Abstract
IMPORTANCE
Glioblastoma multiforme (GBM) remains almost invariably fatal despite optimal surgical and medical therapy. The association between the extent of tumor resection (EOR) and outcome remains undefined, notwithstanding many relevant studies.
OBJECTIVE
To determine whether greater EOR is associated with improved 1- and 2-year overall survival and 6-month and 1-year progression-free survival in patients with GBM.
DATA SOURCES
Pubmed, CINAHL, and Web of Science (January 1, 1966, to December 1, 2015) were systematically reviewed with librarian guidance. Additional articles were included after consultation with experts and evaluation of bibliographies. Articles were collected from January 15 to December 1, 2015.
STUDY SELECTION
Studies of adult patients with newly diagnosed supratentorial GBM comparing various EOR and presenting objective overall or progression-free survival data were included. Pediatric studies were excluded.
DATA EXTRACTION AND SYNTHESIS
Data were extracted from the text of articles or the Kaplan-Meier curves independently by investigators who were blinded to each other’s results. Data were analyzed to assess mortality after gross total resection (GTR), subtotal resection (STR), and biopsy. The body of evidence was evaluated according to Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria and PRISMA guidelines.
MAIN OUTCOME AND MEASURES
Relative risk (RR) for mortality at 1 and 2 years and progression at 6 months and 1 year.
RESULTS
The search produced 37 studies suitable for inclusion (41 117 unique patients). The meta-analysis revealed decreased mortality for GTR compared with STR at 1 year (RR, 0.62; 95%CI, 0.56–0.69; P < .001; number needed to treat [NNT], 9) and 2 years (RR, 0.84; 95% CI, 0.79–0.89; P < .001; NNT, 17). The 1-year risk for mortality for STR compared with biopsy was reduced significantly (RR, 0.85; 95%CI, 0.80–0.91; P < .001). The risk for mortality was similarly decreased for any resection compared with biopsy at 1 year (RR, 0.77; 95%CI, 0.71–0.84; P < .001; NNT, 21) and 2 years (RR, 0.94; 95%CI, 0.89–1.00; P = .04; NNT, 593). The likelihood of disease progression was decreased with GTR compared with STR at 6 months (RR, 0.72; 95%CI, 0.48–1.09; P = .12; NNT, 14) and 1 year (RR, 0.66; 95%CI, 0.43–0.99; P < .001; NNT, 26). The quality of the body of evidence by the GRADE criteria was moderate to low.
CONCLUSION AND RELEVANCE
This analysis represents the largest systematic review and only quantitative systematic review to date performed on this subject. Compared with STR, GTR substantially improves overall and progression-free survival, but the quality of the supporting evidence is moderate to low.
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor in adults and is known for its invasive and aggressive behavior.1,2 The optimal combination of medical, surgical, and radiation therapy for patients with GBM has yet to be defined, and the surgical component of therapy can range from a minimally invasive biopsy to a craniotomy with a goal of gross total resection (GTR).2 Although the advent of 5-aminolevulinic acid–guided intraoperative techniques has increased the extent of resection (EOR) that is surgically possible, not every patient receives an aggressive resection.3 Moreover, variations in treatment protocols have done very little to extend survival, and fierce debate about this topic continues.4–6 Although cytoreductive surgery is the cornerstone of therapy in GBM, no consensus exists regarding the optimal EOR necessary to improve survival.4,7 Even prior meta-analyses on the subject of EOR and overall survival2,8 have provided contradictory results.
The causal association between aggressive tumor resection with tumor-negative margins and improved survival is a venerated belief in the field of surgical oncology despite several spectacular refutations, most notably in the world of breast oncology, where nearly a century of commitment to radical mastectomy ultimately yielded to the long-term results of randomized clinical trials.9–14 In the realm of neuro-oncology, many large retrospective cohort studies have also demonstrated enhanced survival with increased EOR in patients with newly diagnosed GBM, and mathematical modeling of retrospective data suggests incremental improvements in survival with EORs ranging from 78% to 98%.4,5 However, the unique anatomy of the brain and concern about injury to eloquent structures with resulting impairment in quality of life often make the goal of GTR difficult to attain. Moreover, elegant pathologic and radiologic studies have shown that GBM is a diffusely infiltrating and widespread malignant neoplasm that, even at the time of diagnosis, typically invades multiple lobes and both hemispheres of the brain.15–18 For all these reasons, disagreement continues about the optimum EOR, the true risks and benefits of aggressive resection, and how tobalancethem.19,20 As a consequence, most patients do not undergo maximum safe resection, and these controversies, as well as the absence of randomized clinical trials, have prevented neurosurgical and oncologic professional societies from formulating practice guidelines regarding the optimal EOR.20,21
Because of this continuing controversy, widespread practice variation, and considerable deficiencies in the available literature, we sought to determine whether GTR compared with subtotal resection (STR) or biopsy is associated with prolonged overall and progression-free survival using quantitative meta-analytic techniques according to the PRISMA guidelines.11 We also applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria to our results to guide current practice and inform future study.22–24
Methods
Systematic Review
We conducted a systematic literature review using accepted evidence-based techniques under the supervision of an expert librarian with special training in evidence-based literature at the George T. Harrell Health Science Library at the Penn State College of Medicine. The search queried PubMed, CINAHL, and Web of Science using the MeSH term glioblastoma and the non-MeSH terms extent of resection, resection, and survival to broadly capture the existing literature from January 1, 1966, to December 1, 2015. We reviewed individual citations, rejected off-topic citations, and downloaded on topic articles before we reviewed the articles individually. Studies were included if greater than 80% of study patients were adults with newly diagnosed supratentorial GBM, had a comparison group, and provided objective data on 1- or 2-year overall survival or 6-month or 1-year progression-free survival. Abstracts were accepted if they met the same inclusion criteria and the data from the resulting publication were not also included. No language restriction was imposed. Articles were excluded if they focused only on pediatric neoplasms, if greater than 50% of the primary brain tumors included were tumor types other than GBM, if they did not contain a comparison group, or if they did not provide the specified outcome data. Studies that only mentioned EOR as a prognostic factor but did not objectively present data according to our outcomes of interest were excluded. In addition to the systematic review, the bibliographies of qualitative meta-analyses on the subject were searched by hand for relevant articles. Last, we consulted experts in the field for any relevant articles that may have been missed by our search methods. Articles were collected from January 15 until December 1, 2015.
