This systematic review and meta-analysis examines the efficacy and safety of corticosteroids in patients with sepsis.
Key Points
Question
Are corticosteroids associated with a reduction in 28-day mortality in patients with sepsis?
Findings
In this systematic review and meta-analysis of 37 randomized clinical trials that included 9564 patients with sepsis, administration of corticosteroids was associated with reduced 28-day mortality. Corticosteroids were also significantly associated with increased shock reversal at day 7 and vasopressor-free days and with decreased intensive care unit length of stay, the Sequential Organ Failure Assessment score at day 7, and time to resolution of shock.
Meaning
The findings suggest that administration of corticosteroid treatment in patients with sepsis is associated with significant improvement in health care outcomes and thus with reduced 28-day mortality.
Abstract
Importance
Although corticosteroids are widely used for adults with sepsis, both the overall benefit and potential risks remain unclear.
Objective
To conduct a systematic review and meta-analysis of the efficacy and safety of corticosteroids in patients with sepsis.
Data Sources and Study Selection
MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception until March 20, 2018, and updated on August 10, 2018. The terms corticosteroids, sepsis, septic shock, hydrocortisone, controlled trials, and randomized controlled trial were searched alone or in combination. Randomized clinical trials (RCTs) were included that compared administration of corticosteroids with placebo or standard supportive care in adults with sepsis.
Data Extraction and Synthesis
Meta-analyses were conducted using a random-effects model to calculate risk ratios (RRs) and mean differences (MDs) with corresponding 95% CIs. Two independent reviewers completed citation screening, data abstraction, and risk assessment.
Main Outcomes and Measures
Twenty-eight–day mortality.
Results
This meta-analysis included 37 RCTs (N = 9564 patients). Eleven trials were rated as low risk of bias. Corticosteroid use was associated with reduced 28-day mortality (RR, 0.90; 95% CI, 0.82-0.98; I2 = 27%) and intensive care unit (ICU) mortality (RR, 0.85; 95% CI, 0.77-0.94; I2 = 0%) and in-hospital mortality (RR, 0.88; 95% CI, 0.79-0.99; I2 = 38%). Corticosteroids were significantly associated with increased shock reversal at day 7 (MD, 1.95; 95% CI, 0.80-3.11) and vasopressor-free days (MD, 1.95; 95% CI, 0.80-3.11) and with ICU length of stay (MD, −1.16; 95% CI, −2.12 to −0.20), the sequential organ failure assessment score at day 7 (MD, −1.38; 95% CI, −1.87 to −0.89), and time to resolution of shock (MD, −1.35; 95% CI, −1.78 to −0.91). However, corticosteroid use was associated with increased risk of hyperglycemia (RR, 1.19; 95% CI, 1.08-1.30) and hypernatremia (RR, 1.57; 95% CI, 1.24-1.99).
Conclusions and Relevance
The findings suggest that administration of corticosteroids is associated with reduced 28-day mortality compared with placebo use or standard supportive care. More research is needed to associate personalized medicine with the corticosteroid treatment to select suitable patients who are more likely to show a benefit.
