Abstract
Nanoparticles (NPs) promise to advance strategies to treat vascular disease. Since being harnessed by the cancer field to deliver safer and more effective chemotherapeutics, nanoparticles have been translated into applications for cardiovascular disease. Systemic exposure and drug-drug interactions remain a concern for nearly all cardiovascular therapies, including statins, antithrombotic, and thrombolytic agents. Moreover, off-target effects and poor bioavailability have limited the development of completely new approaches to treat vascular disease. Through the rational design of nanoparticles, nano-based delivery systems enable more efficient delivery of a drug to its therapeutic target or even directly to the diseased site, overcoming biological barriers and enhancing a drug’s therapeutic index. In addition, advances in molecular imaging have led to the development of “theranostic” NPs that may simultaneously act as carriers of both therapeutic and imaging payloads. The following is a summary of nanoparticle therapy for atherosclerosis, thrombosis, and restenosis, and an overview of recent major advances in the targeted treatment of vascular disease.
Introduction
Cardiovascular disease is the number one cause of death globally.1 The low delivery efficiency, poor target specificity and/or off-target activity of our therapies has contributed to the challenges we face in cardiovascular medicine.2 Nanoparticles, on the scale of less than 0.1 microns in at least one dimension, have emerged as a powerful tool to increase the targeting selectivity of a drug and limit its distribution throughout the body. Their tunable shape, size, and surface chemistry enables nanoparticles to be “programmed” for site-specific delivery.3 A central goal of nanotherapy is to enhance the efficacy of a therapy and minimize side effects caused by freely delivered drug.4 Nanoparticles achieve this through rational design, frequently incorporating knowledge of normal vs. diseased biology to optimize residence time in the diseased tissue.
Oncology was the first field to leverage the properties of nanoparticles for drug delivery with Doxil, a liposome-encapsulated doxorubicin formulation approved by the FDA in 1995 for the treatment of Kaposi’s sarcoma.5 Now widely used for the treatment of multiple myeloma and other malignancies, the nanoformulation enables preferential uptake by cancer cells and limits exposure to the heart, reducing the risk of doxorubicin-induced cardiotoxicity and heart failure.6 Currently, more than 50 nanoparticle-based therapies are used for a variety of indications including infections, chronic kidney disease, and even psychiatric conditions.7
Nanotechnology has also expanded into the realm of cardiovascular disease. Currently marketed nanoformulations of fenofibrate are used in patients with hypertriglyceridemia to help overcome challenges with drug solubility and absorption. A number of delivery systems are under development to therapeutically target pathways of vascular disease (Figure 1). Additionally, multi-functional “theranostic” NPs hold promise for combined delivery of therapeutic and imaging agents. These theranostic NPs can serve to blend treatment with information from one or even multiple imaging modalities to more comprehensively assess disease. Prior work has highlighted the status of nanomaterials in cardiovascular imaging, including their potential to separately identify “vulnerable” plaques at risk for rupture.8 This review discusses advances in the application of nanoparticles for the treatment of vascular disease, their potential translation to the clinic, and challenges in their development. Greater emphasis is placed on nanoparticle-directed therapy for atherosclerosis and its associated complications, including thrombosis and restenosis, given their role in ischemic heart disease (Table 1).
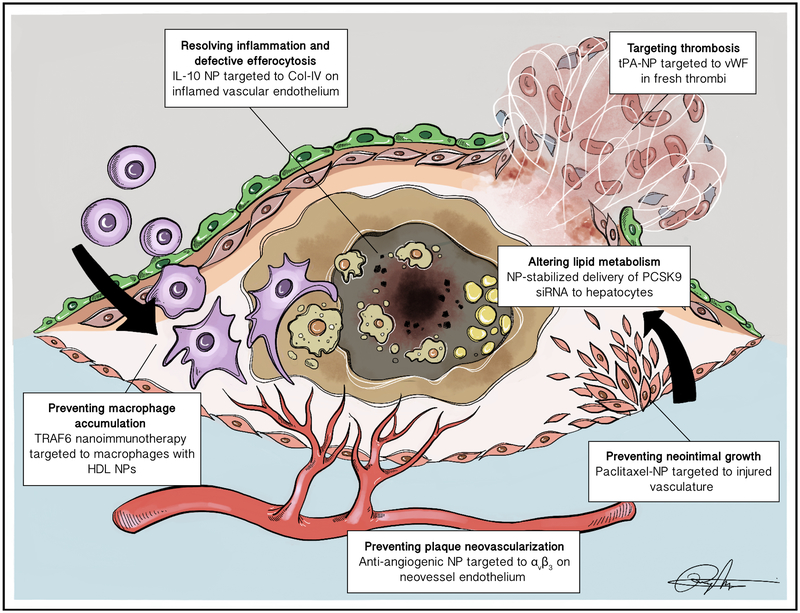
Figure 1:
Targeted therapeutic strategies enabled by nanoparticles. In order to avoid adverse effects and toxicities due to systemic exposure, targeted nanoparticles have been developed to resolve inflammation specifically at the inflamed plaque (e.g. Collagen-IV targeted IL-10 NPs17), prevent plaque neovascularization (e.g. αvβ3-targed anti-angiogenic NPs65), and deliver anti-proliferative or thrombolytic drugs to address restenosis and atherothrombotic events (e.g. endothelial-targeted NPs encapsulating paclitaxel75, vWF-targeted NPs encapsulating tPA81). Nanoparticles have also enabled cell-specific modulation of molecules that drive atherosclerosis, such as CD40-induced TRAF6 signaling in macrophages45 and regulation of PCSK9 in hepatocytes.55
Table 1.
Examples of vascular nanotherapies in development.
