Abstract
BACKGROUND:
Although induction of durable mixed chimerism is required for murine skin allograft tolerance, renal allograft tolerance has been achieved after induction of only transient mixed chimerism in nonhuman primates (NHPs) and humans. To better define the level/duration of chimerism required for stable renal allograft tolerance, we retrospectively analyzed these parameters and compared them with transplant outcomes in NHP combined kidney and bone marrow transplant (CKBMT) recipients.
METHODS:
Peripheral blood levels and duration of myeloid or lymphoid chimerism were retrospectively analyzed in 34 NHP CKBMT recipients which were divided into 3 groups: Tolerance (TOL), n=10; chronic antibody-mediated rejection (CAMR), n=12; and T cell-mediated rejection (TCMR), n=12.
RESULTS:
All 4 of the recipients that failed to develop any chimerism lost their allografts due to TCMR after discontinuation of immunosuppression (56 ± 3 days). Among 30 recipients who successfully developed multilineage chimerism, 10 achieved immunosuppression-free survival (1258 ± 388 days), 12 eventually developed CAMR (932 ± 155 days), and 8 developed TCMR (82 ± 10 days). The maximum level but not duration of lymphoid chimerism was significantly higher in TOL recipients compared with both CAMR (p=0.0159) and TCMR (p=0.0074). Conversely, the maximum myeloid chimerism was significantly higher in TOL than in TCMR (p=0.0469), but not in CAMR. ROC analyses revealed that lymphoid chimerism levels of 3.1% or greater could reliably predict long-term immunosuppression-free renal allograft survival (p<0.0001).
CONCLUSIONS:
This retrospective study confirmed that induction of chimerism is essential for long-term immunosuppression-free survival, which best correlates with lymphoid chimerism levels higher than 3.1%.
INTRODUCTION
Increasingly efficacious immunosuppression has successfully prevented or treated acute allograft rejection, resulting in significantly improved short-term survival of solid organ transplants (1, 2). Unfortunately, the same cannot be said for long-term survival, since chronic immunosuppression is associated with increased risks of cardiovascular disease (3–5), de novo diabetes (6–8), dyslipidemia (9–12), and neurotoxicity (13, 14), resulting in death with a functional graft in as many as 25% of recipients by 10 years after deceased donor kidney transplantation (KTx) (15). Furthermore, chronic rejection cannot be consistently prevented by currently available immunosuppressive medications. Immune tolerance, therefore, continues to be sought as the ultimate solution to these limitations.
Induction of mixed chimerism via donor bone marrow transplantation (DBMT) is a reproducible and effective approach to achieving allograft tolerance (16, 17). We have developed a conditioning regimen, based on murine studies, that achieves long-term immunosuppression-free renal allograft survival in nonhuman primates (NHPs) (18–22). This approach has been successfully extended to both HLA-matched (23) and -mismatched (24–26) living-donor human KTx recipients, with the longest immunosuppression-free survival currently exceeding 15 years. Although these achievements are encouraging, some patients who initially did well, subsequently developed DSA and CAMR. Two of 7 recipients who successfully discontinued their immunosuppression for longer than 5 years, developed DSA and CAMR at 6 and 8 years, respectively, after transplantation (26). Approximately 50% of NHP CKBMT recipients, from whom immunosuppression was initially withdrawn, eventually developed late onset CAMR with DSA. Therefore, the identification of biomarkers that can reliably predict renal allograft outcome is critically important to the safe withdrawal of immunosuppression from these patients.
In the current study, we evaluated the relationship between transplant outcomes and level/duration of mixed chimerism, by retrospectively examining 34 NHP combined kidney and bone marrow transplant (CKBMT) recipients previously treated with our nonmyeloablative conditioning regimens. Some of these animals have been observed for as long as 11 years after successful induction of transient mixed chimerism.
MATERIALS & METHODS
Animals
A total of 34 cynomolgus monkeys 3–8 kg (including donor animals) (Charles River Primates, Wilmington, MA) that received combined kidney and bone marrow transplantation were included in this retrospective study. Some of the results observed in these transplants have been previously reported (21, 22, 27–29). Donors and recipients were paired based on ABO compatibility and MHC-disparity as previously described (30, 31). All surgical procedures and perioperative care of animals was performed in accordance with National Institutes of Health guidelines for the care and use of primates and approved by the Massachusetts General Hospital (MGH) Institutional Animal Care and Use Committee. In this study, a total of 34 NHP recipients of which detailed chimerism and DSA data are available, were selected among 45 recipients that underwent combined kidney and bone marrow transplantation after 1997 at MGH.
Conditioning Regimen
NHP KTx recipients received DBMT from the kidney donors simultaneously (21, 22) or several months after KTx (27–29) (Fig. 1). The basic conditioning regimens for DBMT included low-dose total body irradiation (TBI: 1.5 Gy on days −6 and −5, relative to DBMT), thymic irradiation (TI: 7 Gy on day −1, relative to DBMT), and peritransplant anti-T cell antibodies, with or without a short course of costimulatory blockade, followed by a 1 month course of calcineurin inhibitor (CNI) (Fig. 1). For the antilymphocyte reagent, equine thymocyte immune globulin (Atgam: Pharmacia and Upjohn, Kalamazoo, MI, 50 mg/kg/day on days −2, −1 and 0, relative to DBMT) with or without anti-CD8 mAb (cM-T807: Centocor, Inc., Horsham, 5 mg/kg on days 0 and 2, relative to DBMT) or Fludarabine (30mg/m2X2, Genzyme, Cambridge, MA) were used. Two recipients (M2211 and M2311) received LFA3-Ig (Alefacept: Astellas Pharma US, Inc., Northbrook, IL, 1 mg/kg on days 1, 5, 12 and 19, relative to DBMT) in place of anti-CD8 mAb. For costimulatory blockade, either anti-CD40L mAb (Hu5C8: provided by K. Reimann DVM, University of Massachusetts, Worcester, MA, 20 mg/kg on days 0 and 2, and 10 mg/kg on days 5, 7, 9 and 12, relative to DBMT) or CTLA4Ig (belatacept: Bristol Meyer Squibb, New York, NY, 20 mg/kg on days 0 and 2, and 10 mg/kg on days 5 and 15, relative to DBMT) were used.
Figure 1: Conditioning regimen for induction of DBMT.
