Abstract
Obesity is increasing in an alarming rate worldwide, which causes higher risks of some diseases, such as type 2 diabetes, cardiovascular diseases, and cancer. Current therapeutic approaches, either pancreatic lipase inhibitors or appetite suppressors, are generally of limited effectiveness. Brown adipose tissue (BAT) and beige cells dissipate fatty acids as heat to maintain body temperature, termed non-shivering thermogenesis; the activity and mass of BAT and beige cells are negatively correlated with overweight and obesity. The existence of BAT and beige cells in human adults provides an effective weight reduction therapy, a process likely to be amenable to pharmacological intervention. Herein, we combed through the physiology of thermogenesis and the role of BAT and beige cells in combating with obesity. We summarized the thermogenic regulators identified in the past decades, targeting G protein-coupled receptors, transient receptor potential channels, nuclear receptors and miscellaneous pathways. Advances in clinical trials were also presented. The main purpose of this review is to provide a comprehensive and up-to-date knowledge from the biological importance of thermogenesis in energy homeostasis to the representative thermogenic regulators for treating obesity. Thermogenic regulators might have a large potential for further investigations to be developed as lead compounds in fighting obesity.
Abbreviations: AKT, protein kinase B; ALDH9, aldehyde dehydrogenase 9; AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; β3-AR, β3-adrenergic receptor; BA, bile acids; BAT, brown adipose tissue; BMP8b, bone morphogenetic protein 8b; cAMP, cyclic adenosine monophosphate; C/EBPα, CCAAT/enhancer binding protein α; cGMP, cyclic guanosine monophosphate; Cidea, cell death-inducing DNA fragmentation factor α-like effector A; CLA, cis-12 conjugated linoleic acid; CRABP-II, cellular RA binding protein type II; CRE, cAMP response element; Dio2, iodothyronine deiodinase type 2; ERs, estrogen receptors; ERE, estrogen response element; FAS, fatty acid synthase; FGF21, fibroblast growth factor 21; GPCRs, G protein-coupled receptors; HFD, high fat diet; LXR, liver X receptors; MAPK, mitogen-activated protein kinase; OXPHOS, oxidative phosphorylation; PDEs, phosphodiesterases; PET-CT, positron emission tomography combined with computed tomography; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-α; PKA, protein kinase A; PPARs, peroxisome proliferator-activated receptors; PPREs, peroxisome proliferator response elements; PRDM16, PR domain containing 16; PTP1B, protein-tyrosine phosphatase 1B; PXR, pregnane X receptor; RA, retinoic acid; RAR, RA receptor; RARE, RA response element; RMR, resting metabolic rate; RXR, retinoid X receptor; SIRT1, silent mating type information regulation 2 homolog 1; SNS, sympathetic nervous system; TFAM, mitochondrial transcription factor A; TMEM26, transmembrane protein 26; TRPs, transient receptor potential cation channels; UCP1, uncoupling protein 1; VDR, vitamin D receptor; VDRE, VDR response elements; WAT, white adipose tissue
KEY WORDS: Thermogenesis, Brown adipose tissue, Beige cells, Obesity, Uncoupling protein 1
Graphical abstract
Brown adipose tissue (BAT) and beige cells dissipates fatty acids as heat, termed non-shivering thermogenesis, which has emerged as a potential therapeutically way to treat obesity. The current review provides a comprehensive and up-to-date of knowledge from the biological importance of thermogenesis to the representative thermogenic regulators for treating obesity.
1. Introduction
Overweight and obesity have reached epidemic proportions worldwide, for both children and adults1. The updated World Health Organization data showed more than 1.9 billion adults aged over eighteen were overweight and over 650 million were obese in 2016, which were almost double those in 1980. Obesity always increases the risk of some complications, such as type 2 diabetes2, atherosclerosis3 and several forms of cancer4., 5., 6.. The mainstay anti-obesity approach remains in having low calorie diet and increasing physical activity. While, the anti-obesity therapeutic agents are the only choice for obese patients who have other comorbid conditions to restrict physical activity, such like hypertension, type 2 diabetes and arthritis. Currently, only five U. S. Food and Drug Administration-approved small molecule drugs for obesity treatment were sold on market. These anti-obesity drugs could be classified into two types, pancreatic lipase inhibitors to reduce intestinal fat absorption, and anorectics to suppress appetite. Most of them have unhappy adverse effects7. Thus, there is still a desperate demand for effective and safe candidates to get the obesity under control.
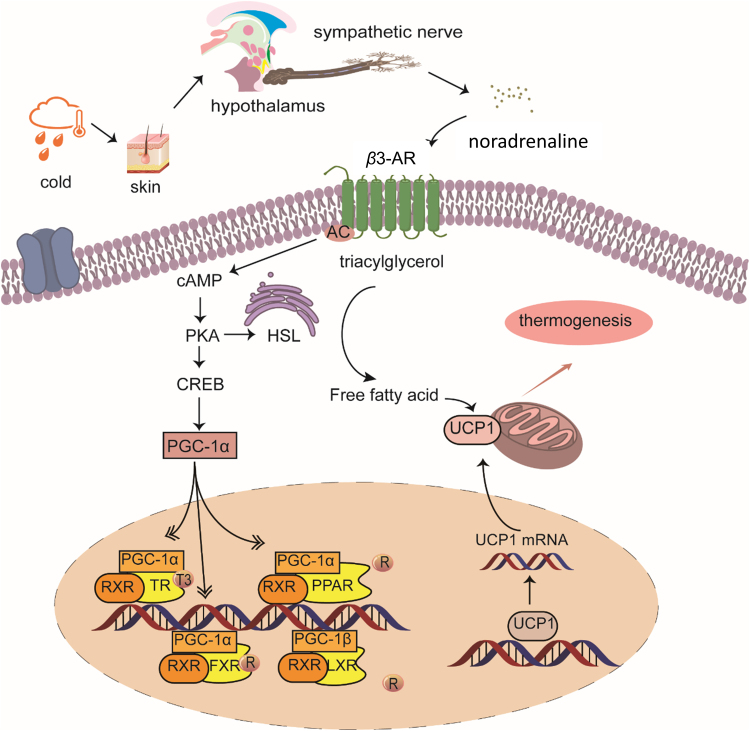
Obesity is characterized by fat mass expansion, occurred via adipocytes hyperplasia (increased number of adipocytes) and hypertrophy (increased size of adipocytes), and dysfunction of adipose tissues. Under positive energy conditions, pre-adipocytes proliferate and differentiate into mature adipocytes (hyperplasia), and excessive lipid stores within adipocytes (hypertrophy). There are 3 types of adipocytes: white adipocytes store excess calories in the form of triglycerides; brown adipocytes contain large amounts of mitochondria and disperse lipids to generate heat by uncoupling protein 1 (UCP1); beige adipocytes express low UCP1 at basal status, which resemble white adipocytes, and have a highly inducible thermogenic capacity upon stimulation8., 9.. Upon cold-stimulus, the sympathetic nervous system (SNS) is activated to release noradrenaline, which binds to β3-adrenergic receptor (β3-AR) on brown and beige adipocytes (Fig. 1). Subsequently, UCP1 is highly expressed and activated in mitochondria, promoting lipid β-oxidation and heat production (Fig. 1)8. Non-shivering thermogenesis in brown and beige adipocytes has been recognized to play a crucial role in energy balance in rodents and humans10. Thermogenic activity of brown and beige adipocytes is positively correlated with energy expenditure, and dysregulation of thermogenesis is linked to obesity in humans11. Studies have disclosed that the “brown” fat in human adults is composed primarily of beige adipocytes9. Therefore, interventions to increase “brown” fat mass and/or activity are attractive strategies for prevention/treatment of obesity.
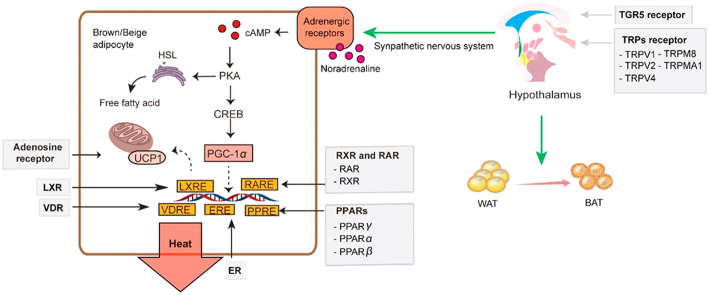
Figure 1.
Schematic diagram of cold-activated thermogenesis. Sympathetic nerve is activated in response to cold exposure to release noradrenaline. Noradrenaline binds to the β3-AR on brown and beige cells to initiate signaling cascades for triglycerides hydrolysis and protein kinase A (PKA) activation. In response to PKA activation, CREB (cAMP response element-binding protein) recruits PGC-1α to facilitate RXR heterodimerization, which then interacts with nuclear receptors, like PPARs and LXR, to enhance UCP1 gene transcription. Then, UCP1 dispersed the released fatty acids to generate heat.
