Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a plasticizer used in a variety of consumer products. This is concerning because DEHP is an endocrine disruptor and ovarian toxicant. Diisononyl phthalate (DiNP) is a DEHP replacement that is a rising human toxicant due to its increased use as a DEHP substitute. However, little is known about the effects of DEHP or DiNP exposure during adulthood on female reproduction. Thus, this study tested the hypothesis that DEHP or DiNP exposure during adulthood has long-term consequences for female reproduction in mice. Adult female CD-1 mice (39–40 days) were orally dosed with vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day) for 10 days. Females were paired with untreated male mice for breeding trials immediately post-dosing and again at 3 and 9 months post-dosing. Immediately post-dosing, DEHP and DiNP did not affect fertility. At 3 months post-dosing, DiNP (20 and 100 µg/kg/day and 200 mg/kg/day) significantly disrupted estrous cyclicity, and DiNP and DEHP (20 µg/kg/day) significantly reduced the ability of females to get pregnant. At 9 months post-dosing, DiNP significantly disrupted estrous cyclicity (100 µg/kg/day), reduced time to mating (100 µg/kg/day–200 mg/kg/day), and borderline reduced percent of females who produced offspring (20 mg/kg/day). At 9 months post-dosing, DEHP (200 µg/kg/day and 200 mg/kg/day) and DiNP (100 µg/kg/day and 20 and 200 mg/kg/day) increased numbers of male-biased litters. These data show that DEHP and DiNP exposure has long-term consequences for female reproduction, even long after cessation of exposure.
Keywords: phthalates, female reproduction, ovary, fertility, endocrine disruptors, reproductive toxicology
Phthalates are chemicals commonly used as plasticizers and stabilizers in a wide variety of consumer products. One of the most common phthalates is di(2-ethylhexyl) phthalate (DEHP), with up to 100 million pounds produced within the U.S. each year (NTP, 2016; U.S. Department of Health and Human Services, PHS, Agency for Toxic Substances and Disease Registry, 2002). DEHP can be found in products such as surgical tubing, blood bags, building materials, clothes, and baby toys (U.S. Department of Health and Human Services, PHS, Agency for Toxic Substances and Disease Registry, 2002). Because DEHP is noncovalently bound to the products it is used in, it can leach out over time and be absorbed via inhalation, ingestion, and dermal contact (Chen et al., 2008; Schettler 2006; U.S. Department of Health and Human Services, PHS, Agency for Toxic Substances and Disease Registry, 2002). Due to the ubiquitous nature of DEHP environmental contamination, the estimated average daily intake of DEHP is 3–30 µg/kg/day (U.S. Department of Health and Human Services, PHS, Agency for Toxic Substances and Disease Registry, 2002).
Widespread and daily exposure of humans to DEHP is concerning because DEHP is a known reproductive toxicant and endocrine disrupting chemical (Chiang et al., 2017; Hannon and Flaws, 2015; Kay et al., 2014; Rattan et al., 2017). DEHP exposure has been shown to reduce testosterone levels and decrease sperm quantity and quality in mice (Barakat et al., 2017; Zhang et al., 2013) and it is associated with decreased sperm concentration and motility in humans (Chang et al., 2017; Hoyer et al., 2018). Further, DEHP exposure disrupts ovarian folliculogenesis and steroidogenesis in female mice (Brehm et al., 2018; Rattan et al., 2018) and it is associated with preterm delivery in women and premature thelarche in girls (Durmaz et al., 2018; Meeker et al., 2009).
Possibly due to increased public awareness of the endocrine disrupting effects of DEHP, some manufacturers have elected to substitute DEHP with other plasticizers. Even with some manufacturers replacing DEHP, the general population is still exposed to DEHP daily, and some populations are exposed to even higher levels due to occupation or medical treatment (Faouzi et al., 1999; Hines et al., 2009; Inoue et al., 2005; Mallow and Fox, 2014). However, few studies have examined how DEHP affects female reproductive parameters overtime. Thus, it is critical to fill this gap in knowledge.
Although the movement to replace DEHP with a less toxic plasticizer is a step in the right direction, often these substitute chemicals have not been rigorously tested for toxicity, especially in regards to endocrine disruption. Diisononyl phthalate (DiNP) is one such understudied DEHP substitute. Few reproductive studies using DiNP have been conducted, but some studies have found that perinatal exposure to DiNP causes histopathological changes in the testes, and prenatal exposure to DiNP disrupts steroidogenesis in the testes in a similar manner to DEHP in rats (Boberg et al., 2011; Borch et al., 2004; Hannas et al., 2011; Li et al., 2015; Masutomi et al., 2003; SCENIHR, 2008). In men, DiNP exposure has been associated with reduced testicular volume, semen volume, and testosterone levels (Axelsson et al., 2015; Joensen et al., 2012; Specht et al., 2014).
Even fewer studies have been conducted on the effects of DiNP on female reproduction. One study found that DiNP reduced corpora lutea number and ovarian and uterine weight at puberty in rats (Masutomi et al., 2003). Although these studies have shed some light on the effects of DiNP on reproduction, they often use doses well above the levels of human exposure. It is imperative to use environmentally relevant doses because effects may not be seen at these higher doses due to endocrine disrupting chemicals sometimes exhibiting nonmonotonic dose response curves (Vandenberg et al., 2012).
In this study, we aim to fill the gaps in knowledge concerning the short- and long-term reproductive consequences of subchronic exposure to DEHP and DiNP in female mice. A previous study in our laboratory has shown that 10 days of exposure to DEHP during adulthood is sufficient to alter folliculogenesis in female mice (Hannon et al., 2016). Thus, this study tested the hypothesis that 10 days of exposure to DEHP or DiNP during adulthood can significantly affect female fertility at time points immediately, 3, and 9 months post-dosing.
MATERIALS AND METHODS
Chemicals
DEHP and DiNP were purchased from Sigma-Aldrich (St Louis, Missouri). Corn oil (vehicle control) was purchased from MP Biomedicals (Solon, Ohio). Dosing solutions were created by using the highest concentration dose of stock solution (200 mg/kg/day for both DEHP and DiNP) in serial dilutions to make lower dose stock solutions (20, 200 µg/kg/day, and 20 mg/kg/day DEHP and 20, 100 µg/kg/day, and 20 mg/kg/day DiNP). Stock solutions for DEHP doses were prepared at concentrations of 133.33, 13.33, 0.13, and 0.013 mg/ml from the highest to lowest dose. Stock solutions for DiNP doses were prepared at concentrations of 133.33, 13.33, 0.067, and 0.013 mg/ml from the highest to lowest dose.
These doses for DEHP were chosen because 20 µg/kg/day is the U.S. Environmental Protection Agency (EPA) reference dose and also falls within the range of estimated daily exposure to DEHP (3–30 µg/kg/day) (Doull et al., 1999; US EPA 2000). The 200 µg/kg/day DEHP dose falls within the estimated range for occupational exposure (143–286 µg/kg/day) (Kavlock et al., 2002). The 20 and 200 mg/kg/day doses of DEHP were chosen because they were used in previous studies and provide a wide range to aid in observation of dose response effects (Hannon et al., 2014, 2016). The doses for DiNP were chosen because 20 µg/kg/day falls within the range of occupational exposure to DiNP (up to 26 µg/kg/day) (Hines et al., 2012). The 100 µg/kg/day dose was chosen because it falls within the range of estimated daily exposure for infants chewing on plastic toys (up to 260 µg/kg/day) (US CPSC, 2001). The 20 and 200 mg/kg/day doses were chosen because they allow for direct comparison of toxicity to DEHP throughout the study.