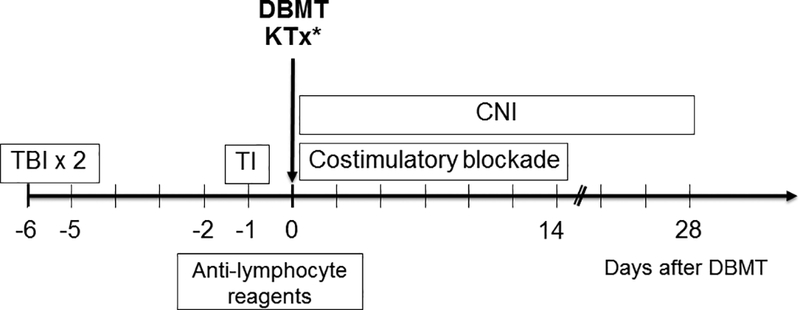
All NHP KTx recipients underwent MHC-mismatched DBMT from the kidney donor either simultaneously with KTx or several months later after KTx. The basic conditioning regimens included low-dose total body irradiation (TBI: 1.5 Gy on days −6 and −5, relative to DBMT), thymic irradiation (TI: 7 Gy on day −1, relative to DBMT), peritransplant anti-T cell antibodies, and a short course of costimulatory blockade. After DBMT, a 1 month course of calcineurin inhibitor (CNI) was administered, after which no immunosuppression was given.
Kidney transplantation (KTx)
The right native kidney is removed first through a midline incision. The aorta and vena cava are identified and skeletonized up to the bifurcation with the iliac vessels. When the donor kidney is available, the recipient vena cava is cross-clamped using a Satinsky clamp and a small venotomy is made. The donor kidney is then brought into the field and an end-of-renal-vein to side-of-vena-cava anastomosis is performed using 8–0 Proline (Ethicon, LLC., Somerville, NJ). After this is completed, the renal vein is occluded using a small vascular clamp, and the Satinsky clamp is released. After assuring hemostasis, the aorta is cross-clamped with the Satinsky clamp and a small arteriotomy is made. The arterial anastomosis between donor and recipient aorta is completed in side-to- end fashion using 7–0 Proline (Ethicon, Somerville, NJ). After completing this anastomosis, the Satinsky clamp is released to allow perfusion of the kidney. When hemostasis has been assured, the kidney is held in place using the previously placed stay sutures that were sutured to the retroperitoneum. The bladder is then opened by dividing the serosa and musculature over a centimeter length, taking care not to open the mucosa. A 3-mm-diameter stoma is made in the most distal mucosa. The bladder mucosa-to-mucosa anastomosis is then completed using 7–0 Chromic gut (Ethicon, Somerville, NJ). Finally, the bladder musculature is closed over the ureter using 5–0 Vicryl (Ethicon, Somerville, NJ). The abdomen is washed with warm saline and then closed (32). The recipient’s left native kidney is removed at the end of the procedure.
Donor bone marrow transplantation (DBMT)
Donor bone marrow cells (DBMCs) obtained from donor iliac bones by multiple percutaneous aspirations are infused immediately after the KTx is completed. If the animal is sacrificed at the time of kidney donation, DBMCs are also recovered from the vertebral bones after euthanasia and frozen for several months until DBMT. The DBMCs are filtered through a nylon mesh, the red cells are lysed with ammonium chloride solution (ACK; Thermo Fisher Scientific, Grand Island, NY), and the preparation is washed with Hank’s balanced salt solution (HBSS; Thermo Fisher Scientific, Cambridge, MA). The DBMCs (1.0–3.0 × 108 mononuclear cells /kg) are then infused intravenously without further processing (18, 21).
Detection of chimerism
After standard water shock treatment, the peripheral blood cells are first stained with donor-specific mAb chosen from a panel of fluorescein isothiothianate (FITC)-conjugated mouse antihuman HLA class I (Bw6) mAb (Miltenyi Biotec, San Diego, CA) that cross-reacts with cynomolgus monkeys. The cells are incubated for 30 min at 4°C and then washed. In all experiments, the percentage of cells stained with mAb is determined from a 1-color fluorescence histogram and compared with those obtained from donor and pretreatment frozen recipient cells, which are used as positive and negative controls, respectively. The percentage of cells considered positive is determined by using the fluorescence level at the beginning of the positive peak for the positive control stain as the cutoff value, and then subtracting from this value the percentage of cells stained with an isotype control. By using forward and 90° light scatter (FSC and SSC, respectively) dot plots, the lymphocyte (FSC- and SSC-low), granulocyte (SSC-high), and monocyte (FSC-high but SSC-low) populations were gated, and chimerism was determined separately for each population. Nonviable cells were excluded by propidium iodide (Thermo Fisher Scientific) staining. The fluorescence of the stained samples was analyzed using FACScan (BD Biosciences, Woburn, MA), FACSverse (BD Biosciences, Woburn, MA), or Accuri flow cytometers (BD Pharmingen) and FlowJo software (Tree Star, Inc., Ashland, OR) (18, 21). Positive chimerism was defined as >0.1% in the lymphoid lineage and >1% in the myeloid lineage.
Detection of donor specific antibody
Antidonor specific alloantibody (DSA) was detected by flow cytometric analysis. Donor PBMCs were first incubated with recipient sera for 30 min. After washing, FITC-conjugated mouse antihuman IgG mAb (Zymed, San Francisco, CA) was added and incubated for another 30 min. PBMCs were washed again and further incubated with PE conjugated anti-CD20 or anti-CD3 mAbs (Pharmingen, San Diego, CA) for 30 min. After washing, the cells were fixed with 2% paraformaldehyde and analyzed on FACScan (BD Biosciences, Woburn, MA), FACSverse (BD Biosciences, Woburn, MA) or Acculi flow cytometers (BD Pharmingen). A positive reaction of the T and B cells was defined in a ratio of test versus negative control values. Pretransplant sera from more than 100 naive nontransfused male monkeys tested with more than 50 different donors were used as negative controls. Sera collected from recipients at different time points posttransplant were tested on donor lymphocytes and the cutoff value for a positive reaction was set 2 Standard Deviations (2SD) over the average of the negative control sera which corresponded to mean channel fluorescence intensity (MFI) values greater than 6.9 for T and 55.2 for B cells. MFI values above these levels were considered positive and the ones below were considered background and therefore subtracted from the final results (33).
