Abstract
Marine sponges of the genus Stelletta are well known as rich sources of diverse and complex biologically relevant natural products, including alkaloids, terpenoids, peptides, lipids, and steroids. Some of these metabolites, with novel structures and promising biological activities, have attracted a lot of attention from chemists seeking to perform their total synthesis in parallel to intensive biological studies towards new drug leads. In this review, we summarized the distribution of the chemically investigated Stelletta sponges, the isolation, synthesis and biological activities of their secondary metabolites, covering the literature from 1982 to early 2018.
KEY WORDS: Marine natural products, Stelletta sponge, Isolation, Total synthesis, Biological activity, Marine drug leads
Graphical abstract
Marine sponges of the genus Stelletta are well known as rich sources of diverse and complex natural products with novel structures and broad biological activities, which have attracted a lot of attention from chemists seeking to perform their total synthesis in parallel to intensive biological studies towards new drug leads. In this review, we summarize the chemical and biological aspects of Stelletta sponges as promising drug sources.
1. Introduction
The ocean is blessed with a great biodiversity bathed in a complex ecological environment with hypersaline, hyperbaric, hypoxia, cryogenic and oligotrophic conditions. Being adapted to such harsh environment, marine organisms have built up unique morphological, ecological and physiological characteristics, along with a capacity to produce diverse and complex functional metabolites, which aroused our deep interest. These compounds, also referred as marine natural products (MNPs), are often characterized by intriguing structures and promising biological properties, which may have a great potential as new drug leads1., 2., 3., 4., 5., 6., 7.. Therefore, the search for small molecules as potential drug candidates from marine organisms has been an impetus to natural product chemists and biologists, leading to the discovery of at least 9 FDA-approved drugs and more than 20 drug candidates at different stages of clinical trials8., 9., 10..
Over the past decades, many species of marine invertebrates (sponges in particular) have been investigated towards potentially bioactive chemical components10., 11., 12., 13., 14., 15.. Numerous studies have indicated that marine sponges constitute one of the richest sources of MNPs with potential therapeutic applications16., 17., 18., 19.. Up to now, 4 out of the 9 FDA-approved marine drugs are indeed derived from marine sponges20.
Marine sponges of the genus Stelletta (Stellettidae) are widely distributed in the marine ecological systems. Triterpenoids, alkaloids, and peptides are the most prominent bioactive secondary metabolites isolated from the title genus, especially from species of the West Pacific coasts. Widespread biological properties, such as cytotoxic, antimicrobial, anti-HIV, and protein-tyrosine phosphatase 1B (PTP1B) inhibitory activities, enable Stelletta-derived MNPs to have a broad range of potential applications and a bright market background in exploiting novel drug leads. Therefore, this review will detail, for the first time, the recent advances of MNPs from Stelletta sponges, and provide an insight into their potential as future medicinal drugs. We will cover topics ranging from the distribution of the sponge to the isolation, synthesis and biological activities of different types of secondary metabolites, with a literature survey from 1982 to early 2018.
2. Structure and properties of MNPs from the Stelletta genus
Stelletta sponges are distributed all over the world seas. Many of them were chemically investigated, especially those from the Sea of Japan, the South China Sea, and off the coast of Australia (the Great Australia Bight, Coral Sea, Torres Strait, and Cape Wilberforce) (Fig. 1). A literature survey revealed that alkaloids, terpenoids, peptides, lipids, and steroids have been isolated from Stelletta21. Alkaloids were mainly discovered from sponges located in Shikinejima and Shikoku Islands of the Japanese Sea, Keomun Island of the South Sea of Korea, the Great Australia Bight, and Bahamas; triterpenoids were mainly obtained from the Hainan sponges, South China Sea; peptides were isolated from sponges of Oshimashinsone, Southern Japan and Torres Strait, Northwestern Australia; lipids were mainly derived from sponges off the coast of Hainan Island, China and Ullung Island, Korea; steroids were found from South China Sea, China, and Oro Island, Japan (Fig. 1). To date, approximately 131 interesting secondary metabolites (1–131) have been isolated and characterized from Stelletta marine sponges, with various biological activities. In this section, the isolation and chemical structures of these compounds will be discussed by different structural types.
Figure 1.
Global distribution of the chemically investigated Stelletta marine sponge according to the structure types of their chemical constituents (red: alkaloids; blue: triterpenoids; green: peptides; brown: lipids; black: steroids).
2.1. Alkaloids
The chemical investigation of Stelletta sponges led to the discovery of alkaloids with novel features. It is worth noting that Fusetani and co-workers22 have made a big contribution to this domain since 1990. From 1990 to 1999, they performed bioassay-guided chemical investigation of the n-BuOH fraction of the methanol extract of Stelletta sp. (undetermined species) collected off the sea area around Japan.
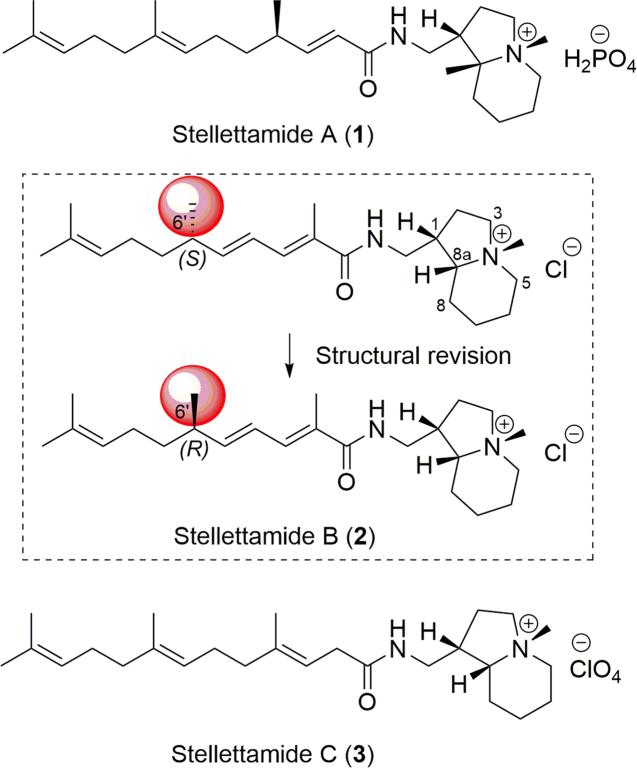
Stellettamides, the novel alkaloids with a farnesyl moiety connected to an indolizidine skeleton through an amide bond, displaying significant bioactivities, were regarded as marine toxins (Fig. 2). The isolation and structural identification of stellettamide A (1), the first indolizidine metabolite, was reported by Fusetani in 199022. The Stelletta sponge was collected in the Shikine-Jima Island (34 °19′25′′ N, 139 °13′19′′ E) from Izu Archipelago, Japan. In antifungal and cytotoxic assays, compound 1 showed significant activities against the fungus Mortierella remannianus and K562 epithelium cancer cells (IC50 = 5.1 μg/mL)22. Later in 1997, the effect of stellettamide A (1) on the contractile system of smooth muscle was investigated by the same group, showing that it inhibited Ca2+/calmodulin-dependent myosin light chain (MLC) phosphorylation, and thus could act as a novel inhibitor of calmodulin23. This interesting activity aroused the interests of chemists to determine the absolute configuration of this natural product by total synthesis, which will be discussed in the synthetic section24., 25..
Figure 2.
Chemical structures of compounds 1–3.
Seven years after the discovery of 1, stellettamide B (2) was obtained from a Stelletta sponge (undetermined species), along with 1 (Fig. 2)26. This dark brown sponge was collected at 20–30 m depth along the offshore of Keomun Island, South Sea, Korea. The isolated compounds were also reported to exhibit moderate antifungal and RNA-cleaving activities26. In this paper, the original structure of 2 was established with an S configuration at the C-6′ position, while the first total synthesis of (−)-stellettamide B led to the revision of the natural product configuration to 6′R27. Stellettamide C (3) was isolated from the same extract as 1 by Fusetani in 1999 (Fig. 2)28. In the bioassay, 3 showed moderate activity against Escherichia coli with an inhibitory zone of 10.5 mm at 20 µg/disk28. In addition, the first asymmetric synthesis of 3 was reported by Aoyagi and co-workers25 in 2008.
Stellettadine A (4), a sesquiterpene amide containing a linear bisguanidinium unit with larval metamorphosis-inducing activity in ascidians, was first isolated from a Stelletta sponge of the Gulf of Sagami, Japan, in 1996 (Fig. 3).29 Later in 1999, two Ca2+/calmodulin-dependent phosphodiesterase inhibitors, bistellettadines A and B (5 and 6), were isolated from a sponge collected off Shikine-jima Island, 200 km South of Tokyo30. It was proposed that a biosynthetic [4π + 2π] Diels—Alder cycloaddition of two dehydro-stellettadines derivatives would generate the bistellettadines (5 and 6) (Fig. 3)30. In 2001, first total synthesis and absolute configuration study of stellettadine A (4) was reported by Mori׳s group31. Three antibacterial homosesquiterpene amides, stellettazoles A−C (7–9), bearing an imidazole moiety and a terminal guanidine chain were isolated from the same extract as bistellettadines28., 32.. In addition, during their study, the acetamide (10) was obtained by the acetylation of stellettazole C (9) with acetic anhydride (Ac2O) (Fig. 3).
Figure 3.
Chemical structures of compounds 4–10, and the proposed biosynthetic pathways of bistellettadines.
As for the biological activities, the tested stellettazole A (7) and bistellettadines (5 and 6) moderately inhibited Ca2+/calmodulin-dependent phosphodiesterase (45% and 40% inhibition at 100 µmol/L, respectively). They were also found to have an antibacterial activity against E. coli and an antifungal activity against yeast at low concentrations, ranging from 10 to 50 µg/disk30. Stellettazoles B and C (8 and 9) were active against the growth of E. coli at 20 µg/6 mm disk, respectively28., 32.. Interestingly, stellettadine A (4) was found to induce the metamorphosis of the ascidian Halocynthia roretzi larvae with an ED100 value of 50 μmol/L29.
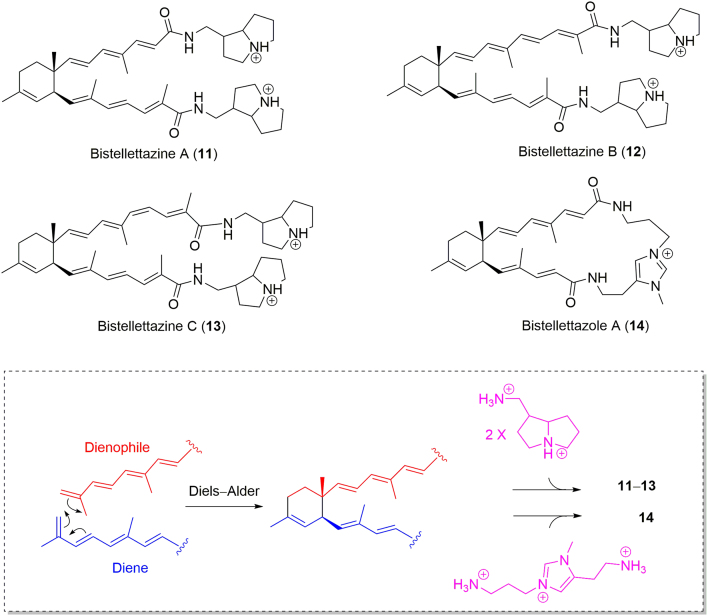
In 2008, Capon׳s group33 reported the discovery of three new terpenyl-pyrrolizidine conjugated alkaloids, bistellettazines A–C (11–13), and a new cyclic terpenyl-imidazole conjugate, bistellettazole A (14), from Southern Australian marine sponge, Stelletta sp. CMB-01936 (Fig. 4), collected in the 90 m depth water of the Great Australian Bight. All the four compounds were isolated as salts. Compounds 11–13 comprise two pyrrolizidines and two terpene-conjugated amide groups, while 14 contains an ansa N-methyl imidazole ring and two terpene-conjugated amide groups. It is obvious that these novel compounds might be generated by a Diels—Alder reaction from the two terpene-conjugated amide groups, followed by the coupling with either two pyrrolizidine groups (for 11–13) or one imidazole moiety (14). The terpene-conjugated amides may be farnesoic acid derivatives, especially 2,6,10-trimethylundeca-2,4,6,8,10-pentaenoic acid.33 In fact, the chemistry and biosynthetic studies of natural occurring pyrrolizidine34 and imidazole35 alkaloids were summarized by Robertson34 and Jin35 in 2016, respectively, providing information for further total synthesis of the novel alkaloids such as 11–14.