Animals and dosing
Adult CD-1 mice were purchased from Charles River Laboratories (Wilmington, Massachusetts) and were allowed to acclimate to the facility prior to the beginning of their dosing at age 39–40 days. Mice were given ad libitum access to reverse osmosis treated water and Teklad Rodent Diet (8604). Facilities were maintained at 21.1°C ± 2.2°C with humidity at 50% ± 20% and 12-h light-dark cycles to provide a consistent and controlled housing environment. All animal handling and procedures used in this study were approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee (Protocol No.: 17079).
Mice were orally dosed with the vehicle control (corn oil), DEHP (20, 200 µg/kg/day, 20, or 200 mg/kg/day), or DiNP (20, 100, 20 µg/kg/day, 20, or 200 mg/kg/day) daily for 10 consecutive days every morning (2 h following the start of the light cycle). Volume of dose was determined using a spreadsheet to find the corresponding volume given the weight of the mouse immediately before dosing each day. Mice were group housed 3 to a cage during the dosing period, with every mouse in a single cage receiving the same treatment. A single cohort of mice was designated for breeding trials and estrous cyclicity monitoring and this cohort was used continuously throughout the study at every time point. The sample size for this cohort was 24 mice in control and 12 mice for every dose of DEHP and DiNP for every time point unless data were missing for any mouse/mice. For tissue collection, each time point used a separate cohort of mice. The sample size for these cohorts of mice designated for tissue collection was 12 mice in control and 6 mice for every dose of DEHP and DiNP for every time point unless data were missing for any mouse/mice.
Estrous cyclicity monitoring
Mice were group housed (3/cage) and vaginally lavaged with 1× phosphate-buffered saline daily (2 h following the start of the light cycle) for 14 days prior to each breeding trial occurring at 3 and 9 months post-dosing (n = 12–24 mice/group per time point unless data were unavailable for any mouse/mice). The stage of the estrous cycle was determined by examining the lavage sample under a light microscope. The type, amount, and condition of the cells present were used to determine if the mouse was in estrus, metestrus, diestrus, or proestrus as previously defined (Hartman, 1944). Metestrus and diestrus were combined in the analyses because of their similarity in behavioral and hormonal profiles. Percent time spent in each stage of the cycle was determined by taking the number of days in each stage (proestrus, estrus, and metestrus/diestrus) divided by 14 and multiplied by 100.
Breeding trials and fertility indices
Females were paired with an untreated CD-1 male mouse (age 7 weeks) in a harem fashion, with 2 females plus a male in a new, clean cage. Females were checked in the morning and afternoon for the presence of copulatory plugs. If a copulatory plug was observed, the female was removed and placed in a new, clean cage. Females were group housed until 3–7 days before predicted birth. If no copulatory plug was observed, the female remained with the male until a maximum of 14 days had passed. If a plug was found the morning immediately following the day of male introduction, the time to mating was calculated as 1 day. From the time of pairing until birth, females were weighed twice a week to monitor weight gain due to pregnancy. If a weight gain of 4 g was observed, the female was considered pregnant. Females were checked morning and afternoon for birth. To calculate the length of gestation, the first day the copulatory plug was observed was considered day 0.5 of the pregnancy. The fertility index was calculated by dividing the number of pregnant females by the number of females who presented with a copulatory plug and multiplying by 100. The gestational index was calculated by dividing the number of females who gave birth to pups by the number of pregnant females and multiplying by 100. The index for females who gave birth was calculated by dividing the number of females who gave birth to pups by the number of total females in each treatment group and multiplying by 100. If a female gave birth, the first day pups were observed was considered to be postnatal day (PND) 0, and total pup number, number of females and males, number of dead pups, and average weight of all live pups were recorded. Male-to-female ratio of pups was calculated by dividing the number of male pups by the number of female pups. Pup mortality was calculated by dividing the number of pups who died by the total number of pups in the litter and multiplying by 100. Average live weight was measured by collectively weighing all live pups on PND 0 and dividing the total weight by the number of live pups. If the number of total pups or the number of males or females could not be determined due to cannibalism, the data for these parameters were not used for that litter.
Tissue collection
Mice were group housed (3/cage) until each cohort was collected immediately following dosing, 3 months following dosing, and 9 months following dosing. Mice were euthanized via CO2 asphyxiation in the diestrous stage of the estrous cycle. Ovaries were collected and the gonadal fat was trimmed away before weighing. The uteri were collected with the oviducts and the cervix attached and fat was trimmed away before weighing. The livers were collected and weighed for each animal.
Statistical analysis
Data were analyzed using SPSS statistical software (SPSS Inc., Chicago, Illinois). Data were presented as means ± SE or as percentages. If data were normally distributed and met the assumption of homogeneity of variances, means were compared using a 1-way analysis of variance (ANOVA) followed by Dunnett’s t test post hoc. If the data did not meet the statistical assumptions required for ANOVA, they were analyzed via Kruskal-Wallis 1-way ANOVA followed by Mann-Whitney U tests. Nominal data were analyzed using a 1-sided Fisher’s exact test. Statistical significance was assigned at p ≤ .05.
RESULTS
Effects of DEHP and DiNP on Body Weight and Organ Weights
Immediately post-dosing, 200 µg/kg/day and 20 mg/kg/day DEHP significantly decreased uterine weight compared with control (Table 1, n = 6–12 mice/group, p ≤ .05). Additionally, 200 mg/kg/day DEHP borderline decreased uterine weight (Table 1, n = 6–12 mice/group, p = .08). No effects of phthalate treatment were seen for body weight, ovarian weight, or liver weight at this time point. Additionally, no doses of DEHP or DiNP affected body weight, ovarian weight, uterine weight, or liver weight at 3 (Table 1, n = 5–12 mice/group) or 9 months post-dosing (Table 1, n = 5–12 mice/group).
Table 1.
