Abstract
The sinoatrial node (SAN) is the primary pacemaker in canine and human hearts. The SAN in both species has a unique 3-dimensional heterogeneous structure characterized by small pacemaker myocytes enmeshed within fibrotic strands, which partially insulate the cells from aberrant atrial activation. The SAN pacemaker tissue expresses a unique signature of proteins and receptors that mediate SAN automaticity, ion channel currents and cell-to-cell communication, which are predominantly similar in both species. Recent intramural optical mapping, integrated with structural and molecular studies, has revealed the existence of up to five specialized SAN conduction pathways that preferentially conduct electrical activation to atrial tissues. Intrinsic heart rate, intranodal leading pacemaker shifts and changes in conduction in response to physiological and pathophysiological stimuli are similar. Structural and/or functional impairments due to cardiac diseases including heart failure (HF) cause SAN dysfunctions (SND) in both species. These dysfunctions are usually manifested as severe bradycardia, tachy-brady arrhythmias, and conduction abnormalities including exit block and SAN reentry, which could lead to atrial tachycardia and fibrillation, cardiac arrest and HF. Pharmaceutical drugs and implantable pacemakers are only partially successful in managing SND, emphasizing a critical need to develop targeted mechanism-based therapies to treat SND. Since several structural and functional characteristics are similar between the canine and human SAN, research in these species maybe mutually beneficial for developing novel treatment approaches. This review describes structural, functional and molecular similarities and differences between the canine and human SAN, with special emphasis on arrhythmias and unique causal mechanisms of SND in diseased hearts.
Keywords: Sinoatrial node dysfunction, fibrosis, sick sinus syndrome, pacemakers, atrial fibrillation
Introduction
The sinoatrial node (SAN), described as the primary cardiac pacemaker, is the source of intrinsic electrical activation consistently driving the coordinated rhythmic contractions of the mammalian heart [1,2]. It initiates the heartbeat via a combination of pacemaker cells which generate spontaneous cellular electrical signals, and specialized conduction pathways, which conduct the electrical impulses from pacemaker cells to adjacent atrial tissue [3,4]. The SAN has the unique ability to match heart rate (HR) with physiological demands, thereby modulating healthy cardiac function and output. It goes without saying that abnormal SAN function can inappropriately accelerate or slow HR, which may result in fatal cardiac arrhythmias. Abnormal SAN function can predispose to heart disease including atrial fibrillation (AF) and heart failure (HF) in humans; on the flip side, preexisting heart disease including AF and HF can induce SAN dysfunction (SND) in human, which can result in syncope and sudden cardiac death [5,6]. Currently, SND in both human and canine is often treated with pharmaceutical interventions and implantable pacemakers with variable success rates [7-9].
Given the central and critical role that the SAN plays in maintaining HR and cardiac function, understanding the complex mechanisms involved in SAN function is of utmost importance in order to diagnose and treat SND in canine and human patients. In fact, the current demographics predict that the number of human SND patients will continue to increase from 78,000 in 2012 to nearly 172,000 by 2060 as a main indication for permanent artificial pacemaker implantation [9]. The prevalence and severity of SND in both canines [7] and humans emphasize the need for more detailed studies that identify and describe structural and functional aspects of the SAN that can be efficiently targeted to treat and/or prevent worsening SND. However, despite several decades of impressive progress in our understanding of the workings of the SAN, we are yet to completely describe its intriguing 3-dimensional (3D) complexity [10-12].
Most of the seminal findings have come from studies using animal models ranging from rabbit, mouse, cat, dog and pig [13]. More recently, direct examinations of the structure and function of the human SAN ex vivo are possible due to the increased availability of explanted human hearts for research purposes [11,14]. Although small animal models such as mouse and rabbit [15-17] have significantly contributed towards our understanding of the SAN, they have multiple limitations primarily due to faster intrinsic heart rhythm and 2-dimensional SAN structure compared to the 3D human SAN. In contrast, the canine SAN closely resembles its human counterpart in many critical aspects including 3D architecture, ultrastructural characteristics and function [4]. Studies using an integrated approach of combining structural, molecular and functional analyses demonstrate that mechanisms known to cause SND in canines and human are predominantly similar [18-20]. Hence studies on the SAN in these two species may prove to be mutually beneficial to understand naturally occurring disease-causing mechanisms, explore novel treatment options including pharmaceutical and/or genetic manipulations, and test outcomes of implantable pacemakers. Of note, the studies on control canine SAN mentioned throughout this review were mainly completed in young adult (~1-4 years old) mongrel canines (~18-25 kg) [19-23]. This review will focus on critical structural, molecular and functional characteristics of the SAN that are common and different between the canine and human SAN, with special emphasis on arrhythmias and unique causal mechanisms of SND in diseased hearts, in order to identify novel therapeutic modalities.
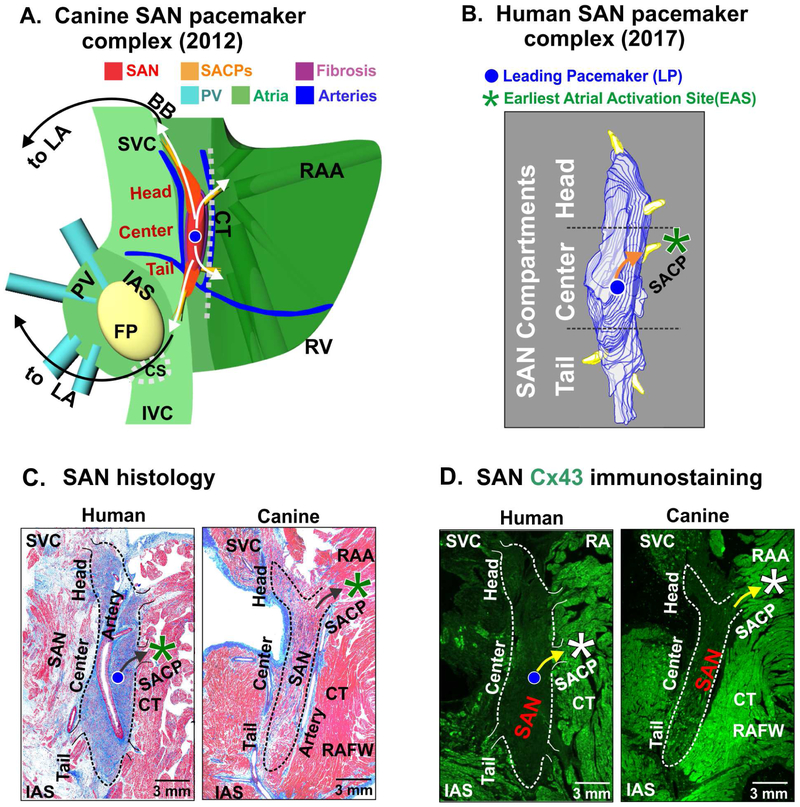
Unique 3D structure of the canine and human SAN
In canine and human hearts, the SAN is typically identified as a compact, slightly elongated 3D intramural “banana” shaped structure located at the junction of the superior vena cava and the right atrium, centered around the SAN artery (Fig. 1A) [1,2,4]. Multiple clusters of small pacemaker cardiomyocytes, fibroblasts, blood vessels and nerves encased within fibrous and fatty deposits form the SAN pacemaker complex. Three dimensional structural reconstruction of the normal human SAN, based on histological and immunochemistry studies, reveal it to be much longer compared to previous descriptions [10,14,24-27]. The human SAN can measure ~12-29 mm in length with a width of ~2-6 mm while the canine SAN is ~11-29 mm long and ~2-5 mm wide [19-23]. Both SANs traverse intramurally to 1-2 mm depth. Functional and structural mapping of the canine and human SAN have revealed that it can be distinguished into head, center and tail compartments (Fig. 1A,B) [11,14,19,27], wherein the head is usually closer to epicardium and tail tilted towards the endocardium. The SAN is typically characterized by dense fibrotic strands compared to the surrounding atria in both species (Fig. 1C) [18,28,29]. Distinctive high expression of specific isoforms of several key ion channel proteins including hyperpolarization-activated cyclic nucleotidegated subunits (HCN), and low expression of main gap junction protein connexin (Cx) 43 compared to atrial tissues are found in the canine and human SAN [30,31]. Expression levels and spatial patterns of these proteins are demonstrated to abruptly change between the SAN and atria and have been used as a specific marker to identify the location of the nodal tissue (Fig. 1D) [11,19]. Importantly, the very heterogeneous compositions of ion channel and gap junction protein expression within the SAN pacemaker complex facilitate its automaticity and can protect it from rhythm failure (see below).
Figure 1: Structural and functional characteristics of the canine and human sinoatrial node (SAN) pacemaker complex.