Increasing evidences have revealed that thermogenic regulators have therapeutic effects towards obesity. With growing demands for treatment of obesity safely and effectively, more and more clinical studies were carried out recent years. Through searching on the data base of clinical registration in USA (https//clinicaltrails.gov), 10 preparations have been involved into clinical trials (Table 1). One (NCT02937298), three (NCT03171415, NCT01783470 and NCT00302276), two (NCT02048215 and NCT00302276) and four (NCT03379181, NCT03269747, NCT03189511 and NCT00781586) products have been involved in phases 1, 2, 3 and 4 clinical trials, respectively. These agents target on treatment of obesity, insulin resistance and hyperthyroidism. Till now, there is still no drug in clinic targeting thermogenesis for treatment of obesity. This review summarized recent research progresses in thermogenic regulators, and speculated their potential as anti-obesity agents.
Table 1.
Thermogenic regulators in clinical trials.
| Name | Identifier | Condition | Phase |
|---|---|---|---|
| Propranolol | NCT03379181 | Hyperthyroidism | 4 |
| NCT01791114 | Insulin sensitivity, obesity | – | |
| Prednisone | NCT03269747 | BAT activity | 4 |
| Fluvastatin | NCT03189511 | Brown fat activity, insulin resistance | 4 |
| RZL-012 | NCT03171415 | Obesity | 2 |
| Caffeine, ephedrine | NCT02048215 | Obesity | 3 |
| β3-AR agonist | NCT01783470 | Obesity | 2 |
| Caffeine | NCT00781586 | Energy expenditure | 4 |
| Zantrex-3 | NCT02937298 | Diet-induced thermogenesis, obesity | – |
| Metobes-compound | NCT00302276 | Obesity | 2 and 3 |
| Tyrosine, green tea, caffeine | NCT02937298 | Diet-induced thermogenesis, obesity | 1 |
–Not applicable.
2. Thermogenic regulators targeting G protein-coupled receptors (GPCRs)
GPCRs, a protein family comprised of more than 600 members, are associated with many physiological and pathological conditions. Thermogenic regulators targeting GPCRs have been widely investigated (Table 2).
Table 2.
Thermogenic regulators targeting GPCRs.
 |
2.1. β3-AR
β3-AR, one isoform of adrenergic receptors, is pivotal in thermogenesis because it׳s selectively expressed in brown and beige adipocytes in both rodents and humans12. Many studies have been focusing on the potential of β3-AR agonists as anti-obesity agents (Table 2). Two β3-AR agonists, BRL-37344 and CL316243, were reported to induce lipolysis and thermogenesis in brown adipocytes from rats13. CL316243 treatment increased brown adipose tissue (BAT) activity and energy expenditure of C57BL/6J mice in a thermoneutral state, but did not reduced adiposity in mice housed below thermoneutrality14. A clinic study showed treatment of CL316243 on healthy men enhanced fat oxidation and insulin-stimulated glucose disposal15. Acute administration of another β3-AR agonist, L-796568, in overweight men significantly increased energy expenditure after 4 h, while chronic administration of this compound for 4 weeks failed to increase energy expenditure16., 17.. CGP-12177A is a β3-AR agonist, which enhanced uncoupling content in BAT and inguinal white adipose tissue (WAT) of NMRI mice18., 19.. Arotinolol, a weak β3-AR agonist, stimulated oxygen consumption in brown adipocytes from hamsters or rats, but did not change thermogenesis in intact animals20. Mirabegron, with a high specific affinity to human β3-AR, is being applied to treat overactive bladder in clinic. High dose of mirabegron was reported to increase resting metabolic rate (RMR) and BAT thermogenesis in healthy young men12.
Surprisingly, several other β3-AR agonists, including ZD7114, ZD2079 and TAK-677, didn׳t change energy expenditure in obese humans21., 22.. The failure of β3-AR agonists to reduce body weight or increase energy expenditure in the clinical trials might be due to the following reasons: 1) the objects, especially those obese patients, lacked brown and beige adipocytes, which led to attenuation of the effect of β3-AR agonists on energy expenditure; 2) the objects were treated with β3-AR agonists for a short period of time in the most of trials, ranging from few hours to a few days; however, the activation of BAT might be observed in a long period of time; 3) the β3-AR expression and function are different in rodents and humans. Most β3-AR agonists were authenticated to be efficient on rodents, but failed in clinical trials. The human setting from in vitro to in vivo need to be addressed. Some β3-AR agonists also showed adverse effects due to insufficient selectivity. The structure and function mechanism of different ARs need to be further investigated to discover and develop more specific β3-AR agonists as a mean of activating brown and beige adipocytes.
2.2. Adenosine receptor
The innate ligand to adenosine receptor is adenosine, which binds to four P1 GPCR subtypes, the inhibitory receptors A1 and A3 and the stimulatory receptors A2A and A2B23. The distribution of the adenine receptor subtypes varies greatly by tissues and species, resulting in distinct response in different tissue contexts. Adenosine and its analogues (including 2-chloroadenosine, 2′-deoxyadenosine, 3′-deoxyadenosine and 2′-deoxyadenosine monophophate) were found to inhibit isoproterenol-induced lipolysis, adenylate cyclase activation and 3′,5′-cyclic monophosphate generation in adipocytes from rodents23., 24.. While, another study suggested adenosine enhanced the thermogenic program in brown and white adipocytes at nanomolar concentrations, either from human or murine; and the effect of adenosine was stronger in brown adipocytes than white adipocytes duo to higher expression of A2A receptor and higher A2A/A1 ratio in brown adipocytes25. These findings indicated the role of adenosine signaling in thermogenesis is still controversial, and more studies are needed.
2.3. G protein-coupled bile acid receptor (TGR5)
G protein-coupled bile acid receptor, named TGR5, is involved in energy homeostasis26., 27.. Administration of bile acids (BA) increased energy expenditure in BAT of mice, through inducing the cyclic adenosine monophosphate (cAMP)-dependent thyroid hormone activation28. Interestingly, the increase of plasma BA concentration in rats was associated with the induction of genes involved in energy metabolism, including Dio2 (iodothyronine deiodinase type 2), Pgc-1α (peroxisome proliferator-activated receptor γ coactivator-1α), and UCP1, in both BAT and abdominal and subcutaneous WAT29. Similarly, chenodeoxycholic acid was found to increase UCP1 expression and activate thermogenesis in human BAT30. BA also has other hormonal actions through the farnesoid X receptor, which makes it not applicable for treatment of obesity.
3. Thermogenic regulators targeting transient receptor potential (TRP) channels
TRP channels are a group of transmembrane cation channels that are relatively non-selective for Ca2+, Mg2+, and Na+ ions31. Unlike the K+ selective ion channels, the TRP channels are constitutively open and are gated by a wide spectrum of physical and chemical stimuli, such as voltage, adenosine triphosphate (ATP), pH, redox agents, and multiple sensory stimuli. Upon stimulation, TRP channels initiate SNS activity, which, in turn, cascade a set of physiological processes, leading to defending responses to environmental changes. All TRP channels family members display six transmembrane α-helical protein domains which are assembled as tetramers to produce the overall functional channel31. Based on sequence and topological differences, the TRP channels family are classified into seven subfamily members, the five group 1 TRPs (TRPC, TRPV, TRPM, TRPN, and TRPA) and two group 2 TRPs (TRPP and TRPML). Among them, TRPV, TRPM and TRPA belong to thermally activated members. TRPV1, TRPV2, TRPV3 and TRPV4 are for warm sensation, and TRPM8 and TRPA1 are for cold sensation.
Upon stimulation from pain, heat, cold, capsaicin, and even mechanical motion, TRP channels receptors are sufficient to activate SNS-noradrenaline-BAT axis to enhance thermogenesis32. However, it׳s still debating whether activation or inhibition of TRP channels has benefit for thermogenesis, central or peripheral expressed TRP channels are the most critical. TRP channels regulators have received considerable attention in the field of obesity and diabetes (Table 3).
Table 3.
Thermogenic regulators targeting TRPs.
 |
|
 |
Capsinoids, including capsiate, dihydrocapsiate, and nordihydrocapsiate, are chemical constituents naturally present in chili peppers. In 2009, a clinical trial on healthy humans showed oral treatment with 6 mg capsinoids each day for 12 weeks caused obvious abdominal fat loss33. In addition, administration of 4 mg/kg capsinoids for 1 month showed enhanced energy expenditure and decreased body weight34. Another trial on healthy humans also showed that 8-week capsinoids treatment (9 mg/kg per day) increased BAT capacity using 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (PET-CT)35. Through activating TRPV1 receptor, capsinoids not only enhances BAT thermogenesis, which always occurs in minutes, but also stimulates browning of WAT, which is adaptive process through increasing capacity of thermogenesis32., 36.. Capsaicin, one principle constituent of hot pepper, was reported to enhance energy expenditure and fatty acid β-oxidation via stimulating TRPV1-SNS axis37. Treatment with high dose of capsaicin (135 mg/day) for 3 months significantly increased fat oxidation without obvious adverse effect38. Importantly, dietary capsaicin activated TRPV1-evoked Ca2+ influx in the process of adipocyte-to-adipocyte communication, which, in turn, promoted lipolysis both in vitro and in vivo, improving visceral fat remodeling39. In 2011, monoacylglycerol was identified as a TRPV1 agonist, which increased UCP1 expression in BAT and prevented visceral fat accumulation in C57BL/6Cr mice40. 10-Oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, was reported to enhance energy metabolism by activation of TRPV141. A 12‐week intervention with nonivamide, a TRPV1 agonist, prevented a dietary‐induced body fat gain and increased peripheral serotonin in moderately overweight subjects42. It is interested to note that most TRPV1 receptor agonists are constituents from edibles, such as Guinea pepper seeds, with high content, which indicate they are safe for long term application. Activation of TRPV1 may mimic chronic cold exposure to increase thermogenesis in BAT that a process for body to adapt the change of environment.