Data Extraction
Our comparisons of interest were differences in 1- or 2-year overall survival or 6-month or 1-year progression-free survival between patients with varying EORs .One-year overall survival was chosen because it is the approximate median overall survival for patients with GBM. Two-year overall survival was chosen as it was likely to reveal significant differences in survival with varying EORs, is a clinically important point from a patient perspective, and is frequently reported in randomized clinical trials in this disease. The 6-month and 1-year progression-free survival end points were selected for the same reasons. The EOR for this study was defined by the authors of the individual studies. Resections were broadly grouped into GTR, STR, or biopsy. The STR group included patients described as undergoing STR and partial resection. Data from most of the studies did not permit more detailed classification, for example, grouping based on the percentage of tumor debulking. When the percentages of surviving patients at our prespecified survival and progression end points were not provided in the text, values were derived from Kaplan-Meier curves using a pixel-coordinate method of mapping the axes of interest and mathematically calculating percentages. Data were extracted independently by members of the study team (T.J.B., M.C.B., E.W.C., N.J.B., K.L.R., E.B.R., and M.G.) who were blinded to each other’s results. Any disagreements were adjudicated by one of us (M.G.).
Evidence Grading
Each article was graded independently by the 7 members of the study team using American Academy of Neurology level of evidence criteria.25,26 Each article included in each meta-analysis was first graded independently by these 7 study team members and was subsequently reviewed by all 7 authors in a consensus meeting. Each article was assigned a grade of I to IV, with I providing the most robust and IV providing the weakest evidence. Disagreements were resolved by group consensus after consulting the study in question.
Once data collection was complete and the meta-analyses had been performed, we evaluated the overall body of evidence using the GRADE system proposed by Guyatt and colleagues22 and others.23,24,27 The GRADE rating scale assigns high, moderate, low, or very low reliability categories to a body of evidence. Each rating reflects the degree of confidence that the magnitude and direction of an estimated effect are correct. This process considers and integrates strength of association, magnitude of effect, and risk for bias.
Meta-analysis
Relative risks (RRs) and 95% CIs for each of our comparisons of interest were calculated using the random effects model in Review Manager (version 5.3; Cochrane Collaboration [http://www.cochrane.org]). Meta-analyses for each of the comparisons were repeated on only class 2 studies (when at least two class 2 studies were available) and on only studies published within the last 10 years. We also repeated each meta-analysis after excluding Surveillance, Epidemiology, and End Results (SEER) data, and again after excluding SEER and Radiation Therapy Oncology Group (RTOG) data to assess the effect of the large sample sizes in those studies and the overall meta analytic estimates. The resulting summary statistics were compared with the original meta-analyses using RR ratios (the RR calculator is found at http://www.hutchon.net). Significance was established using CIs at the level of 95% or P < .05. Publication bias was assessed with funnel plots.
Results
Literature Search
A systematic literature review yielded 1055 citations, of which 1005 were unique citations. Review of titles and publication type allowed 883 citations to be rejected because the corresponding studies lacked a comparison group, failed to provide specified outcome data, or compared treatments other than EOR. One hundred twenty-two articles were deemed potentially suitable for inclusion, retrieved, and analyzed by the group for final eligibility determination. Thirty-seven articles6,7,20,28–62 met our inclusion criteria (41 117 unique patients) and were included in at least 1 comparison in the meta-analysis. Thirty-six studies (References 6, 7, 20, 28, 30–40, 42, 45, 47–51, 53–59, 62) were included in the meta-analyses of overall survival, and 8 articles29,41,43,44,46,52,60,61 were included in the meta-analyses of progression-free survival. Hand searching the bibliographies of recent meta-analyses did not reveal any additional articles fulfilling our inclusion criteria. Three articles were identified from consultation with experts in the field that were ultimately included in our meta-analyses. The landmark study by Lacroix et al4 was excluded because a new, larger study with less risk for bias,34 which included data from the earlier trial, had subsequently been published. The search process and results are outlined in Figure 1.63 Demographic data for included studies are included in eTable 1 in the Supplement.
Figure 1.
