Abstract
Objectives:
Both physical functioning and cognitive abilities are important for well-being, not least in old age. Grip strength is often considered an indicator of general vitality and, as such, may predict cognitive functioning. Few longitudinal studies have examined the relationship between grip strength and cognition, especially where specific cognitive abilities have been targeted.
Method:
Participants ( n = 708, age range: 40–86 years at baseline) came from the population-based longitudinal Swedish Adoption/Twin Study of Aging. We used a longitudinal follow-up of 6 waves during 20 years. For the analyses, we used latent growth modeling, where latent growth trajectories were fitted to the cognitive traits (verbal ability, spatial ability, processing speed, and memory) or to the grip strength values and each, respectively, treated as time-varying covariates of the other trait.
Results:
Results supported a longitudinal influence of grip strength on changes in cognitive function. Grip strength performance was associated with change in the 4 cognitive abilities after age 65 years.
Discussion:
A rather stable connection was found between grip strength and cognitive abilities starting around 65 years of age. The starting period suggests that the association may be due to lifestyle changes, such as retirement, or to acceleration of the aging processes.
Keywords: Cognition, Grip strength, Longitudinal, Time-varying covariates
Two important areas for well-being in older adults are physical functioning and cognitive abilities. They are both related to important outcomes, such as health and mortality (e.g., Cooper et al., 2011 ; Cooper, Kuh, & Hardy, 2010 ; Ghisletta, McArdle, & Lindenberger, 2006 ; Small, Dixon, & McArdle, 2011 ), and they are, in general, declining in old age ( Rönnlund, Nyberg, Bäckman, & Nilsson, 2005 ; Sternäng et al., 2014 ). A connection between the two has been suggested, but the etiology of that relationship has yet to be fully explained. Three main possibilities have been discussed: (a) physical functioning drives age changes in cognition (e.g., Alfaro-Acha et al., 2006 ; Auyeung, Lee, Kwok, & Woo, 2011 ), (b) cognition drives age changes in physical functioning (e.g., Atkinson et al., 2010 ; Charles et al., 2006 ), or (c) a third factor impacts both (e.g., Baltes & Lindenberger, 1997 ; Christensen, Mackinnon, Korten, & Jorm, 2001 ). The connection may also differ depending on the specific physical function and/or cognitive ability under scrutiny. In this study, the focus was on the relationship between grip strength and four cognitive abilities (verbal ability, spatial ability, processing speed, and memory). Grip strength is often used as a marker, not only for muscle strength, but also for biological vitality, since it is sensitive to age-related changes and to changes in biological functioning ( Bohannon, 2008 ).
Results from cross-sectional studies have mainly favored a “common cause” account ( Clouston et al., 2013 ). This account suggests that there is a third factor (a common cause) behind decline in both sensory-motor and cognitive functioning in old age, probably because of declining functioning of the central nervous system ( Baltes & Lindenberger, 1997 ). Support for this argument comes primarily from cross-sectional studies and only partially from longitudinal studies ( Clouston et al., 2013 ).
However, to understand causality, associations should be studied longitudinally. To date, there are only a few longitudinal studies that have examined the relationship between grip strength and cognition, especially where associations with specific cognitive abilities have been examined (e.g., Clouston et al., 2013 ; Deary et al., 2011 ; Kuh, Cooper, Hardy, Guralnik, & Richards, 2009 ). In a review of the relationship between physical functioning and cognition, Clouston and coworkers ( Clouston et al., 2013 ) indicated that changes in grip strength were associated with baseline performance on the Mini-Mental State Examination ( Folstein, Folstein, & McHugh, 1975 ), a rough measure of overall cognitive functioning. There was also a baseline correlation between these two variables, and between grip strength and fluid abilities. This review focused on two connections only: baseline versus baseline and baseline versus change, that is, not change versus change. However, in one study ( Deary et al., 2011 ), participants (79 years of age) were followed up longitudinally with three waves of data on both grip strength and reasoning. Cross-sectional relationships between these two variables were found, but none longitudinally.
This study, with six waves of data on cognition and grip strength, based on data from the population-based longitudinal Swedish Adoption/Twin Study of Aging (SATSA; Finkel & Pedersen, 2004 ) provides an unique opportunity to examine the association between grip strength and cognitive abilities, since it includes repeated measures of four cognitive domains and grip strength over 20 years. The study had two aims: (a) to examine if there was an association between longitudinal changes in grip strength and cognitive abilities in late life and (b) to examine potential mediators and moderators of this association.
