Abstract
Four isoforms of serine/threonine phosphatase type I, PP1α, PP1β, PP1γ1, and PP1γ2, are derived from three genes. The PP1γ1 and PP1γ2 isoforms are alternately spliced transcripts of the protein phosphatase 1 catalytic subunit gamma gene (Ppp1cc). While PP1γ1 is ubiquitous in somatic cells, PP1γ2 is expressed exclusively in testicular germ cells and sperm. Ppp1cc knockout male mice (–/–), lacking both PP1γ1 and PP1γ2, are sterile due to impaired sperm morphogenesis. Fertility and normal sperm function can be restored by transgenic expression of PP1γ2 alone in testis of Ppp1cc (–/–) mice. The purpose of this study was to determine whether the PP1γ1 isoform is functionally equivalent to PP1γ2 in supporting spermatogenesis and male fertility. Significant levels of transgenic PP1γ1 expression occurred only when the transgene lacked a 1-kb 3΄UTR region immediately following the stop codon of the PP1γ1 transcript. PP1γ1 was also incorporated into sperm at levels comparable to PP1γ2 in sperm from wild-type mice. Spermatogenesis was restored in mice expressing PP1γ1 in the absence of PP1γ2. However, males from the transgenic rescue lines were subfertile. Sperm from the PP1γ1 rescue mice were unable to fertilize eggs in vitro. Intrasperm localization of PP1γ1 and the association of the protein regulators of the phosphatase were altered in epididymal sperm in transgenic PP1γ1 compared to PP1γ2. Thus, the ubiquitous isoform PP1γ1, not normally expressed in differentiating germ cells, could replace PP1γ2 to support spermatogenesis and spermiation. However, PP1γ2, which is the PP1 isoform in mammalian sperm, has an isoform-specific role in supporting normal sperm function and fertility.
Keywords: protein phosphatases, spermatogenesis, male fertility, transgenic rescue
PP1γ1 can replace PP1γ2 in testis.
INTRODUCTION
Protein kinases and phosphatases play key roles in the regulation of reproductive cells and gametes. The protein phosphatase 1 (PP1), a serine/threonine phosphatase expressed in nearly every tissue type, is important in mammalian spermatogenesis and sperm function. In mammals PP1 has four isoforms encoded by three genes: PP1α, PP1β, PP1γ1, and PP1γ2 [1]. PP1 isoforms are highly homologous except for their extreme N- and C-termini. PP1 functions in the form of a multimeric enzyme associated with several regulatory subunits. Regulatory proteins define the subcellular localization of the enzyme and catalytic activity against its substrates. More than 60 mammalian PP1 regulatory proteins have been identified [2]. PP1α, PP1β, and PP1γ1 are expressed in all somatic cells. All PP1 isoforms are capable of dephosphorylating the same substrates and their catalytic activities are indistinguishable in vitro. Early attempts to study the isoform-specific roles of different PP1 enzymes through targeted gene deletion revealed that the global loss of PP1β leads to embryonic lethality and the global knockout of PP1α had no observable phenotype. Tissue-specific knockout of PP1β affects cardiac contractility [3]. Similarly, in Drosophila loss of a PP1 isoform resembling mammalian PP1β affects muscle development and function [4]. A gene replacement strategy was utilized to replace PP1 in yeast Saccharomyces cerevisiae with human PP1 isoforms. This study revealed that any mammalian PP1 isoform could support growth and viability of yeast lacking its endogenous PP1, Glc7 [5]. Thus, except for PP1β which appears to have an isoform-specific requirement in cardiac and muscle development, in most contexts, the PP1 isoforms are interchangeable and functionally equivalent both in vivo and in vitro.
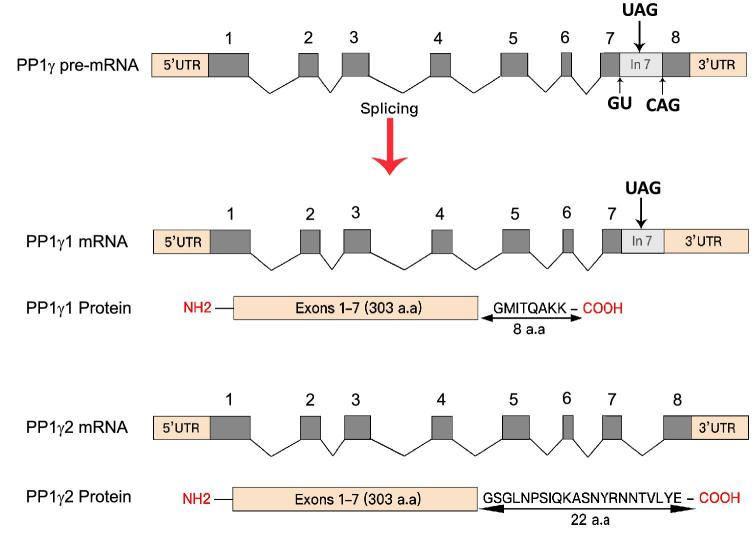
The PP1γ2 isoform, produced by alternative splicing of the protein phosphatase catalytic subunit gamma gene (Ppp1cc) transcript (Figure 1), is the only PP1 isoform expressed in meiotic and postmeiotic germ cells in testis and in spermatozoa. The other three PP1 isoforms, PP1α, PP1β, and PP1γ1, are present in testicular somatic cells; Sertoli and Leydig, and early germ cells; spermatogonia and primary spermatocytes [6, 7]. The only or the predominant PP1 isoform present in sperm is PP1γ2. PP1γ1 and PP1γ2, identical except for a 22-amino-acid C-terminus end in PP1γ2, which replaces a corresponding 7-amino-acid segment of PP1γ1, are expected to be biochemically equivalent. It is noteworthy that PP1γ2 is present only in mammals: sperm from other species contain one of the other PP1 isoforms [6]. The reasons for the preference for the PP1γ2 isoform in mammalian testis and sperm are unknown.
Figure 1.
Schematic of the generation of the two PP1γ isoforms. The Ppp1cc pre-mRNA of approximately 16.91 kb constitutes 5΄ UTR, 3΄ UTR, 8 exons, and 7 introns. The PP1γ1 mature mRNA of approximately 2.3 kb is generated by splicing of introns 1 through 6. Intron 7 is retained in the mature transcript along with exon 8 as a part of its 3΄ UTR. It encodes a protein containing 303 amino acids derived from the 7 exons and 8 amino acids from the extended exon 7 (i.e. initial part of intron 7). In the male meiotic and postmeiotic germ cells, the introns 1–6 along with the 1.1-kb long intron 7 are spliced out, thus producing a shorter PP1γ2 transcript of approximately 1.7 kb. The exon 8 gives PP1γ2 its unique 22 amino acid C-terminus. PP1γ1 and PP1γ2 proteins are identical in structure and their catalytic domain except for the extra C-terminus tail in PP1γ2.
Females lacking the Ppp1cc gene are normal and fertile; however, male mice lacking both products of the Ppp1cc gene, PP1γ1 and PP1γ2, are sterile. Mice lacking Ppp1cc have a negligible concentration of sperm in the epididymis. The few testicular sperm cells bear several morphological abnormalities [8–10]. Similarly, mice with a selective knockout of Ppp1cc in developing male germ cells have the same abnormal phenotype as the global Ppp1cc knockout mice. Thus, Ppp1cc is required only in developing spermatocytes and spermatids for normal spermatogenesis and male fertility. Because Ppp1cc is responsible for both PP1γ1 and PP1γ2, these studies do not show if one or both these isoforms PP1γ are required for spermatogenesis and normal sperm function. Recently, we showed that transgenic expression of PP1γ2 alone in testis of Ppp1cc null mice restores spermatogenesis, normal sperm function, and male fertility. Thus, males are normal and fertile in the global absence of both PP1γ1 and PP1γ2 provided PP1γ2 is present in spermatocytes and spermatids [7]. The ability to direct expression of PP1γ2 exclusively in developing germ cells provided an opportunity to test whether PP1γ1, not normally expressed meiotic and postmeiotic germ cells, can functionally replace PP1γ2. Because PP1γ1 is essentially identical to PP1γ2, it is intriguing that PP1γ2 is the predominant if not the exclusive isoform in mammalian sperm. The goal of this study was to determine if PP1γ1 could substitute for PP1γ2 in developing germ cells and restore fertility in Ppp1cc (–/–) infertile males. In order to test this, we generated Ppp1cc (–/–) knockout mice engineered to transgenically express PP1γ1 in testicular germ cells. Our results show that PP1γ1 can replace PP1γ2 to support completion of spermatogenesis and spermiation. However, PP1γ1 does not substitute for PP1γ2 to support optimal sperm function and fertility. Transgenic expression of PP1γ1 in Ppp1cc (–/–) knockout mouse was comparable to PP1γ2 in wild-type sperm and sperm from PP1γ2 transgenic rescue mice. However, subcellular localization, distribution, and the association of the regulators of PP1γ1 compared to PP1γ2 were altered in sperm. Sperm bearing PP1γ1 have altered motility with significantly attenuated flagellar beat amplitude. Mature caudal epididymal sperm from transgenic mice had low ATP levels compared to normal wild-type sperm. Sperm containing PP1γ1 could not sustain normal levels of ATP even in the presence of energy substrates glucose and lactate and their motility declined during this incubation. Male mice expressing PP1γ1 in developing germ cells were subfertile when bred. Sperm obtained from mice expressing PP1γ1 could not fertilize eggs in vitro. Our data show that PP1γ1 is unable to substitute for PP1γ2 in sperm to support normal function and fertility but can support spermatogenesis and spermiation in testis comparable to normal wild-type mice.
MATERIALS AND METHODS
Generation of the transgenic constructs
PP1γ1 and PP1γ2 are alternatively expressed transcripts of the Ppp1cc gene (Figure 1). Global or testis-specific knockout of this gene prevents the expression of both isoforms. Here we have produced several transgenic mouse lines in an attempt to restore PP1γ1 protein in male germ cells. The mice with the following genotype “Ppp1cc (–/–), PP1γ1” were considered Rescues. The PP1γ1 transgene was expressed under the control of the testis-specific promoter phosphoglycerate kinase 2 (Pgk2) [11] in Rescues I, II, and IV and under the endogenous Ppp1cc promoter in Rescue III.