Effects of DEHP and DiNP on Body Weight and Organ Weights
| Time Point | Treatment | Body Weight (g) | Ovary Weight (g) | Uterine Weight (g) | Liver Weight (g) |
|---|---|---|---|---|---|
| Immediately post-dosing | Control | 24.17 ± 0.55 | 0.0088 ± 0.0003 | 0.1050 ± 0.0065 | 1.3223 ± 0.0399 |
| 20 µg/kg/day DEHP | 24.06 ± 0.64 | 0.0095 ± 0.0008 | 0.0871 ± 0.0101 | 1.2810 ± 0.0726 | |
| 200 µg/kg/day DEHP | 24.38 ± 0.69 | 0.0082 ± 0.0007 | 0.0767 ± 0.0036* | 1.2295 ± 0.0676 | |
| 20 mg/kg/day DEHP | 23.67 ± 0.62 | 0.0082 ± 0.0007 | 0.0722 ± 0.0054* | 1.2490 ± 0.0422 | |
| 200 mg/kg/day DEHP | 23.63 ± 0.65 | 0.0084 ± 0.0003 | 0.0814 ± 0.0079^ | 1.3291 ± 0.0826 | |
| 20 µg/kg/day DiNP | 24.63 ± 0.79 | 0.0082 ± 0.0014 | 0.1034 ± 0.0111 | 1.3205 ± 0.0376 | |
| 100 µg/kg/day DiNP | 23.25 ± 0.46 | 0.0073 ± 0.0014 | 0.1050 ± 0.0072 | 1.2145 ± 0.0549 | |
| 20 mg/kg/day DiNP | 23.02 ± 0.35 | 0.0088 ± 0.0003 | 0.0938 ± 0.0099 | 1.1924 ± 0.0160 | |
| 200 mg/kg/day DiNP | 23.67 ± 0.51 | 0.0085 ± 0.0006 | 0.0920 ± 0.0052 | 1.3561 ± 0.0657 | |
| 3 months post-dosing | Control | 30.95 ± 0.48 | 0.0212 ± 0.0002 | 0.1228 ± 0.0086 | 1.4679 ± 0.0401 |
| 20 µg/kg/day DEHP | 31.47 ± 0.66 | 0.0241 ± 0.0020 | 0.1254 ± 0.0039 | 1.5827 ± 0.0514 | |
| 200 µg/kg/day DEHP | 30.29 ± 0.62 | 0.0199 ± 0.0018 | 0.1363 ± 0.0129 | 1.3443 ± 0.0592 | |
| 20 mg/kg/day DEHP | 31.82 ± 1.76 | 0.0183 ± 0.0007 | 0.1467 ± 0.0183 | 1.5889 ± 0.1343 | |
| 200 mg/kg/day DEHP | 30.97 ± 1.37 | 0.0161 ± 0.0009 | 0.1383 ± 0.0195 | 1.4389 ± 0.1071 | |
| 20 µg/kg/day DiNP | 31.09 ± 0.68 | 0.0199 ± 0.0016 | 0.1207 ± 0.0092 | 1.3353 ± 0.0368 | |
| 100 µg/kg/day DiNP | 30.75 ± 1.42 | 0.0215 ± 0.0023 | 0.1534 ± 0.0141 | 1.3968 ± 0.0615 | |
| 20 mg/kg/day DiNP | 32.56 ± 0.97 | 0.0218 ± 0.0022 | 0.1512 ± 0.0317 | 1.5541 ± 0.0564 | |
| 200 mg/kg/day DiNP | 30.06 ± 0.79 | 0.0210 ± 0.0017 | 0.1492 ± 0.0185 | 1.3395 ± 0.0299 | |
| 9 months post-dosing | Control | 48.78 ± 1.71 | 0.0290 ± 0.0004 | 0.2102 ± 0.0202 | 2.0953 ± 0.0656 |
| 20 µg/kg/day DEHP | 42.38 ± 2.07 | 0.0280 ± 0.0044 | 0.1818 ± 0.0142 | 1.8339 ± 0.1252 | |
| 200 µg/kg/day DEHP | 44.50 ± 3.11 | 0.0244 ± 0.0042 | 0.2083 ± 0.0317 | 1.8429 ± 0.1250 | |
| 20 mg/kg/day DEHP | 48.07 ± 2.99 | 0.0277 ± 0.0043 | 0.1663 ± 0.0165 | 2.0197 ± 0.0931 | |
| 200 mg/kg/day DEHP | 44.78 ± 2.80 | 0.0224 ± 0.0038 | 0.2068 ± 0.0256 | 1.9930 ± 0.0844 | |
| 20 µg/kg/day DiNP | 45.63 ± 3.36 | 0.0294 ± 0.0027 | 0.2036 ± 0.0382 | 1.7889 ± 0.0613 | |
| 100 µg/kg/day DiNP | 46.14 ± 3.81 | 0.0343 ± 0.0091 | 0.2190 ± 0.0385 | 1.9513 ± 0.0497 | |
| 20 mg/kg/day DiNP | 45.86 ± 5.14 | 0.0197 ± 0.0020 | 0.1759 ± 0.0234 | 2.0728 ± 0.2606 | |
| 200 mg/kg/day DiNP | 47.75 ± 4.93 | 0.0236 ± 0.0048 | 0.2060 ± 0.0406 | 1.8549 ± 0.1936 |
Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were euthanized in diestrus either immediately post-dosing (n = 6–12 mice/group), 3 months post-dosing (n = 5–12 mice/group), or 9 months post-dosing (n = 4–12 mice/group) and body weight and organ weights were measured. Data are represented as means ± SE. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*). Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
Effects of DEHP and DiNP on Estrous Cyclicity
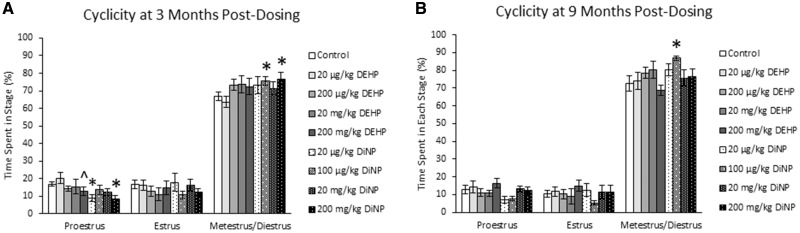
At 3 months post-dosing, DEHP did not significantly affect the time spent in any stage of the estrous cycle. However, the 200 mg/kg/day dose of DEHP borderline decreased the time spent in proestrus compared with control (Figure 1A, n = 12–22 mice/group, p = .09). In contrast, 20 µg/kg/day and 200 mg/kg/day DiNP significantly decreased the time spent in proestrus, and 100 µg/kg/day and 200 mg/kg/day DiNP significantly increased the time spent in metestrus and diestrus compared with control (Figure 1A, n = 12–22 mice/group, p ≤ .05). At 9 months post-dosing, DEHP did not significantly affect the time spent in any stage of the estrous cycle (Figure 1B, n = 11–22 mice/group), but 100 µg/kg/day DiNP significantly increased the time spent in metestrus and diestrus compared with control (Figure 1B, n = 11–22 mice/group, p ≤ .05).
Figure 1.
Effects of DEHP and DiNP on estrous cyclicity. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were vaginally lavaged daily for 2 weeks to assess estrous cyclicity 3 months post-dosing (n = 12–22 mice/group) and again 9 months post-dosing (n = 11–22 mice/group). Data are represented as means ± SE. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*). Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
Effects of DEHP and DiNP on Time to Mating
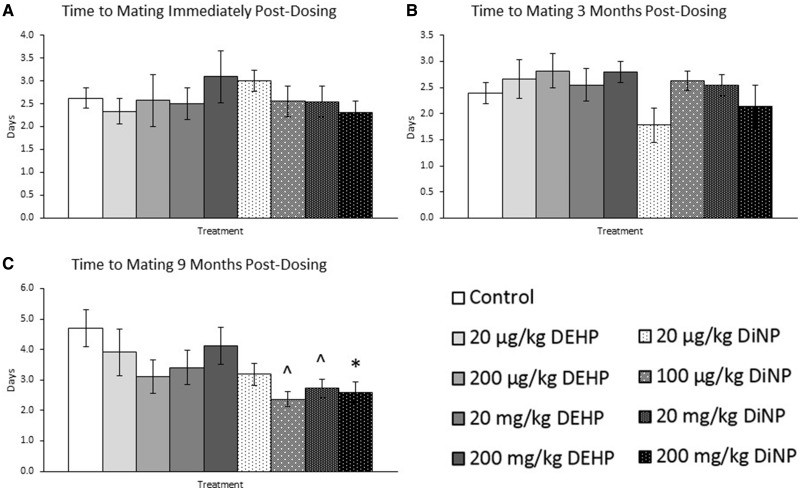
DEHP and DiNP did not significantly affect the time to mating following introduction of the male into the cage for breeding trials immediately post-dosing (Figure 2A, n = 7–21 mice/group) or 3 months post-dosing compared with control (Figure 2B, n = 7–18 mice/group). However, at 9 months post-dosing, 200 mg/kg/day DiNP significantly decreased time to mating (p ≤ .05) and 100 µg/kg/day and 20 mg/kg/day DiNP borderline decreased time to mating compared with control (Figure 2C, n = 10–20 mice/group, .05 < p ≤ .10).
