Abstract
Several mosquito species have been described as vectors for the Zika virus (ZIKV), such as those in the Aedes, Anopheles, Mansonia and Culex genera. Our previous survey studies were found the ZIKV RNA positive in both male, female and larvae of Culex quinquefasciatus Say and Aedes aegypti (L.) mosquitoes collected from active ZIKV infected patients’ homes in Thailand. Therefore, the aims of this study were to investigate whether ZIKV could be vertically transmitted in Cx. quinquefasciatus, Ae. aegypti and Ae. albopictus. Laboratory and field colonies of these mosquito species were maintained and artificially fed with ZIKV in human blood. Fully engorged mosquitoes (F0) were selected and reared for the vertical transmission study. The subsequent mosquito generations were fed with human blood without the virus. ZIKV in the mosquitoes was detected by hemi-nested RT-PCR and sequencing. C6/36 cells were used to isolate ZIKV from samples that tested positive by hemi-nested RT-PCR. Moreover, ZIKV was identified by immunocytochemical staining 7 days after infection in several organs of infected F0 females, including the salivary glands, midguts, yoke granules and facet cells of the eye. The localization of the ZIKV antigen was identified by the presence of the specific antibody in the salivary glands, midguts, yoke granules and facet cells. ZIKV was detected in female and male Cx. quinquefasciatus until the F6 and F2 generations, respectively. The isolated virus showed cytopathic effects in C6/36 cells by 5 days postinfection. The results suggested that the vertical transmission of ZIKV occurs in Cx. quinquefasciatus in the laboratory. However, we were able to detect the presence of ZIKV in Ae. aegypti in only the F1 generation in both male and female mosquitoes, and Ae. albopictus mosquitoes were not able to vertically transmit the virus at all. Data obtained from this study could be valuable for developing a better understanding of the role of Cx. quinquefasciatus as a potential vector for ZIKV transmission in Thailand and may be useful in creating more effective mosquito vector control strategies in the future.
Introduction
Zika virus (ZIKV) is an arbovirus belonging to the Flaviviridae family and the Flavivirus genus, which includes African and Asian lineages1,2. ZIKV infection remains a serious public health threat, especially to pregnant women because of its close association with microcephaly and other severe neurological complications in the developing fetus3. In addition, ZIKV is also associated with Guillain-Barré syndrome (GBS)4. ZIKV in Uganda was first isolated from a febrile sentinel rhesus monkey in 1947 and from pooled specimens of Aedes africanus mosquitoes in 19485. ZIKV is primarily transmitted by Aedes mosquitoes4. Previous studies reported that Aedes mosquitoes such as Ae. africanus, Ae. aegypti, Ae. apicocoargenteus, Ae. furcifer, Ae. vittatus and Ae. luteocephalus are the principal vectors of ZIKV5–9. Diallo et al. (2014) identified several mosquito species as probable vectors of ZIKV in Southeastern Senegal, such as Ae. furcifer, Ae. luteocephalus, Ae. africanus, Ae. vittatus, Ae. taylori, Ae. dalzieli, Ae. hirsutus, Ae. metallicus, Ae. aegypti, Ae. unilineatus, Mansonia uniformis, Culex perfuscus and Anopheles coustani, using virus isolation and reverse transcription-polymerase chain reaction (RT-PCR) techniques10. In Southeast Asia, ZIKV was isolated from wild-caught Ae. aegypti in Malaysia11; moreover, Ae. aegypti12 and Ae. albopictus13 were reported as potential vectors of ZIKV transmission in Singapore. However, knowledge of the vectors of ZIKV transmission in Thailand is limited. In Thailand a total of 686 of confirmed Zika case had been reported between January and November 201614, by the Ministry of Public Health (MoPH), especially in Phitsanulok and Chanthaburi provinces where Cx. quinquefasciatus were found naturally ZIKV infections. In addition, the MoPH also reported the first two indigenous cases of ZIKV-related microcephaly in Thailand15. In 2018, the Bureau of Epidemiology (BoE) of the MoPH revealed that 306 Zika case were reported from 22 provinces in January-August 201816. Our previous studies, ZIKV RNA have been found in 1.85% female, 1.66% male, and 0.29% larva of Cx. quinquefasciatus mosquitoes collected from active ZIKV infected patients’ homes are infected with ZIKV in Thailand17. Therefore, in this study, we determined the potential for the Thai Cx. quinquefasciatus mosquito to vertically transmit ZIKV. The hemi-nested RT-PCR (hnRT-PCR) developed for this study is able to effectively detect ZIKV in mosquitoes. Information obtained from this study provides fundamental data regarding whether Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus are capable of vertically transmitting ZIKV in the laboratory. There are no vaccines or specific therapies against ZIKV. Data regarding ZIKV infection in these mosquitoes will be valuable in developing effective control strategies for ZIKV infection in Thailand.
Results
Vertical transmission of ZIKV in Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus
The mosquitoes were maintained and artificially fed with 1.7 × 105 florescent focus units (FFU)/ml of ZIKV in human blood. The experiments were performed in triplicate. Progeny of Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus exposed to ZIKV were reared to subsequent generations. ZIKV RNA was detected in each pooled generation of mosquitoes.
In field strains of Ae. aegypti, a total of 120 pool consisting of 2,400 F1 female adults and 30 pool consisting of 900 F1 male adults were tested in triplicate. We found that the male and female mosquitoes exposed to ZIKV can maintain the virus for only one generation and were showed infected at rates of 3.3% and 3.3%, respectively. In the laboratory strain, two pools of female F1 progeny were positive with infection rate of 1.7%, and ZIKV was not detected in the F1 generation of the male mosquito (Table 1). In both the laboratory and field strains of Ae. albopictus, the results showed that ZIKV was not detected in the offspring.
Table 1.
