Abstract
In a previous study on camel milk from Kazakhstan, we reported the occurrence of two unknown proteins (UP1 and UP2) with different levels of phosphorylation. Here we show that UP1 and UP2 are isoforms of camel αs2-CN (αs2-CNsv1 and αs2-CNsv2, respectively) arising from alternative splicing events. First described as a 178 amino-acids long protein carrying eight phosphate groups, the major camel αs2-CN isoform (called here αs2-CN) has a molecular mass of 21,906 Da. αs2-CNsv1, a rather frequent (35%) isoform displaying a higher molecular mass (+1,033 Da), is present at four phosphorylation levels (8P to 11P). Using cDNA-sequencing, αs2-CNsv1 was shown to be a variant arising from the splicing-in of an in-frame 27-nucleotide sequence encoding the nonapeptide ENSKKTVDM, for which the presence at the genome level was confirmed. αs2-CNsv2, which appeared to be present at 8P to 12P, was shown to include an additional decapeptide (VKAYQIIPNL) revealed by LC-MS/MS, encoded by a 3′-extension of exon 16. Since milk proteins represent a reservoir of biologically active peptides, the molecular diversity generated by differential splicing might increase its content. To evaluate this possibility, we searched for bioactive peptides encrypted in the different camel αs2-CN isoforms, using an in silico approach. Several peptides, putatively released from the C-terminal part of camel αs2-CN isoforms after in silico digestion by proteases from the digestive tract, were predicted to display anti-bacterial and antihypertensive activities.
Introduction
Recently, combining different proteomic approaches, the complexity of camel milk proteins was resolved to provide a detailed characterization of fifty protein molecules belonging to the 9 main milk protein families, including caseins: κ-, αs2-, αs1- and β-CN and two unknown proteins (UP1 and UP2), exhibiting molecular masses around 23,000 Da1. Since UP1 and UP2 co-eluted in RP-HPLC with αs-CN and displayed different phosphorylation levels, it was tempting to consider that these proteins could originate in CN. However, based on their molecular weight, UP1 and UP2 could be larger isoforms of αs2-CN or smaller isoforms of αs1-CN.
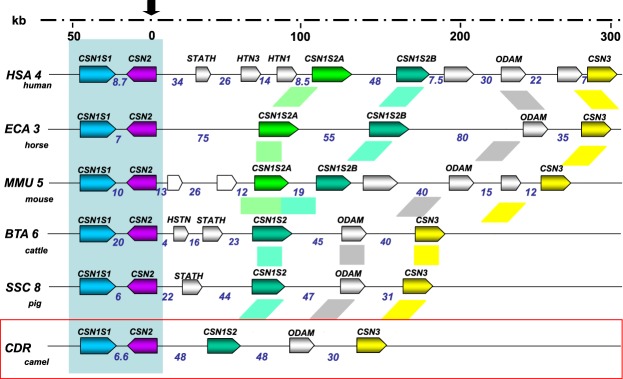
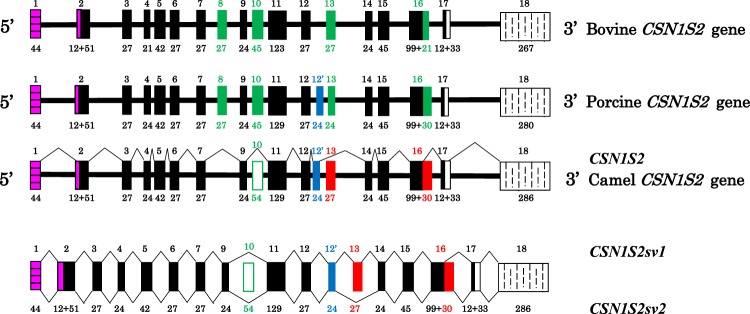
However, the hypothesis of an additional casein in camel milk encoded by a supplementary gene could not be ruled out. Indeed, genes encoding CN are tightly linked on the same chromosome, BTA6 in cattle, CHI6 in goats2,3 and HSA4 in humans4. The evolution of the CN gene cluster (Fig. 1) is postulated to have occurred by a combination of successive intra- and inter-genic exon duplications5–7. In some mammals, including horses, donkeys, rodents and rabbits, there are two αs2-CN encoding genes differentiating in size (CSN1S2-like or CSN1S2A and CSN1S2B), which may have arisen by a gene-duplication event that has occurred prior to the split of Eutherian mammalian species5. The second CSN1S2-like gene was lost in the Artiodactyla, including the camel, while further divergence occurred in both copies in the other species. In humans, there are also two CSN1S2 genes albeit no evidence of protein expression exists6.
Figure 1.
Evolution of the casein locus organization. Casein locus organization of human (Homo sapiens), horse (Equus caballus), mouse (Mus musculus), cattle (Bos taurus), pig (Sus scrofa) and camel (Camelus dromedarius) genomes (adapted from Martin, Cebo and Miranda7 and Lefèvre et al.50 with additional genomic information from the NCBI) was compared. Genes are given as colored arrow boxes, showing the orientation of transcription. Putative genes based on similarity are indicated as empty boxes. Intergenic region sizes are given in kb.
Alternative splicing is a process by which multiple mRNA isoforms are generated. It is a powerful means to extend protein diversity. Such a process which is another possibility to increase the number of molecular species has been frequently reported to occur, as far as caseins are concerned, especially αs-CN8–10, without really knowing whether it is a fortuitous or a scheduled event to expand molecular diversity and functionality of milk proteins. To substantiate the hypothesis according to which UP1 and UP2 might originate in CN and more precisely in αs-CN, we undertook characterizing more precisely these proteins.
In addition to their nutritional value, an increasing number of therapeutic effects and a variety of potential activities11,12 are attributed to milk proteins as well as to milk-derived bioactive peptides encrypted in milk protein sequences13. Caseins, and especially αs-CN, have been shown to be a reservoir of bioactive peptides13–15, it is therefore legitimate to wonder whether these so far unknown and putatively derived αs-CN sequences could be responsible for the occurrence of novel bioactive peptides accounting for the original properties of camel milk. Recent studies have indeed shown that healing properties assigned to camel milk, which is consumed fresh or fermented and traditionally used for the treatment of tuberculosis, gastroenteritis, and allergies, in many countries, are proved16. Whereas there is a substantial literature on bioactive peptides derived from bovine milk proteins13 and more or less comprehensive databases of milk bioactive peptides exist17–20, studies aiming at identifying peptides derived from camel milk proteins having potential health-promoting activities are scarce. Investigations mainly focused on caseins (αs1-, β- and κ-CN), and data available to date mostly concern in vitro and in silico antioxidant, antihypertensive and antimicrobial activities16,21. Therefore, using an in silico approach, we searched for potential biological activities of sequences generated from alternative splicing of primary transcript encoding αs-CN.
Results and Discussion
What gene(s) do UP1 and UP2 arise from
The mass accuracy has allowed distinguishing about fifty protein molecules corresponding to isoforms of 9 protein families (κ-CN, WAP, αs1-CN, α-LAC, αs2-CN, PGRP, LPO/CSA, β-CN and γ2-CN) from LC-MS analysis as shown in Fig. 2. The presence of two unknown proteins UP1 and UP2 with different phosphorylation levels was reported in our previous study8. Regarding UP1, molecular masses ranged between 22,939 and 23,179 Da, whereas UP2 masses ranged between 23,046 Da and 23,366 Da (Table 1), with successive increments of 80 Da (mass of one phosphate group). The eluting range of these two proteins was between 28.53–37.16 min, within the elution times of αs1- and αs2-CN, which confirms our first hypothesis about their αs-CN origin. However, UP1 and UP2 masses exceeded the observed mass of the major isoform of αs2-CN with 8P (21,906 Da) by 1,033 Da and 1,300 Da, respectively, and were lighter than the C variant of αs1-CN-6P (25,773 Da) by 2,834 Da and 2,567 Da, respectively1. Even though it was not possible to exclude a splicing event leading to the inclusion of an additional exon sequence in the αs2-CN mRNA, the most probable hypothesis was the occurrence of exon-skipping event(s) affecting αs1-CN mRNA and, leading to the loss of a peptide sequence accounting for a reduction of at least 2,567 Da. A possible scenario was the skipping of exon 3 on the short isoform of αs1-CN C already impacted by a cryptic splice site usage (∆CAG encoding Q83). The molecular mass of the protein proceeding from such a messenger (23,205 Da) corresponded to the mass of UP2 + 160 Da (23,206 Da). However, sequencing cDNA encoding αs1-CN isoforms failed to reveal the existence of a messenger in which exon 3 was lacking. Therefore, the alternative possibility, in other words the αs2-CN avenue, had to be explored.