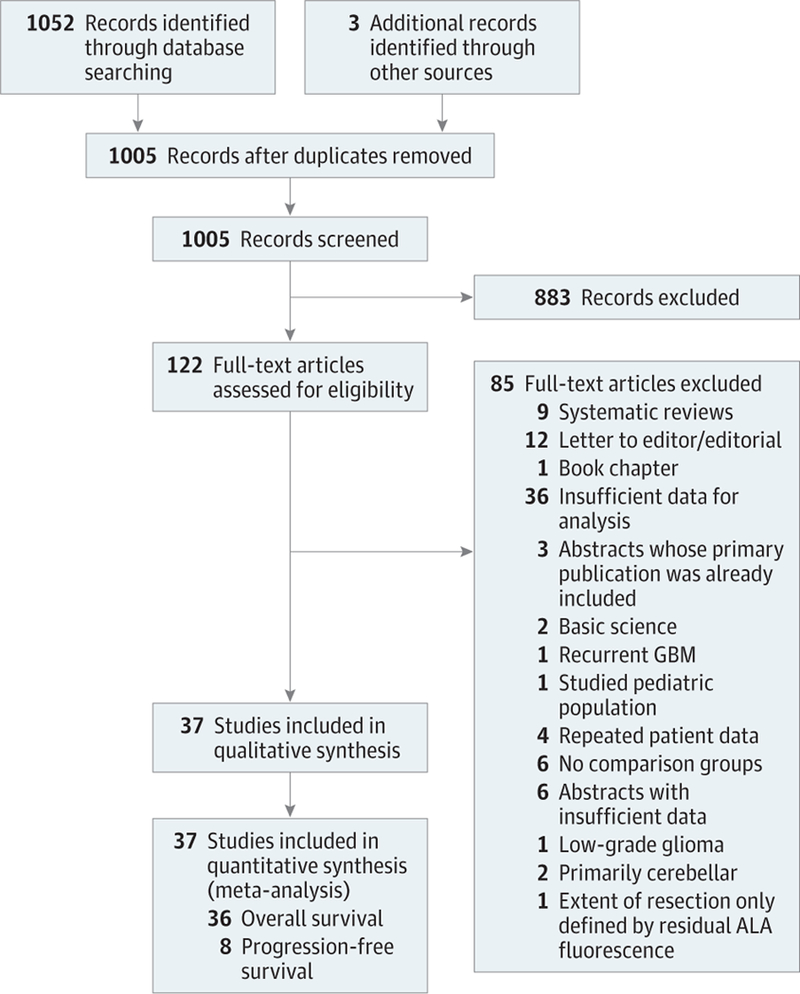
PRISMA Flow Diagram
ALA indicates 5-aminolevulinic acid; GBM, glioblastoma multiforme.
Evidence Grading
Our review failed to discover any class 1 studies. We included four class 2 studies.36,49,54,61 The remaining studies were assigned a class III (15 studies) (References 6, 20, 30, 32, 35, 44, 46, 47, 51–53, 55–57, 60) or class IV (18 studies) (References 7, 28, 29, 31, 33, 34, 37–43, 45, 48, 50, 58, 62) rating (Table). None of the identified studies were prospective or randomized by EOR; however, 1 study36 was prospective and randomized by surgical technique. Many of the studies had substantial differences between treatment groups in prognostically important features, including age, performance status, tumor size, tumor location, multifocality, medical comorbidities, and postoperative treatments. No trial analyzing progression-free survival used masked outcome assessment. Although survival is a relatively robust outcome measure, progression-free survival is notoriously subject to outcome assessment bias. As a result, the progression-free survival end point was further downgraded because of the substantial risk for bias (Table).
Table.
Level of Evidence for Included Studies.
| Source | Level of Evidencea |
Reason for Downgrade |
|---|---|---|
| Ahmadloo et al,45 2013 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Albert et al,29 1994 | 4 | Nonrandom treatment assignment (retrospective); treatment allocation unconcealed; PFS outcome assessor relationship NS |
| Butowski et al,35 2007 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Chaichana et al,55 2011 | 3 | Nonrandom treatment assignment (retrospective, nonconsecutive patients); treatment allocation unconcealed |
| Chaichana et al,57 2014 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Ciric et al,28 1987 | 4 | Treatment group characteristics NS; nonrandom treatment assignment; treatment allocation unconcealed |
| Dea et al,42 2012 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Devaux et al,50 1993 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Ewelt et al,39 2011 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Hrabalek et al,61 2015 | 2 | Nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Jeremic et al,52 1994 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kalita et al,58 2014 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Keles et al,30 1999 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kelly and Hunt,51 1994 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kiwit et al,48 1996 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kreth et al,53 1993 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kreth et al,54 1999 | 2 | Nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kreth et al,46 2013 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Kuhnt et al,40 2011 | 4 | Treatment group characteristics NS; nonrandom treatment assignment; treatment allocation unconcealed |
| Li et al,49 2016 | 2 | Nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Martinez et al,34 2007 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| McGirt et al,20 2009 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Nitta and Sato,7 1995 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Noorbakhsh et al,47 2014 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Oszvald et al,44 2012 | 3 | Treatment group characteristics DS; nonrandom treatment assignment; treatment allocation unconcealed |
| Pichlmeier et al,36 2008 | 2 | Nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Pirotte et al,37 2009 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Salvati et al,43 2012 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Senft et al,38 2010 | 4 | Treatment group characteristics NS; nonrandom treatment assignment; treatment allocation unconcealed |
| Shinoda et al,32 2001 | 3 | Treatment group characteristics DS; nonrandom treatment assignment; treatment allocation unconcealed |
| Simpson et al,6 1993 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Stark et al,33 2005 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Stummer et al,31 2000 | 4 | Treatment group characteristics NS; nonrandom treatment assignment; treatment allocation unconcealed |
| Uzuka et al,56 2012 | 3 | Treatment group characteristics DS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Vuorinen et al,60 2003 | 3 | Treatment group characteristics DS; treatment allocation unconcealed |
| Yamaguchi et al,41 2012 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
| Zinn et al,62 2013 | 4 | Treatment group characteristics NS; nonrandom treatment assignment (retrospective); treatment allocation unconcealed |
Abbreviations: DS, dissimilar; NS, not stated; PFS, progression-free survival.