Percent infected via vertical transmission of ZIKV in each generation in both the field and laboratory strains of Ae. aegypti mosquitoes.
| Strain | Sex (n) per replicate |
Generation:F [Mean ± SD (% ZIKV infection)] | |
|---|---|---|---|
| F1 | F2 | ||
| Laboratory | Female (40) | 0.7 ± 1.2 (1.7) |
0.0 ± 0.0 (0) |
| Male (10) | 0.0 ± 0.0 (0) |
0.0 ± 0.0 (0) |
|
| Field | Female (40) | 1.3 ± 0.6 (3.3) |
0.0 ± 0.0 (0) |
| Male (10) | 0.3 ± 0.6 (3.3) |
0.0 ± 0.0 (0) |
|
In Cx. quinquefasciatus, ZIKV RNA could be detected until the F6 generation in females and the F2 generation in males. The F1 generation had the highest level of ZIKV infection (29.2%), which decreased to 24.2%, 8.3%, 7.5%, 5.8%, 0.83% and 0% in the F2 to F7 generations of female mosquitoes, respectively. However, in male mosquitoes, the transmission of ZIKV was only found by using hnRT-PCR until the F2 generation, decreasing from 20% in F1 to 16.7% in F2 and then undetectable levels in F3 (Table 2). The nucleotide sequences of all positive samples showed 99–100% similarity to the Zika virus/H. sapiens-tc/THA/2014/SV0127-14 (accession number KU681081) that was used to infect the F0 generation; this strain belongs to the Asian lineage of the ZIKV. The entire sequences were assigned GenBank numbers (GenBank: MK028538-MK028557).
Table 2.
Percent infected via vertical transmission of ZIKV in each generation in laboratory strain of Cx. quinquefasciatus mosquitoes.
| Sex (n) per replicate | Generation:F [Mean ± SD (% ZIKV infection)] | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| Female (40) | 12 ± 7.0 (29.2) | 9.7 ± 6.8 (24.2) |
3.3 ± 3.1 (8.3) | 3.0 ± 2.0 (7.5) |
2.3 ± 2.1 (5.8) |
0.3 ± 0.6 (0.83) | 0.0 ± 0.0 (0) |
| Male (10) | 2 ± 2 (20.0) |
1.7 ± 2.9 (16.7) |
0.0 ± 0.0 (0) |
0.0 ± 0.0 (0) |
0.0 ± 0.0 (0) |
0.0 ± 0.0 (0) |
0.0 ± 0.0 (0) |
Isolation of positive ZIKV from each generation of mosquitoes in C6/36 cells
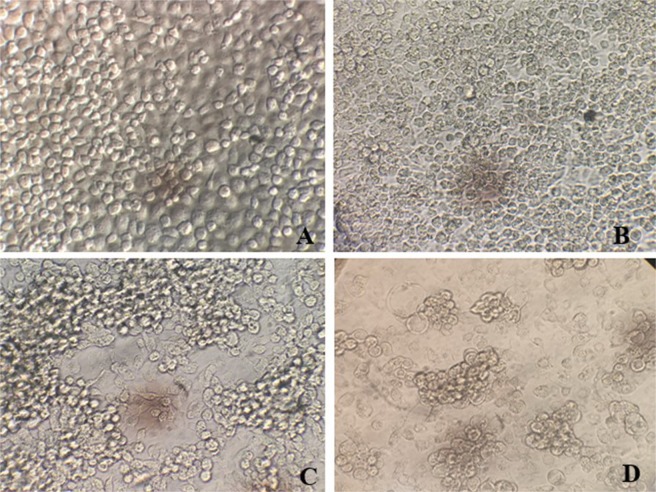
Samples that were positive for ZIKV RNA in each generation of mosquitoes were used to isolate the virus by inoculating C6/36 cells, and the morphological changes of the infected cells were compared with the morphology of uninfected cells (Fig. 1A). The ZIKV infection in C6/36 was monitored microscopically for cytopathic effects (CPEs) at days 3 (Fig. 1B), 5 (Fig. 1C) and 7 (Fig. 1D) after inoculation. CPEs were observed on days 5–7 post-ZIKV inoculation, and ZIKV RNA was detected by hnRT-PCR. The characteristic CPEs of ZIKV infection are a loss of the normal cell shape, cell rounding, multinucleated giant cells, nuclear vacuolization, and degeneration of the cells. The results of this study suggest that ZIKV from mosquitoes can replicate in C6/36 cells. Cx. quinquefasciatus is clearly suspected of being able to vertically transmit ZIKV.
Figure 1.
Cytopathic effects in C6/36 cells infected with ZIKV and not infected with ZIKV (A) and in C6/36 cells at postinfection day 3 (B), day 5 (C) and day 7 (D) under an inverted microscope (400x magnification).
Immunocytochemistry (ICC) staining of ZIKV in mosquitoes
The F0 generation of female Cx. quinquefasciatus mosquitoes that were infected with ZIKV were dissected 7 days postinfection to obtain their salivary glands, embryos, midguts, and heads. ZIKV antigens were detected in the salivary glands, midguts, yoke granules and facet cells of the eyes by ICC assay (Fig. 2). The dissection of the heads of the mosquitoes allowed investigation of the salivary glands, midguts and compound eyes. Positive staining for ZIKV is shown as distinct brown staining caused by the oxidation of 3,3′-diaminobenzidine (DAB) by horseradish peroxidase (HRP) within the organs of the mosquitoes (Fig. 2A). In the salivary glands, which are essential for transmission, ZIKV antigens were detected in the three lobes, especially in the distal lateral lobes, which play a major role in the blood feeding process (Fig. 2C). ZIKV can replicate in the midguts (Fig. 2E), yoke granules (Fig. 2G) and facet cells (Fig. 2I), all of which displayed localization of the ZIKV antigen-specific ZIKV-NS1 protein antibody, seen as brownish-red staining (Fig. 2A,C,E,G,I) compared with uninfected mosquitoes (Fig. 2B,D,F,H,J).
