Abstract
Background: Viscum album L (VA, mistletoe) extracts are commonly used in integrative oncology. Here the clinical safety profile of additional VA-treatments to standard care in cancer patients with preexisting autoimmune diseases was analyzed. Methods: In this observational cohort study medical data and recorded adverse events (AEs) of treated patients were retrieved from the Network Oncology registry and a safety analysis was performed. Results: A total of 106 patients (median age 63 years) treated with add-on VA-extracts were analyzed. Most frequent autoimmune diseases were Hashimoto’s thyroiditis (27%), psoriasis (19%), and ulcerative colitis (15%). Seventeen patients (16%) experienced VA-related AEs, but neither long-term side effects nor VA-therapy discontinuations were recorded. In a subgroup of 30 patients receiving long-term VA-therapy no exacerbations or flares of underlying autoimmune diseases were recorded. Additionally, a significant halving of overall AE-rates was observed during VA-treatment periods (p= 0.019). Conclusions: Our findings suggest that add-on VA-therapy in cancer patients with preexisting autoimmune diseases as Hashimoto’s thyroiditis, psoriasis, ulcerative colitis, Grave’s disease, and some rheumatic diseases is safe. No higher rates of VA-associated AEs were observed and the overall AE-rates were significantly lowered in VA-therapy periods. However, results should be interpreted with caution in light of the study’s observational character.
Keywords: autoimmune diseases, cancer, mistletoe, Viscum album L, safety analysis
Background
Effective and safe anticancer treatment still is a big challenge in modern medicine. Conventional therapies such as chemotherapy, radiation, targeted and immune therapy gained advances in tumor defense and overall survival but usually are associated with sometimes severe adverse events (AEs) including the induction of autoimmune reactions.1,2 Complementary and integrative medicines are becoming increasingly helpful for management of AEs in cancer treatment.3 Mistletoe (Viscum album L [VA]) therapy as an add-on therapy is among the most frequently used complementary treatment by oncologic patients in Europe.4 One mechanism underlying effects of VA treatment in cancer therapy is to stimulate the immune system and to support the elimination of tumor cells.5-7 Within integrative anthroposophic medicine settings, VA preparations are also integrated in the treatment concepts for other diseases.8 VA therapy has effectively been utilized with the intention to improve health-related quality of life.9-12 Safety profiles of VA therapy were assessed under standard clinical practice within an integrative setting in Germany, indicating that subcutaneous (s.c.), intravenous (i.v.), as well as intratumoral (i.t.) applications of VA treatments in cancer patients are safe.13-15
There is a bidirectional relationship between cancer and autoimmunity. Cancer has been implicated in some autoimmune disorders, and on the other hand, cancer risk is increased in patients with autoimmune diseases.16,17 For example, an increased incidence of malignant lymphocytic diseases is present in patients with rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, and autoimmune thyroid diseases,18 and in a recent review it was reported that the incidence of gastric neoplasms is higher in patients with autoimmune gastritis compared to the general population.19 Autoimmune diseases affect about 5% to 10% of the population in the developed countries20 and are more common in women than in men.21 Linkage studies revealed a clustering within families, and many patients are diagnosed with more than one autoimmune disease.20 An enhanced susceptibility to the development of cancer may in part be attributed to the immunosuppressive medications administered to autoimmune patients22 and an increase in the incidence of lymphoproliferative disorders and non-melanoma skin cancers with the use of immunosuppressive medications was registered.23 Cancer cells are able to gain control over a number of inhibitory pathways that are important for controlling immune responses and a major challenge of cancer therapy is immune resistance promoting tumor survival.24 The development of novel immunotherapies by targeting immunoregulatory pathways with immune checkpoint inhibitors (ICIs) are increasingly studied and used as successful therapy for a growing number of malignancies.2 Despite the obvious benefits of ICIs, these drugs affect multiple organ systems, and their use can be associated with immune-related adverse effects such as inflammatory arthritis, myositis, vasculitis, alveolitis, and Sicca syndrome, which require appropriate long-term management.25 Therefore, supportive therapies that can relieve immune-related toxicities might be of significant interest. For most clinical cancer trials, patients with preexisting autoimmune diseases are excluded, and likewise, VA therapy seems only possible to a limited extent for patients with allergic, atopic, or autoimmune disorders. So, in real practice, for cancer patients with autoimmune diseases and further comorbidities it is difficult to find suitable treatment options to relieve chronic complaints, alleviate associated disorders, and to minimize the possible plethora of AEs. Thus, real-world data contribute to a first picture of how VA therapy is applied and tolerated in cancer patients with underlying autoimmune disease.
The objective of the present cohort study was the analysis of the use and safety of add-on VA therapy for cancer patients with preexisting autoimmune comorbidities, within an integrative oncological setting. A VA long-term therapy subgroup was further analyzed to determine whether the rates and numbers of overall AEs in periods of VA therapy compared to VA-free therapy intervals were altered.
Methods
Study Design, Data Sources, and Participants
A nonrandomized, noncontrolled, monocentric, observational cohort study was performed within the Network Oncology (NO), a conjoint clinical registry of German hospitals specialized in anthroposophical medicine.26 All patients, seen between January 2011 and December 2017 at the Gemeinschaftskrankenhaus Havelhöhe, with a valid identification number, birth date, gender, cancer diagnosis date with ICD-10 code, recorded preexisting autoimmune diseases, and application of add-on VA, were included in this analysis. Exclusion criteria were the following: no written consent and no add-on VA-application. Disease stages of patients at first diagnosis were classified according to the Union for International Cancer Control (UICC) staging. The number of patients fulfilling all inclusion criteria determined the sample size. Follow-up was performed routinely and individually, depending on the courses of diseases and comorbidities.
