Abstract
Background:
To investigate whether human adipose-derived stem cells (hADSCs) seeded on multilayered poly (l-lactide-co-ɛ-caprolactone) (PLCL) sheets improve bladder function in a rat model of detrusor smooth muscle-removed bladder.
Methods:
Male rats were randomly divided into 4 groups: Normal, injury (detrusor smooth muscle-removed bladder), PLCL (detrusor smooth muscle-removed bladder implanted with PLCL sheets), and PLCL + ADSC (detrusor smooth muscle-removed bladder implanted with PLCL sheets seeded with hADSCs). Four weeks after the treatment, physiological, histological, immunohistochemical, and immunoblot analyses were performed.
Results:
hADSCs were compatible with PLCL sheets. Further, the physiological study of PLCL + ADSC group showed significant improvement in compliance and contractility suggesting the functional improvement of the bladder. Histological, immunohistochemical and immunoblot analyses revealed the uniform distribution of hADSCs in between PLCL sheets as well as differentiation of hADSCs into smooth muscle cells (SMC) which is illustrated by the expression of SMC markers.
Conclusion:
hADSCs seeded on the multilayered PLCL sheets has the potential to differentiate into SMC, thus facilitating the recovery of compliance and contractility of the injured bladder.
Keywords: Urinary bladder, Smooth muscle, Tissue engineering, Stem cells, Compliance
Introduction
Recent data estimated that the new cases of urinary bladder cancer were 7% and bladder cancer-related deaths were 4% out of all cancer [1]. The prevalence of Detrusor underactivity (DU) is reported to be 9–48% [2]. Moreover, bladder diseases such as neurogenic bladder (NGB), neural defect, and trauma induce bladder smooth muscle degeneration [3]. So, these conditions need to be treated to replace or repair the injured site of the bladder. Neobladder or bladder augmentation cystoplasty using a gastrointestinal segment is one of the treatments performed in current clinical practice [4]. However, it leads to complications like stone formation, infection, metabolic abnormalities etc. [5]. Progress in tissue engineering and regenerative medicine has enabled researchers to develop technology leading to the construction of various bladder tissues [6]. Moreover, synthetic, semi-synthetic, or natural scaffolds have been used alone or with cells for bladder tissue engineering [7]. However, these scaffolds cause problem like fibrosis, calcification, and none of them have met the necessary expectations [8].
The scaffolds for bladder tissue engineering should be elastic and strong enough to resist the repeated contraction and relaxation [9]. One of the difficulties of bladder tissue engineering is the absence of a biomaterial with an ideal thickness that serves as a suitable scaffold. Due to the lack of vascular supplies in the inner regions of the thick scaffolds, seeded cells do not survive. On the other hand, thin scaffolds are unable to withstand the bladder pressure [10]. Therefore, we synthesized poly (l-lactide-co-ɛ-caprolactone) (PLCL) sheets of ideal thickness that are biocompatible and highly elastic with minimal toxicity [11]. The other limitation is the ongoing debate on the best source of smooth muscle cells (SMC). Autologous cells might not be available in diseased patients whereas cells from the allogeneic source may lead to immunorejection. So, the adult stem cells are the best alternative [12]. Human adipose-derived stem cells (hADSCs) are multipotent stem cells, with less immunogenicity that differentiates into SMC and thus can have various clinical applications [13].
Here, we hypothesized that hADSCs contribute to repair and regeneration of injured bladder. To demonstrate this, hADSCs were distributed in between the layers of PLCL sheets and implanted into detrusor smooth muscle removed (DSMR) bladder. The improvement in bladder function, as well as expression of SMC markers, demonstrates that hADSCs seeded on PLCL sheets have the potential to recover injured bladder functions.
Materials and methods
Fabrication of PLCL sheets
PLCL sheets were fabricated as described earlier [14, 15]. Briefly, PLCL was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (Sigma-Aldrich, St. Louis, MO, USA). This solution was delivered at a flow rate of 1 ml/h. Electrospun sheets were collected onto a grounded stainless steel plate followed by vacuum drying. Next, the sheets were coated with collagen, type I (Advanced Biomatrix, Carlsbad, CA, USA), cut into 1 cm2 dimension and sterilized by soaking in 70% ethanol under ultraviolet light.
