Abstract
An important goal of the mycotoxin research community is to develop comprehensive strategies for mycotoxin control and detoxification. Although significant progress has been made in devising such strategies, yet, there are barriers to overcome and gaps to fill in order to design effective mycotoxin management techniques. This is in part due to a lack of understanding of why fungi produce these toxic metabolites. Here we present cumulative evidence from the literature that indicates an important ecological role for mycotoxins, with particular focus on Fusarium mycotoxins. Further, we suggest that understanding how mycotoxin levels are regulated by microbial encounters can offer novel insights for mycotoxin control in food and feed. Microbial degradation of mycotoxins provides a wealth of chemical information that can be harnessed for large-scale mycotoxin detoxification efforts.
Keywords: Mycotoxins–Fusarium, bacterial-fungal interaction (BFI), mycotoxin ecological role, microbial interaction, microbial communication
Introduction
The term mycotoxin refers to harmful secondary metabolites produced by fungi in food and feed products that negatively impact animal and human health, by themselves or through synergistic interactions with each other. Initially thought to be waste products, fungal secondary metabolites are now considered as important players in ecological settings. Some metabolites provide protection from physical damage. For example, spore melanins have been demonstrated to provide protection against ionizing radiation as well as oxidizing agents in addition to acting as virulence factors (Eisenman and Casadevall, 2012). Some fungal metabolites provide protection against other microbes, helping the producing fungus to secure its environmental niche. Gliotoxin, an antifungal produced by several fungi, is a virulence factor of the human pathogen Aspergillus fumigatus (Scharf et al., 2016).
This notion of ecological function is applicable to all fungal secondary metabolites including mycotoxins (Figure 1A). Fusarium species comprise a well-known group of soil-borne microbes that are infamous for their ability to make many potent mycotoxins (Table 1). In soil and host environments, Fusarium spp. engage in intimate associations with other microbes. This review will examine when, where and why mycotoxins are made, highlighting the ecological importance of mycotoxins with a special emphasis on the involvement of Fusarium mycotoxins in bacterial-fungal interactions.
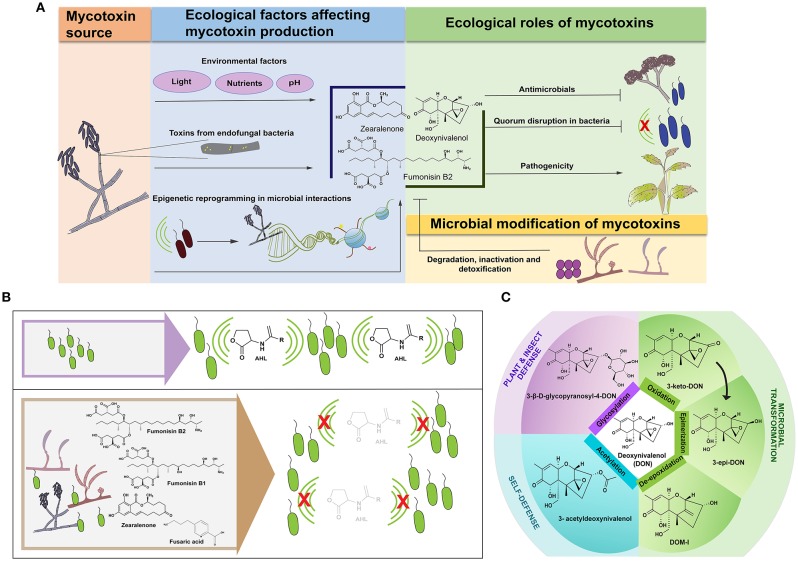
Figure 1.
Mycotoxins in microbial interactions. (A) Mycotoxins in the ecological landscape—Bacterial-fungal interactions influence mycotoxin production in addition to environmental factors (blue box). In some cases, the toxin may be produced by an endofungal bacterium (shown as yellow circular bacteria residing in the gray hypha). Microbial interactions may alter epigenetic modifications (indicated by DNA wrapped around blue histones where the yellow and pink shapes represent epigenetic modifications in the histone tail) that regulate mycotoxin production. These secreted mycotoxins in turn play vital roles in shaping ecological niches (green box) by acting as antimicrobials in addition to inhibiting bacterial quorum-based communication (bacterial communication is represented with green arcs; the red “X” indicates disruption of communication). Mycotoxins also alter the pathogenic abilities of the producing fungus that in turn may influence the microbial communities in the niche. In such niches, microbes (represented by fungi and spherical bacteria in the yellow box) can detoxify, degrade, and inactivate mycotoxins. (B) Fusarium mycotoxins inhibit acyl homoserine lactone (AHL)-based quorum sensing in bacteria. (Top panel) The green arcs indicate active quorum-based communication in a bacterial population. (Bottom panel) The left end of the lower box shows Fusarium spp. co-occurring with bacteria. The right end shows mycotoxins produced by Fusarium spp. that contribute to quorum quenching in the bacterial population, indicated by the red “X” over the green arcs. (C) Detoxification processes of deoxynivalenol (DON) and their products that have been tested to show reduced toxicity compared to DON. The arrow from 3-keto-DON to 3-epi-DON indicates that formation of the epimer proceeds with 3-keto-DON as an intermediate.
Table 1.
List of different mycotoxins and their chemical classes, the Fusarium species identified as producers of each mycotoxin, and corresponding reported activities.
| Mycotoxin | Producers identified | Chemical class | Reported activity | References |
|---|---|---|---|---|
| HT2 toxin | F. langsethiae1, F. sporotrichoides1, F. culmorum1, F. poae1, 8, F. sporotrichoides3, F. acuminatum8, F. chlamydosporum8 | Type-A trichothecene | Hematotoxicity25, myelototoxicty25 |
1 Shi et al., 2016 3Thrane, 1986 8Beukes et al., 2017 25Lautraite et al., 1996 |
| T2 toxin | F. langsethiae1, F. sporotrichoides1, F. culmorum1, 8, F. poae1, 8, F. sporotrichoides3, F. acuminatum9, F. chlamydosporum8 | Type-A trichothecene | Hematoxicity35, myelototoxicity35 |
1 Shi et al., 2016 3Thrane, 1986 8Beukes et al., 2017 9Bottalico and Perrone, 2002 35Chilaka et al., 2017 |
| Neosolaniol | F. langsethiae1, F. sporotrichoides1, 3, F. culmorum1, F. poae1, F. meridionale1, F. acuminatum9 | Type-A trichothecene | Hematotoxicity34 |
1 Shi et al., 2016 3 Thrane, 1986 9 Bottalico and Perrone, 2002 34 Janse van Rensburg et al., 1987 |
| Diacetoxyscirpenol | F. langsethiae1, F. sporotrichoides1, 5, F. polyphialadicum1, F. poae1, 5, F. equiseti4, F. chlamydosporum5, F. avenaceum5, F. semitectum5, F. acuminatum5, F. compactum5, F. sambucinum5, F. venenatum5, F. culmorum5, F. graminearum5, F. crookwellense5 | Type-A trichothecene | Hematotoxicity34, teratogenicity39 |
1 Shi et al., 2016 4 Hestbjerg et al., 2002 5 Schollenberger et al., 2007 34 Janse van Rensburg et al., 1987 39 Mayura et al., 1987 |