Ethics Approval
This study is an observational cohort study of the NO registry. The NO registry has been approved by the ethical committee of the Medical Association Berlin (Eth-27/10). Patients gave written consent to be registered in the NO registry.
Data Collection
As described previously, documentation officers extract patient information, cancer diagnoses, comorbidities, oncological therapies, AEs, and disease progress from patient files and record data using the QuaDoSta (Quality management, Documentation and Statistics) software that was developed at Havelhöhe Research Institute.26 The following autoimmune diseases were taken into consideration and classified as follows: intestine diseases (ulcerative colitis [K51], Crohn’s disease [K50], Celiac disease [K90]), thyroid diseases (Hashimoto’s [E06] and Grave’s disease [E05.0]), rheumatic diseases (Sjögren’s syndrome [M35.3], Bechterew’s disease (ankylosing spondylitis [M45]), and further autoimmune diseases (psoriasis [L40], multiple sclerosis [G35], sarcoidosis [D86], lupus erythematosus [H01], vitiligo [L80]). AE reports were collected during visits at the Gemeinschaftskrankenhaus Havelhöhe and associated outpatient practitioners. All AEs were classified as preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA*) Version 15.0 and grouped by System Organ Classes (SOC). In terms of severity, AEs were evaluated according to the Common Terminology Criteria for AEs (CTCAE) v4.03.27 All documented data retrieved from the NO were analyzed retrospectively and compared with previous VA safety studies.13-15 The dates of occurrence and numbers of frequencies, along with demographic and diagnosis data, were extracted for each patient included in this study. Details of VA treatments, including dates, doses, routes of administration, and associated adverse effects, were also recorded and evaluated. All VA-related AEs were evaluated, classified, and documented as described in detail previously.13-15
Classification of Groups
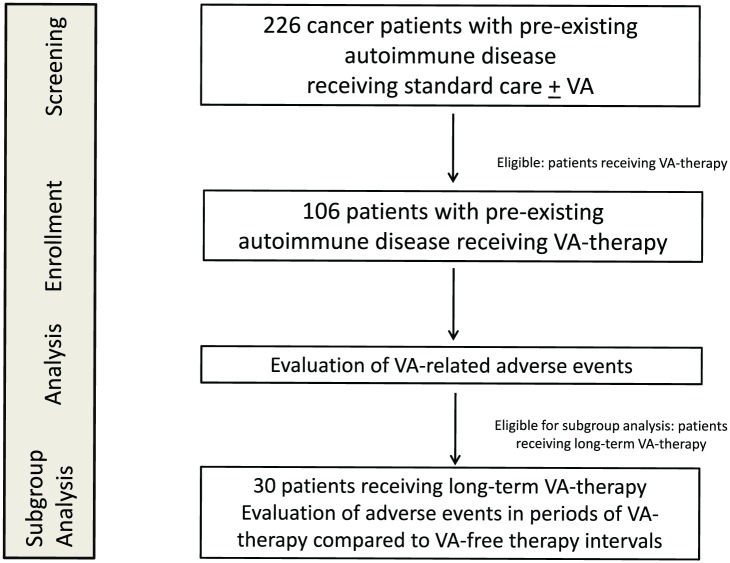
All cancer patients with preexisting autoimmune comorbidities received add-on VA therapy of different preparations and doses. Eligible patients for the VA long-term-subgroup received i.v., i.t., or high s.c. VA applications over a period of at least 6 months. High s.c. VA-applications were preparations from Abnoba >0.2 mg/mL, Iscador ≥1 mg/mL, or Helixor ≥10 mg/mL. All patients receiving shorter than 6 months or only low s.c. VA applications were excluded from subgroup analysis (Figure 1).
Figure 1.
Flow chart of the study population.
VA, Viscum album L; for a detailed definition of VA dose levels and long-term VA therapy see the Methods section.
Endpoints and Statistical Methods
The primary outcome of the study was to analyze the VA-related AEs. All VA-related expected effects and adverse events reported by physicians were assessed by the study center. Expected effects were local reactions <5 cm in size, increased body temperatures <38°C, indurations, and burning sensations. Local reactions, increased temperatures beyond the desired levels, and other AEs were assessed as suspected VA-related AE if a causal relationship between VA and an event was described by physicians as at least a reasonable possibility. The s.c. doses were distinguished into low or high s.c. doses, respectively, as similarly specified previously.14 Experienced effects of VA-related AEs, type of application, dose levels, and the number of VA-related AEs experienced per patient were determined and summarized. Additionally, all other VA-unrelated AEs, which have been reported by physicians and were documented in the registry, were counted per patient, independent of any causality, severity, or length of duration of those events. To reduce reporting bias, we compared the occurrence of overall (VA-unrelated) AEs for different treatment periods per patient. For this purpose, the overall AEs of the patients of the long-term therapy subgroup were discriminated in terms of their occurrence during periods of VA therapy and VA-free intervals, and the AE rates (the number of AEs per patient per month) were calculated. Boxplot, Student’s t test (paired, 2-sided), and Cohen’s d analyses were performed to detect differences between the occurrences of overall AEs in treatment periods. All statistical analyses were performed using the software R (R Version 3.1.2 [2014])28 with the basic R and the “compute.es” packages.
