Abstract
Background
Fucoidan is derived from seaweed widely used in Japanese cuisine, but little is known about its influence on glucose metabolism. To obtain information about the physiological effects of fucoidan on glucose metabolism, the digestive system, and the gustatory system controlling taste sensation in patients with type 2 diabetes, we conducted a randomized, double-blind, placebo-controlled study.
Methods
Thirty patients with type 2 diabetes on diet therapy were recruited from an outpatient clinic (22 men and 8 women aged 59.10 ± 13.24 years, body mass index: 25.18 ± 3.88, hemoglobin A1c: 7.04 ± 1.24%). They were divided into 2 groups and underwent 2 interventions with a 4-week interval. One group received fucoidan for 12 weeks (a daily 60 mL test beverage containing 1,620 mg of fucoidan) and then placebo (60 mL) for the subsequent 12-week period, while the order was reversed in the other group. Evaluation was performed just before and after each intervention. Taste sensitivity was measured for 5 basic tastes by the filter paper disk method and food intake was evaluated with a validated diet questionnaire.
Results
No adverse events occurred during the study period. Despite no change of the diet, stool frequency increased during fucoidan intake (from 7.78 ± 4.64/week in Week 1 to 9.15 ± 5.03/week in Week 5, P < 0.001), and it increased more in lean subjects. In 11 subjects whose stool frequency exceeded the mean value, the thresholds for sweet, salty, bitter and umami tastes were significantly reduced (enhancement of sensitivity) after fucoidan intake. In 14 subjects with normal HOMA-IR (homeostatic model assessment of insulin resistance, < 2.5), hemoglobin A1c decreased after fucoidan intake (from 6.73 ± 1.00 to 6.59 ± 1.00%, P < 0.05), as did the fasting plasma level of GLP-1 (glucagon-like peptide-1, from 6.42 ± 3.52 to 4.93 ± 1.88 pmol/L, P < 0.05).
Conclusion
Sustained fucoidan intake led to alterations of gastrointestinal function, including increased stool frequency and enhanced taste sensitivity, which could contribute to better control of diabetes.
Keywords: dietary fiber, fucoidan, glucagon-like peptide1, taste sensitivity, type 2 diabetes mellitus
In dietary therapy for diabetes, appropriate energy intake, nutritional balance and timing of meals are considered to be important factors.1–3 With regard to nutritional balance, it is important for the diet to contain a certain amount of fiber in addition to appropriate amounts of carbohydrates and lipids.4 To achieve sufficient intake of dietary fiber, it has been reported that premodern firm foods with high dietary fiber content are more suitable than modern Westernized soft foods with low dietary fiber content.5
Regarding dietary fiber, it has been reported that soluble dietary fiber, which seaweed contains in large amounts, slows the absorption of carbohydrate and improves the intestinal environment including bacterial flora.6–10 In particular, microbiota-accessible carbohydrate (MAC) in dietary fiber is involved in growth of the intestinal flora and regulation of the intestinal environment.11 Furthermore, it has been reported that expression of the intestinal sweet taste receptor is altered in type 2 diabetes,12 which is one of the possible links between deterioration of the intestinal environment affecting taste receptors to alter their sensitivity and impairment of carbohydrate/lipid metabolism such as insulin resistance, obesity and diabetes.13, 14
In the present study, we focused on high molecular weight fucoidan extracted from “mozuku,” a seaweed commonly used in healthy Japanese dishes.15, 16 Animal studies have shown that fucoidan with low molecular form influences glucose metabolism, including the promotion of insulin secretion, pancreatic protection, improvement of insulin resistance and inhibiting the progression of diabetic nephropathy.17–23 However, only a few animal studies using high molecular weight fucoidan were performed so far because of its rather labile nature despite an ample history of use as foods in humans. In fact, high molecular weight fucoidan was reported to increase intestinal peristalsis in humans,24 whereas its effects on diabetes and glucose metabolism have not been investigated so far.
Accordingly, to clarify the effects of high molecular weight fucoidan on diabetes when administered for a long period, we planned a randomized, double-blind, placebo-controlled study in patients who had not received pharmacotherapy for diabetes. We investigated the effects of long-term intake of high molecular weight fucoidan on various biochemical parameters of diabetic control. In order to assess the influence of the intestinal environment on taste sensation, we also analyzed the relation between gustatory function and intake of fucoidan.
SUBJECTS AND METHODS
Subjects
Out of the patients with type 2 diabetes attending the outpatient clinic of Hakuai Hospital, Japan from March through April 2015 who were on diet therapy only, 30 patients who gave consent were enrolled in the study. They included 22 men and 8 women aged 59.10 ± 13.24 years (mean ± SD, range: 30–79 years), with a body mass index (BMI) of 25.18 ± 3.88 kg/m2, hemoglobin A1c (HbA1c) of 7.04 ± 1.24% and fasting plasma glucose (FPG) of 7.59 ± 2.21 mmol/L.
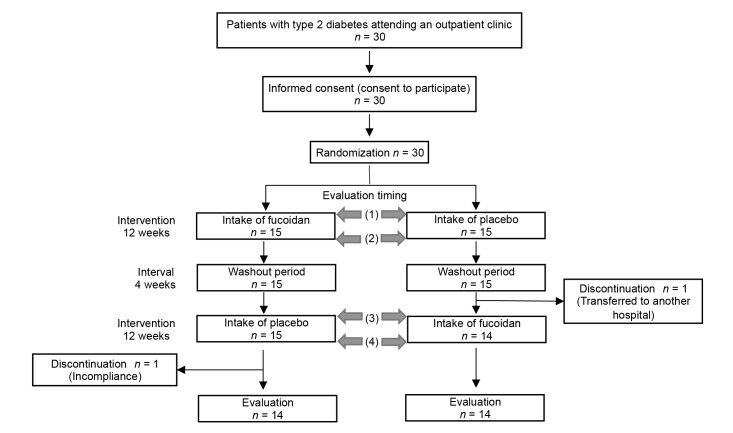
Out of the 30 subjects, two subjects discontinued the study before completion (one subject failed to ingest the study drink as instructed, and the other subject was transferred to another hospital), and 28 subjects were included in the analysis (Fig. 1). Data for discontinued subjects were excluded from analysis.
