Abstract
Depletion of gut T helper 17 (Th17) cells during HIV infection leads to decreased mucosal integrity and increased disease progression. Conversely, T regulatory (Treg) cells may inhibit antiviral responses or immune activation. In HIV elite controllers, a balanced Th17/Treg ratio is maintained in the blood, suggesting a role for these responses in controlling inflammation and viral replication. HIV-infected individuals exhibit a range in responsiveness to combination antiretroviral therapy (cART). Given the link between the Th17/Treg ratio and HIV disease, we reasoned these responses may play a role in cART responsiveness. In this study, we investigated the relationship between the mucosal Th17/Treg ratio to acute simian immunodeficiency virus (SIV) viremia and the response to cART. Nineteen rhesus macaques were infected with highly pathogenic SIVΔB670 virus and cART was initiated 6 weeks postinfection. Mucosal CD4 T cell subsets were assessed by intracellular cytokine staining in the colon and mesenteric lymph nodes. Higher baseline Th17/Treg ratios corresponded with increased acute SIV viremia. Th17/Treg ratios decreased during acute SIV infection and were not restored during cART, and this corresponded to increased gut immune activation (Ki67+), markers of microbial translocation (sCD14), and T cell exhaustion (TIGIT+). Animals that maintained a more balanced mucosal Th17/Treg ratio at the time of cART initiation exhibited a better virological response to cART and maintained higher peripheral CD4 counts. These results suggest mucosal Th17 and Treg homeostasis influences acute viremia and the response to cART, a result that suggests therapeutic interventions that improve the Th17/Treg ratio before or during cART may improve treatment of HIV.
Keywords: Th17, Treg, mucosal immune responses, simian immunodeficiency virus, antiretroviral therapy, rhesus macaque
Introduction
Globally, over 37 million people are infected with HIV, and despite the effectiveness of combination antiretroviral therapy (cART) in limiting viral replication, drug therapies are unable to completely eliminate HIV reservoirs that persist in lymphoid and gut tissues.1 To develop new interventions to improve viral control or eliminate viral reservoirs, a better understanding of how gut CD4 T cell subsets contribute to the effectiveness of cART and HIV persistence in these tissues is needed. In particular, T helper 17 (Th17) and T regulatory (Treg) cells represent two unique CD4+ T cell subsets with opposing regulatory functions.2 In the gut, preferential depletion of Th17 cells during early infection corresponds to a loss of mucosal integrity, increased microbial translocation, immune activation, and disease progression.3,4 In addition, Th17 responses have antiviral effects that could directly contribute to reducing residual virus in the gut.5
The contribution of CD4+ Tregs to HIV disease remains unclear, but it is hypothesized that Tregs may decrease chronic immune activation that can benefit the host in controlling the disease or they can exacerbate disease by inhibiting HIV-specific CD8 T cell activity during HIV infection.6 The differential impact of Tregs on HIV disease may depend on the stage of infection, the immune compartment being analyzed, and the proportion of other T cell subsets observed.7,8
The Th17/Treg ratio, measured in the peripheral blood, has been shown to be a marker of disease progression during HIV. Lower ratios, indicating reduced Th17 cells and elevated Tregs, correlate with more rapid disease progression, whereas higher Th17/Treg ratios are found in elite controllers and HIV-uninfected populations.9,10 During cART, virus persists in the gut mucosa. Therefore, it is important to understand how the mucosal Th17/Treg ratio is modulated during HIV infection and cART treatment, whether there is a relationship between this ratio and progressive HIV disease and responsiveness to therapeutic interventions, and how the dynamics of the mucosal Th17/Treg ratio during acute infection influences disease progression or intervention strategies.
Here, we infected rhesus macaques (RMs) with a highly pathogenic strain of simian immunodeficiency virus (SIV; SIVΔB670) and evaluated the relationship between Th17 and Treg cells in the colon and mesenteric lymph nodes (MLNs) before and during acute infection, and the impact on viremia, the virological response to cART, and the restoration of mucosal CD4+ T cells during cART. We provide evidence that baseline Th17/Treg ratios before infection and maintenance of Th17/Treg ratios in the gut mucosa during acute infection may be important factors influencing acute viremia and the immunological and virological responses to cART.
Materials and Methods
Animals, infection, antiretroviral therapy, and specimen collection
All animals used in this study were housed at the Washington National Primate Research Center (WaNPRC), an accredited institution by the American Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). All experiments performed on the RMs were approved by the University of Washington's Institutional Animal Care and Use Committee (IACUC) and were in compliance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and Animal Welfare. Nineteen male RMs of Indian origin received an intravenous (i.v.) inoculation of 100 TCID50 of cryopreserved SIVΔB670 in 1 mL of RPMI, as previously described.11 Starting at 6 weeks postinfection (wpi), RMs received daily subcutaneous injections of 20 mg/kg 9-(2-phosphoryl-methoxypropyly) adenine (PMPA; Gilead Sciences, Foster City, CA) and 20–30 mg/kg 2′,3′-dideoxy-5-fluroro-3′-thiacytidine (FTC; Gilead Sciences), and twice-daily oral administrations of 150–250 mg per animal of Raltegravir (RAL; Merck & Co., Kenilworth, NJ).
Vital signs of the animals were monitored throughout the course of infection, including weight, complete blood counts, blood CD4 T cell count, and clinical signs indicative of opportunistic infections. WaNPRC guidelines define simian AIDS as (1) >15% weight loss, (2) anemia (sustained hematocrit <15%), (3) blood CD4 counts of <200 cells/μL, and (4) presence of opportunistic infections. Animals were evaluated for all four measures at least once a month and at each specimen collection time point, and more frequently if two or more criteria were filled.
Blood was collected every 1–4 weeks and colonic pinch biopsies were collected by endoscopic biopsy under direct visualization with biopsy forceps, as described12 before infection, during acute SIV infection, and before initiating cART (6 wpi), and 2 and 22 weeks after cART initiation (8 and 28 wpi). In addition, MLNs were collected by laparoscopic technique, as previously described,12 before infection and at 2 and 22 weeks after the start of cART, but not during acute SIV infection. Animals were sedated with an intramuscular injection (10 mg/kg) of ketamine (Ketaset®; Henry Schein) to perform SIV infections and blood collections. For collection of MLN and colon biopsies, animals were sedated with intramuscular injections of ketamine (10 mg/kg) and dexmedetomidine (Dexdormitor®; Zoetis, Inc.) (0.015 mg/kg), and then intubated and maintained on isoflurane gas (Isothesia™; Henry Schein). Animals received i.v. isotonic fluids and heat support during biopsy procedures, and vital signs and anesthetic depth were monitored continuously (jaw tone, heart rate, respiration rate, SPO2, ETCO2, noninvasive blood pressure, and body temperature). Animals received buprenorphine sustained release 0.2 mg/kg subcutaneously (Buprenorphine SR™; SR Veterinary Technologies) immediately before biopsy procedures, and bupivacaine local blocks were provided at each lymph node biopsy site (Marcaine™; Hospira, Inc.). Animals were given atipamezole 0.15 mg/kg intramuscularly (Antisedan®; Zoetis, Inc.) following biopsy procedures to reverse dexmedetomidine sedative.
