Abstract
Background
Polycystic ovary syndrome (PCOS) affects 8% to 13% of reproductive‐aged women and is associated with reproductive and metabolic dysfunction. Obesity worsens the presentation of PCOS and weight management (weight loss, maintenance or prevention of excess weight gain) is proposed as an initial treatment strategy, best achieved through lifestyle changes incorporating diet, exercise and behavioural interventions.
Objectives
To assess the effectiveness of lifestyle treatment in improving reproductive, anthropometric (weight and body composition), metabolic and quality of life factors in PCOS.
Search methods
We searched the Cochrane Gynaecology and Fertility Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, CINAHL and AMED (date of last search March 2018). We also searched controlled trials registries, conference abstracts, relevant journals, reference lists of relevant papers and reviews, and grey literature databases, with no language restrictions applied.
Selection criteria
Randomised controlled trials (RCTs) comparing lifestyle treatment (diet, exercise, behavioural or combined treatments) to minimal or no treatment in women with PCOS.
Data collection and analysis
Two authors independently selected trials, assessed evidence quality and risk of bias, and extracted data. Our primary outcomes were live birth, miscarriage and pregnancy. We used inverse variance and fixed‐effect models in the meta‐analyses. We reported dichotomous outcomes as an odds ratio and continuous outcomes as a mean difference (MD) or standardised mean difference (SMD).
Main results
We included 15 studies with 498 participants. Ten studies compared physical activity to minimal dietary and behavioural intervention or no intervention. Five studies compared combined dietary, exercise and behavioural intervention to minimal intervention. One study compared behavioural intervention to minimal intervention. Risk of bias varied: eight studies had adequate sequence generation, seven had adequate clinician or outcome assessor blinding, seven had adequate allocation concealment, six had complete outcome data and six were free of selective reporting. No studies assessed the fertility primary outcomes of live birth or miscarriage. No studies reported the secondary reproductive outcome of menstrual regularity, as defined in this review.
Lifestyle intervention may improve a secondary (endocrine) reproductive outcome, the free androgen index (FAI) (MD ‐1.11, 95% confidence interval (CI) ‐1.96 to ‐0.26, 6 RCTs, N = 204, I2 = 71%, low‐quality evidence). Lifestyle intervention may reduce weight (kg) (MD ‐1.68 kg, 95% CI ‐2.66 to ‐0.70, 9 RCTs, N = 353, I2 = 47%, low‐quality evidence). Lifestyle intervention may reduce body mass index (BMI) (kg/m2) (‐0.34 kg/m2, 95% CI ‐0.68 to ‐0.01, 12 RCTs, N = 434, I2= 0%, low‐quality evidence). We are uncertain of the effect of lifestyle intervention on glucose tolerance (glucose outcomes in oral glucose tolerance test) (mmol/L/minute) (SMD ‐0.02, 95% CI ‐0.38 to 0.33, 3 RCTs, N = 121, I2 = 0%, low‐quality evidence).
Authors' conclusions
Lifestyle intervention may improve the free androgen index (FAI), weight and BMI in women with PCOS. We are uncertain of the effect of lifestyle intervention on glucose tolerance. There were no studies that looked at the effect of lifestyle intervention on live birth, miscarriage or menstrual regularity. Most studies in this review were of low quality mainly due to high or unclear risk of bias across most domains and high heterogeneity for the FAI outcome.
Keywords: Female, Humans, Life Style, Abdominal Fat, Abdominal Fat/anatomy & histology, Exercise, Insulin Resistance, Obesity, Obesity/complications, Obesity/therapy, Polycystic Ovary Syndrome, Polycystic Ovary Syndrome/complications, Polycystic Ovary Syndrome/rehabilitation, Randomized Controlled Trials as Topic, Virilism, Virilism/therapy, Waist Circumference, Weight Loss
Plain language summary
The effect of a healthy lifestyle for women with polycystic ovary syndrome
Review question
We reviewed the evidence on the effects of lifestyle interventions on reproductive, anthropometric (body measurement), metabolic and quality of life outcomes in women with polycystic ovary syndrome.
Background
Polycystic ovary syndrome (PCOS) is a very common condition affecting 8% to 13% of women. Being overweight worsens all clinical features of PCOS. These clinical features include reproductive issues such as reduced frequency of ovulation and irregular menstrual cycles, reduced fertility, polycystic ovaries on ultrasound and high levels of male hormones such as testosterone, which can cause unwanted facial or body hair growth and acne. PCOS is also associated with metabolic features, with risk factors for diabetes and cardiovascular disease including high levels of insulin or insulin resistance and abnormal cholesterol levels. PCOS affects quality of life and can worsen anxiety and depression either due to its symptoms or due to the diagnosis of a chronic disease. A healthy lifestyle consists of a healthy diet, regular exercise and achieving and maintaining a healthy weight.
Study characteristics
We found 15 studies that included 498 participants. Ten studies compared physical activity to minimal dietary and behavioural intervention or no intervention. Five studies compared combined dietary, exercise and behavioural intervention to minimal intervention. One study compared behavioural intervention to minimal intervention. The risk of bias in the studies varied and was generally unclear. The evidence is current to March 2018.
Key results
There were no studies that investigated the effect of a healthy lifestyle on live birth, miscarriage or regularity of menstrual cycles. Adopting a healthy lifestyle may result in weight loss or reduction in male hormone levels in some individuals. Diet and exercise may not have an effect on the body's ability to maintain normal blood glucose levels.
Quality of the evidence
The evidence was of low quality. The main limitations in the evidence were inconsistent and imprecise findings, and poor reporting of the methods used in the studies.
Summary of findings
Summary of findings for the main comparison. Lifestyle intervention compared to minimal treatment in women with polycystic ovary syndrome.
| Lifestyle intervention compared to minimal treatment in women with polycystic ovary syndrome | ||||
| Patient or population: women with polycystic ovary syndrome Setting: university, medical centre or hospital Intervention: lifestyle intervention Comparison: minimal treatment | ||||
| Outcomes | Anticipated effects | Effect estimate* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
| Fertility: live birth ‐ not reported | No study reported on this outcome | ‐ | ‐ | ‐ |
| Fertility: miscarriage ‐ not reported | No study reported on this outcome | ‐ | ‐ | ‐ |
| Secondary reproductive: menstrual regularity ‐ not reported | No study reported on this outcome in way defined by this review | ‐ | ‐ | ‐ |
| Secondary reproductive: free androgen index (FAI) | Lifestyle intervention may reduce free androgen index (FAI) | MD ‐1.11 (‐1.96 to ‐0.26) | 204 (6 RCTs) | ⊕⊕⊝⊝ LOW a, b |
| Anthropometric: weight (kg) | Lifestyle intervention may reduce body weight by 1.68 kg (reduction of 2.66 kg to 0.7 kg) compared to no intervention. | MD ‐1.68 (‐2.66 to ‐0.70) kg | 353 (9 RCTs) | ⊕⊕⊝⊝ LOW a, b |
| Anthropometric: body mass index (BMI) (kg/m2) | Lifestyle intervention may reduce body mass index (BMI) by 0.34 kg/m2 (reduction of 0.68 to 0.01 kg/m2). | MD ‐0.34 (‐0.68 to ‐0.01) kg/m2 | 434 (12 RCTs) | ⊕⊕⊝⊝ LOW a,c |
| Metabolic: oral glucose tolerance test (OGTT) glucose (mmol/L/minute) | We are uncertain of the effect of lifestyle intervention on oral glucose tolerance test (OGTT) glucose (mmol/L/minute). | SMD ‐0.02 (‐0.38 to 0.33) mmol/L/minute | 121 (3 RCTs) | ⊕⊕⊝⊝ LOW a,c |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
aDowngraded one level for imprecision: the confidence intervals for most studies cross 0 or small number of events (< 400).
bDowngraded one level for inconsistency: high heterogeneity with differing directions of effect.
cDowngraded one level for serious risk of bias: lack of blinding of participants, clinicians or outcome assessors and/or attrition bias in a number of studies.
Background
Description of the condition
Polycystic ovary syndrome (PCOS) is a common condition with a range of clinical features that affects women of reproductive age. These reproductive features include oligo‐anovulation (reduced ovulation), irregular menstrual cycles, biochemical hyperandrogenism (elevated circulating male hormones or androgens such as testosterone), clinical hyperandrogenism (effects of androgens on body tissues including hirsutism or excess hair growth) and infertility (Teede 2011). Metabolic features include increased risk factors for type 2 diabetes mellitus and cardiovascular disease (worsened lipid profile, high blood pressure, worsened blood vessel function) (Heida 2016; Meyer 2005; Paradisi 2001; Rubin 2017), and an increase in the prevalence of the metabolic syndrome (a clustering of risk factors for cardiovascular disease), impaired glucose tolerance or prediabetes, type 2 diabetes and potentially cardiovascular disease (Kakoly 2018; Moran 2010b). The European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) international consensus workshop group have expanded the diagnostic guidelines for PCOS to the Rotterdam criteria, based on presentation with any two of the three criteria of hyperandrogenism, irregular anovulatory cycles or polycystic ovaries on ultrasound, with exclusion of related reproductive disorders (ESHRE/ASRM 2004). Studies reporting PCOS prevalence using ESHRE/ASRM criteria (March 2010), including a systematic review and meta‐analysis of 15 trials, have shown that 8% to 13% of women have PCOS (Bozdag 2016). The recent international evidence‐based guidelines for PCOS revise these criteria in adolescents, now needing both hyperandrogenism and irregular cycles with ultrasound not recommended (International PCOS Guideline 2018).
The aetiology of PCOS is unknown although abnormalities in steroidogenesis (the production of steroid hormones such as reproductive hormones) and gonadotrophin action (the action of hormones that control reproductive hormone production) are implicated. Insulin resistance and compensatory hyperinsulinaemia are proposed as significant aetiological factors and are present in 75% and 95% of lean and overweight women with PCOS respectively (Alebic 2014; Behbourdi‐Gandevani 2016; DeUgarte 2005; Stepto 2013). These play a key role in PCOS through insulin stimulating ovarian androgen production (Barbieri 1986), and decreasing sex hormone‐binding globulin (SHBG) production in the liver (Plymate 1988), resulting in hyperandrogenism. Excess body weight worsens the underlying hormonal disturbances (increasing androgen and insulin levels) and the clinical features evident in women with PCOS.
Description of the intervention
Treatment aims in PCOS include optimising healthy weight, improving underlying hormonal disturbances, prevention of future reproductive and metabolic complications, and improving quality of life. Lifestyle interventions (dietary, exercise, behavioural or combined) are recommended as first‐line management in an international evidence‐based guideline on PCOS (International PCOS Guideline 2018). In addition, medical treatments involve the targeting of biochemical and clinical hyperandrogenism and reproductive and metabolic features. Targeted symptomatic treatment of PCOS includes combination oral contraceptives to improve hyperandrogenism, anti‐androgens for treatment of hirsutism and oral contraceptives or intermittent progestins to regulate menstrual cycles. The first‐line treatment of anovulatory infertility includes ovulation induction agents, second‐line treatment includes gonadotrophins and third‐line treatment includes in vitro fertilisation techniques and laparoscopic ovarian surgery (International PCOS Guideline 2018). Where metabolic features are a concern, preventative and therapeutic use of insulin‐sensitising agents has increasingly been adopted, both in isolation or in combination with other pharmacological options (International PCOS Guideline 2018; Naderpoor 2015; Teede 2007). Overall, the use of lifestyle interventions can present a cost‐effective initial treatment strategy compared to surgical and pharmacological options (Clark 1998). Lifestyle interventions are recommended to prevent excess weight gain, manage weight and prevent future reproductive and metabolic complications (International PCOS Guideline 2018). Prevention opportunities are particularly relevant as women with PCOS have a greater prevalence of overweight and obesity compared to the general population (Kakoly 2018; Lim 2012). Lifestyle intervention may also improve insulin resistance or other features of PCOS independent of weight loss, as demonstrated in the general population (Poehlman 2000; Roberts 2013). As such, there is a strong rationale for lifestyle interventions for both improving underlying hormonal imbalances and management of weight for a large proportion of women with PCOS.
How the intervention might work
Both hyperandrogenism and insulin resistance and hyperinsulinaemia underpin PCOS; women with PCOS are generally more insulin‐resistant and hyperandrogenic, presenting with worsened clinical reproductive and metabolic features than body mass index (BMI)‐matched controls (DeUgarte 2005; Yang 2016). These hormonal imbalances are further worsened by the presence of overweight (defined as a BMI > 25 kg/m2) and obesity (defined as a BMI > 30 kg/m2) (Acien 1999; Lim 2013). Obese and overweight women with PCOS display worsened clinical reproductive (Balen 1995; Kiddy 1990; Lønnebotn 2018) and metabolic features (Ehrmann 2006; Kakoly 2018; Legro 1999). Lifestyle intervention is therefore anticipated to work because a reduction in BMI will be associated with a reduction in insulin resistance, which will, in turn, lead to an improvement in the reproductive and metabolic features of PCOS.
Why it is important to do this review
Despite the high prevalence of PCOS, a strong rationale for lifestyle intervention and the fact that lifestyle intervention is recommended as first‐line treatment in women with PCOS who are overweight, the literature in this area is limited and challenging to interpret. There are a large number of small, uncontrolled trials demonstrating that weight loss achieved through lifestyle management decreases abdominal fat, hyperandrogenism and insulin resistance, and improves lipid profiles, menstrual cyclicity, fertility and risk factors for type 2 diabetes and cardiovascular disease in overweight women with PCOS (Clark 1998; Huber‐Buchholz 1999; Lass 2011; Moran 2003). There is additionally some evidence in both PCOS patients (Hutchison 2011) and the general population (Poehlman 2000; Roberts 2013; Ross 2000) that exercise improves metabolic risk factors in PCOS, even when no weight loss occurs. A Cochrane Review was previously published to summarise these findings (Moran 2011); however, many randomised controlled trials have been published since. This updated review provides the latest summary and assesses the evidence for the effectiveness of lifestyle management in improving reproductive and metabolic features in women with PCOS. Provision of this evidence has significant implications for the treatment of both short‐term reproductive abnormalities and long‐term metabolic morbidity and mortality in PCOS. The aim of this review is, therefore, to provide an update on the effect of lifestyle treatment (defined as a dietary, exercise or behavioural intervention, or a combination) on reproductive, anthropometric (weight and body composition), metabolic and quality of life factors in women with PCOS.
Objectives
To assess the effectiveness of lifestyle treatment in improving reproductive, anthropometric (weight and body composition), metabolic and quality of life factors in PCOS.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) that compare lifestyle intervention to minimal treatment for inclusion in the review. We included cross‐over trials in the review for completeness but we only included data from the first phase in the meta‐analyses as the interventions under study are anticipated to have lasting effects and the cross‐over design is not valid in this context. Quasi‐randomised trials were not included.
Types of participants
Females of reproductive age (postmenarchal and premenopausal) with PCOS. We included studies using any definition of PCOS or overweight in this review, with the trialist's definition of PCOS and overweight described.
We excluded conditions with reproductive symptoms similar to PCOS, including congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinaemia, thyroid disease and androgen‐secreting tumours. Participants were not excluded based on type 2 diabetes, co‐morbidities or medication use for clinical or metabolic features of PCOS, as long as this medication use was not a primary component of the intervention or control arms. In this scenario, we noted type 2 diabetes, co‐morbidities or medication use and assessed the effects on outcome measures. Participants were not excluded based on ethnicity.
Types of interventions
We included RCTs comparing a lifestyle intervention to minimal treatment. Lifestyle intervention was defined as a structured dietary, exercise or behavioural intervention (both those designed to induce weight loss through an energy deficit or not designed to induce weight loss through an energy deficit) while minimal treatment was defined as either no treatment or standard unstructured minimal dietary, exercise or behavioural advice. A structured programme referred to more than one study visit allocated to the implementation of the dietary, exercise or behavioural treatment.
This aimed to include trials examining:
dietary intervention versus minimal treatment;
exercise intervention (resistance or aerobic exercise) versus minimal treatment;
behavioural management techniques for modifying diet or exercise versus minimal treatment;
a combination of dietary, exercise or behavioural intervention versus minimal treatment.
We included all study durations over two weeks.
Types of outcome measures
Primary outcomes
To be measured at the end of intervention (endpoint or change).
Fertility
Live birth and pregnancy, as defined by study authors
Miscarriage, as defined by study authors
Secondary outcomes
To be measured at the end of intervention (endpoint or change).
Secondary reproductive
Menstrual regularity (an initiation of menses or significant shortening of cycle length where possible), ovulation (number of ovulatory menstrual cycles where possible)
Endocrine (total testosterone, sex hormone‐binding globulin (SHBG), free androgen index (FAI) and clinical hyperandrogenism (hirsutism assessed clinically by Ferriman‐Gallwey score)
Anthropometric
Weight, BMI, adiposity distribution (by measures including waist circumference, waist‐to‐hip ratio (WHR))
Metabolic
Oral glucose tolerance test (OGTT), glucose
Fasting glucose
Fasting lipids (total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), triglycerides)
Fasting insulin
Oral glucose tolerance test (OGTT), insulin
Quality of life and participant satisfaction
Search methods for identification of studies
We sought all published and unpublished RCTs of a lifestyle intervention compared to minimal treatment using the following search strategy, without language restriction and in consultation with Cochrane Gynaecology and Fertility Information Specialist. (See the methods of the review: Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7).
Electronic searches
We searched:
the Cochrane Gynaecology and Fertility Specialised Register, PROCITE platform (5 March 2018) (Appendix 1);
the Cochrane Central Register of Controlled Trials; via the Cochrane Register of Studies Online (CRSO Web platform) (searched 5 March 2018) (Appendix 2);
MEDLINE Ovid (1946 to 5 March 2018) (Appendix 3);
Embase Ovid (1980 to 5 March 2018) (Appendix 4);
PsycINFO Ovid(1806 to 5 March 2018) (Appendix 5);
CINAHL EBSCO (1961 to 5 March 2018) (Appendix 6);
AMED Ovid (1985 to 5 March 2018) (Appendix 7).
We also searched the trials registers ClinicalTrials.gov (http://clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/trialsearch/Default.aspx), grey literature in OpenSIGLE (http://opensigle.inist.fr/) and Latin American and Caribbean trials in the LILACS database (http://lilacs.bvsalud.org/en/), using the keywords: polycystic ovary syndrome (PCOS) (April 2018).
Searching other resources
We handsearched the references of relevant reviews, systematic reviews and included studies to locate other potentially eligible studies.
Data collection and analysis
We conducted data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two of four review authors (SKH with SSL, EVR or LJM) undertook the study selection. SSL, EVR, LJM and SKH screened the titles and abstracts of articles found in the search and discarded studies that were clearly ineligible. They aimed to be overly inclusive rather than risk losing relevant studies. We retrieved the full text of all potentially eligible studies. Two review authors independently assessed whether the studies met the inclusion criteria, with disagreements resolved by consensus and discussion with a third author, if necessary. We sought further information from the authors where papers contained insufficient information to make a decision about eligibility.
Data extraction and management
We extracted the following information from the studies included in the review and presented this in the Characteristics of included studies table. Where studies had multiple publications, we used the main trial report as the reference and supplied additional details from secondary papers. Two individuals (SKH with SSL, EVR, LJM or JB) extracted all data independently using data extraction forms designed to Cochrane guidelines. Data from papers written in languages other than English were extracted by translators. We corresponded with study investigators in order to resolve any data queries, as required. We contacted study investigators on a minimum of two occasions (at least once for initial query and at least once for reminder query). We sought additional information on data or trial methodology and actual trial data from the authors of trials that appeared to meet the eligibility criteria but had aspects of methodology that were unclear or data in an unsuitable form for meta‐analysis. We recorded discrepancies in the extracted data and resolved these by consensus.
For each included trial, we collected information regarding the location of the study, methods of the study (design, setting, source of funding), the participants (definition of PCOS, age and BMI range, eligibility criteria, concurrent treatments), the nature of the interventions and data relating to the outcomes specified above. Unit conversion factors are shown in Table 2.
1. Conversion factors.
| Convert from | Convert to | Conversion factor | |
| Total testosterone | ng/dL | nmol/L | 0.03467 |
| SHBG | µg/dL | mmol/L | 34.7 |
| Glucose | mg/dL | mmol/L | 0.056 |
| Cholesterol | mg/dL | mmol/L | 0.026 |
| HDL‐C | mg/dL | mmol/L | 0.0259 |
| LDL‐C | mg/dL | mmol/L | 0.0259 |
| Triglycerides | mg/dL | mmol/L | 0.0113 |
| Standard deviation | Standard error | Standard deviation | Sqrt n |
HDL‐C: high‐density lipoprotein cholesterol, LDL‐C: low‐density lipoprotein cholesterol, SHBG: sex hormone‐binding globulin
Assessment of risk of bias in included studies
We assessed the included studies for risk of bias using the Cochrane 'Risk of bias' tool (Appendix 8). This tool assesses: sequence generation; allocation concealment; blinding of participants, providers and outcome assessors; completeness of outcome data; selective outcome reporting; and other potential sources of bias. Two authors assessed these six domains (SKH with SSL, EVR, LJM or JB), with any disagreements resolved by consensus or by discussing with a third author. We presented the conclusions in the 'Risk of bias' table and figures ('Risk of bias' in Characteristics of included studies table; Figure 1; Figure 2) and incorporated them into the interpretation of the review findings by means of sensitivity analysis (see below).
1.
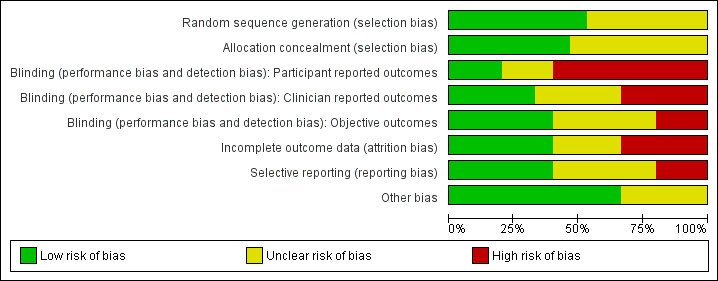
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
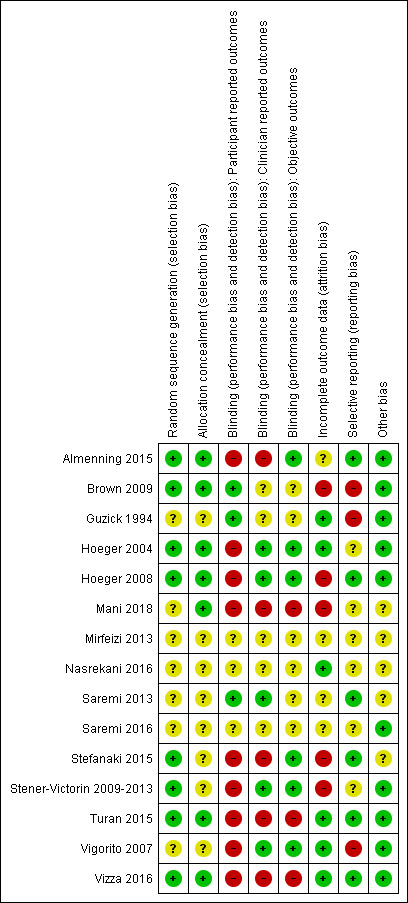
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
For dichotomous data, we used the number of events in the control and intervention groups of each study to calculate a Peto odds ratio (OR). For continuous data, we calculated a mean difference (MD) between treatment groups if all studies reported exactly the same outcomes. If similar outcomes were reported on different scales, we calculated the standardised mean difference (SMD). We presented 95% confidence intervals (CI) for all outcomes.
Unit of analysis issues
The primary analysis was per women randomised. We briefly summarised reported data that did not allow valid analysis (for example 'per cycle' rather than 'per woman' where women contributed to more than one cycle) in an additional table and did not meta‐analyse these data.
Dealing with missing data
We analysed the data on an intention‐to‐treat (ITT) basis, as far as possible, and made attempts to obtain missing data from the original investigators. Where these were unobtainable, we undertook imputation of individual values for the primary outcomes only. If studies reported sufficient detail to calculate mean differences but no information on associated standard deviations (SD), we assumed the outcome to have a standard deviation equal to the highest SD from other studies within the same analysis. For other outcomes, we only analysed the available data. We subjected any imputation undertaken to sensitivity analysis (see below).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic. We took an I2 value greater than 50% to indicate substantial heterogeneity (Higgins 2011). If substantial heterogeneity was detected, we explored possible explanations in sensitivity analyses (see below).
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
Where possible, we combined the data from primary studies using a fixed‐effect model. Meta‐analytic methods for continuous data assume that the underlying distribution of the measurements is normal. Where data were clearly skewed and results were reported in the publication as median and range with non‐parametric tests of significance, we excluded the results from the meta‐analysis. We conducted data management and analysis using Review Manager (RevMan) 5.3 (RevMan 2014). We displayed an increase in the odds of a particular outcome graphically in the meta‐analysis to the right of the centre line and a decrease in the odds of an outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to determine the separate evidence within the following subgroups.
Duration of intervention (short: two to four weeks, medium: four weeks to six months, long: greater than six months).
Component of intervention (dietary alone versus exercise alone versus behavioural intervention alone versus combined intervention).
Weight loss versus weight maintenance studies.
Studies where the inclusion criterion was overweight participants versus studies with no specific inclusion criterion for overweight participants.
Primary fertility outcomes (pregnancy, live birth and miscarriage) were only measured in a subgroup of eligible participants actively seeking pregnancy as part of the inclusion criteria. Where individual‐level data were required for this subgroup analysis, we contacted the authors.
Sensitivity analysis
We performed sensitivity analysis whereby:
eligibility was restricted to studies without a high risk of bias; we assessed only studies with a low risk of bias (assessed by random sequence generation and allocation concealment) in a separate analysis (blinding was not used in the sensitivity analysis as it was not possible to blind participants and the intervention provider due to the interactive nature of the interventions);
studies with outlying results were excluded;
alternative imputation strategies were adopted;
a random‐effects model was adopted.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro and Cochrane methods (GRADEpro GDT 2015; Higgins 2011). This table evaluated the overall quality of the body of evidence for the main review outcomes (live birth, miscarriage, menstrual regularity, FAI, body weight, BMI and glucose tolerance) for the main review comparison (lifestyle intervention versus minimal treatment). We assessed the quality of the evidence using the GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). We made judgements about evidence quality (high, moderate, low or very low) according to these criteria. We justified, documented and incorporated the judgements into our reporting of the results for each outcome.
We extracted study data, formatted our comparisons in data tables and prepared a 'Summary of findings' table before writing the results and conclusions of our review.
Results
Description of studies
(See Characteristics of included studies, and Characteristics of excluded studies tables).
Results of the search
For the current review, we identified a total of 4236 articles from: electronic databases (n = 2587), the Cochrane Gynaecology and Fertility Specialised Register (n = 478), handsearches (n = 261) and web sources of controlled trials (n = 910). After the initial exclusion of articles based on title or abstract, we retrieved 93 full‐text articles for more detailed evaluation. From these, we excluded 80 articles following full‐text screening as they did not meet the inclusion criteria (see Characteristics of excluded studies table). We categorised one article as awaiting classification due to our inability to obtain translated results for data analysis (Gaeini 2012). Six included studies from the original review (Moran 2011) were added to the 14 included studies from the current review. See Figure 3 for a PRISMA study flow diagram of the search and selection process.
3.