Introduction
Sepsis is defined as a life-threatening host response to infection that may culminate in organ failure and death.1,2,3 The incidence of sepsis is 535 cases per 100 000 person-years. The in-hospital mortality in the presence of sepsis ranges from 30% to 45%.4,5,6 Concomitant with early hemodynamic and respiratory support and appropriate antibiotic administration, since the mid-20th century, corticosteroids have been used as adjuvant therapy in the context of sepsis.7,8 Although evaluated in numerous randomized clinical trials (RCTs), both the safety and efficacy of corticosteroids remains controversial.7,8 Various systematic reviews and meta-analyses have either confirmed9,10 or refuted11,12,13 any survival benefit. A recent Cochrane meta-analysis suggested that low-dose corticosteroids may be associated with reduced mortality in patients with sepsis.9 In parallel, an additional systematic review concluded that there is no beneficial effect of high-dose or low-dose corticosteroids for treatment of sepsis.11 The conclusions of both reviews emphasized low9 or very low11 certainty in the evidence, limited by risk of bias,11 inconsistency,9,11 imprecision,9,11 and publication bias.9 Because of the low quality of available evidence, current clinical practice guidelines provide only a weak recommendation for the use of hydrocortisone in patients with septic shock if adequate fluid resuscitation and treatment with vasopressors have not restored hemodynamic stability.8
In 2018, 2 large RCTs14,15 reported comprehensive analyses of the uses of corticosteroids in patients with sepsis. These trials included more than 5000 combined patients, a larger sample than all the previous RCTs. The 2 trials yielded different results. In the Activated Protein C and Corticosteroids for Human Septic Shock (APROCCHSS) trial, hydrocortisone plus fludrocortisone given at low doses reduced 90-day mortality among patients with septic shock.14 In the Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock (ADRENAL) trial, a continuous infusion of hydrocortisone in patients undergoing mechanical ventilation did not result in lower mortality compared with patients receiving a placebo.15 These 2 trials had significant differences in the severity of illness (mortality in the control group, 28.8% vs 49.1%), the type of administered corticosteroids (hydrocortisone plus fludrocortisone vs hydrocortisone), method of drug administration (intermittent boluses vs continuous), and associated medical conditions when sepsis developed (in patient after a surgical admission vs patients with pneumonia).
The uncertainty about the efficacy of corticosteroids among patients with sepsis has resulted in a wide variation in clinical practice.16 This finding was the impetus for this systematic review and meta-analysis of the literature on the efficacy and safety of corticosteroid administration in patients with sepsis.
Methods
Protocol and Guidance
The study protocol was conducted following Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines17 and according to the protocol registered in the PROSPERO database (CRD42018095867). The methods and reporting of the systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.18
Eligibility Criteria
Eligible studies met the following PICOS (participants, interventions, comparators, outcomes, and study design) criteria. The population of interest included adults (age ≥18 years) who were diagnosed with sepsis, severe sepsis, or septic shock, or any combinations thereof.19,20 The intervention included any type of corticosteroid, including but not limited to hydrocortisone, methylprednisolone, betamethasone, and dexamethasone, compared with placebo or standard supportive care (which may have included antibiotics, fluid replacement, inotropic or vasopressor therapy, mechanical ventilation, or dialysis, if needed). The primary outcome was 28-day mortality. In-hospital or intensive care unit (ICU) mortality rates were used to compute the pooled analysis on 28-day mortality unless actual 28-day mortality rates were reported or were obtained from study authors. Secondary outcomes were ICU mortality, in-hospital mortality, and 90-day mortality. Shock reversal at day 7 was also studied, as well as the Sequential Organ Failure Assessment (SOFA) (score range, 0 to 24 with acute change of 2 points indicating organ dysfunction) score at day 7, ICU length of stay, hospital length of stay, health-related quality of life (reported by patients), time to shock reversal, vasopressor-free days to day 28, and ventilation-free days to day 28. Adverse events included any severe adverse event, gastroduodenal bleeding, superinfections, hyperglycemia, and hypernatremia. The definitions of outcomes are presented in eTable 1 in the Supplement. Only RCTs (including quasi-randomized trials and crossover trials) were included.
Studies were excluded if they were case reports, case series, or observational studies; the intervention included topical or inhaled corticosteroids; and all patients received corticosteroids.
Information Sources and Search Strategy
The search strategy was developed and executed in consultation with an experienced research librarian (P.X.) and was independently peer-reviewed by a nonauthor second librarian. MEDLINE, Embase, the Cochrane Central Register of Controlled Trials were searched electronically from inception until March 20, 2018, and updated on August 10, 2018. The World Health Organization International Clinical Trials Registry Platform was consulted regarding any ongoing studies or the availability of completed studies with reported results. The conference proceedings from the Society of Critical Care Medicine, American Thoracic Society, and the European Society of Intensive Care Medicine were also queried. To maximize the search for relevant articles, reference lists of RCTs were reviewed, as well as review articles and systematic reviews on the same topic. Language or publication status restrictions were not used.
The terms corticosteroids, sepsis, septic shock, hydrocortisone, controlled trials, and randomized controlled trial were searched alone or in combination. The details of the search strategy are presented in eTable 2 in the Supplement.