TRPV1 is a temperature sensor which gets activated at 42 °C or over. It suggests that TRPV1 is the transmitter or amplifier to thermogenesis. When initial senor gets the signal of cold and cascade a series of action to initiate thermogenesis, TRPV1 can amplify the effect of thermogenesis. TRPV2 gets activated with an activation temperature threshold of higher than 52 °C. It is notable that loss of TRPV2 in mice showed increased WAT and larger brown adipocytes, and less BAT temperature increase in response to sympathetic activation43. However, it has been reported that activation of TRPV2 with non-selective TRPV2 agonists, 2-aminoethoxydiphenyl borate or lysophosphatidylcholine, inhibited the differentiation of mouse brown adipocytes44. These results suggested that the role of TRPV2 in the treatment of obesity is still remaining elusive.
TRPV4 is highly expressed in adipocytes45. Interestingly, TRPV4 expression is higher in WAT than BAT; and inactivation TRPV4 with its antagonist GSK205 led to WAT browning, while activation of TRPV4 with its agonist, GSK1016790A, repressed thermogenic genes expression46. It inferred that inactivation of TRPV4 might stimulate the formation of beige cells in WAT. Consistently, intravenous blockade of TRPV4 channel with chemical selective antagonists, HC-067047 or RN-1734, caused an increase in core body temperature and oxygen consumption at ambient temperature of 26 °C47. In addition, it is notable intracerebroventricular treatment with RN-1747, a chemical selective agonist of TRPV4, did not cause hypothermia. It indicated that the observed response was indeed due to activation of TRPV4 channels in the periphery47.
At lower experimental temperature like 20 °C, TRPM8 or TRPA1 is more likely to respond to cold stimulation. Previous studies have validated that TRPM8 plays a vital role in the detection of environmental temperature in mammals and is responsible for cold and chemical stimulation like menthol48., 49., 50.. Menthol or 1,8-cineole activates TRPM8 to trigger UCP1-induced non-shivering thermogenesis and locomotor activity51., 52.. Allyl isothiocyannate and cinamaldehyde were reported to enhance thermogenesis and inhibit heat diffusion in mice, through activating TRPA151.
There are controversial results from dietary supplementation of TRP ligands (e.g., capsaicin), either showing beneficial effects on body weight, metabolism, and hormone levels, or no effects. Selectivity of activators or inhibitors should be taken into consideration. Large clinical trials are needed to confirm the role of TRP ligands in the treatment of obesity. TRP channels are expressed in many tissues and organs important for the maintenance of whole body metabolism. Manipulation of TRP with small molecules is a potential strategy for induction of thermogenesis and treatment of obesity.
4. Thermogenic regulators targeting nuclear receptors in adipocyte
Nuclear receptors are a class of proteins directly binding to DNA to regulate expression of specific genes, which are highly related with energy homeostasis and metabolism53. Thermogenic regulators targeting nuclear receptors were listed in Table 4.
Table 4.
Thermogenic regulators targeting nuclear receptors.
 |
 |
 |
4.1. Peroxisome proliferator-activated receptors (PPAR)
PPARs belong to nuclear receptor super family, and so far three PPAR isoforms have been identified54. PPARα and PPARγ are directly linked to thermogenesis. PPARδ has capacity to increase fat acid oxidation55. When activated by specific ligands, PPARs bind to RXR (retinoid X receptor) to form heterodimers, which translocate to nucleus and bind peroxisome proliferator response elements (PPREs) to exert its function56.
Rosiglitazone (Table 4), a PPARγ agonist, was found to promote mitochondrial biogenesis in 3T3-L1 adipocytes, accompanied with increased thermogenesis capacity and browning character57. In addition, chronic treatment of rosiglitazone to human multipotent adipose-derived stem cells showed browning phenotypes by increased UCP1 and CIDEA (cell death-inducing DNA fragmentation factor α-like effector A) mRNA expressions58. Another study showed chronic treatment of rosiglitazone promoted browning in epididymal WAT59. Rosiglitazone induced BAT recruitment and lipolytic mRNA levels independently of tissue innervation status60. However, activation of PPARγ by rosiglitazone strongly exacerbated cold-induced upregulation of thyroid status and PGC-1α and Dio2 in BAT61. Treatment of pioglitazone (Table 4), another PPARγ agonist, not only increased mitochondrial copy number but also enhanced PGC-1α and TFAM (mitochondrial transcription factor A) expressions on human subcutaneous WAT62. PPAR pathway represented an alternative, potent, and fully competent mechanism for BAT recruitment. It is noteworthy that PPARγ induced UCP1 expression involves cross-talk between PKA (protein kinase A) and PPARγ signaling systems. A study showed that rosiglitazone and forskolin synergistically activated the UCP1 promoter involving cross-talk between the signaling systems regulating the cAMP response element (CRE) and PPRE on the promoters63. It suggested that increasing energy expenditure via BAT thermogenesis maybe more potential to stimulate both PKA and PPARγ signaling pathways. However, it should not be neglected that PPARγ is modulated by other factors such as FGF21 (fibroblast growth factor 21)64. Berberine (Table 4) was found to increase energy expenditure and cold tolerance, and enhance BAT activity in db/db mice. In addition, administration of berberine led to increased expressions of UCP1 and thermogenic genes in WAT and primary brown adipocytes via a mechanism involving AMPK (AMP-activated protein kinase) and PGC-1α65. Collectively, PPARγ activation goes prior to functioning on browning in WAT.
PPARα, also known as NR1C1, is a master regulator of fatty acid oxidation, which is highly expressed in tissues consuming fatty acids at a rapid rate66. In term of thermogenesis, PPARα plays an important role in the expression of UCP167. In primary human fat cells, activation of PPARα with its agonist GW7647 (Table 4) resulted in up-regulation of β-oxidation genes and enhanced palmitate oxidation. Particularly, in this process glucose oxidation was decreased. PPARα agonist may stimulate combustion of lipid instead of glucose68. Surprisingly, thiazolidinedione treatment did not show this effect69. In addition, PPARα agonists, GW7647 or WY14643 (Table 4), directly activated PGC-1α and Prdm16 (PR domain containing 16) expression, resulting in induction of thermogenic genes, mitochondrial genes, and lipid oxidation genes in brown fat70. Similarly, oleoylethanolamide (Table 4), a PPARα agonist, enhanced β3-adrenergic-mediated thermogenesis and browning in epididymal WAT in rat, with increased expressions of the mitochondrial (Cox4i1, Cox4i2), thermogenic (FGF21, Prdm16) and fatty-acid β-oxidation related genes71. It suggested PPARα receptor agonists promote adipocyte remodeling in epididymal WAT, and therefore have a potential clinical utility in the treatment of obesity.
PPARδ is a nuclear receptor that governs a variety of biological processes, which ubiquitously distributes in brain, skin, liver, skeletal muscle and adipose tissue. It has been validated PPARδ in WAT plays a role in regulating lipid mobilization and energy storage. To be frustrated, there are only few effective PPARδ agonists and the mechanism remains elusive in terms of thermogenic regulation. Interestingly, previous study showed retinoic acid (RA, Table 4), a vitamin A metabolite, acted as a physiological ligand of PPARδ, participating in cell survival72. RA activated PPARδ in preadipocytes and adipocytes to increase UCP1 and Aldh9 (aldehyde dehydrogenase 9), a key enzyme in fatty acid oxidation73. It suggested activation of PPARδ shifts substrate oxidation towards combustion of lipids. In addition, administration of PPARδ selective agonist GW0742 (Table 4) effectively suppressed adipogenesis and enhanced lipolysis through AKT (protein kinase B) signaling pathway73.
4.2. Liver X receptors (LXR)
LXRs play a vital role in bile acid synthesis, lipid and glucose homeostasis. LXRs present in two isoforms, LXRα and LXRβ. Both isoforms are expressed in mature murine and human adipocytes74. The role of LXR in adipose tissue and obesity is still controversial, due to the complicated interaction among LXR, PPARγ and C/EBPα (CCAAT/enhancer binding protein α)75., 76., 77.. Inhibition of LXR in BAT induced thermogenesis contributing to weight loss. Morin (Table 4), a naturally occurring flavonoid, was found as an LXRα and LXRβ dual antagonist, which reduced body weight gain and white adipocytes size in high fat diet (HFD)-treated mice78. It suggested that LXR suppression has a positive correlation with thermogenesis. Consistently, a report showed TO901317 (Table 4), a potent and selective LXRα agonist, has significant effect on suppression of Dio2 expression in primary brown adipocytes79. Moreover, administration of LXRs agonist GW3965 repressed UCP1 in BAT and browning of subcutaneous WAT80. Although there are still confused findings that activation of LXRs in white adipocytes have positive correlation with fatty acid oxidation, it should be noted that all these results were obtained in white adipocytes81., 82.. Interestingly, rhein (Table 4), a lipophilic anthraquinone derived from a traditional Chinese herbal medicine Rheum palmatum L., was found to maintain energy balance by targeting LXRs and protect against obesity through LXRs-mediated UCP1 upregulation in BAT83. Rhein is a multitarget molecule, which still need further investigation for pharmaceutical application as an anti-obesity agent84.
