model group was distinctly decreased compared to the control group, whereas the resorption activity was increased (p < 0.01). This undesirable reduction of bone turnover was significantly reversed by AKO treatment. AKO increased BMP2 and PICP by 5.38 and 10.83%, respectively, and decreased TRAP and CTX-1 by 7.64 and 12.48%, respectively.
Table 1.
Effects of AKO on bone turnover markers in serum
| Group | BMP2 (ng/mL) | PICP (ng/mL) | CTX-1 (ng/mL) | TRAP (U/L) |
|---|---|---|---|---|
| Control | 18.00 ± 0.48 | 17.88 ± 0.63 | 4.17 ± 0.17 | 30.56 ± 0.95 |
| Model | 16.00 ± 0.56aa | 14.87 ± 0.76aa | 5.85 ± 0.21aa | 36.53 ± 1.81aa |
| ALF | 16.84 ± 0.32aa,bb | 16.80 ± 0.79aa,bb | 4.94 ± 0.18aa,bb | 33.33 ± 1.71aa,bb |
| AKO | 16.86 ± 0.39aa,bb | 16.48 ± 0.56aa,bb | 5.12 ± 0.16aa,bb | 33.74 ± 1.63aa,bb |
Data are presented as the mean ± SD (n = 8 per group). Multiple comparisons were done using one-way ANOVA
aap < 0.01 versus control group
bbp < 0.01 versus model group
AKO Antarctic krill (Euphausia superba) oil
AKO inhibited bone loss and enhanced bone biomechanical strength
BMD is indicative of the bone loss status, thus, it is an effective means to diagnose osteoporosis (Cao et al., 2014). In this study, femur BMD was determined using dual energy X-ray absorptiometry (Fig. 1A). Results showed that dexamethasone administration resulted in a significant decrease of BMD in the model group compared to the control. However, the intervention with AKO clearly rescued the decreased BMD by 11.11%.
Fig. 1.
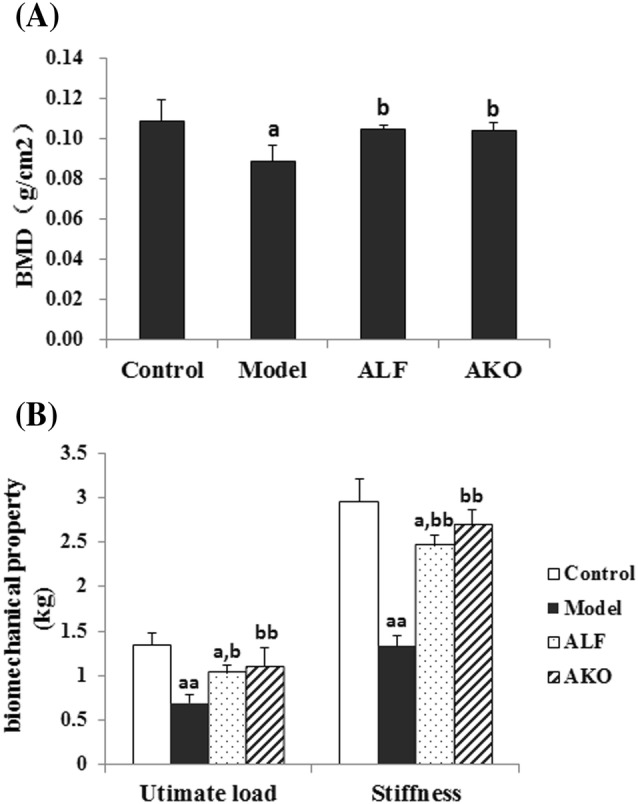
Effects of AKO on BMD and bone biomechanical properties. (A) BMD of mice. Femurs BMD were determined by dual energy X-ray absorptiometry. (B) Bone biomechanical property of mice. A three-point bending test was applied to tibiae for the evaluation of bone stiffness and ultimate load. Data are presented as the mean ± SD (n = 8 per group). Multiple comparisons were done using one-way ANOVA. ap < 0.05; aap < 0.01 versus control group; bp < 0.05; bbp < 0.01 versus model group
Excessive bone loss leads to the increased bone fragility. In the current study, biomechanical strength of tibia, including ultimate load and stiffness, was determined by a three-point bending test (Fig. 1B). Results indicated that the biomechanical strength in the model group was noticeably reduced compared to the control. Nevertheless, treatment with AKO significantly enhanced the biomechanical strength, as evidenced by increased ultimate load and stiffness by 59.42 and 102.26%, respectively.