Isolation and characterization of human adipose-derived stem cells
Human adipose-derived stem cells (hADSCs) were isolated according to the methods previously described by Ra et al. [16]. Briefly, adipose tissue samples were collected with the subject’s consent by simple liposuction from abdominal subcutaneous fat and were digested with collagenase I under gentle agitation for 60 min at 37 °C. The digested tissues were filtered to remove debris and were centrifuged to obtain a pellet. Next, the pellet was resuspended in the Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA)-based media containing 0.2 mM ascorbic acid and 10% fetal bovine serum (FBS). The cell suspension was recentrifuged at 470g for 5 min. The pellet was cultured overnight at 37 °C, 5% CO2 in DMEM based media. After 24 h non-adherent cells were discarded, and adherent cells were cultured in keratinocyte-SFM (Invitrogen)-based media containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/mL epidermal growth factor, and 5% FBS. The cells were maintained for 4–5 days until the cells reached 80% confluency, and they were subcultured in keratinocyte-SFM-based media. hADSCs passages 3 were used for our experiments. hADSCs were directly obtained from K-Stem Cell Co. Ltd., Seoul, Korea (former RNL Bio. Co. Ltd, Seoul, Korea) and the company was collaborator with our group. The procedure for hADSCs preparation was performed under good manufacturing practice (GMP) conditions as per the guidelines.
The characterization and multilineage differentiation of hADSCs were already evaluated by our collaborator as described [16]. Briefly, for immunophenotypic characterization of hADSCs, the surface protein of hADSCs passage 3 was examined by flow cytometry. hADSCs exhibited presence of protein expression for CD29, CD44, CD73, CD90, CD105 and HLA ABC and the absence of hematopoietic and endothelial antigens namely CD31, CD34, CD45 and HLA-DR. Further, to assess the multilineage potential of hADSCs, the cells were cultured into specific differentiation media according to the manufacturer’s instruction. hADSCs in vitro differentiated into adipocytes, osteoblasts, and chondroblasts, assessed by positive Oil Red O staining, Alizarin Red staining, and toluidine blue O staining, respectively.
SEM of multilayered PLCL sheets seeded with hADSCs
The surface morphology of PLCL sheets were analyzed by Scanning electron microscope (SEM, JSM 5410 LV, Jeol, Tokyo, Japan). The multilayered PLCL sheets with or without hADSCs were fixed with 10% formaldehyde for 24 h and sputter-coated with gold in the presence of argon gas at room temperature. Images were taken using SeMAfore software (Jeol, Tokyo, Japan).
Determination of hADSCs viability with PLCL
To evaluate the cell viability, PLCL sheets (1 cm × 1 cm) were transferred into 12-well plates, onto which hADSCs were seeded at a density of 2 × 105 cells per sheet. hADSCs (P3) were cultured in Dulbecco’s modified eagle’s medium (DMEM) with 10% FBS and 1% Penicillin/Streptomycin for 3 days. Cell viability was quantified using resazurin assay (Prestoblue® Cell Viability, Invitrogen, Carlsbad, CA, USA). This kit uses a dye to measure oxidation reduction reactions that principally occurs in the mitochondria of live cells. The dye changes color from dark blue to pink (with absorbance at 570 nm) when reduced by the metabolically active cells. Cell viability was measured at 570 nm and 600 nm using ELISA Reader (Power WaveXS, BioTek Instruments, Winooski, VT, USA) at different time points.
In vivo study
Ten-week-old male Sprague–Dawley rats weighing 250–300 g were obtained from Orient Bio Co., Seongnam, Korea and divided into four groups (n = 7 per group): Normal (Sham operation), Injury (Detrusor smooth muscle-removed (DSMR) bladder), PLCL (DSMR bladder implanted with multilayered PLCL sheets), and PLCL + ADSC (DSMR bladder implanted with multilayered PLCL sheets seeded with hADSCs). All animal experiments were approved by Institutional Animal Care and Use Committee, The Catholic University of Korea, Seoul, Korea (IACUC approval No. 2013-0111-03). All rats were maintained in a temperature and humidity controlled room on a 12 h light/12 h dark cycle during 4 weeks’ period.
DSMR rat model
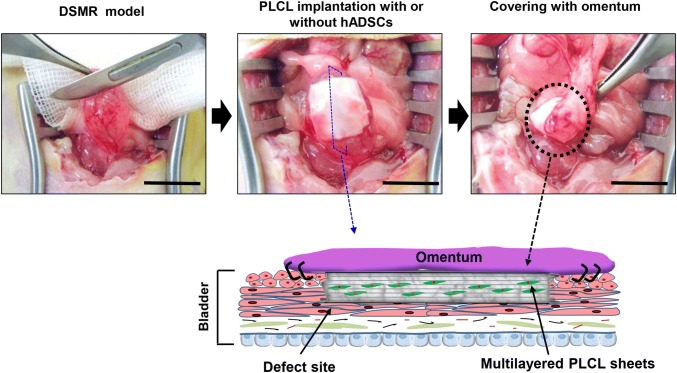
The rats were anesthetized with intraperitoneal injection of (Tiletamine + Zolazepam) 30 mg/kg and Xylazine, 10 mg/kg followed by midline lower abdominal incision. Then the bladder was partially emptied by manual pressure on the bladder. Next, an area of about 1 cm2 of the anterior region of the bladder, below the dome, was scraped using a surgical blade for removing the serosa and smooth muscle layers to develop a DSMR model (Fig. 1A). Finally, the omentum was sutured to the bladder and the abdominal incisions were closed.