Study Selection
Two independent investigators (R.T. and T.L.) screened the titles and abstracts to determine whether the citation met eligibility criteria. They screened the full text for potentially relevant trials when both agreed that a citation met the eligibility criteria. Chance-adjusted interviewer agreement (κ statistic) was calculated. Disagreements between the investigators were resolved by consensus and, if necessary, consultation with a third investigator (F.F.). The corresponding authors were contacted to obtain missing information and unpublished data when needed to assess the inclusion criteria or when suitable data were not available.
Data Collection Process
Two independent investigators (R.T. and T.L.) extracted data from the included RCTs into standardized collection forms and created tables for the evidence and outcomes. Disagreements between the 2 reviewers were resolved by consensus and, if necessary, consultation with a third investigator (F.F.).
Assessment of Risk of Bias and Quality of Evidence
Two independent investigators (R.T. and T.L.) performed risk assessment using the Cochrane Collaboration risk of bias tool.21 The included RCTs were assessed for (1) random-sequence generation, (2) allocation sequence concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) completeness of outcome data, (6) selective reporting, and (7) other sources of bias. Each domain was assessed as low, unclear, or high risk of bias. The highest risk of bias for any criteria was used to reflect the overall risk of bias for the study.
The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach was used to rate the quality of evidence and generate absolute estimates of effect for the outcomes.22 Detailed GRADE guidance was used to assess the overall risk of bias, inconsistency, imprecision, indirectness, and publication bias and to summarize results in an evidence profile.
Data Synthesis
The statistical analyses were performed using RevMan, version 5.3.3 (Cochrane Collaboration), and the meta package in R, version 3.4.3 (R Project for Statistical Computing). The random-effects model was used for all analyses. Dichotomous variables were analyzed using the Mantel-Haenszel method and were expressed as risk ratios (RRs). Continuous variables were analyzed using the inverse variance random-effects model and were expressed as mean differences. A 2-tailed P value of less than 0.05 was set for statistical significance. Heterogeneity was assessed using with the χ2 test and the I2 test, with I2 greater than 50% being considered substantial.23 The possibility of publication bias was assessed by visual estimate of funnel plot and by the regression test of Egger test, Begg test, and Harbord test when 10 or more trials were pooled.24 The approach for incorporating crossover trials was to include only data from the first period.
Trial Sequential Analysis
A trial sequential analysis was conducted to explore whether cumulative data were adequately powered to evaluate outcomes. This analysis was performed using trial sequential analysis software, version 0.9.5.9 (Centre for Clinical Intervention Research).25 The required information size was calculated, and the trial sequential monitoring boundaries were computed using the O’Brien-Fleming approach. An optimal information size was considered as a 2-sided 5% risk of a type I error, 20% risk of a type II error (power of 80%), relative risk reduction of 20%, and the pooled control group event rate across the included studies.
Subgroup Analysis
Subgroup analyses were planned for the following variables: (1) dose of corticosteroid (high dose [defined as ≥400 mg/d of hydrocortisone or equivalent26] and low dose [defined as <400 mg/d]); (2) treatment duration (short [<4 days] and long [≥4 days]); (3) sepsis subtype (sepsis, sepsis and acute respiratory distress syndrome, sepsis and community-acquired pneumonia, septic shock, and severe sepsis); (4) type of corticosteroids used (hydrocortisone, hydrocortisone plus fludrocortisone, dexamethasone, betamethasone, methylprednisolone, or prednisolone); and (5) mortality in the control group (high [≥40%] and low [<40%]).
Sensitivity Analyses
Sensitivity analyses were conducted for the primary outcome by (1) excluding trials only reported as abstracts, (2) excluding trials published before 2000, (3) using fixed-effect models, (4) excluding trials that reported ICU mortality or in-hospital mortality to replace 28-day mortality, (5) excluding trials with non-low risk of bias, (6) excluding trials with fewer than 10 events, (7) excluding trials with fewer than 200 patients, (8) using the adjusted odds ratios, RRs, and hazard ratios with the generic inverse variance method.