Indicates the level of evidence for each study included in at least 1 comparison. One provided the most robust and 4 the weakest evidence.
Publication Bias
A funnel plot was constructed for each meta-analysis to assess publication bias. The largest funnel plot that compared 1-year overall survival in patients with GTR vs STR contained 25 articles. The funnel plot suggests a potential deficit of small trials favoring STR over GTR (eFigure 1 in the Supplement). Although several studies were identified in searching prior meta-analyses that seemed to favor STR over GTR, none of these trials contained data that met our inclusion criteria.64
Body of Evidence Quality (GRADE Rating)
Our final assessment of the quality of the body of evidence using the GRADE criteria was moderate for the overall survival outcome measure and for meta-analyses including only class 2 studies. The assessment was low for all other comparisons.
Meta-analysis for Overall Survival
The meta-analysis revealed substantially improved overall survival after GTR compared with STR at 1 year (RR, 0.62; 95% CI,0.56–0.69;P < .001; number needed to treat [NNT], 9)and 2 years (RR, 0.84; 95% CI, 0.79–0.89; P < .001; NNT, 17) (Figure 2 and Figure 3). A similar improvement was revealed in 1-year overall survival for STR compared with biopsy (RR, 0.85; 95% CI, 0.80–0.91; P < .001). Two-year mortality for STR compared with biopsy was not significantly improved (RR, 0.99; 95% CI, 0.97–1.00; P = .09)(Figure 4). Any resection compared with biopsy alone showed improved 1-year (RR, 0.77; 95% CI, 0.71–0.84; P < .001; NNT, 21) and 2-year (RR, 0.94; 95% CI, 0.89–1.00; P = .04; NNT, 593) mortality (eFigure 2 in the Supplement).
Figure 2.
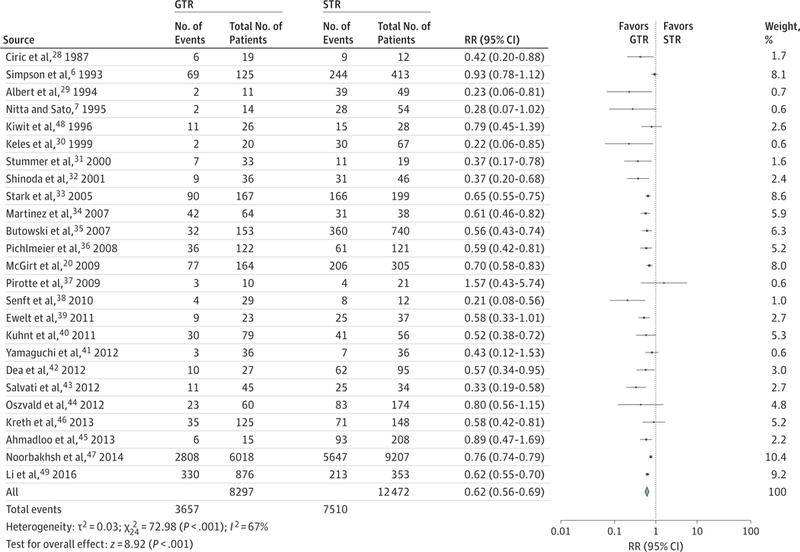
Relative Risk (RR) for 1-Year Mortality for Gross Total Resection (GTR) vs Subtotal Resection (STR)
Forest plots depict RRs (random Mantel-Haenszel test) at 1 year. Twenty-five studies were included in this analysis of 20 769 patients. Overall RR at 1 year is 0.62 (95%CI, 0.56–0.69; P < .001), favoring GTR over STR. Removal of Surveillance, Epidemiology, and End Results (SEER) data produced an RR of 0.60 (95%CI, 0.53–0.67; P < .001). Removal of the SEER and Radiation Therapy Oncology Group data produced an RR of 0.59 (95%CI, 0.53–0.65; P < .001). Marker size indicates the relative weight of the study as it contributes to the results of the overall comparison.
Figure 3.
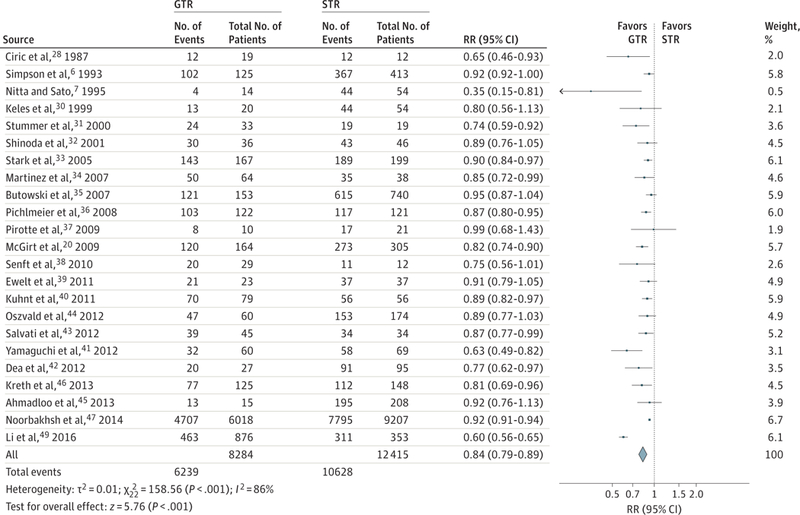
Relative Risk (RR) for 2-Year Mortality for Gross Total Resection (GTR) vs Subtotal Resection (STR)
Forest plots depict RRs (random Mantel-Haenszel test) at 2 years. Twenty-three studies were included in this analysis of 20 699 patients. Overall RR for death at 2 years was 0.84 (95%CI, 0.79–0.89; P < .001), favoring GTR over STR. Removal of the Surveillance, Epidemiology, and End Results (SEER) data produced an RR of 0.83 (95%CI, 0.77–0.89; P < .001). Removal of the SEER and Radiation Therapy Oncology Group data produced an RR of 0.83 (95%CI, 0.77–0.89; P < .001). Marker size indicates the relative weight of the study as it contributes to the results of the overall comparison.