Fig. 1.
Schematic diagram showing detrusor smooth muscle-removed (DSMR) bladder model and quantification of the bladder wall in a rat model. A Graphical image showing the development of DSMR rat model. UB urinary bladder, Pro prostate. The anterior region of the bladder, below the dome, was scraped using a surgical blade for removing the serosa and smooth muscle layers to develop a DSMR model. B Hematoxylin and eosin (H&E) and Masson’s trichrome (MT) staining of the bladder showing the normal and injured (DSMR) region. The injured region of the bladder is illustrated by red arrows. Scale bar: 200 µm. C The normal and injured bladder wall thickness was quantified using ImageJ software (NIH, Bethesda, USA). The data represents mean ± SD (n = 3). Asterisk indicates t test p < 0.05 versus each group
Multilayered PLCL sheets with or without hADSCs implanted onto DSMR bladder
For PLCL group, a 1 cm × 1 cm PLCL sheet was placed over the injured region in the DSMR bladder. The fluid present on the bladder surface helped to attach the sheet to its surface. Then, another sheet was placed over the first and this process was repeated until five PLCL sheets were stacked. In the PLCL + ADSC group, similar procedures were performed except PKH26 labeled 2 × 105 hADSCs/sheet were sandwiched between PLCL sheets. A red fluorescent dye was used as a cell tracker according to the manufacturer’s protocol (PKH26 Red Fluorescent Cell Linker Kit, Sigma-Aldrich, St. Louis, MO, USA). Further steps were performed as described for the DSMR model.
Physiological analysis
Bladder compliance and contractility measurements were performed 4 weeks after the treatment. Bladder compliance was measured as previously described and calculated as the ratio of the maximum bladder capacity to the pressure that triggered voiding minus resting bladder pressure [6].
For contractility studies, the injured bladder section implanted with PLCL with/without hADSCs was harvested. The contractility was evaluated as described earlier and expressed as g tension/g tissue [17]. For both studies, data were collected with a Bridge Amp and a Power Lab 4/30 and analyzed with Lab Chart 5 (AD Instruments, Bella Vista, New South Wales, Australia).
Histological and IHC analyses
Four weeks after the treatment, the bladders were harvested and embedded in paraffin. For histological evaluations, deparaffinized sections were stained with Hematoxylin and eosin (H&E) and Masson’s trichrome (MT) (Sigma-Aldrich, St. Louis, MO, USA) as previously explained [18]. Tissue sections were examined under a light microscope (Olympus, Tokyo, Japan). Next, the immunohistochemistry (IHC) was performed according to a standard protocol [18]. In brief, the sections were incubated overnight at 4 °C with primary antibodies desmin (MAB3430) (1:100, Millipore, CA, USA), α-smooth muscle actin (α-SMA (ab7817)), calponin (ab46794), Smooth muscle myosin heavy chain (SMMHC (ab53219)) (1:500, Abcam, Cambridge, UK) followed by incubation with secondary antibody (1:500, Alexa Fluor®488 IgG, Invitrogen, Eugene, OR, USA) for 1 h at room temperature. Nuclei were stained with DAPI (Vector Labs, Burlingame, CA, USA). Digital images were obtained using a confocal microscope (LSM510 microscope, Zeiss, Jena, Germany) and quantified using Zen software (Zeiss, Jena, Germany).
Immunoblot analysis
Immunoblot analysis was performed as previously described [19]. Briefly, the membrane was blocked and incubated overnight with primary antibodies: α-SMA, calponin, and Transforming growth factor β1 (TGFβ1) (ab92486) ((1:1000); Abcam, Cambridge, UK), followed by incubation with secondary antibody (1:5000, Gendepot, TX, USA) for 1 h at room temperature. GAPDH (ab8245) ((1:1000); Abcam, Cambridge, UK) was used as housekeeping gene. The image was developed using an enhanced chemiluminescence kit using Luminescent Image Analyzer LAS 3000 and Multi Gauge Ver3.0 software (Fujifilm, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). All measurements are expressed as the mean ± SD. Data were analyzed using the Student’s t test or ANOVA wherever appropriate.
Results
Characterization of DSMR model
After the DSMR model was prepared, histological differences of the bladder layer were examined (Fig. 1). The injured region of the bladder was confirmed by H&E and MT staining. The uninjured bladder layer has a longitudinally oriented thick layer of smooth muscle, whereas the injured layer has been severely thin (indicated by red arrows). Based on these histological results, the normal and injured bladder wall thicknesses were 625.93 ± 21.45 µm and 118.4 ± 28.86 µm, respectively, giving the difference of 400–500 µm. as measured by ImageJ software (Fig. 1C). Thus, it can be estimated that five PLCL sheets with seeded hADSCs could be suitable to fill the defect site of the injured bladder.