AKO ameliorated cancellous bone microstructure
Bone histological analysis is commonly used to evaluate bone quality. Here, paraffin sections of the distal femur were stained with H&E stain to examine changes in cancellous microstructure. As shown in Fig. 2 that dexamethasone induced dramatic reduction of trabecular number and deterioration of trabecular structure when compared to the control. However, treatment with AKO remarkably recovered the undesirable changes in cancellous microstructure under growth plate.
Fig. 2.
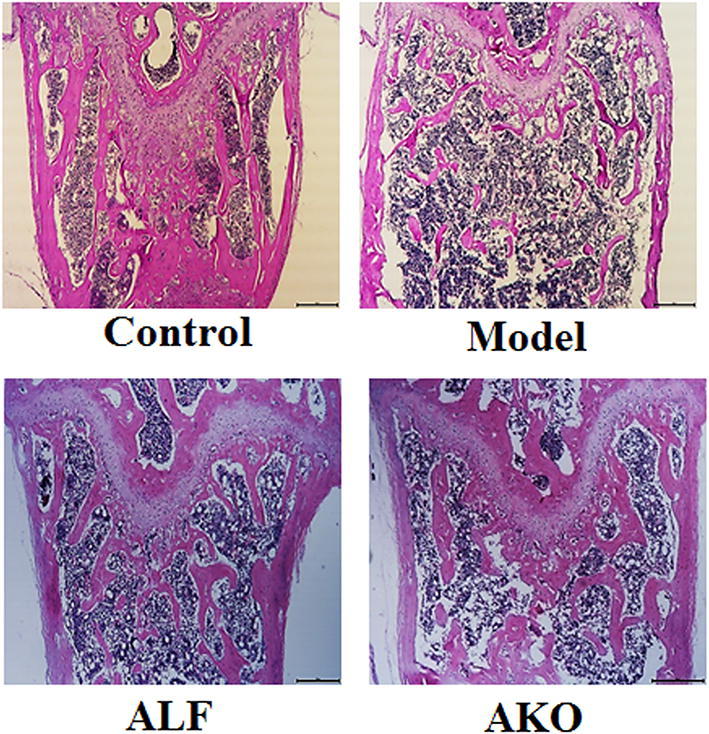
Effects of AKO on cancellous bone microstructure. The distal femur was embedded in paraffin after 4 weeks of decalcification. Paraffin sections of 4 µm thickness were stained with H&E and analyzed using BH-2 microscope equipped with a matching camera software (n = 6, ×4 magnification)
AKO induced new bone formation
Long-term treatment with glucocorticoids could directly induce the apoptosis of osteoblasts and osteocytes, impair the differentiation of osteoblasts, and prolong the life span of osteoclasts (Canalis, 2005; Weinstein et al., 2010; Yun et al., 2009). Deterioration of bone formation activity is a prominent reaction in glucocorticoid-induced osteoporosis (Wu et al., 2011). In the current study, tetracycline/calcein double labeling was performed to monitor dynamic bone formation in vivo (Fig. 3). Results showed that the distance between tetracycline and calcein labels in the model group was noticeably shorter than that in the control group, indicating that the rate of bone formation was reduced by dexamethasone. Treatment with AKO facilitated new bone formation, as evidenced by increased distance between both labels.
Fig. 3.