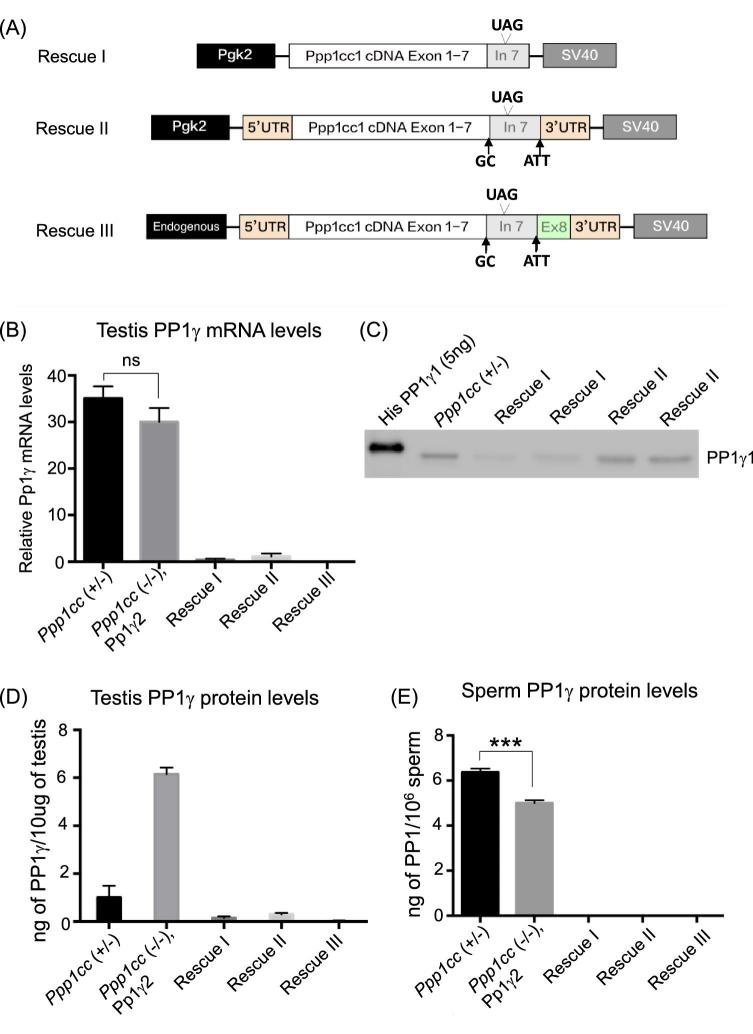
As outlined in Figure 2, to generate the transgenic mouse line Rescue I, the entire coding sequence for mouse Ppp1cc plus the remainder sequence of intron 7 was amplified by RT-PCR from mouse testicular RNA using a Pfu proofreading polymerase (Invitrogen), and was subsequently ligated into the pBluescript SK [2] II vector between the BamHI and XhoI restriction endonuclease sites. A fragment containing the human Pgk2 promoter (hPgk2) was amplified from the plasmid pCR2.1 (kindly provided by Dr John McCarrey, University of Texas, San Antonio, USA) and was subcloned into the pBluescript SK II [2] backbone between the SacI and XbaI restriction endonuclease sites. Finally, a 210-bp SV40 polyA signal was PCR amplified from pcDNA4.0 plasmid and the resultant fragment was subcloned between the XhoI and KpnI sites downstream of the Ppp1cc1 cDNA backbone. The Rescue II construct was generated in a similar manner except that the entire Ppp1cc1 cDNA including both UTRs was ligated into the pBluescript SK [2] II vector. Rescue III construct expression was driven by the Ppp1cc endogenous promoter. A genomic region spanning 2.6-kb upstream of the Ppp1cc transcription start site was amplified using the forward primer 5΄-GCGGCCGCATTGGATTTCAACATTC-3΄ and the reverse primer 5΄-CTATGAATTCATGGCCGCCGACTC-3΄. This 2.6-kb upstream genomic fragment was subcloned between NotI and EcoRI in the vector containing the entire Ppp1cc1 cDNA and the SV40-PolyA tail. Splice donor and accepter sites of the cDNA were mutated to prevent splicing and formation of the transcript for PP1γ2. Rescue IV was generated in a similar manner to Rescue II, however, lacking the untranslated region of intron 7, and with the splice acceptor and donor sites mutated. All of the DNA constructs were sequenced with multiple primers to check for integrity of the reading frame, presence of desired mutations, and absence of unwanted mutations before generating the mice.
Figure 2.
Design of PP1γ1 Rescue constructs. (A) Driven by the Pgk2 promoter is Rescue I construct consisting of PP1γ1 cDNA including a part of intron 7. Constructs of Rescues II and III contain the entire region of intron 7, as well as the 3΄ and 5΄ UTR's driven by Pgk2 and Ppp1cc endogenous promoters, respectively. The arrows in Rescue II and III indicate mutations of the splice donor and acceptor sites GT to GC and CAG to ATT, respectively. (B) Results of RT-qPCR showing very low levels of PP1γ1 mRNA in testes of Rescue I, Rescue II, and Rescue III in comparison to the heterozygous control “Ppp1cc (+/–)” and the PP1γ2 rescue “Ppp1cc (–/–), Pp1γ2”. (C) Western blot analysis of testis extracts along with 5 ng of His-PP1γ1 recombinant protein probed with anti-PP1γ1 antibody. (D) Quantification of the levels of PP1γ1 protein in testis of Ppp1cc (+/–) and rescue I, II, and III in comparison to PP1γ2 of Ppp1cc (–/–), Pp1γ2” estimated by the immunoreactivity intensity comparison with known amounts of recombinant PP1γ1/γ2 protein in western blot analysis from six blots. (E) Levels of PP1γ1 protein incorporated in 2 × 106 sperm based on comparison with recombinant PP1γ1 protein (western blot not shown). All error bars represent the standard error of the mean SEM. ***P < 0.001, n.s indicates not significant.
Generation of transgenic mice
Transgenic and all other mice used in the present study were housed and used at the Kent State University animal facility under a protocol approved by the Institutional Animal Care and Use Committee (IACUC). Production of transgenic mice was carried out at the Case Transgenic and Targeting Facility (Case Western Reserve University, Cleveland, Ohio) with institutional IACUC review.
Genotyping and breeding transgenic lines
Fresh ear punches from mice were resuspended in 50 μl of alkali lysis buffer [7] and denatured at 95°C for 1 h. Next, 50 μl of 40 mM Tris-HCl was added to neutralize it. The samples were centrifuged at 1000 × g, and the supernatant was collected for genotyping PCR. Transgenic mice produced by pronuclear microinjection of DNA constructs and embryo implantation were genotyped for the presence of the transgene with 5΄-GTGGTTGAAGATGGCTATGA-3΄ (exon 6) forward and 5΄-AAGCTGCAATAAACAAGTTGG-3΄ (SV40 internal) reverse primer pairs. Transgenic lines from male founders were established by crossing with Ppp1cc (–/–) females. Offspring were genotyped and the males with the PP1γ1 transgene were further mated with Ppp1cc null females to obtain males of Ppp1cc (–/–), PP1γ1 genotype (Rescue). Transgenic lines from female founders were established by crossing with males heterozygous for Ppp1cc (+/–). The transgene carrying males were then crossed with Ppp1cc (–/–) females to obtain male Rescues.
RNA isolation and RT-PCR
Fresh isolated mouse tissue (100 mg) was homogenized in 1 ml cold Tri reagent (Sigma-Aldrich, St. Louis, MO, USA), 200 μl of chloroform was added to the tissue homogenate and it was incubated for 15 min on ice. The homogenate was centrifuged at 12 000 × g at 4°C for 15 min. The RNA enriched top layer was collected and 500 μl of isopropanol was added before incubation at room temperature for 10 min prior to centrifugation at 10 000 × g for 10 min at 4°C. The supernatant was collected and added to 1 ml of 75% ethanol and vortexed gently. The mixture was centrifuged at 7500 × g for 7 min at 4°C and the supernatant was discarded. The pellet was semidried and resuspended in approximately 50 μl of RNase-free water. Finally, the RNA concentration was measured using a Nanodrop spectrophotometer (ND-1000; Nanodrop Technologies).
The RNA was diluted to a concentration of 200 ng/μl with RNase-free water, and cDNA was prepared using the Qiagen RT-PCR kit. The genomic DNA was eliminated by incubating 600 ng of RNA with RNase-free DNase for 2 min at 42°C. It was then mixed with reverse transcriptase and random hexamers, and incubated in the PCR machine at 42°C for 15 min. Finally, the reverse transcriptase was inactivated at 95°C for 3 min. The cDNA concentration was measured, and its purity was estimated by its 260/280 nm absorbance ratio using Nanodrop spectrophotometer (ND-1000; Nanodrop Technologies). The cDNA was diluted to a concentration of 300 ng/μl. cDNA (600 ng) was used per reaction with 12.5 μl of SYBR green master mix (Qiagen) with the following primers at a concentration of 50 nM (PP1γ Forward: 5΄-CCCATCAGGTGGTTGAAG-3΄ and PP1γ Reverse: 5΄-GTATAAACCGGTGGACGGCA-3΄. The RNA concentration was estimated by comparing the CT value of the target mRNA with that of the GAPDH mRNA (reference mRNA).
Mouse sperm isolation
Mouse caudal epididymis and vas deferens were isolated together; the artery of vas deferens was carefully removed; and the cauda epididymis and vas deferens were rinsed in phosphate-buffered saline (PBS) before being placed in EmbryoMax Human Tubal Fluid (HTF; Millipore). The HTF media had been equilibrated and buffered in a 5% CO2 chamber at 37°C for 2 h prior to sperm isolation. The cauda epididymus while in HTF media was punctured with a needle, and the sperm were squeezed from the vas deferens using surgical tweezers. Throughout this report we refer to sperm isolated for the cauda epididymis and vas deferens as ‘caudal sperm’ in contrast to sperm resident in the caput epididymis. Sperm were allowed to disperse in the media for 5–10 min at 37°C aided with occasional swirling. Upon complete dispersion, the sperm were transferred to 1.5 ml tube using a cut-off 1-ml pipette tip. Sperm cells were counted using a hemocytometer.
Sperm extract preparation
After counting, sperm were pelleted by centrifuging at 700 × g at 4°C. Whole sperm extracts were prepared by resuspending the pellet in 1% sodium dodecyl sulfate (SDS) in water, boiled in a water bath for 7 min, and centrifuged at 16 000 × g at room temperature for 20 min. The supernatant was collected and boiled with Laemmli buffer for SDS-PAGE. Soluble and insoluble fractions of sperm were prepared by sonication in homogenization buffer (HB+; 10 mM Tris [pH 7.2] containing 1 mM EDTA, 1 mM EGTA, 10 mM benzamidine-HCl, 1 mM PMSF, 0.1 mM N-p-tosyl-L-phenylalanine chloromethyl ketone [TPCK], 0.1% (V/V) β-mercaptoethanol, and 1 mM sodium orthovanadate). The sperm suspension was sonicated on ice with three 10-s bursts of a Microson ultrasonic cell disruptor (Misonix) set at level 3. The suspension was then centrifuged at 16 000 × g for 20 min at 4°C. The supernatants were supplemented with 5% glycerol and stored at −20°C.
Testis protein extract preparation and protein estimation
Testes were isolated, weighed, and placed in a test tube with 1 ml HB+ and homogenized using a Tissue Tearor homogenizer on ice at level 4 for three 10-s bursts. The suspension was then centrifuged at 16 000 × g for 20 min at 4°C. The supernatants were collected; protein was estimated and stored at −20°C. Protein concentration was estimated using the DC protein assay kit II (Bio-Rad).
Protein phosphatase assay
Phosphatase activity was measured according to protocol as previously described by [12]. In short, radiolabeled phosphorylase a was used as a substrate to measure catalytic activity of PP1. Recombinant protein phosphatase inhibitor 2 [I-2] (New England BioLabs) was used to inhibit PP1γ and 2 nM okadaic acid to inhibit PP2A. The reaction was terminated by addition of 90 μl 20% trichloroacetic acid, and supernatants were analyzed for 32P released from phosphorylase a. Enzyme activity is expressed in mmol of PO4 released/minute/2 × 105 sperm.