Surface morphology and cell viability of multilayered PLCL sheets
It was observed that, PLCL electrospun sheets have random fiber morphology with nanoscale diameters that were similar to the native extracellular matrix (ECM) structure (Fig. 2A). Next, hADSCs was seeded between the layers of five PLCL sheets (Fig. 2B). hADSCs were adhered and spreaded on the surface of PLCL sheets after 3 days of culture. SEM images confirmed that hADSCs were proliferated between the layers. In addition, the viability of hADSCs seeded on multilayered PLCL sheets showed hADSCs were viable in both culture dish and PLCL sheets (Fig. 2C). This indicated PLCL sheet with seeded hADSCs were compatible showing no toxicity.
Fig. 2.
PLCL electrospinning and cellular behavior of hADSCs culture on multilayered PLCL sheets. A Schematic diagram showing the electrospinning PLCL sheets. Scale bar: 10 µm. B Stacking of PLCL sheets seeded with hADSCs. Below is the SEM micrographs showing the surface morphology of the PLCL nanofiber sheet and hADSCs (indicated by arrows) seeded PLCL multilayered sheets. Scale bar: 50 µm (left image) and 200 µm (right image). C The viability of hADSCs on a culture dish and multilayered PLCL sheets were measured by resazurin assay at different time points. hADSCs human adipose-derived stem cells, PLCL poly (l-lactide-co-ɛ-caprolactone)
hADSCs seeded on scaffold improved the bladder performance
Since we found hADSCs were compatible with PLCL, next we implanted this integrated system onto the DSMR bladder (Fig. 3). After implantation, the omentum was covered to supply blood vessels into the defect site. In addition, the omentum also prevents scaffold movement from the implanted region to the elsewhere. The compliance of the Normal, Injury, PLCL and PLCL + ADSC groups were 0.082 ± 0.006, 0.031 ± 0.052, 0.035 ± 0.001, and 0.069 ± 0.011 ml/cm H2O respectively. The Injury group displayed a significant decrease in compliance indicating that injury had deteriorated bladder performance. There was no statistical difference between the compliance of the Injury and PLCL group. However, compliance of the PLCL + ADSC group was significantly increased (84.26% vs 37.54% and 42.04%, p < 0.01) as compared with the Injury and PLCL group (p < 0.01) (Fig. 4A).
Fig. 3.
In vivo experiment. The bladder was injured using surgical scalpel to create DSMR model. Multilayered PLCL sheets with or without hADSCs were placed over the injured bladder followed by suturing and finally draped with the omentum (as illustrated by dotted circle). Schematic diagram illustrating the arrangement of PLCL and hADSCs over the DSMR bladder and covering of the graft by omentum. Scale bar: 1 cm
Fig. 4.
hADSCs seeded on PLCL improved the physiological function of the injured bladder. The physiological studies were performed after 4 weeks of implantation. The bladder section containing both the injured native bladder and implanted PLCL with/without hADSCs (grafted portion) was used. A Compliance was significantly improved in the PLCL + ADSC group. Compliance of Normal group is set at 100% and each group’s corresponding compliance values are expressed as a percentage of compliance value of the Normal group. B Contractility was also significantly improved in the PLCL + ADSC group. The data represents mean ± SD *(p < 0.05), ** (p < 0.01) and ***(p < 0.005) indicated the statistically significant difference (1-way ANOVA followed by Tukey post hoc test). C The presence of cells in between the multilayered PLCL sheets in the PLCL + ADSC group MT staining of the entire treatment group. A pound sign (#) indicates the PLCL sheets. D H&E staining showed the presence of cells in the PLCL + ADSC group. Scale bar: 250 µm. (Normal (Sham operation), Injury (DSMR bladder), PLCL (DSMR bladder implanted with multilayered PLCL sheets), and PLCL + ADSC (DSMR bladder implanted with multilayered PLCL sheets seeded with hADSCs))
Next, the bladder section containing both the native injured bladder and implanted PLCL with/without hADSCs were analyzed for smooth muscle contractility. The contractile response of the Normal, Injury, PLCL and PLCL + ADSC groups were 8.245 ± 0.220, 2.904 ± 0.514, 3.463 ± 0.314 and 5.302 ± 0.148 g tension/g weight respectively. The contractility of the PLCL + ADSC group was significantly higher compared to that of the Injury and PLCL group (p < 0.01 and p < 0.05) There was no statistical difference between the contractility of the Injury and PLCL groups (Fig. 4B).
hADSCs were evenly distributed on PLCL sheets and expressed SMC markers
For all the analysis, bladder section containing both the injured native bladder and implanted PLCL with/without hADSCs was used. The presence of cells in the treatment groups was determined by histological staining. MT staining showed the smooth muscle content of the bladder wall was reduced in the Injury, PLCL and PLCL + ADSC groups compared to the Normal group. However, in the PLCL + ADSC group, there was the presence of cells in between PLCL sheets (Fig. 4C). Furthermore, for clear inspection, we used the same section for H&E staining, which showed the even distribution of cells in between the layers of PLCL sheets of the PLCL + ADSC group (Fig. 4D).