Figure 2.
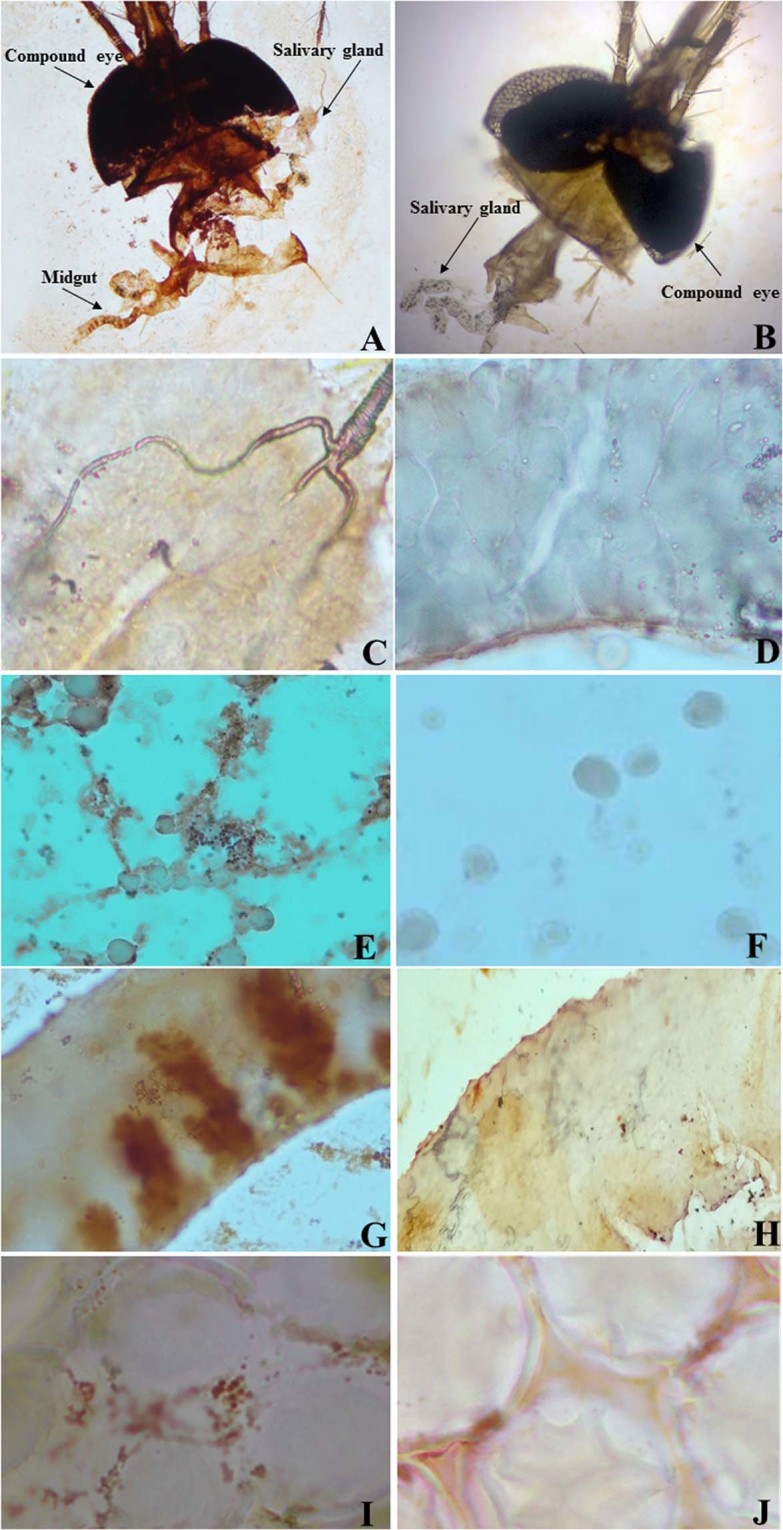
The ZIKV-infected F0 generation of Cx. quinquefasciatus mosquitoes at 7 days postinfection. The mosquitoes were infected via artificial blood feeding. Positive test results for the presence of the ZIKV antigen by using ICC staining of the positive head (A), negative head (B), positive salivary glands (C), negative salivary glands (D), positive midguts (E), negative midguts (F), positive yoke granules (G), negative yoke granules (H), positive facet cells (I) and negative facet cells (J).
ZIKV infection, dissemination and transmission rates in the F0 generations of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus mosquitoes
The ICC results in F0 mosquitoes were used to calculate the infection, dissemination and transmission rates in the F0 generations of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus mosquitoes. Mosquitoes that died before 7 days postinfection were excluded from the study. The Ae. aegypti laboratory strain and field strain had infection, dissemination and transmission rates of 88.2% and 90.5%, 71.1% and 67.4% and 60.8% and 60.0%, respectively. The Ae. albopictus laboratory strain and field strain had infection, dissemination and transmission rates of 53.9% and 51.0%, 41.8% and 42.0% and 21.8% and 22.0%, respectively. The Cx. quinquefasciatus laboratory strain had infection, dissemination and transmission rates of 87.5%, 72.4% and 63.3%, respectively (Table 3).
Table 3.