Method
Participants
The SATSA sample is a subset of twins from the population-based Swedish Twin Registry ( Finkel & Pedersen, 2004 ). The sample consists of same-sex twin pairs reared apart from early age and of matched same-sex twin pairs reared together. The first wave of data collection through questionnaires (Q1) in SATSA was administered in 1984, and the first wave of in-person testing (IPT1) was administered in 1986–1988. The IPT was completed during a single 4-hr visit at a location convenient to the twins and includes biomedical examination and cognitive testing ( Finkel & Pedersen, 2004 ; Pedersen et al., 1991 ). SATSA is an ongoing study and for the present analyses, we used six different IPT waves (IPT1–IPT3 and IPT5–IPT7) conducted between 1986 and 2007. All the IPT waves were administered with 3-year intervals, except IPT5 that occurred after a 7-year interval, since no IPT was administered during Wave 4 ( Finkel & Pedersen, 2004 ). All participants ( n = 832) had at least one grip strength value. Participants diagnosed with dementia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-III and DSM-IV) criteria during the period were excluded ( n = 124) ( Dahl et al., 2010 ). This resulted in a final sample including 708 persons: 397 women and 311 men. Age range at baseline (IPT1) was 40–86 years of age (for descriptives of the sample, see Table 1 ).
Table 1.
Descriptives of the Participants
| Variables | Total | Women | Men |
|---|---|---|---|
| ( n = 708) | ( n = 397) | ( n = 311) | |
| Mean ( SD ) | Mean ( SD ) | Mean ( SD ) | |
| At Time II | |||
| Age (years) | 64.4 (8.4) | 64.4 (9.0) | 64.3 (7.4) |
| Grip strength (kg) | 30.2 (12.0) | 22.4 (6.2) | 41.1 (9.4) |
| Height (cm) | 167.8 (9.8) | 161.2 (6.3) | 176.2 (6.4) |
| Smoking | 44% | 28% | 63% |
| Physical activity | 3.8 (1.6) | 3.7 (1.6) | 3.8 (1.5) |
| SES in childhood | 0.0 (3.7) | 0.0 (3.5) | 0.1 (3.9) |
| SES | 1.4 (0.8) | 1.3 (0.8) | 1.5 (0.8) |
| Chronic disorders | 29% | 30% | 29% |
| At Time I | |||
| Smoking | 50% | 33% | 72% |
| Physical activity | 1.1 (0.8) | 1.0 (0.6) | 1.3 (0.9) |
Note: Time I regards data from questionnaires in the Swedish Twin Registry administered in 1967–1973, and Time II regards data from Q1 (1984) or IPT1 (1986–1988) in SATSA. All measures at Time II were assessed at Q1, except age, grip strength, and height that were assessed at IPT1.
Measures
Cognitive variables
There are four cognitive domains in the SATSA cognitive test battery: verbal abilities, spatial abilities, processing speed, and memory ( Pedersen, Plomin, Nesselroade, & McClearn, 1992 ). Verbal ability measures included the Information Subtest (from the Wechsler Adult Intelligence Scale-Revised [WAIS-R]; Wechsler, 1981 ), Synonyms, and Analogies. Spatial ability tests included Figure Logic, Block Design (WAIS-R), and Card Rotation. Processing speed tests included Symbol Digit and Figure Identification. Memory tests included Digit Span (WAIS-R) and Thurstone’s Picture Memory Task. The reliabilities of the cognitive tests range from 0.82 to 0.96 ( Pedersen et al., 1992 ).
All four cognitive domains were represented in terms of factor scores based on principal component analyses within each domain. The range of factor loadings was from 0.79 to 0.92 and the factor structure was invariant across age and time ( Finkel, Reynolds, McArdle, & Pedersen, 2005 ). The cognitive measures were standardized based on means and variances at IPT1, which means that the factors at each time point (IPT1–IPT7) are comparable. The factor loadings from IPT1 were used to construct the verbal, spatial, speed, and memory factors. All factor scores were later transformed to T -scores using factor means and variances from IPT1. These procedures have been described in earlier papers ( Finkel et al., 2005 ; Sharp, Reynolds, Pedersen, & Gatz, 2010 ).
Independent variables
The main independent variable was grip strength across time (IPT1–IPT7). Grip strength was measured by a Collins hand grip dynamometer at the IPT. The participant made six attempts (three with each hand) ( Coldham, Lewis, & Lee, 2006 ) and the highest score (in kg) was considered as the participant’s grip strength score.