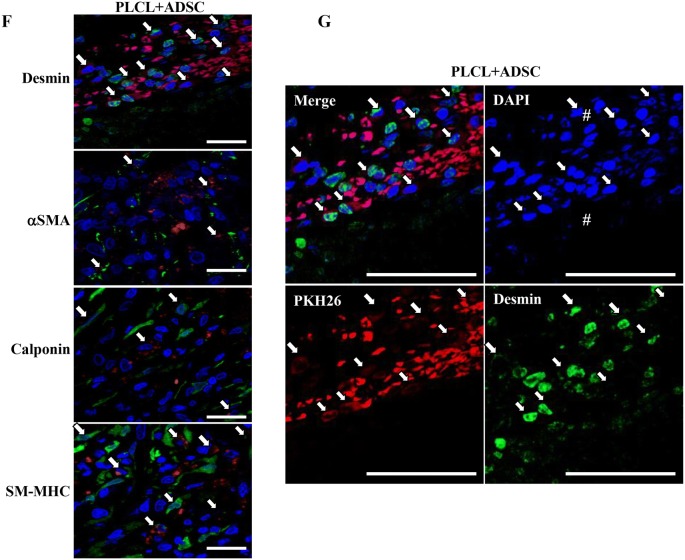
Further, to determine that the proliferated cells are the hADSCs differentiated into SMC, we performed IHC and immunoblot analysis using SMC markers. IHC analysis of early, mid, and late markers of SMC namely α-SMA, Calponin, and SM-MHC were performed. IHC analysis showed higher expression of SMC-specific markers in the Normal group (Mean fluorescence intensity (MFI) of Desmin: 8046.2 ± 708.8; α-SMA: 12,655.0 ± 1774.3; calponin: 9238.0 ± 1138.1 and SM-MHC: 9928.2 ± 1219.9 Arbitrary unit (AU)), whereas expression was remarkably decreased in the Injury group (MFI of Desmin: 362.2 ± 47.94; α-SMA: 196.5 ± 49.06; calponin: 244.6 ± 36.23; and SM-MHC: 156.6 ± 30.08 AU). There was no significant difference between the Injury and PLCL group (MFI of Desmin: 1279.0 ± 760.1; α-SMA: 185.4 ± 445.0; calponin: 311.6 ± 97.5; and SM-MHC: 285.6 ± 53.5 AU). Interestingly, the PLCL + ADSC group (MFI of Desmin: 3270.0 ± 286.4; α-SMA: 2359 ± 273.1; calponin: 2061.4 ± 393.1; and SM-MHC: 3217.6 ± 441.9 AU) showed significantly upregulated expression of SMC markers compared to the Injury and PLCL group. Moreover, the presence of PKH26 labeled hADSCs, which were co-stained with green fluorescence of SMC markers, indicated that seeded hADSCs had differentiated into SMC (Fig. 5A–E). In addition, there was the presence of PKH26 negative SMC signals. High magnification images of desmin, α-SMA, calponin and SM-MHC positive hADSCs which are colocalized with PKH26 labeled is illustrated (Fig. 5F). Co-labeling of desmin expressing cells with PKH26 labeled hADSCs is shown at higher magnification in all the different channels (Fig. 5G).
Fig. 5.
