Abstract
Aquaporins (AQPs) are transmembrane proteins widely distributed in various organisms, and they facilitate bidirectional diffusion of water and uncharged solutes. The catalase-negative bacterium Streptococcus oligofermentans produces the highest H2O2 levels reported to date, which has to be exported to avoid oxidative stress. Here, we report that a S. oligofermentans aquaporin functions as a peroxiporin facilitating bidirectional transmembrane H2O2 transport. Knockout of this aquaporin homolog, So-AqpA, reduced H2O2 export by ∼50% and increased endogenous H2O2 retention, as indicated by the cellular H2O2 reporter HyPer. Heterologous expression of So-aqpA accelerated exogenous H2O2 influx into Saccharomyces cerevisiae and Escherichia coli cells, indicating that So-AqpA acts as an H2O2-transferring aquaporin. Alanine substitution revealed Phe-40 as a key residue for So-AqpA–mediated H2O2 transport. Northern blotting, qPCR, and luciferase reporter assays disclosed that H2O2 induces a >10-fold expression of So-aqpA. Super-resolution imaging showed that H2O2 treatment increases So-AqpA protein molecules per cell by 1.6- to 3-fold. Inactivation of two redox-regulatory transcriptional repressors, PerR and MntR, reduced H2O2-induced So-aqpA expression to 1.8- and 4-fold, respectively. Electrophoretic mobility shift assays determined that MntR, but not PerR, binds to the So-aqpA promoter, indicating that MntR directly regulates H2O2-induced So-aqpA expression. Importantly, So-aqpA deletion decreased oxic growth and intraspecies competition and diminished the competitive advantages of S. oligofermentans over the caries pathogen Streptococcus mutans. Of note, So-aqpA orthologs with the functionally important Phe-40 are present in all streptococci. Our work has uncovered an intrinsic, H2O2-inducible bacterial peroxiporin that has a key physiological role in H2O2 detoxification in S. oligofermentans.
Keywords: aquaporin, hydrogen peroxide, oxidative stress, transcription regulation, bacteria, Streptococcus, H2O2 detoxification, H2O2-facilitator, H2O2-induced expression
Introduction
Aquaporins (AQPs)3 belong to the major intrinsic protein (MIP) family and are widely distributed in all the cellular organisms. They form channels across biological membranes and facilitate bidirectional diffusion of water and small uncharged solutes, such as glycerol and urea (1, 2). Since their first discovery in 1992 (1), numerous studies have demonstrated that human AQPs display important physiological functions and their dysfunctions cause many clinical disorders (3, 4). The plant AQPs are found to be involved in transpiration, root water uptake, seed desiccation, inhibition of self-pollination, and closure of leaf guard cells (5, 6). Phylogenetically, MIPs are clustered into two clades, the water-transporting AQPs and the glycerol-permeable aquaglyceroporins (GLPs) (7). Both clades of the aquaporins possess an Asn-Pro-Ala (NPA) signature motif and an aromatic/arginine (ar/R) substrate selectivity filter; and ar/R consists of one conserved arginine and three other amino acids that are conserved within each subfamily (8). Like many other membrane transporters, the activities of AQPs are subjected to regulation. The eukaryotic water-transporting aquaporins can be regulated by protein trafficking (9), and phosphorylation, pH, or divalent cations mediated gating (10, 11). Recently, it was reported that pH regulates the permeability of an aquaglyceroporin, AQP7, by altering protonation of the key amino acid residues (12).
Probably because of the similar electrochemical properties of H2O and H2O2, some AQPs can transport H2O2 through the H2O channel (6, 13). In recent years, a growing number of animal and plant aquaporin homologs have been verified to transport H2O2, which can promote development of some important physiological characteristics in eukaryotes (14–16). The human AQP3 and AQP8 facilitate H2O2 permeating across the cell membranes (17), which allows mitochondria-generated H2O2, a key molecule in the redox signaling network, to permeate into other cellular compartments and regulate physiological processes (6, 16–19). However, being an oxidant, the H2O2 level has to be strictly controlled to prevent cells from oxidative stress, which would subsequently cause disease and tumorigenesis (5).
Because of the limited studies on prokaryotic AQPs (2, 20, 21), their functions are largely unknown, such as whether they also function to facilitate H2O2 excretion and whether this action has physiological significance. Based on the rate of H2O2 flux, Seaver and Imlay (22) found that a H2O2 transmembrane concentration gradient exists in Escherichia coli. This suggests that H2O2 permeability across the cellular membrane is limited, and the bacterial aquaporins may also function in facilitating H2O2 diffusion. Streptococci, a type of facultative anaerobic bacteria that lack catalase, are known to produce and accumulate high concentrations of H2O2 in cultures, indicating they possess effective H2O2 excretion pathways. Streptococci carry genes that encode two clades of aquaporins, AQPs and GLPs. Interestingly, our previous microarray analysis found increased expression of the AQP genes in H2O2-treated Streptococcus oligofermentans, a bacterium so far known to produce and tolerate the highest levels of H2O2 (23), implying that the AQPs might play a role in H2O2 excretion.
In the present study, we investigated the roles of S. oligofermentans aquaporins in facilitating H2O2 efflux and the regulatory mechanisms. Through the integration of genetic, physiological, biochemical, and single-molecule imaging approaches, we identified a streptococcal peroxiporin, So-AqpA. By using an intracellular-specific H2O2 fluorescence reporter, HyPer, we demonstrated that So-AqpA, with Phe-40 as a key residue, facilitated the bidirectional permeation of H2O2 across the cellular membrane. Northern blotting and quantitative PCR, and photoactivated localization microscopy (PALM) super-resolution imaging determined that H2O2 induced the expression of So-aqpA gene at both transcriptional and translational levels. The two well-known redox transcriptional regulators PerR and MntR are involved in the H2O2-induced expression of So-aqpA. Deletion of the So-aqpA gene caused oxidative stress and reduced the intraspecies and interspecies competitive advantages of S. oligofermentans. This work reports for the first time the physiological roles of a bacterial peroxiporin, which could be a potential target for suppression of streptococci, especially the pathogenic species.
Results
The aquaporin homolog So-aqpA encodes an H2O2 facilitator
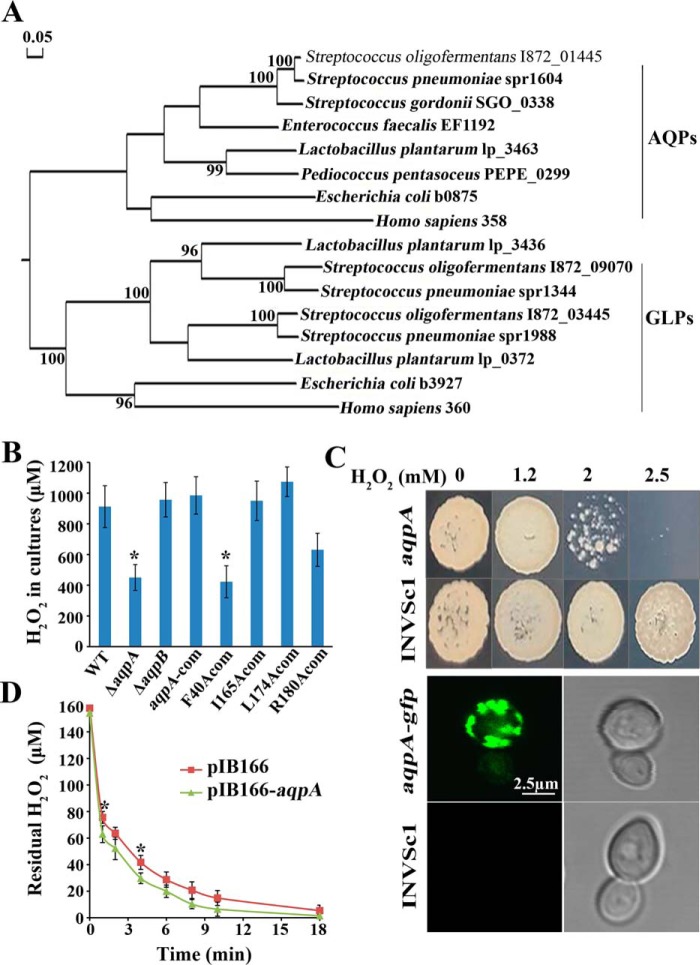
The S. oligofermentans genome carries three MIP family homologous genes: I872_01445 encodes an aquaporin, so was designated as So-aqpA and the encoded protein designated as So-AqpA; I872_09070 and I872_03455 encode glycerol uptake facilitator proteins and are designated as So-aqpB and So-aqpC, respectively, and the encoded proteins were designated as So-AqpB and So-AqpC. So-aqpC belongs to a three-gene operon for glycerol metabolism; thereby it was predicted to facilitate glycerol uptake. Phylogenetically, So-AqpA is related to the E. coli aquaporin Z (b0875), whereas the two aquaglyceroporins (So-AqpB and So-AqpC) are related to the E. coli aquaglyceroporin GlpF (b3927) and the human aquaporin AQP3 (360) (Fig. 1A). To determine whether these MIP family proteins function as H2O2 transporters in S. oligofermentans, So-aqpA and So-aqpB were deleted and the mutants were designated as ΔaqpA and ΔaqpB, respectively. Given that streptococci produce and accumulate endogenous H2O2 under oxic conditions, the two mutants and WT strain were cultured statically, and then the exponential phase cells were resuspended in fresh BHI medium. After incubated at 37 °C for 1 h, H2O2 concentrations in the cultures were measured as described in “Experimental procedures.” 113 ± 16 μm H2O2 was determined in the WT strain culture, whereas 64 ± 9 μm and 110 ± 13 μm H2O2 were determined in the ΔaqpA and ΔaqpB cultures, respectively. Next these three strains, and the So-aqpA complemented strain (aqpA-com) were cultured under higher oxygen supplies in 10 ml BHI in a 100-ml triangle flask for higher endogenous H2O2 production. Then H2O2 contents in the cultures were measured. We found that while about half amount of H2O2 was produced by the ΔaqpA, similar H2O2 yields were measured in the aqpA-com, the WT, and ΔaqpB strains (Fig. 1B). This suggests that So-AqpA is a H2O2 facilitator, but So-AqpB is not.