Based on previous work on grip strength ( Stenholm, Tiainen, et al., 2012 ; Sternäng et al., 2014 ) and cognition (e.g., Langlois et al., 2013 ; Melrose et al., 2014 ; Rönnlund & Nilsson, 2009 ; Swan & Lessov-Schlaggar, 2007 ), the variables below were selected as possible mediators or moderators of the associations between grip strength and the cognitive abilities. Smoking and physical activity were selected as possible mediators since they have been found to be significantly associated with both grip strength and cognition in previous studies (e.g., Langlois et al., 2013 ; Stenholm, Tiainen, et al., 2012 ; Sternäng et al., 2014 ; Swan & Lessov-Schlaggar, 2007 ) and they have the possibility to act as dynamic mediators. Smoking and physical activity together with height, chronic disorders, and socio-economic status (SES) were also tested as possible moderators. To the best of our knowledge, longitudinal research on these moderator associations does not exist, and it is reasonable to believe (and to test) that the associations between grip strength and cognition may differ for different levels of physical activity, smoking, height, chronic disorders, or SES. Data for the tested mediator and moderator variables came from questionnaires in the Swedish Twin Registry ( Lichtenstein et al., 2002 ) administered in 1967–1973 (referred to as Time I), and from Q1 (1984) or IPT1 (1986–1988) in SATSA (referred to as Time II). Even if some of the possible mediators or moderators (e.g., smoking and physical activity) were measured at two time points, they were used as two separate cross-sectional variables in the analyses (e.g., smoking at Time I and smoking at Time II).
Smoking at Time I
A dichotomous variable coded as 1 if the participant has answered yes to the question—“Have you together in your life smoked more than 5–10 packs of cigarettes?” Otherwise it was coded 0.
Smoking at Time II
A dichotomous variable coded as 1 if the participant had ever smoked and otherwise 0.
Physical activity at leisure time at Time I
This variable included four alternatives: hardly any exercise (0), light exercise (1), regular exercise (2), and hard exercise (3).
Physical activity at leisure time at Time II
This variable considered the physical activity at leisure time during the year as a whole. The alternatives were: (0) Never, can’t walk, (1) I hardly get any exercise at all, (2) I get very little exercise, (3) I get little exercise, (4) I don’t get very much exercise, (5) I get quite a lot of exercise, (6) I get a lot of exercise, and (7) I get very much exercise.
Height at Time II
Height was median centered at 161cm for women and at 176cm for men.
Chronic disorders at Time II
This self-reported variable regarding chronic disorders was coded as 1 if the participant had answered yes to having had any of the following chronic disorders: (a) heart failure, (b) angina pectoris, (c) heart attack, (d) circulatory disorders in arms and legs, (e) stroke, (f) emphysema, (g) seizure, (h) Parkinson’s disease, (i) multiple sclerosis, (j) rheumatoid arthritis, (k) diabetes, (l) gout, (m) cancer or leukemia, (n) phlebitis, (o) bronchitis, or (p) kidney disease. Otherwise it was coded as 0.
SES in childhood
A variable based upon a battery of retrospective questions regarding the participant’s living conditions in childhood (the rearing home) administered at Time II. The questions regarded, for example, educational and occupational level of parents, rooms per person, the family’s situation compared to others, and if the family’s money could meet the family’s needs. All items were standardized and then summed into a total. Higher scores indicated higher SES in childhood.
SES at Time II
Information about the participant’s subjective SES was taken from the following question in a questionnaire, “Does your family income cover your needs?” The four alternatives were: very well (0), quite well (1), quite badly (2), and badly (3).
Statistical Analyses
For the main analyses, we used latent growth modeling ( McArdle & Epstein, 1987 ) in the SAS Proc Mixed procedure (version 9.2). Alpha level was set at .05. To avoid bias due to drop out, all available data from participants was used. Instead of imputation, we used full information modeling with maximum likelihood estimation. Twin pair dependencies were taken into account by estimating within and between pair random effects.
The basic model included grip strength as a time-varying covariate and sex, because of the well-known gender differences in grip strength ( Stenholm, Härkänen, Sainio, Heliövaara, & Koskinen, 2012 ; Sternäng et al., 2014 ). Sex was controlled for both as main effect and as interaction with grip strength and the slopes. The outcome variable was the cognitive domain of interest. The models were run using age as basis. Linear and quadratic models were tested. In line with previous work in SATSA on cognition ( Reynolds, Gatz, Prince, Berg, & Pedersen, 2010 ) and grip strength ( Sternäng et al., 2014 ), a linear 2-spline model with a turning point at age 65 was chosen for the calculations. The slope (i.e., the change with age over time) before the turning point was called slope A and after the turning point slope B. This basic model was also reversed to test if cognition (as a time-varying covariate) instead predicted grip strength.
To study mediating effects, the mediator of interest was introduced to the basic model, to examine if the effect between grip strength and cognition disappeared. Possible moderator effects were analyzed by the interactions with the potential moderator of interest.