Figure 2.
Effects of DEHP and DiNP on time to mating. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately post-dosing (Panel A, n = 7–21 mice/group), 3 months post-dosing (Panel B, n = 7–18 mice/group), and 9 months post-dosing (Panel C, n = 10–20 mice/group) and checked every morning and afternoon until a copulatory plug was observed or until 14 days had elapsed. Time to mating was calculated as the days between male introduction and observation of a copulatory plug. Data are represented as means ± SE. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*). Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
Effects of DEHP and DiNP on Fertility Index
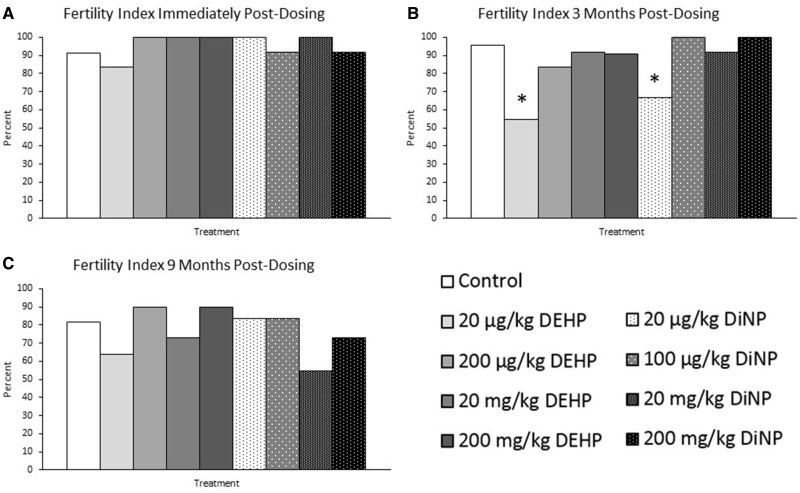
DEHP and DiNP did not significantly affect the ability of females to become pregnant after a successful mating immediately post-dosing (Figure 3A, n = 12–23 mice/group) or at 9 months post-dosing compared with control (Figure 3C, n = 10–22 mice/group). At 3 months post-dosing, however, the lowest doses of DEHP (20 µg/kg/day) and DiNP (20 µg/kg/day) significantly reduced the ability of females to become pregnant compared with control (Figure 3B, n = 11–22 mice/group, p ≤ .05). Approximately half of successfully mated females treated with 20 µg/kg/day DEHP and one-third of females treated with 20 µg/kg/day DiNP were unable to achieve pregnancy, whereas 95% of females achieved pregnancy in the control group (Figure 3B, n = 11–22 mice/group).
Figure 3.
Effects of DEHP and DiNP on fertility index. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day – 200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately post-dosing (Panel A, n = 12–23 mice/group), 3 months post-dosing (Panel B, n = 11–22 mice/group), and 9 months post-dosing (Panel C, n = 10–22 mice/group). The fertility index was calculated by dividing the number of females who successfully achieved pregnancy by the number of females who presented with a copulatory plug and multiplying by 100. Data are represented as percentages. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*).
Effects of DEHP and DiNP on Gestational Index
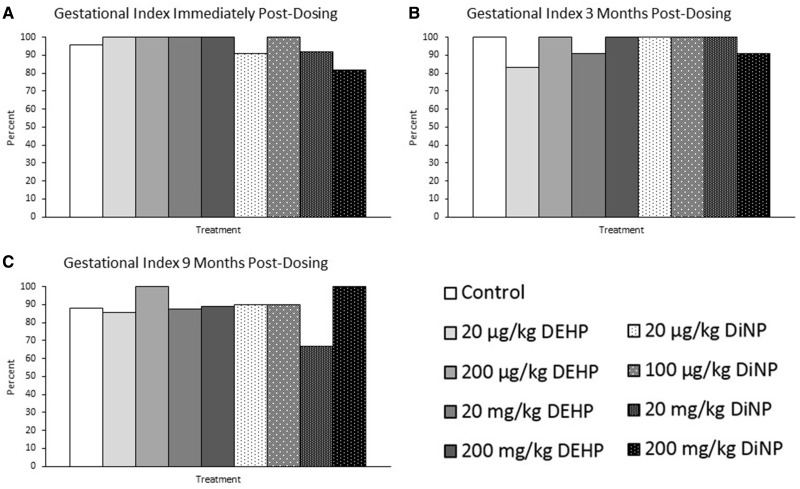
Treatment did not affect the gestational index. Specifically, DEHP and DiNP did not significantly affect the ability of females to carry their pregnancy to term immediately post-dosing (Figure 4A, n = 11–23 mice/group), 3 months post-dosing (Figure 4B, n = 6–21 mice/group), or 9 months post-dosing compared with control (Figure 4C, n = 6–18 mice/group).
Figure 4.
Effects of DEHP and DiNP on gestational index. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately post-dosing (Panel A, n = 11–23 mice/group), 3 months post-dosing (Panel B, n = 6–21 mice/group), and 9 months post-dosing (Panel C, n = 6–18 mice/group). The gestational index was calculated by dividing the number of females who gave birth to pups by the number of females who successfully achieved pregnancy and multiplying by 100. Data are represented as percentages.
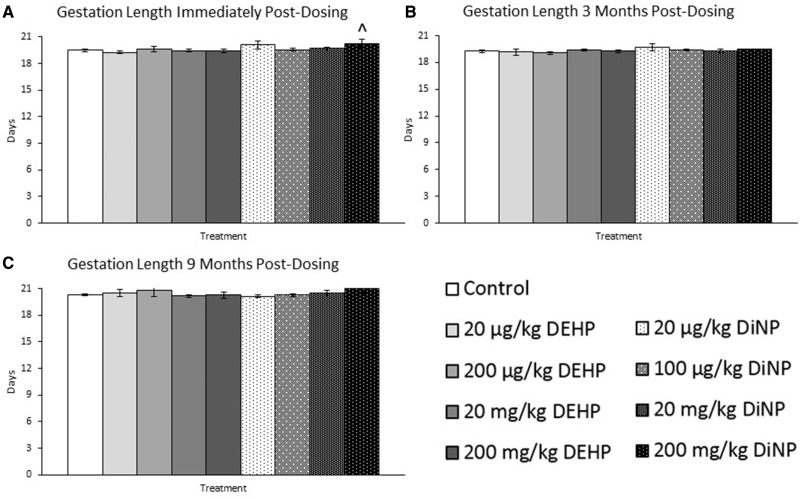
Effects of DEHP and DiNP on Gestation Length
Immediately post-dosing, 200 mg/kg/day DiNP borderline increased length of gestation compared with control (Figure 5A, n = 8–20 mice/group, p = .07). DEHP and DiNP did not significantly affect length of gestation at 3 months post-dosing (Figure 5B, n = 3–17 mice/group) or at 9 months post-dosing compared with control (Figure 5C, n = 6–14 mice/group).
Figure 5.