hADSCs seeded in between PLCL sheet differentiated into smooth muscle cells (SMC). The bladder section containing both the injured native bladder and implanted PLCL with/without hADSCs (grafted portion) was used. A Immunohistochemistry analysis shows positive SMC-specific protein markers such as desmin, α-SMA, calponin, and SM-MHC (1st, 2nd, 3rd, and 4th rows, respectively). Nuclei were stained with 4,6-diamidino-2-phenyl-indole (DAPI) (blue fluorescence) and red fluorescence was emitted by PKH26 labeled hADSCs. In the PLCL + ADSC group, the arrows indicate desmin, α-SMA, calponin and SM-MHC positive hADSCs which are PKH26 labeled (4th column). Scale bar: 50 µm. A pound sign (#) represent the PLCL sheets. B–E Mean fluorescence intensity of desmin, α-SMA, calponin, and SM-MHC respectively in all the groups is illustrated. The data represents mean ± SD. *(p < 0.05), **(p < 0.01) and ***(p < 0.005) indicated the statistically significant difference (1-way ANOVA followed by Tukey post hoc test). (Normal (Sham operation), Injury (DSMR bladder), PLCL (DSMR bladder implanted with multilayered PLCL sheets), and PLCL + ADSC (DSMR bladder implanted with multilayered PLCL sheets seeded with hADSCs)). F High magnification IHC images of desmin, α-SMA, calponin and SM-MHC positive hADSCs which are PKH26 labeled Scale bar: 20 µm. G High magnification inset of desmin expressing cells. The bladder section containing both the injured native bladder and implanted PLCL with/without hADSCs (grafted portion) was used. IHC staining of PKH26 labeled hADSCs for desmin in DSMR bladder. DAPI was used to stain the nuclei (blue fluorescence) and red fluorescence was emitted by PKH26 labeled hADSCs. Merged image shows seeded hADSCs differentiated into SMC. The arrows indicate desmin positive hADSCs. A pound sign (#) represents the PLCL sheets. Scale bar: 50 µm
The IHC data were further supported by immunoblot analysis which showed significantly higher expression of SMC markers in the PLCL + ADSC group (PI: α-SMA: 1.081 ± 0.044, calponin: 0.619 ± 0.026 and TGF β1: 0.590 ± 0.028 Densitometry unit (DU)%, compared to the Injury and PLCL group (p < 0.05). There was no significant difference between the Injury (PI: α-SMA: 0.558 ± 0.062, calponin: 0.505 ± 0.058 and TGF β1: 0.185 ± 0.021 DU), and PLCL group (PI: α-SMA: 0.793 ± 0.160, calponin: 0.504 ± 0.012 and TGF β1: 0.165 ± 0.007 DU). The expression of α-SMA and calponin was increased only in the lesional region of the PLCL + ADSC group whereas expression in the non-lesional region in all group remained unchanged. In addition, hADSCs differentiated into SMC via the TGFβ1-Smad pathway [20]. We found the TGFβ1 expression was increased only in the lesional region of the PLCL + ADSC group compared to other groups whereas expression in the non-lesional region in all group remained unchanged (Fig. 6).
Fig. 6.
Expression of α-SMA, calponin, and TGFβ1 increased only in the lesional region of the bladder. The bladder section containing both the injured native bladder and implanted PLCL with/without hADSCs (grafted portion) was used. A Immunoblot analysis. NL non-lesional region of the bladder and L lesional region of the bladder. B–D The relative band density of α-SMA, calponin, and TGF 1 respectively, in the lesional region from all the four groups is illustrated. The bands were quantified using ImageJ software (NIH, Bethesda, USA) and normalized to GAPDH. DU: Densitometry unit. The data shows mean ± SD. *(p < 0.05), **(p < 0.01) and ***(p < 0.005) indicated the statistically significant difference (1-way ANOVA followed by Tukey post hoc test). (Normal (Sham operation), Injury (DSMR bladder), PLCL (DSMR bladder implanted with multilayered PLCL sheets), and PLCL + ADSC (DSMR bladder implanted with multilayered PLCL sheets seeded with hADSCs))
Discussion
The bladder tissue cannot be easily substituted due to the difficulty in mimicking natural elasticity and urothelial permeability [21]. The bladder tissue engineering has focused on scaffolds with or without cells to support tissue formation [22]. Direct injection of the cell without scaffolds shows difficulty in controlling the localization of transplanted cells [21]. Usually, in defect area, bladder acellular matrix (BAM) have been shown to encourage ingrowth of endogenous uroepithelial cells, SMC, endothelial cells, however, smooth muscle regeneration and neovascularization of the graft were limited and disorganized leading to bladder fibrosis [23, 24]. However, the result of bladder repair was positive in short term, but on increasing the time frame to 22 weeks, shrinkage rate of BAM was very high to 48% [25]. Similarly, Small intestinal submucosa (SIS) induces immune reactions as foreign DNA was detected. Furthermore, BAM and SIS both possess batch to batch variations due to different processing techniques leading to unstable result after the implantation [26]. Electrospun nanofibers are attractive due to their resemblance to the structural dimensions of the native Extracellular matrix (ECM) [27]. Nanofibers like poly (glycolic acid) and poly (lactide-co-glycolide) could provide a sufficient space for seeding cells but they have the limitation of urine leakage directly into smooth muscle layer [28]. Poly (ε-caprolactone) was not a promising scaffold for bladder regeneration due to its poor flexibility [18]. To overcome various limitations of the scaffold in prior studies, we investigated the potential use of multilayered scaffolds.