| Therapeutic agent | Nanoparticle | Delivery | Model | In vivo findings | Reference |
|---|---|---|---|---|---|
| Resolving inflammation and defective efferocytosis | |||||
| Annexin A1 mimetic peptide | Type IV collagen-targeted copolymers of PLGA-PEG | Systemic | Ldlr −/− mice | ↓: plaque area, necrotic core, lesional oxidative stress ↑: fibrous cap thickness |
16 |
| IL-10 | Type IV collagen-targeted copolymers of PLGA-PEG | Systemic | Ldlr −/− mice | ↓: necrotic core and lesional oxidative stress ↑: lesional efferocytosis, fibrous cap thickness |
17 |
| Superoxide dismutase mimetic agent and hydrogen-peroxide-eliminating compound | Cyclodextrin-based polysaccharide | Systemic | apoE −/− mice | ↓: ROS-induced inflammation, lesional cholesterol crystals, necrotic core | 18 |
| Prednisolone* | MRI detectable liposomes | Systemic | Hypercholesterolemic rabbits with repeated balloon aortic injury | ↓: plaque inflammation, lesional macrophage content Delivery visualized by MRI Anti-inflammatory effect validated by 18F-FDG/PET-CT and MRI |
19 |
| VCAM1, ICAM1 and 2, E- and P-selectin siRNA | PEI polymer | Systemic | Coronary ligation of apoE −/− mice | ↓: post-myocardial infarction leukocyte recruitment, plaque inflammation, necrotic core, lesion size ↑: fibrous cap thickness |
23 |
| Preventing plaque neovascularization | |||||
| Fumagillin* | avb3 integrin–targeted paramagnetic NPs | Systemic | Hypercholesterolemic rabbits | ↓: aortic plaque angiogenesis, expansion of the vasa vasorum Aortic neovascular delivery and treatment response assessed by MRI |
32 |
| Fumagillin* | avb3 integrin–targeted paramagnetic NPs | Systemic NP delivery, followed by oral statin treatment | Hypercholesterolemic rabbits | Marked anti-angiogenic effect of NPs sustained by statin therapy Angiogenesis of aortic wall assessed by MRI |
33 |
| Targeting macrophages | |||||
| Pitavastatin | PLGA | Systemic | angiotensin II-infused apoE −/− mice | ↓: plaque monocyte infiltration ↑: plaque stability and protection from rupture |
37 |
| CCR2 siRNA* | Lipid NPs | Systemic | apoE −/− mice | ↓: monocyte migration and accumulation, lesion size Changes in biodistribution over time evaluated by FMT-CT |
38 |
| Simvastatin* | Reconstituted HDL | Systemic | apoE −/− mice | ↓: lesion area due to reduced plaque macrophage content, mRNA expression levels of monocyte recruitment and pro-inflammatory genes No effect on serum lipid levels Biodistribution evaluated by ex vivo NIRF Plaque macrophage targeting and treatment efficacy assessed by MRI and FMT/CT |
40 |
| Simvastatin* | Reconstituted HDL | Short-term systemic NP delivery, followed by long-term oral statin treatment | apoE −/− mice | ↓: plaque macrophage proliferation and subsequent plaque inflammation Rapid suppression of inflammation by NP intervention, with sustained effects by oral statin therapy Treatment efficacy assessed by MRI and ex vivo NIRF |
41 |
| Liver X receptor agonist | Type IV collaged-targeted PLA-PEG NPs | Systemic | Ldlr −/− mice | ↓: plaque macrophage content and cholesterol efflux No change in plasma lipid levels and hepatic lipid metabolism |
42 |
| Macrophage scavenger receptor-blocking sugars | Amphiphilic NPs with PEG core | Systemic | apoE −/− mice | ↓: oxidized LDL uptake by macrophages, lipid burden, and overall plaque development | 43 |
| Pioglitazone | PLGA | Systemic | angiotensin II-infused apoE −/− mice | ↓: peripheral inflammatory monocytes, aortic M1 macrophage polarization, plaque rupture ↑: plaque stabilization No effect on plaque macrophage content or lesion area |
44 |
| TRAF6 inhibitor | Reconstituted HDL | Systemic | apoE −/− mice Cynomolgus monkeys |
↓: monocyte recruitment, plaque macrophage content Lack of immune suppressive side effects Liver and spleen accumulation demonstrated by PET/MRI Short-term safety in mice and non-human primates |
39, 45 |
| Near-infrared fluorophore* | Light-activated iron-oxide NPs | Systemic, with local photodynamic therapy | apoE −/− mice | ↓: lesional macrophage content following local light therapy ↑: plaque macrophage apoptosis Plaque localization demonstrated by intravital fluorescence microscopy |
48 |
| Photothermal ablation* | Single-walled carbon nanotubes | Systemic, with local activation by photoluminescence | Mice carotid artery ligation |
In vivo delivery visualized by FMT Ex vivo near-infrared imaging and photothermal ablation of vascular macrophages |
49 |
| Altering lipid metabolism | |||||
| apoB siRNA | Liposomes | Systemic | Cynomolgus monkeys | ↓: apoB mRNA expression in the liver, serum cholesterol, and LDL levels sustained for 11 days following single dose | 52 |
| apoB siRNA | Liposomes | Systemic | Ldlr CETP +/− mice | ↓: apoB protein expression in the liver and serum LDL levels sustained for 3 weeks following single dose | 53 |
| PCSK9 siRNA | Lipidoid-PEG formulation | Systemic | Wild-type mice, rats, cynomolgus monkeys | ↓: plasma PCKS9 protein and LDL levels sustained for 3 weeks following single bolus | 55 |
| TRPV1 antibodies* | Copper sulfide NPs | Systemic, with local photothermal activation | apoE −/− mice | ↓: plaque lipid storage, VSMC foam cell formation, lesion formation ↑: autophagy and cholesterol efflux Vessel delivery monitored by photoacoustic imaging |
61 |
| Preventing neointimal growth | |||||
| Rapamycin | Albumin NPs | Local catheter delivery | Porcine femoral artery balloon injury | ↓: luminal stenosis at 28 days | 64 |
| Rapamycin* | avb3-targeted paramagnetic NPs | Local catheter delivery | Rabbit femoral artery balloon injury | ↓: vascular stenosis, neointimal formation at 14 days No delay in endothelial healing Intramural delivery and luminal changes assessed by MRI |
65 |
| NOX2 siRNA | Lysine-based NPs | Open delivery onto adventitia | Rat carotid artery balloon injury | ↓: ROS production, neointima formation at 14 days | 66 |
| Nitric oxide | Liposomes | Local catheter delivery | Rabbit carotid artery balloon injury | ↓: neointimal proliferation at 14 days | 67 |
| Paclitaxel | Stent-targeted magnetic NPs | Local delivery, with targeting by magnetic exposure | Rat carotid artery stenting | ↓: in-stent neointimal growth at 14 days | 68 |
| Imatinib (PDGF tyrosine kinase inhibitor) | PLGA NP-eluting stents | Coated on stent | Porcine coronary artery stenting | ↓: in-stent restenosis at 28 days No effect on endothelial proliferation |
70 |
| Pitavastatin | PLGA NP-eluting stents | Coated on stent | Porcine coronary artery stenting | ↓: in-stent restenosis at 28 days Lack of delay in endothelial healing |
71 |
| Paclitaxel | Albumin NPs | Systemic | Rabbit iliac artery stenting | ↓: in-stent neointimal growth sustained for 28 days ↑: endothelialization and healing |
74 |
| Paclitaxel | Type IV collagen-targeted lipid-polymeric NPs | Systemic | Rat carotid artery balloon injury | ↓: neointimal proliferation at 14 days | 75 |
| Alendronate (bisphosphonate) | Liposomes | Systemic | Rabbit iliac artery stenting | ↓: blood monocyte count, arterial macrophage infiltration, in-stent neointimal formation at 28 days | 76 |
| Nitric oxide | Nanofibers | Systemic | Rat carotid artery balloon injury | ↓: neointimal hyperplasia sustained for 7 months | 78 |
| Prednisolone | Subendothelial-targeted liposomes | Systemic | Rabbit iliac artery stenting | ↓: in-stent stenosis at 42 days | 79 |
| Targeting thrombosis | |||||
| tPA | PAA-coated iron oxide magnetic NPs | Local catheter delivery, with magnetic targeted thrombolysis | Rat iliac artery embolus | ↑: aortoiliac blood flow, hind limb perfusion | 80 |
| tPA | vWF-targeted gelatin NP complex | Systemic, with drug release enhanced by local ultrasound | Porcine myocardial infarction | ↑: coronary recanalization and left ventricular ejection fraction compared to free tPA | 81 |
| tPA* | Iron oxide PLGA NPs | Systemic | Rat aorta thrombosis | ↑: thrombolytic efficiency Thrombus targeting and thrombolytic activity assessed by MRI |
82 |
| Streptokinase | GPIIb/IIIa and P-selectin-targeted liposomes | Systemic | Mouse carotid artery thrombosis | ↑: delay in thrombus growth, time to vessel occlusion Minimal effect on tail bleeding time Targeting to activated platelets demonstrated by intravital microscopy |
83 |
| Urokinase | Iron oxide magnetic NPs | Local catheter delivery, with magnetic targeted thrombolysis | Rat carotid artery and jugular vein thrombosis | ↑: thrombus dissolution Minimal changes in systemic plasminogen activity and tail bleeding time |
85 |
| Thrombin inhibitor* | PFC NPs | Systemic | apoE −/− mice carotid artery injury | ↓: thrombin-induced inflammatory and pro-coagulant molecules ↑: time to carotid occlusion, restoration of endothelium No change in activated partial thromboplastin time Ex vivo assessment of delivery and endothelial damage by magnetic resonance spectroscopy |
87 |
Nanoparticles designed as theranostic agents.
