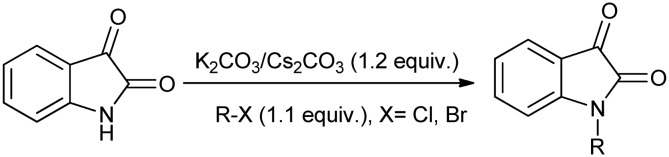 Isatin (1H-indole-2,3-dione) and its derivatives represent an important class of heterocyclic compounds that can be used as precursors for drug synthesis.
Isatin (1H-indole-2,3-dione) and its derivatives represent an important class of heterocyclic compounds that can be used as precursors for drug synthesis.
Abstract
Isatin (1H-indole-2,3-dione) and its derivatives represent an important class of heterocyclic compounds that can be used as precursors for drug synthesis. Since its discovery, a lot of research work has been done regarding the synthesis, chemical properties, and biological and industrial applications of isatin. In this review, we have reported several novel methods for the synthesis of N-, C2-, and C3-substituted and spiro derivatives of isatin. The isatin moiety also shows important chemical reactions such as oxidation, ring expansion, Friedel–Crafts reaction and aldol condensation. These reactions, in turn, produce several biologically viable compounds like 2-oxindoles, tryptanthrin, indirubins, and many more. We have also summarized some recently reported biological activities exhibited by isatin derivatives, like anti-cancer, anti-bacterial, anti-diabetic and others. Special attention has been paid to their anti-cancer activity, and various anti-cancer targets such as histone deacetylase, carbonic anhydrase, tyrosine kinase, and tubulin have been discussed in detail. Other applications of isatin derivatives, such as in the dye industry and in corrosion prevention, have also been discussed.
Introduction
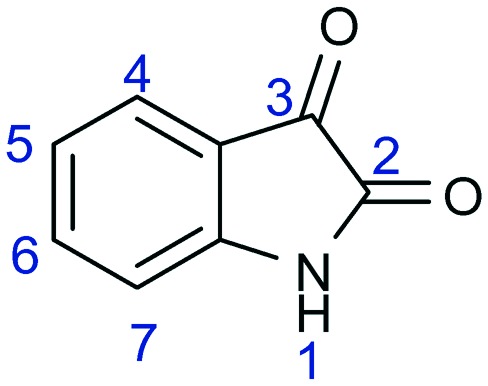
Heterocyclic compounds are an important class of organic compounds having vivid biological and pharmacological properties.1,2 Isatin (1H-indole-2,3-dione), also known as indenedione and indole quinone, is one such biologically active heterocyclic moiety. It has a nitrogen atom at position 1 and two carbonyl groups at positions 2 and 3 (Fig. 1). It comprises two cyclic rings, one of which is six-membered and the other is five-membered. Both of the rings are planar. The six-membered ring has an aromatic character, whereas the five-membered ring possesses an anti-aromatic character.
Fig. 1. Structure of isatin (1H-indole-2,3-dione).
Isatin was first isolated by Erdmann and Laurent as an oxidation product of indigo using nitric and chromic acids. It crystallizes from water, alcohol, or acetic acid in the form of orange-red monoclinic prism crystals melting at 200 °C. In human beings, it is found as a metabolic derivative of the adrenaline hormone and is also a component of secretion from the parotid gland of Bufo frogs. Various derivatives of isatin also occur naturally in plants. For example, methoxy phenylpentyl isatins (the melosatin alkaloids) were isolated from Melochia tomentosa, a Caribbean tumorigenic plant, 5-(3′-methylbut-2′-yl)isatin was isolated from Chaetomium globosum and 6-(3′-methylbuten-2′-yl)isatin was found in Streptomyces albus.3
The Sandmeyer, Stolle, Gassman and Martinet procedures are the conventional methods used for the preparation of various isatin derivatives. Apart from these, various novel and environmentally benign synthetic methods for synthesizing isatin derivatives have been reported lately and are reviewed here. Furthermore, derivatives of isatin display certain chemical reactions such as oxidation, ring expansion, Friedel–Crafts reaction, and aldol condensation. These chemical reactions form the basis for the synthesis of other biologically important derivatives such as tryptanthrin, indirubins, and 2-oxindoles. Isatin derivatives also have various industrial applications; for example, they are used as corrosion inhibitors, as fluorescent sensors and also in the dye industry.
Literature surveys revealed that isatin and its derivatives show various biological activities like anti-cancer, anti-bacterial, anti-fungal, anti-diabetic, anti-convulsant, anti-tubercular, anti-HIV, neuroprotective,4 anti-oxidant,5 anti-glycation,6 anti-malarial,7 anti-inflammatory,8 analgesic,9 and anti-anxiety activities.10 In this work, we have reviewed in detail some of the biological activities that have been reported in the past decade. Special attention has been paid to its anti-cancer activity, and various anti-cancer targets such as histone deacetylase, carbonic anhydrase, tyrosine kinase, and tubulin have also been discussed in detail.
The chemistry3 and a few biological applications11–13 of isatin have been already reviewed. In this review, we have summarized the most recent literature for the synthesis, chemistry, biological and industrial applications of isatin and its derivatives.
Synthesis of isatin derivatives
The Sandmeyer synthesis is one of the oldest and most frequently used methods for the synthesis of isatin and its derivatives.14 However, it works only for simple analogs. Several other synthetic methods such as Martinet, Stolle, Gassman, and metalation of anilide derivatives have also been reviewed earlier.3 However, there is a problem of low yields and inseparable mixtures of regioisomeric products are formed using substrates containing m-substituted or electron-withdrawing substituents.
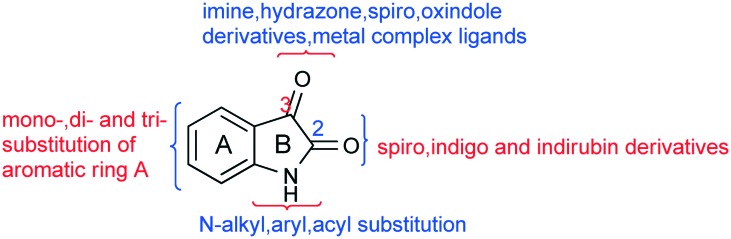
Fig. 2 shows the substitution of the aryl ring (A), alkylation of the nitrogen atom in the moiety (B) and also modifications at its C2 and/or C3 carbonyl functionalities, which lead to the formation of a number of biologically important derivatives. Hence various schemes involving the synthesis of these derivatives have been reviewed here.
Fig. 2. The various substitutions possible on the isatin scaffold.
N-Substituted isatin derivatives
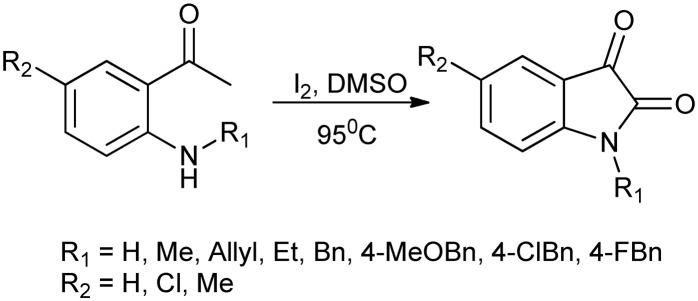
N-Substituted isatin derivatives show many biological applications. They are effective inhibitors of carbonic anhydrase isoform IX (CA IX),15 the form which is found to be over-expressed in a large number of solid tumors. Several novel synthetic routes have been designed for the synthesis of N-substituted isatins. One such effort involves a metal-free synthesis that uses I2–DMSO as a catalyst. The scheme involves the synthesis of N-alkylated and N-arylated isatins from 2-amino acetophenones via C–H bond activation and subsequent internal cyclization (Scheme 1).16
Scheme 1. Synthesis of N-alkylated/arylated isatin derivatives from 2-amino acetophenones.
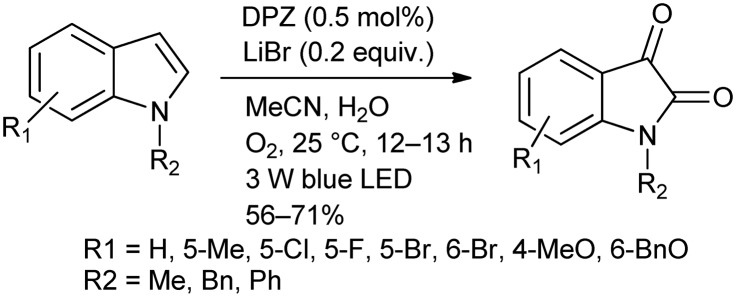
Oxidation of indole derivatives is one of the modern methods used for the synthesis of N-alkylated isatins. Zhang et al.17 developed an environmentally benign method, which involves the oxidation of indole using O2 as the oxidizing agent in the presence of a photosensitizer, a dicyanopyrazine derivative (DPZ) (Scheme 2).
Scheme 2. Formation of N-alkylated isatins via photocatalytic aerobic oxidation of indoles.
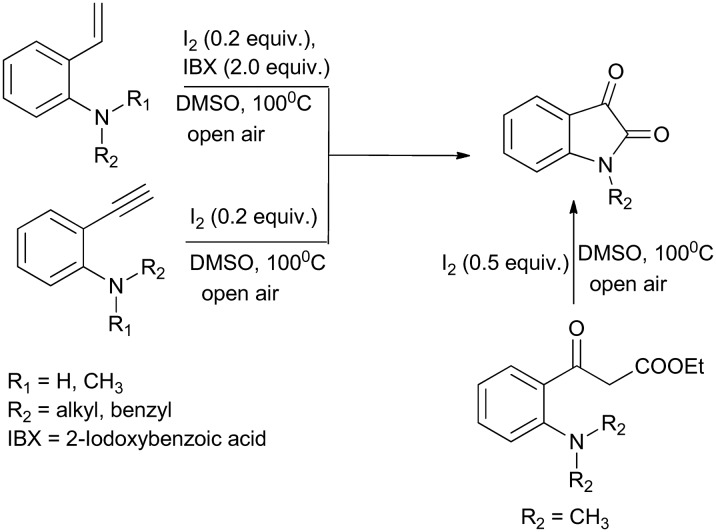
A Unified Multisubstrate Domino Approach for the synthesis of N-alkylated derivatives has been reported. It involves the direct amidation of 2′-aminophenylacetylenes, 2′-aminostyrenes, and 2′-amino-β-ketoesters (Scheme 3). The major advantage of this protocol is the metal-free and peroxide-free synthesis of N-alkylated isatin derivatives.18
Scheme 3. Direct amidation of 2′-aminophenylacetylene, 2′-aminostyrenes, and 2′-amino-β-ketoesters (in open air, 100 °C, DMSO) to form isatins.
C2-Substituted isatin derivatives
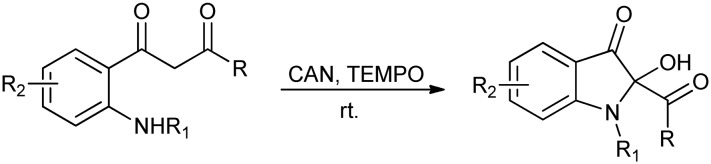
2-Hydroxy-2-substituted indol-3-ones bearing a C2-quaternary center represent an important class of C2-substituted isatin derivatives. These derivatives are components of many natural and bioactive molecules and also serve as key intermediates in their synthesis. Recently, 2-hydroxy-2-substituted indol-3-ones bearing a C2-quaternary center have been synthesized via the oxidative cyclization of 2-aminophenyl-1,3-dione using ceric ammonium nitrate (CAN) and 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as oxidants (Scheme 4).19
Scheme 4. Synthetic routes to 2-hydroxy-2-substituted-indol-3-ones.
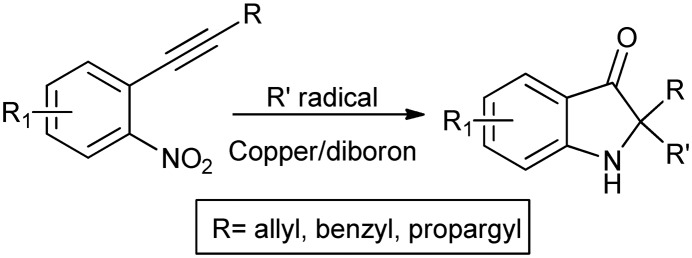
Pseudoindoxyls form another important class of C-2-substituted compounds having a quaternary stereo center. These are present as important fragments in various biologically active molecules such as (+)-austamide, cephalinone, lipidgreen, etc. Syntheses of pseudoindoxyls have been reported via the Claisen rearrangement20,21 and photocatalytic aerobic oxidation/semipinacol rearrangement.22 However, in one study, a unique method employing a Cu/B2pin2 system for intramolecular oxygenation of alkynes has been reported (Scheme 5).23
Scheme 5. Synthesis of pseudoindoxyls via intramolecular oxygenation of alkynes using a Cu/B2pin2 system.
C3-Substituted isatin derivatives
A wide range of C3-substituted isatins, like thiosemicarbazones, oxindoles and their derivatives, imines and hydrazones, have been synthesized.
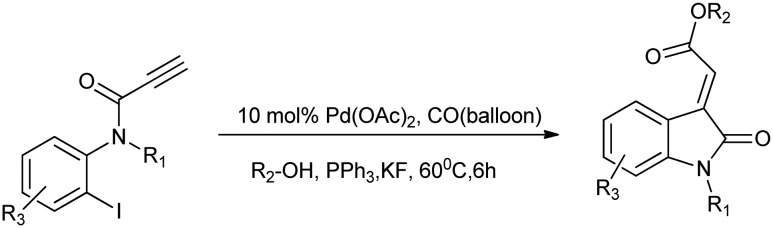
Among these derivatives, the 3-ylideneoxindole moiety is an important constituent of many pharmaceutically essential compounds such as sunitinib and hesperidin, which possess the capacity to bind the receptor tyrosine kinase (RTK) and Aurora B with high affinity (Dar). Therefore, many recent efforts have been made for its effective synthesis, and one such work reports the use of a tandem Pd-catalyzed Heck and alkoxycarbonylation reaction for the stereoselective synthesis of (E)-oxindolylidene acetates. These compounds were found to show potent anticancer activities against a variety of human cancer cell lines (Scheme 6).24
Scheme 6. Synthesis of (E)-oxindolylidene acetates via a Pd-catalyzed Heck and alkoxycarbonylation reaction.
Flourine-18 labeled thiosemicarbazone derivatives of isatin have been synthesized recently via the route shown in Scheme 7. These derivatives showed inhibition towards P-glycoprotein (P-gp), which is one of the main proteins involved in the active efflux from the brain to blood and helps in maintaining the blood–brain barrier (BBB). Their radioactivity is used for imaging P-gp expression using PET, which is otherwise difficult to measure.25
Scheme 7. Synthesis of F-18 labeled C-3-substituted thiosemicarbazone derivatives of isatin.
Spiro derivatives
Spiro compounds have been found to show prominent pharmacological activity, especially in the chemistry of natural products. Various studies have been reported on the synthesis of spiro derivatives of isatin at the C-3 position.
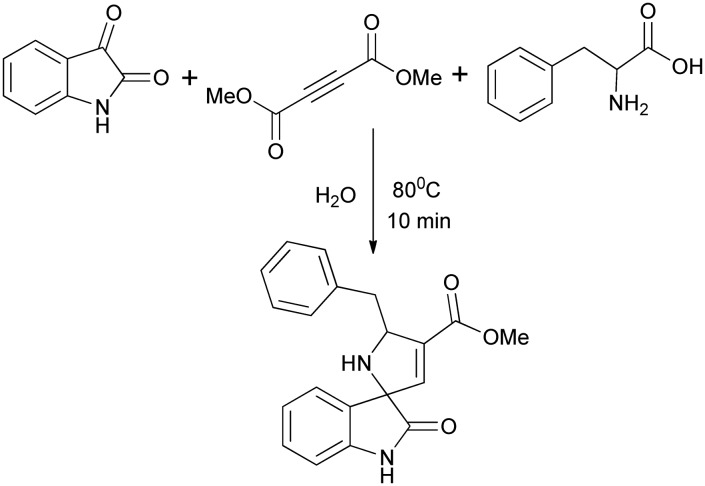
Meshram and co-workers reported the synthesis of isatin derivatives having the spirooxindole moiety by a three component reaction, viz. isatin (1 mmol), amino acid (1.2 mmol), and but-2-ynedioates (1 mmol), using microwave irradiation (150 W power) under catalyst-free and base-free conditions in aqueous medium (Scheme 8).26 This protocol has several advantages, such as efficiency and simplicity, environmentally friendly conditions, excellent yields, and short reaction time.
