Abstract
Fusion of lipid bilayers to deliver genetic information is a process common to both viral infection and fertilization, and the two share common molecular mechanisms. Now, identification of fusion-facilitators shows that plants have their own unique slant on the fusion process.
Discerning the mechanisms that underlie gamete membrane fusion has been challenging. For many animals, including mouse and human, a gamete membrane fusogen — a molecule that facilitates the fusion of two membranes — has yet to be identified. Studies of fertilization in protists, unicellular algae, invertebrates and higher plants, however, are more advanced and have revealed a conserved membrane fusion reaction as well as a conserved fusogen1, the broadly conserved, single-pass transmembrane protein HAP2 (also called GCS1). Its distribution across kingdoms indicates that HAP2 is an ancient protein and was likely used for gamete fusion by the first sexual eukaryotes2. HAP2 is a structural homologue of the class II fusion proteins used by many viruses, including Dengue and Zika. Like its viral counterparts3, HAP2 functions uni-directionally. Its single transmembrane domain anchors it in the membrane of one gamete (for example a sperm) and during fusion a distal segment inserts itself into the target cell (an egg’s) membrane4–6.
Although the structure and core functions of HAP2 and viral class II fusion proteins are conserved, their working conditions are profoundly different. The sole function of the viral envelope is to display its fusion protein at maximum density in preparation for fusing with the target cell membrane to deliver the viral genome. In contrast, HAP2 is just one of many proteins on the dynamic and multi-functional plasma membrane of gametes of eukaryotic organisms. In this issue of Nature Plants, Cyprys et al.7 report that HAP2-based fusion in Arabidopsis does indeed differ from viral class II fusion mechanisms, and that the fusion reaction works better with a little help from two conserved, short, multi-pass integral membrane proteins DMP8 and DMP9. Fertilization is reduced over 2-fold in Arabidopsis mutants lacking sperm-specific DMP8 and DMP9.
In a related paper, Takahashi et al.8 also uncovered a role for DMP9 in fertilization, showing that RNAi-induced knockdown of DMP9 led to a modest (~20%), but consistent, reduction in double fertilization. Both groups found that the dmp mutant sperm that had failed to fuse underwent normal adhesion with the egg cell. Cyprys et al. further showed that HAP2 expression was not affected in the sperm pair of the double mutant, lending support to their model that the DMPs function after delivery of sperm from the pollen tube to the ovule.
Consideration of viral class II fusion mechanisms offers insights into possible steps that might need help in HAP2-based gamete fusion (Fig. 1). In contrast to the complex plasma membranes of gametes, the envelopes of all viruses that use class II fusogens are simple and composed primarily of the fusogen itself. After endocytosis by a target cell, the low pH of the endosome triggers the viral fusogens to transition from homo- or hetero-dimers, to monomers. Concomitantly, a previously hidden hydrophobic segment, the fusion loop, is exposed and inserts into the outer leaflet of the membrane of the target cell. Almost immediately, the monomers, which are now bridging the two membranes, assemble into trimers. This oligomeric rearrangement is accompanied by a dramatic conformational change, in which each monomer of the trimer folds back on itself, bringing the two membranes into intimate contact. As a consequence of the fold-back of several clustered trimers, both membranes become distorted, forming closely apposed membrane dimples. According to current models, the lipids of the outer leaflets of the two bilayers first become continuous (hemifusion), followed by merger of the inner leaflets to create a so-called fusion pore. The pore subsequently expands to accomplish complete coalescence of the two membrane-bound compartments.
Fig. 1 |. Possible functions for DMP8 and 9 during gamete membrane fusion.
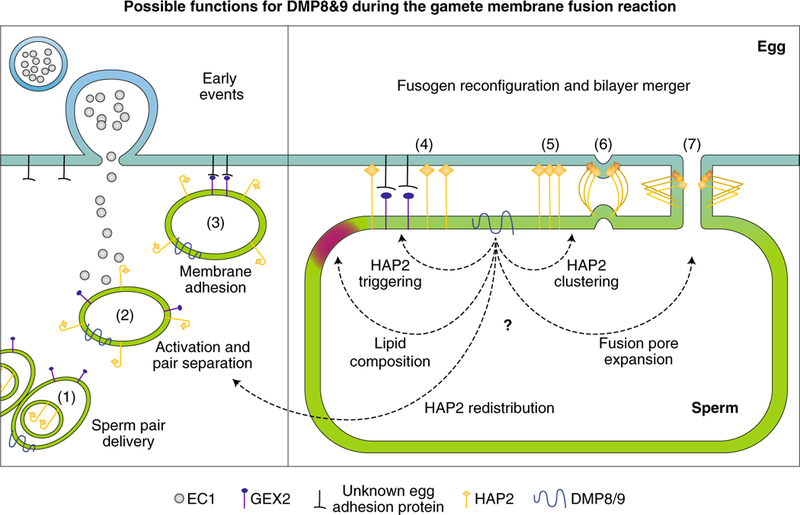
Time course (left to right) of fusion-related events at the egg (blue) and sperm cell (green) membranes. The events shown for the egg are likely also true for the central cell. DMP8 and 9 are at the sperm plasma membrane when the sperm pair are delivered to the ovule (1). HAP2, with its cryptic fusion loop, moves to the sperm surface only under the influence of egg-cell-secreted EC1 (2), a step that could be influenced by DMPs. EC1 also provides the impetus for separation of the sperm pair. Adhesion protein GEX2 on the sperm membrane then binds to an unidentified GEX2-binding protein on the egg (3). At some point after adhesion, HAP2 is triggered to first expose, then insert, its fusion loop into the egg membrane and, based on mechanistic evidence from viral class II fusion proteins, bridges the two membranes as an extended monomer (4). DMPs could regulate lipid properties of the sperm membrane or influence initial oligomeric changes of HAP2 that allow fusion loop exposure/insertion. Class II fusion proteins then form clusters of trimers (5), another possible DMP-influenced event, and change their conformation to induce membrane dimples (6). Complete fold-back generates a fusion pore (7), which expands to consummate cell–cell fusion. Fusion-pore expansion is yet another poorly understood step that could be influenced by DMP proteins.
