Abstract
Tumor cells along with a small proportion of cancer stem cells exist in a stromal microenvironment consisting of vasculature, cancer-associated fibroblasts, immune cells and extracellular components. Recent epidemiological and clinical studies strongly support that vitamin D supplementation is associated with reduced cancer risk and favorable prognosis. Experimental results suggest that vitamin D not only suppresses cancer cells, but also regulates tumor microenvironment to facilitate tumor repression. In this review, we have outlined the current knowledge on epidemiological studies and clinical trials of vitamin D. Notably, we summarized and discussed the anticancer action of vitamin D in cancer cells, cancer stem cells and stroma cells in tumor microenvironment, providing a better understanding of the role of vitamin D in cancer. We presently re-propose vitamin D to be a novel and economical anticancer agent.
Abbreviations: 1,25(OH)2D3, 1α,25-dihydroxyvitamin D3; 25(OH)D, 25-hydroxyvitamin D; CAF, cancer-associated fibroblast; CRC, colorectal cancer; CSC, cancer stem cell; DBP/GC, vitamin D-binding protein; ESCC, esophageal squamous cell carcinoma; GI, gastrointestinal; NSCLC, non-small cell lung cancer; PC, pancreatic adenocarcinoma; PG, prostaglandin; PSC, pancreatic stellate cells; TDEC, tumor derived endothelial cell; TME, tumor microenvironment; TIC, tumor initiating cell; TIL, tumor-infiltrating lymphocyte; VDR, vitamin D receptor; VDRE, VDR element; VEGF, vascular endothelial growth factor
KEY WORDS: Vitamin D; 1α,25-Dihydroxyvitamin D3; Tumor microenvironment; Cancer stem cell; Tumor-infiltrating lymphocyte; Tumor-derived endothelial cell; Cancer-associated fibroblast
Graphical abstract
Supplementation of vitamin D is associated with reduced cancer risk and favorable prognosis. Vitamin D not only suppresses cancer cells and cancer stem cells, but also regulates tumor microenvironment, demonstrating the promise of the benefit of vitamin D in cancer prevention and treatment.
1. Introduction
Tumor microenvironment (TME) is comprised of various stromal cells (e.g., endothelia, cancer-associated fibroblasts, and immune cells) and extracellular components (e.g., cytokines and extracellular matrix), which surround tumor cells and are nourished by a vascular system. The pivotal roles of TME during tumor initiation, progression, and metastasis have been recently reviewed in detail elsewhere1, 2. Importantly, TME greatly influences therapeutic efficacy and emerges as a new target for treating tumors3, 4, 5.
Vitamin D is a multifunctional precursor of the potent steroidal hormone calcitriol (1α,25-dihydroxyvitamin D3, 1,25(OH)2D3). As most foods contain little vitamin D, there are certain diseases which need vitamin D as a dietary supplement to replenish the deficiency, especially for the elderly and children to maintain adequate vitamin D store for bone health and autoimmunity6. Recently, epidemiological and clinical observations strongly suggest that vitamin D deficiency or low circulating 25-hydroxyvitamin D (25(OH)D) level in serum increases the risk of developing multiple malignancies. Experimental evidence also showed that 1,25(OH)2D3, the active form of vitamin D, can exhibit anticancer actions through different signaling pathways, including inhibition of proliferation, induction of cell apoptosis and differentiation, as well as suppression of metastasis and angiogenesis in various cancers. Notably, vitamin D was found to not only have profound effects on normal cancer cells, but also on cancer-associated stromal cells and cancer stem cells within the TME. Regarding the important role of TME in cancer initiation, progression, metastasis and recurrence, vitamin D might be used as a therapeutic agent targeting the TME to assist clinical treatment and prognosis for many kinds of cancer. Herein, this review summarized the anticancer action of vitamin D in the TME of cancer cells, stroma cells and cancer stem cells. We also outlined the epidemiological studies and clinical trials of vitamin D, providing a better understanding of the role of vitamin D in cancer treatment to re-propose vitamin D as a novel anticancer agent.
2. Potentiating vitamin D as an anticancer agent
2.1. Vitamin D metabolism and signaling
2.1.1. Vitamin D metabolism
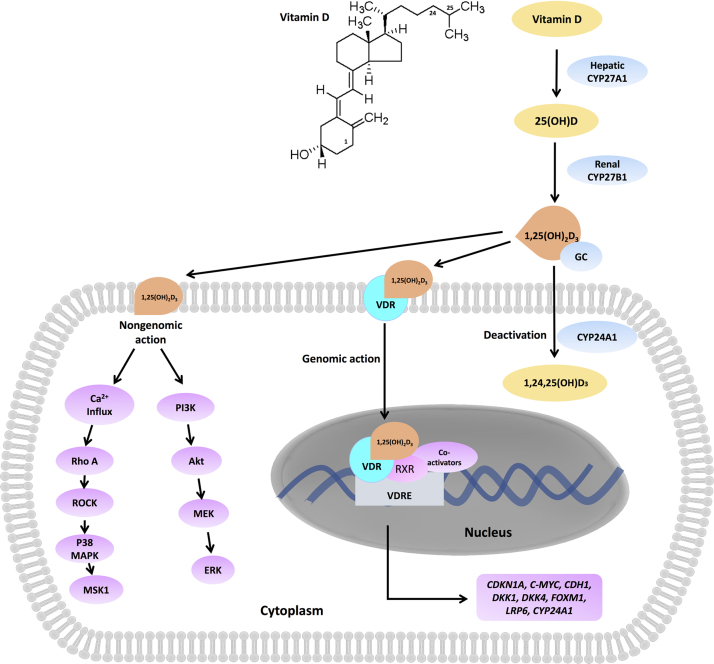
After absorption, vitamin D binds to vitamin D-binding protein (DBP, also named as GC) in the circulation, and is transported to the liver. There are three main enzymes, i.e., CYP27A1, CYP27B1 and CYP24B1, involved in vitamin D metabolism. The metabolic process of vitamin D in human is depicted in Fig. 1. Briefly, vitamin D can be hydroxylated by CYP27A1, and converted into 25(OH)D in the liver. Further, 25(OH)D is catalyzed by CYP27B1 in the kidney, which results in the active form 1,25(OH)2D3. 1,25(OH)2D3 is mainly responsible for the biological function of vitamin D in humans. However, CYP24A1 functions inversely, converting 1,25(OH)2D3 into an inactive form. In fact, the expression of CYP24A1 is highly induced by 1,25(OH)2D3, which is thus the limiting step of vitamin D activation. CYP24A1 is usually highly expressed in cancer tissues, and CYP24A1-induced vitamin D insufficiency might also promote cancer progression. A study of 99 CRC patients demonstrated that CYP24A1 expression was higher in cancer specimens than paired normal tissue, and high expression level of CYP24A1 strongly correlated with tumor invasion, lymph node metastasis, decreases in overall survival (P = 0.026) and advanced CRC recurrences (P = 0.32)7. In addition, Shiratsuchi et al.8 has recently observed that knockdown of CYP24A1 significantly restrained lung cancer xenograft in vivo, suggesting that CYP24A1 could be identified as a potential oncogene in lung cancer. It was found that a protein kinase CK2 inhibitor, 4,5,6,7-tetrabromobenzimidazole, could enhance the antitumor effect of 1,25(OH)2D3 on prostate cancer through directly inhibiting the CYP24A1 expression9. Thus, the inhibition of CYP24A1 expression or activity and exploration of CYP24A1 inhibitors might be a good way to increase the anticancer action of 1,25(OH)2D3.
Figure 1.
Vitamin D metabolism and signaling. After absorption, vitamin D is hydroxylated by hepatic CYP27A1 into 25(OH)D, which is further catalyzed by CYP27B1 in the kidney resulting in the active form 1,25(OH)2D3. 1,25(OH)2D3 triggers genomic or non-genomic actions. The binding of 1,25(OH)2D3 to vitamin D receptor (VDR) triggers VDR translocation into nuclear and transcriptionally induces the downstream target genes such as CDKN1A, C-MYC, CDH1, DKK4, FOXM1, LRP6 and CYP24A1. Alternatively, 1,25(OH)2D3 induces rapid upregulation of cytosolic Ca2+ concentration and activates Rho-ROCK-p38MAPK-MSK1 pathway. In certain cancer cells, 1,25(OH)2D3 is capable of stimulating PI3K/Akt/ERK1/2/MAPK pathway.
2.1.2. Vitamin D signaling
The biological actions of 1,25(OH)2D3 are mainly mediated by vitamin D receptor (VDR) via genomic pathway, although a 1,25(OH)2D3-induced non-genomic pathway has been identified recently (Fig. 1). The binding of 1,25(OH)2D3 to VDR triggers VDR translocation into the nucleus and transcriptionally induces the downstream target genes such as CDKN1A, C-MYC, CDH1, and CYP24A1. Thus, the expression level of VDR in cancers is always associated with vitamin D response. Notably, high VDR expression has been found in many cancer types, such as breast cancer and papillary thyroid carcinoma10, 11. There was also a similar trend of CYP27B1 expression in cancers, which could provide a possible explanation for VDR overexpression in cancer cells12, 13. High level of VDR expression has been associated with improvement in prognosis of patients with lung, prostate, pancreatic, and colorectal cancers, indicating that VDR expression might be a prognostic biomarker in these cancers14, 15, 16, 17. Recently, it was demonstrated that higher expression level in stroma cells in cancer adenocarcinoma could facilitate larger actions for vitamin D, thus improving therapeutic outcome18, 19. Moreover, we recently demonstrated that higher expression of VDR was found in gastric cancer tissue than that in normal gastric tissue, and 1,25(OH)2D3 displayed inhibitory effect in gastric cancer cells without influencing normal epithelial cells20. Taken together with the current findings, overexpression of VDR in cancer cells and tissues or in surrounding stroma cells would pose an advantage of using vitamin D as an anticancer agent with an enhanced therapeutic window against cancer. However, although VDR expression has a predominant role for vitamin D action, VDR may potentially function as an oncogene. One study reported that knockdown of VDR evidently induced apoptosis in breast and prostate cancer cells21, indicating a controversial role for VDR.
2.2. Epidemiological studies
The serum concentration of the vitamin D metabolite 25(OH)D is commonly used as the index of vitamin D status in body. In particular, circulating 25(OH)D level in human lower than 20 ng/mL, from 20 to 30 ng/mL, and higher than 30 ng/mL indicate a deficiency, a relative insufficiency and a sufficiency of vitamin D, respectively22, 23. Although controversial findings exist, most epidemiological studies have indicated that serum vitamin D status is correlated with multiple types of cancer risk, including colon, prostate, breast, and gastric cancers. Patients with low vitamin D levels are usually diagnosed with poorer survival and higher cancer mortality, suggesting that vitamin D might protect individuals against late stages of carcinogenesis.
2.2.1. Prostate cancer
An early prediagnostic study demonstrated that there was a prospective decrease in prostate cancer risk in men at age above 57 years with high level of serum 25(OH)D24. A 13-year-follow-up study involved 129 cases and 167 controls showed that vitamin D supplement and increased plasma 25(OH)D concentration significantly correlated with prostate cancer susceptibility25. A recent study included 1066 men with prostate cancer within the US Physicians׳ Health Study between 1982 and 2000, and 1618 matched healthy men as controls26. Results suggested that improving vitamin D status through increased exposure to sunlight and vitamin D supplement might reduce prostate cancer risk, particularly in men with the FokI ff genotype26. However, there was a nested case-control study showing no association between serum 25(OH)D level and prostate cancer risk27. A meta-analysis found a significant association between higher 25(OH)D level and increased prostate cancer risk28. It seemed that results in prostate cancer were somewhat inconsistent, which might be due to the limitations of selected population and control. Considering that the anticancer action of vitamin D has been well elucidated in prostate cancer cells in vitro and in vivo29, more thorough epidemiological studies on vitamin D and prostate cancer risk may be needed.
2.2.2. Breast cancer
The relationship between vitamin D intake and breast cancer susceptibility has been well studied. Pooled analysis on breast cancer supported the inverse correlation between vitamin D and calcium intake and breast cancer risk, while some of the studies showed no association30, 31, 32, 33. Although the results are complex and inclusive, it is expected that vitamin D supplement still benefits for breast cancer prevention. In a sectional study, high vitamin D levels significantly improved clinical outcomes in Brazilian postmenopausal women with breast cancer34. Moreover, it seemed that the association was stronger in premenopausal vs. postmenopausal women27, 35, 36. Recently, a cohort study including 1666 women diagnosed with breast cancer seemed highly convincing. They found that elevations in serum 25(OH)D concentrations were associated with superior overall survival especially among premenopausal women37, strongly suggesting the use of serum vitamin D level as a prognostic marker for breast cancer. Moreover, among African American women, data showed that vitamin D deficiency increased breast cancer susceptibility by approximately 23%38, indicating vitamin D intake could be consider as a preventive factor for breast cancer incidence.
2.2.3. Colorectal cancer
Epidemiological studies conducted on colorectal cancer (CRC) provided strong support for inverse association between serum 25(OH)D level and colon cancer risk in both men and women39, 40, 41, 42. A cross-sectional study indicated that low concentration of 25(OH)D in serum was significantly associated with adenomas or colon polyps43. A meta-analysis showed that higher circulating 25(OH)D levels predicted decrease cancer death of CRC patients44. indicating that restoration of vitamin D level to normal levels in vitamin D deficient patients might benefit for a better prognosis of CRC. Notably, the association between vitamin D status and CRC risk sometimes varied with multiple factors. For example, a nest case-control study showed that high concentrations of plasma 25(OH)D was correlated with decreased CRC incidence in individuals with intense immune reaction but not that with lower-level immune responses (Odds ratio = 0.10; 95% CI: 0.03 to 0.35; P < 0.001)45. The association between dietary calcium and vitamin D supplementation and decreased CRC risk was also observed in a Japanese population with VDR gene polymorphism Apa I but not Bsm I and Taq I46.
