Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease associated with progressive loss of cognitive function, personality, and behavior. The present study evaluates neuronal and astroglial metabolic activity, and neurotransmitter cycle fluxes in AβPP-PS1 mouse model of AD by using 1H-[13C]-nuclear magnetic resonance (NMR) spectroscopy together with an infusion of either [1,6-13C2]glucose or [2-13C]acetate. The levels of N-acetyl-aspartate (NAA) and glutamate were found to be decreased in the cerebral cortex and hippocampus in AβPP-PS1 mice, when compared with wild type controls. The cerebral metabolic rate of acetate oxidation was increased in the hippocampus and cerebral cortex of AβPP-PS1 mice suggesting enhanced astroglial activity in AD. AβPP-PS1 mice exhibit severe reduction in glutamatergic and gamma-amino butyric acid (GABA)ergic neuronal metabolic activity and neurotransmitter cycling fluxes in the hippocampus, cerebral cortex, and striatum as compared with controls. These data suggest that metabolic activity of excitatory and inhibitory neurons is compromised across brain in AβPP-PS1 mouse model of AD.
Keywords: Gamma-amino butyric acid, glutamate, 13C nuclear magnetic resonance spectroscopy, neurodegeneration, neuronal–glial interaction
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder associated with gradual deterioration of cognitive functions, personality and memory.1 The cause and pathogenesis of the disease remains complex but has been shown to be associated with gray matter atrophy, disruption of neuronal function, and formation of neurofibrillary tangles and neuritic plaques in the medial temporal limbic regions and isocortex.2 The degradation of amyloid precursor protein (AβPP) is central to the pathological cascade that eventually leads to AD.3 Deficits in numerous neurotransmitters are suggested to build up with progression of the disease.3
Quantitative analyses have suggested loss of 25-35% synapses in the postmortem cortical tissue of AD patients.4 Several positron emission tomography studies have shown glucose hypo-metabolism across brain under mild cognitive impairment and in late onset AD patients.5 The reduced rates of neuronal glucose oxidation was also shown at an early age in AβPP-presenilin (PS1) mouse model of AD.6 Glutamate and gamma-amino butyric acid (GABA) are the major excitatory and inhibitory neurotransmitters, respectively, in the mature central nervous system.7 Majority of brain energy is utilized to sustain the processes associated with these neurotransmitters, which are involved in behavior, cognition, emotion and memory.8 Neurons and astroglia work in coordination for the trafficking of neurotransmitters, commonly known as glutamate-glutamine and GABA-glutamine neurotransmitter cycling, to maintain normal functioning of brain. It has been established that rates of neurotransmitter cycling and neuronal glucose oxidation are stochiometrically coupled.9,10 Moreover, the oxidative glucose metabolism and neurotransmitter cycle rates are shown to increase with brain activity. In vivo 13C nuclear magnetic resonance (NMR) spectroscopy and functional magnetic resonance imaging measurements have established relationships between neurometabolism and neuronal activity that provide novel insights into the nature of brain function.11 Therefore, neuronal glucose oxidation and neurotransmitter cycling are of prime importance for understanding brain activity and cognitive function. The perturbation in neuronal activity in AD is expected to affect neurotransmitter cycling and metabolic activity of neurons. Hence, a comprehensive understanding of neuronal and astroglial metabolic activities in AD condition is expected to provide better insight about the AD pathology.
AβPP-PS1 mice have been developed by inserting mutants of APP and presenilin at a single locus under the control of mouse prion promoter.12 These mice exhibit memory impairment and severe amyloid plaque loading, a hall mark of AD, in the cerebral cortex and hippocampus at the age of 12 months. The present study assessed the neuronal and astroglial metabolic activity, and neurotransmitter cycling in AβPP-PS1 mice at the age of 12 months by using 1H-[13C]-NMR spectroscopy in conjunction with infusion of [1,6-13C2]glucose or [2-13C]acetate. Our results indicate compromised neuronal metabolic activity, and enhanced astroglial activity in AD condition.
Materials and methods
All the experimental procedures with mice were approved by the Institutional Animals Ethics Committee of Centre for Cellular and Molecular Biology (CCMB), Hyderabad, India, and were conducted in accordance with the guidelines established by Committee for the Purpose of Control and Supervision on Experiments on Animals, Ministry of Environment and Forests, Government of India. ARRIVE guidelines were followed in the preparation of the manuscript. In order to get rid of the complication of hormonal cycling on the neurometabolism, only male mice has been used. Male AβPP-PS1 (n = 23) mice and wild-type littermates (n = 24) of age 12 months as confirmed by genotyping for the presence and absence of mutant AβPP and PS1 genes were used for the study.
Assessment of learning and memory in AβPP-PS1 mouse
Learning and memory of mice were evaluated using Morris Water Maze (MWM) test.13 MWM consists of a circular tank with height and diameter, 50 and 150 cm, respectively, and virtually divided into four equal quadrants with different clues provided on the wall for spatial map of the pool. The pool was filled with water to a depth of 30 cm, and an escape platform (10 cm in diameter) was submerged 0.5 cm under water level in the fourth quadrant. During the training period, mice were left in each quadrant for 90 s to explore the maize. The movement path of mice was video recorded and analyzed by the Ethovision software. Following this period, animals were guided towards the hidden platform that was placed in the fourth quadrant. The training was continued for four days from each quadrants. The memory was assessed on 7 and 8 day with and without the platform. The escape latency to reach the platform, and frequency of crossing over the platform zone were measured.
Infusion of [1,6-13C2]glucose and [2-13C]acetate
Metabolic study was carried out in overnight fasted mice. Animals were anesthetized with urethane (1.5 g/kg, i.p.), and the lateral tail vein was cannulated for administration of 13C labeled substrates. Urethane produces a long-lasting steady level of anesthesia with physiologic and pharmacologic behaviors similar to those observed in unanesthetized condition. The body temperature of animals was maintained at 37℃. [1,6-13C2]Glucose was administered for 10, 30, 60 and 90 min in mice using bolus variable infusion rate protocol.14 A bolus of 590 µmol/kg of labeled glucose (0.225 mol/L, dissolved in water) was administered in 15 s, there after the infusion rate was stepped down exponentially by decreasing the rate every 30 s. The infusion rate of [1,6-13C2]glucose at steady state (≥8.25 min) was 15 µmol/kg/min. At least four mice were used for each time point. In addition, mice were also infused with [2-13C]acetate + glucose for 15 min (WT, n = 6; AβPP-PS1, n = 5) and 90 min (WT, n = 4; AβPP-PS1, n = 4) to evaluate the astroglial metabolic activity and Vcyc/Vtca(n), respectively. The [2-13C]acetate (1 mol/L) was dissolved in water and pH adjusted to 7.0 using HCl. Initially, [2-13C]acetate was administered a bolus of 1.25 mmol/kg in 15 s. The acetate infusion rate was stepped down every 4 min, and the infusion rate at steady state (≥8 min) was maintained to 0.2 mmol/kg/min.15 Blood was collected from the retro-orbital sinus artery during the last minute of the experiment, and centrifuged to separate plasma. Blood plasma was stored at −80℃ for further analysis. At the end of the experiment, animal head was frozen in situ using liquid nitrogen.