Fig. 1.
Study design. This was a randomized, double-blind, placebo-controlled, cross-over study. Evaluations were conducted at times (1) to (4). Evaluations included the diet history, number of bowel movements, stool characteristics, clinical parameters, biochemistry tests and assessment of the taste threshold (sweetness, saltiness, sourness, bitterness and umami).
Study design and intervention
This was a randomized, double-blind, placebo-controlled, cross-over study (Clinical trial registration number: UMIN 000031171).
The subjects received numbers in the order of giving informed consent, and subjects with odd numbers were assigned to Group A while those with even numbers were assigned to Group B. Group A ingested fucoidan for the first intervention, followed by placebo for the second intervention, while the order was reversed in Group B. The first intervention involved daily intake of fucoidan or placebo for 12 weeks and was followed by a 4-week washout period, after which the study treatments were switched for the second 12-week intervention period (Fig. 1).
Study treatments (fucoidan and placebo)
Fucoidan
The fucoidan beverage (Fucomin, Marine Products Kimuraya , Sakaiminato, Japan) used as the study drug contained 810 mg (dry weight) of high molecular weight fucoidan (molecular weight: 240,000, sulfate content: 13%) per 30 mL.15, 16 One dose of this beverage (30 mL) was taken before breakfast and dinner (60 mL/day). The safety of fucoidan was previously confirmed up to 4,050 mg/day in an overdose study conducted by Abe et al.25
Placebo
The placebo beverage (30 mL twice daily= 60 mL/day) contained purified water and starch for viscosity. Its color was adjusted with synthetic colorant until the appearance was identical to the active beverage. Because fucoidan does not have any taste or smell, taste was not adjusted.
Parameters evaluated
Evaluation of the following parameters was conducted before and after the first and second interventions (Fig. 1).
Food intake
Food intake was evaluated by using the brief-type self-administered diet history questionnaire. This questionnaire assesses food intake over a 1-month period, and the mean time required to answer it is approximately 15 minutes.26, 27 Based on the responses submitted, a nutritional value program was used to calculate the daily intake of 30 different nutrients and 50 different foods for each subject.
Bowel movements and stool characteristics
During the intervention period, the presence/absence of abdominal signs, number of bowel movements, and stool characteristics were documented by the subjects using record sheets. The number of bowel movements was evaluated on a weekly basis. Stool characteristics were recorded by referring to illustrations showing scales for hardness and color (darkness).28 Hardness was classified into 6 grades ranging from 1 (soft) to 6 (hard), while color was classified into 7 grades ranging from light brown (1) to dark brown (7). The stool record sheets were collected every 4 weeks.
Clinical data and biochemical parameters
Clinical data were collected from the medical records, including the height, weight, BMI, body fat, abdominal circumference and blood pressure. A fasting morning blood sample was collected to measure baseline levels of HbA1c, fasting plasma glucose (FPG), triglycerides, total cholesterol, high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), aspartate transaminase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), baseline glucagon-like peptide-1 (GLP-1), high molecular weight (HMW) adiponectin, insulin, leptin and zinc.
The plasma GLP-1 level was measured by a solid-phase enzyme immunoassay kit (GLP-1 Active Form Assay Kit-IBL, Immuno-Biological Laboratories, Fujioka, Japan) using a blood sample collected in a tube containing an anticoagulant and protease inhibitors (BDTM P800 Blood Collection System, NJ). Serum HMW adiponectin and leptin levels were measured with enzyme-linked immunosorbent assay kits (High Molecular Weight Adiponectin ELISA Kit, Otsuka Pharmaceutical, Tokyo, Japan; and Human Leptin Quantikine ELISA Kit, R&D Systems, MN, respectively).
The homeostatic model assessment of insulin resistance (HOMA-IR) was used as the index to measure insulin resistance. HOMA-IR was calculated as fasting insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5.
Taste threshold
Thresholds for recognition of the 5 basic tastes (sweetness, saltiness, sourness, bitterness and umami) were evaluated by the filter paper disk method. A filter paper disk (5 mm in diameter) soaked with a taste solution was applied to the tip of the tongue, which is innervated by the chorda tympani nerve, for 3 seconds with the mouth open. Taste solutions of 5 different concentrations were applied in order from low to high, and the taste recognition threshold was defined as the concentration that the subject identified correctly. The threshold was classified as Concentration 1 to Concentration 5 in the order of low to high concentrations, and the result was recorded as Concentration 6 if a subject could not identify the taste at Concentration 5.
Assessment was conducted in the order of sweetness, saltiness, sourness, umami and bitterness. To eliminate residual taste, the subjects gargled with water and a one-minute interval was set between each taste test.
Taste Discs (Sanwa Kagaku Kenkyusho, Nagoya, Japan) were used to test sweetness, saltiness, sourness and bitterness.29–31 For umami, Ajinomoto (Ajinomoto, Tokyo, Japan) containing 97.5% monosodium L-glutamate and 2.5% 5-ribonucleotide sodium was used (Table 1).
Table 1.
Reagents and solutions for the taste test
| Concentration levels | ||||||
| Taste type | Reagent | 1 | 2 | 3 | 4 | 5 |
| Sweetness | Purified white sugar (g/dL) | 0.3 | 2.5 | 10 | 20 | 80 |
| Saltiness | Sodium chloride (g/dL) | 0.3 | 1.25 | 5 | 10 | 20 |
| Sourness | Tartaric acid (g/dL) | 0.02 | 0.2 | 2 | 4 | 8 |
| Bitterness | Quinine hydrochloride (g/dL) | 0.01 | 0.02 | 0.1 | 0.5 | 4 |
| Umami | Monosodium glutamate (g/dL) | 0.03 | 0.1 | 0.25 | 0.5 | 1 |
Subjects were asked to fast from 21:00 on the day before testing. Smoking was prohibited from 30 minutes prior to testing. In order to reduce the influence of environmental factors, the test was done in a quiet room at a temperature of 25ºC.
