Abstract
A diverse group of toxicants has been identified that cause injury to the lung including gases (eg, ozone, chlorine), particulates/aerosols (eg, diesel exhaust, fly ash, other combustion products, mustards, nanomaterials, silica, asbestos), chemotherapeutics (eg, bleomycin), and radiation. The pathologic response to these toxicants depends on the dose and duration of exposure and their physical/chemical properties. A common response to pulmonary toxicant exposure is an accumulation of proinflammatory/cytotoxic M1 macrophages at sites of tissue injury, followed by the appearance of anti-inflammatory/wound repair M2 macrophages. It is thought that the outcome of the pathogenic responses to toxicants depends on the balance in the activity of these macrophage subpopulations. Overactivation of either M1 or M2 macrophages leads to injury and disease pathogenesis. Thus, the very same macrophage-derived mediators, released in controlled amounts to destroy injurious materials and pathogens (eg, reactive oxygen species, reactive nitrogen species, proteases, tumor necrosis factor α) and initiate wound repair (eg, transforming growth factor β, connective tissue growth factor, vascular endothelial growth factor), can exacerbate acute lung injury and/or induce chronic disease such as fibrosis, chronic obstructive pulmonary disease, and asthma, when released in excess. This review focuses on the role of macrophage subsets in acute lung injury and chronic fibrosis. Understanding how these pathologies develop following exposure to toxicants, and the contribution of resident and inflammatory macrophages to disease pathogenesis may lead to the development of novel approaches for treating lung diseases.
Keywords: Macrophages, lung injury, fibrosis, inflammatory mediators, cytokines, oxidants
The idea that macrophages accumulating at sites of injury play a role in the pathogenic response to xenobiotics was proposed more than 100 years ago by Eli Metchnikoff. Describing the inflammatory response as a “salutary reaction against some injurious influence,” he hypothesized that “ferments” released by cells at inflammatory sites might contribute to tissue damage (Metchnikoff, 1968). Since that time, there have been numerous publications supporting this concept in all tissues of the body. In this review, we focus on the role of macrophages and inflammatory mediators they release in acute and chronic lung injury and disease pathogenesis induced by pulmonary toxicants. For brevity, we have not included discussion of the impact of exposure dose, duration or frequency, animal species, or mechanism of action. Although we recognize that these factors influence the inflammatory response, herein we present a general overview that may be more broadly applicable to multiple toxicants with distinct mechanisms of action.
INFLAMMATORY MACROPHAGES
Inflammatory macrophages are mononuclear phagocytes that play an essential role in host defense and in innate immune responses to noxious stimuli. In contrast to resident tissue macrophages (eg, alveolar macrophages), which originate mainly from embryonic precursors during development and self-renew during adulthood, inflammatory macrophages are largely derived from blood and bone marrow precursors. They express specific chemokine receptors such as CCR2 or CX3CR1 and accumulate in tissues in response to chemokines released from injured cells and tissues (Tsou et al., 2007; Vannella and Wynn, 2017). Inflammatory macrophages also express the integrin CD11b, a marker of migratory cells. Once localized at sites of tissue injury, these newly recruited macrophages become activated by mediators they encounter in the tissue microenvironment developing into subpopulations exhibiting varying levels of pro-inflammatory/cytotoxic (M1) or anti-inflammatory/wound repair (M2) activity (Arora et al., 2018; Hussell and Bell, 2014; Laskin et al., 2011) (Figure 1). The process of macrophage activation into M1 and M2 subsets is tightly controlled and involves specific signaling pathways and transcription factors, and posttranslational regulatory networks (Lawrence and Natoli, 2011; Wang et al., 2014a) (Table 1).
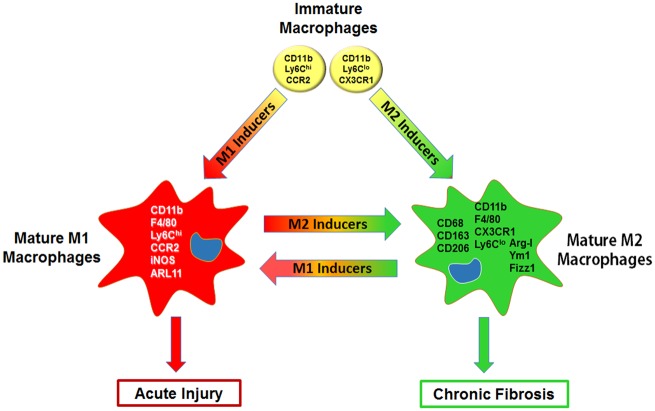
Figure 1.
Proinflammatory/cytotoxic (M1) and anti-inflammatory/wound repair (M2) macrophages in acute injury and chronic fibrosis. Immature inflammatory mouse macrophages originating from blood and precursors express the integrin CD11b, high or low levels of the surface antigen Ly6C, and chemokine receptors, CCR2 or CX3CR1, respectively. In response to environmental cues (eg, cytokines, growth factors, TLR agonists, and lipids) and intracellular/nuclear regulatory pathways (eg, kinases, transcription factors, epigenetic regulators, metabolic factors) that are activated, these CD11b+ cells mature into F4/80+Ly6ChiCCR2+ proinflammatory/cytotoxic M1 macrophages, which express markers such as inducible nitric oxide synthase (iNOS) and ARL11, or F4/80+Ly6CloCX3CR1+ anti-inflammatory/wound repair M2 macrophages which express CD68, CD163, CD206, Arginase (Arg)-1, Ym1, and/or Fizz1. The extent to which macrophages develop into these subpopulations depends on the activities of M1 and M2 inducers/regulatory factors. As these vary over the course of the inflammatory response, macrophages exist along a continuum with subpopulations expressing varying levels of M1 and M2 markers and activities. The process of M1 and M2 macrophage activation is also highly dynamic; thus, as environmental cues, signaling molecules, transcription factors, and cellular metabolism change, macrophages readily modify their phenotype and function. The outcome of inflammatory responses to tissue injury depends on the relative activities of M1 and M2 macrophage subpopulations. In this context, overactivation of M1 macrophages and/or aberrant M2 anti-inflammatory/wound repair activity can exacerbate and perpetuate acute injury, while excessive activity of M2 macrophages can lead to chronic diseases such as fibrosis.
Table 1.
Regulators of Macrophage Activation and Polarization
| Regulator | M1 | M2 | Supplementary References |
|---|---|---|---|
| Extracellular | |||
| Cytokines | IL-1β, IL-6, IL-12, IL-23, IFNγ, TNFα, MIP-1α | IL-4, IL-13, IFNα, IL-1 RA | Martinez and Gordon (2014), Amici et al. (2017), Murray (2017), Parisi et al. (2018) |
| Growth factors | GM-CSF | M-CSF, TGFβ | Martinez and Gordon (2014), Wynn and Vannella (2016) |
| Eicosanoids/bioactive lipids | LTB4; 12-HETE, 5-HETE | Lipoxins, resolvins, thromboxane, PGI2 | Masoodi et al. (2015), Robb et al. (2016), Amici et al. (2017) |
| TLR-4 agonists | DAMPs, PAMPs, LPS | Martinez and Gordon (2014) | |
| NOD agonists | Peptidoglycans | Zhou et al. (2015) | |
| Intracellular | |||
| Oxidative stress | ROS, RNS | Martinez and Gordon (2014), Amici et al. (2017), Murray (2017), Parisi et al. (2018) | |
| Metabolism | Anaerobic glycolysis, glucose uptake, fatty acid synthesis | Oxidative glucose metabolism, fatty acid oxidation, and uptake | Jha et al. (2015), Murray (2017); Van den Bossche et al. (2017); Van den Bossche and Saraber (2018) |
| Signaling pathways | AKT2, NOTCH1/2 | AKT1, RTKs | Amici et al. (2017) |
| Nuclear | |||
| Transcription factors | NFκB, AP-1, STAT1, IRF1, IRF5, IRF8, HIF-1α | IRF4, KLF-4, c-myc, PPARγ, RXRs, LXRs, STAT3, STAT6, IRF3, IRF4, HIF2α | Escribese et al. (2012), Pello et al. (2012), Martinez and Gordon(2014); Roszer (2015), Chistiakov et al. (2018), Parisi et al. (2018), Saradna et al. (2018) |
| Epigenetic | |||
| MicroRNA | miRNA-155, miRNA-125b, miRNA-27b, miRNA-127, miRNA-223, miRNA-106a | miRNA-146a/b miRNA-21, miRNA-511-3p, miRNA-124, miRNA-125a/b, miRNA-24, miRNA-34a, let-7c | Ponomarev et al. (2011), Amici et al. (2017), Parisi et al. (2018), Saradna et al. (2018), Shapouri-Moghaddam et al. (2018) |
| Histone modifications | HDAC3 | ||
| DNA Methylation | DNMT3b, DNMT1 | H3K27 demethylase, DNMT3a, DNMT3al | |
Abbreviations: AKT, protein kinase B; AP, activator protein; DAMP, damage-associated molecular pattern; DNMT, DNA methyltransferase; GM-CSF, granulocyte-macrophage colony stimulating factor; HDAC, histone deacetylase; HETE, hydroxyeicosatetraenoic acid; HIF, hypoxia-inducible factor; IFN, interferon; IL, interleukin; IRF, interferon regulatory factor; KLF, Kruppel-like factors; LPS, lipopolysaccharide; LT, leukotriene; LXR, liver X receptor; M-CSF, macrophage colony stimulating factor; MIP, macrophage inflammatory protein; miRNA, micro-RNA; NF-κB, nuclear factor-kappa B; NOTCH, neurogenic locus notch homolog protein; PAMP, pathogen-associated molecular pattern; PG, prostaglandin; PPAR, peroxisome proliferator-activated receptor; RNS, reactive nitrogen species; ROS, reactive oxygen species; RTK, receptor tyrosine kinase; RXR, retinoid X receptor; STAT, signal transducer and activator of transcription; TGF, transforming growth factor; TNF, tumor necrosis factor.
Proinflammatory/cytotoxic M1 macrophages, also referred to as classically activated macrophages, develop in response to interferon (IFN)γ alone, or in concert with toll-like receptor (TLR) 4 agonists (eg, lipopolysaccharide [LPS]; high-mobility group protein [HMGB1]) or other cytokines (eg, tumor necrosis factor [TNF]α and granulocyte macrophage-colony stimulating factor [GM-CSF]). Activated M1 macrophages release proinflammatory cytokines (eg, TNFα, interleukin [IL]-1β, IL-6, IL-12, IL-15, IL-23), and generate cytotoxic reactive oxygen species (ROS) and reactive nitrogen species (RNS), proteolytic enzymes, and bioactive lipids. The activity of M1 macrophages is balanced by M2 macrophages, which downregulate inflammation and initiate wound repair. These actions are mediated by anti-inflammatory cytokines (eg, IL-4, IL-10, IL-13), pro-resolving eicosanoids (eg, lipoxins, resolvins), and growth factors (eg, transforming growth factor [TGF]β, vascular endothelial growth factor [VEGF], epidermal growth factor [EGF], connective tissue growth factor [CTGF], fibroblast growth factor [FGF], platelet-derived growth factor [PDGF]). M2 macrophages have been subdivided into M2a macrophages, activated by IL-4 and IL-13, M2b macrophages, activated by immune complexes and LPS, M2c macrophages, activated by IL-10, TGFβ, or glucocorticoids, and M2d macrophages, activated by IL-6 and adenosines (Roszer, 2015). Although acute lung injury and persistent inflammation involves a prolonged or exaggerated response of M1 macrophages and defective M2 macrophage-mediated tissue repair, the development of chronic diseases such as fibrosis and cancer is thought to be a consequence of hyper-responsive subpopulations of M2 macrophages.