Figure 1.
An aquaporin homolog So-aqpA encodes a H2O2 facilitator. A, phylogenetic analysis was performed for the S. oligofermentans aquaporin (I872_01445) and aquaglyceroporins (I872_09070 and I872_03455) with the homologs from representative lactic acid bacteria, E. coli, and humans. Protein sequences were retrieved from the NCBI protein database. The phylogenetic tree was constructed with DNAMAN using the neighbor-joining method and the bootstrap value was set as 1000. The bar of 0.05 represents the evolution distance. B, the WT strain, deletion mutants of So-aqpA and So-aqpB, and various point-mutated So-aqpA strains were cultured in 10-ml BHI broth in a 100-ml flask, and H2O2 yields in the stationary phase cultures were determined. The average ± S.D. from triplicate cultures of each strain are shown. *, data are statistically significant compared with those of the WT strain and the So-aqpA point mutation strains, as verified by one-way ANOVA followed by Tukey's post hoc test (p <0.05). C, the So-aqpA gene either fused with the sfGFP gene or alone was integrated into the pYES2 plasmid and transformed into S. cerevisiae INVSc1. The INVSc1-So-aqpA, -So-aqpA-gfp, and -pYES2 strains were grown in SC-Ura-glucose for overnight, and then shifted to galactose to induce the So-aqpA gene expression. After a 6- to 8-h induction, 10 μl diluted INVSc1-So-aqpA and -pYES2 cultures (OD600 0.01) were spotted on SC-Ura galactose agar plate which is supplemented with various concentrations of H2O2 (upper panel). The INVSc1-So-aqpA-gfp and -pYES2 cells were visualized under a confocal laser scanning microscope (Leica TCS SP8, Leica Microsystems) with excitation at 488 nm, and emission was collected from a range of 500 to 600 nm (lower panel). The representative GFP fluorescence (left) and differential interference contrast pictures (right) were shown. D, the So-aqpA gene was cloned into pIB166 and heterogeneously expressed in E. coli. Mid–exponential phase LB cultures (OD600 ∼0.60) of the pIB166-aqpA and vacant pIB166 strains were collected by centrifugation and washed twice with PBS. Cells were then diluted into 10 ml PBS to OD600 0.1, and a final concentration of 150 μm H2O2 was added. The residual H2O2 in PBS was then determined at the indicated time points. Average ± S.D. of triplicate experiments is shown. *, data were statistically significantly different from that of pIB166-aqpA strain at the corresponding time point (Student's t test, p <0.05).
To further validate that So-AqpA acts as a H2O2 facilitator, its gene was heterogeneously expressed in Saccharomyces cerevisiae by the vector pYES2 and in E. coli by the vector pIB166 (24). As shown in Fig. 1C, good growth of the S. cerevisiae INVSc1 strain carrying a vacant vector occurred on the galactose agar plates that contained H2O2 up to 2.5 mm, whereas the So-aqpA–expressing strain grew poorly at 2 mm and no growth was shown at 2.5 mm H2O2. Accordingly, H2O2 minimal inhibitory concentration value was determined to be 3 mm for the So-aqpA–expressing strain compared with 6 mm for the empty vector–expressing S. cerevisiae. In addition, a green fluorescence protein (sfGFP)–So-aqpA fusion was introduced into strain INVSc1, and the GFP fluorescence around the cytoplasmic membrane was observed under a confocal laser scanning microscope, confirming the expression of So-aqpA in S. cerevisiae (Fig. 1C). Subsequently, So-aqpA facilitated H2O2 permeation into E. coli cells was tested by monitoring the dynamics of exogenous H2O2 reduction based on the cytoplasmic catalase scavenging. The mid-exponential phase Luria broth (LB) cultures of E. coli DH5α expressing pIB166-aqpA and the vacant pIB166 were suspended in PBS, and by using 150 μm H2O2 as the initial concentration, the residual H2O2 amounts were measured over time. As shown in Fig. 1D, So-aqpA expression increased about 8% H2O2 uptake rate of E. coli until 4 min, and about 6% increase until 8 min. Using quantitative RT-PCR, the transcript copies of So-aqpA and 16S rRNA in E. coli were quantified as 369,564 ± 94,966/μg cDNA and 335,341 ± 81,442 × 104/μg cDNA, respectively, thus, So-aqpA transcription in E. coli was determined as 0.11 ± 0.01 copies/1000 16S rRNAs. Taken together, these experiments demonstrated that So-AqpA is an H2O2 facilitator.
So-AqpA facilitates H2O2 efflux and influx
To test the direct involvement of So-AqpA in efflux of the S. oligofermentans endogenous H2O2, a HyPer fluorescent protein was used as an intracellular H2O2 reporter. The HyPer protein was constructed by Belousov et al. (25) by inserting the fluorescent protein cpYFP into the regulatory domain of the E. coli H2O2-sensing protein OxyR. When H2O2 oxidizes Cys-199 and Cys-208 to form a disulfide bond, HyPer emits green fluorescence. The S. oligofermentans lactate dehydrogenase promoter-HyPer gene fusion was inserted into the streptococci–E. coli shuttle vector pDL278 (26) and introduced into E. coli DH5α cells. A similar peak of HyPer fluorescence, as reported previously (27), was observed in the E. coli–HyPer strain after 1-min treatment by 20 μm H2O2 (Fig. S1A). Following that, the pDL278-HyPer plasmid was introduced into the S. oligofermentans WT strain, ΔaqpA and the pyruvate oxidase deletion mutant (Δpox) (28) to generate cellular H2O2 real-time reporter strains WT-HyPer, ΔaqpA-HyPer, and Δpox-HyPer, respectively.
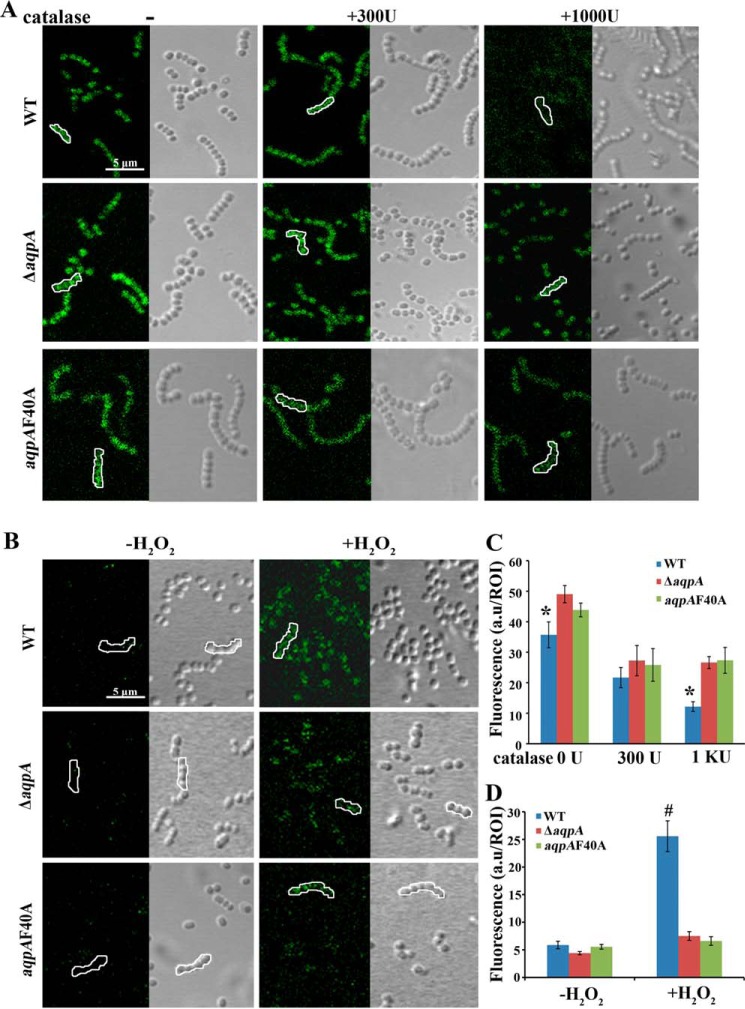
Next, So-AqpA-facilitated H2O2 export was examined in the three H2O2 real-time reporter strains that were cultured statically in BHI broth supplemented with 0 units, 300 units, and 1000 units catalase, respectively. Catalase was used to hydrolyze excreted H2O2 during growth so as to create an H2O2 gradient across cell membrane. The exponential phase cells were collected, and HyPer fluorescence was observed under a confocal laser scanning microscope. Fig. 2A showed significantly higher HyPer fluorescence intensity (∼28 a.u. per region of interesting (ROI)) in 1000 units catalase-treated ΔaqpA-HyPer cells compared with the WT-HyPer strain (∼12 a.u. per ROI), whereas only slightly higher intensities were observed in 0 units and 300 units catalase-treated mutant cells (Fig. 2C). No HyPer fluorescence was observed at all in the Δpox-HyPer cells at the earlier growth phase (Fig. S1B); this is consistent with the fact that the pox gene encoded pyruvate oxidase contributes to H2O2 production at the earlier growth phase of S. oligofermentans (28). This demonstrated that the S. oligofermentans aqpA gene encodes an H2O2 facilitator protein, and absence of the protein led to endogenous H2O2 retention. Although antibiotics can induce H2O2 production in bacteria, the observed HyPer fluorescence difference should be attributed to the studied genes, because all of the tested HyPer reporter strains were grown in BHI broth plus spectinomycin to maintain the shuttle plasmid.