Results
Patient Characteristics
In total, 226 cancer patients with recorded preexisting autoimmune diseases were treated between 2011 and 2017 at the Cancer Center Gemeinschaftskrankenhaus Havelhöhe. Eligibility for analysis was characterized by receipt of VA therapy, and for 106 patients complete data were retrieved from the NO registry (Figure 1). Table 1 shows the main characteristics of analyzed patients, 74 (70%) women and 32 (30%) men. At first diagnosis of cancer, the median age was 63 years, with an interquartile range of 54 to 71 years. The median body mass index (BMI) was 23, and 14 (13%) patients were underweight. In Table 1, the numbers of patients with regard to autoimmune diseases and cancer diagnoses are summarized. For 5 patients (5%) more than a single autoimmune disease was documented. The majority suffered from thyroid or intestinal autoimmune diseases. The most frequent autoimmune diseases were Hashimoto’s thyroiditis (n = 29, 27%) followed by psoriasis (n = 20, 19%) and ulcerative colitis (n = 16, 15%). Rare cases of lupus erythematosus, sarcoidosis, Bechterew’s disease, and vitiligo are summarized in Table 1 under other autoimmune diseases. With regard to cancer diagnoses, mainly breast (n = 23, 22%), lung (n = 21, 20%), and colorectal (n = 19, 18%) cancers were diagnosed in the patients. With regard to gender, the most common cancer entities were breast cancer (n = 23, 31%) for female patients and lung cancer in men (n = 11, 34%). As far as available histological data, all different UICC tumor stages were represented. At the time of first cancer diagnosis most patients had early stage cancers (Table 1). VA extracts of different manufacturers were given (for details, see Table 2). Ninety-seven patients (92%) received s.c. injections. 46 patients (43%) received i.v. injections, and 9 (8%) i.t. off-label VA treatments, generally accompanied by s.c. VA applications.
Table 1.
Baseline Characteristics of Cancer Patients at Day of First Diagnosisa.
| Number of patients, n (%) | 106 (100) |
| Age (years), median (IQR) | 63 (54-71) |
| BMI, median (IQR) | 23 (21-26) |
| Underweight (BMI < 18.5), n (%) | 14 (13) |
| Normal weight (18.5 < BMI < 25.0), n (%) | 53 (50) |
| Overweight (BMI > 25.0), n (%) | 30 (28) |
| NA, n (%) | 9 (8) |
| Gender, n (%) | |
| Male | 32 (30) |
| Female | 74 (70) |
| Autoimmune diseases, n (%) | |
| Hashimoto’s thyroiditis | 29 (27) |
| Grave’s disease | 8 (8) |
| Ulcerative colitis | 16 (15) |
| Crohn’s disease | 9 (8) |
| Celiac disease | 6 (6) |
| Psoriasis | 20 (19) |
| Rheumatic diseases | 11 (10) |
| Multiple sclerosis | 3 (3) |
| Others | 9 (8) |
| Cancer disease, n (%) | |
| Lung | 21 (20) |
| Breast | 23 (22) |
| Colorectal | 19 (18) |
| Lymphoma | 8 (8) |
| Uterine, ovarian, cervical | 5 (5) |
| Stomach | 6 (6) |
| Pancreas | 5 (5) |
| Liver | 3 (3) |
| Other | 16 (15) |
| UICC stage, n (%) | |
| 0 | 2 (2) |
| I | 15 (14) |
| II | 21 (20) |
| III | 19 (18) |
| IV | 18 (17) |
| NA | 31 (29) |
Abbreviations: IQR, interquartile range; BMI, body mass index; UICC, Union for International Cancer Control; TNM, tumor, node, metastasis.
TNM staging according to the UICC. Number and portions (%) in columns do not necessarily add to 106 (100%), as patients may have various combinations of diseases.
Table 2.
Characteristics of VA Therapy and Occurrence of AEs (N = 106)a.
| VA Application |
|||
|---|---|---|---|
| Subcutaneous | Intravenous | Intratumoural | |
| Number of patients, n (%) | 97 (92) | 46 (43) | 9 (8) |
| Abnoba | 60 (57) | 13 (12) | 8 (8) |
| Helixor | 13 (12) | 37 (35) | 1 (1) |
| Iscador | 38 (36) | 1 (1) | 0 |
| Iscucin | 6 (6) | 0 | 0 |
| VA-related AEs, n (%) | |||
| None | 89 (84) | ||
| 1-3 | 16 (15) | ||
| 4-9 | 0 | ||
| >9 | 1 (1) | ||
| AEs (VA-unrelated), n (%) | |||
| None | 33 (31) | ||
| 1-3 | 27 (25) | ||
| 4-9 | 20 (19) | ||
| >9 | 26 (25) | ||
Abbreviations: VA, Viscum album L; AE, adverse event.
Characteristics of VA therapy applied additionally to standard of care (n = 106). Numbers and portions (%) in columns do not necessarily add to 106 (100%), as patients may have received various combinations of preparations and applications respectively. The number of documented AEs, discriminated into VA-related and -unrelated AEs were counted per patient and each summarized into no, 1 to 3, 4 to 9, and >9 AEs, respectively.
Subcutaneous VA Therapy
For 97 patients (92%), it was documented in the NO registry that they received s.c. VA applications (Table 2). Total periods of time for which patients received VA therapy ranged from 1 day to 6 years. As similarly described in a previous report,14 the majority (91 patients, 86%) received s.c. VA applications 2 to 3 times per week. When i.v. or i.t. VA applications were also applied, s.c. VA applications were accompanying or applied in a later course. VA extracts from Abnoba were the most frequently used (60 patients, 57%), followed by Iscador (38 patients, 36%) and Helixor (13 patients, 12%; Table 2). In the beginning of VA therapy, low doses were s.c. injected and in the later course of the therapy VA doses were increased. Applied VA products varied markedly, and as outlined in detail in the Methods section were classified into low or high s.c. doses, respectively. The analysis revealed for 28 patients (26%) that they received high s.c. VA doses repeatedly, and at least over a period of 6 months or longer.