(A) 3 dimensional model of the canine SAN based on structural and functional data from intramural optical mapping. Compact SAN (red) is isolated from the surrounding atrial myocardium (green) by bifurcating coronary arteries (blue) and fibrotic insulation (purple). Preferential sinoatrial conduction pathways (SACPs) are depicted by yellow bundles and arrows that electrically connect SAN to the atrium. (B) 3-dimensional reconstruction of the human SAN showing intranodal pacemaker compartments and 5 SACPs. (C) Histological features of the canine and human SAN pacemaker complex revealed by Masson’s trichrome staining. (D) Immunostaining identifies the SAN by negative connexin 43 (Cx43) expression. [(A) Data modified from Fedorov et al. Am J Physiol Heart Circ Physiol 2012 [37]; (B) (C) and (D) Figures modified from Li et al. Sci Transl Med 2017 (Human) [11] and Lou et al. Circulation 2014 (Canine) [19]]. BB: Bachmann bundle; CT: crista terminalis; EAS: earliest atrial activation site; FP: fat pad; IAS: interatrial septum; PV: pulmonary veins; RA: right atrium; RAA: right atrial appendage; RAFW: right atrial free wall; SAN: sinoatrial node; SVC: superior vena cava.
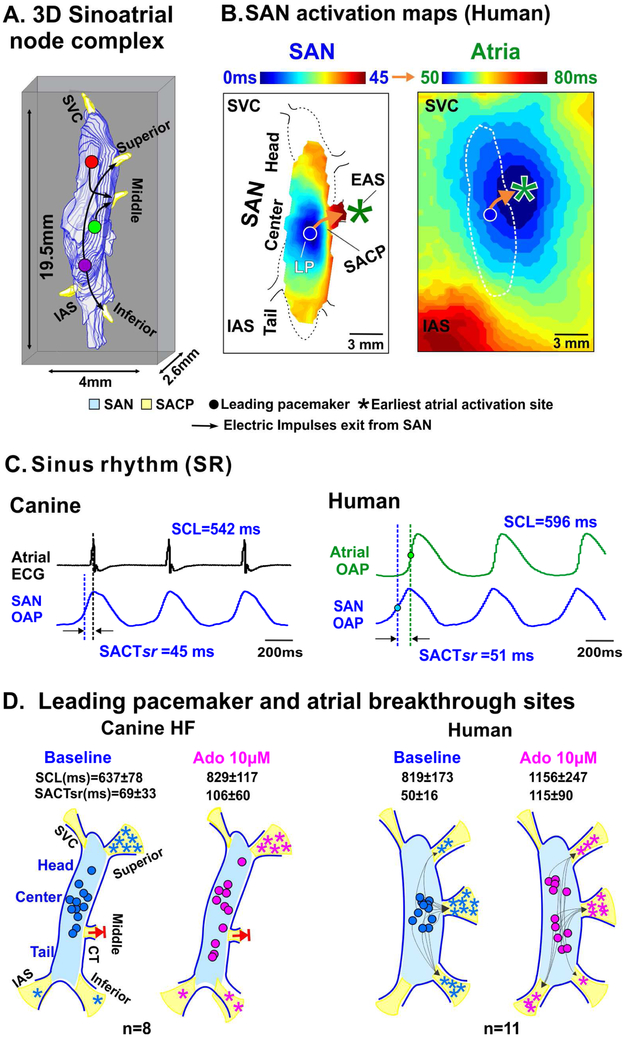
In addition to similar structural features, near-infrared optical mapping of explanted canine and human SAN demonstrates that the functional indices of SAN activation and conduction including sinus cycle length and SAN conduction time during sinus rhythm are remarkably similar (Fig. 2B,C). Intrinsic HR is ~90-140 beats per minute in the canine heart [32] which is very close to the ~60-125 beats per minute in humans [11,33,34]. The “leading pacemaker” site, or the first site of SAN activation, is typically located in the central part of the SAN in both species (Fig. 1,2) [11,19].
Figure 2: Atrial activation pattern is determined by the sinoatrial conduction pathway (SACP) during sinus rhythm.
(A) 3-dimensional view of the sinoatrial node (SAN) complex (B) Intranodal and atrial activation patterns revealed by high resolution optical maps. (C) Traces of SAN and atrial Electrocardiograms (ECG) or optical action potentials (OAP) from canine and human during sinus rhythm. (D) Schematic representation of canine and human SAN depicting shift in leading pacemaker sites and earliest atrial activation site at baseline and after adenosine (Ado). Blocked arrows indicate nonfunctional mid-lateral conduction pathway in canine heart failure (HF) preparations. [(A-D) Sinoatrial node figures and traces were modified from Li et al. Sci Transl Med 2017 (Human) [11] and Lou et al. Circulation 2014 (Canine) [19]]. CT: crista terminalis; EAS: earliest atrial activation site; IAS: interatrial septum; IVC: inferior vena cava; LAA: left atrial appendage; LP: leading pacemaker; OAP: optical action potential; PV: pulmonary veins; RA: right atrium; RAA: right atrial appendage; RAFW: right atrial free wall; SACPs: sinoatrial conduction pathway; SACTsr: sinoatrial conduction time during sinus rhythm; SAN: sinoatrial node; SCL: sinus cycle length; SVC: superior vena cava.
Sinoatrial Conduction Pathways (SACPs): critical facilitators of SAN function and electrical conduction
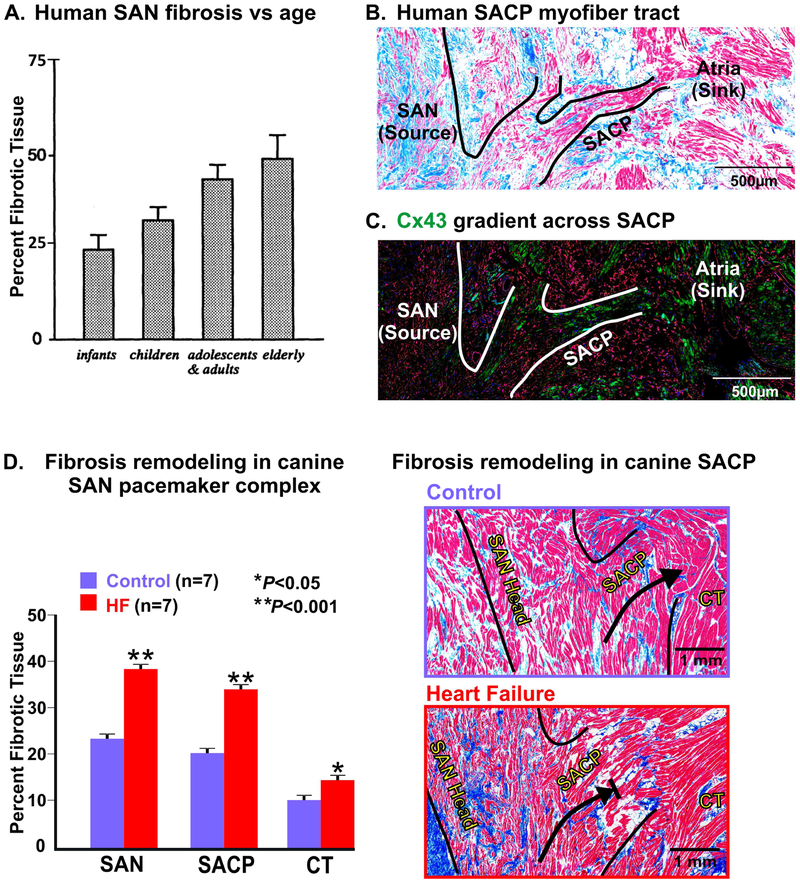
Although several structural and functional studies have suggested the existence of discrete myofiber connections to conduct excitation from the SAN to the surrounding atria, contradicting hypotheses regarding SAN conduction mechanisms such as diffuse interdigitations of the SAN border connecting the SAN to the atrial myocardium, have been debated for many years, primarily due to lack of high resolution structural and functional data [14]. Using atrial epicardial multielectrode mapping, the Schuessler and Boineau group were the first to demonstrate that during normal sinus rhythm, sites of earliest atrial activation or “breakthroughs”, in canine in 1980 [35] and then in human hearts in 1988 [28], occurred well beyond the length of the anatomical SAN body. These findings suggested the existence of an extended “atrial pacemaker complex” beyond the SAN. They also suggest that widely distributed earliest epicardial or endocardial atrial activation sites could represent the exit points of SAN intramural activation through SACPs, rather than by multiple independent atrial pacemakers in intercaval regions. However, electrode surface mapping could not resolve intramural SAN activation [36]; only recent near-infrared optical mapping studies in canine and human SAN, integrated with detailed 3D structural analysis could clarify intramural SAN activation. These studies demonstrated that the observed atrial breakthrough sites do not indicate the specific sites of leading pacemakers but actually indicate the locations where SAN electrical wave propagation exits through the SACPs [11,19]. Near-infrared optical mapping is the only currently available approach, which can reveal intramural SAN activation in canine and human hearts [11,19]. Atrial activation patterns in humans and canines can result from intramural SAN excitation waves exiting either from the superior or inferior SACPs, and not due to two separate leading pacemakers [28,37]. Since then, high-resolution optical mapping studies, integrated with 3D histological analyses, have demonstrated the existence of three to five discrete branching myofiber tracts, located near the superior vena cava, interatrial septum, and laterally (referred to as superior, middle and inferior SACPs, as indicated in Fig. 2A,D), that form uninterrupted physical SACP myobundles between the SAN and atria, in both canines and humans [11,19,37].These branching myofiber tracts are composed of cells that show transitional morphology between SAN and atrial cells, and a progressive transition in Cx43 expression: negative in SAN pacemaker cells to intermediate expression in the SACP region and higher in the atria (Fig. 5C).