Figure 2.
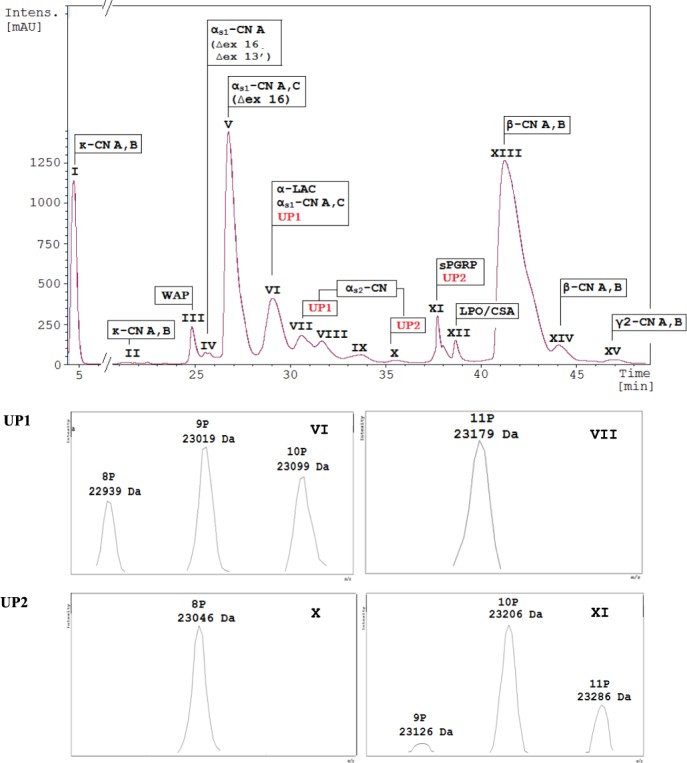
LC-ESI-MS profile of dromedary milk proteins. The chromatogram displays the presence of 15 major milk protein fractions labeled from I to XV, with retention times from 4.50 to 48.71 min, respectively. Deconvolution of multicharged ions spectra with emphasis on phosphorylation degrees (P) of two unknown proteins (UP1 and UP2) which are related to chromatographic peaks VI and VII, X and XI respectively.
Table 1.
Analysis of molecular masses contained in peaks V–XI of dromedary milk sample from the Shymkent region.
| Peak | Ret.Time, min | Observed Mr, Da | Theoretical Mr, Da | Protein description | UniProt accession | Intensity |
|---|---|---|---|---|---|---|
| V | 26,31 | 24,547 | 24,547 | αs1-CN C -short isoform (∆ex 16), 5P, splice variant (∆Q83) | 3,954 | |
| 24,561 | 24,561 | αs1-CN A - short isoform (∆ex 16), 5P, splice variant (∆Q83) | 4,385 | |||
| 24,627 | 24,627 | αs1-CN C - short isoform (∆ex 16), 6P, splice variant (∆Q83) | 16,348 | |||
| 24,640 | 24,641 | αs1-CN A - short isoform (∆ex 16), 6P, splice variant (∆Q83) | 17,422 | |||
| 24,675 | 24,675 | αs1-CN C - short isoform (∆ex 16), 5P | 7,758 | |||
| 24,689 | 24,689 | αs1-CN A - short isoform (∆ex 16), 5P | 8,004 | |||
| 24,722 | 24,721 | αs1-CN A - short isoform (∆ex 16), 7P, splice variant (∆Q83) | 4,453 | |||
| 24,755 | 24,755 | αs1-CN C - short isoform (∆ex 16), 6P | K7DXB9 | 34,653 | ||
| 24,768 | 24,769 | αs1-CN A - short isoform (∆ex 16), 6P | O97943-2 | 37,452 | ||
| 24,835 | 24,835 | αs1-CN C - short isoform (∆ex 16), 7P | 5,026 | |||
| 24,849 | 24,849 | αs1-CN A - short isoform (∆ex 16), 7P | 4,851 | |||
| VI | 28.80 | 14,430 | 14,430 | α-LAC | P00710 | 12,948 |
| 22,939 | n/a* | UP1 | n/a | 2,676 | ||
| 23,019 | n/a | UP1, +80 Da | 2,408 | |||
| 23,099 | n/a | UP1, +160 Da | 958 | |||
| 25,645 | 25,645 | αs1-CN C, 6P, splice variant (∆Q83) | 1,736 | |||
| 25,659 | 25,659 | αs1-CN A, 6P, splice variant (∆Q83) | 1,057 | |||
| 25,693 | 25,693 | αs1-CN C, 5P | 916 | |||
| 25,772 | 25,773 | αs1-CN C, 6P | 5,014 | |||
| 25,787 | 25,787 | αs1-CN A, 6P | O97943-1 | 1,509 | ||
| VII | 30.07 | 21,826 | 21,825 | αs2-CN, 7P | 709 | |
| 21,906 | 21,905 | αs2-CN, 8P | O97944 | 4,222 | ||
| 21,985 | 21,986 | αs2-CN, 9P | 289 | |||
| 23,179 | n/a | UP1, +240 Da | 1,430 | |||
| VIII | 31.26 | 21,986 | 21,985 | αs2-CN, 9P | O97944 | 866 |
| 22,066 | 22,065 | αs2-CN, 10P | 3,682 | |||
| IX | 33.04 | 22,066 | 22,065 | αs2-CN, 10P | 120 | |
| 22,146 | 22,145 | αs2-CN, 11P | 1,408 | |||
| X | 34.85 | 22,226 | 22,225 | αs2-CN, 12P | 806 | |
| 23,046 | n/a | UP2 | n/a | 295 | ||
| XI | 37.15 | 19,143 | 19,143 | PGRP | Q9GK12 | 3,659 |
| 23,126 | n/a | UP2, +80 Da | 150 | |||
| 23,206 | n/a | UP2, +160 Da | 1,162 | |||
| 23,286 | n/a | UP2, +240 Da | 940 |
n/a - not applicable.
UP1 and UP2: new camel αs2-CN splicing variants
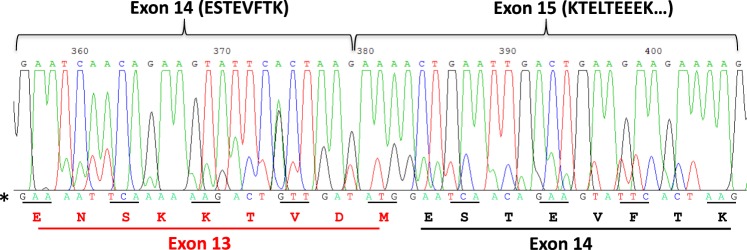
Amplification of camel αs2-CN cDNA revealed the presence of a major PCR fragment (ca. 620 bp) and several minor PCR products differing in size between ca. 670 bp and 710 bp (Supplementary Data S1). Sequencing of PCR fragments generated two different nucleotide sequences: first identical from the forward primer to nucleotide 359, and then overlapping and shifted by 27 nucleotides (Fig. 3). The main sequence corresponded to the 193-aa αs2-CN (including the signal peptide) reported by Kappeler et al.22. The second sequence, with weaker signals, showed the insertion of the following sequence: GAA AAT TCA AAA AAG ACT GTT GAT ATG, between exons 12′ and 14. Thus, this insertion introduced an additional peptide sequence (ENSKKTVDM), identical to the aa sequence encoded by exon 13 in the bovine CSN1S2 gene (Fig. 4). The level of exon 13 conservation in both species appeared to be extremely high. This exon is also present in the predicted sequence of the CSN1S2 gene from the Camelus ferus genome (NCBI Reference Sequence: XP_014418048.1) and the lama gene transcript (GenBank: LK999989.1) with two point mutations. The first mutation concerning the fourth codon (AAA = >AAT) is silent and the second one, that is a missense mutation, regards the last codon (ACG = >ATG), leading to T = >M substitution23. Exon 13 is present in one of the two copies of the CSN1S2 gene of most mammalian species. In mice, rats and rabbits the aa sequence encoded by this exon is present in CSN1S2-like (or CSN1S2A) protein but not in CSN1S2B24. The insertion of this sequence leads to the increasing of the molecular mass of αs2-CN by 1,033 Da, exactly the mass difference observed between αs2-CN-8P and UP1.