Preparation of brain extracts
Frozen brain was chiseled out and dissected in a cryostat maintained at −20℃ to isolate the cerebral cortex, hippocampus, and striatum based on mouse brain atlas. The cortical region was separated as the outermost layer after removing the skull, and striatal region was identified as the sub-cortical structure posterior to the prefrontal region. Hippocampus appears as a wing-like structure, with completely different tissue contrast than neighboring tissues, is well distinguished, and easily dissected. Metabolites were extracted from frozen tissue as described previously.16 The frozen weighed tissues were ground with 0.1 N HCl in methanol (1:2 w/v) in a dry ice/ethanol bath. [2-13C]Glycine was added as an internal concentration reference. [2-13C]Glycine has been used as a concentration reference in several studies.14,15,17 The tissue powder was homogenized with ethanol, and clarified by centrifugation at 20,000 g. The tissue extracts were passed through Chelex column (Biorad), and pH adjusted to 7.0. The lyophilized extracts were dissolved in deuterium oxide containing sodium 3-trimethylsilyl[2,2,3,3-D4]-propionate (TSP) as a chemical shift reference.
Preparation and NMR analysis of blood plasma
Blood plasma was mixed with sodium phosphate buffer, which was prepared in deuterium oxide containing formate, and filtered using a 10-kD cut off centrifugal filter to remove macromolecules. The concentrations and fractional 13C enrichments of glucose and acetate were measured in 1H NMR spectrum of plasma using formate as an internal reference. The percent 13C labeling of glucose-C1α (5.2 ppm) and acetate-C2 (1.9 ppm) was calculated by dividing the intensity of the 13C with the total (12C + 13C).
NMR analysis of brain extract
1H-[13C]-NMR spectra of brain tissue extracts were recorded at 600 MHz NMR spectrometer (Bruker Biospin, Germany).18 The concentrations of metabolites were determined relative to [2-13C]glycine. The 13C atom percentage enrichment of various metabolites at different carbon positions was determined as the ratio of the peak areas in the 1H-[13C]-NMR difference spectrum (2 × 13C only) to the non-edited spectrum (12C + 13C), and was corrected for the natural abundance (1.1%).
Determination of acetate oxidation
Acetate is transported specifically into astrocytes by monocarboxylate transporters19 and oxidized therein.15 Oxidation of [2-13C]acetate in astrocytes labels astroglial glutamineC4, which in turn transfers 13C label into glutamateC4 and GABAC2 via corresponding neurotransmitter cycling.15 The cerebral metabolic rate of acetate oxidation (CMRAc(Ox)) was calculated as
| (1) |
where [Gln] and [Glu] are the concentration of glutamine and glutamate, respectively, while GlnC4 refers the 13C fractional enrichment of glutamine. GluC4(g) represents fractional enrichment of astroglial glutamate-C4, and was assumed to be same as the enrichment of glutamine-C4. ‘fGluA’, represents the fraction of glutamate in astroglia.14,17
Determination of Vcyc/VTCA from steady state [2-13C]acetate experiment
Steady state 13C labeling of amino acids from [2-13C]acetate was used to determine the ratios, Vcyc/VTCA(n) for glutamatergic and GABAergic neurons as described earlier in detail.14,17 The ratio Vcyc(Glu-Gln)/VTCA(Glu) was calculated as follows
| (2) |
where GlnC4 and GluC4 are the steady state labeling of astroglial glutamine-C4 and neuronal glutamate-C4, respectively. The 13C labeling of GluC4 and GlnC4 from [1-13C]/[6-13C]glucose that was formed from [2-13C]acetate was corrected by subtracting [3-13C]lactate labeling.
The ratio Vcyc(GABA-Gln)/VTCA(GABA) was determined as follows
| (3) |
where GABAC2 is the steady state 13C enrichments of [2-13C]GABA from [2-13C]acetate.
Determination of metabolic rates
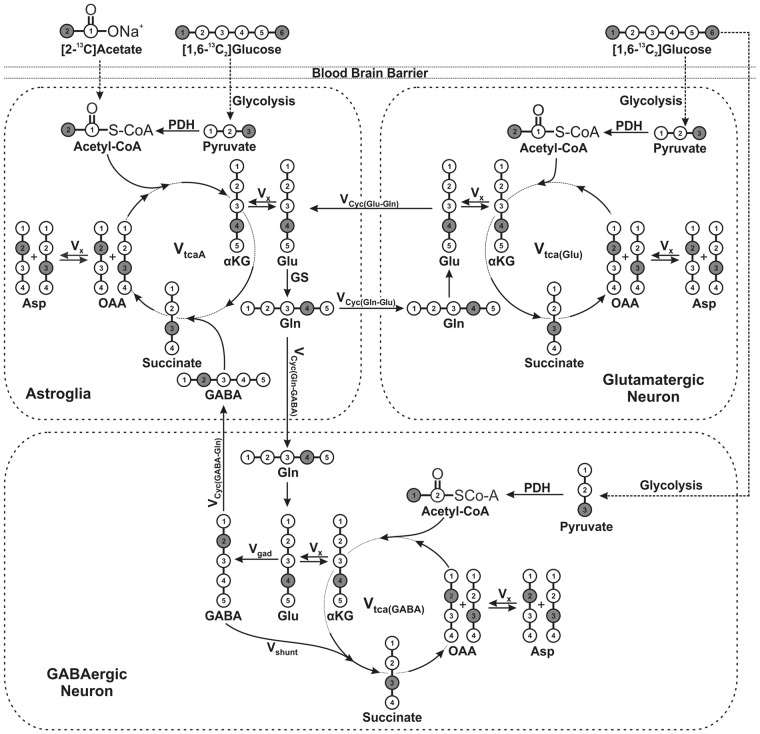
The 13C labeling of brain amino acids from [1,6-13C2]glucose was used to generate 13C turnover curve of amino acids. A three compartment metabolic model (Figure 1) was fitted to 13C turnover curves to determine metabolic rates.17 The metabolic model is based on series of mass and 13C isotope balance equations describing 13C labeling of brain metabolites from [1,6-13C2]glucose into neurons and astroglia14,17 using a CWave software package running in Matlab.20 The measured concentrations of aspartate, GABA, glutamate, and glutamine in brain tissue were used for metabolic modeling (Table S1). The glutamate pool was divided among GABAergic neurons (2%), astroglia (16%), and glutamatergic neurons (82%).14 Aspartate was distributed into astroglia, GABAergic neurons, and glutamatergic neurons in the proportion 16:42:42. The GABA and glutamine were assumed to be localized into GABAergic neurons and astrocytes, respectively. The ratios, Vcyc(Glu-Gln)/VTCA(Glu) and Vcyc(GABA-Gln)/VTCA(GABA) (Table 1) obtained from the steady state measurements from [2-13C]acetate were used as constraints during fitting of the model to 13C turnover curve of cerebral amino acids from [1,6-13C2]glucose. The Runge-Kutta algorithm was used to solve the differential equations, and the fitting was carried out using a Levenberg-Marquardt algorithm.21 The cerebral metabolic rates were determined from the best fit of the metabolic model to the 13C turnover curves of amino acids by using a simulated annealing algorithm. The fitted rates included glutamatergic TCA cycle (Vtca(Glu)), GABA shunt (Vshunt), net GABAergic TCA cycle flux (VTCA(GABA)Net), astroglial TCA cycle (Vtca(A)), and exchange rate between α-ketoglutarate-glutamate (Vx) and different dilution fluxes.
