Abstract
Over the last decades, the incidence of human papilloma virus (HPV) positive head and neck squamous-cell carcinoma (HNSCC) has significantly increased. Infection with high-risk HPV types drives tumourigenesis through expression of the oncoproteins E6 and E7. Currently, the primary treatment of HNSCC consists of radiotherapy, often combined with platinum-based chemotherapeutics. One of the common features of HNSCC is the occurrence of tumour hypoxia, which impairs the efficacy of radiotherapy and is a negative prognostic factor. Therefore, it is important to detect and quantify the severity of hypoxia, as well as develop strategies to specifically target hypoxic tumours. HPV-positive tumours are remarkably radiosensitive compared to HPV-negative tumours and consequently the HPV-positive patients have a better prognosis. This provides an opportunity to elucidate mechanisms of radiation sensitivity, which may reveal targets for improved therapy for HPV-negative head and neck cancers. In this review, we will discuss the differences between HPV-positive and HPV-negative head and neck tumours and methods of hypoxia detection and targeting in these disease types. Particular emphasis will be placed on the mechanisms by which HPV infection impacts radiosensitivity.
Introduction
Head and neck squamous-cell carcinoma (HNSCC) is a disease marked by its aggressiveness and likelihood of recurrence. The most prominent risk factors include longstanding alcohol and tobacco consumption and chronic infection with human papilloma virus (HPV). Radiotherapy, often combined with platinum-based chemotherapeutics, is considered to be the standard care for treating HNSCC. Primary care for HNSCC includes radiotherapy, as it has been shown to have a high efficacy as well as being a preferable option for organ conservation.1 Unfortunately, the efficacy of radiotherapy is strongly attenuated by the presence of low oxygen (hypoxia) within tumour tissue. Hypoxia can arise due to an imbalance between oxygen supply and demand, caused by an altered tumour metabolism, as well as aberrant tumour vasculature or poor vessel perfusion.2 Tumour hypoxia contributes to radioresistance, primarily due to decreased radiation-induced DNA damage in the absence of oxygen.3–5 HPV-positive tumours, which represent an increasing subset of HNSCC, display marked radiosensitivity compared to HPV-negative tumours. This is reflected in the patient population, as HPV-positive HNSCC patients have a 3 year survival rate of 82.4% as compared to 57.1% in HPV-negative patients.6
HPV infection in HNSCC
While both alcohol-/tobacco-induced HNSCC and HPV-associated HNSCC are subject to the same treatment regimen, it is important to acknowledge the differences between the disease types (Figure 1). Patients with HPV-associated HNSCC are younger, more often Caucasian and male, and have a higher number of sexual partners.7 Recently, the rising incidence of HPV-positive HNSCC has led to an increasing number of reports evaluating the role of HPV in the treatment of HNSCC.8 HPV is a member of the papillomaviridae family, which are unencapsulated circular double stranded DNA viruses comprised of 6 to 8 genes. Of over 200 types of HPV have been identified, only some of which have been identified as “high-risk”, meaning that these types contribute to oncogenesis. Among these, HPV-16 and HPV-18 are the most commonly found types in HNSCC.9 The HPV-16 virus is an 8 Kb virus that encodes 2 late genes (L1 and L2) and 6 early genes (E1, E2, E4, E5, E6, and E7). The early genes facilitate viral genome replication, while the late genes are required for the production of viral capsid proteins that are required for effective viral entry into future host cells.10, 11 Of the HPV genes, the E6 and E7 genes are known to contribute most significantly to oncogenesis. The E6 protein is able to bind the tumour suppressor protein p53 and target it for ubiquitination and subsequent proteasomal degradation.12 Similarly, E7 binds to Retinoblastoma (Rb), also a tumour suppressor, and facilitates proteasomal degradation.12 As a result of proteolysis of p53 and Rb, the host cell loses the ability to enter into apoptosis or senescence, or to arrest the cell cycle, creating an ideal environment for viral production and oncogenesis. Notably, E6 and E7 proteins have been shown to interact with dozens of other cellular proteins, indicating that there may be other contributing pathways.13 HPV-positive tumours are characterised by a mutational landscape that is distinct from HPV-negative tumours. Overall the mutation rate is lower in HPV-positive tumours and they are specifically characterised by a lower number of p53 mutations. As E6 can effectively abrogate p53 function, there is less evolutionary pressure for HPV-positive tumour cells to select for p53 mutations.14, 15 In HNSCC, particularly in oropharyngeal squamous-cells carcinomas (OPSCC), HPV positivity has both prognostic and clinical implications.16–18 Numerous studies have shown a strong prognostic effect of HPV positivity with superior loco-regional tumour control and overall/event-free survival both in the primary and post-operative radiotherapy of HNSCC.19–23 Data from the Danish Head-And-Neck Cancer Study Group (DAHANCA) demonstrated a significantly superior loco-regional tumour control, event-free and overall survival in HPV-positive OPSCCs and recently, the results from four randomised trials were reported (RTOG9003, DAHANCA6&7, RTOG0129, ARTSCAN), showing a significantly better progression-free and overall survival with an absolute survival increase at 10 years of 31.2% in HPV-positive tumours following radiotherapy.24–26 Taken together, there is a significant amount of evidence to support that HPV-positive tumours are more radiosensitive.
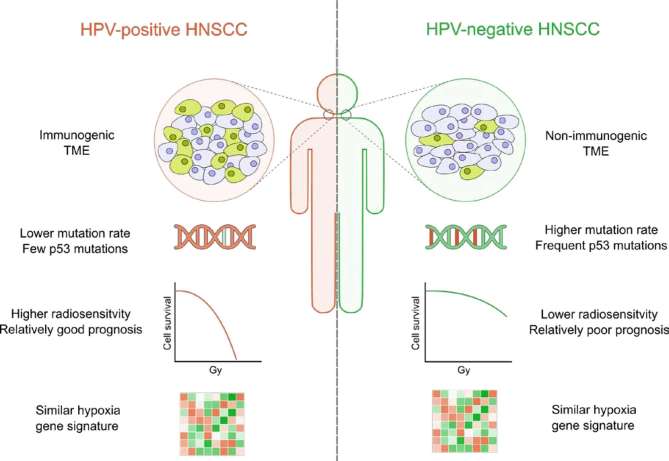
Figure 1.
Overview of the key characteristics of HPV-positive and HPV-negative HNSCC can be divided into HPV-positive and HPV-negative subgroups, with each having unique characteristics. Overall, patients with HPV-positive tumours have a better prognosis and are more radiosensitive. The overall mutation rate and frequency of p53 mutations is higher in HPV-negative tumours. Despite their different aetiology, both HPV-negative and HPV-positive tumours display a similar degree of hypoxia. HNSCC, head and neck squamous-cell carcinoma; HPV, human papilloma virus; TME, tumour microenvironment.