Histopathology
Protocol renal biopsies were obtained every 100 days after DBMT in recipients with stable function as well as whenever a rise in serum creatinine occurred. Tissue was processed for routine microscopy and a portion was frozen for immunofluorescence staining. Other organs obtained surgically (lymph nodes, native kidney and spleen) were similarly processed. Following euthanasia of any monkey, complete autopsies were performed for histopathologic examination of the renal allograft, lymph nodes, heart, lung, liver, pancreas, thymus, and skin.
Statistical analysis
Statistical analysis was performed with GraphPad PRISM 7.01 (GraphPad Software, Inc, San Diego, CA). We used the Mantel-Cox log-rank test to analyze the survival of different groups, and ANOVA with Tukey’s post hoc test for the lymphoid and myeloid chimerism data. Two-group comparisons between different costimulatory blockade treatments were performed with the Mann-Whitney U test. P values < than 0.05 were considered statistically significant.
RESULTS
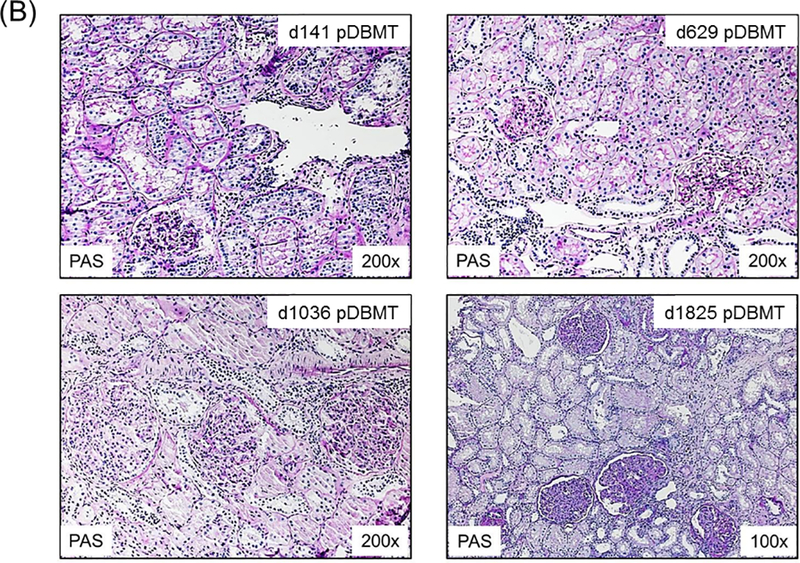
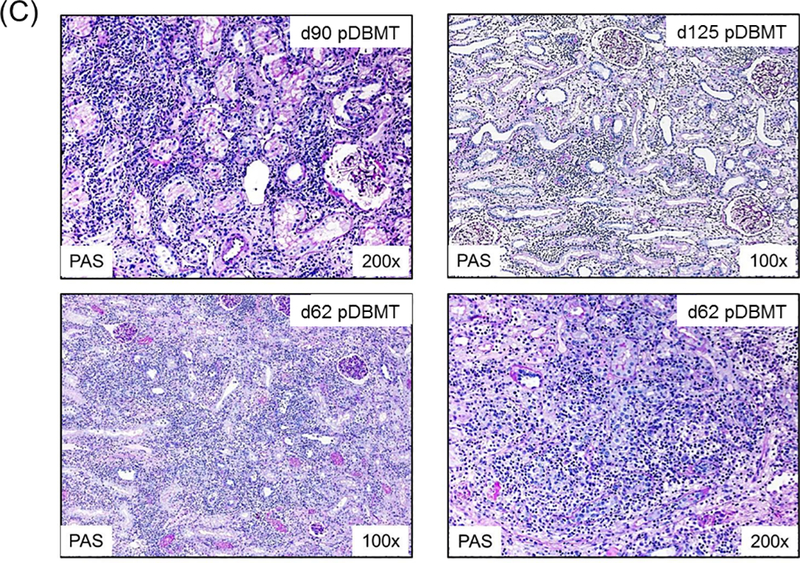
A total of 34 CKBMT recipients, with detailed chimerism and DSA data available retrospectively, were included in this study. These animals were divided into 3 groups based on renal allograft outcome: tolerance (TOL), chronic antibody-mediated rejection (CAMR), and T cell-mediated rejection (TCMR). Recipients in the TOL group (n=10) achieved long-term immunosuppression-free survival without evidence of rejection or DSA over the entire observation period (>1258 ± 388 days) (Fig. 2A). Since DSA was consistently became detectable by 300 days in recipients with CAMR, only recipients followed for longer than 1 year were categorized as TOL. The recipients in the CAMR group (n=12) also achieved immunosuppression-free survival but with minimal interstitial infiltrate in the allograft and eventually developed DSA with C4d deposition leading to allograft failure by CAMR at 932 ± 155 days after DBMT (Fig. 2B). The TCMR group (n=12) lost their allografts at 73 ± 8 days after DBMT as shown in biopsies from representative animals (Fig. 2C).
Figure 2: Histopathological findings in NHP renal allografts after DBMT.
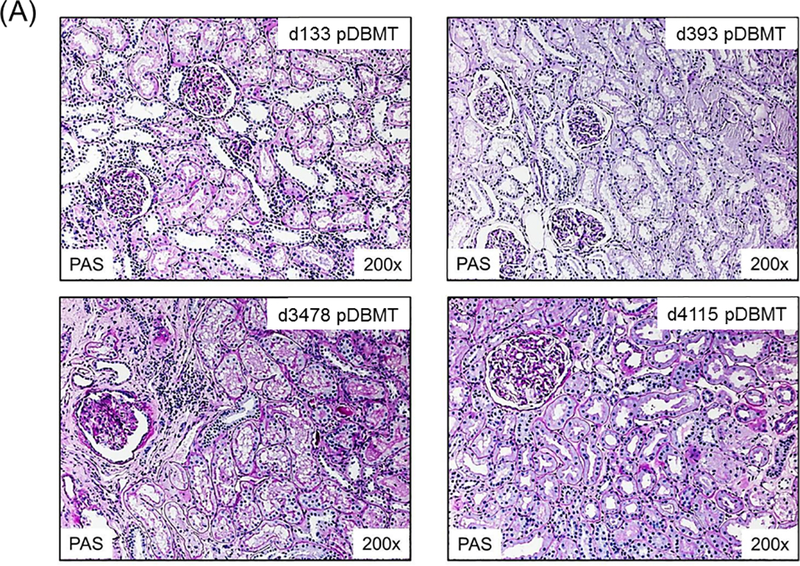
A: A representative recipient in the TOL group showed no evidence of rejection on days 133 (upper left), 393 (upper right), 3478 (lower left), and 4115 (lower right) post-DBMT. A minimal infiltrate in a focal area of fibrosis was present at day 3478.