Although originally considered two phenotypically and functionally distinct subpopulations at opposite ends of the activation spectrum, it is now recognized that macrophages exist on a continuum with subpopulations in between expressing varying levels of M1 and M2 markers and activities. Moreover, the extent of macrophage activation toward an M1 or M2 phenotype is regulated not only by environmental cues including cytokines, growth factors, TLR agonists, and lipids, but also by various signaling pathways (ie, kinases), downstream transcription factors (eg, nuclear factor-kappa B [NF-κB], activator protein [AP]-1, interferon regulatory factor [IRF]s, signal transducer and activator of transcription [STAT]s, peroxisome proliferator-activated receptor [PPAR]γ), and epigenetic regulators (eg, microRNAs, chromatin modifiers) that are activated within the cells, as well as by posttranslational regulatory networks, and changes in intracellular metabolism (Murray, 2017) (Table 1). It should be noted, however, that M1 and M2 macrophage activation is a highly dynamic process; thus, as environmental cues, signaling molecules, transcription factors, epigenetic regulators, and cellular metabolism change in response to pathophysiologic conditions, macrophages readily modify their phenotype and function. Accordingly, cells that initially promote proinflammatory and cytotoxic reactions, can undergo phenotypic switching, subsequently participating in the resolution of inflammation and injury (Porcheray et al., 2005; Parisi et al., 2018). In this context, the transition of macrophages from a proinflammatory M1 to an anti-inflammatory/wound repair M2 phenotype appears to be critical for the progression of normal wound healing and tissue regeneration. This is exemplified by findings that noxious pulmonary stimuli such as hypoxia impair M1 to M2 macrophage phenotypic switching, a response associated with prolonged tissue injury (Faulknor et al., 2017). Also of importance, is the observation that while there are some unique characteristics of the activation profile of M1 and M2 macrophages, there are also many overlapping activities. These findings underscore the dynamic nature of the macrophage activation process and the notion that within any inflamed tissue, mixed phenotype macrophages co-exist with M1 and M2 macrophages, their specific function depending on the balance of activating and inhibiting activities and the tissue microenvironment (Martinez and Gordon, 2014).
INFLAMMATORY MACROPHAGES IN ACUTE LUNG INJURY AND REPAIR
Acute injury to the lung is associated with disruption of endothelial and epithelial barriers (Muller-Redetzky et al., 2014; Tam et al., 2011). It is characterized by an accumulation of protein-rich edema fluid, sloughing of the bronchial epithelium, the appearance of necrotic or apoptotic type I cells, denuded basement membrane, enlarged edematous interstitium, injured endothelial cells, and an accumulation of cellular debris in the tissue. Cellular characteristics include loss of alveolar-capillary membrane integrity, transepithelial migration of neutrophils and macrophages, and increases in proinflammatory/cytotoxic proteins. The outcome of acute lung injury depends on the nature of the toxicant, the dose and duration of exposure and the specific tissue location. Although after some exposures, lung structure and function return to normal, in other instances, there is persistence and/or progression of injury, leading to pulmonary vascular destruction, fibrosing alveolitis, multiple organ failure and death.
Initial evidence suggesting a role of macrophages in acute lung injury was based on findings that numbers of these cells increased in the tissue following exposure of animals to diverse pulmonary toxicants (eg, ozone, particulate matter, bleomycin, endotoxin, nanomaterials, mustard vesicants, silica, asbestos, and radiation) (Bekki et al., 2016; Hiraiwa and van Eeden, 2013; Laskin et al., 2011; Weinberger et al., 2011). In addition, macrophages accumulating in the lung early after injury induced by pulmonary toxicants were found to be activated toward a proinflammatory M1 phenotype, as evidenced by morphologic changes including increased size and vacuolization, and expression of inducible nitric oxide synthase (iNOS) and TNFα, prototypical marks of proinflammatory/cytotoxic macrophages (Aggarwal et al., 2014; Laskin et al., 2011; Malaviya et al., 2017; Sugiura and Ichinose, 2011). Macrophages responding to toxicant-induced injury also generated increased quantities of cytotoxic and proinflammatory mediators, which induce and/or amplify the pathogenic response, including ROS, RNS, IL-1, IL-6, IL-18, TNFα, chemokines, eicosanoids, proteases, and bioactive lipids.
Direct evidence for a role of proinflammatory/cytotoxic M1 macrophages in acute lung injury comes from findings that tissue damage is directly correlated with macrophage functional status. Thus, in a number of experimental models using a variety of approaches (eg, pharmacologic, genetic, or macrophage-deficient mice), pulmonary damage is ameliorated or prevented by suppressing or depleting macrophages (Table 2). For example, when M1 macrophage cytotoxic/proinflammatory activity is blocked with anti-inflammatory steroids, lung damage induced by ozone, silica, residual oil fly ash, bleomycin, sulfur mustard, endotoxin, oleic acid, or hydrogen sulfide is reduced (Al-Harbi et al., 2016; Chen et al., 2006; Geng et al., 2018; Huang et al., 2014; Laskin et al., 2011; Samet et al., 2000; Wigenstam et al., 2009). Similarly, the accumulation of macrophages in the lung and subsequent toxicity of ozone, cigarette smoke, carbon nanotubes, particulate matter, radiation, and silica are mitigated by pretreatment of animals with gadolinium chloride, which blocks M1 macrophage activation or clodronate liposomes, administered intravenously, which depletes newly recruited inflammatory macrophages (Frank et al., 2015; Laskin et al., 2011; Marchini et al., 2016; Nemmar et al., 2005; Perez-Rial et al., 2013; Poole et al., 2012). Bleomycin-induced acute lung injury and expression of iNOS are also abrogated in CCR4 knockout mice, which cannot generate M1 macrophages (Trujillo et al., 2008). Similar protective effects against dust have been described in IL-18R knockout mice, and against ozone in TNFR1 and galectin-3 knockout mice, which also display defective M1 macrophage development (Bauer et al., 2013; Laskin et al., 2011; Sunil et al., 2015). Production of inflammatory mediators and lung injury induced by endotoxin are also reduced in mice lacking CD40, a cell surface receptor required for M1 macrophage activation and iNOS expression (Hashimoto et al., 2004). Mice deficient in TLR4, a key receptor/signaling pathway regulating M1 inflammatory macrophage activation, are also protected from toxicity induced by ozone, endotoxin, and particulate matter (Connor et al., 2012; Hollingsworth et al., 2004). Likewise, loss of downstream TLR4 signaling molecules including MyD88 or NF-κB, protects animals from lung injury induced by ozone, bleomycin, silica, and particulate matter (He et al., 2016; Laskin et al., 2011; Re et al., 2014).
Table 2.
Approaches for Assessing the Role of Macrophages in Acute Lung Injury and Fibrosis
|
Proinflammatory/cytotoxic M1 macrophages in acute injury | |||
|---|---|---|---|
| Approach | M1 Macrophage Suppression | Outcome: Decreased Injury | Supplementary References |
| Pharmacologic | Glucocorticoids | Ozone, silica, PM, bleomycin, mustard, endotoxin, oleic acid, hydrogen sulfide | Haddad et al. (1995), DiMatteo and Reasor (1997), Samet et al. (2000), Chen et al. (2003b), Chen et al. (2006), Wigenstam et al. (2009), Huang et al. (2014), Wang et al. (2014a), Al-Harbi et al. (2016); Geng et al. (2018) |
| Gadolinium chloride | Ozone, hyperoxia, PM, endotoxin | Jankov et al. (2003), Arimoto et al. (2005), Laskin et al. (2011), Duke-Novakovski et al. (2013) | |
| Chlodronate liposomes | Cigarette smoke, carbon nanotubes, endotoxin, PM, silica | Elder et al. (2004), Nemmar et al. (2005), Laskin et al. (2011), Dhaliwal et al. (2012), Poole et al. (2012), Perez-Rial et al. (2013), Frank et al. (2015), Marchini et al. (2016) | |
| Cyclophosphamide | Ozone, silica | Bhalla et al. (1992), Bassett et al. (2001), Nemmar et al. (2005) | |
| Anti-CCR2 antibody | Endotoxin | Dhaliwal et al. (2012) | |
| CCl2-specific inhibitor | Radiation | Wiesemann et al. (2018) | |
| Genetic | CCR4−/− mice | Bleomycin | Trujillo et al. (2008) |
| IL-18R−/− mice | PM | Bauer et al. (2013) | |
| TNFα−/− or TNFR−/− mice | Ozone | Cho et al. (2007), Fakhrzadeh et al. (2008b) | |
| Galectin-3−/− mice | Ozone | Sunil et al. (2015) | |
| CD40−/− mice | Endotoxin | Hashimoto et al. (2004) | |
| TLR2−/− and TLR4−/− mice | Ozone, endotoxin, PM | Kleeberger et al. (2000, 2001), Gilmour et al. (2004), Hollingsworth et al. (2004), Cho et al. (2005), Williams et al. (2007), Garantziotis et al. (2010), Bauer et al. (2011), Li et al. (2011), Connor et al. (2012); Connor et al. (2013) | |
| MyD88−/− mice | Ozone, PM, bleomycin, silica | Gasse et al. (2007), Williams et al. (2007), Li et al. (2011), Bauer et al. (2013), Re et al. (2014), He et al. (2016a) | |
| NF-κBp50−/− mice | Ozone | Fakhrzadeh et al. (2004b) | |
| CD11b-DT transgenic mice | Endotoxin | Dhaliwal et al. (2012), Lu et al. (2018) | |
| CCR2−/− mice | Ozone, radiation, bleomycin, endotoxin | Moore et al. (2001), Gharaee-Kermani et al. (2003), Maus et al. (2003), Okuma et al. (2004), Yang et al. (2010), Francis et al. (2017), Wiesemann et al. (2018) | |
| IL1R−/− mice | Bleomycin | Gasse et al. (2007) | |
| IL13−/− mice | Hyperoxia | Bhandari et al. (2007) | |
| Macrophage-deficient mice | MAFIA | Ricin | Lindauer et al. (2009) |
| Approach | M1 Macrophage Activation | Outcome: Increased Injury | Supplementary References |
| Pharmacologic | Endotoxin | Endotoxin, radiation, ozone | Wollert et al. (1994), Johnston et al. (2004), Haque et al. (2009) |
| Bacillus Calmette-Guerin | Endotoxin, bleomycin | Chyczewska et al. (1993), Tasaka et al. (1995) | |
| Anti-IL-13 antibody | Endotoxin | Matsukawa et al. (2000) | |