Statistical Analysis
SPSS ver. 21.0 was used for statistical analysis. Results are expressed as the mean ± SD. The paired t-test was used for comparison between before and after intervention, while the unpaired t-test was used for intergroup comparisons. Multivariate analysis (multiple regression analysis) was conducted using HbA1c as a dependent variate. In all analyses, the level of significance was 5%.
Ethical considerations
Patients received written and oral explanations about the purpose and methods of this study, and written informed consent was obtained from all subjects prior to enrolment.
This study was approved by the Institutional Review Board of Tottori University Faculty of Medicine (February 2015, Approval ID: 2642) and was performed according to the Declaration of Helsinki.
RESULTS
Food intake
Daily food intakes including seaweeds did not show any significant difference between fucoidan and placebo groups (seaweeds: 12.37 ± 10.88 vs 9.87 ± 11.96 g/day). Intake of fucoidan or placebo did not lead to any significant changes of food intake in the subjects.
Bowel movements and stool characteristics
The number of bowel movements increased significantly with fucoidan intake (7.78 ± 4.64 /week in Week 1, 9.15 ± 5.03 /week in Week 5, P < 0.001). The change in the number of bowel movements (difference between Weeks 1 and 5) was also significantly larger with fucoidan intake (+1.37 ± 1.86 /week with fucoidan intake versus +0.20 ± 1.61 /week with placebo intake, P < 0.05, Table 2). Stool hardness and color were not significantly different between fucoidan and placebo intake.
Table 2.
Changes of bowel movements and stool characteristics during the intervention period
| Intervention | n | Week 1 | Week 5 | Week 5 - Week 1 | |
| No. of bowel movements (times/w) | Fucoidan intake | 27 | 7.78 ± 4.64** | 9.15 ± 5.03** | +1.37 ± 1.86* |
| Placebo intake | 26 | 6.89 ± 2.69 | 7.23 ± 3.00 | +0.20 ± 1.61* | |
| Stool hardness (grades 1–6) | Fucoidan intake | 27 | 3.15 ± 0.94 | 3.24 ± 0.72 | +0.24 ± 0.81 |
| Placebo intake | 25 | 3.30 ± 0.86 | 3.20 ± 0.99 | +0.08 ± 0.78 | |
| Stool color (grades 1–7) | Fucoidan intake | 26 | 3.13 ± 1.28 | 3.15 ± 1.02 | -0.19 ± 1.18 |
| Placebo intake | 26 | 3.20 ± 0.97 | 3.07 ± 1.05 | -0.30 ± 0.63 |
*: P < 0.05, **: P < 0.005
The number of bowel movements per week is shown, and stool hardness and color are the mean results for 1 week.
Clinical data and biochemical parameters
Analysis of the overall study population
After intake of fucoidan or placebo, there were no significant changes of the BMI, blood pressure, fasting plasma glucose, HbA1c, serum lipids, hepatic function and zinc. After fucoidan intake, a significant decrease of the baseline GLP-1 and HDL-C levels was observed. The leptin level was significantly increased after placebo intervention (Table 3).
Table 3.
Parameters before and after each intervention (overall study population)
| Parameter | n | Fucoidan intake | n | Placebo intake | |||
| Before | After | Before | After | ||||
| Clinical | Body weight (kg) | 28 | 69.00 ± 13.42 | 68.71 ± 13.28 | 26 | 70.30 ± 13.32 | 69.96 ± 13.36 |
| BMI (kg/m2) | 23 | 25.01 ± 4.03 | 24.89 ± 4.07 | 22 | 25.94 ± 3.77 | 25.77 ± 3.69 | |
| Body fat (%) | 22 | 28.15 ± 6.24 | 28.17 ± 6.40 | 22 | 29.79 ± 6.58 | 29.90 ± 6.28 | |
| Waist circumference (cm) | 22 | 88.92 ± 11.22 | 89.20 ± 13.40 | 22 | 89.67 ± 10.54 | 89.76 ± 11.56 | |
| Systolic blood pressure (mmHg) | 19 | 135.00 ± 14.82 | 135.74 ± 17.85 | 19 | 136.63 ± 18.25 | 134.53 ± 15.06 | |
| Diastolic blood pressure (mmHg) | 19 | 77.68 ± 14.96 | 76.74 ± 11.74 | 19 | 79.74 ± 10.86 | 77.63 ± 11.62 | |
| Biochemical | FPG (mmol/L) | 28 | 7.60 ± 2.33 | 7.22 ± 1.67 | 26 | 7.05 ± 1.61 | 7.33 ± 1.78 |
| HbA1c (%) | 28 | 6.99 ± 1.31 | 6.93 ± 1.21 | 27 | 6.85 ± 1.06 | 6.89 ± 1.12 | |
| Insulin (pmol/mL) | 28 | 57.60 ± 46.20 | 58.20 ± 39.00 | 26 | 63.60 ± 64.80 | 53.40 ± 28.20 | |
| HOMA-IR | 28 | 3.08 ± 2.36 | 3.07 ± 2.02 | 26 | 3.33 ± 3.36 | 2.90 ± 1.61 | |
| Leptin (nmol/L) | 28 | 0.45 ± 0.38 | 0.50 ± 0.38 | 26 | 0.46 ± 0.34* | 0.52 ± 0.35* | |
| HMW-adiponectin (μmol/L) | 28 | 0.21 ± 0.18 | 0.21 ± 0.20 | 26 | 0.19 ± 0.21 | 0.19 ± 0.19 | |
| GLP-1 (pmol/L) | 28 | 6.29 ± 3.47* | 5.18 ± 2.70* | 26 | 5.95 ± 1.98 | 5.71 ± 2.31 | |
| LDL-C (mmol/L) | 25 | 3.18 ± 0.85 | 3.15 ± 0.70 | 24 | 3.13 ± 0.70 | 3.18 ± 0.72 | |
| HDL-C (mmol/L) | 24 | 1.56 ± 0.54* | 1.48 ± 0.44* | 23 | 1.49 ± 0.49 | 1.53 ± 0.49 | |
| Triglyceride (mmol/L) | 24 | 1.51 ± 0.88 | 1.57 ± 0.99 | 23 | 1.75 ± 1.30 | 1.68 ± 1.05 | |
| T-cholesterol (mmol/L) | 15 | 5.61 ± 1.19 | 5.46 ± 0.80 | 13 | 5.46 ± 0.67 | 5.48 ± 0.75 | |
| AST (U/L) | 18 | 23.17 ± 12.56 | 28.06 ± 22.52 | 15 | 30.07 ± 21.98 | 29.53 ± 20.62 | |
| ALT (U/L) | 19 | 29.68 ± 25.30 | 32.26 ± 30.48 | 15 | 37.73 ± 33.34 | 36.93 ± 27.46 | |
| γ-GTP (U/L) | 19 | 49.84 ± 44.46 | 52.21 ± 59.88 | 15 | 54.60 ± 55.60 | 57.27 ± 65.73 | |
| Zinc (mmol/L) | 28 | 12.48 ± 1.29 | 12.03 ± 1.35 | 26 | 12.76 ± 1.62 | 12.58 ± 1.45 | |
Data are the mean ± SD; *: P < 0.05.