Scheme 8. Synthesis of a spiro compound under microwave irradiation in aqueous medium.
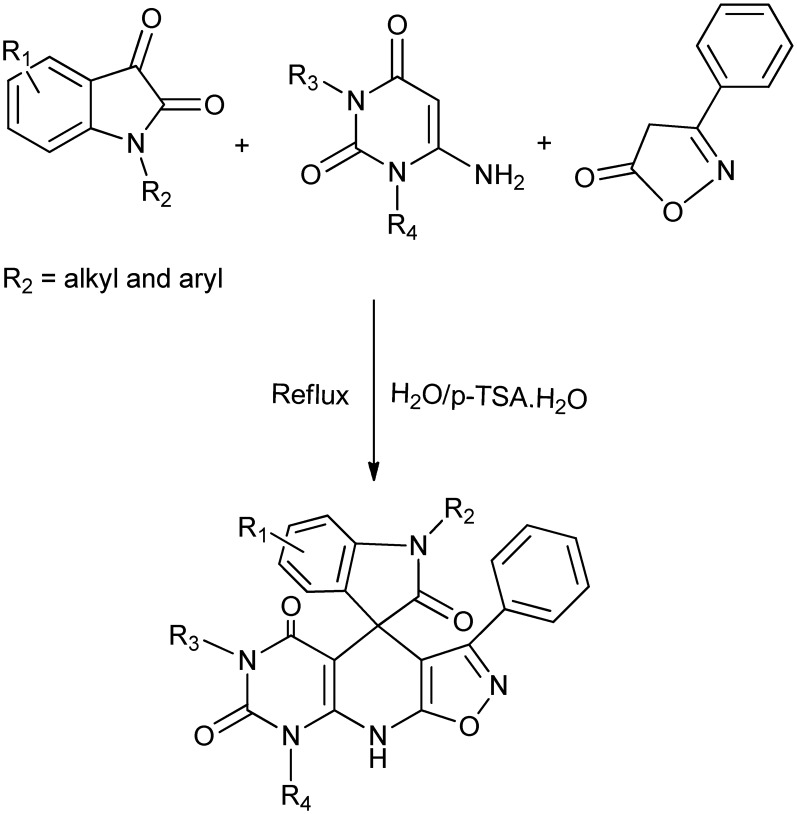
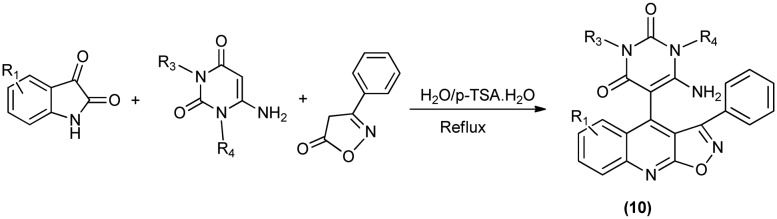
A novel one-pot multicomponent reaction for the regioselective synthesis of a diverse spirooxindole-based framework has been reported. The multicomponent reaction involves isatins, aminouracils and isooxazolones as substrates. The formation of spirooxindoles was found to be favored only when N-alkyl/aryl/benzyl-substituted isatins were used otherwise the side product isoxazoloquinolines were obtained predominantly (Scheme 9).27
Scheme 9. One-pot multicomponent synthesis of regioselective spirooxindoles.
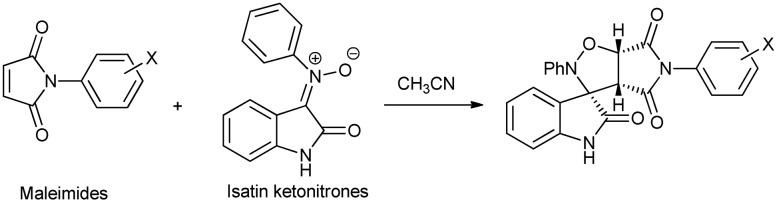
Novel spiro[oxindole-isoxazolidine] derivatives have been synthesized via 1,3-dipolar cycloaddition reactions of variously substituted maleimides with isatin ketonitrone (Scheme 10). The reactants, substituted maleimides, were prepared separately using maleic anhydride and aniline, whereas isatin ketonitrone was prepared via multiple steps using isatin, aniline, and phenyl hydroxylamine.28
Scheme 10. Synthesis of spiro[oxindole-isoxazolidine] via 1,3-dipolar cycloaddition of reactants.
Synthesis of some other pharmaceutically relevant isatin derivatives
Synthesis of 1-methylisoindigo
1-Methylisoindigo, or meisoindigo, is a well-known indirubin derivative known for its activity against various forms of leukemia, such as chronic myelogenous leukemia (CML), acute promyelocytic leukemia (APL), and acute myeloid leukemia (AML).29
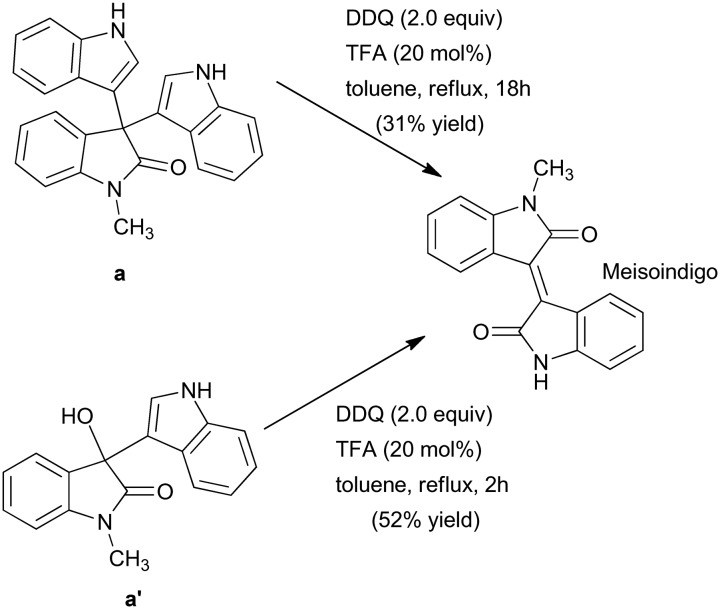
In general, meisoindigo has been synthesized via acid-catalyzed condensation reaction of isatin with 2-oxindoles.30 However, in a recent approach, Seo and co-workers carried out an intramolecular oxidative coupling reaction of 3,3-diindolyl-2-oxindole (a) with DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) in the presence of p-TsOH or trifluoroacetic acid (TFA). The reaction yielded meisoindigo in 31% yield, whereas carrying out a similar synthesis using 3-indolyl-2-oxindole (a′) instead of (a) yielded meisoindigo in 52% yield (Scheme 11).31
Scheme 11. Synthesis of meisoindigo in different yields by using different starting reactants.
Synthesis of bromo derivatives
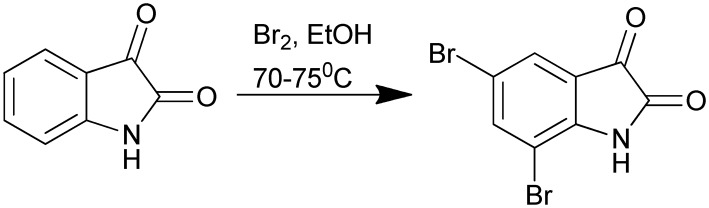
The halogenated derivatives of isatin, especially the bromo-substituted ones, are found to exhibit anti-cancer activity against the human lymphoma cell line.32 Several synthetic methods have been reported for the mono-, di-, and tri-bromo-substituted derivatives. Vine et al.33 have reported one such synthesis where different conditions of bromination produced different substituted bromo derivatives such as 5,7-dibromoisatin (Scheme 12), 5,6-dibromoisatin, and 5,6,7-tribromoisatin. For instance, the 5,7-dibromo derivative was synthesized by refluxing isatin in ethanol and adding bromine drop-wise, while maintaining the temperature at 70–75 °C (Scheme 12). In continuation of this work, Krishnegowda and co-workers used 5,7-dibromoisatin as the starting material and further synthesized its N-bromo derivatives.34
Scheme 12. Synthesis of the 5,7-dibromoisatin derivative.
Synthesis of C-5-substituted isatin derivatives
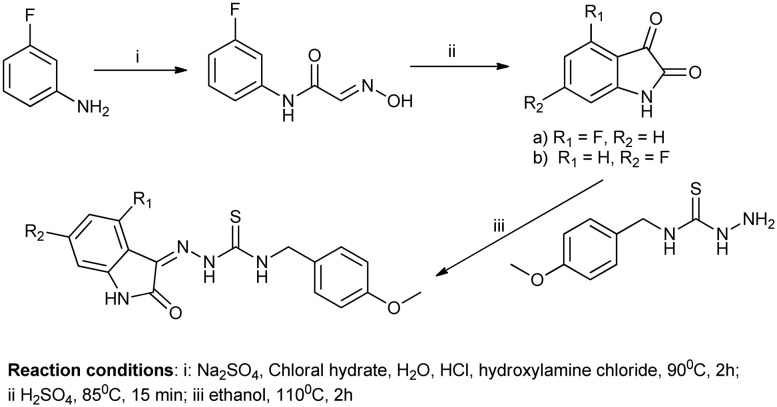
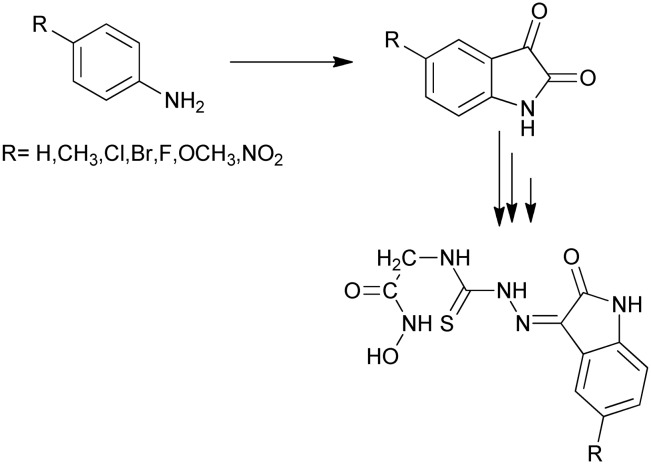
The isatin moiety substituted at the C-5 position is an important scaffold in the designing of effective anti-cancer drugs,35 whereas substitution with the methoxy group at C-5 results in effective carbonic anhydrase inhibitors.36 Various methods have been reported for the synthesis of C-5 isatin derivatives. One such method involves the reaction between chloral hydrate, sodium sulfate, and a solution of substituted anilines (Scheme 13). The solution was heated to 80 °C and this temperature was maintained for 10 min to give the C-5-substituted isatins. Further, the reaction continued to synthesize some novel hybrids of isatin.35
Scheme 13. Synthesis of C-5-substituted isatin derivatives from substituted anilines; further reaction also produces hybrids of these derivatives.
Structural characteristics
Tautomerization
In 1882, Baeyer first proposed that isatin exists as two tautomeric forms, lactam (1) and lactim (2), in which a proton transfer between the nitrogen atom and the oxygen atom present at the second carbon occurs.37 In the solid state, isatin predominantly exists in the lactam structure. Formation of O-alkyl ethers (3) and isatin-α-chloride (4) supports the existence of the lactim form.38 Furthermore, the 1H NMR spectrum of isatin in CD3OD shows signals for both lactam and lactim forms, whereas in DMSO-d6, only the signal due to the lactam form appears.39 In one of our previous reports, a theoretical study on the stability of the various conformers and tautomers of isatin-3-thiosemicarbazone in the gas phase and in the aqueous phase was reported. It was found that tautomer 5 is the key tautomer and one of its conformers accounts for approximately 87% of the population in the gas phase.40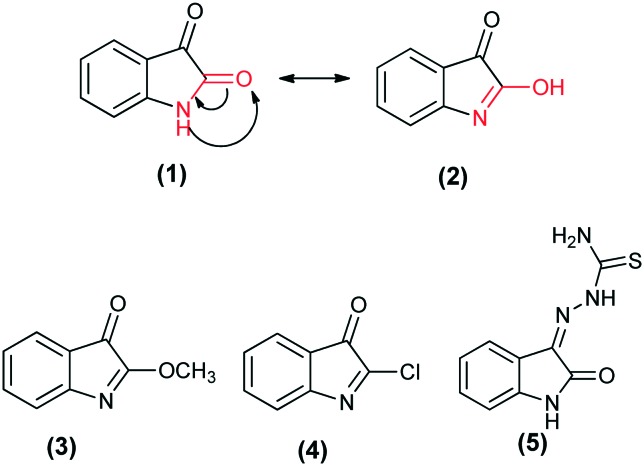
Spectral studies
The UV-visible spectrum of isatin shows an absorption maximum in the region of 260 nm to 350 nm corresponding to a π → π* transition due to the aromatic ring. The absorption maximum and band intensity in this region depend on the donor/acceptor ability of the aromatic ring, where the maxima band shifts bathochromically with the increase in donor ability of the ring. A relatively weak absorption band in the region of 350 nm to 600 nm corresponds to the n → π* and intramolecular charge transfer (ICT) transitions of the free electron pairs of nitrogen and oxygen. In basic medium, the long-wavelength absorption bands in the 350 nm to 600 nm region disappear and a new bathochromically shifted band in the region of 400 nm to 750 nm appears. This new band arises due to the formation of an azanion (6).41
The 1H-NMR spectrum of isatin (7) shows a doublet at δ 7.47 ppm and 6.86 ppm corresponding to Hb and He respectively. The hydrogen atom (Ha) attached to nitrogen appears as a singlet at approximately δ 11.03 ppm. The protons Hc and Hd show triplets at δ 7.05 ppm and 7.57 ppm, respectively. Deprotonation of NH in the isatin moiety leads to a downfield shift for the azanion's protons (Hb, Hc, Hd, and He) in the 1H-NMR spectrum.41
Furthermore, the IR spectrum of isatin shows two strong bands at 1740 and 1620 cm–1, representing the carbonyl stretching vibrations. A broad band accompanied by some sub-bands occurs at 3188 cm–1 corresponding to N–H stretching, which moves to 2370 cm–1 on deuteration of N–H.42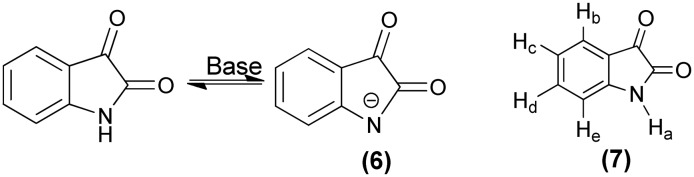
Reactions of isatin
Oxidation
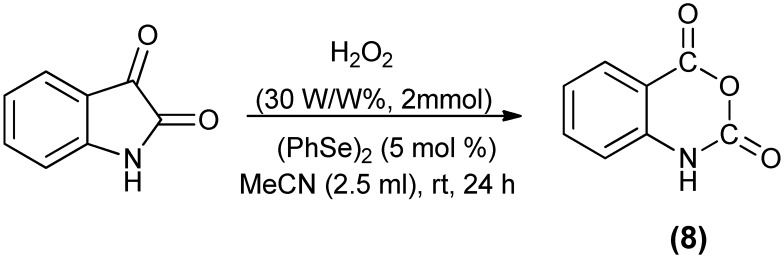
Isatoic anhydride (8), an abundantly employed compound in herbicide production and in medicinal chemistry, has been obtained through the oxidation of isatin. Isatin reacts with chromic acid in acetic acid solution to yield isatoic anhydride.38 Yu et al.43 reported the selective organoselenium-catalyzed clean oxidation of isatin by H2O2 under mild and neutral conditions to give isatoic anhydride (Scheme 14).