PLGA: poly lactide-co-glycolide. PEG: polyethylene glycol. IL-10: interleukin-10. ROS: reactive oxygen species. VCAM1: vascular cell adhesion molecule 1. ICAM1: intercellular adhesion molecule 1. PEI: polyethyleneimine. MRI: magnetic resonance imaging. 18F-FDG/PET-CT: 2-deoxy-2-[fluorine-18]fluoro-D-glucose positron emission tomography-computed tomography. PLA: polylactic acid. LDL: low density lipoprotein. HDL: high density lipoprotein. NIRF: near-infrared fluorescence. FMT-CT: fluorescence molecular tomography-computed tomography. CCR2: C-C chemokine receptor type 2. TRAF6: tumor necrosis factor receptor-associated factor 6. apoB: apolipoprotein B. PCSK9: proprotein convertase subtilisin/kexin type 9. TRPV1: transient receptor potential cation channel subfamily V member 1. VSMC: vascular smooth muscle cell. NOX2: NADPH oxidase 2. PDGF: platelet-derived growth factor. GPIIb/IIIa: glycoprotein IIb/IIIa. vWF: von Willebrand factor. tPA: tissue plasminogen activator. PFC: perfluorocarbon.
Resolving inflammation and defective efferocytosis
Atherosclerosis is an inflammatory disease characterized by the accumulation of lipids, diseased cells, and necrotic debris. Pro-inflammatory leukocytes and cytokines act at different stages during the formation of the atherosclerotic plaque.9 Heightened inflammation is driven, in part, by the failure to clear apoptotic tissue from the diseased vessel wall due to a defect in efferocytosis (programmed cell removal), such that apoptotic cells accumulate, become secondarily necrotic, and release their pro-inflammatory intracellular contents.10, 11 Importantly, this non-resolving inflammation drives clinically dangerous lesions that are at increased risk of rupture and thrombosis. The recent CANTOS (Canakinumab Anti-inflammatory Therapy Outcomes Study) trial demonstrated the benefit of suppressing inflammation on cardiovascular disease in high-risk patients.12, 13 However, targeting inflammation systemically also has significant potential to inhibit innate immunity and compromise host defense against infections.14 Indeed, deaths due to infection or sepsis were more common among CANTOS trial patients who received the systemic anti-inflammatory treatment. Because of their ability to achieve local delivery, atherosclerosis-targeted nanoparticles may be able to address these risks.
Type IV collagen is a major sub-endothelial basement membrane protein that is exposed upon vascular injury and inflammation.15 When combining a Type IV-collagen targeting peptide and pro-resolving peptide derived from Annexin A1, there was a 70% increase in selectivity of the NPs for atherosclerotic lesions, relative to the spleen and liver.16 The targeted, inflammation-resolving NPs enhanced resolution to a much greater extent than the free-resolving peptide, where NP treatment effectively suppressed plaque oxidative stress, necrosis, and fibrous cap thinning. In another study using a similar Type IV-collagen targeting system, NPs that incorporate the anti-inflammatory cytokine IL-10 were engineered.17 IL-10 nanotherapy had similar protective effects on advanced atherosclerosis in Ldlr−/− mice, in addition to enhancing macrophage-mediated clearance of apoptotic debris. Resolving local inflammation thus also appeared to have a pro-efferocytic effect. Short-term toxicity studies revealed no alterations in blood cytokine levels, suggesting the IL-10 nanotherapy was specific to sites of inflammation and may not compromise host defense. In a study using an NP designed to attenuate inflammation due to the production of reactive oxygen species (ROS), delivery of a free-radical scavenging payload led to a decrease in cell apoptosis within the plaques of apoE−/− mice. Following internalization by macrophages and vascular smooth muscle cells (VSMCs), the “ROS-scavenging” NPs decreased cellular oxLDL uptake and subsequent transformation to foam cells. NPs were thus able to overcome the rapid elimination and short retention time of the free therapeutic agent in atherosclerotic plaques. Additionally, their benefit on plaque progression and stability was importantly observed without side effects, indicated by normal clinical chemistry, hematology, and viability of mice following treatment.18
Inflammation-targeting nanoparticles have also been formulated as theranostic NPs. In a rabbit model of atherosclerosis, magnetic resonance imaging (MRI)-detectable liposomes were developed for delivery of prednisolone to the inflamed vessel wall.19 Liposomal encapsulation improved the pharmacokinetics of prednisolone and prolonged its circulating half-life, without systemic toxicity. After a single dose, rapid and sustained decreases in plaque inflammation were observed by MRI and correlated with 18F-FDG-positron emission tomography/computed tomography (PET/CT), a validated method of tracking inflammation in atherosclerosis imaging.20 Decreases in plaque inflammation were attributable to a decrease in monocyte chemoattracts and lesional macrophage density, effects that were observed to a much lesser degree in rabbits treated with the free corticosteroid. Investigators then executed a pharmaceutical development program in which they optimized a scaled up synthesis method and formed a purified and storage-stable good manufacturing practice (GMP)-grade product.21 Following pharmacokinetic and toxicologic evaluation in healthy rats and rabbits, the prednisolone-containing liposomes failed to induce measurable effects on arterial wall inflammation in Phase I/II trials.22 Optimizing the dose and treatment schedule in larger animal models may have led to a more thorough understanding of the therapeutic margin and dose required to achieve efficient target engagement. Despite the lack of treatment benefit, multi-modal imaging demonstrated that the nanoparticles accumulated in plaque macrophages without adverse effects, thus serving as a guide for imaging-based efficacy measures and demonstrating the feasibility of targeting nanoparticles to human atherosclerotic areas.