| Genetic | SP-D−/− mice | Ozone, radiation, bleomycin | Casey et al. (2005), Kierstein et al. (2006), Groves et al. (2012), Groves et al. (2013), Malaviya et al. (2015a) |
| IL-10−/− micea | Ozone | Backus et al. (2010) | |
| CD206-DT transgenic mice | Endotoxin | Kambara et al. (2015) | |
|
Anti-inflammatory/profibrotic M2 macrophages in fibrosis | |||
|---|---|---|---|
| Approach | M2 Macrophage Suppression | Outcome: Decreased Fibrosis | Supplementary References |
| Pharmacologic | Chlodronate liposomes | Bleomycin | Gibbons et al. (2011) |
| Anti-CSF-1R antibody | Radiation | Meziani et al. (2018) | |
| Serum amyloid P | Bleomycin | Pilling et al. (2007), Laskin et al. (2011) | |
| Glucocorticoid | Bleomycin | Chaudhary et al. (2006) | |
| Liposome-encapsulated spironolactone | Bleomycin | Ji et al. (2016) | |
| IL-13-recombinant immunotoxin | Silica | Ferreira et al. (2013) | |
| Anti-IL-33 antibody | Bleomycin | Li et al. (2014) | |
| Genetic | IL-4−/− or IL-4R−/− mice | Silica | Huaux et al. (2003), Laskin et al. (2011) |
| IL-10−/− mice | Silica | Huaux et al. (1998), Barbarin et al. (2004) | |
| IL-13Rα2 overexpression | Bleomycin | Lumsden et al. (2015) | |
| IL-33−/− or IL-33R (ST2)-/- mice | Carbon nanotubes, bleomycin | Li et al. (2014), Wang et al. (2014b) | |
| Fizz-1−/− mice | Bleomycin | Liu et al. (2014) | |
| C/EBP−/− mice | Bleomycin | Buck and Chojkier (2011), Yao et al. (2016) | |
| CCR2 or CCL2−/− miceb | Bleomycin, radiation | Moore et al. (2001), Gharaee-Kermani et al. (2003), Okuma et al. (2004), Baran et al. (2007), Groves et al. (2018) | |
| CX3CR1−/− mice | Bleomycin | Tighe et al. (2011), Ishida et al. (2017) | |
| CCR4−/− mice | Bleomycin | Trujillo et al. (2008) | |
| IL-18−/− or IL18Rα−/− mice | Bleomycin | Hoshino et al. (2009) | |
| MyD88−/− mice | Bleomycin | Gasse et al. (2007) | |
| IL1R−/− mice | Bleomycin | Gasse et al. (2007) | |
| M-CSF−/− mice | Bleomycin | Baran et al. (2007) | |
| Macrophage-deficient mice | MAFIA | Bleomycin | Redente et al. (2014) |
| Approach | M2 Macrophage Activation | Outcome: Increased Fibrosis | Supplementary References |
| Pharmacologic | IL-33 | Bleomycin | Luzina et al. (2013), Li et al. (2014) |
| Genetic | IL-10 over-expressing mice | Silica | Barbarin et al. (2005) |
| MyD88−/− mice | Silica | Re et al. (2014) | |
| SP-D−/− mice | Bleomycin | Casey et al. (2005), Kierstein et al. (2006), Groves et al. (2012, 2013), Malaviya et al. (2015a) | |
PM, particulate matter (ash, dust, diesel exhaust).
M1 macrophage activation secondary to loss of M2 macrophages.
Indirect suppression.
Abbreviations: CCL, chemokine ligand; CCR, chemokine receptor; C/EBP, CCAAT/enhancer-binding protein; CSF-1R, colony-stimulating factor-1 receptor; DT, diphtheria toxin; Fizz, found in inflammatory zone; IL, interleukin; LPS, lipopolysaccharide; MAFIA, macrophage Fas-induced apoptosis; M-CSF, macrophage-colony stimulating factor; SP-D, surfactant protein-D; TLR, Toll-like receptor; TNF, tumor necrosis factor; TNFR, tumor necrosis factor receptor.
Protection against damage induced by ozone has also been described in animals treated with cyclophosphamide, which depletes bone marrow-derived monocytic precursors (Bhalla et al., 1992), consistent with the origin of proinflammatory macrophages. Analogous protection against LPS-induced lung toxicity, has been observed in CD11b-diptheria toxin (DT) receptor mice, depleted of blood monocyte precursors by administration of DT (Dhaliwal et al., 2012; Lu et al., 2018). CCR2 knockout mice, which are defective in M1 macrophage migratory activity to sites of injury, are also protected from oxidative stress and tissue injury induced by ozone, radiation, bleomycin, and endotoxin (Francis et al., 2017; Gharaee-Kermani et al., 2003; Laskin et al., 2011; Wiesemann et al., 2018; Yang et al., 2010).
Additional support for proinflammatory M1 macrophage involvement in the pathogenesis of acute lung injury comes from findings that activation of these cells exacerbates tissue damage induced by pulmonary toxicants. Hence, pretreatment of rodents with macrophage activators such as LPS or Bacillus Calmette-Guerin augments acute lung injury induced by endotoxin, radiation, bleomycin, and ozone (Laskin et al., 2011). Similar increases in toxicity in response to radiation, ozone, and bleomycin have been observed in mice lacking the pulmonary collectin, surfactant protein D, which under homeostatic conditions, functions to suppress lung macrophage proinflammatory activity (Casey et al., 2005; Groves et al., 2012; 2013; Malaviya et al., 2015a).
The resolution of acute injury and the return to normal lung structure and function is an active coordinated process. Evidence suggests that it is largely mediated by M2 macrophages, which stimulate counter-regulatory mechanisms that suppress the release of proinflammatory mediators and activate tissue repair processes. Anti-inflammatory/wound repair M2 macrophages have been reported to increase in the lung following exposure of animals to ozone, mustards, bleomycin, particulate matter, radiation, endotoxin, cigarette smoke, silica and asbestos (Arora et al., 2018; Duru et al., 2016; Francis et al., 2017; Kambara et al., 2015; Laskin et al., 2011; Malaviya et al., 2015b; Murthy et al., 2015; Venosa et al., 2016; Xiang et al., 2016). However, their appearance is delayed relative to M1 macrophages, consistent with a role for these cells in tissue repair. These macrophages express prototypical M2 markers including CD68, CD163, CD206, Ym-1, Arg-1, and Fizz-1.
Increases in M2 macrophages in the lung following exposure to ozone, endotoxin, mustard, diesel exhaust, asbestos, silica, or hyperoxia are correlated with upregulation of IL-4, IL-10, and IL-13 (Hussell and Bell, 2014; Laskin et al., 2011; Venosa et al., 2016). These cytokines dampen macrophage production of proinflammatory/cytotoxic mediators and stimulate the generation of extracellular matrix proteins and growth factors important in wound healing (Wynn and Vannella, 2016). Notably, administration of IL-13, which is key for M2 macrophage development, protects mice from lethal endotoxemia, whereas anti-IL-13 antibodies significantly reduce survival of these mice (Matsukawa et al., 2000). Additionally, cytotoxicity and mortality are increased in response to hyperoxia in IL-13 null mice (Bhandari et al., 2007). Increases in susceptibility to ozone have also been described in mice deficient in IL-10, a potent anti-inflammatory cytokine known to activate M2 macrophages (Backus et al., 2010). The importance of M2 macrophages in suppressing inflammation and initiating wound repair is also evidenced by findings that depletion of these cells by administration of DT to CD206-DT receptor transgenic mice is associated with increased expression of proinflammatory cytokines including TNFα, IL-1β, IL-6, and MCP-1/CCL2 following endotoxin administration, a response correlated with exacerbation of lung inflammation and injury (Kambara et al., 2015).
The origin of M2 macrophages has not been clearly established. The fact that their appearance in injured tissues is, like M1 macrophages, dependent in large part on CCR2/CCL2, suggests that they are derived from bone marrow and monocyte precursors. However, whether they originate from M1 macrophages via phenotypic switching or from a separate monocytic precursor pool is unclear. In this regard, recent studies have demonstrated an accumulation of bone marrow-derived macrophages in the lung following ozone inhalation which co-express the M2 macrophage chemokine receptor, CX3CR1, and CD206 (Francis et al., 2017). It has also been suggested that at least some populations of M2 repair macrophages are derived from proliferating resident lung macrophages (Alber et al., 2012; Tighe et al., 2011). In this regard, while both monocyte and tissue-derived M2 macrophages express Arg1, Ym-1, and Fizz-1, only monocyte-derived M2 macrophages express CX3CR1, CD206, Raldh2, and PD-L2 (Gundra et al., 2014).
INFLAMMATORY MACROPHAGES AND PULMONARY FIBROSIS
Chronic pulmonary fibrosis refers to a range of disorders characterized by irreversible destruction and remodeling of lung architecture that occurs as a consequence of excess deposition of collagen and other extracellular matrix components. This leads to scarring of the airways and difficulty in breathing. Evidence suggests that dysregulated wound repair is a key factor contributing to pulmonary fibrosis, and that this is due, at least in part, to an imbalance in the actions of M1 and M2 macrophages after prolonged inflammation or pneumonitis (Alber et al., 2012; Byrne et al., 2016; Herold et al., 2011) (Figure 1). This imbalance is associated with reduced production of antifibrotic cytokines (eg, CXCL10) and MMPs by M1 macrophages, which promote the resolution of scarring and matrix degradation, and excessive release of profibrotic mediators (eg, TGFβ, CTGF) by M2 macrophages, which induce fibroproliferative tissue remodeling.
The importance of overactive macrophages in the development of pulmonary fibrosis has been recognized since the mid-1980s, and macrophage phenotype has more recently emerged as an important contributor to the process (Laskin et al., 2011). This is largely based on findings that macrophages are persistently increased in the lung in close proximity to collagen producing fibroblasts, during the aberrant wound healing phase of fibrogenesis in animals treated with bleomycin, asbestos, silica, mustards, carbon nanotubes, or radiation (Duke and Bonner, 2018; Huang et al., 2017; Laskin et al., 2011; Malaviya et al., 2015b; Murthy et al., 2015), and that these macrophages express markers of an anti-inflammatory/profibrotic M2 phenotype including Arg-1, Fizz-1, Ym-1, CD68, CD163, and/or mannose receptor (CD206). In a number of these experimental models, macrophages are also enlarged and foamy in appearance, a characteristic feature of a profibrotic phenotype (Romero et al., 2015; Venosa et al., 2016). Increases in M2 macrophages in areas of fibrotic tissue have similarly been noted in lungs of patients with idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease and cystic fibrosis; moreover, number of these cells in the tissue correlate directly with a worsening prognosis.