Lymphocyte isolation from gut tissues
Intraepithelial and lamina propria lymphocytes were isolated from colon biopsies following enzymatic digestion, as previously described.13 Lymphocytes were isolated from whole MLN biopsies by straining through a 70-μm filter in supplemented RPMI-1640 media. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using an ACCUSPIN™–Histopaque® technique. After isolation, cells were resuspended in freezing media (90% fetal bovine serum +10% dimethyl sulfoxide), then placed in a Mr. Frosty™ container at −80°C overnight, and then transferred to liquid nitrogen freezer for long-term storage.
Plasma viral load quantification
SIVΔB670 plasma viral RNA was evaluated by quantitative real time polymerase chain reaction (RT-PCR) using previously described primers.11,14 All viral loads were determined by the Virology Core at the WaNPRC.
Complete blood counts
Blood CD4 counts and CD4/CD8 ratios were determined from the complete blood counts as determined on a Beckman Coulter® AC*T™ 5diff hematology analyzer.
Intracellular cytokine staining
Cells isolated from colon biopsies, PBMCs, and MLNs were left unstimulated or stimulated overnight with 10 ng/mL PMA (Sigma) and 1 μg/mL ionomycin (Life Technologies) in supplemented RPMI-1640. All samples were treated overnight with 1 μg/mL brefeldin A (Sigma) and in the presence of CD107a antibody (eBioH4A3; eBioscience). Viability was assessed using a live/dead stain (Life Technologies) and cells were stained using the surface markers identified in Supplementary Table S1. Cells were fixed and permeabilized with True-Nuclear Transcription Buffer Set (BioLegend) and stained for the intranuclear transcription factor FoxP3 (206D; BioLegend), and for intracellular cytokines shown in Supplementary Table S1. Samples were acquired on an LSRII (BD Biosciences) and analyzed using FlowJo software version 9.9.4 (FlowJo; LLC). Gating schemes are detailed in Supplementary Figure S1. Briefly Tregs were identified by coexpression of CD25 and FoxP3 within the CD4+ T cell subset from unstimulated samples, Th17 cell frequencies were measured by gating on CD4 T cell subsets expressing IL-17 and subtracting levels measured in unstimulated controls, and polyfunctionality of Th17 cells (IL-22, IFNγ, TNFα, or IL-2) was evaluated by Boolean gating.
TIGIT staining
Cryopreserved PBMCs were thawed in cold R10 media and rested at 37°C and 5% CO2 for 4 h. Cells were washed twice with phosphate-buffered saline and stained with a Live/Dead amine dye (Life Technologies). Next, cells were stained for CCR7 (3D12; BD Biosciences) at 37°C and 5% CO2 for 15 min. Cells were then stained for the surface markers shown in Supplementary Table S2 at 4°C for 20 min in the dark. Gating schemes are detailed in Supplementary Figure S2. Samples were fixed in 1% paraformaldehyde and acquired on an LSRII (BD Biosciences), and then analyzed using FlowJo software version 9.9.4 (FlowJo; LLC).
Soluble CD14 ELISA
Soluble CD14 (sCD14) was quantified in plasma using the Quantikine ELISA Human CD14 Immunoassay kit (R&D Systems) as per the manufacturer's instruction. Results were analyzed using a 4-PL (four-parameter logistic) function for fitting standard curves using Prism version 6.0h (GraphPad).
Statistical analyses
Nonparametric statistical methods were employed for all comparisons. Specifically, paired Wilcoxon tests were used to compare changes in values between the following time points: before infection versus acute SIV infection (4–6 wpi); before infection versus 2 weeks on cART; acute SIV infection versus 22–24 weeks on cART; and 2 weeks on cART versus 22–26 weeks on cART (results shown in Figs. 1, 2, and 4 and Supplementary Figs. 3 and 4) for PBMC and endoscopic biopsy (p-values summarized in Supplementary Table S3). Since MLN samples were not collected during acute infection, Wilcoxon tests were used to compare changes in values between the following time points: before infection versus 2 weeks on cART and 2 weeks on cART versus 22–24 weeks on cART (shown in Supplementary Fig. S5, p-values summarized in Supplementary Table S3). Parameters in the blood and plasma were compared as follows: before infection versus acute SIV infection (4–6 wpi); before infection versus 2–4 weeks on cART; acute SIV infection versus 22–26 weeks on cART; and 2–4 weeks on cART versus 22–26 weeks on cART (Supplementary Table S3). Spearman rank-transformed correlation analyses were conducted to evaluate correlation robustly (Figs. 3 and 4). All analyses were conducted using two-sided tests. A value of p ≤ 0.05 was considered statistically significant. All statistical measures were performed using Prism version 6.0h (GraphPad). A partial permutation test was used to determine significance between pie charts (Fig. 2C) using SPICE software (version 5.3), as previously described.15
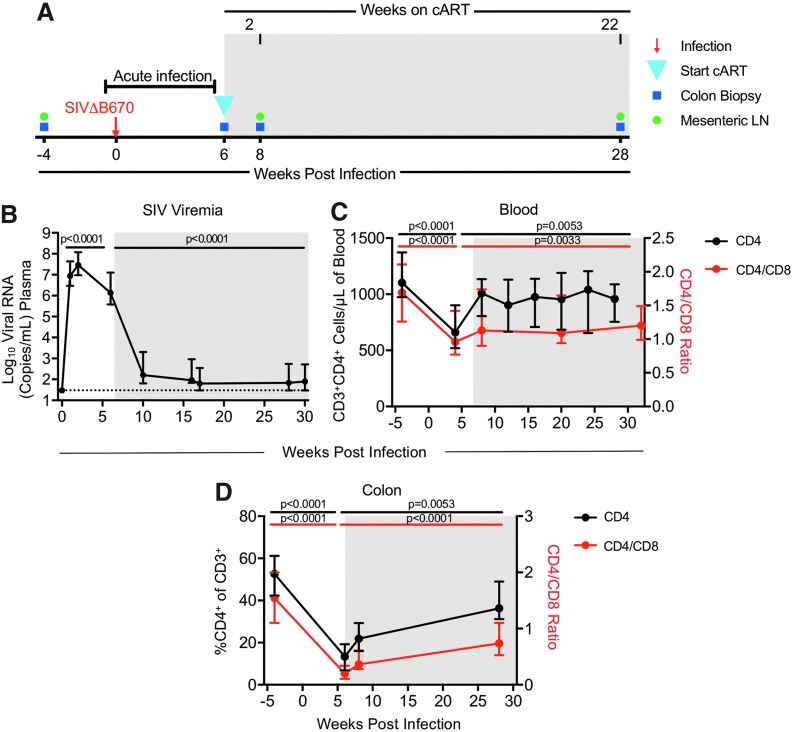
FIG. 1.