Results
Study Selection and Study Characteristics
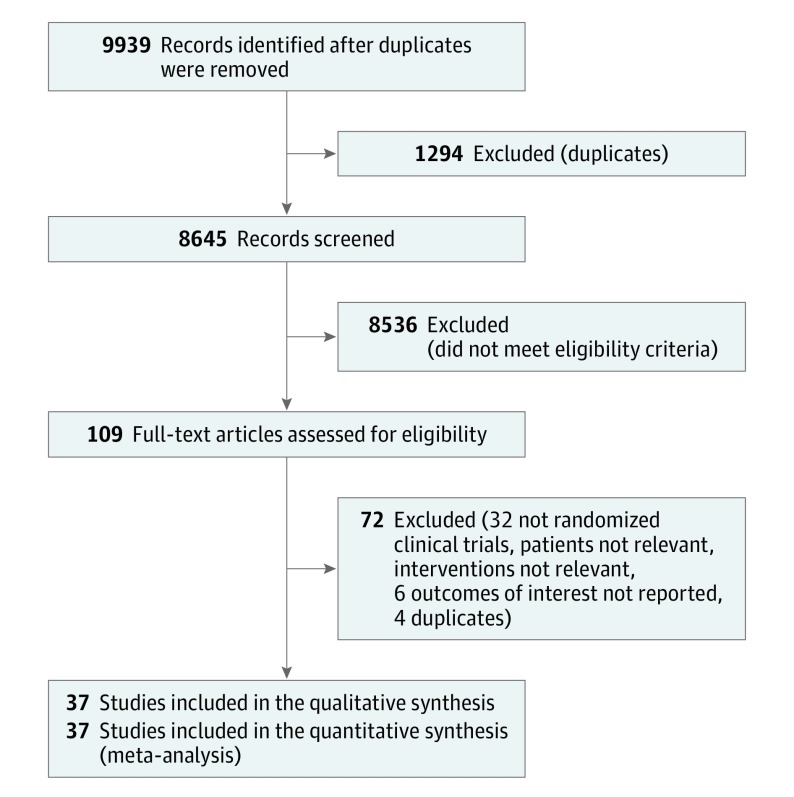
Figure 1 shows the study selection process. Of the 9939 results, 37 RCTs14,15,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62 that enrolled a total of 9564 patients were included in the final meta-analysis. There was close agreement between the reviewers on the review of full-text articles (κ = 0.78).
Figure 1. Flow of the Search Strategy and Included Studies.
Table 1 and eTable 4 in the Supplement present the main characteristics of selected studies. The studies were published from 1963 to 2018. Population sizes ranged from 26 to 3800 patients. Fifteen trials were multicenter.14,15,29,30,31,33,36,39,44,46,50,51,55,59,63 Two trials35,41 were published as abstracts. Eighteen trials14,15,28,29,33,34,35,36,38,40,41,43,46,47,48,55,58,61 included patients with septic shock. One trial42 used a standard therapy as the control to compare with corticosteroids, and others used a placebo. Thirty-two trials14,15,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,64,65 investigated the use of low-dose corticosteroids, whereas 7 trials27,28,29,30,31,32,63 studied the effects of high-dose corticosteroids. Twenty-one trials15,33,34,35,38,39,40,41,42,46,47,48,51,53,55,56,58,59,60,61,63 investigated the use of hydrocortisone; 3 trials,14,36,45 hydrocortisone plus fludrocortisone; 4 trials,30,32,44,54 prednisolone; 4 trials,43,50,64,65 dexamethasone; 6 trials,30,31,32,44,54,57 methylprednisolone; and 1 trial,27 betamethasone. Two trials28,29 tested effects of dexamethasone and methylprednisolone. The daily dose of corticosteroid varied between 30 mg/kg and 600 mg/kg of hydrocortisone (or equivalent26).