B. A recipient in the CAMR group showed minimal interstitial infiltrate and focal capillaritis without C4d deposition on days 141 (upper left) and 629 post-DBMT (upper right). (right upper panel). Transplant glomerulopathy and glomerulitis without C4d deposition was present at d1036 post-DBMT (lower left). Extensive transplant glomerulopathy and focal C4d deposition (not shown) was present at day 1825 post-DBMT (lower right). This was associated with the development of DSA.
C: A recipient that initially developed a low level of chimerism developed TCMR with dense interstitial inflammation and tubulitis on day 90 post-DBMT (left upper panel) and then progressed to terminal acute cellular rejection (Banff Type II: g0, i3, t2, v*, cg0, ci0, ct0, cv0, ptc0, C4d0) characterized by extensive interstitial inflammation, edema, tubulitis, and endarteritis (not shown) without C4d depositions on d125 post-DBMT (right upper panel). A recipient that failed to develop mixed chimerism developed acute cellular rejection (Banff Type I: g3, i3, t3, v*, cg, ci0, ct0, cv0, ptc2, C4d0) without C4d deposition at d62 post-DBMT, right after serum cyclosporine became undetectable (lower panels).
Development of mixed hematopoietic chimerism and transplant outcomes.
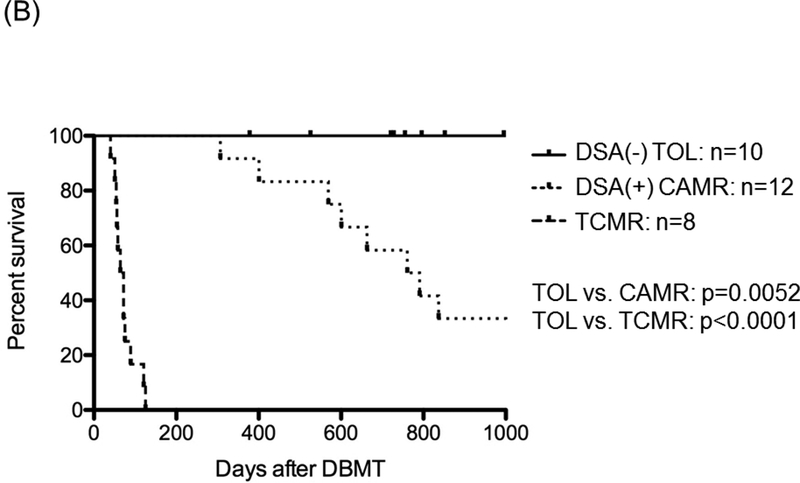
Among these 34 recipients, the 4 recipients that failed to develop any chimerism rejected their allograft by day 64 (Table 1 and Fig. 3A). Thirty animals developed multilineage mixed chimerism in both myeloid and lymphoid lineages (Table 1). Among these, 10 recipients in the TOL group achieved significantly longer immunosuppression-free renal allograft survival than those observed in either the CAMR or TCMR group (Fig. 3B).
Table 1:
Summary of survival after donor bone marrow transplantation and myeloid and lymphoid chimerism is described.
| * Animal | Treatment | Outcome | GST | BM | Myeloid Chimerism | Lymphoid Chimerism | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-T cell treatment | CoB | Pathology | DSA | (days) | Source/dose (X108/kg) |
Max (%) | Duration (days) | Max (%) | Duration (days) | |
| M2800 | ATG+aCD8 | aCD40L | TOL | - | >4328 | V/1.8 | 91.5 | 49 | 4.2 | 31 |
| M5898 | ATG | aCD40L | TOL | - | >2497 | I/0.62 | 65.3 | 21 | 3.3 | 31 |
| M8907 | ATG+aCD8 | aCD40L | TOL | - | >995 | V/3.0 | 86.4 | 40 | 16.7 | 40 |
| M4403 | ATG+aCD8 | aCD40L | TOL | - | >852 | V/3.0 | 97.2 | 47 | 4.6 | 27 |
| M8010 | ATG | CTLA4Ig | TOL | - | >796 | V/3.0 | 96 | 47 | 3.1 | 47 |
| M200 | ATG | aCD40L | TOL | - | >755 | I/0.5 | 89.9 | 42 | 2.3 | 35 |
| M4808 | ATG | aCD40L | TOL | - | >728 | V/3.0 | 92.6 | 32 | 5.9 | 35 |
| M2108 | ATG+aCD8 | aCD40L | TOL | - | >721 | I/0.8 | 86.7 | 81 | 7.9 | 137 |
| M2211 | ATG+alefacept | aCD40L | TOL | - | >526 | V/3.0 | 84.1 | 75 | 3.4 | 49 |
| M2611 | ATG | CTLA4Ig | TOL | - | >378 | V/3.0 | 93.2 | 24 | 3.5 | 28 |
| M504 | ATG+Fludarabine | - | CAMR | + | 2023 | I/1.5 | 94 | 35 | 0.7 | 7 |
| M1902 | ATG+aCD8 | aCD40L | CAMR | + | 1825 | I/0.7 | 59.5 | 29 | 3.3 | 35 |
| M6007 | ATG+aCD8 | aCD40L | CAMR | + | 1260 | V/3.0 | 96.2 | 66 | 3.8 | 80 |
| M5710 | ATG | CTLA4Ig | CAMR | + | 1143 | V/3.9 | 91.6 | 69 | 1.8 | 51 |
| M1900 | ATG | aCD40L | CAMR | + | 837 | V/3.4 | 96 | 52 | 2.4 | 42 |
| M4507 | ATG | aCD40L | CAMR | + | 791 | V/3.0 | 90.9 | 32 | 2.5 | 21 |
| M3208 | ATG+aCD8 | aCD40L | CAMR | + | 761 | V/3.0 | 68.9 | 21 | 1.3 | 9 |
| M8110 | ATG+alefacept | aCD40L | CAMR | + | 663 | I/1.0 | 80.1 | 55 | 1.5 | 23 |
| M6601 | ATG+aCD8 | aCD40L | CAMR | + | 601 | V/5.9 | 66.1 | 32 | 0.9 | 17 |