Figure 4.
Chemical structures of compounds 11–14, and their possible biosynthetic pathway.
Except for the above mentioned alkaloids conjugated to linear terpenes, three new cyclic alkaloids, named nordercitin (15), dercitamine (16), and dercitamide (17) with a pyrido[4,3,2-mn]thiazolo[3,2-β]acridinium-9-ethyl skeleton were isolated in 1989 from a red sponge Stelletta sp. from Bahamas, at a depth of 70 m (Fig. 5)36. All the new compounds inhibited the growth of murine P388 leukemia cells in vitro with good to moderate IC50 of 4.79, 26.7, and 12.0 µmol/L, respectively36. In 1994, the chemical investigation of the marine sponge S. maxima, collected off the Sata Peninsula, Shikoku, resulted into six novel cyclostellettamines (A−F, 18−23) with macrocyclic bis-pyridine structures linked through two Cl2-Cl4 alkyl chains37. In the bioassay, the new compounds inhibited the binding of [3H]-methyl quinuclidinyl benzilate (QNB) which is a selective antagonist to muscarinic receptors37. It is worth mentioning that muscarinic acetylcholine receptors were found to play important roles in various physiological functions including memory and learning38. It was reported that muscarinic receptors are known to be correlated with some disease states, and their agonists or antagonists may be potential drugs39.
Figure 5.
Chemical structures of compounds 15–23.
To date, a variety of marine alkaloids and new derivatives have been isolated and characterized from marine sponges40. Some of these interesting bioactive marine alkaloids, represented by marine guanidines, may be the most promising for future medicinal applications41., 42.. In the Stelletta genus of sponge, 23 novel alkaloids have been discovered and characterized by either extensive spectroscopic analysis or chemical synthesis. The typical Stelletta-derived alkaloids were mainly terpene and alkaloid hybrids, featured by a long acyclic terpenyl side chain connected to the alkaloid moieties, such as pyrrolizidine, imidazole, indolizidine, and guanidine groups (Figure 2, Figure 3, Figure 4, Figure 5). The unique structures of such alkaloids might due to the special living ecological system, which also dowered them with significant pharmacological values. It is worth mentioning that the isolation process of alkaloids is always more challenging than the other metabolites due to their high polarity, while most of Stelletta alkaloids exist as salt forms. Therefore, n-BuOH extracts were mainly investigated in the search for bioactive alkaloids from Stelletta sponges and various column chromatography or HPLC conditions were used for their isolation and purification. For instance, the addition of either formic acid or trifluoroacetic acid (TFA) during the reverse phase isolation could optimize the chromatographic peaks to a certain extent32. The novel structures and special isolation methods for these novel alkaloids and their broad biological activities stimulated natural product chemists and biologists for further studies of Stelletta-derived natural products, as will be discussed in the following sections.
2.2. Triterpenoids
Isomalabaricane-type triterpenoids are a class of unusual triterpenoids with a 6,6,5-fused tricyclic ring system. The first discovery of such triterpenoid from the sponge Stelletta was reported by Clardy and co-workers43 in 1982. The fresh brown sponge was collected off Mogadishu, Somalia. Its chemical investigation resulted into pure yet unstable yellow triterpenoid pigment 24, of which structure was determined by spectroscopic analysis and X-ray single crystal diffraction (Fig. 6)43. This novel pigment was also obtained 12 years later, in 1994, by Zeng and co-workers44 from the Hainan sponge S. tenuis Lindgren (Stellettidae). During this work, another new isomalabaricane-type triterpenoid was isolated and designated as stellettin A (25), while compound 24 was named stellettin B at the same time (Fig. 6). Both compounds comprise a 6,6,5-fused tricyclic ring system and a terminal 3-methyl-2-pyranone moiety, which are connected via a conjugated triene. During biological assays, the authors found that stellettin A (25) exhibited significant cytotoxicity against P388 leukemia cells with an ED50 value of 0.001 μg/mL44.
Figure 6.
Chemical structures of compounds 24–53.
In 1996, four new cytotoxic isomalabaricane triterpenes, namely stellettins C−F (26–29)46, along with the three known related compounds, stellettins A (25)44, B (24)43, and G (30)45 were isolated from the CCl4 extract of the yellow sponge Stelletta sp. (Fig. 6), which was guided by the cytotoxicity on human tumor cell lines in vitro. The sponge was collected off the shore North of Cape Wilberforce at the depth of 15 m, Australia. In fact, these compounds are all unstable, of which 26 and 30 could be rapidly equilibrate to 27 and 31, respectively, due to the E/Z isomerization of the exocyclic olefin46. Thus, the cytotoxic potential of the mixtures of each pair of interconverting compounds was evaluated against sensitive cell lines including leukemia, central nervous system (CNS), and renal lines. These mixtures generally showed promising activities with GI50 concentrations in the low-to-mid nanomolar range46. In fact, during purification, a methylation step of the polar fraction containing stellettins with free carboxylic acid groups made them much easier to be isolated. By using such strategy, compounds 28–30 were initially methylated during the isolation process to afforded compounds 31–33, which could be rapidly isolated by common purification methods46.
Eleven years later in 2007, stellettins L and M (34 and 35), two new cytotoxic triterpenes, were isolated by Lin׳s group47 from S. tenuis collected in Sanya Bay, Hainan Province, China (Fig. 6). In fact, the crude extract of this sponge exhibited obvious activity against the HL-60 human leukemia cell line (IC50 < 0.01 µg/mL). The cytotoxicity-guided fractionation of the CH2Cl2 fraction of the EtOH extract led to the isolation and characterization of 34 and 35, which were further tested for their cytotoxicity against human lung (A549), liver (SMMC-7721), stomach (AGS), and colon (HT-29) cancer cell lines. Both compounds showed selective cytotoxicity against AGS cancer cells, with IC50 values of 3.9 and 2.1 µmol/L, respectively47.
Despite the rich chemical diversity of these isomalabaricane triterpenes, among various biological activities, only their cytotoxicity had been reported44., 46., 47.. Thus, it was necessary to explore additional biological functions for these metabolites. In 2013, Guo׳s group48 made a breakthrough when searching for MNPs with protein-tyrosine phosphatase 1B (PTP1B) inhibitory activity. Several isomalabaricane triterpenes were isolated from the Hainan sponge Stelletta sp. YAL-3, which was collected off Lingshui Bay, Hainan Province, China48. After a careful chemical investigation of the Et2O fraction of its acetone extract, a new isomalabaricane triterpene, stellettin N (36), was isolated, together with five known analogues, stellettin H (37), rhabdastrellic acid A (38), stellettin G (30), stellettin D (27), and 22,23-dihydrostellettin D (39) (Fig. 6). The biological evaluation revealed that the known compound 27 exhibited significant PTP1B inhibitory activity with an IC50 value of 4.1 ± 0.9 μmol/L48. As discussed above that compounds 26 and 27 could be rapidly equilibrate to each other46, the PTP1B inhibitory activity of 27 thus could possibly be the activity of the mixture of these two compounds as well.
The chemical investigation of the marine sponge S. tenuis Lindgren, collected in Sanya, Hainan, led to the isolation of three new cytotoxic isomalabaricane triterpenes, named stellettin N (40), stellettin O (41) and stellettin P (42)49, and two known related compounds, stellettins C and D (26 and 27)46 (Fig. 6). Among all the isolated compounds, only the new ones were tested for cytotoxic activities against three human cancer cell lines (A549, AGS and U-251MG). Biological assays showed that compounds 40–42 were cytotoxic to AGS cells, with IC50 values of 4.52, 9.61 and 7.44 μmol/L, respectively49. Intriguingly, the authors identified two naturally occurring α-pyrones, namely gibepyrones C and F (43 and 44) (Fig. 6), along with the above triterpenoids49. As parts of some isomalabaricane triterpenes, these α-pyrones might be the oxidation products of the co-occurring stelletins. In fact, gibepyrone F had previously been isolated from the fungal plant pathogen Gibberella fujikuroi50, and occasionally from sponges51. Therefore, it is questionable if these two compounds are derived from the sponge catabolism or that of associated microorganisms.
Except for alkaloids, Fusetani׳s group52 was also engaged in the discovery of bioactive isomalabaricane triterpenes in 1996. Based on bioassay-guided isolation, four new cytotoxic triterpenes, named globostellatic acids A−D (45–48), were isolated from the MeOH extract of the marine sponge S. globostellata Carter, 1883 collected at depths of 3–10 m off Mage Island near Kagoshima in southern Japan (Fig. 6). All the four isolated compounds exhibited cytotoxicity against P-388 murine leukemia cells with IC50 values ranging from 0.1 to 0.46 µg/mL52. After their pioneering discovery of bioactive compounds from Stelletta sponges, Fusetani and co-workers53 continued their study by investigating metabolites by a different biological assay. The new assay system, using rat 3Y1 fibroblasts, was applied to find compounds involved in the regulation of cytoskeleton formation. It was reported that metabolites from the sponge S. globostellata induced unusual morphological changes in the cells. The sponge S. globostellata was collected using scuba at a depth of 3 m off Mage-jima Island (30 °43′ N, 130 °52′ E). Three new isomalabaricane triterpenes, 29-hydroxystelliferin D (49), 3-epi-29-hydroxystelliferin E (50), and 3-epi-29-hydroxystelliferin A (51), together with two known ones, stelliferin A (52) and stelliferin D (53), were isolated from the MeOH fraction of the EtOH extract of S. globostellata (Fig. 6)53.
Isomalabaricane-type triterpenoids from Stelletta sponges are attractive due to their highly conjugated structures and significant biological activities, which might be influenced by the environment of sponges originated from completely different collections. However, there are still many challenges in the study of such triterpenoids. On the isolation aspect, many highly conjugated isomalabaricane triterpenes were photochemically unstable, and all the extraction and isolation procedures have to be performed under lightproof condition to avoid artifacts. This may cause difficulties in both new compound discovery and biological assays. Therefore, strategies of chemical modification towards more stable compounds could be a future challenge, so as to explore their biological function. In addition, it is interesting to note that sponges from Hainan Island are the main source of new structures of isomalabaricane triterpenoids. The extensive chemical investigation of Stelletta collected from this sea area may not only expand the diversity and complexity scope of triterpenes in this genus, but also provide evidence for chemical ecological relationships between Stelletta sponges and their marine environment.