Results
An association between grip strength and cognition was found. Comparisons between the two directions “grip strength to cognition” and “cognition to grip strength” showed that the direction “grip strength to cognition” showed better model fit for three of the four studied cognitive abilities (verbal ability, spatial ability, and processing speed) according to criteria (see Table 2 ). For memory, the direction “memory to grip strength” showed better overall model fit, but no significant associations was found for the slopes in that direction, only in the direction “grip strength to memory” (see below). The focus in the present study is, therefore, on the direction “grip strength to cognition”.
Table 2.
Model Fit of the Two Directions
| Models | Verbal ability | Spatial ability | Processing speed | Memory | ||||
|---|---|---|---|---|---|---|---|---|
| (AIC) | (BIC) | (AIC) | (BIC) | (AIC) | (BIC) | (AIC) | (BIC) | |
| Grip strength to cognition | ||||||||
| Spline at 65 | 14169.8*** | 14270.5*** | 14746.2*** | 14842.9*** | 15749.5*** | 15846.2*** | 16577.0*** | 16669.6*** |
| Cognition to grip strength | ||||||||
| Spline at 65 | 15538.2*** | 15630.6*** | 15014.7*** | 15106.9*** | 16158.2*** | 16250.6*** | 16071.6*** | 16164.0*** |
Notes: AIC (Akaike Information Criteria) and BIC (Bayesian Information Criteria) were used for assessing model fit. Lower values indicate better model fit.
*** p < .001.
The mean trajectories for the four cognitive domains can be seen in Table 3 . The slope A (decline before age 65) was significant for processing speed, whereas the slope B (decline after age 65) was significant for three of the four cognitive domains (the p value for verbal ability was .053). The two fluid abilities demonstrated the steepest rates of decline, processing speed (−0.68 T -score units/year) and spatial ability (−0.46 T -score units/year).
Table 3.
Mean Trajectories in the Cognitive Domains ( SE s within parentheses).
| Parameters | Verbal ability ( T -scores) | Spatial ability ( T -scores) | Processing speed ( T -scores) | Memory ( T -scores) |
|---|---|---|---|---|
| Intercept a | 52.88 (0.76)*** | 53.38 (0.84)*** | 52.02 (0.81)*** | 49.82 (0.86)*** |
| Slope A b | −0.01 (0.08) | −0.12 (0.10) | −0.29 (0.11)** | −0.19 (0.12) |
| Slope B n | −0.08 (0.04) | −0.46 (0.05)*** | −0.68 (0.05)*** | −0.23 (0.06)*** |
Notes: a At 65 years of age.
b Slope A refers to the slope before age 65, and slope B refers to the slope after age 65.
** p < .01, *** p < .001.
Grip strength across time was associated with the intercept (i.e., the overall effect of grip strength on the cognitive ability at 65 years of age) in verbal and spatial ability (see Table 4 ). For each unit higher grip strength, the increase was +0.08 T -score units ( p = .003) in verbal ability and +0.09 T -score units ( p = .011) in spatial ability. Grip strength across time did not predict slope A in any cognitive domain. However, it was associated with slope B (>65 years of age) in all four cognitive abilities. For each unit higher grip strength, the participant was expected to have less decline with +0.01 T -score units per year in all four cognitive domains ( p = .025 for verbal ability, p = .009 for spatial ability, p = .021 for processing speed, and p = .004 for memory) compared to the average decline. For example, a person with a 5kg higher grip strength performance would be expected to have a decline of −0.41 T -score units per year in spatial ability instead of the average −0.46 T -score units per year.
Table 4.
Effects of Grip Strength on the Four Cognitive Abilities ( SE s within parentheses)
| Parameters | Verbal ability ( T -scores) | Spatial ability ( T -scores) | Processing speed ( T -scores) | Memory ( T -scores) |
|---|---|---|---|---|
| Intercept a | 0.078 (0.026)** | 0.089 (0.035)* | −0.012 (0.035) | 0.080 (0.042) |
| Slope A b | 0.006 (0.004) | −0.002 (0.005) | −0.001 (0.005) | 0.007 (0.006) |
| Slope B b | 0.006 (0.003)* | 0.009 (0.004)** | 0.008 (0.003)* | 0.012 (0.004)** |
Notes: a At 65 years of age.
b Slope A refers to the slope before age 65, and slope B refers to the slope after age 65.
* p < .05, ** p < .01.
The significant associations for the intercept in verbal and spatial ability were bidirectional (see Tables 4 and 5 ). However, the significant associations during slope B were unidirectional (grip strength to cognition), except between grip strength and processing speed, which was bidirectional.
Table 5.
