ABBREVIATIONS
- aGPCR
adhesion family of G protein-coupled receptor
- ChIP
chromatin immuno-precipitation
- GBM
glioblastoma
- GSC
glioblas-toma stem cell
- Hif
hypoxia inducible transcription factor
- PPN
pseudopalisading necrosis
Glioblastoma (GBM) is an incurable brain malignancy in dire need of novel therapeutic targets.1 A critical component of GBM’s resistance to conventional therapies is a stem-like tumor cell population that employs cell-intrinsic and microenvironment-mediated mechanisms to support tumor progression.2-7 Among microenvironmental factors that promote GBM stem cell (GSC) phenotypes is intratumoral hypoxia,8,9 a naturally occurring consequence of vascular thrombosis in these tumors. While many of the effects of hypoxia on tumor progression are mediated by hypoxia-inducible transcription factors (Hif),9,10 no effective Hif inhibitors have been developed. In our effort to identify novel targetable mediators of hypoxia-driven tumor progression, we recently published on the role that GPR133 (ADGRD1),11,12 an orphan member of the adhesion family of G protein-coupled receptors (aGPCRs),13-17 plays in GBM progression. Here, we describe evidence that GPR133 is selectively expressed in hypoxic regions of GBM via direct transcriptional upregulation by Hif1α. Furthermore, we show that GPR133 knockdown abrogates tumor initiation. This compelling evidence suggests that GPR133 plays an important protumorigenic role in GBM, particularly in the context of hypoxia, and argues that it represents a novel therapeutic target.
This research was approved by NYU School of Medicine's IRB (Protocol 12–01130) and IACUC (protocol 160503).
FINDINGS
GPR133 is Expressed Within Hypoxic Regions of GBM
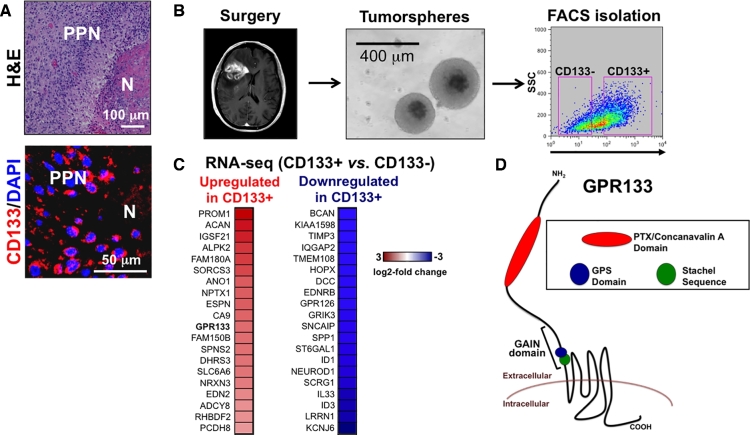
Our immunohistochemical analysis indicated that the GSC marker CD133 (PROM1) is enriched in the hypoxic regions of pseudopalisading necrosis (PPN) within human GBM biospecimens (Figure 1A). In order to identify novel genes involved in hypoxia-driven growth of GBM, we performed an RNA-seq comparison of FACS-sorted CD133+ and CD133– cells from a patient-derived human GBM culture (Figures 1B and 1C). One of the top genes enriched in the CD133+ tumor population was GPR133 (ADGRD1), an orphan member of the aGPCR family (Figure 1D).
FIGURE 1.
Identification of GPR133 enrichment in the CD133+ cell population of human GBM. A, CD133 (PROM1) is predominantly expressed in the hypoxic regions of PPN in human GBM biospecimens. B, Protocol for FACS isolation of CD133+ and CD133– cells. Human surgical specimens are cultured as tumorspheres in serum-free media supplemented with epidermal growth factor and fibroblast growth factor 2. CD133+ and CD133– populations can be FACS-isolated by incubating dissociated tumor cells with anti-CD133 antibody (Miltenyi) conjugated to a fluorophore. C, Top 20 genes upregulated or downregulated in CD133+ vs CD133– cells in a patient-derived GBM culture. D, Membrane topology of human GPR133. The extracellular domain contains unique (PTX/concanavalin A domain) and preserved domains (GAIN and GPS13,17), which are found throughout the aGPCR family. The Stachel sequence acts as an endogenous agonist.15 PPN, pseudopalisading necrosis; N, necrosis; H&E, hematoxylin and eosin; PTX, pentraxin; GPS, GPCR proteolysis site; GAIN, GPCR-autoproteolysis-inducing domain. Adapted from reference12, published OA-CC-BY-4.0.