cART reduces CD4 decline in blood and colon in SIV-infected macaques. (A) Rhesus macaques (N = 19) were infected i.v. with SIVΔB670 and put on daily cART starting 6 wpi (blue triangle). Blood, colon biopsies (blue squares), and MLN (green circles) were collected before and after SIV infection. (B) Plasma viral RNA levels at each time point were measured by qRT-PCR. The dotted line indicates the limit of detection (30 copies/mL of plasma). (C) CD3+CD4+ T cells (black line/left axis) and the CD4/CD8 (CD3+CD4+/CD3+CD8+) ratio (red line/right axis) in the blood were quantified by CBC using a hematology analyzer. (D) The frequency of CD4+ T cells (black line/left axis) within CD45+ cells and the CD4/CD8 ratio (red line/right axis) from colon biopsies was determined by flow cytometry. (B–D) Shown are medians with interquartile ranges. Shaded regions indicate periods of cART treatment. p-Values were determined using paired Wilcoxon tests, with p-values ≤0.05 considered significant. cART, combination antiretroviral therapy; CBC, complete blood count; i.v., intravenously; MLN, mesenteric lymph node; qRT-PCR, quantitative RT-PCR; SIV, simian immunodeficiency virus; wpi, weeks postinfection.
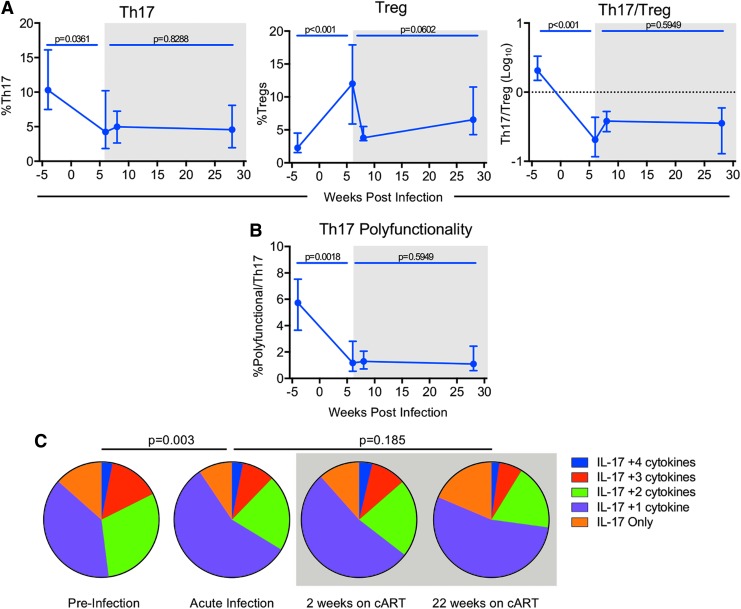
FIG. 2.
Th17/Treg homeostasis is disrupted during acute SIV infection and not restored during cART. (A) The percentage of Th17 (IL-17+) (left panel), stimulated with PMA and ionomycin, or unstimulated Tregs (FoxP3+CD25+) (center panel) of CD4+ T cells in the colon was determined by flow cytometry (as described in Materials and Methods section). The Th17/Treg ratio (right panel) was determined by taking the percentage of IL-17+CD4+ cells and dividing it by the percentage of FoxP3+CD4+ cells. The ratio is given as a log10 scale with the dotted line (1) indicating equilibrium of Th17 and Treg cells. (B) Polyfunctional Th17 cells were identified as the percentage IL-17+ cells that produced at least two additional cytokines (IL-22, IFNγ, TNFα, and/or IL-2) as determined by Boolean gating, following in vitro stimulation with PMA and ionomycin. Medians with interquartile ranges (N = 19) are shown in (A, B). Paired Wilcoxon p-values are indicated, p ≤ 0.05 is considered significant. (C) Pie charts show the relative average proportion (N = 19) of Th17 cells producing IL-17 and two or more cytokines (IL-22, IFNγ, TNFα, and/or IL-2) before infection (baseline), during acute infection, but before initiated cART (6 wpi) and after 2 (8 wpi) and 22 (28 wpi) weeks on cART. Percentages were calculated by Boolean gating in FlowJo. Pie Permutation tests were determined using SPICE. Approximate p-values are indicated, values ≤0.05 are considered significant. Shaded boxes indicate time points after cART imitation. Th17, T helper 17; Treg, T regulatory.
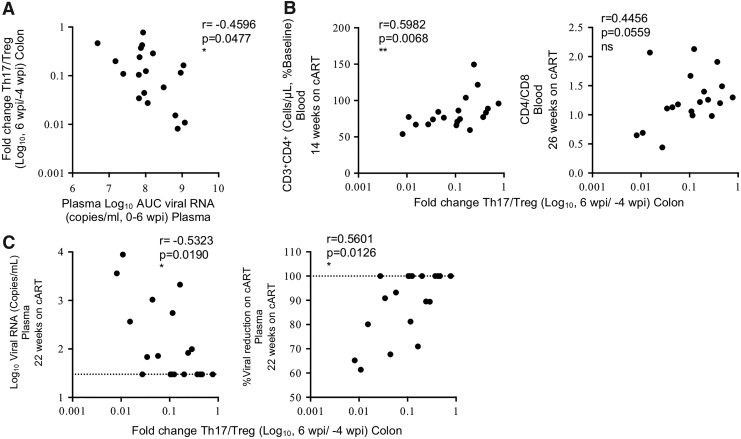
FIG. 4.
Maintenance of the gut Th17/Treg ratio correlates with improved virological response to cART. (A) Correlation between acute SIV plasma viral load (viral RNA copies/mL), measured as AUC 1–6 wpi, versus log10-fold change in the Th17/Treg ratio during acute infection compared to preinfection levels (log10 6 wpi/−4 wpi). (B) Correlations between log10-fold change in Th17/Treg ratio during acute infection (6 wpi/−4 wpi) versus restoration of peripheral blood CD4 counts as percent of preinfection levels during cART (left panel) or CD4/CD8 ratio (right panel) (14–26 wpi). (C) Correlations between log10-fold change in Th17/Treg ratio during acute infection (6 wpi/−4 wpi) versus plasma viral RNA (left panel) or percent viral reduction in the plasma (right panel) after 22 weeks on cART. Percent viral reduction on cART using the limit of viral detection for the assay of 30 copies/mL of plasma was calculated as follows: [(log FVL/IVL)/(log 30/IVL)]*100, where FLV = final viral load measured after 22 weeks on cART, IVL = initial viral load measured at 6 wpi when cART was first initiated. The dotted line indicates the limit of detection of the assay (left panel) or maximum percentage (100%) of viral reduction (right panel). Spearman's rank correlation coefficients are shown, with p-values ≤0.05 considered significant. AUC, area under the curve.