| M2311 | ATG+alefacept | aCD40L | CAMR | + | 569 | V/3.0 | 91.1 | 57 | 1.9 | 32 |
| M4498 | ATG | aCD40L | CAMR | + | 401 | I/1.9 | 83.8 | 35 | 2.3 | 22 |
| M5598 | ATG | aCD40L | CAMR | + | 307 | V/2.4 | 85 | 42 | 2.4 | 18 |
| M3809 | ATG | CTLA4Ig | TCMR | - | 125 | V/3.0 | 91.6 | 67 | 1.5 | 59 |
| M1005 | ATG | CTLA4Ig | TCMR | + | 121 | I/0.8 | 94.2 | 24 | 1.1 | 21 |
| M905 | ATG | CTLA4Ig | TCMR | - | 89 | I/1.2 | 85.9 | 32 | 0.3 | 6 |
| M5807 | ATG+aCD8 | aCD40L | TCMR | - | 75 | V/3.0 | 75.2 | 18 | 1.2 | 23 |
| M2297 | ATG+splenectomy | - | TCMR | - | 72 | V/2.5 | 58.1 | 21 | 0.4 | 14 |
| M3505 | ATG+aCD8 | aCD40L | TCMR | - | 72 | V/3.0 | 2.27 | 9 | 0.2 | 5 |
| M300 | ATG | aCD40L | TCMR | - | 58 | V/3.5 | 76 | 45 | 1.4 | 35 |
| M3997 | ATG+splenectomy | - | TCMR | - | 40 | V/1.5 | 50.4 | 18 | 0.2 | 3 |
| M1102 | ATG+aCD8 | aCD40L | TCMR | - | 64 | V/6.9 | - | - | - | - |
| M1605 | ATG | CTLA4Ig | TCMR | - | 56 | V/3.0 | - | - | - | - |
| M3304 | ATG+Fludarabine | - | TCMR | - | 54 | I/1.4 | - | - | - | - |
| M6600 | ATG | aCD40L | TCMR | - | 51 | I/1.0 | - | - | - | - |
GST: graft survival time, TOL: tolerance, CAMR: chronic antibody mediated rejection, TCMR: T cell mediated rejection, DSA: donor-specific antibodies.
V: processed from 5–7 vertebral bones, I: aspirated from iliac bones, BM dose: 108/kg MNC
Myeloid and lymphoid chimerism percentage specifies quantity of donor cells in recipients. Duration of chimerism is shown in days.
Figure 3: Renal allograft survival mediated by transient mixed chimerism.
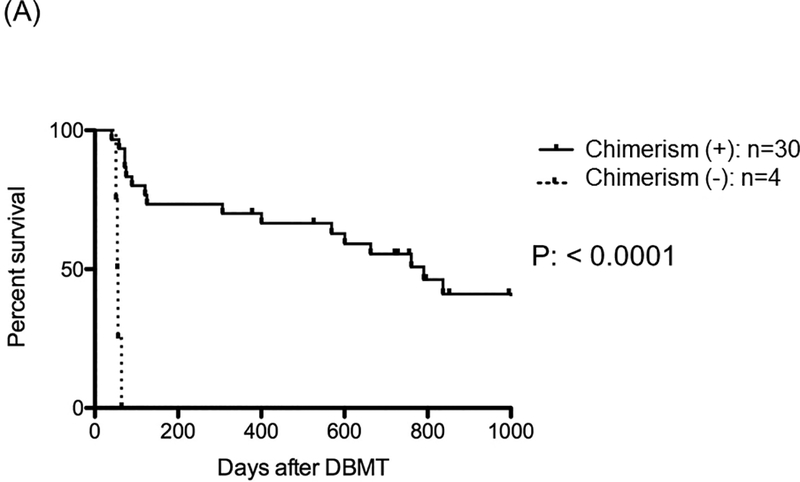
A: In 34 NHPs that received KTx and DBMT, 30 recipients successfully developed transient multilineage mixed chimerism [Chimerism (+)], but 4 recipients failed to develop multilineage mixed chimerism [Chimerism (–)]. Recipients that developed multilineage mixed chimerism showed significantly longer renal allograft survival, compared with those without chimerism.
B: The 30 recipients that developed transient mixed chimerism were then categorized in 3 groups based on the transplant outcome: 10 recipients never developed rejection during the entire observation period (Group TOL) with a graft survival time (GST) > 1258 ± 388 days; 12 recipients eventually developed chronic antibody mediated rejection (Group CAMR) with GST 932 ± 155 days. The last group consisted of 8 recipients that developed T cell mediated rejection (Group TCMR) with GST 73 ± 8 days. Renal allograft survival was significantly longer in TOL recipients than in CAMR (p=0.0052) and TCMR (p<0.0001).
The maximum levels of lymphoid chimerism significantly correlated with renal allograft outcome.
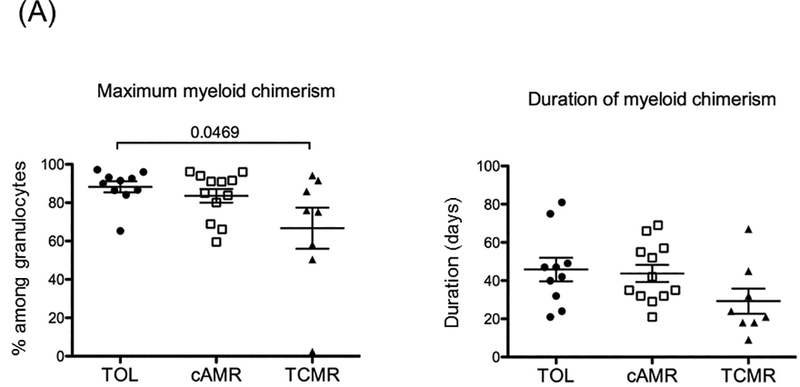
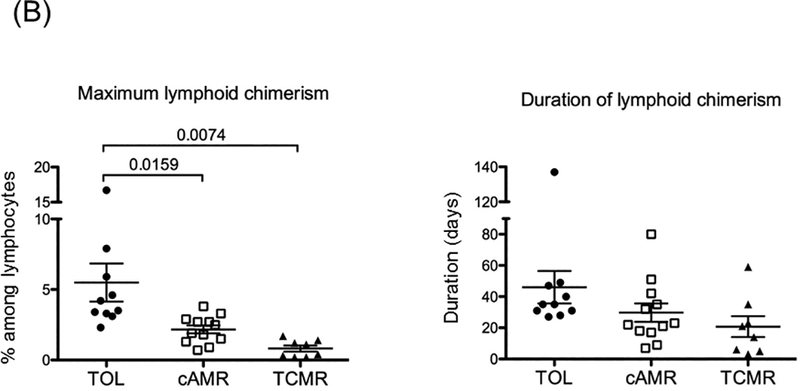
The duration and maximum levels of chimerism in the lymphoid and myeloid lineages in each group were compared (Fig. 4). In the myeloid lineage, the maximum chimerism levels were significantly higher in TOL than those in TCMR (p=0.0469), but not in CAMR. No difference was observed in the duration of chimerism (Fig. 4A). The maximum chimerism levels in the lymphoid lineage were significantly higher in TOL than those in both CAMR (p=0.0159) and TCMR (p=0.0074) (Fig. 4B). There were no significant differences observed in the duration of lymphoid chimerism among the 3 groups (Fig. 4B). The level of maximal lymphoid chimerism was not influenced by the use of different costimulatory blockade treatments (Figure S1A). Likewise, the source of bone marrow did not determine the level of lymphoid chimerism (Figure S1B).