Scheme 14. Organoselenium-catalyzed oxidation of isatin to isatoic anhydride.
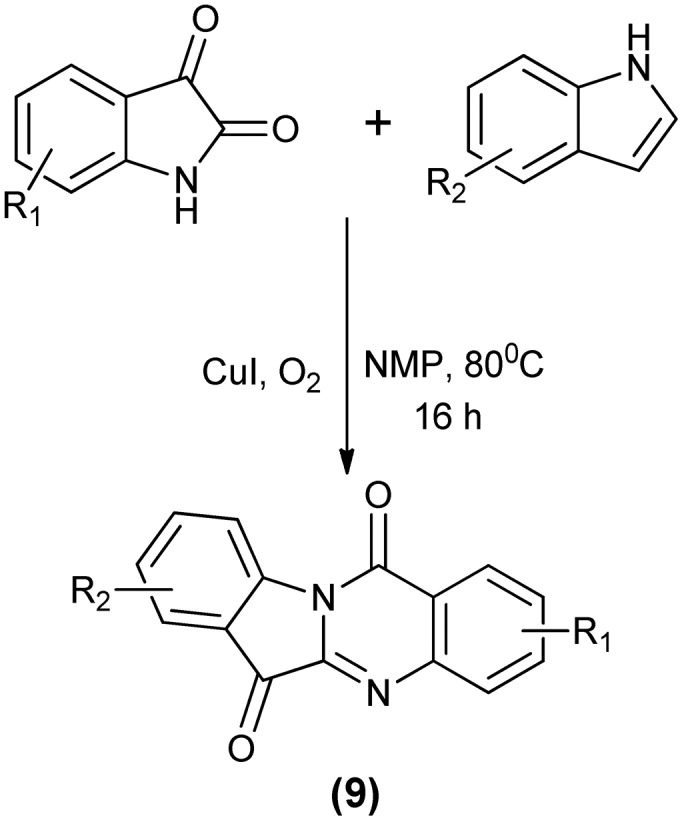
Another biologically active compound, tryptanthrin (9), can be synthesized by the oxidation of isatin. Moskovkina et al.44 reported the formation of tryptanthrin through the oxidation of isatin and its 5-substituted analogs using potassium permanganate in anhydrous acetonitrile. In another study, tryptanthrin and its derivatives were obtained via CuI-catalyzed oxidative condensation between isatins and indoles (Scheme 15).45
Scheme 15. Cu-Catalyzed aerobic oxidation of isatins to tryptanthrin derivatives.
Ring expansion reactions
Ring expansion reactions are valuable in the field of organic synthesis, since ring expansion allows access to larger rings, which are otherwise difficult to synthesize by other methods. The strong electrophilic nature of the C3 carbon of isatin allows it to undergo ring expansion reactions. Li et al.46 reported the catalytic asymmetric ring expansion reaction of isatins and α-alkyl-α-diazoesters to synthesize 2-quinolone derivatives.
Recently, a novel one-pot multicomponent reaction for the regioselective synthesis of a diverse isoxazoloquinoline (10)-based framework has been designed (Scheme 16). It involves cleavage of the isatin C–N bond, followed by a ring expansion reaction using the environmentally benign p-toluene sulfonic acid as the catalyst. The multicomponent reaction involves isatins, aminouracils and isooxazolones as substrates (Scheme 16). Isoxazoloquinoline is obtained in the desired amount when the nitrogen atom of isatin ring is unsubstituted.27
Scheme 16. Formation of isoxazoloquinolines through one-pot synthesis.
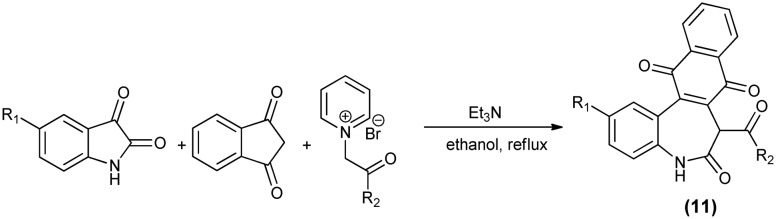
A unique two-carbon ring expansion of the isatin ring to construct the functionalized dibenzo[b,d]azepin-6-one (11) scaffold has been reported (Scheme 17).47N-Substituted pyridinium bromide and indene-1,3-dione are the sources of the two carbon atoms needed in the ring expansion.
Scheme 17. Synthesis of dibenzo[b,d]azepin-6-one scaffold via the two-carbon ring expansion of isatin.
Friedel–Crafts reactions
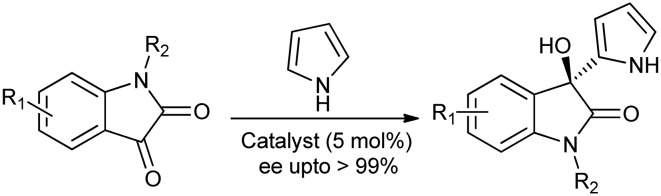
Friedel–Crafts reactions are an important class of organic syntheses used to form highly functionalized aromatic compounds, which can in turn generate important pharmaceutically relevant compounds.48,49 The asymmetric Friedel–Crafts alkylation of isatin with electron-rich aromatic compounds gives the biologically interesting and optically active 3-aryl-3-hydroxy-2-oxindoles. Franz and co-workers reported the first and only successful asymmetric Friedel–Crafts alkylation of isatins with pyrroles to give oxindoles.50 Further, in order to improve the enantioselectivity of the oxindoles formed, Wang and co-workers used a tridentate Schiff base/Cu as a catalyst and hexafluoroisopropanol as a crucial additive agent (Scheme 18).51
Scheme 18. Friedel–Crafts alkylation of isatins with pyrroles to give oxindoles.
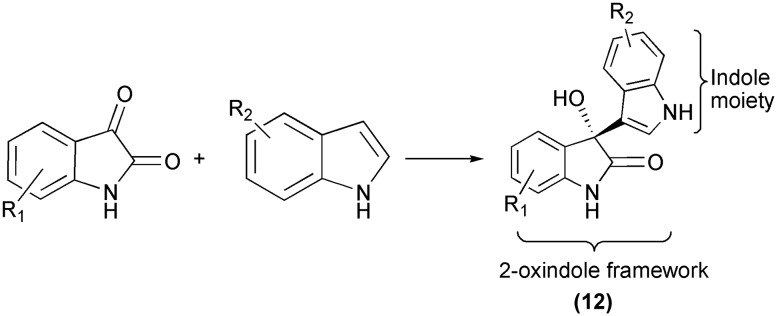
In another reaction (Scheme 19), enantioselective 2-oxindoles (12) were synthesized via the Friedel–Crafts alkylation of isatins with indoles using cuprine as the catalyst. The resulting molecules have great potential for biological activities.52 Hence, Friedel–Crafts reactions of isatins prove to be important synthetic pathways.
Scheme 19. Friedel–Crafts alkylation of isatins with indoles to give 2-oxindoles.
Aldol reactions
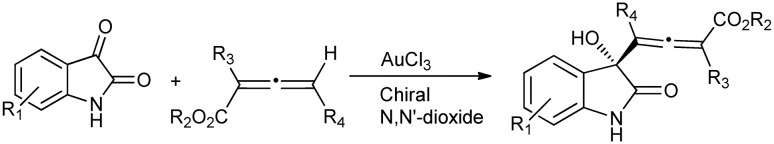
Aldol reactions produce β-hydroxyl carbonyl compounds, which serve as important intermediates in the synthesis of biologically active derivatives. The strong H-bond acceptor behavior of isatin makes it a good substrate for the condensation reactions. The first diastereospecific and enantioselective alleno-aldol reaction of isatins with allenic esters produces tri- and tetra-substituted carbinol allenoates using a metal complex as the catalyst (Scheme 20).53
Scheme 20. Alleno-aldol condensation of isatins with allenic esters to give carbinol allenoates.
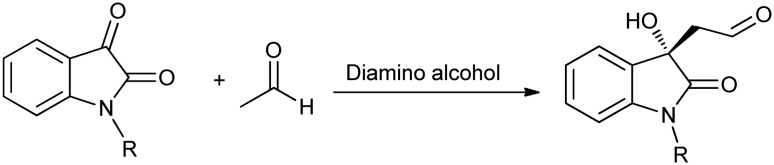
Reddy et al.54 reported cross aldol reactions of isatin and its derivatives with acetaldehyde to form biologically active 3-substituted 3-hydroxyindolin-2-ones (Scheme 21). The reported method offers a rapid, enantioselective, protecting-group-free, and metal-free synthesis of various anti-tumor and anti-viral agents.
Scheme 21. Cross aldol reaction of isatins with acetaldehyde.
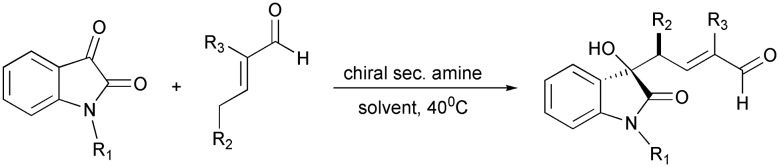
Additionally, isatin and its derivatives also undergo the vinylogous aldol reaction, which produces α,β-unsaturated δ-hydroxy carbonyl compounds, which form components of many natural compounds. The direct vinylogous aldol reaction of isatin and its derivatives with α-substituted α,β-unsaturated aldehydes forms biologically important 3-substituted 3-hydroxyoxindoles (Scheme 22).55
Scheme 22. Vinylogous aldol reaction of isatins with α-substituted α,β-unsaturated aldehydes.
Miscellaneous reactions
Alkylation of isatin is a synthetically viable reaction that uses an alkylating agent, generally an alkyl or aryl halide, in the presence of a base like Cs2CO3 or K2CO3 (Scheme 23). The rate of reaction depends on the reactivity of the alky halide used and thus the reactions with more reactive alkyl halides require less time for completion.56
Scheme 23. Alkylation of isatin in the presence of a base.
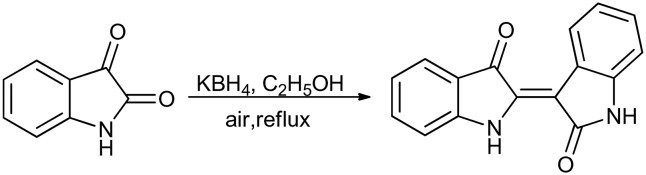
The dimerization reaction of isatin or its derivatives with 3-acetoxyindole in the presence of catalytic Na2CO3 in methanol produces indirubin.57 It is the red component of indigo pigments and a well-known cytotoxic compound. Derivatives of indirubin have been reported as inhibitors of cyclin-dependent kinase 1 (CDK1).58 However, this dimerization approach has been hampered by the limited availability of 3-acetoxyindole and the poor overall yield. To overcome these limitations, in a recently reported study, indirubins were synthesized through dimerization of isatins (1 equiv.) with KBH4 (0.5 equiv.) in ethanol or methanol (Scheme 24).59
Scheme 24. Dimerization of isatin to produce indirubin.
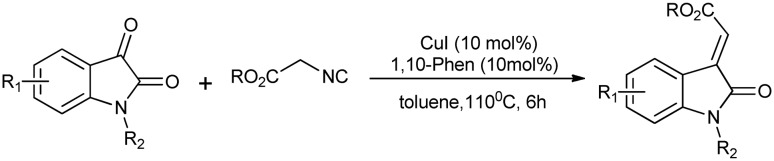
Recently, a novel reaction has been studied that involves isatin and ethyl isocyanoacetate as reactants and Cu(i) as a catalyst. It is 1,3 dipolar cycloaddition that produces an intermediate, which undergoes inverse dipolar reaction to give 3-ylidene oxindoles, which are an important class of compounds (Scheme 25).60
Scheme 25. 1,3-Dipolar/inverse dipolar cycloaddition reaction of isatin with ethyl isocyanoacetate.
Biological applications of isatin
Anti-cancer activity
In the present scenario, cancer has become a fast growing threat across the globe. According to the WHO global health observatory report of 2015, about 8.8 million people worldwide have died from cancer. It is projected that by 2030, there will be ∼26 million new cancer cases worldwide and 17 million cancer deaths per year. Therefore, it is a huge challenge for researchers to develop novel effective anti-cancer agents that offer both selectivity and lower toxicity.
The anti-cancer activity of isatin and its derivatives has been widely explored by researchers. Interestingly, isatin is an important pharmacophore unit in two clinically approved anti-cancer drugs: sunitinib V (13) and toceranib phosphate (14). In this section, we will discuss the in vivo, in vitro, and in silico anti-cancer/anti-tumor activity of several reported isatin derivatives and the different targets available for designing novel isatin-based anti-cancer drugs.
Isatins as histone deacetylase (HDAC) inhibitors
Histone deacetylases (HDACs) are the enzymes that catalyze deacetylation of a specific lysine residue in the histone tails. Literature surveys reveal that HDAC is an attractive target for the development of novel anti-cancer drugs, and HDAC inhibitors represent a promising class of anti-cancer agents.61 A majority of HDAC inhibitors share a common pharmacophore structure: a metal/zinc binding head group (ZBG) that interacts with the Zn2+ ion at the active site of the enzyme, a saturated or unsaturated hydrophobic linker, and a surface recognition group (a cap group) that interacts with the amino acid residues at the surface of the HDAC protein.
