Colistin-based combination therapy has become an important strategy to combat the carbapenem-resistant Acinetobacter baumannii (CRAB). However, the optimal dosage regimen selection for the combination with maximum efficacy is challenging.
KEYWORDS: Acinetobacter baumannii, PK/PD modeling, carbapenem resistance, colistin, combination therapy, meropenem
ABSTRACT
Colistin-based combination therapy has become an important strategy to combat the carbapenem-resistant Acinetobacter baumannii (CRAB). However, the optimal dosage regimen selection for the combination with maximum efficacy is challenging. Checkerboard assay was employed to evaluate the synergy of colistin in combination with meropenem, rifampin, fosfomycin, and minocycline against nine carbapenem-resistant A. baumannii isolates (MIC of meropenem [MICMEM], ≥32 mg/liter) isolated from Chinese hospital-acquired pneumonia (HAP) patients. A static time-kill assay, in vitro dynamic pharmacokinetic/pharmacodynamic (PK/PD) model, and semimechanistic PK/PD modeling were conducted to predict and validate the synergistic effect of the most efficacious combination. Both checkerboard and static time-kill assays demonstrated the superior synergistic effect of the colistin-meropenem combination against all CRAB isolates. In the in vitro PK/PD model, the dosage regimen of 2 g meropenem daily via 3-h infusion combined with steady-state 1 mg/liter colistin effectively suppressed the bacterial growth at 24 h with a 2-log10 decrease, compared with the initial inocula against two CRAB isolates. The semimechanistic PK/PD model predicted that more than 2 mg/liter colistin combined with meropenem (2 g, 3-h infusion) was required to achieve the killing below the limit of detection (<LOD; i.e., 1 log10CFU/ml) at 24 h with an MICMEM of ≥32 mg/liter. Colistin combined with meropenem exerted synergistic killing against CRAB even with an MICMEM of ≥32 mg/liter and MIC of colistin (MICCST) of ≤1 mg/liter. However, it is predicted that a higher concentration of colistin combined with meropenem was crucial to kill bacteria to <LOD. Our study provides important PK/PD information for optimization of the colistin and meropenem combination against CRAB.
TEXT
Acinetobacter baumannii is a key nosocomial pathogen responsible for multiple infections, including hospital-acquired pneumonia (HAP), urinary tract infections, wound infections, meningitis, and bacteremia (1–3). It is classified by the Infectious Diseases Society of America as one of the six most important multidrug-resistant (MDR) microorganisms in hospitals worldwide (4). It is classified as “critical priority” in the urgency of need for new antibiotics in 2017 on the published World Health Organization (WHO) antibiotic-resistant “priority pathogens” list (5). The resistance rate of A. baumannii has reached up to 70% for carbapenems in a number of countries (6). In China, the incidence of hospital-acquired pneumonia caused by A. baumannii has a rising trend in the patients infected with Gram-negative bacilli isolated from the respiratory tract (7). It has become the second most common pathogen in respiratory infections and accounts for 17.3% of all the isolated respiratory infectious pathogens in 2017 (http://www.chinets.com).
Facing the dilemma of lacking new and effective antibiotics for the carbapenem-resistant A. baumannii (CRAB), colistin has become a last resort in the clinic; however, the nephrotoxicity of colistin limited its dose selection. Moreover, colistin heteroresistance was reported in clinics worldwide (8). Hence, combination therapy was put forward to alleviate the resistance development. It was reported in a meta-analysis (9) that colistin in combination with carbapenems (including meropenem and doripenem) or rifampin produced >50% of synergistic rates against colistin-resistant strains in vitro. Additionally, colistin in combination with minocycline can also produce a synergistic killing effect (10). Such a high percentage of synergy effect indicated a great potential for clinical use to treat the carbapenem-resistant A. baumannii. Although the checkerboard and static time-kill assays were the most commonly used methods to test the synergy of combinations, only part of the clinical situation was reflected due to the static drug concentration in vitro, which is dynamically changing over time in vivo.
Tangden et al. (11) investigated the combination of colistin and meropenem against A. baumannii and Pseudomonas aeruginosa using the dynamic pharmacokinetic/pharmacodynamic (PK/PD) model in 8 h and showed a significant bacterial reduction after treatments. In our previous in vitro PK/PD model study, a colistin and meropenem combination therapy demonstrated a synergistic effect against CRAB (12). Computational models (13) have been successfully developed from the dynamic PK/PD experiments to describe the time-kill curves of colistin combinations against MDR bacteria and to predict the optimal dosage regimens. Lenhard et al. (14) predicted using a hollow-fiber infection model that polymyxin B (maximum concentration of free, unbound drug in serum [fCmax], 2.41 mg/liter) combined with 2 g meropenem every 8 h produced a >2.5-log10 decrease with regrowth by 72 h, while the combination with 8 g meropenem every 8 h achieved complete eradication by 336 h.
In the present study, carbapenem-resistant A. baumannii with MIC of meropenem of ≥32 mg/liter and MIC of colistin of ≤1 mg/liter was chosen to test the synergistic effect of colistin combinations by checkerboard assay. Colistin and meropenem combinations were further investigated by static time-kill assay and dynamic in vitro PK/PD model. A semi-mechanism-based PK/PD model was developed to predict the efficacy of colistin and meropenem dosage regimens.
RESULTS
Susceptibilities, checkerboard, and time-kill studies.