Figure 2.
S. oligofermentans So-AqpA facilitates H2O2 transmembrane diffusion. A, the cellular H2O2 reporter HyPer fluorescence protein gene was introduced into all tested strains. The WT strain, So-aqpA deletion mutant (ΔaqpA), and its Phe-40 substitution mutant (aqpAF40A) were cultured statically in BHI broth containing 0 units (−), 300 units, and 1000 units catalase, respectively. The exponential phase cells were collected, washed, and resuspended in 100 μl PBS, and after a 30-min air exposure in dark, 40 μl cells were visualized under a confocal laser scanning microscope (Leica TCS SP8, Leica Microsystems) with excitation at 488 nm and emission collected from a range of 500 to 600 nm. Bar, 5 μm. B, mid–exponential phase anaerobically grown cells were resuspended in 100 μl PBS and pulsed by 0.5 mm H2O2. 40 μl of cells were sampled for visualization under a confocal laser scanning microscope before and 5 min after H2O2 pulsing. The results shown are representative of three independent experiments. Left and right panels in each picture show the cells with HyPer fluorescence and differential interference contrast, respectively. Bar, 5 μm. C and D, cell HyPer fluorescence intensity was measured using the Leica Application Suite Advanced Fluorescence software. At least five images were captured for each sample, and 25 ROI (framed) by each, including five cells in panels A (C) and B (D), were measured for calculation of the average fluorescence intensities. For images with too weak fluorescence to observe the cells, ROI in the corresponding differential interference contrast picture was framed and the fluorescence intensity was measured in the same ROI of the fluorescence image. Average fluorescence intensities were calculated and expressed as arbitrary unit (a.u) per ROI. * and #, data are statistically significantly different between the wild strain and the mutants as verified by one-way ANOVA analysis followed by Tukey's post hoc test (p <0.05).
To explore the role of So-AqpA in facilitating H2O2 influx, 2 ml mid–exponential phase cells of anaerobically grown WT-HyPer and ΔaqpA-HyPer cells were collected and resuspended in PBS. Cells were first exposed to air for 15 min and then pulsed with 0.5 mm H2O2. Fluorescence of the WT-HyPer cells was observed at 5 min post pulsing under a confocal laser scanning microscope. However, only weak fluorescence was observed in the ΔaqpA mutant (Fig. 2B), which was 5-fold lower than that in the WT strain (Fig. 2D). These results demonstrate that So-AqpA functions as a H2O2 facilitator for its bidirectional diffusion across the cellular membrane.
The key amino acid residues of So-AqpA for H2O2 permeation
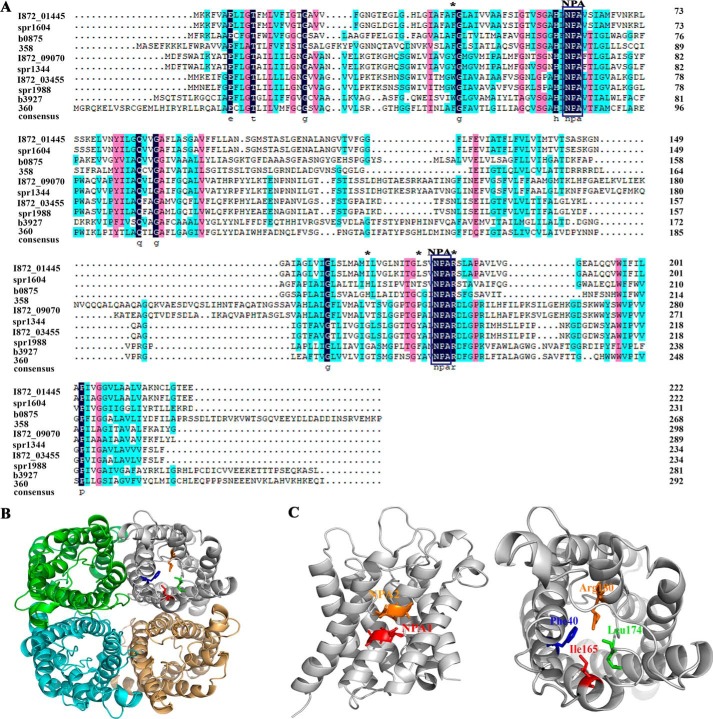
To probe the key amino acids of So-AqpA that are involved in H2O2 transport, we first performed an amino acid sequence alignment with the phylogenetic orthologs from eukaryotic and prokaryotic species. As shown in Fig. 3A, So-AqpA possesses the two characteristic NPA motifs and the four conserved amino acid residues for AQP substrate binding (29). Homology modeling of the So-AqpA protein was performed by automatic selection of the Archaeoglobus fulgidus aquaporin (identity 44%) as a template via the SWISS-MODEL web service. Fig. 3B shows that So-AqpA is a tetramer with each monomer forming a barrel shape, and the conserved Phe-40, Ile-165, Leu-174, and Arg-180 are situated at the substrate-binding sites, the two NPA motifs meet at the central part of the channel (Fig. 3C). Next, alanine substitution was performed for the four substrate-binding residues, and each of the residue-substituted So-AqpA mutants was introduced into the ΔaqpA strain by shuttle vector pIB166. Using the same approach as described above, we determined that alanine substitution of Phe-40 (F40A) substantially and Arg-180 (R180A) moderately reduced H2O2 excretion, respectively (Fig. 1B). Mutation of the remaining two had no effect on excreted H2O2 content.
Figure 3.
Structural analysis of the S. oligofermentans aquaporin protein So-AqpA. A, amino acid sequence alignment of the MIPs from S. oligofermentans (I872_01445, I872_09070, I872_03455), S. pneumoniae (spr1604, spr1344, spr1988), E. coli (b0875, b3927), and humans (358 and 360). The ar/R selective filter residues, FH(I)XR (X, small uncharged residues) in AQPs or WGFR in GLPs are labeled with asterisks, and the two NPA motifs are framed. B and C, structure modeling shows So-AqpA as a tetramer (B), the conserved ar/R selective filter residues Phe-40, Ile-165, Leu-174, and Arg-180 are situated at the substrate-binding sites, and the two NPA motifs meet at the central part of the channel (C).
The effect of So-AqpA F40A mutation on H2O2 excretion was also tested using the HyPer fluorescence reporter. To accomplish this, F40A mutation was introduced into the chromosome at the So-aqpA locus to construct aqpAF40A strain, and then the HyPer fluorescence reporter was introduced into aqpAF40A strain to construct an aqpAF40A-HyPer strain. By comparing the HyPer fluorescence intensity with those of the WT-HyPer and ΔaqpA-HyPer strains, failure of H2O2 efflux and influx was observed for the aqpAF40A-HyPer strain. As shown in Fig. 2, either by suspending the cells in 1000 units catalase-contained fresh BHI or by pulsing with 0.5 mm H2O2, similar HyPer fluorescence intensities were found for the aqpAF40A and ΔaqpA strain. Collectively, these experimental evidences demonstrate that Phe-40 is a key residue for So-AqpA to facilitate H2O2 transport.
H2O2 induces transcription of the So-aqpA gene
To investigate the link between So-AqpA's function as a H2O2 facilitator and its role in detoxification for the bacterium, H2O2 induction of the So-aqpA expression was detected using Northern blotting and quantitative real-time PCR (qPCR) assays. A final concentration of 40 μm H2O2 was added into the mid–exponential phase cultures of S. oligofermentans that were grown anaerobically, and the same volumes of H2O were added to the non-H2O2 treatment controls. By using a DNA fragment of the So-aqpA gene as probe (Table S1), Northern blotting detected an RNA of about 0.7 kb only in H2O2-treated cells, indicating that 40 μm H2O2 significantly induced So-aqpA transcription (Fig. 4A); consistently, the qPCR assay also determined a 10.5-fold elevated abundance of the So-aqpA mRNA in response to 40 μm H2O2 (Fig. 4B). To test whether the endogenous H2O2 affected So-aqpA expression, its transcription was compared in the statically cultured WT strain and a double-gene deletion mutant of pox and lox, which encode the two primary H2O2 production proteins, pyruvate and lactate oxidase (28). As expected, qPCR determined a 4.3-fold reduced expression of the So-aqpA gene in the pox/lox double mutant (Fig. 4B).
Figure 4.