As a result of these clinical observations, several groups have investigated the in vitro mechanisms of radiosensitivity in HNSCC and specifically the contribution of HPV. A number of studies have now demonstrated that HPV-positive HNSCC cell lines are more sensitive to irradiation in vitro. 27–33 Understanding why HPV-positive HNSCC are more susceptible to irradiation, or HPV-negative more resistant, may lead to improved therapeutic approaches for both types. For OPSCCs it has been shown that the cellular mechanism underlying the increased radiosensitivity of HPV-positive HNSCC cells is, at least in part, due to a reduced capacity for repairing radiation-induced double strand breaks (DSB), as shown by a delayed resolution of yH2AX and 53BP1 foci (Figure 2).34 Interestingly, key components of base excision and single strand repair, including XRCC1, DNA polymerase β, PNKP and PARP-1, were found to be upregulated in HPV-positive OPSCC suggesting an increased ability to repair certain DNA lesions.34 Together, this could indicate that the increased basal radiosensitivity in HPV-positive HNSCC is specifically due to impaired DSB repair, as opposed to the repair of all types of damage. The deficiency in DSB repair combined with increased levels of PARP-1 observed in HPV-positive OPSCC cells suggested that PARP-1 inhibition might radiosensitise HPV-positive cells. Unexpectedly, olaparib increased in vitro radiosensitivity of HPV-negative OPSCC cells compared to HPV-positive cells where the PARP-1 inhibitor had no significant effect on tumour cell survival.34 Several other studies, which investigated the cause of radiosensitivity in HPV-positive HNSCC, found altered DNA damage repair pathways as well as differentially regulated cell cycle control in HPV-positive HNSCC. The viral oncoprotein E7 not only leads to loss of Rb, but also the degradation of the acetyltransferase Tip60, which is required for ATM activation upon DNA damage.35 In addition, E6 contributes, through an as yet undescribed mechanism, to the hypermethylation of the SMG-1 promoter. The consequential reduction of SMG-1 protein expression results in enhanced radiosensitivity, as SMG-1 is a DNA damage signalling transducer. In support of a role for SMG-1 in radiosensitivity, depletion of SMG-1 in HPV-negative HNSCC also increased radiosensitivity.36 E7 was also found to contribute to altered cell cycle regulation and DNA damage response (DDR). E7-mediated degradation of Rb leads to accumulation of p16, as it is no longer under transcriptional repression of Rb/E2F.37 The overexpression of p16 has been shown to be an important feature in HPV-associated radiosensitivity, and is commonly used in clinical practice to determine HPV status.38 Specifically, it was demonstrated that p16 overexpression leads to activation of the TRIP12-RNF168-53BP1 axis, and that by repressing TRIP12, an E3 ubiquitin-protein ligase, p16 overexpression ultimately leads to a delayed DDR.39 In another study, p16 expression was found to impair the homologous recombination (HR) pathway, the most faithful DSB repair mechanism, by preventing the recruitment of Rad51.28 Other studies found additional evidence for altered, delayed, or deficient DDR in HPV-positive HNSCC, and it was shown that expression of E7 correlated with increased yH2AX foci, albeit in human keratinocytes.40 Similarly, it has been demonstrated that HPV-positive cell lines accumulate more 53BP1 and yH2AX foci and display a marked G2/M arrest in response to radiation.30 This observation was supported by a subsequent study, where it was shown that E7-transgenic mice had retained 53BP1 and yH2AX foci without alterations in ATM or ATR levels.41 A further study observed a deficiency in DNA damage repair as shown by a delayed resolution of yH2AX foci, and attributed this to a decreased BRCA2 and DNA-PK expression, which resulted in aberrant non-homologous end joining (NHEJ) and HR signalling. Additionally, it could be shown that, while DNA damage repair effector proteins are affected, DNA damage sensing mechanisms are still intact.42 An alternative or complementary explanation for the increased radiosensitivity in HPV-positive HNSCC could be that, while E6 mediates p53 degradation, there is still residual activity of the p53 protein. This hypothesis was supported by the observation that further knockdown of p53 was possible in HPV-positive HNSCC cell lines and that this increased radiation resistance in HPV-positive cell lines.33 Taken together, the evidence demonstrates that HPV-positive HNSCC have altered DNA repair mechanisms, including NHEJ, HR, and mismatch repair, which strongly contributes to radiosensitivity. The roles that E6 and E7 play in this process are evident; however it is possible that other HPV driven mechanisms may have been overlooked.
Figure 2.
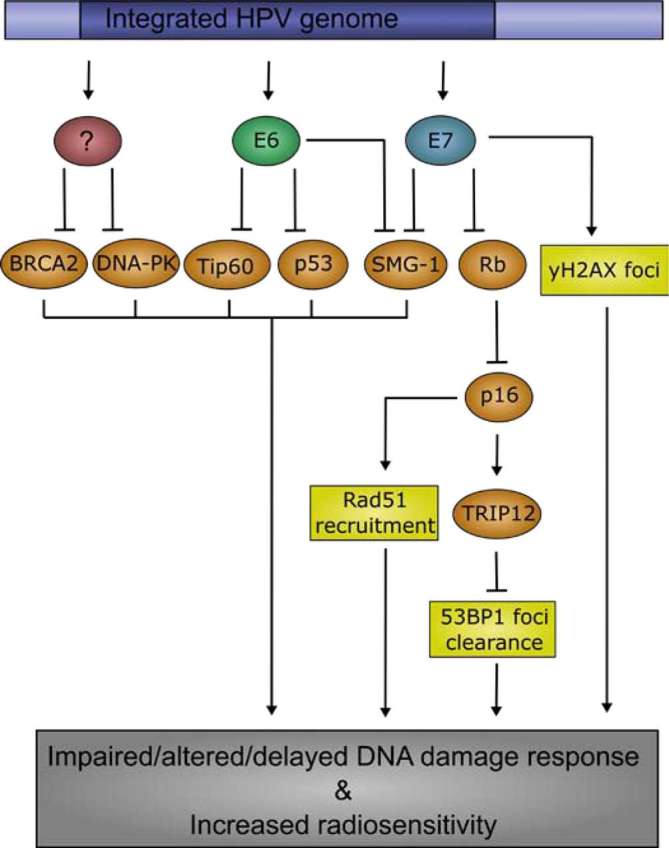
Mechanisms contributing to an altered DDR and increased radiosensitivity in HPV-positive HNSCC Infection with HPV, and subsequent expression of E6 and E7, contribute to an altered DDR in HNSCC tumour cells. E6, E7, and possibly other un-described mechanisms, repress DNA damage signalling transducers and effectors, such as SMG-1, p53, and BRCA2. Irradiation of HPV-positive tumour cells causes significantly more yH2AX and RAD51 foci than in HPV-negative tumour cells. Additionally, it is believed that HPV-positive tumour cells have a delay in DSB repair, as measured by 53BP1 foci clearance, indicating that HPV-positive tumour cells have impaired DSB repair mechanisms, contributing to their intrinsic radiosensitivity. DDR, DNA damage response; DSB, double strand breaks; HNSCC, head and neck squamous-cell carcinoma; HPV, human papilloma virus.