In a study specifically aiming to interfere with leukocyte recruitment into the atherosclerotic plaque, Sager and colleagues combined small interfering RNA (siRNA) targeting multiple cell adhesion molecules into a polymer-based NP.23 Made up of a variety of synthetic or natural polymers, polymeric NPs are more resistant to degradation and offer a more tunable architecture than liposomes.24 In apoE−/− mice that underwent coronary ligation, treatment with NPs encapsulating five siRNAs targeting leukocyte adhesion molecules significantly reduced vascular inflammation after myocardial infarction.23 The resultant decrease in leukocyte accumulation led to a decrease in tissue injury and necrotic core formation following ischemic insult. Altogether, these studies exemplify the exciting possibility that plaque inflammation and apoptotic cell accumulation can be directly addressed using targeted NPs.
Preventing plaque neovascularization
Advanced atherosclerotic plaques frequently display extensive adventitial and neointimal neovascularization. In humans, increased plaque vascularity has been observed in lesions from patients with acute coronary syndrome and symptomatic carotid stenosis, relative to individuals with stable or asymptomatic disease.25, 26 These data suggest that plaque neovascularization may have an important role in atherogenesis and intraplaque hemorrhage.27 Angiogenesis is coordinated by a number of cytokines, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor.28 Anti-VEGF therapies and other anti-angiogenic agents have successfully been used to promote regression of tumor vessels and prolong survival in cancer patients in some studies29, 30, but come with an increased risk of arterial thromboembolic events that is further compounded in patients with a history of cardiovascular disease.31 Based on prior work demonstrating that high dose anti-angiogenic therapy reduces plaque development in apoE−/− mice, investigators developed a targeted theranostic NP in efforts to avoid the drug’s neurocognitive effects and combine an imaging agent for serial monitoring of neovessel formation.32 Using a ligand for the αvβ3-integrins that are up-regulated during angiogenesis, hyperlipidemic rabbits treated with the MRI-detectable NPs exhibited a reduction in αvβ3-related signal enhancement in the aorta. T1-weighted MRI signal in the aorta correlated with the degree of neovessel formation in the atherosclerotic aorta. Interestingly, this benefit occurred at a dosage of 50,000 times less when the anti-angiogenic agent was encapsulated as a nanoformulation than when the therapy was delivered alone. In a follow-up study, combining their αvβ3-targeted anti-angiogenic treatment with atorvastatin achieved a greater and more sustained decrease in MR signal and plaque neovessel count of hyperlipidemic rabbits.33 This finding is in line with work suggesting that statins inhibit endothelial proliferation and VEGF production, potentially explaining the synergistic effect on plaque neovessel formation.34 By leveraging plaque biology, these studies highlight the potential of NPs to feature an imaging/therapeutic payload and prevent disease in ways that were previously inaccessible due to off-target effects.
Targeting macrophages
Macrophages have a key role in atherosclerosis, from lesion initiation, foam cell formation, and by contributing to the pool of apoptotic cells that affect plaque size and vulnerability.35, 36 Importantly, in advanced lesions, defective efferocytic activity by lesional macrophages is what causes the build-up of toxic intracellular material and subsequent plaque necrosis. Several nanotherapies have been described that target plaque monocyte recruitment and infiltration37–39, macrophage proliferation40, 41, cholesterol metabolism42, 43, and polarization to a less inflammatory M2 phenotype.44 In a study by Lewis and colleagues, sugar-based NPs were designed to block oxidized LDL uptake from macrophage scavenger receptors (SR) by both direct inhibition and long-term downregulation of SR expression on the cell surface.43 Binding to macrophage SRs was shown to directly correlate with targeting of NPs to established atherosclerotic plaques. They reported that treatment resulted in markedly reduced lipid burden and overall plaque occlusion in the aorta of apoE−/− mice. In another recent study, a targeted nanoimmunotherapy was developed to block CD40-induced tumor necrosis receptor-associated factor 6 (TRAF6) in monocytes and macrophages, thereby preventing monocyte recruitment into the arterial wall.39, 45 While systemic inhibition of the CD40-TRAF6 axis results in serious complications such as thromboembolic events and immune suppression, investigators selectively targeted TRAF6 in monocytes by incorporating TRAF6 inhibitors into recombinant HDL NPs (TRAF6i-HDL). As an extension of the group’s experience with HDL particles in atherosclerosis nanotherapy40 and in vivo imaging46, TRAF6i-HDL was shown to both hamper the initiation of disease in young apoE−/− mice with no atherosclerosis and induce a more stable plaque phenotype in animals with established disease. Following incorporation with fluorescent dyes or radiolabeled molecules, TRAF6i-HDL was shown to accumulate primarily in the liver and spleen of apoE−/− mice and non-human primates. Investigators also provided evidence of the short-term safety of the nanoimmunotherapy in mice and cynomolgus monkeys.45 These safety experiments revealed that TRAF6i-HDL did not elicit adverse immune responses, alter major serological parameters, or cause any organ toxicity, although an acute increase in alkaline phosphatase levels and reticulocyte count was noted in mice treated for 1 week. In mice that received long-term treatment, these changes were not observed, although interestingly, cholesterol levels and white blood cell counts were both elevated in mice treated for 6 weeks.39 Because the HDL NPs primarily accumulate in the liver and spleen, further toxicological studies are needed for this promising nanoimmunotherapy.
The preferential uptake of NPs by inflammatory monocytes and macrophages has also served as a means for focal therapy of inflamed lesions. Iron-oxide NPs undergo uptake by macrophages (>75%) and to a lesser degree by neutrophils and other vascular cells.47 When modified with a near-infrared fluorophore, irradiation of the atheroma resulted in focal ablation of the macrophage-rich plaques of mice following carotid ligation.48 However, the authors note the phototoxicity also may affect other cell types with an affinity for the NPs, including VSMCs and endothelial cells. Subjecting these cell types to photothermal therapy could potentially make the plaques more dangerous and prone to rupture. Nanoparticles that may be more cell-specific are under development, including single-walled carbon nanotubes (SWNTs, Figure 2A).49 SWNTs undergo highly selective uptake by inflammatory Ly-6Chi monocytes.50 Within the diseased vessels of carotid-ligated mice, SWNTs co-localized with lesional macrophages, with negligible amounts observed in VSMCs.49 Further exploration of in vivo efficacy and therapy-loading ability is needed. Given that inflammatory monocytes represent the majority of circulating cells recruited to the atherosclerotic plaque, there is compelling rationale for the use of SWNTs and other NPs that afford similar cell-specific drug delivery. Moreover, SWNTs and other carbon nanomaterials exhibit a natural photoacoustic contrast and near-infrared fluorescence signal.49, 51 These intrinsic imaging capabilities make carbon nanomaterials particularly useful for theranostic strategies to detect and treat the inflamed “vulnerable” plaque.