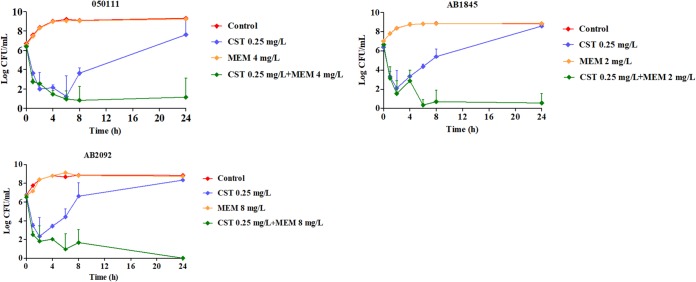
All MDR strains contained both the blaOXA-51 gene and blaOXA-23 gene associated with carbapenem resistance (see Fig. S1 in the supplemental material). MIC results were shown in Table S1 in the supplemental material. All the nine HAP isolates were susceptible to colistin (CST) with an MIC of ≤1 mg/liter and were resistant to meropenem (MEM) with quite high MICs (MIC of meropenem [MICMEM], ≥32 mg/liter). Eight strains were susceptible to minocycline (MIN). There were no breakpoints for fosfomycin (FOF) and rifampin (RIF) against A. baumannii, which cannot be decided as susceptible or resistant. In the checkerboard assay, the synergy rates of CST-MEM, CST-RIF, and CST-MIN were 44%, 56%, and 33% (see Table S2 in the supplemental material), respectively. The CST-FOF combination is not effective for the HAP isolates, with only one strain showing synergy. The CST-RIF and CST-MEM combinations had good synergy effects, with approximately half isolates showing synergy. Rifampin was commonly used as an antitubercular agent and may cause hepatotoxicity (15). Therefore, the combination of colistin and meropenem was chosen for the static time-kill study. The strains 050111, AB1845, and AB2092 were chosen for the static time-kill assay. In the time-kill study, drug concentrations were chosen when the lowest fractional inhibitory concentration indexes (FICIs) were acquired. The time-kill curves and the ΔlogCFU0–24 of each regimen were shown in Fig. 1 and Table 1.
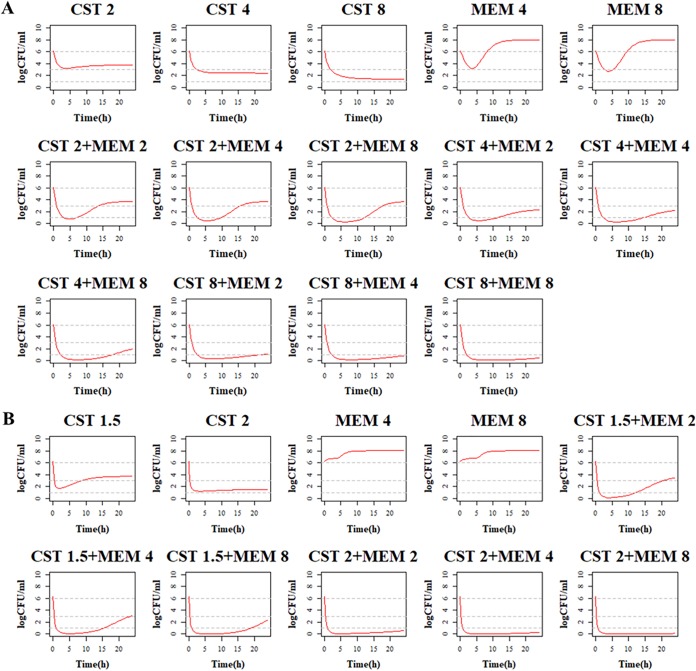
FIG 1.
Static time-kill curves show the bactericidal effect of colistin (blue), meropenem (orange), and their combination (green) against carbapenem-resistant A. baumannii isolates (mean ± SD, n = 3). CST, colistin; MEM, meropenem.
TABLE 1.
Δ logCFU0–24 values of colistin and meropenem as monotherapy and in combination
| Strain (MICCST/MICMEM) | ΔlogCFU0–24 by antibiotic therapy (mean ± SD) (n = 3) |
|||
|---|---|---|---|---|
| No drug | Colistin | Meropenem | Combination | |
| 050111 (0.5/128) | 2.62 ± 0.12 | 1.13 ± 1.50 | 2.69 ± 0.24 | −5.25 ± 1.93 |
| AB1845 (0.5/32) | 1.81 ± 0.08 | 2.19 ± 0.17 | 1.86 ± 0.09 | −6.1 ± 0.92 |
| AB2092 (1/128) | 2.11 ± 0.10 | 1.58 ± 0.30 | 2.04 ± 0.14 | −6.52 ± 0.24 |
Due to the high MICs of meropenem, there was almost no killing effect when all the bacterial strains were treated with meropenem alone. When one-half MIC or one-fourth MIC of colistin was treated to the three strains, they were both killed at the first 2 to 6 h, and regrowth happened afterward. The ΔlogCFU0–24 was the same as the control group at 24 h. However, the combination of colistin and meropenem demonstrated great synergistic effect, with ΔlogCFU0–24 being −5.25 ± 1.93, −6.1 ± 0.92, and −6.52 ± 0.24 for strains 050111, AB1845, and AB2092.
In vitro PK/PD model.
The concentrations of meropenem under a dosage regimen of 2 g with 3-h infusion was determined by a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method. The results confirmed meropenem concentrations in the PK/PD study (see Table S3 and Fig. S2 in the supplemental material).
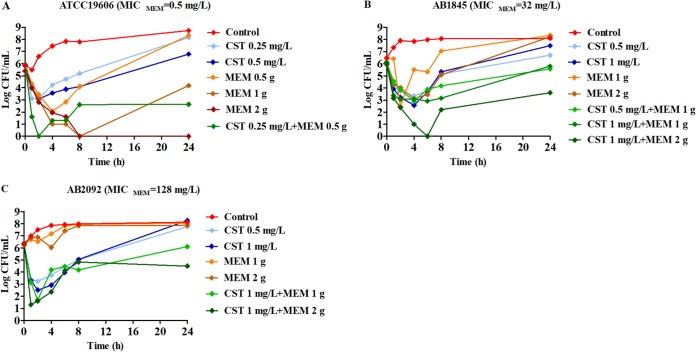
Time-kill curves under different regimens of colistin and meropenem alone or in combination were shown in Fig. 2. The ΔlogCFU0–24 values were shown in Fig. 3. Colistin monotherapy (0.25, 0.5, and 1 mg/liter) showed rapid bacterial killing and reached the maximum bactericidal killing with ΔlogCFU0–t of −3.5 for AB1845 and −3.7 for AB2092 within 2 to 4 h (Fig. 2B and C). However, there was regrowth after 6 h and no difference at 24 h between the control group and colistin monotherapy. In meropenem monotherapy, there were different bactericidal effects among the susceptible strain ATCC 19606 and resistant strains AB1845 and AB2092. For susceptible ATCC 19606, 2 g meropenem killed bacteria and no bacterial colony was detected at 24 h. For AB1845 (MICMEM, 32 mg/liter), it produced a >3-log10 decrease in the first 2 h, and regrowth occurred after 4 h while 1 g meropenem with 3-h infusion was used. The %T>MIC (time for which the drug concentration remained above the MIC of the strain as a percentage of the dosing interval [8 h]) for 1 g or 2 g meropenem with 3-h infusion was 38% and 51%, respectively (Table 2). For AB2092 (MICMEM, 128 mg/liter), meropenem monotherapy did not show a bactericidal effect.