In a recent study, 5-substituted isatin capped hydroxamic acid derivatives (15) have been reported as HDAC inhibitors and their anti-proliferative activity against cervical tumor cell lines has been evaluated. SAR showed that halogenation of the parent compound (15a) produced the most active compounds of the series (15c, 15d, and 15e). Out of the halogenated compounds, the 5-chloro-substituted derivative (15d) was found to be the most potent, with IC50 = 0.97 ± 0.26 μM. Furthermore, in order to analyze the binding modes of these compounds, they were docked into the active site of the HDAC enzyme (PDB code: ; 1ZZ1). It was concluded that the isatin moiety acts like a capping group, whereas the hydroxamic acid binds the Zn2+ ion.35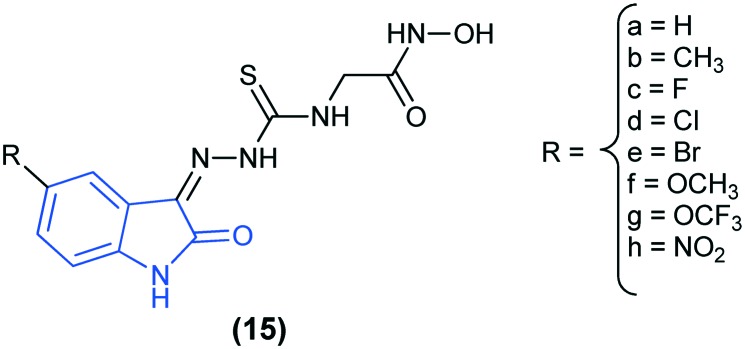
Novel hybrid derivatives, where the N-hydroxybenzamide group and oxindole groups are linked by a triazole moiety or by a methylene linker (16 and 17), have been synthesized. Oxindole is a C-3-substituted isatin derivative known for its vivid biological activities, including anti-cancer activity.62 The compounds were screened against four human cancer cell lines: SW620, PC3, AsPC-1, and NCI-H23. The in vivo inhibitory activity against the HDAC2 enzyme has also been reported, and the results revealed that, except 17a, all the other compounds exhibited promising activity. The introduction of an electron withdrawing or donating group at position 5 or 7 leads to an increase in the inhibitory activity; however, substitution at position 5 is more favorable. The introduction of –Br at position 5 results in the best cytotoxic compound of the series (17d). Furthermore, in order to explore the binding interaction of derivatives 16 and 17 with the HDAC2 enzyme (PDB ID: ; 4LXZ), docking studies were performed. The results showed that these compounds show similar interaction profiles to the bound inhibitor, SAHA.63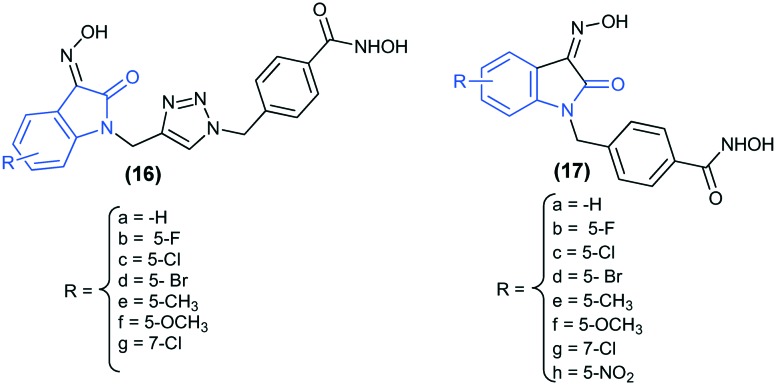
Isatins as carbonic anhydrase inhibitors
Carbonic anhydrases (CAs) belong to a family of metalloenzymes containing Zn2+ ions at the active site of the enzyme. They are involved in pH buffering of extra- and intracellular spaces by catalyzing the rapid reversible hydration of carbon dioxide to the bicarbonate anion and proton (CO2 + H2O ⇌ HCO3– + H+). Recent studies have highlighted the clinical relevance of these enzymes in cancer therapy. Among the sixteen isoforms known in humans (hCAs), two trans-membrane CA isoforms, namely hCA IX and hCA XII, are involved in tumor progression and metastasis formation. Thus, these enzymes are efficient drug targets for the development of new cancer therapeutics against hypoxic tumors.64
Recently, the inhibitory activities of two novel series of 4/3-((4-oxo-5-(2-oxoindolin-3-ylidene)thiazolidin-2-ylidene)amino) benzenesulfonamides (18 and 19) have been reported against four isoforms of CAs (I, II, IV, and IX). The results revealed that all the synthesized compounds effectively inhibit the tumor-associated isoform hCA IX and the physiological domain isoform hCA II. A better selectivity for hCA IX over hCA II was observed for the meta-substituted derivatives (18m). Moreover, docking studies and MM-GBSA calculations predicted the binding modes and Gibbs energies of these inhibitors against the isoforms hCA II (PDB ID: ; 5LJT) and hCA IX (PDB ID: ; 5FL4).65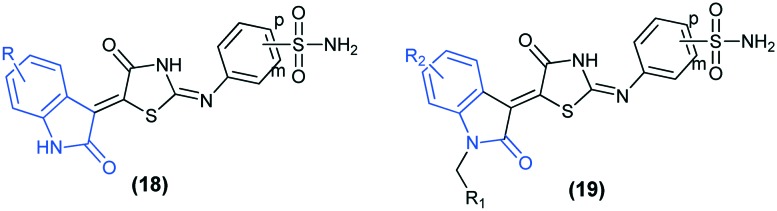
The inhibitory activities of three novel series of isatin-pyrazole-benzenesulfonamide hybrids (20, 21, and 22) were analyzed against four isoforms CAs (I, II, IX, and XII). The introduction of the –NO2 group at position 5 of the isatin moiety led to more selective compounds (21e and 22e), which preferentially inhibited hCA IX (Ki = 15.7 and 7.4 nM) and hCA XII (Ki = 3.7 and 5.4 nM) over hCA I (Ki = 49.5 and 70.4 nM) and hCA II (Ki = 31.3 and 23.1 nM). Furthermore, docking studies of the compounds (21e and 22e) revealed that the 5-NO2 group of the isatin ring can form electrostatic interaction with Asp132 during inhibition of hCA IX and with Lys67/Asp130 in hCA XII and hence shows selectivity against these two isoforms over the others. In addition, the isatin moiety in the two derivatives forms additional H-bond interaction with the hCA XII active site and thus might be responsible for the lower Ki values of these derivatives against hCA XII compared to hCA IX.66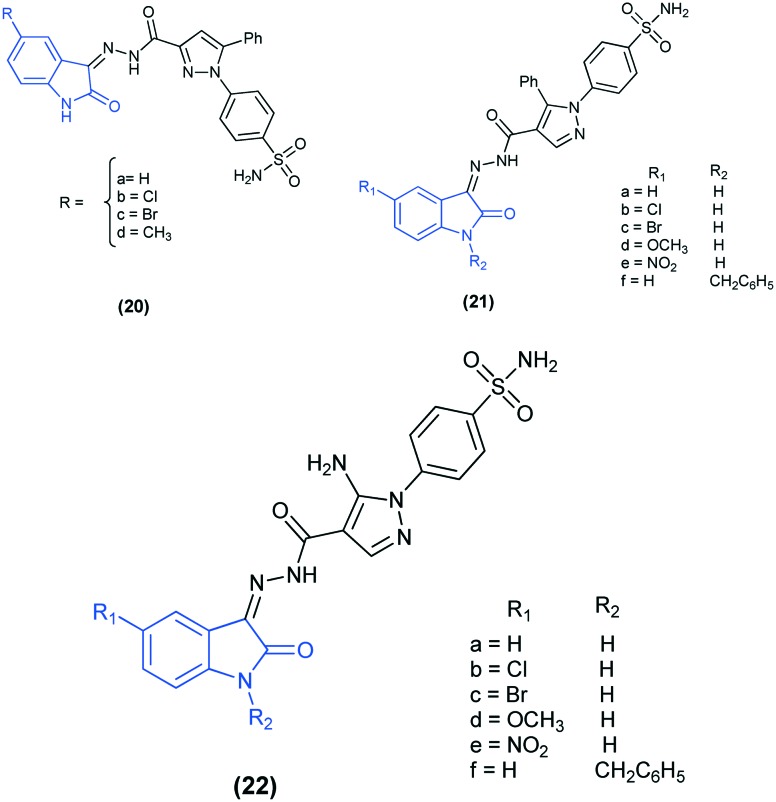
A series of 2/3/4-[(2-oxo-1,2-dihydro-3H-indol-3-ylidene)amino]benzenesulfonamides (23) were evaluated as CA inhibitors and they showed interesting inhibitory profiles against hCA IX and hCA XII isoforms. Among these, the derivative with R1 = CH3, R2 = H, and o-substituted SO2NH2 displayed the highest selectivity for the tumor-associated isozymes hCA IX and hCA XII over the cytosolic off-target isoforms hCA I and hCA II.67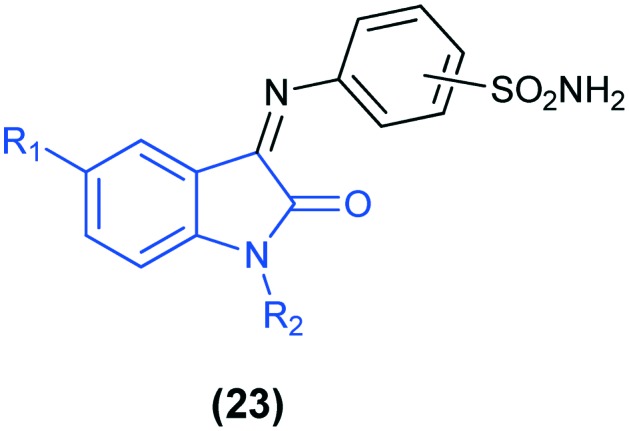
Isatins as epidermal growth factor receptor-tyrosine kinase (EGFR-TK) inhibitors
The epidermal growth factor receptor family of receptor tyrosine kinase is highly expressed in many types of cancers, especially in the breast, colon, and bladder cancers, and thus represents a promising target for the design of new anti-cancer drugs. There are various tyrosine kinase inhibitors which are in clinical trials, such as SU5416 (24) and SU6668 (25), consisting of the isatin scaffold.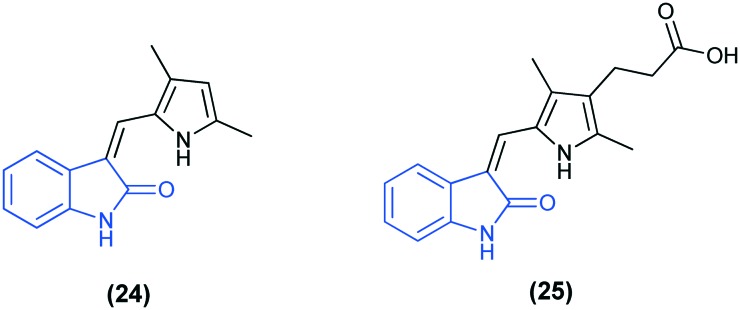
A series of quinazolinone compounds incorporating the isatin moiety (26) were synthesized and their anti-tumor efficacy was assessed against the human breast cancer cell line MDA-MB-231 and the colon cancer cell line LOVO. The results revealed that most of the compounds displayed high activity against the MDA-MB-231 breast cell line. Moreover, compounds 26(d–h), 26j, and 26(l–s) also exhibited strong activity against the LOVO colon cancer cell line (IC50: 9.91–17.87 μM). Among the tested compounds, 26p showed the highest potency toward MDA-MB-231 cells and LOVO cells. The enzyme activity assay for compound 26p showed that at a concentration of 10 μM, it exhibited good selectivity and efficient inhibitory effect against EGFR-TK and induced apoptosis in MDA-MB-231 cells. Furthermore, molecular docking simulations of the binding site of the EGFR kinase (PDB code: ; 1M17) found that the most active compound 26p showed a similar binding mode as shown by the bound inhibitor erlotinib. The quinazoline ring of 26p typically overlaps with the corresponding ring of erlotinib without clashing with the surrounding amino acids, while the weakly active compound 26m binds in a different manner.68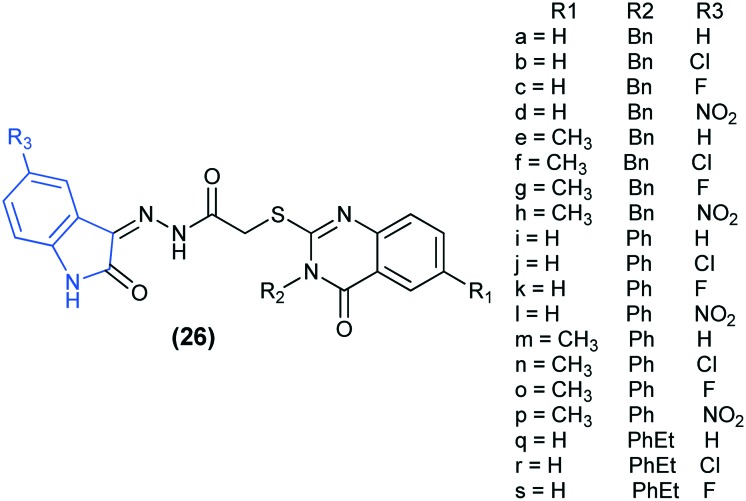
In another study, the anti-cancer activity, in silico docking, and virtual ADME studies for a series of isatin analogs (27) have been reported. Among the tested compounds, four compounds (27b, 27c, 27e, and 27f) displayed demonstrative anti-proliferative effects on the MCF-7 breast cancer cell line. SAR studies highlighted the importance of electron withdrawing halogen groups and the combination of an electron withdrawing group with a hydrophobic electron releasing methyl group in improving the activity. All the synthesized compounds were docked into the ATP-binding site of EGFR-TKs (PDB ID: ; 1M17). Based on the docking results, compound 27f emerged as the most active compound. The isatin moiety of 27f interacts with multiple amino acid residues, Met769, Leu820, Leu764, Ala719, Lys721, Thr766, Thr830, and Gly722. In addition, a strong H-bond interaction was found between the C
O group of the isatin ring and the NH group of residue Met793.69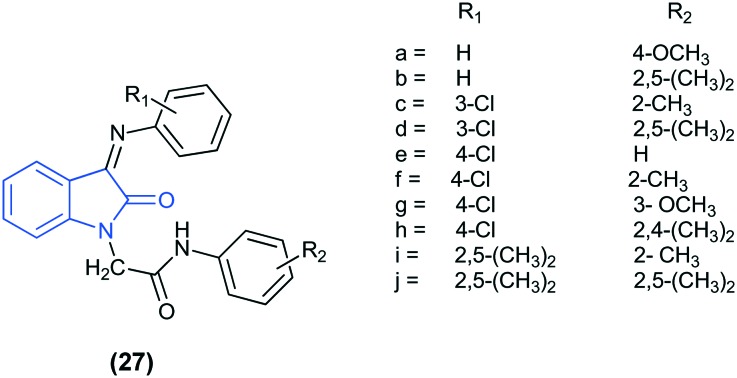
Isatins as tubulin inhibitors
Microtubules, the key cytoskeletal filaments, play an important role in many cellular processes, such as in maintaining the cell structure and shape, together with mitosis and cell division. A variety of antimitotic agents interfere with the dynamics of microtubules by targeting tubulin, which is a major protein component of microtubules and hence is one of the most important strategic targets for developing novel anti-cancer drugs. Six distinct families of tubulin have been identified so far: α-, β-, γ-, δ-, ε-, and ζ-tubulin. However, microtubules only consist of α- and β-tubulin, and hence designing inhibitors for them can help in cancer therapy.70,71
Recently, triazole tethered isatin–coumarin molecular hybrids (28) have been reported as novel anti-tubulin agents. The unsubstituted isatin ring (R = H) showed the highest cytotoxic effects, and the overall preference order for R and n (chain length) is: H > F > Cl > Br > I > NO2 > OCH3 and 1 > 2 > 3 > 4, respectively. Compound 28a (R = H; n = 1) emerged as the most potent anti-tubulin agent, followed by 28b (R = F; n = 1) and 28a (R = H; n = 2). The results thus support the fact that these hybrid molecules exert their cytotoxic effects through tubulin inhibition. The docking study of the most active compound 28a (n = 1) revealed that it is well incorporated at the interface of the β1 and α2 subunits of tubulin (PDB ID: ; 1SA0) and is stabilized by polar and van der Waals interactions. The isatin moiety lies in the hydrophobic cavity formed by residues Val177β1, Pro222β1, Thr223β1 and Thr224β1.72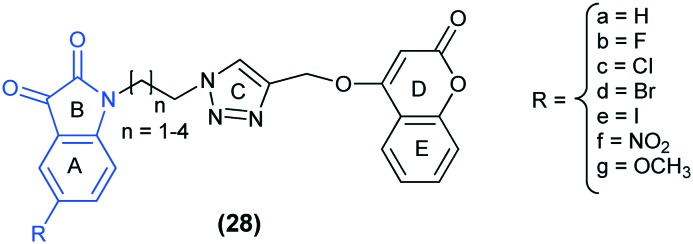