AST, aspartate transaminase; ALT, alanine aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; γ-GTP, γ-glutamyl transpeptidase; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein-cholesterol; HMW, high molecular weight; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low density lipoprotein-cholesterol; T-cholesterol, total cholesterol.
Taste thresholds of the 28 subjects before the intervention were compared with those of 108 healthy adults reported in a literature,32 which showed significant elevation in the subjects with diabetes for sweetness (3.36 ± 1.50 vs 2.44 ± 1.12, P < 0.01) and sourness (3.18 ± 1.31 vs 2.59 ± 1.07, P < 0.05).
Analysis of subjects whose bowel movements increased during fucoidan intake
Since there were marked individual variations in the response of bowel movements to fucoidan, clinical data were compared before and after fucoidan intake in 11 subjects (7 men and 4 women) in whom the mean increase of bowel movements was above average. No significant changes of the parameters occurred after placebo intervention, but fucoidan intervention resulted in a significant increase of leptin and a significant decrease of the baseline GLP-1 level (leptin: before: 0.36 ± 0.30 nmol/L, after: 0.51 ± 0.43 nmol/L, baseline GLP-1: before: 5.70 ± 1.76 pmol/L, after: 4.76 ± 2.22 pmol/L, Table 4).
Table 4.
Parameters before and after intervention (subgroup with an increase of bowel movements after fucoidan)
| Parameter | n | Fucoidan intake | n | Placebo intake | |||
| Before | After | Before | After | ||||
| Clinical | Body weight (kg) | 11 | 65.44 ± 7.48 | 64.76 ± 8.04 | 11 | 65.64 ± 7.52 | 65.28 ± 7.89 |
| BMI (kg/m2) | 11 | 24.95 ± 2.71 | 24.69 ± 2.81 | 11 | 25.03 ± 2.76 | 25.02 ± 2.79 | |
| Body fat (%) | 9 | 28.24 ± 6.23 | 27.90 ± 6.43 | 11 | 28.78 ± 7.97 | 29.41 ± 7.36 | |
| Waist circumference (cm) | 10 | 86.12 ± 5.97 | 87.28 ± 6.64 | 11 | 87.16 ± 5.76 | 86.00 ± 7.49 | |
| Systolic blood pressure (mmHg) | 9 | 135.22 ± 17.06 | 135.22 ± 16.97 | 5 | 147.00 ± 22.55 | 141.40 ± 19.27 | |
| Diastolic blood pressure (mmHg) | 9 | 80.33 ± 16.78 | 75.67 ± 14.35 | 5 | 77.80 ± 8.90 | 72.60 ± 13.45 | |
| Biochemical | FPG (mmol/L) | 11 | 8.10 ± 2.05 | 7.83 ± 1.83 | 11 | 7.55 ± 2.00 | 7.99 ± 2.16 |
| HbA1c (%) | 11 | 7.58 ± 1.08 | 7.38 ± 1.00 | 11 | 7.36 ± 1.07 | 7.35 ± 1.20 | |
| Insulin (pmol/mL) | 11 | 43.20 ± 39.00 | 46.20 ± 26.40 | 11 | 45.00 ± 39.00 | 43.80 ± 25.20 | |
| HOMA-IR | 11 | 2.49 ± 1.90 | 2.62 ± 1.39 | 11 | 2.55 ± 2.16 | 2.61 ± 1.58 | |
| Leptin (nmol/L) | 11 | 0.36 ± 0.30* | 0.51 ± 0.43* | 11 | 0.40 ± 0.33 | 0.47 ± 0.39 | |
| HMW-adiponectin (μmol/L) | 11 | 0.23 ± 0.21 | 0.19 ± 0.19 | 11 | 0.21 ± 0.21 | 0.22 ± 0.23 | |
| GLP-1 (pmol/L) | 11 | 5.70 ± 1.76* | 4.76 ± 2.22* | 11 | 5.33 ± 1.75 | 5.12 ± 2.59 | |
| LDL-C (mmol/L) | 9 | 3.15 ± 0.85 | 3.10 ± 0.65 | 10 | 2.95 ± 0.80 | 3.13 ± 0.80 | |
| HDL-C (mmol/L) | 9 | 1.59 ± 0.75 | 1.57 ± 0.62 | 10 | 1.51 ± 0.59 | 1.57 ± 0.62 | |
| Triglyceride (mmol/L) | 9 | 1.76 ± 1.41 | 1.63 ± 1.41 | 10 | 1.80 ± 1.80 | 1.68 ± 1.05 | |
| T-cholesterol (mmol/L) | 6 | 5.51 ± 1.19 | 5.38 ± 0.80 | 4 | 4.73 ± 0.49 | 5.04 ± 0.75 | |
| AST (U/L) | 3 | 22.67 ± 12.50 | 27.33 ± 15.37 | 5 | 22.60 ± 9.13 | 23.60 ± 4.72 | |
| ALT (U/L) | 3 | 32.33 ± 18.77 | 36.33 ± 22.23 | 5 | 26.20 ± 16.00 | 26.80 ± 7.52 | |
| γ-GTP (U/L) | 3 | 42.00 ± 18.33 | 38.67 ± 20.43 | 5 | 34.40 ± 18.88 | 33.60 ± 14.28 | |
| Zinc (mmol/L) | 11 | 12.45 ± 0.87 | 12.01 ± 1.42 | 11 | 12.96 ± 1.47 | 12.59 ± 1.70 | |
Data are the mean ± SD; *: P < 0.05.