Figure 5: Fibrosis and structural remodeling of the canine and human sinoatrial node (SAN) pacemaker complex.
(A) Graph showing increasing percentage of age-related fibrous tissue volume to the total SAN volume, mean value, and standard deviation. (B) Histological Masson’s trichrome staining of human SAN with lateral SAN conduction pathways (SACP) outlined. (C) A sister section to panel (B) immunostained for connexin 43 (Cx43; green) and vimentin (red) showing increasing gradient of Cx43 expression from SAN to atria. (D) Summary data and Masson’s trichrome staining of fibrosis (blue) in the canine SAN pacemaker complex and SACP in control vs. heart failure (HF). [(A) From Shiraishi et al 1992 [42] (used with permission); (B,C) Data modified from Csepe et al Prog Biophys Mol Biol. 2016 [14] (D) Data adapted from Lou et al Circulation. 2014 [19]]. CT: crista terminalis.
Moreover, conduction via the SACPs is also suggested to overcome “source-sink” mismatch between the relatively small SAN intranodal pacemakers (“source”) characterized by a positive resting potential (~ −60 mV), which must activate a much larger atrial myocardium with more negative resting potential (~ −85 mV) and hence a bigger “sink” of current [14,20]. Restricting electrical delivery only through the branching myofibers of SACPs ensures slow conduction from the SAN thereby allowing sufficient time to reach threshold and build up charge, which can in turn excite the larger atria. Hence, myofiber discontinuity in the SACPs, due to aging and cardiac diseases, is also a potential cause of conduction abnormalities and SND [14]. Sinoatrial node conduction is known to vary from 25 to 350 ms due to autonomic stimulations and has a significant contribution to heart rhythm control even in healthy human [11] and canines [19], and hence should be considered for heart rate variability analysis. Moreover, in diseased human hearts, SAN conduction could be further slowed down to ~900 ms [14] which should be considered during diagnosis of SND.
Redundant intranodal pacemakers and SACPs: robust protectors of SAN function
Sinoatrial node conduction in canine and humans can be affected by many factors including sympathetic and parasympathetic agonists, antiarrhythmic drugs and naturally occurring metabolites including adenosine. These stimuli are known to induce rapid shifts in the location of the intranodal leading pacemaker to either superior or inferior sites within the SAN, and also induce a preferential use of either the superior or inferior SACPs [11,19,31]. Furthermore, the shifts in preferential SACPs are independent from intranodal leading pacemaker shifts which could potentially ensure a viable pathway if conduction through others is temporally blocked or suppressed. Intramural mapping studies revealed that conduction via the inferior septal SACP can be potentially preferred in some human hearts only during adenosine mediated bradycardia, and not at baseline (Fig. 2D) [11]. These data could suggest that the septal SACP may serve as a backup pathway specifically recruited to maintain conduction if all lateral SACPs are suppressed. These intriguing backup redundant mechanisms found in the human SAN have been proposed to provide robust failsafe protection against SAN arrest in diseased or aging hearts [11]. In diseased hearts, the availability and/or the preference for a SACP can change. As demonstrated in Fig. 2D, in HF canine SAN, although the leading pacemakers shift to head and tail regions, the mid-lateral conduction pathway was found to be completely non-functional both at baseline and with adenosine, thereby decreasing the number of functional SACPs, which predisposed these hearts to exit block occurrence [19]. Interestingly, despite impaired robustness (diminished redundancy) due to structural/molecular remodeling, even diseased human SAN can maintain normal heart rhythm at baseline conditions. However, they experience complete arrest during adenosine challenge mainly due to the absence of functional back-up pacemakers and SACPs [11].
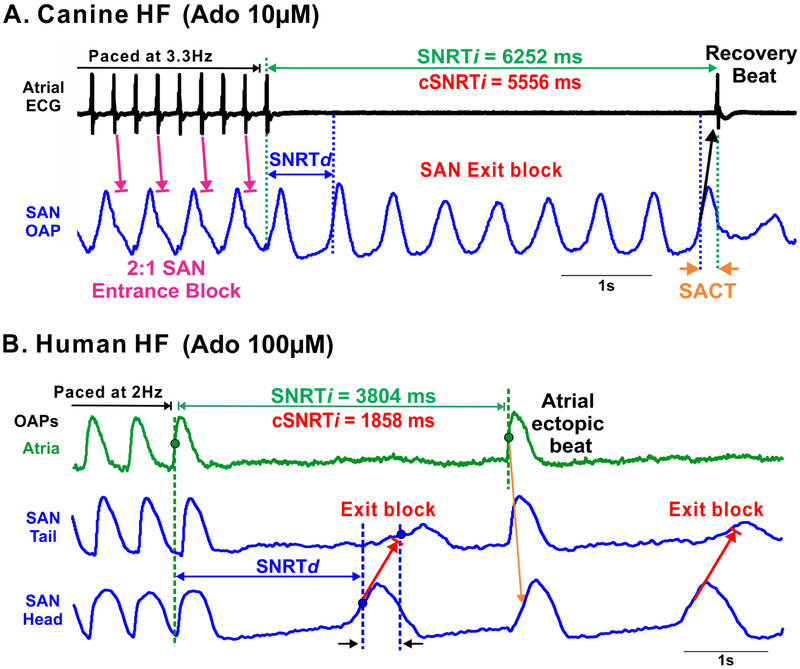
In addition to conduction, SACPs are known to act as a low-pass filter preventing atrial activation of the SAN by creating entrance blocks which avert overdrive suppression of the SAN during fast atrial pacing and AF. The mechanism responsible for SAN entrance block during fast atrial rhythm is likely related to differences in refractoriness and excitability between the SAN pacemaker complex and surrounding atria [23,38]. Canine and human SAN are also susceptible to conduction impairments including block of electrical activation within the SACPs, known as exit block, which causes prolonged atrial pauses, especially in failing hearts [11,19-21]. As shown in Fig. 3A, prolonged atrial pauses induced by pacing in the presence of adenosine in HF canine hearts, could primarily be due to SAN exit blocks rather than depressed SAN automaticity, as depression of the intranodal pacemakers could be prevented by the protective role of SAN entrance block [11,19]. As shown in Fig. 3B, in the absence of entrance blocks, overdrive pacing could exaggerate the negative chronotropic effects of adenosine and lead to both SAN automaticity suppression and exit blocks which contribute to post-pacing atrial pauses. Notably, while exit block is always considered pathologic in human patients, exit block in the canine SAN may occur naturally during resting conditions due to high vagal tone [22].
Figure 3: Pacing induced automaticity depression and sinoatrial node (SAN) exit blocks during adenosine (Ado) perfusion in failing canine and human hearts.
(A) Sinoatrial node optical action potential (OAP) and atrial electrocardiogram (ECG) recordings during and after pacing showing 2:1 SAN entrance block and subsequent exit blocks in a canine heart failure (HF) model. (B) Atrial and SAN OAPs during and after pacing showing exit blocks. [Data modified from (A) Lou et al. Circulation 2014 [19] and (B) Li et al. Sci Transl Med 2017 [11]]. SACT: sinoatrial conduction time; (c)SNRTi/d: (corrected) sinus node recovery time, indirect/direct.