Figure 4.
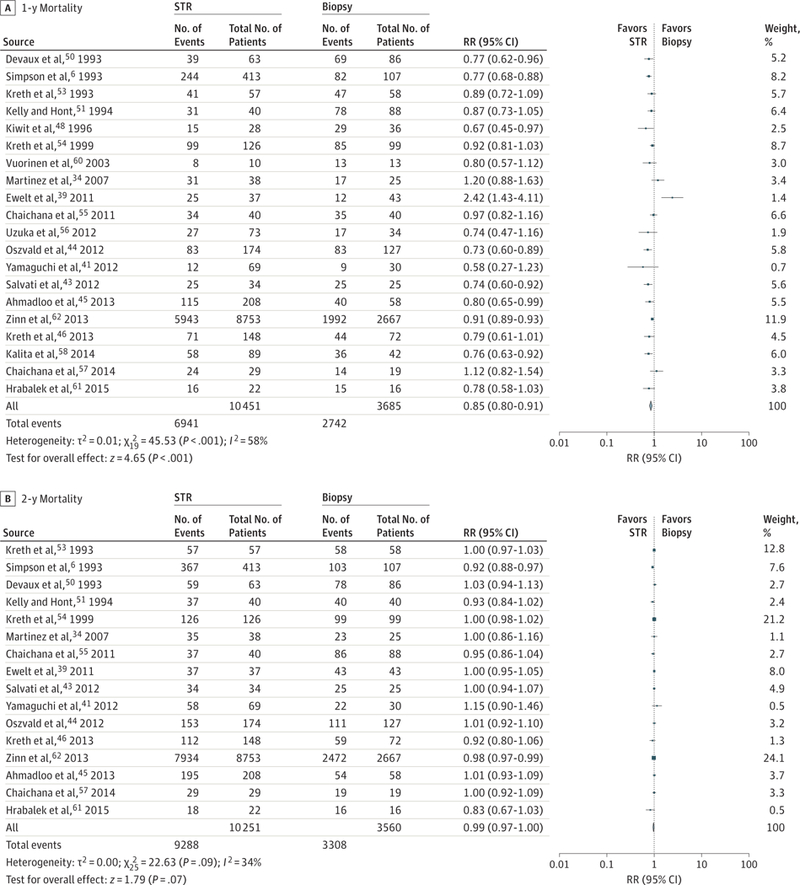
Relative Risk (RR) for Mortality for Subtotal Resection (STR) vs Biopsy
Forest plots depict RRs (random Mantel-Haenszel test) at 1 and 2 years. A, Twenty studies were included in this analysis of 14 136 patients. The RR of mortality at 1 year with STR is 0.85 (95%CI, 0.80–0.91; P < .001). Removal of the Surveillance, Epidemiology, and End Results (SEER) data produced an RR of 0.85 (95%CI, 0.78–0.92; P < .001) compared with biopsy. B, Sixteen studies were included in this analysis of 13 811 patients. The RR of mortality at 2 years did not significantly differ between STR and biopsy in this analysis (RR, 0.99; 95%CI, 0.97–1.00; P = .09).We hypothesized that the narrower differences between STR and biopsy result from the wide percentages of resections that constitute STR in the primary literature and perhaps some overlap between biopsy and STR, particularly in the setting of smaller tumors. Marker size indicates the relative weight of the study as it contributes to the results of the overall comparison.
Additional Meta-analyses
For the end points of 1- and 2-year overall survival, the meta-analyses were repeated including only the class 2 trials, with results similar to those of the larger meta-analyses. For comparison of GTR with STR, the RRs for death at 1 and 2 years and NNTs to achieve one additional 1- or 2-year survivor were 0.62 (95% CI, 0.55–0.69; P < .001; NNT, 5) and 0.72 (95% CI, 0.49–1.07; P = .11; NNT, 7), respectively. We also calculated RRs for 1- and 2-year mortality with STR vs biopsy and any resection vs biopsy. However, these comparisons only included 2 studies54,61 and constituted an identical data set for both comparisons. Although the RRs for STR vs biopsy suggest decreased mortality, statistical significance was not achieved, perhaps because of the very small number of patients included in these 2 studies (eTable 2 in the Supplement).
We then repeated the meta-analyses after removing the very large single study based on SEER data32 as a sensitivity analysis to avoid distortion by a single large study and again after removing the SEER study and the study derived from composite RTOG data.36 In both instances, essentially no change was found in the meta-analytic summary statistics for any comparison (eTable 2 in the Supplement).32,36 Comparison of the RR ratios after the removal of these large data sets confirmed that no effect was found on the meta-analytic results (eFigure 3 in the Supplement). Finally, the meta-analyses were repeated after excluding studies that analyzed patients accrued before 2000 and before 2005.Once again, we found no effect on any meta-analytic result (eTable 2 in the Supplement).
Progression-Free Survival
Relatively few articles met inclusion criteria for the secondary end point of progression-free survival at 6 months. Eight studies29,41,43,44,46,52,60,61 were identified from the systematic review and were included in at least 1 progression analysis. At 6 months, the RR for progression when comparing GTR with STR was 0.72 (95% CI, 0.48–1.09; P = .12; NNT, 14), a statistically nonsignificant finding favoring GTR. At 1 year, the RR for progression significantly favored GTR at 0.66 (95% CI, 0.43–0.99; P < .001; NNT, 26) (eFigure 4A and B in the Supplement).