We aimed to determine whether hADSCs seeded on PLCL sheets implanted onto DSMR bladder promotes hADSCs differentiation into SMC, thus, improving bladder function. PLCL has been used in a number of medical applications, including scaffolds for tissue engineering [29]. Also, it promotes cellular interactions and has biodegradable properties with minimal toxicity and has been reported in urology tissue engineering [11, 30]. We used hADSCs, as it has been reported to contribute to spontaneous myotube formation and myogenic differentiation [28]. Furthermore, hADSCs may have antioxidant properties; secrete multiple angiogenic and anti-apoptotic cytokines, thus supporting tissue regeneration [31]. We used allogeneic hADSCs in the rats because they possess less immunogenicity and have immunosuppressive properties [32].
The cell viability showed that PLCL is biocompatible with hADSCs. Compliance is important for bladder reconstruction since the increase in compliance is desired in patients suffering from NGB or extrophy [7]. There is a significant increase in compliance in the PLCL + ADSC group. Similarly, contractility of smooth muscle is a significant factor for voiding function in the bladder. After the injury, the detrusor muscle is replaced with fibrous tissue which lacks elasticity, thus causing loss of contractility. The improved compliance and contractility may be due to the elastic nature of PLCL, proliferation and differentiation of seeded hADSCs into SMC and other proteins that are related with contractility.
The histological studies showed the presence of cells in between the multilayered scaffold implanted over the DSMR bladder in the PLCL + ADSC group. Next, the IHC and immunoblot analyses revealed that hADSCs were differentiated to SMC significantly in the PLCL + ADSC group. In IHC analysis, the expression of SM-MHC, the late marker of SMC, also convince transdifferentiation of hADSCs. Moreover, the IHC analysis of other SMC markers also showed that the PKH26 labeled hADSCs had been differentiated into SMC. However, there was the presence of PKH26 unlabeled SMC signals, which could be due to the transdifferentiation of surrounding resident cells, or migration of neighboring host SMC to the injured sites. In addition, hADSCs secrete growth factors that induce the migration of host cells into the scaffolds, influences tissue microenvironments by creating suitable conditions which enhance tissue regeneration, cell survival, and endogenous repair [33].
The advantage of the present model is their reproducibility and the safe implantation of the scaffolds. The multilayered arrangement of PLCL has high surface area to volume ratio, which provides more area for cell attachment. This approach could be useful in the treatment of bladder diseases like NGB and DU. The future research should focus on examining the effect of hADSCs seeded on multilayered PLCL sheets for a longer duration to confirm its complete degradation, modulating the inflammatory response provide strong evidence of bladder smooth muscle regeneration, as well a neuronal and vascular supply to the implanted area.
hADSCs seeded on the multilayered PLCL sheets has the potential to differentiate into SMC, thus facilitating the recovery of compliance and contractility of the injured bladder. This approach using hADSCs and PLCL may have promising clinical application for the treatment of bladder disease like NGB, DU, and severe cases of bladder cancer.
Acknowledgement
This research was supported by the National Research Foundation of Korea grant funded by the Korea government (MSIP) (No. 2011-0030075).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in The Catholic University of Korea, Seoul, Korea (IACUC Approval No. 2013-0111-03).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Osman NI, Chapple CR, Abrams P, Dmochowski R, Haab F, Nitti V, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389–398. doi: 10.1016/j.eururo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Shakhssalim N, Dehghan MM, Moghadasali R, Soltani MH, Shabani I, Soleimani M. Bladder tissue engineering using biocompatible nanofibrous electrospun constructs: feasibility and safety investigation. Urol J. 2012;9:410–419. [PubMed] [Google Scholar]