4.3. Retinoid X receptor (RXR) and RA receptor (RAR)
RAR and RXR are members of the steroid/thyroid hormone receptor superfamily. RAR is activated by binding either all-trans RA or 9-cis RA (Table 4); while RXR is activated only by 9-cis RA but not all-trans RA. Cellular responses to RA are mediated by RAR and RXR, which are activated to form dimeric transcriptional factors that bind to specific RA response element (RARE) to regulate thermogenesis in adipocytes85. Twenty years ago, RA was found to activate primary brown preadipocytes, which stimulated UCP1 gene expression through a RA-responsive region but independent of adrenergic pathway86. Puigserver et al.87 firstly reported that administration of all-trans-RA or 9-cis-RA led to an increase in the BAT specific UCP1 content in mice, as well as in HIB1B brown adipocytes. Dietary vitamin A (Table 4) supplementation increased UCP1 expression in BAT of mice88. Feeding a vitamin A-deficient diet triggered opposite effects to those of all-trans-RA treatment, including increased body weight and reduced BAT thermogenic potential89. In addition, either synthetic RAR-specific agonist, p-[(E)-2-(5,6,7,8,-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (Table 4), or RXR-specific agonist, methoprene (Table 4), increased the expression of UCP1 mRNA and the activity of chloramphenicol acetyltransferase expression vectors driven by the UCP1 promoter90. It indicated both RAR- and RXR-dependent signaling pathways mediate the induction of UCP1 in BAT by retinoids. It is noteworthy that RA׳s effects on induction of UCP1 might associate with p38 mitogen-activated protein kinase (p38MAPK) activation. Inhibition of p38MAPK activity with PD169316 hindered retinoid on induction of UCP1, suggesting p38MAPK participating in this process91. RA suppressed adipogenesis in vivo by activating the cellular PPARδ associated binding proteins cellular RA binding protein type II (CRABP-II)/RARγ path in preadipocytes92. These findings indicated RA as common dietary supplements help to counteract diet-induced obesity. Fenretinide (Table 4), a synthetic retinoid, was found to prevent obesity and insulin resistance in HFD-fed mice and completely suppress 3T3-L1 preadipocyte differentiation93. It is promising to apply RAR agonist to counteract diet-induced obesity. It has been reported that all-trans RA induced UCP1 expression in mouse white and brown adipocytes, but not in human adipocyte cell lines or primary human white adipocytes94. More studies on human adipocytes are needed to verify RAs as thermogenesis inducers.
4.4. Vitamin D receptor
The vitamin D receptor (VDR), a member of the steroid/thyroid/retinoid nuclear receptor superfamily, dimerizes with RXRα, and binds to VDR response elements (VDREs). It was reported that VDR is expressed in adipose tissue and dynamically up-regulated during adipocytes differentiation95., 96.. The hormonal form of vitamin D, 1,25-dihydroxy vitamin D3 (Table 4), suppressed the expression of UCP197, and vitamin D or VDR deficiency decreased adiposity and increased UCP1 in rodents98., 99., 100., 101.. In addition, adipocytes from humans with hereditary vitamin D resistant rickets showed increased UCP1 expression and a browning phenotype102. To be frustrated, there is no data reported about VDR antagonist on thermogenesis. On the other hand, vitamin D׳s positive effect on adipogenesis might be suitable for some special populations like cachexia patients.
4.5. Pregnane X receptor
The primary function of pregnane X receptor (PXR) is to sense the presence of xenobiotic substances and respond to detoxification and clearance of these substances from the body103. PXR activation is associated with thermogenesis104. Pregnenolone 16α-carbonitrile (Table 4) enhanced thermogenesis by induction the mRNA expression of Dio2, PGC-1α, PGC-1β and Cidea in BAT of mice105. However, there was no significant increase in UCP1 mRNA in adipocytes. The mechanism of PXR on thermogenesis is still unclear.
4.6. Estrogen receptors (ERs)
The rat, mouse and human ERs exist as two subtypes, ERα and ERβ106. A decade ago, 17β-estradiol was found to promote mitochondrial biogenesis and thermogenic function in primary brown adipocytes107. It is notable that 17β-estradiol negatively modulated the ATP synthase activity through direct binding to the oligomycin sensitive-conferring protein108, which may result in decreasing of mitochondrial ATP generation. ERs are highly expressed in the hypothalamus109 and estradiol upregulated BAT thermogenesis via hypothalamic AMPK110. In the brown fat, diethylstilbestrol increased the expression of Bmp8b (bone morphogenetic protein 8b) and FGF family genes involved in BAT activity111. A 3-day-treatment with a selective ERβ agonist, LY3201 (Table 4), induced browning of subcutaneous abdominal fat pad in obese female mice112. Consistently, acute 17β-estradiol or estradiol benzoate (Table 4) treatment led to thermogenesis in female sheep but not chronic estrogen treatment113. One possible explanation was that the distribution of ER subtypes varies by tissues and species, resulting in distinct response in different tissue contexts. Taken together, a stable estrogen level or ER agonist is essential to keep thermogenesis in BAT and energy homeostasis in female.
5. Miscellaneous
Some other small molecules have also been reported to induce thermogenesis (Table 5).
Table 5.
Miscellaneous small molecules inducing thermogenesis.
 |
Phosphodiesterases (PDEs) hydrolyze cGMP (cyclic guanosine monophosphate) and cAMP. Chronic treatment with sildenafil, a PDE-5 inhibitor, resulted in increased energy expenditure114. Surprisingly, the UCP1 level was significantly lower in BAT from sildenafil-treated mice114. While, short-term sildenafil treatment showed no change on UCP1 or PGC-1α levels in BAT; however, it caused an increase of UCP1 and PGC-1α expressions in WAT and browning features like appearance of multilocular adipocytes within WAT115. It suggested that beige cells are responsible for sildenafil induced thermogenesis.
FAS (fatty acid synthase) is a multi-enzyme protein that catalyzes fatty acid synthesis, especially the synthesis of palmitate. Inhibition of FAS by its inhibitor C75 activated sympathetic outflow and thermogenesis in BAT, indicating FAS might serve as a potential target of thermogenesis116.
Low dosage of trans-10, cis-12 conjugated linoleic acid (CLA) increased browning in overweight SV129 mice. CLA led to reduction in percentage of body fat, and increased UCP1 level and fatty acid oxidation117., 118. 12,13-Dihydroxy-9Z-octadecenoic acid (12,13-diHOME), a lipid to stimulate BAT activity, is negatively correlated with body-mass index. A study showed the injection of 12,13-diHOME activated BAT fuel uptake, enhanced cold tolerance and decreased levels of serum triglycerides through promoting the membrane translocation of the fatty acid transporters fatty acid transport protein 1 and CD36119. The identification of BAT-specific lipid utilization may spark potential way to unlock the maximum therapeutic potential of brown fat in humans. R-(+)-citronellal and β-citronellol from citronella oil, one of the most famous Indonesian essential oils, have ability to increase temperature and sympathetic nerve activity in BAT120. In another study, the major component of Zingiber zerumbet, zerumbone, was found to enhance sympathetic nerve activity and temperature in BAT121. Some oral anti-diabetic drugs showed thermogenic effect. Miglitol, α-glucosidase inhibitor, was able to increase energy expenditure by upregulating UCP1 in BAT. Miglitol has capability in enhancement of β3-adrenergic signaling122. However, it needs further investigation to fully elucidate the thermogenic effect of miglitol. Another study showed paradol analogues increased energy metabolism in the BAT via the activation of sympathetic nerve activity; and the length of the acyl chain of the paradol analogues had a significant impact on the extent of UCP1 expression level123. Using a transgenic animal model expressing luciferase to mimics endogenous UCP1 expression, a potential modulator WWL113 was discovered with capacity to increase UCP1 expression and thermogenic response without significant change in locomotor activity, food intake, or heartbeat124. Resveratrol increased energy expenditure and the expression of thermogenic markers through activating SIRT1 (silent mating type information regulation 2 homolog 1)125. However, another group reported resveratrol induced thermogenesis through SIRT1 independent pathway126. The role of SIRT1 in resveratrol induced thermogenesis remains elusive. Experiments using transgenic mice overexpressing UCP1 in metabolic tissues showed that locally uncoupling oxidative phosphorylation (OXPHOS) could combat obesity127. A novel chemical uncoupler, CZ5, was found to elevate energy expenditure without change UCP1 level128.
6. Conclusions
Direct ways to evaluate thermogenic capacity are mainly comprised of determination of expressions of thermogenic genes, oxygen consumption rate and mitochondrial function in brown adipocytes, as well as measurement of core temperature, locomotor activity, energy expenditure and sympathetic nerve activity in animals. Each the above method has limitation; and results from one or few assays might cause misleading. A systemic evaluation including in vitro and in vivo models should be carried out to authenticate thermogenic regulators.