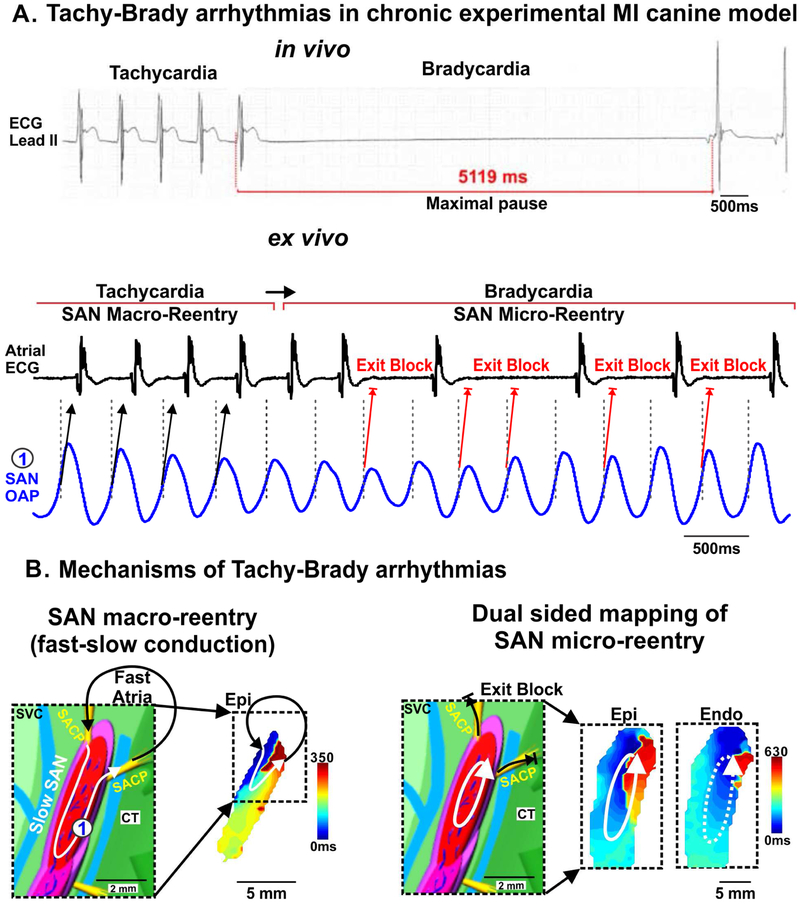
Several arrhythmias are common in both species and linked to SND in diseased hearts [39,40]. For instance, SND induced in experimental canine myocardial infarction (MI) models is characterized by tachy-brady arrhythmias, bradycardia, prolonged atrial pauses and SAN exit blocks and reentry (Fig. 4A) [22], which are also reported in human SND. Intramural optical mapping of canine models of chronic MI reveals that SAN macro- and micro reentries can induce significant alternations of heart rate, including both tachy- and bradycardia (Fig. 4B) [19,22]. Although MI is uncommon as a spontaneous disease in canines, similarities in arrhythmias and major arrhythmic mechanisms including SAN exit blocks and reentry between humans and these experimental canine MI models emphasize the importance of canines as suitable experimental models to study human SND.
Figure 4: Sinoatrial node (SAN) reentry could lead to both tachycardia and bradycardia in experimental chronic canine myocardial infarction (MI) and heart failure (HF) models.
(A) In vivo electrocardiogram (ECG) recordings reveal maximum atrial pauses in post-MI canines at rest and ex vivo atrial ECG and SAN optical action potential (OAP) representing tachycardia and bradycardia due to SAN macro- and micro-reentry, respectively. (B) (Left) SAN model and epicardial activation map of fast-slow SAN macro-reentry in a canine model of chronic experimental MI. (Right) Micro-reentry within SAN and exit block in sinoatrial conduction pathways (SACPs) in HF model. [(A) Data modified from Glukhov et al. Circ Arrhythm Electrophysiol 2013 [22] (B-D) Data and models modified from Lou et al. Circulation 2014 [19]]. CT: crista terminalis; Endo: endocardium; Epi: epicardium; SACPs: sinoatrial conduction pathway; SVC: superior vena cava.
Fibrosis: a structural modulator of SAN automaticity and conduction
Structurally, fibrotic strands and dense connective tissue are an inherent feature of the canine and human SAN (Fig. 1C) [18]. These dense fibrotic strands characterized by fibroblasts, collagen and elastin fibers increase the compactness of the SAN. The amount of fibrotic content appears to be correlated with the size of the heart wherein smaller hearts, e.g. mouse, show relatively lower levels (10-17%) compared to a bigger heart e.g. cat (~27%) [18,41]. Since bigger hearts are normally characterized by slower HR, the higher levels of fibrotic strands in these SANs may play a role in slowing HR in addition to other intrinsic cellular features. In this regard, the healthy canine SAN is composed of 20-30% fibrotic content while the human node is composed of 35-55% fibrosis [42], which could also contribute to the lower HR in humans compared to canine models. Intranodal fibrotic content is known to increase with age from ~24% to ~70% even in healthy human hearts (Fig. 5A) [41,42]. Fibrotic strands are proposed to help electrically insulate SAN automaticity from hyperpolarizing effects of the adjacent atrial tissues [43] and to facilitate the predominantly unidirectional conduction of electrical signals from the SAN to the atria (Fig. 5B,C) [14]. Furthermore, dense collagen networks can provide a scaffolding structural support within which the SAN cardiomyocytes and other components including blood vessels, nerve fibers and other supporting cell types can be stably compartmentalized. This enhanced dense collagen can also provide mechanical protection of SAN pacemaker cells from overstretching by the strongly contracting surrounding atrial myocardium [18]. However, diseases including HF and MI in human and experimental canine models have been shown to increase fibrosis within the SAN and SACPs (Fig. 5D) [18,19,22,44]. This pathological increase in fibrosis is known to interrupt the tight coupling between SAN cardiomyocytes and affect their mutual entrainment, a vital necessity for the robust pacemaking of the SAN, often resulting in bradycardia, conduction blocks and reentry [18].
Unique SAN protein expression patterns: molecular signatures that mediate SAN automaticity and electrical conduction
Sinoatrial node cardiomyocytes are characterized by a unique profile of ion channels and receptors that facilitate and support its specialized function [30]. Although several ion channels and receptors can be predicted to be differentially expressed in pacemaker myocytes [30], in this review, we will focus on the spatial and functional characteristics of critical: (1) ion-channels that mediate intrinsic automaticity - HCN isoforms [45]; proteins and receptors that mediate (2) intracellular calcium (Ca2+) cycling in response to ion currents [46], (3) cellular signaling pathways via metabolic ligands - adenosine receptor (AR) isoforms [47], and autonomic signaling via G protein-coupled inwardly-rectifying potassium channel (GIRK) isoforms [48], (4) transport of sodium (Na+) ions across cell membranes - Na+ channels [30] and (5) proteins that form gap junctions between adjacent myocytes - Cx isoforms [31].
Hyperpolarization-activated cyclic nucleotide-gated channels and the funny current: key players in SAN automaticity
Hyperpolarization-activated cyclic nucleotide-gated channels have been shown to play an important role in generating spontaneous activation in pacemaker myocytes [49-52]. They are voltage-gated cation channels responsible for conducting a mixed Na+-potassium inward current. This current, also known as the “funny current” (If) is shown to facilitate spontaneous diastolic depolarization in pacemaker myocytes [49-52]. Blocking If with Ivabradine, a pharmaceutical blocker of the HCN channel [53], decreases the automaticity of pacemaker cells thereby establishing the central and important role that these channels might play in the intrinsic automaticity of the SAN. Furthermore, loss-of-function mutations in HCN4 gene are also known to affect If and are associated with SND in humans indicating its role in SAN function [54].
There are four known isoforms of HCN channels, HCN 1-4 [55]; HCN1, 2 and 4 are expressed in the mammalian heart [30]. While HCN2 and HCN4 are found to be prevalent isoforms in pacemaker cells, HCN4 has been identified as the main isoform responsible for the If current. In the canine SAN, Zicha et al [56] found mRNA and protein levels of HCN2 and 4 to be significantly higher relative to the right atrial (RA) tissue. They also showed that HF-induced SAN remodeling and dysfunction in canines could be due to down-regulation of both HCN2 and HCN4.
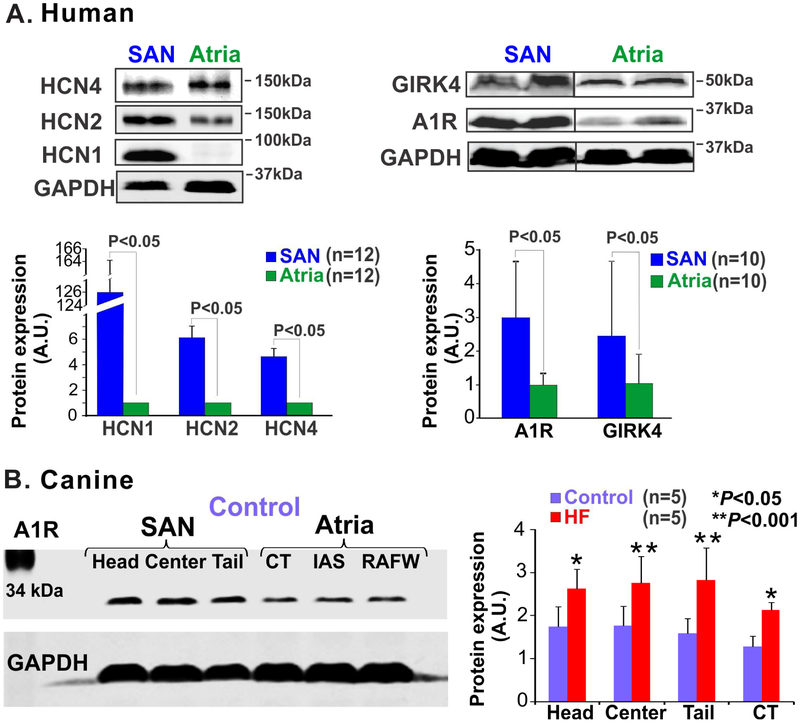
Verkerk et al [57] demonstrated that If is functionally present in human SAN myocytes and could contribute to pacemaking as well. In healthy human SAN, similar to findings from canine SAN, mRNA profiles confirm the presence of HCN2 and 4 isoforms [30]. Our group recently reported that protein expression of HCN2 and 4 isoforms were significantly higher in the human SAN compared to surrounding atria (Fig. 6A, left panel) [58]. Of note, in contrast to canine and small mammals, where HCN4 is highly expressed in the SAN and latent pacemaker cells but not in atrial myocardium, in humans, HCN4 expression is also present in RA myocytes and thus may not be used as a distinct marker for identifying SAN pacemaker cells [30,58].