Figure 4: The maximum lymphoid chimerism was significantly higher in TOL recipients.
The maximum levels and duration of both myeloid and lymphoid chimerism were analyzed.
A: Maximum myeloid chimerism was significantly higher in TOL than in TCMR (p=0.0469), but no CAMR.
B: Maximum lymphoid chimerism was significantly higher in TOL than in both CAMR (p=0.0159) and TCMR (p=0.0074). However, no difference was observed in the duration of chimerism among the 3 groups.
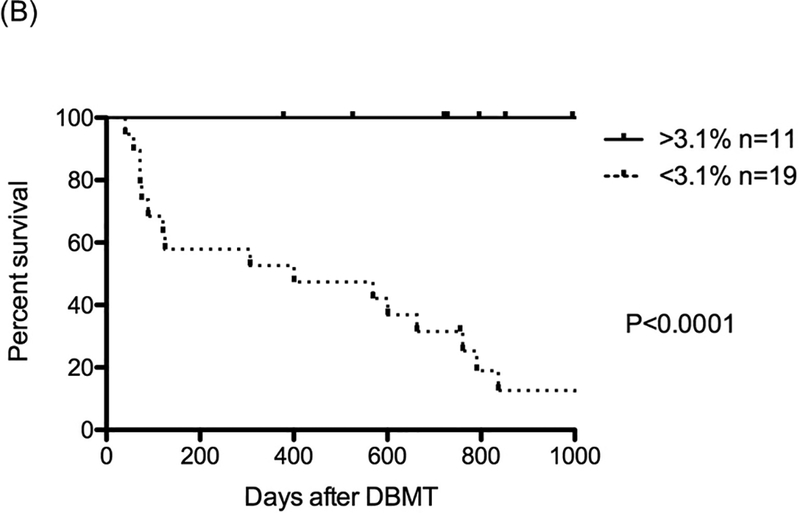
We also performed receiver operating characteristic curve (ROC) analysis to identify what percentage of chimerism detected in the recipient’s peripheral blood after DBMT is predictive of long-term renal allograft survival (Fig. 5A). ROC analysis showed that maximum level of lymphoid chimerism was higher than any other parameter and reliably predicted transplant outcome with an AUC of 0.94, 95%CI of 0.87–1.02, and optimal cutoff of 3.1% (Fig. 5A), (Fig. 5A and Figure S1C). Statistical analysis of renal allograft survival in recipients with greater than 3.1% lymphoid chimerism (n=11) compared to those with less than 3.1% (n=19) lymphoid chimerism revealed a significant difference (Fig. 5B). None of the recipients with greater than 3.8% maximum lymphoid chimerism developed DSA, as reflected in 100% survival without CAMR in this group (Figure S2).
Figure 5: ROC analysis of maximum lymphoid chimerism revealed an excellent AUC for renal allograft tolerance.
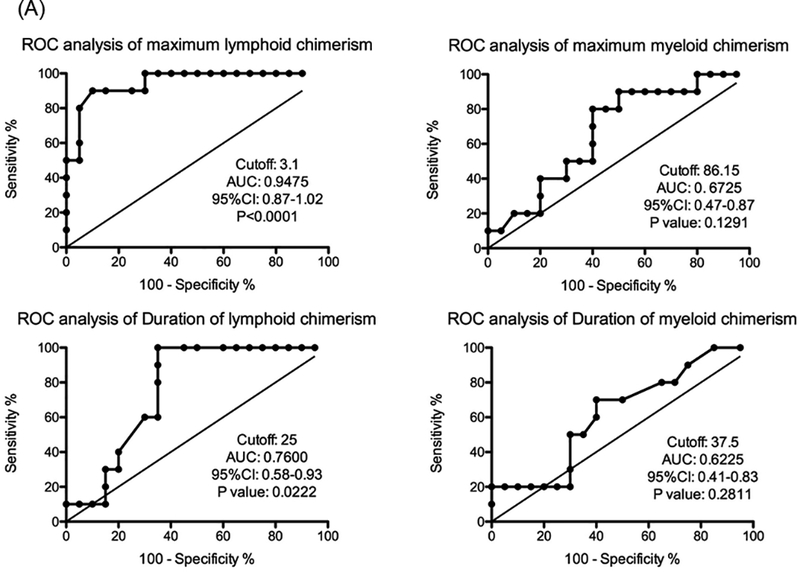
A: ROC curve analyses of the maximum and minimum duration of both myeloid and lymphoid chimerism were performed. ROC analysis showed that the maximum level of lymphoid chimerism reliably predicted transplant outcome, with AUC 0.94, 95%CI 0.87–1.02, and a cut off value of 3.1%
B: With cutoff values greater than 3.1% of maximum lymphoid chimerism (n=11), significantly prolonged renal allograft survival was observed, compared with those that were less than 3.1% (n=19)
DISCUSSION
In contrast to mice, persistent mixed chimerism has been extremely difficult to achieve via MHC-mismatched DBMT in NHPs (21, 22) and humans (26, 34). Mixed chimerism either transforms to full donor chimerism or disappears, depending upon the intensity of the conditioning regimen (24, 34, 35). Transient mixed chimerism, on the other hand, is effective and indeed necessary for long-term immunosuppression-free renal allograft survival. The transient nature of our approach to chimerism induction rules out the possibility of using chimerism as a biomarker for stable tolerance. Therefore, it is critically important to find other reliable biomarkers to monitor the long-term (years) outcome of renal allografts. In our decades-long experience with chimerism induction in NHPs, we have observed that recipients who develop a more robust chimerism seem to achieve better allograft survival, but the precise level or duration of chimerism required for stable tolerance induction have not been previously determined. In the current study, we retrospectively analyzed chimerism levels in recipients who achieved robust tolerance without developing DSA or CAMR and compared these findings with recipients who eventually developed CAMR or TCMR.