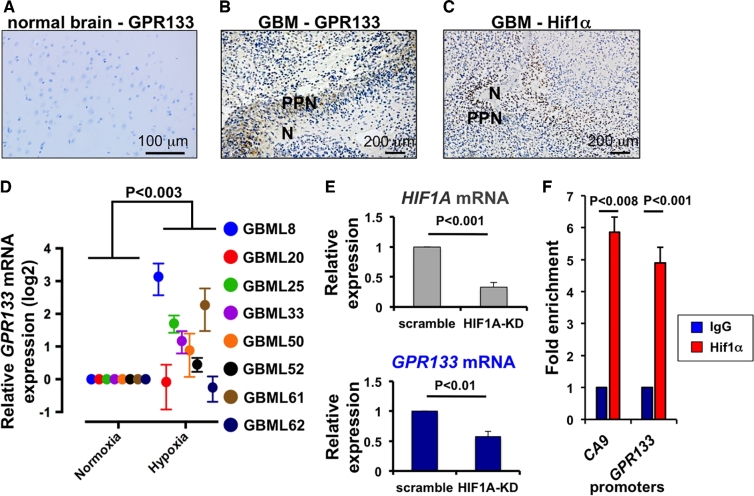
To test the expression pattern of GPR133 in human GBM, we analyzed histological specimens from 9 GBM patients and observed that all tumors expressed GPR133. No staining was observed in normal human brain specimens (Figure 2A). Within each tumor, GPR133 immunostaining was specific to areas of PPN, which also expressed the hypoxia marker Hif1α, an Hif (Figures 2B and 2C).18
FIGURE 2.
GPR133 is upregulated in hypoxia via Hif1α. A-C, Immunohistochemical staining from normal brain (A) and GBM tissue (B and C) shows that GPR133 selectively localizes to areas of PPN, which also express Hif1α. No GPR133 is detected in normal brain tissue. D, GPR133 mRNA is upregulated in hypoxia in 6/8 primary GBM cultures in vitro (ANOVA F(1/12) = 14.82). E, Knockdown of HIF1A results in reduced GPR133 mRNA (t-tests). F, ChIP-PCR assays, using anti-Hif1α antibody, indicate increased binding of Hif1α to the GPR133 promoter (t-test), 571 bp upstream of transcriptional start site. The promoter of Carbonic Anhydrase 9 (CA9), a hypoxic marker and known target of Hif1α, was used as positive control (t-test). IgG alone was used as negative control. PPN, pseudopalisading necrosis; N, necrosis. Adapted from reference12, published OA-CC-BY-4.0.
This observation raised the possibility that GPR133 expression is regulated by hypoxia and Hif1α. To investigate this hypothesis, we subjected 8 patient-derived human GBM cultures to hypoxia (1% O2) in vitro for 24 h. Six of 8 primary GBM cultures showed upregulation of GPR133 mRNA after hypoxia (Figure 2D). Furthermore, knockdown of Hif1α with lentiviral shRNA reduced GPR133 mRNA (Figure 2E) and chromatin immunoprecipitation (ChIP)-PCR analysis demonstrated direct binding of Hif1α to the GPR133 promoter (Figure 2F). These findings indicated that GPR133 is predominantly expressed in hypoxic areas of human GBM via direct transcriptional upregulation by transcription factor Hif1α.
GPR133 Promotes Tumor Growth In Vitro and In Vivo
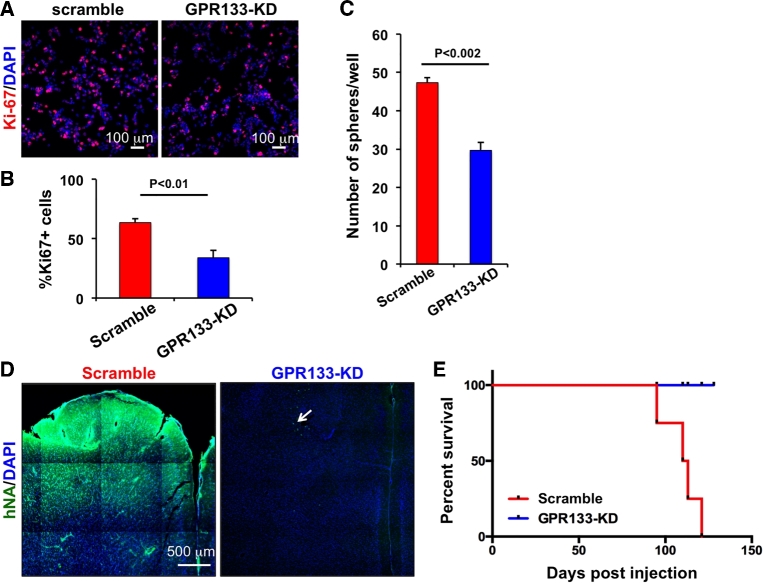
Our next question was whether GPR133 promotes or suppresses tumor growth. To answer this question, we employed lentiviral shRNA-mediated knockdown of GPR133 in patient-derived GBM cultures. Tumor cells with GPR133 knockdown (GPR133-KD) showed reduced proliferation compared to scrambled shRNA control, using Ki67 immunofluorescence staining (Figures 3A and 3B). In addition, the ability of GPR133-KD cells to form spheres was significantly reduced relative to controls (Figure 3C). These in vitro findings were reproduced with 2 different shRNA constructs, suggesting lack of nonspecific effects.