UCP proteins are able to mediate directly adaptive non-shivering thermogenesis and metabolic inefficiency129., 130., 131.. Among them, UCP1 protein is the most important marker to predict thermogenesis capacity. At the mitochondrial inner membrane, the energy of nutrients such as glucose and lipids is converted into a proton gradient, but instead of storing the potential energy in the generation of ATP, UCP1 catalyzes an inducible proton leak to release the energy of the proton gradient directly as heat. It should be noted that UCP1 does not primarily evolve as an anti-obesity protein but as a means of quickly generating heat. Over-activated UCP1 posed a threat on thermogenic response when confronted with acute cold stimulation. Activation of UCP1 is not an automatic process and requires extra stimulation such as hormones, chemical agents, nutritional or even environmental factors. Therefore, additional variables including housing temperature, mouse strain and diet should be accurately controlled. No exact evidence showed there is a definite link between the expression of UCP1 and basal brown adipocyte metabolic rate. On the contrary, a previous work showed enhancement in UCP1 expression was accompanied with no difference in basal energy expenditure124. To analyze thermogenic capacity, measurements of UCP1 at both mRNA and protein levels with functional and metabolic assessments are necessary.
There are some indications of alternative uncoupling mechanisms besides UCP1, such as the creatine kinase cycle132 and calcium cycle133. UCP1 knockdown animals was found to be acclimated to cold temperature134 and WAT contributes to UCP1-independent thermogenesis135. Evidences have showed that beige cells have higher respiratory capacity than brown adipocytes, which are supposed to occupy high level of UCP19. In addition, beige cells express beige-selective marker, TMEM26 (transmembrane protein 26), CD137, and other thermogenic markers including mitochondrial genes Cox7a1 and Cox8b, transcriptional coregulatory PRDM16 and PGC-1β, and the thermogenic hormone FGF219. Using a brown adipocyte culture system, PPAR activation was found to represent a nonadrenergic, potent, and fully competent mechanism for BAT recruitment59. The complementary ways to increase energy expenditure in BAT remain to be unexplored.
The major brown fat deposits in adult humans are composed of beige adipocytes, which express distinct gene profiles9. It׳s also notable that classic brown fat exists in adult human, mainly distributes in the cervical, supraclavicular, axillary, and paravertebral regions; it may be involved in protecting the brain by warming up the blood supplied to the brain136. While, greater proportion of brown adipocytes and less proportion of beige cells exist in adult rodents. Most of the thermogenic regulators in previous studies were investigated on either in vitro brown adipocytes or in vivo murine models. It might explain why some thermogenic inducers did not show activity in humans. Human WAT derived beige cells should be recruited to screen thermoregulatory molecules and investigate underlying mechanisms. The content and function of brown adipocytes and the beige cells are declined with age, contributing to an obesity-prone character in aged objects10., 137.. The design of clinical trials in future need to include a broader age range, both genders, and diverse genetic or ethnic backgrounds to reveal important information for stratified therapeutic approaches.
Uncontrolled thermogenic treatments can produce excessive heat, promote cachexia, and muscle waste, similar to victims of severe burns and cancer138. Thus, developing pharmacological brakes for thermogenesis also has an important therapeutic value.
There are a constantly expanding numbers of regulatory nodes and pathways that integrate BAT function with physiological changes. Some endogenous molecules have been identified to control thermogenesis. The increasing levels of key endogenous molecules, such as irisin and FGF21, are associated with metabolically beneficial in obese states, which might be potential targets of some of the molecules described in this review. The metabolic changes in certain disease states are disproportionately inhibiting thermogenesis; thus, identifying the molecular pathways other than thermogenesis is likely to supply new therapeutic opportunities.
The allosteric regulation triggering the protein׳s functional activity via conformational changes is an intrinsic function of protein under many physiological and pathological conditions, including metabolism139., 140.. Protein-tyrosine phosphatase 1B (PTP1B) is the prototype for the superfamily of PTPs involving in regulation of insulin, leptin and adiponectin to govern food intake and energy metabolism141. Either a whole-body or whole-brain deletion of PTP1B causes lean, leptin-hypersensitive and resistant to HFD-induced obesity in mice142., 143.. PTP1B allosteric inhibitors prevent formation of the active form of PTP1B by blocking mobility of the catalytic loop, thereby exploiting a general mechanism used by tyrosine phosphatases144. However, it remains elusive how PTP1B allosteric inhibition regulates energy metabolism. Modern allosteric drug discovery faces considerable challenges; in particular, there is the vast majority of allosteric sites in proteins which are undiscovered145., 146.. Thus, the allosteric regulation might be a potential pathway for discovery of thermogenic regulators.
The key for the medicinal utilization of small molecules targeting thermogenesis is specificity and efficacy. The unique qualities of brown adipocytes with unique regulatory systems will help address the issue of specificity. It׳s more difficult to elucidate the complex central regulatory mechanisms that sense heat production and modulate sympathetic nervous stimulation of thermogenesis. There should be key nuclei that integrate information on temperature and energy availability, which might be specific targets to control BAT activation. The approaches of thermogenic regulation at multiple levels are likely to be the most effective.
Given the growing world-wide prevalence and increasing healthcare burden of obesity and associated diseases and the current lack of effective treatment strategies, new anti-obesity therapies are urgently needed. Emerging evidences have indicated BAT, mostly beige adipocytes, is present in human adults, and activation of BAT is inversely associated with obesity and metabolic disease. In either rodent models or clinical trials, several pharmacological approaches increasing thermogenic capacity have been proven to effectively prevent obesity, facilitate weight reduction, and ameliorate insulin resistance. Although, there are still many issues to be solved for the therapeutic agents targeting activation or expansion of BAT, including: 1) the effectiveness of a thermogenic enhancer in treating obesity and insulin resistance is still unstable; 2) compensating mechanisms, such as increased appetite, could reduce the benefits of this approach; 3) the risks of drugs in central nervous system and sympathetic nerve activation should also be considered. The pharmacological approaches targeting stimulation of BAT activity and increase of energy expenditure would provide exciting new options in obesity therapy.
Acknowledgments
Financial support by Science and Technology Development Fund, Macao SAR, China (FDCT 102/2017/A) and the Research Fund of University of Macau, China (MYRG2017-00109-ICMS) are gratefully acknowledged.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher E.J., LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovren F., Teoh H., Verma S. Obesity and atherosclerosis: mechanistic insights. Can J Cardiol. 2015;31:177–183. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Deng T., Lyon C.J., Bergin S., Caligiuri M.A., Hsueh W.A. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 5.Himbert C., Delphan M., Scherer D., Bowers L.W., Hursting S., Ulrich C.M. Signals from the adipose microenvironment and the obesity–cancer link—a systematic review. Cancer Prev Res. 2017;10:494–506. doi: 10.1158/1940-6207.CAPR-16-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J., Morley T.S., Kim M., Clegg D.J., Scherer P.E. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daneschvar H.L., Aronson M.D., Smetana G.W. FDA-approved anti-obesity drugs in the United States. Am J Med. 2016;129:879. doi: 10.1016/j.amjmed.2016.02.009. e1-6. [DOI] [PubMed] [Google Scholar]
- 8.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 9.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 12.Cypess A.M., Weiner L.S., Roberts-Toler C., Franquet Elia E., Kessler S.H., Kahn P.A. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atgie C., D׳Allaire F., Bukowiecki L.J. Role of β1- and β3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. Am J Physiol. 1997;273:C1136–C1142. doi: 10.1152/ajpcell.1997.273.4.C1136. [DOI] [PubMed] [Google Scholar]
- 14.Xiao C., Goldgof M., Gavrilova O., Reitman M.L. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 degrees C. Obesity. 2015;23:1450–1459. doi: 10.1002/oby.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weyer C., Tataranni P.A., Snitker S., Danforth E., Jr., Ravussin E. Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective β3-adrenoceptor agonist in humans. Diabetes. 1998;47:1555–1561. doi: 10.2337/diabetes.47.10.1555. [DOI] [PubMed] [Google Scholar]