Table 1. Characteristics of the 37 Randomized Clinical Trials Included in the Meta-analysis of Corticosteroids vs Placebo or Standard Supportive Care in Adults With Sepsis.
| Study | Sites, No. | Patients, No. | Mean Age, y | Women, % | Type of Patient Population | Experimental Intervention | Primary Outcome |
|---|---|---|---|---|---|---|---|
| Klastersky et al,27 1971 | 1 | 85 | NR | CS: 39.1; PC: 51.3 | Severe sepsis and advanced cancer | Betamethasone 0.5 mg/kg every 12 h for 3 d | 30-d Mortality |
| Schumer,28 1976 | 1 | 172 | 50 | 3.5 | Septic shock | Group 1: methylprednisolone (30 mg/kg); group 2: dexamethasone (3 mg/kg); dose was repeated once in both groups after 4 h and had to be initiated at the time of diagnosis | Hospital mortality |
| Sprung et al,29 1984 | 2 | 59 | CS: 58; PC: 55 | CS: 14.3; PC: 18.2 | Septic shock | Group 1: methylprednisolone (30 mg/kg); group 2: dexamethasone (6 mg/kg); dose was repeated once in both groups after 4 h if shock persisted | Hospital mortality |
| Bone et al,30 1987 | 19 | 382 | CS: 53.0; PC: 53.6 | 38.5 | Sepsis or septic shock | Methylprednisolone bolus (30mg/kg) repeated every 6 h for 24 h | 14-d Septic shock |
| VASSCSG et al,31 1987 | 10 | 223 | CS: 60.9; PC: 60.6 | NR | Sepsis or septic shock | Methylprednisolone bolus (30 mg/kg) followed by 5 mg/kg/h for 9 h | 14-d Mortality |
| Luce et al,32 1988 | 1 | 75 | NR | NR | ARDS and sepsis | Methylprednisolone (30 mg/kg) every 6 h, 4 times | Prevention of ARDS |
| Bollaert et al,33 1998 | 2 | 41 | CS: 66; PC: 56 | CS: 31.8; PC: 36.8 | Septic shock | Hydrocortisone bolus (100 mg) every 8 h for 5 d, then tapered over 6 d | Shock reversal |
| Briegel et al,34 1999 | 1 | 40 | CS: 47; PC: 51 | CS: 55; PC: 40 | Septic shock | Hydrocortisone bolus (100 mg), followed by a continuous infusion of 0.18 mg/kg per hour until shock reversal, then tapered off | Shock reversal |
| Chawla and Kupfer,35 1999 | 1 | 44 | NR | NR | Septic shock | Hydrocortisone (100 mg) every 8 h for 3 d, then tapered over 4 d | Shock reversal |
| Annane et al,36 2002 | 19 | 300 | CS: 62; PC: 60 | CS: 36; PC: 30 | Septic shock | Hydrocortisone bolus (50 mg) every 6 h and fludrocortisone (50 μg) taken orally every 24 h for 7 d | 28-d Mortality |
| Yildiz et al,37 2002 | 1 | 40 | CS: 57.8; PC: 56.5 | CS: 43.8; PC: 56.3 | Sepsis, severe sepsis or septic shock | Prednisolone intravenous boluses 2 times daily at 6:00 am (5 mg) and at 6:00 pm (2.5 mg) for 10 d | 28-d Mortality |
| Keh et al,38 2003 | 1 | 40 | 52 | 65 | Septic shock | Hydrocortisone bolus (100 mg) followed by a continuous infusion of 10 mg/h for 3 d | Immune response |
| Confalonieri et al,39 2005 | 6 | 46 | CS: 60.4; PC: 66.6 | CS: 26.0; PC: 34.8 | Sepsis and community-acquired pneumonia | Hydrocortisone bolus (200 mg), followed by a continuous infusion of 10 mg/h for 7 d, then tapered off over 4 d | Improvement in Pao2:FIo2 |
| Oppert et al,40 2005 | 1 | 40 | CS: 59; PC: 47 | CS: 27.8; PC: 17.4 | Septic shock | Hydrocortisone bolus (50 mg), followed by continuous infusion of 0.18 mg/kg per hour up to cessation of vasopressor for ≥1 h, reduced to a dose of 0.02 mg/kg per hour for 24 h, then reduced by 0.02 mg/kg per hour every d | Time to cessation of vasopressor support |