Antibodies
Anti-PP1γ2 and anti-PP1γ1 were prepared against the carboxy terminal regions of both proteins by Zymed Laboratories, and their ability to recognize respective isoforms is well documented [13]. protein phosphatase 1 regulatory inhibitor subunit 2 (PPP1R2), protein phosphatase 1 regulatory subunit 7 (PPP1R7) and protein phosphatase 1 regulatory inhibitor subunit 11 (PPP1R11) were made in house. Anti-Beta actin (1:1000 dilution, Sigma, catalog #A2228), anti-Beta Tubulin (1:6000 dilution, Abcam, catalog #ab52901), phospho glycogen synthase kinase 3 alpha (pGSK3α) (1:1000 dilution, Cell signaling, catalog# 9331S), and GSK3α (1:1000 dilution, Cell signaling, catalog# 4337S), and GSK3α/β (1:1000 dilution, Invitrogen, catalog# 44610) primary antibodies were commercially purchased.
Western blot analysis
Testis and sperm extracts were separated on 12% polyacrylamide slab gels in a Mini-Protean II system (Bio-Rad Laboratories, Hercules, CA). After electrophoresis, proteins were electrophoretically transferred to Immobilon-P, PVDF membrane (Millipore Corp., Bedford, MA) and blocked with 5% nonfat dry milk in TTBS (TTBS; 0.2 M Tris [pH 7.4], 1.5 M NaCl, 0.1% thimerosol, and 0.5% Tween 20). The blots were washed with TTBS and incubated with primary antibody at 4°C overnight: anti-PP1γ1 (2 μg/ml), anti-PP1γ2 (0.4 μg/ml), anti-Beta actin (1:1000), and anti-Beta Tubulin (1:6000). Blots were washed in TTBS and incubated with the appropriate secondary antibody (Amersham, Piscataway, NJ) conjugated to horseradish peroxidase at 1:5000 dilution for 1 h at room temperature. They were then washed with TTBS twice 15 min each and four times 5 min each. Finally, the blots were developed with enhanced chemiluminescence kit (Amersham).
Motility analysis
Sperm were isolated into HTF media buffered in a CO2 chamber, washed by centrifugation, and resuspended in HTF to a concentration of 2 × 107/ml. Motility was analyzed on 100-μm capillary slides at ×10 magnification by computer-assisted sperm motility analyzer (CASA) of Hamilton Thorne Biociences installed with the CEROS version 12.2 g software. Three random fields each of 90 frames at 60 frames per second were chosen for each sample and analyzed with the following settings: minimum contrast 30, minimum cell size 4 pixels, static cell size 8 pixels, static cell intensity 60, minimum VAP 50 μ/s, minimum STR 50%, VAP cut-off 10 μ/s, and VSL cut off 0 μ/s [14].
Histology
After removal, testes were immediately fixed in freshly prepared 4% paraformaldehyde in PBS for 12–16 h. Fixed tissues were dehydrated by washing in a graded series of ethanol (70%, 95%, and 100%) for 45 min each, and then permeabilized in CitriSolv (Fisher Scientific) for 30 min. Tissues were then embedded in paraffin, and 8-mm thick sections were transferred to poly-L-lysine coated slides. Testis sections were de-paraffinized in CitriSolv and rehydrated by washing in a graded series of decreasing ethanol concentrations (100%, 95%, 80%, and 70%). Sections were stained for 2 min with PAS stain (Abcam, ab150680). Slides were washed with distilled water twice for 2 min, mounted, and viewed under a ×20 lens.
Immunofluorescence
De-paraffinized, rehydrated testis sections were boiled in citrate buffer (10 mM citric acid, 0.05% Tween20, pH 6.0) for antigen retrieval for 1 min followed by a resting time of 2 min and then boiled once again for 1 min. Slides were allowed to cool down while in citrate buffer for 30 min at room temperature. Then washed in ddH2O for 5 min. Sections were blocked by incubation in a blocking solution (5% goat serum, 5% BSA in 1x PBS) for 1 h at room temperature in a humidified chamber. Sections were then incubated with primary antibodies at 1:200 dilutions (in blocking solution) at 4°C overnight in a humidified chamber followed by three 5-min washes in 1x PBS. Negative control sections were incubated in blocking solution instead of primary antibody. Sections were then incubated with goat anti-rabbit Cy3-conjugated secondary antibody (Jackson Immunoresearch) for 1 h at room temperature in a dark humidified chamber. Finally, sections were washed three times for 5 min each in 1X PBS, counterstained with Hoechst or DAPI in 1X PBS for 10 min, washed a final wash in 1X PBS, and mounted with anti-fade mounting media (Prolong Live Antifade Reagent, P36975 Thermo Fisher Scientific), then examined using a fluorescence microscope (IX81, Olympus).
Measurement of ATP levels
Mouse cauda epididymis and vas deferens were isolated in PBS warmed to 37°C. Following sperm extraction, the suspension was mixed gently to obtain a uniform mixture before being divided equally into three sets; sperm with no energy substrate, sperm supplemented with glucose (10 mM), or sperm supplemented with lactate (25 mM). ATP was measured following a 10-min incubation period at 37°C. A 50-μl aliquot of each sperm suspension was removed and diluted into individual tubes containing 450 μl of boiling Tris-EDTA buffer (0.1 M Tris-HCl and 4 mM EDTA; pH 7.7), further boiled for 5 min and then frozen in liquid nitrogen. Upon thawing, each sample was centrifuged at 15 000 × g for 5 min at room temperature. A 100-μl aliquot of the supernatant was utilized for quantifying ATP. The ATP content was determined using the ATP Bioluminescence Assay Kit CLS II (Roche Applied Science) according to the manufacturer's protocol.
In vitro fertilization
Sperm were collected from each cauda epididymis of 3- 5-month-old male mice in a 300-μl drop of HTF media covered with mineral oil in a 35-mm dish. The tissue was incised with the edge of a 26G injection needle to allow the sperm to swim out and disperse for 10 min at 37°C under 5% CO2 in a humidified incubator. To obtain mature eggs, 21- 35-day-old female mice were super ovulated by intraperitoneal injection (IP) of 5 IU of equine chorionic gonadotropin (PMSG; Sigma). Forty six to fifty hours after PMSG injection, females were injected with 5 IU of human chorionic gonadotropin (HCG; Sigma) intraperitoneally. Thirteen to fifteen hours following HCG administration, the female mice were euthanized with CO2; their oviducts were aseptically removed and dissected free from surrounding tissues and placed in a culture dish containing 2 ml of PBS medium warmed at 37°C. The cumulus–oocyte complexes were removed from the ampullae using microdissecting forceps. Fifteen microliters of the sperm suspension was added to a 250-μl drop of HTF covered with mineral oil. The isolated cumulus masses were then transferred to the drop of HTF containing the sperm and incubated at 37°C in humidified atmosphere of 5% CO2 in air for 4 h. The eggs were then washed to eliminate excess sperm and cultured overnight in a 250-μl drop of HTF medium 37°C in humidified atmosphere of 5% CO2 in air. The following day the number of two-cell embryos was counted. The extent of fertilization was determined by the percentage of two-cell stage embryos scored 24 h after insemination.
RESULTS
Expression of PP1γ1 in rescue lines using cDNA of PP1γ1 mRNA
Transgenic mice were produced to determine if the phenotype in Ppp1cc (–/–) mice can be rescued by PP1γ1 expressed as a transgene in male germ cells. The PP1γ1 transcript is approximately 2.4 kb. Removal of the last intron 7 by splicing results in the 1.4-kb PP1γ2 transcript: in the PP1γ1 transcript this segment remains as extended exon 7, which results in the 7-amino-acid C-terminus in PP1γ1 (Figure 1). Three lines of PP1γ1 rescue mice were made (Figure 2A). The first, Rescue I, was made with a transgene construct consisting of PP1γ1 cDNA which included 300 bp of the 3΄ UTR of PP1γ1 transcipt following the stop codon of the coding sequence. The transgene was driven by the testis-specific Pgk2 promoter [11]. Due to low level of transgene expression in this line, two additional lines were generated using the cDNA of the entire PP1γ1 transcript as the transgene. Constructs of Rescues II and III contained the entire region of intron 7, as well as the 3΄ and 5΄ UTRs, that is the cDNA corresponding to the entire 2.4-kb PP1γ1 transcript. Transgene expression was driven by the Pgk2 promoter or by the endogenous Ppp1cc promoter which was previously shown to direct testis-specific expression of PP1γ2 [7]. Quantitative PCR analysis of mRNA isolated from the testis of PP1γ1 rescue mice lines I, II and III shows low levels of transgenic PP1γ1 mRNA compared to the heterozygous control Ppp1cc (+/–) and the PP1γ2 rescue mice refered to as Ppp1cc (–/–), Pp1γ2 (Figure 2B). Testes from all the three transgenic rescue mice lines showed low levels of PP1γ1 transgene protein expression. Among the three lines Rescue II had the highest level of PP1γ1 protein; yet this level was 20-fold less PP1γ1 protien compared to PP1γ2 in the testis of PP1γ2 rescue mice Ppp1cc (–/–), Pp1γ2 (Figure 2C and D). PP1γ1 was undetectable in extracts of sperm from all three lines of rescue mice (Figure 2E).
Despite low levels of PP1γ1 expression in the testis of the rescue mice, there was partial recovery of spermatogenesis and rescue male mice from all three lines were infertile. Average testis weights as well as sperm counts were significantly low compared to heterozygous control mice. Cauda epididymal sperm were completley immotile, showing several morphological abnormalities (data not shown).