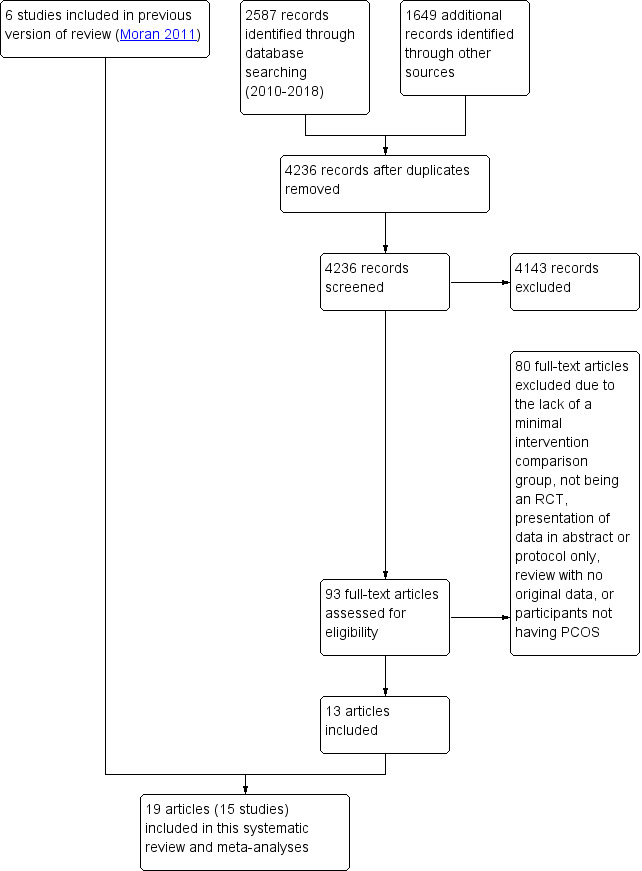
Study flow diagram.
Included studies
Design
We included 15 RCTs (from 19 articles) in this review (Almenning 2015; Brown 2009; Guzick 1994; Hoeger 2004; Hoeger 2008; Mani 2018; Nasrekani 2016; Saremi 2013; Saremi 2016; Stefanaki 2015; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vizza 2016; Vigorito 2007; Mirfeizi 2013). Trial characteristics are presented in the Characteristics of included studies table. One of the studies (Stener‐Victorin 2009‐2013) was reported in five articles (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013).
We contacted all corresponding authors of included trials for additional information, with seven authors providing some or all of the additional requested information (Almenning 2015; Brown 2009; Hoeger 2004; Hoeger 2008; Mani 2018, Stener‐Victorin 2009‐2013; Vigorito 2007) and the authors of one study unable to provide additional information due to the timing and location of the study (Guzick 1994).
All studies were reported as RCTs with a total number of 498 participants completed and analysed. Sample sizes ranges from 12 to 100 in each trial (N = 12 (Guzick 1994); N = 13 (Hoeger 2004; Vizza 2016); N = 18 (Hoeger 2008); N = 20 (Brown 2009; Nasrekani 2016); N = 22 (Saremi 2013); n=24 (Mirfeizi 2013); N = 25 (Almenning 2015); N = 28 (Saremi 2016); N = 30 ( Turan 2015); N = 38 (Stefanaki 2015); N = 45 (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013); N = 90 (Vigorito 2007); N = 100 (Mani 2018)).
The reported dropout rates for the arms studied were: 0% at 8 to 16 weeks (Guzick 1994; Nasrekani 2016; Saremi 2013; Vigorito 2007); 6% at 8 weeks (Turan 2015); 7% at 8 weeks (Saremi 2016); 12% at 16 weeks (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013); 13% at 12 weeks (Vizza 2016); 17% at 8 weeks (Stefanaki 2015); 18% at 24 weeks (Hoeger 2008); 19% at 10 weeks (Almenning 2015); 23% at 12 weeks (Mirfeizi 2013); 35% at 48 weeks (Hoeger 2004); 42% at 12 months (Mani 2018); and 46% at 16 weeks (Brown 2009).
Most studies (13/15) were of medium duration (four weeks to six months), while the remaining two studies were of long duration (beyond six months) (Hoeger 2004; Mani 2018).
The studies were conducted at university, hospital or clinical research centres in the USA (Brown 2009; Guzick 1994; Hoeger 2004; Hoeger 2008), Australia (Vizza 2016), Greece (Stefanaki 2015), Iran (Nasrekani 2016; Saremi 2013; Saremi 2016), Norway (Almenning 2015), Turkey (Turan 2015), the UK (Mani 2018), Sweden (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013) or Italy (Vigorito 2007).
Study durations were: 8 weeks (Saremi 2013; Saremi 2016; Stefanaki 2015; Turan 2015); 10 weeks (Almenning 2015); 12 weeks (Brown 2009;Mirfeizi 2013; Guzick 1994; Nasrekani 2016; Vigorito 2007; Vizza 2016); 16 weeks ( Stener‐Victorin 2009‐2013); 24 weeks (Hoeger 2008); 48 weeks (Hoeger 2004); and 12 months (Mani 2018). There were no short‐term studies; 15 were medium‐term and two were long‐term studies.
The lifestyle intervention was either a structured physical activity intervention (Almenning 2015; Brown 2009; Nasrekani 2016; Saremi 2013; Saremi 2016; Stener‐Victorin 2009‐2013; Turan 2015; Vigorito 2007; Vizza 2016), a combined dietary and exercise intervention (Guzick 1994; Hoeger 2004; Hoeger 2008; Mani 2018; Mirfeizi 2013), or a behavioural intervention (Stefanaki 2015). Interventions were designed to specifically induce weight loss (Guzick 1994; Hoeger 2004; Hoeger 2008), not specifically induce weight loss (Almenning 2015; Mani 2018; Mirfeizi 2013; Nasrekani 2016; Saremi 2013; Saremi 2016; Stefanaki 2015; Turan 2015; Vigorito 2007; Vizza 2016), or to be about weight maintenance (Brown 2009; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013).
As detailed below, under the inclusion criteria, studies specifically assessed overweight participants (Guzick 1994; Hoeger 2004; Hoeger 2008; Mani 2018; Vigorito 2007), or did not specifically assess overweight participants. Control groups either received no lifestyle advice (Brown 2009; Guzick 1994; Hoeger 2004; Nasrekani 2016; Stefanaki 2015; Vizza 2016), or minimal lifestyle advice (Almenning 2015; Mani 2018; Hoeger 2008; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007).
Participants
The PCOS diagnosis was either consistent with the ESHRE/ASRM criteria (ESHRE/ASRM 2004): two of three of hyperandrogenism, oligo‐ or anovulation or ultrasound polycystic ovary (PCO) morphology (Almenning 2015; Jedel 2011; Leonhardt 2015; Mani 2018; Nasrekani 2016; Saremi 2013; Saremi 2016; Stefanaki 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007); or consistent with the National Institutes of Health (NIH) (Zawadski 1992) two criteria of anovulation and hyperandrogenism (clinical or biochemical) (Brown 2009; Guzick 1994; Hoeger 2004; Hoeger 2008), with the exclusion of other disorders; or as confirmed by a general practitioner or specialist (Vizza 2016).
The main inclusion criteria were as follows.
Overweight or obese (Guzick 1994; Hoeger 2004; Hoeger 2008; Mani 2018; Mirfeizi 2013; Vigorito 2007).
Aged one year postmenarchal 12 to 18 years (Hoeger 2008), 15 to 40 years (Stefanaki 2015), 18 to 37 years (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013), 18 to 42 years (Vizza 2016), 18 to 50 years (Brown 2009), 18 to 49 years (Mani 2018), 20 to 40 years (Guzick 1994) or not specified (Almenning 2015; Hoeger 2004; Nasrekani 2016; Saremi 2013; Saremi 2016; Turan 2015; Vigorito 2007).
The main exclusion criteria were as follows.
Pregnancy (Almenning 2015; Brown 2009; Hoeger 2004; Mani 2018; Vigorito 2007) or recent breastfeeding (Brown 2009; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Stefanaki 2015).
Impaired glucose tolerance (Vigorito 2007), type 2 diabetes (known or elevated fasting glucose) (Hoeger 2004; Mani 2018; Turan 2015) or fasting hyperglycaemia (Brown 2009).
Pre‐study use of medication: oral contraceptives, glucocorticoids, anti‐androgens, ovulation induction agents, anti‐diabetic, anti‐obesity, other hormonal drugs within the previous six months or during study (Vigorito 2007), hormonal medication (Hoeger 2004), oral contraceptives, oestrogen or progestin or other drugs known to affect lipoprotein metabolism within two months of the study (Hoeger 2008) or hormonal contraceptive use, antiandrogen therapy, use of medications known to affect carbohydrate metabolism (metformin and thiazolidinediones) within past 90 days (Brown 2009) or medication use for less than three months (Stener‐Victorin 2009‐2013).
Concurrent treatments: no use of insulin sensitisers, anti‐androgen therapy, anti‐hypertensives, corticosteroid or statin therapy (Almenning 2015; Hoeger 2008; Mani 2018) or drugs that are known to affect gonadotrophin secretion or ovulation (Almenning 2015; Hoeger 2008; Saremi 2013) during the study. For Hoeger 2004, one control took progestin for 10 days for unscheduled heavy bleeding at week 16.
Neoplastic disease (Brown 2009; Guzick 1994; Vigorito 2007), cardiovascular disorder (Guzick 1994; Saremi 2013; Turan 2015; Vigorito 2007), cerebrovascular disease (Guzick 1994), concurrent medical illness (e.g. heart failure, lung) (Vigorito 2007; Turan 2015), significant ovarian surgery (Hoeger 2008), psychiatric disorders (Guzick 1994; Stefanaki 2015).
Abnormal kidney or liver function (Guzick 1994; Hoeger 2004; Hoeger 2008; Saremi 2013; Turan 2015; Vigorito 2007).
Regular exercise (Almenning 2015; Brown 2009; Hoeger 2008; Saremi 2013).
Smoking (Hoeger 2008; Saremi 2016), current alcohol use or history of substance abuse (Hoeger 2008).
At baseline, participants in the intervention and control groups were comparable with the exception of Brown 2009, where intervention participants were significantly older.
With regards to comparisons between studies, overweight was an inclusion criterion in six studies (Guzick 1994; Hoeger 2004; Hoeger 2008; Mani 2018; Mirfeizi 2013; Vigorito 2007). As impaired glucose tolerance was excluded in participants in five studies (Brown 2009; Hoeger 2004; Mani 2018; Turan 2015; Vigorito 2007), this potentially introduced participants with worsened glucose tolerance for the remaining studies, which could impact on the baseline or end score glucose. In Hoeger 2008, one participant had type 2 diabetes, six impaired glucose tolerance and four impaired fasting glucose, although it was not stated whether these were from the intervention, control or additional arms of the study.
Interventions
The studies assessing structured physical activity interventions consisted of the following.
Three supervised 40‐minute training sessions/week at 60% to 70% VO2 max (Vigorito 2007).
Individualised prescriptions (average of 228 minutes/week at 40% to 60% peak VO2) (Brown 2009).
Thirty to 45 minutes of moderate exercise beyond daily physical activity with pulse frequency above 120 beats/minute (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013).
Three weekly sessions of high‐intensity interval training (90% to 95% individual heart rate maximum or strength training of eight drills with a resistance of 75% of one repetition maximum with 10 reps and three sets) (Almenning 2015).
Three supervised sessions per week of aerobic training at 40% to 65% maximum heart rate reserve (Nasrekani 2016).
Three sessions of 40 to 60 minutes per week of an aerobic training programme (Saremi 2013).
Three supervised sessions per week of resistance exercise with placebo or calcium supplementation (Saremi 2016).
Three supervised sessions per week (50 to 60 minutes per session) of a structured exercise programme (Turan 2015).
Two supervised progressive resistance training sessions per week (60 minutes per session) (Vizza 2016).
For studies assessing a combined lifestyle intervention, the interventions were as follows:
Meal replacement formula diet (Optifast) with meals and multivitamin supplements (energy intake 4200 to 5040 kJ/day), behavioural modification training and individualised energy expenditure goals in a non‐supervised environment (Guzick 1994).
Dietary, exercise and behavioural intervention aiming for a 7% to 10% weight loss through individual and group dietitian and exercise physiologist meetings weekly from weeks 0 to 24 and bi‐weekly from weeks 25 to 48 and individualised meal (2100 to 4200 kJ/day energy deficit) and exercise plans (150 minutes/week) (Hoeger 2004). Hoeger 2008 was based on the methodology of Hoeger 2004, with weekly group or individual training classes for diet, exercise and behavioural modification skills with overall therapy goals of a weight loss of 5% to 7% and a weekly level of exercise of at least 150 minutes/week.
A single session consisting of seven hours of interactive group discussion that included diet and physical activity and self‐management in PCOS (Mani 2018).
An eight‐week mindfulness stress management programme through a 30‐minute audio CD. Participants were required to participate daily in the programme (Stefanaki 2015).
No studies reported any financial compensation received by the study participants or costs incurred by participants.
The number of visits often differ between intervention and control groups, with more visits in the intervention groups:
24 versus six visits (Hoeger 2008).
36 versus 12 visits (Hoeger 2004).
39 versus three visits (Vigorito 2007).
24 versus one visit (Turan 2015).
There were also other potential confounders in the intervention duration, type or delivery. In Brown 2009 the study duration was longer for the intervention group compared to the controls. In addition, in Hoeger 2004 both the intervention and control groups received a placebo. The participant population in the study Hoeger 2008 was adolescents as opposed to adults and participants also attended the dietary, exercise and behavioural visits with a parent or guardian. In Stefanaki 2015, participants included both adolescents and adults.
Intervention compliance was reported for Almenning 2015 (87% compliance in the strength‐training group and 90% compliance in the high‐intensity interval training group), Mani 2018 (77% attended sessions, none recorded time duration for physical activities), Vizza 2016 (76% adherence to progressive resistance training and 43% adherence to home‐based callisthenics training, Hoeger 2008 (for the intervention group four participants attended < 50% of the lifestyle sessions and the remaining attended at least 75% of sessions), Vigorito 2007 (exercising women attended an average or 28 ± 2 sessions with an accuracy of 0.78 indicating number of expected sessions/effective sessions performed) and Brown 2009 (mean adherence rate, defined as minutes of exercise at a prescribed heart rate completed divided by minutes prescribed, of 89.8%).
Outcomes
For the primary fertility outcomes, two studies reported on pregnancy data (two pregnancies in the lifestyle arm (Hoeger 2004) and three pregnancies in the entire study (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013). In Hoeger 2004 only 2 of 20 participants were actively seeking pregnancy prior to the intervention and in Stener‐Victorin et al (Stener‐Victorin 2009‐2013) no participants were actively seeking pregnancy prior to the intervention. For these studies, pregnancy was not a defined outcome or aim of the study. We therefore excluded this variable from the analysis.
No studies reported live birth or miscarriage.
For the secondary reproductive outcomes, four studies reported on menstrual regularity (Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007). Three studies reported on ovulation (Guzick 1994; Hoeger 2004; Hoeger 2008).
For the secondary endocrine outcomes, 10 studies reported on total testosterone (Almenning 2015; Guzick 1994; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Mani 2018; Mirfeizi 2013; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007; Vizza 2016), nine studies reported on sex hormone‐binding globulin (SHBG) (Almenning 2015; Guzick 1994; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Mani 2018; Mirfeizi 2013; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007; Vizza 2016), and six studies reported on free androgen index (FAI) or Ferriman‐Gallwey score (Almenning 2015; Vizza 2016; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007).
For secondary anthropometric factors, nine studies reported on weight (Almenning 2015; Hoeger 2008; Mani 2018; Nasrekani 2016; Saremi 2013; Saremi 2016; Stener‐Victorin 2009‐2013; Vigorito 2007; Vizza 2016), with separate analyses performed due to the use of different scales (% change in weight (Hoeger 2004) and kg in weight (Almenning 2015; Hoeger 2008; Mani 2018; Nasrekani 2016; Saremi 2013; Vigorito 2007; Vizza 2016). Twelve studies reported on BMI (Almenning 2015; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Mani 2018; Nasrekani 2016; Saremi 2013; Saremi 2016; Stefanaki 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007; Vizza 2016). Eight studies reported on adiposity distribution (Almenning 2015; Guzick 1994; Hoeger 2008; Jedel 2011; Leonhardt 2015; Saremi 2013Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007; Vizza 2016), with separate analyses performed due to the use of different scales (waist circumference (Almenning 2015; Hoeger 2008; Saremi 2013; Turan 2015; Vigorito 2007; Vizza 2016) and waist to hip ratio (WHR) (Guzick 1994; Jedel 2011; Leonhardt 2015; Saremi 2013; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007).
For secondary metabolic outcomes, three studies reported on area under the curve (AUC) insulin (Hoeger 2004; Hoeger 2008; Vigorito 2007) or glucose tolerance (OGTT glucose) (Hoeger 2004; Hoeger 2008; Vigorito 2007), with the meta‐analysis calculated using the SMD for the endpoint 120‐minute or 180‐minute AUC glucose or insulin. Nine studies reported on endpoint lipids (Almenning 2015; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Mani 2018; Saremi 2013; Saremi 2016; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007). Eleven studies reported on fasting glucose or fasting insulin (Almenning 2015; Guzick 1994; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Mani 2018; Saremi 2013; Saremi 2016; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vigorito 2007; Vizza 2016).
Three studies reported quality of life (Jedel 2011; Leonhardt 2015; Stefanaki 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vizza 2016). No studies reported participant satisfaction data.
Excluded studies
We excluded a total of 80 studies after full‐text consideration. The reasons for excluding 49 studies are presented in the Characteristics of excluded studies table (Asemi 2015; Atiomo 2009; Azadi‐Yazdi 2017; Beena 2016; Bruner 2006; Curi 2012; Ebrahimi 2014; Foroozanfard 2017; Fux Otta 2010; Giallauria 2008; Glueck 2006; Gower 2015; Hamayeli 2010; Hutchison 2012; Jakubowicz 2013; Jiskoot 2017; Johnson 2015; Kim 2013; Konopka 2015; Legro 2015; Marzouk 2015; Mehrabani 2012; Moran 2006; Moran 2010a; Nidhi 2012; Nybacka 2013; Orio 2008; Orio 2016; Ornstein 2011; Palomba 2008; Palomba 2010; Panico 2014; Papakonstantinou 2016; Pasquali 1986; Pasquali 2000; Pekhlivanov 2006; Roessler 2013; Sa 2016; Sordia‐Hernandez 2016; Sorensen 2012; Sprung 2013; Talluto 2002; Tang 2006; Thomson 2008; Thomson 2016; Toscani 2011; Turner‐McGrievy 2014; Wong 2016; Zarrinkoub 2005).
In total, we excluded 62 due to the absence of a comparator group with minimal intervention; six due to the study not being an RCT, five due to presentation of data in an abstract or protocol only, two due to participants not having PCOS, one because we were unable to obtain full texts, one that was a review with no original data, one because there were no results from PCOS patients, one that was a duplicate report and one due to an irrelevant outcome.
Risk of bias in included studies
Our assessment of risk of bias in the included studies is presented in Figure 1 and Figure 2.
Allocation
Sequence generation
Eight studies described adequate methods of generating randomised sequences (computer generation) (Almenning 2015; Brown 2009; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Stefanaki 2015Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Turan 2015; Vizza 2016), whereas insufficient information was provided for the other studies, which may introduce selection bias (Guzick 1994; Mani 2018; Mirfeizi 2013; Nasrekani 2016; Saremi 2013; Saremi 2016; Vigorito 2007).
Allocation concealment
Seven studies described adequate allocation concealment from both the participants and investigators (Almenning 2015; Brown 2009; Hoeger 2004; Hoeger 2008; Mani 2018; Turan 2015; Vizza 2016), whereas there was a lack of information provided for the other studies to determine whether adequate allocation concealment occurred, which may introduce selection bias (Guzick 1994; Jedel 2011; Leonhardt 2015; Mirfeizi 2013; Nasrekani 2016; Saremi 2013; Saremi 2016; Stefanaki 2015Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007).
Blinding
Blinding of participants and personnel
In all studies, participant or treatment provider blinding was not possible as it is difficult to blind participants and treatment providers to behavioural interventions. This could be a potential source of performance and detection bias in favour of the treatment group. This is more relevant for participant‐reported outcomes such as menstrual diaries (Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007) and quality of life measures (Jedel 2011; Leonhardt 2015; Stefanaki 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vizza 2016). As no participant‐reported outcomes occurred for Guzick 1994 and Brown 2009 the lack of participant blinding is unlikely to introduce bias in these studies. Clinician blinding occurred in five studies (Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Saremi 2013; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007). No blinding occurred in five studies (Almenning 2015; Mani 2018; Stefanaki 2015; Turan 2015; Vizza 2016). Insufficient information was provided to determine whether blinding occurred in six studies (Brown 2009; Guzick 1994; Saremi 2013; Saremi 2016; Mirfeizi 2013; Nasrekani 2016).
Blinding of outcome assessors
Outcome assessor or data analyst blinding occurred in six studies (Almenning 2015; Hoeger 2004; Hoeger 2008; Jedel 2011; Leonhardt 2015; Stefanaki 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007). There was a lack of blinding of outcome assessors in three studies (Mani 2018; Turan 2015; Vizza 2016), which may introduce detection bias. Lack of blinding in outcome assessors may introduce bias for the clinician‐ or assessor‐reported outcomes of hirsutism, weight, BMI and adiposity distribution and the objective outcomes of ovulation or biochemical data.
Incomplete outcome data
Three studies reported an intention‐to‐treat (ITT) analysis ( Jedel 2011; Leonhardt 2015; Mani 2018; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vizza 2016) and no dropouts were reported in five studies (Guzick 1994; Jedel 2011; Leonhardt 2015; Nasrekani 2016; Saremi 2013; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vigorito 2007). One study had outcome data reported for only a subset of study completers (5/17 physical activity and 7/11 controls) (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013). However, these missing data (n = 12 physical exercise, n = 6 control) are unbalanced between the intervention and control groups. Four studies reported a higher dropout rate for the intervention group than the control group (50% versus 39%) (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013); 27% versus 9% (Hoeger 2008); 62% versus 25% (Brown 2009) and 42% versus 33% (Mani 2018), indicating that the reason for missing outcome data was likely to be related to true outcome and a higher dropout rate for the intervention versus control group potentially giving an over‐exaggeration of treatment effect. The lack of blinding may have contributed to higher levels of dropout from the intervention group. One study reported a higher dropout rate in the control group (35% versus 0%; Stefanaki 2015), possibly related to the control group not receiving any intervention. In Guzick 1994 incomplete outcome data were reported for reproductive parameters for two studies (ovulation data were reported for 10/12 participants), however, the missing outcome data were balanced in numbers across intervention groups with similar reasons for the missing data and we considered this a low risk of bias. For Hoeger 2004, although the dropout rate was higher in the intervention group than in the control group at week 48 (45% versus 22%), two pregnancies occurred in the intervention group indicating a potential positive intervention effect (Hoeger 2004).
Selective reporting
For six studies there was insufficient information to permit a judgement on selective reporting (Hoeger 2004; Mani 2018; Mirfeizi 2013; Nasrekani 2016; Saremi 2016; Stener‐Victorin 2009‐2013). Six studies had a low risk of reporting bias, three of which are registered clinical trials (Almenning 2015; Hoeger 2008; Stefanaki 2015) and the remaining three had reported all pre‐specified outcomes that are of interest to this review (Saremi 2013; Turan 2015; Vizza 2016). Three studies had a high risk of reporting bias due to selective reporting (Brown 2009; Guzick 1994; Vigorito 2007). However, from the results section of these papers, all outcomes that are of interest in the review were reported in the prespecified way with the exception of menstrual data for the controls (Vigorito 2007), weight for the controls (Guzick 1994), and total cholesterol for all participants (Brown 2009).
Other potential sources of bias
We identified no other serious potential sources of bias from the included studies.
Effects of interventions
See: Table 1
We conducted analyses for each defined primary or secondary outcome. We excluded Brown 2009 from all meta‐analyses because the data were skewed and reported as median and range with non‐parametric tests of significance. Brown 2009 compared lipid outcomes between intervention and control groups and found that the lifestyle intervention significantly improved triglycerides. We therefore included a total of 14 studies (N = 478) in the meta‐analyses. For studies with ITT, the final number of participants may reflect imputed data instead of the number of completers (Jedel 2011; Leonhardt 2015; Mani 2018; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vizza 2016). We combined both endpoint and change data in the meta‐analysis as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Primary outcome measures
We found no studies that had looked at live birth, miscarriage or pregnancy.
Secondary outcome measures
1.1. Secondary reproductive: menstrual regularity and ovulation
No studies reported menstrual regularity as defined in the review methods (initiation of menses or significant shortening of cycle length). The studies reported data for this outcome in different ways. Hoeger 2004 reported the data as mean ± SD menstrual cycles for lifestyle versus minimal treatment (24 weeks: 2.88 ± 1.7 versus 2.85 ± 1.6, mean difference (MD) 0.03, 95% confidence interval (CI) ‐1.64 to 1.70, P = 0.97; and 48 weeks: 5.4 ± 3.6 versus 4.3 ± 2.1, MD 1.10, 95% CI ‐2.17 to 4.37, P = 0.51). Vigorito 2007 reported 27 of 45 participants (60%) in the treatment group having normal menstrual cycles with no reported data for the control group. Hoeger 2008 reported an average of 2.3 versus 2.5 cycles per 24 weeks for the lifestyle group compared to controls. In one study, changes in menstrual bleeding pattern were not provided for the intervention group compared with the control group (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013) (Analysis 1.1).
1.1. Analysis.
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 1 Secondary reproductive: menstrual regularity and ovulation.
| Secondary reproductive: menstrual regularity and ovulation | ||
|---|---|---|
| Study | Menstrual regularity | Ovulation |
| Brown 2009 | No data | No data |
| Guzick 1994 | No data | 4/6 people ovulatory versus 1/6 people ovulatory OR 6.59, 95% CI 0.73 to 59.34, P = 0.09 |
| Hoeger 2004 |
Menstrual cycles per participant (mean ± SD) 24 weeks: 2.88 ± 1.7 versus 2.85 ± 1.6 MD 0.03, 95% CI ‐1.64 to 1.70, P = 0.97 48 weeks: 5.4 ± 3.6 versus 4.3 ± 2.1 MD 1.10, 95% CI ‐2.17 to 4.37, P = 0.51 Number of menstrual events (mean): 48 weeks: 6.2 versus 4.7 |
Ovulations per participant (mean±SD) 24 weeks: 2.25 ± 1.7 versus 2.23 ± 2.1 MD 0.02, 95% CI ‐1.93 to 1.97, P = 0.98 48 weeks: 6.0 ± 3.6 versus 2.8 ± 2.9 based on n = 4 intervention and n = 6 control patients MD 3.20, 95% CI ‐1.02 to 7.42, P = 0.14 Ovulations (mean): 48 weeks: 3.5 versus 2.7 Ovulations/menstrual cycle 24 weeks: ovulations/menstrual cycle: 18/23 (78.2%) versus 16/20 (80%) 48 weeks: ovulations/menstrual cycle: 25/29 (86.2%) versus 17/28 (60.7%) |
| Hoeger 2008 | Mean 2.3 versus 2.5 cycles/24 weeks No reported significant difference in menstrual cycles for lifestyle versus minimal treatment |
60% ovulatory cycles versus 50% ovulatory cycles No comment on significance of comparison for lifestyle versus minimal treatment |
| Stener‐Victorin 2009‐2013 |
Change in menstruation frequency (month) after 16 weeks of intervention (mean ± SD) Intervention: 0.14 ± 0.33 Control: ‐0.04 ± 0.007 Change in menstruation frequency (month) at 16 weeks follow‐up (16 weeks after last intervention treatment) Intervention: 0.11 ± 0.36 Control: ‐0.04 ± 0.07 No comment on significance of comparison for lifestyle versus minimal treatment |
|
| Vigorito 2007 | 27/45 (60%) of treatment normal menstrual cycles No data reported for minimal treatment group No comment on significance of comparison for lifestyle versus minimal treatment |
No data |
No studies reported ovulation as defined in the review methods (number of ovulatory menstrual cycles). The studies again reported data in different ways. Hoeger 2004 reported the data as mean ± SD ovulations for lifestyle versus minimal treatment (24 weeks: 2.25 ± 1.7 versus 2.23 ± 2.1, MD 0.02, 95% CI ‐1.93 to 1.97, P = 0.98; and 48 weeks: 6.0 ± 3.6 versus 2.8 ± 2.9, MD 3.20, 95% CI ‐1.02 to 7.42, P = 0.14). Guzick 1994 reported 4/6 versus 1/6 participants as ovulatory in the lifestyle group versus the control group (odds ratio (OR) 6.59, 95% CI 0.73 to 59.34, P = 0.09). Hoeger 2008 reported 60% versus 50% ovulatory cycles for the lifestyle group versus the control group (Analysis 1.1).