Figure 1.
A three compartment metabolic model depicting the 13C labeling of brain metabolites from [1,6-13C2]glucose and [2-13C]acetate.17 Metabolism of [1,6-13C2]glucose via neuronal TCA cycle labels GluC4, which is converted to GABAC2 by enzyme GAD in GABAergic neurons. Labeling of GlnC4 occurs from GluC4 and GABAC2 via glutamate–glutamine and GABA–glutamine cycle, respectively. [2-13C]Acetate is selectively transported and metabolized in astroglia and labels GlnC4. Neuronal GluC4 and GABAC2 are labeled from GlnC4 via glutamine-glutamate and glutamine-GABA cycling, respectively. α-KG: α-ketoglutarate; OAA: oxaloacetate; Asp: aspartate; GABA: γ-aminobutyric acid; Glu: glutamate; GAD: glutamate decarboxylase; Gln: glutamine; Vcyc(GABA-Gln): GABA-glutamine cycling flux; Vcyc(Glu-Gln): glutamate-glutamine cycling flux; Vgad: glutamate decarboxylase flux; Vgln: glutamine synthesis rate; Vshunt: flux of GABA shunt; Vtca(A): astroglial TCA cycle flux; Vtca(GABA): GABAergic TCA cycle flux; Vtca(Glu): glutamatergic TCA cycle flux; Vx: exchange rate between α-ketoglutarate and glutamate. The filled circle represents the position of 13C carbon.
Table 1.
Percent 13C labeling of brain amino acids from [2-13C]acetate in AβPP-PS1 and wild-type (WT) mice.
| Brain region | Infusion time (min) | Groups | Amino acids |
CMRAc(ox) (µmol/g/min) |
Vcyc/VTCA(N) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| GluC4 | GABAC2 | GlnC4 | AspC3 | GluC3 | Glu’ergic | GABAergic | ||||
| Cortex | 15 | WT | 3.8 ± 0.2 | 3.2 ± 0.7 | 10.4 ± 0.8 | 2.3 ± 0.2 | 0.9 ± 0.2 | 0.051 ± 0.005 | – | – |
| AβPP-PS1 | 3.9 ± 0.1 | 3.4 ± 0.9 | 11.9 ± 1.4 | 2.4 ± 0.5 | 0.9 ± 0.2 | 0.064 ± 0.007* | – | – | ||
| 90 | WT | 8.3 ± 0.8 | 7.4 ± 1.0 | 17.0 ± 1.2 | 6.9 ± 0.6 | 7.6 ± 2.4 | – | 0.43 ± 0.14 | 0.51 ± 0.14 | |
| AβPP-PS1 | 9.3 ± 2.2 | 7.7 ± 1.8 | 19.0 ± 3.0 | 7.4 ± 2.8 | 6.5 ± 3.7 | – | 0.44 ± 0.06 | 0.60 ± 0.08 | ||
| Hippocampus | 15 | WT | 3.9 ± 0.3 | 2.4 ± 0.4 | 12.5 ± 1.0 | ND | ND | 0.051 ± 0.003 | – | – |
| AβPP-PS1 | 3.7 ± 0.4 | 2.2 ± 0.8 | 13.2 ± 0.5 | ND | ND | 0.056 ± 0.004* | – | – | ||
| 90 | WT | 7.3 ± 1.6 | 6.8 ± 1.7 | 16.3 ± 2.1 | ND | ND | – | 0.39 ± 0.14 | 0.50 ± 0.11 | |
| AβPP-PS1 | 7.6 ± 0.9 | 6.8 ± 0.7 | 17.7 ± 0.9 | ND | ND | – | 0.48 ± 0.13 | 0.64 ± 0.16 | ||
| Striatum | 15 | WT | 4.2 ± 0.1 | 2.2 ± 0.1 | 12.2 ± 1.3 | ND | ND | 0.051 ± 0.007 | – | – |
| AβPP-PS1 | 3.5 ± 0.7 | 1.5 ± 0.4 | 10.7 ± 1.6 | ND | ND | 0.051 ± 0.003 | – | – | ||
| 90 | WT | 7.5 ± 1.7 | 6.2 ± 1.6 | 15.5 ± 3.2 | ND | ND | – | 0.38 ± 0.04 | 0.46 ± 0.12 | |
| AβPP-PS1 | 8.7 ± 2.0 | 7.3 ± 2.1 | 18.6 ± 3.3 | ND | ND | – | 0.44 ± 0.06 | 0.49 ± 0.08 | ||
ND: not determined.
Mice were administered [2-13C]acetate for 15 and 90 min. The 13C labeling of amino acids was measured in brain tissue extracts using 1H-[13C]-NMR spectroscopy. CMRAc(ox) and Vcyc/VTCA(N) were calculated using equations (1) to (3). Values represent mean ± SEM. *p< 0.05 when compared with respective controls.
Statistics
The Data Analysis Tool package of Microsoft Excel 2007 was used for the Statistical analysis. Analysis of variance (ANOVA) analysis was carried out to determine the significance of differences in amino acids labeling between AβPP-PS1 and wild-type mice within same brain region. One-way ANOVA was carried out to find the significance of differences in the concentrations and metabolic rates among different groups. The post hoc Tukey honest test was performed to identify the significance of difference between groups.
Results
Learning and memory in AβPP-PS1 mice
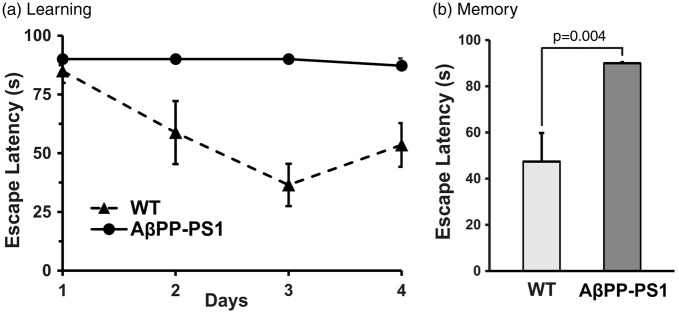
Learning and memory in mice were assessed using MWM test. Wild type (WT) mice showed a good learning pattern during four days of training but AβPP-PS1 mice could not locate the platform even after four days of intense training (Figure 2(a)). Memory tests carried out two days after the training, revealed that WT mice had good memory retention, and could locate the platform in 47 ± 11 s. In contrast, the escape latency in AβPP-PS1 mice was more than 90 s suggesting impaired memory in AD mice (Figure 2(b)).
Figure 2.
Learning and memory in AβPP-PS1 and control mice. Learning and memory were evaluated using Morris Water Maze test. (a) Learning curve of AβPP-PS1 and wild type mice during training period. (b) The memory of AβPP-PS1 and control mice: The escape latency of mice to reach the platform was evaluated on the seventh day.