Intravenous VA Therapy
For 46 patients (43%), it was documented that they received i.v. VA applications (Table 2). VA applications from Helixor were the most frequently used i.v. preparation (37 patients, 35%), followed by Abnoba (13 patients, 12%) and Iscador (1 patient, 1%; Table 2). Administered doses and periods of i.v. treatments varied markedly and in most cases were applied in addition to chemotherapy and accompanied by s.c. VA applications, similar as described in our previous report.13 Nine patients (9%) received just a single VA i.v. application and 8 patients (8%) received high i.v. applications, >300 mg. Twenty patients (19%) received i.v. VA applications at least 6 months or longer including s.c. VA applications and therapy intermissions.
Intratumoral VA Therapy
In total, 9 cancer patients (9%; 4 pancreatic, 3 liver, 1 stomach, and 1 lung cancer) with preexisting autoimmune comorbidities received i.t. VA injections. In 7 of these patients, i.t. VA injections were accompanied by s.c. VA applications. Preparations from Abnoba (8 patients, 8%) were the most commonly applied. Similar to that reported in our previous publication,15 i.t.-administered VA doses ranged from 30 to 200 mg. One patient (1%) received 3 i.t. injections and 5 patients (5%) received only a single i.t. injection.
Adverse Events
For the study cohort, all reported and documented AEs were retrieved from the NO registry and were described by frequency (see Table 2). For 89 patients (84%) of the study cohort, no VA-related AEs were documented, for 16 patients (15%) 1 to 3 VA-related AEs, and for 1 patient (1%) 10 VA-related AEs were retrieved from the registry (Table 2). Additionally, all other VA-unrelated AEs were retrieved from the NO registry and described by frequency (Table 2). For 33 patients (31%) none AEs, for 27 patients (25%) 1 to 3 AEs, for 20 patients (19%) 4 to 9 AEs, and for 26 patients (25%) >9 VA-unrelated AEs were documented (Table 2).
Regarding VA-related AEs, in total, for 17 patients (16%) 37 VA-related AEs were documented (Table 3). Twenty VA-related AEs were expected, such as local reactions <5 cm in size, indurations, or burning sensations. For 12 patients (11%) in total 17 unexpected VA-related AEs were documented (for details see Table 3). All 17 patients with VA-related AEs were female. All VA-related AEs were mild or moderate (CTCAE, version 4.03; grade 1 or 2),27 and in all 17 patients the VA therapy was continued immediately or after a break of 1 to 11 months. In only 3 patients (3%; patient numbers h, n, o) was the VA dose lowered; in all the other 14 patients (13%) the VA dose was maintained (8 patients) or increased (6 patients) after experiencing a VA-related AE. No patient has completely stopped VA therapy. For 3 patients (3%) VA-related AEs resulted from i.v. application (patient numbers h, i, m), one (1%) from i.t. application (patient number g), and all other reported VA-related AEs resulted from s.c. applications. For one cervical carcinoma patient (patient number h), diagnosed with rheumatoid arthritis, Hashimoto’s thyroiditis, Sjögren’s syndrome, and 5 further comorbidities, multiple AEs were reported, among them one VA-related AE with hypotension and short-term fainting during ambulant i.v. VA application. After VA application was interrupted, the patient was treated with i.v. Fenistil (antihistamine) and recovered fully with normalization of circulatory parameters. The patient was discharged home on the same day and no further treatment was required. According to CTCAE, version 4.03,27 this event was classified as grade 2 AE. Six weeks later the patient received and tolerated very low doses of s.c. VA preparations, and in the period 2 to 5 years thereafter, further s.c. applications of low VA doses were documented for this patient. No further VA-related AEs were documented. For the 17 patients for whom VA-related AEs were reported, numerous further VA-unrelated AEs were documented. For 2 patients (patient numbers k and p) no other AEs, for 6 patients (patients numbers b, e, g, I, j, n, q) 1 to 3 AEs, for 3 patients (patients numbers a, c, l) 4 to 9 AEs, and for 5 patients (patient numbers d, f, h, m, o) >9 VA-independent AEs were documented.
Table 3.
Recorded Adverse Drug Reactions Attributed to VA Therapy.
| Event | Patients, n | Events, n | Dose Level | Patients, No. |
|---|---|---|---|---|
| Erythema | ||||
| Local reaction <5 cmb | 8 | 11 | 7 high s.c. | b, f, l, m, qc |
| 4 low s.c. | a, e, n, l | |||
| Local reaction >5 cm | 3 | 3 | 1 high s.c. | qc |
| 2 low s.c. | k, j | |||
| Indurationb | 4 | 5 | 3 high s.c. | b, qc |
| 2 low s.c. | c, e | |||
| Burning sensationb | 3 | 4 | 3 high s.c. | d, qc |
| 1 low s.c. | e | |||
| Local reaction, unspecified | 3 | 4 | 1 high s.c. | qc |
| 3 low s.c. | n, o | |||
| Hot flushes | 2 | 2 | 1 high s.c. | qc |
| 1 low s.c. | p | |||
| Pain | 2 | 2 | 1 i.t. | g |
| 1 high s.c. | qc | |||
| Pyrexia | 1 | 2 | 2 i.v. | i |
| White cell blood count reduction | 1 | 1 | 1 high s.c. | d |
| Swelling | 1 | 1 | 1 i.v. | m |
| Swollen lymph nodes | 1 | 1 | 1 high s.c. | f |
| Allergic reaction | 1 | 1 | 1 i.v. | h |
Abbreviations: s.c., subcutaneous; i.v., intravenous; i.t., intratumoral; VA, Viscum album L; AE, adverse event.