2.3. Peptides
2.3.1. Ciliatamides
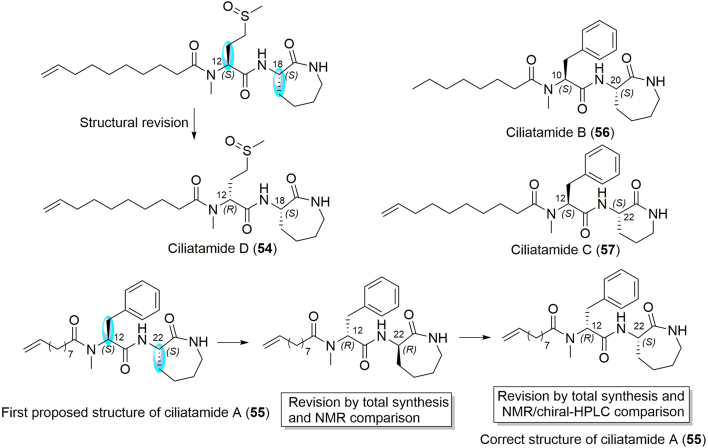
The chemical diversity and complexity of the genus Stelletta was not only exemplified by isomalabaricane triterpenes and alkaloids, but also by novel peptides, such as lipopeptides and depsipeptides. In 2013, two uncommon lipopeptides, ciliatamide D (54) containing a N-methyl-l-methionine sulfone moiety, and ciliatamide A (55), were isolated from the EtOH extract of a marine sponge Stelletta sp., collected from Oshima-shinsone, southern Japan (Fig. 7)54. The absolute configuration of these two peptides were determined by the isolation team by using Marfey׳s analysis, showing that they contained two l-amino acids residues54.
Figure 7.
Chemical structures of compounds 54–57.
However, after lipopeptides ciliatamides A–C were first reported in 2008 from the sponge Aaptos ciliata55, Lindsley and co-workers56 shortly performed their total synthesis and made a stereochemical revision in the same year. The stereochemistry was thus revised from (12S,22S) to (12R,22R). Moreover, Lindsley and co-workers56 built up a library of all possible stereoisomers of ciliatamides A–C and the corresponding optical rotations were tested. However, it is surprising that the challenging relative configuration of the naturally occurring ciliatamide A remained incorrect in these two seminal papers. Finally, Matsunaga and co-workers57 resolved the problems of the relative and absolute configuration for ciliatamide A, by utilizing NMR and chiral-phase HPLC analyses. Four synthetic isomers were compared with an authentic natural sample of ciliatamide A, allowing its structure to be revised for the second time, with an (12R,22S) configuration, highlighting the presence of a d-phenylalanine moiety. Furthermore, the absolute configuration of the methionine sulfoxide residue in ciliatamide D (54) was also revised to the d form54.
Although the HPLC separation of Stelletta ciliatamides was carried out by using a bioassay-guided approach, the purified compounds showed no inhibitory activity on cathepsin B nor cytotoxicity against HeLa cells at 50 μg/mL54. Actually, after their first isolation from the sponge Aaptos ciliata in 200855, ciliatamides A and C (55 and 57) were found to be antileishmanial (50% and 45.5% growth inhibition at 10 µg/mL on Leishmania major, respectively). Ciliatamides A−C also inhibited the growth of HeLa cells at IC50 values of 50, 4.5, and 50 µg/mL, respectively55.
2.3.2. Depsipeptides
Among marine organisms58., 59., marine sponges have played an important role in anti-HIV natural products discovery60. In particular, depsipeptides (also known as cyclodepsipeptides) may be the most promising molecules61 as lead compounds in anti-HIV drug research and development60., 61., 62.. Structurally, these compounds often incorporate unique amino acids in their structures63., 64.. After literature survey, only seven depsipeptides were found in the genus Stelletta by now, thus leaving opportunities to further explore the chemical and biological diversity of Stelletta-based depsipeptides.
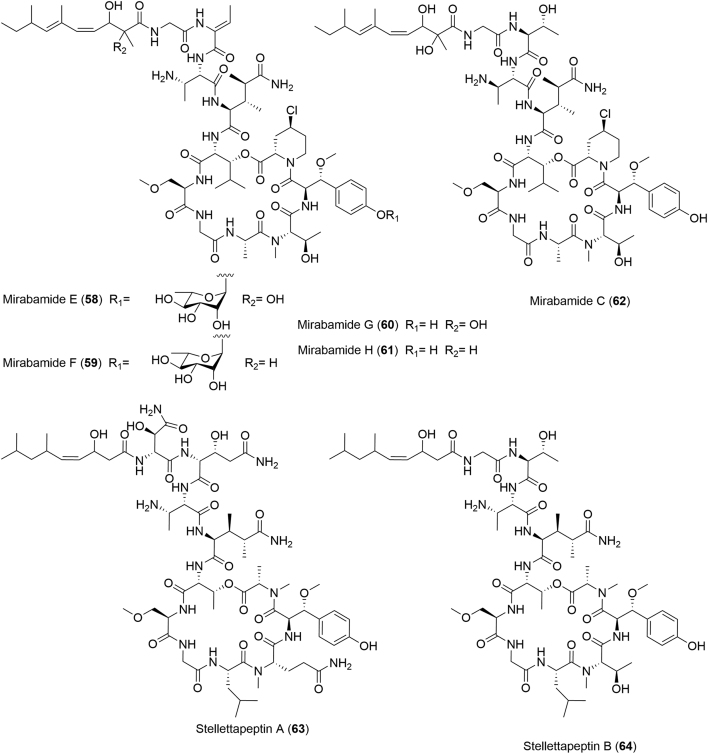
In 2011, four new HIV-inhibitory depsipeptides, mirabamides E–H (58–61), along with the known mirabamide C (62) were isolated from the MeOH extract of S. clavosa, collected from the interlagoon seabed areas in the Torres Strait (Fig. 8)65. This was the first report on depsipeptides from a Stelletta sponge. These novel compounds displayed significant inhibitory activities against HIV-1 with potent IC50 values ranging from 42 to 121 nmol/L65. In light of these promising activities, the subsequent research reported in 2015 by Gustafson and co-workers66 from a Stelletta sponge collected from northwestern Australia resulted into two HIV-inhibitory cyclic depsipeptides, named stellettapeptins A and B (63 and 64) with potent EC50 values of 23 and 27 nmol/L, respectively (Fig. 8). However, direct cytotoxicity of 63 and 64 against the host cells was observed at low IC50 values of 367 and 373 nmol/L, respectively.
Figure 8.
Chemical structures of compounds 58–64.
2.4. Lipids
Modified lipids are among the most abundant secondary metabolites found in sponges67. During the last 35 years, over 30 lipids were discovered from marine Stelletta sponges. These lipids showed both novel structural features and significant biological activities.
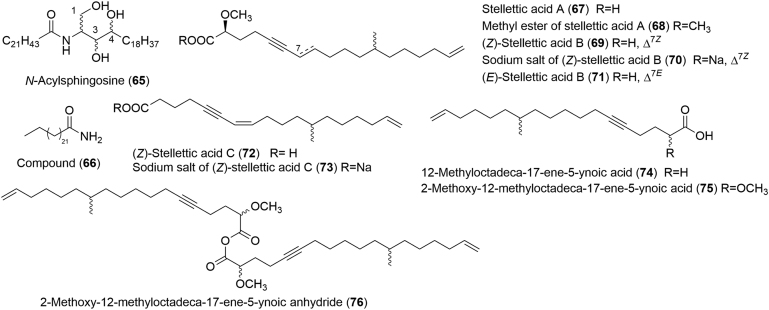
Chemical study of the EtOAc fraction of EtOH extract from the sponge S. tenuis (Lindgren) collected off Hainan Island led to the isolation of a new N-nervonoyl-d-sphingosine derivative (65, the first example of lipids found in the title sponge) and a saturated amide (66), respectively (Fig. 9)68. Actually, compound 65 possesses the two hydroxyl groups of sphingosine at C-1 and C-3, respectively, and an additional uncommon one at C-4. As reported, from a biogenetical point of view, the hydroxyl group at C-4 would possibly be introduced by hydration of sphingosine68.
Figure 9.
Chemical structures of compounds 65–76.
Acetylenic acids are a series of metabolites featuring an acetylenic or an enyne moiety, and usually, a terminal double bond. New acetylenic acids including stellettic acid A (67), its methyl ester (68) (Z)-stellettic acid B (69), its sodium salt (70), (E)-stellettic acid B (71), (Z)-stellettic acid C (72) and its sodium salt (73), were obtained from the MeOH extract of a Stelletta sponge by Jung in 2003 (Fig. 9)69. The sponges were collected off the coast of Ullung Island, Korea. Since the MeOH extract displayed toxicity to brine shrimp larvae (LD50 = 296 µg/mL), bioactivity guided isolation was used during the whole separation procedure. In vitro cytotoxic activities against five human tumor cell lines, including human solid tumor cells, human lung cancer cell A549, human ovarian cancer cell SK-OV-3, human skin cancer cell SK-MEL-2, human CNS cancer cell XF498, and human colon cancer cell HCT15, indicated the significant cytotoxicity of the aforementioned purified compounds with ED50 values ranging from 3.4 to 30 μg/mL. In fact, compound 72 was found to be the most cytotoxic metabolite against above described cancer cell lines, with ED50 values ranging from 3.4 to 4.8 μg/mL69. Interestingly, three years later, the potential of compound 72 to induce apoptosis in human leukemic U937 cells and its inhibition on telomerase activity were further investigated due to its significant cytotoxicity70.
In 2003, Shin and co-workers71 reported the discovery of three new acetylenic metabolites, 12-methyloctadeca-17-ene-5-ynoic acid (74), 2-methoxy-12-methyloctadeca-17-ene-5-ynoic acid (75), and 2-methoxy-12-methyloctadeca-17-ene-5-ynoic anhydride (76), from the marine sponge Stelletta sp., which was collected off the shore of GaguDo Island, Korea (Fig. 9). Although the crude extract of these sponges only showed weak cytotoxicity against the human leukemia cell-line K562 with LC50 value of 490 µg/mL, the great potential of sponges from Korean water to produce novel metabolites encouraged the authors to further chemically investigate these animals. Thus, the authors introduced the 1H NMR spectra guided isolation for new lipid-type compound discovery and finally obtained acetylenic acids 74–76 (Fig. 9). However, although their chemical structures were close to acetylenic acids reported by Jung׳s group69 (67–73), unlike compounds 67–73 showing potent cytotoxic properties, compounds 74–76 displayed no significant cytotoxic effects. They only exhibited weak cytotoxic activity against the human leukemia cell-line K562 with LC50 values of 43.5, 51.3, and 62.5 µg/mL, respectively71.
From the same extract, Jung and co-workers72 characterized two new lysophosphatidylcholines (77 and 78) and four new monoglycerides (79–82), along with two known monoglycerides (83 and 84) in 2003 (Fig. 10). These metabolites were also evaluated for their cytotoxicity against the same five human tumor cell lines as mentioned above. Unfortunately, only 77 and 78 exhibited moderate cytotoxicity (ED50 values ranging from 4.8 to 30 μg/mL), while the new monoglycerides were all inactive (ED50 value over 30 μg/mL).72 The same year, the bioactivity-guided isolation of the same marine sponge extract further gave a new glycerol ether (85) with significant cytotoxicity (Fig. 10)73. Compound 85 was established as the first reported glycerol ether from a natural source. The cytotoxicity evaluation of this compound against the same five tumor cell lines, showed that 85 exhibited a potent cytotoxic effect with ED50 values ranging from 4.4 to 8.7 μg/mL73.
Figure 10.
Chemical structures of compounds 77–89.
In 2003, the Jung׳s group74 reported the isolation of norsarcotride A (86), another new cyclitol derivative, along with three known related compounds, sarcotrides A–C (87–89) (Fig. 10). Since the previously reported compounds showed promising cytotoxicity against several human cell lines, the new compound was also tested for its cytotoxicity and showed significant effects against previously described five human solid tumor cells, with ED50 values of 4.3, 5.1, 5.3, 4.4, and 3.9 μg/mL, respectively74.