Level of neurochemicals in AβPP-PS1
Neurochemical profile obtained using ex vivo 1H NMR spectroscopy in different regions of brain indicated that the levels of neurometabolites were significantly perturbed in AβPP-PS1 mice when compared with age-matched WT controls. The levels of glutamate and aspartate were significantly (p < 0.05) lower in hippocampal and cortical regions in AβPP-PS1 mice (Table S1). Additionally, the level of N-acetyl-aspartate (NAA) was found to be decreased in hippocampal (AβPP-PS1 6.6 ± 0.2 µmol/g; WT 7.6 ± 0.2 µmol/g, p < 0.01) and cortical (AβPP-PS1 7.0 ± 0.1 µmol/g; WT 7.5 ± 0.2 µmol/g, p < 0.01) regions in AβPP-PS1 mice. Interestingly, there was significant increase in the level of myo-inositol in hippocampal (WT 7.3 ±0.2 µmol/g; AβPP-PS1 8.5 ± 0.2 µmol/g, p < 0.01) and cortical (AβPP-PS1 7.8 ± 0.2 µmol/g; WT 7.2 ±0.2 µmol/g, p < 0.01) regions in AβPP-PS1 mice. There were no significant changes in the levels of other metabolites in different brain regions in AβPP-PS1 mice (Supplementary Table S1).
Astroglial activity in AβPP-PS1 mice
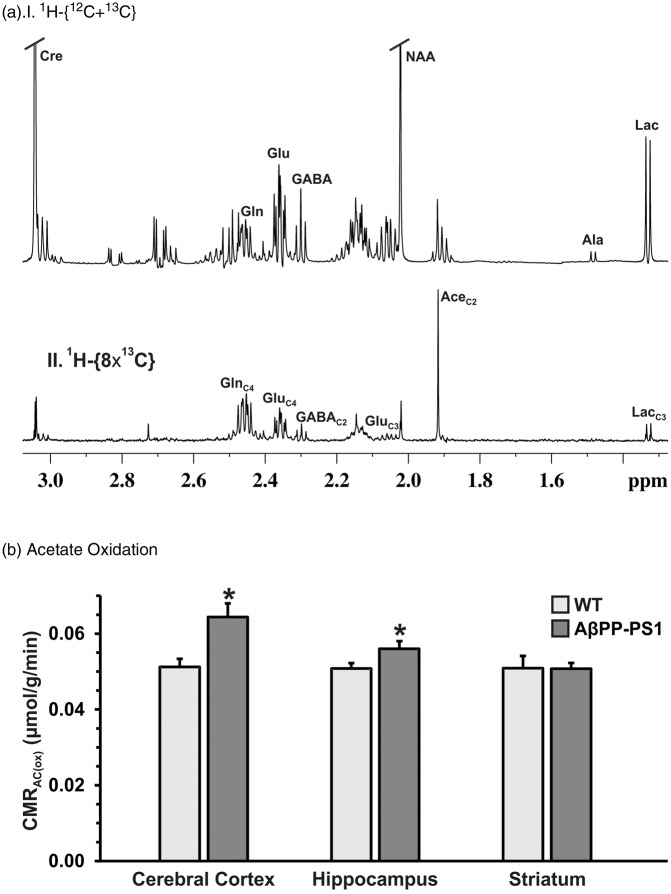
The astroglial metabolic activity was measured by following the 13C labeling of cerebral amino acids from a 15-min infusion of [2-13C]acetate in mice (Figure 3(a)). The concentration of GlnC4 was found to be increased in the hippocampal (0.65 ± 0.06 vs. 0.57 ± 0.03 µmol/g, p < 0.01) and cortical (0.71 ± 0.08 vs. 0.54 ± 0.05 µmol/g, p < 0.01) regions in AβPP-PS1 mice when compared with WT controls. The striatal region did not exhibit significant change in the GlnC4 labeling from [2-13C]acetate (0.57 ± 0.04 vs. 0.55 ± 0.08, p > 0.05). The increased 13C labeling of GlnC4 from [2-13C]acetate indicates enhanced astroglial metabolic activity in the hippocampus and cerebral cortex of AβPP-PS1 mice (Figure 3(b), Table 1).
Figure 3.
(a) 1H-[13C]-NMR spectrum depicting 13C labeling of hippocampal amino acids from [2-13C]acetate in AβPP-PS1 mice. Mice were infused with [2-13C]acetate for 15 min. Metabolites were extracted from frozen hippocampal tissue. 1H-[13C]-NMR spectra were recorded in hippocampal extracts. (b) The cerebral metabolic rate of acetate oxidation (CMRAc(ox)) in AβPP-PS1 and control mice. The 13C labeling of brain amino acids were measured using 1H-[13C]-NMR spectroscopy in tissue extracts. The CMRAc(ox) was calculated using equation (1). *p < 0.5 when compared with the respective controls.
Ratio Vcyc/Vtca in AβPP-PS1 mice
For the measurement of ratio of neurotransmitter cycle to TCA cycle flux, an infusion of [2-13C]acetate was carried out in mice for 90 min so that 13C labeling of brain amino acids have attained an isotopic steady state. No significant change (p > 0.1) in the steady state labeling of GlnC4, GluC4 and GABAC2 from [2-13C]acetate was observed between AβPP-PS1 and control mice (Table 1). The ratio of neurotransmitter cycle flux to corresponding TCA cycle of different neurons was calculated using equations (2) and (3), respectively. The ratios, Vcyc(Glu-Gln)/VTCA(Glu) and Vcyc(GABA-Gln)/VTCA(GABA), in AβPP-PS1 mice were not significantly (p > 0.16) different when compared with controls (Table 1).