| Deoxynivalenol | F. graminearum1, F. culmorum9, F. acuminatum8, F. crookwellense8, F. pseudograminearum8, F. semitectum8 | Type-B trichothecene | Cytotoxicity28, endocrine disruption26, immune modulation26, developmental and reproductive toxicity26, genotoxicity26 |
1 Shi et al., 2016 8 Beukes et al., 2017 9 Bottalico and Perrone, 2002 26 Knutsen et al., 2017 28 Alassane-Kpembi et al., 2013 |
| Nivalenol |
F. culmorum1, 9, F. poae1, 8, F. meridionale1, 8, F. graminearum8, F. equiseti4, F. crookwellense9, F. pseudograminearum8, F. semitectum8, F. acaciae-mearnsii8, F. brasilicum8, F. cortaderiae8 |
Type-B trichothecene | Cytotoxicity28, hematotoxicity35, immunotoxicity35 |
1 Shi et al., 2016 4 Hestbjerg et al., 2002 8 Beukes et al., 2017 9 Bottalico and Perrone, 2002 28 Alassane-Kpembi et al., 2013 35 Chilaka et al., 2017 |
| Fusarenon-X | F. culmorum1, 8, F. poae1, 8, F. meridionale1, F. graminearum3, F. equiseti30, F. crookwellense9, F. pseudograminearum8 | Type-B trichothecene | Genotoxicity28, cytotoxicity28 | 1. Shi et al., 2016 3 Thrane, 1986 8 Beukes et al., 2017 9 Bottalico and Perrone, 2002 38 Alassane-Kpembi et al., 2013 30Jestoi, 2008 |
| 15-ADON | F. graminearum1, 8, F. boothi8 | Type-B trichothecene | Cytotoxicity28 |
1 Shi et al., 2016 8 Beukes et al., 2017 28 Alassane-Kpembi et al., 2013 |
| 3-ADON | F. graminearum1, 8, F. culmorum4, F. acaciae-mearnsii8, F. brasilicum8, F. cortaderiae8 | Type-B trichothecene | Cytotoxicity28 |
1 Shi et al., 2016 4 Hestbjerg et al., 2002 8 Beukes et al., 2017 28 Alassane-Kpembi et al., 2013 |
| Beauvericin |
F. acuminatum8, F. anthophilum8, F. avenaceum8, F. globosum8, F. fujikuroi8, F. nygamai8, F. oxysporum8, F. poae8, F. proliferatum8, F. semitectum8, F. subglutinans8, F. temperatum8, F. verticillioides8, F. acutatum30, F. beomiforme30, F. circinatum30, F. concentricum30, F. dlamini30, F. equiseti30, F. guttiforme30
F. konzum30, F. langsethiae30, F. longipes30, F. pseudoanthophilum30, F. sambucinum30, F. sporotrichioides30, F. tricinctum30 |
Non- ribosomal peptide | Antimicrobial activity30, insecticidal activity30, cytotoxicity30, genotoxicity27 |
8 Beukes et al., 2017 30 Jestoi, 2008 27 Mallebrera et al., 2018 |
| Enniatins |
F. merismoides8, F. acuminatum30, F. arthrosporioides30, F. avenaceum30, F. compactum30, F. culmorum30, F. equiseti30, F. kyushuense30, F. langsethiae30, F. lateritium30, F. oxysporum30, F. poae30, F. sambucinum30, F. scirpi30, F. sporotrichioides30, F. torulosum30, F. tricinctum30, F. venenotum30 |
Non- ribosomal peptide | Antimicrobial activity30, insecticidal activity30, cytotoxicity30, phytotoxicity30 |
8 Beukes et al., 2017 30 Jestoi, 2008 |
| Fusaric acid | F. proliferatum1, F. verticillioides1, F. fujikuroi1, F. solani1, F. temperatum1, F. subglutinans1, 8, F. musae1, F. tricinctum1, F. oxysporum1, F. equiseti1, F. sacchari1, F. concentricum1, F. andiyazi1, F. thapsinum8, F. moniliforme20 | Polyketide | Neurotoxicity20, antibacterial activity21, phytotoxicity19 |
1 Shi et al., 2016 8 Beukes et al., 2017 19 Stipanovic et al., 2011 20 Porter et al., 1995 21 Bacon et al., 2006 |
| Fusarin C | F. avenaceum9, F. verticillioides8, F. moniliforme42, F. graminearum43, F. culmorum43, F. crookwellense43, F. sporotrichioides43, F. poae43, F. tricinctum43, F. avenaceum43 | Polyketide | Estrogenic agonist40, carcinogenicity40 |
8 Beukes et al., 2017 9 Bottalico and Perrone, 2002 42 Gelderblom et al., 1984 43 Thrane, 1988 40 Sondergaard et al., 2011 |
| Equisetin | F. equiseti2, F. pallidoroseum2, F. heterosporum | Polyketide | Antibacterial activity46, phytotoxicity2, antiviral activity24 cytotoxicity24, fungicidal activity24 |
2 Wheeler et al., 1999 24 Burke et al., 2005 46 Vesonder et al., 1979 |
| Fumonisins | F. proliferatum1, 8, F. verticillioides1, 8, F. fujikuroi1, 8, F. solani1, F. andiyazi8, F. anthophilum8, F. globosum8, F. napiforme8, F. nygamai8, F. oxysporum8, F. pseudonygamai8, F. subglutinans8, F. thapsinum8, F. temperatum8 | Polyketide | Carcinogenicity23, neurotoxicity23, hepatotoxicity23 |
1 Shi et al., 2016 8 Beukes et al., 2017 23 Schertz et al., 2018 |
| Fusaproliferin | F. globosum30, F. guttiforme30, F. konzum30, F. proliferatum30, F. pseudocircinatum30, F. pseudonygamai30, F. subglutinans30, F. verticillioides30 | Sesquiterpene | Phytotoxicity30, insecticidal activity30, cytotoxicity30, teratogenicity30 |
30 Jestoi, 2008 |
| Culmorin |
F. culmorum44, F. graminearum44, F. crookwellense44, F. venenatum44, Fusarium praegraminearum44 |
Sesquiterpene | antifungal and phytotoxic properties44, weak cytotoxicity44, weak teratogenicity44 | 44 Weber et al., 2018 |
| Zearalenone | F. culmorum1, 3, 4, 9, F. meridionale1, F. graminearum1, 3, F. equiseti3, 4, F. crookwellense9, F. oxysporum8, F. pseudograminearum8, F. semitectum8 | β-resorcyclic acid lactone | Non-steroidal estrogen14, immunotoxicity14, hepatocarcinogenicity45, nephropathy45, hematotoxicity45 |
1 Shi et al., 2016 3 Thrane, 1986 4 Hestbjerg et al., 2002 8 Beukes et al., 2017 9 Bottalico and Perrone, 2002 14 Kuiper-Goodman et al., 1987 45 Buranatragool et al., 2015 |
| Butenolide | F. culmorum3, F. sporotrichoides3, F. tricinctum3, F. graminearum10 | Lactones | Cytotoxicity11 |
3 Thrane, 1986 10 Harris et al., 2007 11 Wang et al., 2006 |
| Moniliformin | F. avenaceum3, 9, F. acuminatum8, F. anthophilum8, F. chlamydosporum8, F. culmorum8, F. fujikuroi8, F. napiforme8, F. nygamai8, F. oxysporum8, F. proliferatum8, F. pseudonygamai8, F. semitectum8, F. subglutinans8, F. thapsinum8, F. temperatum8, F. verticillioides8, F. acutatum30, F. arthrosporioides30, F. begoniae30, F. beomiforme30, F. bulbicola30, F. concolor30, F. denticulatum30, F. dlaminii30, F. equiseti30, F. fusarioides30, F. lactis30, F. nisikadoi30, F. phyllophilum30, F. pseudoanthophilum30, F. pseudocircinatum30, F. ramigenum30, F. redolens30, F. retuculatum30, F. sacchari30, F. sambucinum30, F. sporotrichioides30, F. tricinctum30 | Cyclobutane | Phytotoxicity30, cytotoxicity30 |
3 Thrane, 1986 8 Beukes et al., 2017 9 Bottalico and Perrone, 2002 30 Jestoi, 2008 |
Why are Mycotoxins Made?