FIG 2.
Dynamic in vitro PK/PD killing kinetics of ATCC 19606 (A), AB1845 (B), and AB2092 (C) in different dosage regimens. Meropenem dose of 0.5 g is infused for 0.5 h, and 1 or 2 g is for 3-h infusion. CST, colistin; MEM, meropenem.
FIG 3.
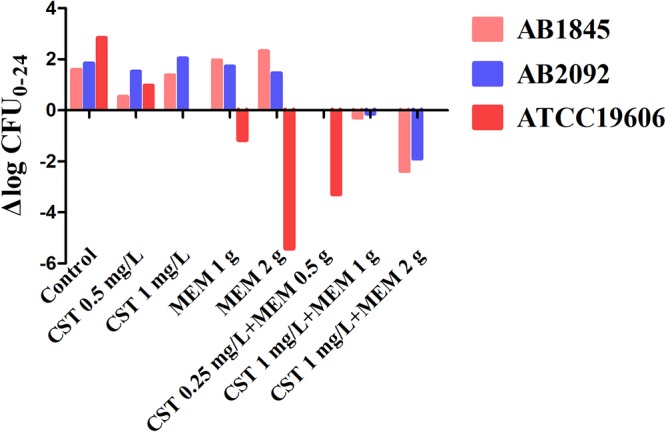
The values of ΔlogCFU0–24 for ATCC 19606, AB1845, and AB2092 after each monotherapy and the combination.
TABLE 2.
Values of %T>MIC, %T>2MIC, and %T>3MIC for AB2092 with different dosing regimensa
| Meropenem dosage (g) | Infusion value (mg/liter) | MIC at an infusion duration (h) of: |
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 8 | ||
| 1 | %T>MIC (8) | 25 | 32 | 38 | 0 |
| %T>2MIC (16) | 16 | 18 | 3.6 | 0 | |
| %T>3MIC (24) | 9.4 | 0 | 0 | 0 | |
| 2 | %T>MIC (8) | 37 | 44 | 51 | 90 |
| %T>2MIC (16) | 26 | 32 | 38 | 0 | |
| %T>3MIC (24) | 20 | 25 | 27 | 0 | |
MIC values refer to those in the checkerboard assay.
Combination therapy demonstrated a stronger bactericidal effect against both AB1845 and AB2092 than monotherapy. For AB1845, 1 mg/liter colistin in combination with 1 g meropenem for 3-h infusion produced 3-log10 killing during 8 h and, yet, regrowth occurred at 24 h. When meropenem was increased to 2 g in the combination, there was no viable colony at 6 h, and ΔlogCFU0–24 was −2.38 at 24 h. For AB2092, the combination therapy produced a strong bactericidal effect in the first 4 h and the ΔlogCFU0–24 was −0.13 and −1.89, respectively, when 1 g and 2 g meropenem was used in combination.
Semimechanistic PK/PD model.
The static time-kill curve data were applied in the semimechanistic PK/PD model. Both experimental data and modeling simulation curves were shown in Fig. S3 in the supplemental material, which showed the time-kill curves were adequately described by the proposed PK/PD model. Parameter estimates were provided in Table S4 in the supplemental material. The comparison of parameters maximum effect (Emax) and 50% effective concentration (EC50) among three strains were shown in Fig. S4 in the supplemental material. The values of EC50 and Emax of colistin were not significantly different when treated alone or in combination. However, the EC50 of meropenem was lower when treated by the combination than the meropenem monotherapy for AB2092, which was 1.85 mg/liter versus 5.5 mg/liter.
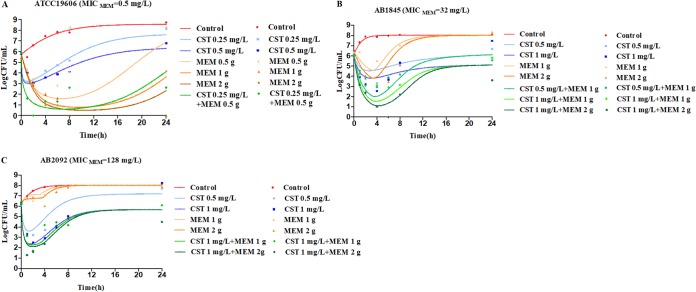
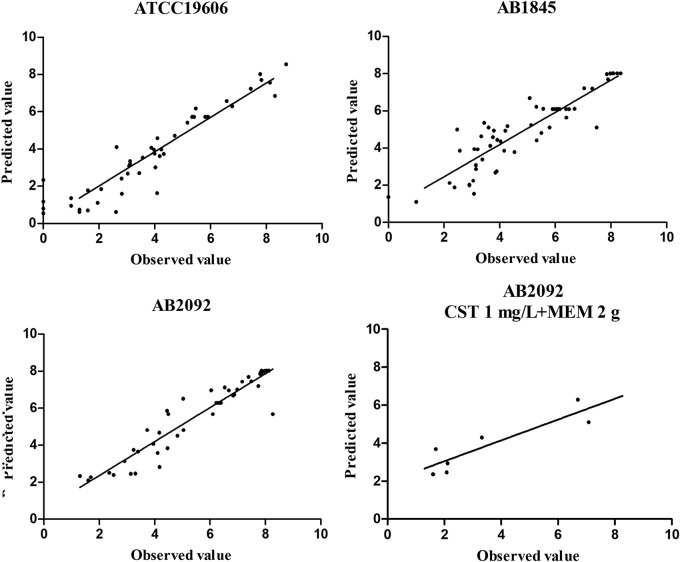
In the dynamic in vitro PK/PD model, all experimental data were fitted for parameter estimation (Fig. 4). Typical parameter estimates were presented in Table 3. Compared with meropenem susceptible strain ATCC 19606, resistant strains AB1845 and AB2092 had a lower Emax in meropenem monotherapies. There were no significant differences in EC50_MEM among the three strains. Diagnostic plots of predicted versus actual values indicated well fitted results, with data points distributed symmetrically and close to the regression line (Fig. 5).