- 16.Larsen T.M., Toubro S., van Baak M.A., Gottesdiener K.M., Larson P., Saris W.H. Effect of a 28-d treatment with L-796568, a novel β3-adrenergic receptor agonist, on energy expenditure and body composition in obese men. Am J Clin Nutr. 2002;76:780–788. doi: 10.1093/ajcn/76.4.780. [DOI] [PubMed] [Google Scholar]
- 17.van Baak M.A., Hul G.B., Toubro S., Astrup A., Gottesdiener K.M., DeSmet M. Acute effect of L-796568, a novel β3-adrenergic receptor agonist, on energy expenditure in obese men. Clin Pharmacol Ther. 2002;71:272–279. doi: 10.1067/mcp.2002.122527. [DOI] [PubMed] [Google Scholar]
- 18.Oliver P., Pico C., Martinez N., Bonet M.L., Palou A. In vivo effects of CGP-12177 on the expression of leptin and uncoupling protein genes in mouse brown and white adipose tissues. Int J Obes Relat Metab Disord. 2000;24:423–428. doi: 10.1038/sj.ijo.0801174. [DOI] [PubMed] [Google Scholar]
- 19.Pico C., Bonet M.L., Palou A. Stimulation of uncoupling protein synthesis in white adipose tissue of mice treated with the β3-adrenergic agonist CGP-12177. Cell Mol Life Sci. 1998;54:191–195. doi: 10.1007/s000180050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J., Golozoubova V., Cannon B., Nedergaard J. Arotinolol is a weak partial agonist on β3-adrenergic receptors in brown adipocytes. Can J Physiol Pharmacol. 2001;79:585–593. [PubMed] [Google Scholar]
- 21.Buemann B., Toubro S., Astrup A. Effects of the two β3-agonists, ZD7114 and ZD2079 on 24 h energy expenditure and respiratory quotient in obese subjects. Int J Obes Relat Metab Disord. 2000;24:1553–1560. doi: 10.1038/sj.ijo.0801452. [DOI] [PubMed] [Google Scholar]
- 22.Redman L.M., de Jonge L., Fang X., Gamlin B., Recker D., Greenway F.L. Lack of an effect of a novel β3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled randomized study. J Clin Endocrinol Metab. 2007;92:527–531. doi: 10.1210/jc.2006-1740. [DOI] [PubMed] [Google Scholar]
- 23.Schimmel R.J., McCarthy L. Role of adenosine as an endogenous regulator of respiration in hamster brown adipocytes. Am J Physiol. 1984;246:C301–C307. doi: 10.1152/ajpcell.1984.246.3.C301. [DOI] [PubMed] [Google Scholar]
- 24.Fain J.N., Pointer R.H., Ward W.F. Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem. 1972;247:6866–6872. [PubMed] [Google Scholar]
- 25.Gnad T., Scheibler S., von Kugelgen I., Scheele C., Kilic A., Glode A. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 26.Ding L., Yang L., Wang Z., Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135–144. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y., Liu H., Zhang M., Guo G.L. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B. 2016;6:409–412. doi: 10.1016/j.apsb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 29.Liaset B., Hao Q., Jorgensen H., Hallenborg P., Du Z.Y., Ma T. Nutritional regulation of bile acid metabolism is associated with improved pathological characteristics of the metabolic syndrome. J Biol Chem. 2011;286:28382–28395. doi: 10.1074/jbc.M111.234732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broeders E.P., Nascimento E.B., Havekes B., Brans B., Roumans K.H., Tailleux A. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab. 2015;22:418–426. doi: 10.1016/j.cmet.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Venkatachalam K., Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneshiro T., Saito M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr Opin Clin Nutr Metab Care. 2013;16:625–631. doi: 10.1097/MCO.0b013e3283653ee1. [DOI] [PubMed] [Google Scholar]
- 33.Snitker S., Fujishima Y., Shen H., Ott S., Pi-Sunyer X., Furuhata Y. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr. 2009;89:45–50. doi: 10.3945/ajcn.2008.26561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue N., Matsunaga Y., Satoh H., Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids) Biosci Biotechnol Biochem. 2007;71:380–389. doi: 10.1271/bbb.60341. [DOI] [PubMed] [Google Scholar]
- 35.Nirengi S., Homma T., Inoue N., Sato H., Yoneshiro T., Matsushita M. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy. J Biomed Opt. 2016;21:091305. doi: 10.1117/1.JBO.21.9.091305. [DOI] [PubMed] [Google Scholar]
- 36.Yoneshiro T., Aita S., Kawai Y., Iwanaga T., Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr. 2012;95:845–850. doi: 10.3945/ajcn.111.018606. [DOI] [PubMed] [Google Scholar]
- 37.Whiting S., Derbyshire E., Tiwari B.K. Capsaicinoids and capsinoids. a potential role for weight management? A systematic review of the evidence. Appetite. 2012;59:341–348. doi: 10.1016/j.appet.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Lejeune M.P., Kovacs E.M., Westerterp-Plantenga M.S. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr. 2003;90:651–659. doi: 10.1079/bjn2003938. [DOI] [PubMed] [Google Scholar]
- 39.Chen J., Li L., Li Y., Liang X., Sun Q., Yu H. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. 2015;14:22. doi: 10.1186/s12933-015-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki Y., Tamura Y., Inayoshi K., Narukawa M., Kobata K., Chiba H. TRPV1 agonist monoacylglycerol increases UCP1 content in brown adipose tissue and suppresses accumulation of visceral fat in mice fed a high-fat and high-sucrose diet. Biosci Biotechnol Biochem. 2011;75:904–909. doi: 10.1271/bbb.100850. [DOI] [PubMed] [Google Scholar]
- 41.Kim M., Furuzono T., Yamakuni K., Li Y., Kim Y.I., Takahashi H. 10-Oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017;31:5036–5048. doi: 10.1096/fj.201700151R. [DOI] [PubMed] [Google Scholar]
- 42.Hochkogler C.M., Lieder B., Rust P., Berry D., Meier S.M., Pignitter M. A 12-week intervention with nonivamide, a TRPV1 agonist, prevents a dietary-induced body fat gain and increases peripheral serotonin in moderately overweight subjects. Mol Nutr Food Res. 2017;61:1600731. doi: 10.1002/mnfr.201600731. [DOI] [PubMed] [Google Scholar]
- 43.Sun W., Uchida K., Suzuki Y., Zhou Y., Kim M., Takayama Y. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016;17:383–399. doi: 10.15252/embr.201540819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun W.P., Uchida K., Takahashi N., Iwata Y., Wakabayashi S., Goto T. Activation of TRPV2 negatively regulates the differentiation of mouse brown adipocytes. Pflugers Arch. 2016;468:1527–1540. doi: 10.1007/s00424-016-1846-1. [DOI] [PubMed] [Google Scholar]
- 45.Liedtke W., Choe Y., Marti-Renom M.A., Bell A.M., Denis C.S., Sali A. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye L., Kleiner S., Wu J., Sah R., Gupta R.K., Banks A.S. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vizin R.C., Scarpellini Cda S., Ishikawa D.T., Correa G.M., de Souza C.O., Gargaglioni L.H. TRPV4 activates autonomic and behavioural warmth-defence responses in Wistar rats. Acta Physiol. 2015;214:275–289. doi: 10.1111/apha.12477. [DOI] [PubMed] [Google Scholar]
- 48.Voets T., Owsianik G., Janssens A., Talavera K., Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- 49.Colburn R.W., Lubin M.L., Stone D.J., Jr., Wang Y., Lawrence D., D׳Andrea M.R. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Bautista D.M., Siemens J., Glazer J.M., Tsuruda P.R., Basbaum A.I., Stucky C.L. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 51.Masamoto Y., Kawabata F., Fushiki T. Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci Biotechnol Biochem. 2009;73:1021–1027. doi: 10.1271/bbb.80796. [DOI] [PubMed] [Google Scholar]
- 52.Ma S., Yu H., Zhao Z., Luo Z., Chen J., Ni Y. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. 2012;4:88–96. doi: 10.1093/jmcb/mjs001. [DOI] [PubMed] [Google Scholar]
- 53.Evans R.M., Mangelsdorf D.J. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Bilsen M., van der Vusse G.J., Gilde A.J., Lindhout M., van der Lee K.A. Peroxisome proliferator-activated receptors: lipid binding proteins controling gene expression. Mol Cell Biochem. 2002;239:131–138. [PubMed] [Google Scholar]
- 55.Roberts L.D., Murray A.J., Menassa D., Ashmore T., Nicholls A.W., Griffin J.L. The contrasting roles of PPARδ and PPARγ in regulating the metabolic switch between oxidation and storage of fats in white adipose tissue. Genome Biol. 2011;12:R75. doi: 10.1186/gb-2011-12-8-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castelein H., Gulick T., Declercq P.E., Mannaerts G.P., Moore D.D., Baes M.I. The peroxisome proliferator activated receptor regulates malic enzyme gene expression. J Biol Chem. 1994;269:26754–26758. [PubMed] [Google Scholar]
- 57.Wilson-Fritch L., Burkart A., Bell G., Mendelson K., Leszyk J., Nicoloro S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elabd C., Chiellini C., Carmona M., Galitzky J., Cochet O., Petersen R. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009;27:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 59.Petrovic N., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARγ agonist. Am J Physiol Endocrinol Metab. 2008;295:E287–E296. doi: 10.1152/ajpendo.00035.2008. [DOI] [PubMed] [Google Scholar]
- 60.Festuccia W.T., Blanchard P.G., Richard D., Deshaies Y. Basal adrenergic tone is required for maximal stimulation of rat brown adipose tissue UCP1 expression by chronic PPAR-γ activation. Am J Physiol Regul Integr Comp Physiol. 2010;299:R159–R167. doi: 10.1152/ajpregu.00821.2009. [DOI] [PubMed] [Google Scholar]