Mycotoxins in Fungal-Bacterial Battles
The advances in sequencing and bioinformatic technologies have shown that fungi, including the Fusarium spp. possess a large number of biosynthetic gene clusters (BGCs) with the potential to produce a myriad of secondary metabolites. One of the most powerful ways to activate BGCs is by co-cultivation or mixed fermentation of two or more microbes. Microbial interactions that have led to up-regulation of known metabolites or to discovery of novel natural products are numerous (Schroeckh et al., 2009; Bao et al., 2017). Co-cultivation of F. tricinctum and F. begoniae has led to the identification of subenniatins A and B, which have been reported to be the precursors of enniatins (Wang et al., 2013). Co-cultivation of F. tricinctum and Bacillus subtilis also led to the identification of a few novel metabolites such as macrocarpon C, 2-(carboxymethylamino)benzoic acid and citreoisocoumarinol [(Ola et al., 2013), and references therein]. F. oxysporum MSA 35 is an antagonist to wilt-causing F. oxysporum species. The antagonism has been at least in-part attributed to small volatile organic compounds produced by the fungus only when it is in association with ectosymbiotic bacteria (Minerdi et al., 2011) The exposure of Serratia plymuthica, to volatiles produced by F. culmorum upregulated the production of the volatile bacterial terpene sodorifen (Schmidt et al., 2017).
Several emerging studies place mycotoxins directly in the microbial battleground. One elaborate interaction involves the wilt-causing phytobacterium Ralstonia solanacearum and F. fujikuroi, the causal agent of foolish seedling disease in rice. The bacterium produces the lipopeptide ralsolamycin, which induces developmental changes in many fungal species resulting in chlamydospore formation. These chlamydospores are subsequently colonized by Ralstonia (Spraker et al., 2016). In response to this invasion, the F. fujikuroi responds with an increase in localized production of bikaverin and beauvericin, which together show additive antibacterial activity against Ralstonia (Spraker et al., 2018). Fusaric acid, a mycotoxin produced by numerous Fusarium species, has antibacterial activity (Table 1). Fusaric acid can sequester iron which has been suggested as a mechanism of toxicity to bacteria (Ruiz et al., 2015). Production of the siderophores, pyoverdine, and ennantio-psychelin, by Pseudomonas protegens has been demonstrated to contribute to the resistance of the bacterium to fusaric acid (Ruiz et al., 2015). Further, pyoverdine has been shown to contribute to successful survival in soil (Drehe et al., 2018). Fusaric acid has also been reported to repress the expression of biosynthetic genes involved in the production of 2,4-diacetylphloroglucinol, an antimicrobial polyketide made by Pseudomonas fluorescens, both in vitro as well as in the wheat rhizosphere (Notz et al., 2002). Pseudomonas protegens exhibits antibiosis against F. verticillioides which has been primarily attributed to the production of pyrrolnitrin, rhizoxin, and 2,4-diacetylphloroglucinol. Fusaric acid has been shown to reduce the antibiosis by P. protegens against F. verticillioides (Quecine et al., 2016). Thus, mycotoxins form an integral part of microbial interactions where they may offer protection from competing or invading microbes.
Mycotoxins as Communication Signals in Quorum and Biofilm Formation
Quorum sensing is an important mechanism by which bacteria and fungi regulate developmental programs including biofilm formation and expression of virulence proteins through alteration of gene expression patterns based on population densities. Several studies have demonstrated how other microbes and their metabolites can interfere in quorum sensing and biofilms. Fungal secondary metabolites, including those produced by Fusarium spp., are involved in disrupting quorum signaling in bacteria (Martín-Rodríguez et al., 2014) (Figure 1B). Fusaric acid acts as a quorum quencher of acyl homoserine lactone molecules at low concentrations against the biocontrol agent Pseudomonas chlororaphis (van Rij et al., 2005). At higher concentrations, fusaric acid inhibits the production of the antifungal metabolite phenazine-1-carboxamide by the bacterium (van Rij et al., 2005). In addition, two other mycotoxins, zearalenone and fumonisin, have been demonstrated to inhibit quorum sensing in the bacterium Chromobacterium violaceum (Bacon et al., 2017). Diketopiperazines derived from gram-negative bacteria have been shown to regulate quorum-dependent phenotypes (Holden et al., 2002), possibly implicating diketopiperazine-like mycotoxins (gliotoxin, roquefortines among others) as additional quorum modulating molecules (Bacon et al., 2017). Taken together with section Mycotoxins in Fungal-Bacterial Battles, these instances indicate that mycotoxins may be synthesized in response to microbial signals in the ecological landscape, while also serving as interspecies signals themselves.
Mixed bacterial-fungal biofilms have increasingly come under scrutiny, especially in clinical settings. Candida spp. of fungi contribute to the majority of infections related to medical implant devices, where biofilm formation is a major contributor (Wargo and Hogan, 2006). A recent study has reported a bacterial exopolysaccharide offering antifungal resistance to Candida in an oral biofilm (Kim et al., 2018). Pseudomonas aeruginosa and Aspergillus fumigatus have also been reported to form mixed biofilms (Zheng et al., 2015). Phenazine-derived metabolites from the bacterium have been shown to regulate the developmental shifts of the fungus in co-cultured biofilms (Zheng et al., 2015). The Fusarium mycotoxin zearalenone has been shown to reduce Candida biofilm formation (Rajasekharan et al., 2018) and the Penicillium expansum mycotoxin patulin has been reported to modulate biofilm formation by P. aeruginosa and Achromobacter sp. (Liaqat et al., 2010). Therefore, mycotoxins may play vital roles in communication and/or microbial assembly processes that lead to successful formation of mixed biofilms in varied niches.
Mycotoxins in Intra-kingdom Fungal Interactions
A significant increase in the levels of deoxynivalenol (DON) and zearalenone produced by F. culmorum has been reported upon co-culture with the fungus Alternaria tenuissima (Müller et al., 2012). An endophytic strain of F. verticilloides has been shown to reduce the corn smut disease caused by Ustilago maydis (Lee et al., 2009) which has in part been correlated to the fusaric acid-mediated repression of growth of U. maydis (Jonkers et al., 2012). Trichoderma species are well-known mycoparasites of several fungi including the Fusarium spp. (Chérif, 1990). Trichoderma spp. secrete cell wall degrading enzymes like chitinases and glucanases to aid in parasitism (de la Cruz et al., 1995). DON production by F. culmorum and F. graminearum strains has been reported to repress expression of the chitinase gene (encoding the N-acetyl-β-d-glucosaminidase) in Trichoderma atroviride (Lutz et al., 2003). Paraconiothyrium variabile is a plant endophytic fungus that is antagonistic to F. oxysporum (Combès et al., 2012). This antagonism has been attributed to F. oxysporum-induced production of 13-oxo-9,11-octadecadienoic acid by the endophyte. This metabolite downregulated the production of beauvericin in F. oxysporum (Combès et al., 2012). In the soil environment, it has been shown that F. oxysporum can repress the production of aflatoxin by Aspergillus flavus leading to a higher accumulation of the Fusarium mycotoxin fumonisin (Falade et al., 2016). Thus, mycotoxins may be involved in specific interactions of fungi with each other where they may offer ecological advantages to the interacting species.