High s.c. VA applications were products from Abnoba >0.2 mg/mL, Iscador ≥1 mg/mL, or Helixor ≥10 mg/mL; all other s.c. preparations were classified as low s.c. doses.
Expected VA-related AEs.
Patient number q experienced multiple VA-related AEs. The patient numbers h, n, and o reduced their VA doses after experiencing VA-related AEs.
Subgroup Analysis
Thirty patients (28%) were eligible for subgroup analysis and received repeatedly i.v., i.t., or high-dose s.c. VA applications, over a period of at least 6 months. Seventy-six patients (72%) were not eligible for subgroup analysis. Of these patients, 17 (16%) still were under follow-up at cutoff time, 9 patients (9%) died within 6 months after onset of VA therapy, 6 patients (6%) received only low VA applications, 16 patients (15%) were lost for follow-up, and for 28 patients (26%) less than 6 months or unspecified VA applications were recorded.
For the VA long-term subgroup of 30 patients, the median overall length of VA therapy was 21 months (interquartile range was 11-34 months) and the mean length was 23 months (standard deviation of 14 months) and VA therapy was interrupted by VA-free intervals. All characteristics of this subgroup and details about different VA applications and preparations are listed in Table 4. With respect of age, BMI, and gender, the 30 patients of the VA long-term subgroup were comparable to those 106 patients of the entire study cohort. Regarding the range of distribution of autoimmune comorbidities, no significant differences between subgroup and entire groups were observed (compare Table 1 with Table 4). However, while 9 patients (8%) of the entire group had Crohn’s disease just a single (3%) patient of the VA long-term-group suffered from Crohn’s disease. Similarly, 3% of the entire but none of the VA long-term-group suffered from multiple sclerosis (compare Table 1 with Table 4).
Table 4.
Characteristics of the Subgroupa.
| Number of patients, n (%) | 30 (100) |
| Age (years), median (IQR) | 62 (55-69) |
| BMI, median (IQR) | 24 (21-28) |
| Underweight (BMI < 18.5), n (%) | 4 (13) |
| Normal weight (18.5 < BMI < 25.0), n (%) | 12 (40) |
| Overweight (BMI > 25.0), n (%) | 11 (37) |
| NA, n (%) | 3 (10) |
| Gender, n (%) | |
| Male | 8 (27) |
| Female | 22 (73) |
| Autoimmune disease, n (%) | |
| Hashimoto’s thyroiditis | 9 (30) |
| Grave’s disease | 3 (10) |
| Ulcerative colitis | 6 (20) |
| Crohn’s disease | 1 (3) |
| Celiac disease | 1 (3) |
| Psoriasis | 5 (17) |
| Rheumatic diseases | 6 (20) |
| Multiple sclerosis | 0 |
| Others | 2 (7) |
| VA applications, n (%) | |
| s.c. | 28 (93) |
| i.v. | 20 (67) |
| i.t. | 2 (7) |
| VA preparations, n (%) | |
| Abnobaviscum | 19 (63) |
| Helixor | 19 (63) |
| Iscador | 10 (33) |
| VA-related AEs, n (%) | |
| None | 22 (73) |
| 1-3 | 7 (23) |
| 4-9 | 0 |
| >9 | 1 (3) |
| AEs (VA-unrelated), n (%) | |
| None | 5 (17) |
| 1-3 | 5 (17) |
| 4-9 | 8 (27) |
| >9 | 12 (40) |
Abbreviations: IQR, interquartile range; BMI, body mass index; VA, Viscum album L; s.c., subcutaneous; i.v., intravenous; i.t., intratumoral; AE, adverse event.
Characteristics of the 30 patients of the subgroup. Numbers and portions (%) in columns do not necessarily add to 30 (100%), as patients may have various combinations of autoimmune diseases and received various combinations of VA preparations and applications, respectively. The number of documented AEs, discriminated into VA-related and VA-unrelated AEs were counted per patient and each summarized into no, 1 to 3, 4 to 9, and >9 AEs, respectively.
For 22 patients of the subgroup (73%), no VA-related AEs were reported. For 7 (23%) patients (patient numbers a, d, f, g, i, k, l in Table 3) 1 to 3 VA-related AEs were reported, and for 1 patient (3%, patient number q in Table 3) 10 VA-related AEs were documented. Furthermore, also the occurrence of overall VA-unrelated AEs was evaluated (Table 4). For 5 patients (17%) 1 to 3 VA-unrelated AEs, for 8 patients (27%) 4 to 9 VA-unrelated AEs, and for 12 patients (40%) >9 VA-unrelated AEs were documented, while for 5 patients (17%) of the subgroup no overall AEs were documented during their entire observed treatment periods (Table 4). No exacerbations or flares of the underlying autoimmune diseases during VA treatment were recorded for any of these 30 patients. For 22 patients (73%) of the subgroup AEs were documented for VA-free intervals, while for 18 patients (60%) AEs during VA therapy periods were reported. For all patients of the subgroup, in total 281 AEs were recorded. For the 25 patients with documented AEs, the median entire length of VA-treatment periods was 11 months (inter quartile range was 6-26 months) and the median entire length of VA-free observation periods was 12 months (inter quartile range was 6-19 months). A total of 172 VA-unrelated AEs (61% of the total AEs) were documented for VA-free intervals versus 109 VA-unrelated AEs (39% of the total AEs) during VA-treatment periods. In Figure 2, for the 25 patients with documented AEs, boxplots for the number of VA-unrelated AEs for VA therapy and VA-free-intervals are shown. Apparently, fewer overall AEs were recorded for VA-therapy periods. Calculation of Pearson’s χ2 test and Cohen’s d analyses revealed a medium effect, d[95% confidence interval] = 0.54 [−0.05, 1.14], with p(d) = 0.07, for this reduction. Considering the relative lengths of the VA treatment and VA-free periods by calculation of number of AEs per patient per month revealed for the mean values 0.66 AEs per month (standard deviation of 0.69 AEs per month) for VA-free intervals versus 0.27 AEs per month (standard deviation of 0.3 AEs per month) for VA-therapy periods. Student’s t test (2-tailed, paired) calculation revealed a significant halving of the AE rates for VA-treatment periods (P = .019).