Our study clearly shows that higher maximum levels of lymphoid chimerism are statistically correlated with more robust renal allograft tolerance. The ROC analysis revealed that lymphoid chimerism levels greater than 3.1% were exclusively associated with renal allograft tolerance. In contrast, no significant difference was found in the myeloid lineage with respect to chimerism levels between the TOL and CAMR groups, consistent with observations reported in a NHP BMT model by Larsen et al (36).
Correlations between levels of chimerism and allograft tolerance have also been studied in murine models. Taniguchi et al (37) found that level of thymic deletion of donor reactive T cells correlated with levels of chimerism. They further determined that greater than 30% peripheral chimerism was required for a significant reduction in donor reactive Vβ11+ CD4+T cells in order to achieve skin allograft tolerance. In the skin allograft tolerance model induced by antilymphocyte serum and DBMT, Hale et al (38) showed that higher chimerism levels (8–10%) in the spleen induced by a sufficient number of DBM administrations (150 × 106) resulted in consistent induction of skin allograft tolerance, whereas half of the recipients who developed lower chimerism levels (<5%) after low DBM (75 × 106) administration eventually lost chimerism, resulting in rejection of the skin allograft. In the neonatal tolerance model, Chen et al (39) reported that peripheral chimerism was linearly related to thymic chimerism and that greater than 3.0% chimerism at the time of skin grafting consistently correlated with long-term allograft acceptance. In contrast, chimerism levels of 0.2%−3.0% provided only a 34% chance of long-term skin tolerance and less than 0.2% chimerism never induced skin allograft tolerance. An interesting observation was that the skin allograft tolerance persisted despite the eventual loss of chimerism in the majority of recipients, which is very similar to our observations in renal allograft tolerance in NHPs. In summary, both studies suggest that higher (>3%) hematopoietic chimerism is necessary for induction of allograft tolerance, but persistent chimerism is not necessary for the maintenance of tolerance. It is unclear why a higher chimerism level is necessary for the induction phase of tolerance. Chen et al found that peripheral chimerism levels are linearly related to chimerism levels in the thymus. We also found migration of donor dendritic cells in the thymus after DBMT in NHPs (21), which suggests that robust thymic deletion of donor reactive T cells may be initially required for induction of allograft tolerance.
The mechanisms involved in maintaining tolerance also remain undefined. In these murine studies, it was suggested that the significantly higher regulatory T cells (Tregs) that developed in tolerant recipients resulted in the maintenance of tolerance. In our NHP studies, we also regularly observed expansion of peripheral blood-induced Tregs (iTregs) in tolerant recipients after mixed leukocyte reaction (MLR) with donor antigens (40). Enrichment of Tregs in the renal allograft, itself, has also been observed in both humans (24) and NHPs (manuscript in preparation). We hypothesize that naïve T cells may be converted to iTregs upon encountering donor antigens expressed in the renal allograft, leading to enrichment of Tregs in the renal allograft.
In the current study, we were not able to isolate a specific chimeric lymphocyte subset that was crucial for induction of renal allograft tolerance, since the levels and duration of lymphoid chimerism were too limited for detailed analysis. It is possible that some lymphoid cells, for example, immature dendritic cells or B cells, could function as antigen presenting cells and therefore would be important to the induction of antigen-specific tolerance. Analysis from another study of a single recipient that developed durable chimerism revealed clear dominance of donor CD3-CD20+ B cells, which express HLA-DR+ at 1 month after DBMT (data not shown). Circulating donor B cells which express donor MHC class II antigens may migrate to the thymus and participate in thymic deletion as has been reported in murine models (41). These chimeric B cells may also serve as antigen presenting cells and induce specific tolerance/anergy of the peripheral T cells in the presence of costimulatory blockade (42). In light of the need for future human studies, it will be critical to determine the levels of chimerism in specific lymphocyte subsets that are relevant to tolerance induction. Although chimerism has typically been assessed by STR analysis in clinical trials, flow cytometric analysis, which is capable of more detailed chimerism evaluation, would be more useful in the clinical setting.
Although this study is limited by the retrospective nature of the design, our findings emphasize the apparent critical importance of multilineage mixed chimerism, especially lymphoid chimerism, for long-term immunosuppression-free renal graft survival. Identification of specific lymphoid cell subsets that are capable of inducing allograft tolerance is the next critical step in elucidating the mechanism of renal allograft tolerance in our NHP model.
Supplementary Material
ACKNOWLEDGMENTS
The present work was supported by grant U19AI102405, part of the NIH NHP Transplantation Tolerance Cooperative Study Group sponsored by the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases.
Funding sources: This work was supported by NIH grant U19 AI102405–01.
Abbreviations:
- ATG:
antithymocyte globulin
- CAMR:
chronic antibody mediated rejection
- CNI:
calcineurin inhibitor
- CKBMT:
combined kidney and bone marrow transplantation
- DBMT:
donor bone marrow transplantation
- DSA:
donor specific antibody
- KTx:
kidney transplantation
- MHC:
major histocompatibility complex
- NHP:
nonhuman primate
- ROC:
receiver operating characteristi
- STR:
short tandem repeat
- TBI:
total body irradiation
- TCMR:
T cell mediated rejection
- TI:
thymic irradiation
- TOL:
toleran
Authorship :
- CCT:
Data analysis, Writing the manuscript
- TO:
Perform Research, Data analysis, Writing the manuscript
- HS:
Perform Research
- AD:
Perform Research
- MM:
Perform Research
- IR:
Perform Research
- ABC:
Writing the manuscript
- TK:
Design the study, Perform Research, Data analysis, Writing the manuscript
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation. This manuscript was also not prepared or funded by any commercial organization.