Mycotoxins in Improving Pathogen Fitness and Pathogenicity
Although secondary metabolites are not “required” for the growth and development of fungi, they function as fitness factors. Mycotoxins have been shown to contribute to the pathogenicity, aggressiveness and/or virulence of fungi. Fusaric acid has been reported to enhance the virulence of F. oxypsorum in both plant and animal hosts (López-Díaz et al., 2018). Mutants of F. avenaceum that lacked the ability to synthesize enniatin showed decreased virulence when infected on potato tubers (Herrmann et al., 1996). On the contrary, it has also been reported that the ability of Fusarium oxysporum f. sp. melonis isolates to synthesize beauvericin or enniatin B does not contribute to virulence in melons (Moretti et al., 2002). DON has been shown to be produced several fold-higher in infected host tissue compared to in vitro cultures in F. graminearum and F. pseudograminearum (Mudge et al., 2006) and functions as an important virulence factor (Proctor et al., 1995). F. culmorum, F. graminearum, and F. pseudograminearum cause fusarium head blight as well as fusarium crown rot. However, the former two pathogens are more aggressive pathogens in head blight while F. pseudograminearum shows enhanced fitness as the pathogen of crown rot. This has been attributed to the differential production of DON in the different tissues (stem base vs. wheat heads; Tunali et al., 2012). In the early stages of the hemibiotrophic lifestyle of the pathogen F. graminearum, DON has been demonstrated to inhibit apoptosis-like programmed cell death in Arabidospis thaliana (Diamond et al., 2013). This suggests that mycotoxins may play a vital role in modulating host defense responses. Upon colonization and establishment of an intracellular hyphal network, DON is specifically induced during wheat spike colonization by F. graminearum (Voigt et al., 2007). A hypothetical model for the role of DON in establishment of infection by F. graminearum has been proposed (Audenaert et al., 2013). An intimate cross-kingdom interaction between Burkholderia glumae, a seed-borne bacterium and F. graminearum has been recently identified. Co-cultivation of the two microbes resulted in an increase in sporulation and DON production in F. graminearum, which is at least partially in response to a toxic bacterial metabolite. An overall increase in disease severity was observed upon co-infection of rice with the two pathogens. The two microbes were also found to be physically attached upon microscopic observations after co-cultivation (Jung et al., 2018). These instances highlight the strong correlation between mycotoxin production and virulence/fitness of Fusarium spp. It is necessary to note here that microbial interactions can also reduce the virulence of Fusarium species on their plant hosts, as discussed in section Microbial Interactions in Detoxification and Degradation of Mycotoxins.
How do Microbial Interactions Modulate Mycotoxin Levels?
Regulation of Epigenetic Modifiers During Bacterial-Fungal Interactions
Epigenetics has been steadily gaining momentum in the last few decades in the world of transcriptional regulation. There is now growing evidence that microbial communication regulates epigenetic modifiers that in turn control mycotoxin biosynthesis. The SAGA complex, conserved across eukaryotes, induces transcription of genes by mediating histone acetylation of the corresponding promoters. A study that isolated the bacterium, Pseudomonas piscium, from the wheat head microbiome has shown that the bacterium secretes an antifungal agent, phenazine, against F. graminearum. Phenazine, upon entering the fungal cell, inhibits the histone acetyl transferase module of the SAGA complex which subsequently leads to an inhibition of fungal growth and pathogenicity in addition to a complete suppression of DON biosynthesis (Chen et al., 2018). Another similar instance has been reported in Aspergillus nidulans—Streptomyces rapamycinicus association where the bacterium induces histone modification mediated by the SAGA complex which results in production of orsellinic acid and its derivatives by the fungus (Nutzmann et al., 2011). It is indeed fascinating that microbes have evolved such well-tuned, intricately regulated mechanisms of interaction.
Microbial Interactions in Detoxification and Degradation of Mycotoxins
The literature supports the idea that mycotoxins can be important players in shaping microbial communities and their interaction with hosts. Signaling molecules are regulated “coinage” and need to be recycled—through various chemical transformation processes—to maintain homeostasis in the community. Thus, it is not surprising that there are several examples of degradation of Fusarium mycotoxins mediated by microbes, plants and insects (Figure 1C).
Bacteria have been shown to contribute to reduction of Fusarium mycotoxin accumulation in grains. Preventative application of Psuedomonas fluorescens strain before inoculation with F. culmorum resulted in a significant reduction in Fusarium head blight as well as DON levels in infected wheat grains (Khan and Doohan, 2009). Endophytes belonging to Paenibacillus polymyxa, isolated from wild teosinte, have been shown to produce fusaridins which contribute to the antifungal activity against F. graminearum. Co-existence of these bacteria with F. graminearum in grains during storage at room temperature resulted in a significant decrease in DON accumulation (Mousa et al., 2015). A recent review summarizes the different bacteria and fungi that can degrade mycotoxins including zearalenone and DON (Vanhoutte et al., 2016).
Understanding the mechanisms underlying chemical transformation of mycotoxins could pave the way toward evolving novel techniques for mycotoxin decontamination in food and feed. Several mechanisms of detoxification of DON have been studied as summarized in Figure 1C. Aspergillus tubingenesis NJA-1, a soil isolate, has been shown to convert DON into a less-toxic product that has been speculated to be the result of hydrolysis, based on differences in the mass of the metabolites (He et al., 2008). Agrobacterium–Rhizobium strain E3-39 converts DON into 3-keto DON (Shima et al., 1997); Nocardioides WSN05-2 forms the non-toxic epimer, 3-epi-DON (Ikunaga et al., 2011), Deviosa insulae forms 3-keto-DON (Wang et al., 2019) and Devosia mutans 17-2-E-8 forms both 3-keto-DON and 3-epi-DON (He et al., 2015). A recent work has provided evidence that the formation of the epimer from zearalenone proceeds with 3-keto-DON as an intermediate (Hassan et al., 2017). Rumen-associated bacteria can inactivate DON by de-epoxidation since the epoxy group is vital for the toxicity of DON. Eggerthella spp., isolated from chicken intestine, has been reported to de-epoxify DON over a wide range of temperatures and pH (Gao et al., 2018). The gene Tri101, encoding 3-O-acetyltransferase for 3-O-acetylation of the trichothecene ring has been characterized in F. graminearum and has been further identified in other Fusarium species (Kimura et al., 1998; Khatibi et al., 2011). DON-glucosides have been reported to be formed as a result of plant metabolism (Sewald et al., 1992) as well as insect metabolism (De Zutter et al., 2016). In Arabidopsis, it has been reported that the UDP-glycosyltransferase catalyzes transfer of glucose from UDP-glucose to the hydroxyl group at the 3-C position in DON (Poppenberger et al., 2003). Whether acetylation and glycosylation can be considered detoxification is subject to debate since these forms can be hydrolyzed to regenerate the toxins in the animal gut (Ji et al., 2016).
Zearalenone mimics estrogen upon ingestion in animals and humans resulting in sexual and reproductive abnormalities. Microbes possess the ability to degrade and inactivate zearalenone, as reviewed in Ji et al. (2016). Clonostachys rosea has been reported to produce a zearalenone-specific lactonase that catalyzes the hydrolysis of the lactone ring, which is followed by spontaneous decarboxylation (Utermark and Karlovsky, 2007). This has been demonstrated o be responsible for the resistance of C. rosea to zearalenone. Trichosporon mycotoxinivorans has been shown to convert zearalenone into ZOM-1 which is characterized by the opening of the ring structure at the ketone group positioned at C6' (Vekiru et al., 2010). Further, ZOM-1 has been shown to have lost the estrogenic activity (Vekiru et al., 2010). Rhizopus arrhizus catalyzes sulfation of the hydroxyl group at the C4 position resulting in the formation of zearalenone-4-O-sulfate conjugate (el-Sharkaway et al., 1991).
Species of Pseudomonas (Altalhi, 2007), Bacillus (Cho et al., 2010; Yi et al., 2011; Hsu et al., 2018), Rhodoccous, and Streptomyces (De Mets et al., 2018) have been reported to degrade zearalenone. Degradation may not always result in detoxification. Acinetobacter has been shown to secrete extracellular enzymes that oxidize zearalenone into smaller estrogenic products (Yu et al., 2011). Interestingly, a mixed culture of bacteria enriched from a coal gasification site completely degraded zearalenone but lost the capability upon purification (Megharaj et al., 1997). Although reports of degradation have emerged, the degradation products as well as biochemical and genetic mechanisms underlying these processes remain unclear. El-Nezami et al. have shown that no degradation products were observed upon culturing Lactobacillus strains with zearalenone although the bacteria removed the mycotoxin from the cultures. The authors were able to recover zearalenone from the bacterial cultures and suggest that the bacteria bind zearalenone in a density-dependent manner (El-Nezami et al., 2002). Lysinibacillus sp. isolated from chicken intestine can remove zearalenone from cultures and the process has been shown to be significantly reduced upon heat treatment. The authors suggest a potential enzymatic process that may be involved in the interaction between the bacterium and zearalenone (Wang et al., 2018). Pseudomonas putida ZEA-1 utilizes zearalenone as a carbon source (Altalhi, 2007).