A series of C-3-substituted isatin derivatives, viz. (Z)-3-((2-(phenylamino)pyridin-3-yl)methylene)indolin-2-one (29), were evaluated for their anti-proliferative and anti-tubulin activity. The SAR study revealed that the compounds possessing electron withdrawing substituents on rings A and D and electron donating substituents on ring B showed better cytotoxic activity. Also, it was found that the compounds bearing halides at the 5th position exhibit better cytotoxic activity as compared to compounds having halides at the 6th position. The molecular docking study of 29r and 29y at the colchicine binding site of tubulin (PDB ID: ; 3HKC) highlighted that these compounds fit well at the α–β interface of the protein.73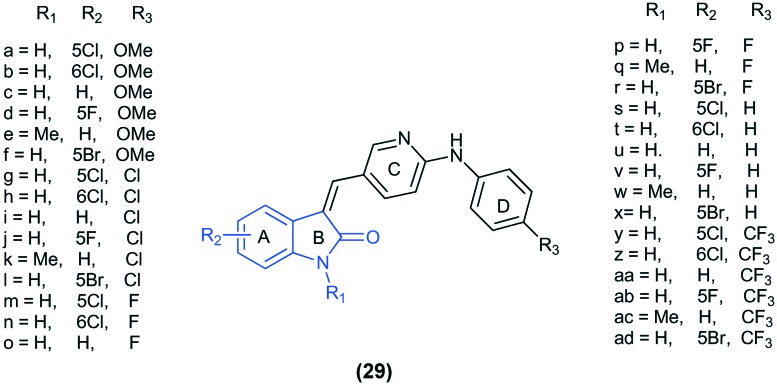
The 5,7-dibromoisatin analogs (30) have been evaluated as dual inhibitors of tubulin polymerization and the Akt pathway. Among these, compounds 30d and 30e inhibited tubulin polymerization to the same extent as the anti-cancer drug vinblastine sulfate, whereas significantly better activity than vinblastine sulfate has been reported for compounds 30j and 30l.34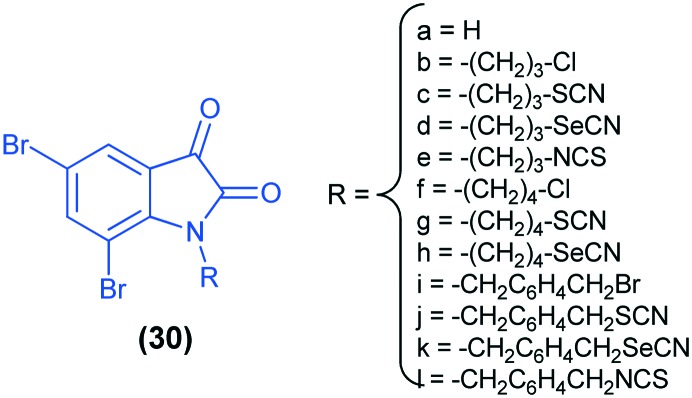
Recent studies
Isatin analogues bearing an α,β-unsaturated ketone moiety (31 and 32) have been reported as potential anti-cancer compounds. Their SAR and QSAR analyses confirmed that the inhibition ability is correlated with the electron-donating substituents on the benzyl ring and with the α,β-unsaturated ketone.74 1H-1,2,3-Triazole-linked isatin–ferrocene (33), ferrocenylmethoxy–isatin (34), and isatin–ferrocenyl–chalcone (35) conjugates have been evaluated for their anti-proliferative activities against MCF-7 and MDA-MB-23. Of all the tested conjugates, isatin–ferrocene proved to be the most potent against MCF-7.75 A series of 1H-1,2,3-triazol-4-yl tethered 3-pyrrolylisatins (36) have been evaluated for anti-breast cancer activity. Docking studies of the most active compound of the series revealed that it fits well into the ATP pocket of topoisomerase II and forms four hydrogen bonds with Ile141, Thr147, Ser148 and Ser149.76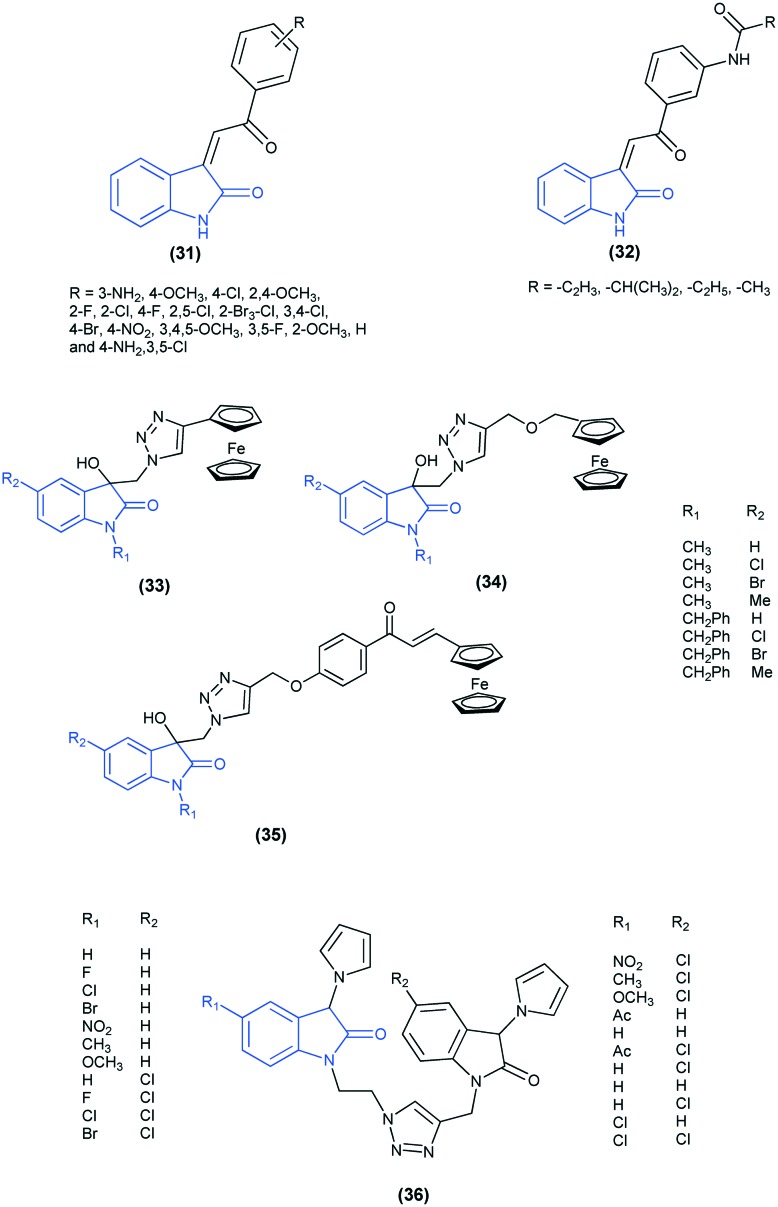
Anti-bacterial activity
Isatin derivatives display therapeutic potential against a variety of pathogenic microbes and are being widely explored by researchers for anti-bacterial activity. In several studies, Schiff bases and Mannich bases of isatin and its derivatives were reported to exhibit significant anti-bacterial activity.77–79 SAR studies of various isatin derivatives revealed that 5-halogenation, N-alkylation, and N-Mannich bases are also effective in causing marked enhancement in the anti-bacterial activity.
A series of Schiff bases of isatin (37 and 38) were synthesized and screened for their in vitro anti-bacterial and anti-fungal activities against Gram positive (Staphylococcus aureus and Bacillus subtilis) and Gram negative (Escherichia coli and Proteus vulgaris) bacteria and fungi (Candida albicans and Aspergillus niger). Results revealed that these compounds showed significant anti-bacterial activity against B. subtilis, S. aureus and E. coli. In addition to the anti-bacterial activity, compound 38f showed significant anti-fungal activity comparable to the standard drug clotrimazole.80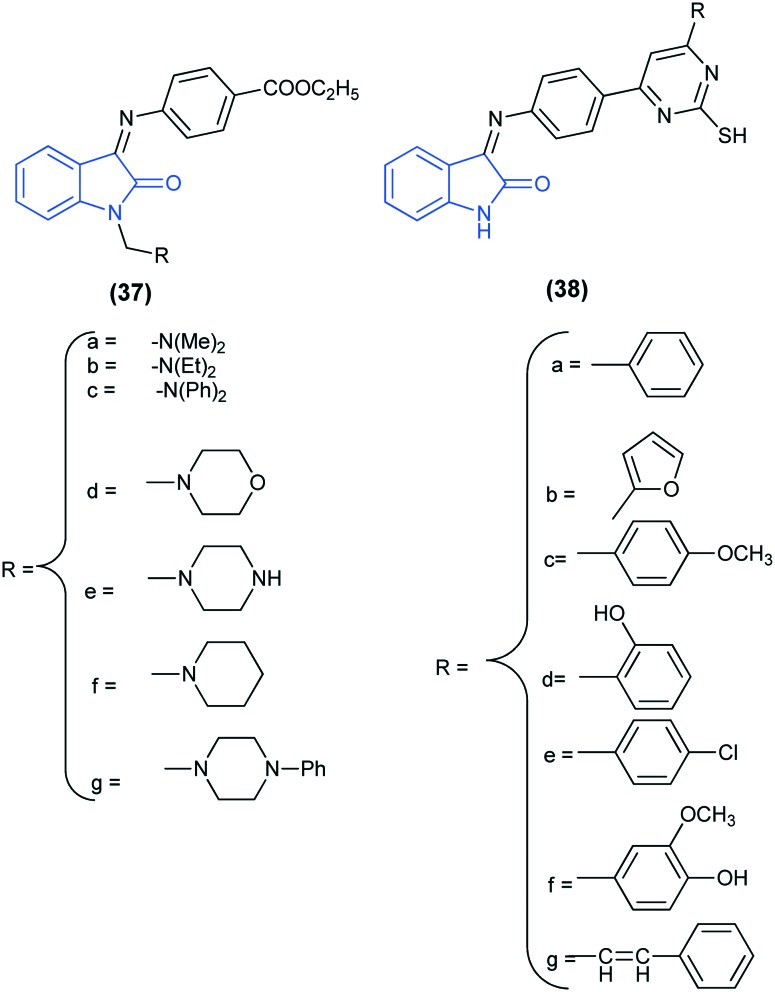
In another work, seven isatin derivatives (39) were tested against E. coli, P. aeruginosa, B. subtilis and S. aureus, taking kanamycin and chloromycetin as the standard drugs. Results showed that the compounds 39c (R = 3-Cl) and 39d (R = 3-Br) exhibited better anti-bacterial activities against S. aureus, whereas compound 39g (R = 3-F) exhibited better anti-bacterial activity against E. coli and P. aeruginosa. The compounds were docked into the active site of filamentous temperature-sensitive protein Z (FtsZ) (PDB code: ; 2VAM, ; 2VAW, 3SVO8, ; 1S1S), which is a major prokaryotic cell division protein that serves as an important component in bacterial cell division, and its inhibition can restrain bacterial cellular fission. Docking studies revealed that there are mainly two types of interactions, hydrogen bond and π–cation interactions that exist between FtsZ and compound 39.81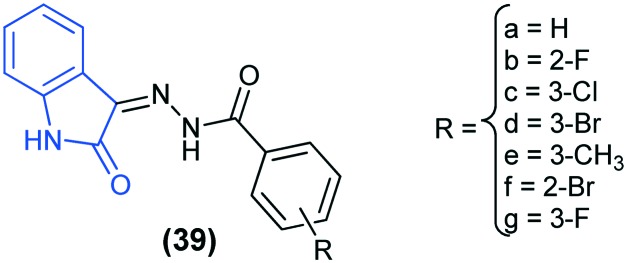
Various rhodanine and furan derivatives have been reported to have promising anti-bacterial activities.82,83 Therefore, combination of these pharmacophores with isatin can develop new active molecules. With this view, Baharfar et al.84 reported the anti-bacterial activity of novel 5-isatinylidenerhodanine-based furan derivatives. The results showed that the compounds were effective against bacterial growth retardation activity for E. coli.
Anti-diabetic activity
Diabetes mellitus (DM), commonly referred to as diabetes, is a syndrome characterized by disordered metabolism and inappropriately high blood sugar (hyperglycemia) resulting either from low levels of the insulin hormone or from abnormal resistance to insulin's effect.85
The anti-diabetic activity of the novel compound 1-(4-(dimethylamino)benzylidene)-5-(2-oxoindolin-3-ylidene)-thiocarbohydrazone (40) has been reported. Administration of the compound with a single dose of 50 and 100 mg kg–1 to diabetic rats showed a significant reduction in the blood glucose levels in a dose dependent manner.86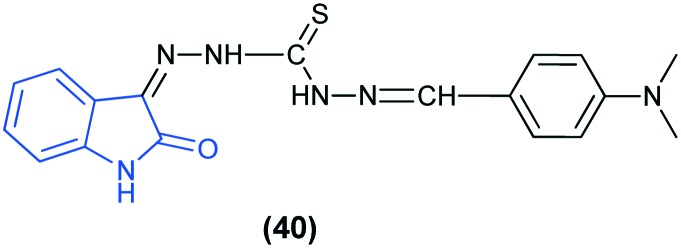
Type 2 diabetes is a more common form of diabetes and accounts for around 90% of all cases worldwide. α-Glucosidase, a carbohydrate enzyme secreted from the intestinal chorionic epithelium, is a therapeutic target for type 2 diabetes.87 A number of chromone88,89 and isatin90 derivatives have been reported as α-glucosidase inhibitors. Therefore, combination of these two scaffolds in a single molecule can result in improved pharmacological activity. In this direction, a novel series of chromone–isatin derivatives (41) were synthesized and screened to evaluate their in vitro α-glucosidase inhibitory activity. All the compounds exhibited excellent inhibitory activity, but compound 41j, with a hydroxyl group at the 7-position of chromone and a 4-bromobenzyl group at the N1-position of isatin, was the most active. Furthermore, to understand the binding interaction of these compounds with α-glucosidase, a molecular docking study was performed.91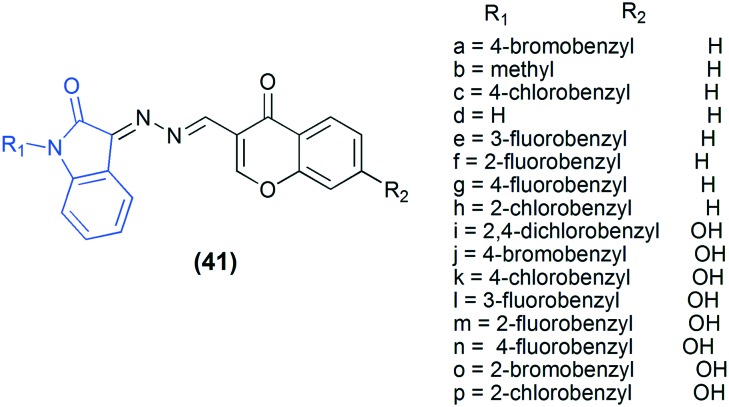
Anti-convulsant activity
Epilepsy or convulsion is the most common neurological disorder characterized by frequent unprovoked seizures. It usually begins during childhood and happens due to some sort of electrical activity mishap in the brain.92 The commonly used drugs for the treatment of epilepsy are vigabatrin, zonisamide, topiramate, etc. The available antiepileptic drugs (AEDs) show various side effects, such as ataxia, drowsiness, gingival hyperplasia, gastrointestinal disturbances, and megaloblastic anemia, and hence new anti-convulsants with less toxic effects are required.93,94
The conformational analysis of the well-known AEDs revealed that the essential structural features responsible for interaction with the receptor sites are a hydrogen acceptor/donor unit (HAD), one electron donor atom (D) and a hydrophobic domain (A) (aryl ring substituted/unsubstituted).95,96 Saravanan et al.97 reported the anti-convulsant activity of novel 1-(morpholinomethyl)-3-substituted isatin derivatives (42 and 43). Among the synthesized compounds, isoxazole-substituted isatin derivatives (43) exhibit better activity than the corresponding acryloylphenylimino-substituted isatin derivatives (42). The increase in the activity may be due to the presence of an extra electron donor atom (D) on the isoxazole ring, which might be responsible for additional bonding with the binding site. In addition, the compounds bearing electron donating groups showed better activity in comparison with compounds having electron withdrawing groups and therefore, 43f emerged as the most active compound.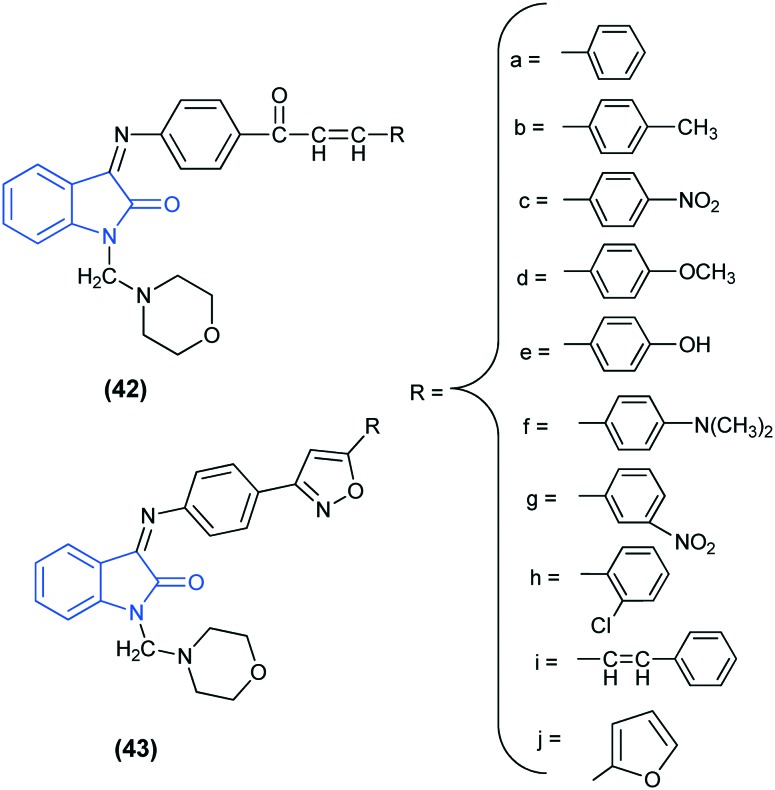
Isatin-1-N-phenylacetamide derivatives (44) were synthesized using isatin as the starting compound. The compounds were screened for their in vivo anti-convulsant activity against maximal electroshock seizure (MES) and evaluated for their neurotoxicity by the rotarod test. It was found that the compounds substituted with electron donating groups at the ortho-position have better anti-convulsant activity than the para- and meta-substituted ones. For the ortho-halogen substituted derivatives, the order for the anti-convulsant activity was found to be –F > –Cl > –Br. Among the series, compound 44b has the maximum activity, with a median effective dose (ED50) of 91.3 mg kg–1 and a median toxic dose (TD50) of >1000 mg kg–1.98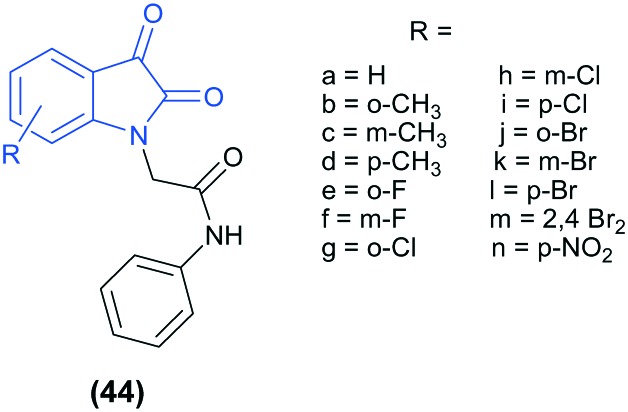
Anti-tuberculosis activity
Tuberculosis (TB), which is caused mainly by Mycobacterium tuberculosis (MTB), is a deadly disease and appears as the second leading cause of mortality due to infectious diseases worldwide. Unfortunately, due to the increasing cases of multi-drug-resistant TB (MDR-TB) and extensively drug-resistant TB, the currently available drugs are becoming less effective. Therefore, there is an urgent need to develop novel anti-TB drugs that can show activity against its hard-to-kill MDR and other latent forms.