Expression of PP1γ1 rescue mice with a cDNA construct resembling PP1γ2 cDNA
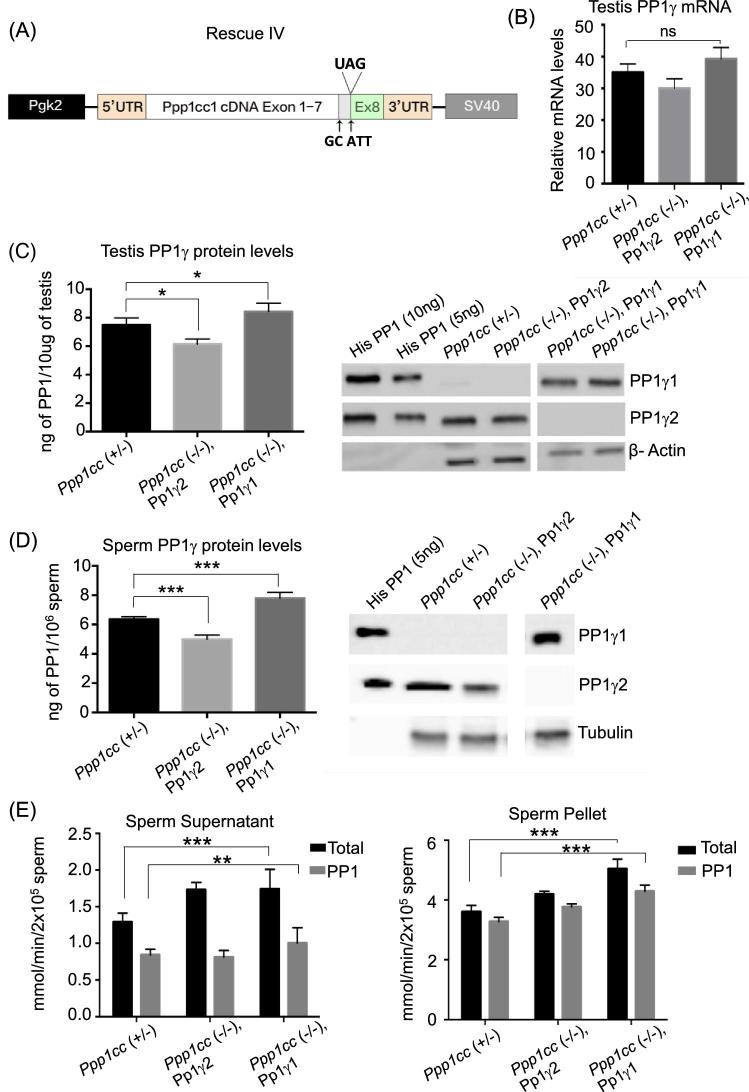
We suspected that poor transgenic expression of PP1γ1 could be due to instability of the mRNA for PP1γ1 in developing germ cells in testis. A fourth line of rescue mice (Rescue IV) was generated using cDNA lacking the 1-kb portion of the 3΄ UTR following the translation stop site of the PP1γ1 transcript as the transgene. Splice donor and acceptor sites were also mutated (GT to GC and CAG to ATT respectively) to prevent possible splicing which could produce PP1γ2. Messenger RNA from this construct will mimic the transcript for PP1γ2 but when translated should yield the PP1γ1 protein (Figure 3A). The transgene construct was sequenced with multiple primers to check for integrity of the reading frame, presence of desired mutations, and absence of unwanted mutations before generating the mice. The transgene was injected into one-cell embryo and we obtained 11 transgene positive founders. They were crossed with Ppp1cc (–/–) mice followed by repeated back crossing until the Ppp1cc (–/–), Pp1γ2 rescue mice were obtained from each of the founder. The results from the highest transgenic PP1γ1 expressing line out of the 11 established lines are shown. The comparison of all 11 lines is shown in Supplementary Table S1. Quantitative PCR analysis of mRNA isolated from the testes of highest PP1γ1expressing rescue mice line IV referred to as “Ppp1cc (–/–), Pp1γ1” shows high levels of transgenic PP1γ mRNA that is comparable to PP1γ levels of heterozygous control Ppp1cc (+/–) and the PP1γ2 rescue Ppp1cc (–/–), Pp1γ2 (Figure 3B). The tissue-specific expression of the Pgk2 promoter-driven Pp1γ1expression in this line was confirmed by western blot analysis (Supplementary Figure S1A and B). Quantitation of PPγ1 protein in the testes of the transgenic line Rescue IV was made by comparison with recombinant His-PP1γ1 and His-PP1γ2 (Figure 3C). The blots were probed with anti-PP1γ1 and anti-PP1γ2 antibodies. The expression levels in Ppp1cc (–/–), Pp1γ1 were approximately 8 ng of PP1γ1 protein per 10 μg of testis protein extracts. These levels are comparable to the levels of PP1γ2 expressed in the testis of Ppp1cc (+/–) and Ppp1cc (–/–), Pp1γ2, a transgenic PP1γ2 rescue line, where spermatogenesis and fertility were comparable to wild-type mice [7].
Figure 3.
Expression of PP1γ1 in Rescue IV. (A) Design of PP1γ1 Rescue IV construct mimicking PP1γ2 mRNA. The transgene bears exons 1–7 of PP1γ1 and exon 8 of PP1γ2 with 5΄ and 3΄ UTRs. The arrows indicate the mutations GT to GC and CAG to ATT. The region between the arrows is the extended exon 7 sequence specific to PP1γ1 mRNA. It is driven by the Pgk2 promoter for testis-specific expression. (B) Results of RT-qPCR showing comparable levels of PP1γ mRNA in testes of Rescue IV “Ppp1cc (–/–), Pp1γ1” in comparison to the heterozygous control “Ppp1cc (+/–)” and the PP1γ2 rescue “Ppp1cc (–/–), Pp1γ2”. Error bars indicate the standard error of the mean SEM (n = 3). (C) Quantification of PP1 levels expressed per 10 μg of testis extract of control and rescue mice was obtained by intensity analysis of immunoreactive bands of western blot. Panel on the right shows the western blot analysis of Rescue IV testis. Ten micrograms of testis extracts from Ppp1cc (+/–), Ppp1cc (–/–), Pp1γ2, and Rescue IV “Ppp1cc (–/–), Pp1γ1” along with 10 and 5 ng of His-PP1γ1 and His-PP1γ2 were analyzed by western blot and probed with anti-PP1γ1 and anti-PP1γ2 antibodies. The blots were re-probed with antibodies against β-Actin to confirm equal protein loading. The blots are representative of three different experiments. (D) Quantification of PP1 levels expressed per 2 × 106 sperm of control and rescue mice was obtained by intensity analysis of immunoreactive bands of western blot. Panel on the right shows the western blot analysis of Rescue IV sperm extracts. A total of 2 × 106 sperm from Ppp1cc (+/–), Ppp1cc (–/–), Pp1γ2, and Rescue IV “Ppp1cc (–/–), Pp1γ1” along with 5 ng of His-PP1γ1 and His-PP1γ2 were analyzed by western blot and probed with anti-PP1γ1 and anti-PP1γ2 antibodies. The blots were re-probed with antibodies against β-Tubulin to confirm equal protein loading. The blots are representative of three different experiments. (E) Phoshatase activity in sperm. Protein phosphatase activity in the soluble fraction (supernatant) and insoluble fraction (pellet) of sperm from Ppp1cc (+/–), Ppp1cc (–/–), Pp1γ2, and Ppp1cc (–/–), Pp1γ1 rescue mice expressed in millimoles/minute/extract. Total phosphatase activity represented as black bars is the combined phosphatase activity of PP1γ and PP2A. Activity of PP1γ was measured by deducting the activity remaining after incubation with recombinant I2 from the total activity. The phosphatase activity values are mean ± SEM obtained from animals aged 3–5 months. Ppp1cc (+/–) (n = 6), Ppp1cc (–/–), Pp1γ2 (n = 2), Ppp1cc (–/–), Pp1γ1 (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 and n.s indicates not significant.
Sperm extracts of Ppp1cc (+/–), Ppp1cc (–/–), Pp1γ2 and Ppp1cc (–/–), Pp1γ1 along with recombinant His-tagged PP1γ1 and PP1γ2 were analyzed by western blot analysis using anti-PP1γ1 and anti-PP1γ2 antibodies (Figure 3D). PP1 protein quantification in extracts from 2 × 106 sperm from the Ppp1cc (–/–), Pp1γ1 mice showed approximately 8 ng of PP1γ1 compared to 6 and 5 ng of PP1γ2 in Ppp1cc (+/–) and Ppp1cc (–/–), Pp1γ2 mice. Thus, levels of PP1γ1 in sperm were comparable to PP1γ2 levels in sperm from heterozygous mice or the trangenic PP1γ2 rescue mice. Next we determined the catalytic activity of PP1 in sperm from PP1γ1 rescue mice. Results in Figure 3E show that protein phosphatase catalytic activity of Ppp1cc (–/–), Pp1γ1 in soluble sperm extracts is similar to both PP1γ2 rescue and but higher compared to normal sperm. The insoluble fraction of PP1γ1 rescue sperm has significantly higher phosphatase activity than both PP1γ2 of Ppp1cc (+/–) and (–/–), Pp1γ2 mice.
Transgenic expression of PP1γ1 within cross sections of seminiferous tubules matches the expression of endogenous PP1γ2
PP1γ2 has a distinct cellular localization in wild-type mouse testes sections, present exclusively in meiotic and postmeiotic germ cells: spermatocytes, round spermatids, elongated spermatids, and testicular spermatozoa [6]. To examine the expression pattern of transgenic PP1γ1 in the testes of rescue males, testis sections from Ppp1cc (+/–) and PP1γ1 rescue mice Ppp1cc (–/–), PP1γ1 were subjected to immunohistochemical staining using specific antibodies against PP1γ2 or PP1γ1. Figure 4A shows the presence of PP1γ2 in spermatocytes, spermatids, and testicular spermatozoa of Ppp1cc (+/–) mice. Figure 4B shows that cellular localization of PP1γ1 in the testes of rescue males is comparable to the localization of PP1γ2 in testes of control mice Ppp1cc (+/–). This staining pattern of transgenic PP1γ1 is similar to PP1γ2 localization seen in Figure 4B and also as shown in testis of Ppp1cc (+/+) and Ppp1cc (–/–) PP1γ2 rescue mice [6, 7]. Multiple approaches including northern and western blot of testis RNA and extracts show that PP1γ2 expression is initiated from primary spermatocytes starting at day 14–15 in postnatal developing testis. [6, 7, 10, 15]. To confirm the absence of PP1γ2 in rescue testis, the sections were stained with PP1γ2 antibody as well but PP1γ2 was not detected (Figure 4C).
Figure 4.
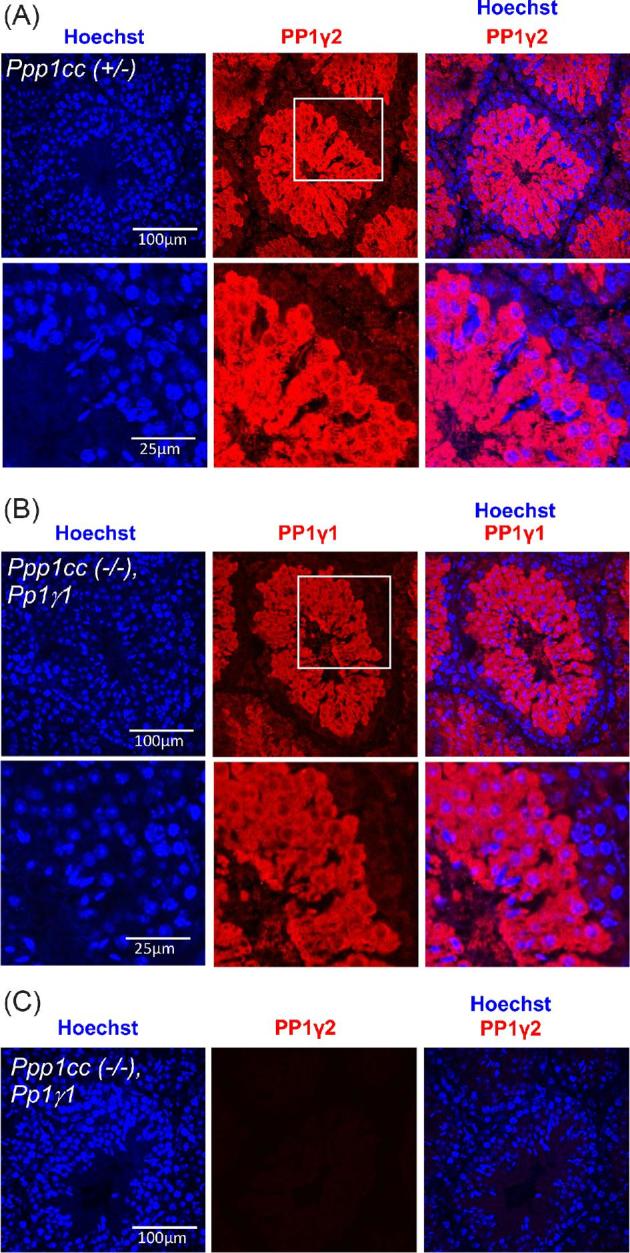
Immunohistochemisrty showing the expression and localization of PP1γ2 in Ppp1cc (+/–) and PP1γ1 in Ppp1cc (–/–), Pp1γ1 testis. (A) Ppp1cc (+/–) testis sections stained with anti-PP1γ2 antibody (red) and counterstained with Hoechst for nuclei (blue). PP1γ2 staining appears in meiotic and postmeiotic germ cells but absent from premeiotic spermatogonial cells. The region highlighted with white box is enlarged and shown in the bottom panel. (B) Ppp1cc (–/–), Pp1γ1 testis sections stained with anti-PP1γ1 antibody (red) and counterstained with Hoechst for nuclei (blue). PP1γ1 staining appears in meiotic and postmeiotic germ cells but absent from premeiotic germ cells near the basal membrane similar to the expression pattern of PP1γ2 in Ppp1cc (+/–) control testis. The region highlighted with white box is enlarged and shown in the bottom panel. (C) Ppp1cc (–/–), Pp1γ1 testis sections stained with anti-PP1γ2 antibody show no detectable signal (red) indicating the absence of PP1γ2.