Figure 3.
Sequence of C. dromedarius αs2-CN cDNA spanning exons 14 and 15 (main sequence). A secondary sequence (*) identified by manual reading of overlapping weak signals is given below the main sequence, showing the existence of transcripts, in which exon 13 is included. The corresponding aa sequence is given below. cDNA sequences encoding CSN1S2sv1 were submitted to NCBI Genbank with the following submission IDs: BankIt2160486 Seq. 1 MK077758 (C. bactrianus) and BankIt2160533 Seq. 1 MK077759 (C. dromedarius).
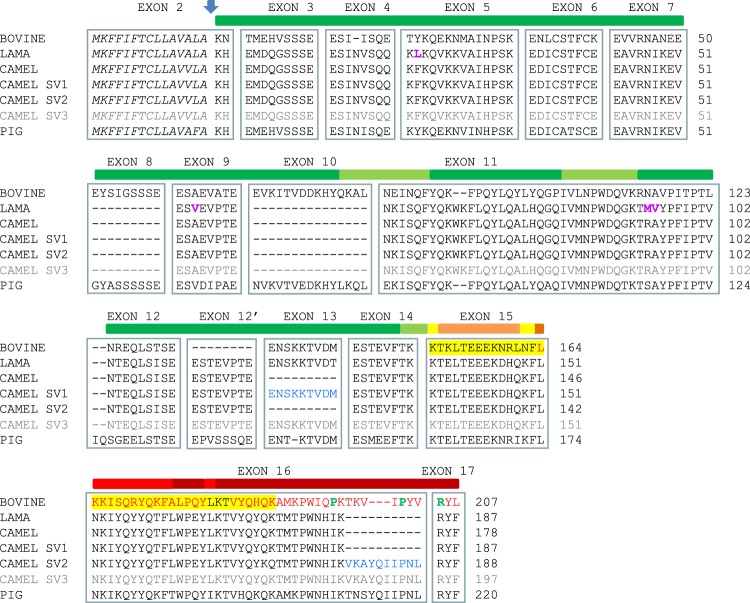
Figure 4.
Multiple alignment of αs2-CN protein sequences from different Artiodactyls species. Bos taurus (M16644), C. dromedarius (O97944 and splicing variants identified in the present study), Lama glama (A0A0D6DR01), and Sus scrofa (X54975) protein sequences are compared. Camel αs2-CN putative isoform (αs2-CNsv3) comprising both additional sequences, encoded by exon 13 and exon16 extension, is in grey. Sequences are split into blocks of amino acid residues to visualize the exon modular structure of the protein as deduced from known splice junctions of the bovine gene51. Exon numbering (top of blocks) is that of the bovine gene taken as reference for Artiodactyls. Amino-acid sequences characterizing UP1 (αs2-CNsv1) and UP2 (αs2-CNsv2) encoded by exon 13 and the extension of exon 16, respectively, are given in blue. Italics indicate the signal peptides, for which the vertical blue arrow points out the cleavage site. Dashes indicate missing aa residues. Amino acid mutations distinguishing camel and lama αs2-CN are in fuchsia. The highest sequence antimicrobial peptide density is indicated by red on a heat map above the bovine protein sequence. The regions of Bioactive peptides encrypted in bovine αs2-CN f(150–188) with antibacterial activities reported by Zucht et al.39 are highlighted in yellow, while two antibacterial domains f(164–179) and f(183–207) described by Recio and Visser40 are indicated in red. Amino acid residues increasing significantly antibacterial potency are in green. Full-length mature CSN1S2sv1 and CSN1S2sv2 aa sequences were submitted to Expasy UniProtKB database as splicing variants of C. dromedarius CSN1S2 with the following submission IDs: SPIN200013828 and SPIN200013835, respectively.
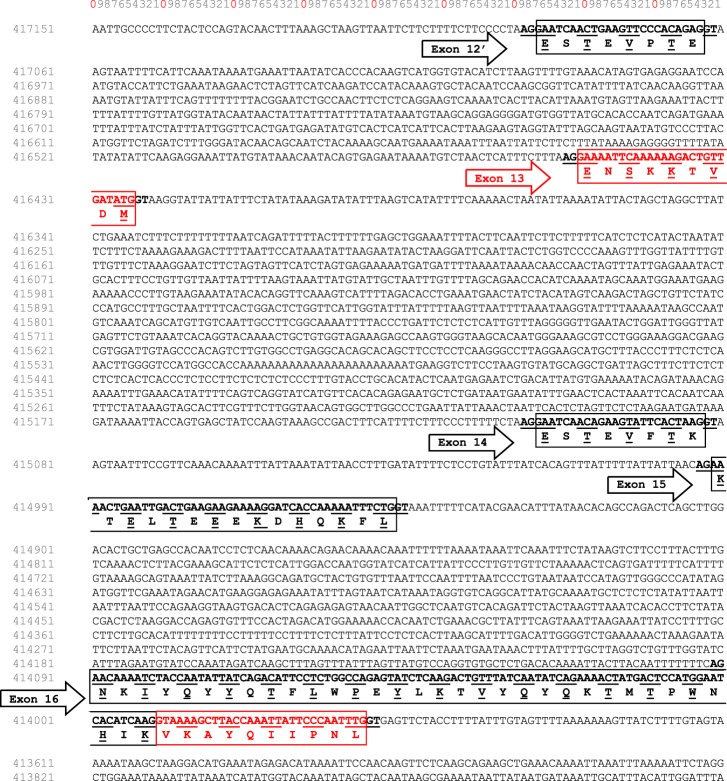
A deep and comprehensive analysis of the dromedary camel CSN1S2 gene sequence available in GenBank (gi|742343530|ref|NW_011591251.1|), overlaying exon 12′ (ESTEVPTE) to exon 14 (ESTEVFTK) allowed identifying a 27-nucleotide sequence corresponding to exon 13 (Fig. 5). This sequence is flanked with consensus splice sites at the beginning (GTG/AAG) and end (polypyrimidine tract followed by XAG) of intron sequences. Therefore, this exon is included or not during the course of camel αs2-CN pre-mRNA processing. This is possibly due to the weakness (presence of purine in the polypyrimidine tract at the 3′-end of the upstream intron) of the acceptor splice sequence. The short transcript (without exon 13) encodes the 193 aa residues (including the signal peptide) described by Kappeler et al.22 and the long transcript (with exon 13) codes for UP1 (202 aa including signal peptide). The mature protein corresponding to UP1 is named thereafter αs2-CNsv1.
Figure 5.
Nucleotide sequence view (from 417151 to 413731) of C. dromedarius (breed Arabia) taken from the unplaced genomic scaffold of CSN1S2 (LOC105090951). Already known exons 12′, 14, 15 and 16 are given in black, and additional exon 13 and extension of 30 additional nucleotides of exon 16 are in red. Exon subdivisions are boxed with amino acid sequences beneath. Intron donor and acceptor splice sites are underlined.
To confirm such an additional exon 13 hypothesis, detection of αs2-CN peptides after trypsin action was performed using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). A tryptic peptide composed of 12 aa residues TVDMESTEVFTK (Fig. 6), identified through the Bubalus bubalis αs2-CN sequence (UniProt KB accession number E9NZN2), was attributed to two coherent arranged sequences (ENSKKTVDM and ESTEVFTK) encoded by exons 13 and 14, respectively. The sequence is identical to that of the Bos taurus (UniProt KB accession number P02663). The presence of a TVDM peptide sequence confirmed the existence of transcripts having included exon 13 during the course of pre-mRNA processing. Therefore, the existence of an exon 13 alternatively spliced in the camel CSN1S2 gene was successfully confirmed both at the protein (LC-MS and LC-MS/MS) and at the nucleotide (cDNA sequencing and genome data) levels. The same cDNA sequences encoding αs2-CN with and without a 27-nucleotide additional sequence (exon 13) were found in all individual samples analyzed, including C. bactrianus, C. dromedarius, and hybrids.
Figure 6.