M2 macrophages accumulating in the lung during fibrogenesis in both humans and rodents have been identified as the major source of a number of key profibrotic mediators (eg, TGFβ, CTGF, and CCL18), which stimulate fibroblast proliferation and collagen synthesis (Table 3) (Bickelhaupt et al., 2017; Prasse et al., 2006; Pulichino et al., 2008; Shvedova et al., 2005). M2 macrophages are also thought to promote fibrosis through the release of TNFα, IL-1, IL-10, IL-13, IL-33, PDGF, FGF, and fibronectin, which are increased in patients and animals with fibrotic lung disease (Wynn and Vannella, 2016). Arginase, which favors polyamine and proline biosynthesis and promotes cell growth and collagen formation, is also upregulated in M2 macrophages localized in fibrotic lesions (Murthy et al., 2015).
Table 3.
Macrophage Mediators Implicated in Lung Injury and Fibrosis
| Mediator | Toxicant | Supplementary References |
|---|---|---|
| Proinflammatory/cytotoxic M1 macrophage mediators | ||
| ROS (eg, superoxide anion, hydrogen peroxide, hydroxide radicals) | Ozone | Fakhrzadeh et al. (2004a), Connor et al. (2012), Sunil et al. (2012), Kumarathasan et al. (2015), Zhu et al. (2016), Francis et al. (2017) |
| Radiation | Rabbani et al. (2005), Lee et al. (2015) | |
| Endotoxin | Dang et al. (2018) | |
| Bleomycin | Yamazaki et al. (1998), Gu et al. (2015), Zhou et al. (2018) | |
| PM | Morio et al. (2001), Laskin et al. (2003), Li et al. (2003), Naota et al. (2010), Shvedova et al. (2013), Bekki et al. (2016), He et al. (2017) | |
| Asbestos | Simeonova and Luster (1995), Fattman et al. (2006), Blake et al. (2007), Nagatomo et al. (2007), He et al. (2011) | |
| Silica (Talc) | Rimal et al. (2005), Hu et al. (2006), Kaewamatawong et al. (2006), Nagatomo et al. (2006), Langley et al. (2011), Shim et al. (2015) | |
| Nanoparticles | Shvedova et al. (2005), Chou et al. (2008), Coccini et al. (2012) | |
| Chemical warfare agents | Malaviya et al. (2010), McGovern et al. (2011), Malaviya et al. (2015b), Lam et al. (2016), Elfsmark et al. (2018) | |
| RNS (eg, NO, peroxynitrite) | Ozone | Pendino et al. (1995), Kleeberger et al. (2001), Laskin et al. (2001), Fakhrzadeh et al. (2002), Fakhrzadeh et al. (2004b), Malaviya et al. (2010), Sunil et al. (2012) |
| Radiation | Nozaki et al. (1997), Malaviya et al. (2015a) | |
| Endotoxin | Pendino et al. (1993b), Wizemann et al. (1994), Kristof et al. (1998), Moncao-Ribeiro et al. (2011), Tu et al. (2017), Dang et al. (2018) | |
| Bleomycin | Yamazaki et al. (1998), Gurujeyalakshmi et al. (2000), Chen et al. (2003a), Genovese et al. (2005) | |
| PM | Morio et al. (2001), Becher et al. (2007), Kumarathasan et al. (2015) | |
| Asbestos | Tanaka et al. (1998), Dorger et al. (2002), Zeidler and Castranova (2004) | |
| Silica | Srivastava et al. (2002), Zeidler et al. (2004), Rimal et al. (2005), Cruz et al. (2016) | |
| Nanoparticles | Coccini et al. (2012), Lee et al. (2012) | |
| Chemical warfare agents | Martin et al. (2003), Malaviya et al. (2010), Venosa et al. (2016), Weinberger et al. (2016) | |
| Proinflammatory cytokines and chemokines (eg, TNFα, IL-1, IL-2, IL-6, IL-12, IL-18, CCL2, CCL3, CCL4, CCL5, CCL17, CXCL1, CXCL2, CXCL5, CXCL10) | Ozone | Johnston et al. (2000), Kenyon et al. (2002), Yu et al. (2002), Fakhrzadeh et al. (2004b), Cho et al. (2007), Hollingsworth et al. (2007), Fakhrzadeh et al. (2008a), Backus et al. (2010), Tighe et al. (2011), Gabehart et al. (2014), Francis et al. (2017) |
| Radiation | Zhang et al. (2008), Lee et al. (2015), Sohn et al. (2015), Li et al. (2017) | |
| Endotoxin | Jordan et al. (2001), Moncao-Ribeiro et al. (2011), Takashima et al. (2014), Tu et al. (2017), Dang et al. (2018) | |
| Bleomycin | Failla et al. (2006), Baran et al. (2007), Gasse et al. (2007), Trujillo et al. (2008), Hoshino et al. (2009), Yu et al. (2015), Wei et al. (2016), Zhou et al. (2018) | |
| PM | Morio et al. (2001), Becher et al. (2007), Gowdy et al. (2008), Churg et al. (2009), Naota et al. (2010), Hiraiwa and van Eeden (2013), Shvedova et al. (2013), Snow et al. (2014), Bekki et al. (2016), He et al. (2016b), He et al. (2017) | |
| Asbestos | Simeonova and Luster (1995), Fisher et al. (2000) | |
| Silica | Srivastava et al. (2002), Zeidler et al. (2004), Barbarin et al. (2005), Rimal et al. (2005), Hu et al. (2006), Langley et al. (2011), Ferreira et al. (2013), Kawasaki (2015), Cruz et al. (2016) | |
| Nanoparticles | Shvedova et al. (2005), Chou et al. (2008), Hsieh et al. (2012), Frank et al. (2015), Sager et al. (2016) | |
| Chemical warfare agents | Sunil et al. (2011), Malaviya et al. (2015b), Venosa et al. (2016), Elfsmark et al. (2018) | |
| Proteases (eg, MMPs, TIMPs, cathepsin) | Ozone | Kenyon et al. (2002), Sunil et al. (2012) |
| Radiation | Flechsig et al. (2010), Lee et al. (2015), Li et al. (2017) | |
| Endotoxin | Franco et al. (2002), Moncao-Ribeiro et al. (2011), Takashima et al. (2014) | |
| Bleomycin | Yu et al. (2015), Liu et al. (2017) | |
| PM | Adamson et al. (2003), Zhang et al. (2010), Shimada et al. (2015), Bekki et al. (2016) | |
| Asbestos | Tan et al. (2006) | |
| Silica | Langley et al. (2011), Cruz et al. (2016) | |
| Nanoparticles | Hsieh et al. (2012) | |
| Chemical warfare agents | Guignabert et al. (2005), Anderson et al. (2009), Malaviya et al. (2010), Sunil et al. (2014), Venosa et al. (2016) | |
| Hydrogen sulfide | Wang et al. (2014a) | |
| Oleic acid | Yeh et al. (2009) | |
| Bioactive lipids (eg, lipid peroxides, PAF, prostaglandins and leukotrienes) | Ozone | Giri et al. (1975), Tan and Bethel (1992), Pendino et al. (1993a), Kaneko et al. (1995), Stevens et al. (1995), Devlin et al. (1996), Kinney et al. (1996), Nakano et al. (2000), Fakhrzadeh et al. (2002), Connor et al. (2012), Sunil et al. (2012), Francis et al. (2017) |
| Radiation | Lee et al. (2015) | |
| Endotoxin | Ermert et al. (2000), Inoue et al. (2004a) | |
| Bleomycin | Chen et al. (2003a), Chen et al. (2006), Failla et al. (2006) | |
| PM | Kuhn et al. (1993), Samet et al. (2000), Shvedova et al. (2013), Yanamala et al. (2013), Bekki et al. (2016), Salama et al. (2016) | |
| Silica | Mohr et al. (1992) | |
| Nanoparticles | Shvedova et al. (2005), Coccini et al. (2012), Lee et al. (2012) | |
| Chemical warfare agents | Wigenstam et al. (2009), Malaviya et al. (2010), McGovern et al. (2011), Sunil et al. (2014), Venosa et al. (2016) | |
| Anti-inflammatory/profibrotic M2 macrophage mediators | ||
| Profibrotic cytokines and Chemokines (eg, IL-4, IL-10, IL-13, IL-17, IL-33 CXCL-9, fractalkine, CCL-18) | Ozone | Backus et al. (2010), Zhu et al. (2016) |
| Radiation | Inoue et al. (2004b), Chung et al. (2016), Groves et al. (2016), Zhang et al. (2018) | |
| Hyperoxia | Bhandari et al. (2007) | |
| Endotoxin | Matsukawa et al. (2000), Hocke et al. (2006), D’Alessio et al. (2016), Tu et al. (2017) | |
| Bleomycin | Pochetuhen et al. (2007), Li et al. (2014), Wei et al. (2016) | |
| PM | Gowdy et al. (2008) | |
| Silica | Barbarin et al. (2004), Barbarin et al. (2005), Ferreira et al. (2013) | |
| Nanoparticles | Shvedova et al. (2005) | |
| Chemical warfare agents | Venosa et al. (2016) | |
| Growth factors (eg, CTGF, TGFβ, TGFα, PDGFα, M-CSF) | Radiation | Rube et al. (2000), Chung et al. (2014), Lee et al. (2015), Sohn et al. (2015), Bickelhaupt et al. (2017), Li et al. (2017), Zhang et al. (2018) |
| Endotoxin | Tu et al. (2017) | |
| Bleomycin | Baran et al. (2007), Yu et al. (2015), Wei et al. (2016), Yao et al. (2016), Liu et al. (2017), Xie et al. (2017) | |
| PM | Yanamala et al. (2013) | |
| Asbestos | Nishimura et al. (2013), Murthy et al. (2015) | |
| Silica | Barbarin et al. (2004), Barbarin et al. (2005), Ferreira et al. (2013), Cruz et al. (2016) | |
| Nanoparticles | Shvedova et al. (2005) | |
| Chemical warfare agents | Sunil et al. (2011), Venosa et al. (2016) | |
Abbreviations: CCL, chemokine ligand; CTGF, connective tissue growth factor; IL, interleukin; M-CSF, macrophage-colony stimulating factor; MMPs, matrix metalloproteinases; NO, nitric oxide; PAF, platelet-activating factor; PDGF, platelet-derived growth factor; PM, particulate matter; RNS, reactive nitrogen species; ROS, reactive oxygen species; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor.