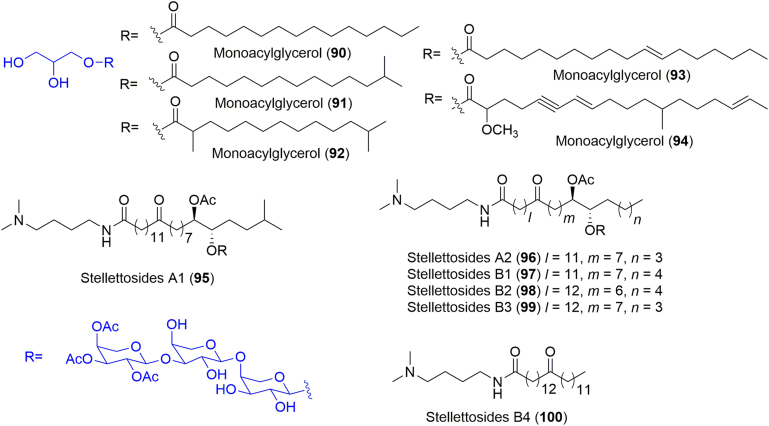
The isolation from the MeOH extract of a marine sponge Stelletta sp. by Hong and co-workers75 gave five new monoacylglycerols (MAGs, 90–94) (Fig. 11). As for the isolation procedure, the animals were extracted by MeOH and the resulting extract was partitioned between H2O and CH2Cl2, and the organic solvent layer was further partitioned between MeOH and n-hexane to yield two portions. The MeOH portion was subjected to HPLC for isolation, with the four purified metabolites being identified as MAGs 90−94 by positive ion fast atom bombardment mass spectrometry (FAB-MS)75.
Figure 11.
Chemical structures of compounds 90–100.
Glycolipids from natural origins, such as rhamnolipids are regarded as promising bioactive molecules due to their novel structures and diverse biological functions.76 In 2015, Matsunaga and co-workers77 reported the chemical investigation of a Stelletta sponge, which was collected at a depth of 170 m at the seamount “Oshima-shinsone” in 2008. In this research, LC—MS analysis combined with cytotoxicity assays of the extract resulted in the isolation of several glycosylated fatty acid derived amides, including two isomers, stellettosides A1 (95) and A2 (96), as well as stellettosides B1–B4 (97−100) (Fig. 11). The stellettosides contain an N,N-dimethylputrescine moiety as the amine fragment and an erythro diol on the fatty acid moiety, which is acetylated and glycosylated. Besides, stellettosides comprise l-arabinose units as the sugar components. The biological assays indicated that the mixture of stellettosides B1–B4 exhibited moderate cytotoxic activity against HeLa cells with an IC50 value of 9 μmol/L, whereas the mixture of stellettosides A1 and A2 was not active at a concentration of 10 μmol/L77.
2.5. Steroids
It has been wildly reported that steroids are among the most frequently discovered metabolites from marine organisms78., 79.. In sponges of the genus Stelletta, 30 new sterols were isolated and characterized. The first sterols and sterones were discovered from a sponge Stelletta sp., collected at depth of 700 m in the deep Coral Sea, southeast of Noumea in 198880. In fact, these compounds are the first examples of stigmastane steroids with a Δ24., 25. from marine origin. The structures were elucidated as stigmasta-4,24(25)-dien-3-one (101), stigmasta-4,6,24(25)-trien-3-one (102), stigmasta-4,24(25)-diene-3,6-dione (103), 6β-hydroxystigmasta-4,24(25)-dien-3-one (104), acetate of compound 101 (105), stigmasta-5,24(25)-dien-3β-ol (106), and compound 107. In addition, two ketone analogues were also obtained probably by chemical oxidation (108 and 109) (Fig. 12). As demonstrated by the authors, the stigmastanes are the only steroids of this sponge, which were more likely to function as stabilizers of sponge cell walls80.
Figure 12.
Chemical structures of compounds 101–131.
In 1992, 24-methylene-27-methylcholesterol (110) was identified from a marine Stelletta collected in the South China Sea by Deng and co-workers81. Although this compound was a known one, its structural assignment was accomplished for the first time. Stellettasterol (111), a new 9,11-secosterol, was isolated from a Japanese marine sponge, Stelletta sp.82, collected off Shikine-jima Island of the Izu Archipelago, Japan. In fact, this new compound was the C-3 isomer of known ichtyotoxic secosterol 112 isolated from the marine sponge Dysidea herbacea83. It was found to have an antifungal activity against M. remannianus with an MIC value of 12.5 μg/mL, while not cytotoxic against P388 leukemia cells at 2 μg/mL82. In 2001, Su and co-workers84 carried out the chemical investigation of the South China Sea derived sponge S. tenuis Lindgren, which was collected off Xisha Island. The sponge gave two known steroids, 3β-hydroxy-stigmasta-5-en-7-one (113) and 3β-hydroxy-cholesta-5-en-7-one (114). Besides, eleven steroids (115–125) were also detected by GC–MS from the same extract (Fig. 12). During the search for natural anti-cancer drugs from marine invertebrates, Higuchi and co-workers85 reported a novel sterol named orostanal (126) and its synthetic analog, KPN-2001 (127), which induced apoptosis in HL-60 cells at 10 μg/mL. Moreover, the cytotoxic activity indicated that compounds 126 and 127 were highly toxic with IC50 values of 1.7 and 2.2 μmol/L, respectively. Bioactive compound 126 was isolated from a Japanese marine sponge, S. hiwasaensis, which was collected off Oro Island, Fukuoka Prefecture, Japan. Structurally, the absolute configuration of 126 was determined by comparison of its CD spectra with that of a synthetic analogs, KPN-2001 (127)85. Such uncommon sterol with 6/5/6/5 fused rings had only been previously found in taiwaniasterols, which were isolated from the leaves of Taiwania cryptomerioides86.
In 2007, Lin and co-workers46 isolated six steroid derivatives from the marine sponge S. tenuis, including three new ones, 24-methylene-27-methylcholestane-3β,5α,6β-triol (128), 24-methylene-27-methylcholest-5-ene-3α,7R-diol (129), and 24-methylene-27-methylcholest-5-ene-3α,7α-diol (130) (Fig. 12). The structure determination of the new sterols and the structural revision of a known steroid (131) were reported in this paper, but no biological assay46.
2.6. MNPs from Stelletta-derived microorganisms
As we know that chemistry drives many biological interactions between the microorganisms and their host animals, yet it is often challenging to identify the chemicals involved. Many of the sponge-microbial associates are symbionts involved in nutrient cycling and may also play a role in the sponge׳s chemical defense mechanisms87., 88.. Probably by this reason, several Stelletta-derived microorganisms have been isolated and chemically studied, leading to the discovery of a total of 28 secondary metabolites (132–159) from four marine-derived strains (two bacteria and two fungi), which were isolated from the inner tissues of the sponge Stelletta sp. (Figure 13, Figure 14)89., 90., 91., 92.. Among them, the most intriguing compounds were the anti-inflammatory sesquiterpenoids (135–148) from Stelletta-derived fungus Acremonium sp.91 Acremofuranones A (136) and B (137) possess unprecedented cyclic skeleton and were evaluated by measuring the inhibitory effects on the production of pro-inflammatory mediators (NO, IL-6, and TNF-α) in RAW 264.7 murine macrophage cells. Among the tested compounds, 141 and 143 inhibited the production of NO and TNF-α at the concentration of 100 µmol/L, while compounds 145 and 146 showed selective inhibition of NO production at the same concentration91.
Figure 13.
Chemical structures of compounds 132–148.
Figure 14.
Chemical structures of compounds 149–159.
Over comparison of the secondary metabolites from Stelletta sponge and their associate microorganisms, the most possible chemicals involved between them might be depsipeptides, since they shared the similar structure skeletons. While, the typical and intriguing alkaloids or triterpenoids from Stelletta sponge have not yet been found any possible relation to their associate microorganisms.
3. Synthetic achievements on Stelletta MNPs
The complex structures and extensive biological activities of MNPs from Stelletta sponges have attracted many synthetic chemists toward their total syntheses, which either allowed the determination or revision of their absolute configuration or provided sufficient amounts of compounds for further biological evaluation. In this section, all synthetic intermediates will be numbered as Sn (n = 1, 2, 3…).
3.1. Stellettadine A
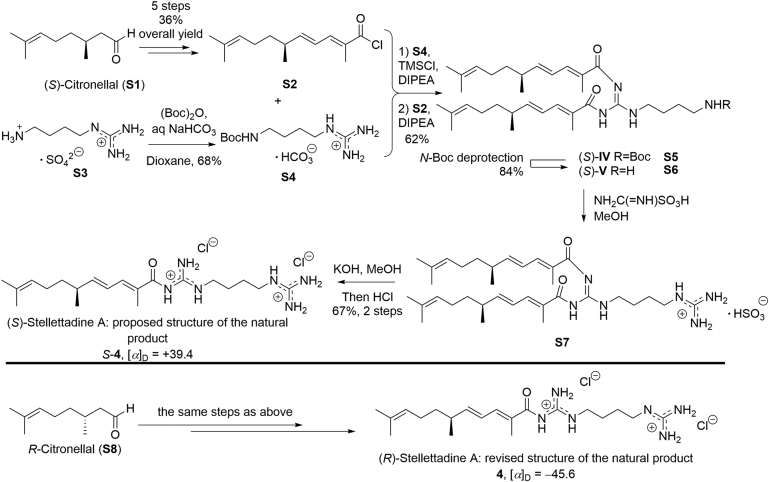
In light of the interesting biological activity of stellettadine A (4), the total synthesis of 4 and its enantiomer (ent-4) was achieved by Mori and co-workers31 in 2000, ending with structure revision of 4. As shown in Scheme 1, for the synthesis of (S)-stellettadine A and (S)-citronellal (S1) was used as the starting material, which underwent five steps, including a Wittig reaction, a palladium-catalyzed oxidation, a second Wittig reaction, an ester hydrolysis, and a chlorination to afford conjugated acyl chloride S2, with 36% overall yield. Meanwhile, after some synthetic attempt, the authors selected the commercially available agmatine sulfate (S3) as the guanidine starting material to be reacted with S2, after t-butoxycarbonyl (Boc) protection of the non-guanidine primary amino group towards S4. The key acylation of S4 in the presence of two equiv.alents of S2 gave rise to diacylated product S5 in 42% yield over two steps. As for the acylation conditions, the authors did many attempts, and finally used Ottenheijm׳s method93 by treating S4 with trimethylsilyl chloride in the presence of diisopropylethylamine (DIPEA) to yield N-silylated intermediate, which then reacted with S2 in the presence of DIPEA to afford S5. The Boc group was removed by p-TsOH in 2-methyl-2-butene and MeOH towards S6 in 84% yield, and the second guanidine group at the N-terminal position was installed by treating S6 with aminoiminomethanesulfonic acid in MeOH to give S7. The extra acyl group was removed by methanolysis in the presence of KOH to afford ent-4 as its dihydrochloride after HCl treatment, in 67% yield over two steps, with [α]D25 +39.4 (c 1.01, MeOH). Therefore, the total synthesis of (S)-stellettadine A was achieved in 10 steps from S-citronellal, with an overall yield of 8.5%. By using the same sequence, and starting from R-citronellal (S8), (R)-stellettadine A (4) was obtained with [α]D25 −45.6 (c 1.37, MeOH) (Scheme 1). By comparing the specific rotations, the natural product {[α]D25 −42.7 (c 0.092, MeOH)} was revised to be (R)-stellettadine A.
Scheme 1.
Total synthesis of stellettadine A and its enantiomer.