Figure 6: Heterogeneous protein expression in the sinoatrial node (SAN) vs. adjacent atrial tissue.
(A) Protein expression of hyperpolarization-activated cyclic nucleotide-gated channel (HCN) 1, 2 and 4 isoforms (left) and adenosine A1 receptor (A1R) and G protein-coupled inwardly rectifying potassium channel (GIRK4) (right), and summary data below show that these proteins are significantly higher in the SAN relative to atrial tissues; (B) Left, immunoblots showing protein expression pattern of A1R in the SAN compartments and in the atrial myocardium of control canine hearts ; right, summary of these data shows that A1R expression is significantly upregulated in the SAN compartments in heart failure (HF). [(A) Li et al. Circ Arrhythm Electrophysiol. 2015 [58] and Li et al. Sci Transl Med 2017 [11]; (B) Data modified from Lou et al. Circulation 2014 [19] (left)]. CT: crista terminalis; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; IAS: interatrial septum; RAFW: right atrial free wall.
We also found HCN1 protein to be highly enriched specifically in the SAN pacemaker tissues, emphasizing a potential key role for this isoform in maintaining the pacemaker current in the human SAN [58]. Currently, no data exist on the expression of HCN1 at the protein level in the canine SAN. In the absence of conclusive data, potential differences in the ratios of HCN isoform expression between canine and human SAN should be taken into account while comparing their individual contribution towards the pacemaker current and rhythm maintenance in canine and human SAN.
Hyperpolarization-activated cyclic nucleotide-gated channels have been utilized as a therapeutic target to treat AF, inappropriate SAN tachycardia and potentially SND. One approach was to block HCN channels and hence decrease If, which was shown to have significant anti-arrhythmic properties. Sinus rate reducing studies using Ivabradine [59] have shown that AF inducibility as well as the duration of AF can be decreased in canine models of age-related AF [60]. Ivabradine has also been clinically tested as an alternate to conventional drugs to treat inappropriate SAN tachycardia and was found to be more effective compared to β-blockers [61]. A second therapeutic strategy is to restore HCN channel expression in diseased SAN, as aging and AF are known to induce a downregulation of HCN channel expression in dogs which can lead to SND [62,63]. Hence, HCN has been studied as a molecular candidate to develop a “biological pacemaker” as an alternative to implantable pacemakers for SND treatment [64]. Targeted adenoviral expression of HCN2 protein in canine atria was able to generate an alternate source of pacemaking current sufficient to maintain low ventricular heart rate for few weeks, which demonstrated the potential of HCN channels as gene-therapy for canine and potentially human SND [65]. Human embryonic stem cell-derived myocytes and mesenchymal stem cells have also been demonstrated as valid gene-delivery platforms for HCN channels in cultures as well as in the whole heart [66,67]. Overall, these pioneering studies provide a very promising prognosis for utilizing HCN gene therapy as a targeted treatment for canine and human SND.
Membrane ion channels and Ca2+ handling proteins: complementary partners of SAN automaticity
In addition to If, several key membrane ion channels including voltage-gated Ca2+ channels, the sodium-calcium exchanger and several voltage-gated potassium channels are known to contribute to spontaneous action potentials (AP) in SAN myocytes, which represent cellular level of SAN robustness [46]. Since these ion channel activation and inactivation kinetics interact to sustain a self-perpetuating time and voltage-dependent cycle, they are proposed to constitute a “membrane clock” [46]. More recently, an independent system, composed of intracellular Ca2+ cycling parameters, has also been implicated in the intrinsic activation of SAN myocytes [46]. Spontaneous and rhythmic local releases of Ca2+ from the sarcoplasmic reticulum (SR) via the ryanodine receptor during diastole, the subsequent en-masse release of Ca2+ from the SR coinciding with the upstroke of the AP, calcium re-uptake by the SR Ca2+ ATPase (SERCA) pump back into the SR, along with Ca2+ extrusion by the sodium-calcium exchanger at the next diastole complete the intracellular Ca2+ cycle [46]. Since these events are self-perpetuating and cyclical, they are collectively said to constitute the “Ca2+ clock” [46]. The membrane and Ca2+ clocks may be completely independent of each other or can be entrained by interacting at certain nodal points during their spontaneous cycling. The tight interaction between the two coupled clocks could maintain flexibility and robustness of the SAN, and provide mutual fail-safe mechanisms against rhythm failure [68].
Whether the Ca2+ clock plays a role in canine and human SAN cells is yet to be determined. Gao et al [69] showed that voltage and Ca2+ clocks exist in canine SAN cells and cooperate to maintain automaticity, both at baseline and during adrenergic stimulation. However, Sosunov et al [70] found that blocking the Ca2+ clock with ryanodine in canine SAN did not affect basal pacemaker activity while it only inhibited isoproterenol mediated acceleration of SAN automaticity. These findings might underscore an important role for the Ca2+ clock in sinus acceleration during β-adrenergic stimulation rather than basal automaticity in canine SAN. Currently, Verkerk et al [57] is the only reliable study that demonstrates the role of If in spontaneously beating isolated human SAN myocytes (cycle length 828 +/− 15 ms). Although gene expression of several Ca2+ handling proteins have been demonstrated in the human SAN [30], detailed studies in adult human isolated pacemaker cells are warranted to determine if Ca2+ dependent mechanisms contribute to human SAN automaticity.
Adenosine Receptors and GIRK channels: critical modulators of SAN function, conduction and robustness
Adenosine, an endogenous metabolite, is a well-known modulator of negative chronotropic effects on sinus rhythm [71]. It is increasingly released by myocytes specifically during metabolic stress e.g. ischemia and in HF [72]. Adenosine activates ARs which facilitate the potassium current IK,Ado, via downstream GIRK1/4 channels. IK,Ado is also known as IK,ACh since acetylcholine, working through the muscarinic M2 receptors , activates the same downstream GIRK1/4 channels [48]. GIRK channels in turn conduct IK,Ado/IKACh leading to membrane hyperpolarization and AP suppression in pacemaker cells and other conducting tissues within the heart [11,19]. Additionally, adenosine and acetylcholine affect membrane excitability by opposing adrenergic signaling via decreased cyclic adenosine monophosphate production, which suppresses If and ICa,L [73].
Of their known isoforms (A1R, A2A, A2B and A3 and GIRK1-4), A1R, and GIRK1 and GIRK4 have been primarily studied in the context of HR regulation [11,19]. In the normal canine heart, A1R is predominantly expressed in the SAN relative to the surrounding atrial tissues [11,19]. Moreover, its heterogeneous expression is found in the head, center and tail compartments, thereby establishing a strong role for A1R in pacemaking in the healthy canine SAN [19]. Increased levels of adenosine and AR signaling, which decreases HR and metabolic energy consumption, are predicted to be energetically protective during ischemic conditions. On the other hand, altered expression of A1Rs has been shown to play important roles in impaired SAN function and conduction abnormalities in HF [11,19]. Our group [19] demonstrated that a decrease in SAN function in failing canine hearts may be mediated by A1R upregulation. We showed that expression of A1R and GIRK4 is upregulated in the SAN and atria in a tachypacing induced HF canine model, which could amplify adenosine-mediated conduction abnormalities and correlated with AP duration shortening and adenosine induced AF (Fig. 6B) [19]. Adenosine acting on the upregulated A1Rs induced SAN automaticity depression, post-pacing atrial pauses and shortened atrial repolarization leading to AF. These findings indicate that increased A1R expression may increase sensitivity to adenosine in failing canine atria, which could in turn, increase AF inducibility by activating downstream GIRK channel signaling. Moreover, since adenosine antagonizes adrenergic signaling by decreasing cyclic adenosine monophosphate production, increased A1R could also affect conduction by suppressing If and ICa,L, especially during adrenergic stimulation.