Labeling of brain amino acids from [1,6-13C2]glucose
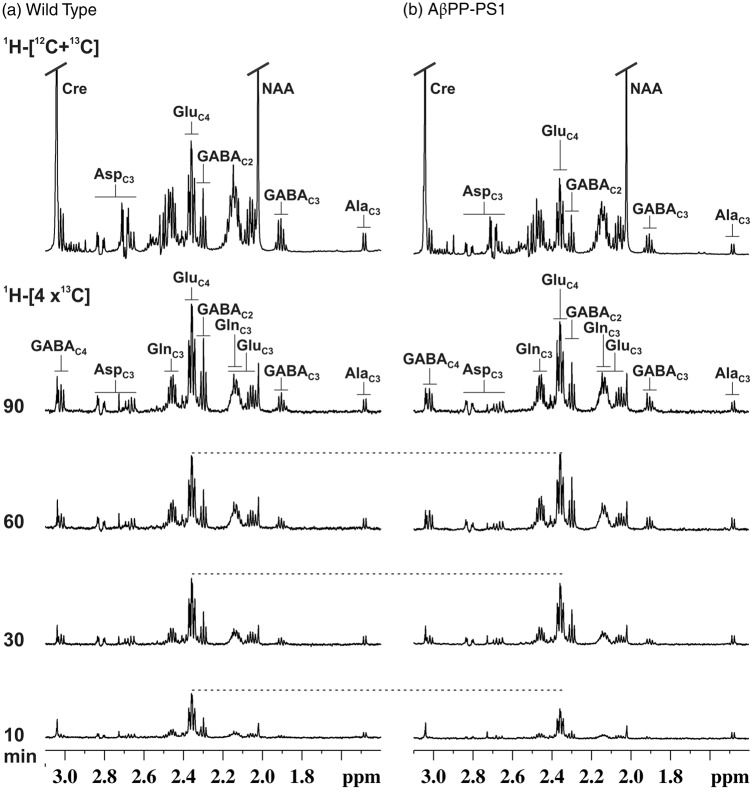
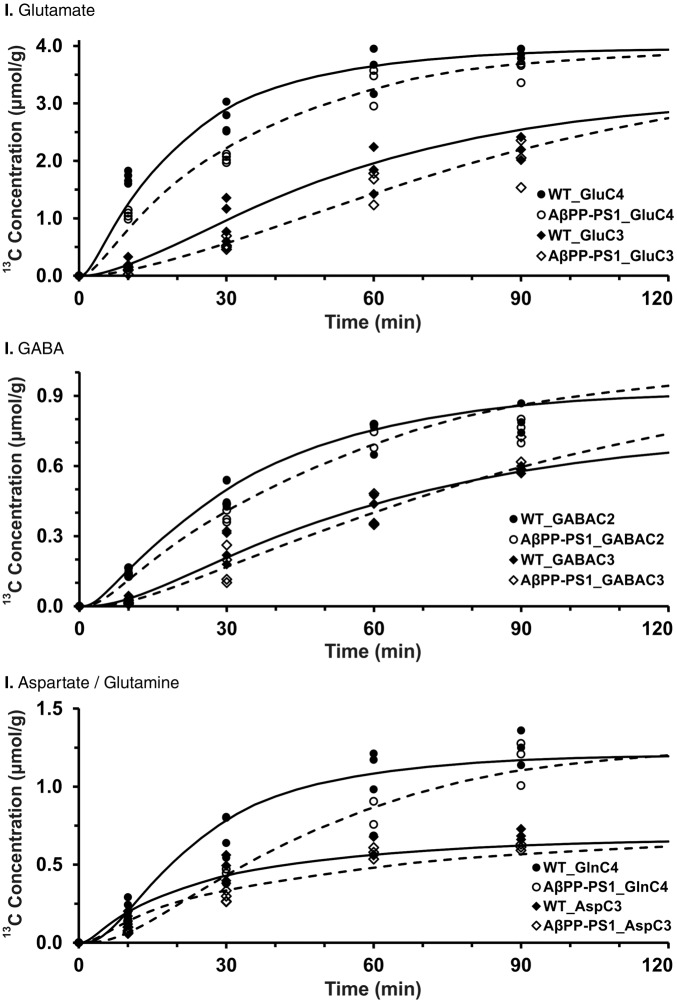
To derive the 13C turnover of brain amino acids, [1,6-13C2]glucose was infused for different time points ranging from 10 to 90 min, and percent 13C enrichment of brain amino acids was measured in tissue extracts using 1H-[13C]-NMR spectroscopy. The 1H-[13C]-NMR spectral time course depicting the 13C labeling of hippocampal metabolites in control mice is presented in Figure 4(a). The 13C labeled GluC4 and GABAC2 could be seen at the early infusion time (10 min). Additionally, resonances from GluC3, AspC3, GABAC3, and GABAC4 which are labeled in the second turn of TCA cycle could be seen in the spectra recorded at 30 min onward. A similar pattern was seen for the AβPP-PS1 mice (Figure 4(b)). However, the intensity of 13C labeled signal at earlier time points (10 and 30 min) is lower in AβPP-PS1 mice when compared with controls, suggesting a slower turnover of metabolites in transgenic mice (Figure 4). The 13C turnover curves of amino acids from [1,6-13C2]glucose were created by plotting the percent 13C labeling of amino acids with time (Figure 5), and were used for metabolic flux analysis.
Figure 4.
Representative 1H-[13C]-NMR spectra of hippocampal tissue extracts in (a) Wild type, and (b) AβPP-PS1 mice. [1,6-13C2]Glucose was administered in mice for pre-defined time, and 1H-[13C]-NMR spectra were recorded from hippocampal tissue extracts. The top spectrum was used for the quantification of concentrations of metabolites, while lower spectra (1H-[4x13C]) were used to determine the 13C turnover of brain amino acids. Peaks labeling are: AlaC3, alanine-C3; AspC3, asparate-C3; GABAC2, GABA-C2; GlnC4, glutamine-C4; GluC4, glutamate-C4.
Figure 5.
Fit of a three-compartment metabolic model to 13C turnover of hippocampal amino acids in (I) glutamate, (II) GABA, and (III) glutamine/aspartate. [1,6-13C2]Glucose was administered in mice for pre-defined time. 13C concentrations of amino acids were measured in hippocampal extracts in 1H-[13C]-NMR spectra. Symbols depict to the measured labeling, while lines represent the best fit of the metabolic model to the measured values.
Glutamatergic and GABAergic fluxes in AβPP-PS1 mice brain
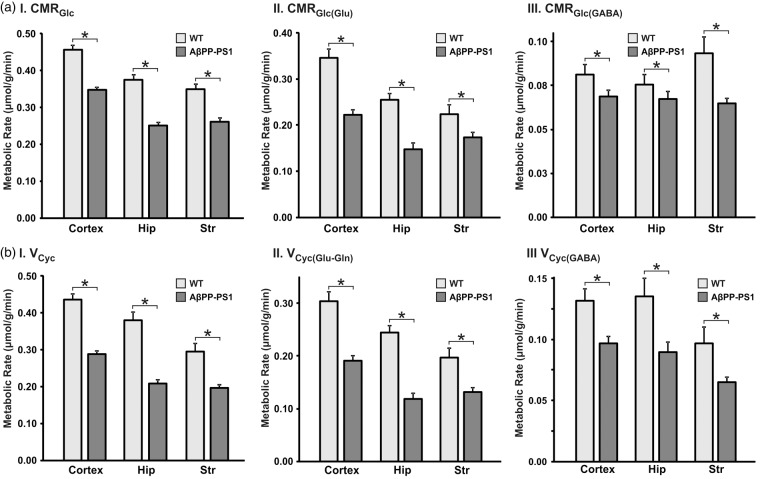
The metabolic fluxes associated with glutamatergic and GABAergic neurons were obtained by analysis of 13C labeling of amino acids from [1,6-13C2]glucose. The best fit of the metabolic model to the hippocampus data is depicted in Figure 5. The rates of neuronal glucose oxidation (CMRGlc(Ox)) and neurotransmitter cycle (Vcyc) thus obtained in different brain regions in AβPP-PS1 and age-matched controls are presented in Figure 6.
Figure 6.
Metabolic rates in AβPP-PS1 and WT mice. (a.I) Cerebral metabolic rate of glucose oxidation; (a.II) Rate of glucose oxidation in glutamatergic neurons; (a.III) rate of glucose oxidation in GABAergic TCA cycle; (b.I) neurotransmitter cycle; (b.II) glutamate-glutamine cycle; (b.III) GABA-glutamine cycle. Metabolic rates were determined by fitting a three compartment model to the measured 13C turnover of amino acids from [1,6-13C2]glucose. Values represent mean ± SEM. *p < 0.001.