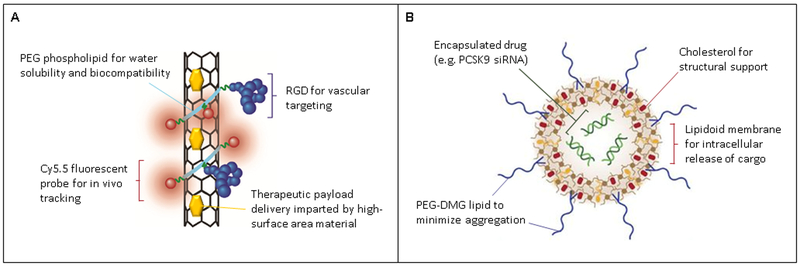
Figure 2:
Schematic of nanoparticles functionalized with agents that control their stability and interactions with the biological environment. A. Single-walled carbon nanotube (SWNT) tailored for vessel delivery of a therapeutic payload by coating the SWNT with polyethylene glycol (PEG) chains linked to arginine-glycine-aspartic acid (RGD), a potent αvβ3 integrin-binding peptide. B. Lipidoid nanoparticles that are formulated with phospholipids and cholesterol to facilitate intracellular delivery of siRNA for potent gene knockdown. Figures modified from [50] and [60].
Altering lipid metabolism
Nanoparticle delivery systems have particularly impacted the ability to target liver cholesterol metabolism using RNA interference (RNAi). A liposomal formulation of apolipoprotein B (apoB) siRNA resulted in specific silencing of apoB in hepatocytes and reductions in apoB, LDL, and total cholesterol levels in rodents and monkeys.52, 53 The opportunity to target apoB is particularly exciting given genetic and epidemiological studies that suggest the clinical benefit of lowering LDL may depend on a corresponding reduction in apoB levels.54
Most recently, siRNA silencing of proprotein convertase subtilisin/kexin type 9 (PCSK9) shows promise as a future strategy for reducing LDL cholesterol in a potent and convenient manner. The field of RNAi therapy has historically been impeded by the instability of naked siRNA in the bloodstream and their inability to cross the cell membrane. RNA silencing of PCKS9 using lipid-based nanoparticles, termed “lipidoids” (Figure 2B), has recently been shown to efficiently target and suppress PCSK9 synthesis in the liver. Unlike the currently available PCSK9 antibodies (i.e. Evolucumab) that require bi-weekly injections, the novel nanoformulations of PCSK9 siRNA (Inclisiran) caused rapid and durable effects after a single dose. First shown in rodents and monkeys55, Inclisiran effectively reduced levels of PCSK9 and LDL for at least 6 months in Phase 1/2 trials.56–59 Further evaluation is ongoing in Phase III trials (NCT03399370). This development is a major advance in cardiovascular medicine, and was driven by nanotechnology that enabled stable delivery of RNAi therapeutics.60
In another recent study, antibodies against the VSMC-expressed ion channel, transient receptor potential vanilloid subfamily 1 (TRPV1), were conjugated to copper sulfide (CuS) NPs.61 TRPV1 induces autophagy in VSMCs, reduces lipid accumulation, and prevents foam cell formation. Upon irradiation of the aortic arch of apoE−/− mice, the local increase of temperature opened TRPV1 channels and allowed an influx of calcium ions to activate autophagy. Excitingly, NPs provided highly precise, non-invasive treatment under image-guidance due to the characteristic near infrared absorption of CuS NPs that generates a strong photoacoustic signal.
Preventing neointimal growth
High restenosis and reintervention rates are still a significant limitation of revascularization procedures, particularly among patients with peripheral arterial disease. A number of NPs have been identified that enhance retention of anti-restenotic agents in the local vascular bed62, 63. These include NPs that are delivered locally via catheter, implanted in stents, and even systemically administered surrounding the time of revascularization.
Using an endovascular microinfusion catheter for local delivery, albumin-bound rapamycin NPs were concentrated in the adventitial and medial layers of the arterial wall, and reduced luminal stenosis in a porcine femoral artery balloon angioplasty model.64 Rapamycin levels in the femoral artery remained >100 times higher in the perivascular tissues than in the blood for 8 days. Importantly, this period was when cell proliferation rates were the highest in control animals, suggesting that the NPs promote drug retention during a particularly critical period of neointima formation. In another study, Cyrus and colleagues developed αvβ3-targeted paramagnetic NPs for delivery of rapamycin to balloon injured femoral arteries of rabbits.65 Local infusion of vessel wall-targeted NPs resulted in a decrease in neointimal formation, and their retention in the injured walls was amenable to MRI due to the high contrast potential of paramagnetic nanomaterials. Highlighting the tunable properties of NPs, nitric oxide gas and RNAi components have also been encapsulated within NPs and delivered locally to the diseased arterial wall.66, 67
The surfaces of stents may also be loaded or targeted with NPs. In a study by Chorny et al., “stent-targeted” magnetic NPs were designed.68 Following stent placement in the carotid artery of rats, these paclitaxel-loaded NPs were infused in the isolated artery and were targeted to the stent by applying a magnetic field to the body surface. Treatment effectively inhibited in-stent stenosis at drug doses below those provided by paclitaxel-eluting stents. Innovative NP-eluting stents have also been developed to prevent stenosis after stent implantation.69–71 Importantly, these stents were found to prevent in-stent restenosis without delayed endothelial healing, which is a central reason for late stent thrombosis following percutaneous interventions.
Rather than requiring stent placement or advancement of a catheter for local delivery, targeted NPs may enable systemic therapy for restenosis. Although a promising albumin-stabilized nanoformulation of paclitaxel failed to show efficacy in early clinical trials72–74, this work highlighted the existing space for more targeted or “precision medicine” approaches to restenosis nanotherapy. Using lipid-polymeric NPs that were surface modified with Type IV-collagen targeting peptides, Chan and colleagues reported the efficacy of paclitaxel-encapsulated NPs that preferentially localized to the denuded vessel wall.75 Systemically administered NPs led to a reduction in arterial stenosis in a rat carotid injury model. While 15 mg/kg doses of free paclitaxel induced signs of toxicity, NPs enabled higher 35 mg/kg doses that were well-tolerated in animals. Another group described a liposomal formulation of the bisphosphonate alendronate that reduced neointimal formation by transiently suppressing circulating monocyte levels in rabbits that had undergone iliac artery stenting.76 Early-phase clinical trials supported the safety of liposomal alendronate for infusion at the time of percutaneous coronary intervention. Although there was no difference in restenosis rates between the treatment and placebo groups, the anti-inflammatory NP led to a significant reduction in in-stent late loss in an “inflammatory patient” subgroup with elevated baseline monocyte counts (NCT02645799).77 Other systemic nanoformulations that have prevented restenosis include nitric-oxide containing nanofibers78 and glucocorticoid-encapsulated NPs that are targeted to subendothelial matrix proteins79. Taken together, NP-based delivery may be a useful adjunct to revascularization procedures, particularly for patient-personalized treatment and for those with diffuse disease and other challenging lesion patterns.