Rates of ZIKV infection, dissemination and transmission in the F0 generation of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus mosquitoes collected in Thailand.
| Strains | Mosquitoes tested | 7 dpi | ||
|---|---|---|---|---|
| Infection rate | Dissemination rate | transmission rates | ||
| Laboratory | Cx. quinquefasciatus | 98:112 (87.5%) | 71:98 (72.4%) | 62:98 (63.3%) |
| Ae. aegypti | 97:110 (88.2%) | 69:97 (71.1%) | 59:97 (60.8%) | |
| Ae. albopictus | 55:102 (53.9%) | 23:55 (41.8%) | 12:55 (21.8%) | |
| Field | Ae. aegypti | 95:105 (90.5%) | 64:95 (67.4%) | 57:95 (60.0%) |
| Ae. albopictus | 50:98 (51.0%) | 21:50 (42.0%) | 11:50 (22.0%) | |
Calculation of the filial infection rate (FIR) of ZIKV in progeny
The FIR of ZIKV in the progeny was tested using pooled populations of mosquitoes; these pools consisted of 2,400 and 900 filial female and male adult mosquitoes, respectively. To calculate the FIR, the total number of pools was divided by the number of positive pools. Each positive pool indicated that one or more of the filial progeny in the pool were infected with ZIKV. In this study, female Cx. quinquefasciatus mosquitoes had an FIR for ZIKV of 1:66 for F1, which decreased to 1:2,400 in F6. Male Cx. quinquefasciatus mosquitoes had an FIR for ZIKV of 1:150 for F1, which decreased to 1:180 for F2. In the Ae. aegypti laboratory strain, the female mosquitoes had an FIR for F1 of 1:1,200, while the Ae. aegypti field strain female and male mosquitoes had FIRs for F1 of 1:600 and 1:900, respectively. The FIR results for ZIKV infection for the progeny are shown in Table 4.
Table 4.
Filial infection rate (FIR) of ZIKV in the progeny of Ae. aegypti and Cx. quinquefasciatus mosquitoes.
| Strains | Mosquitoes tested | Sex | Filial infection rate (s) | |||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |||
| Laboratory | Cx. quinquefasciatus | Female | 1:66 | 1:83 | 1:240 | 1:267 | 1:345 | 1:2,400 |
| Male | 1:150 | 1:180 | — | — | — | — | ||
| Ae. aegypti | Female | 1:1,200 | — | — | — | — | — | |
| Male | — | — | — | — | — | — | ||
| Field | Ae. aegypti | Female | 1:600 | — | — | — | — | — |
| Male | 1:900 | — | — | — | — | — | ||
Discussion
Our results demonstrated that vertical transmission of ZIKV occurs in the Cx. quinquefasciatus mosquito. The study was based on a molecular technique (hnRT-PCR) for ZIKV RNA detection, the isolation of viruses in cell culture and an ICC assay for the localization of a ZIKV-specific antigen. Culex mosquito species have been found to be able to vertically transmit West Nile (WNV)18 and Japanese encephalitis (JE)19 viruses. The role of the vertical transmission of ZIKV is still under investigation. Several reports have suggested that ZIKV could be transovarially transmitted to progeny in both laboratory and field experiments. Guo et al. (2016) revealed that Cx. pipiens quinquefasciatus clearly demonstrates the potential to be a vector for ZIKV in Southern China20. The Cx. quinquefasciatus laboratory colonies had detectable ZIKV in the midgut and salivary glands after artificial blood feeding with ZIKV; moreover, field-caught Cx. quinquefasciatus tested positive for ZIKV by RT-qPCR in Brazil21. In addition, Cx. quinquefasciatus had detectable ZIKV in the salivary glands at 7 and 15 days postfeeding in Northeast Brazil22.
In contrast, several reports have shown evidence of a lack of competence of Culex species for ZIKV23,24. For example, ZIKV isolated from Cx. pipiens and Cx. quinquefasciatus mosquitoes from the United States were unable to replicate, as determined by a plaque assay25,26. Similar reports showed that C. pipiens and other Culex species from Brazil27, Germany28, Tunisia29, Italy30 and Australia31 had no detectable ZIKV transmission. Factors that affect the competence of mosquito vectors include the following. (1) Differences in geographic regions mean that mosquito colonies from various areas have unique genetic backgrounds; therefore, mosquitoes collected from different areas may have varying degrees of viral competence32–34. This genetic variation may affect the morphology of mosquito organs and processes that are involved in virus replication and dissemination, such as mosquito immune responses, small RNA-based interferon (RNAi) pathways35–37 and the midgut and salivary gland barriers29,38. (2) Mosquitoes collected from different geographic regions also have different microbiomes and microviromes39. Microbiomes have been shown to interfere with viral replication in mosquito vectors; therefore, different microbiomes would affect the competency of the mosquitoes for ZIKV40–42. With regard to intracellular bacteria in mosquitoes, Wolbachia is another factor that affects viral replication in mosquitoes. A novel strategy to interfere with arbovirus transmission in mosquitoes using Wolbachia pipientis (wPip) has been proposed43,44. However, a study by Lourenço-de-Oliveira, et al. (2018) showed that no ZIKV was found in Cx. quinquefasciatus lines whether or not they contained Wolbachia38. The effects of the microbiome and Wolbachia in Cx. quinquefasciatus lines in Thailand could be investigated in the future. (3) The differences in the ZIKV strains used for the experiments, including the genotype, titer, and number of passages, could affect the ability of the ZIKV to enter mosquito organs, resulting in differences in replication and dissemination in the mosquitoes32–44. (4) The techniques used in the experiments, such as the mode of ZIKV infection of the mosquitoes (oral, intrathoracic) and the ZIKV detection method (plague assay, molecular techniques, immunological techniques), which have different sensitivities and specificities32–44, might also result in different findings.