In recent years, the isatin scaffold has provided a boost in the discovery of a number of novel anti-tubercular agents. A number of isatin derivatives have been reported to show significant anti-TB activity.99,100 Recently, Hu et al.101 reported the synthesis and in vitro anti-mycobacterial activity of a series of 1H-1,2,3-triazole-tethered ciprofloxacin (CPFX) isatin conjugates (45) against Mycobacterium smegmatis and Mycobacterium tuberculosis (MTB) H37Rv. The preliminary results showed that all hybrids exhibited considerable activity against M. smegmatis and displayed excellent activity against the MTB H37Rv strain. Also, the hybrids having the carbonyl group (44a–d) were more active than the corresponding methyloxime hybrids (45e–h) against MTB H37Rv.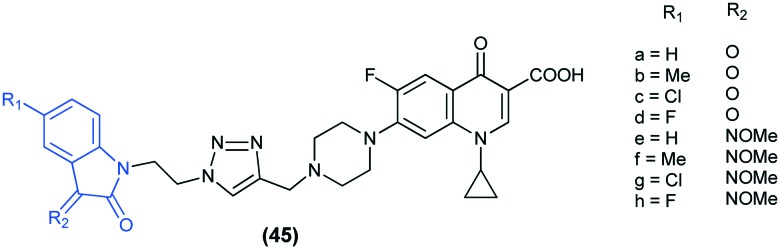
In another study, a novel series of ethylene tethered isatin–coumarin hybrids (46) were synthesized and evaluated for their in vitro anti-mycobacterial activities against MTB H37Rv and MDR-TB. Results showed that all the analogs displayed potential anti-mycobacterial activities against the two strains. The hybrids having 5-F substitution at R1 (46f–j) were more potent than the corresponding unsubstituted analogs (46a–e). Furthermore, substituents at the C-3 position of the isatin motif also have a great influence on the activity, which follows the order: NOMe > NNHCSNH2 > O > NOEt > NOH.102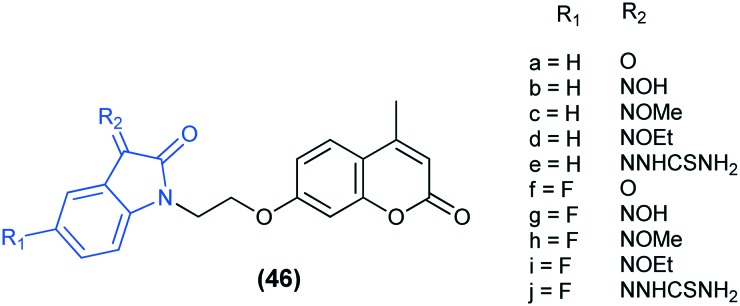
Docking studies of isatin–quinoline hybrids (47) as potential anti-tubercular agents against the enoyl-ACP reductase enzyme (PDB ID: ; 4TZK) have been reported. The results revealed that the compound 47h fits well in the active site of the enzyme and has the highest binding affinity. This compound shows strong hydrophobic interaction with Tyr158 and the cofactor NAD 500, which are the pivotal residues responsible for the enzyme activity.103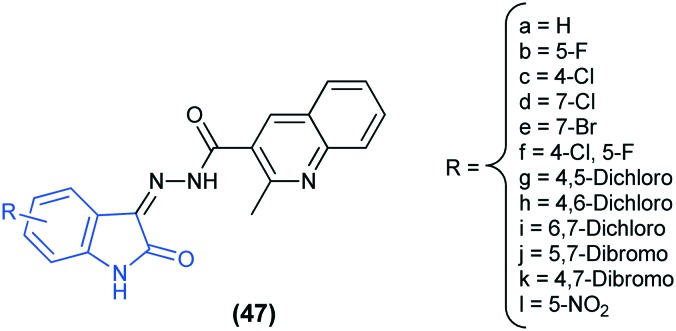
Anti-viral activity
HIV-1 (human immunodeficiency virus type 1) is the most widespread type of virus. The current approved treatment for this infection is based on the highly active anti-retroviral therapy (HAART), which is a combination of many antiviral agents, targeting different steps of the virus replication cycle.104 HIV-reverse transcriptase (HIV-RT) plays a key role in the replication cycle of HIV-1. It is associated with dual important enzymatic activities, namely DNA/RNA dependent polymerase (DDDP and RDDP) and ribonuclease H (RNase H). Hence, designing inhibitors for HIV-RT can help in treating the dual targets. Isatin-based molecular hybrids have been reported as dual inhibitors of RT-associated enzymatic functions.105,106 In a recent study, isatin–thiazoline hybrids (48) have been reported as dual inhibitors of HIV-1 reverse transcriptase (RT).107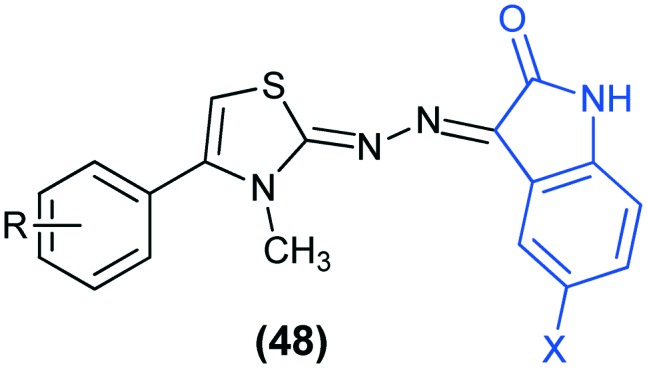
Isatin derivatives are known to show anti-viral activity against various other viruses such as coxsackievirus B3 (CVB3), which is an enterovirus of the Picornaviridae family. It is a primary agent for causing viral myocarditis, particularly in children and young adolescents.108 Four isatin derivatives (49a–c, 50) were synthesized and screened for their anti-CVB3 activity and compound 49a was found to be the most potent anti-CVB3 agent.109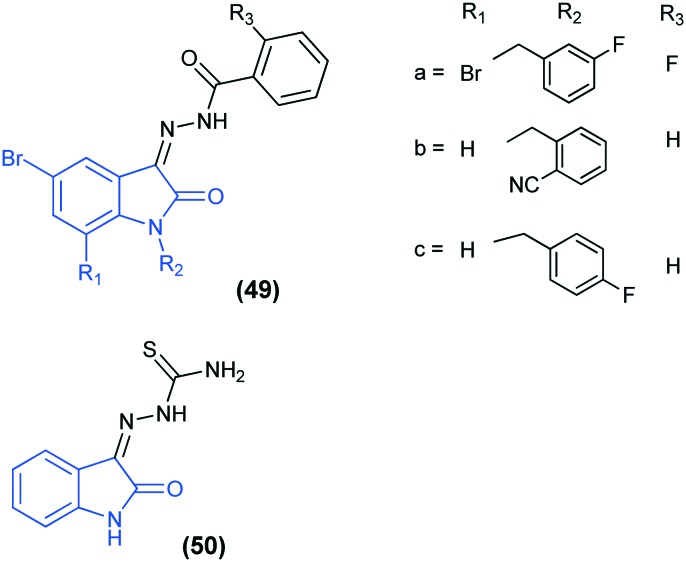
In another study, the anti-viral activities of isatin-β-thiosemicarbazone hybrids with an imidazole derivative against the chikungunya virus (CHIKV) have been reported. In this investigation, 1-[{1-[(2-methylbenzimidazol-1-yl)methyl]-2-oxo-indolin-3-ylidene}amino]thiourea (MBZM-N-IBT) (51) emerged as a potent anti-viral molecule against CHIKV.110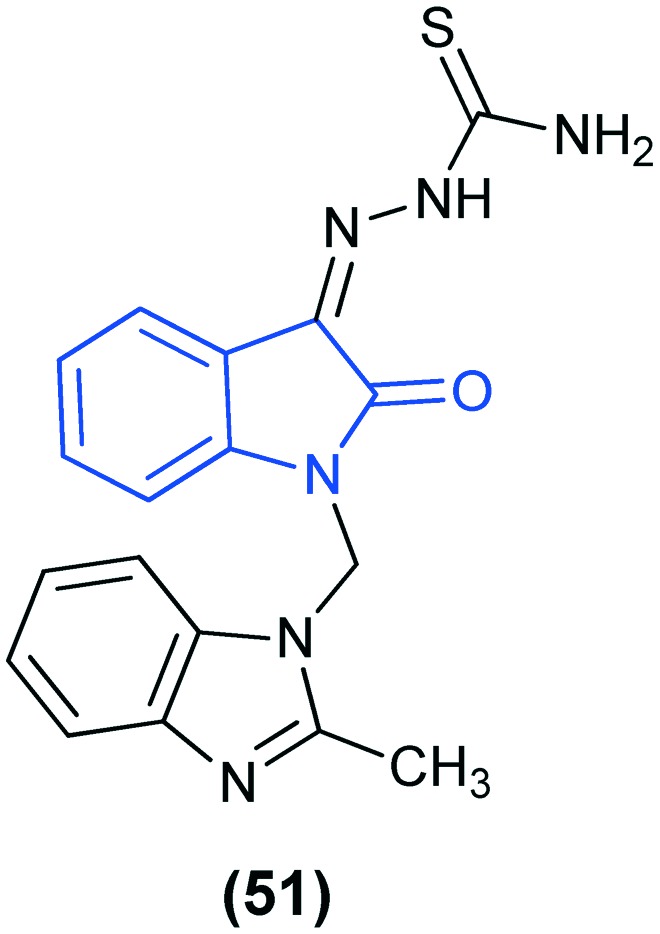
The anti-viral activities of isatin derivatives against the pox virus,111 vaccinia virus,112 rhinovirus,113 Moloney leukemia virus,114 and SARS virus115,116 have also been reported.