Subtotal resection also significantly reduced the risk for progression at 6 months when compared with biopsy (RR,0.72; 95% CI, 0.51–1.00; P = .05; NNT, 321) (eFigure 4C in the Supplement). At 1 year, the risk for progression was not significantly different between the 2 interventions (RR, 0.96; 95% CI, 0.79–1.17; P = .69). Last, any resection appeared to reduce the risk for progression compared with biopsy alone at 6 months (RR, 0.61; 95% CI, 0.44–0.84; P = .003; NNT, 330) (eFigure 4D in the Supplement). At 1 year, the risk for progression was not significantly different (RR, 0.84; 95% CI, 0.69–1.01; P = .07).
Discussion
This report is, to our knowledge, the largest systematic review and the only quantitative meta-analysis to date to examine the association between EOR and overall and progression free survival. We found that patients with newly diagnosed GBM undergoing GTR were 61% more likely to survive 1 year (RR, 0.62; 95% CI, 0.56–0.69; P < .001),19% more likely to survive 2 years (RR, 0.84; 95% CI, 0.79–0.89; P < .001), and 51% more likely to be progression free at 12 months (RR, 0.66; 95% CI,0.43–0.99; P < .001) compared with patients receiving only an STR. These benefits translate into NNTs of 9, 17, and 26, respectively. When only class 2 studies are analyzed, the NNTs to achieve one additional 1- or 2-year survivor (again comparing GTR with STR) are an even more impressive 5 and 7, although the 2-year end point was not statistically significant. For comparison with widely accepted neurosurgical and neurologic interventions, the NNTs for endarterectomy for stroke prevention in the setting of severe and moderate symptomatic stenosis are 12 and 75; for warfarin compared with aspirin for primary stroke prevention and compared with placebo for secondary stroke prevention in the setting of atrial fibrillation, 57 and 8; for intravenous tissue plasminogen activator within 6 hours of an ischemic event, with the end point alive and independent at 6 months, 19; and for clopidogrel for secondary prevention of vascular events, 196.65–68 Our findings were unchanged after excluding all class 3 and 4 studies, after excluding studies accruing patients more than 10 and 15 years ago, and after excluding 2 large studies based on aggregate data. Subtotal resection also produced superior 1-year overall survival compared with biopsy, but this benefit was less substantial than for GTR, possibly because of variable and imprecise definitions of STR and biopsy in many studies, potentially leading to very heterogeneous populations with overlapping EORs.
These findings must be interpreted in the context of several important caveats. First, the GTR and STR cohorts differed with respect to prognostically important variables (especially age, performance status, tumor size and topography, eloquent location, medical comorbidities, and postoperative therapies) in many trials. Studies providing the highest class of evidence (class 2 in this analysis) attempted to account for this serious risk for confounding. Unfortunately, we were not able to extract patient-level data regarding these variables, and no randomized clinical trials or carefully designed prospective registries address this issue to allow meta-regression or an analysis using propensity score matching. Should data of this type become available, a more robust analysis based on these variables could be performed. Second, small studies favoring STR or biopsy may be lacking, which suggests possible publication bias. Several trials were uncovered in our systematic review that favored less extensive resection; however, these studies failed to meet prespecified inclusion criteria for our study. Finally, EOR was defined by the authors of the individual studies, often imprecisely and almost always in an unblended fashion. Two retrospective studies used mathematical modeling to estimate such a threshold and suggested that at least a 78% reduction in preoperative tumor volume is necessary to increase survival and that incremental benefit accrues to increasingly more complete resections up to 98%.4,48
Conclusions
Although the available studies are retrospective and mostly carry a high risk for bias and confounding, an overwhelming consistency of the evidence (including three class 2 studies) supports the superiority of GTR over STR and biopsy. We therefore suggest, based on three class II studies and many consistent class 3 studies, that GTR probably increases the likelihood of 1-year survival compared with STR by about 61% and increases the likelihood of 2-year survival by about 19%. Similar improvements (51%) are seen in 12-month progression-free survival. Therefore, when clinically feasible, the body of literature favors GTR in all patient switch newly diagnosed GBM. In light of the existing evidence, we further suggest that additional retrospective cohort trials will not contribute additional useful data and should not be performed or published. Randomized clinical trials have not proved feasible. A high-quality, audited, prospective registry of patients with GBM represents a valuable alternative for identifying factors that affect patient outcomes such as EOR, adjuvant therapies, molecular data, preoperative and postoperative imaging, tumor size, topography, location, and medical comorbidities, and should be a critical priority for the neurosurgical and oncology communities.
Supplementary Material
Key Points
Question
Does increasing the extent of resection improve the likelihood of overall survival in glioblastoma multiforme?
Findings
In this meta-analysis of 37 studies, gross total resection was significantly associated with a lower relative risk for mortality at 1 and 2 years compared with subtotal resection. Overall, a dose-dependent reduction in mortality was seen with increasing extent of resection.
Meaning
These findings support the use of gross total resection for glioblastoma multiforme for reducing 1- and 2-year mortality.
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentations: This paper was presented at the 20th Annual Meeting of the Society for Neuro-oncology; November 21, 2015; San Antonio, Texas; and was published in part as an abstract in conjunction with the Annual Meeting of the American Society of Clinical Oncology; June 3–7, 2016; Chicago, Illinois.