FIG 4.
Observed (symbols) and model fitted (lines) viable counts for the dynamic in vitro PK/PD model experiments with colistin or meropenem alone and the combination against A. baumannii ATCC 19606 (A), AB1845 (B), and AB2092 (C). Meropenem dose of 0.5 g is infused for 0.5 h, and 1 or 2 g is for 3-h infusion. CST, colistin; MEM, meropenem.
TABLE 3.
Parameter estimates for the in vitro PK/PD model
| Parameter | Explanation | Value for strain: |
||
|---|---|---|---|---|
| ATCC 19606 | A. baumannii 1845 | A. baumannii 2092 | ||
| kgrowth (h−1) | Rate constant of bacterial net growth | 0.250 | 1.01 | 0.640 |
| Bmax (log10CFU/ml) | Bacterial count in the stationary phase | 8.56 | 8.02 | 8.03 |
| Emax_CST (h−1) | Maximum achievable kill rate constant by colistin | 7.21 | 0.998 | 4.66 |
| EC50_CST (mg/liter) | Colistin concentration that results in 50% of Emax | 0.0715 | 0.374 | 0.730 |
| Emax_MEM (h−1) | Maximum achievable kill rate constant by meropenem | 1.11 | 0.947 | 0.095 |
| EC50_MEM (mg/liter) | Meropenem concentration that results in 50% of Emax | 15.5 | 14.8 | 13.5 |
| γCST | Hill factor for colistin | 1.27 | 1.05 | 1.55 |
| γMEM | Hill factor for meropenem | 0.306 | 0.773 | 7.30 |
| f | Maximal adaptation factor | 290 | 3.60 | 9.54 |
| k | Rate of adaptation | 1.06 | 0.156 | 0.444 |
| Int | Parameter describing drug interaction | 0.190 | 0.0118 | 0.0117 |
| fval (%) | Residual error fraction | 5.99 | 3.77 | 2.23 |
FIG 5.
Diagnostic plots of fitting results for ATCC 19606, AB1845, and AB2092 and validation results for AB2092. Meropenem was dosed at 2 g every 8 h with 8-h infusion.
Model validation and prediction.
In the multidose combination therapy, the dosing regimen of colistin (1 mg/liter) and meropenem (2 g with 8-h infusion) was simulated. The model-predicted result which was shown via a solid line was close to observed values; only the regrowth after 8 h was lower than the experimental data (Fig. 6). Predictions of bactericidal effects of new therapies were conducted based on parameter estimates from dynamic experiments (Fig. 7). For AB1845, 8 mg/liter colistin alone killed most bacteria, while meropenem efficacy was not increased significantly with enhanced dose. When combining the two drugs, more rapid bactericidal effects were predicted for all dosages. Bacterial counts were almost close to limit of detection (LOD) at 24 h for the highest concentration of 8 mg/liter colistin in combination with 2 g meropenem as a 3-h infusion. For AB2092, 2 mg/liter colistin resulted in quite a strong bactericidal effect, while meropenem can hardly kill bacteria even with the highest dosage. At 24 h, bacterial counts were below the LOD (1 log10 CFU/ml) when 2 mg/liter colistin was combined with 2 g meropenem as a 3-h infusion.
FIG 6.
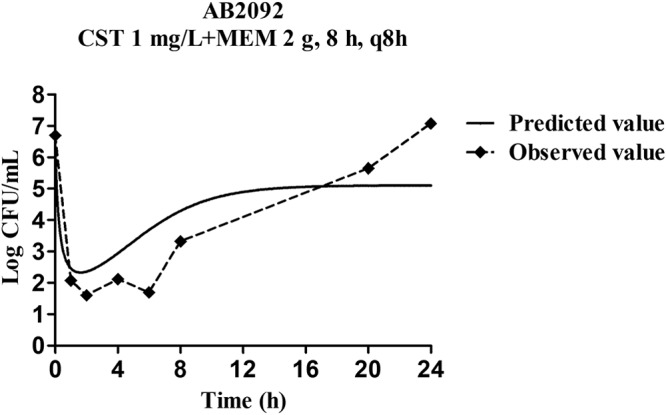
Validation of the PK/PD modeling for the regimen of 1 mg/liter colistin in combination with 2 g meropenem every 8 h with 8-h infusion.
FIG 7.
Pharmacodynamic predictions of colistin and meropenem mono- and combination therapy against A. baumannii AB1845(A) and AB2092(B). The units for colistin and meropenem are mg/liter at steady state and g with 3-h infusion, respectively. LOD, 1 log10 CFU/ml.
DISCUSSION
In this study, a semimechanistic PK/PD model was developed for colistin and meropenem in combination against A. baumannii based on both static and dynamic time-kill studies. The model well described and predicted the time course of bacterial killing by both antibiotics, facilitating the optimization of this important combination therapy in the clinic.