2.2.4. Upper gastrointestinal cancer
Epidemiological studies on upper gastrointestinal (GI) cancers are limited. In the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers, a study examined the association between the level of serum 25(OH)D and upper GI cancers (esophageal and gastric cancer). In the subgroup of Asian, but no Caucasians, there was a significant decrease of GI cancer risk associated with low concentration of 25(OH)D (Odds ratio = 0.53, 95% CI: 0.31 to 0.93; P = 0.003)47. Another study only found prospective evidence in esophageal squamous cell carcinoma (ESCC) but not in gastric carcinogenesis48. In a study of 197 gastric cancer patients, the cancer center of Sun Yat-sen University demonstrated that vitamin D deficiency might contribute to decreases in overall survival (P=0.019)49. Until now, the relationship between vitamin D supplements and upper gastrointestinal cancer risk has not been unsubstantiated, and the protective and prognostic role of serum 25(OH)D concentrations need to be further analyzed.
2.2.5. Other types of cancer
Some studies have reported an association of bladder cancer risk with vitamin D insufficiency, suggesting a protective role of vitamin D in bladder cancer50, 51, 52. In addition, the incidences of other types of cancer (e.g., lung cancer, ovarian cancer, and melanoma) showed controversial outcomes or indeterminate results between 25(OH)D concentrations and cancer risk, and the association might be varied in different cancers53, 54, 55.
Taken together, epidemiological evidence for the association between vitamin D supplements and cancer incidence are inclusive, and the beneficial role of vitamin D sufficiency is not well established in all cancer types. More well-controlled studies may be needed. Based on previous studies, additional factors such as district should be taken into consideration and further assessed. For example, there might be no statistical significance between plasma 25(OH)D levels and pancreatic cancer risk in European populations, whereas high circulating vitamin D levels showed protective effect on pancreatic cancer prognosis in USA cohorts56, 57.
2.3. Clinical trials of vitamin D and its analogues for treatment of cancers
2.3.1. Vitamin D and 1,25(OH)2D3
Initial trials of vitamin D have been conducted using its original form. It has been found that 400 IU is the optimal and safe dose of vitamin D for adults to prevent cancers, while high-dose higher than 2000 IU may inversely promote cancer growth in multiple cancer types such as gastric, pancreatic, colorectal and breast cancers58. The selection of proper doses might be one of the reasons for the controversial role of vitamin D in cancer prevention and treatment. In one study, vitamin D intake (≥ 400 IU) daily significantly decreased risks of lung cancer among non-smoking participants59. However, a clinical trial including 2303 subjects found that vitamin D intake (2000 IU/day) was not associated with lower cancer risk compared with the placebo control group in all cancer types60. Furthermore, it is seemed that high dose supplementation of calcium and vitamin D was correlated with undesirable results. For example, daily intake of calcium (1200 mg/day) and vitamin D (1000 IU/day) continuous for 3–5 years could increase the incidence of serrated adenomas in colon among the participants with one or more colon polyps61.
Clinical trials performed in most cases were supplied with 1,25(OH)2D3 other than vitamin D to achieve the higher serum concentrations, expecting to produce the maximum antitumor effects of 1,25(OH)2D3. Clinical studies of 1,25(OH)2D3 plus other anticancer drugs such as docetaxel, estramustine, and carboplatin in patients with cancers indicated that the high dose (20 IU/kg) of 1,25(OH)2D3 was safe and well-tolerated62, 63, 64, 65. Some of the trials exhibited promising antitumor activity, while some did not. A phase II trial of in androgen-independent prostate cancer with high-dose of 1,25(OH)2D3 (480 IU three days per week) for at least one month plus dexamethasone revealed encouraging antitumor activity66. It was demonstrated in a phase II study in advanced pancreatic cancer that 1,25(OH)2D3 might have enhanced activity in combination with docetaxel but not with gemcitabine or erlotinib67. However, in another phase II trial of weekly intravenous 1,25(OH)2D3 at dose of 2960 IU in combination with dexamethasone for castration-resistant prostate cancer, no positive outcomes were found68, 69. DN-101, a high-dose calcitriol formulation, was developed to increase the bioavailability of 1,25(OH)2D3. The maximum tolerated dose (MTD) of DN-101 was 1800 IU by weekly oral administration, while hypercalcemia was observed in patients treated at 2400 IU weekly65. A phase II study in recurrent prostate cancer, combination therapy with weekly 1,25(OH)2D3 (DN-101, 1800 IU) and daily naproxen was associated with prolonged the PSA doubling time, indicating the encouraging benefit of 1,25(OH)2D370. However, a randomized phase III trial of androgen-independent prostate cancer study of calcitriol enhancing taxotere (ASCENT, DN-101 1800 IU per week) unexpectedly revealed that shorter overall survival was found in 1,25(OH)2D3 treatment group compared to placebo group71. Consequently, even when a high dose of 1,25(OH)2D3 was studied, the outcomes were not always promising. In particular, optimization of the dosage of 1,25(OH)2D3 might be critical for effective treatment of cancers.
2.3.2. Vitamin D analogues
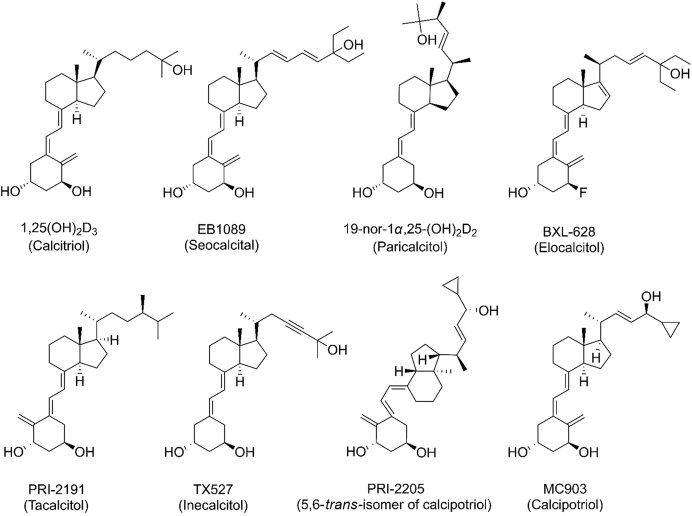
Normally, picomolar concentrations of 1,25(OH)2D3 are sufficient to maintain bone homeostasis, while vitamin D in cancer treatment sometimes necessitates relatively higher dosages, which simultaneously increases the risk of hypercalcemia and hypercalciuria. Moreover, 1,25(OH)2D3 is chemically unstable, and easily converted into the inactive form by CYP24A1, limiting the efficacy of vitamin D as a therapeutic agent. To diminish the side effects and increase the therapeutic effect of 1,25(OH)2D3 for cancer, vitamin D analogues (Fig. 2), including EB1089, 19-nor-1α,25-(OH)2D2 (paricalcitol), BXL-628 (elocalcitol), calcipotriol, PRI-2191 (tacalcitol, 1,24-dihydroxyvitamin D3), PRI-2205 (5,6-trans-isomer of calcipotriol), have been developed and investigated. The side chain attached to C17 is the main region involved in development for vitamin D analogues, because it is responsible for the binding to vitamin D receptor and is the main target for degradation by CYP24A1. Generally, vitamin D analogues showed similarly or extensively enhanced tumor effect than 1,25(OH)2D3 and mostly yield less calcemic.
Figure 2.
Structures of vitamin D analogues.
Among the most of these clinically studied analogues is EB1089. This drug (also named as seocalcitol) is characterized by an altered side chain structure featuring 26,27-dimethyl groups and two double bonds. It was synthesized to overcome the side effect of hypercalcemia. EB1089 was shown to inhibit prostate cancer metastasis in vivo with significantly weaker calcemic side effect compared with 1,25(OH)2D372. EB1089 has been shown to induce cell cycle arrest, cell differentiation and apoptosis in various cancer types including colon, prostate, breast and hepatocellular cancer (without hypercalcemia) both in vitro and in vivo73, 74, 75, 76, 77. Notably, EB1089 was shown to be more effective than 1,25(OH)2D3 in inducing apoptosis through suppressing BCL-2 and this agent also induced autophagic cell death in breast cancer cell line MCF778, 79. EB1089 was also significantly less calcemic than 1,25(OH)2D3 and exhibited anti-metastatic effects on prostate cancer72. A phase II clinical study has shown that it was well tolerated at a daily dose of 10–20 μg, which is significantly less calcemic than 1,25(OH)2D380. A phase II study of EB1089 conducted on patients with inoperable hepatocellular carcinoma indicated promising antitumor activity81.
Another less calcemic vitamin D analogue, paricalcitol, was applied in a phase I/II study in advanced androgen-insensitive prostate cancer patients with i.v. receiving paricalcitol at escalating doses of 5 to 25 μg82. Serum parathyroid hormone (PTH) level, a common index of advanced prostate cancer, was significantly decreased by paricalcitol, potentiating it as a therapeutic agent for improving cancer outcome. Accordingly, oral paricalcitol was also proved to be associated with low serum PTH level in patients with metastatic breast cancer83.
Inecalcitol, which has been identified as a VDR antagonist, more effectively activates VDR expression than 1,25(OH)2D3 and exerts much stronger inhibitory effect on prostate cancer84, 85. In a phase I study, in metastatic castration-resistant prostate cancer patients, the maximum dose of inecalcitol was 4000 μg per day combined with docetaxel, which showed antiproliferative activity and 100-fold lower hypercalcemic activity than 1,25(OH)2D386.
These findings show that there is still limited utility of vitamin D and 1,25(OH)2D3 for clinical use due to hypercalcemia induced by high-dose administration. The efficacy of low-dose vitamin D on cancer prevention or treatment requires additional future study. On the other hand, less calcemic vitamin D analogues may play an important role in cancer treatment. Moreover, new combination treatments of vitamin D analogues and chemotherapeutics for cancer therapy may be discovered. Current knowledge on clinical trials of vitamin D and its analogues has been summarized in Table 159, 60, 64, 67, 68, 71, 86, 87, 88, 89, 90, 91, 92, 93.
Table 1.
Representative clinical trials of vitamin D intake for cancer prevention or treatment.
| Subject | Dosage regimen | Aim | Main finding | Ref. |
|---|---|---|---|---|
| 128,779 participants | Oral 400 IU vitamin D plus 1 g calcium per day | The association between vitamin D intake and lung cancer | Among non-smokers, vitamin D intake significantly benefited for decreasing risks of lung cancer when compared with control. | 59 |
| 2303 healthy women | Oral 2000 IU vitamin D plus 1500 mg calcium per day | Cancer risk | Vitamin D supplementation did not reduce cancer risk. | 60 |
| 250 patients | i.v. docetaxel 36 mg/m2 per week for a 4-week cycle in combination with 45 μg DN-101 or placebo orally per day before docetaxel | Comparison of the efficacy and safety of DN-101 and docetaxel with placebo and docetaxel on AIPC | Oral taken DN-101 prolonged survival of AIPC patients compared with placebo. | 87 |
| 19 patients | Oral DN-101 180 μg on day 1 and i.v. mitoxantrone 12 mg/m2 on day 2 every 21 days with daily prednisone 10 mg orally for a maximum of 12 cycles | To evaluate efficacy and safety of DN-101 combined with mitoxantrone and glucocorticoids in AIPC | The addition of DN-101 does not appear to increase the toxicity of mitoxantrone in AIPC. | 64 |
| 25 patients | Oral 1,25(OH)2D3 0.5 µg/kg on day 1, followed by docetaxel 36 mg/m2 i.v. on day 2 per week for three consecutive weeks, followed by 1-week non-treatment | To assess safety and efficacy of weekly high-dose oral 1,25D3 and docetaxel in patients with non-resectable, incurable pancreatic cancer | Weekly oral intake of 1,25(OH)2D3 and docetaxel might prevent pancreatic cancer progression. | 67 |
| 18 patients | i.v. 1,25(OH)2D3 weekly at a dose of 74 µg and dexamethasone in patients with CRPC | CRPC treatment and prevention | 1,25(OH)2D3 supplement did not achieve favorable clinical outcomes. | 68 |
| 953 patients | ASCENT (45 μg DN-101, 36 mg/m2 docetaxel, and 24 mg dexamethasone weekly for 3 of every 4 weeks); control (5 mg prednisone twice daily with 75 mg/m2 docetaxel and 24 mg dexamethasone every 3 weeks) | To compare the efficacy and safety of docetaxel plus DN-101 to docetaxel plus prednisone in a phase III trial | ASCENT treatment decreased prostate cancer survival compared with control. | 71 |
| 66 patients | Daily oral supplementation of vitamin D (400, 10,000, or 40,000 IU per day) | Ki67 staining in prostate cancer tissue | Vitamin D intake decreased Ki67 level in prostate cancer tissue. | 88 |
| 23 patients | Oral administration of 0.5 µg/kg 1,25(OH)2D3 in 4 divided doses over on day 1 of each treatment week, docetaxel 36 mg/m2 i.v. infusion on day 2 of each treatment week and zoledronic acid 4 mg i.v. on day 2 of the first and fifth week of each cycle | To evaluate the efficacy and safety of combination of high dose 1,25(OH)2D3, docetaxel and zoledronic acid in CRPC | Prostate-specific antigen response was detected. | 89 |
| 54 patients | Inecalcitol at eight dose levels (40–8000 μg) daily combined with docetaxel | Prostate cancer treatment and prevention | Inecalcitol in combination with docetaxel encouraged PSA response of prostate cancer. | 86 |
| 1107 patients | 0.5 μg 1,25(OH)2D3 plus 75 mg acetylsalicylic acid and 1250 mg calcium carbonate (n = 209), or placebo (n = 218) | Adenoma recurrence after three-year treatment | Supplement with 1,25(OH)2D3 did not reduce the risk of CRC recurrence. | 90 |
| 64 cases and 64 controls | Diclofenac sodium 3% gel, 1,25(OH)2D3 3 μg/g ointment | BCC progression | Combination of diclofenac and 1,25(OH)2D3 treatment inhibited BCC proliferation. | 91 |
| 104 CRC patients | Calcium (1200 mg daily) alone, vitamin D (1000 IU daily) alone and in combination or placebo | APC/β-catenin pathway in normal colorectal mucosa | Vitamin D intake significantly suppressed APC/β-catenin pathway. | 92 |
| 2259 participants with colon adenoma | Daily oral 1000 IU vitamin D or 1200 mg calcium carbonate, or both or placebo | Colon adenoma recurrence | Vitamin D prevented colon cancer recurrence among individuals with AA genotype in VDR rs7969585 polymorphism. | 93 |
AIPC, androgen-independent prostate cancer; ASCENT, AIPC Study of Calcitriol Enhancing Taxotere; BCC, basal cell carcinoma; CRPC, castration-resistant prostate cancer; DN-101, a new high-dose oral formulation of 1,25(OH)2D3; i.v., intravenous administration; PSA, prostate specific antigen.