Burkholderia ambifaria, a novel bacterium isolated from barley rhizosphere has been reported to be able to utilize fusaric acid as a sole carbon source (Simonetti et al., 2018). Other examples of detoxification include conversion of fusaric acid to—fusarinol by Aspergillus tubingensis (Crutcher et al., 2014), 4-butyl-2-carboxy-pyrimidine by Colletotrichum sp (Fakhouri et al., 2003), and hydroxyfusaric acid by Mucor rouxii (Crutcher et al., 2017).
A significant understanding of the biochemical pathways involved in detoxification processes (Carere et al., 2018) along with the biotechnological advancements may pave the path toward novel detoxification methodologies that are feasible and economical.
Concluding Statement
The mycotoxigenic fungal species live in complex and nutrient-deficient environments—be it in soil, plant or animal hosts. The soil micro-environment often fluctuates with variations in water availability, air, light, and temperature, among other abiotic factors. Now add to this, a complex cocktail of microbes and hosts that are integral to the environment where survival of microbes heavily depends on active community participation. Mycotoxins play a significant role in the defensive strategies of mycotoxigenic fungi against the resident microbes. Interactions between microbes in such environments may involve competition or compromise where mycotoxins may serve as essential chemical language mediating communication. The host environments are usually unfriendly, thus requiring special adaptations in order for the fungi to thrive in such conditions. Several studies support a view that mycotoxins may act as signaling molecules that modulate host responses and promote successful colonization. Reports of microbes that can metabolize and detoxify mycotoxins are aplenty, highlighting the importance of examining microbial interactions to uncover strategies for mycotoxin detoxification.
In this review chapter, we have summarized existing literature that accentuate the ecological significance of mycotoxins with focus on Fusarium spp. The evolving knowledge on molecular and genetic mechanisms that govern mycotoxin production provides us with valuable tools to study the ecological roles of mycotoxins. This is not only an achievable goal but also has the potential to be highly rewarding. Such knowledge can facilitate development of novel strategies to control infections of mycotoxigenic fungi as well as mycotoxin contamination in food and feed.
Author Contributions
NV along with NK conceptualized and drafted the theme of the review. NV wrote the article and NK reviewed, edited, and refined the manuscript along with NV.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by in part by support by the National Institute of Food and Agriculture, United States Department of Agriculture, Hatch project 1012878 to NK and part by R01GM112739-01 to NK.
References
- Alassane-Kpembi I., Kolf-Clauw M., Gauthier T., Abrami R., Abiola F. A., Oswald I. P., et al. (2013). New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 272, 191–198. 10.1016/J.TAAP.2013.05.023 [DOI] [PubMed] [Google Scholar]
- Altalhi A. D. (2007). Plasmid-mediated detoxification of mycotoxin zearalenone in Pseudomonas Sp. ZEA-1. Am. J. Biochem. Biotechnol. 3, 150–158. 10.3844/ajbbsp.2007.150.158 [DOI] [Google Scholar]
- Audenaert K., Vanheule A., Höfte M., Haesaert G. (2013). Deoxynivalenol: a major player in the multifaceted response of fusarium to its environment. Toxins 6, 1–19. 10.3390/toxins6010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C. W., Hinton D. M., Hinton A. (2006). Growth-inhibiting effects of concentrations of fusaric acid on the growth of Bacillus mojavensis and other biocontrol Bacillus species. J. Appl. Microbiol. 100, 185–194. 10.1111/j.1365-2672.2005.02770.x [DOI] [PubMed] [Google Scholar]
- Bacon C. W., Hinton D. M., Mitchell T. R. (2017). Is quorum signaling by mycotoxins a new risk-mitigating strategy for bacterial biocontrol of Fusarium verticillioides and other endophytic fungal species? J. Agric. Food Chem. 65, 7071–7080. 10.1021/acs.jafc.6b03861 [DOI] [PubMed] [Google Scholar]
- Bao J., Wang J., Zhang X.-Y., Nong X.-H., Qi S.-H. (2017). New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 14:e1600327. 10.1002/cbdv.201600327 [DOI] [PubMed] [Google Scholar]
- Beukes I., Rose L. J., Shephard G. S., Flett C. F., Viljoen A. (2017). Mycotoxigenic Fusarium species associated with grain crops in South Africa – a review. S. Afr. J. Sci. 113:20160121 10.17159/sajs.2017/20160121 [DOI] [Google Scholar]
- Bottalico A., Perrone G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 108, 611–624. 10.1023/A:1020635214971 [DOI] [Google Scholar]
- Buranatragool K., Poapolathep S., Isariyodom S., Imsilp K., Klangkaew N., Poapolathep A. (2015). Dispositions and tissue residue of zearalenone and its metabolites α-zearalenol and β-zearalenol in broilers. Toxicol. Rep. 2, 351–356. 10.1016/J.TOXREP.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke L. T., Dixon D. J., Ley S. V., Rodríguez F. (2005). Total synthesis of the Fusarium toxin equisetin. Org. Biomol. Chem. 3, 274–280. 10.1039/B411350K [DOI] [PubMed] [Google Scholar]
- Carere J., Hassan Y. I., Lepp D., Zhou T. (2018). The enzymatic detoxification of the mycotoxin deoxynivalenol: identification of DepA from the DON epimerization pathway. Microb. Biotechnol. 11, 1106–1111. 10.1111/1751-7915.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wang J., Yang N., Wen Z., Sun X., Chai Y., et al. (2018). Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 9:3429. 10.1038/s41467-018-05683-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chérif M. (1990). Cytochemical aspects of chitin breakdown during the parasitic action of a Trichoderma sp. on Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 80:1406 10.1094/Phyto-80-1406 [DOI] [Google Scholar]
- Chilaka C., De Boevre M., Atanda O., De Saeger S., et al. (2017). The status of fusarium mycotoxins in sub-saharan Africa: a review of emerging trends and post-harvest mitigation strategies towards food control. Toxins 9:19. 10.3390/toxins9010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. J., Kang J. S., Cho W. T., Lee C. H., Ha J. K., Song K., Bin (2010). In vitro degradation of zearalenone by Bacillus subtilis. Biotechnol. Lett. 32, 1921–1924. 10.1007/s10529-010-0373-y [DOI] [PubMed] [Google Scholar]
- Combès A., Ndoye I., Bance C., Bruzaud J., Djediat C., Dupont J., et al. (2012). Chemical communication between the endophytic fungus paraconiothyrium variabile and the phytopathogen Fusarium oxysporum. PLoS ONE 7:e47313. 10.1371/journal.pone.0047313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher F. K., Liu J., Puckhaber L. S., Stipanovic R. D., Duke S. E., Bell A. A., et al. (2014). Conversion of fusaric acid to fusarinol by Aspergillus tubingensis: a detoxification reaction. J. Chem. Ecol. 40, 84–89. 10.1007/s10886-013-0370-4 [DOI] [PubMed] [Google Scholar]
- Crutcher F. K., Puckhaber L. S., Bell A. A., Liu J., Duke S. E., Stipanovic R. D., et al. (2017). Detoxification of fusaric acid by the soil microbe Mucor rouxii. J. Agric. Food Chem. 65, 4989–4992. 10.1021/acs.jafc.7b01655 [DOI] [PubMed] [Google Scholar]