Expression of A1R, GIRK1 and GIRK4 proteins have been shown to be higher in the RA compared to the left atrium in humans [74]. This finding is of particular significance since it could explain why adenosine augments RA vs. left atrium repolarization sensitivity and induces AF with a higher activation frequency in RA than in the left atrium. Li et al [11] recently showed that A1R and GIRK4 are highly expressed in the human SAN relative to the atria (Fig 6A, right panel), and that this difference in humans is even higher than previously found in canine SAN. The reported relatively higher A1R expression might underlie the higher incidence of adenosine-induced SAN arrest in humans but not in canine hearts [11,19]. However, the expression patterns of A1R are very heterogeneous and vary among the different SAN compartments and hence the sensitivity of the pacemakers to adenosine in these regions are also different (Fig 2D). These disparities actually create redundant protection mechanisms for the SAN so that even when adenosine levels are high enough to depress the highly sensitive central pacemaker, the less sensitive head and tail pacemakers can continue to maintain a slower rhythm and prevent complete SAN arrest [11].
Therapeutic approaches to treat Adenosine and Vagal mediated SND and AF
The data presented above collectively emphasize that adenosine plays a double-edged role in regulating the SAN with protective as well as impairing effects. To combat its role in exacerbating SND, blocking A1R and GIRK channels have been explored as effective therapeutic approaches to treat SND [11,19]. In particular, theophylline, a specific A1R blocker, has been used as a pharmaceutical intervention to treat canine and human patients [75] with varying outcomes. We showed that theophylline can effectively abolish SAN dysfunction and AF in a canine HF model [19]. Theophylline has also been tested in human HF and SND patients. Although clinical trials at various phases found beneficial results with AR blockade, off-target side effects due to the ubiquitous expression of ARs limit its long-term utility in humans [76]. Therefore, expression and/or activity of A1R should be locally targeted within the SAN and SACP, to harness the beneficial effects of adenosine in ameliorating bradycardia without affecting its role in reducing metabolic stress, in the ventricular myocardium. Alternately, studies in ex-vivo human atria revealed that blocking GIRK channels with the selective blocker tertiapin prevents adenosine induced SND and AF [11,74], indicating that GIRK channel blockade can present a potential treatment approach for both adenosine and vagal mediated SND and AF in humans and canines.
Sodium channels: undecided but important players in canine and human SAN function
Voltage-gated Na+ channels (Nav) and the resultant current (INa) play an important role in facilitating rapid membrane depolarization by the influx of Na+ ions into the cell which generates the upstroke of the atrial and ventricular AP. Voltage-gated Na+ channel 1.5, the major human cardiac alpha subunit expressed by the sodium voltage-gated channel alpha subunit 5 gene has been extensively studied. Since it plays a key role in cardiac excitability, ~450 mutations affecting its structure and/or function have been associated with several human diseases including cardiomyopathies and severe arrhythmias including AF, premature ventricular beats and SND [77].
More recently, expression of neuronal Nav channel isoforms including Nav1.1, 1.3 and 1.6, which were previously suggested to be exclusively expressed in central nervous system and skeletal tissues, have been demonstrated in cardiac tissues [30,78]. Pharmacologically, neuronal channels are increasingly sensitive to tetrodotoxin and can be inhibited at nanomolar concentrations while the Nav1.5 channels require micromolar concentrations which allow to distinguish functional contributions of neuronal and cardiac Nav channels to membrane potentials [79]. However, the presence and role of INa in SAN pacemaking has been long debated since the majority of Na+ channels would be predicted to stay inactivated due to low negative maximal diastolic potentials (~−55 to −60 mV) and long diastolic depolarization phase [80]. In contrast to other cardiac myocytes wherein the AP upstroke is very fast, the slow AP upstroke (~2 V/sec) in central SAN cells is postulated to depend only on the ICa,L rather than INa [81]. However, expression of several Nav isoforms has been demonstrated specifically in the SAN, with species- specific differences, and has introduced a potential role for Nav channels in SAN pacemaking and conduction [82].
In the canine heart, Protas et al [80] found INa to be present at all ages with the highest nanomolar sensitivity to tetrodotoxin in adult canine SAN cells. Although no functional role was demonstrated within physiological conditions, INa in SAN is suggested to protect against excessive vagal slowing of the HR especially in the newborn. Direct studies on the role of INa in the human SAN, and expression of Na channel isoforms are scarce. However, several sodium voltage-gated channel alpha subunit 5 mutations are strongly associated with sinus bradycardia which emphasize a role for the INa in the human SAN [83]. Recently, Chandler et al [30] showed that expression of Nav1.5 mRNA is lowest in the human SAN pacemaker cells compared to surrounding atrial myocardium. Furthermore, Verkerk et al [84] have demonstrated potential INa in a small number of human SAN myocytes. Preliminary data from ex-vivo human heart studies also support the presence of functional cardiac and neuronal Nav channels in the SAN [85].These findings collectively begin to establish the presence of INa in both canine and human SAN cells and that INa may not play a significant role within baseline functional ranges. However, Nav channels could be recruited by hyperpolarization as a fail-safe mechanism against sinus bradycardia and SAN conduction blocks. Future studies should embark on more extensive investigations in both canine and human SAN tissues to document changes in Nav protein levels and corresponding electrophysiological adaptations especially in diseased or failing hearts, in order to determine if INa plays a beneficial role against sinus bradycardia.
Connexin expression across the SAN and SACP
Connexins form gap junctions between adjacent cells and play an important role in cell-to-cell impulse propagation as well as throughout the heart [86]. More than 20 Cx genes have been identified from mice through humans. Of these, mRNA of isoforms 40, 43, 45 have been reported in the mammalian heart [87]. Amongst the cardiac isoforms, Cx43 is the predominant isoform in atrial and ventricular cardiomyocytes [87]. However, Cx43 expression is not detected in the central SAN pacemaker compartment in both canine [20] and human [11,30]. In canine SAN, Davis et al [88] demonstrated Cx40 and Cx45 expression, with a complete absence of Cx43. Kwong et al [89] found that the canine SAN was composed of 3 populations of pacemaker cells: 30-35% that expressed Cx43, Cx40 and Cx45, ~55% that expressed only Cx40 and ~10-15% that did not express any Cx. In the human SAN, Davis et al [90] reported expression of Cx45, low levels of Cx40 and absence of Cx43. Importantly, in studies mentioned above, mainly immunohistochemistry were used, which often suffers from non-specific staining. At the present, protein expression of only Cx43 levels has been demonstrated across canine [19] and human SAN complexes [11,58]. Further rigorous studies are required to reliably demonstrate expression pattern of Cx isoforms in different compartments of canine and human SAN.
Conclusions and Future Directions
In summary, several critical aspects of normal and dysfunctional SAN activation and conduction are very similar in canines and humans. Currently, available treatment options to treat SND are still limited in canine and human patients. Pharmaceutical drugs and implantable pacemakers are only partially successful in managing SND [7], and these interventions are unable to cure/or prevent progression of the disease. Hence there is a critical need not only to identify novel causal mechanisms of SND but also to develop targeted therapies to treat and/or manage SND. Moreover, the distinct structural and functional similarities between the canine and human SAN also suggest that studies investigating sources of dysfunction and novel targeted treatments in these two species can be mutually beneficial. Recent studies exploring the viability and applicability of biological pacemakers have demonstrated the potential utility of viral, gene and/or cell based therapies to treat canine and human SND [64]. Importantly, since SAN rhythm regulation depends not only on nodal pacemakers but also on robust availability and functioning SACPs [11], future therapies should incorporate targeted treatment of SACPs as well in canine and human SND patients. Novel approaches to remove fibrotic lesions and/or to prevent excessive fibrotic remodeling within the SAN and SACPs in diseased hearts would be ideal to restore normal function to the SAN complex. Our increasing knowledge of how the SAN works in these two species continues to strengthen the potential possibility of treating SND with improved, targeted therapies.
Acknowledgments
Sources of Funding
This work was supported by NIH R01 HL135109 and HL115580, and American Heart Association Grant in Aid #16GRNT31010036 (VVF)
Glossary
- AF
atrial fibrillation
- AP
action Potential
- AR
adenosine receptor
- Cx
connexin
- GIRK
G protein-coupled inwardly-rectifying potassium (channel)
- HCN
hyperpolarization-activated cyclic nucleotide-gated (channel)
- HF
heart failure
- HR
heart rate
- MI
myocardial infarction
- RA
right atrium
- SACP
sinoatrial conduction pathway
- SAN
sinoatrial node
- SND
sinoatrial node dysfunction
- SR
sinus rhythm
- 3D
three dimensional
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
The authors do not have any conflicts to disclose.