This study is the first report of vertically transmitted ZIKV in Cx. quinquefasciatus and Ae. aegypti mosquitoes in Thailand. We also successfully isolated infectious ZIKV from a C6/36 cell line that had been infected with ZIKV from Cx. quinquefasciatus and and Ae. aegypti. Cx. quinquefasciatus mosquitoes are widely distributed in tropical and subtropical areas45. In Thailand, there have been reports of JE virus isolation from Cx. quinquefasciatus using C6/36 cells46. However, ZIKV in Cx. quinquefasciatus has never been studied in Thailand. In this study, we investigated the localization of ZIKV antigens in the organs of mosquitoes by ICC. The salivary gland, midguts, yoke granules and facet cells showed a reaction between the ZIKV antigen and its specific antibody. The salivary glands of mosquitoes play an important role in the transmission of pathogens and an essential role in the transmission cycle47. This study demonstrated that ZIKV also infects the salivary glands and midguts; therefore, we hypothesized that ZIKV was replicating in the salivary glands and midguts at 7 days postinfection. Several reports have shown the presence of ZIKV in the salivary glands and midguts of female mosquitoes such as Ae. aegypti48, Ae. albopictus49, Cx. quinquefasciatus21, Cx. coronator, and Cx. tarsalis50. The ICC assay was first performed on the facet cells and yoke granules of mosquitoes orally infected with ZIKV. The facet cells of the mosquito eyes showed brownish-red staining in the ICC assay. The ZIKV infection, dissemination and transmission rates in the F0 generations of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus mosquitoes were calculated as shown in Table 3. Based on these results, there were no differences in the infection, dissemination or transmission rates in the F0 generations of the laboratory and field strains in both Ae. aegypti and Ae. albopictus. However, Ae. aegypti has higher infection, dissemination and transmission rates than Ae. albopictus, which is a similar finding to that in a recent report by Liu et al. (2017). High infection, dissemination and transmission rates in Ae. aegypti were also reported by Liu et al. (2017) and Main et al.24,51. In this study, we found lower infection, dissemination and transmission rates in Ae. albopictus than in Ae. aegypti, which is similar to the results of the study by Liu et al. (2017). Interestingly, the infection, dissemination and transmission rates of ZIKV in Cx. quinquefasciatus mosquitoes were higher in this study than in previous reports24,51. As discussed previously, several factors could affect the competency of mosquitoes for ZIKV, and future studies should be conducted to investigate the factors influencing these results. However, in this study we determined the transmission rates by examining virus infection in salivary glands, not in the expectorated saliva; therefore, the transmission rates in this study may over estimated. Further investigation for transmission rate in expectorated saliva should be perform for obtaining more accurate data.
The FIRs for ZIKV in the F1 generation of Ae. aegypti in both the laboratory and field strains in this study were lower than those in the previous reports by Thangamani et al.52 and Ciota et al.53, which had FIRs of 1:290 and 1:84, respectively. However, for Cx. quinquefasciatus mosquitoes, our results showed that the FIR for ZIKV in the F1 generation was high (1:66) and that it decreased to 1:2,400 in the F6 generation. The high FIR for ZIKV is related to the vertical transmission phenomenon.
The results of this study provide more information about the transmission dynamics of ZIKV in mosquitoes and could be used to explain the natural pathogenesis of ZIKV infection in wild mosquitoes. The presence of ZIKV antigens in the yoke granules may be associated with the vertical transmission of ZIKV, as ZIKV may infect the germinal tissues of the female mosquito and may occur in the fully formed egg during oviposition54,55. However, the mechanism of transovarian transmission is still unclear. ZIKV detected in the facet cells could imply that ZIKV also infects the nervous system organs of the mosquitoes.
Unlike Cx. quinquefasciatus, Ae. aegypti had detectable ZIKV in only the F1 generation, and ZIKV was not detected in the F2 generation in either the field strain or the laboratory strain (Table 1). ZIKV was detected in only the F0 generation of Ae. albopictus mosquitoes in both the field and laboratory strains. Vertical transmission of ZIKV in Ae. aegypti mosquitoes was reported by Thangamani et al. (2016); they found that ZIKV was transmitted to the F1 generation. The current study also demonstrated that ZIKV was not found in any F1 Ae. albopictus52. We therefore conclude that vertical transmission occurred in both Cx. quinquefasciatus and Ae. aegypti and not in Ae. albopictus. Further research should be conducted to explore the factors that might affect these results.
Cx. quinquefasciatus prefers to feed on animal blood, and some studies have suggested that the blood found in Cx. quinquefasciatus is 50% human, 32% bird, 12% dog, and less than 3% cat56. In Thailand, Cx. quinquefasciatus is found in urban and suburban areas. Feeding behavior studies in Koh Chang, Thailand showed that only 0.98% of the blood meals ingested by Cx. quinquefasciatus were human57. Data regarding the vertical transmission of ZIKV obtained from this study together with the feeding pattern of Cx. quinquefasciatus in Thailand indicate that Cx. quinquefasciatus mosquitoes may be a potential vector for ZIKV transmission in Thailand. Therefore, vector control strategies for addressing ZIKV outbreaks by managing mosquitoes should not focus only on Aedes mosquitoes; in particular, the larval control strategies should also focus on the breeding sites of Cx. quinquefasciatus. The development of vector control measures for ZIKV outbreaks in Thailand should consider both Aedes and Culex mosquitoes.
Materials and Methods
Ethics statement
The study was approved by the animal research ethics committee of Chulalongkorn University and adhered to the Animal Care and Use Protocol (CU-ACUP). The Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (COA No. 023/2560) approved this study, which abided by the Animals for Scientific Purposes Act and all relevant institutional policies and regulations regarding animal care and use at Chulalongkorn University. The use of hazardous agents was only initiated after approval from the institutional animal care and use committee (IACUC), Institutional Biosafety Committee (IBC), and/or Environmental Health and Safety Department. The use of human blood was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (COA No. 724/2015), and the study was conducted in compliance with the international guidelines for human research protection as stated in the Declaration of Helsinki, The Belmont Report, the Council for International Organizations of Medical Sciences (CIOMS) guidelines and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP).