The strongest evidence supporting a role of hyperactive M2 macrophages in the development of pulmonary fibrosis comes from findings that the response to fibrogenic toxicants (eg, bleomycin, radiation, silica, carbon nanotubes) is exacerbated in animals overexpressing IL-10 or IL-13, or by exogenous administration of IL-33, which promote M2 macrophage activation (Table 3) (Fulkerson et al., 2006; Laskin et al., 2011; Li et al., 2014; Lumsden et al., 2015; Luzina et al., 2013; Wang et al., 2014b). Conversely, collagen production and fibrosis are reduced in animals lacking M-CSF or those treated with chlodronate liposomes or anti-CSF1R antibody, which deplete M2 macrophages (Baran et al., 2007; Gibbons et al., 2011; Meziani et al., 2018; Murray et al., 2011), or serum amyloid P, which inhibits the development of M2 macrophages in the lung (Laskin et al., 2011; Murray et al., 2011; Pilling et al., 2007), and in mice deficient in the M2 macrophage-inducing cytokines IL-4, IL-10, IL-13, IL-18, IL-33, or their receptors (Hoshino et al., 2009; Huaux et al., 1998, 2003; Laskin et al., 2011; Li et al., 2014; Liu et al., 2004; Lumsden et al., 2015; Wang et al., 2014b; Zhao et al., 2018), chemokine receptor CCR4 (Trujillo et al., 2008), Fizz-1, which induces M2 macrophage recruitment and myofibroblast differentiation (Liu et al., 2014), or C/EBP homologous protein, which is required for M2 macrophage activation and TGFβ production (Buck and Chojkier, 2011; Yao et al., 2016). A role for IL-1R1 and MyD88 signaling has also been described in bleomycin-induced lung fibrosis (Gasse et al., 2007).
There is also some experimental evidence that proinflammatory monocyte-derived M1 macrophages drive pulmonary fibrosis (Byrne et al., 2016; Gibbons et al., 2011). Thus, prednisone is effective in reducing TGFβ production and collagen deposition in bleomycin-induced fibrosis, but only if it is administered beginning one day after bleomycin administration, a time associated with a prominent proinflammatory/cytotoxic M1 macrophage response (Chaudhary et al., 2006). Additionally, selective depletion of circulating proinflammatory monocytes, defined in mice by expression of high levels of the surface marker Ly6C (Ly6Chi), leads to decreases in M2 macrophages in the lung, and abrogation of bleomycin-induced pulmonary fibrosis; moreover, adoptive transfer of these cells during fibrogenesis exacerbates fibrosis (Gibbons et al., 2011; Ji et al., 2016). Studies with mice lacking CCR2 or CCL2, or wild-type mice treated with MCP-1 (CCL2) neutralizing antibodies, which have blunted M1-monocyte-derived macrophage emigration to sites of injury, have confirmed the importance of these cells in the development of fibrosis. Thus, in these experimental models, significantly fewer M2 macrophages are observed in the lung following administration of bleomycin or radiation, a response correlated with reduced TGFβ, CTGF, and fibrosis (Baran et al., 2007; Gharaee-Kermani et al., 2003; Gibbons et al., 2011; Groves et al., 2018). In contrast, when proinflammatory monocytes are adoptively transferred into mice during the acute reversible inflammatory response to bleomycin, they have a resolution promoting role (Gibbons et al., 2011). These data demonstrate that there is considerable heterogeneity in lung macrophage functioning during different phases of the fibrogenic process (Misharin et al., 2013).
A clear causal link between inflammatory monocyte-derived lung macrophages and the pathobiology of fibrosis has recently been established by Misharin et al. (2017). Using novel lineage tracking systems, these investigators showed that lung fibrosis is ameliorated when inflammatory monocytes are driven to necroptosis during their differentiation into macrophages. A similar attenuation of pulmonary fibrosis was observed using an inducible transgenic system, which specifically depletes proinflammatory M1 macrophages (McCubbrey et al., 2018). These data provide support for the notion that profibrotic M2 macrophages are largely derived from proinflammatory M1 macrophages. This is in accord with reports that proinflammatory monocytes are also precursors of circulating fibrocytes, which have been shown to be important in pulmonary fibrosis (Gibbons et al., 2011). It should be noted, however, that at present, direct evidence for a precursor relationship between proinflammatory M1-like (Ly6ChiCCR2+) monocytes and M2 macrophages in the lung during fibrosis is limited. In this context, it cannot be ruled out that M2 macrophages are derived from anti-inflammatory monocytes, which express low levels of Ly6C (Ly6Clo) and chemokine receptor CX3CR1, and migrate into the lung from the blood (Gibbons et al., 2011). The fact that reduced number of M2 macrophages are observed in the lungs of bleomycin-treated mice lacking CX3CR1 and that fibrosis is blunted, are consistent with this idea (Ishida et al., 2017). It is also possible that, at least some M2 macrophages originate from proliferating resident alveolar macrophages. This idea is in accord with reports that M2 macrophage accumulation in the lung is reduced in bleomycin-treated CSF-1 knockout mice, which have defective resident macrophage proliferation relative to wild-type mice, a response characterized by reduced CTGF expression and collagen deposition (Baran et al., 2007).
Of note are findings that the M1 macrophage marker, iNOS, as well as the M1-associated chemokine, CXCL10/IP10, are increased in M2-suppressed animals, consistent with the idea that M2 macrophages are important in downregulating M1 macrophage activity, and that it is a balance in the activity between these cell populations that dictates the outcome of the response to fibrogenic stimuli. This is supported by findings that the development of fibrosis in a granulomatous lung disease model is associated with upregulation of arginase-positive M2 macrophages, and that administration of the proinflammatory cytokine, IL-12, results in increases in M1 macrophages, overexpression of iNOS, and reduced fibrosis (Hesse et al., 2000).
Evidence suggests that proinflammatory cytokines produced by M1 macrophages including IL-1 and TNFα play a role in the development of pulmonary fibrosis. Thus, following administration of silica, bleomycin, or nitrogen mustard, IL-1 and TNFα are rapidly upregulated in the lung (Byrne et al., 2015). Moreover, administration of IL-1β or transient overexpression of IL-1β in the lung recapitulates many of the features of bleomycin-induced fibrosis in mice, while blockade or loss of IL-1R1 reduces fibrosis (Byrne et al., 2016; Gasse et al., 2007). Silica-induced fibrosis in rodents is also blunted by neutralization of IL-1β (Guo et al., 2013). Similarly, although infusion of recombinant TNFα produces alterations characteristic of pulmonary fibrosis including fibroblast proliferation, collagen deposition, and cell necrosis, blockade or loss of TNFα activity early in the pathogenic process mitigates fibrosis induced by bleomycin, silica, asbestos, and mustard vesicants, a response associated with decreased expression of TGFβ (Laskin et al., 2011; Liu and Brody, 2001; Malaviya et al., 2015b; Ortiz et al., 1998). Conversely, pulmonary delivery of TNFα to mice with established fibrosis reduces their fibrotic burden and improves lung function and architecture, a response linked to decreases in profibrotic M2 macrophages (Redente et al., 2014). These data demonstrate that the contribution of M1 macrophages to fibrosis is distinct during different phases of the fibrogenic process.
MACROPHAGE-DERIVED MEDIATORS IMPLICATED IN ACUTE LUNG INJURY AND CHRONIC FIBROSIS
Mediators released by inflammatory macrophages are generally not target specific. Thus, their actions depend on the amount and location of release and their persistence in the tissue. In this regard, they may exacerbate acute tissue injury and/or promote chronic fibrosis. Among the more well-established proinflammatory/cytotoxic mediators implicated in tissue injury are ROS (eg, superoxide anion, hydrogen peroxide, hydroxyl radicals) and RNS (eg, nitric oxide, peroxynitrite), proteases (eg, MMPs, TIMPs), lipid mediators (eg, lipid peroxides, PGE2, PAF), and cytokines (eg, TNFα, IL-1, IL-6, IL-12, IL-18)/chemokines (eg, CCL2, CCL3, CCL4, CCL5, CXCL1). Macrophage mediators implicated in chronic fibrosis include cytokines (eg, IL-4, IL-13, IL-33) and growth factors (eg, TGFβ, CTGF, VEGF). As illustrated in Table 3, similar macrophage-derived mediators contribute to the pathogenic effects of diverse toxicants with distinct mechanisms of action. Complicating the understanding of the role of macrophage mediators in the response to toxicants is the fact that they likely act in concert to promote tissue damage and fibrosis. It should also be noted that macrophages are not the only source of proinflammatory/cytotoxic and profibrotic mediators in the lung. Thus, following toxicant exposure, airway and alveolar epithelial cells and fibroblasts also have the capacity to generate cytotoxic/proinflammatory and profibrotic mediators, a response due in part to macrophage-derived products (Hiraiwa et al., 2013). The contribution of mediators released by these cells to pulmonary toxicity, relative to macrophages, remains to be established.
RESIDENT ALVEOLAR MACROPHAGES IN ACUTE LUNG INJURY AND FIBROSIS
Phenotypically and functionally distinct subpopulations of macrophages are localized throughout the lung of healthy individuals and experimental animals; these resident tissue macrophages have been identified as alveolar macrophages, interstitial macrophages, pleural macrophages, intravascular macrophages, and airway macrophages (reviewed in Laskin et al., 2015). The largest and most well characterized are alveolar macrophages, which play a key role in recycling of surfactant. Resident alveolar macrophages are also central to pulmonary immune defense, serving as sentinels, strategically located to respond to invading pathogens and inhaled toxicants. Like other tissue-resident macrophages, they possess an M2-like phenotype, which is key to their role in maintaining homeostasis and protecting against injury and infection (Alber et al., 2012; Morales-Nebreda et al., 2015). In healthy lung, alveolar macrophages are sustained in a restrained state by their interaction with the alveolar epithelium and molecules such as surfactant protein D, CD200, IL-10, TGFβ and a transmembrane glycoprotein, MUC-1, via macrophage receptors like TLR4, CD200R, and SIRPα (Herold et al., 2011; Morales-Nebreda et al., 2015; Snelgrove et al., 2011). Disturbances in the alveolar macrophage-epithelium contact are considered critical events in early inflammatory signaling (Alber et al., 2012). Evidence suggests that resident alveolar macrophages play a role in triggering acute inflammatory responses to noxious stimuli. They have the capacity to recognize danger signals (eg, DAMPs) released from injured and/or necrotic epithelial cells and initiate the inflammatory phase of wound healing. It has been suggested that following injury, epithelial cells release calcium which upregulates macrophage NADPH oxidase leading to hydrogen peroxide production; this triggers the release of HMGB1 and other DAMPs (eg, ATP, hyaluronan, heat shock proteins, heparan sulfate) from necrotic cells (Minutti et al., 2017). DAMPs are recognized by macrophage TLR4 and purigenic receptors; binding to these receptors initiates a signaling process resulting in the release of inflammatory mediators and chemokines and the recruitment of inflammatory cells to sites of injury.
Resident alveolar macrophage activation following pulmonary exposure to noxious stimuli can also occur via inflammasomes (eg, NLRP3). These are cytosolic multiprotein complexes that oligomerize and activate caspase-1 resulting in the generation and release of IL-1 and IL-18, which promote inflammation. Full activation of the NLRP3 inflammasome requires both priming and activating stimuli. Although priming is initiated by DAMPs binding to pattern recognition receptors (PRR) such as TLR4, which causes upregulation of pro-IL-1 and pro-IL-18 and other components of the inflammasome (adaptor protein ASC, procaspase-1), activation involves the assembly of these components into the inflammasome structure followed by production of proinflammatory interleukins. In sterile inflammation, NLRP3 activation occurs following phagocytosis of crystals (eg, cholesterol, urate), particles (eg, silica, titanium), or nanomaterials (eg, carbon nanotubes) (Hosseinian et al., 2015; Nakayama, 2018). Studies suggest that the NLRP3 inflammasome contributes to acute lung injury induced by ozone, titanium nanoparticles, asbestos, silica, and particulate matter (Che et al., 2016; Kim et al., 2017; Michaudel et al., 2016; Sayan and Mossman, 2016).