Effects of the Four Cognitive Abilities on Grip Strength ( SE s within parentheses)
| Parameters | Verbal ability (kg) | Spatial ability (kg) | Processing speed (kg) | Memory (kg) |
|---|---|---|---|---|
| Intercept a | 0.140 (0.046)** | 0.146 (0.040)*** | 0.010 (0.042) | 0.105 (0.038)** |
| Slope A b | 0.010 (0.007) | −0.007 (0.006) | −0.003 (0.007) | 0.006 (0.006) |
| Slope B b | 0.005 (0.004) | 0.006 (0.004) | 0.009 (0.004)* | 0.006 (0.004) |
Notes: a At 65 years of age.
b Slope A refers to the slope before age 65, and slope B refers to the slope after age 65.
* p < .05, ** p < .01, *** p < .001.
Mediators
In a second step, the variables smoking at Time I, smoking at Time II, physical activity at Time I, and physical activity at Time II were tested as possible mediators of the associations between grip strength and the cognitive abilities. The effects of grip strength on cognitive functions were, however, not mediated by the four tested variables.
Moderators
The variables smoking at Time I, smoking at Time II, physical activity at Time I, physical activity at Time II, height, chronic disorders at Time II, SES in childhood, and SES at Time II were tested as possible moderators of the effects of grip strength on the cognitive abilities. We found that only height moderated the associations between grip strength and the intercept in verbal abilities (−0.006 T -score units, p = .02). In Figure 1 , this interaction effect is demonstrated by the four combinations: (a) low (−1 SD ) height and low grip strength, (b) low height and high (+1 SD ) grip strength, (c) high height and low grip strength, and (d) high height and high grip strength. Focusing on slopes, those who were functioning well on grip strength maintained (high height plus high grip) and even showed small gains (low height plus high grip) in verbal abilities well into later life, but those who had low grip strength showed declines regardless of height.
Figure 1.
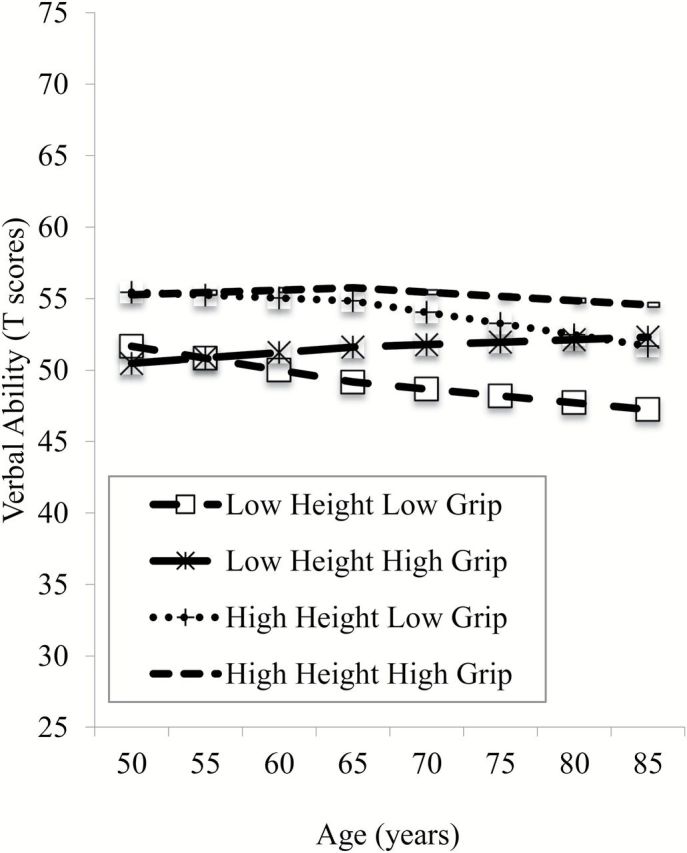
Interaction effects of grip strength and height on verbal ability.
Discussion
From our population-based longitudinal study, where most of the second half of the life span was covered, and where grip strength and cognition were followed over 20 years, we found a longitudinal relationship between changes in grip strength and cognition. This result underlines the importance of staying healthy physically in old age to maintain cognitive functions. The result is also in partial agreement with previous research, where some studies found that grip strength predicted cognition (e.g., Alfaro-Acha et al., 2006 ; Auyeung et al., 2011 ), but others indicate that cognition predicts grip strength (e.g., Atkinson et al., 2010 ; Charles et al., 2006 ), and there is also the possibility of a third factor, a common cause (e.g., Baltes & Lindenberger, 1997 ; Christensen et al., 2001 ). However, even with a dominant direction, it is plausible that it is a mixture between all three alternatives in old age. The relationship may also differ during the life span. Proposed mechanisms for the relationship between grip strength and cognition in healthy adults are brain-aging processes, such as the functioning of the central nervous system or changes in white matter integrity ( Baltes & Lindenberger, 1997 ; Christensen et al., 2001 ).