Mosquitoes
Laboratory and field colonies of Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus mosquitoes were maintained in the Biology and Ecology Laboratory of the National Institute of Health (NIH), Department of Medical Sciences, Nonthaburi Province, Thailand, under standard conditions as follows: 28 ± 2 °C, 65–85% relative humidity, and a 12/12-hour light/dark cycle. These mosquitoes were originally obtained from eggs laid in Nonthaburi Province in Central Thailand in 2007. The populations of Cx. quinquefasciatus, Ae. aegypti and Ae. albopictus had been reared for 274, 232 and 215 generations, respectively. Field strains of Ae. aegypti and Ae. albopictus were obtained from eggs laid in Nonthaburi Province, and the F4 of those lines were used in this study. Adult mosquitoes were maintained ad libitum with a mixture of 5% sucrose and 5% vitamin B complex (w/v), while larvae were maintained in plastic trays and fed on minced commercial mouse food until reaching the pupal stage.
Virus strain
The virus was provided by the National Science and Technology Development Agency of Thailand. This strain was named Zika virus/H. sapiens-tc/THA/2014/SV0127-14, and it was isolated from the blood of a patient in Thailand in 2014 and used to infect the Toxorhynchites splendens mosquito (1 passage). Following isolation, the virus was passed once in Ae. albopictus C6/36 cells (1 passage). This strain is from an Asian lineage. The complete genome is accession number KU681081. Viral stocks were then produced in C6/36 cells and stored at −80 °C until further use.
Mosquito infection
Prior to artificial oral infection, the viral titer of the virus stock was calculated by fluorescent focus assay in C6/36 cells and was determined to be 1.7 × 106 FFU/ml. Four- to five-day-old female Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus mosquitoes were deprived of food for 24 hours prior to being provided with a blood meal. Starved females were fed with expired human blood from deidentified donors58, which was obtained from the National Blood Center, Thai Red Cross Society, Bangkok, Thailand, with ZIKV added at a concentration of 1.7 × 105 FFU/ml; the mosquitoes were fed via artificial blood feeding. The female mosquitoes were allowed to feed for 45 minutes. Non-engorged females were removed, and engorged females were reared in a cage and maintained with a 5% sucrose and 5% vitamin B complex (w/v). Three days after receiving the blood meal, water containing a black plastic bow was placed into the mosquito cage for 3 days for oviposition. Mosquitoes were collected for virus detection 7 days after blood feeding (F0). Eggs were collected and allowed to hatch in a plastic tray. Larvae were reared to the pupal stage and then adulthood to obtain the subsequent progeny (F) and for the detection of ZIKV. Starting with the F1 generation, the mosquitoes were fed blood without ZIKV under the same conditions described previously.
Viral detection in mosquitoes
There were forty pools per replicate of female (20 adults/pool) and ten pools per replicate of male (30 adults/pool) Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus mosquitoes. The experiments were performed in triplicate. Each pool was ground in 1X phosphate-buffered saline (PBS) and centrifuged at 14,000 g at 4 °C for 10 minutes. The supernatant was transferred to minimum essential media (MEM) (Gibco, US) containing 2% fetal bovine serum (FBS) (Gibco, US) and maintained at −80 °C. Viral RNA was extracted from the pellets of the pooled mosquito samples, ground in a lysis solution (provided with the kit) and centrifuged; then, the supernatant was processed for RNA extraction using the Invisorb Spin Virus RNA Mini Kit (Invitec GmbH, Germany). The viral RNA samples from the mosquitoes were amplified to test for ZIKV infection by hnRT-PCR. Primers for the NS5 gene were modified from Moureau et al.59. The RT-PCR amplification reaction was set up in a final volume of 25 μl using the Superscript III One-Step RT-PCR kit, and the nested PCR was performed with 2 μl from the first reaction and 1 unit of Taq DNA polymerase (Fermentas, USA). Ae. aegypti, Ae. albopictus and Cx. quinquefasciatus mosquitoes that had fed on human blood without ZIKV were used as negative controls. The PCR products were analyzed via 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized with Quantity One Quantification Analysis Software version 4.5.2 (Gel DocEQ System; Bio-Rad, Hercules, CA). The positive PCR products were recovered from the gel and purified using the Agarose Gel DNA Purification Kit: Invisorb Fragment CleanUp (STRATEC Molecular GmbH, Germany) following the manufacturer’s instructions. The purified DNA was sent to Macrogen, Inc. (Macrogen, South Korea) for direct DNA sequencing for confirmation of the identification of ZIKV.
ZIKV isolation and propagation
The supernatants of the samples that tested positive by hnRT-PCR were filtered through a 0.2 µm sieve and spread in a 12-well plate with a monolayer of Ae. albopictus C6/36 cells (ATCC CRL-1660) for 1 hour. After discarding the supernatant and refreshing with 2 ml of MEM (Gibco, US) containing 10% FBS (Gibco, US), 1% penicillin (100 U/ml; Sigma-Aldrich, US), and streptomycin (100 μg/ml; Sigma-Aldrich, US) (P/S), the cells were maintained at 37 °C with 5% CO2. The cultures were incubated for 7 days. CPEs were checked every 24 hours for 7 days after the initial 24-hour incubation period, with observations made under an inverted microscope (Olympus, Japan).
Mosquito salivary glands, yoke granules and eye samples
The salivary glands, midguts, yoke granules and eyes were collected at 7 days postinfection from females orally exposed to ZIKV. Anesthetized individual mosquitoes (30–40 mosquitoes per replicate) were dissected in a drop of 1X PBS on a glass slide under a stereomicroscope (Olympus, Japan). The salivary glands, embryos and head were transferred onto SuperFrost Plus microscope slides (Thermo Scientific, USA).
ICC staining
The salivary glands, embryos and heads of infected and uninfected mosquitoes were transferred to SuperFrost Plus microscope slides (Thermo Scientific, USA), which were then air dried and fixed in 100% cold acetone before being rehydrated in graded absolute ethanol. The slides were stained with the primary rabbit-Zika virus NS1 protein antibody (GeneTex, USA) and the HRP-conjugated anti-rabbit IgG secondary antibody (Abcam, MA). The color was developed using DAB and counterstained with hematoxylin (Dako, CA), and the specimens were then examined under a light microscope (Olympus, Japan) at 100X magnification.