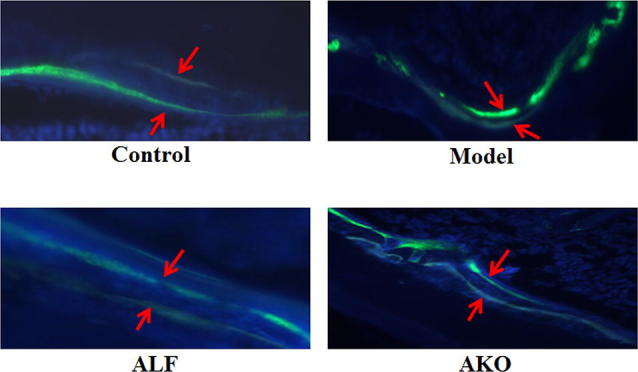
Effects of AKO on new bone formation. Mice were treated with tetracycline (20 mg/kg body weight) and calcein (20 mg/kg body weight) by intraperitoneal injection 13 and 3 days before sacrifice, respectively. The distal femur was collected and decalcified in 10% EDTA for 3 h. The decalcified tissue was embedded in paraffin and serially sectioned at 10 μm thickness. After dewaxing with xylene and addition of fluorescence quenching agent, the tissue sections were immediately analyzed under a fluorescence microscope (n = 6, × 40 magnification)
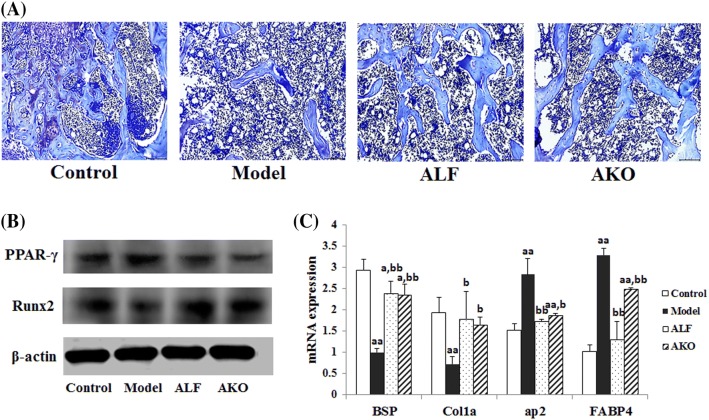
AKO facilitated the formation of trabecula and inhibited the generation of adipocyte in marrow cavity
Osteogenesis is mediated by recruiting BMSCs, and they can differentiate into osteoblasts. BMSCs exist mainly in bone marrow and subcutaneous fat, can differentiate into osteoblasts, adipocytes and chondrocytes (Fink et al., 2011; Mosna et al., 2010). Glucocorticoids disturb osteogenic differentiation capacity in bone tissue and stimulate BMSCs differentiation into adipose cells in marrow cavity (Rauch et al., 2010; Yao et al., 2008). Toluidine blue staining of bone marrow (Fig. 4A) showed that there were more adipocytes and fewer trabeculae in the model group compared to the control group. Correspondingly, the mRNA expressions of osteogenic marker genes BSP and Col1a were decreased, and the expression of adipogenic marker genes ap2 and FABP4 were increased in the model group (Fig. 4C). Nevertheless, AKO treatment remarkably increased the number of trabeculae in bone marrow. In addition, AKO decreased the mRNA expression of ap2 and FABP4 genes by 33.92 and 24.32%, respectively, and increased the expression of BSP and Col1a by 135.00 and 127.78%, respectively, when compared to the model group.
Fig. 4.
Effects of AKO on directional differentiation of BMSCs in bone tissue. The distal femur was embedded in paraffin after 4 weeks of decalcification. Paraffin sections of 4 µm thickness were stained with toluidine blue and analyzed using BH-2 microscope equipped with a matching camera software (n = 6, × 10 magnification). The proximal femurs were used for real-time PCR and Western blot experiments. (A) Toluidine blue staining of marrow cavity in the femur. (B) The expressions of Runx2 and PPARγ proteins. (C) The mRNA expressions of BSP, Col1a, ap2, and FABP4. Data are presented as the mean ± SD (n = 6 per group). Multiple comparisons were done using one-way ANOVA. ap < 0.05; aap < 0.01 versus control group; bp < 0.05; bbp < 0.01 versus model group
Osteogenic and adipogenic differentiation of MSCs is reversely regulated by critical differentiation regulators. Runx2 is the key transcription factor for BMSCs differentiation into osteoblasts, whereas PPARγ stimulates adipogenesis via inhibiting transcriptional activity of Runx2 (Jeon et al., 2003; Kawai and Rosen, 2010; Shi et al., 2000). The results in Fig. 4B showed that AKO significantly increased the expression of Runx2 protein but decreased PPARγ expression, suggesting that AKO promoted the directional osteoblastic differentiation of BMSCs in bone marrow.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The primers used for determination of mRNA expression (DOCX 15 kb)
Acknowledgements
This study is financially supported by Primary Research & Development Plan of Shandong Province (2016YYSP017), Key S&T Special Projects of Shandong Province (2015ZDZX05003) and National Key Research and Development Program (2017YFF0207800).