AST, aspartate transaminase; ALT, alanine aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; γ-GTP, γ-glutamyl transpeptidase; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein-cholesterol; HMW, high molecular weight; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low density lipoprotein-cholesterol; T-cholesterol, total cholesterol.
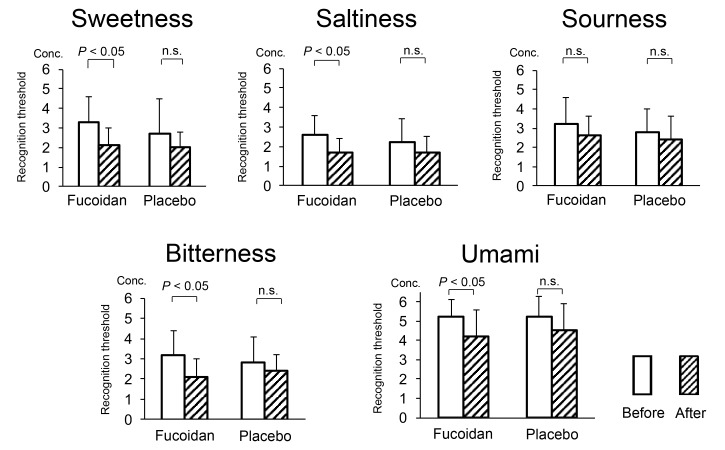
Regarding the taste threshold, significant decreases of the following thresholds were observed only after fucoidan intervention: sweetness (before: 3.30 ± 1.34, after: 2.10 ± 0.88), saltiness (before: 2.60 ± 0.97, after: 1.70 ± 0.67), bitterness (before: 3.20 ± 1.23, after: 2.10 ± 0.88) and umami (before: 5.20 ± 0.92, after: 4.20 ± 1.40, Fig. 2).
Fig. 2.
Comparison of the taste threshold before and after intervention (subgroup with an increase of bowel movements after fucoidan). Conc., Concentration; n.s., not significant.
Analysis of the subgroup with HOMA-IR < 2.5 before intervention
In the group with an above average increase of bowel movements, HOMA-IR values were lower before intervention compared to those of the group with a below average increase of bowel movements (group with above average increase of bowel movement: 2.62 ± 1.39 times/week, group with below average increase of bowel movement: 2.99 ± 2.14 times/week). Accordingly, clinical parameters were compared in 14 subjects (10 men and 4 women) in whom HOMA-IR was not above the normal range (< 2.5).
A significant decrease of HbA1c and the baseline GLP-1 level was observed only after fucoidan intake (HbA1c: before: 6.73 ± 1.00%, after: 6.59 ± 1.00%, baseline GLP-1: before: 6.42 ± 3.52 pmol/L, after: 4.93 ± 1.88 pmol/L). The leptin level increased significantly after intake of both fucoidan and placebo (Table 5).
Table 5.
Clinical parameters before and after intervention (subgroup with HOMA-IR < 2.5)
| Parameter | n | Fucoidan intake | n | Placebo intake | |||
| Before | After | Before | After | ||||
| Clinical | Body weight (kg) | 14 | 61.11 ± 7.94 | 60.31 ± 8.27 | 13 | 62.39 ± 8.62 | 62.31 ± 8.99 |
| BMI (kg/m2) | 11 | 22.92 ± 2.53 | 22.44 ± 2.17 | 13 | 23.35 ± 2.53 | 23.32 ± 2.54 | |
| Body fat (%) | 10 | 25.24 ± 5.86 | 24.75 ± 5.63 | 10 | 27.11 ± 5.88 | 27.30 ± 5.85 | |
| Waist circumference (cm) | 10 | 81.02 ± 7.67 | 81.08 ± 8.58 | 11 | 84.20 ± 8.95 | 83.59 ± 10.87 | |
| Systolic blood pressure (mmHg) | 12 | 131.00 ± 12.49 | 130.92 ± 14.64 | 11 | 134.00 ± 15.31 | 130.36 ± 12.40 | |
| Diastolic blood pressure (mmHg) | 12 | 75.67 ± 10.79 | 75.33 ± 10.59 | 11 | 78.18 ± 9.89 | 73.64 ± 6.96 | |
| Biochemical | FPG (mmol/L) | 14 | 7.05 ± 1.72 | 6.77 ± 1.72 | 13 | 7.16 ± 1.72 | 7.11 ± 1.94 |
| HbA1c (%) | 14 | 6.73 ± 1.00* | 6.59 ± 1.00* | 14 | 6.74 ± 1.00 | 6.61 ± 1.00 | |
| Insulin (pmol/mL) | 14 | 28.80 ± 10.20 | 36.60 ± 16.20 | 13 | 28.80 ± 9.60 | 34.20 ± 18.60 | |
| HOMA-IR | 14 | 1.47 ± 0.48 | 1.79 ± 0.77 | 13 | 1.52 ± 0.50 | 1.55 ± 1.14 | |
| Leptin (nmol/L) | 14 | 0.22 ± 0.16* | 0.31 ± 0.29* | 13 | 0.23 ± 0.16* | 0.31 ± 0.22* | |
| HMW-adiponectin (μmol/L) | 14 | 0.28 ± 0.27 | 0.27 ± 0.26 | 13 | 0.23 ± 0.26 | 0.21 ± 0.23 | |
| GLP-1 (pmol/L) | 14 | 6.42 ± 3.52* | 4.93 ± 1.88* | 13 | 6.01 ± 3.48 | 5.08 ± 2.41 | |
| LDL-C (mmol/L) | 13 | 3.15 ± 0.98 | 3.15 ± 0.75 | 12 | 3.03 ± 0.85 | 3.18 ± 0.72 | |
| HDL-C (mmol/L) | 13 | 1.86 ± 0.65 | 1.76 ± 0.49 | 11 | 1.81 ± 0.67 | 1.78 ± 0.59 | |
| Triglyceride (mmol/L) | 13 | 1.23 ± 0.87 | 0.99 ± 0.37 | 12 | 1.16 ± 0.87 | 1.11 ± 0.67 | |
| T-cholesterol (mmol/L) | 12 | 5.69 ± 1.19 | 5.38 ± 0.8 | 7 | 5.28 ± 0.91 | 5.25 ± 0.8 | |
| AST (U/L) | 3 | 16.00 ± 1.73 | 19.33 ± 2.08 | 4 | 21.00 ± 10.10 | 25.50 ± 5.69 | |
| ALT (U/L) | 4 | 20.25 ± 1.50 | 20.75 ± 3.30 | 4 | 22.00 ± 2.94 | 25.75 ± 5.85 | |
| γ-GTP (U/L) | 4 | 36.75 ± 10.69 | 34.75 ± 15.69 | 4 | 48.50 ± 22.75 | 58.00 ± 31.44 | |
| Zinc (mmol/L) | 14 | 12.50 ± 1.06 | 11.80 ± 1.04 | 13 | 12.47 ± 1.27 | 12.10 ± 1.38 | |
Data are the mean ± SD; *: P < 0.05.