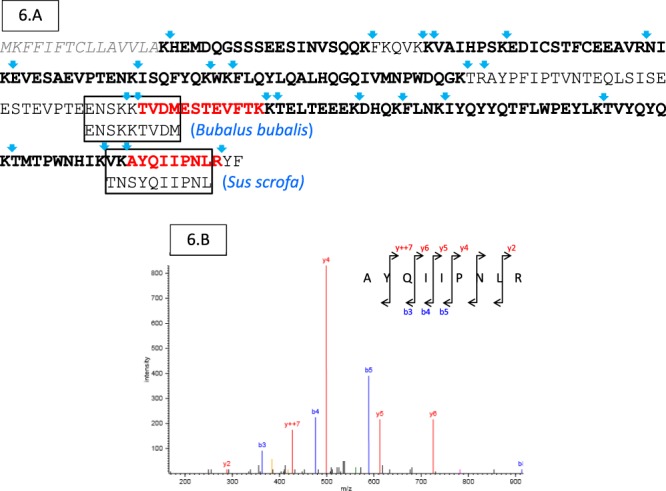
Identification and characterization of UP1 and UP2 as splicing variants of αs2-CN by LC-MS/MS analysis. (A) Camel αs2-CN full-length sequence is given and its coverage (81%) from peptides identified by LC-MS/MS analysis is in bold. Blue arrows indicate a cleavage of camel αs2-CN by trypsin. Tryptic peptides indicating the presence of exon 13 and extension of exon 16 are in red. Camel αs2-CN peptide sequences encoded by exon 13 and by the extension of exon 16 matching with Bubalus bubalis (UniProt KB accession number E9NZN2) and Sus scrofa (UniProt KB accession number P39036) are framed. The signal peptide is in italics and in grey. (B) Validation of the additional peptide sequence (AYQIIPNLR) with five and three ions from the “y” (including y7 double charged: y + + 7) and “b” series, respectively.
Concerning the second unknown protein detected (UP2) that showed molecular masses comprised between 23,046 Da and 23,286 Da with n and n + 3 phosphate groups, in LC-ESI-MS, the mass difference observed was 1,140 Da, relative to the 8P-11P αs2-CN protein reported by Kappeler and co-workers22. LC-MS/MS analysis revealed the occurrence of a 9 aa-long peptide (AYQIIPNLR) matching with the C-terminal sequence of Sus scrofa αs2-CN (NP_001004030.1), strongly suggesting that mRNA described by Kappeler et al.22 was in fact the result of a cryptic splice site usage occurring in the antepenultimate exon of the camel CSN1S2 gene (Fig. 6).
Examination of intron sequence downstream of exon 16 (Fig. 5) highlighted a 30-nucleotide segment: GTA AAA GCT TAC CAA ATT ATT CCC AAT TTG encoding 10 aa residues (VKAYQIIPNL). The intron donor splice site following the previously considered ending sequence of exon 16 CACATCAAG│GTAAA was recognized by the spliceosome machinery to generate the protein described by Kappeler et al.22. Alternatively, a second downstream intron donor splice site (CCC AAT TTG│GTGAG), which also fulfils all requirements of a splicing recognition signal, may also be used as well (Fig. 5). As a result, this alternative splicing event is responsible for the occurrence of two mature peptide chains, the first one made of 178 aa residues (21,906 Da with 8P), and the second one 10 aa residues longer (23,046 Da with 8P). The mature protein corresponding to UP2 is named thereafter αs2-CNsv2. Interestingly, the 10 aa residue peptide (VKAYQIIPNL) included in the C-terminal part of the camel protein due to this alternative splicing event was highly similar with the porcine (TNSYQIIPNL) and donkey (TNSYQIIPVL) αs2-CN sequences. Recently a shorter αs2-CN isoform, in which a deletion of the heptapeptide YQIIPVL, was reported in donkey milk25,26.
Cross-species comparison of the gene encoding αs2-CN and primary transcript maturation
Comparative analysis of camel CSN1S2 gene organization with orthologous bovine and pig genes is illustrated in Fig. 7. The first camel αs2-CN sequence published by Kappeler et al.22 lacks three peptide sequences encoded in cattle by exons 8 (EYSIGSSSE), 10 (EVKITVDDKHYQKAL), and 13 (ENSKKTVDM) composed of 27, 45 and 27 nucleotides, respectively. By contrast, exon 12′ that encodes in camel and lama a peptide of 8 aa residues (ESTEVPTE), was believed to be missing in the bovine counterpart, while it was present in the porcine genome, coding for the EPVSSSQE peptide. Surprisingly, we succeed in finding a putative exon 12′, encoding the octapeptide VSANSSQE, in intron 12 of the bovine gene. However, the downstream GTAAG donor splice site flanking this putative exon 12′ is mutated in GCAAG, apparently preventing its recognition as such as an exon. On the other hand, we failed to find a putative exon 8 in intron 7 of the camel gene. Exon 10 is present both in bovine and pig CSN1S2 genes. In addition, it is also present in intron 9 of the camel gene, being 9 nucleotides longer than in the other species (Fig. 7), and bounded upstream and downstream by canonical intron consensus sequences. However, even though it seems to be perfectly eligible for splicing, we did not find any transcript nucleotide sequence, nor tryptic peptides at the protein level, signing its presence in multiple mRNA encoding αs2-CN. By contrast, as demonstrated in the present study, exon 13 was actually present in some camel CSN1S2 transcripts, as well as the peptide sequence it is coding for in isoform αs2-CNsv1. Finally, the camel CSN1S2 gene, just as its lama counterpart23, is made up of at least 17 exons, since we have no objective demonstration of the usage of exon 10, whereas its bovine and porcine counterparts are made up of 18 and 19 exons, respectively. Since a further exon sequence (exon 7′) occurs in the Equidaes CSN1S2B gene (not in CSN1S2-like A), we can hypothesize that the CSN1S2 gene can comprise up to 20 exons with different combinatory splicing schemes across species. Interestingly, sequence alignments revealed that within the bovine intron 7, as well as in camels and pigs, the sequence corresponding to horse and donkey exon 7′ is partially deleted.
Figure 7.
Structural organization of the bovine, porcine and camel CSN1S2 transcription units and splicing patterns for camel (CSN1S2, CSN1S2sv1 and CSN1S2sv2). CSN1S2 corresponds to the splicing pattern characterized by Kappeler et al.22. Solid bars represent introns, and exons are depicted by blocks: 5′UTR and noncoding sequence are given in pink, leader peptide and coding frame are in black, exons absent from the camel protein are in green, exons absent from the bovine protein are in blue, exons found in our study are in red, and 3′UTR in white. Exons and exon sequences present in bovine and porcine CSN1S2 but which were absent from the camel until now are highlighted in green, while exons present in the camel and pig are in blue. Exon 13 and the extension of exon 16 identified in this study are in red. Exon numbering (referring to bovine) and sizes (in bp) are indicated at the top, and at the bottom of the structures, respectively.
Genomic and mRNA analyses carried out previously demonstrated that deletions of aa residues in CN across species occurred essentially by exon skipping during the processing of the primary transcripts7,8,23,27–29. This event, leading to a shortening of the peptide chain length, is caused by weaknesses in the consensus sequences, either at the 5′ and/or 3′ splice junctions or at the branch point, or both7. Therefore, alternative splicing has to be regarded as a frequent event, mainly in αs-CN encoding genes, for which the coding region is divided into many short exons. Usage of cryptic splice sites is also responsible for the occurrence of multiple transcripts and finally for generating a protein molecular diversity. For example, the peptide sequence (VKAYQIIPNL) encoded by the “extension” of 30 nucleotides at the 3′ end of exon 16, not previously detected in camel nor in lama αs2-CN, was shown here to be alternatively included in camel CSN1S2 transcripts. Extending the comparison to other species including ruminants, pigs and Equidaes, we show that the true donor splice site (GTGAG…) defining the end of exon 16 and common to the considered species (Fig. 8), is located 30 nt downstream of that preferentially used in Camelidaes. In other words, the isoform corresponding to UP2/αs2-CNsv2 is the genuine protein, whereas the isoform first described22 corresponds to the protein arising from the usage of a cryptic splice site internal to an exon.
Figure 8.