- 61.Festuccia W.T., Blanchard P.G., Oliveira T.B., Magdalon J., Paschoal V.A., Richard D. PPARγ activation attenuates cold-induced upregulation of thyroid status and brown adipose tissue PGC-1a and D2. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1277–R1285. doi: 10.1152/ajpregu.00299.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogacka I., Xie H., Bray G.A., Smith S.R. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 63.Chen H.Y., Liu Q., Salter A.M., Lomax M.A. Synergism between cAMP and PPARγ signalling in the initiation of UCP1 gene expression in HIB1B brown adipocytes. PPAR Res. 2013;2013:476049. doi: 10.1155/2013/476049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dutchak P.A., Katafuchi T., Bookout A.L., Choi J.H., Yu R.T., Mangelsdorf D.J. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z., Zhang H., Li B., Meng X., Wang J., Zhang Y. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 66.Rigano D., Sirignano C., Taglialatela-Scafati O. The potential of natural products for targeting PPARa. Acta Pharm Sin B. 2017;7:427–438. doi: 10.1016/j.apsb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barbera M.J., Schluter A., Pedraza N., Iglesias R., Villarroya F., Giralt M. Peroxisome proliferator-activated receptor a activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–1493. doi: 10.1074/jbc.M006246200. [DOI] [PubMed] [Google Scholar]
- 68.Lee J.Y., Hashizaki H., Goto T., Sakamoto T., Takahashi N., Kawada T. Activation of peroxisome proliferator-activated receptor-alpha enhances fatty acid oxidation in human adipocytes. Biochem Biophys Res Commun. 2011;407:818–822. doi: 10.1016/j.bbrc.2011.03.106. [DOI] [PubMed] [Google Scholar]
- 69.Ribet C., Montastier E., Valle C., Bezaire V., Mazzucotelli A., Mairal A. Peroxisome proliferator-activated receptor-alpha control of lipid and glucose metabolism in human white adipocytes. Endocrinology. 2010;151:123–133. doi: 10.1210/en.2009-0726. [DOI] [PubMed] [Google Scholar]
- 70.Hondares E., Rosell M., Diaz-Delfin J., Olmos Y., Monsalve M., Iglesias R. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARγ coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suarez J., Rivera P., Arrabal S., Crespillo A., Serrano A., Baixeras E. Oleoylethanolamide enhances β-adrenergic-mediated thermogenesis and white-to-brown adipocyte phenotype in epididymal white adipose tissue in rat. Dis Model Mec. 2014;7:129–141. doi: 10.1242/dmm.013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schug T.T., Berry D.C., Shaw N.S., Travis S.N., Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berry D.C., Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/δ and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juvet L.K., Andresen S.M., Schuster G.U., Dalen K.T., Tobin K.A., Hollung K. On the role of liver X receptors in lipid accumulation in adipocytes. Mol Endocrinol. 2003;17:172–182. doi: 10.1210/me.2001-0210. [DOI] [PubMed] [Google Scholar]
- 75.Seo J.B., Moon H.M., Kim W.S., Lee Y.S., Jeong H.W., Yoo E.J. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor γ expression. Mol Cell Biol. 2004;24:3430–3444. doi: 10.1128/MCB.24.8.3430-3444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hummasti S., Laffitte B.A., Watson M.A., Galardi C., Chao L.C., Ramamurthy L. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J Lipid Res. 2004;45:616–625. doi: 10.1194/jlr.M300312-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Zheng F., Zhang S., Lu W., Wu F., Yin X., Yu D. Regulation of insulin resistance and adiponectin signaling in adipose tissue by liver X receptor activation highlights a cross-talk with PPARγ. PLoS One. 2014;9:e101269. doi: 10.1371/journal.pone.0101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu M., Zhang Y., Liu C., Wang D., Feng L., Fan S. Morin, a novel liver X receptor alpha/β dual antagonist, has potent therapeutic efficacy for nonalcoholic fatty liver diseases. Br J Pharmacol. 2017;174:3032–3044. doi: 10.1111/bph.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shu L., Hoo R.L., Wu X., Pan Y., Lee I.P., Cheong L.Y. A-FABP mediates adaptive thermogenesis by promoting intracellular activation of thyroid hormones in brown adipocytes. Nat Commun. 2017;8:14147. doi: 10.1038/ncomms14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korach-Andre M., Archer A., Barros R.P., Parini P., Gustafsson J.A. Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc Natl Acad Sci U S A. 2011;108:403–408. doi: 10.1073/pnas.1017884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stenson B.M., Ryden M., Steffensen K.R., Wahlen K., Pettersson A.T., Jocken J.W. Activation of liver X receptor regulates substrate oxidation in white adipocytes. Endocrinology. 2009;150:4104–4113. doi: 10.1210/en.2009-0676. [DOI] [PubMed] [Google Scholar]
- 82.Dib L., Bugge A., Collins S. LXRalpha fuels fatty acid-stimulated oxygen consumption in white adipocytes. J Lipid Res. 2014;55:247–257. doi: 10.1194/jlr.M043422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheng X., Zhu X., Zhang Y., Cui G., Peng L., Lu X. Rhein protects against obesity and related metabolic disorders through liver X receptor-mediated uncoupling protein 1 upregulation in brown adipose tissue. Int J Biol Sci. 2012;8:1375–1384. doi: 10.7150/ijbs.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun H., Luo G., Chen D., Xiang Z. A comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front Pharmacol. 2016;7:247. doi: 10.3389/fphar.2016.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabelo R., Reyes C., Schifman A., Silva J.E. A complex retinoic acid response element in the uncoupling protein gene defines a novel role for retinoids in thermogenesis. Endocrinology. 1996;137:3488–3496. doi: 10.1210/endo.137.8.8754778. [DOI] [PubMed] [Google Scholar]
- 86.Alvarez R., de Andres J., Yubero P., Vinas O., Mampel T., Iglesias R. A novel regulatory pathway of brown fat thermogenesis. retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem. 1995;270:5666–5673. doi: 10.1074/jbc.270.10.5666. [DOI] [PubMed] [Google Scholar]
- 87.Puigserver P., Vazquez F., Bonet M.L., Pico C., Palou A. In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem J. 1996;317:827–833. doi: 10.1042/bj3170827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar M.V., Sunvold G.D., Scarpace P.J. Dietary vitamin A supplementation in rats: suppression of leptin and induction of UCP1 mRNA. J Lipid Res. 1999;40:824–829. [PubMed] [Google Scholar]
- 89.Bonet M.L., Oliver J., Pico C., Felipe F., Ribot J., Cinti S. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J Endocrinol. 2000;166:511–517. doi: 10.1677/joe.0.1660511. [DOI] [PubMed] [Google Scholar]
- 90.Alvarez R., Checa M., Brun S., Vinas O., Mampel T., Iglesias R. Both retinoic-acid-receptor- and retinoid-X-receptor-dependent signalling pathways mediate the induction of the brown-adipose-tissue-uncoupling-protein-1 gene by retinoids. Biochem J. 2000;345:91–97. [PMC free article] [PubMed] [Google Scholar]
- 91.Teruel T., Hernandez R., Benito M., Lorenzo M. Rosiglitazone and retinoic acid induce uncoupling protein-1 (UCP-1) in a p38 mitogen-activated protein kinase-dependent manner in fetal primary brown adipocytes. J Biol Chem. 2003;278:263–269. doi: 10.1074/jbc.M207200200. [DOI] [PubMed] [Google Scholar]
- 92.Berry D.C., DeSantis D., Soltanian H., Croniger C.M., Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61:1112–1121. doi: 10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McIlroy G.D., Tammireddy S.R., Maskrey B.H., Grant L., Doherty M.K., Watson D.G. Fenretinide mediated retinoic acid receptor signalling and inhibition of ceramide biosynthesis regulates adipogenesis, lipid accumulation, mitochondrial function and nutrient stress signalling in adipocytes and adipose tissue. Biochem Pharmacol. 2016;100:86–97. doi: 10.1016/j.bcp.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murholm M., Isidor M.S., Basse A.L., Winther S., Sorensen C., Skovgaard-Petersen J. Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. BMC Cell Biol. 2013;14:41. doi: 10.1186/1471-2121-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamei Y., Kawada T., Kazuki R., Ono T., Kato S., Sugimoto E. Vitamin D receptor gene expression is up-regulated by 1,25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 1993;193:948–955. doi: 10.1006/bbrc.1993.1717. [DOI] [PubMed] [Google Scholar]
- 96.Fu M., Sun T., Bookout A.L., Downes M., Yu R.T., Evans R.M. A nuclear receptor atlas: 3T3-l1 adipogenesis. Mol Endocrinol. 2005;19:2437–2450. doi: 10.1210/me.2004-0539. [DOI] [PubMed] [Google Scholar]
- 97.Ricciardi C.J., Bae J., Esposito D., Komarnytsky S., Hu P., Chen J. 1,25-Dihydroxyvitamin D3/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. Eur J Nutr. 2015;54:1001–1012. doi: 10.1007/s00394-014-0778-9. [DOI] [PubMed] [Google Scholar]