Targeting thrombosis
Platelet activation, the coagulation cascade, and fresh thrombus include unique factors that enable targeted delivery of therapeutic agents. Thrombus-targeted NPs have been developed for delivery of thrombolytic agents and anti-coagulants, including tissue plasminogen activator (tPA)80–82 streptokinase83, 84, urokinase85, 86, and direct thrombin inhibitors.87 By encapsulating a von Willebrand factor-binding protein within NPs, investigators effectively targeted tPA to thrombi induced in swine coronary arteries.81 Intravenous delivery of NPs resulted in reperfusion and vessel recanalization in 90% of animals. Interestingly, NPs were designed for controlled release of tPA using transthoracic ultrasound, where application of ultrasound led to greater tPA off-loading and thrombolytic activity at the affected artery. In another study, anti-thrombin theranostic NPs directly attenuated plaque coagulant activity within the injured arteries of apoE−/− mice.87 Detectable by magnetic resonance spectroscopy, systemically administered NPs were retained within the plaques and exerted rapid inactivation of any locally produced thrombin. These effects were observed without altering activated partial thromboplastin time or other systemic effects on coagulation. Moreover, focal inhibition of plaque thrombin reduced the expression of plaque inflammatory molecules and enhanced restoration of the disrupted vascular endothelium, suggesting the anti-thrombin NPs promoted plaque stability. Taken together, these studies illustrate the broad potential that NPs have for reperfusion therapy and anticoagulation with decreased bleeding consequences.
Conclusions and future directions
Target-driven NPs have opened the door to improved and even novel treatment options for patients with vascular disease. Those that have advanced into early clinical trials provide important lessons, namely the need for (1) a well-defined patient population most likely to benefit from therapy, and (2) a thorough understanding of a formulation’s therapeutic margin. In addition to implementing clinically relevant animal models, these considerations will greatly draw from the use of biomarker and molecular imaging based-strategies to enable patient selection and assessment of drug accumulation and response.
Nonetheless, there are important limitations and challenges in cardiovascular nanomedicine that should be noted (Table 2). First, NPs are inherently heterogeneous in composition and can present challenges in synthesis of large volumes that adhere to pharmaceutical GMP guidelines (e.g. sterility, stability, and purity). It will be important to develop methods to scale-up production with high reliability and reproducibility, reasonable cost, and time-efficiency for successful translation from bench-to-bedside. Moreover, stability in circulation and during storage is a common pitfall of nanotechnology. Although conventional methods for quality control exist, each nanoformulation is a specialized unit and requires unique measurements of physicochemical properties and drug release rates. In particular, there is a need to develop a drug release profile under both physiological and storage conditions, such that the drug is not released too slowly, prematurely in circulation, or even during storage. In addition, although some nanoformulations use disease-targeting ligands, several NPs have been described to have an intrinsic affinity for certain cell types (e.g. intraplaque macrophages). The distinct mechanisms by which such NPs are taken up by cells are poorly understood and is an intriguing area for future study. Lastly, there is a major gap in the evaluation of the long-term safety of nanoformulations in vivo. Many currently available nanoformulations were developed to reduce the side effects of a loaded drug, but some nanoparticles undergoing preclinical development have retroactively been discovered to be cytotoxic and/or immunogenic.88 In an ideal situation these studies will be pursued in parallel as a means of informing decisions earlier in the development process. Coating NPs with polyethylene glycol (PEG), or “PEGylation” is a commonly used approach for reducing their systemic toxicity and interaction with plasma proteins and circulating cells.89 Further optimization of methods to reduce unwanted nano-bio interactions may be necessary.
Table 2.
Advantages and limitations of nanotherapy for vascular disease.
| Advantages | Limitations | |
|---|---|---|
| Targeted delivery to site of disease (e.g. atherosclerotic plaque) or therapeutic target (e.g. PCSK9 in the liver) | Challenges and cost of scaled-up production | |
| Increased efficacy per drug dose | Gap in knowledge of clinical safety | |
| Avoidance of systemic exposure and/or off-target effects | Requirement for formulation-specific methods for characterization of composition and purity | |
| Potential for combining treatment, diagnosis, and monitoring disease in a single formulation | Long-term stability of loaded agents | |
| Ability to be loaded with molecular recognition agents for “precision” therapy | ||
While there has been substantial progress, the global epidemic of vascular disease and resultant deaths are predicted to increase over the next decades, affecting developed and developing nations alike.90 Lipid-lowering therapies are the dominant treatment for atherosclerosis, but there is much room for improvement with complementary therapies. Our understanding of plaque biology has evolved to include inflammatory mechanisms and impaired efferocytosis as causal drivers of lesion progression and instability. However, anti-inflammatory therapies are known to cause immunosuppression. Additionally, reactivating efferocytosis has the drawback of inducing the off-target clearance of healthy tissues under some conditions. For example, in addition to efficiently restoring phagocytosis and preventing atherosclerosis, pro-efferocytic therapy also caused a clinically relevant anemia due to clearance of red blood cells in the spleen.91, 92 To overcome the toxicities associated with anti-inflammatory and pro-efferocytic approaches, it is likely that nanoparticles will serve as the innovative delivery systems that will enable targeting of these important pathways specifically within the vulnerable plaque.
Supplementary Material
Highlights.
Nanoparticles have uniquely appealing features that enable them to be programmed as cell- and tissue-specific delivery systems, thus overcoming the low drug delivery and off-target effects which commonly impede developments in cardiovascular medicine.
Nanotechnology is driving efforts to develop novel and more effective treatments for vascular disease, namely by targeting chronic inflammation, resolving defective efferocytosis, and producing more potent lipid-lowering therapies.
This review discusses advances in the application of nanoparticles for the treatment of vascular disease, their potential translation to the clinic, and challenges in their development.
Acknowledgements
We thank Ryoko Hamaguchi for her assistance with the illustration of Figure 1.
Sources of Funding
A.M. Flores is a Howard Hughes Medical Institute Research Fellow. K.-U. Jarr is supported by Deutsche Forschungsgemeinschaft grant JA 2869/1–1:1. This manuscript was supported by NIH/NHLBI grant R01 HL123370 (to N.J. Leeper), a 2018 Fondation Leducq Award (to N.J. Leeper), a Catalyst Award from the Dr. Ralph and Marian Falk Medical Research Trust, and the American Heart Association Transformational Project grant (to B.R. Smith and N.J. Leeper).
Abbreviations
- NPs
Nanoparticles
- ROS
Reactive oxygen species
- VSMCs
Vascular smooth muscle cells
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- CT
Computed tomography
- GMP
Good manufacturing practice
- siRNA
small interfering RNA
- VEGF
Vascular endothelial growth factor
- SR
Scavenger receptor
- TRAF6
Tumor necrosis receptor-associated factor 6
- HDL
high-density lipoprotein
- SWNTs
Single-walled carbon nanotubes
- RNA
RNA interference
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- TRPV1
Transient receptor potential vanilloid subfamily 1
- CuS
Copper sulfide
- tPA
Tissue plasminogen activator
- PEG
Polyethylene glycol
Footnotes
Disclosures
Dr. Leeper is a co-founder and holds equity interest in 47 Incorporated. A.M. Flores, J. Ye, B.R. Smith, and N.J. Leeper are co-inventors on patents regarding vascular nanotherapies that have been licensed to Stanford University.