Alignment of nucleotide sequences of exon 16 3′-end and downstream intron across nine species. Accession numbers of different species are: camel (NCBI Gene ID: 105090951), pig (NCBI Gene ID: 445515), donkey (NCBI Gene ID: 106835119), horse (NCBI Gene ID: 100327035), rabbit (NCBI Gene ID: 100009288), bovine (NCBI Gene ID: 282209), goat (NCBI Gene ID: 100861229), sheep (NCBI Gene ID: 443383), and buffalo (NCBI Gene ID: 102395699). Exon sequences are in bold, intron sequences are in italics. Perfectly conserved nucleotides are dark-grey shaded. Nucleotides identical in more than eight animal species are light-grey shaded. Dashes in ruminants indicate missing nucleotides that are highlighted in yellow in the other species. The dinucleotide GT, highlighted in green in the camel sequence, generates the preferential site of splicing occurring within exon 16 that leads to the main αs2-CN isoform first described by Kappeler et al.22.
The combination of both splicing events such as exon skipping and cryptic splice site usage generates more transcript isoforms in the same species and is responsible for the differences across species in the aa sequences of αs2-CN. However, regarding αs2-CN in camels we were not able to detect any transcript in which both exon 13 and the extension of exon 16 were present (αs2-CNsv3). That does not mean that this structure does not exist, even though the protein corresponding to both events was not detected in LC-MS profiling. Therefore, given that such an isoform is putatively present at a very low level, cloning PCR fragments and screening of a significant number of clones should probably make it possible to identify such a transcript.
Phosphorylation level enhances camel αs2-CN isoform complexity
The non-phosphorylated peptide chain of the mature αs2-CN protein, which comprises 178 aa residues, yields a molecular weight of 21,266 Da22. Compared with other Ca-sensitive CNs, αs2-CN is the most phosphorylated with 12 potential phosphorylation sites and it is therefore likely to be the major transporter of Ca-phosphate.
Structural characterization of the αs2-CN fraction and relevant mRNA analyses has demonstrated that camel αs2-CN should be theoretically present in milk as a mixture of at least 18 isoforms derived from three mature peptide chains comprising 178 (αs2-CN), 187 (αs2-CNsv1, UP1) and 188 (αs2-CNsv2, UP2) aa residues originating from alternative splicing phenomena (Fig. 4). Each splicing variant should display six phosphorylation levels ranging between 7 and 12P groups. Based on LC-ESI-MS data, we identified 14 phosphorylation isoforms. Surprisingly, even though an additional peptide sequence does not provide further phosphorylation sites, the predominant phosphorylation level of each peptide isoform was not the same: 8P for αs2-CN, 8P for αs2-CNsv1, and 10P for αs2-CNsv2. The addition of 10 aa residues in the C-terminal part of αs2-CNsv2 might induce conformational changes in the protein facilitating the modification of definite phosphorylable sites. Multiple non-allelic variants produced from at least three different mRNA were shown to occur in all thirty Kazakh individuals analyzed, apparently indicating a stabilized mechanism for the production of protein isoforms of different lengths, structures and possibly biological activities.
With 11 potentially phosphorylated aa residues matching the S/T-X-A motif, camel αs2-CN displays the highest phosphorylation level, as mentioned by Ryskaliyeva et al.8. To reach such a phosphorylation level, besides the nine SerP, two putative Threonine residues (T118 and T132) should be phosphorylated. However, in all the Kazakh milk samples analyzed in LC-ESI-MS we found αs2-CN with up to 12P groups. This means that at least another S/T residue that does not match the canonical sequence recognized by the mammary kinase(s), is potentially phosphorylated. According to Allende et al.30 the sequence S/T-X-X-A is in agreement with the minimum requirements for phosphorylation by the CN-kinase II (CK2). In this regard, it is critical to highlight that the A residue in this site, usually E or D, can be replaced by SerP or ThrP. Two T residues, namely T39 and T129 in the camel αs2-CN fully meet the requirements of the above-mentioned motif and might be phosphorylated. Such an event is the only possible hypothesis to reach 12P for camel αs2-CN. Since these two kinases are very likely secreted, the idea that phosphorylation at T39/T129 may occur in the extracellular environment cannot be excluded. This warrants further investigation. Fam20C, which is very likely the major secretory pathway protein kinase31, might be responsible for the phosphorylation of S and T residues within the S/T-X-A motif, whereas a CK2-type kinase might be responsible for phosphorylation of the T residue within an S/T-X-X-A motif. This was in agreement with the hypothesis put forward by Bijl et al.32 and Fang et al.33, who suggest, from phenotypic correlations and hierarchical clustering, the existence of at least two regulatory systems for phosphorylation of αs-CN. Interestingly, twelve phosphorylation sites were also predicted in llama αs2-CN23, including two Threonine residues at T118 (instead of T114 as erroneously mentioned) and T141 (also T141 in camel αs2-CNsv1). Phosphorylation sites matching the S/T-X-A motif in llama αs2-CN are actually 12. Indeed, S122 (llama’s numbering) that has been predicted as phosphorylated23 does not meet the criteria required by the S/T-X-A consensus motif and cannot be phosphorylated. By contrast, T128, which is substituted by a methionine residue (M) in the camel αs2-CNsv1, is potentially phosphorylated provided S130 has been phosphorylated before. On the contrary, sites potentially phosphorylated by a second kinase (CK2-type) identified in the camel sequence are also present in the llama sequence and therefore the phosphorylation level that could be reached in this species is potentially13P.
Alternate splicing isoforms of camel αs2-CN increase its ability to generate potential bioactive peptides
A growing number of genes encoding milk proteins displays complex patterns of splicing, thus increasing their coding capacity to generate an extreme protein isoform diversity from a single gene. It is well established that milk proteins represent a reservoir of biologically active peptides13,34,35, capable of modulating different functions. Therefore, beside genetic polymorphisms, the molecular diversity generated by differential splicing mechanisms can increase its content.
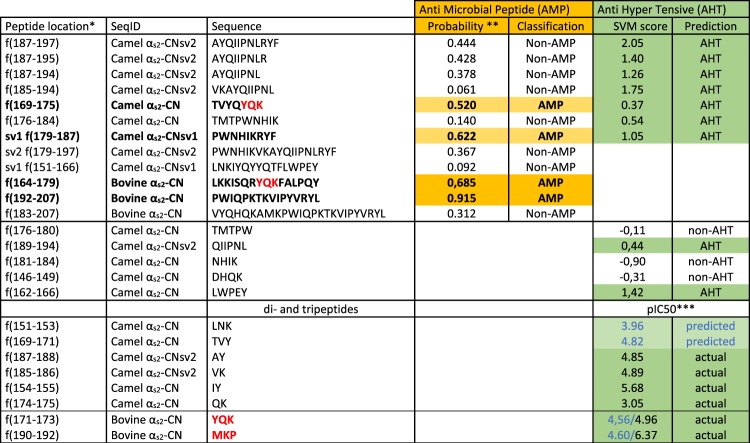
To evaluate this possibility, we undertook to search for bioactive peptides encrypted in the different camel αs2-CN isoforms, using an in silico approach. Since alternative splicing events impact the C-terminal part of the molecule (f(150–197)) which seems, in addition, to be the most accessible domain of the bovine protein15,36, we therefore focused our attention on this region. Previous studies performed on the bovine αs2-CN have demonstrated that this casein is the least accessible in the micelles and that a limited number of tryptic peptides were released from its C-terminal part37,38; of which some were subsequently shown to display antibacterial properties15. The first antibacterial peptide isolated from bovine αs2-CN (f(150–188) of the mature protein), inhibiting the growth of Escherichia coli and Staphylococcus carnosus, was called casocidin-I39. Two distinct antibacterial domains f(164–179) and f(183–207), also located in the C-terminal part of the molecule, were subsequently isolated from a peptic hydrolysate of bovine αs2-CN40. It is worth noting that in our prediction analyses (Fig. 9), the bovine peptide f(164–179) displays a rather high probability (0.685) to have an antimicrobial (AMP) activity; whereas peptide f(183–207) for which a probability of 0.312 was found, would not have such an activity. In contrast, peptide f(192–207) is by far the one with the highest probability (0.915) to exhibit an AMP activity.
Figure 9.