Hypoxia detection and patient stratification
In order to therapeutically exploit tumour hypoxia, overcome hypoxia-related radiation resistance, or select patients for hypoxia-modifying therapy, feasible and reliable methods of detection of clinically relevant tumour hypoxia are required. Approaches to detect tumour hypoxia in the clinic have historically included methods such as polarographic needle electrodes, measurement of endogenous or exogenous hypoxia tissue markers, and hypoxia imaging.43 Needle electrode measurements using oxygen probes provide a direct approach to detect oxygen partial pressure within the tumour, and have been shown to be prognostic in HNSCC.44 However, as the use of needle electrodes is an invasive procedure, they are limited to accessible tumours.45 In the clinical setting, this approach is also limited by methodological issues such as poor spatial resolution, oxygen consumption by the microelectrodes and biological heterogeneity of oxygenation within tumours. A solution to this is the use of hypoxia markers in biopsies or surgical material, obtained from the primary tumour or distant metastases. The most commonly investigated endogenous hypoxia markers include hypoxia inducible factor 1α (HIF-1α), glucose transporter 1 (GLUT-1), carbonic anhydrase IX (CAIX), vascular endothelial growth factor (VEGF), and the serological marker, osteopontin (OPN). HIF-1α overexpression has been associated with poor prognosis, advanced disease, an aggressive cancer phenotype and poor response to radiotherapy in a number of malignancies, including HNSCC.46 There is clear clinical utility to using hypoxia-related proteins such as CAIX or HIF-1α as surrogate markers of tumour hypoxia as they can be easily detected in tissue samples, have low assay costs, offer the potential of marker co-detection and the possibility of repeatedly detecting and monitoring biomarkers when samples are available. Extrinsic markers of hypoxia such as EF5 and pimonidazole are non-physiologic substances that are injected into the body where they accumulate under hypoxic conditions as a result of chemical reduction and covalent binding to macromolecules. EF5 and pimonidazole offer high spatial resolution, delineating hypoxia from anoxia/necrotic areas in which exogenous hypoxia markers do not accumulate, and were shown to be of prognostic value in patients with HNSCC.47, 48 While these hypoxia tissue markers, with the exception of serological OPN, provide an excellent spatial resolution as well as information on the chronic or acute state of hypoxia, markers such as OPN and CAIX are not entirely hypoxia-specific and are subject to the influence of other components of the tumour microenvironment including local acidity and the immune infiltrate.49 Hypoxia-related gene signatures have also been developed, which consist of a number of genes that are significantly upregulated in response to hypoxia. Evidence for the prognostic and predictive value of specific hypoxic gene signatures is increasing.50–52 These data demonstrated inferior clinical outcome in 323 HNSCC patients whose tumours were classified hypoxic according to a 15-gene hypoxia classifier previously determined in human squamous cell carcinoma xenograft tumours.53 This was later evaluated using alternative gene signatures in an independent HNSCC patient cohort (n = 302) and showed a prognostic impact of all three of the signatures tested and a successful discrimination of patients with low and high tumour hypoxia.54 Interestingly, the clinical outcome of patients with hypoxic tumours could be improved by the addition of the hypoxia modifier, nimorazole, in patients classified according to hypoxic gene signatures.53 As both oxygen electrode measurements and tissue/blood-based hypoxia markers only inform on tumour oxygenation at a specific time point, they require repeated readings or biopsies in order to monitor tumour oxygenation throughout treatment. Thus, non-invasive approaches such as positron emission tomography (PET) imaging with exogenous or endogenous hypoxia tracers are more realistic methods for clinical practice.55 PET, using the hypoxic tracer 18fluoromisonidazole (18F-MISO), has been shown to be a feasible and reproducible approach for visualising tumour hypoxia, offering a high correlation with tumour oxygenation in many types of human cancers including HNSCC.56–59 Moreover, 18F-MISO-PET was able to identify HNSCC patients who benefitted from additional treatment with the hypoxia-activated cytotoxin, tirapazamine (TPZ), and successfully predicted the risk for tumour recurrence after radiotherapy.60 Recently, 18F-MISO-PET was used to detect tumour hypoxia during radiotherapy of HNSCC and this subsequently identified patients at high risk for local recurrence.61 Other imaging-based methods for hypoxia detection include visualisation through single photon emission computed tomography-computed tomography with the use of radioactive tracers, consisting of a 111In/89Zr labelled antibody directed against endogenous hypoxic markers such as CAIX.62 While showing promising in vitro and in vivo data, the prognostic value of these agents in patients remain to be determined.