There are also data showing that the inflammasome and inflammasome-linked cytokines released from resident alveolar macrophages are involved in the development of chronic lung diseases including asthma, COPD, and fibrosis (Grailer et al., 2014). In this regard, acute pulmonary fibrotic changes are associated with increased levels of IL-1β and IL-18 in alveolar macrophages from patients with idiopathic pulmonary fibrosis, as well as asbestos-induced fibrosis (Sayan and Mossman, 2016). Fibrogenic toxicants including cigarette smoke, asbestos, silica, carbon nanotubes, engineered nanomaterials, and bleomycin have all been shown to directly activate the NLRP3 inflammasome in alveolar-resident macrophages leading to IL-1β secretion (Sayan and Mossman, 2016). IL-1β stimulates the release of TGFβ, which triggers activation, proliferation, and differentiation of epithelial cells and fibroblasts into collagen-producing myofibroblasts (dos Santos et al., 2012). Recent studies have demonstrated that depletion of resident macrophages just prior to the onset of bleomycin-induced fibrosis did not alter the severity of the pathology (Misharin et al., 2017). These data suggest that the contribution of resident macrophages to fibrogenesis is more prominent in early phases of the disease process.
Consistent with a role of resident alveolar macrophages in early responses to toxicants and pathogens are recent findings demonstrating that these cells are prone to undergo regulated cell death (ie, pyroptosis, necroptosis, METosis) following toxic exposures or infections (Doster et al., 2018; Ginhoux et al., 2017). These tightly regulated cell death pathways are highly inflammatory and immunogenic. They can be induced by a variety of factors including TLR4 ligands (eg, LPS, HMGB1), RAGE activation, inflammasomes and cytokines like IL-1β and TNFα (Li et al., 2016; Xu et al., 2014), and involve caspase-1 receptor-interacting serine/threonine-protein kinases (RIPK1, RIPK3), mixed lineage kinase domain-like protein, and gasdermin D (Doster et al., 2018; Ginhoux et al., 2017). Resident alveolar macrophage death is thought to be key in triggering the recruitment of circulating monocytes and neutrophils to sites of injury and infection and initiating inflammatory responses (Fan and Fan, 2018). There is increasing recognition that macrophage death and inflammation reciprocally affect one another, forming an auto-amplification loop which exaggerates inflammation (Linkermann et al., 2014).
CONCLUSIONS AND FUTURE DIRECTIONS
Because the respiratory tract is in direct contact with the external environment, it is particularly vulnerable to the adverse effects of inhaled pathogens and toxicants. Macrophages represent an essential host immune defense mechanism against these harmful xenobiotics. However, effective host defense, wound healing, and restoration of homeostasis requires that the activity of macrophages be carefully controlled. In the absence of operative control mechanisms, macrophages become overactivated, resulting in exacerbation of acute injury and/or the development of chronic lung disease. This is complicated by the fact that macrophages do not consist of one homogeneous cell population; rather subpopulations with unique phenotypic and functional properties. Moreover, as macrophages are highly plastic cells, they have the capacity to rapidly change their phenotype. The use of approaches that allow identification and characterization of individual macrophages within a mixed population (eg, single cell RNAseq or western blotting), as well a conditional knockdown of macrophages (eg, Cre-lox) at different times during the pathogenic process will be particularly valuable for assessing the role of these cells in the development of lung disease. Also important will be a focus on the interactions between lung macrophages and epithelial cells in the response to toxicants. In this regard, recent studies demonstrating that epithelial cell-derived microvesicles generated following hyperoxia or toxicant-induced lung injury are key in proinflammatory activation of macrophages, represent an exciting new avenue of research (Lee et al., 2016, 2017). Another area of interest for future investigations is the role of extracellular traps (fibers composed of DNA and proteins) released from inflammatory macrophages (METs) in response to ROS, proteases, and TNFα in the pathogenic response to pulmonary toxicants (Boe et al., 2015; Doster et al., 2018). It has been suggested that a failure to remove METs may prolong inflammatory responses contributing to chronic tissue injury and disease (Boe et al., 2015; King et al., 2017). Understanding pathways regulating macrophage activation and the mediators they release may lead to more efficacious approaches for treating lung diseases and disorders caused by inhaled toxicants.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institutes of Health (U54AR055073, R01ES004738, and P30ES005022).
Supplementary Material
REFERENCES
- Aggarwal N. R., King L. S., D’Alessio F. R. (2014). Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L709–L725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harbi N. O., Imam F., Al-Harbi M. M., Ansari M. A., Zoheir K. M. A., Korashy H. M., Sayed-Ahmed M. M., Attia S. M., Shabanah O. A., Ahmad S. F. (2016). Dexamethasone attenuates LPS-induced acute lung injury through inhibition of NF-κB, COX-2, and pro-inflammatory mediators. Immunol. Invest. 45, 349–369. [DOI] [PubMed] [Google Scholar]
- Alber A., Howie S. E., Wallace W. A., Hirani N. (2012). The role of macrophages in healing the wounded lung. Int. J. Exp. Pathol. 93, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S., Dev K., Agarwal B., Das P., Syed M. A. (2018). Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 223, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus G. S., Howden R., Fostel J., Bauer A. K., Cho H.-Y., Marzec J., Peden D. B., Kleeberger S. R. (2010). Protective role of interleukin-10 in ozone-induced pulmonary inflammation. Environ. Health Perspect. 118, 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran C. P., Opalek J. M., McMaken S., Newland C. A., O’Brien J. M., Hunter M. G., Bringardner B. D., Monick M. M., Brigstock D. R., Stromberg P. C., et al. (2007). Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C., Kielian T., Wyatt T. A., Romberger D. J., West W. W., Gleason A. M., Poole J. A. (2013). Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 48, 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekki K., Ito T., Yoshida Y., He C., Arashidani K., He M., Sun G., Zeng Y., Sone H., Kunugita N., et al. (2016). PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ. Toxicol. Pharmacol. 45, 362–369. [DOI] [PubMed] [Google Scholar]
- Bhalla D. K., Daniels D. S., Luu N. T. (1992). Attenuation of ozone-induced airway permeability in rats by pretreatment with cyclophosphamide, FPL 55712, and indomethacin. Am. J. Respir. Cell Mol. Biol. 7, 73–80. [DOI] [PubMed] [Google Scholar]
- Bhandari V., Choo-Wing R., Homer R. J., Elias J. A. (2007). Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J. Immunol. 178, 4993–5000. [DOI] [PubMed] [Google Scholar]
- Bickelhaupt S., Erbel C., Timke C., Wirkner U., Dadrich M., Flechsig P., Tietz A., Pfohler J., Gross W., Psechke P., et al. (2017). Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J. Natl. Cancer Inst. 109, djw339. [DOI] [PubMed] [Google Scholar]
- Boe D. M., Curtis B. J., Chen M. M., Ippolito J. A., Kovacs E. J. (2015). Extracellular traps and macrophages: new roles for the versatile phagocyte. J. Leukoc. Biol. 97, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Chojkier M. (2011). C/EBPβ-Thr217 phosphorylation signaling contributes to the development of lung injury and fibrosis in mice. PLoS One 6, e25497.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne A. J., Maher T. M., Lloyd C. M. (2016). Pulmonary macrophages: A new therapeutic pathway in fibrosing lung disease? Trends Mol. Med. 22, 303–316. [DOI] [PubMed] [Google Scholar]
- Byrne A. J., Mathie S. A., Gregory L. G., Lloyd C. M. (2015). Pulmonary macrophages: key players in the innate defence of the airways. Thorax 70, 1189–1196. [DOI] [PubMed] [Google Scholar]
- Casey J., Kaplan J., Atochina-Vasserman E. N., Gow A. J., Kadire H., Tomer Y., Fisher J. H., Hawgood S., Savani R. C., Beers M. F. (2005). Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am. J. Respir. Crit. Care Med. 172, 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N. I., Schnapp A., Park J. E. (2006). Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. Am. J. Respir. Crit. Care Med. 173, 769–776. [DOI] [PubMed] [Google Scholar]
- Che L., Jin Y., Zhang C., Lai T., Zhou H., Xia L., Tian B., Zhao Y., Liu J., Wu Y., et al. (2016). Ozone-induced IL-17A and neutrophilic airway inflammation is orchestrated by the caspase-1-IL-1 cascade. Sci. Rep. 6, 18680.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Gong L., Zhang L., Wang H., Qi X., Wu X., Xiao Y., Cai Y., Liu L., Li X., et al. (2006). Short courses of low dose dexamethasone delay bleomycin-induced lung fibrosis in rats. Eur. J. Pharmacol. 536, 287–295. [DOI] [PubMed] [Google Scholar]
- Connor A. J., Laskin J. D., Laskin D. L. (2012). Ozone-induced lung injury and sterile inflammation. Role of toll-like receptor 4. Exp. Mol. Pathol. 92, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal K., Scholefield E., Ferenbach D., Gibbons M., Duffin R., Dorward D. A., Morris A. C., Humphries D., MacKinnon A., Wilkinson T. S., et al. (2012). Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am. J. Respir. Crit. Care Med. 186, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos G., Kutuzov M. A., Ridge K. M. (2012). The inflammasome in lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L627–L633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster R. S., Rogers L. M., Gaddy J. A., Aronoff D. M. (2018). Macrophage extracellular traps: A scoping review. J. Innate Immun. 10, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke K. S., Bonner J. C. (2018). Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10, e1498.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru N., Wolfson B., Zhou Q. (2016). Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World J. Biol. Chem. 7, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan E. K. Y., Fan J. (2018). Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 19, 50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulknor R. A., Olekson M. A., Ekwueme E. C., Krzyszczyk P., Freeman J. W., Berthiaume F. (2017). Hypoxia impairs mesenchymal stromal cell-induced macrophage M1 to M2 transition. Technology 5, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M., Groves A. M., Sun R., Cervelli J. A., Choi H., Laskin J. D., Laskin D. L. (2017). CCR2 regulates inflammatory cell accumulation in the lung and tissue injury following ozone exposure. Toxicol. Sci. 155, 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. A., Birch M. E., Yadav J. S. (2015). MyD88 mediates in vivo effector functions of alveolar macrophages in acute lung inflammatory responses to carbon nanotube exposure. Toxicol. Appl. Pharmacol. 288, 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulkerson P. C., Fischetti C. A., Hassman L. M., Nikolaidis N. M., Rothenberg M. E. (2006). Persistent effects induced by IL-13 in the lung. Am. J. Respir. Cell Mol. Biol. 35, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasse P., Mary C., Guenon I., Noulin N., Charron S., Schnyder-Candrian S., Schnyder B., Akira S., Quesniaux V. F. J., Lagente V., et al. (2007). IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Invest. 117, 3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng P., Ma T., Xing J., Jiang L., Sun H., Zhu B., Zhang H., Xiao H., Wang J., Zhang J. (2018). Dexamethasone ameliorates H2S-induced acute lung injury by increasing claudin-5 expression via the PI3K pathway. Hum. Exp. Toxicol. 37, 626–635. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M., McCullumsmith R. E., Charo I. F., Kunkel S. L., Phan S. H. (2003). CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 24, 266–276. [DOI] [PubMed] [Google Scholar]