2.4. Vitamin D-related gene polymorphism
Polymorphisms in genes related with vitamin D metabolism, including VDR, CYP27A1, CYP27B1, CYP24A1 and GC, have been associated with cancer risk and progression, and may function as a valuable factor in cancer prognosis. Here, we summarized the epidemiological and genetic studies performed in recent years depicting the association of gene polymorphisms with cancer risk, progression and prognosis in Table 257, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119.
Table 2.
The association of vitamin D-related gene polymorphisms with cancer risk or mortality.
| Gene | Polymorphism | Study | Main finding | Ref. |
|---|---|---|---|---|
| CYP27B1 | rs10877012 (G > T); rs4646536 (C > T) | A follow-up study with 528 CRC patients and 605 cancer-free controls | Reduced CRC risk in GG carriers of rs10877012 and CC genotype of rs4646536; higher CRC risk in GT + TT carries of rs10877012 | 94, 95 |
| CYP27A1 | rs4674343 (G > A); rs6436086 (T > C) | 1260 men with prostate cancer and 1331 controls | Positive association of rs4674343 and rs6436086 with prostate cancer risk | 96 |
| rs964293 | 10,835 postmenopausal women (5419 CRC cases and 5416 controls) | Modify the association between combined oestrogen-progestogen hormone treatment and CRC incidence | 97 | |
| rs6068816; rs2181874 | 426 NSCLC cases and 445 controls from China | Reduction of NSCLC susceptibility by rs6068816 in both smokers and non-smokers; reduction of NSCLC risk in smokers with mutated homozygous rs6068816; increased NSCLC risk in those with mutated homozygous rs2181874 | 98 | |
| rs4809957 (A > G) | 528 CRC cases and 605 cancer-free controls in northeast China | Worse prognosis of CRC patients with CYP24A1 A>G (rs4809957) | 94 | |
| rs2296241 | 582 ESCC patients and 569 controls in a Northern Chinese population | Positive correlation between ESCC and rs2296241 | 99 | |
| GC | rs12512631; rs2239182 | 3566 primary melanoma cases | Longer cutaneous melanoma overall survival in those with rs12512631 and rs2239182 in GC | 100, 101 |
| rs7041 | 426 NSCLC cases and 445 controls from China; 1777 breast cancer cases and 1839 controls | Significant association of decreased NSCLC incidence with rs7041; increased risk of breast cancer in rs7041 TT genotype | 98, 102 | |
| rs4588 | 522 metastatic CRC patients | Longer CRC patients overall survival in AA carriers of rs4588 | 103 | |
| VDR | rs11568820 | 1598 patients with stage I to III CRC | Interaction of rs11568820 genotype with serum 25(OH)D concentration, modulating the risk for CRC-specific and all-cause mortality | 104 |
| rs7299460 | 493 pancreatic cancer patients from five prospective US cohorts | Better pancreatic cancer prognosis in rs7299460, while no interaction with pancreatic cancer risk | 57 | |
| Apa I | 340 patients (201 chronic hepatitis, 47 cirrhosis and 92 HCC) and 100 healthy controls | Association of CC genotype in Apa I with HCC progression | 105 | |
| Apa I | 302 RCC patients and 302 healthy controls | Increased susceptibility of RCC in AA and AC genotypes in Apa I variants compared with CC genotypes in Chinese populations | 106 | |
| rs2228570/Fok I | 378 participants: 78 CRC cases and 230 non-cancer controls | rs2228570/Fok I associated with CRC risk in African American populations | 107 | |
| rs2228570/Fok I | Meta-analysis including 9720 prostate cancer patients and 9710 control subjects; 10,486 prostate cancer cases and 10,400 controls | rs2228570/Fok I correlated with prostate cancer risk and progression in Caucasian | 108, 109 | |
| rs2228570/Fok I (C>T) | Meta-analysis with 209 tobacco-related cancers (cases) and 418 controls | Increase risk of smoking-related cancers in AA genotype of Fok I polymorphism | 110 | |
| rs2228570/Fok I (C>T) | 1820 white ovarian cancer cases and 3479 controls; 4152 cases and 6693 controls of Caucasian populations | Association of risk of ovarian cancer with rs2228570/Fok I | 111, 112 | |
| rs1989969 | 330 cases and 608 controls | Association with increased gastric cancer risk and development, especially in the younger and alcohol drinking Chinese population | 113 | |
| Bsm I; Apa I; Fok I; Poly (A) | Meta-analysis with 26,372 breast cancer cases and 32,883 controls | Significant association with breast cancer susceptibility | 114 | |
| rs7975232/Apa I; rs731236/Taq I | 100 cases of Egyptian females with breast cancer; 498 patients with breast cancer with a mean age at diagnosis of 61 years from Saarland, Germany | Strong correlation of ATT genotype in ApaI and TaqI polymorphism with susceptibility of breast cancer carcinogenesis in Egyptians | 115, 116 | |
| rs1544410/Bsm I; rs731236/Taq I | 3336 incident primary melanoma cases | Positively association with favorable overall survival melanoma patients exposed with high UVB | 117 | |
| rs1544410/Bsm I | A population-based cohort of 531 CRC patients in Newfoundland and Labrador, Canada | Significant correlation with the overall survival of CRC patients; Decreased overall survival in the G-allele in rs1544410 compared with the A-allele | 118 | |
| rs7968585 | A randomized clinical trial conducted at 11 clinical centers in the United States including 2259 participants | Reduced colorectal cancer recurrence risk among patients with AA genotype of rs7968585 | 93 | |
| rs1544410/BsmI; rs7975232/ApaI; rs2228570/Fok I | Meta-analyses with total 44,165 cases from 64 studies | Associated of cancer prognosis with rs1544410/Bsm I and rs7975232/Apa I polymorphisms; Correlation of lung cancer mortality with rs2228570/Fok I polymorphism | 119 |
CRC, colorectal cancer; ESCC, esophageal squamous cell carcinoma; HCC, hepatocellular carcinoma; GC, vitamin D binding protein; NSCLC, non-small cell lung cancer; RCC, Renal Cell Carcinoma; VDR, vitamin D receptor.
A meta-analysis revealed that Bsm I, Apa I, Fok I and Poly (A) polymorphisms in VDR gene might be related to breast cancer progression114. Another study which involved 3336 incident primary melanoma cases found that VDR single nucleotide polymorphisms (SNPs), rs1544410/Bsm I and rs731236/Taq I, significantly correlated with melanoma survival among subjects exposed to high UVB117. A case-control study including 528 CRC patients and 605 cancer-free controls and a follow-up study with 317 cases, which were conducted in northeast China, suggested that two polymorphisms in CYP27B1 (rs10877012 and rs4646536) are associated with decreased CRC risk while CYP24A1 (rs4809957) polymorphism might lead to a worse prognosis of CRC94. It was also reported that the CYP24A1 variant rs964293 modulated the association between combined oestrogen-progestogen (E+P) hormone therapy and CRC risk, suggesting the important role of CYP24A1 polymorphism in cancer progression97. Equal importantly, GC polymorphisms might be a predictive marker for outcome of chemotherapies. AA genotypes of GC rs4588 polymorphism was strongly associated with longer overall survival in CRC patients with treatment of irinotecan/cetuximab, whereas treatment with irinotecan/bevacizumab were associated with the converse103. These data support the use of specific gene polymorphisms as potential markers for cancer risk and cancer mortality.
3. Mechanisms of action of vitamin D within the tumor microenvironment
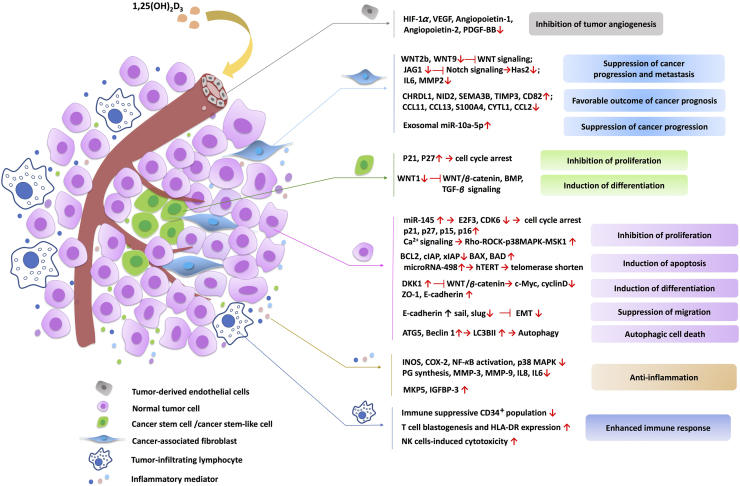
The antitumor activity of 1,25(OH)2D3, the active form of vitamin D, has been widely studied in a number of cancer types. By binding to VDR, 1,25(OH)2D3 exerted antitumor efficacy through regulating target gene expression or nongenomic actions related to different signaling pathways in both normal cancer cells and cancer stem cells. The anticancer actions of 1,25(OH)2D3 include the induction of cell cycle arrest, cell differentiation, cell apoptosis, autophagic cell death, as well as inhibition of metastasis tumor angiogenesis. Importantly, vitamin D could modulate stromal cells to suppress tumor angiogenesis, progression and metastasis in TME. Recent studies also demonstrate an anti-inflammatory role of vitamin D within TME. The schematic illustration of mechanisms related to the anticancer action of vitamin D within the TME is shown in Fig. 3.
Figure 3.
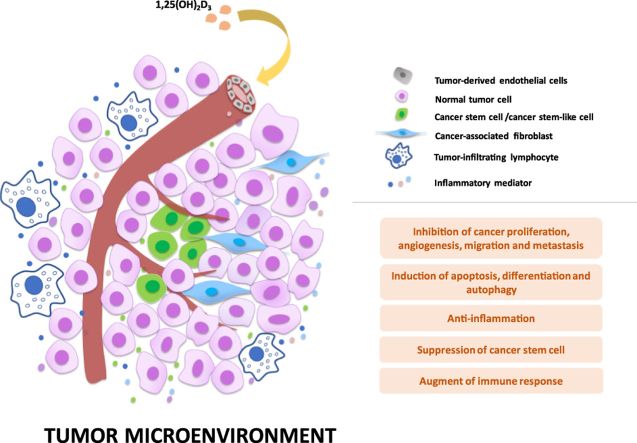
Mechanisms of anticancer action of vitamin D within tumor microenvironment (TME). The active form of vitamin D, 1,25(OH)2D3, not only suppresses cancer cell growth, but also regulates a range of stromal cells, including cancer-associated fibroblasts, tumor-derived endothelial cells, cancer stem cells and infiltrating immune cells, within TME to facilitate cancer suppression. 1,25(OH)2D3 also exhibits anti-inflammatory effect within TME. The representative signal transduction pathways are displayed.