In silico analyses of αs2-CN peptides for antimicrobial (yellow) and antihypertensive (green) activities. *Peptide location is given in the longest camel amino acid sequence (putative as2-CNsv3). **Probability > 0.5 = Predicted AMP. ***pIC50 = −logIC50 with IC50 = peptide concentration (μmol/L) necessary to inhibit the angiotensin converting enzyme (ACE) activity by 50%. SVM (support vector machine) score: threshold = 017. Tripeptides YQK and MKP recently identified as an antihypertensive peptide43,52 are bolded and in red.
The picture is less positive with regard to the corresponding camel sequences, since peptides f(179–197) and f(179–187), according to the splicing variant (αs2-CN sv2 and αs2-CN sv1, respectively), as well as f(151–166) from αs2-CN sv1, compared with the bovine αs2-CN f(164–179), gave more contrasted results (Fig. 9). Given the magnitude of the splicing events occurring in the camel αs2-CN pre-mRNA, it is not surprising that it would impact biological properties of αs2-CN C-terminal peptides, including antimicrobial activity, since several aa residues of this region were shown to be essential regarding AMP activity39,40. Indeed, the importance of specific amino acids (P and R residues) at the C-terminus of the bovine milk-derived αs2-CN f(183–207) peptide for its antibacterial activity against the food-borne pathogens Listeria monocytogenes and Cronbacter sakazakii, was recently demonstrated41. Nevertheless, this in silico screening remains a predictive approach, aimed at identifying sequences that would be potentially bioactive. It is therefore necessary to confirm experimentally, and possible discordances may occur between in silico and in vitro results. It is not because the sequence of a peptide is predicted as potentially bioactive that it will be actually active in vitro and if it is active in vitro, this does not mean that even though it will be active in vivo. McCann et al.42 identified 5 peptides from chymosin digests of a bovine sodium caseinate, all being once again from the C-terminal end of αs2-CN, including f(164–207), f(175–207) and f(181–207), and showing in vitro antibacterial activity against Listeria innocua. However, they stressed that it was not excluded that these cationic peptides may lose their antibacterial activity in vivo. From all these studies it appears, nevertheless, that the C-terminal part of αs2-CN was predicted to yield peptides with defensin-like activity, which may aid the immune system in fighting bacteria15.
Interestingly, further bioactive peptides with different properties such as AHT (Anti Hyper Tensive) activity were identified from camel αs2-CN (Fig. 9). Indeed, according to the splicing patterns, including or not exon 16 extension, two peptide sequences (KTMTPWNHIKRYF and KTMTPWNHIKVKAYQIIPNLRYF) occur within the C-terminal part of the molecule (Fig. 4), thus giving rise to different peptides after digestion by proteolytic enzymes from the digestive tract, including pepsin, trypsin and chymotrypsin (Supplementary Data S2). Several peptides, related to the inserted VKAYQIIPNL decapeptide characterizing camel αs2-CNsv2, were in silico identified as AHT peptides involved in the angiotensin I-converting enzyme (ACE) inhibitory activity, with SVM (Support Vector Machine) scores >1 (Fig. 9). Two ACE-inhibitory dipeptides (f(185–186): VK and f(187–188): AY) were found exclusively in camel αs2-CNsv2 (and in the putative camel αs2-CNsv3). Interestingly, the AY dipeptide was also found in the B variant of the camel αs1-CN21. A novel ACE inhibitory peptide (YQK) exhibiting an IC50 of 11.1 µM was recently isolated from a pepsin and trypsin hydrolysate of bovine αs2-CN43. An oral administration, using a rodent hypertensive model, revealed a significant decrease of systolic blood pressure, thus demonstrating its AHT effects. Such a tripeptide sequence also occurs in the C-terminal part of the camel αs2-CN.
To summarize, the data reported here allowed identifying UP1 and UP2 detected in our previous study1 as splicing isoforms of αs2-CN (αs2-CNsv1 and αs2-CNsv2, respectively). These isoforms arise from different processing of the CSN1S2 primary transcript, giving rise to the insertion of exon 13 in αs2-CNsv1 and a downstream extension of exon 16 in αs2-CNsv2. Thus, αs2-CN was shown to be a mixture of at least 16 isoforms differing in polypeptide chain length and phosphorylation levels, identified in both Camelus species (C. bactrianus and C. dromedarius), as well in hybrids. Such a situation is not specific to Camelids and is frequently observed in most of the mammalian species, particularly in small ruminants and Equidae. Little is known about the mechanisms identifying alternatively spliced exons. Do those deletions/insertions in camel αs2-CN simply reflect the lack of accuracy of an intricate processing mechanism whenever mutations induce conformational modifications of pre-mRNA, preventing the normal progress of the splicing process? There are more and more evidences to support the hypothesis that cis-acting sequences, both in introns and exons, are involved in the control of this process.
Despite the extreme conservation of the organization of the “casein” locus during the course of evolution (Fig. 1), the sequences of the proteins encoded by each of the genes that compose this locus have rapidly evolved. Given the exon modular structure of messenger RNAs, the real similarity between αs2-CN across species is significantly higher than it appears at first whether the exon modular structure is taken into account (Fig. 4). The apparent divergence is in fact largely due to a splicing combinatorial assembly of exons specific of each species, as previously suggested by Martin et al.44, as far as αs1-CN is concerned. Therefore, differential splicing, as well as genetic polymorphisms as described with camel αs1-CN21, generate a molecular diversity of sequences increasing the ability of camel caseins to generate potentially bioactive encrypted peptides.
Methods
Ethics Statements
All animal studies were carried out in compliance with European Community regulations on animal experimentation (European Communities Council Directive 86/609/EEC) and with the authorization of the Kazakh Ministry of Agriculture. Milk sampling was supervised by a veterinarian accredited by the French Ethics National Committee for Experimentation on Living Animals.
Milk Sample Collection and Preparation
Raw milk samples were collected during morning milking on healthy dairy camels belonging to two species: C. bactrianus (n = 72) and C. dromedarius (n = 65), and hybrids (n = 42) at different lactation stages, ranging between 30 and 90 days postpartum. Camels grazed on four various natural pastures from different regions of Kazakhstan, namely Almaty (AL), Shymkent (SH), Kyzylorda (KZ), and Atyrau (ZKO). Whole-milk samples were centrifuged at 3,000 g for 30 min at 4°C (Allegra X-15R, Beckman Coulter, France) to separate fat from skimmed milk. Samples were quickly frozen and stored at −80 °C (fat) and −20 °C (skimmed milk) until analysis.
Selection of Milk Samples
Thirty milk samples: C. bactrianus (n = 10), C. dromedarius (n = 10), and hybrids (n = 10)) were selected for LC-ESI-MS analysis from the 179 camel milks collected in a previous study1, based on lactation stages and number of parities (from 2 to 14). The most representative eight milk samples (C. bactrianus, n = 3, C. dromedarius, n = 3, and hybrids, n = 2) were analyzed by LC-MS/MS (LTQ-Orbitrap Discovery, Thermo Fisher Scientific) after a tryptic digestion of bands, excised from each track, between 20 and 30 kDa of SDS-PAGE.
RNA Extraction from Milk Fat Globules
Total RNA was extracted from MFG using TRIzol® and TRIzol® LS solutions (Invitrogen, Life Technologies), respectively, according to the original manufacturer’s protocol modified as described by Brenaut et al.45.
First-Strand cDNA Synthesis and PCR Amplification
First-strand cDNA was synthesized from 5 to 10 ng of total RNA primed with oligo(dT)20 and random primers (3:1, vol/vol) using Superscript III reverse transcriptase (Invitrogen Life Technologies Inc., Carlsbad, CA) as described previously1. Primer pairs, purchased from Eurofins (Eurofins genomics, Germany), were designed using published Camelus nucleic acid sequences (NCBI, NM_001303566.1 for αs1-CN and NM_001303561.1 for αs2-CN). The forward primers for αs1-CN and αs2-CN amplification were 5′-CTTACCTGCCTTGTGGCTGT-3′ (starting from nucleotide 61, located in exon 2 of αs1-CN mRNA) and 5′-TCATTTTTACCTGCCTTTTGGCTGT-3′ (starting from nucleotide 71, located in exon 2 of αs2-CN mRNA), respectively. The reverse primers were 5′-GTGGAGGAGAAATTTAGAGCAT-3′ (terminating at nucleotide 751 of αs1-CN mRNA located in the last exon) and 5′-CGATTTTCCAGTTGAGCCATA-3′ (terminating at nucleotide 692 of αs2-CN mRNA located in the last exon), respectively. Thus, the amplified fragments cover regions of 691 nucleotides for αs1-CN and 622 nucleotides for αs2-CN, including the sequence coding the mature proteins, with genomic reference to the published sequences (NCBI, NM_001303566.1 for αs1-CN and NM_001303561.1 for αs2-CN). Five (two C. bactrianus, one C. dromedarius, and two hybrids) samples representative of the 30 camel milks analyzed in LC-MS, were selected for amplification of αs1-CN and αs2-CN cDNA by RT-PCR and sequencing. Amplicons were sequenced from both strands with primers used for PCR according to the Sanger method by Eurofins (Eurofins genomics, Germany).