The work was performed at the Department of Physiology and Cell Biology, Dorothy M. Davis Heart & Lung Research Institute, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Reference List
- [1].Keith A, Flack M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J Anat Physiol 1907;41:172–89. [PMC free article] [PubMed] [Google Scholar]
- [2].Lewis T, Oppenheimer A, Oppenheimer BS. The site of origin of the mammalian heart beat: the pacemaker in the dog. Heart 1910;II:147–69. [Google Scholar]
- [3].Schuessler RB, Boineau JP, Bromberg BI. Origin of the Sinus Impulse. J Cardiovasc Electrophysiol 1996;7:263–74. [DOI] [PubMed] [Google Scholar]
- [4].James TN, Sherf L, Fine G, Morales AR. Comparative ultrastructure of the sinus node in man and dog. Circulation 1966;34:139–63. [DOI] [PubMed] [Google Scholar]
- [5].Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989;80:1675–80. [DOI] [PubMed] [Google Scholar]
- [6].Thiyagarajah A, Lau DH, Sanders P. Atrial fibrillation and conduction system disease: the roles of catheter ablation and permanent pacing. J Interv Card Electrophysiol 2018;52:395–402. [DOI] [PubMed] [Google Scholar]
- [7].Ward JL, DeFrancesco TC, Tou SP, Atkins CE, Griffith EH, Keene BW. Outcome and survival in canine sick sinus syndrome and sinus node dysfunction: 93 cases (2002-2014). J Vet Cardiol 2016;18:199–212. [DOI] [PubMed] [Google Scholar]
- [8].Alboni P, Menozzi C, Brignole M, Paparella N, Gaggioli G, Lolli G, Cappato R. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome the THEOPACE study: a randomized controlled trial. Circulation 1997;96:260–6. [DOI] [PubMed] [Google Scholar]
- [9].Jensen PN, Gronroos NN, Chen LY, Folsom AR, deFilippi C, Heckbert SR, Alonso A. Incidence of and risk factors for sick sinus syndrome in the general population. J Am Coll Cardiol 2014;64:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sanchez-Quintana D, Cabrera JA, Farre J, Climent V, Anderson RH, Ho SY. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 2005;91:189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li N, Hansen BJ, Csepe TA, Zhao J, Ignozzi AJ, Sul LV, Zakharkin SO, Kalyanasundaram A, Davis JP, Biesiadecki BJ, Kilic A, Janssen PML, Mohler PJ, Weiss R, Hummel JD, Fedorov VV. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Csepe TA, Zhao J, Sul LV, Wang Y, Hansen BJ, Li N, Leota S, Ignozzi AJ, Bratasz A, Powell KA, Kilic A, Mohler PJ, Janssen PML, Hummel JD, Simonetti OP, Fedorov VV. Novel Application of 3D Contrast Enhanced CMR to Define Fibrotic Structure of the Human Sinoatrial Node In-vivo . Eur Heart J Cardiovasc Imaging 2017;18:862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Opthof T The mammalian sinoatrial node. Cardiovasc Drugs Ther 1988;1:573–97. [DOI] [PubMed] [Google Scholar]
- [14].Csepe TA, Zhao J, Hansen BJ, Li N, Sul LV, Lim P, Wang Y, Simonetti OP, Kilic A, Mohler PJ, Janssen PM, Fedorov VV. Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways. Prog Biophys Mol Biol 2016;120:164–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res 1980;46:11–22. [DOI] [PubMed] [Google Scholar]
- [16].Vinogradova TM, Bogdanov KY, Lakatta EG. Novel perspectives on the beating rate of the heart. Circ Res 2002;91 :e3. [DOI] [PubMed] [Google Scholar]
- [17].Bucchi A, Barbuti A, DiFrancesco D, Baruscotti M. Funny Current and Cardiac Rhythm: Insights from HCN Knockout and Transgenic Mouse Models. Front Physiol 2012;3:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Csepe TA, Kalyanasundaram A, Hansen BJ, Zhao J, Fedorov VV. Fibrosis: a structural modulator of sinoatrial node physiology and dysfunction. Front Physiol 2015;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lou Q, Hansen BJ, Fedorenko O, Csepe TA, Kalyanasundaram A, Li N, Hage LT, Glukhov AV, Billman GE, Weiss R, Mohler PJ, Gyorke S, Biesiadecki BJ, Carnes CA, Fedorov VV. Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation 2014;130:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fedorov VV, Schuessler RB, Hemphill M, Ambrosi CM, Chang R, Voloshina AS, Brown K, Hucker WJ, Efimov IR. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res 2009;104:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lou Q, Glukhov AV, Hansen B, Hage L, Vargos-Pinto P, Billman GE, Carnes CA, Fedorov VV. Tachy-brady arrhythmias: The critical role of adenosine-induced sino-atrial conduction block in post-tachycardia pauses. Heart Rhythm 2013;10:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Glukhov AV, Hage LT, Hansen BJ, Pedraza-Toscano A, Vargas-Pinto P, Hamlin RL, Weiss R, Carnes CA, Billman GE, Fedorov VV. Sinoatrial node reentry in a canine chronic left ventricular infarct model: role of intranodal fibrosis and heterogeneity of refractoriness. Circ Arrhythm Electrophysiol 2013;6:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fedorov VV, Chang R, Glukhov AV, Kostecki G, Janks D, Schuessler RB, Efimov IR. Complex interactions between the sinoatrial node and atrium during reentrant arrhythmias in the canine heart. Circulation 2010;122:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].James TN. Anatomy of the human sinus node. Anat Rec 1961;141:109–39. [DOI] [PubMed] [Google Scholar]
- [25].Truex RC, Smythe MQ, Taylor MJ. Reconstruction of the human sinoatrial node. Anat Rec 1967;159:371–8. [DOI] [PubMed] [Google Scholar]
- [26].Chandler N, Aslanidi O, Buckley D, Inada S, Birchall S, Atkinson A, Kirk D, Monfredi O, Molenaar P, Anderson R, Sharma V, Sigg D, Zhang H, Boyett M, Dobrzynski H. Computer three-dimensional anatomical reconstruction of the human sinus node and a novel paranodal area. Anat Rec (Hoboken) 2011;294:970–9. [DOI] [PubMed] [Google Scholar]
- [27].Fedorov VV, Glukhov AV, Chang R, Kostecki G, Aferol H, Hucker WJ, Wuskell JP, Loew LM, Schuessler RB, Moazami N, Efimov IR. Optical mapping of the isolated coronary-perfused human sinus node. J Am Coll Cardiol 2010;56:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boineau JP, Canavan TE, Schuessler RB, Cain ME, Corr PB, Cox JL. Demonstration of a widely distributed atrial pacemaker complex in the human heart. Circulation 1988;77:1221–37. [DOI] [PubMed] [Google Scholar]
- [29].Boineau JP, Schuessler RB, Canavan TE, Corr PB, Cain ME, Cox JL. The human atrial pacemaker complex. J Electrocardiol 1989;22 Suppl:189–97. [DOI] [PubMed] [Google Scholar]
- [30].Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation 2009;119:1562–75. [DOI] [PubMed] [Google Scholar]
- [31].Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 2000;47:658–87. [DOI] [PubMed] [Google Scholar]
- [32].Evans JM, Randall DC, Funk JN, Knapp CF. Influence of cardiac innervation on intrinsic heart rate in dogs. Am J Physiol 1990;258:H1132–7. [DOI] [PubMed] [Google Scholar]
- [33].Jose AD, Taylor RR. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J Clin Invest 1969;48:2019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 1970;4:160–7. [DOI] [PubMed] [Google Scholar]
- [35].Boineau JP, Schuessler RB, Hackel DB, Miller CB, Brockus CW, Wylds AC. Widespread distribution and rate differentiation of the atrial pacemaker complex. Am J Physiol 1980;239:H406–15. [DOI] [PubMed] [Google Scholar]
- [36].Bromberg BI, Hand DE, Schuessler RB, Boineau JP. Primary negativity does not predict dominant pacemaker location: implications for sinoatrial conduction. Am J Physiol 1995;269:H877. [DOI] [PubMed] [Google Scholar]
- [37].Fedorov VV, Glukhov AV, Chang R. Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias. Am J Physiol Heart Circ Physiol 2012;302:H1773–83. [DOI] [PubMed] [Google Scholar]
- [38].Fedorov VV, Glukhov AV, Chang R, Kostecki G, Alferol H, Wuskell J, Loew LM, Moazami N, Efimov IR. The origin of heartbeat in the human sinus node: evidence of sino-atrial exit pathways. Circulation 2009;120:S673–4. [Google Scholar]
- [39].Monfredi O, Boyett MR. Sick sinus syndrome and atrial fibrillation in older persons - A view from the sinoatrial nodal myocyte. J Mol Cell Cardiol 2015;83:88–100. [DOI] [PubMed] [Google Scholar]
- [40].Alpert MA, Flaker GC. Arrhythmias associated with sinus node dysfunction. Pathogenesis, recognition, and management. JAMA 1983;250:2160–6. [PubMed] [Google Scholar]
- [41].Alings AM, Abbas RF, Bouman LN. Age-related changes in structure and relative collagen content of the human and feline sinoatrial node. A comparative study. Eur Heart J 1995;16:1655–67. [DOI] [PubMed] [Google Scholar]
- [42].Shiraishi I, Takamatsu T, Minamikawa T, Onouchi Z, Fujita S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation 1992;85:2176–84. [DOI] [PubMed] [Google Scholar]