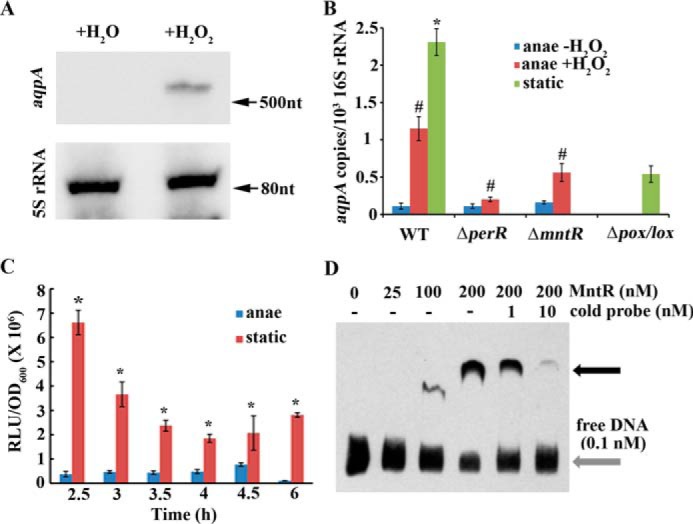
H2O2 induces the expression of the So-aqpA gene. A, anaerobically grown mid–expansion phase cells were collected and divided into two aliquots. One aliquot was treated with 40 μm H2O2 (+H2O2) for 20 min and the other with the same volume of H2O (+H2O) used as a control. Total RNA was extracted from both aliquots, and Northern blotting was performed using a biotin-labeled So-aqpA DNA fragment (Table S1) as the probe. 5S rRNA was used as an internal control (details are described in “Experimental procedures”). B, the tested strains were cultured and treated with 40 μm H2O2 as described in A. Quantitative RT-PCR was performed to quantify the transcript copies of the So-aqpA gene in anaerobically grown (anae −H2O2) and 40 μm H2O2-pulsed (anae +H2O2) wild strain (WT), deletion mutants of perR (ΔperR) and mntR (ΔmntR), and statically grown (static) wild strain and pox and lox double deletion mutant (Δpox/lox) (details are described in “Experimental procedures”). Triplicate measurements were performed for three batches of cultures, and the averages ± S.D. are shown. * and #, data are statistically significantly different in comparison between statically grown WT strain and Δpox/lox mutant (Student's t test, p <0.05) and H2O2 induction on anaerobically grown WT strain and mutants as verified by one-way ANOVA analysis followed by Tukey's post hoc test (p <0.05), respectively. C, a luciferase reporter strain, PaqpA-luc, in which the So-aqpA promoter was fused to the luciferase gene, was grown in BHI broth anaerobically or statically. At the indicated time points during growth, 100 μl cultures were collected in 1.5-ml Eppendorf tubes, and after 5 min exposure to air at room temperature, the luciferase activities (RLU, relative light units) were measured as described in “Experimental procedures.” OD600 was measured in parallel. Triplicate measurements were performed for three batches of cultures, and the averages ± S.D. are shown. *, data are statistically significant compared with those of anaerobically grown WT strain as verified by Student's t test (p <0.05). D, a DNA fragment of the So-aqpA promoter was PCR amplified with 5′-end biotin-labeled primers (Table S1). 0.1 nm biotin-labeled DNA was mixed with various concentrations of MntR protein in the EMSA-binding mixture and run in a native PAGE gel. Black arrow indicates the protein-DNA complex. Addition of increasing nonlabeled DNA (cold probe) decreased the protein-DNA complex.
A luciferase reporter was then used to test the So-aqpA expression profile in response to endogenous H2O2 during growth. The So-aqpA gene promoter was fused to the luc gene on pFW5-luc (30), and luciferase activities were determined during growth of the statically or anaerobically cultured S. oligofermentans. Fig. 4C shows that under static growth condition, relatively higher expression of the So-aqpA gene occurred at the earlier exponential phase, corresponding to the peak expression periods of the pox gene and H2O2 production (28). Luciferase reporter determined 2.7- to 26.7-fold higher activities in the statically grown cells than those in the anaerobically grown cells during the entire growth period, consistent with qPCR assayed up-regulation of So-aqpA in the static culture (Fig. 4B). This demonstrated that when cells accumulated relative high H2O2, expression level of the So-aqpA gene would be enhanced to fulfill its task in excreting endogenous H2O2 and attenuating oxidative stress in Streptococcus.
Super-resolution PALM imaging reveals numerous So-AqpA protein molecules in H2O2-induced cell membrane
Subsequently, we investigated how many So-AqpA protein molecules are present in one bacterial cell so as to meet the requirement for efficient endogenous H2O2 efflux. To accomplish this, the state-of-the-art super-resolution imaging method of PALM (31) was employed to obtain high-resolution cell images. First, a chromosomal AqpA-mMaple3 fusion was constructed in which the So-aqpA gene was tagged with the monomeric photoactivatable fluorescent protein mMaple3 (32). To visualize the So-AqpA protein expression in different growth phases and also in response to H2O2, the AqpA-mMaple3 strain was cultured statically and anaerobically, respectively. Cells of the static culture were collected at its earlier and mid-exponential growth phases and suspended in PBS buffer after washing. For the anaerobic culture, the mid-exponential phase cells were collected, and one aliquot was treated with 40 μm H2O2 for 20 min, whereas another was treated with the same volume of H2O. Fig. 5A shows the representative PALM images of the So-AqpA-mMaple3 fusion fluorescent proteins on cell membrane, each image included a cell chain consisting of three or four single cells. The mMaple3 signals indicated abundant So-AqpA protein molecules distributed on the cytoplasmic membrane. By using the Insight3 software, the protein numbers were counted on 18 cells for each sample (33), and the calculated So-AqpA protein molecules were 131 ± 22 and 122 ± 17 per cell in the earlier and mid–exponential phase statically grown culture, respectively. However, only an average of 41 ± 9 So-AqpA molecules per cell were counted in the anaerobic culture; and the protein number was increased by 1.6-fold upon H2O2 treatment (Fig. 5B). The super-resolution imaging not only reveals that S. oligofermentans possesses numerous transmembrane peroxiporin molecules, but also validates H2O2 induction of the So-aqpA gene expression. This robust approach is particularly powerful for detection of the membrane proteins that are difficult to be assayed by Western blotting.
Figure 5.
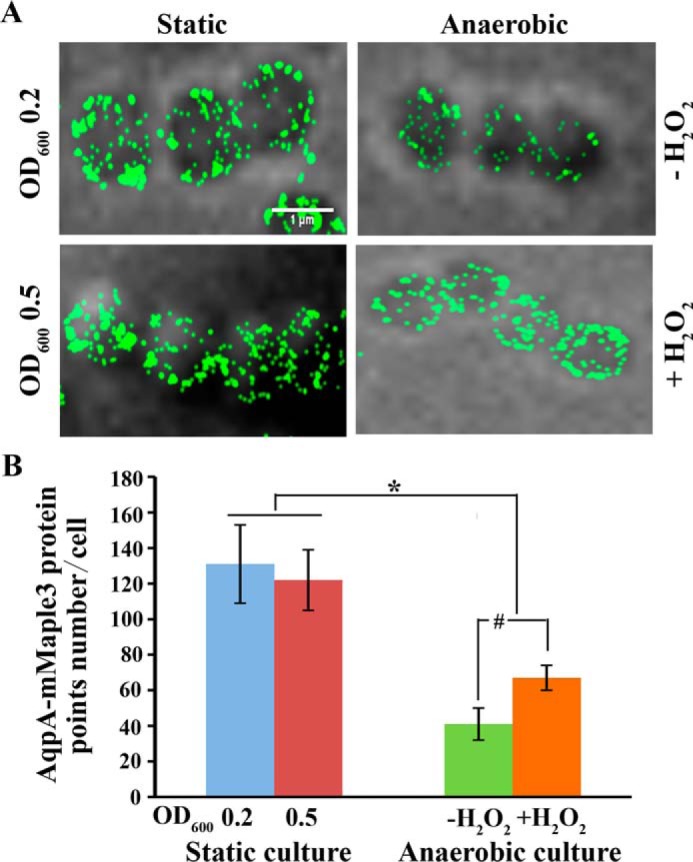
Representative PALM images show H2O2-induced So-AqpA protein abundance. S. oligofermentans with a chromosomal AqpA-mMaple3 fusion was grown statically and anaerobically, respectively. The static cultures were collected at the earlier (OD600 ∼0.2) and mid-exponential phase (OD600 ∼0.5) and suspended in PBS after washing. The anaerobically cultured cells were collected at mid-exponential phase (OD600 ∼0.5), by one aliquot treated with 40 μm H2O2 for 20 min before observation. A, cells were observed using PALM super-resolution imaging. B, for each sample, at least three images were captured, and 18 representative cells were selected for quantification of So-AqpA protein by using the custom-written MATLAB scripts. # and *, data are statistically significant, as verified by one-way ANOVA analysis followed by Tukey's post hoc test (p <0.05), compared with that of anaerobically grown WT strain without H2O2 treatment, and between the static and anaerobic cultures, respectively.
MntR and PerR are involved in regulation of H2O2-induced expression of So-aqpA
To further explore the regulatory mechanisms that mediate H2O2 induction of the So-aqpA expression, we tested the possible involvement of two known redox regulatory repressors, the metalloregulator MntR (34) and the peroxide-responsive repressor PerR (35). Our unpublished transcriptomic data4 also showed that by deletion of either mntR or perR, H2O2-induced So-aqpA expression appeared to be attenuated. ΔmntR and ΔperR strains were then cultured anaerobically, and one aliquot of the mid–exponential phase cell was treated with 40 μm H2O2 while another was treated with the same volume of H2O. After incubation at 37 °C for 20 min, the So-aqpA transcript copies were quantified by qPCR. As shown in Fig. 4B comparing with the >10-fold induction of So-aqpA expression by H2O2, the induction was reduced to 4- and 1.8-fold in the mntR and perR deletion mutants, respectively. This confirmed a regulatory involvement of the two regulators in H2O2-induced So-aqpA transcription.