- Gibbons M. A., MacKinnon A. C., Ramachandran P., Dhaliwal K., Duffin R., Phythian-Adams A. T., van Rooijen N., Haslett C., Howie S. E., Simpson A. J., et al. (2011). Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 184, 569–581. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Bleriot C., Lecuit M. (2017). Dying for a cause: Regulated necrosis of tissue-resident macrophages upon infection. Trends Immunol. 38, 693–695. [DOI] [PubMed] [Google Scholar]
- Grailer J. J., Canning B. A., Kalbitz M., Haggadone M. D., Dhond R. M., Andjelkovic A. V., Zetoune F. S., Ward P. A. (2014). Critical role for the NLRP3 inflammasome during acute lung injury. J. Immunol. 192, 5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. M., Gow A. J., Massa C. B., Hall L., Laskin J. D., Laskin D. L. (2013). Age-related increases in ozone-induced injury and altered pulmonary mechanics in mice with progressive lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L555–L568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. M., Gow A. J., Massa C. B., Laskin J. D., Laskin D. L. (2012). Prolonged injury and altered lung function after ozone inhalation in mice with chronic lung inflammation. Am. J. Respir. Cell Mol. Biol. 47, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. M., Johnston C. J., Williams J. P., Finkelstein J. N. (2018). Role of infiltrating monocytes in the development of radiation-induced pulmonary fibrosis. Radiat. Res. 189, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundra U. M., Girgis N. M., Ruckerl D., Jenkins S., Ward L. N., Kurtz Z. D., Wiens K. E., Tang M. S., Basu-Roy U., Mansukhani A., et al. (2014). Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123, e110–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Gu N., Chen J., Shi T., Zhou Y., Rong Y., Zhou T., Yang W., Cui X., Chen W. (2013). Neutralization of interleukin-1β attenuates silica-induced lung inflammation and fibrosis in C57BL/6 mice. Arch. Toxicol. 87, 1963–1973. [DOI] [PubMed] [Google Scholar]
- Hashimoto N., Kawabe T., Imaizumi K., Hara T., Okamoto M., Kojima K., Shimokata K., Hasegawa Y. (2004). CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 30, 808–815. [DOI] [PubMed] [Google Scholar]
- He M., Ichinose T., Song Y., Yoshida Y., Bekki K., Arashidani K., Yoshida S., Nishikawa M., Takano H., Shibamoto T., et al. (2016). Desert dust induces TLR signaling to trigger Th2-dominant lung allergic inflammation via a MyD88-dependent signaling pathway. Toxicol. Appl. Pharmacol. 296, 61–72. [DOI] [PubMed] [Google Scholar]
- Herold S., Mayer K., Lohmeyer J. (2011). Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2, 65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M., Cheever A. W., Jankovic D., Wynn T. A. (2000). NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am. J. Pathol. 157, 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa K., van Eeden S. F. (2013). Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013, 619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth J. W., Cook D. N., Brass D. M., Walker J. K. L., Morgan D. L., Foster W. M., Schwartz D. A. (2004). The role of Toll-like receptor 4 in environmental airway injury in mice. Am. J. Respir. Crit. Care Med. 170, 126–132. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Okamoto M., Sakazaki Y., Kato S., Young H. A., Aizawa H. (2009). Role of proinflammatory cytokines IL-18 and IL-1β in bleomycin-induced lung injury in humans and mice. Am. J. Respir. Cell Mol. Biol. 41, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinian N., Cho Y., Lockey R. F., Kolliputi N. (2015). The role of the NLRP3 inflammasome in pulmonary diseases. Ther. Adv. Respir. Dis. 9, 188–197. [DOI] [PubMed] [Google Scholar]
- Huang B., Wang D. X., Deng W. (2014). Protective effects of dexamethasone on early acute lung injury induced by oleic acid in rats. Int. J. Clin. Exp. Med. 7, 4698–4709. [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhang W., Yu F., Gao F. (2017). The cellular and molecular mechanism of radiation-induced lung injury. Med. Sci. Monit. 23, 3446–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F., Liu T., McGarry B., Ullenbruch M., Phan S. H. (2003). Dual roles of IL-4 in lung injury and fibrosis. J. Immunol. 170, 2083–2092. [DOI] [PubMed] [Google Scholar]
- Huaux F., Louahed J., Hudspith B., Meredith C., Delos M., Renauld J. C., Lison D. (1998). Role of interleukin-10 in the lung response to silica in mice. Am. J. Respir. Cell Mol. Biol. 18, 51–59. [DOI] [PubMed] [Google Scholar]
- Hussell T., Bell T. J. (2014). Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 14, 81–93. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Kimura A., Nosaka M., Kuninaka Y., Hemmi H., Sasaki I., Kaisho T., Mukaida N., Kondo T. (2017). Essential involvement of the CX3CL1-CX3CR1 axis in bleomycin-induced pulmonary fibrosis via regulation of fibrocyte and M2 macrophage migration. Sci. Rep. 7, 16833.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W.-J., Ma Y.-Q., Zhang X., Zhang L., Zhang Y.-D., Su C.-C., Xiang G.-A., Zhang M.-P., Lin Z.-C., Wei L.-Q., et al. (2016). Inflammatory monocyte/macrophage modulation by liposome-entrapped spironolactone ameliorates acute lung injury in mice. Nanomedicine 11, 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara K., Ohashi W., Tomita K., Takashina M., Fujisaka S., Hayashi R., Mori H., Tobe K., Hattori Y. (2015). In vivo depletion of CD206+ M2 macrophages exaggerates lung injury in endotoxemic mice. Am. J. Pathol. 185, 162–171. [DOI] [PubMed] [Google Scholar]
- Kim B. G., Lee P. H., Lee S. H., Park M. K., Jang A. S. (2017). Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol. Res. 9, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. T., Sharma R., O’Sullivan K. M., Callaghan J., Dousha L., Thomas B., Ruwanpura S., Lim S., Farmer M. W., Jennings B. R., et al. (2017). Deoxyribonuclease 1 reduces pathogenic effects of cigarette smoke exposure in the lung. Sci. Rep. 7, 12128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin D. L., Malaviya R., Laskin J. D.. 2015. Pulmonary macrophages In Comparative Biology of the Normal Lung (Parent R. A., Ed.), pp. 629–649. Academic Press, San Diego, CA. [Google Scholar]
- Laskin D. L., Sunil V. R., Gardner C. R., Laskin J. D. (2011). Macrophages and tissue injury: agents of defense or destruction? Ann. Rev. Pharmacol. Toxicol. 51, 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T., Natoli G. (2011). Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761. [DOI] [PubMed] [Google Scholar]
- Lee H., Zhang D., Wu J., Otterbein L. E., Jin Y. (2017). Lung epithelial cell-derived microvesicles regulate macrophage migration via microRNA-17/221-induced integrin β1 recycling. J. Immunol. 199, 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Zhang D., Zhu Z., Dela Cruz C. S., Jin Y. (2016). Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 6, 35250.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Guabiraba R., Besnard A.-G., Komai-Koma M., Jabir M. S., Zhang L., Graham G. J., Kurowska-Stolarska M., Liew F. Y., McSharry C., et al. (2014). IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 134, 1422–1432.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Scott M. J., Fan E. K., Li Y., Liu J., Xiao G., Li S., Billiar T. R., Wilson M. A., Jiang Y., et al. (2016). Tissue damage negatively regulates LPS-induced macrophage necroptosis. Cell Death Differ. 23, 1428–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A., Stockwell B. R., Krautwald S., Anders H. J. (2014). Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat. Rev. Immunol. 14, 759–767. [DOI] [PubMed] [Google Scholar]
- Liu J. Y., Brody A. R. (2001). Increased TGF-β1 in the lungs of asbestos-exposed rats and mice: reduced expression in TNF-α receptor knockout mice. J. Environ. Pathol. Toxicol. Oncol. 20, 97–108. [PubMed] [Google Scholar]
- Liu T., Jin H., Ullenbruch M., Hu B., Hashimoto N., Moore B., McKenzie A., Lukacs N. W., Phan S. H. (2004). Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: Role of IL-4/IL-13 and mediation via STAT-6. J. Immunol. 173, 3425–3431. [DOI] [PubMed] [Google Scholar]
- Liu T., Yu H., Ullenbruch M., Jin H., Ito T., Wu Z., Liu J., Phan S. H. (2014). The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One 9, e88362.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. L., Huang X. Y., Luo Y. F., Tan W. P., Chen P. F., Guo Y. B. (2018). Activation of M1 macrophages plays a critical role in the initiation of acute lung injury. Biosci. Rep. 38, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden R. V., Worrell J. C., Boylan D., Walsh S. M., Cramton J., Counihan I., O’Beirne S., Medina M. F., Gauldie J., Fabre A., et al. (2015). Modulation of pulmonary fibrosis by IL-13α2. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L710–L718. [DOI] [PubMed] [Google Scholar]
- Luzina I. G., Kopach P., Lockatell V., Kang P. H., Nagarsekar A., Burke A. P., Hasday J. D., Todd N. W., Atamas S. P. (2013). Interleukin-33 potentiates bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 49, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Gow A. J., Francis M., Abramova E. V., Laskin J. D., Laskin D. L. (2015a). Radiation-induced lung injury and inflammation in mice: Role of inducible nitric oxide synthase and surfactant protein D. Toxicol. Sci. 144, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Sunil V. R., Venosa A., Verissimo V. L., Cervelli J. A., Vayas K. N., Hall L., Laskin J. D., Laskin D. L. (2015b). Attenuation of nitrogen mustard-induced pulmonary injury and fibrosis by anti-tumor necrosis factor-α antibody. Toxicol. Sci. 148, 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R., Laskin J. D., Laskin D. L. (2017). Anti-TNFα therapy in inflammatory lung diseases. Pharmacol. Ther. 180, 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini T., Wolf D., Michel N. A., Mauler M., Dufner B., Hoppe N., Beckert J., Jackel M., Magnani N., Duerschmied D., et al. (2016). Acute exposure to air pollution particulate matter aggravates experimental myocardial infarction in mice by potentiating cytokine secretion from lung macrophages. Basic Res. Cardiol. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F. O., Gordon S. (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa A., Hogaboam C. M., Lukacs N. W., Lincoln P. M., Evanoff H. L., Strieter R. M., Kunkel S. L. (2000). Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J. Immunol. 164, 2738–2744. [DOI] [PubMed] [Google Scholar]