Advances in modifying and targeting tumour hypoxia in HNSCC
Through technological advances in radiation oncology, specifically the implementation of new treatment strategies for HNSCC such as intensity-modulated or volumetric-modulated arc radiotherapy, treatment-related toxicity has been significantly reduced and further reduction may come from the introduction of proton therapy.63–65 Treatment modification, such as addition of concomitant chemotherapy and the use of altered fractionation regimes (i.e. hyperfractionation), has improved clinical outcome and tumour control significantly in patients with locally advanced HNSCC.66 Moreover, the integration of biological targeted therapy such as EGFR-inhibition (cetuximab) and the use of hypoxic cell radiosensitisers in selected patients further increased overall survival and locoregional tumour control.53, 67 Strategies to target and modify hypoxia-mediated radiation resistance include hyperbaric oxygenation, the use of bioreductive compounds such as TPZ and accelerated radiotherapy with carbogen and nicotinamide.60, 68 Evidence shows a clinical impact of hypoxic modification on therapeutic outcome, predominantly on (loco-) regional tumour control and disease-free survival.69, 70 Hypoxia offers an opportunity to target and exploit tumour biology in the treatment of cancer. The principle behind hypoxia-activated prodrugs (HAP), which include N-oxides, metal complexes, nitro compounds and quinones, is that these inactive prodrugs undergo enzymatic reduction at low oxygen concentrations leading to the generation of cytotoxic species selectively in hypoxic cells.71–73 TPZ, which is an aromatic N-oxide HAP, showed favourable tumour control and increased failure-free overall survival when added to primary radiotherapy of locally advanced HNSCC, although there was evidence of augmented hematologic toxicity in hypoxic tumours.60, 74,75 Late phase clinical trials failed to demonstrate a significant survival benefit when TPZ was combined with radiotherapy and cisplatin for HNSCC thereby highlighting the necessity of patient stratification for hypoxia modulating therapies.76 Among the nitro-based HAPs, nimorazole can be regarded as the only hypoxic radiosensitiser that has been translated into clinical practice. The results of a large phase III clinical trial of the DAHANCA demonstrated significantly improved loco-regional tumour control at 4 years (49% vs 33% without nimorazole, p = 0.002) and reduced cancer-related deaths (52% vs 41%, p = 0.002), and lead to the implementation of nimorazole as the standard of care in Danish HNSCC cases.77 Currently, a multicentre phase III trial (NIMRAD) is accruing patients to confirm the DAHANCA findings (NCT01950689). Results from this trial are likely to dictate whether hypoxia modification using nimorazole is adopted in combination with radiotherapy for both HPV- and HPV + HNSCC. Related strategies in this field include the development of DNA-targeting HAP such as TH-302 and PR-104, which induce cell death through DNA crosslinking.73, 78,79 Despite promising data on TH-302 in combination with radiotherapy in other cancer types, preclinical data using HNSCC cells as tumour xenografts showed these cells were resistant to TH-302 alone and in combination with radiation.80 Recently, it was reported for HNSCC that there is higher expression of cytochrome P450 oxidoreductase (POR) in HPV-negative tumours. Knockdown of POR results in reduced sensitivity to TH-302 under hypoxia, suggesting POR as a potential predictive biomarker of HAP sensitivity and a possible relationship between HPV status and HAP sensitivity.81 Importantly, not only do a considerable number of HNSCC cases express significant levels of POR, but a subset of these carcinomas was classified as hypoxic by 18-F-MISO PET, confirming coincidence of the two targets, hypoxia and POR.81
An alternative method for targeting solid tumours is the use of non-pathogenic anaerobic or facultative anaerobic bacteria such as Clostridia, Salmonella or Bifidobacteria.82, 83 The rationale behind this approach is that the unique properties of the tumour microenvironment including hypoxia, anoxia and necrosis can be used as a selective target since they provide favourable conditions for anaerobic bacteria to colonise.84 Moreover, anaerobic bacteria may be used as delivery vehicles for gene-based therapies in order to express anti-cancer proteins within solid tumours.85 However, studies in HNSCC have yet to be carried out and the efficacy and persistence of gene transfer and bloodstream stability remain major challenges.86 Notably, hypoxia may not be the only element of the tumour microenvironment that is responsible for selective bacterial colonisation in solid tumours, as immune infiltrate, tumour vasculature and pH constitute other crucial factors that play an important role in anaerobic bacterial targeting of solid tumours.87
HPV and the tumour microenvironment
Aside from the intrinsic cellular radiosensitivity of HPV-positive HNSCC, it is important to recognise that other factors, such as the tumour microenvironment, contribute to the in vivo response to radiation. HPV-positive tumours have been found to have striking differences in the tumour microenvironment. E6 and E7 specific antibodies were detected in sera of HPV-positive HNSCC patients, and correlated with improved survival, indicating that there is an active immune response against HPV in patients.88, 89 Multiple studies show that in HPV-positive HNSCC, a shift towards more CD4+ and CD8+ effector T-cells (as opposed to naïve T-cells) could be observed. This HPV-mediated shift in immune response was also associated with increased production of pro-inflammatory cytokines TNF-α and IFN-γ, and a distinct B-cell signature.90–92 While these studies did not establish a causal link for increased radiosensitivity, it has been hypothesised that in HPV-positive HNSCC, cell injury and local inflammation after irradiation may effectively contribute to increased antigen presentation and enhanced recruitment of cytotoxic immune cells.27 Taken together, it is clear that the immune system is involved in the systemic and local anti-tumour response in terms of radiotherapy response. While strong causal relationships are still lacking, several studies suggest that the immune cell rich tumour microenvironment of HPV-positive HNSCC may contribute to their increased radiosensitivity.
Clinical data suggest there is no significant difference in the level, nor distribution of hypoxia in HPV-positive and HPV-negative tumours, as measured by a 15-gene hypoxia classifier and 18F-MISO PET.53, 93 To date, the expression of two of the key viral proteins, E6 and E7, have been shown to be altered in response to hypoxic conditions. In response to hypoxia, the interaction between E6, the ubiquitin ligase E6AP and p53 is abrogated, and therefore p53 can be stabilised in response to hypoxia in HPV-positive cell lines.94 More recently, exposure to hypoxia was also found to repress the expression of both E6 and E7 in a range of cervical cell lines.95 Interestingly, repression of E6 and E7 did not induce senescence in these cells, yet rather forced them into a dormant state, which upon reoxygenation was quickly reversed. It was suggested that the repression of E6 and E7 could provide a mechanism to escape E6/E7 specific therapeutic approaches, such as E6/E7 vaccines, or perhaps anti-tumour immune cells, as well as that the dormant tumour cells may serve as a reservoir for repopulation after reoxygenation.95
In HPV-positive tumours there is evidence for immune-related phenomena, such as increased intratumoural immune-cell infiltration, that could further potentiate the response to therapy including radiotherapy.96 However, there are also data suggesting that tumour infiltrating lymphocytes may not be limited to HPV-positive tumours.97 This underlines the necessity for further studies evaluating the prognostic impact of the immune component on the outcome after radiotherapy for HNSCC, specifically in the context of HPV infection. Hypoxia can directly affect the immune cells present in the tumour and their function. Exposure to hypoxia alters IL-6, IL-10 and TGF-β cytokine levels and the expression of PDL1, the last of which is mediated by HIF-1α.98 Yet, there is no evidence that HPV-positivity is associated with HIF-1α expression in OPSCC and the prognostic impact of other hypoxia-related parameters such as CAIX or microvascular density seems to be independent of HPV-status.99, 100 Additionally, hypoxic tumours appear to contain various types of immune cells, such as regulatory T-cells, tumour-associated macrophages, myeloid- derived suppressor cells, which together, exert an immunosuppressive function.101 While it has not yet directly been investigated, it is highly likely that hypoxia will affect the immune-permissive tumour microenvironment of HPV-positive tumours, and shift its balance towards a more immunosuppressive state. Further investigation of both the altered DDR and effects of the immune system in HPV-positiveHNSCC could lead to useful therapeutic targets/strategies for the treatment of HPV-negative tumours.