3.2. Stellettamides A−C
As discussed in the isolation section, stellettamides A–C (1–3) exhibited strong cytotoxicity and antifungal activities, which attracted the attention of synthetic chemists. In 1997, Carreira and co-workers24 reported an enantioselective synthesis of ent-stellettamide A (ent-1), involving an asymmetric diazoalkane dipolar cycloaddition, to establish its absolute stereochemistry (Scheme 2). They first used dipolarophile S9 to react with a solution of 2.2 equiv. of Me3SiCHN2, affording the pyrazoline cycloadducts as a 93:7 mixture of C2/C3 diastereomers. The unpurified mixture was further treated with EtO2CCl and AgOTf towards S10 and S11, with 71% and 6% yield, respectively. S10 was then reduced to the corresponding primary alcohol with LiAlH4 in 91%, followed by Swern oxidation with 93% yield to produce aldehyde S12. It was further treated by the acetylide derived from TBSOCH2C≡CH to afford a secondary propargyl alcohol. Without purification, this alcohol underwent mesylation to obtain S13 with 68% yield over two steps. S13 was converted to cis-alkene S14 by the hydrogenolysis of the propargyl mesylate followed by partial hydrogenation of the alkyne, with 72% yield over two steps. S14 was treated by aqueous Ba(OH)2 to hydrolyze its ethyl carbamate and the silyl ether, to give a pyrazoline which was selectively protected towards the N-Boc-carbamate, followed by mesylation of the remaining alcohol to afford S15 with 80% overall yield. Dissolution of S15 in 10% concentrated H2SO4/dioxane resulted in hydrolysis of the carbamate, and the reaction mixture was then added to a 2 mol/L NaOH solution to give bicyclic intermediate S16 (83%, two steps). This compound was then treated with Raney Ni/H2 to reduce C=C and C=N unsaturations and cleave the N-N bond to afford a diamine, which was further selectively protected as the corresponding N-trifluoroacetamide (S17) with 85% yield over two steps. Deprotection of the benzyl ether followed by treatment of the resulting primary alcohol with CBr4 and Ph3P led to ring closure94, of which the alkaline hydrolysis of the trifluoroacetamide gave the indolizidine core of stellettamide S18. Since the isolation report did not describe the relative configuration of the side chain22, both (R)- and (S)-trienoic acids S19 and ent-S19 were synthesized95. Each of these two carboxylic acids was coupled with S18 in the presence of DCC and DMAP to yield the diastereomeric indolizidines with 72% yield, which were separately methylated (MeI, DMF) to afford stellettamide A and its C-4′ epimer with 95% yield. After spectroscopic analysis of each product, the diastereomer ent-1 displayed identical NMR data and retention time in HPLC as natural stellettamide A, except for their opposite optical rotation. Therefore, ent-1 was determined as ent-stellettamide A. In summary, the authors achieved the total synthesis of ent-stellettamide A in 15 steps with 11% overall yield. During the synthesis, a new asymmetric [3 + 2] cycloaddition by using TMSCHN2 was developed for the rapid access to optically active pyrazolines, which could be further applied for the construction of similar skeletons.
Scheme 2.
Total synthesis of ent-stellettamide A.
The structure of stellettamide B (2) differed from that of stellettamide A (1) on the side chain. In 2001, Kibayashi׳s group27 reported the total synthesis of (−)-stellettamide B by a totally different way as Carreira׳s synthesis for ent-1, especially for the construction of the indolizidine core (Scheme 3). The synthesis of the indolizidine ring started by the treatment of enantiomeric 2-(1-aminoethyl)phenol (S20) with glutaric anhydride (S21) towards glutarimide S22, which was then reduced to tricyclic N,O-acetal S23 as a single diastereomer by Vitride® followed by HCl treatment. S23 was further submitted to allylation with allyltrimethylsilane (3 equiv.) and TiCl4 (3 equiv.) at 50 °C to afford the allylated products S24 and S25 in a ratio of 6:1 favoring the desired (6R)-allyl-2-piperidinone S24. This intermediate underwent methylation of the phenolic hydroxyl group, followed by Lemieux-Johnson oxidative cleavage of the olefin (OsO4, NaIO4) to give aldehyde S26 (Scheme 3). It was further functionalized by lactam reduction, chiral auxiliary cleavage, N-Boc protection, PDC oxidation of the aldehyde, and esterification to give 2-[(2R)-piperidinyl]acetate S27. Then, the diastereoselective allylation of ester S27 was performed by using allyl bromide after deprotonation by LiHMDS, to obtain the desired product S28 (84% de, 76% yield)27., 96.. Another oxidative cleavage of S28, followed by N-Boc deprotection led to compound S29 as a TFA salt, which underwent an intramolecular reductive amination in the presence of Pd-C/H2 in MeOH to promote ring closure towards indolizidine S30 as a single isomer. It was then converted into nitrile S31 by treatment with trimethylaluminum-NH4Cl, which was further transformed into aminomethylindolizidine S32 by LiAlH4 reduction in diisopropyl ether. After completion of the cyclic part, the linear moiety S35 was achieved by the Horner—Emmons olefination of linear aldehyde S33 with the appropriate 4-phosphono-2-methylcrotonate, followed by hydrolysis of the ester. The two fragments S32 and S35 were coupled by using DCC and DMAP, furnishing the desired amide S37 in 83% yield, which further underwent quarternization of the indolizidine part with iodomethane. Final exposure to AgCl resulted into methylammonium chloride 6′-epi-2 in 84% yield. However, the NMR data of 6′-epi-2 did not completely match those of the natural product stellettamide B, and more strikingly, the values of their specific rotation were of the opposite sign. Therefore, 6′-epi-2 should be an isomer of natural stellettamide B. Finally, the authors performed the same synthetic sequence from S34 to achieve the total synthesis of (−)-stellettamide B (2). It was confirmed to be identical to the natural product by NMR and displayed similar specific rotation. In conclusion, Kibayashi and co-workers25 reported another synthetic strategy for the construction of the indolizidine ring and further achieved the total synthesis of natural (−)-stellettamide B and its structure revision. In 2008, by using a similar synthetic route, Yamazaki and co-workers25 achieved the asymmetric total synthesis of (+)-stellettamide A and (−)-stellettamide C.
Scheme 3.
Total synthesis of (−)-stellettamide B.
3.3. Ciliatamides A−C
Ciliatamides A and B (55 and 56) exhibited significant antileishmanial activity, which led them to become valuable targets for total synthesis and further biological evaluation towards new and structurally less complex antileishmanial drugs. With this aim, in 2008, Lindsley and co-workers55 reported the total synthesis of ciliatamides A–C (55–57) and their structure revision (Scheme 4). They started from N-Boc-N-Me-l-Phe S39, by an amide coupling with either (S)-3-aminoazepan-2-one S41 (for 55 and 56) or (S)-3-aminopiperid-2-one S40 (for 57) in the presence of PS-DCC and HOBt, followed by Boc-deprotection with HCl in dioxane, leading to cyclic precursors S43 and S42, respectively. S43 underwent a second amide coupling either with fatty acid S44 in the presence of EDC, HOBt and DIPA in DMF to give 55, or with acyl chloride S45 in the presence of NMM, DMAP, in DMF under microwave at 160 °C to afford 56, in 56% and 58% yield, respectively. With the same procedure as for 55, ciliatamide C (57) was achieved by coupling S42 and S44. Due to the physicochemical properties of 55–57 and the poor chromatographic performance of such lipopeptides, the overall yields were lower than expected. Therefore, the authors developed a solution phase parallel synthesis approach to synthesize ciliatamides A (55) and C (57), involving only polymer supported reagents, scavengers and ion-exchange chromatography to avoid normal and reverse-phase chromatography.97 This strategy improved the synthesis, giving 55 and 57 in overall yields of more than 75% for the three steps with over 95% purity (Scheme 4). The NMR data of synthetic ciliatamides A–C overlaid with those of the natural products, while their optical rotations were of opposite sign. On the basis of this fact, the authors synthesized all stereoisomers (S,S), (S,R), (R,S), and (R,R) of ciliatamides A–C, employing the solid phase route depicted in Scheme 4. Finally, 55–57 were determined to be (R,R)-ciliatamide A, (R,R)-ciliatamide B, and (R,R)-ciliatamide C, respectively, by comparing their NMR data and optical rotation with those reported for the natural products. In is worth mentioning that the authors further synthesized more than 50 unnatural ciliatamide analogs in order to evaluate their antileishmanial activity and the structure-activity relationships, while the biological results were not reported in that paper.
Scheme 4.
Total synthesis of ciliatamides A–C.
In fact, the total synthesis of ciliatamide A (55) and its three isomers were also accomplished by Takada and co-workers56 with a similar synthetic strategy as that of Lindsley, However, through the comparison of NMR data, optical rotation, and more convincingly chiral HPLC of the four synthetic compounds with those of the natural product, the authors also revised the structure of natural ciliatamide A 55 to be of 12R,22S configuration. In addition, by using Marfey׳s method, the absolute configuration of the methionine sulfoxide residue in ciliatamide D (54) was also revised to be d-form. This research reminds us that, in certain cases, the direct comparison of synthetic compounds with the natural product for their absolute configuration determination is crucial.
In summary, synthetic efforts were mainly focused on Stelletta alkaloids or peptides and, in most cases, led to structure revision of the natural products. Some synthetic methodologies have also been developed during these syntheses, such as Carreira׳s asymmetric [3+2] cycloaddition for the rapid access to optically active pyrazolines. However, the authors do not seem to have taken advantage of the large quantity of natural products obtained from total synthesis to gain more deepened biological results. Future work should thus be conducted toward the intensive biological evaluation of these natural products and their analogs derived by rapid synthesis.
4. Bioactivities of stellettins A and B
Among all the metabolites identified in marine Stelletta sponge, the triterpenoids stellettins A and B (25 and 24) have been intensively studied for their anticancer activities by several research groups, which is worth to be detailed discussed herein so as to understand how the bioactive Stelletta-derived compounds being well studied towards potential drug leads.
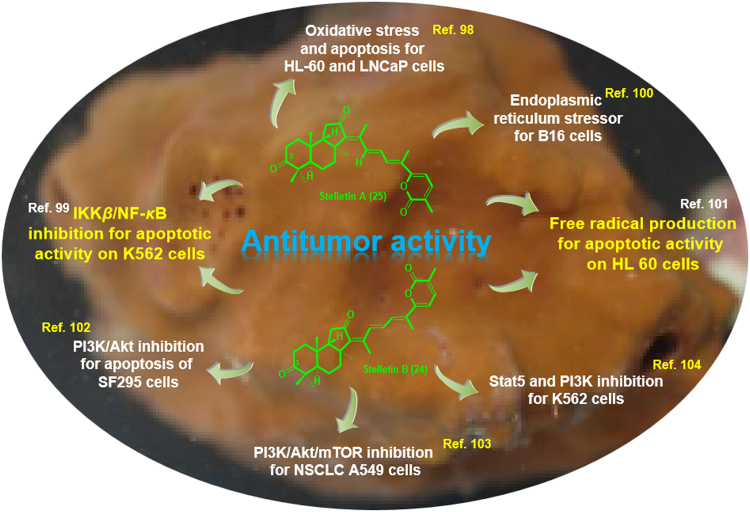
Although the selective cytotoxicity of both stellettins A and B were reported from 1997 to 2002, the underlying mechanism of stellettin A (25) was only reported in 2006 by Liu׳s group98. It was suggested that 25 was able to induce oxidative stress and apoptosis in HL-60 human leukemia and LNCaP prostate cancer cell lines. More specifically, they found that 25 induced more potent oxidative stress in HL-60 cells than in LNCaP cells, resulting into cell death through a FasL-caspase-3-apoptotic pathway (Fig. 15). In 2009, Folmer et al.99 investigated the cytotoxic effects of several natural products, including stellettins A and B, and their inhibition of TNF-α-induced NF-κB activation. After structure-activity relationship (SAR) analysis, they suggested that for both compounds 24 and 25, the α,β-unsaturated lactone rings might be responsible for their interference with IKKβ and with the binding of NF-κB to DNA99. In 2012, Liu׳s group100 reported that stellettin A (25) could act as an endoplasmic reticulum stressor to inhibit the growth of B16 melanoma cells by induction of abnormal protein glycosylation and autophagy. They also demonstrated that 25 was able to induce the upregulation of the unfolded protein chaperone, glucose-regulated protein 78, in a dose-dependent manner (Fig. 15).