1.2 Secondary reproductive: endocrine
Lifestyle treatment may result in a slightly greater decrease in total testosterone (MD ‐0.12 nmol/L, 95% CI ‐0.23 to ‐0.02, 10 studies, N = 392, I2 = 5%) and a greater increase in sex hormone‐binding globulin (SHBG) (MD 2.52 nmol/L, 95% CI 0.22 to 4.82, 9 studies, N = 364 participants, I2 = 63%) for lifestyle treatment compared to minimal treatment, with high heterogeneity. Lifestyle intervention may improve the free androgen index (FAI) (MD ‐1.11, 95% CI ‐1.96 to ‐0.26, 6 studies, N = 204, I2 = 71%, low‐quality evidence) (Analysis 1.2; Figure 4).
1.2. Analysis.
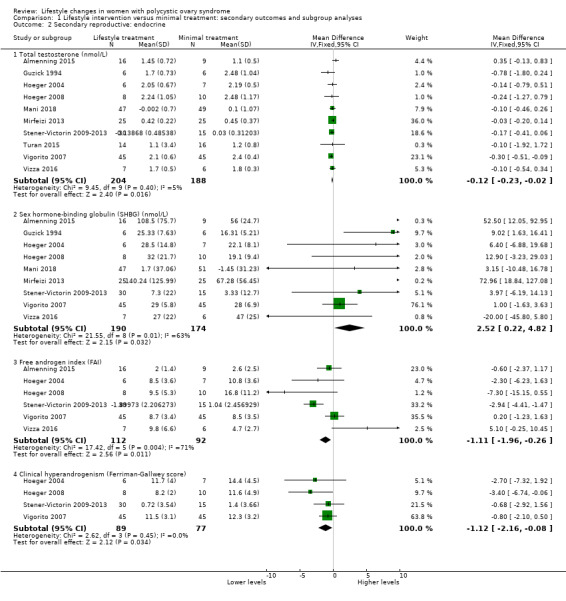
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 2 Secondary reproductive: endocrine.
4.
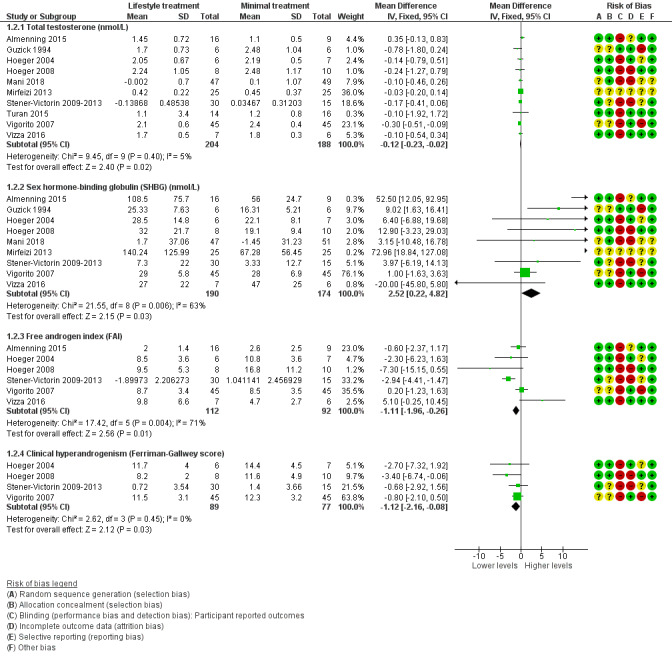
Forest plot of comparison: 1 Lifestyle intervention versus minimal treatment: Combined data, outcome: 1.2 Secondary reproductive outcomes.
For SHBG, total testosterone and clinical hyperandrogenism (Ferriman‐Gallwey score), mean change instead of post‐intervention data were used for Mani 2018 and Stener‐Victorin 2009‐2013.
For FAI, mean and SD values for Stener‐Victorin 2009‐2013 were calculated from group means and SD for T and SHBG.
There may be a slightly greater reduction in hirsutism (Ferriman‐Gallwey score) (MD ‐1.12, 95% CI ‐2.16 to ‐0.08, 4 studies, N = 166, I2 = 0% ) for lifestyle treatment compared to minimal treatment (Analysis 1.2; Figure 4).
1.3 Anthropometric
There may be greater weight loss (kg) with lifestyle treatment compared to minimal treatment (MD ‐1.68 kg, 95% CI ‐2.66 to ‐0.70, 9 studies, N = 353, I2 = 47%, low‐quality evidence) (Analysis 1.3; Figure 5). There may be a greater reduction in body mass index (BMI) with lifestyle treatment compared to minimal treatment (MD ‐0.34 kg/m2, 95% CI ‐0.68 to ‐0.01, 12 studies, N = 434, I2 = 0%, low‐quality evidence). There may be a greater reduction in waist circumference (MD ‐0.97 cm, 95% CI ‐1.80 to ‐0.14; 7 studies, N = 243, I2 = 22%) (Analysis 1.3; Figure 5) and waist‐hip ratio (MD ‐0.04, 95% CI ‐0.07 to ‐0.01, 4 studies, N = 135, I2 = 0%) (Analysis 1.3; Figure 5) with lifestyle treatment compared to minimal treatment.
1.3. Analysis.
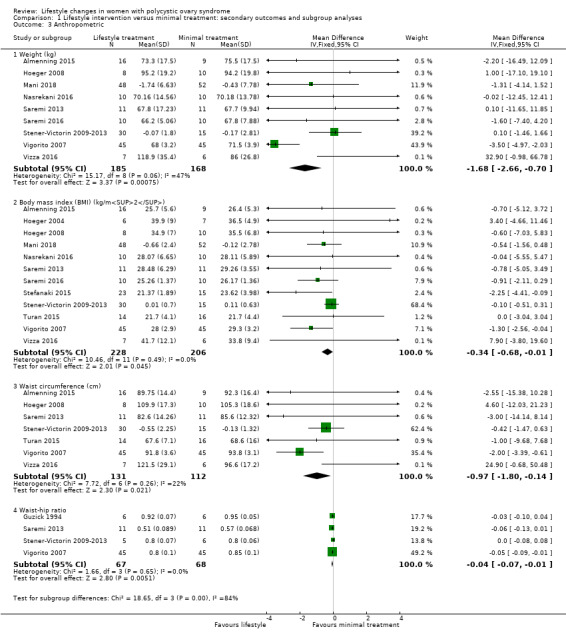
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 3 Anthropometric.
5.
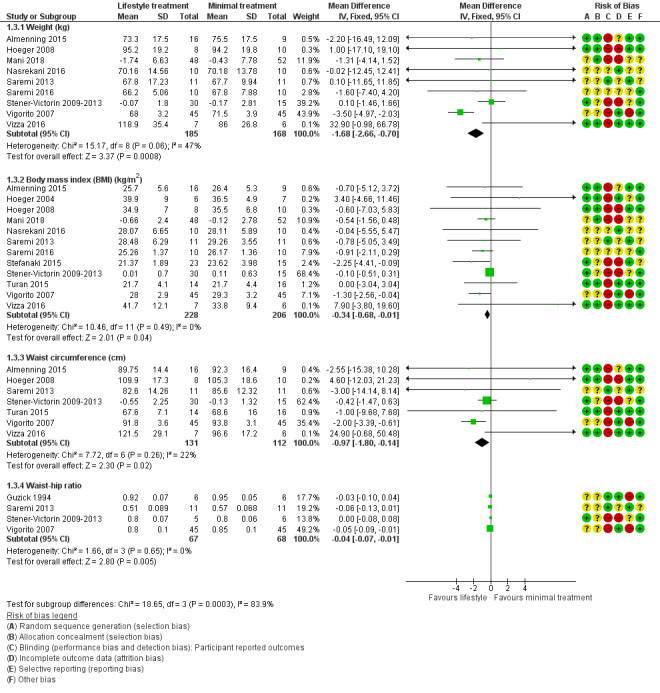
Forest plot of comparison: 1 Lifestyle intervention versus minimal treatment: Combined data, outcome: 1.3 Anthropometric outcomes. For weight, BMI and waist circumference, mean change instead of post‐intervention data were used for Mani 2018 and Stener‐Victorin 2009‐2013.
1.4 to 1.8. Metabolic outcomes
We are uncertain what the effect of lifestyle treatment is on oral glucose tolerance test (OGTT) glucose (SMD ‐0.02, 95% CI ‐0.38 to 0.33, 3 studies, N = 121, I2 = 0%, low‐quality evidence) (Analysis 1.4; Figure 6) or fasting glucose (MD ‐0.07 mmol/L, 95% CI ‐0.15 to 0.01, 11 studies, N = 354, I2=27%) (Analysis 1.5) compared to minimal treatment.
1.4. Analysis.
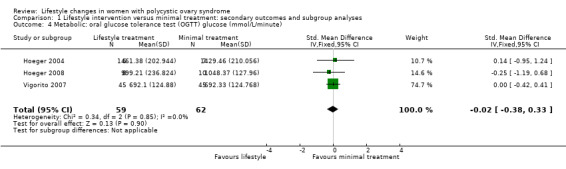
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 4 Metabolic: oral glucose tolerance test (OGTT) glucose (mmol/L/minute).
6.
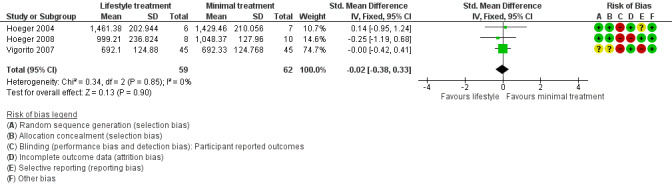
Forest plot of comparison: 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, outcome: 1.4 Metabolic: oral glucose tolerance test (OGTT) glucose (mmol/L/minute).
1.5. Analysis.
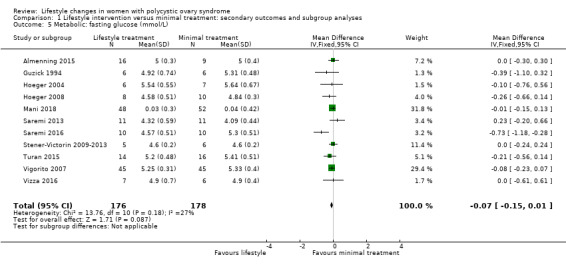
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 5 Metabolic: fasting glucose (mmol/L).
There may be a greater reduction in total cholesterol (MD ‐0.14 mmol/L, 95% CI ‐0.25 to ‐0.02; 9 studies, N = 331, I2 = 0%) and low‐density lipoprotein cholesterol (MD ‐0.16 mmol/L, 95% CI ‐0.29 to ‐0.03; 9 studies, N = 326, I2 = 29%) with lifestyle treatment compared to minimal treatment. Lifestyle treatment may make little or no difference to high‐density lipoprotein cholesterol (MD 0.01 mmol/L, 95% CI ‐0.03 to 0.05, 9 studies, N = 327, I2 = 59%) and triglycerides (MD ‐0.02 mmol/L, 95% CI ‐0.06 to 0.02, 9 studies, N = 328, I2 = 21%) (Analysis 1.6) compared to minimal treatment.
1.6. Analysis.
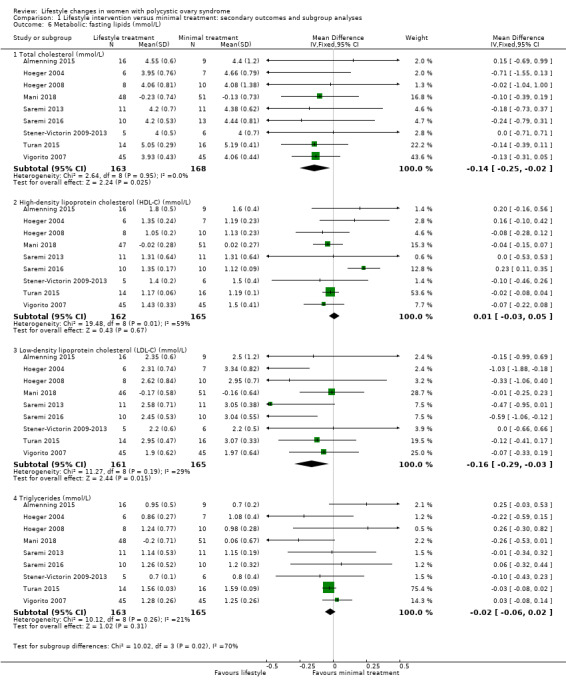
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 6 Metabolic: fasting lipids (mmol/L).
Fasting insulin (MD ‐1.42 µU/mL, 95% CI ‐2.44 to ‐0.39, 10 studies, N = 321, I2 = 0%) (Analysis 1.7) and OGTT insulin (standardised mean difference (SMD) ‐1.32, 95% CI ‐1.73 to ‐0.92, 3 studies, N = 121, I2 = 74% ) (Analysis 1.8) may be further reduced with lifestyle treatment compared to minimal treatment, although there was high heterogeneity for the OGTT insulin result.
1.7. Analysis.
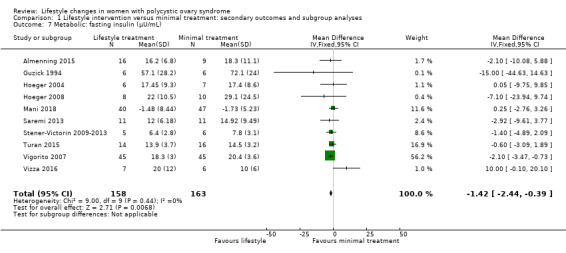
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 7 Metabolic: fasting insulin (µU/mL).
1.8. Analysis.
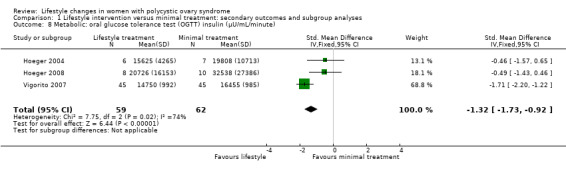
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 8 Metabolic: oral glucose tolerance test (OGTT) insulin (µU/mL/minute).
1.9. Quality of life outcomes
There may be a small beneficial effect on Polycystic Ovary Syndrome Questionnaire (PCOSQ) scores in the domains of emotions (MD 0.77, 95% CI 0.30 to 1.23, 3 studies, N = 95, I2 = 92%) and infertility (MD 0.68, 95% CI 0.21 to 1.14, 3 studies, N = 95, I2 = 87%) with lifestyle treatment compared to minimal treatment, although the heterogeneity was high (Analysis 1.9). Due to the wide confidence intervals, we are uncertain of the effect of lifestyle treatment on PCOSQ scores in the domains of weight (MD ‐0.11, 95% CI ‐0.71 to 0.49; 3 studies, N = 95, I2 = 88%), hirsutism (MD ‐0.01, 95% CI ‐0.57 to 0.56; 3 studies, N = 95, I2 = 56%) and menstrual regularity (MD 0.25, 95% CI ‐0.24 to 0.75; 3 studies, N = 95, I2 = 85%) compared to minimal treatment.
1.9. Analysis.
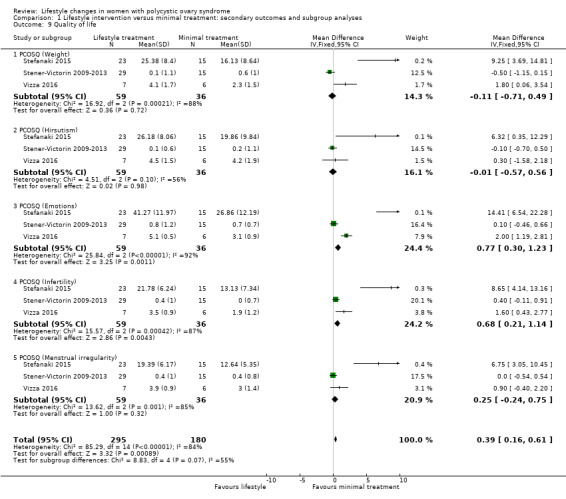
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 9 Quality of life.
Subgroup analysis
We conducted subgroup analyses for SHBG, FAI, OGTT insulin, high‐density lipoprotein (HDL) cholesterol and quality of life to explore possible sources of statistically significant heterogeneity (I2 value greater than 50%) in these outcomes according to the subgroups determined a priori in the Methods section, such as duration of intervention, types of intervention, weight loss versus weight maintenance studies and participants characteristics (i.e. whether a study included only overweight or obese participants).
There were insufficient studies for meaningful subgroup comparisons for the quality of life outcomes.
For SHBG, studies with a combined diet and physical activity intervention (MD 8.73, 95% CI 3.27 to 14.19; 5 studies, N = 191, I2 = 38%) may result in greater improvement compared with physical activity‐only interventions (MD 1.11, 95% CI ‐1.39 to 3.61; 4 studies, N = 173, I2 = 66%) (Analysis 1.10).
1.10. Analysis.
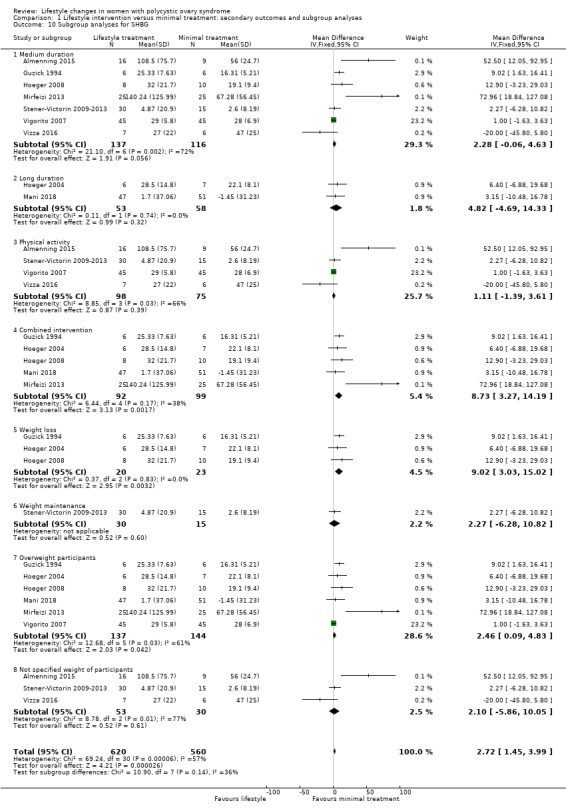
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 10 Subgroup analyses for SHBG.
There were no subgroup differences found for FAI (Analysis 1.11) and HDL cholesterol (Analysis 1.12).
1.11. Analysis.
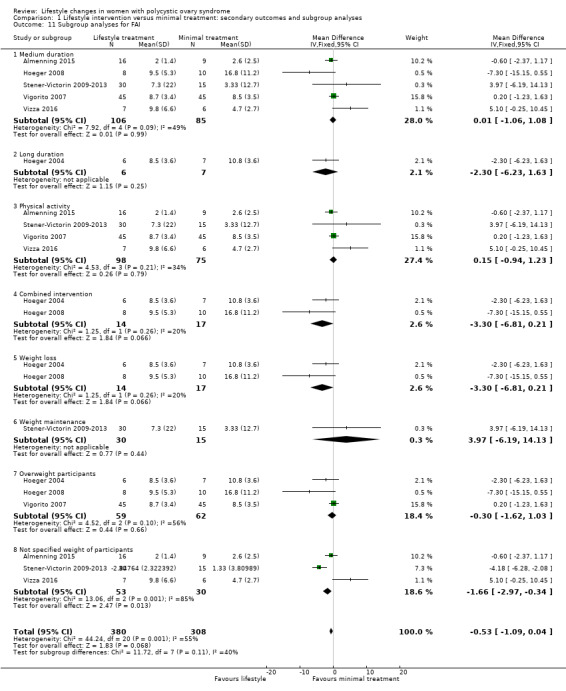
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 11 Subgroup analyses for FAI.
1.12. Analysis.
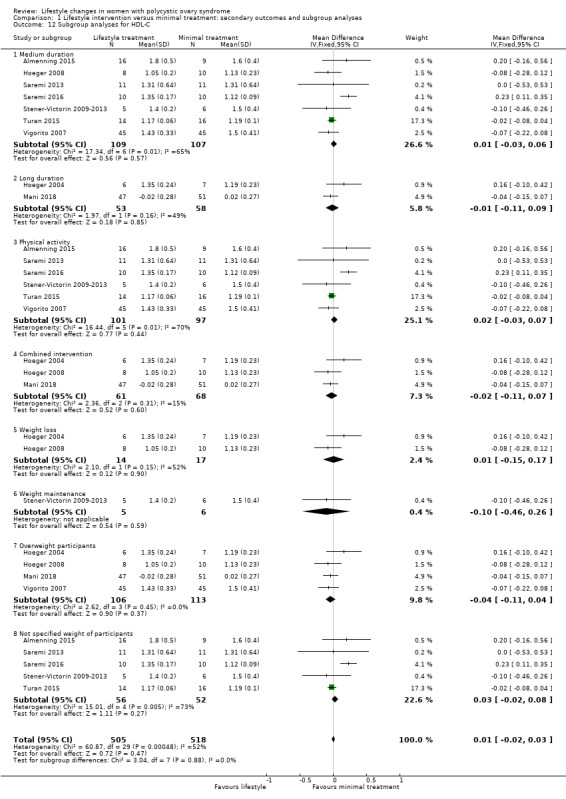
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 12 Subgroup analyses for HDL‐C.
For OGTT insulin, a physical activity‐only intervention (SMD ‐1.71, 95% CI ‐2.20 to ‐1.22, 1 study, N = 90) may result in a small but significantly greater decrease compared to combined interventions (SMD ‐0.48, 95% CI ‐1.20 to 0.24, 2 studies, N = 31, I2 = 0%) (Analysis 1.13).
1.13. Analysis.
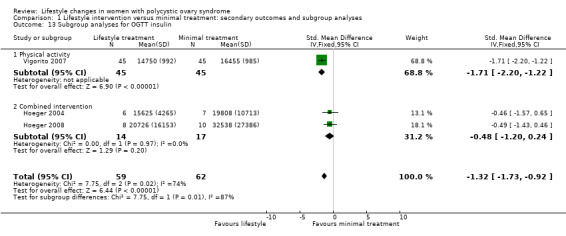
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 13 Subgroup analyses for OGTT insulin.
For the quality of life measures, behavioural intervention (Stefanaki 2015) may result in greater improvement than physical activity interventions (Stener‐Victorin 2009‐2013; Vizza 2016) in all PCOSQ domains (Analysis 1.14).
1.14. Analysis.
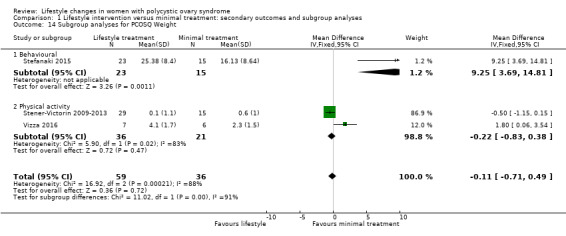
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 14 Subgroup analyses for PCOSQ Weight.
Sensitivity analysis
We conducted sensitivity analyses for BMI and FAI due to their clinical significance. We only included studies with low risk of bias for sequence generation and allocation concealment in the sensitivity analyses. Due to the wide confidence intervals, we are uncertain of the effect of lifestyle intervention on BMI (MD 0.29, 95% CI ‐1.91 to 2.49, 5 studies, N = 99, I2 = 0%) (Analysis 1.15) and FAI (MD ‐0.64, 95% CI ‐2.16 to 0.87, 4 studies, N = 69, I2 = 62%) (Analysis 1.16).
1.15. Analysis.
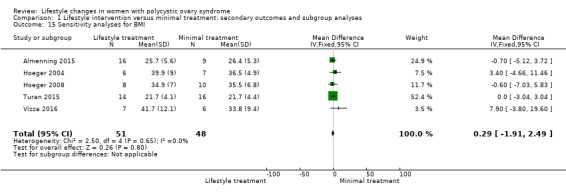
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 15 Sensitivity analyses for BMI.
1.16. Analysis.
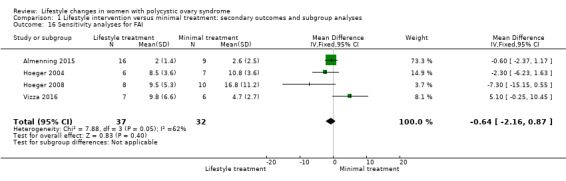
Comparison 1 Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses, Outcome 16 Sensitivity analyses for FAI.
Discussion
Summary of main results
This Cochrane Review supports the benefits of lifestyle treatment in women with polycystic ovary syndrome (PCOS). We were not able to perform a meta‐analysis for primary fertility outcomes such as live birth, miscarriage and pregnancy or secondary reproductive outcomes such as menstrual regularity and ovulation due to a lack of data or reporting of data in a form inappropriate for meta‐analysis. In terms of secondary outcomes, lifestyle intervention may improve free androgen index, weight and body mass index (BMI). We are uncertain of the effect of lifestyle intervention on glucose tolerance. The recent guideline recommends free testosterone, bioavailable testosterone or free androgen index (FAI) to be used to assess biochemical hyperandrogenism (International PCOS Guideline 2018); however, FAI includes markers of insulin resistance such as sex hormone‐binding globulin (SHBG). The current review suggests that lifestyle treatment may improve biochemical and clinical hyperandrogenism. This is consistent with the widespread international recommendations that lifestyle treatment improves fertility and reproductive outcomes in PCOS (International PCOS Guideline 2018; Moran 2009).
This review reports that lifestyle treatment may improve a number of anthropometric markers (weight and BMI) in women with PCOS, with a mean difference in weight for lifestyle compared to minimal treatment of 1.68 kg. A 5% to 10% weight loss is considered clinically significant and is associated with metabolic, reproductive and psychological health benefits (International PCOS Guideline 2018). A 2 kg to 3 kg weight loss is associated with reductions in impaired glucose tolerance prevalence with improvements in risk factors for cardiovascular disease and type 2 diabetes in the general population (Aziz 2015). Overall this review indicates a modest reduction in weight and improvement in abdominal obesity with lifestyle treatment.
We are uncertain of the effect of lifestyle intervention on glucose tolerance and fasting glucose. Fasting glucose is an inferior predictor of abnormal glucose metabolism in PCOS compared to a glucose tolerance test (Vrbikova 2014); however, we also observed the null effect for oral glucose tolerance test (OGTT) glucose. The exclusion criterion of glucose intolerance in a number of studies suggests that where normal glucose tolerance is present, glucose tolerance improvements are less likely to be induced by lifestyle treatment.
This review reports that lifestyle treatment may improve lipid profile, specifically total cholesterol and low‐density lipoprotein (LDL) cholesterol despite the modest weight loss achieved (< 5 kg). As most of the studies reporting lipid outcomes were not weight loss studies and involved only physical activity, these findings are consistent with a recent systematic review and meta‐analysis, which reported that exercise training improves lipid profiles in women with PCOS (Benham 2018). However, significant heterogeneity that was not explained in the subgroup analyses highlights the complex relationship between PCOS and cardiovascular risk (Gunning 2017). The different cardio‐metabolic risks associated with different PCOS phenotypes (Daan 2014) may have also contributed to the heterogenous response to lifestyle intervention.
Insulin resistance is a key aetiological factor in PCOS, associated with increasing severity of PCOS (Androulakis 2014; Landay 2009; Stepto 2013), and it is an independent predictor of impaired glucose tolerance, type 2 diabetes and cardiovascular disease in the general population (Salazar 2016). Improvements in insulin resistance are associated with improvements in the clinical features of PCOS (Moran 2003; Morley 2017) and are therefore potential surrogate markers for lifestyle intervention success in PCOS. We report that lifestyle intervention may reduce surrogate markers of insulin resistance compared to minimal treatment, as evident from improvements in waist circumference and OGTT insulin. Given the role of insulin resistance in the pathophysiology and co‐morbidities of PCOS, the improvement in these markers is an important finding of this review.