Contributor Information
Timothy J. Brown, Department of Medicine, University of Texas Southwestern Medical Center, Dallas.
Matthew C. Brennan, Ann Barshinger Cancer Center, Lancaster General Health, Lancaster, Pennsylvania.
Michael Li, Department of Neurosurgery, University of Rochester Medical Center, Pittsford, New York.
Ephraim W. Church, Department of Neurosurgery, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
Nicholas J. Brandmeir, Department of Neurosurgery, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
Kevin L. Rakszawski, Department of Medicine, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
Akshal S. Patel, Department of Neurosurgery, Swedish Cerebrovascular Institute, Seattle, Washington.
Elias B. Rizk, Department of Neurosurgery, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
Dima Suki, Division of Surgery, Department of Neurosurgery, University of Texas MD Anderson Cancer Center, Houston.
Raymond Sawaya, Division of Surgery, Department of Neurosurgery, University of Texas MD Anderson Cancer Center, Houston.
Michael Glantz, Department of Neurosurgery, Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2011; 115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 3.Aldave G, Tejada S, Pay E, et al. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid–guided surgery. Neurosurgery 2013;72(6):915–920. [DOI] [PubMed] [Google Scholar]
- 4.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95(2): 190–198. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, Mirzadeh Z, Polley M-Y, Berger MS. The value of glioblastoma extent of resection: a volumetric analysis of 500 patients. J Neurosurg 2010;113(2):A433. [Google Scholar]
- 6.Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 1993;26(2):239–244. [DOI] [PubMed] [Google Scholar]
- 7.Nitta T, Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer 1995;75(11): 2727–2731. [DOI] [PubMed] [Google Scholar]
- 8.Quigley MR, Maroon JC. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas. Neurosurgery 1991;29(3):385–388. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 1985;312(11):665–673. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347(16): 1233–1241. [DOI] [PubMed] [Google Scholar]
- 11.Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol 2012; 13(4):412–419. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347(16):1227–1232. [DOI] [PubMed] [Google Scholar]
- 13.Halsted WSI. The results of radical operations for the cure of carcinoma of the breast. Ann Surg 1907;46(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patey DH, Dyson WH. The prognosis of carcinoma of the breast in relation to the type of operation performed. Br J Cancer 1948;2(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger PC, Dubois PJ, Schold SC Jr, et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg 1983;58(2): 159–169. [DOI] [PubMed] [Google Scholar]
- 16.Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg 1988;68(5):698–704. [DOI] [PubMed] [Google Scholar]
- 17.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 18.Earnest F IV, Kelly PJ, Scheithauer BW, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 1988;166(3):823–827. [DOI] [PubMed] [Google Scholar]
- 19.Gulati S, Jakola AS, Nerland US, Weber C, Solheim O. The risk of getting worse: surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg 2011;76(6):572–579. [DOI] [PubMed] [Google Scholar]
- 20.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 21.Litofsky NS, Bauer AM, Kasper RS, Sullivan CM, Dabbous OH; Glioma Outcomes Project Investigators. Image-guided resection of high-grade glioma: patient selection factors and outcome. Neurosurg Focus 2006;20(4):E16. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines, 3: rating the quality of evidence. J Clin Epidemiol 2011;64(4):401–406. [DOI] [PubMed] [Google Scholar]
- 24.Kavanagh BP. The GRADE system for rating clinical guidelines. PLoS Med 2009;6(9):e1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronseth G, French J. Practice parameters and technology assessments: what they are, what they are not, and why you should care. Neurology 2008;71(20):1639–1643. [DOI] [PubMed] [Google Scholar]
- 26.Gross RA, Johnston KC. Levels of evidence: taking neurology to the next level. Neurology 2009;72(1):8–10. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciric I, Ammirati M, Vick N, Mikhael M. Supratentorial gliomas: surgical considerations and immediate postoperative results: gross total resection versus partial resection. Neurosurgery 1987;21(1):21–26. [DOI] [PubMed] [Google Scholar]
- 29.Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 1994;34(1):45–60. [DOI] [PubMed] [Google Scholar]
- 30.Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol 1999;52(4):371–379. [DOI] [PubMed] [Google Scholar]
- 31.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid–induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 2000;93(6): 1003–1013. [DOI] [PubMed] [Google Scholar]
- 32.Shinoda J, Sakai N, Murase S, Yano H, Matsuhisa T, Funakoshi T. Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection. J Neurooncol 2001;52(2):161–171. [DOI] [PubMed] [Google Scholar]
- 33.Stark AM, Nabavi A, Mehdorn HM, Blömer U. Glioblastoma multiforme—report of 267 cases treated at a single institution. Surg Neurol 2005;63(2):162–169. [DOI] [PubMed] [Google Scholar]
- 34.Martinez R, Janka M, Soldner F, Behr R. Gross-total resection of malignant gliomas in elderly patients: implications in survival. Zentralbl Neurochir 2007;68(4):176–181. [DOI] [PubMed] [Google Scholar]
- 35.Butowski N, Lamborn KR, Berger MS, Prados MD, Chang SM. Historical controls for phase II surgically based trials requiring gross total resection of glioblastoma multiforme. J Neurooncol 2007;85(1):87–94. [DOI] [PubMed] [Google Scholar]