Fusion of gametes in flowering plants is a yet more complex and elaborate process, and not just because two sets of gametes must fuse9,10. HAP2 is not present on the surface of either of the two interconnected sperm cells, which are enclosed in a common membrane in the cytoplasm of the pollen tube. On arrival at the ovule, the pollen tube bursts, delivering the sperm, which separate and come into close contact with the the egg and the central cell. The proximity of the sperm prompts the egg to release EC1, a recently discovered protein that induces redistribution of HAP2 from the intracellular membranes to the sperm plasma membrane11.
Cyprys et al. uncovered a new role for EC1, which is to induce separation of the two sperm cells. Each of the, now single, sperm cells then adheres to the egg or central cell with the participation of a sperm-specific adhesion protein, GEX2 (ref. 12). Within a short time after adhesion, one sperm cell fuses with the egg to generate the embryo, while the other sperm fuses with the central cell to generate the endosperm. Both of the new papers show that sperm with reduced DMP9 expression8 or completely absent DMP8 and DMP9 expression7 retained a higher capacity for fusion with the central cell than with the egg cell. These results likely are not a reflection of an inherent difference in the fusion capacity of the two female gametes, since the egg cell is preferentially fertilized in other fertilization-related mutants13,14.
What is the molecular function of these fusion-facilitators? Although HAP2 expression on the dmp mutant sperm pair still in the pollen tube was normal, the DMPs could be important for the EC1-induced trafficking of HAP2 to the sperm plasma membrane. Additionally, we still do not know the ‘trigger’ that initiates the oligomeric and conformational changes of HAP2, which is essential to the fusion mechanism. Although the ‘trigger’ could be low pH as in viruses, another possibility is that membrane adhesion receptor interactions, which could even differ between the egg and central cells, are somehow linked through DMPs to the activation of HAP2. Alternatively, DMPs could function a bit later in the fusion pathway. For example, these multi-pass membrane proteins could form interconnected assemblies similar to those of another family of multi-pass, short membrane proteins, the tetraspanins15, one of which, CD9, is essential for sperm–egg fusion in mice16. DMPs could also aid in concentrating or promoting a supramolecular organization of HAP2 on the sperm membrane, analogous to the organization of class II fusion proteins on the viral envelope. Finally, DMP-regulated changes in the physical properties of the lipid bilayer might facilitate the membrane bending associated with dimple formation in the sperm lipid bilayer, or DMPs could promote the still poorly understood expansion of the fusion pore after the membrane fusion reaction is complete.
Perhaps a triple dmp7 dmp8 dmp9 mutant (as proposed by Cyprys et al.) will show that these DMP assistants have become essential for HAP2 function. Certainly, evolution has done its tinkering with fusion mechanisms. Fungi and chordates have seemingly abandoned HAP2 completely, and nematodes have put their own stamp on class II fusogens. Caenorhabditis elegans uses the class II fusion protein EFF-1 to form a syncytium, but with the fundamental difference that EFF-1 is incapable of functioning uni-directionally17. Building on these new findings, it will be exciting to learn whether the membrane fusion reactions that drive fertilization in other organisms have evolved similar, or entirely different, HAP2 fusion-facilitators.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Clark T PLoS Biol 16, e3000007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong JL & Johnson MA Trends Cell Biol 20, 134–141 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Modis Y Adv. Exp. Med. Biol 790, 150–166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valansi C et al. J. Cell Biol 216, 571–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinello JF et al. Curr. Biol 27, 651–660 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedry J et al. Cell 168, 904–915 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cyprys P, Lindemeier M & Sprunck S Nat. Plants http://doi.org/10/1038/s41477-019-0382-3 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T et al. Development 145, dev170076 (2018). [Google Scholar]
- 9.Hamamura Y, Nagahara S & Higashiyama T Curr. Opin. Plant Biol 15, 70–77 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Dresselhaus T, Sprunck S & Wessel GM Curr. Biol 26, 125–139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprunck S et al. Science 338, 1093–1097 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Igawa T, Tamiya G, Miyagishima SY & Berger F Curr. Biol 24, 170–175 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Iwakawa H, Shinmyo A & Sekine M Plant J 45, 819–831 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Nowack MK et al. Nat. Genet 38, 63–67 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Charrin S et al. Biochem. J 420, 133–154 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Miyado K et al. Science 287, 321–324 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Podbilewicz B et al. Dev. Cell 11, 471–481 (2006). [DOI] [PubMed] [Google Scholar]