3.1. Tumor cells
3.1.1. Regulation of proliferation
1,25(OH)2D3 has been found to inhibit cell proliferation through cell cycle arrest in most cancer cells, which plays an important role in cancer prevention76. The effect of 1,25(OH)2D3 on cell cycle distribution was mainly dependent on induction of p21 and p27 expression. p21 and p27 were identified as the targets of 1,25(OH)2D3 and were critical for G1 phase cell cycle arrest and inhibition of cancer cell proliferation120, 121. It was reported that the activation of JNK and ERK1/2 MAPK signaling pathways by 1,25(OH)2D3 were required for the induction of p21 expression122. Notably, p53 was involved in the regulation of p21 by 1,25(OH)2D3. There were multiple binding sites for p53 located in the promoter of p21 to cooperate with VDR to regulate transcriptional activity of p21120. We recently found that 1,25(OH)2D3 can induce p21expression and G1 phase cell cycle arrest in a mutant p53 and VDR dependent manner in gastric cancer cells20. At the meantime, p27 could up-regulated by 1,25(OH)2D3, although no canonical VDR element (VDRE) was identified in the p27 promoter123. It is demonstrated that VDR could not directly bind to the p27 promoter but interacts with sp1 to regulate the promoter activity of p27123. Also, 1,25(OH)2D3 up-regulated p27 expression through inhibition of Thr187 phosphorylation-dependent degradation in prostate cancer and increase the stability of p27 through down-regulation of ubiquitin pathway in ovarian cancer.124, 125 Furthermore, some other cyclin-dependent kinase inhibitors such as p15 and p16 may be induced by 1,25(OH)2D3121, 126, 127.
3.1.2. Induction of apoptosis
1,25(OH)2D3 can induce cell apoptosis in several cancer cells, mainly in breast, prostate and squamous carcinoma cells, effects which often require lengthy exposures to 1,25(OH)2D3. 1,25(OH)2D3-mediated cell apoptosis was demonstrated to be through mitochondrial-dependent pathway, in which the release of cytochrome c and the protein of BCL-2 family were involved, resulting in the suppression of the anti-apoptotic protein (BCL-2, BCL-XL) and the induction of the apoptotic protein (such as BAX, BAK, BAD)77, 128, 129, 130, 131, 132. In addition, a study demonstrated that 1,25(OH)2D3 could induce apoptosis in ovarian cancer cells through down-regulation of telomerase activity and telomerase reverse transcriptase (hTERT) expression after 6 days treatment with 1,25(OH)2D3, contributing to shortening the telomere length133, 134. It was also found that suppression of hTERT expression by 1,25(OH)2D3 was regulated by microR-498, showing that microRNA played an important role in vitamin D regulation in cancers133, 134. This sheds light on a new perspective in 1,25(OH)2D3-induced cancer cell apoptosis. At the meantime, 1,25(OH)2D3 could accelerate chemotherapeutics-induced apoptosis, thus enhancing the antitumor activity of chemotherapeutic drugs such as paclitaxel, gemcitabine and dexamethasone135, 136, 137, 138. In in vitro studies, it has been reported that vitamin D, in synergy with cisplatin, the histone deacetylase inhibitor trichostatin A, sodium butyrate or the methylation inhibitor 5-aza-2′-deoxycytidine, could induce apoptosis in gastric cancer cells139, 140. This indicated that 1,25(OH)2D3, the normal dietary supplement, could be considered for combination treatment for cancer.
3.1.3. Induction of differentiation
Induction of differentiation has been reported to involve in 1,25(OH)2D3-induced cancer suppression. 1,25(OH)2D3 is considered as a differentiating agent for leukemia cells. It could promote myeloid leukemia cells differentiating into mature myeloid cells, and has been used in clinical trials for acute myeloid leukemia141, 142. Furthermore, 1,25(OH)2D3 can induce differentiation in cancer cells, mostly in colon cancer, through repression of WNT/β-catenin signaling. Activation of WNT/β-catenin signaling is commonly found in colon cancer, and is associated with undifferentiated properties and malignance of cancers143. 1,25(OH)2D3-induced VDR translocation can bind β-catenin to the VDRE of vitamin D target genes, thereby inhibiting the transcriptional activity of WNT downstream targets144. It was also found that 1,25(OH)2D3 inhibited WNT/β-catenin signaling through induction of E-cadherin and the WNT inhibitor DKK1 in colon cancer cells, functioning as a multilevel suppressor of WNT/β-catenin signaling pathway145, 146, 147, 148, 149. E-cadherin is associated with epithelial phenotype of cancer cells and loss of E-cadherin causes invasiveness of cancer cells. Induction of E-cadherin and other differentiated epithelial marker ZO-1 by 1,25(OH)2D3 and inhibition of WNT/β-catenin downstream targets such as c-Myc and cyclin D finally restrain the cancer cell differentiation, which is also associated with tumor angiogenesis, migration and invasion.
3.1.4. Inhibition of angiogenesis and metastasis
The vitamin D signaling pathway is also associated with tumor angiogenesis, which is one of the mechanisms contributing to the anticancer action of 1,25(OH)2D3. Vascular endothelial growth factor (VEGF) is a growth factor that stimulates the formation of blood vessels, and its overexpression in cancer cells promotes angiogenesis and metastasis150. Suppression of hypoxia-inducible factor 1 (HIF-1α)-mediated VEGF expression and signaling by 1,25(OH)2D3 was detected in many kinds of cancer cells including prostate, breast and colon cancers151, 152. NF-κB signaling, the nuclear protein Forkhead box M1 (FOXM1) and DKK4 have been suggested to mediate the anti-angiogenesis effects of 1,25(OH)2D3. It was found that 1,25(OH)2D3 inhibited angiogenesis through reduction of NF-κB mediated interleukin-8 (IL-8) secretion in prostate cancer cells151. Treatments with 1,25(OH)2D3 or the vitamin D analogue EB1089 or activation of VDR remarkably suppressed pancreatic ductal adenocarcinoma cells proliferation, self-renewal abilities and metastasis through down-regulation of FOXM1. The latter is an oncogene that plays a key role in cell cycle regulation and carcinogenesis153. In addition, DKK4, a known downstream target of vitamin D signaling and an inhibitory Wnt ligand, was reported to inhibit tumor angiogenesis, migration and invasion in colon cancer cells in vitro. These findings further support the anti-angiogenesis and anti-invasiveness roles for vitamin D in cancer prevention147. Finally, 1,25(OH)2D3 also suppressed epithelial-mesenchymal transition of SKOV-3 ovarian cancer cells through reduction of Slug and Snail and upregulation of E-cadherin, thus resulting in the inhibition of migration and invasion of SKOV-3 cells154.
3.1.5. Induction of autophagy
Induction of autophagy by vitamin D was initially studied in the immune system. Vitamin D can induce autophagy in many kinds of immune cells through the host defense peptide LL37 for bacteria eradication. Accumulating evidences (best substantiated in breast cancer) indicates that vitamin D, as well as its analogue EB1089, suppresses cancer progression through autophagic-induced cell death. Initially, it was found that EB1089 triggers massive autophagy in MCF-7 breast cancer cells by upregulating the tumor suppressor gene Beclin-1, thereby inducing breast cancer cell death79. Recent evidence showed that the vitamin D-induced autophagic effect was found specifically in luminal-like breast cancer cells and benefited a lot in combination with hydroxychloroquine (HCQ), an inhibitor of autolysosome acidification for suppression of breast cancer growth in vivo155. Studies also indicated that the autophagy produced by either vitamin D or its analogue EB1089 could also modulate radiation sensitivity in breast cancer and non-small cell lung cancer (NSCLC) cells. Vitamin D or EB1089 increased radiation efficiency through promotion of autophagy in p53 existing breast cancer156 and NSCLC cells157 but not in p53-null cells, which indicates an important role of p53 in vitamin D-induced autophagy. Above all, vitamin D functions as an autophagic switch in human health maintenance and various types of cancer resistance, providing a novel perspective by magnifying the radiation responses in human malignancies.
3.1.6. Nongenomic effect
Nongenomic actions of 1,25(OH)2D3 are rapid and not dependent on VDR-mediated transcriptional activation (Fig. 2), recently reviewed by Hii and Ferrante158. The most well-established nongenomic effect of 1,25(OH)2D3 is the rapid intestinal absorption of Ca2+. It has been demonstrated that alteration of intracellular Ca2+ homeostasis is associated with tumor initiation, angiogenesis, progression and metastasis159. Based on this, a new mechanism of 1,25(OH)2D3 in regulation of E-cadherin has been found in colon cancer cells. Rho-ROCK-p38MAPK-MSK1 activation was mediated by 1,25(OH)2D3-induced upregulation of cytosolic Ca2+ concentration ([Ca2+]cyt), which was required for genes expression of CYP24A1 and E-cadherin160. In squamous cell carcinoma cells, 1,25(OH)2D3 provoked apoptosis through the rapid nongenomic activation of PI3K/Akt/ERK1/2/MAPK signaling and inhibition of the anti-apoptotic protein cIAP and XIAP161. These findings dual actions of VDR and expands the understanding that the nongenomic activation of VDR might synergize with the VDR-dependent genomic pathway to produce antitumor effect of 1,25(OH)2D3.
3.2. Stromal cells
The tumor stroma cell is one important part of developing tumor microenvironment, promoting expansive proliferation, metastatic capacities and chemoresistance of solid tumors. Stroma cells contain many cell types including epithelial cells, tumor-associated fibroblast, tumor-derived endothelial cells and infiltrating immune cells. More and more studies show that different stromal cells types in tumor microenvironment influence a lot of hallmarks of cancer. For example, endothelial cells trigger tumor angiogenesis, while cancer-associated fibroblasts (CAFs) are involved in activating tumor invasion and metastasis, proliferation and in resisting cell death162, 163. Emerging evidence suggests that vitamin D participates in modulating the gene signature of tumor stroma cells to regulate tumor angiogenesis, progression, and metastasis. Such findings suggest that vitamin D is a promising therapeutic for cancer.
3.2.1. Tumor-derived endothelial cell
Both in vitro and in vivo studies have demonstrated that 1,25(OH)2D3 is able to inhibit tumor derived endothelial cells (TDECs). It is reported that 1,25(OH)2D3 and its analogs inhibited TDECs proliferation without affecting normal aortic endothelial cells at concentrations comparable to those required to suppress cancer cells152, 164. Recently, emerging evidence showed that 1,25(OH)2D3 repressed tumor angiogenesis in some in vivo models such as the TRAMP-2 prostate tumor models and breast cancer models. 1,25(OH)2D3 inhibited TDECs in TRAMP-2 tumor with wild-type VDR but not in VDR knockout mice and enhanced tumor blood vessel density in VDR-knockout mice when compared with tumors from wild-type mice165. These results indicate the importance of VDR signaling on TDECs in the regulation of prostate cancer angiogenesis. It should be noted that 1,25(OH)2D3 could induce apoptosis of VEGF sprouting endothelial cells, thereby decreasing the blood vessel density in breast cancer xenograft tumors152, 166. Therefore, 1,25(OH)2D3 may function as an important regulator in alteration of pathologic angiogenesis to regulate the tumor microenvironment.
3.2.2. Cancer-associated fibroblast
The effect of 1,25(OH)2D3 on CAFs has been mainly investigated in pancreatic adenocarcinoma (PC) and CRC. VDR overexpression was found in tumor stromal cells of PC including pancreatic stellate cells (PSCs) existing in the extracellular matrix and CAFs19. 1,25(OH)2D3 suppressed pancreatic cancer metastasis through inhibiting secretion of metastasis supportive factors in PSCs including the WNT ligand (WNT2B), Notch receptor (JAG1), cytokines (IL6) and growth factors19. In a recent report, the activation of vitamin D signaling pathway in pancreatic CAFs suppressed the transmission of certain exosomal oncomiR such as miR-10a-5p from CAF to PC cells and thus diminished its protumoral effects on PC cells167, which provided a new perspective on how vitamin D regulates CAFs. Furthermore, it is reported that 1,25(OH)2D3 exhibited protective effect against CRC through inhibiting CAFs independently of VDR expression in CRC cells, while higher VDR expression in CAFs predicted longer overall survival in CRC patients168. Although promising results have been achieved on the regulation of CAFs by vitamin D, the underlying mechanisms remain to be evaluated, especially in different cancer types.
3.2.3. Tumor-infiltrating lymphocyte
Recently, a number of clinical and preclinical studies showed that tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment play a significant role in cancer progression or tumor suppression and may be identified as a new therapeutic target for cancer treatment. Effects of vitamin D on immune cells has been well studied, especially in host defense against bacteria such as the clearance of Mycobacterum tuberculosis. It was reported that the antibacterial activity of vitamin D on M. tuberculosis was mediated by autophagy through the upregulation of LL37 and the activation of Beclin-1 and ATG5 in monocytes and macrophage169, 170. Vitamin D supplements promoted INF-γ related antimicrobial effects of human macrophages171. On the other hand, vitamin D plays a significant role in adaptive immunity, and vitamin D deficiency might disrupt CD8 effector differentiation and the response of memory CD8 T cells for viral or bacterial infection in mice172. It was also found that vitamin D-induced tolerogenic dendritic cells (DC) increased population of CD4+CD25+Foxp3+ T cells, CD4+IL-10+ T cells, and CD19+CD5+CD1d+ B cells, resulting in attenuated experimental autoimmune encephalomyelitis173. Moreover, vitamin D induced regulatory T cell (Treg/Th17) differentiation through TGF-β signaling in a VDR-dependent manner, which indicated that vitamin D uptake might benefit for organ transplantation recipients174. Sun et al.175 found that vitamin D could modulate IL-17 and RANKL expression through VDR-mediated NF-κB signaling, resulting in the inhibition of Th17 cell differentiation in both in vitro and mice models. Vitamin D indeed plays an important role in both innate and adaptive immune responses. There is increasing evidence related to the important role of vitamin D on immune cells such as DC, NK cells, T cells, B cells as well as TILs in cancer and inflammation. For example, vitamin D can modulate tumor cell sensitivity to NK cells through suppression of miR-302c and miR-520c in breast cancer cells, resulting in increased NK cells-induced cytotoxicity and decreased breast cancer cell viability176.