The deleterious effect of PCOS on quality of life, anxiety and depression is increasingly recognised and these are important issues to treat in conjunction with anthropometric, reproductive and metabolic outcomes. Lifestyle intervention may improve quality of life scores in the domains of emotions and infertility in PCOS, as reported in this review. The studies that reported quality of life outcomes studied physical activity (Jedel 2011; Leonhardt 2015; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vizza 2016) or stress management (Stefanaki 2015) interventions. A significant association between physical activity and positive emotions has been observed in the general population (Richards 2015), which may explain the improvement in the emotions domain of the PCOSQ score resulting from these interventions. Lifestyle interventions in this review also resulted in significant improvements in the infertility domain of the PCOSQ score. It is unclear if this reflected actual improvements in menstrual cyclicity, which were poorly reported in these studies. No studies reported on patient satisfaction, which indicates a considerable gap in the research literature.
Overall completeness and applicability of evidence
The identified studies are not sufficient to address the effect of lifestyle intervention on the primary outcomes of this review. The lack of fertility outcomes (live birth, miscarriage and pregnancy) as a specified endpoint in any of the studies means that no statement can be made as to the effects of lifestyle treatment on these primary outcomes. There is also insufficient evidence to address a number of secondary outcomes of the review; for example, there was a lack of reporting or incomplete reporting of menstrual regularity and ovulation. Participant satisfaction was not a measured outcome in any of the included literature.
We aimed to assess a range of intervention durations (short, medium and long‐term), types (exercise, behaviour, diet or combined; and weight loss or weight maintenance) and participants (overweight or not overweight) to consider the clinical utility of lifestyle treatment according to participant preference and available resources. Subgroup differences were observed for SHBG and triglycerides, with greater intervention effects in weight loss studies, studies with combined diet and physical activity interventions and studies of longer duration (over six months). Given the limited number of studies identified and the small sample sizes for other outcomes, subgroup analysis according to these predefined criteria was not feasible and the inclusion of these ranges of methodologies resulted in clinical heterogeneity. The limited duration of most trials in this review (13 of 15 with a duration of six months or less) may have impacted on effect size especially for weight loss. We also noted clinical heterogeneity in the intensity of the control group intervention, with this differing as to whether this was no intervention or standard minimal advice. However, the studies are similar enough to make meaningful comparisons and we still observed the effect of lifestyle intervention on a number of outcomes, indicating its likely utility in a range of settings. It is also recommended in the International Evidence‐based Guidelines for Assessment and Management of PCOS (International PCOS Guideline 2018). With regards to current international clinical practice, the effects of lifestyle treatment for PCOS on biochemical and clinical hyperandrogenism, adiposity and adiposity distribution, metabolic outcomes, surrogate markers of insulin resistance and quality of life are supported by this review. In areas where direct evidence is lacking, the recent guidelines for PCOS strongly recommend healthy eating and physical activity in women with PCOS to improve hormonal outcomes, general health and quality of life based on clinical consensus (International PCOS Guideline 2018). With regards to the application of types of lifestyle treatment or specific subpopulations within PCOS, further targeted research is required. This is of particular interest when generalising the results of the review to specific populations, interventions and settings, given that not all women with PCOS are overweight or obese. In these settings, the effect of lifestyle treatment independent of weight loss is of great clinical interest.
There are a range of other issues potentially affecting clinical heterogeneity that were not assessed in this review. All studies assessed adults except two, which included adolescents (Hoeger 2008; Stefanaki 2015). In one of these studies, participants also attended the lifestyle visits with a parent or guardian, which could indicate improved compliance and motivation (Hoeger 2008). Reproductive and metabolic features may differ between women with PCOS diagnosed by different criteria, with the National Institutes of Health (NIH) diagnosed women considered to have more severe disease than those diagnosed with the European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM criteria). Hence, the variability in diagnostic criteria may have introduced further clinical heterogeneity (Moran 2009b). The different country settings and populations studied may also introduce cultural or ethnic heterogeneity (Essah 2008).
Factors relating to the implementation of a lifestyle intervention in research or clinical settings, including intervention intensities, visit numbers, training of intervention providers and group versus individual treatment formats, may impact on the effectiveness of the intervention. This indicates the need for caution in extrapolating research results to clinical practice.
Quality of the evidence
In this review, we assessed 15 studies with a total of 498 participants. The majority of the studies had small sample sizes (11 to 45 participants) with the exception of two studies with sample sizes of 90 to 100 women (Mani 2018; Vigorito 2007). This has implications for the power of all analyses. For the meta‐analyses, we were only able to include 14 studies with a total of 478 participants due to the skewed data in Brown 2009. Three studies carried out intention‐to‐treat (ITT) analyses ( Jedel 2011; Leonhardt 2015; Mani 2018; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013; Vizza 2016), only seven studies had predefined power calculations (Almenning 2015; Brown 2009; Jedel 2011; Leonhardt 2015; Mani 2018; Nasrekani 2016; Saremi 2013; Saremi 2016; Stener‐Victorin 2009; Stener‐Victorin 2012; Stener‐Victorin 2013), and high dropout rates were reported for the majority of studies, with the exception of Guzick 1994 and Vigorito 2007, indicating methodological weaknesses and the need for caution in results interpretation.
Inconsistent outcomes were reported with few studies covering all predefined endpoints. We observed imprecision for several outcomes with large confidence intervals and different directions of effect reported (Table 1). There was significant heterogeneity in the findings for SHBG, FAI, high‐density lipoprotein (HDL) cholesterol, OGTT insulin and quality of life, suggesting the need for caution in interpreting the pooled results. We did not meet the predefined objective of examining outcomes according to subgroup by intervention duration and type due to the lack of data in the primary outcome analysis. Regarding the risk of bias, we noted lack of evidence of adequate allocation concealment or random sequence generation for nine studies and lack of evidence of adequate clinician, outcome or data analyst blinding for 12 studies. We also noted incomplete outcome data for five studies mostly due to higher dropout rates from the intervention group compared to the minimal treatment group, potentially exaggerating treatment effects. We also noted selective outcome reporting for four studies. All of these introduce selection bias, performance bias, detection bias and attrition bias, which compromises the quality of the evidence. It is acknowledged that there are inherent challenges in reducing performance bias in trials designed to assess lifestyle interventions compared to no treatment and in reducing detection bias for clinical outcomes that rely on participants' self‐report, such as menstrual diaries. As a result of these challenges in addition to the shortcomings of the trial design as described above, the overall the quality of the evidence was low.
We conducted sensitivity analyses of studies with low risk of bias for sequence generation and allocation concealment. Due to the wide confidence intervals we could not conclude with certainty what the effect of lifestyle intervention was on BMI and FAI.
Potential biases in the review process
This review was conducted according to Cochrane methodology, with a predefined protocol. We aimed to minimise reporting bias by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. Funnel plots were largely symmetrical for total testosterone, BMI, weight, fasting glucose and fasting insulin, suggesting a low risk of publication bias. We were unable to examine whether publication bias was present for other outcomes as we were not able to construct a funnel plot due to the small number of identified studies. We also attempted to address within‐trial bias through carefully assessing studies for within‐study reporting bias, such as trials failing to report obvious outcomes or reporting them in insufficient detail to allow inclusion, and we have included this level of detail in the 'Risk of bias' tables (Characteristics of included studies). As the systematic review included a narrow range of studies according to the eligibility criteria, a large number of studies that provide useful information on the topic of lifestyle management in PCOS were excluded. In particular, there were many studies that compared different dietary approaches or interventions that include medications, but these were not included in the present review as it did not allow for the assessment of the effect of lifestyle modification by itself. We were also unable to include the results of one study due to the results being reported as median and interquartile range. This study reported significant improvements in triglycerides after the exercise intervention compared to the control treatment (Brown 2009).
Agreements and disagreements with other studies or reviews
We identified two other systematic reviews on the effect of lifestyle treatment in PCOS (Domecq 2013; Haqq 2014; Haqq 2015). We identified an international position statement by the Androgen Excess PCOS Society (AEPCOS), which consisted of a systematic search strategy but included studies of all levels of evidence with no systematic review of methodological quality (Moran 2009). This paper reviewed a number of intervention studies on lifestyle treatment in PCOS that were of a lower quality of evidence. Study designs consisted of non‐comparison intervention studies in PCOS with no minimal treatment arm, comparisons with different non‐PCOS populations or comparisons to different types of lifestyle interventions. We also identified an international evidence‐based guideline on the assessment and management of PCOS for which the lifestyle section was based on the previous version of this review (International PCOS Guideline 2018; Moran 2011).
Past reviews have generally indicated the positive effects of lifestyle treatment on anthropometric, reproductive (biochemical and clinical hyperandrogenism, menstrual function, ovulation, pregnancy and conception), metabolic (fasting insulin, fasting glucose, glucose tolerance, lipid profiles, surrogate markers of insulin resistance) and quality of life endpoints. In this current review, we found that lifestyle treatment may improve reproductive (biochemical and clinical hyperandrogenism), anthropometric (adiposity, adiposity distribution) and metabolic features (markers of insulin resistance and cholesterol levels) and quality of life (emotions and infertility). We are unable to confirm the effects of lifestyle intervention on reproductive fertility outcomes, menstrual regularity and ovulation as these were not reported in a format that could be assessed in this review. In contrast with previous reviews, we could not conclude with certainty what the effect of lifestyle treatment was on fasting glucose (Domecq 2013). The inclusion of more recent studies in the current review with differing effects may have contributed to the discrepant conclusions. Similar to the previous review, we found little or no effect of lifestyle intervention on HDL cholesterol and triglycerides (Haqq 2015).
Authors' conclusions
Implications for practice.
Lifestyle intervention may improve free androgen index (FAI), weight and body mass index (BMI) in women with polycystic ovary syndrome (PCOS). We are uncertain of the effect of lifestyle intervention on glucose tolerance. There were no studies that looked at the effect of lifestyle intervention on live birth, miscarriage and menstrual regularity. Most studies in this review were of low quality, mainly due to high or unclear risk of bias across most domains and high heterogeneity for the FAI outcome.
Implications for research.
Future research should focus on well‐designed, adequately powered studies of sufficient long‐term duration for lifestyle intervention in PCOS. Future studies should also be designed to reduce the risk of attrition and detection bias through blinding of treatment allocation and the use of blinded, measured objective endpoints. The benefits of diet and exercise for weight loss have been demonstrated in women with PCOS in low‐quality studies, but this remains to be confirmed in higher‐quality studies. Future research should also explore the effect of lifestyle modification on various PCOS phenotypes. The effect of various intervention characteristics including intervention type, duration, intensity and other aspects of implementation on various outcomes should also be considered in future trials. Optimal study design inclusion of a minimal treatment arm is vital for all further research in this area. This review focused on the comparison of lifestyle intervention to minimal treatment. Extrapolation of these findings to comparisons with standard pharmacological therapy would also be of clinical interest. High dropout rates were observed for the majority of the reviewed studies. In the future, strategies should be employed to minimise dropouts and intention‐to‐treat analysis applied to account for the dropouts. In terms of outcomes, there is a significant research gap for ovulation, menstrual and fertility outcomes and glucose levels. Of key importance is the future assessment of primary reproductive outcomes and participant satisfaction with lifestyle intervention in PCOS. Future research also needs to increase consistency in the reporting of data on menstrual regularity and ovulation. It is not possible to state from the existing research whether the lack of an intervention effect on glucose outcomes is due to the degree of weight loss achieved, to clinical heterogeneity or to the small sample size and moderate study durations available for review. Future research should, therefore, assess the effect of a range of weight losses on primary and secondary outcomes to determine optimal weight loss for all clinical improvements. Cost‐benefit analysis should also be included as an outcome to be compared with other commonly used pharmacological and surgical treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 29 March 2019 | Amended | Correction in abstract |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 24 August 2018 | New citation required and conclusions have changed | This review has been updated, with slight changes to the conclusions due to the incorporation of new findings. |
| 24 August 2018 | New search has been performed | We updated the review and included 10 new studies (Almenning 2015; Brown 2009; Guzick 1994; Hoeger 2004; Hoeger 2008; Mani 2018; Mirfeizi 2013; Nasrekani 2016; Saremi 2013; Saremi 2016; Stefanaki 2015; Stener‐Victorin 2009‐2013; Turan 2015; Vigorito 2007; Vizza 2016). Studies awaiting classification: Gaeini 2012. |
| 11 May 2011 | New citation required but conclusions have not changed | The abstract has been edited. |
| 11 February 2010 | Amended | Primary outcomes of menstrual regularity and ovulation modified to report all data. |
| 11 February 2010 | Amended | The title, inclusion criteria and methodology have been altered to include both overweight and not overweight women with PCOS and to include both studies designed and not designed to induce weight loss. Introduction modified to include new research. |
| 19 May 2008 | Feedback has been incorporated | Substantive amendment. |
Acknowledgements
The authors gratefully acknowledge the Australasian Cochrane Centre and Cochrane Gynaecology and Fertility for assistance in preparing this manuscript. We would also like to thank all the authors who provided additional data on methodology or results. We would like to thank Dr Shayesteh Jahanfar, Dr Parvin Abedi and Mahnaz Bahri Khomami for assistance in the translation of data into the English language.
The authors of the 2018 update thank Dr Julie Brown and Dr Anju Joham for their contributions to early drafts of the updated review.
The authors would like to thank the following peer reviewers for their time and commitment: Andrew Watson, Jack Wilkinson, Gabriela Cooper and Tommy Tang.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register (CGFG) search strategy
Searched 5 March 2018
PROCITE platform
Keywords CONTAINS "polycystic ovary syndrome" or "PCOS" or Title CONTAINS or "polycystic ovary syndrome" or "PCOS"
AND
Keywords CONTAINS "*Lifestyle" or "lifestyle change" or "lifestyle modification" or "lifestyle program" or "Weight Loss" or "diet therapy" or "diet" or "dietary intervention" or "dietary soy counseling" or "dietary" or "exercise" or "Exercise Therapy" or "physical performance" or "physical well being" or "behavioral therapy" or "behavioral coping strategies" or "cognitive behavioral therapy" or "walking" or "yoga" or "aerobic exercise" or "Psychological therapies" or "psychological therapy" or "psychosocial therapy" or "psychosocial treatment" or "Psychotherapy or "counseling" or "counselling" or Title CONTAINS"*Lifestyle" or "lifestyle change" or "lifestyle modification" or "lifestyle program" or "Weight Loss" or "diet therapy" or "diet" or "dietary intervention" or "Psychological therapies" or "psychological therapy" or "Psychotherapy" or "counseling" or "exercise" or "Exercise Therapy" (178 hits)
Appendix 2. CENTRAL via Cochrane Register of Studies Online (CRSO) search strategy
Searched 5 March 2018
Web platform
1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES (967)
2 ( Polycystic Ovar*):TI,AB,KY (2078)
3 ( PCOS or PCOD):TI,AB,KY (1665)
4 ( sclerocystic adj3 ovar*):TI,AB,KY (0)
5 (stein leventhal):TI,AB,KY (3)
6 1 OR 2 OR 3 OR 4 OR 5 (2270)
7 MESH DESCRIPTOR Diet Therapy EXPLODE ALL TREES (4987)
8 diet*:TI,AB,KY (57124)
9 MESH DESCRIPTOR Diet, Reducing EXPLODE ALL TREES (1871)
10 MESH DESCRIPTOR Weight Loss EXPLODE ALL TREES (4463)
11 MESH DESCRIPTOR Weight Reduction Programs EXPLODE ALL TREES (467)
12 (weight loss):TI,AB,KY (11332)
13 (weight reduc*):TI,AB,KY (7364)
14 (body mass index adj2 (loss or reduc* or decreas*)):TI,AB,KY (328)
15 (bmi adj2 (loss or reduc* or decreas*)):TI,AB,KY (563)
16 MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES (10239)
17 exercis*:TI,AB,KY (59238)
18 MESH DESCRIPTOR Sports EXPLODE ALL TREES (13167)
19 (run* or jog*):TI,AB,KY ( 12834)
20 (sport* or walk*):TI,AB,KY (20388)
21 (swim* or train*):TI,AB,KY (56805)
22 (fitness or yoga):TI,AB,KY (7697)
23 MESH DESCRIPTOR Cognitive Therapy EXPLODE ALL TREES (6974)
24 MESH DESCRIPTOR Relaxation Therapy EXPLODE ALL TREES (1658)
25 (cogniti* adj2 therap*):TI,AB,KY (12601)
26 MESH DESCRIPTOR Psychotherapy EXPLODE ALL TREES (19415)
27 Psychotherap*:TI,AB,KY (8859)
28 psychosocial*:TI,AB,KY (7659)
29 MESH DESCRIPTOR Behavior Therapy EXPLODE ALL TREES (13461)
30 (Behavio?r adj2 therap*):TI,AB,KY (8904)
31 (behavio?r adj2 modif*):TI,AB,KY (773)
32 (behavio?r adj2 manage*):TI,AB,KY (282)
33 CBT:TI,AB,KY (3935)
34 MESH DESCRIPTOR Life Style EXPLODE ALL TREES (4122)
35 (life*style adj2 change*):TI,AB,KY (1120)
36 (life*style adj2 intervention*):TI,AB,KY (2551)
37 counselling:TI,AB,KY (2680)
38 MESH DESCRIPTOR Social Support EXPLODE ALL TREES (2882)
39 (social adj2 support):TI,AB,KY (5026)
40 relaxation:TI,AB,KY (7182)
41 MESH DESCRIPTOR Self Efficacy EXPLODE ALL TREES (2473)
42 (self efficacy):TI,AB,KY (6105)
43 MESH DESCRIPTOR Health Promotion EXPLODE ALL TREES (5081)
44 (Health adj2 Promotion*):TI,AB,KY (6412)
45 MESH DESCRIPTOR Health Education EXPLODE ALL TREES (16558)
46 (motivation* adj2 therap*):TI,AB,KY (509)
47 (Health* adj2 Education*):TI,AB,KY (8588)
48 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 OR 41 OR 42 OR 43 OR 44 OR 45 OR 46 OR 47 (212251)
49 6 AND 48 (432)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 5 March 2018
OVID platform
1 exp Polycystic Ovary Syndrome/ (12559) 2 Polycystic Ovar$.tw. (14041) 3 (PCOS or PCOD).tw. (9306) 4 (sclerocystic adj3 ovar$).tw. (99) 5 stein leventhal.tw. (608) 6 or/1‐5 (16842) 7 exp Diet Therapy/ (48859) 8 diet$.tw. (487118) 9 exp Weight Loss/ (35993) 10 (weight adj2 lose).tw. (3446) 11 Weight Loss.tw. (73205) 12 (weight adj3 reduc$).tw. (30573) 13 ((body mass index adj2 loss) or reduc$ or decreas$).tw. (4401121) 14 ((BMI adj2 loss) or (BMI adj2 reduc) or (BMI adj2 decreas$)).tw. (2519) 15 exp Exercise Therapy/ (41561) 16 (exercise$ or exercising).tw. (254068) 17 exp sports/ or exp bicycling/ or exp running/ or exp swimming/ or exp walking/ (176840) 18 (run$ or jog$).tw. (164981) 19 (sport$ or walk$).tw. (157282) 20 swim$.tw. (34407) 21 train$.tw. (440545) 22 fitness.tw. (59669) 23 yoga.tw. (3525) 24 exp cognitive therapy/ or exp relaxation techniques/ (30104) 25 (cognitive adj2 therap$).tw. (16565) 26 exp Psychotherapy/ (178245) 27 Psychotherapy.tw. (29898) 28 psychosocial.tw. (79410) 29 exp Behavior Therapy/ (65028) 30 (Behavio?r adj2 therap$).tw. (7095) 31 behavio?r modif$.tw. (2844) 32 (behavio?r adj2 manage$).tw. (2052) 33 CBT.tw. (8291) 34 exp life style/ or exp life change events/ (79697) 35 ((life*style adj2 change$) or intervention$).tw. (802804) 36 counselling.tw. (22827) 37 social support/ (62628) 38 (social adj2 support).tw. (32109) 39 relaxation.tw. (106728) 40 exp self efficacy/ (16694) 41 self efficacy.tw. (21044) 42 exp Health Promotion/ (67559) 43 (Health adj2 Promotion).tw. (26138) 44 exp Health Education/ (222221) 45 (Health$ adj2 Education).tw. (37503) 46 (motivation$ adj2 therap$).tw. (643) 47 or/7‐46 (6457272) 48 6 and 47 (6244) 49 randomised controlled trial.pt. (454849) 50 controlled clinical trial.pt. (92204) 51 randomized.ab. (404382) 52 placebo.tw. (191916) 53 clinical trials as topic.sh. (182777) 54 randomly.ab. (285994) 55 trial.ti. (178689) 56 (crossover or cross‐over or cross over).tw. (75504) 57 or/49‐56 (1162984) 58 exp animals/ not humans.sh. (4430952) 59 57 not 58 (1069796) 60 48 and 59 (1164)
Appendix 4. Embase search strategy
Searched from 1980 to 5 March 2018
OVID platform
1 exp Diet Therapy/ (310653) 2 diet$.tw. (590690) 3 ((weight adj3 loss) or (weight adj3 lose)).tw. (121239) 4 (weight adj3 reduc$).tw. (40928) 5 (body mass index adj2 loss).tw. (516) 6 (body mass index adj2 reduc$).tw. (940) 7 (body mass index adj2 decreas$).tw. (1081) 8 (BMI adj2 loss).tw. (1321) 9 (BMI adj2 reduc$).tw. (3085) 10 (BMI adj2 decreas$).tw. (3742) 11 exercis$.tw. (323114) 12 exp sports/ or exp bicycling/ or exp running/ or exp swimming/ or exp walking/ (222193) 13 (run$ or jog$).tw. (209631) 14 (sport$ or walk$).tw. (205224) 15 swim$.tw. (39325) 16 train$.tw. (555945) 17 (fit or fitness).tw. (185779) 18 exp cognitive therapy/ or exp relaxation techniques/ (50976) 19 (cognitive adj2 therap$).tw. (23682) 20 exp Psychotherapy/ (224673) 21 Psychotherapy.tw. (38441) 22 exp Behavior Therapy/ (41912) 23 (Behavio?r adj2 therap$).tw. (9809) 24 CBT.tw. (12487) 25 (behavio?r adj2 manage$).tw. (2438) 26 behavio?r modif$.tw. (3212) 27 exp life style/ or exp life change events/ (133701) 28 ((life*style adj2 change$) or intervention$).tw. (1082857) 29 social support/ (76878) 30 (social adj2 support).tw. (39360) 31 counselling.tw. (34265) 32 relaxation.tw. (108745) 33 kinesiotherapy/ (27939) 34 exp weight control/ or exp weight reduction/ (151665) 35 exp self concept/ (170129) 36 exp health promotion/ (87093) 37 exp health education/ (285721) 38 (health$ adj2 promotion$).tw. (31256) 39 (health$ adj2 education).tw. (41685) 40 or/1‐39 (3832956) 41 exp ovary polycystic disease/ or exp stein leventhal syndrome/ (22972) 42 Polycystic Ovar$.tw. (19367) 43 (PCOS or PCOD).tw. (14084) 44 (sclerocystic adj3 ovar$).tw. (97) 45 stein leventhal.tw. (402) 46 41 or 42 or 43 or 44 or 45 (26576) 47 Clinical trial/ (962537) 48 Randomized controlled trials/ (140047) 49 Random Allocation/ (73840) 50 Single‐Blind Method/ (29077) 51 Double‐Blind Method/ (121142) 52 Cross‐Over Studies/ (47847) 53 Placebos/ (250001) 54 Randomi?ed controlled trial$.tw. (175245) 55 RCT.tw. (27377) 56 Random allocation.tw. (1747) 57 Randomly allocated.tw. (28945) 58 Allocated randomly.tw. (2291) 59 (allocated adj2 random).tw. (791) 60 Single blind$.tw. (20345) 61 Double blind$.tw. (179235) 62 ((treble or triple) adj blind$).tw. (749) 63 Placebo$.tw. (262971) 64 Prospective Studies/ (331626) 65 or/47‐64 (1767706) 66 Case study/ (52324) 67 Case report.tw. (346377) 68 Abstract report/ or letter/ (1020578) 69 or/66‐68 (1410943) 70 65 not 69 (1717764) 71 animal/ (1828109) 72 human/ (19121849) 73 71 not 72 (1390128) 74 70 not 73 (1688418) 75 40 and 46 and 74 (1654)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 5 March 2018
OVID platform
1 exp Diets/ (11601) 2 diet$.tw. (39555) 3 (weight adj3 lo$).tw. (18180) 4 (weight adj3 reduc$).tw. (3570) 5 (body mass index adj2 loss).tw. (31) 6 (body mass index adj2 reduc$).tw. (114) 7 (body mass index adj2 decreas$).tw. (107) 8 (BMI adj2 loss).tw. (59) 9 (BMI adj2 redu$).tw. (322) 10 (BMI adj2 decreas$).tw. (317) 11 exercise$.tw. (60379) 12 (run$ or jog$).tw. (42403) 13 (sport$ or walk$).tw. (55472) 14 (swim$ or cycl$).tw. (71804) 15 train$.tw. (300072) 16 fit$.tw. (83920) 17 (cognitive adj2 therap$).tw. (29367) 18 Psychotherapy.tw. (95158) 19 (Behavio*r adj2 therap$).tw. (18073) 20 ((life*style adj2 change$) or intervention$).tw. (336060) 21 therap$.tw. (381004) 22 social support/ (32765) 23 (social adj2 support).tw. (46428) 24 relaxation.tw. (15286) 25 exercise$.tw. (60379) 26 exp weight control/ or exp weight reduction/ (6740) 27 exp self concept/ (67828) 28 exp health promotion/ (22179) 29 exp health education/ (17214) 30 (health$ adj2 promotion$).tw. (18608) 31 (health$ adj2 education).tw. (15386) 32 exp Exercise/ or exp Diets/ or exp Nutrition/ or exp Walking/ or exp Eating Behavior/ or exp Dietary Restraint/ (61413) 33 exp Respiration/ or exp Yoga/ or exp Muscle Relaxation/ or exp Meditation/ or exp Systematic Desensitization Therapy/ or exp Progressive Relaxation Therapy/ or exp Relaxation/ or exp Relaxation Therapy/ or exp Biofeedback/ or exp Hypnosis/ (29900) 34 exp Cognitive Therapy/ (12898) 35 exp Education/ or exp Exercise/ or exp Physical Activity/ or exp Walking/ (351481) 36 exp Sports/ (23588) 37 exp Swimming/ (1618) 38 exp Running/ (1759) 39 exp psychotherapy/ (205466) 40 exp Behavior Therapy/ (18933) 41 exp Lifestyle/ (10672) 42 or/1‐41 (1552673) 43 exp endocrine sexual disorders/ (1128) 44 Polycystic Ovar$.tw. (371) 45 pco$.tw. (841) 46 (sclerocystic adj3 ovar$).tw. (1) 47 stein leventhal.tw. (2) 48 or/43‐47 (1963) 49 42 and 48 (748) 50 random.tw. (52311) 51 control.tw. (403643) 52 double‐blind.tw. (21298) 53 clinical trials/ (10820) 54 placebo/ (5068) 55 exp Treatment/ (707355) 56 or/50‐55 (1099613) 57 49 and 56 (425)
Appendix 6. CINAHL search strategy
Searched from 1961 to 5 March 2018
EBSCO platform
1 (MM "Polycytic Ovary Syndrome") (1,684)
2 TX Polycytic Ovary Syndrome (2,557)
3 TX PCOS (2,359)
4 1 OR 2 OR 3 (3,789)
5 (MM "Life Style Changes") (2,760)
6 (MM "Behavioral Changes") (3,430)
7 (MH "Diet+") (89,659)
8 TX diet* (186,208)
9 (MM "Exercie+") (53,616)
10 (MM "Weight Loss+") (8,899)
11 TX weight loss (28,493)
12 (MM "Cognitive Therapy+") (10,363)
13 TX cognitive therap* (19,448)
14 (MM "Pychotherapy+") (79,503)
15 (MM "Support, Pychoocial") (19,910)
16 (MM "Nutritional Counseling") OR (MM "Counseling") (12,749)
17 TX counselling (20,102)
18 TX counseling (57,271)
19 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 (416,078)
20 4 AND 19 (513)
21 (MH "Clinical Trials+") (235,949)
22 PT Clinical trial ( 85,872)
23 TX clinic* n1 trial* (221,376)
24 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) (939,703)
25 TX randomi* control* trial* (144,353)
26 (MH "Random Asignment") (45,897)
27 TX random* allocat* (8,330)
28 TX placebo* (50,027)
29 (MH "Placebo") (10,689)
30 (MH "Quantitative studie") (18,737)
31 TX allocat* random* (8,330)
32 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 OR 31 (1,210,612)
33 20 AND 32 (170)
Appendix 7. AMED search strategy
Searched from 1985 to 5 March 2018
OVID platform
1 Polycystic Ovar$.tw. (70) 2 (PCOS or PCOD).tw. (48) 3 (sclerocystic adj3 ovar$).tw. (0) 4 stein leventhal.tw. (1) 5 or/1‐4 (79) 6 exp Diet Therapy/ (1894) 7 diet$.tw. (6811) 8 exp Weight Loss/ (374) 9 (weight adj3 loss).tw. (794) 10 (weight adj3 reduc$).tw. (438) 11 (weight adj2 lose).tw. (34) 12 weight losing.tw. (2) 13 ((body mass index adj2 loss) or reduc$ or decreas$).tw. (33838) 14 ((BMI adj2 loss) or reduc$ or decreas$).tw. (33836) 15 exp Exercise Therapy/ (7314) 16 exercise$.tw. (24249) 17 (run$ or jog$).tw. (3711) 18 (sport$ or walk$).tw. (15848) 19 (swim$ or cycl$).tw. (6746) 20 train$.tw. (17556) 21 fit$.tw. (5926) 22 (cognitive adj2 therap$).tw. (1464) 23 CBT.tw. (255) 24 Psychotherapy.tw. (2377) 25 (Behavio?r adj2 therap$).tw. (1299) 26 (behavio?r adj2 manage$).tw. (111) 27 psychosocial.tw. (3619) 28 exp life style/ or exp life change events/ (1464) 29 ((life*style adj2 change$) or intervention$).tw. (23297) 30 yoga.tw. (668) 31 social support/ (2093) 32 (social adj2 support).tw. (3010) 33 relaxation.tw. (2401) 34 exp self efficacy/ (552) 35 self efficacy.tw. (1289) 36 exp Health Promotion/ (2055) 37 (Health adj2 Promotion).tw. (2394) 38 exp Health Education/ (2463) 39 (Health$ adj2 Education).tw. (1142) 40 ((motivation$ adj2 therap$) or interview$).tw. (9726) 41 exp Psychotherapy/ or exp Biofeedback/ or exp Relaxation/ or exp Hypnosis/ or exp Psychosomatic therapies/ (11155) 42 exp Cognitive therapy/ (1120) 43 exp Behavior therapy/ (3177) 44 or/6‐42 (115165) 45 5 and 44 (32)
Appendix 8. Cochrane 'Risk of bias' tool
| Domain | Description | Review authors’ judgement |
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
| Blinding of participants and personnel. Assessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
| Blinding of outcome assessment. Assessments should be made for each main outcome (or class of outcomes). | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Detection bias due to knowledge of the allocated interventions by outcome assessors. |
| Incomplete outcome data. Assessments should be made for each main outcome (or class of outcomes). | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Attrition bias due to the amount, nature or handling of incomplete outcome data. |
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Reporting bias due to selective outcome reporting. |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool. If particular questions/entries were pre‐specified in the review's protocol, responses should be provided for each question/entry. | Bias due to problems not covered elsewhere in the table. |
Data and analyses
Comparison 1. Lifestyle intervention versus minimal treatment: secondary outcomes and subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary reproductive: menstrual regularity and ovulation | Other data | No numeric data | ||
| 2 Secondary reproductive: endocrine | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Total testosterone (nmol/L) | 10 | 392 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.23, ‐0.02] |
| 2.2 Sex hormone‐binding globulin (SHBG) (nmol/L) | 9 | 364 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [0.22, 4.82] |
| 2.3 Free androgen index (FAI) | 6 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐1.11 [‐1.96, ‐0.26] |
| 2.4 Clinical hyperandrogenism (Ferriman‐Gallwey score) | 4 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐2.16, ‐0.08] |
| 3 Anthropometric | 13 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Weight (kg) | 9 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐1.68 [‐2.66, ‐0.70] |
| 3.2 Body mass index (BMI) (kg/m2) | 12 | 434 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.68, ‐0.01] |
| 3.3 Waist circumference (cm) | 7 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.80, ‐0.14] |