Hippocampus
The cerebral metabolic rates of neuronal glucose oxidation (CMRGlc) and neurotransmitter cycle was significantly lower (p < 0.01) in the hippocampus of AβPP-PS1 mice when compared with WT controls (Figure 6). The rate of glucose oxidation by glutamatergic neurons (CMRGlc(Glu)) was found to be reduced significantly (p < 0.001) in hippocampus (0.148 ± 0.003 µmol/g/min) of AβPP-PS1 mice when compared with controls (0.255 ± 0.005 µmol/g/min) (Figure 6(a)II). Likewise, the glutamate–glutamine neurotransmitter cycling flux was found to be reduced in AβPP-PS1 mice (control: 0.245 ± 0.005; AβPP-PS1: 0.118 ± 0.002 µmol/g/min, p < 0.001) (Figure 6(b)II). The rate of glucose oxidation by GABAergic neurons (CMRGlc(GABA)) was found to be reduced by 17% (0.086 ± 0.002 vs. 0.106 ± 0.002 µmol/g/min, p < 0.001) (Figure 6(a)III). Similar to glutamatergic neurotransmission, the GABA-glutamine neurotransmitter cycle flux was found to be decreased in AβPP-PS1 mice (0.090 ± 0.002 vs. 0.135 ± 0.003 mol/g/min, p < 0.001) (Figure 6(b)III).
Cerebral cortex
The CMRGlc(Glu) was found to be reduced by 36% (control: 0.346 ± 0.007; AβPP-PS1: 0.223 ± 0.002 µmol/g/min, p < 0.001), while CMRGlc(GABA) exhibited a reduction of only 18% (control 0.110 ± 0.002; AβPP-PS1 0.090 ± 0.002 µmol/g/min, p < 0.01). Additionally, the rates of glutamate–glutamine (0.191 ± 0.002 vs. 0.305 ± 0.003 µmol/g/min) and GABA-glutamine (0.097 ± 0.001 vs. 0.132 ±0.002 µmol/g/min) were reduced significantly in AβPP-PS1 mice when compared with WT controls (Figure 6(b)II/III).
Striatum
Similar to the hippocampus and cerebral cortex, the CMRGlc(ox) of glutamatergic neurons was found to be decreased in the striatum of AβPP-PS1 mice (0.173 ± 0.002, p < 0.01, −23%) when compared with WT controls (0.224 ± 0.006 µmol/g/min). The glutamate–glutamine neurotransmitter cycle rate was also found to be reduced (−33%) in AβPP-PS1 mice (0.132 ± 0.002 vs. 0.197 ± 0.004 µmol/g/min). Similarly, CMRGlc(GABA) was reduced ( − 36%) in AβPP-PS1 (0.063 ± 0.001 vs. 0.099 ± 0.002 µmol/g/min) as compared with controls (Figure 6(a)III). Moreover, GABA-glutamine neurotransmitter cycle flux was reduced (−33%) in AβPP-PS1 (0.065 ± 0.001 µmol/g/min) mice when compared with age matched controls (0.097 ± 0.002 µmol/g/min) (Figure 6(b)III).
These data indicate that AβPP-PS1 mice exhibit decreased excitatory and inhibitory neurotransmission across brain. It is noteworthy that reduction in glutamatergic fluxes was higher than GABAergic rates.
Discussion
The quantitative significance of brain energy metabolism in AD has not been explored. Present study evaluates for the first time, the excitatory and inhibitory neurotransmitter cycling flux, and energy demand of different neural cell types (neurons and astroglia) by using a three compartment metabolic model across brain at the stage of high plaque loading in AβPP-PS1 mice. Our analysis indicates that astroglial activity is increased while neuronal function is decreased in AD mice.
The current study involved the use of AβPP-PS1 mice for investigation of neuronal and astroglial metabolic rates in AD. The plaque pathology, memory and neurotransmission have been studied in details in these mice. There was no detectable plaque pathology observed in hippocampus at five months of age.22 There was no impairment in conditional learning in these mice at the age of five months. The electrophysiological studies have suggested impaired neuronal activity in these mice. The whole-cell patch clamp recording of CA3 neurons in AβPP-PS1 mice at the age of six months indicated reduction in the amplitude of excitatory post synaptic currents associated with glutamatergic neurons.23 Moreover, the long-term potentiation of excitatory post synaptic currents of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor was absent in AβPP-PS1 mice, further suggesting impairment of neurotransmission in these mice. The amyloid deposition was clearly visible in AβPP-PS1 mice during the age 6–9 months,24 and increased severely beyond 12 months. Moreover, our immunohistological examination using Aβ-antibody has suggested huge burden of plaques in the cerebral cortex and hippocampus in AβPP-PS1 mice at the age of 12 months (Supplementary Figure S1). The memory analysis using different paradigms has shown impairment in learning and memory at and beyond six months of age in AβPP-PS1 mice.25 Our analysis using MWM paradigm has also indicated reduction in the learning and memory in AβPP-PS1 mice at the age of 12 months. The 1H MRS analysis of neurochemical profile has indicated a reduction in the levels of N-acetyl aspartate and glutamate in these mice with advancing age.26 Moreover, an increase in the level of myo-inositol with age in AβPP-PS1 mice was also reported. These age-dependent changes in the levels of neurometabolites in AβPP-PS1 mice agree well with 1H MRS studies in human AD. These findings suggest that AβPP-PS1 mice closely mimic the pathology of human AD condition.
Neurochemical homeostasis in AD brain
Neurochemical profile has been investigated under AD condition in human27 as well as in animal models. These studies have shown reduction in level of NAA in different brain regions of AD patients.28 A recent study has also indicated decreased glutamate level in AD patients.27 Moreover, a reduction in the level of NAA has been reported at the age of 12 months in the hippocampus of AβPP-PS1 mice.26 The findings of decreased level of glutamate and NAA in cortical and hippocampal regions of AβPP-PS1 in the current study are in good accordance with these reports. The reduction in NAA level in the cerebral cortex and hippocampus is suggestive of reduced neuronal viability and/or density in AD brain. Hippocampus is known to be responsible for contextual and declarative memory. Glutamate is linked with long-term potentiation and memory,29 and NAA is considered to be the neuronal viability and density marker.30 Hence, reduction in the number of glutamatergic neurons in the hippocampus may be a plausible cause for the loss in memory in AD subjects. The level of myo-inositol has been shown to increase with age in AβPP-PS1 mice.26 Myo-inositol has been believed to be a glial marker.31 Our findings of increased level of myo-inositol in the cerebral cortex and hippocampus of AβPP-PS1 mice suggest gliosis like condition in the AD brain.
Neuronal and astroglial metabolic activity in AD
There are conflicting reports about cerebral metabolism in rodent models of AD. The 13C labeling of brain amino acids were reported to increase in three-month old P301L mice32 and in seven-month old triple transgenic mice suggesting hyper metabolic state at early stage of AD.33 However, metabolic analysis conducted at later age (13 months) suggested hypo-metabolism in triple transgenic mice.34 Our earlier measurements in AβPP-PS1 at pre-symptomatic stage (6 months) has revealed decreased metabolic rates of excitatory and inhibitory neurons.6 The data reported from the current study suggest the neuronal hypo-metabolism observed at pre-symptomatic stage persist at the stage of high plaque loading in AβPP-PS1 mice.