Figure 2.
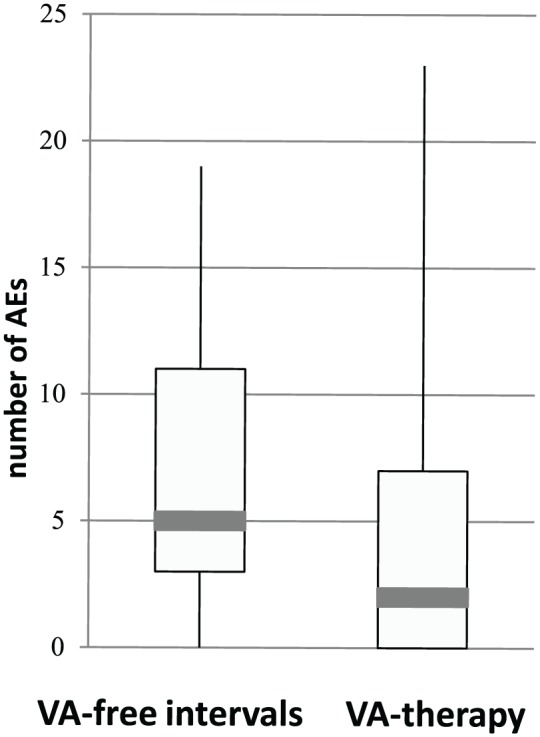
Number of overall AEs in periods of VA therapy and VA-free intervals.
VA, Viscum album L; AE, adverse event. For 25 patients of the subgroup, which experienced AEs, the number of documented VA-unrelated AEs was counted per patient. In boxplots, for periods of VA therapy and VA-free intervals the respective number of AEs is compared.
Discussion
The present study assessed 106 oncological patients with preexisting autoimmune comorbidities and receiving VA applications. The most common autoimmune diseases were Hashimoto’s thyroiditis, ulcerative colitis, and psoriasis. Seventeen patients (16%) of this study cohort experienced VA-associated AEs, which is comparable to VA-related AE rates of other VA-treated cancer patients, published previously.13,14 In a VA long-term subgroup of 30 patients, the rates of VA-unrelated AEs during periods of VA therapy were significantly halved. Hence, VA therapy for cancer patients with preexisting autoimmune disorders appears to be a safe treatment.
The administered doses of s.c. or i.v. VA applications were comparable as reported previously.13,14 VA preparations are most commonly applied via s.c. injections, starting at a low dose, which is slowly increased over time, based on the individual patient’s responses.29 Accordingly, all patients of this study started with low VA concentrations. With regard to i.t. applications, lower amounts of applications than reported previously15 were observed. Previously we reported that observed VA-related AEs were dose-dependent29 and believed to be related to the immune-stimulating, pharmacological activity of VA preparations.14 The rates and severity of observed VA-related AEs, expected or unexpected, were in the same ranges as reported in our previous reports.13-15 Only 17 (16%) of all cancer patients with preexisting autoimmune comorbidities that received VA applications experienced a VA-related AE and only 3 patients (3%) reduced their VA doses, but all 17 VA-related AE patients (16%) continued their VA therapy (Table 3) after experiencing a VA-related AE. VA-associated side effects consisted mainly of dose-dependent local reactions at the injection site, and various mild unspecific effects.6,30 For the patients of the present analysis it was difficult to assess the causality of observed AEs. For example, pain, nausea, and vomiting could also be the symptoms of advanced-stage pancreatic or liver cancers. Furthermore, it is suggested that robust immune responses are required in VA treatment for beneficial therapeutic responses to occur, and some mild or moderate VA-related AEs are expected and desired events.31 So, the 20 documented expected VA-related AEs (local reactions <5 cm in size, indurations, and burning sensations) in Table 3 could be considered as intended events.
Most autoimmune diseases occur significantly more frequently in women than in men.21 Accordingly, in our study cohort 70% was female. For none of the 32 male patients was any VA-related AE reported. A higher risk of AEs among female subjects compared to males was also found previously.14 The reasons for female sex as being a risk factor to experience an adverse drug reaction may include gender-related differences in pharmacokinetic, immunological, and hormonal factors but are not entirely understood.32,33 In this study, VA therapy for cancer patients with preexisting comorbidities such as Hashimoto’s thyroiditis, psoriasis, ulcerative colitis, Grave’s disease, or Sjögren’s syndrome was a safe treatment. Nevertheless, the proportion of patients with autoimmune diseases, Crohn’s disease, and multiple sclerosis were lowered; thus, no further conclusions can be drawn.