PD-1 and PD-L1 function as blockers of immune responses and anti-PD-1 immunotherapy achieves rapid and effective treatments on a variety of cancers. Although PD-L1 suppresses the anti-tumor activity of T cells through immune checkpoint blockade, the anti-immunity role of PD-L1 might show protective effect in inflammation. It was found that vitamin D directly induced PD-L1 and PD-L2 expression in human epithelial cells and myeloid cells and treatment with vitamin D significantly suppress the inflammatory cytokine expression177, suggesting the anti-inflammatory role of vitamin D in immune responses and the protective role of vitamin D by reducing human malignancy initiation. One phase 1B study including patients with head and neck squamous cell carcinoma indicated that daily oral administration of vitamin D metabolite 25(OH)D3 decreased immune suppressive cells CD34+ population and had favorable immune responses including promoting T-cell blastogenesis and HLA-DR expression178. As the functional role of vitamin D in cancer immunotherapy is still limited, more studies on vitamin D in anti-cancer immunity are needed. Since cancer immunotherapy is now becoming a hot topic and benefits increasing number of cancer patients worldwide, it is expected that vitamin D might enhance cancer immunoprevention and increase the activity of immune cells to kill cancer cells.
3.3. Cancer stem cells
Cancer stem cells (CSCs) or tumor initiating cells (TICs) are critically responsible for tumorigenesis, progression, metastasis, and tumor recurrence, but they account for only a small proportion of cancer cells. Regarding the important role of the low-proportion CSCs in cancer microenvironment, targeting CSCs might be an efficient and promising method for cancer suppression. Recent studies have demonstrated that vitamin D may suppress cancer progression through targeting CSCs compartment. The role of vitamin D in regulating CSCs in gastrointestinal cancers has been thoroughly reviewed in our previous report179. Here we further summarized the suppressive action of vitamin D on CSCs in other cancers such as breast and prostate cancers.
In breast cancer, CD44 was identified as a breast cancer stem cell marker and CD44+/CD24–/low cells and CD49f+/CD24–/low were identified as tumor-initiating cells180. In MCF10A ductal carcinoma in situ (DCIS) cells, CD44 was significantly down-regulated by a Gemini vitamin D analog, BXL1024180. BXL1024 reduced the breast cancer stem-like cells population including CD44+/CD24–/low and CD49f+/CD24–/low subpopulation of MCF10A DCIS cells, and suppressed the stem cell markers expression (CD44 and CD49f)180. Further study has shown that the suppressive effect of 1,25(OH)2D3 or its analogue on breast CSCs in MCF10A DCIS was dependent in Hes1-mediated inhibition of Notch signaling181. In mammosphere, OCT4 and KLF4, which is associated with stem cell self-renewal and undifferentiated phenotype, were reduced by 1,25(OH)2D3 or BXL1024182. It is reported that another vitamin D analogue EB1089 in cooperation with BRAC1 exhibited anti-mammosphere formating ability in breast cancer cells183. Notably, Jeong et al.184 found that 1,25(OH)2D3 or oral administration with vitamin D could inhibit mouse TICs in MMTV-WNT1 mammary tumors through WNT/β-catenin signaling in vivo. 1,25(OH)2D3 inhibited spheroids formation of mouse TICs in 3D tumor spheroid culture assay in vitro184. However, some studies showed that VDR was down-regulated in breast cancer mammosphere, potentially indicating less vitamin D action on breast CSCs183, 185. Despite the controversy, the current findings still provided a new strategy for vitamin D targeting breast CSCs and give us a deeper understanding of vitamin D effect in cancer.
Although the anticancer effect of vitamin D on prostate cancer cells has been widely explored both in vitro and in vivo, the exact pharmacological action of vitamin D on prostate CSCs is not well understood. Existing evidence showed that the mechanism involved in the regulation of prostate CSCs by vitamin D was partly similar to that of prostate cancer cells. Maund et al.186 demonstrated that 1,25(OH)2D3 could induce p21 and p27 expression as well as cell cycle arrest in mouse prostate CSCs, and its anti-proliferative effect was mainly mediated by upregulation of IL-1α. In addition, the micro-array data indicated that the CSCs signaling (e.g., BMP and TGF-β) participated in the regulatory action of 1,25(OH)2D3 on prostate CSCs186. Taken together, the mechanisms underlying vitamin D regulation of CSCs seem similar to that of normal cancer cells, i.e., mainly through WNT/β-catenin, BMP, Notch and TGF-β signaling mechanisms.
3.4. Inflammatory mediators
Inflammation plays an important role in tumorigenesis187. Studies have indicated that vitamin D exhibits anti-inflammatory effects within TME to inhibit cancer initiation and progression188. Animal studies showed that dietary supplement of vitamin D significantly decreased colon cancer incidence in mice, which might be due to the decrease of MAPK and NF-κB signaling as well as the reduction of pro-inflammatory cytokines in colonic epithelial cells189. It is reported that 1,25(OH)2D3 may restrain inflammatory progression through downregulation of MMP-3, INOS and COX-2 levels and upregulation of inflammatory mediators including IL-1β, IL-6, IL-8, and prostaglandins (PGs) in macrophage190, 191. COX-2 is involved in pathogenesis of many inflammatory diseases192, and 1,25(OH)2D3 is able to decrease COX-2 expression in many kinds of cancers such as breast cancer, colon cancer, ovarian cancer and prostate cancer, thereby suppressing cancer growth193, 194, 195, 196. In a clinical trial, higher levels of vitamin D were correlated with lower COX-2 level in prostate cancer cells and stroma cells197. Moreover, inflammatory factors including TNF-α, IL-6 and IL-8 were significantly inhibited by 1,25(OH)2D3 in prostate primary epithelial cells, indicating the beneficial role of anti-inflammatory action of vitamin D in prostate cancer197. Above all, vitamin D exerts anti-inflammatory effects through suppression of the production and action of inflammatory mediators such as cytokines, chemokines and PGs, and inhibition of MAPK and NF-κB signalings in cancer cells, macrophages as well as the epithelial cells, all of which might contribute to the prevention of cancer progression and inflammatory progress.
4. Conclusions/perspectives
Accumulating evidence strongly supports the notion that vitamin D deficiency is associated with elevated cancer risk and poor prognosis. Vitamin D supplementation can exert profound suppression of cancers, in particular via targeting components of the TME. Importantly, the emerging role of vitamin D in the regulation of the TME provides a mechanistic basis for its potential efficacy in treating cancer. Although the preclinical and epidemiologic data are persuasive and provide supportive evidence for continued development of vitamin D or its analogues for cancer therapy, no well-designed clinical trial of vitamin D has been carried out. In particular, the optimum dosage of vitamin D supplementation to reduce cancer risk or treat cancer is still unclear. Future clinical trials that treat cancer patients with suitable doses of vitamin D, or new analogues as well as combination therapy may finally demonstrate the promise for use of a classical vitamin with low and known toxicity in humans. Such results may lead to the development of an effective medicine for the prevention and treatment of malignancies in an economical and efficient manner.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Nos. 81770562, 81602166 and 81703807) and grants from the Science and Technology Planning Project of Luzhou, Sichuan Province, China (Nos. 2016LZXNYD-Z04 and 2017LZXNYD-J02).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Jing Shen, Email: crystal_stray@126.com.
Mingxing Li, Email: star.lee@hotmail.com.
References
- 1.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mlecnik B., Bindea G., Kirilovsky A., Angell H.K., Obenauf A.C., Tosolini M. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 3.Albini A., Sporn M.B. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 4.Pitt J.M., Marabelle A., Eggermont A., Soria J.C., Kroemer G., Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X., Chen R., Liu M., Feng J., Chen J., Hu K. Remodeling the blood–brain barrier microenvironment by natural products for brain tumor therapy. Acta Pharm Sin B. 2017;7:541–553. doi: 10.1016/j.apsb.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688SS. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 7.Sun H., Wang C., Hao M., Sun R., Wang Y., Liu T. CYP24A1 is a potential biomarker for the progression and prognosis of human colorectal cancer. Hum Pathol. 2016;50:101–108. doi: 10.1016/j.humpath.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Shiratsuchi H., Wang Z., Chen G., Ray P., Lin J., Zhang Z. Oncogenic potential of CYP24A1 in lung adenocarcinoma. J Thorac Oncol. 2017;12:269–280. doi: 10.1016/j.jtho.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Luo W., Yu W.D., Ma Y., Chernov M., Trump D.L., Johnson C.S. Inhibition of protein kinase CK2 reduces Cyp24a1 expression and enhances 1,25-dihydroxyvitamin D3 antitumor activity in human prostate cancer cells. Cancer Res. 2013;73:2289–2297. doi: 10.1158/0008-5472.CAN-12-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich M., Axt-Fliedner R., Villena-Heinsen C., Tilgen W., Schmidt W., Reichrath J. Analysis of vitamin D-receptor (VDR) and retinoid X-receptor a in breast cancer. Histochem J. 2002;34:35–40. doi: 10.1023/a:1021343825552. [DOI] [PubMed] [Google Scholar]
- 11.Izkhakov E., Somjen D., Sharon O., Knoll E., Aizic A., Fliss D.M. Vitamin D receptor expression is linked to potential markers of human thyroid papillary carcinoma. J Steroid Biochem Mol Biol. 2016;159:26–30. doi: 10.1016/j.jsbmb.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Khadzkou K., Buchwald P., Westin G., Dralle H., Akerstrom G., Hellman P. 25-hydroxyvitamin D3 1a-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J Histochem Cytochem. 2006;54:355–361. doi: 10.1369/jhc.5A6734.2005. [DOI] [PubMed] [Google Scholar]
- 13.Cross H.S., Bareis P., Hofer H., Bischof M.G., Bajna E., Kriwanek S. 25-Hydroxyvitamin D3-1a-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66:287–292. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson W.K., Flavin R., Kasperzyk J.L., Fiorentino M., Fang F., Lis R. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. 2011;29:2378–2385. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S.H., Chen G., King A.N., Jeon C.K., Christensen P.J., Zhao L. Characterization of vitamin D receptor (VDR) in lung adenocarcinoma. Lung Cancer. 2012;77:265–271. doi: 10.1016/j.lungcan.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K., Dong M., Sheng W., Liu Q., Yu D., Dong Q. Expression of vitamin D receptor as a potential prognostic factor and therapeutic target in pancreatic cancer. Histopathology. 2015;67:386–397. doi: 10.1111/his.12663. [DOI] [PubMed] [Google Scholar]
- 17.Momen-Heravi F., Masugi Y., Qian Z.R., Nishihara R., Liu L., Smith-Warner S.A. Tumor expression of calcium sensing receptor and colorectal cancer survival: results from the nurses׳ health study and health professionals follow-up study. Int J Cancer. 2017;141:2471–2479. doi: 10.1002/ijc.31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer-Mayorga G., Gomez-Lopez G., Barbachano A., Fernandez-Barral A., Pena C., Pisano D.G. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman M.H., Yu R.T., Engle D.D., Ding N., Atkins A.R., Tiriac H. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M., Li L., Zhang L., Hu W., Shen J., Xiao Z. 1,25-Dihydroxyvitamin D3 suppresses gastric cancer cell growth through VDR- and mutant p53-mediated induction of p21. Life Sci. 2017;179:88–97. doi: 10.1016/j.lfs.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y., Trivedi T., Lin R.C., Fong-Yee C., Nolte R., Manibo J. Loss of the vitamin D receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of Wnt/β-catenin signaling. Bone Res. 2017;5:17023. doi: 10.1038/boneres.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick M.F. Vitamin D deficiency. New Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 23.Malabanan A., Veronikis I., Holick M. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 24.Corder E.H., Guess H.A., Hulka B.S., Friedman G.D., Sadler M., Vollmer R.T. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomar Prev. 1993;2:467–472. [PubMed] [Google Scholar]
- 25.Deschasaux M., Souberbielle J.C., Latino-Martel P., Sutton A., Charnaux N., Druesne-Pecollo N. A prospective study of plasma 25-hydroxyvitamin D concentration and prostate cancer risk. Br J Nutr. 2016;115:305–314. doi: 10.1017/S0007114515004353. [DOI] [PubMed] [Google Scholar]