Traditionally, glucose metabolism in human subject has been measured using positron emission tomography in conjunction with administration of 18F-labeled fluorodeoxyglucose. This approach provides information about glucose phosphorylation / consumption in brain. A widespread reduction in local cerebral glucose consumption has been reported in the posterior cingulated, lateral temporo-parietal, and occipital cortices of AD patients.35 Additionally, hypo-glucose metabolism has also been observed in the posterior cingulate and temporo-parietal regions in mild cognitive impaired subjects.36 The cerebral metabolic rate of glucose utilization (CMRGlc) in the current study was found to be decreased in the hippocampus (−37%), cerebral cortex (−23%), and striatum (−29%) in AβPP-PS1 mice, and agrees very well with human AD studies. Analysis of pyruvate dehydrogenase flux at the cellular level suggested that the hypo-metabolism of glucose in AβPP-PS1 mice brain is due to reduced activity of both glutamatergic and GABAergic neurons across brain.
The stochiometric coupling between neuronal glucose oxidation and neurotransmitter cycling has been well established in healthy brain.9,10 Qualitative analysis of energy metabolism in mouse model of AD6,34 has shown a reduction in the labeling of GluC4/C3, GABAC2/C3, and GlnC4/C3 labeling from [1-13C]glucose/[1,6-13C2]glucose. These studies suggest that coupling between neurotransmitter cycling and neuronal glucose oxidation is also maintained under disease conditions. The findings of current study indicate that the flux through neurotransmitter and neuronal glucose oxidation are coupled in AD. Therefore, measurement of neuronal glucose oxidation may be used to assess the neurotransmitter cycling under various neurological conditions.
The reactive microglia are shown to be enhanced in AβPP-PS1 mice.37 Moreover, the degree of microglial activation increases with AD severity, and with Aβ plaque load.38 Though glia have been associated with neuro-inflammation, there is no quantitative report pertaining to the glial metabolic activity in AD condition. Acetate is specifically metabolized in astrocytes because monocarboxylate transporters, which are used to transport acetate from blood to brain, are exclusively localized on glial cells.19 Therefore, acetate has been used extensively to explore the astroglial metabolic activity in different neurological conditions.39 The astroglial activity was reported to be un-perturbed in a triple transgenic mouse model of AD,33 while it was reduced in rat model.40 Our findings of increased oxidation of acetate in cortical and hippocampal region in AβPP-PS1 mice as compared with controls (Figure 3(b)) suggest that the astroglial activity is increased in AD.
The astrolglial metabolic activity is used to sustain different cellular processes such as efflux of Na+ that is co-transported with glutamate into astrocytes, pyruvate carboxylation, and conversion of glutamate (plus GABA) to glutamine. Moreover, astrogliosis has been implicated in neuro-inflammatory condition. The observed metabolic activity of astroglia indicates the sum total of energy requirements of above mentioned fluxes. The pyruvate carboxylation has been shown to be reduced in AβPP-PS1 mice at the age of 20 months.41 Therefore, the observed increase in astroglial activity measured using [2-13C]acetate in the current study suggests the dominance of neuro-inflammation over other two pathways (glutamine synthesis and pyruvate carboxylation) in AD condition.
Excitatory and inhibitory neurotransmission in AD
Glutamate, the major excitatory neurotransmitter in the central nervous system, is involved in learning, memory, and cognition. Though level of glutamate has been reported to be lower in the cerebral cortex and hippocampus in transgenic models of AD in mice,26,40 there is no quantitative information pertaining to flux associated with trafficking of glutamate between neuron and astrocytes, commonly known as neurotransmitter cycling, is available. A recent study conducted in transgenic rat model of AD has shown small reduction in 13C labeling of glutamate and GABA in hippocampus while cortical region was unperturbed.42 Additionally, the 13C labeling of amino acids in whole brain extract was shown to be decreased in a triple transgenic mouse model of AD.34 These studies qualitatively suggested hypo-metabolism and reduced neurotransmitter cycling in AD condition. Our findings suggest that excitatory activity associated with glutamatergic neurons are severely impaired in hippocampus followed by cerebral cortex and striatum, while the inhibitory activity comprising of GABAergic neurons is decreased highest in the striatum, suggesting differential perturbations in excitatory and inhibitory neurotransmission in AD brain. The severe impairment in glutamatergic neurotransmission across brain points towards reduced firing of excitatory neurons in AD. Since synaptic transmission associated with glutamatergic neurons is implicated in long-term potentiation and memory,29 the reduction in glutamatergic neurotransmission in hippocampus explains loss of memory in AD patients.
Relevance of neurometabolic measurement in diagnosis of AD
Our analysis in AβPP-PS1 mice at the age of six month has indicated no significant perturbation in neurometabolites homeostasis. However, reduction in the 13C labeling of amino acids in the cerebral cortex and hippocampus from labeled glucose has suggested severe reduction in neurotransmitter energy metabolism at pre-symptomatic stage of AD.6 The finding from present study revealed that neurometabolites homeostasis in AβPP-PS1 mice is perturbed in the cerebral cortex and hippocampus at stage of high plaque loading. Additionally, findings of reduction in glutamatergic and GABAergic neurometabolic activity indicates decreased neurotransmission at the pre-symptomatic stage as well as at the high plaque loading. Interestingly, astroglial metabolic activity that was unperturbed at pre-symptomatic stage was found to be increased in AβPP-PS1 mice at the stage of high plaque loading. These data point towards differential perturbation of neural metabolic activity with the progress of AD, which may be useful for better understanding of different stages of disease.
Summary
1H-NMR analysis revealed perturbation in homeostasis of neurometabolites across brain. Levels of glutamate and NAA are reduced in cortical and hippocampal regions, while no change was noted in striatal region. Interestingly, neuronal glucose oxidation and neurotransmitter cycling flux of glutamatergic and GABAergic neurons were found to be impaired across brain in AβPP-PS1 mice. In contrast, the astroglial metabolic rate was increased in the cerebral cortex and hippocampus in AD mice. Previous study in AD mice at pre-symptomatic stage has indicated impairment of neuronal metabolic activity without any perturbation in neurometabolite homeostasis.6 Therefore, analysis of neuronal and astroglial metabolic rates in addition to levels of neurometabolites would provide a comprehensive status of brain functions, which have potential for specific diagnosis of AD.