Identification of proteins and validation of peptides by LC-MS/MS Analysis
In order to identify the different αs1-CN and αs2-CN isoforms, mono dimensional electrophoresis (1D SDS-PAGE), followed by trypsin digestion and LC-MS/MS analysis, was used. After a long migration (10 cm) in 1D SDS-PAGE, bands (1.5 mm3) migrating in the range of 20–30 kDa, were cut on each of the eight gel lanes, and analyzed as described by Henry et al.46 and Saadaoui et al.47.
LC-ESI-MS
Fractionation of camel milk proteins and determination of their molecular masses were performed by coupling RP-HPLC to ESI-MS (micrOTOFTM II focus ESI-TOF mass spectrometer; Bruker Daltonics). Twenty μL of skimmed milk samples were clarified by addition of 230 μL of clarification solution 0.1 M bis-Tris buffer pH 8.0, containing 8 M urea, 1.3% trisodium citrate, and 0.3% DTT. Clarified milk samples (25 μL) were directly injected onto a Biodiscovery C5 reverse phase column (300 Å pore size, 3 µm, 150 × 2.1 mm; Supelco, France) and analyzed as described by Miranda et al.48.
In silico release of Peptides using PeptideCutter and BIOPEP analyses
Protein sequences of αs2-CN from Bos taurus (entry P02663), Lama glama (entry A0A0D6DR01) and Camelus dromedarius (entry O97944 and new sequences identified in the present study) were selected from the Protein Knowledge Base (UniProtKB, ExPASy Bioinformatics Resource Portal) available at www.uniprot.org. Each sequence was then subjected to in silico release of peptides by pepsin (pH 1.3), pepsin + trypsin and pepsin + trypsin + chymotrypsin using “PeptideCutter”, a resource available at www.expasy.org. Thereafter, each αs2-CN sequence was entered in the “PeptideCutter”. After cutting the sequences, a list of probable peptides with cleavage sites, length and amino acid sequence of peptides was established. BIOPEP analyses were then performed at https://omictools.com/biopep-tool by selecting the available option “Peptide Prediction Software Tools”. Peptide Structure Prediction/AHTpin17 and Antimicrobial Peptide Prediction/Antimicrobial Peptide Scanner49 (AMP Scanner Vr.2) sections were used one by one for prediction of the peptides with the sought properties.
Supplementary information
Acknowledgements
The study was carried out within the Bolashak International Scholarship of the first author, funded by the JSC «Center for International Programs» (Kazakhstan). The research was partly supported by a grant from the Ministry of Education and Science of the Republic of Kazakhstan under the name “Proteomic investigation of camel milk” #1729/GF4, which is duly appreciated. The authors thank all Kazakhstani camel milk farms and Moldir Nurseitova with Ali Totaev for rendering help in sample collection, as well as PAPPSO and @BRIDGe teams at INRA (Jouy-en-Josas, France) for providing necessary facilities and technical support. The authors would like also to thank warmly Wendy Brand-Williams for English language editing.
Author Contributions
A.R. carried out the study, collected milk samples, performed the experiments, and interpreted the data. C.H. performed LC-MS/MS analysis and analyzed the data. G.M. performed LC-ESI-MS analysis and analyzed the data. B.F. and G.K. provided funding. P.M. conceived and supervised the research, interpreted the data. The manuscript was written by A.R., revised and approved by P.M. All authors reviewed and contributed to the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41649-5.
References
- 1.Ryskaliyeva, A. et al. Combining different proteomic approaches to resolve complexity of the milk protein fraction of dromedary, Bactrian camels and hybrids, from different regions of Kazakhstan. PLoS One13 (2018). [DOI] [PMC free article] [PubMed]
- 2.Threadgill DW, Womack JE. Genomic analysis of the major bovine milk protein genes. Nucleic Acids Res. 1990;18:6935–42. doi: 10.1093/nar/18.23.6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes H, Petit E, Bouniol C, Popescu P. Localization of the αS2-casein gene (CASAS2) to the homoeologous cattle, sheep, and goat chromosomes 4 by in situ hybridization. Cytogenet. Genome Res. 1993;64:281–285. doi: 10.1159/000133593. [DOI] [PubMed] [Google Scholar]
- 4.Menon RS, Chang YF, Jeffers KF, Jones C, Ham RG. Regional localization of human β-casein gene (CSN2) to 4pter-q21. Genomics. 1992;13:225–226. doi: 10.1016/0888-7543(92)90227-J. [DOI] [PubMed] [Google Scholar]
- 5.Groenen MAM, Dijkhof RJM, Verstege AJM, van der Poel JJ. The complete sequence of the gene encoding bovine α2-casein. Gene. 1993;123:187–193. doi: 10.1016/0378-1119(93)90123-K. [DOI] [PubMed] [Google Scholar]
- 6.Rijnkels M, Elnitski L, Miller W, Rosen JM. Multispecies comparative analysis of a mammalian-specific genomic domain encoding secretory proteins. Genomics. 2003;82:417–432. doi: 10.1016/S0888-7543(03)00114-9. [DOI] [PubMed] [Google Scholar]
- 7.Martin, P., Cebo, C. & Miranda, G. In Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th Edition 387–429, 10.1007/978-1-4614-4714-6_13 (2013).
- 8.Leroux C, Mazure N, Martin P. Mutations away from splice site recognition sequences might cis-modulate alternative splicing of goat α(s1)-casein transcripts. Structural organization of the relevant gene. J. Biol. Chem. 1992;267:6147–6157. [PubMed] [Google Scholar]
- 9.Ramunno L, et al. Characterization of two new alleles at the goat CSN1S2 locus. Anim. Genet. 2001;32:264–268. doi: 10.1046/j.1365-2052.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramunno L, et al. Comparative analysis of gene sequence of goat CSN1S1 F and N alleles and characterization of CSN1S1 transcript variants in mammary gland. Gene. 2005;345:289–299. doi: 10.1016/j.gene.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Marcone S, Belton O, Fitzgerald DJ. Milk-derived bioactive peptides and their health promoting effects: a potential role in atherosclerosis. British Journal of Clinical Pharmacology. 2017;83:152–162. doi: 10.1111/bcp.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohanty DP, Mohapatra S, Misra S, Sahu PS. Milk derived bioactive peptides and their impact on human health – A review. Saudi Journal of Biological Sciences. 2016;23:577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meisel, H. Multifunctional peptides encrypted in milk proteins. In BioFactors, 10.1002/biof.552210111 (2004). [DOI] [PubMed]
- 14.Clare, D. A. & Swaisgood, H. E. Bioactive Milk Peptides: A Prospectus. J. Dairy Sci., 10.3168/jds.S0022-0302(00)74983-6 (2000). [DOI] [PubMed]
- 15.Farrell HM, Malin EL, Brown EM, Mora-Gutierrez A. Review of the chemistry of αS2-casein and the generation of a homologous molecular model to explain its properties. J. Dairy Sci. 2009;92:1338–1353. doi: 10.3168/jds.2008-1711. [DOI] [PubMed] [Google Scholar]
- 16.Mati A, et al. Dromedary camel milk proteins, a source of peptides having biological activities – A review. International Dairy Journal. 2017;73:25–37. doi: 10.1016/j.idairyj.2016.12.001. [DOI] [Google Scholar]
- 17.Kumar, R. et al. An in silico platform for predicting, screening and designing of antihypertensive peptides. Sci. Rep., 10.1038/srep12512 (2015). [DOI] [PMC free article] [PubMed]
- 18.Minkiewicz, P., Dziuba, J., Iwaniak, A., Dziuba, M. & Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. in Journal of AOAC International, 10.1093/bib/bbl035 (2008). [PubMed]
- 19.Théolier, J., Fliss, I., Jean, J. & Hammami, R. MilkAMP: A comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. Technol., 10.1007/s13594-013-0153-2 (2014).