When analyzing the direction “grip strength to cognition” further, significant effects of grip strength on the intercepts were found only for verbal and spatial ability. This finding is partly in line with studies showing baseline associations between grip strength and mainly fluid abilities ( Clouston et al., 2013 ; Deary et al., 2011 ). The association between grip strength and verbal ability was also moderated by height. In our previous study on grip strength ( Sternäng et al., 2014 ), height had a clear effect on baseline grip strength. Intuitively, the effect of height could have had something to do with gender differences in height. However, in the present study, sex was controlled for in the model and height was also centered differently for women and men. The effect of height may in part reflect nutrition, especially in early life ( Gatz et al., 2006 ).
For the slopes, the results indicated a small but stable relationship (very few of the selected potential mediators/moderators had any effect on the associations) between decreasing grip strength and decreasing cognitive performance starting after 65 years of age in all four cognitive abilities. This result differs from previous studies, with other methodological approaches, since they did not find an association between change in grip strength and change in fluid or crystallized cognition ( Clouston et al., 2013 ). It is interesting to note the timing and effects (at greater than 65 years of age), since people are retiring around that age in Sweden, which might indicate that this is an issue of change in lifestyle. That the associations were absent before age 65 indicates also the possibility that this connection might be an effect of general aging processes. Around age 65 might be for many when the aging process intensifies and starts to accelerate. These intensified aging processes may result in greater individual differences and also in qualitative changes that will affect the relationships between the variables.
There is little research about grip strength and cognition connected to the earlier parts of the life span, and it is probable that a longitudinal relationship might not exist earlier in the adult life span until age 65. There are, for example, studies showing that cognition in childhood does not seem to be correlated with grip strength later in life ( Deary et al., 2011 ; Kuh et al., 2009 ).
This study had some strong features, but there are also limitations worth mentioning. First, our method using grip strength and cognition as time-varying covariates was a strength, but at the same time, it could not investigate the temporal ordering or causality in more detail. Other methods, such as bivariate/multivariate dual change score modeling ( McArdle & Hamagami, 2001 ), could be used to investigate this question further. We can only conclude that our results are consistent with temporal hypotheses. Second, to prioritize statistical power, the results were controlled for by gender, but not analyzed with regard to gender differences. Such differences exist in both grip strength and cognition ( de Frias, Nilsson, & Herlitz, 2006 ; Sternäng et al., 2014 ), and to get a better understanding of gender differences in underlying mechanisms, that would be a natural continuation of this study. Third, missing data might influence the results. Both men and women participated in an average of 3.7 waves of testing (out of six), and only 84 participants have just one grip strength measure during the follow-up period. To avoid bias due to attrition, we made use of all available grip strength and cognitive data from the participants with full maximum likelihood estimation. Fourth, some of the measures tested as mediators or moderators (e.g., smoking, physical activity, and SES) were rather crude which limit the ability to draw more nuanced conclusions.
Conclusions
In this longitudinal study with 20 years of follow-up, we found a longitudinal association between grip strength and cognition. The starting period (around 65 years of age) of the rather stable connection between changes in grip strength and cognitive performance indicates that something crucial for these abilities is happening around that part of the lifespan. The timing suggests that association may be due to lifestyle changes, such as retirement, or to intensified aging processes. While the salience of grip performance to cognitive performance change was supported in the current study, future work should evaluate bidirectional connections or possible third factors underlying associations between both grip strength and cognitive abilities.
Funding
This work was supported by National Institute of Aging (AG04563, AG10175, AG08724); The MacArthur Foundation Research Network on Successful Aging; the Swedish Council for Working Life and Social Research (97:0147:1B, 2009-0795); and Swedish Research Council (825-2007-7460, 825-2009-6141). A. K. Dahl Aslan was supported by Future Leaders of Aging Research in Europe postdoctoral grant (FORTE 2010-1852).