To determine whether the two regulatory repressors directly regulate the expression of So-aqpA, EMSA was performed to detect the associations of overexpressed MntR and PerR proteins with 5′-biotin–labeled So-aqpA promoter fragment. As shown in Fig. 4D, a protein-DNA complex appeared at a 1000:1 ratio of MntR to DNA, a comparable affinity to its regulated manganese transporting protein mntABC promoter in a parallel experiment (Fig. S2); this complex disappeared with addition of the competing nonlabeled cold probe. This indicates that MntR may directly regulate the expression of So-aqpA. Additionally, a putative MntR-binding sequence (TATTATAACCTAAAAATT, subscript letters indicate non-conserved bases) was found at the So-aqpA promoter region, and this is consistent with a ∼1.5-fold enhanced expression of So-aqpA detected in the mntR deletion mutant (Fig. 4B). We previously demonstrated that MntR is inactivated by H2O2 via cysteine oxidation (34), which may explain MntR-dependent H2O2 induction of the So-aqpA expression. However, direct association of PerR with So-aqpA promoter was not found (data not shown), and the regulatory mechanisms of PerR on So-aqpA expression need to be further explored.
So-AqpA–based H2O2 export alleviates oxidative stress and provides S. oligofermentans with both intraspecies and interspecies competitive advantages
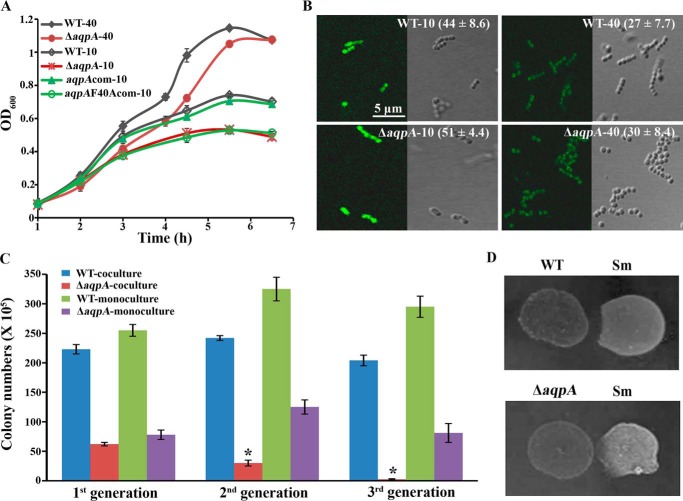
Because the WT strain produced significantly higher H2O2 (912 ± 136 μm) in 10 ml than that (255 ± 108 μm) in 40 ml BHI broth, to investigate the physiological significance of So-AqpA, the So-aqpA mutants and WT strain were statically grown in 10 ml or 40 ml medium in a 100-ml flask to create relatively high and low oxygen contents, respectively. As expected, no large growth difference was observed for the ΔaqpA and WT strains in 40 ml culture. However, in 10 ml cultures, severely retarded growth was found for the ΔaqpA- and F40A-complemented strains, but little inhibition was found for the WT and aqpA-complemented strain aqpA-com (Fig. 6A). Growth inhibition was not found for the aqpA-L174Acom, aqpA-I165Acom, and aqpA-R180Acom strains (Fig. S3).
Figure 6.
So-AqpA promotes the oxic growth and intraspecies and interspecies competitive advantage of S. oligofermentans. A, growth profiles were determined for the WT strain, So-aqpA mutant (ΔaqpA), So-aqpA complemented (aqpAcom), and the Phe-40 substitution complemented (aqpAF40Acom) strains that were statically cultured in 10 ml or 40 ml BHI broth. Overnight BHI cultures of the tested strains were 1:30 inoculated into fresh BHI medium and incubated at 37 °C, and OD600 was measured at the indicated time. The results are expressed as averages ± S.D. of three independent experiments. Suffix numbers after strain names indicate the culture volumes. B, exponential growing cells from the cultures above were collected, and HyPer fluorescence was observed and measured using the same procedure as described in Fig. 2. Representative images of three independent experiments are shown and fluorescence intensity a.u. per ROI is shown inside the parentheses in each image. Bar, 5 μm. C, competition between the wild strain and So-aqpA mutant was determined for three successive subcultured generations under high oxygen content. The same amounts of the two strains were co-inoculated or mono-inoculated into 10 ml BHI broth contained in a 100-ml flask, and the co-culture and mono-culture were subcultured for three successive generations. Colony-forming units (cfu) of each culture in each generation were counted on BHI agar plate; BHI plate containing 1 mg/ml kanamycin was used to count cfu of the ΔaqpA mutant in co-cultures. The results are averages ± S.D. of three independent experiments. *, significantly different from the So-aqpA deletion mutant in the first generation of co-culture as verified by one-way ANOVA analysis followed by Tukey's post hoc test (p <0.05). D, growth suppression of S. mutans (Sm) by the S. oligofermentans WT strain and So-aqpA mutant (ΔaqpA) was tested on TPYG plate (0.5% tryptone, 0.5% peptone, 1% yeast, 1% glucose, 4% salt solution) (23). Overnight cultures of the tested strains were collected and adjusted to the same OD600. 10 μl of each strain were spotted side-by-side on the plates and incubated at 37 °C in the candle jar for 24 h. The experiments were repeated three times, and one representative experiment is shown.
To link growth inhibition with endogenous H2O2, cellular H2O2 levels were monitored using HyPer reporter. The mid–exponential phase cells of the WT-HyPer and ΔaqpA-HyPer strains were examined under a confocal microscope. As shown in Fig. 6B, in the cells from either 10 ml or 40 ml culture, higher HyPer fluorescence intensities were always observed in ΔaqpA-HyPer compared with the WT-HyPer strain, whereas for each strain, higher HyPer fluorescence intensity was always detected in 10 ml compared with 40 ml cultures. The growth and cellular H2O2 measurements indicated that the aquaporin protein plays a significant role in protecting the bacterium from oxidative stress that is imposed by its own H2O2 production.
To examine the survivability of the ΔaqpA mutant among its parental WT strain, we co-cultured the two under higher and lower O2 contents, respectively, and monocultures of the two strains were included as controls. By inoculating the same cell amounts of ΔaqpA and the WT strain into 40 ml and 10 ml BHI broth contained in a 100-ml flask, respectively, and until the stationary phase, the co-cultures and monocultures in 10-fold serial dilutions were plated on BHI agar plate with or without 1 mg/ml kanamycin. Significantly lower cell numbers were counted for the aqpA mutant (62 ± 3 × 105 cells/ml) in the 10 ml co-culture compared with the WT strain (22 ± 8 × 106 cells/ml). No significant difference was found between the ΔaqpA (60 ± 12 × 106 cells/ml) and WT strain (65 ± 15 × 106 cells/ml) in the 40 ml BHI co-culture. Furthermore, upon three consecutive subcultures in 10 ml BHI, the percentage of the So-aqpA mutant gradually decreased from 21.7 to 1.2% in the first and third subculture of the co-culture (Fig. 6C), whereas cell numbers in the monospecies culture remained unchanged during subculturing. This further emphasizes the physiological significance of the aquaporin, which endows the bacterial cells with a selective advantage in intraspecies competition.
Previously, we found that S. oligofermentans overcompeted the caries-pathogen Streptococcus mutans using excreted H2O2, which is produced from the ample lactate generated by S. mutans so establishes a counterattack strategy (23). To determine the contribution of So-AqpA to this interspecies competition, the WT strain and ΔaqpA mutant were respectively spotted adjacent to S. mutans on TPYG plates. Fig. 6D shows that the ΔaqpA mutant grew poorer and only slightly suppressed S. mutans compared with the WT strain. To further quantify the inhibition of WT and ΔaqpA strain against S. mutans, the two strains were co-incubated with S. mutans as a mix-species culture, by including S. mutans single-species culture as control. After 24 h incubation, the cfu of S. mutans was counted based on its different colony appearance from that of S. oligofermentans (23). Compared with the cell number (18 ± 2 × 107 cells/ml) in monoculture, only 45% of live S. mutans (81 ± 11 × 106 cells/ml) was detected in the co-culture with S. oligofermentans WT strain, whereas the live cells (145 ± 13 × 106 cells/ml) were about 80% in the co-culture with ΔaqpA, thus the H2O2 excreted through So-AqpA can enhance about 35% inhibition effect of S. oligofermentans.
Discussion
In recent years, the physiological importance of aquaporin-facilitated transmembrane diffusion of H2O2, particularly in redox signaling, has been acknowledged in animals and plants (14–16). Yet, such information about the prokaryotic AQPs is almost missing. In this study, by using the ample H2O2-producing bacterium S. oligofermentans as a model, we demonstrated that a bacterial aquaporin, So-AqpA, functions to facilitate H2O2 transmembrane diffusion. The function not only detoxifies the endogenous H2O2 but also promotes the bacterium's intraspecies and interspecies competitive abilities. Notably, H2O2 significantly induces So-aqpA expression at both the mRNA and protein levels, and two redox transcriptional regulators, PerR and MntR, are involved in the H2O2 induction. Therefore, So-AqpA is most likely an intrinsic peroxiporin as named by Henzler and Steudle (36) and is the first reported bacterial AQP with physiological importance.