The synergistic bactericidal effect was reflected in both time-kill studies and the semimechanistic PK/PD model parameter estimates. The EC50 of meropenem was lower in the combination (1.85 mg/liter) for AB2092 than meropenem monotherapy (5.5 mg/liter). This synergism could be based on their mechanisms of action. Meropenem interferes with the synthesis of peptidoglycan in the cell wall by acylating the penicillin binding proteins (PBPs) (16). Colistin kills bacteria by membrane disruption which simultaneously increases meropenem entrance into bacteria (17). It had been reported that the synergy of colistin and carbapenems (doripenem) was time-dependent inhibition of different metabolic pathways in a metabolomics study (18). Colistin induced disruption of bacterial lipids and metabolic changes via pentose phosphate pathway metabolism in early stages. Carbapenem caused peptidoglycan biosynthesis metabolites to decrease in later stages. This mechanistic finding supported combination therapy in the clinic for maintaining a persistent antibacterial effect and minimizing the potential bacterial regrowth due to colistin monotherapy. Although it was reported that the colistin and meropenem combination was not superior to colistin alone against carbapenem-resistant bacteria in severe infections (19), the subgroup analysis showed less clinical failure in combination among patients with ventilator-associated pneumonia, HAP, and bloodstream infections. The high clinical failure rate (not antimicrobial failure rate), not validated outcome and high MIC of colistin, could affect the interpretation of the results.
Meropenem is a time-dependent antibiotic, and the target value of %T>MIC is 40% for Klebsiella pneumoniae (20). Under a 1-g dose with 3-h prolonged infusion, the probability of target attainment (PTA) was over 90% only for meropenem-susceptible or -intermediate strains, with MICs lower than 4 mg/liter (20). Roberts et al. (21) conducted Monte Carlo simulations on different meropenem dosages. With the high-level resistance to carbapenems (MIC, ≥32 mg/liter), the PK/PD target was unlikely to be attained even with high-dose extended infusion schemes (2 g, 3 h). Two cohort studies (22, 23) pointed out that the colistin and meropenem combination significantly decreased mortality rates only when carbapenem MIC was ≤8 mg/liter. Those studies indicated that meropenem MICs had significant influence on PTA and clinical efficacy. In this study, the maximum concentrations of meropenem regimen with 1 g and 2 g with 3-h prolonged infusion reached 16.5 and 32.9 mg/liter (24). It is very hard to achieve the PK/PD target for the highly resistant strains with an MIC of ≥32 mg/liter (25). The synergistic combination therapy can decrease the apparent MICs of both antibiotics and, therefore, increase the possibility of reaching targets for A. baumannii. In the present study, the apparent MICMEM decreased from 128 to 8 mg/liter when meropenem was alone and in combination with colistin (0.25 mg/liter) for AB2092. However, it is controversial to use %T>MIC (40%) as the target. It was reported in the clinical study that %T>4MIC should be over 40% to achieve clinical success (25, 26). This study showed meropenem 2 g with 8-h infusion (%T>MIC, 90%) combined with colistin did not show better efficacy at 24 h compared with 3-h infusion (%T>MIC, 51%) using apparent MIC calculation for each regimen. The %T>2MIC or %T>3MIC value of meropenem 2 g with 8-h or 3-h infusion combined with colistin regimen was 0% and about 30%, respectively, which could be an appropriate index for meropenem.
The protein binding of colistin in human plasma is approximately 50% (27). For the strains with colistin MIC equal to or less than 1 mg/liter, it showed that the free colistin maintained at 1 mg/liter combined with 2 g meropenem with 3-h infusion could result in 2- to 3-log10 reduction compared to the initial inoculum. However, in pharmacodynamic prediction by the developed PK/PD model, 8 mg/liter free colistin combined with 2 g meropenem adopting prolonged infusion kept colony counts near the LOD for AB1845 at 24 h. While for AB2092, 2 mg/liter free colistin alone produced a >4-log10 decrease of the colony counts, and it was below the LOD if adding 2 g meropenem to the colistin monotherapy. In vivo experiments are warranted to validate the efficacy of each dosage regimen.
The experimental data showed that AB1845 had a slightly higher growth rate than AB2092. In the PK/PD model, the estimated values of growth rate for AB1845 and AB2092 were 1.01 and 0.64/h, respectively. It has been proven that the growth rate of induced resistant strains decreased when compared with that of their susceptible parental strains (28, 29). It was shown in the prediction section that bacteria with a higher growth rate were more difficult to be killed to the limit of detection level. Therefore, higher doses were needed for the bacteria with higher growth rate instead of higher MICs.
These prediction results indicated a higher concentration of free colistin (>1 mg/liter) was needed for a complete bactericidal effect at 24 h. The pharmacokinetics of colistin methanesulfonate (CMS) in healthy Chinese subjects after single and multiple intravenous doses was previously investigated. The Cmax was 1.17 ± 0.22 mg/liter, and steady state concentration was 1.03 ± 0.16 mg/liter after multiple doses at day 7 (2.5 mg CBA/kg, twice daily for 7 days). The Cmax ranged from 2 to 2.65 mg/liter after loading dose (2 MIU or 9 MIU) or at steady state (30–32) in critically ill patients. Nevertheless, the intersubject variations in colistin maximum plasma concentration had been reported, which could range from 0.95 to 5.1 mg/liter (mean, 2.65 mg/liter) after 9 MIU loading dose, or range from 0.48 to 9.38 mg/liter (median, 2.36 mg/liter) after 2.5- to 13.6-MIU dose (31). These data suggested the possibility of reaching higher concentration of colistin in vivo to achieve a better bactericidal effect. Whereas, the higher concentration of colistin could lead to higher possibility of nephrotoxicity (33). The colistin plasma concentration and nephrotoxicity should be closely monitored if a higher dose or concentration of colistin is being used.
MATERIALS AND METHODS
Bacterial strains and resistance gene, medium, and antibiotics.