Effects of DEHP and DiNP on gestation length. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately post-dosing (Panel A, n = 8–20 mice/group), 3 months post-dosing (Panel B, n = 3–17 mice/group), and 9 months post-dosing (Panel C, n = 6–14 mice/group). Gestation length was calculated by using the day a copulatory plug was observed as day 0.5 of pregnancy. Data are represented as means ± SE. Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
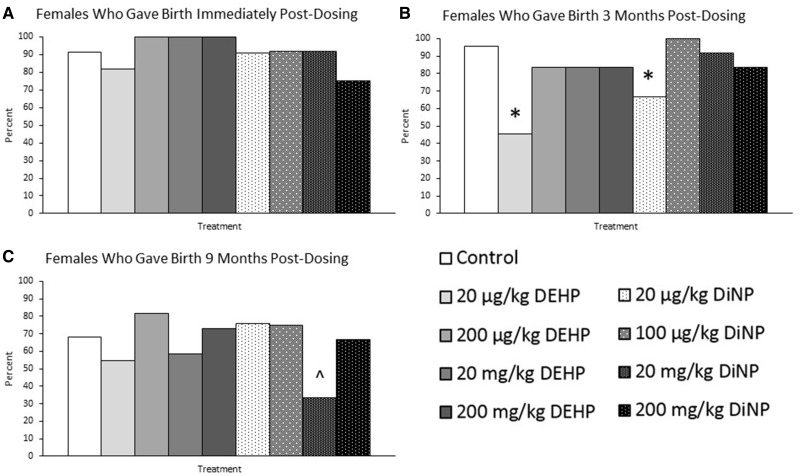
Effects on DEHP and DiNP on the Ability of Females to Produce Pups
Immediately post-dosing, DEHP and DiNP did not significantly affect the overall ability of females to produce offspring compared with control (Figure 6A, n = 11–23 mice/group). Strikingly, after 3 months post-dosing, females in the lowest treatment groups (20 µg/kg/day) for DEHP and DiNP had a significantly impaired ability to give birth to pups compared with control (Figure 6B, n = 11–22 mice/group, p ≤ .05). Additionally, at 9 months post-dosing, females in the 20 mg/kg/day DiNP group had a borderline impaired ability to produce offspring compared with control (Figure 6C, n = 11–22 mice/group, p = .07).
Figure 6.
Effects on DEHP and DiNP on the ability of females to produce pups. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately post-dosing (Panel A, n = 11–23 mice/group), 3 months post-dosing (Panel B, n = 11–22 mice/group), and 9 months post-dosing (Panel C, n = 11–22 mice/group). The percent of females who gave birth was calculated by dividing the number of females who produced pups by the total number of females in that treatment group and multiplying by 100. Data are represented as percentages. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*). Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
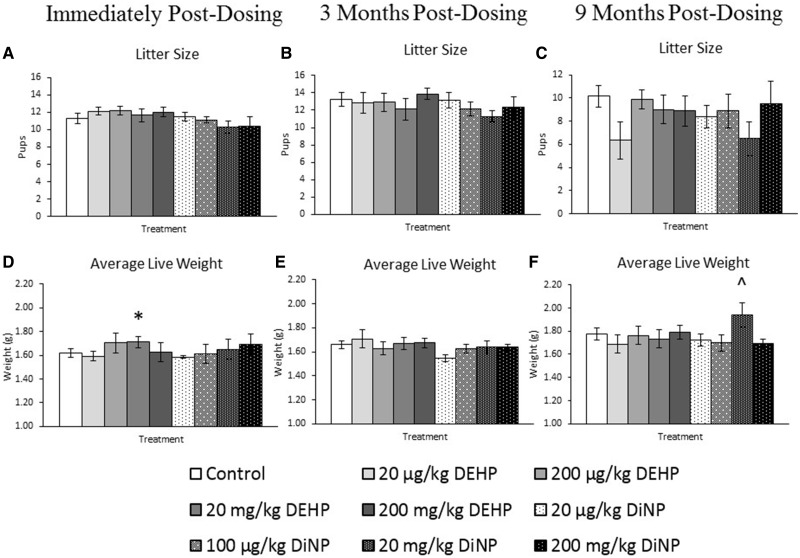
Effects of DEHP and DiNP on Litter Size and Average Live Pup Weight
DEHP and DiNP did not significantly affect litter size immediately post-dosing (Figure 7A, n = 8–19 mice/group), 3 months post-dosing (Figure 7B, n = 5–18 mice/group), or 9 months post-dosing compared with control (Figure 7C, n = 4–14 mice/group). Immediately post-dosing, the average live weight of pups on PND 0 was significantly increased by treatment with 20 mg/kg/day DEHP compared with control (Figure 7D, n = 8–19 mice/group, p ≤ .05). At 9 months post-dosing, treatment with 20 mg/kg/day DiNP borderline increased average live weight of pups on PND 0 compared with control (Figure 7F, n = 4–15 mice/group, p = .06). DEHP and DiNP did not significantly affect the average live weight of pups on PND 0 at 3 months post-dosing compared with control (Figure 7E, n = 5–16 mice/group).
Figure 7.
Effects of DEHP and DiNP on litter size and average live pup weight. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately post-dosing, 3 months post-dosing, and 9 months post-dosing. Live and dead pups were counted to determine the litter size on PND 0 (Panels A–C, n = 8––19 mice/group, n = 5–18 mice/group, and n = 4–14 mice/group, respectively). Live pups were weighed collectively and an average was taken on PND 0 to determine the average live weight (Panels D–F, n = 8–19, 5–16, and 4–15 mice/group, respectively). Data are represented as means ± SE. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*). Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
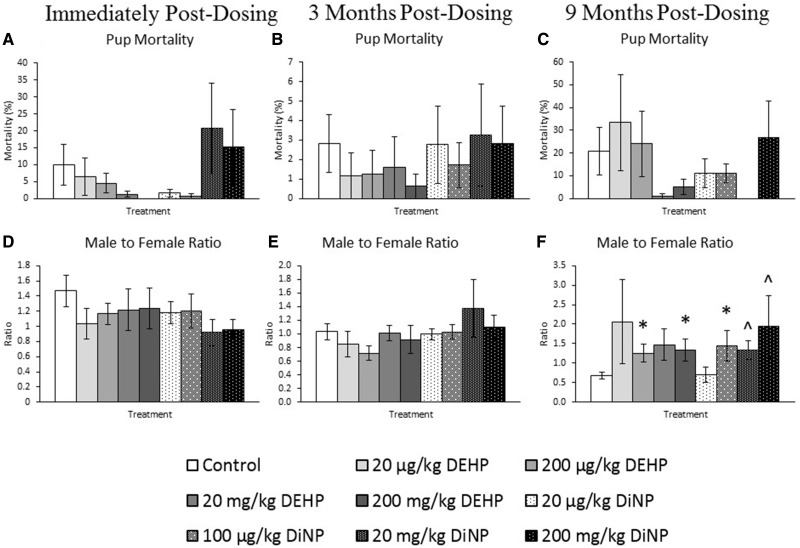
Effects of DEHP and DiNP on Pup Mortality and Sex Ratio
DEHP and DiNP did not significantly affect pup mortality immediately post-dosing (Figure 8A, n = 9–19 mice/group), 3 months post-dosing (Figure 8B, n = 5–20 mice/group), or 9 months post-dosing compared with control (Figure 8C, n = 3–13 mice/group). Additionally, DEHP and DiNP did not significantly affect the male-to-female ratio of pups immediately post-dosing (Figure 8D, n = 9–18 mice/group) or at 3 months post-dosing compared with control (Figure 8E, n = 4–14 mice/group). However, at 9 months post-dosing, treatment with 20 and 200 mg/kg/day DEHP and 100 µg/kg/day DiNP significantly increased the male-to-female ratio of pups compared with control (Figure 8F, n = 4–11 mice/group, p ≤ .05). Further, treatment with 20 and 200 mg/kg/day DiNP borderline significantly increased the ratio of male-to-female pups at 9 months post-dosing compared with control (Figure 8F, n = 4–11 mice/group, .05 < p ≤ .10).