For the entire study cohort, observed VA-related AEs were mostly mild to moderate in intensity. However, in one case after off-label i.v. VA application one patient experienced a short period of hypotension and fainting (CTCAE grade 2 reactions). A closer inspection of the original medical records for this patient revealed that VA therapy with low s.c. doses was started already 2 years before this AE occurred, without any adverse reactions. For this patient with multiple rheumatic and autoimmune diseases, further hypersensitivity toward other drugs, light, and copper were documented. Two weeks after the described AE, VA treatment with very low s.c. concentrations and subsequent onset of anti-autoimmune therapy was performed under clinical control. During continued VA therapy no further VA-related AEs were reported for this patient in the following years. Kienle et al34 summarized that hypersensitivity might occur under intravenous VA treatment in a dose-dependent fashion or when the infusion rate is too high. Therefore, if in the later course no further VA-specific allergic reactions can be observed, such dose-dependent vascular dilatation can be designated as non–immune-mediated pseudo-allergic reaction.34,35
For none of the 30 patients of the VA long-term subgroup was any exacerbation or flare of underlying autoimmune diseases during VA treatment recorded and no increase of the rate of VA-unrelated AEs was observed. In the analysis by Bock et al,36 it was concluded that considerably fewer adverse effects and treatment-associated symptoms were attributed to conventionally treated cancer patients when concomitant VA treatments were applied. In line with those findings, in the present study we observed a significant halving of rates of overall (VA-unrelated) AEs in periods of VA therapy compared to VA-free periods. Thus, for the subgroup of cancer patients analyzed here with certain preexisting autoimmune comorbidities, VA therapy was safe and, in addition, led to a significant reduction of the overall AE-rates.
The management of immune-related AEs, with regard to the utilization of ICIs, plays an increasingly important role. ICIs have improved the treatment of various types of cancers, but can cause severe immune-related AEs, leading to the development of autoimmune phenomena, rarely even with lethal consequences.2,37 In most clinical trials of ICIs, patients with preexisting autoimmune diseases were excluded, so there are only limited data on how these drugs affect this group of patients. In a recent review of several case studies, it was summarized that exacerbations of autoimmune diseases were frequently reported in patients with autoimmune diseases so that the use of ICIs in those patients is limited.37 Interestingly in this context, we recently published clinical safety analyses of combined treatment of ICIs with VA extracts showing good safety profiles.38 To date there are limited possibilities to treat immune-related AEs. Add-on VA therapy may possibly offer an option in the management of immune-related AEs.
There is a lack of safety data for the patients analyzed here, since the indication for VA therapy in patients with allergic, atopic, or especially autoimmune disorders is limited.6 Therefore, in the present study, use and safety data of VA preparations applied in cancer patients with preexisting autoimmune diseases were collected from usual clinical practice. It represents real-world data, taking into account the individual treatment approach by physicians according to needs and preferences of these patients. However, in the present study, various reporting and documentation bias cannot be excluded. Since patients with preexisting autoimmune diseases differ significantly from each other with respect to their cancer diagnoses, various comorbidities, and demographic issues, no general conclusions for all autoimmune comorbidities can be drawn here. Several further unwanted biases may have been introduced due to the observational, nonrandomized, and noncontrolled character of the study. Due to the low case number of some diseases, such as multiple sclerosis or lupus erythematosus enrolled in this study, the present analysis is limited to first observations on safety aspects of concomitant VA treatment in patients with autoimmune comorbidities such as Hashimoto’s thyroiditis, ulcerative colitis, and psoriasis. Nevertheless, the real-world data presented here complement the existing base of safety aspects of add-on VA therapy in oncological patients.
Conclusions
Our findings suggest that the add-on VA use in cancer patients with preexisting autoimmune diseases is safe. No higher rates of AEs were observed. In a VA long-term subgroup a significant halving of the overall AE rates were observed for VA-treatment periods. The available data were of observational nature. Further studies with larger study cohorts on the assessment of safety aspects in cancer patients with preexisting autoimmune diseases and VA therapy are needed.
Acknowledgments
We would like to thank all medical documentation officers at the GKH and the FIH involved in the present work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The Network Oncology was partially funded by Iscador AG Arlesheim, Switzerland; Abnoba GmbH Pforzheim, Germany; and Helixor Heilmittel GmbH Rosenfels, Germany. By contract, researchers were independent from the funder. Friedemann Schad reports grants from Helixor Heilmittel GmbH, grants from Abnoba GmbH, grants from Iscador AG, outside the submitted work. Grants from Helixor Heilmittel GmbH include travel costs and honoraria for speaking. Matthias Kröz received honoraria for lectures from Helixor Heilmittel GmbH outside the submitted work. All other authors declare that no competing financial interests exist.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11:e0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cappelli LC, Shah AA, Bingham CO., 3rd Immune-related adverse effects of cancer immunotherapy—implications for rheumatology. Rheum Dis Clin North Am. 2017;43:65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and meta-analysis. Integr Cancer Ther. 2012;11:187-203. [DOI] [PubMed] [Google Scholar]
- 4. Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;(2):CD003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bussing A. Immune modulation using mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung. 2006;56(6A):508-515. [DOI] [PubMed] [Google Scholar]