H2O2, as a by-product, is generated in all metabolic pathways with the involvement of oxygen, but this oxidant molecule is scavenged rapidly by catalase in aerobic organisms (22, 37, 38). However, in the catalase-negative lactic acid bacteria like lactobacilli and streptococci, H2O2 efflux is an effective approach for detoxification. Although these bacteria carry genes encoding both AQPs and GLPs, so far only one in vitro study indicates that three GLPs from Lactobacillus plantarum promote H2O2 sensitivity when they are heterogeneously expressed in yeast (21). Whether these GLPs act as H2O2 facilitators and their physiological importance in bacteria remain unknown. The present study demonstrated a water-facilitator type of AQP in an ample H2O2-producing streptococcus acting as a H2O2 facilitator and contributing to H2O2 detoxification. However, inactivation of the gene did not improve streptococcus hypertonic growth (data not shown), thus suggesting that So-AqpA is not primarily a water facilitator.
Remarkably, H2O2 induces the expression of So-aqpA, so indicating that it is an intrinsic peroxiporin (Figs. 4 and 5). Two transcriptional regulators, PerR and MntR, which are specifically involved in regulation of the cellular redox state, are involved in the H2O2 induction, and MntR is determined to be a direct regulator. It is also found that H2O2 induces a human water facilitator AQP, aquaporin-4; however, the oxidant appears not to directly induce the AQP synthesis but through H2O2-promoted phosphorylation of Cav1, which can indirectly modulate AQP4 subcellular distribution (39). In contrast, H2O2 induction of the So-aqpA expression appears to be at the transcriptional level and is mediated by the global H2O2-responsive regulators. Thus, H2O2-induced So-AqpA synthesis could be a strategy used by the catalase-lacking streptococci for dealing with the endogenous H2O2; when at higher levels, H2O2 can be speedily exported for detoxification with the assistance of large amounts of So-AqpA. Noticeably, a relatively lower H2O2 induction was found on synthesis of So-AqpA protein than the transcription of the gene (Figs. 4 and 5). This discrepancy could be because in general the cellular proteins have longer life spans than mRNAs, so larger alteration could be detected for transcript abundance when bacteria encounter changed environments. In addition, posttranscriptional or posttranslational regulation could occur for bacterial AQPs gene expression, similar as reported for the eukaryotic AQPs (9, 10, 11).
The substrate selectivity of AQPs is based on the proper substrate size and formation of energetically favorable hydrogen bonds between substrates and AQP amino acid residues in the substrate path (8, 40). Because water and H2O2 molecules possess similar physicochemical properties (41), it is predicted that the water-permeable AQPs also facilitate H2O2 diffusion. This prediction is supported by some families of AQPs, such as the human AQP8 and AQP1, that are primarily water facilitators but also function in H2O2 permeation through cell membranes (42). In addition, the two molecules appear to pass through the same substrate channel, as transports of them are inhibited by the same amino acid mutations in eukaryotic AQPs (14). However, we cannot conclude that all AQPs transport H2O2, as the plant AQPs AtPIP2;3, AtPIP2;6, and AtPIP2;8 do not transport H2O2. Similarly, E. coli aqpZ (b0875) also does not transport H2O2 (20, 43, 44). So-AqpA may therefore represent a distinct type of AQP that acts primarily as a H2O2 facilitator, and its homologs are present in all the Streptococcus spp. and other catalase-negative bacteria like Enterococcus and Lactococcus. Such type of AQPs may also occur in plants, as expression of the Solanaceae XIP genes in yeast induce a high sensitivity to exogenous H2O2, even though they have no significant water-transport ability (45). Therefore, the distinct characteristics of the H2O2 facilitators need to be investigated but not limited to comparative analyses of the protein sequences.
Consistent with the majority of AQPs, So-AqpA is a six-transmembrane protein and possesses the conserved Asn-Pro-Ala motif which constitutes the substrate channel. Similar with most water-transporting AQPs, So-AqpA uses Phe-40 as one of the residues in the ar/R-selective filter, which was demonstrated to be the key residue for So-AqpA–mediated H2O2 transport. Differently, So-AqpA protein has no cysteine residue, which is present in almost all the eukaryotic AQPs (29, 46). This unique feature of the streptococcal aquaporins implies that it could be serve as a potential drug target for controlling infection of the pathogenic streptococci.
Collectively, this work reports a bacterial aquaporin that functions as a dedicated H2O2 facilitator (peroxiporin) and has important physiological roles for streptococci by detoxifying the endogenous H2O2 and endowing it intraspecies and interspecies competitive advantages.
Experimental procedures
Bacterial strains and culture conditions
S. oligofermentans AS 1.3089 (47) and its derivative strains (Table S1) were grown in brain heart infusion (BHI) broth (BD Difco, Franklin Lakes, NJ) statically or anaerobically under 100% N2. Spectinomycin (1 mg ml−1) or kanamycin (1 mg ml−1) was used to select transformants.
Construction of genetic strains
All primers used in this study are listed in Table S1. So-aqpA and So-aqpB deletion strains were constructed using the PCR ligation method (48). The H2O2 reporter HyPer gene was amplified from the pHyPer-N1 plasmid, which was kindly provided by Prof. Jiangyun Wang at the Institute of Biophysics, Chinese Academy of Sciences, and fused to the S. oligofermentans lactate dehydrogenase gene (ldh) promoter by overlapping PCR; meanwhile, the So-aqpA promoter and coding gene were PCR amplified. The purified PCR products were integrated into the compatible sites of pIB166 or pDL278 plasmids, and the correct recombinant plasmids pDL278-HyPer or pIB166-aqpA were transformed into the WT strain or ΔaqpA mutant to produce HyPer or So-aqpA ectopically expressed strains. So-AqpA F40A, I165A, L174A, or R180A mutations were introduced into the pIB166-aqpA plasmid using a site-directed gene mutagenesis kit (Beyotime Biotechnology Co., Shanghai, China), and the correct constructs were transformed into the ΔaqpA mutant. The PCR-amplified monomeric gene sequence of the photoactivatable fluorescent protein mMaple3, which was kindly provided by Xiaowei Zhuang (Harvard University), was 3′ fused to the So-aqpA gene by overlapping PCR. Meanwhile, the F40A mutated So-aqpA gene was PCR amplified from the pIB166-aqpAF40A plasmid, and the purified PCR products were integrated into the S. oligofermentans WT genomic DNA via double crossover homologous recombination using kanamycin, amplified from plasmid pALH124 (49), as a selective marker to obtain aqpAF40A and aqpA-mMaple3 strains. pDL278-HyPer was transformed into the aqpAF40A strain to obtain aqpAF40A-HyPer.
Detection of intracellular hydrogen peroxide by HyPer imaging
Mid-exponential phase HyPer reporter cells were pelleted, washed with PBS twice, and resuspended in 100 μl of PBS in a 1.5 ml Eppendorf tube. After exposure to air for 30 min in the dark at room temperature, 40 μl of cells were placed on a glass slide (25 × 75 mm, 1- to 1.2-mm thick), covered with a coverslip (14 × 14 mm, 0.17-mm thick), and then visualized under a confocal laser scanning microscope (Leica TCS SP8, Leica Microsystems, Buffalo Grove, IL). Excitation was provided at 488 nm, with emission collected from a range of 500 to 600 nm. The gray values of 25 ROI by each containing five cells from each sample were measured using Leica Application Suite (LAS) Advanced Fluorescence software.
PALM imaging
The aqpA-mMaple3 strain was cultured statically and anaerobically. Cells of the static culture were collected at the indicated time by centrifugation, washed twice, and suspended in PBS. The anaerobic cultured cells were collected when OD600 reached ∼0.5. One aliquot was treated with 40 μm H2O2 for 20 min and another aliquot was treated with the same volume of H2O and used as a control. After PBS washing twice, cells were resuspended in PBS. All cells were exposed to air for 30 min in the dark at room temperature, then the cells were fixed with 4% paraformaldehyde for 15 min at room temperature, washed three times with PBS, and observed using PALM imaging. PALM imaging was performed using a Nikon TiE inverted microscope equipped with a 100 × oil-immersion objective (Nikon, PLAN APO, 1.49 NA) and an EMCCD camera (Andor-897). A 405-nm laser (Coherent, 100 milliwatt), 488-nm laser (Coherent, 100 milliwatt), and 561-nm laser (Coherent, 50 milliwatt) were used to either photoconvert or excite the fluorophores. AqpA-mMaple3 was activated with a continuous 405-nm laser, which was slowly increased for optimal photoconversion rates and excited with a constant 561-nm laser (∼2 kilowatts/cm2). 100 nm Tetraspeck beads (Invitrogen) were used to calibrate for stage drift during data acquisition. Construction of super-resolution images was performed using Insight3 software, kindly provided by Dr. Bo Huang (University of California San Francisco). PALM data analysis such as drift correction and image rendering was carried out using custom-written MATLAB scripts.
Determination of the excreted hydrogen peroxide in culture
Hydrogen peroxide (H2O2) in culture suspension was quantified as described previously (35). Briefly, 650 μl of culture supernatant was added to 600 μl of solution containing 2.5 mm 4-amino-antipyrine (4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one; Sigma) and 0.17 m phenol. The reaction proceeded for 4 min at room temperature; horseradish peroxidase (Sigma) was then added to a final concentration of 50 milliunits/ml in 0.2 m potassium phosphate buffer (pH 7.2). After 4 min incubation at room temperature, optical density at 510 nm was measured with a Unico 2100 visible spectrophotometer (Shanghai, China). A standard curve was generated with known concentrations of chemical H2O2.