- McCubbrey A. L., Barthel L., Mohning M. P., Redente E. F., Mould K. J., Thomas S. M., Leach S. M., Danhorn T., Gibbings S. L., Jakubzick C. V., et al. (2018). Deletion of c-FLIP from CD11bhi macrophages prevents development of bleomycin-induced lung fibrosis. Am. J. Respir. Cell Mol. Biol. 58, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metchnikoff E. 1968. Lectures on the Comparative Pathology of Inflammation Delivered at the Pasteur Institute in 1891. Dover, New York. [Google Scholar]
- Meziani L., Mondini M., Petit B., Boissonnas A., Thomas de Montpreville V., Mercier O., Vozenin M. C., Deutsch E. (2018). CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur. Respir. J. 51, doi: 10.1183/13993003.13902120-13992017. [DOI] [PubMed] [Google Scholar]
- Michaudel C., Couturier-Maillard A., Chenuet P., Maillet I., Mura C., Couillin I., Gombault A., Quesniaux V. F., Huaux F., Ryffel B. (2016). Inflammasome, IL-1 and inflammation in ozone-induced lung injury. Am. J. Clin. Exp. Immunol. 5, 33–40. [PMC free article] [PubMed] [Google Scholar]
- Minutti C. M., Knipper J. A., Allen J. E., Zaiss D. M. (2017). Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 61, 3–11. [DOI] [PubMed] [Google Scholar]
- Misharin A. V., Morales-Nebreda L., Mutlu G. M., Budinger G. R., Perlman H. (2013). Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 49, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin A. V., Morales-Nebreda L., Reyfman P. A., Cuda C. M., Walter J. M., McQuattie-Pimentel A. C., Chen C.-I., Anekalla K. R., Joshi N., Williams K. J. N., et al. (2017). Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 214, 2387–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Nebreda L., Misharin A. V., Perlman H., Budinger G. R. (2015). The heterogeneity of lung macrophages in the susceptibility to disease. Eur. Respir. Rev. 24, 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Redetzky H. C., Suttorp N., Witzenrath M. (2014). Dynamics of pulmonary endothelial barrier function in acute inflammation: Mechanisms and therapeutic perspectives. Cell Tissue Res. 355, 657–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. A., Chen Q., Kramer M. S., Hesson D. P., Argentieri R. L., Peng X., Gulati M., Homer R. J., Russell T., van Rooijen N., et al. (2011). TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int. J. Biochem. Cell Biol. 43, 154–162. [DOI] [PubMed] [Google Scholar]
- Murray P. J. (2017). Macrophage polarization. Annu. Rev. Physiol. 79, 541–566. [DOI] [PubMed] [Google Scholar]
- Murthy S., Larson-Casey J. L., Ryan A. J., He C., Kobzik L., Carter A. B. (2015). Alternative activation of macrophages and pulmonary fibrosis are modulated by scavenger receptor, macrophage receptor with collagenous structure. Faseb J. 29, 3527–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M. (2018). Macrophage recognition of crystals and nanoparticles. Front. Immunol. 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemmar A., Nemery B., Hoet P. H., Van Rooijen N., Hoylaerts M. F. (2005). Silica particles enhance peripheral thrombosis: Key role of lung macrophage-neutrophil cross-talk. Am. J. Respir. Crit. Care Med. 171, 872–879. [DOI] [PubMed] [Google Scholar]
- Ortiz L. A., Lasky J., Hamilton R. F., Holian A., Hoyle G. W., Banks W., Peschon J. J., Brody A. R., Lungarella G., Friedman M. (1998). Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp. Lung Res. 24, 721–743. [DOI] [PubMed] [Google Scholar]
- Parisi L., Gini E., Baci D., Tremolati M., Fanuli M., Bassani B., Farronato G., Bruno A., Mortara L. (2018). Macrophage polarization in chronic inflammatory diseases: Killers or builders? J. Immunol. Res. 2018, 8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rial S., del Puerto-Nevado L., Terron-Exposito R., Giron-Martinez A., Gonzalez-Mangado N., Peces-Barba G. (2013). Role of recently migrated monocytes in cigarette smoke-induced lung inflammation in different strain of mice. PLoS One 8, e72975.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D., Roife D., Wang M., Ronkainen S. D., Crawford J. R., Travis E. L., Gomer R. H. (2007). Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J. Immunol. 179, 4035–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole J. A., Gleason A. M., Bauer C., West W. W., Alexis N., van Rooijen N., Reynolds S. J., Romberger D. J., Kielian T. L. (2012). CD11c+/CD11b+ cells are critical for organic dust-elicited murine lung inflammation. Am. J. Respir. Cell Mol. Biol. 47, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheray F., Viaud S., Rimaniol A. C., Leone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. (2005). Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasse A., Pechkovsky D. V., Toews G. B., Jungraithmayr W., Kollert F., Goldmann T., Vollmer E., Müller-Quernheim J., Zissel G. (2006). A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am. J. Respir. Crit. Care Med. 173, 781–792. [DOI] [PubMed] [Google Scholar]
- Pulichino A.-M., Wang I.-M., Caron A., Mortimer J., Auger A., Boie Y., Elias J. A., Kartono A., Xu L., Menetski J., et al. (2008). Identification of transforming growth factor β1-driven genetic programs of acute lung fibrosis. Am. J. Respir. Cell Mol. Biol. 39, 324–336. [DOI] [PubMed] [Google Scholar]
- Re S. L., Giordano G., Yakoub Y., Devosse R., Uwambayinema F., Couillin I., Ryffel B., Marbaix E., Lison D., Huaux F. (2014). Uncoupling between inflammatory and fibrotic responses to silica: Evidence from MyD88 knockout mice. PLoS One 9, e99383.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redente E. F., Keith R. C., Janssen W., Henson P. M., Ortiz L. A., Downey G. P., Bratton D. L., Riches D. W. H. (2014). Tumor necrosis factor-α accelerates the resolution of established pulmonary fibrosis in mice by targeting profibrotic lung macrophages. Am. J. Respir. Cell Mol. Biol. 50, 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero F., Shah D., Duong M., Penn R. B., Fessler M. B., Madenspacher J., Stafstrom W., Kavuru M., Lu B., Kallen C. B., et al. (2015). A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am. J. Respir. Cell Mol. Biol. 53, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszer T. (2015). Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet J. M., Ghio A. J., Costa D. L., Madden M. C. (2000). Increased expression of cyclooxygenase 2 mediates oil fly ash-induced lung injury. Exp. Lung Res. 26, 57–69. [DOI] [PubMed] [Google Scholar]
- Sayan M., Mossman B. T. (2016). The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre Toxicol. 13, 51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova A. A., Kisin E. R., Mercer R., Murray A. R., Johnson V. J., Potapovich A. I., Tyurina Y. Y., Gorelik O., Arepalli S., Schwegler-Berry D., et al. (2005). Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L698–L708. [DOI] [PubMed] [Google Scholar]
- Snelgrove R. J., Godlee A., Hussell T. (2011). Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 32, 328–334. [DOI] [PubMed] [Google Scholar]
- Sugiura H., Ichinose M. (2011). Nitrative stress in inflammatory lung diseases. Nitric Oxide 25, 138–144. [DOI] [PubMed] [Google Scholar]
- Sunil V. R., Francis M., Vayas K. N., Cervelli J. A., Choi H., Laskin J. D., Laskin D. L. (2015). Regulation of ozone-induced lung inflammation and injury by the β-galactoside-binding lectin galectin-3. Toxicol. Appl. Pharmacol. 284, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A., Wadsworth S., Dorscheid D., Man S. F., Sin D. D. (2011). The airway epithelium: more than just a structural barrier. Ther. Adv. Respir. Dis. 5, 255–273. [DOI] [PubMed] [Google Scholar]
- Tighe R. M., Li Z., Potts E. N., Frush S., Liu N., Gunn M. D., Foster W. M., Noble P. W., Hollingsworth J. W. (2011). Ozone inhalation promotes CX3CR1-dependent maturation of resident lung macrophages that limit oxidative stress and inflammation. J. Immunol. 187, 4800–4808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Trujillo G., O’Connor E. C., Kunkel S. L., Hogaboam C. M. (2008). A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 172, 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou C.-L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007). Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannella K. M., Wynn T. A. (2017). Mechanisms of organ injury and repair by macrophages. Annu. Rev. Physiol. 79, 593–617. [DOI] [PubMed] [Google Scholar]
- Venosa A., Malaviya R., Choi H., Gow A. J., Laskin J. D., Laskin D. L. (2016). Characterization of distinct macrophage subpopulations during nitrogen mustard-induced lung injury and fibrosis. Am. J. Respir. Cell Mol. Biol. 54, 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Liang H., Zen K. (2014a). Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 5, 614.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Shannahan J. H., Brown J. M. (2014b). IL-33 modulates chronic airway resistance changes induced by multi-walled carbon nanotubes. Inhal. Toxicol. 26, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger B., Laskin J. D., Sunil V. R., Sinko P. J., Heck D. E., Laskin D. L. (2011). Sulfur mustard-induced pulmonary injury: Therapeutic approaches to mitigating toxicity. Pulm. Pharmacol. Ther. 24, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesemann A., Ketteler J., Slama A., Wirsdorfer F., Hager T., Rock K., Engel D. R., Fischer J. W., Aigner C., Jendrossek V., et al. (2018). Inhibition of radiation-induced Ccl2 signaling protects lungs from vascular dysfunction and endothelial cell loss. Antioxid. Redox Signal., doi: 10.1089/ars.2017.7458. [DOI] [PubMed] [Google Scholar]
- Wigenstam E., Rocksen D., Ekstrand-Hammarstrom B., Bucht A. (2009). Treatment with dexamethasone or liposome-encapsuled vitamin E provides beneficial effects after chemical-induced lung injury. Inhal. Toxicol. 21, 958–964. [DOI] [PubMed] [Google Scholar]
- Wynn T. A., Vannella K. M. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang G.-A., Zhang Y.-D., Su C.-C., Ma Y.-Q., Li Y.-M., Zhou X., Wei L.-Q., Ji W.-J. (2016). Dynamic changes of mononuclear phagocytes in circulating, pulmonary alveolar and interstitial compartments in a mouse model of experimental silicosis. Inhal. Toxicol. 28, 393–402. [DOI] [PubMed] [Google Scholar]
- Xu J., Jiang Y., Wang J., Shi X., Liu Q., Liu Z., Li Y., Scott M. J., Xiao G., Li S., et al. (2014). Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 21, 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Tong L., Wang D., Wang Y., Wang X., Bai C. (2010). Roles of CC chemokine receptors (CCRs) on lipopolysaccharide-induced acute lung injury. Respir. Physiol. Neurobiol. 170, 253–259. [DOI] [PubMed] [Google Scholar]
- Yao Y., Wang Y., Zhang Z., He L., Zhu J., Zhang M., He X., Cheng Z., Ao Q., Cao Y., et al. (2016). Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol. Ther. 24, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Okamoto Y., Asano Y., Ishimaru K., Aki S., Yoshioka K., Takuwa N., Wada T., Inagaki Y., Takahashi C., et al. (2018). Sphingosine-1-phosphate receptor-2 facilitates pulmonary fibrosis through potentiating IL-13 pathway in macrophages. PLoS One 13, e0197604.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.