One reference strain, A. baumannii ATCC 19606, and nine clinical multidrug-resistant (MDR) strains were investigated in our study. These strains were isolated from HAP patients in a multicenter clinical trial in 2014. Strains AB1845 and AB2092 were isolated from one HAP patient before and after cefoperazone-sulbactam therapy (2:1, 3 g every 8 h with 1-h infusion) (34). Genomes of AB1845 and AB2092 were sequenced with the Illumina HiSeq 2500 platform, and there was 99.98% similarity between the two isolates. The differences were found in 33 synonymous single nucleotide polymorphisms (SNPs) and three copies of blaOXA-23 in AB2092 versus AB1845. Isolate 050111 was from an HAP patient in The Second Hospital of Jilin University.
Cation-adjusted Muller-Hinton broth (CAMHB; Becton, Dickinson, Sparks, MD) was used for checkerboard and time-kill assays. Colistin (sulfate; Sigma-Aldrich, St. Louis, MO), meropenem, rifampin, fosfomycin supplemented with glucose-6-phosphate (G6P; 25 mg/liter; Sigma-Aldrich), (35), and minocycline (National Institutes for Food and Drug Control, Beijing, China) were used for the MIC tests.
Checkerboard assays.
The MICs of tested antibiotics were carried through the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI, 2017). The checkerboard tests were conducted to evaluate the synergistic effect of different colistin-based combinations. The concentrations of colistin were 0.0625 to 4 mg/liter, and the concentrations of the other antibiotic were 0.125 to 128 mg/liter. The 96-well broth microdilution plates were inoculated with each strain to yield the appropriate density (105 CFU/ml) in a 100-μl final volume and incubated at 35°C for 18 to 22 h. Escherichia coli ATCC 25922 was used as the quality-control strain.
The fractional inhibitory concentration index (FICI) was calculated using the following formula: FICI = FICIA + FICIB, where FICIA is MIC of drug A in combination/MIC of drug A alone and FICIB is MIC of drug B in combination/MIC of drug B alone. The interpretation of FICI against A. baumannii was as follows: synergy as FICI of ≤0.5, indifference as 4 ≥ FICI > 0.5, and antagonism as FICI of >4 (36).
Static time-kill assays.
Time-kill assays were performed for the combination of colistin and meropenem on the strains of 050111, AB1845, and AB2092. Time-kill analysis was conducted according to previously published methods (37). The initial inoculum of the bacteria was approximately 106 CFU/ml, and there was a 2-h preculture before the addition of antibiotics and sampling at 0, 1, 2, 4, 6, 8, and 24 h. The assay was performed in triplicates. Bactericidal effect was defined as a ≥3-log10 reduction in the colony counts relative to the initial inoculum (38). Synergy was interpreted as a ≥2-log10 decrease in colony counts for antimicrobial combination compared with the most active single agent at 24 h. Indifference was defined as a ≤2-log10 increase or decrease in colony counts at 24 h for the combination compared with the most active drug alone. Antagonism was defined as a ≥2-log10 increase in colony counts at 24 h for the combination compared with the most active drug alone (37, 39).
In vitro PK/PD model experiments.
The in vitro PK/PD model (see Fig. S5 in the supplemental material) was used to conduct dynamic time-kill experiments (12, 40). Pharmacokinetics of both antibiotics was simulated by the PK/PD model by adjusting the flow rate of the medium pumped into the central compartment. Meropenem concentration was simulated according to pharmacokinetic parameters from Chinese healthy volunteers which was best described with a two-compartment model (24), and pharmacokinetic parameters were provided in Table S5 in the supplemental material.
Although the commonly used clinical dose of meropenem is 1 g every 8 h (q8h), it was reported that meropenem dosed at 2 g, q8h with prolonged infusion was more likely to achieve the desired %T>MIC target (41–43). Hence, the dose of meropenem in the PK/PD model was 1 g or 2 g with 3-h infusion. Colistin concentration was maintained constant at 0.5 or 1 mg/liter to mimic the steady-state concentration from the critically ill patients receiving colistin methanesulfonate (31). The bacteria were inoculated into the central compartment, and central compartment was sealed by a filter membrane (0.45 μm) to prevent bacterial loss. Samples were obtained from the central compartment with a syringe, and bacterial counts were determined using Mueller-Hinton agar plates.
Semi-mechanistic PK/PD modeling.
A model scheme was shown in Fig. S6 in the supplemental material. Bacterium B is in a self-replication state with kg as the net growth rate. Colistin and meropenem act on bacteria by decreasing the bacterial counts with the rate of kdrug. CCST and CMEM represent the real-time concentration of colistin and meropenem, and ke is the in vivo elimination rate of meropenem.
Bacterial growth modeling.
Bacterial growth is a self-limiting process. A logistic function was applied to describe this characteristic using the equation 1 (44):
| (1) |
Bmax is the carrying capacity or the maximum bacterial counts in the system. B is the log colony counts of bacteria.
Killing effects of colistin, meropenem and the combination.
The change of bacterial counts could be described as equation 2 (45).
| (2) |
The killing effects of the antibiotics are characterized using sigmoid Emax function.
| (3) |
| (4) |
ECST is the killing rate of colistin. Emax_CST is the maximum achievable killing rate constant while EC50_CST is the antibiotic concentration that results in 50% of Emax_CST. An adaptation factor α that is dependent on time as well as drug concentration is introduced in equation 3 to explain the adaptive resistance of colistin. The parameter of f is the maximal adaptation factor and k is the rate of adaptation (equation 4) (45). The increase of α could result in an increase in EC50_CST.
The killing effect of meropenem against A. baumannii was consistent with the following sigmoid Emax model (where EC50_MEM is the antibiotic concentration that results in 50% of Emax_MEM):
| (5) |
| (6) |
In the combination therapy, the drug interaction was evaluated using the following empirical interaction function (13)
| (7) |
where E is the combined killing rate constant of colistin and meropenem; Int represents the interaction effect. The Int value indicates the effect of synergy, indifference, and antagonism. Zero suggests no interaction, a positive value suggests a synergistic effect, and a negative value suggests an indifference or antagonism effect.