Quantitative PCR
Total RNA was extracted from the mid-log-phase (OD600, ∼0.4 to 0.5) cultures of tested strains using TRIzol reagent (Invitrogen) as recommended by the suppliers. After quality confirmation on 1% agarose gel, RNA extracts were treated with RNase-free DNase (Promega). cDNAs were generated from 2 μg of total RNA with random primers using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega) according to the supplier's instructions and used for qPCR amplification with the corresponding primers (Table S1). Amplifications were performed with a Mastercycler ep realplex2 (Eppendorf AG, Hamburg, Germany). To estimate copy numbers of the So-aqpA mRNA, a standard curve of the So-aqpA gene was generated by quantitative PCR using 10-fold serially diluted PCR product as the template. The 16S rRNA gene was used as the biomass reference. The number of copies of So-aqpA transcript per 1000 16S rRNA copies is shown. All the measurements were done for triplicate samples and repeated at least three times.
Northern blotting
Total RNA was run on 5% Urea-PAGE for 100 min at 250 volts on ice. RNAs in the gel were transferred to positively charged nylon membranes (GE Healthcare) and cross-linked by using GS Gene Linker™ UV Chamber. The membranes were pre-hybridized in buffer (5 × SSC, 5 × Denhardt's, 50% (v/v) deionized formamide, 0.5% (m/v) SDS, and 200 μg/ml salmon sperm DNA) for 4 h, and then hybridized with biotin-labeled NoraqpA (Table S1) at 42 °C overnight. The So-aqpA transcript was then detected using Chemiluminescent Nucleic Acid Detection Module Kit (Thermo Fisher Scientific).
Construction of So-aqpA luciferase reporter strain and assay of luciferase activity
The So-aqpA luciferase reporter was constructed by inserting the promoter fragment of So-aqpA gene into the compatible sites on plasmid pFW5-luc (30) which carries luciferase reporter gene. The recombinant plasmid pFW5-PaqpA-luc was then transformed into the WT strain to produce PaqpA-luc strain. For luciferase activity assay, 100 μl of PaqpA-luc cells were collected into 1.5 ml-Eppendorf tubes, exposed to air for 5 min at room temperature, and 25 μl of 1 mm d-luciferin (Sigma-Aldrich) solution (in 1 mm citrate buffer, pH 6.0) was added, and then the assay was performed with a TD 20/20 luminometer (Turner Biosystems, Sunnyvale, CA). The optical density of the samples (OD600) was measured using a 2100 visible spectrophotometer (Unico, Shanghai, China) and used to normalize the luciferase activity. All the measurements were done on triplicate samples and repeated at least three times.
EMSA
The So-aqpA promoter fragment was PCR amplified using a biotin-labeled primer pair of aqpAEMSAF/aqpAEMSAR (Table S1). EMSA was performed using Light Shift Chemiluminescent EMSA Kit (Pierce). Briefly, 0.1 nm biotin-labeled dsDNA probe was mixed with various amounts of MntR protein (0–200 nm) in the binding buffer (10 mm Tris-HCl, pH 8.0, 5% glycerol, 50 mm NaCl, 10 μg/ml BSA, 2 ng/μl poly (dI-dC) and 0.1 mm MnCl2). The reaction mixtures stayed at 30 °C for 30 min, and then were electrophoresed on 8% polyacrylamide gel on ice. The DNA-protein complex was transferred onto a nylon membrane and detected by Chemiluminescent Nucleic Acid Detection Module kit (Thermo Scientific).
Heterologous expression in Saccharomyces cerevisiae and observation of GFP fluorescence
The S. oligofermentans So-aqpA gene was PCR amplified or fused to the green fluorescence protein (sfGFP) gene. Purified PCR products were double digested by HindIII and BamHI, and then integrated into the compatible sites of the pYES2 yeast expression vector (Thermo Fisher). Correct recombinant pYES2-So-aqpA, pYES2-So-aqpA-gfp, and pYES2 were transformed into S. cerevisiae INVSc1 using a yeast transformation kit (Labest Company, Beijing, China) and selected on SC-Ura medium (Coolaber Company, Beijing, China). Correct transformants were verified by plasmid extraction, PCR, and sequencing.
S. cerevisiae strains carrying pYES2-So aqpA-gfp or pYES2 were grown in SC-Ura galactose medium to induce the So-aqpA-gfp gene expression. Then 500 μl cells were pelleted, washed with distilled water twice, and resuspended in 100 μl of distilled water in a 1.5 ml Eppendorf tube. After 30-min exposure to air in the dark at room temperature, 40 μl of cells were placed on a glass slide (25 × 75 mm, 1- to 1.2-mm thick), covered with a coverslip (14 × 14 mm, 0.17-mm thick), and then visualized under a confocal laser scanning microscope (Leica TCS SP8, Leica Microsystems, Buffalo Grove, IL). Excitation was provided at 488 nm, and emission was collected from a range of 500 to 600 nm.
Hydrogen peroxide sensitivity assay of S. cerevisiae
Overnight cultures of S. cerevisiae INVSc1 strains carrying pYES2-So-aqpA or empty vector pYES2 grown in SC-Ura glucose were diluted into SC-Ura galactose medium to a final OD600 of 0.4, and then induced at 30 °C for 6 h to allow So-aqpA gene expression. After induction, the two strains were diluted to OD600 of 0.01, and 10 μl of the dilutions were spotted onto SC-Ura galactose agar plates containing various concentrations of H2O2. For minimal inhibitory concentration assay, 100-μl dilutions were added into a 96-well cell culture plate (Nunc), and 100 μl of 24 mm H2O2 were added to the first well and mixed, then 100 μl were taken out and added into the second well. Therefore, 2-fold serially diluted H2O2 concentration until 0.09 mm was generated. Growth was recorded after 6 days at 30 °C.
Assay of So-AqpA promoting Escherichia coli to uptake H2O2
Overnight cultures of E. coli carrying recombinant pIB166-aqpA or vacant vector pIB166 were 1:100 diluted into fresh LB and grown at 37 °C. After OD600 reached ∼0.60, cells were collected by centrifugation at 5000 rpm for 10 min and washed twice with PBS. Cells were then diluted to OD600 0.1 with 10 ml of PBS, and H2O2 was added to a final concentration of 150 μm. 750 μl of cell suspension were centrifuged at 12,000 rpm for 2 min at indicated time points, and the residual H2O2 content in the supernatant was determined.
Statistical analysis
One-way ANOVA followed by Tukey's post hoc test or Student's t test was performed by using PASW Statistics 18 or Excel, respectively. The level of significance was determined at p <0.05.
Author contributions
H. T., F. B., and X. D. conceptualization; H. T. and X. D. formal analysis; H. T. funding acquisition; H. T., X. W., Y. D., Q. H., Z. Z., Y. Z., and L. D. investigation; H. T. methodology; H. T., X. W., Y. D., Q. H., Z. Z., Y. Z., F. B., and X. D. writing-original draft; H. T., F. B., and X. D. writing-review and editing; X. W., Y. D., and Q. H. data curation; X. D. supervision; X. D. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Jiangyun Wang at the Institute of Biophysics, Chinese Academy of Sciences for providing pHyPer-N1 plasmid; Dr. Yu Fu at Institute of Microbiology, CAS for providing S. cerevisiae; and Dr. Xiaolan Zhang at the Institute of Microbiology, CAS for help in confocal laser scanning microscopy imaging.
This study was supported by the National Natural Science Foundation of China Grant No. 31370098. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S3 and Table S1.
H. Tong, X. Wang, Y. Dong, Q. Hu, Z. Zhao, Y. Zhu, L. Dong, F. Bai, X. Dong, unpublished data.
- AQP
- aquaporin
- qPCR
- quantitative PCR
- MIP
- major intrinsic protein
- GLP
- glycerol-permeable aquaglyceroporin
- PALM
- photoactivated localization microscopy
- BHI
- brain heart infusion
- a.u.
- arbitrary units
- ROI
- region of interesting
- cfu
- colony-forming unit
- ANOVA
- analysis of variance.