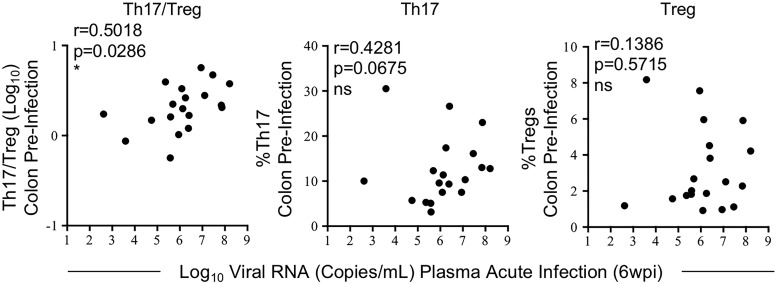
FIG. 3.
Higher acute SIV viremia is associated with higher preinfection Th17/Treg ratios. Correlations between plasma viral load measured before initiation of cART at 6 wpi during acute SIV infection and preinfection Th17/Treg ratios (left panel) and Th17 (center panel) or Treg (right panel) frequencies in the colon. Spearman's rank correlation coefficients are shown, p ≤ 0.05 is considered significant.
Results
Study design and dynamics of SIV viremia and CD4 T cells in blood and mucosal tissues
Nineteen RMs were infected intravenously with SIVΔB670, a highly pathogenic primary isolate that results in high viral loads and generally leads to AIDS in untreated RMs within 11 months postinfection.11,16 Combination ART, consisting of PMPA, FTC, and RAL (see Materials and Methods section), was initiated at 6 wpi (Fig. 1A). Blood was collected every 1–4 weeks to measure plasma SIV viremia and blood T cell counts, and PBMCs were collected for analysis of immune responses. Lymphocytes from colonic gut mucosal tissue biopsies and MLNs were collected before SIV infection and at 2 and 22 weeks after cART initiation, and also from the colon during acute SIV infection/before initiating cART at 6 wpi (Fig. 1A). Peak SIV plasma viremia of 1.93–370 × 106 copies/mL occurred 1–2 wpi, a result that is consistent with in vivo virus loads previously reported in RMs for this challenge stock.11 SIV viral burden was dramatically reduced by 39%–100% within the first 4 weeks and by 93%–100% by 22 weeks after cART initiation (Fig. 1B), with 8 of the 19 animals (42.1%) reaching complete viral suppression at levels below the limit of detection.
CD4+ T cells in the blood declined 2%–64%, and in the colon 35%–89% during acute infection by 4–6 wpi, but were significantly restored to 59%–104% in the blood and 48%–155% in the colon of preinfection baseline levels after 22–26 weeks on cART (Fig. 1C, D and Supplementary Fig. S3A). The frequencies of CD4+ T cells and central memory (CM) CD4+ T cells in the PBMCs also declined up to 60% in the first 6 weeks of infection (Supplementary Figs. S3A, S4A), a result that is consistent with findings in HIV-infected patients.17 Furthermore, similar to HIV-infected patients,18 levels were restored to that of baseline within 22 weeks on cART. The MLN was not sampled during acute infection, but after 22 weeks on cART, CD4+ T cell levels in the MLN were comparable to preinfection levels (>91%) (Supplementary Fig. S5A).
The blood CD4/CD8 ratio is a biomarker of HIV disease progression, and individuals with a ratio <1.0 have a higher rate of AIDS-related comorbidities.19,20 Following SIV infection, blood and colon CD4/CD8 ratios declined, 11%–78% and 49%–95%, respectively, within the first 6 weeks of infection. Blood CD4/CD8 ratios were partially restored to 54%–95% of the preinfection levels after 24 weeks on cART, whereas restoration of colonic CD4/CD8 ratios was highly variable with levels restored to 25%–171% of preinfection levels (Fig. 1C, D). Overall, loss of CD4+ T cell frequencies and CD4/CD8 ratios followed by highly variable restoration in the gut mucosa mirrored the dynamics of T cell loss and recovery in the blood and in the PBMC. No relationship was observed between the level of viral suppression after 22 weeks on cART and the levels or amount of restorations of CD4+ T cells or the CD4/CD8 ratio in the blood or colon before/after initiating cART, indicating these parameters did not significantly influence responsiveness to cART.
Th17/Treg homeostasis is disrupted during acute SIV infection and not restored during cART
Dysregulation of the peripheral Th17/Treg ratio is negatively associated with AIDS progression and HIV viremia.9,21,22 To determine if a similar relationship occurs in tissues of SIV-infected macaques and the impact of cART on this ratio, we measured the frequency of Th17 and Treg CD4+ T cell subsets in the colon and MLN during acute infection and cART. During the first 6 weeks of SIV infection, frequencies of Th17 cells in the colon significantly declined by a median of 2.5-fold concurrent with a 4.4-fold median increase in Treg cell frequencies, resulting in an overall 8.7-fold median decline in the Th17/Treg ratio when compared to baseline levels (Fig. 2A), indicating a substantial disruption in the balance between Th17 and Tregs in the gut. A similar pattern of CD4 dysregulation was also observed in the PBMC within the first 6 weeks of infection (Supplementary Fig. S3B). After 22 weeks on cART, frequencies of Tregs were reduced (p = 0.0602) by a median of 51%, but there was no impact on Th17 cells in the colon (p = 0.8288), leading to no significant improvement in the Th17/Treg ratio (p = 0.5949) (Fig. 2A). Similar results were seen in the MLN (Supplementary Fig. S5B).
Loss of gut Th17 polyfunctionality during HIV and SIV infection is associated with increased disease progression and HIV/SIV persistence.23–25 To determine the impact of acute SIV infection and cART on Th17 polyfunctionality in the gut, Boolean gating was used to measure the frequency of polyfunctional Th17 cells co-producing two or more cytokines, including IL-22, IFNγ, TNFα, and/or IL-2. Polyfunctional Th17 cells declined by a median of 3.5-fold during acute SIV infection and were not restored during cART with an overall 6-fold median decline in the frequency of Th17 cells after 22 weeks on cART (Fig. 2B, C). The decline in Th17 polyfunctionality was not due to the loss of any specific effector function and was consistent with an overall decline in frequencies of other cytokine-producing (IFNγ+, TNFα+, or IL-2+) CD4+ T cells in the colon (data not shown), demonstrating a generalized loss in T cell polyfunctionality that is not restored during cART. Together these results show that SIV infection leads to severe loss of Th17/Treg cell homeostasis and immune function in the gut that, despite partial-to-full restoration of CD4+ T cell counts and the CD4/CD8 ratio, is not restored after >5 months of cART therapy.