- [43].Joyner RW, van Capelle FJ. Propagation through electrically coupled cells. How a small SA node drives a large atrium. Biophys J 1986;50:1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J 2015;36:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baruscotti M, Difrancesco D. Pacemaker channels. Ann N Y Acad Sci 2004;1015:111–21. [DOI] [PubMed] [Google Scholar]
- [46].Monfredi O, Maltsev VA, Lakatta EG. Modern concepts concerning the origin of the heartbeat. Physiology (Bethesda ) 2013;28:74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Szentmiklosi AJ, Galajda Z, Cseppento A, Gesztelyi R, Susan Z, Hegyi B, Nanasi PP. The Janus face of adenosine: antiarrhythmic and proarrhythmic actions. Curr Pharm Des 2015;21:965–76. [DOI] [PubMed] [Google Scholar]
- [48].Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem 2000;267:5830–6. [DOI] [PubMed] [Google Scholar]
- [49].Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature 1979;280:235–6. [DOI] [PubMed] [Google Scholar]
- [50].DiFrancesco D Pacemaker mechanisms in cardiac tissue. [Review]. Annual Review of Physiology 1993;55:455–72. [DOI] [PubMed] [Google Scholar]
- [51].Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev 2008;88:919–82. [DOI] [PubMed] [Google Scholar]
- [52].Baruscotti M, Barbuti A, Bucchi A. The cardiac pacemaker current. J Mol Cell Cardiol 2010;48:55–64. [DOI] [PubMed] [Google Scholar]
- [53].Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol 1996;118:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Verkerk AO, Wilders R. Pacemaker Activity of the Human Sinoatrial Node: An Update on the Effects of Mutations in HCN4 on the Hyperpolarization-Activated Current. Int J Mol Sci 2015;16:3071–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature 1998;393:587–91. [DOI] [PubMed] [Google Scholar]
- [56].Zicha S, Fernandez-Velasco M, Lonardo G, L'Heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 2005;66:472–81. [DOI] [PubMed] [Google Scholar]
- [57].Verkerk AO, Wilders R, van Borren MM, Peters RJ, Broekhuis E, Lam K, Coronel R, de Bakker JM, Tan HL. Pacemaker current (I(f)) in the human sinoatrial node. Eur Heart J 2007;28:2472–8. [DOI] [PubMed] [Google Scholar]
- [58].Li N, Csepe TA, Hansen BJ, Dobrzynski H, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Rosen MR, Biesiadecki BJ, Fedorov VV. Molecular Mapping of Sinoatrial Node HCN Channel Expression in the Human Heart. Circ Arrhythm Electrophysiol 2015;8:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bucchi A, Tognati A, Milanesi R, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol 2006;572:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li YD, Ji YT, Zhou XH, Jiang T, Hong YF, Li JX, Xing Q, Xiong J, Yusufuaji Y, Tang BP. Effects of ivabradine on cardiac electrophysiology in dogs with age-related atrial fibrillation. Med Sci Monit 2015;21:1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Annamaria M, Lupo PP, Foresti S, De AG, de RE, Sciarra L, Cappato R, Calo L. Treatment of inappropriate sinus tachycardia with ivabradine. J Interv Card Electrophysiol 2016;46:47–53. [DOI] [PubMed] [Google Scholar]
- [62].Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, Comtois P, Wang Z, Kuo CT, Nattel S. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation 2009;119:1576–85. [DOI] [PubMed] [Google Scholar]
- [63].Li YD, Hong YF, Zhang Y, Zhou XH, Ji YT, Li HL, Hu GJ, Li JX, Sun L, Zhang JH, Xin Q, Yusufuaji Y, Xiong J, Tang BP. Association between reversal in the expression of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel and age-related atrial fibrillation. Med Sci Monit 2014;20:2292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Boink GJ, Robinson RB. Gene therapy for restoring heart rhythm. J Cardiovasc Pharmacol Ther 2014;19:426–38. [DOI] [PubMed] [Google Scholar]
- [65].Qu J, Plotnikov AN, Danilo P Jr., Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circulation 2003;107:1106–9. [DOI] [PubMed] [Google Scholar]
- [66].Potapova I, Plotnikov A, Lu Z, Danilo P Jr., Valiunas V, Qu J, Doronin S, Zuckerman J, Shlapakova IN, Gao J, Pan Z, Herron AJ, Robinson RB, Brink PR, Rosen MR, Cohen IS. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res 2004;94:952–9. [DOI] [PubMed] [Google Scholar]
- [67].Chauveau S, Brink PR, Cohen IS. Stem cell-based biological pacemakers from proof of principle to therapy: a review. Cytotherapy 2014;16:873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 2010;106:659–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gao Z, Chen B, Joiner ML, Wu Y, Guan X, Koval OM, Chaudhary AK, Cunha SR, Mohler PJ, Martins JB, Song LS, Anderson ME. I(f) and SR Ca(2+) release both contribute to pacemaker activity in canine sinoatrial node cells. J Mol Cell Cardiol 2010;49:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sosunov EA, Anyukhovsky EP. Differential effects of ivabradine and ryanodine on pacemaker activity in canine sinus node and purkinje fibers. J Cardiovasc Electrophysiol 2012;23:650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Watt AH. Sick sinus syndrome: an adenosine-mediated disease. Lancet 1985;325:786–8. [DOI] [PubMed] [Google Scholar]
- [72].Funaya H, Kitakaze M, Node K, Minamino T, Komamura K, Hori M. Plasma adenosine levels increase in patients with chronic heart failure. Circulation 1997;95:1363–5. [DOI] [PubMed] [Google Scholar]
- [73].Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 1987;325:321–6. [DOI] [PubMed] [Google Scholar]
- [74].Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Van Wagoner DR, Kilic A, Mohler PJ, Janssen PM, Biesiadecki BJ, Hummel JD, Weiss R, Fedorov VV. Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart. Circulation 2016;134:486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Saito D, Matsubara K, Yamanari H, Obayashi N, Uchida S, Maekawa K, Sato T, Mizuo K, Kobayashi H, Haraoka S. Effects of oral theophylline on sick sinus syndrome. J Am Coll Cardiol 1993;21:1199–204. [DOI] [PubMed] [Google Scholar]
- [76].Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 2006;5:247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Amin AS, Asghari-Roodsari A, Tan HL. Cardiac sodium channelopathies. Pflugers Arch 2010;460:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci USA 2002;99:4073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kaufmann SG, Westenbroek RE, Maass AH, Lange V, Renner A, Wischmeyer E, Bonz A, Muck J, Ertl G, Catterall WA, Scheuer T, Maier SKG. Distribution and function of sodium channel subtypes in human atrial myocardium. J Mol Cell Cardiol 2013;61:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Protas L, Oren RV, Clancy CE, Robinson RB. Age-dependent changes in Na current magnitude and TTX-sensitivity in the canine sinoatrial node. J Mol Cell Cardiol 2010;48:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kodama I, Nikmaram MR, Boyett MR, Suzuki R, Honjo H, Owen JM. Regional differences in the role of the Ca2+ and Na+ currents in pacemaker activity in the sinoatrial node. Am J Physiol 1997;272:H2793–806. [DOI] [PubMed] [Google Scholar]
- [82].Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, Catterall WA, Scheuer T. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci USA 2003;100:3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Remme CA. Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J Physiol 2013;591:4099–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Verkerk AO, Wilders R, van Borren MM, Tan HL. Is sodium current present in human sinoatrial node cells? Int J Biol Sci 2009;5:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Li N, Hansen BJ, Ssekayombya PS, Helfrich KM, Gyorke S, Biesiadecki BJ, Whitson B, Janssen PML, Mohler PJ, Accornero F, Fedorov V. Functional and molecular evidence of the presence of neuronal and cardiac sodium channels in the human sinoatrial node. Heart Rhythm 2018;15:S392. [Google Scholar]
- [86].Jansen JA, van Veen TA, de Bakker JM, van Rijen HV. Cardiac connexins and impulse propagation. J Mol Cell Cardiol 2010;48:76–82. [DOI] [PubMed] [Google Scholar]
- [87].van Veen AA, van Rijen HV, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res 2001;51:217–29. [DOI] [PubMed] [Google Scholar]
- [88].Davis LM, Kanter HL, Beyer EC, Saffitz JE. Distinct gap junction protein phenotypes in cardiac tissues with disparate conduction properties. Journal of the American College of Cardiology 1994;24:1124–32. [DOI] [PubMed] [Google Scholar]
- [89].Kwong KF, Schuessler RB, Green KG, Laing JG, Beyer EC, Boineau JP, Saffitz JE. Differential expression of gap junction proteins in the canine sinus node. Circ Res 1998;82:604–12. [DOI] [PubMed] [Google Scholar]
- [90].Davis LM, Rodefeld ME, Green K, Beyer EC, Saffitz JE. Gap junction protein phenotypes of the human heart and conduction system. J Cardiovasc Electrophysiol 1995;6:813–22. [DOI] [PubMed] [Google Scholar]