HPV, hypoxia and radiation response
An in vitro study showed that, as expected, both HPV-positive and HPV-negative HNSCC cell lines display decreased radiosensitivity when irradiated in hypoxic conditions. The oxygen enhancement ratios were found to be similar (2.3–2.9) when both HPV-positive and HPV-negative cell lines were irradiated under hypoxia.29 As previously mentioned, it has been confirmed that HPV-positive tumours have a similar degree and distribution of hypoxia compared to HPV-negative tumours.17, 53,93 In vivo it has been demonstrated that in HPV-positive xenograft tumours in nude mice, cell proliferation decreased significantly upon irradiation, as opposed to HPV-negative tumours. Furthermore, after irradiation, the hypoxic fraction was reduced over time in HPV-positive tumours.102 This observation is supported by earlier observations that irradiation of HPV-positive cell lines induces a G2/M arrest, and can thereby effectively repress cell proliferation and thus oxygen consumption.30, 33 Together, this suggests that the higher radiosensitivity of HPV-positive tumours may be caused by a radiation-induced decrease of the hypoxic fraction and proliferating cells.102 These mechanisms, while not yet fully understood, may prove to be essential in determining the optimal use of hypoxia modification therapy in HNSCC. While in vitro, hypoxic HPV-positive cell lines could be radiosensitised by nimorazole, there is no clinical evidence of its efficacy in patient populations.29 Indeed, clinical studies have shown that patients have improved locoregional control when combining irradiation with nimorazole, but that this effect is limited to HPV-negative HNSCC patients.18, 53,103 This suggests hypoxia modification may be a less effective and unnecessary treatment option for HPV-positive HNSCC. Further studies will be required to determine the added value of hypoxia modification in HNSCC and to bring more clarity to the mechanisms involved. Additionally, these future studies would require a consistent and well-defined manner of measuring tumour hypoxia and detecting HPV status.
Summary
HNSCC can be divided in two distinct tumour types based on HPV infection, and the incidence of HPV-positive HNSCC has been increasing over the last decades. It has been conclusively demonstrated that patients with HPV-positive tumours have a better overall prognosis, and that these tumours are more sensitive to radiotherapy. However, so far there have been no changes in the treatment strategy for either HPV-positive or HPV-negative HNSCC. By investigating the mechanisms causing HPV-positive tumours to be more sensitive, possible targets for treatment of HPV-negative HNSCC could be identified. HPV-positive tumours express the E6 and E7 oncoproteins, which are a causal factor for oncogenesis. In addition, the tumour microenvironment of HPV-positive tumours contains elevated numbers of immune cells and pro-inflammatory cytokines, which may contribute to a more efficient tumour clearance after irradiation. Hypoxia, an overall negative prognostic marker, affects HPV-positive and HPV-negative HNSCC equally, reducing the effectiveness of radiotherapy. Several methods to detect and quantify levels of hypoxia have been established, and can be used clinically to predict treatment response. Concurrently, hypoxia-targeting or hypoxia-modifying therapies have been developed which can effectively radiosensitise hypoxic tumour cells. Despite observations that HPV-positive tumours have an equal hypoxic fraction as HPV-negative tumours, hypoxia-modifying strategies such as nimorazole treatment have been shown to be ineffective in HPV-positive HNSCC. This contributes to the notion that HPV-positive and HPV-negative HNSCC are two distinct disease types, which require individual treatment optimisation. Further, and larger, studies are needed in the future to determine mechanisms that contribute to radiosensitivity, optimal treatment strategies, and the effect hypoxia has on this.
ACKNOWLEDGMENTS
Thank you to Dr Ishna Mistry for critical feedback on the manuscript. EMH is supported by Cancer Research UK (C9720/A18513). CO is supported by the German Society of Radiation Oncology and the German Cancer Society.
Contributor Information
Christian Ostheimer, Email: Eva-Leonne.Gottgens@radboudumc.nl.
Paul N Span, Email: Paul.Span@radboudumc.nl.
Jan Bussink, Email: christian.ostheimer@oncology.ox.ac.uk.
Ester M Hammond, Email: ester.hammond@oncology.ox.ac.uk.
REFERENCES
- 1. Poole ME, Sailer SL, Rosenman JG, Tepper JE, Weissler MC, Shockley WW, et al. Chemoradiation for locally advanced squamous cell carcinoma of the head and neck for organ preservation and palliation. Arch Otolaryngol Head Neck Surg 2001; 127: 1446–50. [DOI] [PubMed] [Google Scholar]
- 2. Vaupel P, Kelleher DK, Höckel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 2001; 28(2 Suppl 8): 29–35. [DOI] [PubMed] [Google Scholar]
- 3. Olcina M, Lecane PS, Hammond EM. Targeting hypoxic cells through the DNA damage response. Clin Cancer Res 2010; 16: 5624–9. doi: 10.1158/1078-0432.CCR-10-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 2008; 8: 180–92. doi: 10.1038/nrc2344 [DOI] [PubMed] [Google Scholar]
- 5. Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res 2012; 18: 5585–94. doi: 10.1158/1078-0432.CCR-12-0858 [DOI] [PubMed] [Google Scholar]
- 6. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015; 33: 3235–42. doi: 10.1200/JCO.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mallen-St Clair J, Alani M, Wang MB, Srivatsan ES. Human papillomavirus in oropharyngeal cancer: the changing face of a disease. Biochim Biophys Acta 2016; 1866: 141–50. doi: 10.1016/j.bbcan.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 9. Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer 2017; 123: 2219–29. doi: 10.1002/cncr.30588 [DOI] [PubMed] [Google Scholar]
- 10. Pereira R, Hitzeroth II, Rybicki EP. Insights into the role and function of L2, the minor capsid protein of papillomaviruses. Arch Virol 2009; 154: 187–97. doi: 10.1007/s00705-009-0310-3 [DOI] [PubMed] [Google Scholar]
- 11. Schuck S, Ruse C, Stenlund A. CK2 phosphorylation inactivates DNA binding by the papillomavirus E1 and E2 proteins. J Virol 2013; 87: 7668–79. doi: 10.1128/JVI.00345-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruttkay-Nedecky B, Jimenez Jimenez AM, Nejdl L, Chudobova D, Gumulec J, Masarik M, et al. Relevance of infection with human papillomavirus: the role of the p53 tumor suppressor protein and E6/E7 zinc finger proteins (Review). Int J Oncol 2013; 43: 1754–62. doi: 10.3892/ijo.2013.2105 [DOI] [PubMed] [Google Scholar]
- 13. Münger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 2001; 20: 7888–98. doi: 10.1038/sj.onc.1204860 [DOI] [PubMed] [Google Scholar]