FIGURE 3.
GPR133 knockdown impairs tumor growth in vitro and in vivo. A, Representative stainings for Ki67 obtained from a patient-derived GBM culture treated with control (scramble) and GPR133 (GPR133-KD) shRNA lentivirus. B, GPR133 knockdown reduced the fraction of Ki67-positive cells in vitro (t-test). C, GPR133 knockdown impaired the clonogenic ability of GBM cells in hypoxia in vitro (t-test). D, Representative microscopic images show that GPR133 knockdown prevented tumor formation in the brain of NOD.SCID mice. The nuclei of tumor cells were stained for human nuclear antigen (hNA). The arrow indicates a few remaining GBM cells bearing the GPR133-KD construct. However, these cells failed to generate a tumor. E, Kaplan–Meier survival curves indicate that GPR133 knockdown prevented death of implanted mice (n = 4 mice/group; logrank test, P < .05). DAPI, nuclear counterstain. Adapted from reference12, published OA-CC-BY-4.0.
We then tested the effects of GPR133 knockdown on in vivo tumorigenicity. Human GBM cells bearing either scrambled or GPR133-KD shRNA were injected into the brains of immunocompromised NOD.SCID mice. We found that GPR133 knockdown prevented tumor initiation and, therefore, death in implanted mice (Figures 3D and 3E). This robust phenotype indicated that GPR133 is critical for tumorigenicity.
GPR133 Expression Correlates With Poor Prognosis in GBM
In order to understand the clinical relevance of our findings, we used the UCSC Cancer Genome Browser to analyze existing RNA-seq data from 160 GBM samples in the TCGA database.19 After ranking the samples according to expression levels of GPR133, we divided the samples into 2 groups: GPR133 low (percentile 1-50, blue) and GPR133 high (percentile 51-100, red; Figure 4). Then, we analyzed the survival of these 2 groups using Kaplan–Meier survival curves. We found that, in agreement with our mouse data, high GPR133 expression correlates with poor prognosis and reduced survival (Figure 4). These data confirm that GPR133 has pro-tumorigenic function and suggest that its inhibition represents an attractive and novel therapeutic approach.
FIGURE 4.
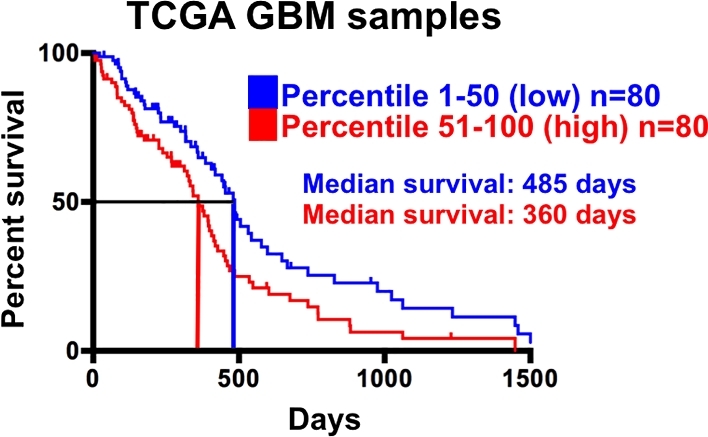
GPR133 expression in human GBM tumors inversely correlates with survival. GBM tumors in the TCGA database (n = 160) were ranked by GPR133 mRNA expression and dichotomized into 2 groups: high and low. Kaplan–Meier survival curves indicated a significant difference in survival between the high and low groups (logrank P < .001). Adapted from reference12, published OA-CC-BY-4.0.