Acknowledgements
This study was supported by National Science and Technology Development Agency (Thailand) (Grant No. P-16-50702), the Research Chair Grant and National Research Council of Thailand, Health Systems Research Institute (Grant No. 61-003), Rachadapisek Sompote Fund (RA/MF 2/62), and the World Health Organization Thailand (WHO-Thailand), and the United States Agency for International Development (USAID) grant.
Author Contributions
Conceived and designed the experiments: A.P., J.C. and P.S. Performed the experiments: A.P., J.C., P.I., R.B. and Y.J. Analysed the data: A.P. and P.S. Contributed reagents/materials/analysis tools: S.W., S.B., A.T. and U.T. Wrote the paper: A.P. and P.S.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Musso D, Gubler DJ. Zika virus. Clin. Microbiol. Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang A, et al. Zika Virus Genome Biology and Molecular Pathogenesis. Emerg. Microbes. Infect. 2017;6(3):e13. doi: 10.1038/emi.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrs C, et al. Zika Virus and Pregnancy: A Review of the Literature and Clinical Considerations. Am. J. Perinatol. 2016;33(7):625–639. doi: 10.1055/s-0036-1580089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parra B, et al. Guillain-Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016;375(16):1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 5.Haddow AJ, et al. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda Forest. Bull. World. Health. Organ. 1964;31:57–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Lanciotti RS, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerging. Infect. Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans. R. Soc. Trop. Med. Hyg. 1958;52:263–268. doi: 10.1016/0035-9203(58)90085-3. [DOI] [PubMed] [Google Scholar]
- 8.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J. Hyg. (Lond) 1979;83:213–219. doi: 10.1017/S0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans. R. Soc. Trop. Med. Hyg. 1982;76:552–562. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 10.Diallo D, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS. One. 2014;9:e109442. doi: 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 12.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS. Negl. Trop. Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong PS, et al. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS. Negl. Trop. Dis. 2013;7(8):e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ProMed-mail. PRO/MBDS > Zika virus-Thailand (09) [Accessed 4 May. Available at, https://www.promedmail.org/post/4631824 (2018).
- 15.World Health Organization (WHO) Emergencies: situation report- Zika virus microcephaly Guillain-Barré syndrome. Available at, http://www.who.int/emergencies/zika-virus/situation-report/6-october-2016/en/ [Accessed 4 May, 2018].
- 16.Weekly Epidemiological Surveillance Report, Thailand. Available at, http://www.wesr.moph.go.th/wesr_new/ [Accessed 30 October, 2017].
- 17.Phumee, A. et al. Molecular epidemiology and genetic diversity of Zika virus from field-caught mosquitoes in various regions of Thailand. Pathogens. 8(1), 30, 10.3390/pathogens8010030 (2019). [DOI] [PMC free article] [PubMed]
- 18.Baqar S, Hayes CG, Murphy JR, Watts DM. Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. Am. J. Trop. Med. Hyg. 1993;48:757–762. doi: 10.4269/ajtmh.1993.48.757. [DOI] [PubMed] [Google Scholar]
- 19.Rosen L, Lien JC, Shroyer DA, Baker RH, Lu LC. Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am. J. Trop. Med. Hyg. 1989;40:548–556. doi: 10.4269/ajtmh.1989.40.548. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, et al. Culex pipiens quinquefasciatus: a potential vector to transmit Zika virus. Emerg. Microbes. Infect. 2016;5(9):e102. doi: 10.1038/emi.2016.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guedes DR, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes. Infect. 2017;6(8):e69. doi: 10.1038/emi.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franca RFO, Neves MHL, Ayres CFJ, Melo-Neto OP, Filho SPB. First International Workshop on Zika Virus held by Oswaldo Cruz Foundation FIOCRUZ in Northeast Brazil March 2016-a meeting report. PLoS. Negl. Trop. Dis. 2016;10:e0004760. doi: 10.1371/journal.pntd.0004760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epelboin Y, Talaga S, Epelboin L, Dusfour I. Zika virus: An updated review of competent or naturally infected mosquitoes. PLoS. Negl. Trop. Dis. 2017;11(11):e0005933. doi: 10.1371/journal.pntd.0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, et al. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus Mosquitoes as Zika Virus Vectors, China. Emerg. Infect. Dis. 2017;23(7):1085–1091. doi: 10.3201/eid2307.161528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aliota MT, Peinado SA, Osorio JE, Bartholomay LC. Culex pipiens and Aedes triseriatus Mosquito Susceptibility to Zika Virus. Emerging. Infect. Dis. 2016;22(10):1857–1859. doi: 10.3201/eid2210.161082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenney JL, et al. Transmission Incompetence of Culex quinquefasciatus and Culex pipiens pipiens from North America for Zika Virus. Am. J. Trop. Med. Hyg. 2017;96(5):1235–1240. doi: 10.4269/ajtmh.16-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes RS, et al. Culex quinquefasciatus from Rio de Janeiro Is Not Competent to Transmit the Local Zika Virus. PLoS. Negl. Trop. Dis. 2016;10(9):e0004993. doi: 10.1371/journal.pntd.0004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heitmann A, et al. Experimental Transmission of Zika Virus by Mosquitoes from Central Europe. Euro. Surveill. 2017;22(2):30437. doi: 10.2807/1560-7917.ES.2017.22.2.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amraoui, F. et al. Culex mosquitoes are experimentally unable to transmit Zika virus. Euro. Surveill. 21(35), 10.2807/1560-7917.ES.2016.21.35.30333 (2016). [DOI] [PMC free article] [PubMed]
- 30.Boccolini, D. et al. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Euro. Surveill. 21(35), 10.2807/1560-7917.ES.2016.21.35.30328 (2016). [DOI] [PMC free article] [PubMed]