Figure 15.
Summary of cytotoxicity mechanism studies of stelletins A and B (25 and 24).
Undoubtedly, due to the highly similar structures of stellettins A and B, stellettin B (24) was also found to be active on several cell lines. Liu and co-workers101 investigated the apoptotic activity of stellettins A and B. The study indicated that a ketone group at the C-3 position of isomalabaricane triterpenes 24 and 25 was necessary for free radical production and dissipation of mitochondrial membrane potential during the induction of leukemia HL60 cell death. Recently, a more systematical in vitro cytotoxicity study of stellettin B on human glioblastoma cancer SF295 cells was reported (Fig. 15)102. The authors investigated the cytotoxic activities on 39 human cancer cell lines, showing significant cytotoxic effects against the human glioblastoma cell line SF295, with a GI50 value of 0.01 μmol/L. Moreover, compared to the cytotoxic activity against several normal human cell lines including HMEC, RPTEC, NHBE and PrEC with GI50 values of 10 μmol/L, compound 24 possessed relatively selective cytotoxicity against human cancer cells. Further flow cytometric analysis showed that stellettin B induced apoptosis of SF295 cells in a concentration-dependent manner. The treatment of SF295 cells with stellettin B also increased ROS production, caspase 3/7 activity, and cleavage of PARP, all of which were known to be involved in apoptosis. To better understand the molecular mechanism for cell proliferation inhibition and apoptosis induction by stellettin B, its effect on the phosphorylation of several signal proteins of PI3K/Akt and RAS/MAPK pathways was examined. The targeted compound inhibited the phosphorylation of Akt, without any activity on p-ERK and p-p38, indicating that inhibition of PI3K/Akt pathway might be involved in the antiproliferative and apoptosis-inducing effect (Fig. 15). However, homogenous time-resolved fluorescence (HTRF) assays indicated that stellettin B did not inhibit PI3K, suggesting that the direct target might be a signal protein upstream of Akt pathway other than PI3K.
Another recent biological study of stellettin B isolated from the marine sponge Jaspis stellifera demonstrated that stellettin B induced G1 arrest, apoptosis and autophagy at low concentrations in human non-small cell lung cancer (NSCLC) A549 cells103. The findings indicated that the cytotoxicity of stellettin B on NSCLC was triggered by targeting PI3K/Akt/mTOR pathway (Fig. 15). G1 arrest by stellettin B might be attributed to the reduction of cyclin D1 and enhancement of p27 expression. Several assays including monodansylcadaverine (MDC) staining, transmission electron microscopy (TEM), tandem mRFP GFP-LC3 fluorescence microscopy, and western blot detection of the autophagy markers of LC3B, p62 and Atg5 were used to demonstrate autophagy inducing ability of stellettin B in A549 cells. The apoptosis induction of stellettin B in A549 cells might be related to the cleavage of PARP and increase of ROS generation. Meanwhile, stellettin B inhibited the expression of PI3K-p110, and the phosphorylation of PDK1, Akt, mTOR, p70S6K, as well as GSK-3β, suggesting a correlation with PI3K/Akt/mTOR pathway inhibition in the above cytotoxicity. Thus, the cytotoxic effects of stellettin B on human NSCLC A549 cells, including G1 cell cycle arrest, apoptosis and autophagy induction was well investigated during the research.
The most recent biological research on stellettin B was reported by Kong and co-workers104 in 2017. They investigated in vitro anti-leukemia activity of stellettin B on human CML K562 and KU812 cells. 24 inhibited K562 and KU812 cell proliferation with IC50 as 0.035 and 0.95 μmol/L respectively. After treatment by stellettin B, apoptosis was induced in K562 cells and it was described that stellettin B-induced apoptosis was possibly related in mitochondrial pathway to increased BAD and BAX, decreased BCL-2 and caspase-9 activation. In addition, the authors demonstrated that the inhibition of Stat5 and PI3K might be involved in the apoptosis-inducing effect based on a set of experimental results, including the inhibition of Stat5 phosphorylation, inhibition of the expression of 4 PI3K catalytic isoforms, and inhibition of the phosphorylation of downstream effectors including PDK1 and Akt (Fig. 15). Furthermore, the synergistic effect of stellettin B and Imatinib occurred with ratio as IC50 (stellettin B): 5×IC50 (Imatinib), which made stellettin B a promising candidate against chronic myeloid leukemia therapy alone or together with Imatinib. As for the SAR analysis of these two stellettins, although 24 and 25 shared similar structural features and bioactivities, their mechanism action are different. It seems that the configuration at Δ13., 14. significantly altered the binding sites of the compounds.
In summary, the mechanisms of anticancer activity of 24 and 25 were studied by several groups. They provided clues for the understanding of the signal pathway or target protein of both compounds towards cancer cells. Further in vivo study should be conducted to evaluate the druggability of these two potential cytotoxicity natural products.
5. Conclusion and perspectives
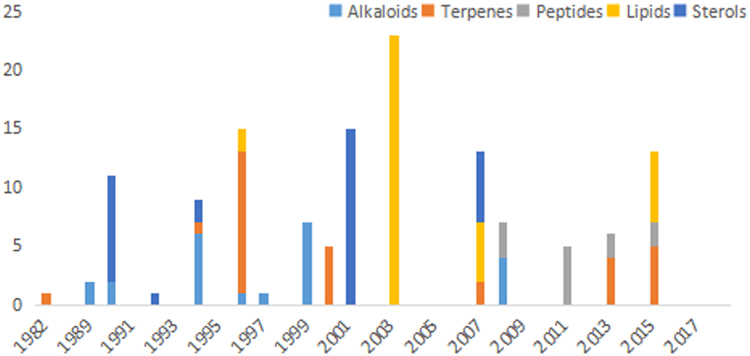
Great efforts have been made for the chemical investigation of marine Stelletta sponges all over the world sea from 1982 to early 2018. During the 35 years, more than 130 diverse secondary metabolites, including alkaloids, terpenoids, peptides, lipids and steroids, were discovered and characterized with different ratio of structure types varied by years (Fig. 16), and broad biological activities, such as antimicrobial, RNA cleavage activating, cytotoxic and anti-HIV effects. It is worth noting that, as have been demonstrated in Fig. 1, the skeletons of Stelletta metabolites also differed according to the collection locations. For instance, steroids and triterpenoids were mainly discovered in South China Sea, whereas alkaloids and lipids were mostly isolated from Japanese Sea. Such observation indicated that marine Stelletta sponges were capable of producing different kind of secondary metabolites to adapt to the various living environments. On this chemo-ecological point of view, future work would be interesting to focus on the discovery of targeted bioactive molecules, such as highly cytotoxic triterpenoids, from certain sea areas. Another outlook worth to mention is the possible biogenetic relationship between some metabolites in this genus. It could inspire biomimetic syntheses and compound interconversion, to scale up the available amounts for further pharmacological and toxicological evaluation. Furthermore, we also hold the belief that symbiotic microbes in Stelletta sponges have taken part in the generation of some metabolites, which could be another direction for the future study.
Figure 16.
Stelletta-derived secondary metabolites from 1982 to 2017.
As we all know that the research of natural products is a labor and resource intensive work, novel and more powerful chromatographic methods should be developed in accelerating the discovery process toward new drug leads from Nature, especially from marine environments which have yet seldom been developed. For example, the LC—MS-based metabolomics approach is a promising way to recognize already known metabolites from marine sponges105. It may further become crucial in Stelletta-derived drug candidate discovery. Since the high chemical diversity in Stelletta-derived MNPs was established, tandem mass spectrometry could be applied to build molecular networks for new compound searching. Data analysis of MSn fragmentation can be searched and assigned based on the open access databases, such as Global Natural Products Social Molecular Networking (GNPS)106. All these efforts would speed up the discovery of functional metabolites, not only from Stelletta sponges, but also from all the precious Ocean resources.
Acknowledgments
This paper is dedicated to Prof. Dr. Yuewei Guo on the occasion of his 60th birthday, for his pioneer work on Chinese marine natural product chemistry. This work was supported by the National Natural Science Foundation of China (NSFC, Nos. 41676073, 81520108028), the National Natural Science Foundation of China /Centre National de la Recherché Scientifique (NSFC/CNRS) joint project (No. 81811530284),“Youth Innovation Promotion Association” (No. 2016258) from Chinese Academy of Sciences, the SA-SIBS Scholarship Program, and the National Key Research and Development Program (2017YFE0103100).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Dedication to Prof. Dr. Yue-Wei Guo on the occasion of his 60th birthday, for his pioneer work on Chinese marine natural product chemistry.