Preinfection Th17/Treg ratios in the colon influence early SIV viremia
Previous studies show that HIV-infected, cART-naive individuals, who have higher levels of peripheral Th17 cells during the early stages of infection (i.e., 50–120 days postinfection), exhibit lower viral loads and slower disease progression during chronic infection when compared to rapid progressors,9 suggesting that levels of Th17 cells present early in infection may contribute to improved viral control and slower disease progression. However, it is unclear if slow progressors develop higher Th17 responses during acute HIV infection or if they inherently have higher levels before infection. Since these previous studies evaluated Th17 responses only in the blood, the relationship between disease progression and Th17 levels in the gut is also not clear. Since we observed severe disruption of the Th17/Treg balance during acute SIV infection in RMs, we wanted to determine if there was a relationship between the Th17/Treg ratio before infection or at the earliest stages and acute viremia. Higher colonic Th17/Treg ratios before infection positively correlated with higher acute viral loads at 6 wpi (p = 0.0286, r = 0.5018) and when analyzed separately, higher Th17 (p = 0.0675, r = 0.4281), but not Treg (p = 0.5715, r = 0.1386), preinfection frequencies showed a trend in positively correlating with higher acute viral loads (Fig. 3). In contrast, higher viremia at 6 wpi did not correlate with colonic frequencies of Th17 cells (p = 0.5280, r = 0.1544), Tregs (p = 0.7807, r = 0.0685), or the Th17/Treg ratio (p = 0.7535, r = −0.0772) at the same time point (data not shown). These data suggest that higher frequencies of gut Th17 cells may initially provide a larger SIV target pool, leading to increased acute viremia, and furthermore, preinfection Th17/Treg homeostasis may be a key factor influencing acute viral loads and subsequent disease progression.
Maintenance of the gut Th17/Treg ratio correlates with improved virological responses to cART
During HIV infection, a loss in the balance of Th17 and Treg cells in the periphery is associated with greater immune activation, CD4 decline, and faster HIV disease progression.9,21,26 We therefore investigated if there was a relationship between the early dysregulation of Th17 and Treg cells we observed in the gut of SIV-infected macaques and viral loads during acute infection or cART. Greater SIV viral burden, measured as the area under the curve in the first 6 weeks of infection, positively correlated (p = 0.0477, r = −0.4596) with greater gut dysregulation, measured as the fold change in the Th17/Treg ratio within the first 6 wpi (between preinfection and 6 wpi time points) (Fig. 4A), indicating that greater viral burden during acute infection leads to greater dysregulation of Th17 and Treg cells in the gut.
We next investigated if animals that maintained a more balanced Th17/Treg ratio in the colon during acute SIV infection had improved responses to therapy after >3 months of cART. Maintenance of the Th17/Treg ratio was defined as the fold change in the ratio between preinfection and acute SIV infection (6 wpi) time points. Animals with better maintenance of the colonic Th17/Treg ratio (fold change closer to 1.0) at the time of cART initiation (6 wpi) exhibited higher restoration of whole blood CD4 counts and CD4/CD8 ratios after >3 months of cART (Fig. 4B), but not CM CD4+ T cells in the PBMCs (Supplementary Fig. S4B), when compared to preinfection levels. In correspondence, animals with less Th17/Treg dysregulation in the first 6 weeks of SIV infection had lower plasma viral loads and higher viral reduction after being on cART for 22 weeks (Fig. 4C). Collectively, these data suggest that conservation of the gut Th17/Treg ratio during acute SIV viremia corresponds to a more robust response to cART treatment.
Reduction of the Th17/Treg ratio is associated with increased gut immune activation, microbial translocation, and peripheral immune exhaustion
Despite the effectiveness of cART in reducing HIV viremia, persistent immune activation and microbial translocation still occur in HIV-infected individuals, leading to increased morbidity and mortality.27–31 We therefore investigated if there is a relationship between the Th17/Treg ratio in the colon and the level of immune activation and microbial translocation during acute infection and cART. Immune activation in the colon, measured as the percentage of Ki67+ cells of CD4+ or CD8+ T cells, increased 2.9- and 1.79-fold, respectively, during the first 6 weeks of infection (Fig. 5A), a result that mirrors the increases in immune activation we observed in the PBMC during the same time frame (Supplementary Fig. S3C) and in the MLN after 2 weeks on cART (Supplementary Fig. S5C). After 22 weeks on cART, immune activation in the colon was significantly reduced, reaching levels similar to those found at preinfection (Fig. 5A). However, plasma sCD14 levels, an indicator of microbial translocation,32 significantly increased during acute SIV infection and continued to escalate during the 5 months of cART (Fig. 5B), indicating that although cART reduced gut immune activation, it was unable to protect against progressive mucosal barrier dysfunction and microbial translocation. LPS binding protein (LBP), another measurement of microbial translocation,32 was also evaluated in plasma. However, changes in LBP levels were discerned in only 4 of the 19 animals post-SIV infection (data not shown). Since our analysis of microbial translocation focused on the earliest weeks postinfection, levels of LBP in the blood may still be below threshold levels of detection. Indeed, our results are consistent with previous studies in SIV-infected macaques, which similarly showed variable to no changes in LBP levels within the first 5 weeks of infection.33
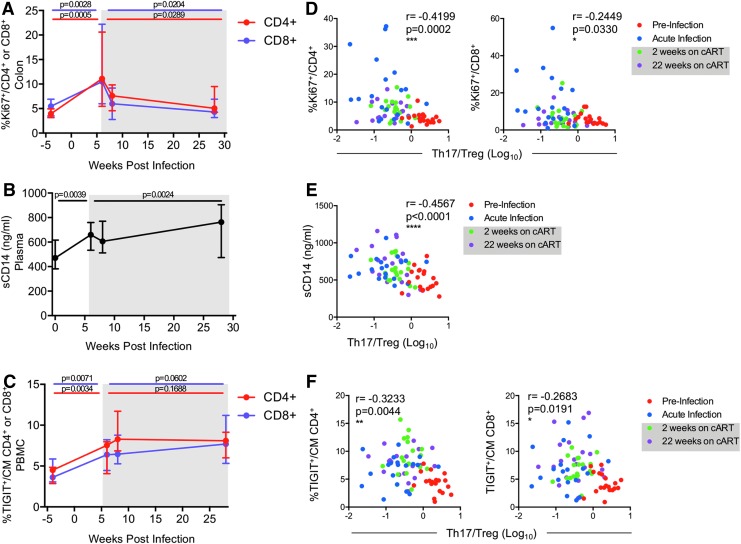
FIG. 5.