- 6. Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L. in animals and humans—systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schink M, Tröger W, Dabidian A, et al. Mistletoe extract reduces the surgical suppression of natural killer cell activity in cancer patients. A randomized phase III trial. Forsch Komplementmed. 2007;14:9-17. [DOI] [PubMed] [Google Scholar]
- 8. Hamre HJ, Pham VN, Kern C, et al. A 4-year non-randomized comparative phase-IV study of early rheumatoid arthritis: integrative anthroposophic medicine for patients with preference against DMARDs versus conventional therapy including DMARDs for patients without preference. Patient Prefer Adherence. 2018;12:375-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beuth J, Schneider B, Schierholz JM. Impact of complementary treatment of breast cancer patients with standardized mistletoe extract during aftercare: a controlled multicenter comparative epidemiological cohort study. Anticancer Res. 2008;28(1B):523-527. [PubMed] [Google Scholar]
- 10. Büssing A, Raak C, Ostermann T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (Iscador): a meta-analysis. Evid Based Complement Alternat Med. 2012;2012:219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eisenbraun J, Scheer R, Kröz M, Schad F, Huber R. Quality of life in breast cancer patients during chemotherapy and concurrent therapy with a mistletoe extract. Phytomedicine. 2011;18:151-157. [DOI] [PubMed] [Google Scholar]
- 12. Ostermann T, Bussing A. Retrolective studies on the survival of cancer patients treated with mistletoe extracts: a meta-analysis. Explore (NY). 2012;8:277-281. [DOI] [PubMed] [Google Scholar]
- 13. Steele ML, Axtner J, Happe A, Kroz M, Matthes H, Schad F. Safety of intravenous application of mistletoe (Viscum album L.) preparations in oncology: an observational study. Evid Based Complement Alternat Med. 2014;2014:236310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Adverse drug reactions and expected effects to therapy with subcutaneous mistletoe extracts (Viscum album L.) in cancer patients. Evid Based Complment Alternat Med. 2014;2014:724258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Use and safety of intratumoral application of European mistletoe (Viscum album L) preparations in oncology. Integr Cancer Ther. 2015;14:140-148. [DOI] [PubMed] [Google Scholar]
- 16. Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16:1049-1057. [DOI] [PubMed] [Google Scholar]
- 17. Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Autoimmune disease and subsequent digestive tract cancer by histology. Ann Oncol. 2012;23:927-933. [DOI] [PubMed] [Google Scholar]
- 18. Ehrenfeld M, Abu-Shakra M, Buskila D, Shoenfeld Y. The dual association between lymphoma and autoimmunity. Blood Cells Mol Dis. 2001;27:750-756. [DOI] [PubMed] [Google Scholar]
- 19. Bizzaro N, Antico A, Villalta D. Autoimmunity and gastric cancer. Int J Mol Sci. 2018;19:E377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forabosco P, Bouzigon E, Ng MY, et al. Meta-analysis of genome-wide linkage studies across autoimmune diseases. Eur J Hum Genet. 2009;17:236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1-6. [DOI] [PubMed] [Google Scholar]
- 22. Vajdic CM, Falster MO, de Sanjose S, et al. Atopic disease and risk of non-Hodgkin lymphoma: an InterLymph pooled analysis. Cancer Res. 2009;69:6482-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg SK, Loftus EV., Jr. Risk of cancer in inflammatory bowel disease: going up, going down, or still the same? Curr Opin Gastroenterol. 2016;32:274-281. [DOI] [PubMed] [Google Scholar]
- 24. Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother. 2016;65:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sosa A, Cadena EL, Olive CS, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10:1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schad F, Axtner J, Happe A, et al. Network Oncology (NO)—a clinical cancer register for health services research and the evaluation of integrative therapeutic interventions in anthroposophic medicine. Forsch Komplementmed. 2013;20:353-360. [DOI] [PubMed] [Google Scholar]
- 27. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v.4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed February 13, 2019.
- 28. R Foundation. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/ [Google Scholar]
- 29. Schad F, Thronicke A, Merkle A, Matthes H, Steele ML. Immune-related and adverse drug reactions to low versus high initial doses of Viscum album L. in cancer patients. Phytomedicine. 2017;36:54-58. [DOI] [PubMed] [Google Scholar]
- 30. Huber R, Lüdtke H, Wieber J, Beckmann C. Safety and effects of two mistletoe preparations on production of interleukin-6 and other immune parameters—a placebo controlled clinical trial in healthy subjects. BMC Complement Alternat Med. 2011;11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bussing A, Tröger W, Stumpf C, Schietzel M. Local reactions to treatments with Viscum album L. extracts and their association with T-lymphocyte subsets and quality of life. Anticancer Res. 2008;28(3B):1893-1897. [PubMed] [Google Scholar]
- 32. Gochfeld M. Sex differences in human and animal toxicology. Toxicol Pathol. 2017;45:172-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64:999-1004. [DOI] [PubMed] [Google Scholar]
- 34. Kienle GS, Mussler M, Fuchs D, Kiene H. Intravenous mistletoe treatment in integrative cancer care: a qualitative study exploring the procedures, concepts, and observations of expert doctors. Evid Based Complement Alternat Med. 2016;2016:4628287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pichler WJ, Hausmann O. Classification of drug hypersensitivity into allergic, p-i, and pseudo-allergic forms. Int Arch Allergy Immunol. 2016;171:166-179. [DOI] [PubMed] [Google Scholar]
- 36. Bock PR, Friedel WE, Hanisch J, Karasmann M, Schneider B. Efficacy and safety of long-term complementary treatment with standardized European mistletoe extract (Viscum album L.) in addition to the conventional adjuvant oncologic therapy in patients with primary non-metastasized mammary carcinoma. Results of a multi-center, comparative, epidemiological cohort study in Germany and Switzerland [in German]. Arzneimittelforschung. 2004;54:456-466. [DOI] [PubMed] [Google Scholar]
- 37. Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:610-616. [DOI] [PubMed] [Google Scholar]
- 38. Thronicke A, Steele ML, Grah C, Matthes B, Schad F. Clinical safety of combined therapy of immune checkpoint inhibitors and Viscum album L. therapy in patients with advanced or metastatic cancer. BMC Complement Alternat Med. 2017;17:534. [DOI] [PMC free article] [PubMed] [Google Scholar]