- 26.Li H., Stampfer M.J., Hollis J.B., Mucci L.A., Gaziano J.M., Hunter D. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn J., Peters U., Albanes D., Purdue M.P., Abnet C.C., Chatterjee N. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao J., Wei W., Wang G., Zhou H., Fu Y., Liu N. Circulating vitamin D concentration and risk of prostate cancer: a dose-response meta-analysis of prospective studies. Ther Clin Risk Manag. 2018;14:95–104. doi: 10.2147/TCRM.S149325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn J., Park S., Zuniga B., Bera A., Song C.S., Chatterjee B. Vitamin D in prostate cancer. Vitam Horm. 2016;100:321–355. doi: 10.1016/bs.vh.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Garland C.F., Gorham E.D., Mohr S.B., Grant W.B., Giovannucci E.L., Lipkin M. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 31.John E.M., Schwartz G.G., Dreon D.M., Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomar Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 32.Chlebowski R.T., Johnson K.C., Kooperberg C., Pettinger M., Wactawski-Wende J., Rohan T. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin M.H., Holmes M.D., Hankinson S.E., Wu K., Colditz G.A., Willett W.C. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 34.de Sousa Almeida-Filho B., De Luca Vespoli H., Pessoa E.C., Machado M., Nahas-Neto J., Nahas E.A.P. Vitamin D deficiency is associated with poor breast cancer prognostic features in postmenopausal women. J Steroid Biochem Mol Biol. 2017;174:284–289. doi: 10.1016/j.jsbmb.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Lin J., Manson J.E., Lee I.M., Cook N.R., Buring J.E., Zhang S.M. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 36.Anderson L.N., Cotterchio M., Vieth R., Knight J.A. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr. 2010;91:1699–1707. doi: 10.3945/ajcn.2009.28869. [DOI] [PubMed] [Google Scholar]
- 37.Yao S., Kwan M.L., Ergas I.J., Roh J.M., Cheng T.D., Hong C.C. Association of serum level of vitamin D at diagnosis with breast cancer survival: a case-cohort analysis in the pathways study. JAMA Oncol. 2017;3:351–357. doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer J.R., Gerlovin H., Bethea T.N., Bertrand K.A., Holick M.F., Ruiz-Narvaez E.N. Predicted 25-hydroxyvitamin D in relation to incidence of breast cancer in a large cohort of African American women. Breast Cancer Res. 2016;18:86. doi: 10.1186/s13058-016-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garland C., Shekelle R.B., Barrett-Connor E., Criqui M.H., Rossof A.H., Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 40.Garland C.F., Comstock G.W., Garland F.C., Helsing K.J., Shaw E.K., Gorham E.D. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 41.Lee J.E., Li H., Chan A.T., Hollis B.W., Lee I.M., Stampfer M.J. Circulating levels of vitamin D and colon and rectal cancer: the Physicians׳ Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila) 2011;4:735–743. doi: 10.1158/1940-6207.CAPR-10-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCullough M, Zoltick E, Weinstein S, Fedirko V, Wang M, Cook N, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst 2018. Available from: 10.1093/jnci/djy087. [DOI] [PMC free article] [PubMed]
- 43.Hightower J.M., Dalessandri K.M., Pope K., Hernandez G.T. Low 25-Hydroxyvitamin D and myofascial pain: association of cancer, colon polyps, and tendon rupture. J Am Coll Nutr. 2017;36:455–461. doi: 10.1080/07315724.2017.1320951. [DOI] [PubMed] [Google Scholar]
- 44.Mohr S.B., Gorham E.D., Kim J., Hofflich H., Cuomo R.E., Garland C.F. Could vitamin D sufficiency improve the survival of colorectal cancer patients? J Steroid Biochem Mol Biol. 2015;148:239–244. doi: 10.1016/j.jsbmb.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Song M., Nishihara R., Wang M., Chan A.T., Qian Z.R., Inamura K. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65:296–304. doi: 10.1136/gutjnl-2014-308852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeshige N., Yin G., Ohnaka K., Kono S., Ueki T., Tanaka M. Associations between vitamin D receptor (VDR) gene polymorphisms and colorectal cancer risk and effect modifications of dietary calcium and vitamin D in a Japanese population. Asian Pac J Cancer Prev. 2015;16:2019–2026. doi: 10.7314/apjcp.2015.16.5.2019. [DOI] [PubMed] [Google Scholar]
- 47.Chen W., Dawsey S.M., Qiao Y.L., Mark S.D., Dong Z.W., Taylor P.R. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97:123–128. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abnet C.C., Chen Y., Chow W.H., Gao Y.T., Helzlsouer K.J., Le Marchand L. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:94–106. doi: 10.1093/aje/kwq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren C., Qiu M.Z., Wang D.S., Luo H.Y., Zhang D.S., Wang Z.Q. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J Transl Med. 2012;10:16. doi: 10.1186/1479-5876-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y., Chen C., Pan W., Gao M., He W., Mao R. Comparative efficacy of vitamin D status in reducing the risk of bladder cancer: a systematic review and network meta-analysis. Nutrition. 2016;32:515–523. doi: 10.1016/j.nut.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H., Zhang H., Wen X., Zhang Y., Wei X., Liu T. Vitamin D deficiency and increased risk of bladder carcinoma: a meta-analysis. Cell Physiol Biochem. 2015;37:1686–1692. doi: 10.1159/000438534. [DOI] [PubMed] [Google Scholar]
- 52.Ben Fradj M.K., Gargouri M.M., Hammami M.B., Ben Rhouma S., Kallel A., Jemaa R. Bladder cancer is associated with low plasma 25-hydroxyvitamin D concentrations in tunisian population. Nutr Cancer. 2016;68:208–213. doi: 10.1080/01635581.2016.1134598. [DOI] [PubMed] [Google Scholar]
- 53.Dimitrakopoulou V.I., Tsilidis K.K., Haycock P.C., Dimou N.L., Al-Dabhani K., Martin R.M. Circulating vitamin D concentration and risk of seven cancers: mendelian randomisation study. BMJ. 2017;359:j4761. doi: 10.1136/bmj.j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gromowski T., Gapska P., Scott R.J., Kaklewski K., Marciniak W., Durda K. Serum 25(OH)D concentration, common variants of the VDR gene and lung cancer occurrence. Int J Cancer. 2017;141:336–341. doi: 10.1002/ijc.30740. [DOI] [PubMed] [Google Scholar]
- 55.Ong J.S., Cuellar-Partida G., Lu Y., Australian Ovarian Cancer S., Fasching P.A., Hein A. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2016;45:1619–1630. doi: 10.1093/ije/dyw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Duijnhoven F.J.B., Jenab M., Hveem K., Siersema P.D., Fedirko V., Duell E.J. Circulating concentrations of vitamin D in relation to pancreatic cancer risk in European populations. Int J Cancer. 2018;142:1189–1201. doi: 10.1002/ijc.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan C., Qian Z.R., Babic A., Morales-Oyarvide V., Rubinson D.A., Kraft P. Prediagnostic plasma 25-hydroxyvitamin D and pancreatic cancer survival. J Clin Oncol. 2016;34:2899–2905. doi: 10.1200/JCO.2015.66.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omura Y., Lu D., Jones M.K., Nihrane A., Duvvi H., Yapor D. Optimal dose of vitamin D3 400 I.U. for average adults has a significant anti-cancer effect, while widely used 2000 I.U. or higher promotes cancer: marked reduction of taurine & 1a, 25(OH)2D3 was found in various cancer tissues and oral intake of optimal dose of taurine 175 mg for average adults, rather than 500 mg, was found to be a new potentially safe and more effective method of cancer treatment. Acupunct Electrother Res. 2016;41:39–60. doi: 10.3727/036012916x14597946741564. [DOI] [PubMed] [Google Scholar]
- 59.Cheng T.Y., Lacroix A.Z., Beresford S.A., Goodman G.E., Thornquist M.D., Zheng Y. Vitamin D intake and lung cancer risk in the Women׳s Health Initiative. Am J Clin Nutr. 2013;98:1002–1011. doi: 10.3945/ajcn.112.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lappe J., Watson P., Travers-Gustafson D., Recker R., Garland C., Gorham E. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317:1234–1243. doi: 10.1001/jama.2017.2115. [DOI] [PubMed] [Google Scholar]
- 61.Crockett SD, Barry EL, Mott LA, Ahnen DJ, Robertson DJ, Anderson JC, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. 2018. Available from: 10.1136/gutjnl-2017-315242. [DOI] [PMC free article] [PubMed]
- 62.Beer T.M., Garzotto M., Katovic N.M. High-dose calcitriol and carboplatin in metastatic androgen-independent prostate cancer. Am J Clin Oncol. 2004;27:535–541. doi: 10.1097/01.coc.0000136020.27904.9c. [DOI] [PubMed] [Google Scholar]
- 63.Tiffany N.M., Ryan C.W., Garzotto M., Wersinger E.M., Beer T.M. High dose pulse calcitriol, docetaxel and estramustine for androgen independent prostate cancer: a phase I/II study. J Urol. 2005;174:888–892. doi: 10.1097/01.ju.0000169261.42298.e6. [DOI] [PubMed] [Google Scholar]
- 64.Chan J.S., Beer T.M., Quinn D.I., Pinski J.K., Garzotto M., Sokoloff M. A phase II study of high-dose calcitriol combined with mitoxantrone and prednisone for androgen-independent prostate cancer. BJU Int. 2008;102:1601–1606. doi: 10.1111/j.1464-410X.2008.08017.x. [DOI] [PubMed] [Google Scholar]
- 65.Beer T.M., Javle M.M., Ryan C.W., Garzotto M., Lam G.N., Wong A. Phase I study of weekly DN-101, a new formulation of calcitriol, in patients with cancer. Cancer Chemother Pharmacol. 2007;59:581–587. doi: 10.1007/s00280-006-0299-1. [DOI] [PubMed] [Google Scholar]
- 66.Trump D.L., Potter D.M., Muindi J., Brufsky A., Johnson C.S. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106:2136–2142. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- 67.Blanke C.D., Beer T.M., Todd K., Mori M., Stone M., Lopez C. Phase II study of calcitriol-enhanced docetaxel in patients with previously untreated metastatic or locally advanced pancreatic cancer. Invest New Drugs. 2009;27:374–378. doi: 10.1007/s10637-008-9184-6. [DOI] [PubMed] [Google Scholar]
- 68.Chadha M.K., Tian L., Mashtare T., Payne V., Silliman C., Levine E. Phase 2 trial of weekly intravenous 1,25-dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration-resistant prostate cancer. Cancer. 2010;116:2132–2139. doi: 10.1002/cncr.24973. [DOI] [PubMed] [Google Scholar]
- 69.Ramnath N., Daignault-Newton S., Dy G.K., Muindi J.R., Adjei A., Elingrod V.L. A phase I/II pharmacokinetic and pharmacogenomic study of calcitriol in combination with cisplatin and docetaxel in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2013;71:1173–1182. doi: 10.1007/s00280-013-2109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srinivas S., Feldman D. A phase II trial of calcitriol and naproxen in recurrent prostate cancer. Anticancer Res. 2009;29:3605–3610. [PubMed] [Google Scholar]
- 71.Scher H.I., Jia X., Chi K., de Wit R., Berry W.R., Albers P. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol. 2011;29:2191–2198. doi: 10.1200/JCO.2010.32.8815. [DOI] [PubMed] [Google Scholar]
- 72.Lokeshwar B.L., Schwartz G.G., Selzer M.G., Burnstein K.L., Zhuang S.H., Block N.L. Inhibition of prostate cancer metastasis in vivo: a comparison of 1,25-dihydroxyvitamin D (calcitriol) and EB1089. Cancer Epidemiol Biomar Prev. 1999;8:241–248. [PubMed] [Google Scholar]
- 73.Ghous Z., Akhter J., Pourgholami M.H., Morris D.L. Inhibition of hepatocellular cancer by EB1089: in vitro and in vivo study. Anticancer Res. 2008;28:3757–3761. [PubMed] [Google Scholar]
- 74.James S.Y., Mercer E., Brady M., Binderup L., Colston K.W. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. Br J Pharmacol. 1998;125:953–962. doi: 10.1038/sj.bjp.0702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blutt S.E., Polek T.C., Stewart L.V., Kattan M.W., Weigel N.L. A calcitriol analogue, EB1089, inhibits the growth of LNCaP tumors in nude mice. Cancer Res. 2000;60:779–782. [PubMed] [Google Scholar]
- 76.Wu G., Fan R.S., Li W., Ko T.C., Brattain M.G. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15:1555–1563. doi: 10.1038/sj.onc.1201329. [DOI] [PubMed] [Google Scholar]
- 77.Diaz G.D., Paraskeva C., Thomas M.G., Binderup L., Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60:2304–2312. [PubMed] [Google Scholar]
- 78.Simboli-Campbell M., Narvaez C.J., van Weelden K., Tenniswood M., Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1997;42:31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 79.Hoyer-Hansen M., Bastholm L., Mathiasen I.S., Elling F., Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12:1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- 80.Evans T.R., Colston K.W., Lofts F.J., Cunningham D., Anthoney D.A., Gogas H. A phase II trial of the vitamin D analogue seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer. 2002;86:680–685. doi: 10.1038/sj.bjc.6600162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalhoff K., Dancey J., Astrup L., Skovsgaard T., Hamberg K.J., Lofts F.J. A phase II study of the vitamin D analogue seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer. 2003;89:252–257. doi: 10.1038/sj.bjc.6601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz G.G., Hall M.C., Stindt D., Patton S., Lovato J., Torti F.M. Phase I/II study of 19-nor-1a-25-dihydroxyvitamin D2 (paricalcitol) in advanced, androgen-insensitive prostate cancer. Clin Cancer Res. 2005;11:8680–8685. doi: 10.1158/1078-0432.CCR-05-1237. [DOI] [PubMed] [Google Scholar]