REFERENCES
- 1.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–83. [DOI] [PubMed] [Google Scholar]
- 2.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011;11:1226–35. [DOI] [PubMed] [Google Scholar]
- 3.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2:807–18. [DOI] [PubMed] [Google Scholar]
- 4.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496–506. [DOI] [PubMed] [Google Scholar]
- 5.Morales JM, Dominguez-Gil B. Cardiovascular risk profile with the new immunosuppressive combinations after renal transplantation. J Hypertens. 2005;23:1609–16. [DOI] [PubMed] [Google Scholar]
- 6.Scantlebury V, Shapiro R, Fung J, et al. New onset of diabetes in FK 506 vs cyclosporine-treated kidney transplant recipients. Transplant Proc. 1991;23:3169–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Jindal RM, Sidner RA, Milgrom ML. Post-transplant diabetes mellitus. The role of immunosuppression. Drug Saf. 1997;16:242–57. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti P, Navalesi R. The metabolic effects of cyclosporin and tacrolimus. J Endocrinol Invest. 2000;23:482–90. [DOI] [PubMed] [Google Scholar]
- 9.Nemunaitis J, Deeg HJ, Yee GC. High cyclosporin levels after bone marrow transplantation associated with hypertriglyceridaemia: Lancet. 1986. September 27;2:744–5. [DOI] [PubMed] [Google Scholar]
- 10.Jevnikar AM, Petric R, Holub BJ, Philbrick DJ, Clark WF. Effect of cyclosporine on plasma lipids and modification with dietary fish oil. Transplantation. 1988;46:722–5. [DOI] [PubMed] [Google Scholar]
- 11.Abouljoud MS, Levy MF, Klintmalm GB. Hyperlipidemia after liver transplantation: long-term results of the FK506/cyclosporine A US Multicenter Trial. US Multicenter Study Group. Transplant Proc. 1995;27:1121–3. [PubMed] [Google Scholar]
- 12.McCune TR, Thacker LR II, Peters TG, et al. Effects of tacrolimus on hyperlipidemia after successful renal transplantation: a Southeastern Organ Procurement Foundation multicenter clinical study. Transplantation. 1998;65:87–92. [DOI] [PubMed] [Google Scholar]
- 13.Wijdicks EF. Neurotoxicity of immunosuppressive drugs. Liver Transpl. 2001;7:937–42. [DOI] [PubMed] [Google Scholar]
- 14.Ponticelli C, Campise MR. Neurological complications in kidney transplant recipients. J Nephrol. 2005;18:521–8. [PubMed] [Google Scholar]
- 15.Hart A, Smith J, Skeans M, et al. Kidney. Am J Transplant. 2016;16(Suppl 2):11–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sykes M, Sachs DH. Mixed chimerism. Philos Trans R Soc Lond B Biol Sci. 2001;356:707–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eason JD, Wee SL, Kawai T, et al. Recombinant human dimeric tumor necrosis factor receptor (TNFR:Fc) as an immunosuppressive agent in renal allograft recipients. Transplant Proc. 1995;27:554. [PubMed] [Google Scholar]
- 19.Kimikawa M, Kawai T, Sachs DH, Colvin RB, Bartholomew A, Cosimi AB. Mixed chimerism and transplantation tolerance induced by a nonlethal preparative regimen in cynomolgus monkeys. Transplant Proc. 1997;29:1218. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Sachs DH, Cosimi AB. Tolerance to vascularized organ allografts in large-animal models. Curr Opin Immunol. 1999;11:516–20. [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–8. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Ochiai T, Boskovic S, et al. Use of CTLA4Ig for Induction of Mixed Chimerism and Renal Allograft Tolerance in Nonhuman Primates. Am J Transplant. 2014;14:2704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer TR, Sykes M, Tolkoff-Rubin N, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. 2011;91:672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Sachs DH, Sykes M, Cosimi AB. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013. May 9;368:1850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama I, Nadazdin O, Boskovic S, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Yamada Y, Tonsho M, et al. Alefacept promotes immunosuppression-free renal allograft survival in nonhuman primates via depletion of recipient memory T cells. Am J Transplant. 2013;13:3223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor SL, Blasky AJ, Pendley CJ, et al. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pendley CJ, Becker EA, Karl JA, et al. MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics. 2008;60:339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosimi AB, Delmonico FL, Wright JK, et al. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery. 1990;108:406–13; discussion 13–4. [PubMed] [Google Scholar]
- 33.Boskovic S, Kawai T, Smith RN, et al. Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long-term observations in nonhuman primates. Transplantation. 2006;82:819–25. [DOI] [PubMed] [Google Scholar]
- 34.Scandling JD, Busque S, Shizuru JA, et al. Chimerism, graft survival, and withdrawal of immunosuppressive drugs in HLA matched and mismatched patients after living donor kidney and hematopoietic cell transplantation. Am J Transplant. 2015;15:695–704. [DOI] [PubMed] [Google Scholar]
- 35.Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen CP, Page A, Linzie KH, et al. An MHC-defined primate model reveals significant rejection of bone marrow after mixed chimerism induction despite full MHC matching. Am J Transplant. 2010;10:2396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi H, Abe M, Shirai T, Fukao K, Nakauchi H. Reconstitution ratio is critical for alloreactive T cell deletion and skin graft survival in mixed bone marrow chimeras. J Immunol. 1995;155:5631–6. [PubMed] [Google Scholar]
- 38.Hale DA, Gottschalk R, Umemura A, Maki T, Monaco AP. Establishment of stable multilineage hematopoietic chimerism and donor-specific tolerance without irradiation. Transplantation. 2000;69:1242–51. [DOI] [PubMed] [Google Scholar]
- 39.Chen JC, Kuo ML, Ou LS, et al. Characterization of tolerance induction through prenatal marrow transplantation: the requirement for a threshold level of chimerism to establish rather than maintain postnatal skin tolerance. Cell Transplant. 2010;19:1609–22. [DOI] [PubMed] [Google Scholar]
- 40.Hotta K, Aoyama A, Oura T, et al. Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI insight. 2016;1(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frommer F, Waisman A. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PLoS One. 2010;5:e15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snanoudj R, Frangie C, Deroure B, et al. The blockade of T-cell co-stimulation as a therapeutic stratagem for immunosuppression: Focus on belatacept. Biologics. 2007;1:203–13. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