Transgenic PP1γ1 expression in Ppp1cc knockout testes rescues spermiogenesis and fertility
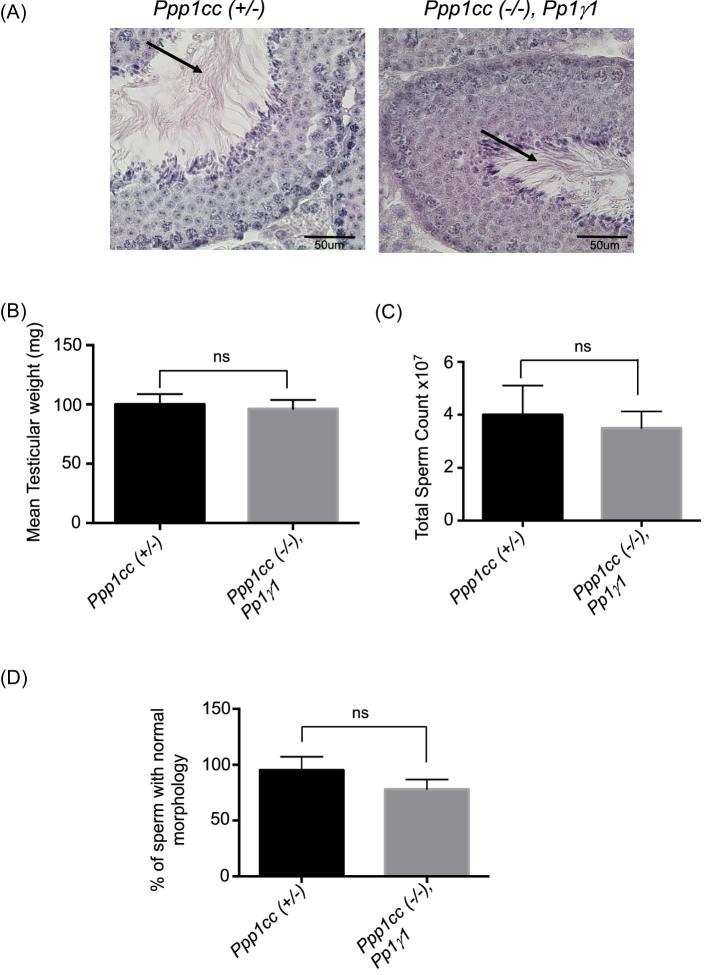
In order to examine spermatogenesis in the testes of Ppp1cc (–/–), Pp1γ1 males, testis sections were subjected to histological analysis following PAS stain [16]. Figure 5A shows testicular spermatozoa in the lumen of seminiferous tubules of PP1γ1 rescue testes, similar to that observed in control testis from Ppp1cc (+/–) mice. Testis weights, total sperm counts, and normal sperm morphology are also restored, showing rescue of spermatogenesis in the Ppp1cc knockout mice with transgenic expression of PP1γ1 (Figure 5B–D).
Figure 5.
Phenotype and fertility of Rescue IV “Ppp1cc (–/–), Pp1γ1”. (A) PAS staining of Ppp1cc (+/–) and Ppp1cc (–/–), Pp1γ1 testis sections showing testicular spermatozoa (indicated by arrows) in the lumen of seminiferous tubules of PP1γ1 rescue testes similar to that observed in normal testis from Ppp1cc (+/–) mice. (B) Comparison of testis weight expressed as the mean value ± standard error of mean (SEM) from seven males showing no significant difference between the groups. (C) Comparison of sperm number (the total number of sperm extracted from two cauda epididymis and two vas deferens of one male). Values are the mean value ± standard error of mean (SEM) from seven males showing no significant difference between the groups. (D) Comparison of sperm morphology (the number of morphologically normal sperm out of 100 counted after fixation). Values are the mean value ± standard error of mean (SEM) from seven males showing no significant difference between the groups. n.s indicates not significant.
Sperm from PP1γ1 rescue mice have poor fertilization ability in vivo and in vitro
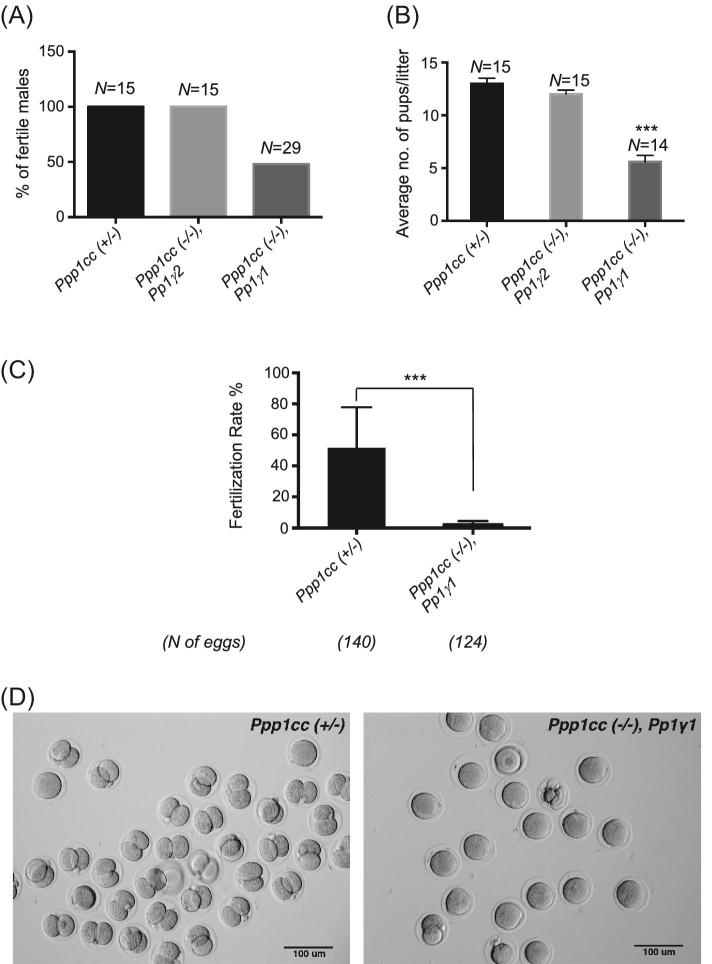
Eight- to nine-week-old male PP1γ1 transgenic rescue mice were tested for the ability to reproduce with 8- 16-week-old female CD1 wild-type mice. Fertility was compared to Ppp1cc (+/–) and (–/–), Pp1γ2 mice (the PP1γ2 rescue line). All males were tested for 4 months. The Rescue IV male mice were set up for fertility testing at the age of approximately 8–9 weeks with CD1 wild-type female mice that were between the ages 8 and 16 weeks. They were tested for 4 months and the Rescue IV male mice which were infertile after 2 months of testing were set up with another CD1 wild-type female mouse to ascertain their infertility. Only 54% of the rescue male mice were fertile (Figure 6A). Litter sizes resulting from the fertile males were significantly lower: an average of 5 pups per litter in the rescue compared to the 13 in heterozygous Ppp1cc (+/–) control mice (Figure 6B). The average time for Ppp1cc (–/–), Pp1γ1 mice to develop pregnancy was 4 to 6 weeks. We performed in vitro fertilization (IVF) using eggs from wild-type females with sperm from PP1γ1 rescue or heterozygous control males. The average fertilization rate in IVF was determined by the number of zygotes that progressed to the two-cell stage (Figure 6C and D) after overnight culture in HTF medium (37˚C, and 5% CO2 in a humidified incubator). There was a significant difference in fertilization rates between PP1γ1 rescue (5%) and heterozygous control males (52%). The fertilization rate of the heterozygous “Ppp1cc (+/–)” control mice, around 43%, was similar to that reported in the literature for CD1 normal wild-type males.
Figure 6.
Subfertility in Rescue IV “Ppp1cc (–/–), Pp1γ1” males. (A) Fertility expressed as the percentage of fertile males. N = the number of total males tested. All males were bred for 4 months, and their fertility was ascertained by testing with two wild-type females. Fourteen of the Rescue IV “Ppp1cc (–/–), Pp1γ1” males were fertile out of 29 tested. (B) Average litter size produced by the fertile males. The rescue males produced an average of 5 pups per litter compared to an average of 13 for the control. (C) In vitro fertilization rate of Ppp1cc (–/–), Pp1γ1 sperm in comparison to control sperm calculated as the percentage of two-cell stage 24 hours after fertilization. The notion “N of eggs” indicates the total number of eggs derived from three females. Error bars indicate the standard error of the mean of three different experiments using sperm from three Ppp1cc (–/–), Pp1γ1 animals. (D) Bright-field images of the two-cell stage embryos resulting from the IVF experiments. Images are representative of the three different experiments. All values are the mean ± SEM. ***P < 0.001.