Model fittings were performed by nonlinear regression analysis using the maximum likelihood algorithm in Matlab 8.3 (Mathwork, Inc., USA). Goodness of fit was assessed by the objective function fval (%) {fval (%) = sum[(simulated data – actual data)2]100/sum(actual data)2} and visual inspection of diagnostic plots (46). The closer the value of fval (%) to zero, the higher goodness of fit was. Parameter estimates were obtained after model fitting.
Model validation.
External validation was conducted to examine the prediction ability of the semi-mechanism-based PK/PD model. The computational model was employed to predict the bacterial counts under the dosage regimen of 1 mg/liter colistin and 2 g meropenem every 8 h with 8-h infusion. The prediction data were compared with the experimental data where a multiple-dose of colistin and meropenem combination was given to AB2092. The figures of fitting curves and predicted versus observed values were plotted to evaluate the predictive power. Berkeley Madonna (version 8.3.18) was used for pharmacodynamic prediction.
Pharmacodynamic prediction of mono- and combination therapy.
Parameter estimates from the dynamic PK/PD model were used, colistin concentration ranged from 1.5 to 8 mg/liter, and meropenem was dosed at 2, 4, or 8 g for a single dose with 3-h infusion. Predictions were conducted on mono- and combination therapies. R3.5.0 was used for plotting.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant identifiers [IDs] 81628015 and 81503114) and Major Research and Development Project of Innovative Drugs, Ministry of Science and Technology of China (2017ZX09304005).
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01989-18.
REFERENCES
- 1.Queenan AM, Pillar CM, Deane J, Sahm DF, Lynch AS, Flamm RK, Peterson J, Davies TA. 2012. Multidrug resistance among Acinetobacter spp. in the USA and activity profile of key agents: results from CAPITAL Surveillance 2010. Diagn Microbiol Infect Dis 73:267–270. doi: 10.1016/j.diagmicrobio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. 2007. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis 44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 3.Diene SM, Rolain JM. 2014. Carbapenemase genes and genetic platforms in Gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin Microbiol Infect 20:831–838. doi: 10.1111/1469-0691.12655. [DOI] [PubMed] [Google Scholar]
- 4.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6.Eliopoulos GM, Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Xia J, Xu Y, Gong M, Zhou Y, Xie L, Fang X. 2016. Biological features of biofilm-forming ability of Acinetobacter baumannii strains derived from 121 elderly patients with hospital-acquired pneumonia. Clin Exp Med 16:73–80. doi: 10.1007/s10238-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 8.Yau W, Owen RJ, Poudyal A, Bell JM, Turnidge JD, Yu HH, Nation RL, Li J. 2009. Colistin hetero-resistance in multidrug-resistant Acinetobacter baumannii clinical isolates from the Western Pacific region in the SENTRY antimicrobial surveillance programme. J Infect 58:138–144. doi: 10.1016/j.jinf.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. 2015. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents 45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Yang YS, Lee Y, Tseng KC, Huang WC, Chuang MF, Kuo SC, Lauderdale TL, Chen TL. 2016. In vivo and in vitro efficacy of minocycline-based combination therapy for minocycline-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 60:4047–4054. doi: 10.1128/AAC.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangden T, Karvanen M, Friberg LE, Odenholt I, Cars O. 2017. Assessment of early combination effects of colistin and meropenem against Pseudomonas aeruginosa and Acinetobacter baumannii in dynamic time-kill experiments. Infect Dis (Lond) 49:521–527. doi: 10.1080/23744235.2017.1296183. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Zhao M, Chen Y, Bian X, Li Y, Shi J, Zhang J. 2016. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents 48:559–563. doi: 10.1016/j.ijantimicag.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed AF, Kristoffersson AN, Karvanen M, Nielsen EI, Cars O, Friberg LE. 2016. Dynamic interaction of colistin and meropenem on a WT and a resistant strain of Pseudomonas aeruginosa as quantified in a PK/PD model. J Antimicrob Chemother 71:1279–1290. doi: 10.1093/jac/dkv488. [DOI] [PubMed] [Google Scholar]
- 14.Lenhard JR, Bulitta JB, Connell TD, King-Lyons N, Landersdorfer CB, Cheah SE, Thamlikitkul V, Shin BS, Rao G, Holden PN, Walsh TJ, Forrest A, Nation RL, Li J, Tsuji BT. 2017. High-intensity meropenem combinations with polymyxin B: new strategies to overcome carbapenem resistance in Acinetobacter baumannii. J Antimicrob Chemother 72:153–165. doi: 10.1093/jac/dkw355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao XF, Wang L, Liu GJ, Wen J, Sun X, Xie Y, Li YP. 2006. Rifampicin plus pyrazinamide versus isoniazid for treating latent tuberculosis infection: a meta-analysis. Int J Tuber Lung Dis 10:1080–1090. [PubMed] [Google Scholar]
- 16.Nicoletti G, Russo G, Bonfiglio G. 2002. Recent developments in carbapenems. Expert Opin Investig Drugs 11:529–544. doi: 10.1517/13543784.11.4.529. [DOI] [PubMed] [Google Scholar]
- 17.Soon RL, Nation RL, Cockram S, Moffatt JH, Harper M, Adler B, Boyce JD, Larson I, Li J. 2011. Different surface charge of colistin-susceptible and -resistant Acinetobacter baumannii cells measured with zeta potential as a function of growth phase and colistin treatment. J Antimicrob Chemother 66:126–133. doi: 10.1093/jac/dkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maifiah MH, Creek DJ, Nation RL, Forrest A, Tsuji BT, Velkov T, Li J. 2017. Untargeted metabolomics analysis reveals key pathways responsible for the synergistic killing of colistin and doripenem combination against Acinetobacter baumannii. Sci Rep 7:45527. doi: 10.1038/srep45527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. 2018. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 20.Vourli S, Tsala M, Kotsakis S, Daikos GL, Tzouvelekis L, Miriagou V, Zerva L, Meletiadis J. 2016. Comparison of short versus prolonged infusion of standard dose of meropenem against carbapenemase-producing Klebsiella pneumoniae isolates in different patient groups: a pharmacokinetic-pharmacodynamic approach. J Pharm Sci 105:1513–1518. doi: 10.1016/j.xphs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64:142–150. doi: 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 22.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P. 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133–2143. doi: 10.1093/jac/dkv086. [DOI] [PubMed] [Google Scholar]