References
- 1.The top 10 causes of death. World Health Organization. 2018 [Google Scholar]
- 2.Fordyce CB, Roe MT, Ahmad T, et al. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol. 2015;65:1567–1582 [DOI] [PubMed] [Google Scholar]
- 3.Smith BR, Gambhir SS. Nanomaterials for In Vivo Imaging. Chem Rev. 2017;117:901–986 [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barenholz Y Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134 [DOI] [PubMed] [Google Scholar]
- 6.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436 [DOI] [PubMed] [Google Scholar]
- 7.Ventola CL. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T. 2017;42:742–755 [PMC free article] [PubMed] [Google Scholar]
- 8.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009;29:992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima Y, Weissman IL, Leeper NJ. The Role of Efferocytosis in Atherosclerosis. Circulation. 2017;135:476–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 13.Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The CANTOS Trial: One Important Step for Clinical Cardiology but a Giant Leap for Vascular Biology. Arterioscler Thromb Vasc Biol. 2017;37:e174–e177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128:2713–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the progression and complications of atherosclerosis. Vasc Med. 2009;14:73–89 [DOI] [PubMed] [Google Scholar]
- 16.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokzhad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamaly N, Fredman G, Fojas JJ, et al. Targeted Interleukin-10 Nanotherapeutics Developed with a Microfluidic Chip Enhance Resolution of Inflammation in Advanced Atherosclerosis. ACS Nano. 2016;10:5280–5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li L, Zhao W, Dou Y, An H, Tao H, Xu X, Jia Y, Lu S, Zhang J, Hu H. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity. ACS Nano. 2018;12:8943–8960 [DOI] [PubMed] [Google Scholar]
- 19.Lobatto ME, Fayad ZA, Silvera S, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm. 2010;7:2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871–878 [DOI] [PubMed] [Google Scholar]
- 21.Lobatto ME, Calcagno C, Otten MJ, Millon A, Ramachandran S, Paridaans MP, van der Valk FM, Storm G, Stroes ES, Fayad ZA, Mulder WJ, Metselaar JM. Pharmaceutical development and preclinical evaluation of a GMP-grade anti-inflammatory nanotherapy. Nanomedicine. 2015;11:1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Valk FM, van Wijk DF, Lobatto ME, et al. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine. 2015;11:1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sager HB, Dutta P, Dahlman JE, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med. 2016;8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan R The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360 [DOI] [PubMed] [Google Scholar]
- 25.Gossl M, Versari D, Hildebrandt HA, Bajanowski T, Sangiorgi G, Erbel R, Ritman EL, Lerman LO, Lerman A. Segmental heterogeneity of vasa vasorum neovascularization in human coronary atherosclerosis. JACC Cardiovasc Imaging. 2010;3:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45:155–159 [DOI] [PubMed] [Google Scholar]
- 27.Guo L, Harari E, Virmani R, Finn AV. Linking Hemorrhage, Angiogenesis, Macrophages, and Iron Metabolism in Atherosclerotic Vascular Diseases. Arterioscler Thromb Vasc Biol. 2017;37:e33–e39 [DOI] [PubMed] [Google Scholar]
- 28.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318 [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342 [DOI] [PubMed] [Google Scholar]
- 31.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, Williams TA, Schmieder AH, Hu G, Allen JS, Lacy EK, Zhang H, Wickline SA, Lanza GM. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2103–2109 [DOI] [PubMed] [Google Scholar]
- 33.Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, Lanza GM. Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging. 2008;1:624–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dulak J, Jozkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5:579–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallat Z Macrophages. Arterioscler Thromb Vasc Biol. 2017;37:e92–e98 [DOI] [PubMed] [Google Scholar]
- 37.Katsuki S, Matoba T, Nakashiro S, Sato K, Koga J, Nakano K, Nakano Y, Egusa S, Sunagawa K, Egashira K. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation. 2014;129:896–906 [DOI] [PubMed] [Google Scholar]
- 38.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seijkens TTP, van Tiel CM, Kusters PJH, et al. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J Am Coll Cardiol. 2018;71:527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang J, Lobatto ME, Hassing L, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv. 2015;1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu M, Amengual J, Menon A, et al. Targeted Nanotherapeutics Encapsulating Liver X Receptor Agonist GW3965 Enhance Antiatherogenic Effects without Adverse Effects on Hepatic Lipid Metabolism in Ldlr(−/−) Mice. Adv Healthc Mater. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis DR, Petersen LK, York AW, Zablocki KR, Joseph LB, Kholodovych V, Prud’homme RK, Uhrich KE, Moghe PV. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc Natl Acad Sci U S A. 2015;112:2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakashiro S, Matoba T, Umezu R, Koga J, Tokutome M, Katsuki S, Nakano K, Sunagawa K, Egashira K. Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE−/− Mice. Arterioscler Thromb Vasc Biol. 2016;36:491–500 [DOI] [PubMed] [Google Scholar]
- 45.Lameijer M, Binderup T, van Leent M, et al. Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nature Biomedical Engineering. 2018;2:279–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Medina C, Binderup T, Lobatto ME, et al. In Vivo PET Imaging of HDL in Multiple Atherosclerosis Models. JACC Cardiovasc Imaging. 2016;9:950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarthy JR, Korngold E, Weissleder R, Jaffer FA. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosuge H, Sherlock SP, Kitagawa T, Dash R, Robinson JT, Dai H, McConnell MV. Near infrared imaging and photothermal ablation of vascular inflammation using single-walled carbon nanotubes. J Am Heart Assoc. 2012;1:e002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith BR, Ghosn EE, Rallapalli H, Prescher JA, Larson T, Herzenberg LA, Gambhir SS. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat Nanotechnol. 2014;9:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De la Zerda A, Zavaleta C, Keren S, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmermann TS, Lee AC, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114 [DOI] [PubMed] [Google Scholar]
- 53.Tadin-Strapps M, Peterson LB, Cumiskey AM, et al. siRNA-induced liver ApoB knockdown lowers serum LDL-cholesterol in a mouse model with human-like serum lipids. J Lipid Res. 2011;52:1084–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of Genetic Variants Related to CETP Inhibitors and Statins With Lipoprotein Levels and Cardiovascular Risk. JAMA. 2017;318:947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A. 2008;105:11915–11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fitzgerald K, White S, Borodovsky A, et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N Engl J Med. 2017;376:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray KK, Stoekenbroek RM, Kallend D, Leiter LA, Landmesser U, Wright RS, Wijngaard P, Kastelein JJ. Effect of an siRNA Therapeutic Targeting PCSK9 on Atherogenic Lipoproteins: Pre-Specified Secondary End Points in ORION 1. Circulation. 2018 [DOI] [PubMed] [Google Scholar]