- de la Cruz J., Pintor-Toro J.A., Benítez T., Llobel A. (1995). Purification and characterization of an endo-1,6-glucanase from trichoderma harzianum that is related to its mycoparasitism. 177, 1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mets L., Audenaert K., De Boevre M., Vidal Corominas A., Van Nieuwerburgh F., De Gelder L. (2018). Microbial degradation of zearalenone by Actinobacteria : mind the toxicity, in Eur. Fusarium Semin. 14th, Abstr. Available online at: https://biblio.ugent.be/publication/8560031 (Accessed November 3, 2018)
- De Zutter N., Audenaert K., Arroyo-Manzanares N., De Boevre M., Van Poucke C., De Saeger S., et al. (2016). Aphids transform and detoxify the mycotoxin deoxynivalenol via a type II biotransformation mechanism yet unknown in animals. Sci. Rep. 6:38640. 10.1038/srep38640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M., Reape T. J., Rocha O., Doyle S. M., Kacprzyk J., Doohan F. M., et al. (2013). The fusarium mycotoxin deoxynivalenol can inhibit plant apoptosis-like programmed cell death. PLoS ONE 8:e69542. 10.1371/journal.pone.0069542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drehe I., Simonetti E., Ruiz J. A. (2018). Contribution of the siderophores pyoverdine and enantio-pyochelin to fitness in soil of Pseudomonas protegens Pf-5. Curr. Microbiol. 75, 1560–1565. 10.1007/s00284-018-1560-7 [DOI] [PubMed] [Google Scholar]
- Eisenman H. C., Casadevall A. (2012). Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 93, 931–940. 10.1007/s00253-011-3777-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nezami H., Polychronaki N., Salminen S., Mykkänen H. (2002). Binding rather than metabolism may explain the interaction of two food-Grade Lactobacillus strains with zearalenone and its derivative (')alpha-earalenol. Appl. Environ. Microbiol. 68, 3545–3549. 10.1128/AEM.68.7.3545-3549.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkaway S. H., Selim M. I., Afifi M. S., Halaweish F. T. (1991). Microbial transformation of zearalenone to a zearalenone sulfate. Appl. Environ. Microbiol. 57, 549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri W., Walker F., Armbruster W., Buchenauer H. (2003). Detoxification of fusaric acid by a nonpathogenic Colletotrichum sp. Physiol. Mol. Plant Pathol. 63, 263–269. 10.1016/J.PMPP.2004.03.004 [DOI] [Google Scholar]
- Falade T. D. O., Syed Mohdhamdan S. H., Sultanbawa Y., Fletcher M. T., Harvey J. J. W., Chaliha M., et al. (2016). In vitro experimental environments lacking or containing soil disparately affect competition experiments of Aspergillus flavus and co-occurring fungi in maize grains. Food Addit. Contam. A 33, 1241–1253. 10.1080/19440049.2016.1198048 [DOI] [PubMed] [Google Scholar]
- Gao X., Mu P., Wen J., Sun Y., Chen Q., Deng Y. (2018). Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 112, 310–319. 10.1016/J.FCT.2017.12.066 [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C. A., Thiel P. G., Marasas W. F. O., Van der Merwe K. J. (1984). Natural occurrence of fusarin C, a mutagen produced by Fusarium moniliforme, in corn. J. Agric. Food Chem. 32, 1064–1067. 10.1021/jf00125a031 [DOI] [Google Scholar]
- Harris L. J., Alexander N. J., Saparno A., Blackwell B., McCormick S. P., Desjardins A. E., et al. (2007). A novel gene cluster in Fusarium graminearum contains a gene that contributes to butenolide synthesis. Fungal Genet. Biol. 44, 293–306. 10.1016/j.fgb.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Hassan Y. I., He J. W., Perilla N., Tang K., Karlovsky P., Zhou T. (2017). The enzymatic epimerization of deoxynivalenol by Devosia mutans proceeds through the formation of 3-keto-DON intermediate. Sci. Rep. 7:6929. 10.1038/s41598-017-07319-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Fan Y., Liu G., Zhang H., He C., Fan Y., et al. (2008). Isolation and identification of a strain of Aspergillus Tubingensis with deoxynivalenol biotransformation capability. Int. J. Mol. Sci. 9, 2366–2375. 10.3390/ijms9122366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. W., Bondy G. S., Zhou T., Caldwell D., Boland G. J., Scott P. M. (2015). Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 84, 250–259. 10.1016/J.FCT.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Herrmann M., Zocher R., Haese A. (1996). Effect of disruption of the enniatin synthetase gene on the virulence of Fusarium avenaceum. Mol. Plant. Microbe. Interact. 9, 226–232. [DOI] [PubMed] [Google Scholar]
- Hestbjerg H., Nielsen K. F., Thrane U., Elmholt S. (2002). Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar: an ecological interpretation. J. Agric. Food Chem. 50, 7593–7599. 10.1021/jf020432o [DOI] [PubMed] [Google Scholar]
- Holden M. T. G., Ram Chhabra S., De Nys R., Stead P., Bainton N. J., Hill P. J., et al. (2002). Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol. Microbiol. 33, 1254–1266. 10.1046/j.1365-2958.1999.01577.x [DOI] [PubMed] [Google Scholar]
- Hsu T.-C., Yi P.-J., Lee T.-Y., Liu J.-R. (2018). Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 13:e0194866. 10.1371/journal.pone.0194866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikunaga Y., Sato I., Grond S., Numaziri N., Yoshida S., Yamaya H., et al. (2011). Nocardioides sp. strain WSN05-2, isolated from a wheat field, degrades deoxynivalenol, producing the novel intermediate 3-epi-deoxynivalenol. Appl. Microbiol. Biotechnol. 89, 419–427. 10.1007/s00253-010-2857-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse van Rensburg D. F., Thiel P. G., Jaskiewicz K. (1987). Short-term effects of two fusarium toxins, diacetoxyscirpenol and neosolaniol monoacetate, in male Wistar rats. Food Chem. Toxicol. 25, 767–71. [DOI] [PubMed] [Google Scholar]
- Jestoi M. (2008). Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—a review. Crit. Rev. Food Sci. Nutr. 48, 21–49. 10.1080/10408390601062021 [DOI] [PubMed] [Google Scholar]
- Ji C., Fan Y., Zhao L. (2016). Review on biological degradation of mycotoxins. Anim. Nutr. 2, 127–133. 10.1016/J.ANINU.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers W., Rodriguez Estrada A. E., Lee K., Breakspear A., May G., Kistler H. C. (2012). Metabolome and transcriptome of the interaction between Ustilago maydis and Fusarium verticillioides in vitro. Appl. Environ. Microbiol. 78, 3656–3667. 10.1128/AEM.07841-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B., Park J., Kim N., Li T., Kim S., Bartley L. E., et al. (2018). Cooperative interactions between seed-borne bacterial and air-borne fungal pathogens on rice. Nat. Commun. 9:31. 10.1038/s41467-017-02430-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. R., Doohan F. M. (2009). Bacterium-mediated control of Fusarium head blight disease of wheat and barley and associated mycotoxin contamination of grain. Biol. Control 48, 42–47. 10.1016/J.BIOCONTROL.2008.08.015 [DOI] [Google Scholar]
- Khatibi P. A., Newmister S. A., Rayment I., McCormick S. P., Alexander N. J., Schmale D. G. (2011). Bioprospecting for trichothecene 3-O-acetyltransferases in the fungal genus Fusarium yields functional enzymes with different abilities to modify the mycotoxin deoxynivalenol. Appl. Environ. Microbiol. 77, 1162–1170. 10.1128/AEM.01738-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Liu Y., Benhamou R. I., Sanchez H., Simón-Soro Á., Li Y., et al. (2018). Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 12, 1427–1442. 10.1038/s41396-018-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Kaneko I., Komiyama M., Takatsuki A., Koshino H., Yoneyama K., et al. (1998). Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. J. Biol. Chem. 273, 1654–1661. 10.1074/JBC.273.3.1654 [DOI] [PubMed] [Google Scholar]