- 24.Zhao CY, Li JT, Zhang L, Zhang P, Liu Y. 2004. Pharmacokinetics of meropenem single dose intravenous in healthy volunteers. Chin J Clin Pharmacol 3:189–192. [Google Scholar]
- 25.Del Bono V, Giacobbe DR, Marchese A, Parisini A, Fucile C, Coppo E, Marini V, Arena A, Molin A, Martelli A, Gratarola A, Viscoli C, Pelosi P, Mattioli F. 2017. Meropenem for treating KPC-producing Klebsiella pneumoniae bloodstream infections: should we get to the PK/PD root of the paradox? Virulence 8:66–73. doi: 10.1080/21505594.2016.1213476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taccone FS, Cotton F, Roisin S, Vincent JL, Jacobs F. 2012. Optimal meropenem concentrations to treat multidrug-resistant Pseudomonas aeruginosa septic shock. Antimicrob Agents Chemother 56:2129–2131. doi: 10.1128/AAC.06389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheah SE, Wang J, Nguyen VT, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 28.Farshadzadeh Z, Taheri B, Rahimi S, Shoja S, Pourhajibagher M, Haghighi MA, Bahador A. 2018. Growth rate and biofilm formation ability of clinical and laboratory-evolved colistin-resistant strains of Acinetobacter baumannii. Front Microbiol 9:153. doi: 10.3389/fmicb.2018.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mu X, Wang N, Li X, Shi K, Zhou Z, Yu Y, Hua X. 2016. The effect of colistin resistance-associated mutations on the fitness of Acinetobacter baumannii. Front Microbiol 7:1715. doi: 10.3389/fmicb.2016.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregoire N, Mimoz O, Megarbane B, Comets E, Chatelier D, Lasocki S, Gauzit R, Balayn D, Gobin P, Marchand S, Couet W. 2014. New colistin population pharmacokinetic data in critically ill patients suggesting an alternative loading dose rationale. Antimicrob Agents Chemother 58:7324–7330. doi: 10.1128/AAC.03508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaiskos I, Friberg LE, Pontikis K, Ioannidis K, Tsagkari V, Galani L, Kostakou E, Baziaka F, Paskalis C, Koutsoukou A, Giamarellou H. 2015. Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 59:7240–7248. doi: 10.1128/AAC.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landersdorfer CB, Nation RL. 2015. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med 36:126–135. doi: 10.1055/s-0034-1398390. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Zheng H, Zhang W, Shen Z, Zhao M, Chen Y, Sun L, Shi J, Zhang J. 2016. Tracking cefoperazone/sulbactam resistance development in vivo in A. baumannii isolated from a patient with hospital-acquired pneumonia by whole-genome sequencing. Front Microbiol 7:1268. doi: 10.3389/fmicb.2016.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao M, Bulman ZP, Lenhard JR, Satlin MJ, Kreiswirth BN, Walsh TJ, Marrocco A, Bergen PJ, Nation RL, Li J, Zhang J, Tsuji BT. 2017. Pharmacodynamics of colistin and fosfomycin: a “treasure trove” combination combats KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother 72:1985–1990. doi: 10.1093/jac/dkx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 37.Petersen PJ, Labthavikul P, Jones CH, Bradford PA. 2006. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother 57:573–576. doi: 10.1093/jac/dki477. [DOI] [PubMed] [Google Scholar]
- 38.Liu B, Liu Y, Di X, Zhang X, Wang R, Bai Y, Wang J. 2014. Colistin and anti-Gram-positive bacterial agents against Acinetobacter baumannii. Rev Soc Bras Med Trop 47:451–456. doi: 10.1590/0037-8682-0081-2014. [DOI] [PubMed] [Google Scholar]
- 39.Principe L, Capone A, Mazzarelli A, D'Arezzo S, Bordi E, Di Caro A, Petrosillo N. 2013. In vitro activity of doripenem in combination with various antimicrobials against multidrug-resistant Acinetobacter baumannii: possible options for the treatment of complicated infection. Microb Drug Resist 19:407–414. doi: 10.1089/mdr.2012.0250. [DOI] [PubMed] [Google Scholar]
- 40.Liang W, Liu XF, Huang J, Zhu DM, Li J, Zhang J. 2011. Activities of colistin- and minocycline-based combinations against extensive drug resistant Acinetobacter baumannii isolates from intensive care unit patients. BMC Infect Dis 11:109. doi: 10.1186/1471-2334-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Kuti JL, Nightingale CH, Nicolau DP. 2006. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J Clin Pharmacol 46:1171–1178. doi: 10.1177/0091270006291035. [DOI] [PubMed] [Google Scholar]
- 42.Jaruratanasirikul S, Sriwiriyajan S, Punyo J. 2005. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob Agents Chemother 49:1337–1339. doi: 10.1128/AAC.49.4.1337-1339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaruratanasirikul S, Kositpantawong N, Jullangkoon M, Aeinlang N, Wongpoowarak W. 2013. Pharmacodynamics of meropenem in critically ill patients with ventilator-associated pneumonia. J Med Assoc Thai 96:1283–1289. [PubMed] [Google Scholar]
- 44.Tam VH, Schilling AN, Nikolaou M. 2005. Modelling time-kill studies to discern the pharmacodynamics of meropenem. J Antimicrob Chemother 55:699–706. doi: 10.1093/jac/dki086. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen EI, Friberg LE. 2013. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol Rev 65:1053–1090. doi: 10.1124/pr.111.005769. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Cao Y, Zhou J, Liu X. 2009. Mechanism-based pharmacokinetic-pharmacodynamic modeling of bidirectional effect of danshensu on plasma homocysteine in rats. Pharm Res 26:1863–1873. doi: 10.1007/s11095-009-9899-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.