- 98.Narvaez C.J., Matthews D., Broun E., Chan M., Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150:651–661. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong K.E., Szeto F.L., Zhang W., Ye H., Kong J., Zhang Z. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296:E820–E828. doi: 10.1152/ajpendo.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong K.E., Kong J., Zhang W., Szeto F.L., Ye H., Deb D.K. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286:33804–33810. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhat M., Noolu B., Qadri S.S., Ismail A. Vitamin D deficiency decreases adiposity in rats and causes altered expression of uncoupling proteins and steroid receptor coactivator3. J Steroid Biochem Mol Biol. 2014;144:304–312. doi: 10.1016/j.jsbmb.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Malloy P.J., Feldman B.J. Cell-autonomous regulation of brown fat identity gene UCP1 by unliganded vitamin D receptor. Mol Endocrinol. 2013;27:1632–1642. doi: 10.1210/me.2013-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brewer C.T., Chen T. PXR variants: the impact on drug metabolism and therapeutic responses. Acta Pharm Sin B. 2016;6:441–449. doi: 10.1016/j.apsb.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He J., Gao J., Xu M., Ren S., Stefanovic-Racic M., O׳Doherty R.M. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes. 2013;62:1876–1887. doi: 10.2337/db12-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma Y., Liu D. Activation of pregnane X receptor by pregnenolone 16 alpha-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS One. 2012;7:e38734. doi: 10.1371/journal.pone.0038734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuiper G.G., Lemmen J.G., Carlsson B., Corton J.C., Safe S.H., van der Saag P.T. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez-Cuenca S., Monjo M., Gianotti M., Proenza A.M., Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17β-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab. 2007;292:E340–E346. doi: 10.1152/ajpendo.00175.2006. [DOI] [PubMed] [Google Scholar]
- 108.Moreno A.J., Moreira P.I., Custodio J.B., Santos M.S. Mechanism of inhibition of mitochondrial ATP synthase by 17β-estradiol. J Bioenerg Biomembr. 2013;45:261–270. doi: 10.1007/s10863-012-9497-1. [DOI] [PubMed] [Google Scholar]
- 109.Shughrue P.J., Lane M.V., Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 110.Martinez de Morentin P.B., Gonzalez-Garcia I., Martins L., Lage R., Fernandez-Mallo D., Martinez-Sanchez N. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grefhorst A., van den Beukel J.C., van Houten E.L., Steenbergen J., Visser J.A., Themmen A.P. Estrogens increase expression of bone morphogenetic protein 8b in brown adipose tissue of mice. Biol Sex Differ. 2015;6:7. doi: 10.1186/s13293-015-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miao Y.F., Su W., Dai Y.B., Wu W.F., Huang B., Barros R.P. An ERβ agonist induces browning of subcutaneous abdominal fat pad in obese female mice. Sci Rep. 2016;6:38579. doi: 10.1038/srep38579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clarke S.D., Clarke I.J., Rao A., Evans R.G., Henry B.A. Differential effects of acute and chronic estrogen treatment on thermogenic and metabolic pathways in ovariectomized sheep. Endocrinology. 2013;154:184–192. doi: 10.1210/en.2012-1758. [DOI] [PubMed] [Google Scholar]
- 114.Ayala J.E., Bracy D.P., Julien B.M., Rottman J.N., Fueger P.T., Wasserman D.H. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes. 2007;56:1025–1033. doi: 10.2337/db06-0883. [DOI] [PubMed] [Google Scholar]
- 115.Mitschke M.M., Hoffmann L.S., Gnad T., Scholz D., Kruithoff K., Mayer P. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 2013;27:1621–1630. doi: 10.1096/fj.12-221580. [DOI] [PubMed] [Google Scholar]
- 116.Cassolla P., Uchoa E.T., Mansur Machado F.S., Guimaraes J.B., Rissato Garofalo M.A., de Almeida Brito N. The central administration of C75, a fatty acid synthase inhibitor, activates sympathetic outflow and thermogenesis in interscapular brown adipose tissue. Pflugers Arch. 2013;465:1687–1699. doi: 10.1007/s00424-013-1301-5. [DOI] [PubMed] [Google Scholar]
- 117.Shen W., Chuang C.C., Martinez K., Reid T., Brown J.M., Xi L. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J Lipid Res. 2013;54:909–922. doi: 10.1194/jlr.M030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shen W., Baldwin J., Collins B., Hixson L., Lee K.T., Herberg T. Low level of trans-10, cis-12 conjugated linoleic acid decreases adiposity and increases browning independent of inflammatory signaling in overweight Sv129 mice. J Nutr Biochem. 2015;26:616–625. doi: 10.1016/j.jnutbio.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lynes M.D., Leiria L.O., Lundh M., Bartelt A., Shamsi F., Huang T.L. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med. 2017;23:631–637. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Batubara I., Suparto I.H., Sa׳diah S., Matsuoka R., Mitsunaga T. Effects of inhaled citronella oil and related compounds on rat body weight and brown adipose tissue sympathetic nerve. Nutrients. 2015;7:1859–1870. doi: 10.3390/nu7031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Batubara I., Suparto I.H., Sadiah S., Matsuoka R., Mitsunaga T. Effect of Zingiber zerumbet essential oils and zerumbone inhalation on body weight of Sprague Dawley rat. Pak J Biol Sci. 2013;16:1028–1033. doi: 10.3923/pjbs.2013.1028.1033. [DOI] [PubMed] [Google Scholar]
- 122.Sugimoto S., Nakajima H., Kodo K., Mori J., Matsuo K., Kosaka K. Miglitol increases energy expenditure by upregulating uncoupling protein 1 of brown adipose tissue and reduces obesity in dietary-induced obese mice. Nutr Metab. 2014;11:14. doi: 10.1186/1743-7075-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haratake A., Watase D., Setoguchi S., Terada K., Matsunaga K., Takata J. Relationship between the acyl chain length of paradol analogues and their antiobesity activity following oral ingestion. J Agric Food Chem. 2014;62:6166–6174. doi: 10.1021/jf500873a. [DOI] [PubMed] [Google Scholar]
- 124.Galmozzi A., Sonne S.B., Altshuler-Keylin S., Hasegawa Y., Shinoda K., Luijten I.H. ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep. 2014;9:1584–1593. doi: 10.1016/j.celrep.2014.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andrade J.M., Frade A.C., Guimaraes J.B., Freitas K.M., Lopes M.T., Guimaraes A.L. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014;53:1503–1510. doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 126.Alberdi G., Rodriguez V.M., Miranda J., Macarulla M.T., Churruca I., Portillo M.P. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141:1530–1535. doi: 10.1016/j.foodchem.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 127.Kopecky J., Clarke G., Enerback S., Spiegelman B., Kozak L.P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Investig. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fu Y.Y., Zhang M., Turner N., Zhang L.N., Dong T.C., Gu M. A novel chemical uncoupler ameliorates obesity and related phenotypes in mice with diet-induced obesity by modulating energy expenditure and food intake. Diabetologia. 2013;56:2297–2307. doi: 10.1007/s00125-013-2987-9. [DOI] [PubMed] [Google Scholar]
- 129.Affourtit C., Crichton P.G., Parker N., Brand M.D. Novel uncoupling proteins. Novartis Found Symp. 2007;287:70–91. [PubMed] [Google Scholar]
- 130.Echtay K.S. Mitochondrial uncoupling proteins—what is their physiological role? Free Radic Biol Med. 2007;43:1351–1371. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 131.Diano S., Horvath T.L. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18:52–58. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 132.Kazak L., Chouchani E.T., Jedrychowski M.P., Erickson B.K., Shinoda K., Cohen P. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ikeda K., Kang Q., Yoneshiro T., Camporez J.P., Maki H., Homma M. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23:1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ukropec J., Anunciado R.P., Ravussin Y., Hulver M.W., Kozak L.P. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1–/– mice. J Biol Chem. 2006;281:31894–31908. doi: 10.1074/jbc.M606114200. [DOI] [PubMed] [Google Scholar]
- 135.Granneman J.G., Burnazi M., Zhu Z., Schwamb L.A. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–E1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 136.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Investig. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abdullahi A., Jeschke M.G. White adipose tissue browning: a double-edged sword. Trends Endocrinol Metab. 2016;27:542–552. doi: 10.1016/j.tem.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu S., Jang H., Gu S., Zhang J., Nussinov R. Drugging Ras GTPase: a comprehensive mechanistic and signaling structural view. Chem Soc Rev. 2016;45:4929–4952. doi: 10.1039/c5cs00911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shen Q., Cheng F., Song H., Lu W., Zhao J., An X. Proteome-scale investigation of protein allosteric regulation perturbed by somatic mutations in 7000 cancer genomes. Am J Hum Genet. 2017;100:5–20. doi: 10.1016/j.ajhg.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsou R.C., Zimmer D.J., De Jonghe B.C., Bence K.K. Deficiency of PTP1B in leptin receptor-expressing neurons leads to decreased body weight and adiposity in mice. Endocrinology. 2012;153:4227–4237. doi: 10.1210/en.2012-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zabolotny J.M., Bence-Hanulec K.K., Stricker-Krongrad A., Haj F., Wang Y., Minokoshi Y. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 143.Bence K.K., Delibegovic M., Xue B., Gorgun C.Z., Hotamisligil G.S., Neel B.G. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 144.Li S., Zhang J., Lu S., Huang W., Geng L., Shen Q. The mechanism of allosteric inhibition of protein tyrosine phosphatase 1B. PLoS One. 2014;9:e97668. doi: 10.1371/journal.pone.0097668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang W., Wang G., Shen Q., Liu X., Lu S., Geng L. ASBench: benchmarking sets for allosteric discovery. Bioinformatics. 2015;31:2598–2600. doi: 10.1093/bioinformatics/btv169. [DOI] [PubMed] [Google Scholar]
- 146.Yang T., Xiao T., Sun Q., Wang K. The current agonists and positive allosteric modulators of alpha7 nAChR for CNS indications in clinical trials. Acta Pharm Sin B. 2017;7:611–622. doi: 10.1016/j.apsb.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]