- Knutsen H. K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., et al. (2017). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 15:4718 10.2903/j.efsa.2017.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper-Goodman T., Scott P. M., Watanabe H. (1987). Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 7, 253–306. [DOI] [PubMed] [Google Scholar]
- Lautraite S., Parent-Massin D., Rio B., Hoellinger H. (1996). Comparison of toxicity induced by HT-2 toxin on human and rat granulo-monocytic progenitors with an in vitro model. Hum. Exp. Toxicol. 15, 208–213. 10.1177/096032719601500304 [DOI] [PubMed] [Google Scholar]
- Lee K., Pan J. J., May G. (2009). Endophytic Fusarium verticillioides â€freduces disease severity caused by Ustilago maydis on maize. FEMS Microbiol. Lett. 299, 31–37. 10.1111/j.1574-6968.2009.01719.x [DOI] [PubMed] [Google Scholar]
- Liaqat I., Bachmann R. T., Sabri A. N., Edyvean R. G. J. (2010). Isolate-specific effects of patulin, penicillic acid and EDTA on biofilm formation and growth of dental unit water line biofilm isolates. Curr. Microbiol. 61, 148–156. 10.1007/s00284-010-9591-8 [DOI] [PubMed] [Google Scholar]
- López-Díaz C., Rahjoo V., Sulyok M., Ghionna V., Martín-Vicente A., Capilla J., et al. (2018). Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 19, 440–453. 10.1111/mpp.12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M. P., Feichtinger G., Ve Défago G., Duffy B. (2003). Mycotoxigenic fusarium and deoxynivalenol production repress chitinase gene expression in the biocontrol agent trichoderma atroviride P1. Appl. Environ. Microbiol. 69, 3077–3084. 10.1128/AEM.69.6.3077-3084.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallebrera B., Prosperini A., Font G., Ruiz M. J. (2018). In vitro mechanisms of Beauvericin toxicity: a review. Food Chem. Toxicol. 111, 537–545. 10.1016/j.fct.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Martín-Rodríguez A., Reyes F., Martín J., Pérez-Yépez J., León-Barrios M., Couttolenc A., et al. (2014). Inhibition of bacterial quorum sensing by extracts from aquatic fungi: first report from marine endophytes. Mar. Drugs 12, 5503–5526. 10.3390/md12115503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayura K., Smith E. E., Clement B. A., Harvey R. B., Kubena L. F., Phillips T. D. (1987). Developmental toxicity of diacetoxyscirpenol in the mouse. Toxicology 45, 245–255. [DOI] [PubMed] [Google Scholar]
- Megharaj M., Garthwaite I., Thiele J. H. (1997). Total biodegradation of the oestrogenic mycotoxin zearalenone by a bacterial culture. Lett. Appl. Microbiol. 24, 329–333. 10.1046/j.1472-765X.1997.00053.x [DOI] [PubMed] [Google Scholar]
- Minerdi D., Bossi S., Maffei M. E., Gullino M. L., Garibaldi A. (2011). Fusarium oxysporum and its bacterial consortium promote lettuce growth and expansin A5 gene expression through microbial volatile organic compound (MVOC) emission. FEMS Microbiol. Ecol. 76, 342–351. 10.1111/j.1574-6941.2011.01051.x [DOI] [PubMed] [Google Scholar]
- Moretti A., Belisario A., Tafuri A., Ritieni A., Corazza L., Logrieco A. (2002). Production of beauvericin by different races of Fusarium oxysporum F. sp. Melonis, The Fusarium Wilt Agent of Muskmelon. Eur. J. Plant Pathol. 108, 661–666. 10.1023/A:1020674929084 [DOI] [Google Scholar]
- Mousa W. K., Shearer C. R., Limay-Rios V., Zhou T., Raizada M. N. (2015). Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 6:805. 10.3389/fpls.2015.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge A. M., Dill-Macky R., Dong Y., Gardiner D. M., White R. G., Manners J. M. (2006). A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 69, 73–85. 10.1016/J.PMPP.2007.01.003 [DOI] [Google Scholar]
- Müller M. E. H., Steier I., Köppen R., Siegel D., Proske M., Korn U., et al. (2012). Cocultivation of phytopathogenic Fusarium and Alternaria strains affects fungal growth and mycotoxin production. J. Appl. Microbiol. 113, 874–887. 10.1111/j.1365-2672.2012.05388.x [DOI] [PubMed] [Google Scholar]
- Notz R., Maurhofer M., Dubach H., Haas D., Défago G. (2002). Fusaric acid-producing strains of Fusarium oxysporum alter 2,4-diacetylphloroglucinol biosynthetic gene expression in Pseudomonas fluorescens CHA0 in vitro and in the rhizosphere of wheat. Appl. Environ. Microbiol. 68, 2229–2235. 10.1128/AEM.68.5.2229-2235.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutzmann H.-W., Reyes-Dominguez Y., Scherlach K., Schroeckh V., Horn F., Gacek A., et al. (2011). Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA. 108, 14282–14287. 10.1073/pnas.1103523108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ola A. R. B., Thomy D., Lai D., Brötz-Oesterhelt H., Proksch P. (2013). Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 76, 2094–2099. 10.1021/np400589h [DOI] [PubMed] [Google Scholar]
- Poppenberger B., Berthiller F., Lucyshyn D., Sieberer T., Schuhmacher R., Krska R., et al. (2003). Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 278, 47905–47914. 10.1074/jbc.M307552200 [DOI] [PubMed] [Google Scholar]
- Porter J. K., Bacon C. W., Wray E. M., Hagler W. M. (1995). Fusaric acid in Fusarium moniliforme cultures, corn, and feeds toxic to livestock and the neurochemical effects in the brain and pineal gland of rats. Nat. Toxins 3, 91–100. [DOI] [PubMed] [Google Scholar]
- Proctor R. H., Hohn T. M., McCormick S. P. (1995). Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant. Microbe. Interact. 8, 593–601. [DOI] [PubMed] [Google Scholar]
- Quecine M. C., Kidarsa T. A., Goebel N. C., Shaffer B. T., Henkels M. D., Zabriskie T. M., et al. (2016). An interspecies signaling system mediated by fusaric acid has parallel effects on antifungal metabolite production by Pseudomonas protegens strain Pf-5 and antibiosis of Fusarium spp. Appl. Environ. Microbiol. 82, 1372–1382. 10.1128/AEM.02574-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan S. K., Lee J.-H., Zhao Y., Lee J. (2018). The mycotoxin zearalenone hinders Candida albicans biofilm formation and hyphal morphogenesis. Indian J. Microbiol. 58, 19–27. 10.1007/s12088-017-0690-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz J. A., Bernar E. M., Jung K. (2015). Production of siderophores increases resistance to fusaric acid in Pseudomonas protegens Pf-5. PLoS ONE 10:e0117040. 10.1371/journal.pone.0117040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf D. H., Brakhage A. A., Mukherjee P. K. (2016). Gliotoxin - bane or boon? Environ. Microbiol. 18, 1096–1109. 10.1111/1462-2920.13080 [DOI] [PubMed] [Google Scholar]
- Schertz H., Dänicke S., Frahm J., Schatzmayr D., Dohnal I., Bichl G., et al. (2018). Biomarker evaluation and toxic effects of an acute oral and systemic fumonisin exposure of pigs with a special focus on dietary fumonisin esterase supplementation. Toxins 10:296. 10.3390/toxins10070296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Jager V., de Zühlke D., Wolff C., Bernhardt J., Cankar K., et al. (2017). Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 7:862. 10.1038/s41598-017-00893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schollenberger M., Drochner W., Müller H.-M. (2007). Fusarium toxins of the scirpentriol subgroup: a review. Mycopathologia 164, 101–118. 10.1007/s11046-007-9036-5 [DOI] [PubMed] [Google Scholar]