| 3.4 Waist‐hip ratio | 4 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 4 Metabolic: oral glucose tolerance test (OGTT) glucose (mmol/L/minute) | 3 | 121 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.38, 0.33] |
| 5 Metabolic: fasting glucose (mmol/L) | 11 | 354 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.15, 0.01] |
| 6 Metabolic: fasting lipids (mmol/L) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Total cholesterol (mmol/L) | 9 | 331 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.25, ‐0.02] |
| 6.2 High‐density lipoprotein cholesterol (HDL‐C) (mmol/L) | 9 | 327 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 6.3 Low‐density lipoprotein cholesterol (LDL‐C) (mmol/L) | 9 | 326 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.29, ‐0.03] |
| 6.4 Triglycerides (mmol/L) | 9 | 328 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 7 Metabolic: fasting insulin (µU/mL) | 10 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐2.44, ‐0.39] |
| 8 Metabolic: oral glucose tolerance test (OGTT) insulin (µU/mL/minute) | 3 | 121 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐1.73, ‐0.92] |
| 9 Quality of life | 3 | 475 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [0.16, 0.61] |
| 9.1 PCOSQ (Weight) | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.71, 0.49] |
| 9.2 PCOSQ (Hirsutism) | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.57, 0.56] |
| 9.3 PCOSQ (Emotions) | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [0.30, 1.23] |
| 9.4 PCOSQ (Infertility) | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | 0.68 [0.21, 1.14] |
| 9.5 PCOSQ (Menstrual irregularity) | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.24, 0.75] |
| 10 Subgroup analyses for SHBG | 9 | 1180 | Mean Difference (IV, Fixed, 95% CI) | 2.72 [1.45, 3.99] |
| 10.1 Medium duration | 7 | 253 | Mean Difference (IV, Fixed, 95% CI) | 2.28 [‐0.06, 4.63] |
| 10.2 Long duration | 2 | 111 | Mean Difference (IV, Fixed, 95% CI) | 4.82 [‐4.69, 14.33] |
| 10.3 Physical activity | 4 | 173 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐1.39, 3.61] |
| 10.4 Combined intervention | 5 | 191 | Mean Difference (IV, Fixed, 95% CI) | 8.73 [3.27, 14.19] |
| 10.5 Weight loss | 3 | 43 | Mean Difference (IV, Fixed, 95% CI) | 9.02 [3.03, 15.02] |
| 10.6 Weight maintenance | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 2.27 [‐6.28, 10.82] |
| 10.7 Overweight participants | 6 | 281 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [0.09, 4.83] |
| 10.8 Not specified weight of participants | 3 | 83 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐5.86, 10.05] |
| 11 Subgroup analyses for FAI | 6 | 688 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.09, 0.04] |
| 11.1 Medium duration | 5 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐1.06, 1.08] |
| 11.2 Long duration | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐6.23, 1.63] |
| 11.3 Physical activity | 4 | 173 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.94, 1.23] |
| 11.4 Combined intervention | 2 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.81, 0.21] |
| 11.5 Weight loss | 2 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐6.81, 0.21] |
| 11.6 Weight maintenance | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.97 [‐6.19, 14.13] |
| 11.7 Overweight participants | 3 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.62, 1.03] |
| 11.8 Not specified weight of participants | 3 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐2.97, ‐0.34] |
| 12 Subgroup analyses for HDL‐C | 9 | 1023 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.03] |
| 12.1 Medium duration | 7 | 216 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.06] |
| 12.2 Long duration | 2 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 12.3 Physical activity | 6 | 198 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 12.4 Combined intervention | 3 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 12.5 Weight loss | 2 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.15, 0.17] |
| 12.6 Weight maintenance | 1 | 11 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 12.7 Overweight participants | 4 | 219 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.11, 0.04] |
| 12.8 Not specified weight of participants | 5 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 13 Subgroup analyses for OGTT insulin | 3 | 121 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐1.73, ‐0.92] |
| 13.1 Physical activity | 1 | 90 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐2.20, ‐1.22] |
| 13.2 Combined intervention | 2 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐1.20, 0.24] |
| 14 Subgroup analyses for PCOSQ Weight | 3 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.71, 0.49] |
| 14.1 Behavioural | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 9.25 [3.69, 14.81] |
| 14.2 Physical activity | 2 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.83, 0.38] |
| 15 Sensitivity analyses for BMI | 5 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐1.91, 2.49] |
| 16 Sensitivity analyses for FAI | 4 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐2.16, 0.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almenning 2015.
| Methods |
Study design: 3‐arm, parallel randomised controlled trial; enrolled from July to October 2013; follow‐up testing was performed from October to December 2013 Duration: 10 weeks Power calculation: yes |
|
| Participants |
Number of participants: HIT, n = 10; ST , n = 11; control, n = 10; analysed: HIT, n = 8; ST, n = 8, control, n = 9 PCOS definition: ESHRE/ASRM Baseline characteristics (mean ± SD), intervention versus controls: Age: not reported Weight: ST 76.5 ± 20.2; HIT 73.5 ± 16.7; CG 74.5 ± 16.1 BMI, kg/m2: ST 27.4 ± 6.9, HIT 26.1 ± 6.5; CG 26.5 ± 5.0 Inclusion criteria: Quote: "PCOS was defined according to the ESHRE/ASRM criteria: A minimum of two of the following: PCO morphology (12 or more 2‐9mm follicle or >10mL in volume, in at least one ovary)". Hyperandrogenism (either clinical signs of hirsutism (Ferriman Gallwey score ≥8) or acne, or biochemical (testosterone >3.0nmol/L, calculated free testosterone >32nmmol/L, SHBG <30nmol/L, or Free Androgen Index (FAI as 100 x testosterone concentration (nmol/L)/SHBG concentration (<30nmol/L) >5%). Oligomenorrhea (intermenstrual interval >35 days and <8 menstrual bleedings in the last year) or amenorrhoea (absent menstrual bleeding or no bleeding in the last 90 days. In women with no prior PCOS diagnosis and only one of the criteria, a vaginal ultrasound was done to confirm diagnosis prior to trial entry. Quote: "Some of the included women were diagnosed by their own gynaecologist. In women who had no prior PCOS diagnosis, we first assessed if they had oligo/amenorrhoea and hyperandrogenism. If they fulfilled only one of these criteria, a vaginal ultrasound was done to confirm the diagnosis before study entry." Exclusion criteria: Quote: "Regular high‐intensity endurance or strength training (defined as > 2 sessions of vigorous exercise per week), physical ailments/injuries that limited exercise performance, on‐going pregnancy, concurrent treatments (insulin sensitizers as metformin and pioglitazone) or drugs known to affect gonadotropin or ovulation, with a wash out period of one month prior to inclusion. The exception was regular use of oral contraceptives, and women were included if they did not change the type or dose > 1 month prior to the study or during the intervention period." Medication use pre and during the study: as in exclusion criteria Groups comparable at study commencement: yes Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: 3 weekly exercise sessions for 10 weeks. At least 1 session per week was supervised by an exercise physiologist. All participants were advised to maintain normal diet. High‐intensity interval training – Quote: "two weekly sessions of four x 4 minute HIT at 90‐95% of individual heart rate maximum separated by three minutes of moderate intensity exercise at 70% of HR, and one weekly session of ten x 1 minute with maximal intensity HIT, separated by one minute of rest/very low activity." Participants could choose from treadmill, outdoor walking/running and/or cycling. Heart rate monitors were used in all sessions, and heart rate data were downloaded once a week to ensure compliance with high intensity protocol. Strength training (n = 11) – Quote: "eight dynamic strength drills with a resistance of 75% of one repetition maximum, with 10 reps and three sets separated by one minute rest between sets. The load was progressively increased once the participant could successfully perform three sets of ten reps." Sessions were conducted at a local fitness centre. b) Control: advised to Quote: "adhere to the recommended at least 150 minutes of weekly moderate‐intensity exercise without any follow‐up during the ten weeks intervention period". Maintain normal diet and physical activity. Compliance with intervention: participants in ST performed 26 ± 6.5 exercise sessions, thereby giving a compliance of 87%. In HIT, participants performed 27 ± 1.9 sessions, thereby an average exercise compliance of 90%. |
|
| Outcomes |
Measurements: baseline and post‐intervention (10 weeks) Endocrine: testosterone, SHBG, FAI Metabolic: total cholesterol, HDL‐C, LDL‐C, triglycerides, glucose, insulin Anthropometric: weight, BMI, waist circumference |
|
| Notes |
Location: Trondheim, Norway Funding: the Norwegian Fund for Research in Sports Medicine. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer random number generator developed and administered at Unit for Applied Clinical Research at the University to randomise the subjects, Baseline testing was done before randomisation." |
| Allocation concealment (selection bias) | Low risk | Third party randomisation and allocation by the University's Unit for Applied Clinical Research |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Quote: "Follow‐up testing was performed from October to December 2013, and these measurements were done non‐blinded to group assignment." An observer blinded to group allocation analysed the FMD data. Menstrual data were self‐reported. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | High risk | Quote: "Follow‐up testing was performed from October to December 2013, and these measurements were done non‐blinded to group assignment." |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Other outcomes are based on biochemistry/lab results so not influenced by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participants dropped out during the intervention period (high‐intensity group 2, strength training 3, control 1). All participants who met for testing after 10 weeks were included in the analyses, regardless of compliance with the intervention protocol, and their outcomes were analysed according to the group to which they were allocated. |
| Selective reporting (reporting bias) | Low risk | Clinical trials pre‐specified outcomes reported in the trial. All pre‐specified outcomes in ClinicalTrial.gov NCT01919281 were reported except menstruation diary secondary outcome |
| Other bias | Low risk | Apart from lower FMD in the HIT group, there were no significant differences in baseline characteristics between groups. |
Brown 2009.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial. Recruitment between April 2003 and April 2005. Duration: 12 weeks Power calculation: yes |
|
| Participants |
Number of participants: N = 37 randomised (n = 21 intervention, n = 16 control), n = 20 completed and analysed (n = 8 intervention, n = 12 control) PCOS definition: 8 or fewer menses per year and clinical or biochemical hyperandrogenism (hirsutism: Ferriman‐Gallwey < 8 or bioavailable testosterone > 8.4 ng/dL, 2 SD above laboratory mean) Baseline characteristics (median ± interquartile range), intervention versus controls: age 36.5 ± 5.0 versus 28.0 ± 11.0 years, weight 89.1 ± 31.5 versus 87.3 ± 40.8 kg, BMI 37.9 ± 9.4 versus 31.3 ± 14.9 kg/m2 Inclusion criteria: PCOS, age 18 to 50 years, sedentary lifestyle (no regular exercise during the usual week), ability to come to study exercise facility for monitored exercise, agreement to maintain current weight/dietary patterns for study Exclusion criteria: menopause, current/planned pregnancy, recent breastfeeding, congenital adrenal hyperplasia, uncontrolled thyroid disease, hyperprolactinaemia, or fasting hyperglycaemia (> 6.9 mmol/L), unresolved medical conditions, history of malignancy other than non‐melanoma skin cancer in the past 5 years, study participation in past 30 days Medication use pre and during the study: no hormonal contraceptive use, anti‐androgen therapy, use of medications known to affect carbohydrate metabolism (metformin and thiazolidinediones) within the past 90 days Groups comparable at study commencement: intervention group significantly older Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: 2 arms a) Intervention: active weight maintenance encouraged, 8‐ to 12‐week ramp‐up followed by a 12‐week moderate‐intensity exercise programme (16 to 24 weeks total, average 228 minutes/week at 40% to 60% peak VO2) b) Control: no change in lifestyle Were the care programmes, other than the trial options, identical? No, study duration different for intervention (20 to 24 weeks) and controls (12 weeks). Control group participants were not contacted during the 12 weeks of their enrolment in the study. Intervention: individualised exercise/week and additional contact from exercise physiologist/dietitian depending on compliance. Average of 228 minutes and 4.4 sessions/week. Compliance with intervention: mean adherence rate (minutes of exercise at prescribed heart rate completed divided by minutes prescribed) of 89.8% |
|
| Outcomes | Measurements: 0 and 20 to 24 weeks for intervention, 0 and 12 weeks for control Endocrine: bioavailable testosterone (Mayo Lab), Ferriman‐Gallwey score (clinician assessed) Metabolic: OGTT glucose, lipid profile (total cholesterol, HDL‐C, LDL‐C, triglycerides by conventional spectrophotometric assays), fasting glucose and insulin, OGTT insulin Anthropometric: weight, BMI, waist/hip circumference |
|
| Notes | Menstrual cycle phase not stated for blood collection Data skewed and reported as median ± IQR. Contacted author to obtain mean ± SD but did not receive results at time of analysis. As previously stated, "Where data were clearly skewed and results reported in the publication as median and range with non‐parametric tests of significance, the results were excluded from the meta‐analysis", all results therefore excluded from meta‐analysis. Location: Duke University Medical Centre, Durham, North Carolina, USA Funding: NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, Quote: "Randomization was accomplished by generating a random sequence of two variables (for instance, As and Bs, representing the two treatment groups) using the online program at http://graphpad.com/quickcalcs/randomize2.cfm". |
| Allocation concealment (selection bias) | Low risk | Investigators blinded to allocation sequence. Quote: "Each group assignment was placed in its own sequentially numbered envelope by an individual not involved in the study". |
| Blinding (performance bias and detection bias) Participant reported outcomes | Low risk | Participant/treatment provider blinding not possible due to interactive nature of intervention. As no self‐reported outcomes, lack of participant blinding unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Unclear risk | Outcome assessor blinding unclear, not stated. Potential for lack of clinical assessor blinding to impact on results reporting and result in bias for clinical reported outcomes of Ferriman‐Gallwey score, weight, BMI, adiposity distribution. |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Outcome assessor blinding unclear, not stated. Potential for lack of outcome assessor blinding to impact on results reporting and result in bias for objective outcomes of biochemical data and ovulation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. Withdrawals: n = 21 intervention and n = 16 control commenced, at study end n = 8/21 intervention (62% dropout) and n = 12/16 control (25% dropout). Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. The lack of blinding may have contributed to higher levels of dropout for the intervention group. Higher dropout rate for intervention versus control potentially giving exaggeration of treatment effect. |
| Selective reporting (reporting bias) | High risk | Total cholesterol measured but not stated. For the remainder of the results, unclear, insufficient information to permit judgement of 'Yes' or 'No', study not registered as clinical trial. From the results section of the paper, all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Guzick 1994.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial. Study start and end dates not provided. Duration: 12 weeks Power calculation: no |
|
| Participants |
Number of participants: 12 recruited, 12 randomised and analysed (n = 6 intervention, n = 6 control) PCOS definition: anovulation or oligo‐ovulation (< 4 bleeding episodes in previous 12 months, anovulation confirmed by weekly progesterone levels and only anovulatory women used) and negative pregnancy test, hyperandrogenism (testosterone > 2.43 mmol/L) Baseline characteristics (mean ± SD), intervention versus controls: age 32.3 ± 12.0 versus 31.2 ± 9.6 years, weight 108.0 ± 31.8 versus 108.0 ± 33.8 kg Inclusion criteria: PCOS, age 20 to 40 years, obese (between 130% and 200% of ideal body weight according to 1983 Metropolitan Height and Weight Tables for women), negative pregnancy test Exclusion criteria: medical conditions that would compromise the safety of very low‐calorie diet including chronic renal failure, cardiovascular or cerebrovascular disease, liver disease, cancer or psychosis, exclusion of specific causes of hyperandrogenism (tumour of the adrenal gland, congenital adrenal hyperplasia, Cushing’s syndrome, acromegaly, hyperprolactinaemia, drug‐induced hyperandrogenism) Medication use pre and during the study: unclear, not stated Groups comparable at study commencement: yes Participants actively seeking pregnancy: no |
|
| Interventions | Comparison: 2 arms a) Intervention: weight loss, 12‐week behavioural weight control programme comprising 8 weeks of very low‐calorie diet (Optifast, additional meals and multivitamin supplement) then reintroduction of foods and gradual increase in energy intake until 4200 to 5040 kJ/day reached. Behaviour modification training around eating behaviours, increasing energy expenditure (1050 kJ/week extra to 4200 kJ/week extra, 2 miles, 5x week) b) Control: no treatment or study visits, 12‐week waiting interval Were the care programmes, other than the trial options, identical? No, the intervention participants attended additional study visits as part of the intervention and therefore had greater study contact (number of visits unknown). Compliance with intervention: not stated |
|
| Outcomes | Measurements at 0 and 12 weeks Ovulation (weekly progesterone, ovulatory is progesterone > 15 mmol/L) Endocrine: total testosterone, SHBG, non‐SHBG testosterone (based on separation of SHBG‐bound titrated from unbound and albumin‐bound testosterone by ammonium sulphate precipitation) Anthropometric: height, weight, body fat distribution (WHR) Metabolic: fasting glucose, insulin |
|
| Notes | Not stated which phase of menstrual cycle measurements occurred in Outcome data for variables total testosterone and glucose presented graphically; Microsoft Paint used to estimate baseline or endpoint data ± SEM and converted to SD Location: University Research Centre, University of Pittsburgh School of Medicine, Pennsylvania, USA Funding: supported in part by Magee Women's Hospital Research Fund, Western Psychiatric Institute and Clinics Seed Monies Fund, National Institute of Health General Clinical Research Centre grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized"; insufficient information about the process to permit a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Not stated; insufficient information about the process to permit a judgement. |
| Blinding (performance bias and detection bias) Participant reported outcomes | Low risk | Participant/treatment provider blinding not possible due to the interactive nature of the intervention. As no self‐reported outcomes, lack of participant blinding unlikely to introduce bias. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Unclear risk | Outcome assessor/data analyst blinding unclear, not stated, insufficient information about the process to permit a judgement. Potential for lack of clinical assessor blinding to impact on results reporting and result in bias for clinician‐reported outcomes of weight, BMI and adiposity distribution. |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Outcome assessor/data analyst blinding unclear, not stated, insufficient information about the process to permit a judgement. Potential for lack of outcome assessor blinding to impact on results reporting and result in bias for objective outcomes of biochemical data and ovulation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT analysis but no dropouts reported. Metabolic/anthropometric: all data reported. Reproductive: all data reported except incomplete ovulation data reported for 10/12 participants. 5/6 for treatment and 5/6 for control, Quote: "all samples were obtained except for one sample in each of the control and treatment groups". Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups unrelated to intervention. No dropouts reported for intervention or control group. Unclear if indicates 0% dropout rate or completers analysis only. |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit a judgement; study not registered as a clinical trial. From results section of paper, all of the studies prespecified (primary and secondary) outcomes that are of interest in the review have been reported in a prespecified way with the exception of weight for the control group, which was only reported as "the control group was not associated with a change in weight" with no numeric data provided. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Hoeger 2004.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial. Study start and end dates not provided. Duration: 48 weeks Power calculation: no |
|
| Participants |
Number of participants: for entire cohort n = 38 randomised. For intervention versus control arms, n = 20 randomised (n = 11 intervention and n = 9 control). At 24 weeks n = 8 intervention and n = 7 control completed and analysed. At 48 weeks n = 6 intervention and n = 7 control completed and analysed. PCOS definition: fewer than 6 menses/year, hyperandrogenism (serum total testosterone > 50 ng/dL, no hirsutism by Ferriman‐Gallwey) Baseline characteristics (mean ± SD), intervention versus controls: age 27.1 ± 4.3 versus 27.1 ± 4.5 years, BMI 40 ± 7.4 versus 37.1 ± 4.6 kg/m2 Inclusion criteria: overweight (BMI > 25 kg/m2), PCOS Exclusion criteria: pregnancy (hCG performed at each visit), DM2 (known or elevated fasting glucose), abnormal liver and kidney function, use of antihypertensives or statin therapy, exclusion of other reproductive disorders (Cushing's syndrome, hyperprolactinaemia, thyroid disease, androgen‐secreting tumours, adrenal disease) Medication use pre and during the study: no hormonal medication within 2 months prior to the study, no use of insulin sensitisers, anti‐androgen therapy, oral contraceptive pill during the study. n = 1 control took 10 days progestin for unscheduled heavy bleeding at week 16. Groups comparable at study commencement: yes Participants actively seeking pregnancy at study commencement: no, n = 2 seeking pregnancy in year prior to study but abandoned pregnancy effort |
|
| Interventions | Comparison: 4 arms a) Metformin 850 mg 2x/day b) Lifestyle modification programme with placebo 2x/day c) Lifestyle modification with metformin 850 mg 2x/day d) Placebo 2x/day For review: a) Intervention: weight loss, lifestyle intervention with placebo defined as aim 7% to 10% weight loss, registered dietitian/exercise physiologists, individualised meal plan with 500 to 1000 calorie deficit/day (50% carbohydrate, 25% protein, 25% fat, low GI foods, individualised exercise plan 150 minutes/week). Group meetings and progress monitoring weekly for 0 to 24 weeks, biweekly for 25 to 48 weeks b) Control: no lifestyle intervention and placebo (no dietary or exercise instruction) Were the care programmes, other than the trial options, identical? No, the intervention participants attended additional study visits as part of the intervention and therefore had greater study contact (36 visits compared to 12 visits). Compliance with intervention: not stated |
|
| Outcomes |
Measurements: 0, 24 and 48 weeks Weekly morning urinary pregnanediol glucuronide (if elevation in urinary pregnanediol glucuronide noted preceding menstrual flow counted as an ovulatory event, consecutive weekly elevated levels were counted as a single ovulatory event), menstrual diaries Endocrine: testosterone, SHBG, FAI, F‐G score (clinician assessed) Anthropometric: height, weight (kg), BMI, waist/hip circumference Metabolic: OGTT 0, 30, 60, 120, 180 minutes glucose (AUC calculated trapezoidally), lipid profile (total cholesterol, HDL‐C, LDL‐C, triglycerides), OGTT 0, 30, 60, 120, 180 minutes insulin (AUC calculated trapezoidally) |
|
| Notes | Not stated which phase of menstrual cycle measurements occurred in Location: General Clinical Research Centre, University of Rochester, New York, USA Funding: supported by Mae Stone Good foundation grant, Women’s Reproductive Health Research Career Centre grant and General Clinical Research Centre grant, National Centre for Research Resources, National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, computer‐generated in blocks by third party (independent pharmacy representative). Quote: "The randomisation schedule was computer generated in blocks by an independent pharmacy representative." |
| Allocation concealment (selection bias) | Low risk | Investigators blinded to allocation sequence. Quote: "and the block schedule was blinded to the investigators." |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Participant/treatment provider blinding not possible due to interactive nature of intervention. Potential for lack of participant blinding could potentially introduce bias for self‐reported outcome of menstrual diaries. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Low risk | Outcome assessors and data analysts unaware of study assignment and therefore reduced risk of bias for clinical reported outcomes of ovulation, hirsutism (Ferriman‐Gallwey), weight, BMI, adiposity distribution. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Outcome assessors and data analysts unaware of study assignment and therefore reduced risk of bias for objective outcomes of biochemical data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT analysis. Withdrawals: n = 11 intervention and n = 9 control commenced, at 24 weeks n = 8/11 intervention (27% dropout) and n = 7/9 control (22% dropout), at 48 weeks n = 6/11 intervention (45% dropout although n = 2 pregnancies indicating potential positive intervention effect) and n = 7/9 control (22% dropout). Metabolic/anthropometric parameters no missing outcome data. Reproductive parameters: no missing outcome data. For 48 weeks, n = 4 for lifestyle and n = 6 for placebo due to missing data. All data points reported for other variables. With exception of pregnancies, missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement; study not registered as a clinical trial. From results section of paper, all of the studies prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Hoeger 2008.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial. Recruitment between August 2002 and September 2003. Duration: 24 weeks Power calculation: no |
|
| Participants |
Number of participants: for entire cohort n = 43 randomised. For intervention versus control arms, n = 22 randomised (n = 11 intervention and n = 11 control), n = 18 completed and analysed (n = 8 intervention and n = 10 control) PCOS definition: irregular menses (> 45‐day menstrual cycles/fewer than 8 menses in the preceding year), hyperandrogenism (acne, hirsutism Ferriman‐Gallwey >7, elevated androgens) Baseline characteristics (mean ± SD), intervention versus controls: age 15.4 ± 1.2 versus 15.4 ± 1.7 years, weight 96.9 ± 18.3 versus 92.0 ± 20.7 kg, BMI 37.8 ± 8.2 versus 36.1 ± 7.5 kg/m2 Inclusion criteria: PCOS, adolescent females, 1 year postmenarchal, ages 12 to 18 years, obese (BMI > 95th percentile) Exclusion criteria: Cushing’s syndrome, hyperprolactinaemia, congenital adrenal hyperplasia, renal or hepatic impairment, exercise > 10 hours/week, smoking > 1 pack cigarettes/week, significant ovarian surgery, current alcohol use or history of substance abuse, other causes of hyperandrogenism or menstrual irregularity Medication use pre and during study: no use of oral contraceptives, oestrogen or progestin or other drugs known to affect lipoprotein metabolism within 2 months of study, no use of drugs known to affect gonadotrophin secretion or ovulation. No use of hormonal support, insulin sensitisers during the study. Groups comparable at study commencement: yes Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: 4 arms a) Metformin 1500 mg/day b) Oral contraceptive 30 µg ethinyl estradiol and 0.15 mg desogestrel c) Lifestyle modification programme with placebo 2x/day d) Placebo For review: a) Intervention: weight loss, lifestyle intervention versus placebo. Closed group intervention format, 5 to 6 members per group, participants and one adult family member (parent or guardian) in structured training classes on diet, exercise and behaviour modification skills with frequent contact, flexible personal strategies, self‐esteem and social support. 16‐session core curriculum group and individual appointments. Therapy goals of a 5% to 7% weight loss and a level of exercise of at least 150 minutes/week. b) Control: standard office advice on nutrition and exercise for healthy living (written information on best lifestyle choices at enrolment but no formal education) and seen monthly. Were the care programmes, other than the trial options, identical? No, the intervention participants attended additional study visits as part of the intervention and therefore had greater study contact (24 visits compared to 6 visits). Compliance with intervention: n = 4 attended < 50% sessions and did not demonstrate weight reduction. Remaining participants attended at least 75% of sessions and met lifestyle goals. |
|
| Outcomes |
Measurements: 0 and 24 weeks Weekly urine pregnanediol assessment for ovulation, menstrual cycle average per 24 weeks Endocrine: total and free testosterone, SHBG, FAI, hirsutism (Ferriman‐Gallwey) Metabolic: OGTT at 0, 30, 60, 120 minutes (AUC glucose), lipid profile (total cholesterol, HDL‐C, LDL‐C, triglycerides), OGTT at 0, 30, 60, 120 minutes (AUC insulin) Anthropometric: BMI, waist circumference |
|
| Notes | Menstrual cycle phase not stated for blood collection Location: General Clinical Research Centre, University of Rochester, New York, USA Funding: NIH, WRHR, GCRC, National Center for Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, Quote: "use of computer generated list of random numbers". |
| Allocation concealment (selection bias) | Low risk | Investigators blinded to allocation sequence "they will be randomised in blocks of 8 by an independent agent in the hospital pharmacy". |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Participant/treatment provider blinding not possible due to the interactive nature of the intervention. Potential for lack of participant blinding to introduce bias for self‐reported outcome of menstrual diaries. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Low risk | Outcome assessors and data analysts unaware of study assignment, therefore reduced risk of bias for clinical reported outcomes of ovulation, hirsutism (Ferriman‐Gallwey), weight, BMI, adiposity distribution. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Outcome assessors and data analysts unaware of study assignment and therefore reduced risk of bias for objective outcomes of biochemical data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. Withdrawals: n = 11 intervention and n = 11 control commenced, at 24 weeks n = 8/11 intervention (27% dropout) and n = 10/11 control (9% dropout). Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. The lack of blinding may have contributed to higher levels of dropout for the intervention group. The higher dropout rate for intervention versus control potentially giving over exaggeration of treatment effect. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the studies prespecified (primary and secondary) outcomes that are of interest in the review have been reported in a prespecified way. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Mani 2018.