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
References
- Appleton KM, Fraser WD, Rogers PJ, Ness AR, Tobias JH. Supplementation with a low-moderate dose of n-3 long-chain PUFA has no short-term effect on bone resorption in human adults. Br. J. Nutr. 2011;105:1145–1149. doi: 10.1017/S0007114510004861. [DOI] [PubMed] [Google Scholar]
- Atkinson A, Siegel V, Pakhomov EA, Jessopp MJ, Loeb V. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep-sea Res. Pt. 2009;I(56):727–740. doi: 10.1016/j.dsr.2008.12.007. [DOI] [Google Scholar]
- Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol. Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet N, Ferrari SL. Effects of long-term supplementation with omega-3 fatty acids on longitudinal changes in bone mass and microstructure in mice. J. Nutr. Biochem. 2011;22:665–672. doi: 10.1016/j.jnutbio.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Canalis E. Mechanisms of glucocorticoid action in bone. Curr. Osteoporos. Rep. 2005;3:98–102. doi: 10.1007/s11914-005-0017-7. [DOI] [PubMed] [Google Scholar]
- Cao PC, Xiao WX, Yan YB, Zhao X, Liu S, Feng J, Zhang W, Wang J, Feng YF, Lei W. Preventive effect of crocin on osteoporosis in an ovariectomized rat model. Evid-based. Compl. Alt. 2014;2014:825181. doi: 10.1155/2014/825181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado-Diaz A, Santiago-Mora R, Dorado G, Quesada-Gomez JM. The omega-6 arachidonic fatty acid, but not the omega-3 fatty acids, inhibits osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: potential implication in osteoporosis. Osteoporosis Int. 2013;24:1647–1661. doi: 10.1007/s00198-012-2138-z. [DOI] [PubMed] [Google Scholar]
- Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health-Syst. Pharm. 2009;66:1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- Fink T, Rasmussen JG, Emmersen J, Pilgaard L, Fahlman A, Brunberg S, Josefsson J, Arnemo JM, Zachar V, Swenson JE, Frobert O. Adipose-derived stem cells from the brown bear (Ursus arctos) spontaneously undergo chondrogenic and osteogenic differentiation in vitro. Stem Cell Res. 2011;7:89–95. doi: 10.1016/j.scr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, Kim SY, Shin CS. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J. Biol. Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ. PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang N, Huang X, Xu J, Fernandes JC, Dai K, Zhang X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis. 2013;3:e832. doi: 10.1038/cddis.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19:1449–1470. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- Pasqualetti S, Congiu T, Banfi G, Mariotti M. Alendronate rescued osteoporotic phenotype in a model of glucocorticoid-induced osteoporosis in adult zebrafish scale. Int. J. Exp. Pathol. 2015;96:11–20. doi: 10.1111/iep.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phleger CF, Nelson MM, Mooney BD, Nichols PD. Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comp. Biochem. Phys. B. 2002;131:733–747. doi: 10.1016/S1096-4959(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Poulsen RC, Moughan PJ, Kruger MC. Long-chain polyunsaturated fatty acids and the regulation of bone metabolism. Exp. Biol. Med. 2007;232:1275–1288. doi: 10.3181/0704-MR-100. [DOI] [PubMed] [Google Scholar]
- Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schutz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–531. doi: 10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola T, Mesenbrink P, Sambrook PN. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- Shi XM, Blair HC, Yang X, McDonald JM, Cao X. Tandem repeat of C/EBP binding sites mediates PPARgamma2 gene transcription in glucocorticoid-induced adipocyte differentiation. J. Cell Biochem. 2000;76:518–527. doi: 10.1002/(SICI)1097-4644(20000301)76:3<518::AID-JCB18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. Qjm. 2005;98:191–198. doi: 10.1093/qjmed/hci029. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RW, Lin TP, Ko JY, Yeh DW, Chen MW, Ke HC, Wu SL, Wang FS. Cannabinoid receptor 1 regulates ERK and GSK-3beta-dependent glucocorticoid inhibition of osteoblast differentiation in murine MC3T3-E1 cells. Bone. 2011;49:1255–1263. doi: 10.1016/j.bone.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58:1674–1686. doi: 10.1002/art.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SI, Yoon HY, Jeong SY, Chung YS. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3beta. J. Bone Miner. Metab. 2009;27:140–148. doi: 10.1007/s00774-008-0019-5. [DOI] [PubMed] [Google Scholar]
- Zhou DA, Zheng HX, Wang CW, Shi D, Li JJ. Influence of glucocorticoids on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. BMC Musculoskel. Dis. 2014;15:1471–2474. doi: 10.1186/1471-2474-15-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The primers used for determination of mRNA expression (DOCX 15 kb)