- 4.Silwal Gautam S, Imamura T, Ishizuka O, Lei Z, Yamagishi T, Yokoyama H, et al. Implantation of autologous adipose-derived cells reconstructs functional urethral sphincters in rabbit cryoinjured urethra. Tissue Eng Part A. 2014;20:1971–1979. doi: 10.1089/ten.tea.2013.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 6.Jack GS, Zhang R, Lee M, Xu Y, Wu BM, Rodríguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 2009;30:3259–3270. doi: 10.1016/j.biomaterials.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HK, Madihally SV, Palmer B, Frimberger D, Fung KM, Kropp BP. Biomatrices for bladder reconstruction. Adv Drug Deliv Rev. 2015;82–83:47–63. doi: 10.1016/j.addr.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol. 2013;9:878–883. doi: 10.1016/j.jpurol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Pokrywczynska M, Jundzill A, Adamowicz J, Kowalczyk T, Warda K, Rasmus M, et al. Is the poly (l-lactide-co-caprolactone) nanofibrous membrane suitable for urinary bladder regeneration? PLoS One. 2014;9:e105295. doi: 10.1371/journal.pone.0105295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 11.Sartoneva R, Haaparanta AM, Lahdes-Vasama T, Mannerström B, Kellomäki M, Salomäki M, et al. Characterizing and optimizing poly-l-lactide co-e-caprolactone membranes for urothelial tissue engineering. J R Soc Interface. 2012;9:3444–3454. doi: 10.1098/rsif.2012.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–2020. doi: 10.1016/j.juro.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafiq M, Jung Y, Kim SH. In situ vascular regeneration using substance P-immobilised poly (l-lactide-co-ε-caprolactone) scaffolds: stem cell recruitment, angiogenesis, and tissue regeneration. Eur Cell Mater. 2015;30:282–302. doi: 10.22203/eCM.v030a20. [DOI] [PubMed] [Google Scholar]
- 15.Jeong SI, Kim BS, Kang SW, Kwon JH, Lee YM, Kim SH, et al. In vivo biocompatibilty and degradation behavior of elastic poly(l-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials. 2004;25:5939–5946. doi: 10.1016/j.biomaterials.2004.01.057. [DOI] [PubMed] [Google Scholar]
- 16.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Lin H, Jiang D, Xu G, Fang X, He L, et al. Co-delivery of VEGF and bFGF via a PLGA nanoparticle-modified BAM for effective contracture inhibition of regenerated bladder tissue in rabbits. Sci Rep. 2016;6:20784. doi: 10.1038/srep20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shrestha KR, Park YH, Choi YS, Kim IG, Piao S, Jung AR, et al. Bladder reconstruction using stem cells seeded on multilayered scaffolds in a mucosa preserving partial cystectomy model. Tissue Eng Reg Med. 2015;12:427–434. doi: 10.1007/s13770-015-9098-2. [DOI] [Google Scholar]
- 19.Soh BS, Ng SY, Wu H, Buac K, Park JH, Lian X, et al. Endothelin-1 supports clonal derivation and expansion of cardiovascular progenitors derived from human embryonic stem cells. Nat Commun. 2016;7:10774. doi: 10.1038/ncomms10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, et al. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355–365. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 21.Mahfouza W, Elsalmya S, Corcosb J, Fayed AS. Fundamentals of bladder tissue engineering. Afr J Urol. 2013;19:51–57. doi: 10.1016/j.afju.2013.01.006. [DOI] [Google Scholar]
- 22.Miano JM. Mammalian smooth muscle differentiation: origins, markers and transcriptional control. In: Brand-Saberi B, editor. Vertebrate myogenesis, results and problems in cell differentiation. Berlin: Springer; 2002. pp. 39–59. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Zhang Y, Zhou L, Sun Z, Zheng J, Chen Y, et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods. 2010;16:1201–1211. doi: 10.1089/ten.tec.2009.0311. [DOI] [PubMed] [Google Scholar]
- 24.Leite MT, Freitas-Filho LG, Oliveira AS, Semedo-Kuriki P, Laks M, Arias VE, et al. The use of mesenchymal stem cells in bladder augmentation. Pediatr Surg Int. 2014;30:361–370. doi: 10.1007/s00383-014-3465-2. [DOI] [PubMed] [Google Scholar]
- 25.Brown AL, Farhat W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23:2179–2190. doi: 10.1016/S0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- 26.Feil G, Christ-Adler M, Maurer S, Corvin S, Rennekampff HO, Krug J, et al. Investigations of urothelial cells seeded on commercially available small intestine submucosa. Eur Urol. 2006;50:1330–1337. doi: 10.1016/j.eururo.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 28.Zambon JP, de Sá Barretto LS, Nakamura AN, Duailibi S, Leite K, Magalhaes RS, et al. Histological changes induced by polyglycolic-acid (PGA) scaffolds seeded with autologous adipose or muscle-derived stem cells when implanted on rabbit bladder. Organogenesis. 2014;10:278–288. doi: 10.4161/org.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burks CA, Bundy K, Fotuhi P, Alt E. Characterization of 75: 25 poly (l-lactide-co-epsilon-caprolactone) thin films for the endoluminal delivery of adipose-derived stem cells to abdominal aortic aneurysms. Tissue Eng. 2006;12:2591–2600. doi: 10.1089/ten.2006.12.2591. [DOI] [PubMed] [Google Scholar]
- 30.Jeong SI, Kim SH, Kim YH, Jung Y, Kwon JH, Kim BS, et al. Manufacture of elastic biodegradable PLCL scaffolds for mechano-active vascular tissue engineering. J Biomater Sci Polym Ed. 2004;15:645–660. doi: 10.1163/156856204323046906. [DOI] [PubMed] [Google Scholar]
- 31.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 32.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi YS, Vincent LG, Lee AR, Dobke MK, Engler AJ. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials. 2012;33:2482–2491. doi: 10.1016/j.biomaterials.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]