Figure 8.
Effects of DEHP and DiNP on pup mortality and sex ratio. Adult female CD-1 mice were orally dosed for 10 days with either vehicle control (corn oil), DEHP (20 µg/kg/day–200 mg/kg/day), or DiNP (20 µg/kg/day–200 mg/kg/day). Females were mated with untreated male mice for breeding trials immediately, 3, and 9 months post-dosing. Pup mortality was calculated by dividing the number of dead pups by the total number of pups within the litter and multiplying by 100 (Panels A–C, n = 9–19, 5–20, and 3–13 mice/group, respectively). The male-to-female ratio was calculated by dividing the number of male pups by the number of female pups for each litter and multiplying by 100 (Panels D–F, n = 9–18, 4–14, and n = 4–11 mice/group, respectively). Data are represented as means ± SE. Statistically significant difference when compared with control (p ≤ .05) is denoted with an asterisk (*). Borderline statistical significance (.05 < p ≤ .10) is denoted with a caret (^).
DISCUSSION
In this study, we investigated how subchronic exposure to DEHP or DiNP during adulthood affected female fertility. Previously, our research group showed that a subchronic exposure to DEHP (10 days) was sufficient to accelerate ovarian folliculogenesis immediately post-dosing and disrupt estrous cyclicity and steroidogenesis up to 9 months post-dosing (Hannon et al., 2014, 2016). This study builds upon this knowledge by examining fertility at additional time points and testing the reproductive toxicity of a common DEHP replacement, DiNP. This study shows that exposure to DEHP and DiNP has negative impacts on female reproductive parameters several months after exposure has stopped. These negative impacts include changes in reproductive organ weights, disruption of estrous cyclicity, reduction in fertility, changes in mating behavior, and changes in litter outcomes.
Immediately post-dosing, 20 mg/kg/day DEHP significantly increased the average live weight of pups at PND 0 without concurrently decreasing litter size. DEHP has been shown to be obesogenic (Pereira-Fernandes et al., 2013). Thus, it is possible that DEHP exposure increases adiposity and subsequently, birth weight of pups. Our finding contrasts with epidemiological data that find associations between phthalate exposure and low birth weight (Song et al., 2018; Strommen et al., 2016). The differences between this study and epidemiological data may be due to different exposure times and species differences.
Immediately post-dosing, some doses of DEHP, but not DiNP, significantly decreased uterine weight. In a previous study in our laboratory, we did not observe any effects of DEHP exposure for 10 days on uterine weight (Hannon et al., 2014). This discrepancy may be due to animals being housed in groups for the current experiment and singly in the previous study. Group housing is known to suppress estrus in rodents, which may cause lower estradiol and subsequently, lower uterine weight (Martin et al., 1973; Whitten, 1959). Additionally, DEHP has been shown to disrupt ovarian steroidogenesis in vivo and in vitro; thus, the observed decrease in uterine weight in this study may also be mediated by DEHP-induced decrease of estradiol (Hannon and Flaws, 2015). Therefore, it is possible that the DEHP-induced reduction in uterine weight in this study was only statistically detectable once animals were group housed, causing them to be in a lower estrogen state than in the previous study.
Three months post-dosing, some doses of DiNP significantly disrupted estrous cyclicity compared with controls. At one dose, these effects persisted for as long as 9 months following dosing. It is possible that DiNP may be affecting levels of sex steroid hormones which dictate the estrous cycle in the mouse (Caligioni, 2009). DiNP has been shown to disrupt steroid hormone production in porcine granulosa cell cultures, indicating that DiNP maybe be targeting ovarian steroidogenesis and subsequently disrupting estrous cyclicity (Mlynarcikova et al., 2007). Other studies have found that perinatal exposure to DiNP does not affect estrous cyclicity during adulthood in rats (Lee et al., 2006; Masutomi et al., 2003). The difference in estrous cyclicity outcomes between these previous studies and this study may be due to the use of different animal models as well as exposure windows. Previous studies assessed cyclicity at postnatal week (PNW) 8–11 or at PNW 8–9 and again at 19–20, whereas the mice in the present study were first monitored at approximately 19 weeks of age and again at approximately 44 weeks of age.
At 3 months post-dosing, we observed that treatment with 20 µg/kg/day of DEHP or DiNP, the lowest dose used in this study, significantly reduced the fertility index of females compared with control. A reduction in the fertility index indicates a reduction in the ability of females to become pregnant from a confirmed mating. Additionally, this significant reduction in the fertility index led to a significant reduction in the percent of females who produced pups. Because the affected females had complete infertility as opposed to reduced fertility (e.g., smaller litter sizes), it is possible that the underlying mechanism involves disruption of global uterine receptivity to implantation. Proper regulation of sex steroid hormones is necessary for the opening of the “implantation window,” the narrow time frame during which the uterus is receptive to embryo implantation (Matsumoto, 2017). In fact, DEHP has been shown to decrease implantation rates in mice by decreasing endometrial receptivity to the embryo (Li et al., 2012). However, one study found that rats exposed to DiNP via the diet prior to mating and during mating had no difficulty achieving pregnancy and producing pups (Waterman et al., 2000). The differences observed between the past study and this study may be due to species differences as well as the use of different doses in these studies.
At 9 months post-dosing, 200 mg/kg/day DiNP significantly reduced time to mating and 100 µg/kg/day and 20 mg/kg/day DiNP borderline reduced time to mating. The effects of DiNP on time to mating reported in the current study appear to be novel, as most studies investigate developmental exposure windows and do not measure time to mating as a fertility parameter. Sex steroid hormones are necessary for maintenance of proper reproductive behavior; thus, it is possible that DiNP disrupts circulating hormones, particularly estradiol and progesterone, in a manner that increases female sexual receptivity to males (Senger, 1997). Although treatment did not affect the fertility index or gestational index of females at 9 months post-dosing, 20 mg/kg/day DiNP borderline decreased the percent of females who gave birth. It is hard to specifically assign the cause of the DiNP-induced reduction in overall fertility to a reduction in ability to achieve pregnancy or ability to carry a pregnancy to term because neither the fertility index nor gestational index were statistically different from controls. It is likely that 20 mg/kg/day DiNP reduced both of these indices enough to cause a cumulative effect that can be seen in the reduction of percent of females who gave birth, but not enough to be statistically detectable for either of the individual fertility indices. It is possible that this overall reduction in fertility could be the beginning of the onset of reproductive senescence in these mice. Previous studies have shown that phthalate exposure has been associated with premature menopause in women as well as increased markers of reproductive aging in female mice (Brehm et al., 2018; Grindler et al., 2015; Hannon et al., 2016).