AST, aspartate transaminase; ALT, alanine aminotransferase; BMI, body mass index; FPG, fasting plasma glucose; γ-GTP, γ-glutamyl transpeptidase; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein-cholesterol; HMW, high molecular weight; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low density lipoprotein-cholesterol; T-cholesterol, total cholesterol.
Multiple regression analysis
Multiple regression analysis was conducted using HbA1c after fucoidan intervention as a dependent variable and other parameters (leptin, HMW-adiponectin, baseline GLP-1 and HOMA-IR) after fucoidan intervention as independent variables. As a result, no parameter was significantly associated with an increase or decrease of HbA1c.
Adverse events
No adverse events were observed during the intervention period, including symptoms, objective signs and abnormal biochemistry data.
DISCUSSION
A relationship between diabetes and the gustatory system has attracted lots of attention since the discovery of taste receptors on the intestinal epithelium where augmentation of insulin secretion is provoked through the incretin pathway.33–37 Accordingly, this randomized, double-blind, placebo-controlled, cross-over study investigated the effects of long-term intake of high molecular weight fucoidan on clinical parameters, the intestinal environment, and the gustatory system controlling taste sensation in patients with type 2 diabetes.
Studies conducted so far have indicated that high intake of fucoidan is safe, and does not induce abnormalities of the gastrointestinal tract or stool condition.25 However, it has been reported that intestinal absorption of minerals, specifically zinc, is inhibited when a large amount of dietary fiber is taken for a long period.38, 39 Therefore, it was a concern that long-term intake of fucoidan, a form of dietary fiber, might reduce the serum zinc level,17 which may in turn lead to the impairment of taste sensation.40 As a results, there was no significant decrease of the serum zinc level with both fucoidan and placebo intake in this intervention study.
On the other hand, HDL-C and the baseline GLP-1 level both decreased significantly after intake of fucoidan. The decrease of GLP-1 is discussed below with the subgroup analysis. While the mechanism of these changes is unclear, the results suggested that both parameters should be monitored when fucoidan is used for a long period. However, none of the changes of clinical and biochemical parameters were classified as adverse events. Accordingly, it was considered that long-term intake of fucoidan is feasible in diabetic patients on diet therapy.
This study also demonstrated a significant increase in the number of bowel movements after fucoidan intake. A previous report suggested that fucoidan intake increases intestinal peristalsis in middle-aged subjects.24 Fucoidan is a water-soluble dietary fiber that provides microbiota-accessible carbohydrate (MAC) to some intestinal bacteria. After that, MAC is digested in the intestine which generates organic acids there. These organic acids reduce the intestinal pH and enhance peristalsis, and the acidic intestinal environment also inhibits growth of undesirable bacteria such as Clostridium welchii and increases the number of beneficial bacteria such as Bifidobacteria.41–43 These changes of the intestinal environment during long-term intake of fucoidan may have led to the increase of bowel movements. Although none of the subjects complained about the increase of bowel movements, this should be explained before patients start long-term intake of fucoidan.
The subgroup with an above average increase in the number of bowel movements showed a decrease of the baseline GLP-1 level, an increase of leptin and increased sensitivity to 4 tastes (except sourness) after intake of fucoidan. Recently, it was demonstrated that taste receptors are expressed throughout the entire gastrointestinal tract epithelium,33–37 and that sweetness receptors in the small and large intestines are involved in the secretion of GLP-1.44–47 In this context, it is possible that the changes of GLP-1 and the taste threshold could be due to modulation of the intestinal environment by fucoidan intake. In short, fucoidan may have altered the intestinal environment as a prebiotics, thus affecting the regulation of GLP-1 secretion via intestinal taste receptors.
Furthermore, the group with an above average increase of bowel movements had lower HOMA-IR values compared to the group with a below average increase. Accordingly, a subgroup without insulin resistance (HOMA-IR < 2.5) was extracted and clinical parameters were compared. Interestingly, a significant decrease of HbA1c and the baseline GLP-1 level was seen after intake of fucoidan in this subgroup. This finding suggests that intestinal changes related to fucoidan intake may lead to improvement of glucose metabolism when insulin sensitivity remains normal.48–51
Decreased taste sensitivity was documented in patients with diabetes observed in this study or reported in literatures,52 which presumably comes from the cause other than diabetic neuropathy. It is believed to have a close link with a possible deterioration in the nutrient sensing and incretin secretion systems both held on the intestinal epithelium to augment insulin secretion.37 In this regard, the enhancement of taste sensitivity and the subtle, but significant decrease in HbA1c following the fucoidan intervention, which have not been documented before as a result of an RCT trial, seem to provide a reasonable assumption that restoration of the intestinal and oral environment by some prebiotics such as fucoidan could ameliorate diabetes control through the nutrient sensing and the incretin secretion systems. Determining postprandial GLP-1 levels could have contributed to it.