| Methods |
Study design: single‐centre, randomised controlled trial Duration: single session intervention, 12 months follow‐up Power calculation: yes ITT analysis conducted |
|
| Participants |
Number of participants: 172 randomised with 86 in each group. At 12 months: 52 in control and 48 in intervention. Completers were analysed. PCOS definition: women with a confirmed diagnosis of PCOS, definitions unclear, referred to ESHRE consensus guideline Baseline characteristics (mean ± SD), intervention versus controls: Control age 33.3 ± 8.1, intervention age 33.4 ± 7.1 Control weight 89.0 ± 19.6, intervention weight 90.9 ± 18.9 Control BMI 33.2 ± 6.2, intervention BMI 34.2 ± 7.2 Inclusion criteria: Quote: "Women with a confirmed diagnosis of PCOS, Body Mass Index (BMI) (≥23 kg/m2 for black and minority; ≥25 kg/m2 for white Europeans), aged 18–49 years inclusive, who had stable PCOS treatment in the previous 6 months were eligible." Exclusion criteria: Pregnancy, diabetes, use of corticosteroids, disabling physical or mental condition and inability to speak English." Medication use pre and during the study: use of corticosteroids is excluded; stable on treatment; number of participants on metformin 15 in control, 17 in intervention Groups comparable at study commencement: yes, although no statistical test was performed to confirm this Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: Quote: "The final programme consisted of 7 hours of interactive discussions including patient and professional story, diet and physical activity, balancing life with PCOS and self‐management plan (Supplementary Table 1, see section on supplementary data given at the end of this article). Each education session was delivered by two trained educators. Each participant received a resource pack including summary of the points from each section as well as the results of their glucose, lipids, BMI and average walking steps from their baseline visit so they could reflect on their results during the programme. In the section ‘Balancing life with PCOS’ (Supplementary Table 1), participants had the opportunity to share their feelings towards PCOS. At the end of the session, participants were asked to reflect on the day." b) Control: the study team did not change the medical treatment for PCOS Both arms continued as before with their own doctors and both received a generic information sheet about PCOS and the benefits of lifestyle change. Compliance with intervention: 65 (77%) women who attended education, Quote: "The activity log indicated that almost half of the participants had taken off their monitors during potentially relevant activities such as swimming, spinning class, running and partying: 47% in the education arm and 50% in the control. None, however, had recorded the time duration for these activities." |
|
| Outcomes |
Measurements: baseline and 12 months follow‐up Endocrine: testosterone, SHBG Metabolic: glucose, total cholesterol, HDL‐C, LDL‐C, triglycerides, insulin Anthropometric: weight, BMI Quality of life (median): PCOSQ, SF‐12 |
|
| Notes |
Location: UK Funding: supported by Diabetes Research Centre, University of Leicester, through funds from National Institute for Health Research (NIHR) and NIHR Collaboration for Leadership in Applied Health Research and Care – East Midlands and the NIHR Leicester – Biomedical Research Centre, a partnership between University Hospitals of Leicester NHS Trust, Loughborough University and the University of Leicester. ‘Early Career Grant’ from Society for Endocrinology (HM). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation occurred after the baseline visit by an independent administrator. Method of randomisation not stated." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation occurred after the baseline visit by an independent administrator." Quote: "Participants were blind to their baseline step‐counts." |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | The study was un‐blinded due to the nature of the intervention. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | High risk | Not blinded due to the nature of the intervention but endpoints objective. |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Not blinded due to the nature of the intervention but endpoints objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Described: unequal loss after randomisation and after allocation. Reasons provided for losses after allocation. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not published but was registered in ClinicalTrials.gov; identifier: NCT01462864 |
| Other bias | Unclear risk | The study appears to be free of other sources of bias. |
Mirfeizi 2013.
| Methods |
Study design: randomised controlled trial Duration: 3 months Power calculation: unclear |
|
| Participants |
Number of participants: 25 in control, 25 in intervention PCOS definition: women with a confirmed diagnosis of PCOS, definitions unclear Baseline characteristics (mean ± SD), intervention versus controls: unclear Inclusion criteria: Quote: "Participants have body mass index = 25 kg/m2; All Participants treat with same dose of oral contraceptives pill and spirinolactone; All Participants have menstrual pattern as oligomenorrhea (irregular bleeding episodes with intervals occur more than 35 days); All Participants have suffered some degree of hirsutism." Exclusion criteria: Quote: "1‐ Use of any drug other than the standard drug for the treatment of polycystic ovary syndrome, which is the same for everyone. (Including drugs to treat infertility, diabetes, hormonal drugs, reduce appetite and etc); 2‐ Smoking; 3‐ Being in pregnancy and lactation period or pregnancy decision; 4‐ Having cardiovascular, renal, liver, respiratory diseases, diabetes, uncontrolled hypertension, malignancy or any other acute or chronic disease; 5‐ Prohibition of the exercise due to illness or any other cause according to physician order; 6‐ Participating in regular exercise before the start of the study; 7‐ Having a separate diet programs before the start of the study." Groups comparable at study commencement: unclear Participants actively seeking pregnancy at study commencement: not stated |
|
| Interventions | Comparison: a) Intervention: Quote: "Diet plan: at the beginning of study a check list prepared for each individual and weekly regimen recommended separately based on feeding patterns and individual facilities. The method of regimen calculation based on total energy for each individual (TEE). We set the countable energy for reducing weight 1000 grams per week. Reduced the amount of energy and some energy into the physical activity to lose weight fraction are obtained. The combination of energy, including 40% carbohydrate, 30% fat (less than 8% saturated fatty acids was) and 30% protein is high physiological value. Fitness program: exercise sessions that included 24 meeting which duration per session was variable according to the program. For improving aerobic energy system and strengthen cardiovascular system a series exercises (slow jogging) will be used. At the beginning of the first practice sessions will be used slow intensity aerobic exercise. The first training sessions with 5 minutes of times, and 50 percent of maximum heart rate of cholera in the form alternating between resting half the time repeating the exercise. In each session we were trying to follow the principle of overload for improving cardiovascular efficiency. In higher stages (after the fifth session) of the repetition of exercises with some 60 percent of maximum heart rate is achieved. Of the ninth session, training intensity reaches to 70 percent of maximum heart rate and in last sessions with 80 percent maximum heart rate do their exercise. In each session, subjects in the first 10 minutes to warm the body and also the final 10 minutes of cooling down exercises and return to the initial state." b) Control: not stated Compliance with intervention: not stated |
|
| Outcomes |
Measurements: baseline and 3 months Endocrine: testosterone, SHBG |
|
| Notes |
Location: Iran Funding: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) Participant reported outcomes | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
Nasrekani 2016.
| Methods |
Study design: randomised controlled trial Duration: 12 weeks Power calculation: yes |
|
| Participants |
Number of participants: 20 randomised with 10 in each group. At 3 months: 10 in control and 10 in intervention. Completers were analysed. PCOS definition: meeting 2 from 3 criteria as follows: 1) anovulation or low ovulation (having oligo‐menorrhoea, amenorrhoea or poly‐menorrhoea), 2) elevation of androgenic hormones in the body or having hirsutism and ratio of LH/FSH>2, 3) having polycystic ovaries in the ultra‐sonography Baseline characteristics (mean ± SD), intervention versus controls: Control age 29.8 ± 4.75, intervention age 30.9 ± 7.14 Control weight 70.13 ± 13.84, intervention weight 71.11 ± 15.05 Control BMI 28.1 ± 5.92, intervention BMI 28.43 ± 6.78 Inclusion criteria: Healthy women according to the Health Questionnaire, not under medication, non‐smokers, infertility, not participated in any exercise programme and having PCOS according to 2 from the 3 criteria mentioned above. Medication use pre and during the study: not reported Groups comparable at study commencement: yes, although no statistical test was performed to confirm this Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: 12 weeks, 3 days/week aerobic training with the intensity of 40% to 65% maximum heart rate reserve b) Control: there was no intervention in the control group Compliance with intervention: not reported |
|
| Outcomes |
Measurements: pre and post intervention Anthropometric: weight, BMI |
|
| Notes |
Location: Iran Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Third party randomisation and allocation by the University's Unit for Applied Clinical Research; allocation concealment not stated. |
| Blinding (performance bias and detection bias) Participant reported outcomes | Unclear risk | Blinding not stated |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Unclear risk | In the methods section, the authors mentioned that they measured the percentages of body fat and waist/hip ratio, but in the results section there were no data on these 2 variables. |
| Other bias | Unclear risk | Information not available |
Saremi 2013.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial Duration: 8 weeks Power calculation: yes |
|
| Participants |
Number of participants: excluded and analysed (not stated) ‐ randomised: 11 intervention and 11 control PCOS definition: ESHRE/ASRM Baseline characteristics (mean ± SD), intervention versus controls: weight (kg) exercise 69.60 ± 18.16, controls 68.50 ± 9.37; body mass index (BMI) exercise 28.29 ± 5.73, controls 28.40 ± 2.70 Inclusion criteria: irregular periods of less than 21 days or more than 31 days, polycystic ovaries on ultrasound, hyperandrogenism – hirsutism and acne Exclusion criteria: infection, metabolic diseases, cardiovascular, renal and adrenal, and extracranial, liver, thyroid disease; oral contraceptive use and metformin, pregnancy, abnormal prolactin and participation in regular exercise Medication use pre and during the study: OCP metformin excluded pre‐study Groups comparable at study commencement: not stated Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: an 8‐week aerobic training programme consisting of aerobic training 3 days per week for 8 weeks, for 40 to 60 minutes each. Each session involved 5 to 7 minutes of warm up, 30 to 50 minutes of main exercises on the treadmill (starting at 40% to 45% of heart rate building up to 60% to 65% of heart rate by the end of the 8th week), finishing with cooling down exercises. b) Control: asked not to do more physical activity than they used to and not to start any physical activity without informing research group Compliance with intervention: not reported |
|
| Outcomes |
Measurements: Endocrine: total testosterone Metabolic: HDL‐C, LDL‐C, triglycerides, fasting glucose, fasting insulin Anthropometric: weight, BMI, waist circumference, WHR |
|
| Notes |
Location: Iran Funding: University of Arak |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | They were randomly divided into 2 11‐person groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Participant reported outcomes | Low risk | Blinding of participants and study personnel would not have been possible due to the nature of the intervention, however outcomes are biochemical assessments of hormone and triglycerides and unlikely to be influenced by knowledge of group allocation. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Low risk | Not blinded but biochemical outcomes unlikely to be affected by blinding as they are objective measures. |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | No details provided of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Manuscript states that 11 women were allocated to each group with no further details of any attrition. |
| Selective reporting (reporting bias) | Low risk | No clinical trial reported but all prespecified outcomes reported. |
| Other bias | Unclear risk | Appears free of other sources of bias, based on translation (original article in Persian). |
Saremi 2016.
| Methods |
Study design: 3‐arm, parallel‐group controlled trial Duration: 8 weeks Power calculation: yes. Based on previous studies, estimated sample size to achieve 5 units difference between groups with a power of 0.8, alpha 0.05 was 8 and allowing dropouts, 10 people per group were included |
|
| Participants |
Number of participants: out of 30 participants in the study, 2 were excluded (1 because of not taking supplement and 1 because of getting sick) PCOS definition: different definition to standard Baseline characteristics (mean ± SD), intervention versus controls: Age: exercise and placebo 25.71 ± 5.1; control 29.81 ± 5.2 Weight: exercise and placebo 65.20 ± 5.3; control 67.4 ± 7.6 BMI: exercise and placebo 24.88 ± 1.23; control 26.02 ± 1.37 Inclusion criteria: at least 3 of the 4 following symptoms: menstrual irregularity, hirsutism, acne and polycystic ovary morphology on the abdominal sonography Exclusion criteria: smoking, infection and taking any medication that could affect laboratory assessments Medication use pre and during the study: taking any medication that could affect laboratory assessments excluded Groups comparable at study commencement: yes, based on numbers above Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: 1. Resistance exercise with placebo (10 people) 2. Resistance exercise with calcium supplements (10 people) b) Control: maintain their routine lifestyle over the study. Controls reported their diet and physical activity to investigators weekly, over the study period. Compliance with intervention: no information about compliance with exercise and compliance results not reported. To be sure the participants were taking supplements and placebo and to calculate to what extent they were adherent to taking capsules, they were asked to hand over the capsule boxes, then capsules for the next 2 days were given to them. Participants were asked to avoid any change to their usual diet and the dose of capsules. |
|
| Outcomes |
Measurements: baseline and 8 weeks Reproductive: none Metabolic: glucose, total cholesterol, HDL‐C, LDL‐C, triglycerides and insulin Anthropometric: weight, BMI |
|
| Notes |
Location: Department of Exercise Physiology, Faculty of Humanities, University of Arak, Arak, Iran Funding: sponsored by the University of Arak |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states "quasi‐experimental" and that eligible women were randomly assigned to groups, but no further information is provided. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to enable a judgement. |
| Blinding (performance bias and detection bias) Participant reported outcomes | Unclear risk | It states that the study was blind but there are no specific details of who was blinded. Presumably the participants due to the use of placebo and calcium supplementation. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Unclear risk | It states that the study was blind but there are no specific details of who was blinded. Presumably the participants due to the use of placebo and calcium supplementation. |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | All biochemical outcomes, therefore likely to be low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Out of 30 participants in the study, 2 were excluded (1 because of not taking the supplement and 1 because of getting sick); not sure which group. |
| Selective reporting (reporting bias) | Unclear risk | Appears free of selective reporting bias: all pre‐specified outcomes are reported. Difficult to judge based on Google translate of Persian article. |
| Other bias | Low risk | At baseline, none of the anthropometric variables was significantly different (P > 0.05) (Table I, II). |
Stefanaki 2015.
| Methods |
Study design: parallel‐group, 2‐arm, randomised controlled trial. November 2012 to May 2013. Duration: 8 weeks Power calculation: no |
|
| Participants |
Number of participants: 46 eligible, n = 23 intervention, n = 23 control, excluded (lost to follow‐up) n = 8 control, analysed n = 23 intervention, n = 15 controls PCOS definition: ESHRE/ASRM criteria Baseline characteristics (mean ± SD), intervention versus controls: Inclusion criteria: pre‐menopausal women age 15 to 40, diagnosed with PCOS by ESHRE/ASRM criteria (that is have at least 2 of the following: Quote: "(a) chronic anovulation, (b) clinical and/or biochemical hyperandrogenism and (c) polycystic ovaries on ultrasound after exclusion of related disorders). For adolescents, at least 2 years must have elapsed since menarche". Exclusion criteria: Quote: "Pregnancy, genetic or endocrine disorder, neuropsychiatric disorders requiring psychotropic medication (eg antipsychotics, antidepressants, or anticonvulsants), practice of stress management techniques within 2 months of study enrolment, simultaneous participation in other trials, inability to read or write in Greek". Medication use pre and during the study: no psychotropic medication Groups comparable at study commencement: yes Age: intervention group n = 23, 23.4 ± 4.62; control group n = 15, 28.3 ± 7.20 Weight: intervention group n = 23, 59.5 ± 6.87; control group n = 15, 65.2 ± 14.8 BMI: intervention group n = 23, 21.53 ± 2.15; control group n = 15, 23.7 ± 4.4 Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: an 8‐week mindfulness stress management programme, which consisted of a 30‐minute audio CD of directed mindfulness and diaphragmatic breathing exercises that participants were required to undertake daily, preferably before bedtime. Participants were monitored by the principal investigator via a scheduled meeting or telephone call. b) Control: control group with no intervention. Participants underwent salivary cortisol collection and questionnaires as per intervention group but did not have the mindfulness stress management programme. Compliance with intervention: not reported |
|
| Outcomes |
Measurements: baseline and 8 weeks (before and after intervention) Reproductive: none Metabolic: none Anthropometric: BMI Quality of life: PCOSQ |
|
| Notes |
Location: Athens, Greece Funding: equipment (salivary cortisol devices) funded by the Medical School of Athens University, and the First Department of Pediatrics of the National & Kapodistrian University of Athens, Greece. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An online randomisation internet site (www.random.org) was used to assign the participants to intervention and control groups." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Random allocation sequence was implemented by a designated clinical assistant who was not otherwise associated with the trial." |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Cannot blind participants; questionnaires are susceptible to bias, but salivary cortisol is an objective measure. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | High risk | No concealment was used within the groups. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Quote: "The fellow researcher who administered the questionnaires and obtained the salivary cortisol devices at the end of the 8‐week period was blinded (not aware of the assigned group of the patients.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was 35% in the control group (8/23 women dropped out of this group). There were no losses to follow‐up in the intervention group. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes (from trial registration ANZCTS) were reported in the paper. |
| Other bias | Unclear risk | There were no significant differences between the 2 groups for the majority of characteristics apart from total life satisfaction and its subscale, general satisfaction scores. |
Stener‐Victorin 2009‐2013.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial. Recruitment between November 2005 and January 2008. Duration: 16 weeks Power calculation: yes ITT analysis conducted |
|
| Participants |
Number of participants: for entire cohort n = 84 randomised. For intervention versus control N = 51 randomised (n = 34 intervention, n = 17 control), n = 45 completed and analysed (n = 30 intervention, n = 15 control). For paper, n = 5 intervention and n = 6 control reported (subset). PCOS definition: PCO (at least 12 follicles, 2 mm to 9 mm and/or increased ovarian volume > 10 mL by 2D ultrasound on one or both ovaries) and one of following (oligomenorrhoea with an intermenstrual interval > 35 days and/or clinical and/or biochemical signs of hyperandrogenism (hirsutism or acne)) Baseline characteristics (mean ± SD), intervention versus controls: age 30.4 ± 5.5 versus 31.0 ± 3.2 years, BMI 26.8 ± 4.8 kg/m2 versus 28.0 ± 6.2 kg/m2 Inclusion criteria: PCOS, age 18 to 37 years Exclusion criteria: breastfeeding < 6 months prior, known endocrine or neoplastic causes of hyperandrogenism including adrenal secreting tumours, Cushing’s syndrome, congenital adrenal hyperplasia and hyperprolactinaemia Medication use pre and during the study: women on medications < 3 months prior to study commencement excluded Groups comparable at study commencement: yes Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: 3 arms a) Acupuncture b) Physical exercise c) Control For review a) Intervention: weight maintenance, exercise: instructed to do 30 to 45 minutes 3x per week moderate exercise beyond daily physical activity (brisk walking, cycling, aerobic) with pulse frequency above 120/minute and weekly follow‐up and guidance b) Control: no exercise, given same information about importance of physical activity and diet as physical activity group in one session by a physiotherapist and given option to phone study co‐ordinator at any point. Were the care programmes, other than the trial options, identical? No, intervention participants contacted weekly, control participants option of contacting co‐ordinators at any time point. Compliance with intervention: not reported |
|
| Outcomes |
Measurements: 0 and 16 weeks Menstrual pattern by daily recordings of basal body temperature, 12‐week documentation of menstrual pattern pre‐study, menstrual bleeding patterns confirmed by daily recordings of basal body temperature throughout the entire study period and via interviews by gynaecologists and gynaecological assessment Endocrine: total testosterone, SHBG, free testosterone (radioimmunoassay), FAI, F‐G score (clinician assessed), Metabolic: lipid profile (total cholesterol, HDL‐C, LDL‐C, triglycerides), fasting glucose and insulin Anthropometric: height, weight, BMI, sagittal abdominal diameter, WHR Quality of life: PCOSQ |
|
| Notes | Blood samples taken independent of follicular phase Location: Sahlgrenska University Hospital, Goteborg, Sweden Funding: Swedish Medical Research Council, Novo Nordisk Foundation, Wilhelm and Martina Lundgren’s Science Fund, Hjalmar Svensson Foundation, Tore Nilson Foundation, Ake Wiberg Foundation, Adlerbert Research Foundation, Ekhaga Foundation, Swedish Federal Government, regional research and development agreement |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised, Quote: "Randomization performed by study coordinator according to a computerized list with stratification for age and BMI. Block randomised". |
| Allocation concealment (selection bias) | Unclear risk | Not stated, insufficient information about the process to permit a judgement. |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Participant/treatment provider blinding not possible due to the interactive nature of the intervention. Potential for lack of participant blinding to introduce bias for the self‐reported outcome of menstrual diaries. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Low risk | Quote: "Independent observers and with blind, independent analysis", outcome assessor and data analyst blinded and reduced risk of bias for clinician‐reported outcomes of hirsutism (Ferriman‐Gallwey), weight, body mass index, adiposity distribution. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Quote: "Independent observers and with blind, independent analysis", outcome assessor and data analyst blinded and reduced risk of bias for objective outcomes of biochemical data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT analysis. On contacting the author data will be presented as ITT in future publications. Withdrawals: n = 34 intervention and n = 17 control commenced, at study end n = 17/34 intervention (50% dropout) and n = 11/18 control (39% dropout). Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. The lack of blinding may have contributed to higher levels of drop out for the intervention group. Data only provided for n = 5 physical exercise and n = 6 control given with no dropout rate for microneurography component. Reason for missing data (n = 12 physical exercise, n = 6 control) due to data currently under analysis and to be included in future publication but is unbalanced between intervention and control. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is available and all of the studies prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way with the exception of: health‐related quality of life, progesterone values mentioned in methods but not results and potential data for ovulation. On contacting authors these outcomes are to be included in future analysis. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Turan 2015.