References
- 1.Cheung R.C., Ng T.B., Wong J.H. Marine peptides: bioactivities and applications. Mar Drugs. 2015;13:4006–4043. doi: 10.3390/md13074006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jesus Raposo M.F., de Morais A.M., de Morais R.M. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. 2015;13:2967–3028. doi: 10.3390/md13052967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S., Ye F., Zhu Z., Huang H., Mao S., Guo Y. Cembrane-type diterpenoids from the South China Sea soft coral Sarcophyton mililatensis. Acta Pharm Sin B. 2018 doi: 10.1016/j.apsb.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang H.K., Seo C.H., Park Y. Marine peptides and their anti-infective activities. Mar Drugs. 2015;13:618–654. doi: 10.3390/md13010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y.Q., Miao Z.H. Marine-derived angiogenesis inhibitors for cancer therapy. Mar Drugs. 2013;11:903–933. doi: 10.3390/md11030903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q., Sun J., Chen J., Zhang H., Guo Y.W., Wang H. Terpenoids from marine soft coral of the genus Lemnalia: chemistry and biological activities. Mar Drugs. 2018;16:320. doi: 10.3390/md16090320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S.X., Zhang X.S., Guan H.S., Wang W. Potential anti-HPV and related cancer agents from marine resources: an overview. Mar Drugs. 2014;12:2019–2035. doi: 10.3390/md12042019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman D.J., Cragg G.M. Drugs and drug candidates from marine sources: an assessment of the current “state of play”. Planta Med. 2016;82:775–789. doi: 10.1055/s-0042-101353. [DOI] [PubMed] [Google Scholar]
- 9.Russo P., Kisialiou A., Lamonaca P., Moroni R., Prinzi G., Fini M. New drugs from marine organisms in Alzheimer׳s disease. Mar Drugs. 2016;14:5. doi: 10.3390/md14010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer A.M., Glaser K.B., Cuevas C., Jacobs R.S., Kem W., Little R.D. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Ebada S.S., Proksch P. Chemical and pharmacological significance of natural guanidines from marine invertebrates. Mini Rev Med Chem. 2011;11:225–246. doi: 10.2174/138955711795049862. [DOI] [PubMed] [Google Scholar]
- 12.Grienke U., Silke J., Tasdemir D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014;142:48–60. doi: 10.1016/j.foodchem.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Menna M. Important classes of bioactive alkaloids from marine ascidians: structures, isolation and bioactivity. Curr Top Med Chem. 2014;14:207–223. doi: 10.2174/1568026613666131213155813. [DOI] [PubMed] [Google Scholar]
- 14.Bordbar S., Anwar F., Saari N. High-value components and bioactives from sea cucumbers for functional foods-a review. Mar Drugs. 2011;9:1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehbub M.F., Lei J., Franco C., Zhang W. Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar Drugs. 2014;12:4539–4577. doi: 10.3390/md12084539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Ruiz C., Sarabia F. Chemistry and biology of bengamides and bengazoles, bioactive natural products from Jaspis sponges. Mar Drugs. 2014;12:1580–1622. doi: 10.3390/md12031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W., Mun B., Lee Y., Venkat Reddy M., Park Y., Lee J. Bioactive sesterterpenoids from a Korean sponge Monanchora sp. J Nat Prod. 2013;76:170–177. doi: 10.1021/np300573m. [DOI] [PubMed] [Google Scholar]
- 18.Youssef D.T., Shaala L.A., Alshali K.Z. Bioactive hydantoin alkaloids from the Red Sea marine sponge Hemimycale arabica. Mar Drugs. 2015;13:6609–6619. doi: 10.3390/md13116609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mioso R., Marante F.J., Bezerra R.S., Borges F.V., Santos B.V., Laguna I.H. Cytotoxic compounds derived from marine sponges. A review (2010–2012) Molecules. 2017;22:E208. doi: 10.3390/molecules22020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindequist U. Marine-derived pharmaceuticals-challenges and opportunities. Biomol Ther. 2016;24:561–571. doi: 10.4062/biomolther.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z.L., Zhang H.J., Lin H.W., Sun J.B., Liu G.L., Zhang C. Secondary metabolites from marine sponges Stelletta and their bioactivities. Chin J Mar Drug. 2004;23:42–47. [Google Scholar]
- 22.Hirota H., Matsunaga S., Fusetani N. Stellettahide A, an antifungal alkaloid from a marine sponge of the genus Stelletta. Tetrahedron Lett. 1990;31:4163–4164. [Google Scholar]
- 23.Abe Y., Saito S.Y., Hori M., Ozaki H., Fusetani N., Karaki H. Stellettamide-A, a novel inhibitor of calmodulin, isolated from a marine sponge. Br J Pharmacol. 1997;121:1309–1314. doi: 10.1038/sj.bjp.0701282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitlock G.A., Carreira E.M. Enantioselective synthesis of ent-Stellettamide A via a novel dipolar cycloaddition reaction of (trimethylsilyl)diazomethane. J Org Chem. 1997;62:7916–7917. doi: 10.1021/jo971571l. [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki N., Suzuki T., Yoshimura Y., Kibayashi C., Aoyagi S. Asymmetric synthesis of Stellettamides A and C. Heterocycles. 2008;75:285–290. [Google Scholar]
- 26.Shin J., Seo Y., Cho K.W., Rho J.R., Sim C.J. Stellettamide B, a new indolizidine alkaloid from a sponge of the genus Stelletta. J Nat Prod. 1997;60:611–613. doi: 10.1021/np970041h. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki N., Dokoshi W., Kibayashi C. Total synthesis of (–)-stellettamide B and determination of its absolute stereochemistry. Org Lett. 2001;3:193–196. doi: 10.1021/ol0003228. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga S., Yamashita T., Tsukamoto S., Fusetani N. Three new antibacterial alkaloids from a marine sponge Stelletta species. J Nat Prod. 1999;62:1202–1204. doi: 10.1021/np990161k. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamoto S., Kato H., Hirota H., Fusetani N. Stellettadine A: a new acylated bisguanidinium alkaloid which induces larval metamorphosis in ascidians from a marine sponge Stelletta sp. Tetrahedron Lett. 1996;37:5555–5556. [Google Scholar]
- 30.Tsukamoto S., Yamashita T., Matsunaga S., Fusetani N. Bistellettadines A and B: two bioactive dimeric stellettadines from a marine sponge Stelletta sp. J Org Chem. 1999;64:3794–3795. [Google Scholar]
- 31.Nozawa D., Takikawa H., Mori K. Synthesis and absolute configuration of stellettadine A: a marine alkaloid that induces larval metamorphosis in ascidians. Bioorg Med Chem Lett. 2001;11:1481–1483. doi: 10.1016/s0960-894x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto S., Yamashita T., Matsunaga S., Fusetani N. Stellettazole A: an antibacterial guanidinoimidazole alkaloid from a marine sponge Stelletta sp. Tetrahedron Lett. 1999;40:737–738. [Google Scholar]
- 33.El-Naggar M., Piggott A.M., Capon R.J. Bistellettazines A−C and bistellettazole A: new terpenyl-pyrrolizidine and terpenyl-imidazole alkaloids from a Southern Australian marine sponge, Stelletta sp. Org Lett. 2008;10:4247–4250. doi: 10.1021/ol8016512. [DOI] [PubMed] [Google Scholar]
- 34.Robertson J., Stevens K. Pyrrolizidine alkaloids: occurrence, biology, and chemical synthesis. Nat Prod Rep. 2017;34:62–89. doi: 10.1039/c5np00076a. [DOI] [PubMed] [Google Scholar]
- 35.Jin Z. Muscarine, imidazole, oxazole and thiazole alkaloids. Nat Prod Rep. 2016;33:1268–1371. doi: 10.1039/c6np00067c. [DOI] [PubMed] [Google Scholar]
- 36.Gunawardana G.P., Kohmoto S., Burres N.S. New cytotoxic acridine alkaloids from two deep water marine sponges of the family Pachastrellidae. Tetrahedron Lett. 1989;30:4359–4362. [Google Scholar]
- 37.Fusetani N., Asai N., Matsunaga S., Honda K., Yasumuro K. Cyclostellettamines A—F, pyridine alkaloids which inhibit binding of methyl quinuclidinyl benzilate (QNB) to muscarinic acetylcholine receptors, from the marine sponge, Stelletta maxima. Tetrahedron Lett. 1994;35:3967–3970. [Google Scholar]
- 38.Nathanson N.M. Molecular properties of the muscarinic acetylcholine receptor. Ann Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- 39.Yazawa H., Honda K. The M3-muscarinic cholinoceptor subtype in rat prostate and its down regulation by aging. Jpn J Pharmacol. 1993;61:319–324. doi: 10.1254/jjp.61.319. [DOI] [PubMed] [Google Scholar]
- 40.Sfecci E., Lacour T., Amade P., Mehiri M. Polycyclic guanidine alkaloids from Poecilosclerida marine sponges. Mar Drugs. 2016;14:77. doi: 10.3390/md14040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J., Li X.W., Guo Y.W. Recent advances in the isolation, synthesis and biological activity of marine guanidine alkaloids. Mar Drugs. 2017;15:324. doi: 10.3390/md15100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berlinck R.G., Bertonha A.F., Takaki M., Rodriguez J.P. The chemistry and biology of guanidine natural products. Nat Prod Rep. 2017;34:1264–1301. doi: 10.1039/c7np00037e. [DOI] [PubMed] [Google Scholar]
- 43.McCabe T., Clardy J., Minale L., Pizza C., Zollo F., Riccio R. A triterpenoid pigment with the isomalabaricane skeleton from the marine sponge Stelletta sp. Tetrahedron Lett. 1982;23:3307–3310. [Google Scholar]
- 44.Su J.Y., Meng Y.H., Zeng L.M., Fu X., Schmitz F.J. Stellettin A, a new triterpenoid pigment from the marine sponge Stelletta tenuis. J Nat Prod. 1994;57:1450–1451. doi: 10.1021/np50112a017. [DOI] [PubMed] [Google Scholar]
- 45.Ravi B.N., Wells R.J., Croft K.D. Malabaricane triterpenes from a Fijian collection of the sponge Jaspis stellifera. J Org Chem. 1981;46:1998–2001. [Google Scholar]
- 46.McCormick J.L., McKee T.C., Cardellina J.H., II, Leid M., Boyd M.R. Cytotoxic triterpenes from a marine sponge, Stelletta sp. J Nat Prod. 1996;59:1047–1050. doi: 10.1021/np960541v. [DOI] [PubMed] [Google Scholar]
- 47.Lin H.W., Wang Z.L., Wu J.H., Shi N., Zhang H.J., Chen W.S. Stellettins L and M, cytotoxic isomalabaricane-type triterpenes, and sterols from the marine sponge Stelletta tenuis. J Nat Prod. 2007;70:1114–1117. doi: 10.1021/np070069l. [DOI] [PubMed] [Google Scholar]
- 48.Xue D.Q., Mao S.C., Yu X.Q., Guo Y.W. Isomalabaricane triterpenes with potent protein-tyrosine phosphatase 1B (PTP1B) inhibition from the Hainan sponge Stelletta sp. Biochem Syst Ecol. 2013;49:101–106. [Google Scholar]
- 49.Li Y., Tang H., Tian X., Lin H., Wang M., Yao M. Three new cytotoxic isomalabaricane triterpenes from the marine sponge Stelletta tenuis. Fitoterapia. 2015;106:226–230. doi: 10.1016/j.fitote.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Barrero A.F., Oltra J.E., Herrador M.M., Cabrera E., Sanchez J.F., Quílez J.F. Gibepyrones: α-pyrones from Gibberella fujikuroi. Tetrahedron. 1993;49:141–150. [Google Scholar]
- 51.Tang S., Xu R., Lin W., Duan H. Jaspiferin A and B: two new secondary metabolites from the South China Sea sponge Jaspis stellifera. Rec Nat Prod. 2012;6:398–401. [Google Scholar]
- 52.Ryu G., Matsunaga S., Fusetani N. Globostellatic acids A−D, new cytotoxic isomalabaricane triterpenes from the marine sponge Stelletta globostellata. J Nat Prod. 1996;59:512–514. doi: 10.1021/np9601317. [DOI] [PubMed] [Google Scholar]
- 53.Oku N., Matsunaga S., Wada S.I., Watabe S., Fusetani N. New isomalabaricane triterpenes from the marine sponge Stelletta globostellata that induce morphological changes in rat fibroblasts. J Nat Prod. 2000;63:205–209. doi: 10.1021/np990333d. [DOI] [PubMed] [Google Scholar]
- 54.Imae Y., Takada K., Okada S., Ise Y., Yoshimura H., Morii Y. Isolation of ciliatamide D from a marine sponge Stelletta sp. and a reinvestigation of the configuration of ciliatamide A. J Nat Prod. 2013;76:755–758. doi: 10.1021/np300878b. [DOI] [PubMed] [Google Scholar]