Supplementary Material
Acknowledgments
We thank Dr Robin A de Graff, Yale University for providing the 1H-[13C]-NMR pulse sequence, Mr Jedy Jose for breeding mice, and Mr Bhargidhar Babu for assistance in animal studies, Dr Swati Maitra for help in carrying out MWM experiments. Authors would like to thank Prof. Subhash C Lakhotia, Banaras Hindu University for the critical review and suggestions for the manuscript. The Behavioral facility is dully acknowledged for MWM test. All NMR experiments were performed at NMR Microimaging and Spectroscopy Facility, CCMB, Hyderabad, India. This study was supported by grants from the Department of Biotechnology (BT/PR14064/Med/30/359/2010), Department of Science and Technology (CO/AB/013/2013), and CSIR network project BSC0208.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
ABP designed research; VT and PV performed research; ABP, VT, and KS analyzed data; ABP and KS prepared figures; ABP and VT interpreted the data; ABP, VT, and KS wrote the paper; and ABP supervised and directed the overall project. All authors reviewed the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science 2006; 314: 777–781. [DOI] [PubMed] [Google Scholar]
- 2.Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 1999; 52: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002; 297: 353–356. [DOI] [PubMed] [Google Scholar]
- 4.DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol 1992; 39: 563–607. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 2010; 133: 512–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari V, Patel AB. Impaired glutamatergic and GABAergic function at early age in AβPPswe-PS1dE9 mice: implications for Alzheimer’s disease. J Alzheimer’s Dis JAD 2012; 28: 765–769. [DOI] [PubMed] [Google Scholar]
- 7.Mattson MP, Kater SB. Excitatory and inhibitory neurotransmitters in the generation and degeneration of hippocampal neuroarchitecture. Brain Res 1989; 478: 337–348. [DOI] [PubMed] [Google Scholar]
- 8.Ottersen OP, Storm-Mathisen J. Excitatory amino acid pathways in the brain. Adv Exp Med Biol 1986; 203: 263–284. [DOI] [PubMed] [Google Scholar]
- 9.Patel AB, de Graaf RA, Mason GF, et al. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J Cereb Blood Flow Metab 2004; 24: 972–985. [DOI] [PubMed] [Google Scholar]
- 10.Sibson NR, Dhankhar A, Mason GF, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A 1998; 95: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothman DL, Behar KL, Hyder F, et al. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev Physiol 2003; 65: 401–427. [DOI] [PubMed] [Google Scholar]
- 12.Jankowsky JL, Fadale DJ, Anderson J, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 2004; 13: 159–170. [DOI] [PubMed] [Google Scholar]
- 13.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari V, Ambadipudi S, Patel AB. Glutamatergic and GABAergic TCA cycle and neurotransmitter cycling fluxes in different regions of mouse brain. J Cereb Blood Flow Metab 2013; 33: 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel AB, de Graaf RA, Rothman DL, et al. Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J Cereb Blood Flow Metab 2010; 30: 1200–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AB, Rothman DL, Cline GW, et al. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res 2001; 919: 207–220. [DOI] [PubMed] [Google Scholar]
- 17.Patel AB, de Graaf RA, Mason GF, et al. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A 2005; 102: 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Graaf RA, Brown PB, Mason GF, et al. Detection of [1,6-13C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn Reson Med 2003; 49: 37–46. [DOI] [PubMed] [Google Scholar]
- 19.Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci 1998; 18: 5225–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason GF, Rothman DL. Basic principles of metabolic modeling of NMR 13C isotopic turnover to determine rates of brain metabolism in vivo. Metab Eng 2004; 6: 75–84. [DOI] [PubMed] [Google Scholar]
- 21.Alcolea A, Carrera J and Medina A. A hybrid marquadrt-simulated annealing method for solving the groundwater inverse problem. Calibration and reliability in groundwater modeling. In: Model-CARE 999 conference, Zurich, Switzerland, September 1999, pp.157–163.
- 22.Dineley KT, Xia X, Bui D, et al. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem 2002; 277: 22768–22780. [DOI] [PubMed] [Google Scholar]
- 23.Viana da Silva S, Haberl MG, Zhang P, et al. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat Commun 2016; 7: 11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankowsky JL, Fadale DJ, Anderson J, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet 2004; 13: 159–170. [DOI] [PubMed] [Google Scholar]
- 25.Wiesmann M, Jansen D, Zerbi V, et al. Improved spatial learning strategy and memory in aged Alzheimer AbetaPPswe/PS1dE9 mice on a multi-nutrient diet. J Alzheimer’s Dis JAD 2013; 37: 233–245. [DOI] [PubMed] [Google Scholar]
- 26.Marjanska M, Curran GL, Wengenack TM, et al. Monitoring disease progression in transgenic mouse models of Alzheimer’s disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 2005; 102: 11906–11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupsingh R, Borrie M, Smith M, et al. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging 2011; 32: 802–810. [DOI] [PubMed] [Google Scholar]
- 28.Block W, Jessen F, Traber F, et al. Regional N-acetylaspartate reduction in the hippocampus detected with fast proton magnetic resonance spectroscopic imaging in patients with Alzheimer disease. Arch Neurol 2002; 59: 828–834. [DOI] [PubMed] [Google Scholar]
- 29.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci 1989; 9: 3040–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adalsteinsson E, Sullivan EV, Kleinhans N, et al. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet 2000; 355: 1696–1697. [DOI] [PubMed] [Google Scholar]
- 31.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993; 15: 289–298. [DOI] [PubMed] [Google Scholar]
- 32.Nilsen LH, Rae C, Ittner LM, et al. Glutamate metabolism is impaired in transgenic mice with tau hyperphosphorylation. J Cereb Blood Flow Metab 2013; 33: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sancheti H, Patil I, Kanamori K, et al. Hypermetabolic state in the 7-month-old triple transgenic mouse model of Alzheimer’s disease and the effect of lipoic acid: a 13C-NMR study. J Cereb Blood Flow Metab 2014; 34: 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancheti H, Kanamori K, Patil I, et al. Reversal of metabolic deficits by lipoic acid in a triple transgenic mouse model of Alzheimer’s disease: a 13C NMR study. J Cereb Blood Flow Metab 2014; 34: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011; 77: 2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 2003; 30: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 37.Verkhratsky A, Olabarria M, Noristani HN, et al. Astrocytes in Alzheimer’s disease. Neurotherapeutics 2010; 7: 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr 2006; 83: 470S–474S. [DOI] [PubMed] [Google Scholar]
- 39.Melo TM, Nehlig A, Sonnewald U. Metabolism is normal in astrocytes in chronically epileptic rats: a 13C NMR study of neuronal-glial interactions in a model of temporal lobe epilepsy. J Cereb Blood Flow Metab 2005; 25: 1254–1264. [DOI] [PubMed] [Google Scholar]
- 40.Nilsen LH, Melo TM, Saether O, et al. Altered neurochemical profile in the McGill-R-Thy1-APP rat model of Alzheimer’s disease: a longitudinal in vivo 1H MRS study. J Neurochem 2012; 123: 532–541. [DOI] [PubMed] [Google Scholar]
- 41.Tiwari V, Patel AB. Pyruvate carboxylase and pentose phosphate fluxes are reduced in a betaPP-PS1 mouse model of Alzheimer’s disease: a 13C NMR study. J Alzheimer’s Dis 2014; 41: 532–541. [DOI] [PubMed] [Google Scholar]
- 42.Nilsen LH, Melo TM, Witter MP, et al. Early differences in dorsal hippocampal metabolite levels in males but not females in a transgenic rat model of Alzheimer’s disease. Neurochem Res 2014; 39: 305–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.