| Methods |
Study design: parallel‐group controlled trial; March 2011 and May 2014 Duration: 8 weeks Power calculation: no |
|
| Participants |
Number of participants: n = 32 randomised, n = 16 training, n = 16 control, n = 2 excluded due to inadequate attendance, analysed n = 14 training, n = 16 control PCOS definition: ESHRE/ASRM Baseline characteristics (mean ± SD), intervention versus controls: Age 24.45 ± 2.8 years (range: 17 to 34 years). BMI (kg/m2) training group n = 14, 21.8 ± 1.0; control group n = 16, 21.9 ± 1.1 Inclusion criteria: body mass index (BMI) was in the normal range (< 25 kg/m2); diagnosed on the basis of ESHRE/ASRM criteria (2003), which requires the presence of 2 of the following: Quote: "(1) a polycystic ovary, defined as the presence of >10 cysts 2–8 mm in diameter, an ovarian volume >10 cm3, and an echodense stroma on transvaginal or pelvic ultrasonography; ... (2) clinical hyperandrogenism (Ferriman‐Gallwey12) score >8) or biochemical hyperandrogenism (serum testosterone level >3.6 pg/mL in the absence of other causes of hyperandrogenism); and (3) oligomenorrhoea and/or anovulation". Exclusion criteria: Quote: "patients with endocrinological diseases, including diabetes, thyroid, adrenal, or pituitary gland dysfunction; cardiovascular, hepatic, or pulmonary disease; a history of orthopedic or other physical symptoms that would otherwise limit exercise performance; and those who had exercised regularly within the last 6 months". Medication use pre and during the study: not stated Groups comparable at study commencement: yes Participants actively seeking pregnancy at study commencement: no |
|
| Interventions |
a) Intervention: patients participated in a structured exercise programme 3 times per week for 8 weeks. During each session (50 to 60 minutes), the patients performed aerobic and resistance exercises. Supervised by a physiotherapist. b) Control: at the beginning of the study, general dietary and behavioural advice, but not a structured calorie restriction programme, was provided to all study participants. All patients were counselled regarding a healthy, balanced meal plan with regular food and a nutritional composition in which 50% of the calories were from carbohydrates, 25% from protein and 25% from fat. Compliance with intervention: n = 2/16 from training group excluded due to inadequate attendance |
|
| Outcomes |
Measurements: 8 weeks Menstrual cycle Endocrine: total testosterone, free testosterone Metabolic: total cholesterol, HDL‐C, LDL‐C, triglycerides, fasting glucose, fasting insulin Anthropometric: BMI, waist circumference |
|
| Notes |
Location: Physical Therapy and Rehabilitation Fitness Unit of Dokuz Eylul University, Turkey Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out using a computer generated random number table and pre‐labelled, sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "... pre‐labeled, sealed envelopes." |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Unable to blind participants to intervention but objective biochemical measures used. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | High risk | Unable to blind participants to intervention but objective biochemical measures used. |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Unable to blind participants to intervention but objective biochemical measures used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two dropouts in the intervention group: inadequate attendance at > 75% of the total training sessions. |
| Selective reporting (reporting bias) | Low risk | Not stated as registered; all prespecified outcomes reported. Data are presented as mean ± standard error rather than standard deviation. |
| Other bias | Low risk | Groups appear even at baseline, except for BP (the control group appears to have a lower mean BP of 110/70 compared to the intervention groups mean BP of 120/75, and the control group has a higher mean estradiol at 56.7 pmol/L compared to the intervention groups mean estradiol of 36.0 pmol/L. The significance of this is unclear. |
Vigorito 2007.
| Methods |
Study design: single‐centre, parallel‐group randomised controlled trial. Study start and end dates not provided. Duration: 12 weeks Power calculation: no |
|
| Participants |
Number of participants: n = 90 randomised and analysed (n = 45 intervention and n = 45 control) PCOS definition: ESHRE/ASRM criteria, PCO identified by transvaginal ultrasound and hirsutism by Ferriman‐Gallwey score > 8 Baseline characteristics (mean ± SD), intervention versus controls: age 21.7 ± 2.3 versus 21.9 ± 1.9 years, weight 72.3 ± 2.2 versus 71.5 ± 3.6 kg, BMI 29.3 ± 2.9 versus 29.4 ± 3.5 kg/m2 Inclusion criteria: PCOS, overweight (not defined) Exclusion criteria: pregnancy, glucose intolerance (2‐hour OGTT), diabetes, hypothyroidism, hyperprolactinaemia, Cushing's syndrome, non‐classical congential adrenal hyperplasia, neoplastic, hepatic, respiratory, cardiovascular disorder, concurrent medical illness (i.e. heart failure, lung, renal disease), smoking Medication use pre and during the study: no use of oral contraceptives, glucocorticoids, anti‐androgens, ovulation induction agents, anti‐diabetic or anti‐obesity drugs or other hormonal drugs within previous 6 months and during 3 months of study duration Groups comparable at study commencement: yes Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: 2 arms a) Intervention: not specifically aimed to induce weight loss, structured supervised training sessions 3 x/week, 5‐minute warm up and cool down, 30‐minute exercise with 60% to 70% VO2 max bicycle ergometer b) Control: no training programme. Both intervention and control received general dietary and behavioural advice without structured calorie restriction programme, healthy balanced meal plan encouraged (50% carbohydrate, 25% protein, 25% fat, low GI food intake) Were the care programmes, other than the trial options, identical? No, the intervention participants attended additional study visits as part of the intervention and therefore had greater study contact (estimated 39 visits compared to 3 visits). Compliance with intervention: exercising women attended an average or 28 ± 2 sessions with an accuracy of 0.78 indicating the number of expected sessions/effective sessions performed. |
|
| Outcomes |
Measurements: 0 and 3 months Menses diary Endocrine: testosterone, SHBG, FAI, clinical hyperandrogenism (clinician assessed Ferriman‐Gallwey score) Anthropometric: height, weight, BMI, waist circumference, waist/hip ratio Metabolic: AUC OGTT glucose (0, 30, 60, 90, 120, 180 minutes), lipid profile (total cholesterol, HDL‐C, LDL‐C, triglycerides), fasting glucose and insulin, AUC OGTT insulin (0, 30, 60, 90, 120, 180 minutes) |
|
| Notes | Blood measurements were taken in early follicular phase (day 2 to 4) of progesterone‐induced menstrual cycle Location: University Federico II of Naples, School of Medicine, Italy Funding: internal funding from University “Federico II” of Naples, University “Magna Graecia” of Catanzaro, University “Parthenope” of Naples, Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned but not stated how; insufficient information about the process to permit a judgement. Quote: "At study entry, PCOS women were randomly subdivided into two groups composed of 45 patients each". |
| Allocation concealment (selection bias) | Unclear risk | Not stated, insufficient information about the process to permit a judgement. |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Participant/treatment provider blinding not possible due to interactive nature of intervention. Potential for lack of participant blinding to introduce bias for self‐reported outcome of menstrual diaries. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | Low risk | Quote: "All clinical assessments performed by physician blinded to patient allocation into study protocol." Outcome assessor blinded and therefore reduced the risk of bias for the clinical reported outcomes of Ferriman‐Gallwey score, weight, BMI, adiposity distribution. |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Quote: "All clinical assessments performed by physician blinded to patient allocation into study protocol." Outcome assessors were blinded and therefore reduced the risk of bias for objective outcomes of biochemical data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT analysis but no dropouts reported for intervention or control group and no missing outcome data. |
| Selective reporting (reporting bias) | High risk | Insufficient information to permit a judgement, study not registered as a clinical trial. From results section of the paper, all of the studies prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way with the exception of menstrual regularity data for controls. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Vizza 2016.
| Methods |
Study design: single‐centre, parallel‐group, randomised controlled trial. Recruitment 20 February to 21 September 2014. Duration: 12 weeks Power calculation: no ITT analysis conducted |
|
| Participants |
Number of participants: progressive resistance training (PRT) n = 8, excluded 1, analysed 7; control n = 7, excluded 1, analysed 6 PCOS definition: diagnosis of PCOS (confirmed via the participant's general practitioner or specialist) Baseline characteristics (mean ± SD), intervention versus controls: Age: PRT group n = 7, 26 (7); control group n = 6, 29 (3) Weight (kg): PRT group n = 7, 117.4 (36.6); control group n = 6, 86 (27.0) BMI: PRT group n = 7, 41.3 (12.5); control group n = 6, 34.0 (9.4) Inclusion criteria: Quote: "Age 18‐42 years with a diagnosis of PCOS (confirmed via the participant’s general practitioner or specialist. Not currently participating in PRT; (3) not pregnant nor breastfeeding; (4) no history of cardiovascular, kidney, respiratory disease, uncontrolled hypertension or cancer; (5) no use of cigarettes for >6 months; (6) no acute or chronic medical condition that would make assessment and interventions potentially hazardous or any of the outcomes impossible to assess; (7) cognition and English language sufficient to understand research procedures and provide informed consent; and (8) willingness to be randomised and undergo study protocols". Exclusion criteria: not stated Medication use pre and during the study: the most common prescription medication was metformin (n = 4 in the PRT group and n = 1 in the control group) and one participant in each group was prescribed an oral contraceptive. Groups comparable at study commencement: there were no significant differences between groups at baseline, according to the descriptive characteristics presented in Table 1. However, trends were noted for waist circumference (P = 0.06) and hip circumference (P = 0.10). Participants actively seeking pregnancy at study commencement: no |
|
| Interventions | Comparison: a) Intervention: Quote: "Participants in the PRT group were prescribed two supervised training sessions per week on non‐consecutive days for 12 weeks at the university campus. The PRT group also performed two home‐based (unsupervised) exercise sessions consisting of lower‐intensity callisthenics to facilitate habitual movement and behaviour change [44]. Supervised sessions lasted for approximately 60 min, and included a standardized (5 min) warm‐up and cool‐down on exercise cycle or treadmill. PRT exercises included lat pulldown, leg curl, seated row, leg press, calf raise, chest press, split squat, shoulder press, biceps curl, triceps extension and abdominal curl. All sets (except abdominal curl) were performed to neuromuscular fatigue, i.e. 8‐12 repetitions maximum; loads were increased with strength gains. Two sets of each exercise were prescribed in the first 2 weeks of training. From week 3, all exercises except split squats and shoulder press were progressed to 3 sets. The home‐based callisthenics exercises were undertaken on non‐PRT days and included lying external hip rotations (‘clam shells’), side leg raises, push‐ups on knees, wall squats, oblique curls, core stabilization exercises (‘bird dog’ and abdominal hollowing), performed for 3 sets x 10 repetitions each. Participants received a different set of callisthenic home‐based exercises every four weeks. Participants were asked to record the number of repetitions of each exercise performed in a log book. This record was collected weekly." b) Control: Quote: "Participants in the control group did not receive any PRT intervention and were instructed to continue with their current lifestyle and usual healthcare and medical treatments" Compliance with intervention: adherence to training inclusive of the 2 participants in the PRT group who withdrew from training was 76% ± 13% for supervised training, 43% ± 26% for home‐based (unsupervised) callisthenics training and 60% ± 10% overall. Excluding these 2 participants, attendance was 95% ± 6% for supervised training, 51% ± 28% for unsupervised training and 73% ± 6% overall. |
|
| Outcomes |
Measurements: week 0 and week 12 Menstrual cyclicity Endocrine: testosterone, SHBG, FAI Metabolic: glucose, insulin Anthropometric: weight, BMI, waist circumference Quality of life: PCOSQ |
|
| Notes |
Location: Western Sydney University Funding: higher degree research funding provided by Western Sydney University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization assignments were generated via www.randomization.com by an investigator not involved into data collection". |
| Allocation concealment (selection bias) | Low risk | Group assignment was given to participants in sealed envelopes on completion of baseline testing. |
| Blinding (performance bias and detection bias) Participant reported outcomes | High risk | Blinding would not have been possible due to the nature of the intervention. No details of blinding of outcome assessors. |
| Blinding (performance bias and detection bias) Clinician reported outcomes | High risk | Blinding would not have been possible due to the nature of the intervention. No details of blinding of outcome assessors. |
| Blinding (performance bias and detection bias) Objective outcomes | High risk | Menstrual cyclicity was self‐monitored by participants using a standardised menstrual diary. Other outcomes are physiological and may not have been influenced by knowledge of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The overall attrition rate was 38% (29% (2/7) in the PRT group and 50% (3/6) in the control group). Intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Appears to be free of selective reporting bias. All pre‐specified outcomes are reported. |
| Other bias | Low risk | The PRT group have larger mean weight, waist and hip circumference measurements (117.4 kg, 123.6 cm and 133.5 cm versus 86 kg, 96.0 cm and 113.0 cm). |
AUC: area under the curve; BMI: body mass index; BP: blood pressure; CG: control group; ESHRE/ASRM: European Society for Human Reproductive and Embryology/American Society for Reproductive Medicine; FAI: free androgen index; FMD: flow‐mediated vasodilation; F‐G: Ferriman‐Gallwey score; FSH: follicle‐stimulating hormone; GI: glycaemic index; hCG: human chorionic gonadotropin; HDL‐C: high‐density lipoprotein cholesterol; HIT: high‐intensity interval training; IQR: interquartile range; ITT: intention‐to‐treat; LDL‐C: low‐density lipoprotein cholesterol; LH: luteinising hormone; OCP: oral contraceptive pills; OGTT: oral glucose tolerance test; PCO: polycystic ovaries; PCOS: polycystic ovary syndrome; PCOSQ: Polycystic Ovary Syndrome Questionnaire; PRT: progressive resistance training; SD: standard deviation; SEM: standard error of the mean; SHBG: sex hormone‐binding globulin; ST: strength training; WHR: waist to hip ratio
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Pasquali 1986 | No minimal intervention comparison group; hypocaloric diet compared to hypocaloric diet and anti‐androgen therapy. |
| Pasquali 2000 | No minimal intervention comparison group; dietary treatment and placebo compared to dietary treatment and metformin. |
| Talluto 2002 | Abstract only; attempted to contact the author but could not obtain full text and determine eligibility. |
| Zarrinkoub 2005 | Abstract only; attempted to contact the author but could not obtain full text and determine eligibility. |
| Moran 2006 | No minimal intervention comparison group; comparison of 2 different dietary interventions. |
| Tang 2006 | No minimal intervention comparison group; dietary treatment and placebo compared to dietary treatment and metformin. |
| Glueck 2006 | No minimal intervention comparison group; single group study (metformin and diet). |
| Pekhlivanov 2006 | No minimal intervention comparison group; metformin compared to diet. |
| Bruner 2006 | No minimal intervention comparison group; exercise and nutritional counselling versus nutritional counselling. |
| Thomson 2008 | No minimal intervention comparison group; diet compared to diet and aerobic exercise compared to diet and combined aerobic‐resistance exercise. |
| Orio 2008 | No minimal intervention comparison group; exercise training compared to exercise training and detraining. |
| Palomba 2008 | No minimal intervention comparison group; exercise versus diet. Not a randomised controlled trial; treatment allocated by participant choice. |
| Giallauria 2008 | Not a randomised controlled trial; treatment allocated by participant choice. |
| Atiomo 2009 | Control arm is not minimal treatment; low GI versus low‐calorie diets. |
| Moran 2010a | Control arm is not minimal treatment; participants were assigned to either low‐protein or high‐protein diets. |
| Hamayeli 2010 | Control arm is not minimal treatment; participants were assigned to either a weight loss diet (carbohydrates 55%, protein 15%, fat 30%) or a modified diet low glycaemic load (carbohydrates 40%, protein 30%, fat 30%). |
| Palomba 2010 | Control arm is not minimal treatment; the 3 interventions were: SET plus hypocaloric diet for 6 weeks (group A); 2 weeks of observation followed by one cycle of clomiphene citrate (CC) therapy (group B); and SET plus hypocaloric diet for 6 weeks, with one cycle of CC after the first 2 weeks (group C). |
| Fux Otta 2010 | Control arm is not minimal treatment; randomisation was to receive either oral metformin or placebo and all participants were given a nutrition plan. |
| Ornstein 2011 | Control arm is not minimal treatment; women were randomised to either: 1) low carbohydrate diet or 2) hypocaloric National Cholesterol Education Program II diet. |
| Toscani 2011 | Control arm is not minimal treatment; control group diet content was specified: "Normal Protein" group (15% protein, 55% carbohydrate, and 30% lipid). |
| Hutchison 2012 | Not a randomised controlled trial. |
| Sorensen 2012 | Control arm is not minimal treatment; a high‐protein and a standard‐protein diet were compared. |
| Curi 2012 | Control arm is not minimal treatment; control arm received metformin. |
| Mehrabani 2012 | Control arm is not minimal treatment; participants were assigned to either a weight loss diet (carbohydrates 55%, protein 15%, fat 30%) or a modified diet low glycaemic load (carbohydrates 40%, protein 30%, fat 30%). |
| Nidhi 2012 | Control arm is not minimal treatment; the yoga group practised a holistic yoga module while the control group practised a matching set of physical exercises. |
| Sprung 2013 | Not a randomised controlled trial. |
| Nybacka 2013 | Control arm is not minimal treatment; women were randomised to either dietary management, exercise or both. |
| Kim 2013 | Participants did not have PCOS. |
| Jakubowicz 2013 | No minimal intervention comparison group. 2 isocaloric diets prescribed with different meal timing distribution. |
| Roessler 2013 | Control arm is not minimal treatment; cross‐over protocol comparing: a) high‐intensity aerobic exercise with b) group counselling. |
| Turner‐McGrievy 2014 | Control arm is not minimal treatment; women were randomised to vegan or low‐calorie diet. |
| Panico 2014 | Control arm is not minimal treatment; cross‐over protocol, with diet A (low glycaemic load) and diet B (moderately high glycaemic load). |
| Ebrahimi 2014 | No minimal intervention comparison group; the control group received less onion to consume compared to the intervention group. |
| Johnson 2015 | No minimal intervention comparison group. Compared low‐fructose to high‐fructose diets. |
| Marzouk 2015 | No minimal intervention comparison group; control group instructed to follow the same healthy food diet as the first group without restriction in calories. |
| Asemi 2015 | No minimal intervention comparison group; caloric restriction and prescribed diet in both groups. |
| Legro 2015 | No minimal intervention comparison group; compares oral contraceptive to lifestyle modification, to both. |
| Konopka 2015 | Control arm is not minimal treatment; all participants had a standardised, weight‐maintaining diet provided for 3 days (50% carbohydrate, 30% fat, and 20% protein) prior to and during the study days. |
| Gower 2015 | Control arm is not minimal treatment; control group received a specific diet (55:18:27 CHO:protein:fat) for 8 weeks. Cross‐over study. |
| Sa 2016 | Did not report any relevant outcomes. |
| Papakonstantinou 2016 | No minimal intervention comparison group; both groups on structured weight maintenance diet either on 3‐ or 6‐meal pattern. |
| Beena 2016 | Not a randomised controlled trial: quasi‐randomised, sampling technique; multi‐stage random sampling. |
| Orio 2016 | No minimal intervention comparison group; polyvitamin and caloric restriction in all groups. |
| Thomson 2016 | Control arm is not minimal treatment; comparison was made between: 1) diet only, 2) diet and aerobic exercise and 3) diet + combined aerobic/resistance exercise. |
| Wong 2016 | No minimal intervention comparison group; comparing low‐fat or low glycaemic load diet. |
| Sordia‐Hernandez 2016 | No minimal intervention comparison group; both intervention and control groups on calorie‐restricted diets. |
| Foroozanfard 2017 | No minimal intervention comparison group; both intervention (low‐calorie DASH) and control diets consisted of 52% to 55% carbohydrates, 16% to 18% proteins and 30% total fats; both diets were equicaloric. |
| Azadi‐Yazdi 2017 | No minimal comparison group; both intervention (low‐calorie DASH) and control diets consisted of 50% to 55% carbohydrate, 15% to 20% protein and 25% to 30% total fat; both diets were equicaloric. |
| Jiskoot 2017 | Control arm is not minimal treatment; control group had individual counselling about health risks associated with being overweight for both mother and child. |
CC: clomiphene citrate; DASH: Dietary Approach to Stop Hypertention
GI: glycaemic index; PCOS: polycystic ovary syndrome; SET: structured exercise training
Characteristics of studies awaiting assessment [ordered by study ID]
Gaeini 2012.
| Methods |
Study design: randomised controlled trial Duration: 12 weeks Power calculation: not known |
| Participants |
Number of participants: N = 40 randomised (n = 20 lean, n = 20 obese) Inclusion criteria: PCOS, age 18 to 50 years, sedentary lifestyle (no regular exercise during the usual week), ability to come to study exercise facility for monitored exercise, agreement to maintain current weight/dietary patterns for study Exclusion criteria: patients undergoing treatment for menstrual disorders; antiepileptic drugs; contraceptives; pregnancy or weight loss drugs; those with hepatitis; pulmonary‐cardiac disorders; diabetes; pregnancy; hypo‐and hyperthyroidism Cushing's syndrome and adrenal hyperplasia Medication use pre and during study: as per exclusion criteria Groups comparable at study commencement: not known Participants actively seeking pregnancy at study commencement: not known n = 20 obese PCOS and 20 lean PCOS Exclusion criteria: medication for irregular menstruation, epilepsy, use of contraceptive pills, ovulation stimulators, medication for hepatitis, medication for the treatment of coronary and respiratory disease, diabetes, pregnancy, medication for treatment of hypo and hyperthyroid, abnormal TSH, Cushing syndrome, androgen‐producing tumours and adrenal hyperplasia |
| Interventions | Comparison: 2 arms a) Intervention: aerobic exercise for 12 weeks, 3 sessions per week with intensity 65% to 80% of maximal heart rate for 25 to 30 minutes of aerobic exercise b) Control: no intervention Compliance with intervention: not known |
| Outcomes |
Measurements: before and after Endocrine: testosterone (data awaiting clarification from translators) Anthropometric: BMI (data awaiting clarification from translators) |
| Notes |
Location: Iran Funding: not known |
BMI: body mass index; PCOS: polycystic ovary syndrome; TSH: thyroid‐stimulating hormone
Differences between protocol and review
The review assessed studies with inclusion criteria for women with PCOS who were both overweight and not specifically overweight and assessed studies designed both to achieve and not specifically achieve weight loss. This has been modified in the title and methodology. It was not possible to report the secondary reproductive outcomes of menstrual regularity (an initiation of menses or significant shortening of cycle length) and ovulation (number of ovulatory menstrual cycles) as prespecified and data were instead reported in the form reported by the authors. The protocol introduction was modified and expanded to include new research. Following editorial review, the primary reproductive (menstrual regularity and ovulation), primary anthropometric (weight, BMI, adiposity distribution) and metabolic (glucose tolerance) outcomes were changed to secondary outcomes to improve the clarity of the review and the inclusion criteria were expanded to include the first phase of cross‐over trials.
Contributions of authors
SSL: data collection for the review, analysis of data, interpretation of data, writing the review.
EVR: data collection for the review, providing general advice on the review, input into writing the review.
SKH: conceiving the review, designing the review, data collection for the review, writing the review.
RJN: conceiving the review, designing the review, providing general advice on the review, input into writing the review.
HJT: conceiving the review, designing the review, providing general advice on the review, input into writing the review.
LJM: conceiving the review, designing the review, data collection and management for the review, analysis of data, interpretation of data, writing the review.
Sources of support
Internal sources
The Jean Hailes Foundation for Women's Health, Monash Institute of Health Services Research, Monash University, Melbourne, Australia.
The Robinson Centre, Discipline of Obstetrics and Gynaecology, University of Adelaide, Adelaide, Australia.
Australasian Cochrane Centre, Monash University, Victoria, Australia.
External sources
No sources of support supplied
Declarations of interest
SL received a National Health and Medical Research Council (NHMRC) fellowship in support of this work. SL has received reimbursement from the Endocrine Society of Australia to present at an endocrinology conference.
EVR has nothing to disclose.
SKH received Sabbatical Support from Monash Health in support of this work. SKH currently works as a clinician in an Australian public hospital PCOS clinic. SKH has received travel grants to attend PCOS conferences from the Endocrine Society of Australia, NHMRC, the Australian Diabetes Society and the PCOS International Guideline Development Group from the PCOS Centre for Research Excellence.
HJT receive partial or complete salary funding from NHMRC.
LJM received salary funding from the Heart Foundation of Australia. LJM has received reimbursement from the Heart Foundation of Australia to attend endocrinology and PCOS conferences.
RJN has received grants and honoraria from Schering Plough, Merck Serono and Ferring Pharmaceuticals.
LJM and RJN are previous investigators of studies examining weight loss through dietary intervention on reproductive and metabolic outcomes in polycystic ovary syndrome.