- 14. Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res 2008; 14: 366–9. doi: 10.1158/1078-0432.CCR-07-1402 [DOI] [PubMed] [Google Scholar]
- 15. Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011; 333: 1157–60. doi: 10.1126/science.1208130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol 2006; 24: 2606–11. doi: 10.1200/JCO.2006.06.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eriksen JG, Lassen P. Human papilloma virus as a biomarker for personalized head and neck cancer radiotherapy. Recent Results Cancer Res 2016; 198: 143–61. doi: 10.1007/978-3-662-49651-0_7 [DOI] [PubMed] [Google Scholar]
- 18. Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. . HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol 2010; 94: 30–5. doi: 10.1016/j.radonc.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 19. Lassen P, Overgaard J, Eriksen JG. Expression of EGFR and HPV-associated p16 in oropharyngeal carcinoma: correlation and influence on prognosis after radiotherapy in the randomized DAHANCA 5 and 7 trials. Radiother Oncol 2013; 108: 489–94. doi: 10.1016/j.radonc.2013.08.036 [DOI] [PubMed] [Google Scholar]
- 20. Lassen P, Primdahl H, Johansen J, Kristensen CA, Andersen E, Andersen LJ, et al . Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol 2014; 113: 310–6. doi: 10.1016/j.radonc.2014.11.032 [DOI] [PubMed] [Google Scholar]
- 21. Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 2001; 92: 805–13. [DOI] [PubMed] [Google Scholar]
- 22. Tehrany N, Kitz J, Rave-Fränk M, Lorenzen S, Li L, Küffer S, et al. High-grade acute organ toxicity and p16(INK4A) expression as positive prognostic factors in primary radio(chemo)therapy for patients with head and neck squamous cell carcinoma. Strahlenther Onkol 2015; 191: 566–72. doi: 10.1007/s00066-014-0801-3 [DOI] [PubMed] [Google Scholar]
- 23. Lohaus F, Linge A, Tinhofer I, Budach V, Gkika E, Stuschke M, et al . HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother Oncol 2014; 113: 317–23. doi: 10.1016/j.radonc.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 24. Lassen P, Lacas B, Pignon J-P, Trotti A, Zackrisson B, Zhang Q, et al. Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: The MARCH-HPV project. Radiotherapy and Oncology 2017; 126: 107–15. doi: 10.1016/j.radonc.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 25. Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27: 1992–8. doi: 10.1200/JCO.2008.20.2853 [DOI] [PubMed] [Google Scholar]
- 26. Lassen P, Eriksen JG, Krogdahl A, Therkildsen MH, Ulhøi BP, Overgaard M, et al . The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol 2011; 100: 49–55. doi: 10.1016/j.radonc.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 27. Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg 2009; 135: 1137–46. doi: 10.1001/archoto.2009.159 [DOI] [PubMed] [Google Scholar]
- 28. Dok R, Abbasi Asbagh L, Van Limbergen EJ, Sablina A, Nuyts S. Nuclear p16INK4a expression predicts enhanced radiation response in head and neck cancers. Oncotarget 2016; 7: 38785–95. doi: 10.18632/oncotarget.9609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sørensen BS, Busk M, Olthof N, Speel EJ, Horsman MR, Alsner J, et al. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother Oncol 2013; 108: 500–5. doi: 10.1016/j.radonc.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 30. Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol 2013; 107: 242–6. doi: 10.1016/j.radonc.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 31. Nagel R, Martens-de Kemp SR, Buijze M, Jacobs G, Braakhuis BJ, Brakenhoff RH. Treatment response of HPV-positive and HPV-negative head and neck squamous cell carcinoma cell lines. Oral Oncol 2013; 49: 560–6. doi: 10.1016/j.oraloncology.2013.03.446 [DOI] [PubMed] [Google Scholar]
- 32. Arenz A, Ziemann F, Mayer C, Wittig A, Dreffke K, Preising S, et al. Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther Onkol 2014; 190: 839–46. doi: 10.1007/s00066-014-0605-5 [DOI] [PubMed] [Google Scholar]
- 33. Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 2013; 73: 4791–800. doi: 10.1158/0008-5472.CAN-13-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nickson CM, Moori P, Carter RJ, Rubbi CP, Parsons JL. Misregulation of DNA damage repair pathways in HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity. Oncotarget 2017; 8: 29963–75. doi: 10.18632/oncotarget.16265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jha S, Vande Pol S, Banerjee NS, Dutta AB, Chow LT, Dutta A. Destabilization of TIP60 by human papillomavirus E6 results in attenuation of TIP60-dependent transcriptional regulation and apoptotic pathway. Mol Cell 2010; 38: 700–11. doi: 10.1016/j.molcel.2010.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gubanova E, Brown B, Ivanov SV, Helleday T, Mills GB, Yarbrough WG, et al. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res 2012; 18: 1257–67. doi: 10.1158/1078-0432.CCR-11-2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol 2013; 2: 51–61. doi: 10.5539/cco.v2n1p51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prigge ES, Arbyn M, von Knebel Doeberitz M, Reuschenbach M. Diagnostic accuracy of p16INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. Int J Cancer 2017; 140: 1186–98. doi: 10.1002/ijc.30516 [DOI] [PubMed] [Google Scholar]
- 39. Wang L, Zhang P, Molkentine DP, Chen C, Molkentine JM, Piao H, et al. TRIP12 as a mediator of human papillomavirus/p16-related radiation enhancement effects. Oncogene 2017; 36: 820–8. doi: 10.1038/onc.2016.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res 2002; 62: 7075–82. [PubMed] [Google Scholar]
- 41. Park JW, Nickel KP, Torres AD, Lee D, Lambert PF, Kimple RJ. Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother Oncol 2014; 113: 337–44. doi: 10.1016/j.radonc.2014.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weaver AN, Cooper TS, Rodriguez M, Trummell HQ, Bonner JA, Rosenthal EL, et al. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget 2015; 6: 26995–7007. doi: 10.18632/oncotarget.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hammond EM, Asselin MC, Forster D, O'Connor JP, Senra JM, Williams KJ. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol 2014; 26: 277–88. doi: 10.1016/j.clon.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 44. Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997; 38: 285–9. [DOI] [PubMed] [Google Scholar]
- 45. Stone HB, Brown JM, Phillips TL, Sutherland RM. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Summary of a workshop held November 19-20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat Res 1993; 136: 422–34. [PubMed] [Google Scholar]