- 36.Pichlmeier U, Bink A, Schackert G, Stummer W; ALA Glioma Study Group. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 2008;10(6):1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirotte BJ, Levivier M, Goldman S, et al. Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 2009;64(3):471–481. [DOI] [PubMed] [Google Scholar]
- 38.Senft C, Franz K, Blasel S, et al. Influence of iMRI-guidance on the extent of resection and survival of patients with glioblastoma multiforme. Technol Cancer Res Treat 2010;9(4):339–346. [DOI] [PubMed] [Google Scholar]
- 39.Ewelt C, Goeppert M, Rapp M, Steiger H-J, Stummer W, Sabel M. Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neurooncol 2011;103(3):611–618. [DOI] [PubMed] [Google Scholar]
- 40.Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 2011;13 (12):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi S, Kobayashi H, Terasaka S, et al. The impact of extent of resection and histological subtype on the outcome of adult patients with high-grade gliomas. Jpn J Clin Oncol 2012;42(4): 270–277. [DOI] [PubMed] [Google Scholar]
- 42.Dea N, Fournier-Gosselin M-P, Mathieu D, Goffaux P, Fortin D. Does extent of resection impact survival in patients bearing glioblastoma? Can J Neurol Sci 2012;39(5):632–637. [DOI] [PubMed] [Google Scholar]
- 43.Salvati M, Pichierri A, Piccirilli M, et al. Extent of tumor removal and molecular markers in cerebral glioblastoma: a combined prognostic factors study in a surgical series of 105 patients. J Neurosurg 2012;117(2):204–211. [DOI] [PubMed] [Google Scholar]
- 44.Oszvald A, Güresir E, Setzer M, et al. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. J Neurosurg 2012;116(2):357–364. [DOI] [PubMed] [Google Scholar]
- 45.Ahmadloo N, Kani AA, Mohammadianpanah M, et al. Treatment outcome and prognostic factors of adult glioblastoma multiforme. J Egypt Natl Canc Inst 2013;25(1):21–30. [DOI] [PubMed] [Google Scholar]
- 46.Kreth FW, Thon N, Simon M, et al. ; German Glioma Network. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol 2013;24(12): 3117–3123. [DOI] [PubMed] [Google Scholar]
- 47.Noorbakhsh A, Tang JA, Marcus LP, et al. Gross-total resection outcomes in an elderly population with glioblastoma: a SEER-based analysis. J Neurosurg 2014;120(1):31–39. [DOI] [PubMed] [Google Scholar]
- 48.Kiwit JC, Floeth FW, Bock WJ. Survival in malignant glioma: analysis of prognostic factors with special regard to cytoreductive surgery. Zentralbl Neurochir 1996;57(2):76–88. [PubMed] [Google Scholar]
- 49.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 2016;124(4): 977–988. [DOI] [PubMed] [Google Scholar]
- 50.Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms: a retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 1993;78(5): 767–775. [DOI] [PubMed] [Google Scholar]
- 51.Kelly PJ, Hunt C. The limited value of cytoreductive surgery in elderly patients with malignant gliomas. Neurosurgery 1994;34(1):62–66. [PubMed] [Google Scholar]
- 52.Jeremic B, Grujicic D, Antunovic V, Djuric L, Stojanovic M, Shibamoto Y. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol 1994;21(2):177–185. [DOI] [PubMed] [Google Scholar]
- 53.Kreth FW, Warnke PC, Scheremet R, Ostertag CB. Surgical resection and radiation-therapy in the treatment of glioblastoma-multiforme. J Neurosurg 1993;78(5):762–766. [DOI] [PubMed] [Google Scholar]
- 54.Kreth FW, Berlis A, Spiropoulou V, et al. The role of tumor resection in the treatment of glioblastoma multiforme in adults. Cancer 1999;86 (10):2117–2123. [PubMed] [Google Scholar]
- 55.Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol 2011;18(1):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uzuka T, Aoki H, Natsumeda M, Takahashi H, Fujii Y. Effectiveness of maximal safe resection for glioblastoma including elderly and low Karnofsky performance status patients: retrospective review at a single institute. Neurol Med Chir (Tokyo) 2012;52(8):570–576. [DOI] [PubMed] [Google Scholar]
- 57.Chaichana KL, Jusue-Torres I, Lemos AM, et al. The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol 2014;120(3):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalita O, Vaverka M, Hrabalek L, et al. The effect of a surgery rationale on life expectancy within primary glioblastoma multiforme patients: resection compared to sole biopsy, six and half year regular follow-up experiences of one onco-centre database of intracranial tumours (DOIT) of Czech Republic. Neuro-oncol 2014;16(suppl 2):ii97 10.1093/neuonc/nou174.373. [DOI] [Google Scholar]
- 59.Roder C, Bisdas S, Ebner FH, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA–assisted surgery. Eur J Surg Oncol 2014;40(3):297–304. [DOI] [PubMed] [Google Scholar]
- 60.Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people: a randomised study. Acta Neurochir (Wien) 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 61.Hrabalek L, Kalita O, Vaverka M, et al. Resectionversus biopsy of glioblastomas in eloquent brain areas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015;159(1):150–155. [DOI] [PubMed] [Google Scholar]
- 62.Zinn PO, Colen RR, Kasper EM, Burkhardt JK. Extent of resection and radiotherapy in GBM: a 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol 2013; 42(3):929–934. [DOI] [PubMed] [Google Scholar]
- 63.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 64.Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol 2012;3:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325(7):445–453. [DOI] [PubMed] [Google Scholar]
- 66.Barnett HJ, Taylor DW, Eliasziw M, et al. ; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998; 339(20):1415–1425. [DOI] [PubMed] [Google Scholar]
- 67.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348(9038):1329–1339. [DOI] [PubMed] [Google Scholar]
- 68.Bussière M, Wiebe S. The numbers needed to treat for neurological disorders. Can J Neurol Sci 2005;32(4):440–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