Edited (no change to conclusions)
References
References to studies included in this review
Almenning 2015 {published and unpublished data}
- Almenning I, Rieber‐Mohn A, Lundgren KM, Shetelig Løvvik T, Garnæs KK, Moholdt T. Effects of high intensity interval training and strength training on metabolic, cardiovascular and hormonal outcomes in women with polycystic ovary syndrome: a pilot study. PloS One 2015;10(9):e0138793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2009 {published and unpublished data}
- Brown AJ, Setji TL, Sanders LL, Lowry KP, Otvos JD, Kraus WE, et al. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Medicine and Sciences in Sports and Exercise 2009;41(3):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guzick 1994 {published data only (unpublished sought but not used)}
- Guzick DS, Wing R, Smith D, Berga SL, Winters SJ. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertility and Sterility 1994;61(4):598‐604. [PubMed] [Google Scholar]
Hoeger 2004 {published and unpublished data}
- Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48‐week, placebo‐controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertility and Sterility 2004;82(2):421‐9. [DOI] [PubMed] [Google Scholar]
Hoeger 2008 {published and unpublished data}
- Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo‐controlled clinical trials. Journal of Clinical Endocrinology and Metabolism 2008;93(11):4299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mani 2018 {published data only}
- Mani H, Chudasama Y, Hadjiconstantinou M, Bodicoat DH, Edwardson C, Levy MJ, et al. Structured education programme for women with polycystic ovary syndrome: a randomised controlled trial. Endocrine Connections 2018;7(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirfeizi 2013 {published data only}
- Mirfeizi M. Comparison of effects of diet and exercise program to improve clinical symptoms and laboratory tests in obese women with polycystic ovary syndrome (PCOS). Medical Journal of Mashhad University of Medical Sciences 2013;56(2):77‐84. [Google Scholar]
Nasrekani 2016 {published data only}
- Nasrekani ZA, Fathi M. Efficacy of 12 weeks aerobic training on body composition, aerobic power and some women‐hormones in polycystic ovary syndrome infertile women. Iranian Journal of Obstetrics, Gynecology and Infertility 2016;19(5):1‐10. [Google Scholar]
Saremi 2013 {published and unpublished data}
- Saremi A, Nader, Karmali M, Kazemi M. Serum level of anti‐mullerian hormone after exercise training in women with polycystic ovary syndrome: a randomised controlled trial. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;16(64):10. [Google Scholar]
Saremi 2016 {published and unpublished data}
- Saremi A, Yaghoubi MS. Effect of resistance exercises with calcium consumption on level of anti‐mullerian hormone and some metabolic indices in women with polycystic ovarian syndrome. Iranian Journal of Obstetrics, Gynecology and Infertility 2016;18(180):7‐15. [Google Scholar]
Stefanaki 2015 {published and unpublished data}
- Stefanaki C, Bacopoulou F, Livadas S, Kandaraki A, Karacalios A, Chrousos G, et al. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: a randomised controlled trial. Stress (Amsterdam, Netherlands) 2015;18(1):57‐66. [DOI] [PubMed] [Google Scholar]
Stener‐Victorin 2009‐2013 {published and unpublished data}
- Jedel E, Labrie F, Oden A, Holm G, Nilsson L, Janson PO, et al. Impact of electro‐acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: a randomised controlled trial. American Journal of Physiology‐Endocrinology and Metabolism 2011;300(1):E37‐45. [DOI] [PubMed] [Google Scholar]
- Leonhardt H, Hellstrom M, Gull B, Lind AK, Nilsson L, Janson OP, et al. Serum anti‐Mullerian hormone and ovarian morphology assessed by magnetic resonance imaging in response to acupuncture and exercise in women with polycystic ovary syndrome: secondary analyses of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2015;94:279‐87. [DOI] [PubMed] [Google Scholar]
- Stener‐Victorin E, Baghaei F, Holm G, Janson PO, Olivecrona G, Lönn M, et al. Effects of acupuncture and exercise on insulin sensitivity, adipose tissue characteristics, and markers of coagulation and fibrinolysis in women with polycystic ovary syndrome: secondary analyses of a randomized controlled trial. Fertility and Sterility 2012;97(2):501‐8. [DOI] [PubMed] [Google Scholar]
- Stener‐Victorin E, Holm G, Janson PO, Gustafson D, Waern M. Acupuncture and physical exercise for affective symptoms and health‐related quality of life in polycystic ovary syndrome: secondary analysis from a randomized controlled trial. BMC Complementary and Alternative Medicine 2013;13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stener‐Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low‐frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology 2009;297(2):R387‐95. [DOI] [PubMed] [Google Scholar]
Turan 2015 {published and unpublished data}
- Turan V, Mutlu EK, Solmaz U, Ekin A, Tosun O, Tosun G, et al. Benefits of short‐term structured exercise in non‐overweight women with polycystic ovary syndrome: a prospective randomised controlled study. Journal of Physical Therapy Science 2015;27(7):2293‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vigorito 2007 {published and unpublished data}
- Vigorito C, Giallauria F, Palomba S, Cascella T, Manguso F, Lucci R, et al. Beneficial effects of a three‐month structured exercise training program on cardiopulmonary functional capacity in young women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2007;92(4):1379‐84. [DOI] [PubMed] [Google Scholar]
Vizza 2016 {published and unpublished data}
- Vizza L, Smith CA, Swaraj S, Agho K, Cheema BS. The feasibility of progressive resistance training in women with polycystic ovary syndrome: a pilot randomised controlled trial. Sports Science, Medicine and Rehabilitation 2016;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Asemi 2015 {published data only}
- Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs‐CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Hormone and Metabolic Research 2015;47(3):232‐8. [DOI] [PubMed] [Google Scholar]
Atiomo 2009 {published data only}
- Atiomo W, Read A, Golding M, Silcocks P, Razali N, Sarkar S, et al. Local recruitment experience in a study comparing the effectiveness of a low glycaemic index diet with a low calorie healthy eating approach at achieving weight loss and reducing the risk of endometrial cancer in women with polycystic ovary syndrome (PCOS). Contemporary Clinical Trials 2009;30(5):451‐6. [DOI] [PubMed] [Google Scholar]
Azadi‐Yazdi 2017 {published data only}
- Azadi‐Yazdi M, Karimi‐Zarchi M, Salehi‐Abargouei A, Fallahzadeh H, Nadjarzadeh A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. Journal of Human Nutrition and Dietetics 2017;30(3):275‐83. [DOI] [PubMed] [Google Scholar]
Beena 2016 {published data only}
- Beena MR, Thomas Kochuthressiamma. Outcome of interventional programme on quality of life of infertile women with polycystic ovarian syndrome. International Journal of Nursing Education 2016;8(2):27‐33. [Google Scholar]
Bruner 2006 {published data only}
- Bruner B, Chad K, Chizen D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Applied Physiology, Nutrition, and Metabolism 2006;31(4):384‐91. [DOI] [PubMed] [Google Scholar]
Curi 2012 {published data only}
- Curi DD, Fonseca AM, Marcondes JA, Almeida JA, Bagnoli VR, Soares JM Jr, et al. Metformin versus lifestyle changes in treating women with polycystic ovary syndrome. Gynecological Endocrinology 2012;28(3):182‐5. [DOI] [PubMed] [Google Scholar]
Ebrahimi 2014 {published data only}
- Ebrahimi‐Mamaghani M, Saghafi‐Asl M, Pirouzpanah S, Asghari‐Jafarabadi M. Effects of raw red onion consumption on metabolic features in overweight or obese women with polycystic ovary syndrome: a randomized controlled clinical trial. Journal of Obstetrics and Gynaecology Research 2014;40(4):1067‐76. [DOI] [PubMed] [Google Scholar]
Foroozanfard 2017 {published data only}
- Foroozanfard F, Rafiei H, Samimi M, Gilasi HR, Gorjizadeh R, Heidar Z, et al. The effects of dietary approaches to stop hypertension diet on weight loss, anti‐Müllerian hormone and metabolic profiles in women with polycystic ovary syndrome: a randomized clinical trial. Clinical Endocrinology 2017;87(1):51‐8. [DOI] [PubMed] [Google Scholar]
Fux Otta 2010 {published data only}
- Fux Otta C, Wior M, Iraci GS, Kaplan R, Torres D, Gaido MI, et al. Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: a randomized, double‐blind, and placebo control trial. Gynecological Endocrinology 2010;26(3):173‐8. [DOI] [PubMed] [Google Scholar]
Giallauria 2008 {published data only}
- Giallauria F, Palomba S, Maresca L, Vuolo L, Tafuri D, Lombardi AM, et al. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome. Clinical Endocrinology 2008;69(5):792‐8. [DOI] [PubMed] [Google Scholar]
Glueck 2006 {published data only}
- Glueck CJ, Aregawi D, Winiarska M, Agloria M, Luo G, Sieve L, et al. Metformin‐diet ameliorates coronary heart disease risk factors and facilitates resumption of regular menses in adolescents with polycystic ovary syndrome. Journal of Pediatric Endocrinology and Metabolism 2006;19(6):831‐42. [DOI] [PubMed] [Google Scholar]
Gower 2015 {published data only}
- Goss AM, Chandler‐Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Effects of a eucaloric reduced‐carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism: Clinical & Experimental 2014;63(10):1257‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Chandler‐Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Favourable metabolic effects of a eucaloric lower‐carbohydrate diet in women with PCOS. Clinical Endocrinology 2013;79(4):550‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Goss AM. A lower‐carbohydrate, higher‐fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. Journal of Nutrition 2015;145(1):177S‐83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamayeli 2010 {published data only}
- Hamayeli Mehrabani H, Tahbaz F, Salehpour S, Hedayati M, Amiri Z, Ghassemi A. Reproductive hormonal changes following two types of hypocaloric diets in overweight and obese polycystic ovary syndrome women. Iranian Journal of Endocrinology and Metabolism 2010;12(2):160‐200. [Google Scholar]
Hutchison 2012 {published data only}
- Hutchison SK, Teede HJ, Rachon D, Harrison CL, Strauss BJ, Stepto NK. Effect of exercise training on insulin sensitivity, mitochondria and computed tomography muscle attenuation in overweight women with and without polycystic ovary syndrome. Diabetologia 2012;55(5):1424‐34. [DOI] [PubMed] [Google Scholar]
Jakubowicz 2013 {published data only}
- Jakubowicz D, Barnea M, Wainstein J, Froy O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clinical Science 2013;125(9):423‐32. [DOI] [PubMed] [Google Scholar]
Jiskoot 2017 {published data only}
- Jiskoot G, Benneheij SH, Beerthuizen A, Niet JE, Klerk C, Timman R, et al. A three‐component cognitive behavioural lifestyle program for preconceptional weight‐loss in women with polycystic ovary syndrome (PCOS): a protocol for a randomized controlled trial. Reproductive Health 2017;14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2015 {published data only}
- Johnson LK, Holven KB, Nordstrand N, Mellembakken JR, Tanbo T, Hjelmesæth J. Fructose content of low calorie diets: effect on cardiometabolic risk factors in obese women with polycystic ovarian syndrome: a randomized controlled trial. Endocrine Connections 2015;4(3):144‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim C, Pi‐Sunyer X, Barrett‐Connor E, Stentz FB, Murphy MB, Kong S, et al. Diabetes Prevention Program Research Group. Sex hormone binding globulin and sex steroids among premenopausal women in the diabetes prevention program. Journal of Clinical Endocrinology and Metabolism 2013;98(7):3049‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konopka 2015 {published data only}
- Konopka AR, Asante A, Lanza IR, Robinson MM, Johnson ML, Dalla Man C, et al. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes 2015;64(6):2104‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legro 2015 {published data only}
- Legro RS, Dodson WC, Kris‐Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2015;100(11):4048‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marzouk 2015 {published data only}
- Marzouk TM, Sayed Ahmed WA. Effect of dietary weight loss on menstrual regularity in obese young adult women with polycystic ovary syndrome. Journal of Pediatric and Adolescent Gynecology 2015;28(6):457‐61. [DOI] [PubMed] [Google Scholar]
Mehrabani 2012 {published data only}
- Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high‐protein, low‐glycemic‐load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. Journal of the American College of Nutrition 2012;31(2):117‐25. [DOI] [PubMed] [Google Scholar]
Moran 2006 {published data only}
- Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short‐term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. American Journal of Clinical Nutrition 2006;84(1):77‐87. [DOI] [PubMed] [Google Scholar]
Moran 2010a {published data only}
- Moran LJ, Noakes M, Clifton PM, Norman RJ. The effect of modifying dietary protein and carbohydrate in weight loss on arterial compliance and postprandial lipidemia in overweight women with polycystic ovary syndrome. Fertility and Sterility 2010;94(6):2451‐4. [DOI] [PubMed] [Google Scholar]
Nidhi 2012 {published data only}
- Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Effect of holistic yoga program on anxiety symptoms in adolescent girls with polycystic ovarian syndrome: a randomized control trial. International Journal of Yoga 2012;5(2):112‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nybacka 2013 {published data only}
- Nybacka A, Carlstrom K, Fabri F, Hellstrom PM, Hirschberg AL. Serum antimullerian hormone in response to dietary management and/or physical exercise in overweight/obese women with polycystic ovary syndrome: secondary analysis of a randomized controlled trial. Fertility and Sterility 2013;100(4):1096‐102. [DOI] [PubMed] [Google Scholar]
- Nybacka A, Carlstrom K, Stahle A, Nyren S, Hellstrom PM, Hirschberg AL. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertility & Sterility 2011;96(6):1508‐13. [DOI] [PubMed] [Google Scholar]
Orio 2008 {published data only}
- Orio F, Giallauria F, Palomba S, Manguso F, Orio M, Tafuri D, et al. Metabolic and cardiopulmonary effects of detraining after a structured exercise training programme in young PCOS women. Clinical Endocrinology 2008;68(6):976‐81. [DOI] [PubMed] [Google Scholar]
Orio 2016 {published data only}
- Orio F, Muscogiuri G, Giallauria F, Savastano S, Bottiglieri P, Tafuri D, et al. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: a randomized controlled trial. Clinical Endocrinology 2016;85(5):764‐71. [DOI] [PubMed] [Google Scholar]
Ornstein 2011 {published data only}
- Ornstein RM, Copperman NM, Jacobson MS. Effect of weight loss on menstrual function in adolescents with polycystic ovary syndrome. Journal of Pediatric & Adolescent Gynecology 2011;24(3):161‐5. [DOI] [PubMed] [Google Scholar]
Palomba 2008 {published data only}
- Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24‐week pilot study. Human Reproduction 2008;23(3):642‐50. [DOI] [PubMed] [Google Scholar]
Palomba 2010 {published data only}
- Palomba S, Falbo A, Giallauria F, Russo T, Rocca M, Tolino A, et al. Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Human Reproduction 2010;25(11):2783‐91. [DOI] [PubMed] [Google Scholar]
Panico 2014 {published data only}
- Panico A, Lupoli GA, Gelsy A, Cioffi, I, Zacchia, G, Caldara A, et al. Effects of an isocaloric low‐glycemic‐load diet in polycystic ovary syndrome. Nutritional Therapy & Metabolism 2014;32(2):85‐92. [Google Scholar]
Papakonstantinou 2016 {published data only}
- Papakonstantinou E, Kechribari I, Mitrou P, Trakakis E, Vassiliadi D, Georgousopoulou E, et al. Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: a randomised trial. European Journal of Clinical Nutrition 2016;70(5):588‐94. [DOI] [PubMed] [Google Scholar]
Pasquali 1986 {published data only}
- Pasquali R, Fabbri R, Venturoli S, Paradisi R, Antenucci D, Melchionda N. Effect of weight loss and antiandrogenic therapy on sex hormone blood levels and insulin resistance in obese patients with polycystic ovaries. American Journal of Obstetrics and Gynecology 1986;154(1):139‐44. [DOI] [PubMed] [Google Scholar]
Pasquali 2000 {published data only}
- Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D, et al. Effect of long‐term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2000;85(8):2767‐74. [DOI] [PubMed] [Google Scholar]
Pekhlivanov 2006 {published data only}
- Pekhlivanov B, Mitkov M, Kavurdzhikova S. Clinical, hormonal and biochemical changes after treatment with metformin and weight reduction in women with polycystic ovary syndrome. Akusherstvo i Ginekologiia 2006;45(6):29‐35. [PubMed] [Google Scholar]
Roessler 2013 {published data only}
- Roessler KK, Birkebaek C, Ravn P, Andersen MS, Glintborg D. Effects of exercise and group counselling on body composition and VO2max in overweight women with polycystic ovary syndrome. Acta Obstetricia et Gynecologica Scandinavica 2013;92(3):272‐7. [DOI] [PubMed] [Google Scholar]
Sa 2016 {published data only}
- J Sá JC, Costa EC, Silva E, Tamburús NY, Porta A, Medeiros LF, et al. Aerobic exercise improves cardiac autonomic modulation in women with polycystic ovary syndrome. International Journal of Cardiology 2016;202:356‐61. [DOI] [PubMed] [Google Scholar]
Sordia‐Hernandez 2016 {published data only}
- Sordia‐Hernández LH, Ancer Rodríguez P, Saldivar Rodriguez D, Trejo Guzman S, Servín Zenteno ES, Guerrero González G, et al. Effect of a low glycemic diet in patients with polycystic ovary syndrome and anovulation ‐ a randomized controlled trial. Clinical and Experimental Obstetrics & Gynecology 2016;43(4):555‐9. [PubMed] [Google Scholar]
Sorensen 2012 {published data only}
- Sørensen LB, Søe M, Halkier KH, Stigsby B, Astrup A. Effects of increased dietary protein‐to‐carbohydrate ratios in women with polycystic ovary syndrome. American Journal of Clinical Nutrition 2012;95(1):39‐48. [DOI] [PubMed] [Google Scholar]
Sprung 2013 {published data only}
- Sprung VS, Cuthbertson DJ, Pugh CJ, Aziz N, Kemp GJ, Daousi C, et al. Exercise training in polycystic ovarian syndrome enhances flow‐mediated dilation in the absence of changes in fatness. Medicine and Science in Sports and Exercise 2013;45(12):2234‐42. [DOI] [PubMed] [Google Scholar]
Talluto 2002 {published data only}
- Talluto C. The effects of a six‐week aerobic and weight‐resistance training program on infertility patients diagnosed with polycystic ovary syndrome. Fertility and Sterility 2002;78 Suppl 1(3):152. [Google Scholar]
Tang 2006 {published data only}
- Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo‐controlled, double‐blind multicentre study. Human Reproduction 2006;21(1):80‐9. [DOI] [PubMed] [Google Scholar]
Thomson 2008 {published data only}
- Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2008;93(9):3373‐80. [DOI] [PubMed] [Google Scholar]
Thomson 2016 {published data only}
- Thomson RL, Buckley JD, Brinkworth GD. Perceived exercise barriers are reduced and benefits are improved with lifestyle modification in overweight and obese women with polycystic ovary syndrome: a randomised controlled trial. BMC Women's Health 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toscani 2011 {published data only}
- Toscani MK, Mario FM, Radavelli‐Bagatini S, Wiltgen D, Matos MC, Spritzer PM. Effect of high‐protein or normal‐protein diet on weight loss, body composition, hormone, and metabolic profile in southern Brazilian women with polycystic ovary syndrome: a randomized study. Gynecological Endocrinology 2011;27(11):925‐30. [DOI] [PubMed] [Google Scholar]
Turner‐McGrievy 2014 {published data only}
- Turner‐McGrievy GM, Davidson CR, Wingard EE, Billings DL. Low glycemic index vegan or low‐calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutrition Research 2014;34(6):552‐8. [DOI] [PubMed] [Google Scholar]
Wong 2016 {published data only}
- Wong JM, Gallagher M, Gooding H, Feldman HA, Gordon CM, Ludwig DS, et al. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatric Obesity 2016;11(3):210‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zarrinkoub 2005 {published data only}
- Zarrinkoub F, Beigi A. Insulin‐sensitization versus lifestyle modification in the management of adolescent girls with polycystic ovary syndrome. The 21st Annual Meeting of the European Society of Human Reproduction and Embryology. 2005.
References to studies awaiting assessment
Gaeini 2012 {published data only}
- Gaeini AA, Satarifard S, Mohamadi F, Rajaei M. The effect of a twelve‐weeks aerobic exercise on ovarian androgens and body composition of women with polycystic ovary syndrome. Armaghane Danesh Journal 2012;17(5):387‐97. [Google Scholar]
Additional references
Acien 1999
- Acien P, Quereda F, Matallin P, Villarroya E, Lopez‐Fernandez JA, Acien M, et al. Insulin, androgens, and obesity in women with and without polycystic ovary syndrome: a heterogeneous group of disorders. Fertility and Sterility 1999;72:32‐40. [DOI] [PubMed] [Google Scholar]
Alebic 2014
- Alebić MŠ, Bulum T, Stojanović N, Duvnjak L. Definition of insulin resistance using the homeostasis model assessment (HOMA‐IR) in IVF patients diagnosed with polycystic ovary syndrome (PCOS) according to the Rotterdam criteria. Endocrine 2014;47(2):625‐30. [DOI] [PubMed] [Google Scholar]
Androulakis 2014
- Androulakis II, Kandaraki E, Christakou C, Karachalios A, Marinakis E, Paterakis T, et al. Visceral adiposity index (VAI) is related to the severity of anovulation and other clinical features in women with polycystic ovary syndrome. Clinical Endocrinology 2014;81(3):426‐31. [DOI] [PubMed] [Google Scholar]
Aziz 2015
- Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real‐world diabetes prevention programs: learnings from the last 15 years. Implementation Science 2015;15(10):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balen 1995
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Human Reproduction 1995;10:2107‐11. [DOI] [PubMed] [Google Scholar]
Barbieri 1986
- Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. Journal of Clinical Endocrinology and Metabolism 1986;62:904‐10. [DOI] [PubMed] [Google Scholar]
Behbourdi‐Gandevani 2016
- Behboudi‐Gandevani S, Ramezani Tehrani F, Rostami Dovom M, Farahmand M, Bahri Khomami M, Noroozzadeh M, et al. Insulin resistance in obesity and polycystic ovary syndrome: systematic review and meta‐analysis of observational studies. Gynecological Endocrinology 2016;32(5):343‐53. [DOI] [PubMed] [Google Scholar]
Benham 2018
- Benham JL, Yamamoto JM, Friedenreich CM, Rabi DM, Sigal RJ. Role of exercise training in polycystic ovary syndrome: a systematic review and meta‐analysis. Clinical Obesity 2018;8(4):275‐84. [DOI] [PubMed] [Google Scholar]
Bozdag 2016
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta‐analysis. Human Reproduction 2016;31(12):2841‐55. [DOI] [PubMed] [Google Scholar]
Clark 1998
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Human Reproduction 1998;13:1502‐5. [DOI] [PubMed] [Google Scholar]
Daan 2014
- Daan NM, Louwers YV, Koster MP, Eijkemans MJ, Rijke YB, Lentjes EW, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk?. Fertility and Sterility 2014;102:1444‐51. [DOI] [PubMed] [Google Scholar]
DeUgarte 2005
- DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertility and Sterility 2005;83:1454‐60. [DOI] [PubMed] [Google Scholar]
Domecq 2013
- Domecq JP, Prutsky G, Mullan RJ, Hazem A, Sundaresh V, Elamin MB, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2013;98(12):4655‐63. [DOI] [PubMed] [Google Scholar]
Ehrmann 2006
- Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2006;91:48‐53. [DOI] [PubMed] [Google Scholar]
ESHRE/ASRM 2004
- ESHRE/ASRM. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004;81:19‐25. [DOI] [PubMed] [Google Scholar]
Essah 2008
- Essah PA, Nestler JE, Carmina E. Differences in dyslipidemia between American and Italian women with polycystic ovary syndrome. Journal of Endocrinological Investigation 2008;31:35‐41. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University, developed by Evidence Prime, Inc.. GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University, developed by Evidence Prime, Inc., 2015.
Gunning 2017
- Gunning MN, Fauser BCJM. Are women with polycystic ovary syndrome at increased cardiovascular disease risk later in life?. Climacteric 2017;20(3):222‐7. [DOI] [PubMed] [Google Scholar]
Haqq 2014
- Haqq L, McFarlane J, Dieberg G, Smart N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: a systematic review and meta‐analysis. Endocrine Connections 2014;3(1):36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haqq 2015
- Haqq L, McFarlane J, Dieberg G, Smart N. The effect of lifestyle intervention on body composition, glycemic control, and cardiorespiratory fitness in polycystic ovarian syndrome: a systematic review and meta‐analysis. International Journal of Sport Nutrition and Exercise Metabolism 2015;25(6):533‐40. [DOI] [PubMed] [Google Scholar]
Heida 2016
- Heida KY, Bots ML, Groot CJ, Dunné FM, Hammoud NM, Hoek A, et al. Cardiovascular risk management after reproductive and pregnancy‐related disorders: a Dutch multidisciplinary evidence‐based guideline. European Journal of Preventive Cardiology 2016;17:1863‐79. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huber‐Buchholz 1999
- Huber‐Buchholz MM, Carey DG, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. Journal of Clinical Endocrinology and Metabolism 1999;84:1470‐4. [DOI] [PubMed] [Google Scholar]
Hutchison 2011
- Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2011;96(1):E48‐56. [DOI] [PubMed] [Google Scholar]
International PCOS Guideline 2018
- Teede H, Misso M, Costello M, Dokras A, Laven J, Moran L, et al on behalf of the International PCOS Network. International evidence based guideline for the assessment and management of polycystic ovary syndrome. https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence‐Based‐Guidelines_20181009.pdf 2018. [DOI] [PMC free article] [PubMed]
Jedel 2011
- Jedel E, Labrie F, Oden A, Holm G, Nilsson L, Janson PO, et al. Impact of electro‐acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: a randomised controlled trial. American Journal of Physiology‐Endocrinology and Metabolism 2011;300(1):E37‐45. [DOI] [PubMed] [Google Scholar]
Kakoly 2018
- Kakoly NS, Khomami MB, Joham AE, Cooray SD, Misso ML, Norman RJ, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta‐regression. Human Reproduction Update 2018;24(4):455‐67. [DOI] [PubMed] [Google Scholar]
Kiddy 1990
- Kiddy DS, Sharp PS, White DM, Scanlon MF, Mason HD, Bray CS, et al. Differences in clinical and endocrine features between obese and non‐obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clinical Endocrinology 1990;32:213‐20. [DOI] [PubMed] [Google Scholar]
Landay 2009
- Landay M, Huang A, Azziz R. Degree of hyperinsulinemia, independent of androgen levels, is an important determinant of the severity of hirsutism in PCOS. Fertility and Sterility 2009;92(2):643‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lass 2011
- Lass N, Kleber M, Winkel K, Wunsch R, Reinehr T. Effect of lifestyle intervention on features of polycystic ovarian syndrome, metabolic syndrome, and intima‐media thickness in obese adolescent girls. Journal of Clinical Endocrinology and Metabolism 2011;96(11):3533‐40. [DOI] [PubMed] [Google Scholar]
Legro 1999
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. Journal of Clinical Endocrinology and Metabolism 1999;84:165‐9. [DOI] [PubMed] [Google Scholar]
Leonhardt 2015
- Leonhardt H, Hellstrom M, Gull B, Lind AK, Nilsson L, Janson OP, et al. Serum anti‐Mullerian hormone and ovarian morphology assessed by magnetic resonance imaging in response to acupuncture and exercise in women with polycystic ovary syndrome: secondary analyses of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2015;94:279‐87. [DOI] [PubMed] [Google Scholar]
Lim 2012
- Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Human Reproduction Update 2012;18(6):618‐37. [DOI] [PubMed] [Google Scholar]
Lim 2013
- Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta‐analysis. Obesity Reviews 2013;14(2):95‐109. [DOI] [PubMed] [Google Scholar]
Lønnebotn 2018
- Lønnebotn M, Natvig GK, Benediktsdóttir B, Burgess JA, Holm M, Jógi R, et al. Polycystic ovary syndrome, body mass index and hypertensive disorders in pregnancy. Pregnancy Hypertension 2018;11:32‐7. [DOI] [PubMed] [Google Scholar]
March 2010
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Human Reproduction 2010;25(2):544‐51. [DOI] [PubMed] [Google Scholar]
Meyer 2005
- Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. Journal of Clinical Endocrinology and Metabolism 2005;90:5711‐6. [DOI] [PubMed] [Google Scholar]
Moran 2003
- Moran LJ, Noakes M, Clifton PM, Tomlinson L, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2003;88:812‐9. [DOI] [PubMed] [Google Scholar]
Moran 2009
- Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertility and Sterility 2009;92(6):1966‐82. [DOI] [PubMed] [Google Scholar]
Moran 2009b
- Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Human Reproduction Update 2009;15(4):477‐88. [DOI] [PubMed] [Google Scholar]
Moran 2010b
- Moran LJ, Misso M, WIld RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta‐analysis. Human Reproduction Update 2010;16(4):347‐63. [DOI] [PubMed] [Google Scholar]
Morley 2017
- Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin‐sensitising drugs (metformin, rosiglitazone, pioglitazone, D‐chiro‐inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD003053.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naderpoor 2015
- Naderpoor N, Shorakae S, Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta‐analysis. Human Reproduction Update 2015;21(5):560‐74. [DOI] [PubMed] [Google Scholar]
Paradisi 2001
- Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001;103:1410‐5. [DOI] [PubMed] [Google Scholar]
Plymate 1988
- Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone‐binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. Journal of Clinical Endocrinology and Metabolism 1988;67:460‐4. [DOI] [PubMed] [Google Scholar]
Poehlman 2000
- Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. Journal of Clinical Endocrinology and Metabolism 2000;85:2463‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2015
- Richards J, Jiang X, Kelly P, Chau J, Bauman A, Ding D. Don't worry, be happy: cross‐sectional associations between physical activity and happiness in 15 European countries. BMC Public Health 2015;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2013
- Roberts CK, Little JP, Thyfault JP. Modification of insulin sensitivity and glycemic control by activity and exercise. Medicine & Science in Sports & Exercise 2013;45(10):1868‐77. [DOI] [PubMed] [Google Scholar]
Ross 2000
- Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet‐induced weight loss or exercise‐induced weight loss in men. A randomized, controlled trial. Annals of Internal Medicine 2000;133:92‐103. [DOI] [PubMed] [Google Scholar]
Rubin 2017
- Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2017;102(10):3848‐57. [DOI] [PubMed] [Google Scholar]
Salazar 2016
- Salazar MR, Carbajal HA, Espeche WG, Aizpurúa M, Leiva Sisnieguez CE, Leiva Sisnieguez BC, et al. Insulin resistance: the linchpin between prediabetes and cardiovascular disease. Diabetes & Vascular Disease Research 2016;13(2):157‐63. [DOI] [PubMed] [Google Scholar]
Stener‐Victorin 2009
- Stener‐Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low‐frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology 2009;297(2):R387‐95. [DOI] [PubMed] [Google Scholar]
Stener‐Victorin 2012
- Stener‐Victorin E, Baghaei F, Holm G, Janson PO, Olivecrona G, Lönn M, Manners‐Holm L. Effects of acupuncture and exercise on insulin sensitivity, adipose tissue characteristics, and markers of coagulation and fibrinolysis in women with polycystic ovary syndrome: secondary analyses of a randomized controlled trial. Fertility and Sterility 2012;97(2):501‐8. [DOI] [PubMed] [Google Scholar]
Stener‐Victorin 2013
- Stener‐Victorin E, Holm G, Janson PO, Gustafson D, Waern M. Acupuncture and physical exercise for affective symptoms and health‐related quality of life in polycystic ovary syndrome: secondary analysis from a randomized controlled trial. BMC Complementary and Alternative Medicine 2013;13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stepto 2013
- Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic‐hyperinsulaemic clamp. Human Reproduction 2013;28(3):777‐84. [DOI] [PubMed] [Google Scholar]
Teede 2007
- Teede H, Hutchison SK, Zoungas S. The management of insulin resistance in polycystic ovary syndrome. Trends in Endocrinology and Metabolism 2007;18(7):273‐9. [DOI] [PubMed] [Google Scholar]
Teede 2011
- Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, et al. Assessment and management of polycystic ovary syndrome: summary of an evidence‐based guideline. Medical Journal of Australia 2011;195(6):S65‐112. [DOI] [PubMed] [Google Scholar]
Vrbikova 2014
- Vrbikova J, Hill M, Fanta M. The utility of fasting plasma glucose to identify impaired glucose metabolism in women with polycystic ovary syndrome. Gynecological Endocrinology 2014;30(9):664‐6. [DOI] [PubMed] [Google Scholar]
Yang 2016
- Yang R, Yang S, Li R, Liu P, Qiao J, Zhang Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta‐analysis. Reproductive Biology and Endocrinology 2016;14(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zawadski 1992
- Zawadski J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. Polycystic Ovary Syndrome Current Issues in Endocrinology and Metabolism. Boston: Blackwell Scientific, 1992:377‐84. [Google Scholar]
References to other published versions of this review
Moran 2011
- Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007506.pub3] [DOI] [PubMed] [Google Scholar]