- 46. Swartz JE, Pothen AJ, van Kempen PM, Stegeman I, Formsma FK, Cann EM, et al. Poor prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinomas that overexpress hypoxia inducible factor-1α. Head Neck 2016; 38: 1338–46. doi: 10.1002/hed.24445 [DOI] [PubMed] [Google Scholar]
- 47. Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res 2002; 62: 7066–74. [PubMed] [Google Scholar]
- 48. Evans SM, Jenkins WT, Joiner B, Lord EM, Koch CJ. 2-Nitroimidazole (EF5) binding predicts radiation resistance in individual 9L s.c. tumors. Cancer Res 1996; 56: 405–11. [PubMed] [Google Scholar]
- 49. Panisova E, Kery M, Sedlakova O, Brisson L, Debreova M, Sboarina M, et al. Lactate stimulates CA IX expression in normoxic cancer cells. Oncotarget 2017; 8: 77819–35. doi: 10.18632/oncotarget.20836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eustace A, Mani N, Span PN, Irlam JJ, Taylor J, Betts GN, et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin Cancer Res 2013; 19: 4879–88. doi: 10.1158/1078-0432.CCR-13-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toustrup K, Sørensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 2011; 71: 5923–31. doi: 10.1158/0008-5472.CAN-11-1182 [DOI] [PubMed] [Google Scholar]
- 52. Toustrup K, Sørensen BS, Alsner J, Overgaard J. Hypoxia gene expression signatures as prognostic and predictive markers in head and neck radiotherapy. Semin Radiat Oncol 2012; 22: 119–27. doi: 10.1016/j.semradonc.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 53. Toustrup K, Sørensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J, et al . Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 2012; 102: 122–9. doi: 10.1016/j.radonc.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 54. Tawk B, Schwager C, Deffaa O, Dyckhoff G, Warta R, Linge A, et al. Comparative analysis of transcriptomics based hypoxia signatures in head- and neck squamous cell carcinoma. Radiother Oncol 2016; 118: 350–8. doi: 10.1016/j.radonc.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 55. Bussink J, van Herpen CM, Kaanders JH, Oyen WJ. PET-CT for response assessment and treatment adaptation in head and neck cancer. Lancet Oncol 2010; 11: 661–9. doi: 10.1016/S1470-2045(09)70353-5 [DOI] [PubMed] [Google Scholar]
- 56. Spence AM, Muzi M, Swanson KR, O'Sullivan F, Rockhill JK, Rajendran JG, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res 2008; 14: 2623–30. doi: 10.1158/1078-0432.CCR-07-4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eschmann SM, Paulsen F, Reimold M, Dittmann H, Welz S, Reischl G, et al. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med 2005; 46: 253–60. [PubMed] [Google Scholar]
- 58. Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys 1996; 36: 417–28. [DOI] [PubMed] [Google Scholar]
- 59. Bussink J, Kaanders JH, van der Graaf WT, Oyen WJ. PET-CT for radiotherapy treatment planning and response monitoring in solid tumors. Nat Rev Clin Oncol 2011; 8: 233–42. doi: 10.1038/nrclinonc.2010.218 [DOI] [PubMed] [Google Scholar]
- 60. Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, et al . Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006; 24: 2098–104. doi: 10.1200/JCO.2005.05.2878 [DOI] [PubMed] [Google Scholar]
- 61. Löck S, Perrin R, Seidlitz A, Bandurska-Luque A, Zschaeck S, Zöphel K, et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother Oncol 2017; 124: 533–40. doi: 10.1016/j.radonc.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 62. Huizing FJ, Hoeben BAW, Franssen G, Lok J, Heskamp S, Oosterwijk E, et al. Preclinical validation of111 In-girentuximab-F(ab')2 as a tracer to image hypoxia related marker CAIX expression in head and neck cancer xenografts. Radiother Oncol 2017; 124: 521–5. doi: 10.1016/j.radonc.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 63. Eekers DBP, Roelofs E, Jelen U, Kirk M, Granzier M, Ammazzalorso F, et al. Benefit of particle therapy in re-irradiation of head and neck patients. Results of a multicentric in silico ROCOCO trial. Radiother Oncol 2016; 121: 387–94. doi: 10.1016/j.radonc.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 64. Moncharmont C, Vallard A, Mengue Ndong S, Guy JB, Saget C, Méry B, et al. Real-life assessment of volumetric modulated arc therapy (VMAT) toxicity in head and neck squamous cell carcinoma (HNSCC) treatment. Acta Otolaryngol 2016; 136: 181–8. doi: 10.3109/00016489.2015.1101783 [DOI] [PubMed] [Google Scholar]
- 65. Sulman EP, Schwartz DL, Le TT, Ang KK, Morrison WH, Rosenthal DI, et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys 2009; 73: 399–409. doi: 10.1016/j.ijrobp.2008.04.021 [DOI] [PubMed] [Google Scholar]
- 66. Corvò R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother Oncol 2007; 85: 156–70. doi: 10.1016/j.radonc.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 67. Levy A, Blanchard P, Bellefqih S, Brahimi N, Guigay J, Janot F, et al. Concurrent use of cisplatin or cetuximab with definitive radiotherapy for locally advanced head and neck squamous cell carcinomas. Strahlentherapie und Onkologie 2014; 190: 823–31. doi: 10.1007/s00066-014-0626-0 [DOI] [PubMed] [Google Scholar]
- 68. Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 2012; 30: 1777–83. doi: 10.1200/JCO.2011.35.9315 [DOI] [PubMed] [Google Scholar]
- 69. Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck-a systematic review and meta-analysis. Radiother Oncol 2011; 100: 22–32. doi: 10.1016/j.radonc.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 70. Hassan Metwally MA, Ali R, Kuddu M, Shouman T, Strojan P, Iqbal K, et al. IAEA-HypoX. A randomized multicenter study of the hypoxic radiosensitizer nimorazole concomitant with accelerated radiotherapy in head and neck squamous cell carcinoma. Radiother Oncol 2015; 116: 15–20. doi: 10.1016/j.radonc.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 71. Guise CP, Mowday AM, Ashoorzadeh A, Yuan R, Lin WH, Wu DH, et al. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J Cancer 2014; 33: 80–6. doi: 10.5732/cjc.012.10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Phillips RM. Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs. Cancer Chemother Pharmacol 2016; 77: 441–57. doi: 10.1007/s00280-015-2920-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mistry IN, Thomas M, Calder EDD, Conway SJ, Hammond EM. Clinical advances of hypoxia-activated prodrugs in combination with radiation therapy. Int J Radiat Oncol Biol Phys 2017; 98: 1183–96. doi: 10.1016/j.ijrobp.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 74. Lee DJ, Trotti A, Spencer S, Rostock R, Fisher C, von Roemeling R, et al. Concurrent tirapazamine and radiotherapy for advanced head and neck carcinomas: a Phase II study. Int J Radiat Oncol Biol Phys 1998; 42: 811–5. [DOI] [PubMed] [Google Scholar]