Sperm bearing transgenic PP1γ1 show altered motility
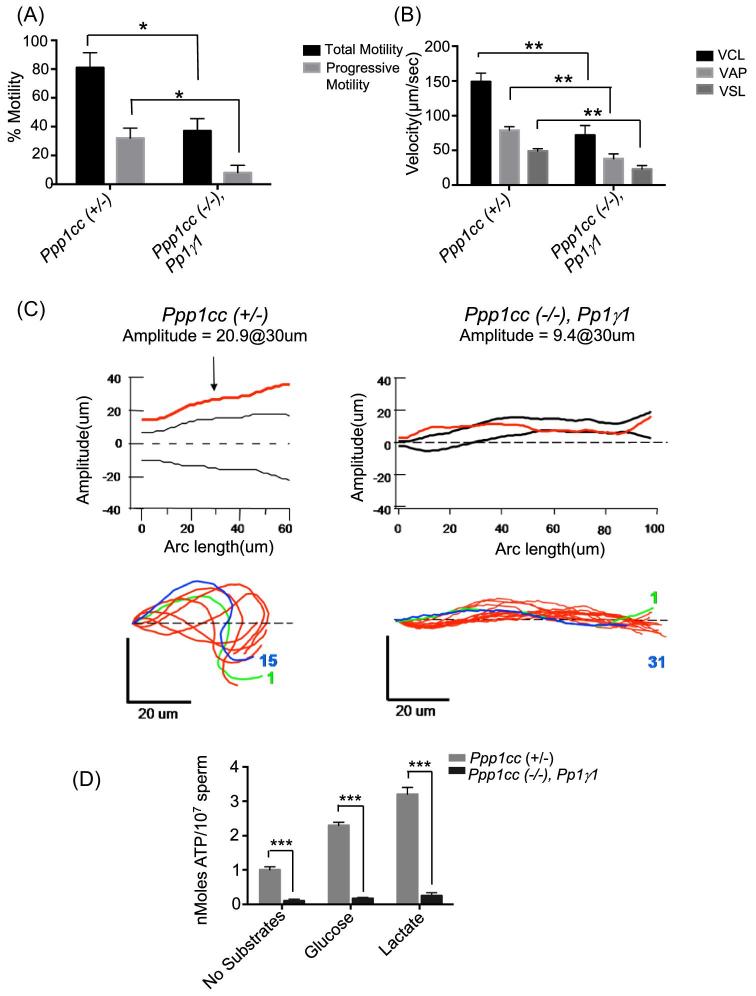
Motility of sperm obtained from the cauda epididymis and vas deferens of the PP1γ1 rescue and control mice was analyzed by CASA. It was observed that sperm from PP1γ1 mice display significantly lower net and progressive motility (Figure 7A). Sperm velocity parameters, such as average path velocity (VAP), curviliear velocity (VCL), and straight line velocity (VSL) as well as lateral head amplitude (ALH), were reduced (Figure 7B and C). Sperm flagellar beat tracings are shown in Figure 7C. The Ppp1cc (+/–) sperm show flagella beat amplitude of 20.9 μm. However, sperm from Ppp1cc (–/–), Pp1γ1 mice show significantly decreased amplitude (9.4 μm). We determined ATP levels in rescue compared to normal sperm. Data in Figure 7D show that even in the presence of glucose and lactate ATP levels are significantly lower in PP1γ1 rescue compared to control sperm. Mean levels of ATP from Ppp1cc (+/–) control sperm were 1.1 nmoles/107 sperm (±0.09, n = 7) while those from PP1γ1 rescue caudal sperm were 0.21 nmoles/107 sperm (±0.06, n = 7) in the absence of any energy substrates. ATP levels of Ppp1cc (+/–) sperm increase significantly upon addition of glucose or lactate. However, PP1γ1 rescue sperm show a poor ability to utilize substrates to increase ATP (Figure 7D).
Figure 7.
Impaired sperm motility in Rescue IV “Ppp1cc (–/–), Pp1γ1”. (A) Computer-assisted sperm motility analysis (CASA) shows a significant decrease in percentage motile sperm (black bars) and progressively motile sperm (gray bars) in the rescue sperm. Sperm with a velocity greater than 50 μm/s were considered to be progressively motile. Motility is expressed as the mean value ± SEM from seven males. (B) The velocity parameters, velocity average path (VAP), velocity curve line (VCL), and velocity straight line (VSL), are significantly lowered in rescue sperm compared to heterozygous “Ppp1cc (+/–)” and PP1γ2 rescue “Ppp1cc (–/–), Pp1γ2” sperm. Bars are representative of the mean value ± SEM from seven males. (C) Flagellar beat wave form of control and Rescue IV mice. (D) ATP levels (expressed in nmoles/107sperm) in caudal sperm of Ppp1cc (+/–) and Ppp1cc (–/–), Pp1γ1 mice. The values shown are the mean ± SEM obtained from seven animals of each type aged 3–5 months following incubation with no energy substrates in PBS, with glucose 10 mM, or lactate 25 mM. The data shown are the average of five separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Rescue sperm show altered subcellular localization of PP1 and its binding partners
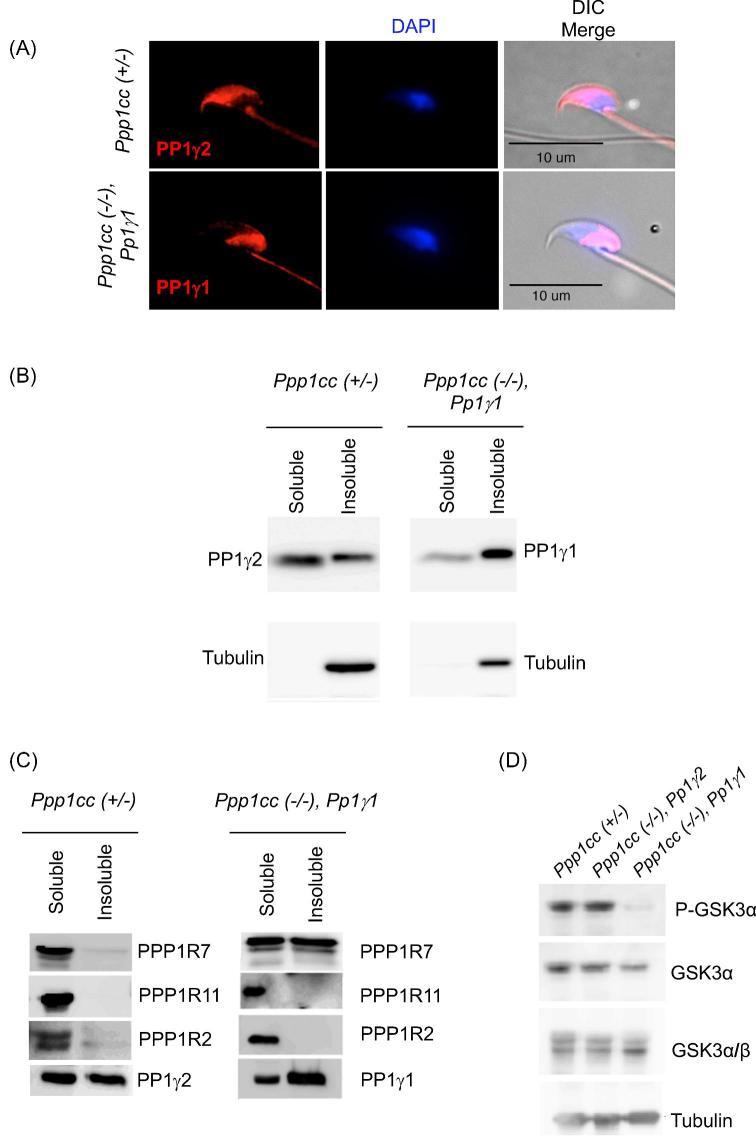
Immunofluorescence of PP1 following immunostaining of fixed sperm with specific antibodies against PP1γ2 and PP1γ1 in heterozygous Ppp1cc (+/–) control mice and Ppp1cc (–/–), Pp1γ1 rescue sperm, respectively, shows different localizations of the two isoforms. PP1γ2 appears to localize in the flagellum and in the equatorial and anterior regions of the sperm head. In rescue sperm similar to PP1γ2, PP1γ1 is also present in the flagellum but unlike PP1γ2 it is present in the postacrosomal region of the head (Figure 8A).
Figure 8.
Intracellular localization of PP1γ in sperm. (A) Representative images of PP1γ2 immunostaining in heterozygous Ppp1cc (+/–) sperm shown in the flagellum and the equatorial region of the sperm head. PP1γ1 in Ppp1cc (–/–), Pp1γ1 sperm is seen in the flagellum and the postacrosomal region of the sperm head. (B) Western blot analysis of soluble and insoluble fractions of RIPA sperm lysates from PP1γ1 rescue males compared to control males. The left panel shows PP1γ2 is almost equally distributed between the soluble and insoluble fractions of control sperm. In the right panel, unlike PP1γ2, PP1γ1 protein appears more abundant in the pellet fraction of Ppp1cc (–/–), Pp1γ1 sperm. All lanes were loaded with 2 × 106 sperm. Blots were reprobed with anti-Tubulin as a control. (C) Western blot anaysis for PP1γ binding proteins PPP1R7, PPP1R11, and PPP1R2 in the soluble and insoluble fractions of RIPA sperm lysates from PP1γ1 rescue males Ppp1cc (–/–), Pp1γ1 compared to the control Ppp1cc (+/–). (D) Detection of Phospho-GSK3α, total GSK3α, and GSK3α/β levels in whole sperm extracts of Ppp1cc (+/–), PP1γ2 rescue “Ppp1cc (–/–)” and PP1γ1 rescue “Ppp1cc (–/–), Pp1γ1” by western blot analysis.
In wild-type and Ppp1cc (+/–) mice, PP1γ2 is present both in the soluble and insoluble fractions of sperm extracts in approximately equal proportions (Figure 8B). However, PP1γ1 of Ppp1cc (–/–), Pp1γ1 rescue sperm is present mostly in the insoluble fraction (pellet) of the sperm lysate, and to a lesser level in the soluble (supernatant) fraction. Next, we examined the distribution of the three PP1 binding regulators (PPP1R2, R7, and R11) in sperm from PP1γ1 rescue mice. PPP1R2 and PPP1R11 are present predominantly in the soluble fraction of the sperm extract similar to control sperm (Figure 8C). This distribution in Ppp1cc (+/–) is also similar to that seen in sperm from wild-type Ppp1cc (+/+) mice. However, the PP1 binding protein PPP1R7, which is present in the soluble fraction of sperm from control mice, is present almost equally in the soluble and the insoluble fractions (Figure 8C) of sperm from PP1γ1 rescue mice. Next we determined the phosphorylation status of GSK3α which was first identified as a regulator of PP1γ2 [17]. Phosphorylation of GSK3α was significantly lower in sperm from PP1γ1 rescue compared to normal to heterozygous and PP1γ2 rescue mice (Figure 8D).
DISCUSSION
Spermatogenesis is associated with a dramatic change in the transcriptome of germ cells, leading to significant increases in the expression of many genes and silencing of others. Some of the proteins such as phospho glycerate kinase 2 (Pgk2) and adenine nucleotide translocase are expressed as testis-specific proteins in differentiating germ cells [18]. The genes corresponding to these proteins are testis-specific, that is, genes expressed only in testis. Others are proteins ubiquitous in somatic cells but are expressed as testis-specific isoforms either by alternative splicing or by the use of alternate transcription start sites. Proteins in this category include some glycolytic enzymes, sperm form of catalytic subunit of protein kinase A, lemur tyrosine kinase 2 (LMTK2), angiotensin converting enzyme (ACE), sodium-potassium ATPase, and the protein phosphate PP1γ2 [19–24]. It is presumed that all these testis-specific proteins, whether encoded by independent genes or derived from alternate splicing or alternate transcription start sites, possess specific biochemical properties that their somatic cell counter parts do not. Whether somatic forms of these proteins can functionally substitute for the testis-specific forms to support normal sperm function has been investigated only in two instances. It was shown that somatic cell form of ACE cannot substitute for the testis-specific isoform [25]. In contrast, it was shown that the testis-specific lactate dehydrogenase isoform could be replaced by somatic lactate dehydrogenase (LDHA) isoform to support normal sperm function and fertility [26].
It is intriguing that the three PP1 isoforms, PP1α, PP1β, and PP1γ1, in most cellular contexts are interchangeable while in developing germ cells in testis the three isoforms are excluded in differentiating germ cells where PP1γ2 is expressed [6]. Northern blot analysis of testicular RNA revealed that PP1γ2 expression increases significantly in the testes of 15-day-old mice coinciding with the onset of meiosis. PP1γ2 is predominant or exclusive in developing germ cells, while PP1γ1 expression is restricted to somatic cells and spermatogonia [10]. Similarly, PP1α and PP1β are also restricted to testicular somatic cells [10]. Targeted disruption of the Ppp1cc gene, causing global loss of both PP1γ1 and PP1γ2, results in male sterility due to impaired spermatogenesis [6, 8]. Subsequent studies showed that PP1γ2 alone in the absence of PP1γ1 can restore spermatogenesis in Ppp1cc (–/–) mice [7]. These data would support the notion that PP1γ2 may have an isoform-specific function in testis and sperm: that is, PP1γ1 and PP1γ2 are functionally non-equivalent. Contrary to this notion, results in this report clearly show that PP1γ1 can substitute for PP1γ2 in testis and that the two isoforms are functionally equivalent at least in supporting spermatogenesis.