There are reports showing suppressive effect of leptin on taste responses,53 which could spoil the fucoidan effect. The results of leptin levels in this study, however, were equivocal. They rose after fucoidan and/or after placebo depending on the subgroup studied. The elevation of leptin by fucoidan may have been related to incidental weight gain by a small number of subjects during the study, because the leptin level reflects body fat mass and it also increased after placebo intake.
There have been interesting reports about improvement of the intestinal microflora by a low fat and high fiber diet, suggesting that foods rich in dietary fiber such as fucoidan may be beneficial.54–56 And also, employment of high molecular weight fucoidan in the present study in humans still has a novelty in regard to its advantage as possible candidates for functional food with health-promoting benefits. That is because it has an ample history of use as foods in humans despite its poorer animal studies than those using low molecular weight fucoidan.
This study had several limitations. First, the number of bowel movements and stool characteristics (color and form) were used as indices for assessment of the intestinal environment, but evaluation of other relevant parameters such as characteristics in intestinal microflora, fatty acid levels, variations in fucoidan dosage, etc., might have influenced the conclusions. Second, only 28 subjects were analyzed in this study. If more subjects had been enrolled for analysis, significant changes of other parameters might have been observed. Third, all of the subjects were Japanese. Since bacterial strains that can utilize seaweed constituents as MAC may be present in the intestinal flora of Japanese individuals due to their high dietary intake of seaweed,57 we must be careful when attempting to apply the conclusions of this study to other ethnic populations. Fourth, there remains the possibility that some food rich in dietary fiber could replace the effect obtained by fucoidan ingestion, whereas fucoidan still has substantial advantages for its ample history of use as dietary fiber in humans as well as for the documented evidence of probability based on the results of the RCT trial in this article. Fifth, the biochemical and gustatory findings in humans observed in the study are not supported by their corresponding findings in animal models because of the lack of study using high molecular weight fucoidan in animals.
Acknowledgments
Acknowledgments: The authors would like to express their sincere appreciation to all those who contributed to the study, especially to Dr. Yosuke Horikoshi, Tottori University, Japan for his advice about data analysis. We would like to thank the staff at Hakuai Hospital, who kindly cooperated with collecting clinical data for this study.
Part of this study was conducted with financial support from JSPS Grant-in-Aid 16K12023 and Kimuraya Research Aid 2015.
Conflicts of interest
Chieko Sakai, Minoru Kouzuki, Hisashi Shimohiro, Yoshie Ota, Hironori Sakinada, Tatsuo Takeuchi, Tsuyoshi Okura and Keiichi Hanaki declare that they have no conflicts of interest. Sunao Abe is an employee of Marine Products Kimuraya Co., Ltd. and Takeshi Kasagi is a consultant for Marine Products Kimuraya Co., Ltd.
REFERENCES
- 1. American Diabetes A. Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 Suppl 1:S61-78. [DOI] [PubMed] [Google Scholar]
- 2. Anderson JW, Randles KM, Kendall CW, Jenkins DJ. Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr. 2004;23:5-17. [DOI] [PubMed] [Google Scholar]
- 3. Koyama T, Yoshita K, Okuda N, Saitoh S, Sakata K, Okayama A, et al. Overall nutrient and total fat intake among Japanese people: The INTERLIPID Study Japan. Asia Pac J Clin Nutr. 2017;26:837-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fujii H, Iwase M, Ohkuma T, Ogata-Kaizu S, Ide H, Kikuchi Y, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson JW, Midgley WR, Wedman B. Fiber and diabetes. Diabetes Care. 1979;2:369-77. [DOI] [PubMed] [Google Scholar]
- 7. Jenkins DJ, Wolever TM, Leeds AR, Gassull MA, Haisman P, Dilawari J, et al. Dietary fibres, fibre analogues, and glucose tolerance: importance of viscosity. Br Med J. 1978;1:1392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Post RE, Mainous AG, 3rd, King DE, Simpson KN. Dietary fiber for the treatment of type 2 diabetes mellitus: a meta-analysis. J Am Board Fam Med. 2012;25:16-23. [DOI] [PubMed] [Google Scholar]
- 9. Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545-50. [DOI] [PubMed] [Google Scholar]
- 10. Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007;167:956-65. doi: 10.1001/archinte.167.9.956 [DOI] [PubMed] [Google Scholar]
- 11. Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL, et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young R, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, et al. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62:3532-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlsson F, Tremaroli V, Nielsen J, Backhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes. 2013;62:3341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J, Jia H. Metagenome-wide association studies: fine-mining the microbiome. Nat Rev Microbiol. 2016;14:508-22. [DOI] [PubMed] [Google Scholar]
- 15. Ohshiro T, Harada N, Kobayashi Y, Miki Y, Kawamoto H. Microbial fucoidan degradation by Luteolibacter algae H18 with deacetylation. Biosci Biotechnol Biochem. 2012;76:620-3. doi: 10.1271/bbb.110911 [DOI] [PubMed] [Google Scholar]
- 16. Yoshimoto M, Higaki K, Nanba E, Ikeguchi M. Anti-Proliferation Activity of Fucoidan in MKN45 Gastric Cancer Cells and Downregulation of Phosphorylated ASK1, a Cell Cycle-Regulated Kinase. Yonago Acta Med. 2015;58:1-7. [PMC free article] [PubMed] [Google Scholar]
- 17. Fitton JH, Stringer DN, Karpiniec SS. Therapies from Fucoidan: An Update. Mar Drugs. 2015;13:5920-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang X, Yu J, Ma Z, Zhang H, Xie F. Effects of fucoidan on insulin stimulation and pancreatic protection via the cAMP signaling pathway in vivo and in vitro. Mol Med Rep. 2015;12:4501-7. [DOI] [PubMed] [Google Scholar]