At 9 months post-dosing, 200 µg/kg/day and 200 mg/kg/day DEHP and 100 µg/kg/day DiNP significantly increased the male-to-female ratio of pups, and 20 and 200 mg/kg/day borderline increased the male-to-female ratio of pups. We find this result particularly interesting because it is unusual for preconception exposure to alter sex ratio, especially in females which are not the heterogametic sex in mice. However, this study is not the first to find that treatment of females can result in altered offspring sex ratio. Multiple animal-based studies have found that females fed diets supplemented with fats produce male-biased litters (Gharagozlou et al., 2016; Rosenfeld et al., 2003). One hypothesis that has existed for decades is that hormonal milieu affects offspring sex ratio in humans (James, 1990). Supporting this hypothesis, one study found that women with fat deposition patterns indicative of higher testosterone were significantly more likely to produce male offspring than their counterparts with fat deposition patterns indicative of lower testosterone levels (Singh and Zambarano, 1997). We speculate that the observed sex selection occurs at the level of the sperm and vaginal mucosa because of evidence that Y-bearing sperm are smaller and more motile and are subsequently able to navigate through cervical mucus faster than their X-bearing counterparts (Martin et al., 1994). Because hormones are known to have a significant impact on mucus production and viscosity within the female reproductive tract, it is possible that DEHP and DiNP may be disrupting hormone levels, leading to a change in the mucosal environment of the female reproductive tract that selects favorably for Y-bearing sperm (Chappell et al., 2014). Further, we do not think the sex selection occurs at the level of the embryo because we did not observe any changes in litter size that would suggest altered resorption of embryos.
In conclusion, this study found that DEHP and DiNP disrupt several aspects of female fertility at time points up to 9 months after cessation of exposure. Many of the results observed in this study may be due to disruption of the hormonal milieu due to treatment with DEHP or DiNP. Thus, future studies should investigate the effects of DEHP and DiNP on sex steroid hormones and gonadotropins. Additionally, future studies should investigate fertility at time points even further out from 9 months post-dosing to follow females through their transition to reproductive senescence and elucidate any effects of these phthalates on the reproductive lifespan. Collectively, these findings suggest that phthalates, which are ubiquitous environmental contaminants that humans are exposed to daily, may have long lasting consequences on the female reproductive system.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by National Institutes of Health (R56 ES 025147, R01 ES 028661, and T32 ES 007326) and University of Illinois (Billie Field Fellowship).
ACKNOWLEDGMENTS
The authors would like to thank the members of the Flaws lab who helped with dosing, collections, and assisted with animal work throughout this project.
REFERENCES
- Axelsson J., Rylander L., Rignell-Hydbom A., Lindh C. H., Jonsson B. A., Giwercman A. (2015). Prenatal phthalate exposure and reproductive function in young men. Environ. Res. 138, 264–270. [DOI] [PubMed] [Google Scholar]
- Barakat R., Lin P. P., Rattan S., Brehm E., Canisso I. F., Abosalum M. E., Flaws J. A., Hess R., Ko C. (2017). Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol. Sci. 156, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J., Christiansen S., Axelstad M., Kledal T. S., Vinggaard A. M., Dalgaard M., Nellemann C., Hass U. (2011). Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod. Toxicol. 31, 200–209. [DOI] [PubMed] [Google Scholar]
- Borch J., Ladefoged O., Hass U., Vinggaard A. M. (2004). Steroidogenesis in fetal male rats is reduced by dehp and DINP, but endocrine effects of dehp are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod. Toxicol. 18, 53–61. [DOI] [PubMed] [Google Scholar]
- Brehm E., Rattan S., Gao L., Flaws J. A. (2018). Prenatal exposure to di(2-ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology 159, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C. (2009). Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. Appendix 4: Appendix-4I [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W. H., Wu M. H., Pan H. A., Guo P. L., Lee C. C. (2017). Semen quality and insulin-like factor 3: Associations with urinary and seminal levels of phthalate metabolites in adult males. Chemosphere 173, 594–602. [DOI] [PubMed] [Google Scholar]
- Chappell C. A., Rohan L. C., Moncla B. J., Wang L., Meyn L. A., Bunge K., Hillier S. L. (2014). The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am. J. Obstet. Gynecol. 211, 226.e1–226.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-L., Chen J.-S., Tang C.-L., Mao I. F. (2008). The internal exposure of Taiwanese to phthalate—an evidence of intensive use of plastic materials. Environ. Int. 34, 79–85. [DOI] [PubMed] [Google Scholar]
- Chiang C., Mahalingam S., Flaws J. A. (2017). Environmental contaminants affecting fertility and somatic health. Semin. Reprod. Med. 35, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull J., Cattley R., Elcombe C., Lake B. G., Swenberg J., Wilkinson C., Williams G., van Gemert M. (1999). A cancer risk assessment of di(2-ethylhexyl)phthalate: Application of the new U.S. EPA risk assessment guidelines. Regul. Toxicol. Pharmacol. 29, 327–357. [DOI] [PubMed] [Google Scholar]
- Durmaz E., Erkekoglu P., Asci A., Akcurin S., Bircan I., Kocer-Gumusel B. (2018). Urinary phthalate metabolite concentrations in girls with premature thelarche. Environ. Toxicol. Pharmacol. 59, 172–181. [DOI] [PubMed] [Google Scholar]
- Faouzi M. A., Dine T., Gressier B., Kambia K., Luyckx M., Pagniez D., Brunet C., Cazin M., Belabed A., Cazin J. C. (1999). Exposure of hemodialysis patients to di-2-ethylhexyl phthalate. Int. J. Pharm. 180, 113–121. [DOI] [PubMed] [Google Scholar]
- Gharagozlou F., Youssefi R., Akbarinejad V. (2016). Effects of diets supplemented by fish oil on sex ratio of pups in bitch. Vet. Res. Forum 7, 105–110. [PMC free article] [PubMed] [Google Scholar]
- Grindler N. M., Allsworth J. E., Macones G. A., Kannan K., Roehl K. A., Cooper A. R. (2015). Persistent organic pollutants and early menopause in U.S. women. PLoS One 10, e0116057.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Lambright C. S., Furr J., Howdeshell K. L., Wilson V. S., Gray L. E. Jr. (2011). Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol. Sci. 123, 206–216. [DOI] [PubMed] [Google Scholar]
- Hannon P. R., Flaws J. A. (2015). The effects of phthalates on the ovary. Front. Endocrinol. (Lausanne) 6, 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Niermann S., Flaws J. A. (2016). Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol. Sci. 150, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Peretz J., Flaws J. A. (2014). Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol. Reprod. 90, 136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman C. G. (1944). Some new observations on the vaginal smear of the rat. Yale J. Biol. Med. 17, 99–112. [PMC free article] [PubMed] [Google Scholar]
- Hines C. J., Hopf N. B., Deddens J. A., Silva M. J., Calafat A. M. (2012). Occupational exposure to diisononyl phthalate (DINP) in polyvinyl chloride processing operations. Int. Arch. Occup. Environ. Health 85, 317–325. [DOI] [PubMed] [Google Scholar]