References
- Alfaro-Acha A. Al Snih S. Raji M. A. Kuo Y. F. Markides K. S. , & Ottenbacher K. J . ( 2006. ). Handgrip strength and cognitive decline in older Mexican Americans . The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 61 , 859 – 865 . doi:10.1093/gerona/61.8.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson H. H. Rapp S. R. Williamson J. D. Lovato J. Absher J. R. Gass M. , … Espeland M. A . ( 2010. ). The relationship between cognitive function and physical performance in older women: Results from the women’s health initiative memory study . The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 65 , 300 – 306 . doi: 10.1093/gerona/glp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung T. W. Lee J. S. Kwok T. , & Woo J . ( 2011. ). Physical frailty predicts future cognitive decline - a four-year prospective study in 2737 cognitively normal older adults . The Journal of Nutrition, Health & Aging , 15 , 690 – 694 . doi:10.1007/s12603-011-0110-9 [DOI] [PubMed] [Google Scholar]
- Baltes P. B. , & Lindenberger U . ( 1997. ). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging , 12 , 12 – 21 . doi:10.1037/0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- Bohannon R. W . ( 2008. ). Hand-grip dynamometry predicts future outcomes in aging adults . Journal of Geriatric Physical Therapy , 31 , 3 – 10 . doi:10.1519/00139143-200831010-00002 [DOI] [PubMed] [Google Scholar]
- Charles L. E. Burchfiel C. M. Fekedulegn D. Kashon M. L. Ross G. W. Sanderson W. T. , & Petrovitch H . ( 2006. ). Occupational and other risk factors for hand-grip strength: The Honolulu-Asia Aging Study . Occupational and Environmental Medicine , 63 , 820 – 827 . doi: 10.1136/oem.2006.027813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. Mackinnon A. J. Korten A. , & Jorm A. F . ( 2001. ). The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis . Psychology and Aging , 16 , 588 – 599 . doi:10.1037/0882-7974.16.4.588 [DOI] [PubMed] [Google Scholar]
- Clouston S. A. Brewster P. Kuh D. Richards M. Cooper R. Hardy R. , … Hofer S. M . ( 2013. ). The dynamic relationship between physical function and cognition in longitudinal aging cohorts . Epidemiologic Reviews , 35 , 33 – 50 . doi:10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldham F. Lewis J. , & Lee H . ( 2006. ). The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects . Journal of Hand Therapy , 19 , 318 – 326 ; quiz 327. doi:10.1197/j.jht.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Cooper R. Kuh D. Cooper C. Gale C. R. Lawlor D. A. Matthews F. , … Team, HALCyon Study . ( 2011. ). Objective measures of physical capability and subsequent health: A systematic review . Age and Ageing , 40 , 14 – 23 . doi:10.1093/ageing/afq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. Kuh D. , & Hardy R . ( 2010. ). Objectively measured physical capability levels and mortality: Systematic review and meta-analysis . British Medical Journal , 341 , c4467 . doi:10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl A., Hassing L. B., Fransson E., Berg S., Gatz M., Reynolds C. A., Pedersen N. L . ( 2010. ). Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life . The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 65 , 57 – 62 . doi: 10.1093/gerona/glp035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary I. J. Johnson W. Gow A. J. Pattie A. Brett C. E. Bates T. C. , & Starr J. M . ( 2011. ). Losing one’s grip: A bivariate growth curve model of grip strength and nonverbal reasoning from age 79 to 87 years in the Lothian Birth Cohort 1921 . The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences , 66 , 699 – 707 . doi: 10.1093/geronb/gbr059 [DOI] [PubMed] [Google Scholar]
- de Frias C. M. Nilsson L. G. , & Herlitz A . ( 2006. ). Sex differences in cognition are stable over a 10-year period in adulthood and old age . Neuropsychology, Development, and Cognition, Section B: Aging, Neuropsychology and Cognition , 13 , 574 – 587 . doi: 10.1080/13825580600678418 [DOI] [PubMed] [Google Scholar]
- Finkel D. , & Pedersen N. L . ( 2004. ). Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging . Aging, Neuropsychology and Cognition , 11 , 325 – 345 . doi:10.1080/13825580490511152 [Google Scholar]
- Finkel D., Reynolds C. A., McArdle J. J., Pedersen N. L . ( 2005. ). The longitudinal relationship between processing speed and cognitive ability: Genetic and environmental influences . Behavior Genetics , 35 , 535 – 549 . doi: 10.1007/s10519-005-3281-5 [DOI] [PubMed] [Google Scholar]
- Folstein M. F. Folstein S. E. , & McHugh P. R . ( 1975. ). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician . Journal of Psychiatric Research , 12 , 189 – 198 . doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gatz M. Mortimer J. A. Fratiglioni L. Johansson B. Berg S. Reynolds C. A. , & Pedersen N. L . ( 2006. ). Potentially modifiable risk factors for dementia in identical twins . Alzheimer’s & Dementia , 2 , 110 – 117 . doi:10.1016/j.jalz.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Ghisletta P. McArdle J. J. , & Lindenberger U . ( 2006. ). Longitudinal cognition-survival relations in old and very old age – 13-year data from the Berlin aging study . European Psychologist , 11 , 204 – 223 . doi:10.1027/1016-9040.11.3.204 [Google Scholar]