- 75. Le QT, Taira A, Budenz S, Jo Dorie M, Goffinet DR, Fee WE, et al. Mature results from a randomized Phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable Stage IV head and neck squamous cell carcinomas. Cancer 2006; 106: 1940–9. doi: 10.1002/cncr.21785 [DOI] [PubMed] [Google Scholar]
- 76. Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 2010; 28: 2989–95. doi: 10.1200/JCO.2009.27.4449 [DOI] [PubMed] [Google Scholar]
- 77. Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol 1998; 46: 135–46. [DOI] [PubMed] [Google Scholar]
- 78. Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, et al. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2015; 33: 1475–81. doi: 10.1200/JCO.2014.55.7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peeters SG, Zegers CM, Biemans R, Lieuwes NG, van Stiphout RG, Yaromina A, et al. TH-302 in combination with radiotherapy enhances the therapeutic outcome and is associated with pretreatment [18F]HX4 hypoxia PET imaging. Clin Cancer Res 2015; 21: 2984–92. doi: 10.1158/1078-0432.CCR-15-0018 [DOI] [PubMed] [Google Scholar]
- 80. Nytko KJ, Grgic I, Bender S, Ott J, Guckenberger M, Riesterer O, et al. The hypoxia-activated prodrug evofosfamide in combination with multiple regimens of radiotherapy. Oncotarget 2017; 8: 23702–12. doi: 10.18632/oncotarget.15784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hunter FW, Young RJ, Shalev Z, Vellanki RN, Wang J, Gu Y, et al. Identification of P450 oxidoreductase as a major determinant of sensitivity to hypoxia-activated prodrugs. Cancer Res 2015; 75: 4211–23. doi: 10.1158/0008-5472.CAN-15-1107 [DOI] [PubMed] [Google Scholar]
- 82. Theys J, Barbé S, Landuyt W, Nuyts S, Van Mellaert L, Wouters B, et al. Tumor-specific gene delivery using genetically engineered bacteria. Curr Gene Ther 2003; 3: 207–21. [DOI] [PubMed] [Google Scholar]
- 83. Wei MQ, Mengesha A, Good D, Anné J. Bacterial targeted tumour therapy-dawn of a new era. Cancer Lett 2008; 259: 16–27. doi: 10.1016/j.canlet.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 84. Wei MQ, Ellem KA, Dunn P, West MJ, Bai CX, Vogelstein B. Facultative or obligate anaerobic bacteria have the potential for multimodality therapy of solid tumours. Eur J Cancer 2007; 43: 490–6. doi: 10.1016/j.ejca.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 85. Alimoradi H, Matikonda SS, Gamble AB, Giles GI, Greish K. Hypoxia responsive drug delivery systems in tumor therapy. Curr Pharm Des 2016; 22: 2808–20. [DOI] [PubMed] [Google Scholar]
- 86. Ryan RM, Green J, Williams PJ, Tazzyman S, Hunt S, Harmey JH, et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther 2009; 16: 329–39. doi: 10.1038/gt.2008.188 [DOI] [PubMed] [Google Scholar]
- 87. Morrissey D, O'Sullivan GC, Tangney M. Tumour targeting with systemically administered bacteria. Curr Gene Ther 2010; 10: 3–14. [DOI] [PubMed] [Google Scholar]
- 88. Zumbach K, Hoffmann M, Kahn T, Bosch F, Gottschlich S, Görögh T, et al. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in patients with head-and-neck squamous-cell carcinoma. Int J Cancer 2000; 85: 815–8. [DOI] [PubMed] [Google Scholar]
- 89. Dahlstrom KR, Anderson KS, Cheng JN, Chowell D, Li G, Posner M, et al. HPV serum antibodies as predictors of survival and disease progression in patients with HPV-positive squamous cell carcinoma of the oropharynx. Clin Cancer Res 2015; 21: 2861–9. doi: 10.1158/1078-0432.CCR-14-3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015; 4: e965570. doi: 10.4161/21624011.2014.965570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wood O, Woo J, Seumois G, Savelyeva N, McCann KJ, Singh D, et al . Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 2016; 7: 56781–97. doi: 10.18632/oncotarget.10788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Turksma AW, Bontkes HJ, van den Heuvel H, de Gruijl TD, von Blomberg BM, Braakhuis BJ, et al. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis 2013; 19: 577–84. doi: 10.1111/odi.12037 [DOI] [PubMed] [Google Scholar]
- 93. Trinkaus ME, Hicks RJ, Young RJ, Peters LJ, Solomon B, Bressel M, et al. Correlation of p16 status, hypoxic imaging using [18F]-misonidazole positron emission tomography and outcome in patients with loco-regionally advanced head and neck cancer. J Med Imaging Radiat Oncol 2014; 58: 89–97. doi: 10.1111/1754-9485.12155 [DOI] [PubMed] [Google Scholar]
- 94. Alarcón R, Koumenis C, Geyer RK, Maki CG, Giaccia AJ. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res 1999; 59: 6046–51. [PubMed] [Google Scholar]
- 95. Hoppe-Seyler K, Bossler F, Lohrey C, Bulkescher J, Rösl F, Jansen L, et al. Induction of dormancy in hypoxic human papillomavirus-positive cancer cells. Proc Natl Acad Sci U S A 2017; 114: E990–E98. doi: 10.1073/pnas.1615758114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mirghani H, Amen F, Tao Y, Deutsch E, Levy A. Increased radiosensitivity of HPV-positive head and neck cancers: molecular basis and therapeutic perspectives. Cancer Treat Rev 2015; 41: 844–52. doi: 10.1016/j.ctrv.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 97. Balermpas P, Rödel F, Rödel C, Krause M, Linge A, Lohaus F, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer 2016; 138: 171–81. doi: 10.1002/ijc.29683 [DOI] [PubMed] [Google Scholar]
- 98. Noman MZ, Chouaib S. Targeting hypoxia at the forefront of anticancer immune responses. Oncoimmunology 2014; 3: e954463. doi: 10.4161/21624011.2014.954463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ou D, Garberis I, Adam J, Blanchard P, Nguyen F, Levy A, et al. Prognostic value of tissue necrosis, hypoxia-related markers and correlation with HPV status in head and neck cancer patients treated with bio- or chemo-radiotherapy. Radiother Oncol 2018; 126: 116–24. doi: 10.1016/j.radonc.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 100. Hong A, Zhang M, Veillard AS, Jahanbani J, Lee CS, Jones D, et al. The prognostic significance of hypoxia inducing factor 1-α in oropharyngeal cancer in relation to human papillomavirus status. Oral Oncol 2013; 49: 354–9. doi: 10.1016/j.oraloncology.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 101. Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, et al. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 2015; 309: C569–C579. doi: 10.1152/ajpcell.00207.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sørensen BS, Busk M, Horsman MR, Alsner J, Overgaard J, Kyle AH, et al. Effect of radiation on cell proliferation and tumor hypoxia in HPV-positive head and neck cancer in vivo models. Anticancer Res 2014; 34: 6297–304. [PubMed] [Google Scholar]
- 103. Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol 2010; 28: 4142–8. doi: 10.1200/JCO.2010.29.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]