- 20.Nielsen, S. D., Beverly, R. L., Qu, Y. & Dallas, D. C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem., 10.1016/j.foodchem.2017.04.056 (2017). [DOI] [PMC free article] [PubMed]
- 21.Erhardt G, et al. Alpha S1-casein polymorphisms in camel (Camelus dromedarius) and descriptions of biological active peptides and allergenic epitopes. Trop. Anim. Health Prod. 2016;48:879–887. doi: 10.1007/s11250-016-0997-6. [DOI] [PubMed] [Google Scholar]
- 22.Kappeler S, Farah Z, Puhan Z. Sequence analysis of Camelus dromedarius milk caseins. J. Dairy Res. 1998;65:209–222. doi: 10.1017/S0022029997002847. [DOI] [PubMed] [Google Scholar]
- 23.Pauciullo, A. & Erhardt, G. Molecular characterization of the llamas (Lama glama) casein cluster genes transcripts (CSN1S1, CSN2, CSN1S2, CSN3) and regulatory regions. PLoS One10 (2015). [DOI] [PMC free article] [PubMed]
- 24.Rijnkels M. Multispecies comparison of the casein gene loci and evolution of casein gene family. Journal of Mammary Gland Biology and Neoplasia. 2002;7:327–345. doi: 10.1023/A:1022808918013. [DOI] [PubMed] [Google Scholar]
- 25.Saletti R, et al. MS-based characterization of αs2-casein isoforms in donkey’s milk. in. Journal of Mass Spectrometry. 2012;47:1150–1159. doi: 10.1002/jms.3031. [DOI] [PubMed] [Google Scholar]
- 26.Cunsolo V, et al. Proteins and bioactive peptides from donkey milk: The molecular basis for its reduced allergenic properties. Food Research International. 2017;99:41–57. doi: 10.1016/j.foodres.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Johnsen, L. B., Rasmussen, L. K., Petersen, T. E. & Berglund, L. Characterization of three types of human alpha s1-casein mRNA transcripts. Biochem. J., 10.1042/bj3090237 (1995). [DOI] [PMC free article] [PubMed]
- 28.Matéos A, et al. Equine alpha S1-casein: characterization of alternative splicing isoforms and determination of phosphorylation levels. J. Dairy Sci. 2009;92:3604–15. doi: 10.3168/jds.2009-2125. [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Leroux C. Exon-skipping is responsible for the 9 amino acid residue deletion occurring near the N-terminal of human β-casein. Biochem. Biophys. Res. Commun. 1992;183:750–757. doi: 10.1016/0006-291X(92)90547-X. [DOI] [PubMed] [Google Scholar]
- 30.Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 31.Tagliabracci VS, et al. A Single Kinase Generates the Majority of the Secreted Phosphoproteome. Cell. 2015;161:1619–1632. doi: 10.1016/j.cell.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bijl E, van Valenberg HJF, Huppertz T, van Hooijdonk ACM, Bovenhuis H. Phosphorylation of αS1-casein is regulated by different genes. J. Dairy Sci. 2014;97:7240–7246. doi: 10.3168/jds.2014-8061. [DOI] [PubMed] [Google Scholar]
- 33.Fang ZH, et al. The relationships among bovine αS-casein phosphorylation isoforms suggest different phosphorylation pathways. J. Dairy Sci. 2016;99:8168–8177. doi: 10.3168/jds.2016-11250. [DOI] [PubMed] [Google Scholar]
- 34.Nagpal, R. et al. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food and Function, 10.1039/c0fo00016g (2011). [DOI] [PubMed]
- 35.Weimann, C., Meisel, H. & Erhardt, G. Short communication: Bovine κ-casein variants result in different angiotensin I converting enzyme (ACE) inhibitory peptides. J. Dairy Sci., 10.3168/jds.2008-1671 (2009). [DOI] [PubMed]
- 36.Tauzin, J., Miclo, L., Roth, S., Mollé, D. & Gaillard, J. L. Tryptic hydrolysis of bovine αs2-casein: Identification and release kinetics of peptides. Int. Dairy J., 10.1016/S0958-6946(02)00127-9 (2003).
- 37.Diaz, O., Gouldsworthy, A. M. & Leaver, J. Identification of Peptides Released from Casein Micelles by Limited Trypsinolysis. J. Agric. Food Chem., 10.1021/jf950832u (1996).
- 38.Gagnaire V, Léonil J. Preferential sites of tryptic cleavage on the major bovine caseins within the micelle. Lait. 1998;78:471–489. doi: 10.1051/lait:1998545. [DOI] [Google Scholar]
- 39.Zucht HD, Raida M, Adermann K, Mägert HJ, Forssmann WG. Casocidin-I: a casein-αs2 derived peptide exhibits antibacterial activity. FEBS Lett. 1995;372:185–188. doi: 10.1016/0014-5793(95)00974-E. [DOI] [PubMed] [Google Scholar]
- 40.Recio I, Visser S. Identification of two distinct antibacterial domains within the sequence of bovine α(s2)-casein. Biochim. Biophys. Acta - Gen. Subj. 1999;1428:314–326. doi: 10.1016/S0304-4165(99)00079-3. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Ordóñez A, et al. Structure-activity relationship of synthetic variants of the milk-derived antimicrobial peptide αs2-casein f(183–207) Appl. Environ. Microbiol. 2013;79:5179–5185. doi: 10.1128/AEM.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCann KB, et al. Isolation and characterisation of antibacterial peptides derived from the f(164–207) region of bovine αs2-casein. Int. Dairy J. 2005;15:133–143. doi: 10.1016/j.idairyj.2004.06.008. [DOI] [Google Scholar]
- 43.Xue L, et al. Identification and characterization of an angiotensin-converting enzyme inhibitory peptide derived from bovine casein. Peptides. 2018;99:161–168. doi: 10.1016/j.peptides.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Martin, P., Brignon, G., Furet, J. P. & Leroux, C. The gene encoding α s1 -casein is expressed in human mammary epithelial cells during lactation. Lait, 10.1051/lait:1996641 (2007).
- 45.Brenaut P, et al. Validation of RNA isolated from milk fat globules to profile mammary epithelial cell expression during lactation and transcriptional response to a bacterial infection. J. Dairy Sci. 2012;95:6130–6144. doi: 10.3168/jds.2012-5604. [DOI] [PubMed] [Google Scholar]
- 46.Henry, C., Saadaoui, B., Bouvier, F. & Cebo, C. Phosphoproteomics of the goat milk fat globule membrane: New insights into lipid droplet secretion from the mammary epithelial cell. Proteomics, 10.1002/pmic.201400245 (2015). [DOI] [PubMed]
- 47.Saadaoui B, et al. Combining proteomic tools to characterize the protein fraction of llama (Lama glama) milk. Electrophoresis. 2014;35:1406–1418. doi: 10.1002/elps.201300383. [DOI] [PubMed] [Google Scholar]
- 48.Miranda G, Mahé MF, Leroux C, Martin P. Proteomic tools characterize the protein fraction of Equidae milk. Proteomics. 2004;4:2496–2509. doi: 10.1002/pmic.200300765. [DOI] [PubMed] [Google Scholar]
- 49.Veltri, D., Kamath, U. & Shehu, A. Deep learning improves antimicrobial peptide recognition. Bioinformatics, 10.1093/bioinformatics/bty179 (2018). [DOI] [PMC free article] [PubMed]
- 50.Lefèvre, C. M., Sharp, J. A. & Nicholas, K. R. Characterisation of monotreme caseins reveals lineage-specific expansion of an ancestral casein locus in mammals. Reprod. Fertil. Dev., 10.1071/RD09083 (2009). [DOI] [PubMed]
- 51.Koczan D, Hobom G, Seyfert HM. Genomic organisation of the bovine alpha-S1 casein gene. Nucleic Acids Res. 1991;19:5591–5596. doi: 10.1093/nar/19.20.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada, A. et al. Antihypertensive effect of the bovine casein-derived peptide Met-Lys-Pro. Food Chem., 10.1016/j.foodchem.2014.09.098 (2015). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.