- Hines C. J., Nilsen Hopf N. B., Deddens J. A., Calafat A. M., Silva M. J., Grote A. A., Sammons D. L. (2009). Urinary phthalate metabolite concentrations among workers in selected industries: A pilot biomonitoring study. Ann. Occup. Hyg. 53, 1–17. [DOI] [PubMed] [Google Scholar]
- Hoyer B. B., Lenters V., Giwercman A., Jonsson B. A. G., Toft G., Hougaard K. S., Bonde J. P. E., Specht I. O. (2018). Impact of di-2-ethylhexyl phthalate metabolites on male reproductive function: A systematic review of human evidence. Curr. Environ. Health Rep. 5, 20–33. [DOI] [PubMed] [Google Scholar]
- Inoue K., Kawaguchi M., Yamanaka R., Higuchi T., Ito R., Saito K., Nakazawa H. (2005). Evaluation and analysis of exposure levels of di(2-ethylhexyl) phthalate from blood bags. Clin. Chim. Acta 358, 159–166. [DOI] [PubMed] [Google Scholar]
- James W. H. (1990). The hypothesized hormonal control of human sex ratio at birth–an update. J. Theor. Biol. 143, 555–564. [DOI] [PubMed] [Google Scholar]
- Joensen U. N., Frederiksen H., Blomberg Jensen M., Lauritsen M. P., Olesen I. A., Lassen T. H., Andersson A. M., Jorgensen N. (2012). Phthalate excretion pattern and testicular function: A study of 881 healthy Danish men. Environ. Health Perspect. 120, 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R., Boekelheide K., Chapin R., Cunningham M., Faustman E., Foster P., Golub M., Henderson R., Hinberg I., Little R., et al. (2002). NTP center for the evaluation of risks to human reproduction: Phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 16, 529–653. [DOI] [PubMed] [Google Scholar]
- Kay V. R., Bloom M. S., Foster W. G. (2014). Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 44, 467–498. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Yamanouchi K., Nishihara M. (2006). Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J. Reprod. Dev. 52, 343–352. [DOI] [PubMed] [Google Scholar]
- Li L., Bu T., Su H., Chen Z., Liang Y., Zhang G., Zhu D., Shan Y., Xu R., Hu Y., et al. (2015). Inutero exposure to diisononyl phthalate caused testicular dysgenesis of rat fetal testis. Toxicol. Lett. 232, 466–474. [DOI] [PubMed] [Google Scholar]
- Li R., Yu C., Gao R., Liu X., Lu J., Zhao L., Chen X., Ding Y., Wang Y., He J. (2012). Effects of dehp on endometrial receptivity and embryo implantation in pregnant mice. J. Hazard. Mater. 241-242, 231–240. [DOI] [PubMed] [Google Scholar]
- Mallow E. B., Fox M. A. (2014). Phthalates and critically ill neonates: Device-related exposures and non-endocrine toxic risks. J. Perinatol. 34, 892–897. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Hammel E. A., Harris M., James W. H., Moore J. H., Reddy P. G., Salzano F. M., Thornton R., John W. M. W. (1994). Changing sex ratios: The history of havasupai fertility and its implications for human sex ratio variation [and comments and reply]. Curr. Anthropol. 35, 255–280. [Google Scholar]
- Martin L., Finn C. A., Trinder G. (1973). Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: An autoradiographic study. J. Endocrinol. 56, 133–144. [DOI] [PubMed] [Google Scholar]
- Masutomi N., Shibutani M., Takagi H., Uneyama C., Takahashi N., Hirose M. (2003). Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology 192, 149–170. [DOI] [PubMed] [Google Scholar]
- Matsumoto H. (2017). Molecular and cellular events during blastocyst implantation in the receptive uterus: Clues from mouse models. J. Reprod. Dev. 63, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D., Hu H., Cantonwine D. E., Lamadrid-Figueroa H., Calafat A. M., Ettinger A. S., Hernandez-Avila M., Loch-Caruso R., Tellez-Rojo M. M. (2009). Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ. Health Perspect. 117, 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarcikova A., Fickova M., Scsukova S. (2007). The effects of selected phenol and phthalate derivatives on steroid hormone production by cultured porcine granulosa cells. Altern. Lab. Anim. 35, 71–77. [DOI] [PubMed] [Google Scholar]
- NTP. (2016). Di(2-ethylhexyl) phthalate. Rep. Carcinog. 14, 1–3. [Google Scholar]
- Pereira-Fernandes A., Demaegdt H., Vandermeiren K., Hectors T. L., Jorens P. G., Blust R., Vanparys C. (2013). Evaluation of a screening system for obesogenic compounds: Screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One 8, e77481.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Brehm E., Gao L., Niermann S., Flaws J. A. (2018). Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol. Reprod. 98, 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Zhou C., Chiang C., Mahalingam S., Brehm E., Flaws J. A. (2017). Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 233, R109–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C. S., Grimm K. M., Livingston K. A., Brokman A. M., Lamberson W. E., Roberts R. M. (2003). Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc. Natl. Acad. Sci. U.S.A. 100, 4628–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCENIHR. (2008). Opinion on the safety of medical devices containing dehp plasticized PVC or other plasticizers on neonates and other groups possibly at risk. Available at: https://ec.europa.eu/health/archive/ph_risk/committees/04_scenihr/docs/scenihr_o_014.pdf. Accessed January 17, 2019. [DOI] [PubMed]
- Schettler T. (2006). Human exposure to phthalates via consumer products. Int. J. Androl. 29, 134–139. discussion 181-135. [DOI] [PubMed] [Google Scholar]
- Senger P. L. (1997). Pathways to Pregnancy and Parturition. Current Conceptions, Pullman, WA. [Google Scholar]
- Singh D., Zambarano R. J. (1997). Offspring sex ratio in women with android body fat distribution. Hum. Biol. 69, 545–556. [PubMed] [Google Scholar]
- Song Q., Li R., Zhao Y., Zhu Q., Xia B., Chen S., Zhang Y. (2018). Evaluating effects of prenatal exposure to phthalates on neonatal birth weight: Structural equation model approaches. Chemosphere 205, 674–681. [DOI] [PubMed] [Google Scholar]
- Specht I. O., Toft G., Hougaard K. S., Lindh C. H., Lenters V., Jonsson B. A., Heederik D., Giwercman A., Bonde J. P. (2014). Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ. Int. 66, 146–156. [DOI] [PubMed] [Google Scholar]
- Strommen K., Lyche J. L., Blakstad E. W., Moltu S. J., Veierod M. B., Almaas A. N., Sakhi A. K., Thomsen C., Nakstad B., Braekke K., et al. (2016). Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. Environ. Int. 89-90, 228–234. [DOI] [PubMed] [Google Scholar]
- U.S. CPSC. (2001). Chronic hazard advisory panel on diisononyl phthalate (DINP). Available at: https://www.cpsc.gov/s3fs-public/pdfs/dinp.pdf. Accessed January 17, 2019.
- U.S. Department of Health and Human Services, PHS, Agency for Toxic Substances and Disease Registry. (2002). Toxicological profile for di(2-ethylhexyl)phthalate. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp9.pdf. Accessed January 17, 2019. [PubMed]
- U.S. EPA. (2000). Bis(2-ethylhexyl) phthalate (DEHP).
- Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R. Jr., Lee D. H., Shioda T., Soto A. M., vom Saal F. S., Welshons W. V., et al. (2012). Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman S. J., Keller L. H., Trimmer G. W., Freeman J. J., Nikiforov A. I., Harris S. B., Nicolich M. J., McKee R. H. (2000). Two-generation reproduction study in rats given di-isononyl phthalate in the diet. Reprod. Toxicol. 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Whitten W. K. (1959). Occurrence of anoestrus in mice caged in groups. J. Endocrinol. 18, 102–107. [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Zhang T., Wang L., Zhang H. Y., Chen Y. D., Qin X. S., Feng Y. M., Feng Y. N., Shen W., Li L. (2013). Effects of diethylhexyl phthalate (dehp) given neonatally on spermatogenesis of mice. Mol. Biol. Rep. 40, 6509–6517. [DOI] [PubMed] [Google Scholar]