Reduction of the Th17/Treg ratio is associated with increased gut immune activation, microbial translocation, and peripheral immune exhaustion. (A) Gut immune activation was measured in the colon as the frequency of Ki67+ of CD4+ (red line) or CD8+ (purple line) T cells by flow cytometry. (B) Microbial translocation was measured as soluble CD14 (sCD14) in plasma by ELISA. (C) The frequencies of CM (CD95+CD28+) CD4+ (red line) or CD8+ (purple line) T cells in the PBMC expressing the immune exhaustion marker TIGIT were measured by flow cytometry. (D–F) Longitudinal correlations between the Th17/Treg ratio in the colon measured at the indicated time points and (D) gut immune activation (%Ki67+ of CD4+ or CD8+ T cells), (E) microbial translocation (sCD14 levels), and (F) immune exhaustion of CM T cells in PBMC (%TIGIT+) were determined by Spearman's rank correlation analyses. Lines in (A–C) indicate the medians with interquartile ranges. p-Values were determined using paired Wilcoxon tests, p ≤ 0.05 is considered significant. Shaded gray boxes in (A–F) indicate periods of cART. CM, central memory; PBMC, peripheral blood mononuclear cell.
Elevated frequencies of the immune checkpoint receptor and marker of exhaustion, T cell immunoreceptor with Ig, and ITIM domains (TIGIT) on T cells is associated with disease progression in HIV-infected people.34 Levels of TIGIT+ T cells remain elevated in cART-treated individuals or elite controllers, indicating that immune exhaustion persists despite potent suppression of viremia by cART.34,35 In the SIV-infected RMs in this study, we similarly found that frequencies of TIGIT+ CM (CD95+CD28+) T cells increased in the PBMCs, with up to a 7.6- and 4.4-fold increase in TIGIT+ CM CD4+ and CD8+ T cells, respectively, during the first 6 weeks of infection (Fig. 5C). Combination ART appeared to have no impact on immune exhaustion with a further 11.1- and 7.4-fold median increase in the frequencies of TIGIT+ CM CD4+ and CD8+ T cells, respectively, during cART when compared to preinfection levels (Fig. 5C). Together these data show that, similar to that observed during HIV infection, cART in SIV-infected macaques has limited effects on barrier dysfunction and peripheral immune exhaustion.
To determine if the dysregulation of the Th17/Treg ratio we observed in the colon during SIV infection contributes to persistence of AIDS-related symptoms during cART, we performed longitudinal comparative analyses of these immune responses to the Th17/Treg ratio in the colon measured at key time points during the study: preinfection, acute infection, and after 2 and 22 weeks on cART. The results in Figure 5 collectively show that lower Th17/Treg levels correlated with greater gut immune activation (Ki67+CD4+: p = 0.0002, r = −0.4199; and Ki67+CD8+: p = 0.0330, r = −0.2449) (Fig. 5D), increased microbial translocation (sCD14: p < 0.0001, r = −0.4567) (Fig. 5E), and increased peripheral immune exhaustion (TIGIT+ CM CD4+: p = 0.0044, r = −0.3233; and TIGIT+ CM CD8+: p = 0.0191, r = −0.2683) (Fig. 5F). Taken together, these results show that acute SIV infection results in severe disruption of the Th17/Treg ratio in the colon that is not restored by cART, and this loss may contribute to the generalized gut immune activation, subsequent microbial translocation, and peripheral immune exhaustion that persists during cART.
Discussion
Previous studies in HIV-infected individuals and SIV-infected macaques have investigated the relationship between Th17 and Treg responses in the peripheral blood or mucosa and disease progression. These studies have consistently shown that Th17 cells are primary initial targets of infection and greater loss of Th17 cells in the blood or gut is a strong predictor of higher immune activation, increased microbial translocation, and more rapid disease progression.3,4,9,36–38 Although Tregs modulate immune activation, their role in HIV/SIV disease has been less clear with studies showing HIV/SIV infection increases,9,10,25,39 decreases,23,40–42 or causes no change41,43 in Treg frequencies in blood and mucosal tissues.
Most studies have examined Th17 and Tregs as independent cell subsets. However, Falivene et al., demonstrated that chronic HIV infection, in the absence of cART, results in a significant decline in the peripheral Th17/Treg ratio when compared to healthy controls, and elite controllers maintain higher Th17/Treg ratios than chronically infected individuals,9 indicating these two subsets work together to influence the course of the disease, likely by impacting the kinetics and magnitude of immune activation and the inflammatory response to the infection. Since HIV-infected individuals with higher immune activation and dysfunction generally exhibit a less potent virological response to cART,44 we reasoned that an imbalance between Th17 and Treg responses may similarly influence effectiveness of cART. In this study, we show a profound decline in the mucosal Th17/Treg ratio in the colon of SIV-infected RMs during acute infection, which is not restored during cART. Furthermore, this decline corresponded with the development of higher immune activation, greater microbial translocation, increased peripheral immune exhaustion, and an overall poorer response to cART. These effects were specific to the Th17/Treg ratio since separate Th17, Treg, and CD4 frequencies did not correlate with the virological response to cART. Strikingly, our results also showed that a higher Th17/Treg ratio measured before infection predicted higher postinfection acute SIV viremia. Since Th17 cells are primary targets of HIV/SIV infection, the larger pool of these cells at the time of viral exposure may have contributed to the observed enhanced susceptibility to the infection. This suggests that the frequency of Th17 cells and the balance between Th17 cells versus Tregs at the earliest stages of infection partially determine the rate of disease progression and the virological response to cART. The analysis of the Th17/Treg ratio in our studies was limited to colonic mucosal tissues and these findings may be translatable to other mucosal tissues. In support of this, similar levels of CD4+ T cell and Th17 depletion and immune activation were observed in the jejunum and colon of SIV-infected macaques.3,45,46 However, greater CD4+ T cell depletion has been reported in the jejunum when compared to the colon in HIV-infected individuals,47 and additional studies are needed to determine if the magnitude of Th17/Treg dysregulation in the colon reported here is similar at other mucosal sites.
The early loss of Th17 cells in the blood and gut we observed following SIV infection is consistent with previous studies3,10,42,48,49 and is likely a key factor leading to an imbalance between Th17 and Tregs, and a profound decline in the Th17/Treg ratio at the earliest stages of disease. Th17 cells and Tregs likely work together to exert control over the inflammatory response to the infection, loss of mucosal integrity, and T cell activation. With a significant loss in Th17 cells immediately during acute infection, there becomes an imbalance in these two subsets that may lead to increased immune activation and inflammation, providing a setting for enhanced viral replication that could counteract the effects of potent cART.