References
- 1. Preston G. M., Carroll T. P., Guggino W. B., and Agre P. (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256, 385–387 10.1126/science.256.5055.385 [DOI] [PubMed] [Google Scholar]
- 2. Borgnia M. J., and Agre P. (2001) Reconstitution and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 98, 2888–2893 10.1073/pnas.051628098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agre P., Bonhivers M., and Borgnia M. J. (1998) The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 273, 14659–14662 10.1074/jbc.273.24.14659 [DOI] [PubMed] [Google Scholar]
- 4. Calamita G., Perret J., and Delporte C. (2018) Aquaglyceroporins: Drug targets for metabolic diseases? Front. Physiol. 9, 851 10.3389/fphys.2018.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siefritz F., Tyree M. T., Lovisolo C., Schubert A., and Kaldenhoff R. (2002) PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 14, 869–876 10.1105/tpc.000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrigues O., Reshetnyak G., Grondin A., Saijo Y., Leonhardt N., Maurel C., and Verdoucq L. (2017) Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. U.S.A. 114, 9200–9205 10.1073/pnas.1704754114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zardoya R. (2005) Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 97, 397–414 10.1042/BC20040134 [DOI] [PubMed] [Google Scholar]
- 8. Savage D. F., O'Connell J. D. 3rd, Miercke L. J., Finer-Moore J., and Stroud R. M. (2010) Structural context shapes the aquaporin selectivity filter. Proc. Natl. Acad. Sci. U.S.A. 107, 17164–17169 10.1073/pnas.1009864107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Törnroth-Horsefield S., Hedfalk K., Fischer G., Lindkvist-Petersson K., and Neutze R. (2010) Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 584, 2580–2588 10.1016/j.febslet.2010.04.037 [DOI] [PubMed] [Google Scholar]
- 10. Rodrigues C., Mósca A. F., Martins A. P., Nobre T., Prista C., Antunes F., Cipak Gasparovic A., and Soveral G. (2016) Rat aquaporin-5 is pH-gated induced by phosphorylation and is implicated in oxidative stress. Int. J. Mol. Sci. 17, E2090 10.3390/ijms17122090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verdoucq L., Grondin A., and Maurel C. (2008) Structure-function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. Biochem. J. 415, 409–416 10.1042/BJ20080275 [DOI] [PubMed] [Google Scholar]
- 12. Mósca A. F., de Almeida A., Wragg D., Martins A. P., Sabir F., Leoni S., Moura T. F., Prista C., Casini A., and Soveral G. (2018) Molecular basis of aquaporin-7 permeability regulation by pH. Cells 7, E207 10.3390/cells7110207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dynowski M., Schaaf G., Loque D., Moran O., and Ludewig U. (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 414, 53–61 10.1042/BJ20080287 [DOI] [PubMed] [Google Scholar]
- 14. Bienert G. P., and Chaumont F. (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604 10.1016/j.bbagen.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 15. Al Ghouleh I., Frazziano G., Rodriguez A. I., Csányi G., Maniar S., St. Croix C. M., Kelley E. E., Egaña L. A., Song G. J., Bisello A., Lee Y. J., and Pagano P. J. (2013) Aquaporin 1, Nox1, and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc. Res. 97, 134–142 10.1093/cvr/cvs295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medraño-Fernandez I., Bestetti S., Bertolotti M., Bienert G. P., Bottino C., Laforenza U., Rubartelli A., and Sitia R. (2016) Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxid. Redox Signal. 24, 1031–1044 10.1089/ars.2016.6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller E. W., Dickinson B. C., and Chang C. J. (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. U.S.A. 107, 15681–15686 10.1073/pnas.1005776107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marinho H. S., Real C., Cyrne L., Soares H., and Antunes F. (2014) Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2, 535–562 10.1016/j.redox.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rhee S. G. (2006) H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 10.1126/science.1130481 [DOI] [PubMed] [Google Scholar]
- 20. Calamita G. (2000) The Escherichia coli aquaporin-Z water channel. Mol. Microbiol. 37, 254–262 10.1046/j.1365-2958.2000.02016.x [DOI] [PubMed] [Google Scholar]
- 21. Bienert G. P., Desguin B., Chaumont F., and Hols P. (2013) Channel-mediated lactic acid transport: A novel function for aquaglyceroporins in bacteria. Biochem. J. 454, 559–570 10.1042/BJ20130388 [DOI] [PubMed] [Google Scholar]
- 22. Seaver L. C., and Imlay J. A. (2001) Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183, 7182–7189 10.1128/JB.183.24.7182-7189.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tong H., Chen W., Merritt J., Qi F., Shi W., and Dong X. (2007) Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: A possible counteroffensive strategy for interspecies competition. Mol. Microbiol. 63, 872–880 10.1111/j.1365-2958.2006.05546.x [DOI] [PubMed] [Google Scholar]
- 24. Biswas I., Jha J. K., and Fromm N. (2008) Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology 154, 2275–2282 10.1099/mic.0.2008/019265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belousov V. V., Fradkov A. F., Lukyanov K. A., Staroverov D. B., Shakhbazov K. S., Terskikh A. V., and Lukyanov S. (2006) Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 10.1038/nmeth866 [DOI] [PubMed] [Google Scholar]
- 26. LeBlanc D. J., Lee L. N., and Abu-Al-Jaibat A. (1992) Molecular, genetic, and functional analysis of the basic replicon of pVA380–1, a plasmid of oral streptococcal origin. Plasmid 28, 130–145 10.1016/0147-619X(92)90044-B [DOI] [PubMed] [Google Scholar]
- 27. Lim J. B., Barker K. A., Huang B. K., and Sikes H. D. (2014) In-depth characterization of the fluorescent signal of HyPer, a probe for hydrogen peroxide, in bacteria exposed to external oxidative stress. J. Microbiol. Methods 106, 33–39 10.1016/j.mimet.2014.07.038 [DOI] [PubMed] [Google Scholar]
- 28. Liu L., Tong H., and Dong X. (2012) Function of the pyruvate oxidase-lactate oxidase cascade in interspecies competition between Streptococcus oligofermentans and Streptococcus mutans. Appl. Environ. Microbiol. 78, 2120–2127 10.1128/AEM.07539-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savage D. F., and Stroud R. M. (2007) Structural basis of aquaporin inhibition by mercury. J. Mol. Biol. 368, 607–617 10.1016/j.jmb.2007.02.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Podbielski A., Spellerberg B., Woischnik M., Pohl B., and Lütticken R. (1996) Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177, 137–147 10.1016/0378-1119(96)84178-3 [DOI] [PubMed] [Google Scholar]
- 31. Betzig E., Patterson G. H., Sougrat R., Lindwasser O. W., Olenych S., Bonifacino J. S., Davidson M. W., Lippincott-Schwartz J., and Hess H. F. (2006) Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 10.1126/science.1127344 [DOI] [PubMed] [Google Scholar]
- 32. Wang S., Moffitt J. R., Dempsey G. T., Xie X. S., and Zhuang X. (2014) Characterization and development of photoactivatable fluorescent proteins for single-molecule-based superresolution imaging. Proc. Natl. Acad. Sci. U.S.A. 111, 8452–8457 10.1073/pnas.1406593111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang B., Wang W., Bates M., and Zhuang X. (2008) Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 319, 810–813 10.1126/science.1153529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen Z., Wang X., Yang F., Hu Q., Tong H., and Dong X. (2017) Molecular insights into hydrogen peroxide-sensing mechanism of the metalloregulator MntR in controlling bacterial resistance to oxidative stresses. J. Biol. Chem. 292, 5519–5531 10.1074/jbc.M116.764126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X., Tong H., and Dong X. (2014) PerR-regulated manganese ion uptake contributes to oxidative stress defense in an oral streptococcus. Appl. Environ. Microbiol. 80, 2351–2359 10.1128/AEM.00064-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henzler T., and Steudle E. (2000) Transport and metabolic degradation of hydrogen peroxide in Chara corallina: Model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 51, 2053–2066 10.1093/jexbot/51.353.2053 [DOI] [PubMed] [Google Scholar]
- 37. Imlay J. A. (2013) The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 11, 443–454 10.1038/nrmicro3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faulkner M. J., and Helmann J. D. (2011) Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid. Redox Signal. 15, 175–189 10.1089/ars.2010.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bi C., Tham D. K. L., Perronnet C., Joshi B., Nabi I. R., and Moukhles H. (2017) The oxidative stress-induced increase in the membrane expression of the water-permeable channel aquaporin-4 in astrocytes is regulated by caveolin-1 phosphorylation. Front. Cell Neurosci. 11, 412 10.3389/fncel.2017.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beitz E., Wu B., Holm L. M., Schultz J. E., and Zeuthen T. (2006) Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl. Acad. Sci. U.S.A. 103, 269–274 10.1073/pnas.0507225103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bienert G. P., Møller A. L., Kristiansen K. A., Schulz A., Møller I. M., Schjoerring J. K., and Jahn T. P. (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 10.1074/jbc.M603761200 [DOI] [PubMed] [Google Scholar]
- 42. Almasalmeh A., Krenc D., Wu B., and Beitz E. (2014) Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 281, 647–656 10.1111/febs.12653 [DOI] [PubMed] [Google Scholar]
- 43. Calamita G., Kempf B., Bonhivers M., Bishai W. R., Bremer E., and Agre P. (1998) Regulation of the Escherichia coli water channel gene aqpZ. Proc. Natl. Acad. Sci. U.S.A. 95, 3627–3631 10.1073/pnas.95.7.3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borgnia M. J., Kozono D., Calamita G., Maloney P. C., and Agre P. (1999) Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 291, 1169–1179 10.1006/jmbi.1999.3032 [DOI] [PubMed] [Google Scholar]
- 45. Bienert G. P., Bienert M. D., Jahn T. P., Boutry M., and Chaumont F. (2011) Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 66, 306–317 10.1111/j.1365-313X.2011.04496.x [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y., Cui Y., and Chen L. Y. (2012) Mercury inhibits the L170C mutant of aquaporin Z by making waters clog the water channel. Biophys. Chem. 160, 69–74 10.1016/j.bpc.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tong H., Gao X., and Dong X. (2003) Streptococcus oligofermentans sp. nov., a novel oral isolate from caries-free humans. Int. J. Syst. Evol. Microbiol. 53, 1101–1104 10.1099/ijs.0.02493-0 [DOI] [PubMed] [Google Scholar]
- 48. Lau P. C., Sung C. K., Lee J. H., Morrison D. A., and Cvitkovitch D. G. (2002) PCR ligation mutagenesis in transformable streptococci: Application and efficiency. J. Microbiol. Methods 49, 193–205 10.1016/S0167-7012(01)00369-4 [DOI] [PubMed] [Google Scholar]
- 49. Liu Y., Zeng L., and Burne R. A. (2009) AguR is required for induction of the Streptococcus mutans agmatine deiminase system by low pH and agmatine. Appl. Environ. Microbiol. 75, 2629–2637 10.1128/AEM.02145-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.