- 83.Lawrence J.A., Akman S.A., Melin S.A., Case L.D., Schwartz G.G. Oral paricalcitol (19-nor-1,25-dihydroxyvitamin D2) in women receiving chemotherapy for metastatic breast cancer: a feasibility trial. Cancer Biol Ther. 2013;14:476–480. doi: 10.4161/cbt.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma Y., Yu W.D., Hidalgo A.A., Luo W., Delansorne R., Johnson C.S. Inecalcitol, an analog of 1,25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle. 2013;12:743–752. doi: 10.4161/cc.23846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okamoto R., Delansorne R., Wakimoto N., Doan N.B., Akagi T., Shen M. Inecalcitol, an analog of 1a,25(OH)2D3, induces growth arrest of androgen-dependent prostate cancer cells. Int J Cancer. 2012;130:2464–2473. doi: 10.1002/ijc.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Medioni J., Deplanque G., Ferrero J.M., Maurina T., Rodier J.M., Raymond E. Phase I safety and pharmacodynamic of inecalcitol, a novel VDR agonist with docetaxel in metastatic castration-resistant prostate cancer patients. Clin Cancer Res. 2014;20:4471–4477. doi: 10.1158/1078-0432.CCR-13-3247. [DOI] [PubMed] [Google Scholar]
- 87.Beer T.M., Ryan C.W., Venner P.M., Petrylak D.P., Chatta G.S., Ruether J.D. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 88.Wagner D., Trudel D., Van der Kwast T., Nonn L., Giangreco A.A., Li D. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J Clin Endocrinol Metab. 2013;98:1498–1507. doi: 10.1210/jc.2012-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shamseddine A., Farhat F.S., Elias E., Khauli R.B., Saleh A., Bulbul M.A. High-dose calcitriol, docetaxel and zoledronic acid in patients with castration-resistant prostate cancer: a phase II study. Urol Int. 2013;90:56–61. doi: 10.1159/000343780. [DOI] [PubMed] [Google Scholar]
- 90.Pommergaard H.C., Burcharth J., Rosenberg J., Raskov H. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. 2016;150:114–122. doi: 10.1053/j.gastro.2015.09.010. [e4] [DOI] [PubMed] [Google Scholar]
- 91.Brinkhuizen T., Frencken K.J., Nelemans P.J., Hoff M.L., Kelleners-Smeets N.W., Zur Hausen A. The effect of topical diclofenac 3% and calcitriol 3 μg/g on superficial basal cell carcinoma (sBCC) and nodular basal cell carcinoma (nBCC): a phase II, randomized controlled trial. J Am Acad Dermatol. 2016;75:126–134. doi: 10.1016/j.jaad.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 92.Liu S., Barry E.L., Baron J.A., Rutherford R.E., Seabrook M.E., Bostick R.M. Effects of supplemental calcium and vitamin D on the APC/β-catenin pathway in the normal colorectal mucosa of colorectal adenoma patients. Mol Carcinog. 2017;56:412–424. doi: 10.1002/mc.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barry E.L., Peacock J.L., Rees J.R., Bostick R.M., Robertson D.J., Bresalier R.S. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol. 2017;3:628–635. doi: 10.1001/jamaoncol.2016.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gong C., Long Z., Yu Y., Zhu L., Tian J., Li S. Dietary factors and polymorphisms in vitamin D metabolism genes: the risk and prognosis of colorectal cancer in northeast China. Sci Rep. 2017;7:8827. doi: 10.1038/s41598-017-09356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vidigal V.M., Silva T.D., de Oliveira J., Pimenta C.A.M., Felipe A.V., Forones N.M. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the risk of colorectal cancer. Int J Biol Markers. 2017;32:e224–e230. doi: 10.5301/jbm.5000248. [DOI] [PubMed] [Google Scholar]
- 96.Shui I.M., Mucci L.A., Kraft P., Tamimi R.M., Lindstrom S., Penney K.L. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst. 2012;104:690–699. doi: 10.1093/jnci/djs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Albeniz X., Rudolph A., Hutter C., White E., Lin Y., Rosse S.A. CYP24A1 variant modifies the association between use of oestrogen plus progestogen therapy and colorectal cancer risk. Br J Cancer. 2016;114:221–229. doi: 10.1038/bjc.2015.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu X., Cheng J., Yang K. Vitamin D-related gene polymorphisms, plasma 25-hydroxy-vitamin D, cigarette smoke and non-small cell lung cancer (NSCLC) risk. Int J Mol Sci. 2016;17:1597. doi: 10.3390/ijms17101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang J., Wang H., Ji A., Ma L., Wang J., Lian C. Vitamin D signaling pathways confer the susceptibility of esophageal squamous cell carcinoma in a northern chinese population. Nutr Cancer. 2017;69:593–600. doi: 10.1080/01635581.2017.1299873. [DOI] [PubMed] [Google Scholar]
- 100.Yin J., Liu H., Yi X., Wu W., Amos C.I., Fang S. Genetic variants in the vitamin D pathway genes VDBP and RXRA modulate cutaneous melanoma disease-specific survival. Pigment Cell Melanoma Res. 2016;29:176–185. doi: 10.1111/pcmr.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Orlow I., Reiner A.S., Thomas N.E., Roy P., Kanetsky P.A., Luo L. Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: a population-based study. Carcinogenesis. 2016;37:30–38. doi: 10.1093/carcin/bgv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson L.N., Cotterchio M., Cole D.E., Knight J.A. Vitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in Ontario. Cancer Epidemiol Biomarkers Prev. 2011;20:1708–1717. doi: 10.1158/1055-9965.EPI-11-0300. [DOI] [PubMed] [Google Scholar]
- 103.Berger M.D., Stintzing S., Heinemann V., Cao S., Yang D., Sunakawa Y. A polymorphism within the vitamin D transporter gene predicts outcome in metastatic colorectal cancer patients treated with FOLFIRI/bevacizumab or FOLFIRI/cetuximab. Clin Cancer Res. 2017;24:784–793. doi: 10.1158/1078-0432.CCR-17-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zgaga L., Theodoratou E., Farrington S.M., Din F.V., Ooi L.Y., Glodzik D. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32:2430–2439. doi: 10.1200/JCO.2013.54.5947. [DOI] [PubMed] [Google Scholar]
- 105.Hung C.H., Chiu Y.C., Hu T.H., Chen C.H., Lu S.N., Huang C.M. Significance of vitamin D receptor gene polymorphisms for risk of hepatocellular carcinoma in chronic hepatitis C. Transl Oncol. 2014;7:503–507. doi: 10.1016/j.tranon.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang C., Li J., Li Y., Wu D., Sui C., Jiang Y. The vitamin D receptor gene ApaI polymorphism is associated with increased risk of renal cell carcinoma in Chinese population. Sci Rep. 2016;6:25987. doi: 10.1038/srep25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarkissyan M., Wu Y., Chen Z., Mishra D.K., Sarkissyan S., Giannikopoulos I. Vitamin D receptor FokI gene polymorphisms may be associated with colorectal cancer among African American and Hispanic participants. Cancer. 2014;120:1387–1393. doi: 10.1002/cncr.28565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mi Y.Y., Chen Y.Z., Chen J., Zhang L.F., Zuo L., Zou J.G. Updated analysis of vitamin D receptor gene FokI polymorphism and prostate cancer susceptibility. Arch Med Sci. 2017;13:1449–1458. doi: 10.5114/aoms.2016.61793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang S., Zhao Y., Liu J., Wang L., Zhao G., Chen X. Association of Vitamin D receptor Fok I polymorphism with the risk of prostate cancer: a meta-analysis. Oncotarget. 2016;7:77878–77889. doi: 10.18632/oncotarget.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deschasaux M., Souberbielle J.C., Latino-Martel P., Sutton A., Charnaux N., Druesne-Pecollo N. Prospective associations between vitamin D status, vitamin D-related gene polymorphisms, and risk of tobacco-related cancers. Am J Clin Nutr. 2015;102:1207–1215. doi: 10.3945/ajcn.115.110510. [DOI] [PubMed] [Google Scholar]
- 111.Lurie G., Wilkens L.R., Thompson P.J., Carney M.E., Palmieri R.T., Pharoah P.D. Vitamin D receptor rs2228570 polymorphism and invasive ovarian carcinoma risk: pooled analysis in five studies within the Ovarian Cancer Association Consortium. Int J Cancer. 2011;128:936–943. doi: 10.1002/ijc.25403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen H., Zhu J. Vitamin D receptor rs2228570 polymorphism and susceptibility to ovarian cancer: an updated meta-analysis. J Obstet Gynaecol Res. 2017;44:556–565. doi: 10.1111/jog.13534. [DOI] [PubMed] [Google Scholar]
- 113.Yin J., Pan H., Long T., Lv L., Zhai P., Liu C. Polymorphisms of VDR gene and risk of gastric cardiac adenocarcinoma in Chinese population. Oncotarget. 2017;8:45531–45543. doi: 10.18632/oncotarget.17270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iqbal M.U.N., Khan T.A. Association between Vitamin D receptor (Cdx2, Fok1, Bsm1, Apa1, Bgl1, Taq1, and Poly (A)) gene polymorphism and breast cancer: a systematic review and meta-analysis. Tumour Biol. 2017;39 doi: 10.1177/1010428317731280. :1010428317731280. [DOI] [PubMed] [Google Scholar]
- 115.El-Shorbagy H.M., Mahmoud N.H., Sabet S. Association of vitamin D receptor gene polymorphisms with breast cancer risk in an Egyptian population. Tumour Biol. 2017;39 doi: 10.1177/1010428317727738. :1010428317727738. [DOI] [PubMed] [Google Scholar]
- 116.Perna L., Butterbach K., Haug U., Schottker B., Muller H., Arndt V. Vitamin D receptor genotype rs731236 (Taq1) and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev. 2013;22:437–442. doi: 10.1158/1055-9965.EPI-12-0970-T. [DOI] [PubMed] [Google Scholar]
- 117.Orlow I., Shi Y., Kanetsky P.A., Thomas N.E., Luo L., Corrales-Guerrero S. The interaction between vitamin D receptor polymorphisms and sun exposure around time of diagnosis influences melanoma survival. Pigment Cell Melanoma Res. 2018;31:287–296. doi: 10.1111/pcmr.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y., Wang P.P., Zhai G., Bapat B., Savas S., Woodrow J.R. Vitamin D receptor and calcium-sensing receptor polymorphisms and colorectal cancer survival in the Newfoundland population. Br J Cancer. 2017;117:898–906. doi: 10.1038/bjc.2017.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaughan-Shaw P.G., O׳Sullivan F., Farrington S.M., Theodoratou E., Campbell H., Dunlop M.G. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer. 2017;116:1092–1110. doi: 10.1038/bjc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saramaki A., Banwell C.M., Campbell M.J., Carlberg C. Regulation of the human p21(WAF1/CIP1) gene promoter via multiple binding sites for p53 and the vitamin D3 receptor. Nucleic Acids Res. 2006;34:543–554. doi: 10.1093/nar/gkj460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu M., Lee M.H., Cohen M., Bommakanti M., Freedman L.P. Transcriptional activation of the CDK inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 122.Wu W., Zhang X., Zanello L.P. 1a,25-Dihydroxyvitamin D3 antiproliferative actions involve vitamin D receptor-mediated activation of MAPK pathways and AP-1/p21waf1 upregulation in human osteosarcoma. Cancer Lett. 2007;254:75–86. doi: 10.1016/j.canlet.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang Y.C., Chen J.Y., Hung W.C. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene. 2004;23:4856–4861. doi: 10.1038/sj.onc.1207621. [DOI] [PubMed] [Google Scholar]
- 124.Yang E.S., Burnstein K.L. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and CDK2 mislocalization to the cytoplasm. J Biol Chem. 2003;278:46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 125.Li P., Li C., Zhao X., Zhang X., Nicosia S.V., Bai W. p27Kip1 stabilization and G1 arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through down-regulation of cyclin E/cyclin-dependent kinase 2 and Skp1-Cullin-F-box protein/Skp2 ubiquitin ligase. J Biol Chem. 2004;279:25260–25267. doi: 10.1074/jbc.M311052200. [DOI] [PubMed] [Google Scholar]
- 126.Chiang K.C., Chen T.C. The anti-cancer actions of vitamin D. Anticancer Agents Med Chem. 2013;13:126–139. [PubMed] [Google Scholar]
- 127.Freedman L.P. Transcriptional targets of the vitamin D3 receptor-mediating cell cycle arrest and differentiation. J Nutr. 1999;129:581S–586SS. doi: 10.1093/jn/129.2.581S. [DOI] [PubMed] [Google Scholar]
- 128.Simboli-Campbell M., Narvaez C.J., Tenniswood M., Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 129.Narvaez C.J., Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276:9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 130.James S.Y., Mackay A.G., Colston K.W. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- 131.Guzey M., Kitada S., Reed J.C. Apoptosis induction by 1a,25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–677. [PubMed] [Google Scholar]
- 132.Blutt S.E., McDonnell T.J., Polek T.C., Weigel N.L. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141:10–17. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 133.Jiang F., Bao J., Li P., Nicosia S.V., Bai W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J Biol Chem. 2004;279:53213–53221. doi: 10.1074/jbc.M410395200. [DOI] [PubMed] [Google Scholar]
- 134.Kasiappan R., Shen Z., Tse A.K., Jinwal U., Tang J., Lungchukiet P. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. 2012;287:41297–41309. doi: 10.1074/jbc.M112.407189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hershberger P.A., Yu W.D., Modzelewski R.A., Rueger R.M., Johnson C.S., Trump D.L. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res. 2001;7:1043–1051. [PubMed] [Google Scholar]
- 136.Yu W.D., Ma Y., Flynn G., Muindi J.R., Kong R.X., Trump D.L. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9:3022–3029. doi: 10.4161/cc.9.15.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bernardi R.J., Trump D.L., Yu W.D., McGuire T.F., Hershberger P.A., Johnson C.S. Combination of 1a,25-dihydroxyvitamin D3 with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clin Cancer Res. 2001;7:4164–4173. [PubMed] [Google Scholar]