The animals in our studies were infected with a highly pathogenic strain of SIV, SIVΔB670, that on average results in progression to AIDS in untreated animals within 11 months.50 Previous studies evaluating mucosal dysfunction employed RMs infected with more commonly used SIVmac251 or SIVmac239 strains.3,42,48,49 Animals infected with these strains also rapidly progress to AIDS, but at a slower rate, generally within 14–18 months postinfection.51 The more aggressive nature of SIVΔB670 likely contributes to greater and more accelerated gut dysfunction. In support of this, pigtail macaques infected with SIV progress more rapidly to AIDS and exhibit a more compromised gastrointestinal integrity when compared to RMs.38,52 In addition, the route of challenge (i.e., single bolus i.v. employed in this study, compared to repeated low-dose rectal challenges) may also impact the kinetics and magnitude of mucosal dysfunction. Despite these differences, there is consistency among all nonhuman primate models in demonstrating a correlation between the level of mucosal immune dysfunction and disease progression. Further studies are needed to determine the non-human primate (NHP) model that best resembles HIV gut dysfunction in humans.
We also found that the decline in the Th17/Treg ratio correlated with higher immune activation and an increase in the frequency of T cells with an exhausted phenotype (TIGIT+). Recent studies show that viral-specific Th17 responses can develop during HIV/SIV infection.53,54 In addition, a subset of Tregs with TIGIT coexpression can suppress Th17 responses55; so a loss of SIV-specific Th17 cells coupled with an increase in Th17 and Treg immune exhaustion could also contribute to reduced viral control during cART.
Although the NHP is a valuable model for studying human HIV disease, additional studies are needed to determine if these findings are consistent with HIV infection in humans. For example, SIV infection induces AIDS in nonhuman primates more rapidly than HIV infection in humans. Therefore, viral replication in SIV-infected animals can be difficult to suppress with antiretroviral drugs designed to target HIV. Relative to humans, the period of cART therapy in our macaques is quite short (e.g., several months vs. several years) and restoration of gut homeostasis, and in particular the Th17/Treg ratio, may require longer cART treatment. Indeed, long-term cART (>5 years) in HIV-infected individuals has been shown to provide partial restoration of gut mucosal CD4+ T cells and Th17 cells in a subset of individuals,25,58 suggesting that future studies in long-term cART-treated SIV-infected NHPs and in HIV-infected humans are needed to determine if the Th17/Treg axis can be restored in the gut and confirm its role in the response to cART.
Our studies were also limited to analysis of immune parameters in male NHPs, but sex differences in HIV pathogenesis, including lower plasma viral load, higher CD4 T cell counts, and greater levels of immune activation, have been observed in HIV-infected women versus men,56 and the results reported in this study for males may not fully reflect mucosal dysregulation of CD4+ T cells in females. Consistent with this possibility, in SIV-infected NHPs, differences in mucosal innate immune responses, gut microbiota, and disease progression have been observed between males and females.57 Therefore, it is possible that similar gender differences may occur in regard to mucosal dysregulation of CD4 T cells.
Taken together, our results suggest that Th17 cells and Tregs play a complementary and defining role in the virological response to cART, likely by limiting microbial translocation, immune activation, and immune exhaustion that can enhance viral replication. These data highlight a role for Th17 cells and, in particular, the balance between Th17 and Treg cells in SIV/HIV pathogenesis and in the response to cART, and suggest that monitoring and maintaining the mucosal Th17/Treg ratio during periods of therapeutic intervention may be important for the success of immune-based HIV cure strategies.
Supplementary Material
Acknowledgments
We thank the members of the Fuller laboratory for helpful discussions and technical support and Paul Edlefsen for statistical advice and input. We thank Brian Agricola, Solomon Wangari, Drew May, Joel Ahrens, Naoto Iwayama, Jennifer Lane, and the Washington National Primate Research Center Research Support Services for macaque handling and procedures. This work was supported by National Institutes of Health grant RO1 AI104679 (to D.H.F. and J.I.M.) and R44 AI110315-01 (to K.C.B.), and, in part, by P51 OD010425. M.A.O. was supported by the National Institutes of Health Training Grant T32-AI007140.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Hong FF, Mellors JW: Changes in HIV reservoirs during long-term antiretroviral therapy. Curr Opin HIV AIDS 2015;10:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bettelli E, Carrier Y, Gao W, et al. : Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238 [DOI] [PubMed] [Google Scholar]
- 3. Cecchinato V, Trindade CJ, Laurence A, et al. : Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol 2008;1:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pallikkuth S, Micci L, Ende ZS, et al. : Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog 2013;9:e1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maddur MS, Miossec P, Kaveri SV, Bayry J: Th17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 2012;181:8–18 [DOI] [PubMed] [Google Scholar]
- 6. Kanwar B, Favre D, McCune JM: Th17 and regulatory T cells: Implications for AIDS pathogenesis. Curr Opin HIV AIDS 2010;5:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macatangay BJ, Rinaldo CR: Regulatory T cells in HIV immunotherapy. HIV Ther 2010;4:639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chevalier MF, Weiss L: The split personality of regulatory T cells in HIV infection. Blood 2013;121:29–37 [DOI] [PubMed] [Google Scholar]
- 9. Falivene J, Ghiglione Y, Laufer N, et al. : Th17 and Th17/Treg ratio at early HIV infection associate with protective HIV-specific CD8(+) T-cell responses and disease progression. Sci Rep 2015;5:11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Favre D, Lederer S, Kanwar B, et al. : Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog 2009;5:e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuller DH, Rajakumar P, Che JW, et al. : Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques. PLoS One 2012;7:e33715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smedley J, Macalister R, Wangari S, et al. : Laparoscopic technique for serial collection of para-colonic, left colic, and inferior mesenteric lymph nodes in macaques. PLoS One 2016;11:e0157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munson P, Liu Y, Bratt D, et al. : Therapeutic conserved elements (CE) DNA vaccine induces strong T-cell responses against highly conserved viral sequences during simian-human immunodeficiency virus infection. Hum Vaccines Immunother 2018;14:1820–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demberg T, Florese RH, Heath MJ, et al. : A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 2007;81:3414–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets—Roederer—Cytometry Part A—Wiley Online Library [Internet]. Available at https://onlinelibrary.wiley.com/doi/abs/10.1002/cyto.a.21015 (2011), accessed September17, 2018 [DOI] [PMC free article] [PubMed]
- 16. Murphey-Corb M, Wilson LA, Trichel AM, et al. : Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J Immunol 1999;162:540–549 [PubMed] [Google Scholar]