- Kuh D. Cooper R. Hardy R. Guralnik J. , & Richards M . ( 2009. ). Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study . Psychosomatic Medicine , 71 , 38 – 48 . doi:10.1097/PSY.0b013e31818a1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois F., Vu T. T., Chassé K., Dupuis G., Kergoat M. J., Bherer L . ( 2013. ). Benefits of physical exercise training on cognition and quality of life in frail older adults . The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences , 68 , 400 – 404 . doi: 10.1093/geronb/gbs069 [DOI] [PubMed] [Google Scholar]
- Lichtenstein P. De Faire U. Floderus B. Svartengren M. Svedberg P. , & Pedersen N. L . ( 2002. ). The Swedish Twin Registry: A unique resource for clinical, epidemiological and genetic studies . Journal of Internal Medicine , 252 , 184 – 205 . doi:10.1046/j.1365-2796.2002.01032.x [DOI] [PubMed] [Google Scholar]
- McArdle J. J. , & Epstein D . ( 1987. ). Latent growth-curves within developmental structural equation models . Child Development , 58 , 110 – 133 . doi:10.1111/j.1467–8624.1987.tb03494.x [PubMed] [Google Scholar]
- McArdle J. J. , & Hamagami F . ( 2001. ). Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data . In Collins L. M., Sayer A. G. (Eds.), New methods for the analyses of change (pp. 137 – 175 ). Washington, DC: : American Psychological Association; . [Google Scholar]
- Melrose R. J. Brewster P. Marquine M. J. Mackay-Brandt A. Reed B. Farias S. T. , & Mungas D . ( 2014. ). Early life development in a multiethnic sample and the relation to late life cognition . The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences . doi:10.1093/geronb/gbt126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. L., McClearn G. E., Plomin R., Nesselroade J. R., Berg S., DeFaire U . ( 1991. ). The Swedish Adoption Twin Study of Aging: An update . Acta Geneticae Medicae et Gemellologiae , 40 , 7 – 20 . [DOI] [PubMed] [Google Scholar]
- Pedersen N. L. Plomin R. Nesselroade J. R. , & McClearn G. E . ( 1992. ). A quantitative genetic analysis of cognitive abilities during the second half of the life span . Psychological Science , 3 , 346 – 353 . doi:10.1111/j.1467–9280.1992.tb00045.x [Google Scholar]
- Reynolds C. A., Gatz M., Prince J. A., Berg S., Pedersen N. L . ( 2010. ). Serum lipid levels and cognitive change in late life . Journal of the American Geriatrics Society , 58 , 501 – 509 . doi: 10.1111/j.1532-5415.2010.02739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M., Nilsson L. G . ( 2009. ). Flynn effects on sub-factors of episodic and semantic memory: Parallel gains over time and the same set of determining factors . Neuropsychologia , 47 , 2174 – 2180 . doi: 10.1016/j.neuropsychologia.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Rönnlund M., Nyberg L., Bäckman L., Nilsson L. G . ( 2005. ). Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study . Psychology and Aging , 20 , 3 – 18 . doi: 10.1037/0882-7974.20.1.3 [DOI] [PubMed] [Google Scholar]
- Sharp E. S., Reynolds C. A., Pedersen N. L., Gatz M . ( 2010. ). Cognitive engagement and cognitive aging: Is openness protective? Psychology and Aging , 25 , 60 – 73 . doi: 10.1037/a0018748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small B. J. Dixon R. A. , & McArdle J. J . ( 2011. ). Tracking cognition-health changes from 55 to 95 years of age . The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences , 66 ( Suppl. 1 ), i153 – 161 . doi:10.1093/geronb/gbq093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S., Härkänen T., Sainio P., Heliövaara M., Koskinen S . ( 2012. ). Long-term changes in handgrip strength in men and women–accounting the effect of right censoring due to death . The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 67 , 1068 – 1074 . doi: 10.1093/gerona/gls064 [DOI] [PubMed] [Google Scholar]
- Stenholm S., Tiainen K., Rantanen T., Sainio P., Heliövaara M., Impivaara O., Koskinen S . ( 2012. ). Long-term determinants of muscle strength decline: Prospective evidence from the 22-year mini-Finland follow-up survey . Journal of the American Geriatrics Society , 60 , 77 – 85 . doi: 10.1111/j.1532-5415.2011.03779.x [DOI] [PubMed] [Google Scholar]
- Sternäng O. Reynolds C. A. Finkel D. Ernsth-Bravell M. Pedersen N. L. , & Dahl Aslan A. K . ( 2014. ). Factors associated with grip strength decline in older adults . Age and Ageing , 44 , 269 – 274 . doi: 10.1093/ageing/afu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan G. E., Lessov-Schlaggar C. N . ( 2007. ). The effects of tobacco smoke and nicotine on cognition and the brain . Neuropsychology Review , 17 , 259 – 273 . doi: 10.1007/s11065-007-9035-9 [DOI] [PubMed] [Google Scholar]
- Wechsler D . ( 1981. ). Manual of the Wechsler Adult Intelligence Scale - Revised . San Antonio, TX: : The Psychological Corporation; . [Google Scholar]