Industrial applications
Isatin as a corrosion inhibitor
The corrosion of iron and steel is a fundamental industrial problem. Organic compounds containing heteroatoms like N, O, and S have been reported as effective inhibitors against the corrosion of steel in acidic media.117,118 Isatin derivatives and their Mannich bases have been reported as efficient inhibitors against aluminum and steel corrosion.119,120
The corrosion inhibition efficiency of three newly synthesized isatin derivatives (52a–c) on carbon steel in hydrochloric acid medium has been evaluated. The inhibition performance of these derivatives increased with the increase in concentration. In HCl solution, the compound 52a easily became protonated to form a cation-ionic form and adsorbed at the metal surface through the already adsorbed chloride ions.121 Several N-substituted isatin-based thiosemicarbazones (PITSc) (53) have been reported to show effective corrosion inhibition for the steel surface in 1 M HCl. Also in this case, the corrosion efficiency increased with the increase in concentration of the inhibitor (PITSc).122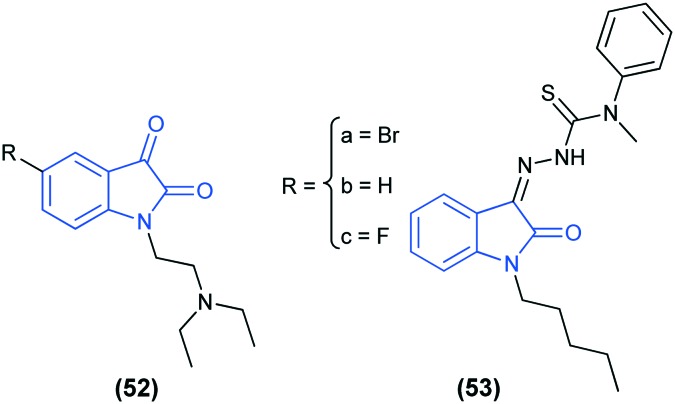
Fluorescent sensor/probe
Detection and quantification of metal ions in biological systems and in the environment is one of the most intense fields of research for chemists. In particular, heavy metal ions such as Hg(ii), Cd(ii) and Pb(ii) are highly toxic pollutants, and therefore, their rapid detection is crucial for the environment, public health and food safety. Recently, isatin-functionalized nanoporous SBA-15 has been reported as a selective fluorescent probe for the detection of Hg(ii) in water. SBA-15 showed a high selectivity for Hg(ii) over a variety of metal ions in aqueous media and was able to detect Hg(ii) in a wide pH range from 4 to 10 and had a detection limit of 3.7 × 10–6 M.123 In another study, a new fluorescent probe based on an isatin–rhodamine hybrid (54) specifically recognized Hg(ii) in an ethanol–Tris buffer medium over various transition metals and heavy metal ions.124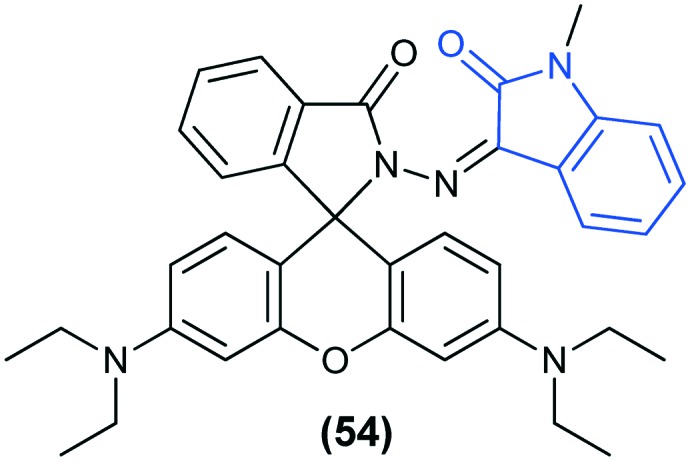
Apart from Hg(ii) detection, an isatin-based Schiff base sensor (55) has been designed for the detection of Cu2+ and S2– in human blood serum, with limits of detection in the range of 2–3 μM and 7–9 μM, respectively.125 Goswami et al.126 reported a rhodamine–isatin-based sensor (RIH) (56) for the detection of Al3+ in aqueous medium. The complex formed (RIH-Al3+) further works as a fluorescence chemo-sensor for the detection of the pyrophosphate anion (P2O74–).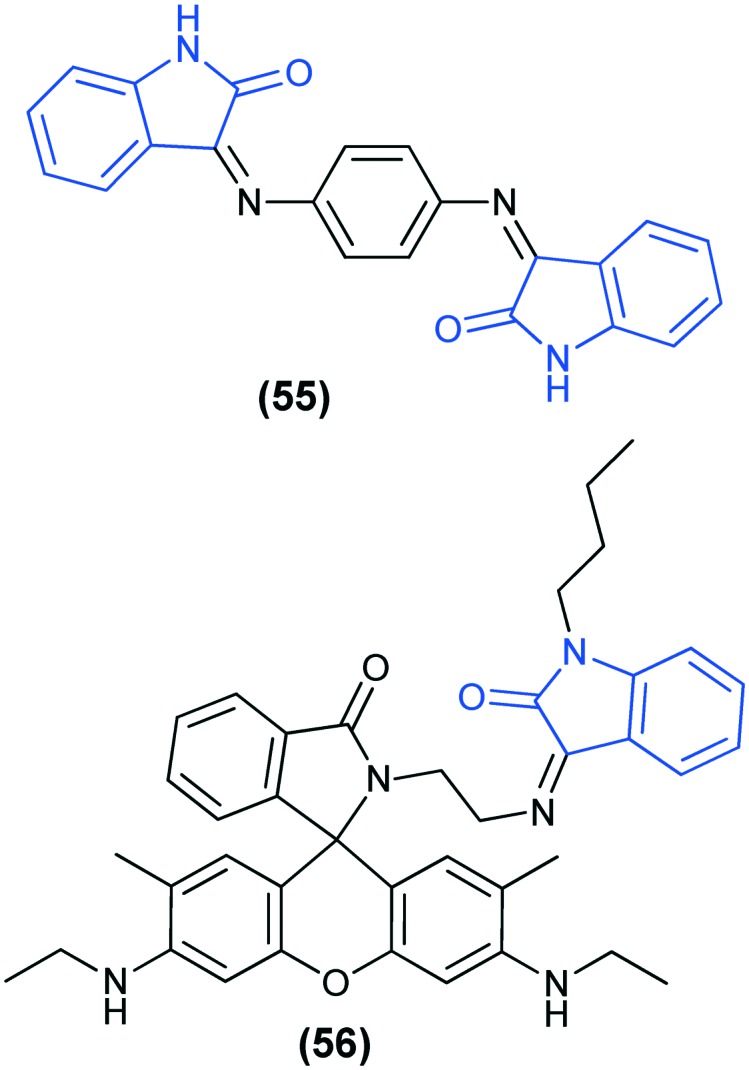
Dyes
Dyes are widely used as coloring agents in the cotton and textile industries and also play an important role in modern electronics. Isatin and its derivatives like indigo (57) (C-2-substituted derivative) and isoindigo (58) (C-3-substituted derivative) are versatile natural dyes. Indigo, probably the oldest and the most famous dye, has been used in the textile industry as a vat dye.127 Indigo and its various derivatives like tyrian purple, indigo carmine and indirubin have applications in field effect transistors due to their electron accepting properties and are also used as food colorants in the food industry.128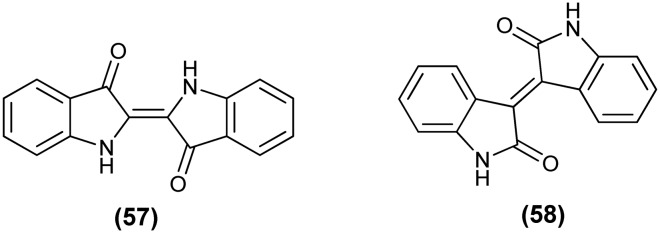
Isoindigo, an isomer of indigo, has been used for the preparation of electro-active materials for organic electronics.129 Incorporation of isoindigo into a π-conjugated polymer scaffold produces materials which are used in the field of organic photovoltaics.130 In this regard, pyrazine-fused isoindigo dye has been reported as a promising building block for construction of donor–acceptor conjugated polymers for optoelectronic applications.131
Conclusions
Isatins continue to be one of the most researched areas in synthetic and medicinal chemistry. Owing to the medicinal use associated with isatin derivatives, organic chemists are coming up with several novel and green ways of synthesizing them. In fact, the chemical reactions shown by isatins are also very crucial, since these reactions in turn produce derivatives such as 2-quinolones, isatoic anhydride, and 2-oxindoles, which are biologically potent. These reactions could be explored further for designing such novel derivatives. Isatin can also be explored further for industrial use, especially as a fluorescent probe. It has been reported as a selective fluorescent probe for the detection of Hg(ii) in water. The novel isatins exhibit promising activities like anti-cancer, anti-viral, anti-convulsant, anti-bacterial, anti-diabetic, anti-TB, etc. All these properties make isatin an important nucleus and open new avenues for future research.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
Authors Varun and Sonam acknowledge financial assistance in the form of Junior Research Fellowships from the Council of Scientific and Industrial Research (CSIR) and the University Grants Commission (UGC), respectively.
Biographies

Varun
Varun received his B.Sc. degree in Chemistry from the University of Delhi in 2015. After obtaining his M.Sc. degree in Physical Chemistry from the University of Delhi in 2017, he started his research work under the guidance of Prof. Rita Kakkar in the Department of Chemistry, University of Delhi. His research interests focus on the designing of novel inhibitors for the human P-glycoprotein.

Sonam
Sonam received her B.Sc. degree in Chemistry from the University of Delhi in 2013. After obtaining her M.Sc. degree in Physical Chemistry from the University of Delhi in 2016, she started her Ph.D. under the guidance of Prof. Rita Kakkar in the Department of Chemistry, University of Delhi. Her research work focuses on the designing of inhibitors for MurB enzymes.

Rita Kakkar
After obtaining a PhD degree in Physical Chemistry from the University of Delhi, Professor Rita Kakkar undertook research on various topics. She has been teaching physical chemistry at the University of Delhi for the past 33 years. She has authored a textbook on Spectroscopy published by Cambridge University Press. Her main research interests are in Computational Chemistry and related fields. She heads a large research group, which is carrying out computational and experimental studies on catalysis by nanomaterials and by enzymes. The focus of the research on nanomaterials is to understand the growth, morphologies and stabilities of nanocrystalline forms of metals and metal oxides, their reactivities, and their catalytic role in various reactions, particularly those involving degradation of organic pollutants. Her research on nanoscale materials also includes theoretical and experimental studies on quantum dots and their size-dependent properties for use as semiconductor devices and sensors. The other area of research is the use of in silico techniques to understand enzyme catalysis and to design enzyme inhibitors. In addition, her research group investigates potential energy surfaces and reaction paths of important organic reactions and studies the role of catalysts in these reactions. Professor Rita Kakkar has over 133 research publications in international journals and chapters in books. She has successfully supervised the work of 40 PhD students. She has delivered invited talks at several scientific conferences and has acted as an International Advisory Member in several of these.
References
- Guzman-Perez A., Webster R. T., Allen M. C., Brown J. A., Buchholz A. R., Cook E. R., Day W. W., Hamanaka E. S., Kennedy S. P., Knight D. R., Kowalczyk P. J., Marala R. B., Mularski C. J., Novomisle W. A., Ruggeri R. B., Tracy W. R., Hill R. J. Bioorg. Med. Chem. Lett. 2001;11:803–807. doi: 10.1016/s0960-894x(01)00059-2. [DOI] [PubMed] [Google Scholar]
- Amr A. E. G. E., Abdel-Latif N. A., Abdalla M. M. Bioorg. Med. Chem. 2006;14:373–384. doi: 10.1016/j.bmc.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Saliva J. F. M., Garden S. J., Pinto A. C. J. Braz. Chem. Soc. 2001;12:273–324. [Google Scholar]
- Medvedev A., Buneeva O., Gnedenko O., Fedchenko V., Medvedeva M., Ivanov Y., Glover V. and Sandler M., in Oxidative Stress and Neuroprotection, Springer, Vienna, 2006, pp. 97–103. [DOI] [PubMed] [Google Scholar]
- Andreani A., Burnelli S., Granaiola M., Leoni A., Locatelli A., Morigi R., Rambaldi M., Varoli L., Cremonini M. A., Placucci G., Cervellati R. Eur. J. Med. Chem. 2010;45:1374–1378. doi: 10.1016/j.ejmech.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Khan K. M., Mughal U. R., Khan A., Naz F., Perveen S., Choudhary M. I. Lett. Drug Des. Discovery. 2010;7:188–193. [Google Scholar]
- Chiyanzu I., Clarkson C., Smith P. J., Lehman J., Gut J., Rosenthal P. J., Chibale K. Bioorg. Med. Chem. 2005;13:3249–3261. doi: 10.1016/j.bmc.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Sharma P. K., Balwani S., Mathur D., Malhotra S., Singh B. K., Prasad A. K., Len C., Eycken E. V., Ghosh B., Richards N. G., Parmar V. S. J. Enzyme Inhib. Med. Chem. 2016;31:1520–1526. doi: 10.3109/14756366.2016.1151015. [DOI] [PubMed] [Google Scholar]
- Prakash C. R., Raja S., Saravanan G. Chin. Chem. Lett. 2012;23:541–544. [Google Scholar]
- Medvedev A., Igosheva N., Crumeyrolle-Arias M., Glover V. Stress. 2005;8(3):175–183. doi: 10.1080/10253890500342321. [DOI] [PubMed] [Google Scholar]
- Grewal A. S. Int. J. Pharm. Res. 2014;6(17):4. [Google Scholar]
- Bhrigu B., Pathak D., Siddiqui N., Alam M. S., Ahsan W. Int. J. Pharm. Sci. Drug Res. 2010;2(4):229–235. [Google Scholar]
- Vine K. L., Matesic L., Locke J. M., Ranson M., Skropeta D. Anti-Cancer Agents Med. Chem. 2009;9:397–414. doi: 10.2174/1871520610909040397. [DOI] [PubMed] [Google Scholar]
- Sandmeyer T. Helv. Chim. Acta. 1919;2:234–242. [Google Scholar]
- Eldehna W. M., Abo-Ashour M. F., Nocentini A., El-Haggar R. S., Bua S., Bonardi A., Al-Rashood S. T., Hassan G. S., Gratteri P., Abdel-Aziz H. A., Supuran C. T. Eur. J. Med. Chem. 2018;162:147–160. doi: 10.1016/j.ejmech.2018.10.068. [DOI] [PubMed] [Google Scholar]
- Reddy M. R., Rao N. N., Ramakrishna K., Meshram H. M. Tetrahedron Lett. 2014;55(34):4758–4762. [Google Scholar]
- Zhang C., Li S., Bures F., Lee R., Ye X., Jiang Z. ACS Catal. 2016;6:6853–6860. [Google Scholar]
- Satish G., Polu A., Ramar T., Ilangovan A. J. Org. Chem. 2015;80:5167–5175. doi: 10.1021/acs.joc.5b00581. [DOI] [PubMed] [Google Scholar]
- Wen S. S., Zhou Z. F., Xiao J. A., Li J., Xiang H., Yang H. New J. Chem. 2017;41:11503–11506. [Google Scholar]
- Wetzel A., Gagosz F. Angew. Chem., Int. Ed. 2011;50:7354–7358. doi: 10.1002/anie.201102707. [DOI] [PubMed] [Google Scholar]
- Xia Z., Hu J., Gao Y.-Q., Yao Q., Xie W. Chem. Commun. 2017;53:7485–7488. doi: 10.1039/c7cc03754f. [DOI] [PubMed] [Google Scholar]
- Ding W., Zhou Q.-Q., Xuan J., Li T.-R., Lu L.-Q., Xiao W.-J. Tetrahedron Lett. 2014;55:4648–4652. [Google Scholar]
- Fu W., Zhou Y., Song Q. Chem. – Asian J. 2018;13:2511–2515. doi: 10.1002/asia.201800500. [DOI] [PubMed] [Google Scholar]
- Lin W. J., Shia K. S., Song J. S., Wu M. H., Li W. T. Org. Biomol. Chem. 2016;14(1):220–228. doi: 10.1039/c5ob01863c. [DOI] [PubMed] [Google Scholar]
- Verbeek J., Eriksson J., Syvänen S., Huisman M., Schuit R. C., Molthoff C. F., Voskuyl R. A., de Lange E. C., Lammertsma A. A., Windhorst A. D. EJNMMI Radiopharm. Chem. 2018;3:11. doi: 10.1186/s41181-018-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P. R., Shirsat P. K., Khomane N., Nayak L., Nanubolu J. B., Meshram H. M. ACS Comb. Sci. 2017;19:633–639. doi: 10.1021/acscombsci.7b00044. [DOI] [PubMed] [Google Scholar]
- Poomathi N., Mayakrishnan S., Muralidharan D., Srinivasan R., Perumal P. T. Green Chem. 2015;17:3362–3372. [Google Scholar]
- Kaur M., Singh B., Singh B., Arjuna A. J. Heterocycl. Chem. 2017;54(2):1348–1354. [Google Scholar]
- Huang M., Lin H. S., Lee Y. S., Ho P. C. Int. J. Oncol. 2014;45:1724–1734. doi: 10.3892/ijo.2014.2548. [DOI] [PubMed] [Google Scholar]
- Wee X. K., Yeo W. K., Zhang B., Tan V. B., Lim K. M., Tay T. E., Go M. L. Bioorg. Med. Chem. 2009;17:7562–7571. doi: 10.1016/j.bmc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Seo D. Y., Roh H. J., Min B. K., Kim J. N. Bull. Korean Chem. Soc. 2017;38:1519–1522. [Google Scholar]
- Westley C. B., Vine K. L. and Benkendorff K., in Indirubin, the Red Shade of Indigo, 2006, pp. 31–44. [Google Scholar]
- Vine K. L., Locke J. M., Ranson M., Pyne S. G., Bremner J. B. Bioorg. Med. Chem. 2007;15:931–938. doi: 10.1016/j.bmc.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Krishnegowda G., Gowda A. P., Tagaram H. R. S., Staveley-O'Carroll K. F., Irby R. B., Sharma A. K., Amin S. Bioorg. Med. Chem. 2011;19:6006–6014. doi: 10.1016/j.bmc.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Raghuwanshi K., Patel V. K., Jain D. K., Veerasamy R., Dixit A., Rajak H. Pharm. Chem. J. 2017;51:366–374. [Google Scholar]
- Eldehna W. M., Fares M., Ceruso M., Ghabbour H. A., Abou-Seri S. M., Abdel-Aziz H. A., Supuran C. T. Eur. J. Med. Chem. 2016;110:259–266. doi: 10.1016/j.ejmech.2016.01.030. [DOI] [PubMed] [Google Scholar]
- Baeyer A., Oekonomides S. Eur. J. Inorg. Chem. 1882;15:2093–2102. [Google Scholar]