- 55.Nakao Y., Kawatsu S., Okamoto C., Okamoto M., Matsumoto Y., Matsunaga S. Ciliatamides A−C, bioactive lipopeptides from the deep-sea sponge Aaptos ciliata. J Nat Prod. 2008;71:469–472. doi: 10.1021/np8000317. [DOI] [PubMed] [Google Scholar]
- 56.Lewis J.A., Daniels R.N., Lindsley C.W. Total synthesis of ciliatamides A−C: stereochemical revision and the natural product-guided synthesis of unnatural analogs. Org Lett. 2008;10:4545–4548. doi: 10.1021/ol801842v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada K., Irie R., Suo R., Matsunaga S. Resolution of the confusion in the assignments of configuration for the ciliatamides, acylated dipeptides from marine sponges. J Nat Prod. 2017;80:2845–2849. doi: 10.1021/acs.jnatprod.7b00684. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X., Liu J., Yang B., Lin X., Yang X.W., Liu Y. Marine natural products with anti-HIV activities in the last decade. Curr Med Chem. 2013;20:953–973. [PubMed] [Google Scholar]
- 59.Anjum K., Abbas S.Q., Akhter N., Shagufta B.I., Shah S.A., Hassan S.S. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem Biol Drug Des. 2017;90:12–30. doi: 10.1111/cbdd.12925. [DOI] [PubMed] [Google Scholar]
- 60.Sagar S., Kaur M., Minneman K.P. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619–2638. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andavan G.S., Lemmens-Gruber R. Cyclodepsipeptides from marine sponges: natural agents for drug research. Mar Drugs. 2010;8:810–834. doi: 10.3390/md8030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemmens-Gruber R., Kamyar M.R., Dornetshuber R. Cyclodepsipeptides-potential drugs and lead compounds in the drug development process. Curr Med Chem. 2009;16:1122–1137. doi: 10.2174/092986709787581761. [DOI] [PubMed] [Google Scholar]
- 63.Plaza A., Gustchina E., Baker H.L., Kelly M., Bewley C.A. Mirabamides A−D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion. J Nat Prod. 2007;70:1753–1760. doi: 10.1021/np070306k. [DOI] [PubMed] [Google Scholar]
- 64.Ramamoorthy G., Acevedo C.M., Alvira E., Lipton M.A. Synthesis and spectroscopic correlation of the diastereoisomers of 2,3-dihydroxy-2,6,8-trimethyldeca-(4Z,6E)-dienoic acid: implications for the structures of papuamides A−D and mirabamides A−D. Tetrahedron: Asymmetry. 2008;19:2546–2554. doi: 10.1016/j.tetasy.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Z., Van Wagoner R.M., Harper M.K., Baker H.L., Hooper J.N., Bewley C.A. Mirabamides E−H, HIV-inhibitory depsipeptides from the sponge Stelletta clavosa. J Nat Prod. 2011;74:185–193. doi: 10.1021/np100613p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin H.J., Rashid M.A., Cartner L.K., Bokesch H.R., Wilson J.A., McMahon J.B. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Tetrahedron Lett. 2015;56:4215–4219. doi: 10.1016/j.tetlet.2015.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dembitsky V.M., Rezanka T., Srebnik M. Lipid compounds of freshwater sponges: family Spongillidae, class Demospongiae. Chem Phys Lipids. 2003;123:117–155. doi: 10.1016/s0009-3084(03)00020-3. [DOI] [PubMed] [Google Scholar]
- 68.Meng Y., Su J., Zeng L. Chemical constituent studies on the marine sponge Stelletta tenuis (Lindgren) Acta Sci Nat Univ Sunyatseni. 1996;35:70–73. [Google Scholar]
- 69.Zhao Q., Lee S.Y., Hong J., Lee C.O., Im K.S., Sim C.J. New acetylenic acids from the marine sponge Stelletta species. J Nat Prod. 2003;66:408–411. doi: 10.1021/np020440z. [DOI] [PubMed] [Google Scholar]
- 70.Park C., Kim G.Y., Kim W.I., Hong S.H., Park D.I., Kim N.D. Induction of apoptosis by (Z)-stellettic acid C, an acetylenic acid from the sponge Stelletta sp., is associated with inhibition of telomerase activity in human leukemic U937 cells. Chemotherapy. 2007;53:160–168. doi: 10.1159/000100809. [DOI] [PubMed] [Google Scholar]
- 71.Lee H.S., Rho J.R., Sim C.J., Shin J. New acetylenic acids from a sponge of the genus Stelletta. J Nat Prod. 2003;66:566–568. doi: 10.1021/np020345q. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Q., Mansoor T.A., Hong J., Lee C.O., Im K.S., Lee D.S. New lysophosphatidylcholines and monoglycerides from the marine sponge Stelletta sp. J Nat Prod. 2003;66:725–728. doi: 10.1021/np0300075. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.Y., Zhao Q., Choi K.T., Hong J.K., Lee D.S., Lee C.O. A new glycerol ether from a marine sponge Stelletta species. Nat Prod Sci. 2003;9:232–234. [Google Scholar]
- 74.Zhao Q., Liu Y., Hong J., Lee C.O., Park J.H., Lee D.S. A new cyclitol derivative from a sponge Stelletta species. Nat Prod Sci. 2003;9:18–21. [Google Scholar]
- 75.Gil J.H., Hong J.Y., Jung J.H., Kim K.J., Hong J. Structural determination of monoacylglycerols extracted from marine sponge by fast atom bombardment tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1264–1270. doi: 10.1002/rcm.2948. [DOI] [PubMed] [Google Scholar]
- 76.Chen J., Wu Q., Hua Y., Chen J., Zhang H., Wang H. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl Microbiol Biotechnol. 2017;101:8309–8319. doi: 10.1007/s00253-017-8554-4. [DOI] [PubMed] [Google Scholar]
- 77.Peddie V., Takada K., Okuda S., Ise Y., Morii Y., Yamawaki N. Cytotoxic glycosylated fatty acid amides from a Stelletta sp. marine sponge. J Nat Prod. 2015;78:2808–2813. doi: 10.1021/acs.jnatprod.5b00795. [DOI] [PubMed] [Google Scholar]
- 78.Li J., Wang Z., Yang F., Jiao W.H., Lin H.W., Xu S.H. Two new steroids with cytotoxicity from the marine sponge Dactylospongia elegans collected from the South China Sea. Nat Prod Res. 2018;4:1–5. doi: 10.1080/14786419.2018.1475385. [DOI] [PubMed] [Google Scholar]
- 79.Fiorucci S., Distrutti E., Bifulco G., D׳Auria M.V., Zampella A. Marine sponge steroids as nuclear receptor ligands. Trends Pharmacol Sci. 2012;33:591–601. doi: 10.1016/j.tips.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Guerriero A., Debitus C., Pietra F. On the first marine stigmastane sterols and sterones having a 24,25-double bond. Isolation from the sponge Stelletta sp. of deep coral sea. Helv Chim Acta. 1991;74:487–494. [Google Scholar]
- 81.Deng S.Z., Liu A.M., Deng F.Y., Zhou H.Q., Ma K., Nakamura H. Structure elucidation of 24-methylene-27-methylcholesterol from South China Sea sponge (Seletta tenuis Lindgren) Chin J Org Chem. 1992;12:501–503. [Google Scholar]
- 82.Li H., Matsunaga S., Fusetani N. A new 9,11-secosterol, stellettasterol from a marine sponge Stelletta sp. Experientia. 1994;50:771–773. [Google Scholar]
- 83.Capon R.J., Faulkner D.J. Herbasterol, an ichthyotoxic 9,11-secosterol from the sponge Dysidea herbacea. J Org Chem. 1985;50:4771–4773. [Google Scholar]
- 84.Yan S.J., Su J.Y., Zhang G.W., Wang Y.H., Li H. Steroids from the marine sponge Stelletta tenuis Lindgren. Acta Sci Nat Univ Sunyatseni. 2001;40:54–57. [Google Scholar]
- 85.Miyamoto T., Kodama K., Aramaki Y., Higuchi R., Van Soest R.W. Orostanal, a novel abeo-sterol inducing apoptosis in leukemia cell from a marine sponge. Stelletta hiwasaensis Tetrahedron Lett. 2001;42:6349–6351. [Google Scholar]
- 86.Lin W.H., Fang J.M., Cheng Y.S. Diterpenoids and steroids from Taiwania cryptomerioides. Phytochemistry. 1998;48:1391–1397. [Google Scholar]
- 87.Smith T.E., Pond C.D., Pierce E., Harmer Z.P., Kwan J., Zachariah M.M. Accessing chemical diversity from the uncultivated symbionts of small marine animals. Nat Chem Biol. 2018;14:179–185. doi: 10.1038/nchembio.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kiran G.S., Sekar S., Ramasamy P., Thinesh T., Hassan S., Lipton A.N. Marine sponge microbial association: towards disclosing unique symbiotic interactions. Mar Environ Res. 2018 doi: 10.1016/j.marenvres.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Oleinikova G.K., Dmitrenok A.S., Voinov V.G., Chaikina E.L., Shevchenko L.S., Kuznetsova T.A. Bacillomycin D from the marine isolate of Bacillus subtilis KMM 1922. Chem Nat Comp. 2005;41:461–464. [Google Scholar]
- 90.Li Z., Peng C., Shen Y., Miao X., Zhang H., Lin H. L, l-Diketopiperazines from Alcaligenes faecalis A72 associated with South China Sea sponge Stelletta tenuis. Biochem Syst Ecol. 2008;36:230–234. [Google Scholar]
- 91.Zhang P., Bao B., Dang H.T., Hong J., Lee H.J., Yoo E.S. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp. J Nat Prod. 2009;72:270–275. doi: 10.1021/np8006793. [DOI] [PubMed] [Google Scholar]
- 92.Liu S., Wang H., Su M., Hwang G.J., Hong J., Jung J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat Prod Res. 2017;31:1682–1686. doi: 10.1080/14786419.2017.1289205. [DOI] [PubMed] [Google Scholar]
- 93.Jetten M., Peters C.A., Van Nispen J.W., Ottenheijm H.C. A one-pot N-protection of l-arginine. Tetrahedron Lett. 1991;32:6025–6028. [Google Scholar]
- 94.Stoilova V., Trifonov L.S., Orahovats A.S. A convenient one-flask synthesis of 1-methylazetidines from 3-aminoalkanols, triphenylphosphine, and carbon tetrachloride. Synthesis. 1979;2:105–106. [Google Scholar]
- 95.Myers A.G., Yang B.H., Chen H., Gleason J.L. Use of pseudoephedrine as a practical chiral auxiliary for asymmetric synthesis. J Am Chem Soc. 1994;116:9361–9362. [Google Scholar]
- 96.Morley C., Knight D.W., Share A.C. Complementary enantioselective routes to the quinolizidine alkaloids lupinine and epilupinine. Tetrahedron: Asymmetry. 1990;1:147–150. [Google Scholar]
- 97.Kennedy J.P., Williams L., Bridges T.M., Daniels R.N., Weaver D., Lindsley C.W. Application of combinatorial chemistry science on modern drug discovery. J Comb Chem. 2008;10:345–354. doi: 10.1021/cc700187t. [DOI] [PubMed] [Google Scholar]
- 98.Liu W.K., Cheung F.W., Che C.T. Stellettin A induces oxidative stress and apoptosis in HL-60 human leukemia and LNCaP prostate cancer cell lines. J Nat Prod. 2006;69:934–937. doi: 10.1021/np058122y. [DOI] [PubMed] [Google Scholar]
- 99.Folmer F., Jaspars M., Solano G., Cristofanon S., Henry E., Tabudravu J. The inhibition of TNF-a-induced NF-κB activation by marine natural products. Biochem Pharmacol. 2009;78:592–606. doi: 10.1016/j.bcp.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 100.Liu W.K., Ling Y.H., Cheung F.W., Che C.T. Stellettin A induces endoplasmic reticulum stress in murine B16 melanoma cells. J Nat Prod. 2012;75:586–590. doi: 10.1021/np2008158. [DOI] [PubMed] [Google Scholar]
- 101.Liu W.K., Ho J.C., Che C.T. Apoptotic activity of isomalabaricane triterpenes on human promyelocytic leukemia HL60 cells. Cancer Lett. 2005;230:102–110. doi: 10.1016/j.canlet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 102.Tang S.A., Zhou Q., Guo W.Z., Qiu Y., Wang R., Jin M. In vitro antitumor activity of stellettin B, a triterpene from marine sponge Jaspis stellifera, on human glioblastoma cancer SF295 cells. Mar Drugs. 2014;12:4200–4213. doi: 10.3390/md12074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang R., Zhang Q., Peng X., Zhou C., Zhong Y., Chen X. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci Rep. 2016;6:27071. doi: 10.1038/srep27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Y., Zhou Q., Zhang L., Zhong Y., Fan G., Zhang Z. Stellettin B induces apoptosis in human chronic myeloid leukemia cells via targeting PI3K and Stat5. Oncotarget. 2017;8:28906–28921. doi: 10.18632/oncotarget.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Urda C., Pérez M., Rodríguez J., Fernández R., Jiménez C., Cuevas C. Njaoamine I, a cytotoxic polycyclic alkaloid from the Haplosclerida sponge Haliclona (Reniera) sp. Tetrahedron Lett. 2018;59:2577–2580. [Google Scholar]
- 106.Wang M., Carver J.J., Phelan V.V., Sanchez L.M., Garg N., Peng Y. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]