The search for PP1γ2 isoform-specific binding proteins in testis used yeast two hybrid [27, 28] and TAP-tag approaches. Three potential PP1γ2 binding proteins identified were endophillin B1t, spermatogenic leucine zipper 1 (Spz1), and Dolichyl-diphosphooligosaccharide-protein glycosyltransferase non-catalytic subunit (Ddost) [29–31]. Other studies did not reveal possible PP1γ2 isoform-specific binding proteins [27]. Whether these testis PP1 binding proteins are involved in the presumed isoform-specific function of PP1γ2 was not known. The fact that PP1γ1 is able to replace PP1γ2 in testis would suggest that the PP1 binding proteins in developing germ cells are able to function with either of the two isoforms.
We suspect that the failure to achieve high PP1γ1 protein expression in three of the four lines could be due to the instability of the PP1γ1 mRNA in developing spermatocytes and spermatids. The PP1γ1 and PP1γ2 transcripts are identical in the regions containing exons 1–7 but they differ at their 3΄ UTRs due to the absence of intron 7 in PP1γ2 mRNA and its presence as extended exon 7 in PP1γ1 mRNA. That is PP1γ1 transcript retains intron 7 as an extended exon while in the PP1γ2 transcript intron 7 is removed by splicing. Analysis of Ppp1cc gene using the bioinformatics algorithms for miRNA target predictions MiRDB and miRanda revealed the presence of several miRNA target sites within intron 7, that is, within the 3΄UTR of PP1γ1 mRNA. Of these putative miRNAs targeting the PP1γ1, miR449 and miR34 are highly expressed in testis. The target sites for miR449 and mir34 are 13 nucleotides in length at the beginning of intron 7 (nucleotides 9–21). From day 14 with the onset of meiosis and formation of spermatocytes, there is an exponential increase in levels of mir449 and mir34c that parallels increased expression of PP1γ2 [32]. This putative mir449 and mir34c binding region was present in the first three PP1γ1 rescue constructs (Rescue I, Rescue II, and Rescue III). Several studies show the crucial role of miRNAs in regulating the spatio-temporal expression of target genes in spermatogenesis [32–34]. Based on this hypothesis supported by bioinformatics evidence, we designed the fourth construct, a cDNA capable of forming a PP1γ1 transcript that lacks the miRNA target region and approximately 1-kb 3΄UTR region following the stop codon of the transcript. This transgene showed high expression levels of PP1γ1 mRNA and protein. However, the role of miRNA target regions in the 3΄UTR in causing instability of the PP1γ1 mRNA should be experimentaly verified in future studies. In these mice despite apparent normal spermatogenesis, fertility of the rescue mice, tested both in vivo and in vitro, was not restored. Sperm containing PP1γ1 had a poor ability to fertilize wild-type eggs in vitro following IVF. The discrepancy between in vivo and in vitro fertility in sperm from PP1γ1 rescue mice could be due to following: (i) Fertilization in vivo occurs with ejaculated sperm whereas IVF is performed with caudal epididymal sperm. (ii). Males used for the IVF experiment were not fertility tested prior to extraction of sperm for the IVF. As seen from Figure 6A, not all males were fertile, in fact greater than 46% of the rescue males were infertile. Thus, it is possible that the rescue males randomly selected for the IVF were infertile and consequently did not give rise to any two-celled embryo in vitro. (iii) Sperm from rescue mice, as evident from the data, have defects in substrate utilization for generating ATP and their motility is impaired (Figure 7). Fertilization impairment due to these defects may be more evident in IVF. The sperm defects may be tolerated during in vivo fertilization within the female reproductive tract where a conducive environment for sperm survival and fertilization should exist. The IVF media (HTF), while somewhat optimized for sperm capacitation and fertilization, does not entirely mimic the environment of the female reproductive tract. A discrepancy between IVF and in vivo fertility has also been previously observed in other KO mice. Mice lacking cysteine-rich secretory protein 1 (CRISP1), the tyrosine kinase FER, and superoxide dismutase (SOD1) were fertile in vivo but sperm from these mice displayed reduced ability to fertilize eggs in vitro [35–37].
The percentage of total motile and progressively motile sperm was low compared to sperm from Ppp1cc (+/–) mice. There was a marked attenuation of flagella beat amplitude affecting velocity parameters. The functional nonequivalence of PP1γ1 and PP1γ2 in sperm would explain why PP1γ1 is not expressed in meiotic and postmeiotic germ cells. In wild-type sperm PP1γ2 was found, by immunostaining, to be present along the flagellum and in the equatorial region of the head. However, PP1γ1 appeared along the flagellum and in the postacrosomal region of the head. In general, phosphatases acquire their specificity by differential subcellular localization. Therefore, the difference in localization of the two isoforms PP1γ2 and PP1γ1 in the sperm head most likely affects the distribution of PP1 binding proteins and proximity of the phosphatase to its substrates. Localization of PPP1R7, a PP1 regulating protein, is altered which could result in altered catalytic activity of PP1γ1 compared to PP1γ2. It is evident that PP1γ1 incorporation into sperm impairs motility. It is known that immotile caput sperm acquire motility in the caudal epididymis. An optimal balance in the activity of phosphatases and kinases is critical for normal sperm motility. PP1γ1 in rescue IV sperm shows higher phosphatase activity both in the supernatant and pellet compared to PP1γ2 in Ppp1cc (+/–) sperm. However, the protein levels of the isoforms are similar in sperm from control and rescue mice. Altered PP1 phosphatase activity likely due altered association with PPP1R7 may also be responsible for decreased levels of phospho-GSK3α resulting in high GSK3 activity. Altered GSK3 activity is known to impair metabolism and fertility of sperm [38, 39].
Sperm rely on both glycolysis and mitochondrial oxidative phosphorylation for ATP production. Among different mouse strains, those with higher oxidative phosphorylation/glycolysis rates have higher ATP content, percentage of motile sperm, and higher sperm velocity parameters [40]. The energy production pathway that is utilized by spermatozoa is dependent on the availability of substrates [41]. The specialized mid-piece mitochondria are believed to be the source of ATP during epididymal transient, while glycolysis appears to be the main source of ATP hydrolyzed by the flagellum during hyperactivated motility [42]. We found lower ATP levels in PP1γ1 rescue sperm and a poor ability to utilize substrates. That could possibly be a consequence of altered energy production, either through low levels of glycolysis or poor oxidative phosphorylation. Inefficient ATP production results in diminished hyperactivation capability and fertility [43]. The possibility that phosphorylation of one or more glycolytic enzymes is altered in PP1γ1 rescue sperm may provide an explanation for the observed results. Little is known about the role of PP1γ2 in metabolism and energy production in spermatozoa. Our data indicate a possible link, yet more investigation is needed to establish this relationship. Efforts to identify the phosphoproteome of sperm from PP1γ1 rescue compared to wild-type mice are underway.
An important question posed by our study is why PP1γ1 does not support normal sperm function while it can replace PP1γ2 to support spermatogenesis. As discussed earlier, localization of PP1γ1 compared to PP1γ2 is altered within sperm as also its association with the PP1 regulators. In the case of PP1γ2, the anticipation so far has been that specific binding proteins for the enzyme exist in sperm. Our previous work thus focused on identifying specific binding proteins for the PP1γ2 isoform in sperm. Surprisingly, all the binding proteins for PP1γ2 we have identified so far are proteins that are ubiquitous and those that are known to bind to all PP1 isoforms [15, 44, 45]: Sds22 (PPP1R7), inhibitor2 (PPP1R2), and inhibitor 3 (PPP1R11). These three protein regulators are ubiquitous but expressed at high levels in testis. They are known to bind PP1γ1, PP1α, and PP1β in the brain and other tissues. It is intriguing that these ubiquitous PP1 regulators participate in the regulation of sperm-specific PP1γ2. Binding of PP1γ1 to the regulators is altered compared to normal wild-type sperm. Why PP1R7 is found, presumably bound to PP1γ1, in the insoluble fraction whereas it is bound to PP1γ2 in the soluble fraction is not known. It is possible that phosphorylation of the inhibitors is altered in sperm from rescue mice. Whether reduced GSK3 phosphorylation, which is expected to increase its catalytic activity, is responsible for the altered phosphorylation of the PP1 regulators remains to be determined.
Data in this report also suggest an alternate explanation for the functional nonequivalence of PP1γ2 and PP1γ1 in sperm. It is likely that the differences in the functions of PP1γ1 of PP1γ2 in sperm are due to differences in the affinities of their binding to scaffolding and regulatory proteins. That is, it appears unlikely that PP1γ2 binds to specific proteins because the binding proteins identified thus far, PPP1R2, PPP1R11, PPP1R7, and protein phosphatase 1 regulatory subunit 36 (PPP1R36), should bind to all PP1 isoforms [46–48]. Thus, PP1γ1 should bind to these same proteins but with differing affinities. It is possible that a consequence of this differential binding affinity is the difference in the distribution of PP1γ1 in the soluble and insoluble fractions of sperm extracts and in the intra sperm localization of PP1γ1 compared to PP1γ2. Altered localization should result in altered catalytic activity which may lead to impaired epididymal sperm maturation and fertilization. Altered phosphorylation of sperm proteins is likely the result of substitution of PP1γ1 for PP1γ2. We predict that similar to PP1γ1, either the PP1α or PP1β isoform should be able to substitute for PP1γ2 in testis to support spermatogenesis but not in sperm. It appears that all three isoforms are excluded from expression in developing spermatocytes and spermatids because they are not functionally identical to PP1γ2 in supporting normal sperm function. This may also be the reason for the presence of PP1γ2 in sperm from all mammals. It is tempting to speculate that PP1γ2 has specific functional roles in supporting optimal sperm function in mammals: epididymal sperm maturation, sperm hyperactivation, and energy generation before and during fertilization. How PP1γ2, but not the other three PP1 isoforms, fulfills these function requires further study. The PP1γ1 transgenic rescue line we have generated should be valuable for these studies.
In summary, we show that PP1γ1 not normally present in developing germ cells when transgenically expressed can support spermatogenesis, whereas the PP1γ2 has isoform-specific functions in supporting normal sperm motility and male fertility.
Supplementary Material
Acknowledgment
We would like to thank Dr Donner Babcock for helping us with sperm motility tracings and insightful discussions.
Notes
Edited by Dr. Kyle Orwig
Footnotes
Grant Support: This study was supported by National Institute of Health Grants R15HD068971 and R21HD086839 (SV).
Supplementary data
Supplementary Figure S1. Testis-specific expression of Pgk2 promoter-driven Rescue IV transgene. (A) Western blot analysis of various tissues form Ppp1cc (–/–), Pp1γ1 mice probed with PP1γ1 antibody shows transgenic PP1γ1 expressed specifically in testis and absent in other tissues. Equal protein was loaded for all tissues. (B) Western blot analysis of various tissues form Ppp1cc (–/–), Pp1γ1 mice and testis from Ppp1cc (+/–) mice probed with PP1γ2 antibody. None of the Ppp1cc (–/–) tissues including testis show PP1γ2 but it abundantly expressed in Ppp1cc (+/–). Equal protein was loaded for all tissues.