- Sumpter W. C. Chem. Rev. 1944;34:393–434. [Google Scholar]
- Jiang K. M., Luesakul U., Zhao S. Y., An K., Muangsin N., Neamati N., Jin Y., Lin J. ACS Omega. 2017;2:3123–3134. doi: 10.1021/acsomega.7b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli E., Arora R., Kakkar R. Can. Chem. Trans. 2014;2:327–342. [Google Scholar]
- Tisovský P., Šandrik R., Horváth M., Donovalová J., Jakusová K., Cigáň M., Sokolík R., Gáplovský A., Gáplovský M., Filo J. Molecules. 2017;22(11):1961. doi: 10.3390/molecules22111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigotto A., Galasso V. Spectrochim. Acta, Part A. 1979;35:725–732. [Google Scholar]
- Yu L., Ye J., Zhang X., Ding Y., Xu Q. Catal. Sci. Technol. 2015;5:4830–4838. [Google Scholar]
- Moskovkina T. V., Denisenko M. V., Kalinovskii A. I., Stonik V. A. Russ. J. Org. Chem. 2013;49:1740–1743. [Google Scholar]
- Wang C., Zhang L., Ren A., Lu P., Wang Y. Org. Lett. 2013;15:2982–2985. doi: 10.1021/ol401144m. [DOI] [PubMed] [Google Scholar]
- Li W., Liu X., Hao X., Cai Y., Lin L., Feng X. Angew. Chem. 2012;124:8772–8775. doi: 10.1002/anie.201204594. [DOI] [PubMed] [Google Scholar]
- Shi R. G., Wang X. H., Liu R., Yan C. G. Chem. Commun. 2016;52:6280–6283. doi: 10.1039/c6cc00525j. [DOI] [PubMed] [Google Scholar]
- Olah G. A., in Across Conventional Lines, World Scientific, 2003, pp. 109–118. [Google Scholar]
- Bandini M., Melloni A., Umani-Ronchi A. Angew. Chem., Int. Ed. 2004;43:550–556. doi: 10.1002/anie.200301679. [DOI] [PubMed] [Google Scholar]
- Gutierrez E. G., Wong C. J., Sahin A. H., Franz A. K. Org. Lett. 2011;13:5754–5757. doi: 10.1021/ol202329s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Guo F., Xu K., Zhang S., Hu Y., Zha Z., Wang Z. Org. Lett. 2014;16:3192–3195. doi: 10.1021/ol501086q. [DOI] [PubMed] [Google Scholar]
- Deng J., Zhang S., Ding P., Jiang H., Wang W., Li J. Adv. Synth. Catal. 2010;352:833–838. [Google Scholar]
- Wang G., Liu X., Chen Y., Yang J., Li J., Lin L., Feng X. ACS Catal. 2016;6:2482–2486. [Google Scholar]
- Reddy S., Venkata U., Chennapuram M., Seki K., Seki C., Anusha B., Kwon E., Okuyama Y., Uwai K., Tokiwa M., Takeshita M. Eur. J. Org. Chem. 2017;26:3874–3885. [Google Scholar]
- Cassani C., Melchiorre P. Org. Lett. 2012;14:5590–5593. doi: 10.1021/ol302711w. [DOI] [PubMed] [Google Scholar]
- Vine K. L., Locke J. M., Ranson M., Pyne S. G., Bremner J. B. J. Med. Chem. 2007;50:5109–5117. doi: 10.1021/jm0704189. [DOI] [PubMed] [Google Scholar]
- Ferandin Y., Bettayeb K., Kritsanida M., Lozach O., Ploychronopoulos P., Magiatis P., Skaltsounis A. L., Meijer L. J. Med. Chem. 2006;49:4638–4649. doi: 10.1021/jm060314i. [DOI] [PubMed] [Google Scholar]
- Marko D., Schätzle S., Friedel A., Genzlinger A., Zankl H., Meijer L., Eisenbrand G. Br. J. Cancer. 2001;84:283–289. doi: 10.1054/bjoc.2000.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yan J., Du M., Burlison J. A., Li C., Sun Y., Zhao D., Liu J. Tetrahedron. 2017;73:2780–2785. [Google Scholar]
- Yuan W. K., Cui T., Wen L. R., Li M. Org. Lett. 2018;20:1513–1516. doi: 10.1021/acs.orglett.8b00217. [DOI] [PubMed] [Google Scholar]
- West A. C., Johnstone R. W. J. Clin. Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddibhotla S. Curr. Bioact. Compd. 2009;5:20–38. [Google Scholar]
- Huan N. V., Cuong L. V., Kim J., Kim Y. G., Han S. B., Nam N. H. Bioorg. Chem. 2017;71:160–169. doi: 10.1016/j.bioorg.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Supuran C. T., Winum J. Y. Future Med. Chem. 2015;7:1407–1414. doi: 10.4155/fmc.15.71. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M., Abo-Ashour M. F., Nocentini A., Gratteri P., Eissa I. H., Fares M., Ismael O. E., Ghabbour H. A., Elaasser M. M., Abdel-Aziz H. A., Supuran C. T. Eur. J. Med. Chem. 2017;139:250–262. doi: 10.1016/j.ejmech.2017.07.073. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. S., Abou-Seri S. M., Tanc M., Elaasser M. M., Abdel-Aziz H. A., Supuran C. T. Eur. J. Med. Chem. 2015;103:583–593. doi: 10.1016/j.ejmech.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Güzel-Akdemir Ö., Akdemir A., Karalı N., Supuran C. T. Org. Biomol. Chem. 2015;13:6493–6499. doi: 10.1039/c5ob00688k. [DOI] [PubMed] [Google Scholar]
- El-Azab A. S., Al-Dhfyan A., Abdel-Aziz A. A. M., Abou-Zeid L. A., Alkahtani H. M., Al-Obaid A. M., Al-Gendy M. A. J. Enzyme Inhib. Med. Chem. 2017;32:935–944. doi: 10.1080/14756366.2017.1344981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath B., Ganguly S. Monatsh. Chem. 2016;147:565–574. [Google Scholar]
- Jordan M. A., Wilson L. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Vindya N. G., Sharma N., Yadav M., Ethiraj K. R. Curr. Top. Med. Chem. 2015;15:73–82. doi: 10.2174/1568026615666150112115805. [DOI] [PubMed] [Google Scholar]
- Singh H., Singh J. V., Gupta M. K., Saxena A. K., Sharma S., Nepali K., Bedi P. M. S. Bioorg. Med. Chem. Lett. 2017;27:3974–3979. doi: 10.1016/j.bmcl.2017.07.069. [DOI] [PubMed] [Google Scholar]
- Sultana F., Shaik S. P., Nayak V. L., Hussaini S. M. A., Marumudi K., Sridevi B., Shaik T. B., Bhattacharjee D., Alarifi A., Kamal A. ChemistrySelect. 2017;2:9901–9910. [Google Scholar]
- Wang J., Yun D., Yao J., Fu W., Huang F., Chen L., Wei T., Yu C., Xu H., Zhou X., Huang Y. Eur. J. Med. Chem. 2018;144:493–503. doi: 10.1016/j.ejmech.2017.12.043. [DOI] [PubMed] [Google Scholar]
- Singh A., Saha S. T., Perumal S., Kaur M., Kumar V. ACS Omega. 2018;3:1263–1268. doi: 10.1021/acsomega.7b01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Gahlyan P., Dwivedi S., Konwar R., Kumar S., Bhandari M., Arora R., Kakkar R., Kumar R., Prasad A. K. ChemistrySelect. 2018;3:5263–5268. [Google Scholar]
- Dileepan A. B., Prakash T. D., Kumar A. G., Rajam P. S., Dhayabaran V. V., Rajaram R. J. Photochem. Photobiol., B. 2018;183:191–200. doi: 10.1016/j.jphotobiol.2018.04.029. [DOI] [PubMed] [Google Scholar]
- Ravichandran V., Mohan S., Kumar K. S. ARKIVOC. 2007;14:51–57. [Google Scholar]
- Patel A., Bari S., Talele G., Patel J., Sarangapani M. Iran. J. Pharm. Res. 2010;5:249–254. [Google Scholar]
- Meenakshi K., Gopal N., Sarangapani M. Int. J. Pharm. Pharm. Sci. 2014;6:318–322. [Google Scholar]
- Lian Z. M., Sun J., Zhu H. L. J. Mol. Struct. 2016;1117:8–16. [Google Scholar]
- Pardasani R. T., Pardasani P., Sherry D., Chaturvedi V. Indian J. Chem. 2001;40:1275–1278. [Google Scholar]
- Khan M. W., Alam M. J., Rashid M. A., Chowdhury R. Bioorg. Med. Chem. 2005;13:4796–4805. doi: 10.1016/j.bmc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Baharfar R., Asghari S., Rassi S., Mohseni M. Res. Chem. Intermed. 2015;41:6975–6984. [Google Scholar]
- American Diabetes Association Diabetes Care. 2013;36(Suppl 1):S67. [Google Scholar]
- Tejasree C., Kiran G., Rajyalakshmi G., Reddy A. R. N. Int. J. Pharm. Sci. Res. 2014;5:2738–2743. [Google Scholar]
- Derosa G., Maffioli P. Arch. Med. Sci. 2012;8:899–906. doi: 10.5114/aoms.2012.31621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao K., Ishikawa R., Sugita Y. Chem. Pharm. Bull. 2014;62:810–815. doi: 10.1248/cpb.c14-00351. [DOI] [PubMed] [Google Scholar]
- Soengas R. G., Silva V. L., Ide D., Kato A., Cardoso S. M., Paz F. A. A., Silva A. M. Tetrahedron. 2016;72:3198–3203. [Google Scholar]
- Rahim F., Malik F., Ullah H., Wadood A., Khan F., Javid M. T., Taha M., Rehman W., Rehman A. U., Khan K. M. Bioorg. Chem. 2015;60:42–48. doi: 10.1016/j.bioorg.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Wang G., Chen M., Qiu J., Xie Z., Cao A. Bioorg. Med. Chem. Lett. 2018;28:113–116. doi: 10.1016/j.bmcl.2017.11.047. [DOI] [PubMed] [Google Scholar]
- Sirven J. I. Cold Spring Harbor Perspect. Med. 2015;5(9):a022848. doi: 10.1101/cshperspect.a022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes Lima J. M. Curr. Pharm. Des. 2000;6:873–878. doi: 10.2174/1381612003400308. [DOI] [PubMed] [Google Scholar]
- Spear B. B. Epilepsia. 2001;42:31–34. doi: 10.1046/j.1528-1157.2001.0420s5031.x. [DOI] [PubMed] [Google Scholar]
- Yogeeswari P., Sriram D., Thirumurugan R., Raghavendran J. V., Sudhan K., Pavana R. K., Stables J. J. Med. Chem. 2005;48:6202–6211. doi: 10.1021/jm050283b. [DOI] [PubMed] [Google Scholar]
- Rajak H., Deshmukh R., Aggarwal N., Kashaw S., Kharya M. D., Mishra P. Arch. Pharm. 2009;342:453–461. doi: 10.1002/ardp.200800213. [DOI] [PubMed] [Google Scholar]
- Saravanan G., Alagarsamy V., Dineshkumar P. Bull. Fac. Pharm. 2014;52:115–124. [Google Scholar]
- Xie C., Tang L. M., Li F. N., Guan L. P., Pan C. Y., Wang S. H. Med. Chem. Res. 2014;23:2161–2168. [Google Scholar]
- Aboul-Fadl T., Bin-Jubair F. A. Int. J. Res. Pharm. Sci. 2010;1:113–126. [Google Scholar]
- Aboul-Fadl T., Abdel-Hamid M. K., Youssef A. F. Der Pharma Chemica. 2015;7:217–225. [Google Scholar]
- Hu Y. Q., Meng L. D., Qiang M., Song X. F. J. Heterocyclic Chem. 2017;54:3725–3729. [Google Scholar]
- Gao T., Zeng Z., Wang G., Sun S., Liu Y. J. Heterocyclic Chem. 2018;55:1484–1488. [Google Scholar]
- Maddela S., Makula A. Anti-Infect. Agents. 2016;14:53–62. [Google Scholar]
- Zhan P., Liu X., Li Z., Pannecouque C., De Clercq E. Curr. Med. Chem. 2009;16:3903–3917. doi: 10.2174/092986709789178019. [DOI] [PubMed] [Google Scholar]
- Meleddu R., Distinto S., Corona A., Bianco G., Cannas V., Esposito F., Artese A., Alcaro S., Matyus P., Bogdan D., Cottiglia F. Eur. J. Med. Chem. 2015;93:452–460. doi: 10.1016/j.ejmech.2015.02.032. [DOI] [PubMed] [Google Scholar]
- Corona A., Meleddu R., Esposito F., Distinto S., Bianco G., Masaoka T., Maccioni E., Menéndez-Arias L., Alcaro S., Le Grice S. F., Tramontano E. PLoS One. 2016;11:e0147225. doi: 10.1371/journal.pone.0147225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleddu R., Distinto S., Corona A., Tramontano E., Bianco G., Melis C., Cottiglia F., Maccioni E. J. Enzyme Inhib. Med. Chem. 2017;32:130–136. doi: 10.1080/14756366.2016.1238366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar S., Liu P. P., Cooper Jr L. T. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. M., Dai H., Hanson P. J., Li H., Guo H., Ye X., Hemida M. G., Wang L., Tong Y., Qiu Y., Liu S. ACS Chem. Biol. 2014;9:1015–1024. doi: 10.1021/cb400775z. [DOI] [PubMed] [Google Scholar]
- Mishra P., Kumar A., Mamidi P., Kumar S., Basantray I., Saswat T., Das I., Nayak T. K., Chattopadhyay S., Subudhi B. B., Chattopadhyay S. Sci. Rep. 2016;6:20122. doi: 10.1038/srep20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Viruses. 2010;2:1322–1339. doi: 10.3390/v2061322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle D. C., Keith K. A., Kern E. R. Antiviral Res. 2006;71:24–30. doi: 10.1016/j.antiviral.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Webber S. E., Tikhe J., Worland S. T., Fuhrman S. A., Hendrickson T. F., Matthews D. A., Love R. A., Patick A. K., Meador J. W., Ferre R. A., Brown E. L. J. Med. Chem. 1996;39:5072–5082. doi: 10.1021/jm960603e. [DOI] [PubMed] [Google Scholar]
- Ronen D., Sherman L., Bar-Nun S., Teitz Y. Antimicrob. Agents Chemother. 1987;31:1798–1802. doi: 10.1128/aac.31.11.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Liu Y., Zhang W., Wei P., Huang C., Pei J., Yuan Y., Lai L. J. Med. Chem. 2006;49:3440–3443. doi: 10.1021/jm0602357. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhu H. M., Niu G. J., Shi E. Z., Chen J., Sun B., Chen W. Q., Zhou H. G., Yang C. Bioorg. Med. Chem. 2014;22:292–302. doi: 10.1016/j.bmc.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi M. A., Jamal D. Mater. Chem. Phys. 2001;68:283–287. [Google Scholar]
- Karthik R., Vimaladevi G., Chen S. M., Elangovan A., Jeyaprabha B., Prakash P. Int. J. Electrochem. Sci. 2015;10:4666–4681. [Google Scholar]
- Singh D. D. N., Singh M. M., Chaudhary R. S., Agarwal C. V. J. Appl. Electrochem. 1980;10:587–592. [Google Scholar]
- Ansari K. R., Quraishi M. A. J. Taiwan Inst. Chem. Eng. 2015;54:145–154. [Google Scholar]
- Kharbach Y., Qachchachi F. Z., Haoudi A., Tourabi M., Zarrouk A., Jama C., Olasunkanmi L. O., Ebenso E. E., Bentiss F. J. Mol. Liq. 2017;246:302–316. [Google Scholar]
- Muralisankar M., Sreedharan R., Sujith S., Bhuvanesh N. S., Sreekanth A. J. Alloys Compd. 2017;695:171–182. [Google Scholar]
- Lashgari N., Badiei A., Ziarani G. M., Faridbod F. Anal. Bioanal. Chem. 2017;409:3175–3185. doi: 10.1007/s00216-017-0258-1. [DOI] [PubMed] [Google Scholar]
- Chen J., Shu W., Wang E. Chem. Res. Chin. Univ. 2016;32:742–745. [Google Scholar]
- Shakir M., Abbasi A. Inorg. Chim. Acta. 2017;465:14–25. [Google Scholar]
- Goswami S., Paul S., Manna A. RSC Adv. 2013;3:10639–10643. [Google Scholar]
- Rondão R., Melo J. S., Schaberle F., Voss G. Phys. Chem. Chem. Phys. 2012;14:1778–1783. doi: 10.1039/c2cp23266a. [DOI] [PubMed] [Google Scholar]
- Kolaczkowski M. A., He B., Liu Y. Org. Lett. 2016;18:5224–5227. doi: 10.1021/acs.orglett.6b02504. [DOI] [PubMed] [Google Scholar]
- Stalder R., Mei J., Graham K. R., Estrada L. A., Reynolds J. R. Chem. Mater. 2013;26:664–678. [Google Scholar]
- Deng P., Zhang Q. Polym. Chem. 2014;5:3298–3305. [Google Scholar]
- Li J. L., Chai Y. F., Wang W. V., Shi Z. F., Xu Z. G., Zhang H. L. Chem. Commun. 2017;53:5882–5885. doi: 10.1039/c7cc01973d. [DOI] [PubMed] [Google Scholar]