- Schroeckh V., Scherlach K., Nutzmann H.-W., Shelest E., Schmidt-Heck W., Schuemann J., et al. (2009). Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA. 106, 14558–14563. 10.1073/pnas.0901870106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewald N., von Gleissenthall J. L., Schuster M., Müller G., Aplin R. T. (1992). Structure elucidation of a plant metabolite of 4-desoxynivalenol. Tetrahedron Asymmetry 3, 953–960. 10.1016/S0957-4166(00)82193-X [DOI] [Google Scholar]
- Shi W., Tan Y., Wang S., Gardiner D. M., De Saeger S., Liao Y., et al. (2016). Mycotoxigenic potentials of fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins 9:9010006. 10.3390/toxins9010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima J., Takase S., Takahashi Y., Iwai Y., Fujimoto H., Yamazaki M., et al. (1997). Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl. Environ. Microbiol. 63, 3825–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti E., Roberts I. N., Montecchia M. S., Gutierrez-Boem F. H., Gomez F. M., Ruiz J. A. (2018). A novel Burkholderia ambifaria strain able to degrade the mycotoxin fusaric acid and to inhibit Fusarium spp. growth. Microbiol. Res. 206, 50–59. 10.1016/J.MICRES.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Sondergaard T. E., Hansen F. T., Purup S., Nielsen A. K., Bonefeld-Jørgensen E. C., Giese H., et al. (2011). Fusarin C acts like an estrogenic agonist and stimulates breast cancer cells in vitro. Toxicol. Lett. 205, 116–121. 10.1016/j.toxlet.2011.05.1029 [DOI] [PubMed] [Google Scholar]
- Spraker J. E., Sanchez L. M., Lowe T. M., Dorrestein P. C., Keller N. P. (2016). Ralstonia solanacearum lipopeptide induces chlamydospore development in fungi and facilitates bacterial entry into fungal tissues. ISME J. 10, 2317–2330. 10.1038/ismej.2016.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker J. E., Wiemann P., Baccile J. A., Venkatesh N., Schumacher J., Schroeder F. C., et al. (2018). Conserved responses in a war of small molecules between a plant-pathogenic bacterium and fungi. MBio 9:e00820-18. 10.1128/mBio.00820-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipanovic R. D., Puckhaber L. S., Liu J., Bell A. A. (2011). Phytotoxicity of fusaric acid and analogs to cotton. Toxicon 57, 176–178. 10.1016/j.toxicon.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Thrane U. (1986). Detection of toxigenic Fusarium isolates by thin layer chromatography. Lett. Appl. Microbiol. 3, 93–96. 10.1111/j.1472-765X.1986.tb01556.x [DOI] [Google Scholar]
- Thrane U. (1988). Screening for Fusarin C production by European isolates of Fusarium species. Mycotoxin Res. 4, 2–10. 10.1007/BF03192082 [DOI] [PubMed] [Google Scholar]
- Tunali B., Obanor F., Erginbaş G., Westecott R. A., Nicol J., Chakraborty S. (2012). Fitness of three Fusarium pathogens of wheat. FEMS Microbiol. Ecol. 81, 596–609. 10.1111/j.1574-6941.2012.01388.x [DOI] [PubMed] [Google Scholar]
- Utermark J., Karlovsky P. (2007). Role of zearalenone lactonase in protection of Gliocladium roseum from fungitoxic effects of the mycotoxin zearalenone. Appl. Environ. Microbiol. 73, 637–642. 10.1128/AEM.01440-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij E. T., Girard G., Lugtenberg B. J. J., Bloemberg G. V. (2005). Influence of fusaric acid on phenazine-1-carboxamide synthesis and gene expression of Pseudomonas chlororaphis strain PCL1391. Microbiology 151, 2805–2814. 10.1099/mic.0.28063-0 [DOI] [PubMed] [Google Scholar]
- Vanhoutte I., Audenaert K., De Gelder L. (2016). Biodegradation of mycotoxins: tales from known and unexplored worlds. Front. Microbiol. 7:561. 10.3389/fmicb.2016.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekiru E., Hametner C., Mitterbauer R., Rechthaler J., Adam G., Schatzmayr G., et al. (2010). Cleavage of zearalenone by Trichosporon mycotoxinivorans to a novel nonestrogenic metabolite. Appl. Environ. Microbiol. 76, 2353–2359. 10.1128/AEM.01438-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesonder R. F., Tjarks L. W., Rohwedder W. K., Burmeister H. R., Laugal J. A. (1979). Equisetin, an antibiotic from Fusarium equiseti NRRL 5537, identified as a derivative of N-methyl-2,4-pyrollidone. J. Antibiot. 32, 759–61. [DOI] [PubMed] [Google Scholar]
- Voigt C. A., Von Scheidt B., Gácser A., Kassner H., Lieberei R., Schäfer W., et al. (2007). Enhanced mycotoxin production of a lipase-deficient Fusarium graminearum mutant correlates to toxin-related gene expression. Eur. J. Plant Pathol. 117, 1–12. 10.1007/s10658-006-9063-y [DOI] [Google Scholar]
- Wang G., Wang Y., Ji F., Xu L., Yu M., Shi J., et al. (2019). Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 276, 436–442. 10.1016/J.FOODCHEM.2018.10.011 [DOI] [PubMed] [Google Scholar]
- Wang J., Lin W., Wray V., Lai D., Proksch P. (2013). Induced production of depsipeptides by co-culturing Fusarium tricinctum and Fusarium begoniae. Tetrahedron Lett. 54, 2492–2496. 10.1016/j.tetlet.2013.03.005 [DOI] [Google Scholar]
- Wang J. Q., Yang F., Yang P. L., Liu J., Lv Z. H. (2018). Microbial reduction of zearalenone by a new isolated Lysinibacillus sp. ZJ-2016-1. World Mycotoxin J. 2018, 1–8. 10.3920/WMJ2017.2264 [DOI] [Google Scholar]
- Wang Y.-M., Peng S.-Q., Zhou Q., Wang M.-W., Yan C.-H., Yang H.-Y., et al. (2006). Depletion of intracellular glutathione mediates butenolide-induced cytotoxicity in HepG2 cells. Toxicol. Lett. 164, 231–238. 10.1016/j.toxlet.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Wargo M. J., Hogan D. A. (2006). Fungal—bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 9, 359–364. 10.1016/J.MIB.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Weber J., Vaclavikova M., Wiesenberger G., Haider M., Hametner C., Fröhlich J., et al. (2018). Chemical synthesis of culmorin metabolites and their biologic role in culmorin and acetyl-culmorin treated wheat cells. Org. Biomol. Chem. 16, 2043–2048. 10.1039/C7OB02460F [DOI] [PubMed] [Google Scholar]
- Wheeler M. H., Stipanovic R. D., Puckhaber L. S. (1999). Phytotoxicity of equisetin and epi-equisetin isolated from Fusarium equiseti and F. pallidoroseum. Mycol. Res. 103, 967–973. 10.1017/S0953756298008119 [DOI] [Google Scholar]
- Yi P.-J., Pai C.-K., Liu J.-R. (2011). Isolation and characterization of a Bacillus licheniformis strain capable of degrading zearalenone. World J. Microbiol. Biotechnol. 27, 1035–1043. 10.1007/s11274-010-0548-7 [DOI] [Google Scholar]
- Yu Y., Qiu L., Wu H., Tang Y., Lai F., Yu Y. (2011). Oxidation of zearalenone by extracellular enzymes from Acinetobacter sp. SM04 into smaller estrogenic products. World J. Microbiol. Biotechnol. 27, 2675–2681. 10.1007/s11274-011-0741-3 [DOI] [Google Scholar]
- Zheng H., Kim J., Liew M., Yan J. K., Herrera O., Bok J. W., et al. (2015). Redox metabolites signal polymicrobial biofilm development via the NapA oxidative stress cascade in Aspergillus. Curr. Biol. 25, 29–37. 10.1016/J.CUB.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]