- 19. Kim KJ, Yoon KY, Lee BY. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia. 2012;83:1105-9. [DOI] [PubMed] [Google Scholar]
- 20. Shan X, Liu X, Hao J, Cai C, Fan F, Dun Y, et al. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol. 2016;82:249-55. [DOI] [PubMed] [Google Scholar]
- 21. Song MY, Ku SK, Kim HJ, Han JS. Low molecular weight fucoidan ameliorating the chronic cisplatin-induced delayed gastrointestinal motility in rats. Food Chem Toxicol. 2012;50:4468-78. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Nie M, Lu Y, Wang R, Li J, Yang B, et al. Fucoidan exerts protective effects against diabetic nephropathy related to spontaneous diabetes through the NF-kappaB signaling pathway in vivo and in vitro. Int J Mol Med. 2015;35:1067-73. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Wang J, Zhao Y, Hu S, Shi D, Xue C. Fucoidan from sea cucumber Cucumaria frondosa exhibits anti-hyperglycemic effects in insulin resistant mice via activating the PI3K/PKB pathway and GLUT4. J Biosci Bioeng. 2016;121:36-42. [DOI] [PubMed] [Google Scholar]
- 24. Miyoshi M, Abe S, Kasagi T, Hiramatsu K, Ikeda T. Effects of Mozuku-Derived High-Molecular-Weight Fucoidan on lntestinal Motility. J Yonago Med Ass. 2013;64:69-77. [Google Scholar]
- 25. Abe S, Hiramatsu K, Ichikawa O, Kawamoto H, Kasagi T, Miki Y, et al. Safety evaluation of excessive ingestion of mozuku fucoidan in human. J Food Sci. 2013;78:T648-51. [DOI] [PubMed] [Google Scholar]
- 26. Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. 2012;22:151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14:1200-11. [DOI] [PubMed] [Google Scholar]
- 28. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-4. [DOI] [PubMed] [Google Scholar]
- 29. Negoro A, Umemoto M, Fujii M, Kakibuchi M, Terada T, Hashimoto N, et al. Taste function in Sjogren’s syndrome patients with special reference to clinical tests. Auris Nasus Larynx. 2004;31:141-7. [DOI] [PubMed] [Google Scholar]
- 30. Tomita H, Horikawa Y. Dissociated taste disorder. Auris Nasus Larynx. 1986;13 Suppl 1:S17-23. [DOI] [PubMed] [Google Scholar]
- 31. Yamashita H, Nakagawa K, Nakamura N, Abe K, Asakage T, Ohmoto M, et al. Relation between acute and late irradiation impairment of four basic tastes and irradiated tongue volume in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:1422-9. [DOI] [PubMed] [Google Scholar]
- 32. Sanwa Kagaku Kenkyusho. Instruction manual for TasteDisk. Nagoya: Sanwa Kagaku Kenkyusho; [Google Scholar]
- 33. Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302-5. [DOI] [PubMed] [Google Scholar]
- 34. Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics?. Trends Endocrinol Metab. 2013;24:92-100. [DOI] [PubMed] [Google Scholar]
- 35. Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822S-5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oda M, Seta Y, Kataoka S, Toyono T, Toyoshima K, Morimoto Y. Expression patterns of T1R3 in duodenal epithelial cells with some gastrointestinal hormone. Arch Histol Cytol. 2010;73:177-85. doi: 10.1679/aohc.73.177 [DOI] [Google Scholar]
- 37. Trivedi BP. Neuroscience: hardwired for taste. Nature. 2012;486:S7-9. [DOI] [PubMed] [Google Scholar]
- 38. Shah M, Chandalia M, Adams-Huet B, Brinkley LJ, Sakhaee K, Grundy SM, et al. Effect of a high-fiber diet compared with a moderate-fiber diet on calcium and other mineral balances in subjects with type 2 diabetes. Diabetes Care. 2009;32:990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wisker E, Nagel R, Tanudjaja TK, Feldheim W. Calcium, magnesium, zinc, and iron balances in young women: effects of a low-phytate barley-fiber concentrate. Am J Clin Nutr. 1991;54:553-9. [DOI] [PubMed] [Google Scholar]
- 40. Matsugasumi M, Hashimoto Y, Okada H, Tanaka M, Kimura T, Kitagawa N, et al. The Association Between Taste Impairment and Serum Zinc Concentration in Adult Patients With Type 2 Diabetes. Can J Diabetes. 2018;42:520-524. [DOI] [PubMed] [Google Scholar]
- 41. Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shang Q, Shan X, Cai C, Hao J, Li G, Yu G, et al. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016;7:3224-32. [DOI] [PubMed] [Google Scholar]
- 43. Yu SH, Wu SJ, Wu JY, Wen DY, Mi FL. Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr Polym. 2015;126:97-107. [DOI] [PubMed] [Google Scholar]
- 44. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rasoamanana R, Darcel N, Fromentin G, Tome D. Nutrient sensing and signalling by the gut. Proc Nutr Soc. 2012;71:446-55. [DOI] [PubMed] [Google Scholar]
- 46. Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C, et al. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr. 2011;30:524-32. [DOI] [PubMed] [Google Scholar]
- 47. Takai S, Yasumatsu K, Inoue M, Iwata S, Yoshida R, Shigemura N, et al. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015;29:2268-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-72. [DOI] [PubMed] [Google Scholar]
- 49. Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Carli L, Gambino R, Lubrano C, Rosato R, Bongiovanni D, Lanfranco F, et al. Impaired taste sensation in type2 diabetic patients without chronic complications:a case-control study. J Endocrinol Invest. 2018;41:765-72. [DOI] [PubMed] [Google Scholar]
- 53. Rodrigues L, Espanca R, Costa AR, Antunes CM, Pomar C, Capela-Silva F, Pinheiro CC, et al. Association between Salivary Leptin Levels and Taste Perception in Children. J Nutr Metab. 2017;2017:7260169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7:2839-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Licht TR, Hansen M, Poulsen M, Dragsted LO. Dietary carbohydrate source influences molecular fingerprints of the rat faecal microbiota. BMC Microbiol. 2006;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G, et al. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908-12. [DOI] [PubMed] [Google Scholar]