- 17. Potter SJ, Lacabaratz C, Lambotte O, et al. : Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: An ANRS EP36 study. J Virol 2007;81:13904–13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muñoz-Calleja C, Costantini A, Silvestri G, et al. : Highly active antiretroviral therapy induces specific changes in effector and central memory T cell sub-populations. AIDS 2001;15:1887–1890 [DOI] [PubMed] [Google Scholar]
- 19. Serrano-Villar S, Sainz T, Lee SA, et al. : HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014;10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buggert M, Frederiksen J, Noyan K, et al. : Multiparametric bioinformatics distinguish the CD4/CD8 ratio as a suitable laboratory predictor of combined T cell pathogenesis in HIV infection. J Immunol 2014;192:2099–2108 [DOI] [PubMed] [Google Scholar]
- 21. Chevalier MF, Didier C, Petitjean G, et al. : Phenotype alterations in regulatory T-cell subsets in primary HIV infection and odentification of Tr1-like cells as the main interleukin 10-producing CD4+ T cells. J Infect Dis 2015;211:769–779 [DOI] [PubMed] [Google Scholar]
- 22. Prendergast A, Prado JG, Kang YH, et al. : HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 2010;24:491–502 [DOI] [PubMed] [Google Scholar]
- 23. Ryan ES, Micci L, Fromentin R, et al. : Loss of function of intestinal IL-17 and IL-22 producing cells contributes to inflammation and viral persistence in SIV-infected rhesus macaques. PLoS Pathog 2016;12:e1005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuetz A, Deleage C, Sereti I, et al. : Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014;10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim CJ, Rousseau R, Huibner S, et al. : Impact of intensified antiretroviral therapy during early HIV infection on gut immunology and inflammatory blood biomarkers. AIDS 2017;31:1529–1534 [DOI] [PubMed] [Google Scholar]
- 26. Li D, Chen J, Jia M, et al. : Loss of balance between T helper type 17 and regulatory T cells in chronic human immunodeficiency virus infection. Clin Exp Immunol 2011;165:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chege D, Sheth PM, Kain T, et al. : Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS 2011;25:741–749 [DOI] [PubMed] [Google Scholar]
- 28. French MA, King MS, Tschampa JM, da Silva BA, Landay AL: Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009;200:1212–1215 [DOI] [PubMed] [Google Scholar]
- 29. Piconi S, Trabattoni D, Gori A, et al. : Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS 2010;24:1991–2000 [DOI] [PubMed] [Google Scholar]
- 30. Hunt PW, Cao HL, Muzoora C, et al. : Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011;25:2123–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandler NG, Wand H, Roque A, et al. : Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 33. Handley SA, Desai C, Zhao G, et al. : SIV infection-mediated changes in gastrointestinal bacterial microbiome and virome are associated with immunodeficiency and prevented by vaccination. Cell Host Microbe 2016;19:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chew GM, Fujita T, Webb GM, et al. : TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog 2016;12:e1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tauriainen J, Scharf L, Frederiksen J, et al. : Perturbed CD8+ T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Sci Rep 2017;7:40354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stieh DJ, Matias E, Xu H, et al. : Th17 cells are preferentially infected very early after vaginal transmission of SIV in macaques. Cell Host Microbe 2016;19:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gordon SN, Cervasi B, Odorizzi P, et al. : Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J Immunol 2010;185: 5169–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klatt NR, Harris LD, Vinton CL, et al. : Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol 2010;3:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jenabian MA, Patel M, Kema I, et al. : Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One 2013;8:e78146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chase AJ, Sedaghat AR, German JR, et al. : Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol 2007;81:12748–12757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. George J, Cofano EB, Lybarger E, et al. : Early short-term antiretroviral therapy is associated with a reduced prevalence of CD8(+)FoxP3(+) T cells in simian immunodeficiency virus-infected controller rhesus macaques. AIDS Res Hum Retroviruses 2011;27:763–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khowawisetsut L, Pattanapanyasat K, Onlamoon N, et al. : Relationships between IL-17(+) subsets, Tregs and pDCs that distinguish among SIV infected elite controllers, low, medium and high viral load rhesus macaques. PLoS One 2013;8:e61264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shaw JM, Hunt PW, Critchfield JW, et al. : Short communication: HIV+ viremic slow progressors maintain low regulatory T cell numbers in rectal mucosa but exhibit high T cell activation. AIDS Res Hum Retroviruses 2013;29:172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hunt PW, Martin JN, Sinclair E, et al. : T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003;187:1534–1543 [DOI] [PubMed] [Google Scholar]
- 45. Ling B, Mohan M, Lackner AA, et al. : The large intestine as a major reservoir for simian immunodeficiency virus in macaques with long-term, nonprogressing infection. J Infect Dis 2010;202:1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veazey RS, DeMaria M, Chalifoux LV, et al. : Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998;280:427–431 [DOI] [PubMed] [Google Scholar]
- 47. Cassol E, Malfeld S, Mahasha P, et al. : Impaired CD4+ T-cell restoration in the small versus large intestine of HIV-1-positive South Africans receiving combination antiretroviral therapy. J Infect Dis 2013;208:1113–1122 [DOI] [PubMed] [Google Scholar]
- 48. Xu H, Wang X, Veazey RS: Th17 cells coordinate with Th22 cells in maintaining homeostasis of intestinal tissues and both are depleted in SIV-infected macaques. J AIDS Clin Res 2014;5:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nigam P, Kwa S, Velu V, Amara RR: Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J Immunol 2011;186:745–753 [DOI] [PubMed] [Google Scholar]
- 50. Trichel AM, Rajakumar PA, Murphey-Corb M: Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J Med Primatol 2002;31:171–178 [DOI] [PubMed] [Google Scholar]
- 51. Ling B, Veazey RS, Luckay A, et al. : SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 2002;16:1489–1496 [DOI] [PubMed] [Google Scholar]
- 52. Klatt NR, Canary LA, Vanderford TH, et al. : Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J Virol 2012;86:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh A, Vajpayee M, Ali SA, Mojumdar K, Chauhan NK, Singh R: HIV-1 diseases progression associated with loss of Th17 cells in subtype “C” infection. Cytokine 2012;60:55–63 [DOI] [PubMed] [Google Scholar]
- 54. Saxena V, Patil A, Tayde R, et al. : HIV-specific CD4+Th17 cells from HIV infected long-term non-progressors exhibit lower CTLA-4 expression and reduced apoptosis. Immunobiology 2018;223:658–662 [DOI] [PubMed] [Google Scholar]
- 55. Joller N, Lozano E, Burkett PR, et al. : Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014;40:569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Addo MM, Altfeld M: Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014;209(Suppl 3):S86–S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ren W, Ma Y, Yang L, et al. : Fast disease progression in simian HIV-infected female macaque is accompanied by a robust local inflammatory innate immune and microbial response. AIDS 2015;29:F1–F8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Macal M, Sankaran S, Chun TW, et al. : Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol 2008;1:475–488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.