- 59.Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N Engl J Med. 2017;376:1430–1440 [DOI] [PubMed] [Google Scholar]
- 60.Whitehead KA, Dorkin JR, Vegas AJ, et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun. 2014;5:4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao W, Sun Y, Cai M, Zhao Y, Cao W, Liu Z, Cui G, Tang B. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat Commun. 2018;9:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noukeu LC, Wolf J, Yuan B, Banerjee S, Nguyen KT. Nanoparticles for Detection and Treatment of Peripheral Arterial Disease. Small. 2018;14:e1800644. [DOI] [PubMed] [Google Scholar]
- 63.Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014;4:175–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gasper WJ, Jimenez CA, Walker J, Conte MS, Seward K, Owens CD. Adventitial nab-rapamycin injection reduces porcine femoral artery luminal stenosis induced by balloon angioplasty via inhibition of medial proliferation and adventitial inflammation. Circ Cardiovasc Interv. 2013;6:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cyrus T, Zhang H, Allen JS, Williams TA, Hu G, Caruthers SD, Wickline SA, Lanza GM. Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arterioscler Thromb Vasc Biol. 2008;28:820–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li JM, Newburger PE, Gounis MJ, Dargon P, Zhang X, Messina LM. Local arterial nanoparticle delivery of siRNA for NOX2 knockdown to prevent restenosis in an atherosclerotic rat model. Gene Ther. 2010;17:1279–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang SL, Kee PH, Kim H, Moody MR, Chrzanowski SM, Macdonald RC, McPherson DD. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J Am Coll Cardiol. 2009;54:652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chorny M, Fishbein I, Yellen BB, Alferiev IS, Bakay M, Ganta S, Adamo R, Amiji M, Friedman G, Levy RJ. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A. 2010;107:8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakano K, Egashira K, Masuda S, Funakoshi K, Zhao G, Kimura S, Matoba T, Sueishi K, Endo Y, Kawashima Y, Hara K, Tsujimoto H, Tominaga R, Sunagawa K. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc Interv. 2009;2:277–283 [DOI] [PubMed] [Google Scholar]
- 70.Masuda S, Nakano K, Funakoshi K, Zhao G, Meng W, Kimura S, Matoba T, Miyagawa M, Iwata E, Sunagawa K, Egashira K. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J Atheroscler Thromb. 2011;18:1043–1053 [DOI] [PubMed] [Google Scholar]
- 71.Tsukie N, Nakano K, Matoba T, Masuda S, Iwata E, Miyagawa M, Zhao G, Meng W, Kishimoto J, Sunagawa K, Egashira K. Pitavastatin-incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model. J Atheroscler Thromb. 2013;20:32–45 [DOI] [PubMed] [Google Scholar]
- 72.Seedial SM, Ghosh S, Saunders RS, Suwanabol PA, Shi X, Liu B, Kent KC. Local drug delivery to prevent restenosis. J Vasc Surg. 2013;57:1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Margolis J, McDonald J, Heuser R, Klinke P, Waksman R, Virmani R, Desai N, Hilton D. Systemic nanoparticle paclitaxel (nab-paclitaxel) for in-stent restenosis I (SNAPIST-I): a first-in-human safety and dose-finding study. Clin Cardiol. 2007;30:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolodgie FD, John M, Khurana C, Farb A, Wilson PS, Acampado E, Desai N, Soon-Shiong P, Virmani R. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002;106:1195–1198 [DOI] [PubMed] [Google Scholar]
- 75.Chan JM, Rhee JW, Drum CL, Bronson RT, Golomb G, Langer R, Farokhzad OC. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci U S A. 2011;108:19347–19352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danenberg HD, Golomb G, Groothuis A, Gao J, Epstein H, Swaminathan RV, Seifert P, Edelman ER. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation. 2003;108:2798–2804 [DOI] [PubMed] [Google Scholar]
- 77.Banai S, Finkelstein A, Almagor Y, Assali A, Hasin Y, Rosenschein U, Apruzzese P, Lansky AJ, Kume T, Edelman ER. Targeted anti-inflammatory systemic therapy for restenosis: the Biorest Liposomal Alendronate with Stenting sTudy (BLAST)-a double blind, randomized clinical trial. Am Heart J. 2013;165:234–240 e231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahnson ES, Kassam HA, Moyer TJ, Jiang W, Morgan CE, Vercammen JM, Jiang Q, Flynn ME, Stupp SI, Kibbe MR. Targeted Nitric Oxide Delivery by Supramolecular Nanofibers for the Prevention of Restenosis After Arterial Injury. Antioxid Redox Signal. 2016;24:401–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joner M, Morimoto K, Kasukawa H, Steigerwald K, Merl S, Nakazawa G, John MC, Finn AV, Acampado E, Kolodgie FD, Gold HK, Virmani R. Site-specific targeting of nanoparticle prednisolone reduces in-stent restenosis in a rabbit model of established atheroma. Arterioscler Thromb Vasc Biol. 2008;28:1960–1966 [DOI] [PubMed] [Google Scholar]
- 80.Ma YH, Wu SY, Wu T, Chang YJ, Hua MY, Chen JP. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials. 2009;30:3343–3351 [DOI] [PubMed] [Google Scholar]
- 81.Kawata H, Uesugi Y, Soeda T, et al. A new drug delivery system for intravenous coronary thrombolysis with thrombus targeting and stealth activity recoverable by ultrasound. J Am Coll Cardiol. 2012;60:2550–2557 [DOI] [PubMed] [Google Scholar]
- 82.Zhou J, Guo D, Zhang Y, Wu W, Ran H, Wang Z. Construction and evaluation of Fe(3)O(4)-based PLGA nanoparticles carrying rtPA used in the detection of thrombosis and in targeted thrombolysis. ACS Appl Mater Interfaces. 2014;6:5566–5576 [DOI] [PubMed] [Google Scholar]
- 83.Pawlowski CL, Li W, Sun M, Ravichandran K, Hickman D, Kos C, Kaur G, Sen Gupta A. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials. 2017;128:94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marsh JN, Senpan A, Hu G, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis. Nanomedicine (Lond). 2007;2:533–543 [DOI] [PubMed] [Google Scholar]
- 85.Bi F, Zhang J, Su Y, Tang YC, Liu JN. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials. 2009;30:5125–5130 [DOI] [PubMed] [Google Scholar]
- 86.Marsh JN, Hu G, Scott MJ, Zhang H, Goette MJ, Gaffney PJ, Caruthers SD, Wickline SA, Abendschein D, Lanza GM. A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine (Lond). 2011;6:605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palekar RU, Jallouk AP, Myerson JW, Pan H, Wickline SA. Inhibition of Thrombin With PPACK-Nanoparticles Restores Disrupted Endothelial Barriers and Attenuates Thrombotic Risk in Experimental Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:446–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, Zhao Y. Safety of Nanoparticles in Medicine. Curr Drug Targets. 2015;16:1671–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mackay J, Mensah GA. The Atlas of Heart Disease and Stroke. World Health Organization; 2004. [Google Scholar]
- 91.Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.