Supplementary Table S1. Phenotypes and fertility of all the Rescue IV lines compared to Ppp1cc (+/–).
References
- 1. Barker HM, Craig SP, Spurr NK, Cohen PT. Sequence of human protein serine/threonine phosphatase 1 gamma and localization of the gene (PPP1CC) encoding it to chromosome bands 12q24.1-q24.2. Biochim Biophy Acta 1993; 1178:228–233. [DOI] [PubMed] [Google Scholar]
- 2. Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 2004; 84:1–39. [DOI] [PubMed] [Google Scholar]
- 3. Liu R, Correll RN, Davis J, Vagnozzi RJ, York AJ, Sargent MA, Nairn AC, Molkentin JD. Cardiac-specific deletion of protein phosphatase 1β promotes increased myofilament protein phosphorylation and contractile alterations. J Mol Cell Cardiol 2015; 87:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raghavan S, Williams I, Aslam H, Thomas D, Szöor B, Morgan G, Gross S, Turner J, Fernandes J, VijayRaghavan K, Alphey L. Protein phosphatase 1beta is required for the maintenance of muscle attachments. Curr Biol 2000; 10:269–272. [DOI] [PubMed] [Google Scholar]
- 5. Gibbons JA, Kozubowski L, Tatchell K, Shenolikar S. Expression of human protein phosphatase-1 in Saccharomyces cerevisiae highlights the role of phosphatase isoforms in regulating eukaryotic functions. J Biol Chem 2007; 282:21838–21847. [DOI] [PubMed] [Google Scholar]
- 6. Chakrabarti R, Kline D, Lu J, Orth J, Pilder S, Vijayaraghavan S. Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biol Reprod 2007; 76:992–1001. [DOI] [PubMed] [Google Scholar]
- 7. Sinha N, Pilder S, Vijayaraghavan S. Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PLoS One 2012; 7:e47623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varmuza S, Jurisicova A, Okano K, Hudson J, Boekelheide K, Shipp EB. Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1cgamma gene. Dev Biol 1999; 205:98–110. [DOI] [PubMed] [Google Scholar]
- 9. Forgione N, Vogl AW, Varmuza S. Loss of protein phosphatase 1c{gamma} (PPP1CC) leads to impaired spermatogenesis associated with defects in chromatin condensation and acrosome development: An ultrastructural analysis. Reproduction 2010; 139:1021–1029. [DOI] [PubMed] [Google Scholar]
- 10. Sinha N, Puri P, Nairn AC, Vijayaraghavan S. Selective ablation of Ppp1cc gene in testicular germ cells causes oligo-teratozoospermia and infertility in mice. Biol Reprod 2013; 89:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang LP, Stroud J, Eddy CA, Walter CA, McCarrey JR. Multiple elements influence transcriptional regulation from the human testis-specific PGK2 promoter in transgenic mice. Biol Reprod 1999; 60:1329–1337. [DOI] [PubMed] [Google Scholar]
- 12. Dudiki T, Kadunganattil S, Ferrara JK, Kline DW, Vijayaraghavan S. Changes in carboxy methylation and tyrosine phosphorylation of protein phosphatase PP2A are associated with epididymal sperm maturation and motility. PLoS One 2015; 10:e0141961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puri P, Myers K, Kline D, Vijayaraghavan S. Proteomic analysis of bovine sperm YWHA binding partners identify proteins involved in signaling and metabolism. Biol Reprod 2008; 79:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiedler SE, Dudiki T, Vijayaraghavan S, Carr DW. Loss of R2D2 proteins ROPN1 and ROPN1L causes defects in murine sperm motility, phosphorylation, and fibrous sheath integrity. Biol Reprod 2013; 88:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng L, Pilder S, Nairn AC, Ramdas S, Vijayaraghavan S. PP1gamma2 and PPP1R11 are parts of a multimeric complex in developing testicular germ cells in which their steady state levels are reciprocally related. PLoS One 2009; 4:e4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meistrich ML, Hess RA. Assessment of spermatogenesis through staging of seminiferous tubules. Methods Mol Biol 2013; 927:299–307. [DOI] [PubMed] [Google Scholar]
- 17. Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, Greengard P. Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biol Reprod 1996; 54:709–718. [DOI] [PubMed] [Google Scholar]
- 18. Danshina PV, Geyer CB, Dai Q, Goulding EH, Willis WD, Kitto GB, McCarrey JR, Eddy EM, O’Brien DA. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod 2010; 82:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H, Brautigan DL. A novel transmembrane Ser/Thr kinase complexes with protein phosphatase-1 and inhibitor-2. J Biol Chem 2002; 277:49605–49612. [DOI] [PubMed] [Google Scholar]
- 20. Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol 2005; 25:292–303. [DOI] [PubMed] [Google Scholar]
- 21. Hagaman JR, Moyer JS, Bachman ES, Sibony M, Magyar PL, Welch JE, Smithies O, Krege JH, O’Brien DA. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci USA 1998; 95:2552–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA 2004; 101:13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, Herr JC. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol 2007; 302:463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey JR, Oh SP, Terada N. Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem 2007; 282:29658–29666. [DOI] [PubMed] [Google Scholar]
- 25. Kessler SP, Rowe TM, Gomos JB, Kessler PM, Sen GC. Physiological non-equivalence of the two isoforms of angiotensin-converting enzyme. J Biol Chem 2000; 275:26259–26264. [DOI] [PubMed] [Google Scholar]
- 26. Tang H, Duan C, Bleher R, Goldberg E. Human lactate dehydrogenase A (LDHA) rescues mouse Ldhc-null sperm function. Biol Reprod 2013; 88:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fardilha M, Esteves SL, Korrodi-Gregorio L, Vintem AP, Domingues SC, Rebelo S, Morrice N, Cohen PT, da Cruz e Silva OA, da Cruz e Silva EF. Identification of the human testis protein phosphatase 1 interactome. Biochem Pharmacol 2011; 82:1403–1415. [DOI] [PubMed] [Google Scholar]
- 28. Silva JV, Freitas MJ, Felgueiras J, Fardilha M. The power of the yeast two-hybrid system in the identification of novel drug targets: building and modulating PPP1 interactomes. Expert Rev Proteomics 2015; 12:147–158. [DOI] [PubMed] [Google Scholar]
- 29. Hrabchak C, Henderson H, Varmuza S. A testis specific isoform of endophilin B1, endophilin B1t, interacts specifically with protein phosphatase-1cγ2 in mouse testis and is abnormally expressed in PP1cγ null mice. Biochemistry 2007; 46:4635–4644. [DOI] [PubMed] [Google Scholar]
- 30. Hrabchak C, Varmuza S. Identification of the spermatogenic zip protein Spz1 as a putative protein phosphatase-1 (PP1) regulatory protein that specifically binds the PP1cgamma2 splice variant in mouse testis. J Biol Chem 2004; 279:37079–37086. [DOI] [PubMed] [Google Scholar]
- 31. MacLeod G, Varmuza S. Tandem affinity purification in transgenic mouse embryonic stem cells identifies DDOST as a novel PPP1CC2 interacting protein. Biochemistry 2012; 51:9678–9688. [DOI] [PubMed] [Google Scholar]
- 32. Comazzetto S, Di Giacomo M, Rasmussen KD, Much C, Azzi C, Perlas E, Morgan M, O’Carroll D. Oligoasthenoteratozoospermia and infertility in mice deficient for miR-34b/c and miR-449 loci. PLoS Genet 2014; 10:e1004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu J, Bao J, Kim M, Yuan S, Tang C, Zheng H, Mastick GS, Xu C, Yan W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc Natl Acad Sci USA 2014; 111:E2851–E2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuan S, Tang C, Zhang Y, Wu J, Bao J, Zheng H, Xu C, Yan W. mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol Open 2015; 4:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ros D, G. V, Munoz MW, Battistone MA, Brukman NG, Carvajal G, Curci L, Gomez-ElIas MD, Cohen DB, Cuasnicu PS. From the epididymis to the egg: participation of CRISP proteins in mammalian fertilization. Asian J Androl 2015; 17:711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alvau A, Battistone MA, Gervasi MG, Navarrete FA, Xu X, Sanchez-Cardenas C, De la Vega-Beltran JL, Da Ros VG, Greer PA, Darszon A, Krapf D, Salicioni AM, Cuasnicu PS et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016; 143:2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsunoda S, Kawano N, Miyado K, Kimura N, Fujii J. Impaired fertilizing ability of superoxide dismutase 1-deficient mouse sperm during in vitro fertilization. Biol Reprod 2012; 87:121. [DOI] [PubMed] [Google Scholar]
- 38. Bhattacharjee R, Goswami S, Dey S, Gangoda M, Brothag C, Eisa A, Woodgett J, Phiel C, Kline D, Vijayaraghavan S. Isoform specific requirement for GSK3alpha in sperm for male fertility. Biol Reprod 2018; 99:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhattacharjee R, Goswami S, Dudiki T, Popkie AP, Phiel CJ, Kline D, Vijayaraghavan S. Targeted disruption of glycogen synthase kinase 3A (GSK3A) in mice affects sperm motility resulting in male infertility. Biol Reprod 2015; 92:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tourmente M, Villar-Moya P, Rial E, Roldan ER. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J Biol Chem 2015; 290:20613–20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takei GL, Miyashiro D, Mukai C, Okuno M. Glycolysis plays an important role in energy transfer from the base to the distal end of the flagellum in mouse sperm. J Exp Biol 2014; 217:1876–1886. [DOI] [PubMed] [Google Scholar]
- 42. du Plessis SS, Agarwal A, Mohanty G, van der Linde M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J Androl 2015; 17:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Odet F, Gabel S, London RE, Goldberg E, Eddy EM. Glycolysis and mitochondrial respiration in mouse LDHC-null sperm. Biol Reprod 2013; 88:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang Z, Khatra B, Bollen M, Carr DW, Vijayaraghavan S. Sperm PP1gamma2 is regulated by a homologue of the yeast protein phosphatase binding protein sds22. Biol Reprod 2002; 67:1936–1942. [DOI] [PubMed] [Google Scholar]
- 45. Korrodi-Gregorio L, Ferreira M, Vintem AP, Wu W, Muller T, Marcus K, Vijayaraghavan S, Brautigan DL, da Cruz ESOA, Fardilha M, da Cruz ESEF. Identification and characterization of two distinct PPP1R2 isoforms in human spermatozoa. BMC Cell Biol 2013; 14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goswami S, Korrodi-Gregorio L, Sinha N, Bhutada S, Bhattacharjee R, Kline D, Vijayaraghavan S. Regulators of the protein phosphatase PP1gamma2, PPP1R2, PPP1R7, and PPP1R11 are involved in epididymal sperm maturation. J Cell Physiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang Z, Myers K, Khatra B, Vijayaraghavan S. Protein 14-3-3zeta binds to protein phosphatase PP1gamma2 in bovine epididymal spermatozoa. Biol Reprod 2004; 71:177–184. [DOI] [PubMed] [Google Scholar]
- 48. Zhang Q, Gao M, Zhang Y, Song Y, Cheng H, Zhou R. The germline-enriched Ppp1r36 promotes autophagy. Sci Rep 2016; 6:24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.