DISCUSSION
Understanding the molecular mechanisms that underlie the response of cancer cells to hypoxia is critical for the discovery of new therapeutic targets, especially in tumors such as GBM, which exhibits extensive hypoxia. Previous literature demonstrated that low oxygen tension evokes stem-like phenotypes that lead to tumor progression.8,9,20-23 Furthermore, several lines of evidence have suggested that the reason for failure of antiangiogenic therapy in GBM lies in induction of hypoxia by vascular regression.24,25 The role of hypoxia-inducible transcription factors Hif1α and Hif2α in the regulation of the response to hypoxia has been well documented.9,10,26 However, pharmacologic targeting of these transcription factors has been elusive to date.
Our experiments provide compelling evidence that GPR133 inhibition represents an appealing strategy for arresting hypoxia-driven tumor progression in GBM. First, its expression within hypoxic PPN areas in tumor biospecimens coincides with that of Hif1α, because, as we demonstrated, the GPR133 gene is a direct transcriptional target of Hif1α. Second, GPR133 is not expressed in normal cerebral hemisphere tissue, indicating a favorable therapeutic window. Finally, genetic knockdown of GPR133 abrogates orthotopic tumor initiation and prevents death of implanted immunocompromised mice. This finding is in agreement with TCGA data that demonstrate an inverse correlation between the amount of tumoral GPR133 mRNA and survival in GBM patients.
Our encouraging data lay the foundation for further translational development of GPR133 as a treatment target. Indeed, we are currently undertaking experiments toward further target validation, as well as toward development of GPR133 inhibitors. Importantly, we are also assessing whether GPR133 inhibition may be beneficial in other extracranial malignancies, in which hypoxia may contribute to disease progression.
Disclosures
Dr Bayin received support from NYSTEM Institutional training grant #CO26880. Dr Zagzag was supported by NIH/NINDS 1R21NS074055-02. Dr Placantonakis received support from NIH/NINDS 1R21NS087241-01, NIH/NINDS 1R21NS088775-01, NIH/NINDS 1R03NS087349-01, NIH/NCI 2P30CA016087-33, NIH/NCATS UL1 TR000038, the B*Cured Foundation, NYU Applied Research Support Fund, NYU OTA Accelerator Fund, and the Grace Jones Richardson Trust. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 2. Singh SK, Hawkins C, Clarke ID et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396-401. [DOI] [PubMed] [Google Scholar]
- 3. Basu-Roy U, Bayin NS, Rattanakorn K et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Comm. 2015;6:6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bayin NS, Modrek AS, Dietrich A et al. Selective Lentiviral gene delivery to CD133-expressing human glioblastoma stem cells. PloS One. 2014;9(12):e116114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bayin NS, Modrek AS, Placantonakis DG. Glioblastoma stem cells: molecular characteristics and therapeutic implications. World J Stem Cells. 2014;6(2):230-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bao S, Wu Q, Sathornsumetee S et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843-7848. [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Li Y, Yu TS et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seidel S, Garvalov BK, Wirta V et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133(pt 4):983-995. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Bao S, Wu Q et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendez O, Zavadil J, Esencay M et al. Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol Cancer. 2010;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayin NS, Frenster J, Kane JR et al. 144 GPR133 promotes glioblastoma growth in hypoxia. Neurosurgery. 2016;63(suppl 1):158-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayin NS, Frenster JD, Kane JR et al. GPR133 (ADGRD1), an adhesion G-protein-coupled receptor, is necessary for glioblastoma growth. Oncogenesis. 2016;5(10):e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamann J, Aust G, Arac D et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev. 2015;67(2):338-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bohnekamp J, Schoneberg T. Cell adhesion receptor GPR133 couples to Gs protein. J Biol Chem. 2011;286(49):41912-41916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liebscher I, Schon J, Petersen SC et al. A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep. 2014;9(6):2018-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monk KR, Hamann J, Langenhan T, Nijmeijer S, Schoneberg T, Liebscher I. Adhesion G protein-coupled receptors: from in vitro pharmacology to in vivo mechanisms. Mol Pharmacol. 2015;88(3):617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Promel S, Frickenhaus M, Hughes S et al. The GPS motif is a molecular switch for bimodal activities of adhesion class G protein-coupled receptors. Cell Rep. 2012;2(2):321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIntyre A, Patiar S, Wigfield S et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res. 2012;18(11):3100-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cline MS, Craft B, Swatloski T et al. Exploring TCGA pan-cancer data at the UCSC Cancer Genomics Browser. Sci Rep. 2013;3:2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177(3):1491-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102(5):789-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hjelmeland AB, Wu Q, Heddleston JM et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18(5):829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Batchelor TT, Gerstner ER, Emblem KE et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110(47):19059-19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiang L, Wu T, Zhang HW et al. HIF-1alpha is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012;19(2):284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]