- 31.Duchemin JB, et al. Zika vector transmission risk in temperate Australia: a vector competence study. Virol. J. 2017;14:108. doi: 10.1186/s12985-017-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodson BL, Rasgon JL. Vector competence of Anopheles and Culex mosquitoes for Zika virus. PeerJ. 2017;5:e3096. doi: 10.7717/peerj.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambrechts L. Quantitative genetics of Aedes aegypti vector competence for dengue viruses: Towards a new paradigm? Trends. Parasitol. 2011;27(3):111–114. doi: 10.1016/j.pt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Tabachnick WJ. Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int. J. Environ. Res. Public. Health. 2013;10(1):249–277. doi: 10.3390/ijerph10010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson KE, Blair CD. Arbovirus-mosquito interactions: RNAi pathway. Curr. Opin. Virol. 2015;15:119–126. doi: 10.1016/j.coviro.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair CD, Olson KE. The role of RNA interference (RNAi) in arbovirus-vector interactions. Viruses. 2015;7:820–843. doi: 10.3390/v7020820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donald CL, Kohl A, Schnettler E. New insights into control of arbovirus replication and spread by insect RNA interference pathways. Insects. 2012;3:511–531. doi: 10.3390/insects3020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lourenço-de-Oliveira R, et al. Culex quinquefasciatus mosquitoes do not support replication of Zika virus. J. Gen. Virol. 2018;99(2):258–264. doi: 10.1099/jgv.0.000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart CE, et al. Zika virus vector competency of mosquitoes, Gulf Coast, United States. Emerg. Infect. Dis. 2017;23(3):559–560. doi: 10.3201/eid2303.161636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saldaña MA, Hegde S, Hughes GL. Microbial control of arthropod-borne disease. Mem. Inst. Oswaldo. Cruz. 2017;112(2):81–93. doi: 10.1590/0074-02760160373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guégan M, et al. The Mosquito Holobiont: Fresh Insight into Mosquito-Microbiota Interactions. Microbiome. 2018;6:49. doi: 10.1186/s40168-018-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alfonso-Parra C, Avila FW. Molecular Responses to the Zika Virus in Mosquitoes. Pathogens. 2018;7(2):49. doi: 10.3390/pathogens7020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mishra N, Shrivastava NK, Nayak A, Singh H. Wolbachia: A prospective solution to mosquito borne diseases. Int. J. Mosq. Res. 2018;5:2. [Google Scholar]
- 44.Huang YS, Higgs S, Vanlandingham DL. Biological control strategies for mosquito vectors of arboviruses. Insects. 2017;8:21. doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gokhale MD, Paingankar MS, Dhaigude SD. Comparison of Biological Attributes of Culex quinquefasciatus (Diptera: Culicidae) Populations from India. ISRN. Entomology. 2013;2013:1–9. doi: 10.1155/2013/451592. [DOI] [Google Scholar]
- 46.Nitatpattana N, et al. First isolation of Japanese encephalitis from Culex quinquefasciatus in Thailand. Southeast. Asian. J. Trop. Med. Public. Health. 2005;36(4):875–878. [PubMed] [Google Scholar]
- 47.Ribeiro J, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6. doi: 10.1186/1471-2164-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa-da-Silva AL, et al. Laboratory strains of Aedes aegypti are competent to Brazilian Zika virus. PloS. One. 2017;12:e0171951. doi: 10.1371/journal.pone.0171951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chouin-Carneiro T, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS. Negl. Trop. Dis. 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elizondo-Quiroga D, et al. Zika Virus in Salivary Glands of Five Different Species of Wild-Caught Mosquitoes from Mexico. Sci. Rep. 2018;8(1):809. doi: 10.1038/s41598-017-18682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Main BJ, et al. Vector competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika virus. PLoS. Negl. Trop. Dis. 2018;12(6):e0006524. doi: 10.1371/journal.pntd.0006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thangamani S, Huang J, Hart CE, Guzman H, Tesh RB. Vertical Transmission of Zika Virus in Aedes aegypti Mosquitoes. Am. J. Trop. Med. Hyg. 2016;95(5):1169–1173. doi: 10.4269/ajtmh.16-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciota AT, Bialosuknia SM, Ehrbar DJ, Kramer LD. Vertical Transmission of Zika Virus by Aedes aegypti and Ae. albopictus Mosquitoes. Emerg. Infect. Dis. 2017;23:880–882. doi: 10.3201/eid2305.162041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosen L. Further observations on the mechanism of vertical transmission of flaviviruses by Aedes mosquitoes. Am. J. Trop. Med. Hyg. 1988;39:123–126. doi: 10.4269/ajtmh.1988.39.123. [DOI] [PubMed] [Google Scholar]
- 55.Lequime S, Lambrechts L. Vertical transmission of arboviruses in mosquitoes: A historical perspective. Infect. Genet. Evol. 2014;28:681–690. doi: 10.1016/j.meegid.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: Blood meal analysis indicates feeding on both humans and birds. J. Insect. Sci. 2004;4:20. doi: 10.1093/jis/4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khaklang S, Kittayapong P. Species composition and blood meal analysis of mosquitoes collected from a tourist island, Koh Chang, Thailand. J. Vector. Ecol. 2014;39(2):448–452. doi: 10.1111/jvec.12122. [DOI] [PubMed] [Google Scholar]
- 58.Pothikasikorn J, Boonplueang R, Suebsaeng C, Khaengraeng R, Chareonviriyaphap T. Feeding response of Aedes aegypti and Anopheles dirus (Diptera: Culicidae) using out-of-date human blood in a membrane feeding apparatus. J. Vector. Ecol. 2010;35(1):149–155. doi: 10.1111/j.1948-7134.2010.00041.x. [DOI] [PubMed] [Google Scholar]
- 59.Moureau G, et al. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector. Borne. Zoonotic. Dis. 2007;7(4):467–477. doi: 10.1089/vbz.2007.0206. [DOI] [PubMed] [Google Scholar]