- 138.Hershberger P.A., McGuire T.F., Yu W.D., Zuhowski E.G., Schellens J.H., Egorin M.J. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1:821–829. [PubMed] [Google Scholar]
- 139.Bao A., Li Y., Tong Y., Zheng H., Wu W., Wei C. 1,25-Dihydroxyvitamin D3 and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. Int J Mol Med. 2014;33:1177–1184. doi: 10.3892/ijmm.2014.1664. [DOI] [PubMed] [Google Scholar]
- 140.Pan L., Matloob A.F., Du J., Pan H., Dong Z., Zhao J. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN upregulation. FEBS J. 2010;277:989–999. doi: 10.1111/j.1742-4658.2009.07542.x. [DOI] [PubMed] [Google Scholar]
- 141.Nowak D., Stewart D., Koeffler H.P. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113:3655–3665. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y. 1a,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981;102:937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- 143.Hlubek F., Brabletz T., Budczies J., Pfeiffer S., Jung A., Kirchner T. Heterogeneous expression of Wnt/β-catenin target genes within colorectal cancer. Int J Cancer. 2007;121:1941–1948. doi: 10.1002/ijc.22916. [DOI] [PubMed] [Google Scholar]
- 144.Larriba M.J., Gonzalez-Sancho J.M., Barbachano A., Niell N., Ferrer-Mayorga G., Munoz A. Vitamin D is a multilevel repressor of Wnt/β-catenin signaling in cancer cells. Cancers. 2013;5:1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xin Y., He L., Luan Z., Lv H., Yang H., Zhou Y., Zhao X. E-cadherin mediates the preventive effect of vitamin D3 in colitis-associated carcinogenesis. Inflamm Bowel Dis. 2017;23:1535–1543. doi: 10.1097/MIB.0000000000001209. [DOI] [PubMed] [Google Scholar]
- 146.Aguilera O., Pena C., Garcia J.M., Larriba M.J., Ordonez-Moran P., Navarro D. The Wnt antagonist DICKKOPF-1 gene is induced by 1a,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis. 2007;28:1877–1884. doi: 10.1093/carcin/bgm094. [DOI] [PubMed] [Google Scholar]
- 147.Pendas-Franco N., Garcia J.M., Pena C., Valle N., Palmer H.G., Heinaniemi M. DICKKOPF-4 is induced by TCF/β-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1a,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 148.Pendas-Franco N., Aguilera O., Pereira F., Gonzalez-Sancho J.M., Munoz A. Vitamin D and Wnt/β-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res. 2008;28:2613–2623. [PubMed] [Google Scholar]
- 149.Palmer H.G., Gonzalez-Sancho J.M., Espada J., Berciano M.T., Puig I., Baulida J. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xiangming G. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bao B.Y., Yao J., Lee Y.F. 1alpha, 25-Dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis. 2006;27:1883–1893. doi: 10.1093/carcin/bgl041. [DOI] [PubMed] [Google Scholar]
- 152.Ben-Shoshan M., Amir S., Dang D.T., Dang L.H., Weisman Y., Mabjeesh N.J. 1alpha,25-Dihydroxyvitamin D3 (calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 153.Li Z., Jia Z., Gao Y., Xie D., Wei D., Cui J. Activation of vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemness. Clin Cancer Res. 2015;21:844–853. doi: 10.1158/1078-0432.CCR-14-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hou Y.F., Gao S.H., Wang P., Zhang H.M., Liu L.Z., Ye M.X. 1alpha,25(OH)(2)D(3) suppresses the migration of ovarian cancer SKOV-3 cells through the inhibition of epithelial-mesenchymal transition. Int J Mol Sci. 2016;17:1285. doi: 10.3390/ijms17081285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tavera-Mendoza L.E., Westerling T., Libby E., Marusyk A., Cato L., Cassani R. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proc Natl Acad Sci U S A. 2017;114:E2186–E2194. doi: 10.1073/pnas.1615015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Demasters G., Di X., Newsham I., Shiu R., Gewirtz D.A. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther. 2006;5:2786–2797. doi: 10.1158/1535-7163.MCT-06-0316. [DOI] [PubMed] [Google Scholar]
- 157.Sharma K., Goehe R.W., Di X., Hicks M.A., 2nd, Torti S.V., Torti F.M. A novel cytostatic form of autophagy in sensitization of non-small cell lung cancer cells to radiation by vitamin D and the vitamin D analog, EB 1089. Autophagy. 2014;10:2346–2361. doi: 10.4161/15548627.2014.993283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hii C.S., Ferrante A. The non-genomic actions of vitamin D. Nutrients. 2016;8:135. doi: 10.3390/nu8030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cui C., Merritt R., Fu L., Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ordonez-Moran P., Larriba M.J., Palmer H.G., Valero R.A., Barbachano A., Dunach M. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697–710. doi: 10.1083/jcb.200803020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma Y., Yu W.D., Kong R.X., Trump D.L., Johnson C.S. Role of nongenomic activation of phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase 1/2 pathways in 1,25D3-mediated apoptosis in squamous cell carcinoma cells. Cancer Res. 2006;66:8131–8138. doi: 10.1158/0008-5472.CAN-06-1333. [DOI] [PubMed] [Google Scholar]
- 162.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 164.Bernardi R.J., Johnson C.S., Modzelewski R.A., Trump D.L. Antiproliferative effects of 1a,25-dihydroxyvitamin D3 and vitamin D analogs on tumor-derived endothelial cells. Endocrinology. 2002;143:2508–2514. doi: 10.1210/endo.143.7.8887. [DOI] [PubMed] [Google Scholar]
- 165.Chung I., Han G., Seshadri M., Gillard B.M., Yu W.D., Foster B.A. Role of vitamin D receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–975. doi: 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mantell D.J., Owens P.E., Bundred N.J., Mawer E.B., Canfield A.E. 1a,25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 167.Kong F, Li L, Wang G, Deng X, Li Z, Kong X. VDR signaling inhibits cancer-associated-fibroblasts' release of exosomal miR-10a-5p and limits their supportive effects on pancreatic cancer cells. Gut 2018. Available from: 10.1136/gutjnl-2018-316627. [DOI] [PubMed]
- 168.Ferrer-Mayorga G., Gomez-Lopez G., Barbachano A., Fernandez-Barral A., Pena C., Pisano D.G. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yuk J.M., Shin D.M., Lee H.M., Yang C.S., Jin H.S., Kim K.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 170.Campbell G.R., Spector S.A. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8:1523–1525. doi: 10.4161/auto.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fabri M., Stenger S., Shin D.M., Yuk J.M., Liu P.T., Realegeno S. Vitamin D is required for IFN-γ-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra2. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yuzefpolskiy Y., Baumann F.M., Penny L.A., Studzinski G.P., Kalia V., Sarkar S. Vitamin D receptor signals regulate effector and memory CD8 T cell responses to infections in mice. J Nutr. 2014;144:2073–2082. doi: 10.3945/jn.114.202895. [DOI] [PubMed] [Google Scholar]
- 173.Xie Z., Chen J., Zheng C., Wu J., Cheng Y., Zhu S. 1,25-dihydroxyvitamin D3-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology. 2017;152:414–424. doi: 10.1111/imm.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou Q., Qin S., Zhang J., Zhon L., Pen Z., Xing T. 1,25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC-γ1/TGF-β1/pathway. Mol Immunol. 2017;91:156–164. doi: 10.1016/j.molimm.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 175.Sun D, Luo F, Xing JC, Zhang F, Xu JZ, Zhang ZH. 1,25(OH)2D3 inhibited Th17 cells differentiation via regulating the NF-kappaB activity and expression of IL-17. Cell Prolif 2018. Available from: 10.1111/cpr.12461. [DOI] [PMC free article] [PubMed]
- 176.Min D., Lv X.B., Wang X., Zhang B., Meng W., Yu F. Downregulation of miR-302c and miR-520c by 1,25(OH)2D3 treatment enhances the susceptibility of tumour cells to natural killer cell-mediated cytotoxicity. Br J Cancer. 2013;109:723–730. doi: 10.1038/bjc.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dimitrov V., Bouttier M., Boukhaled G., Salehi-Tabar R., Avramescu R.G., Memari B. Hormonal vitamin D up-regulates tissue-specific PD-L1 and PD-L2 surface glycoprotein expression in humans but not mice. J Biol Chem. 2017;292:20657–20668. doi: 10.1074/jbc.M117.793885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lathers D.M., Clark J.I., Achille N.J., Young M.R. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–430. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li M.X., Li L.F., Zhang L., Xiao Z.G., Shen J., Hu W. Vitamin D and cancer stem cells in the gastrointestinal tract. Curr Med Chem. 2017;24:918–927. doi: 10.2174/0929867324666170214110633. [DOI] [PubMed] [Google Scholar]
- 180.So J.Y., Lee H.J., Smolarek A.K., Paul S., Wang C.X., Maehr H. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol. 2011;79:360–367. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.So J.Y., Wahler J., Das Gupta S., Salerno D.M., Maehr H., Uskokovic M. HES1-mediated inhibition of Notch1 signaling by a Gemini vitamin D analog leads to decreased CD44+/CD24–/low tumor-initiating subpopulation in basal-like breast cancer. J Steroid Biochem Mol Biol. 2015;148:111–121. doi: 10.1016/j.jsbmb.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wahler J., So J.Y., Cheng L.C., Maehr H., Uskokovic M., Suh N. Vitamin D compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. J Steroid Biochem Mol Biol. 2015;148:148–155. doi: 10.1016/j.jsbmb.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pickholtz I., Saadyan S., Keshet G.I., Wang V.S., Cohen R., Bouwman P. Cooperation between BRCA1 and vitamin D is critical for histone acetylation of the p21waf1 promoter and growth inhibition of breast cancer cells and cancer stem-like cells. Oncotarget. 2014;5:11827–11846. doi: 10.18632/oncotarget.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jeong Y., Swami S., Krishnan A.V., Williams J.D., Martin S., Horst R.L. Inhibition of mouse breast tumor-initiating cells by calcitriol and dietary vitamin D. Mol Cancer Ther. 2015;14:1951–1961. doi: 10.1158/1535-7163.MCT-15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pervin S., Hewison M., Braga M., Tran L., Chun R., Karam A. Down-regulation of vitamin D receptor in mammospheres: implications for vitamin D resistance in breast cancer and potential for combination therapy. PLoS One. 2013;8:e53287. doi: 10.1371/journal.pone.0053287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maund S.L., Barclay W.W., Hover L.D., Axanova L.S., Sui G., Hipp J.D. Interleukin-1a mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer Res. 2011;71:5276–5286. doi: 10.1158/0008-5472.CAN-10-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Allavena P., Garlanda C., Borrello M.G., Sica A., Mantovani A. Pathways connecting inflammation and cancer. Cur Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 188.Krishnan A., Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 189.Meeker S., Seamons A., Paik J., Treuting P., Brabb T., Grady W. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014;74:4398–4408. doi: 10.1158/0008-5472.CAN-13-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Q., He Y., Shen Y., Zhang Q., Chen D., Zuo C. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem. 2014;289:11681–11694. doi: 10.1074/jbc.M113.517581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fan P., He L., Hu N., Luo J., Zhang J., Mo L. Effect of 1,25-(OH)2D3 on proliferation of fibroblast-like synoviocytes and expressions of pro-inflammatory cytokines through regulating microRNA-22 in a rat model of rheumatoid arthritis. Cell Physiol Biochem. 2017;42:145–155. doi: 10.1159/000477123. [DOI] [PubMed] [Google Scholar]
- 192.Kirwen E.M., Batra T., Karthikeyan C., Deora G.S., Rathore V., Mulakayala C. 2,3-Diaryl-3H-imidazo[4,5-b]pyridine derivatives as potential anticancer and anti-inflammatory agents. Acta Pharm Sin B. 2017;7:73–79. doi: 10.1016/j.apsb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thill M., Reichert K., Woeste A., Polack S., Fischer D., Hoellen F. Combined treatment of breast cancer cell lines with vitamin D and COX-2 inhibitors. Anticancer Res. 2015;35:1189–1195. [PubMed] [Google Scholar]
- 194.Refaat B., El-Shemi A., Kensara O., Mohamed A., Idris S., Ahmad J. Vitamin D3 enhances the tumouricidal effects of 5-fluorouracil through multipathway mechanisms in azoxymethane rat model of colon cancer. J Exp Clin Cancer Res. 2015;34:71. doi: 10.1186/s13046-015-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Harten-Gerritsen A., Balvers M., Witkamp R., Kampman E., van Duijnhoven F. Vitamin D, inflammation, and colorectal cancer progression: a review of mechanistic studies and future directions for epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2015;24:1820–1828. doi: 10.1158/1055-9965.EPI-15-0601. [DOI] [PubMed] [Google Scholar]
- 196.Moreno J., Krishnan A., Feldman D. Molecular mechanisms mediating the anti-proliferative effects of vitamin D in prostate cancer. J Steroid Biochem Mol Biol. 2005;97:31–36. doi: 10.1016/j.jsbmb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 197.Giangreco A., Dambal S., Wagner D., Van der Kwast T., Vieth R., Prins G. Differential expression and regulation of vitamin D hydroxylases and inflammatory genes in prostate stroma and epithelium by 1,25-dihydroxyvitamin D in men with prostate cancer and an in vitro model. J Steroid Biochem Mol Biol. 2015;148:156–165. doi: 10.1016/j.jsbmb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]