| Tandan and Guleria,41 2005 | 1 | 51 | 51 | NR | Septic shock and adrenal insufficiency | Hydrocortisone (stated low dose but actual dose and duration NR) | 28-d Mortality or survival to hospital discharge |
| Rinaldi et al,42 2006 | 1 | 40 | CS68; PC: 66 | NR | Severe sepsis | Hydrocortisone (300 mg) daily as a continuous infusion for 6 d, then tapered off | Effect of steroids on urinary albumin/creatinine ratio |
| Cicarelli et al,43 2007 | 1 | 29 | CS: 69; PC: 61 | CS: 57.1; PC: 53.3 | Septic shock | Dexamethasone (0.2 mg/kg) given 3 times at intervals of 36 h | Improvement in Pao2:FIo2 |
| Meduri et al,44 2007 | 5 | 91 | CS: 59.1; PC: 54.5 | CS:29; PC:31 | ARDS and sepsis | Methylprednisolone loading dose of 1 mg/kg, followed by continuous infusion of 1 mg/kg per day from days 1-14, then 0.5 mg/kg per day from days 15-21, then 0.25 mg/kg per day from days 22-25, then 0.125 mg/kg per day from days 26-28 | Improvement in lung injury score at 7 d |
| Aboab et al,45 2008 | 1 | 23 | CS:55; PC:56 | CS: 60; PC: 61 | Septic shock | Hydrocortisone bolus (50 mg) every 6 h and fludrocortisone (50 μg) taken orally every 24 h for 7 d | Low- and high-normalized frequency components |
| Sprung et al,46 2008 | 52 | 499 | CS:63; PC:63 | CS: 34; PC: 33 | Septic shock | Hydrocortisone (50 mg) every 6 h for 5 d, then 50 mg every 12 h for 3 d, then 50 mg once daily for 3 d | 28-d Mortality |
| Hu et al,47 2009 | 1 | 77 | CS:56; PC:54 | CS: 39.5; PC: 35.9 | Septic shock | Hydrocortisone (50 mg) every 6 h for the first 7 d, 50 mg every 8 h for the next 3 d, then 50 mg every 12 h for 2 d and 50 mg once daily for 2 d | Time on norepinephrine and lactate clearance |
| Arabi et al,48 2010 | 1 | 75 | CS: 60.6; PC: 59.3 | CS: 44; PC: 44 | Cirrhosis and septic shock | Hydrocortisone bolus (50 mg) every 6 h until shock resolution | 28-d Mortality |
| Snijders et al,49 2010 | 1 | 213 | CS: 63.0; PC: 64.0 | CS: 47.1; PC: 36.7 | Community-acquired pneumonia and sepsis | Prednisolone (40 mg) intravenous once daily for 7 d | Rate of treatment failure at 7 d and 30 d |
| Meijvis et al,50 2011 | 2 | 304 | CS:64.5; PC:62.5 | CS:84; PC:87 | Community-acquired pneumonia and sepsis | Dexamethasone (5 mg) intravenously for 4 d | Length of hospital stay |
| Sabry,51 2011 | 3 | 80 | 63 | 56.1 | Community-acquired pneumonia and sepsis | Hydrocortisone bolus (200 mg) followed by intravenous dose of 300 mg daily for 7 d | Unclear |
| Yildiz et al,52 2011 | 1 | 55 | CS: 75; PC: 64 | CS: 22.2; PC: 45.5 | Sepsis or septic shock | Prednisolone intravenous boluses 3 times daily at 6 am (10 mg), 2 pm (5 mg), and 10 pm (5 mg) for 10 d | 28-d Mortality |
| Liu et al,53 2012 | 1 | 26 | CS: 69.8; PC: 55.9 | CS: 25.0; PC: 28.6 | ARDS and sepsis | Hydrocortisone bolus (100 mg) followed by 100 mg every 8 h for 3 d | 28-d Mortality |
| Rezk and Ibrahim,54 2013 | 1 | 27 | NR | NR | ARDS and sepsis | Methylprednisolone loading dose of 1 mg/kg, followed by continuous infusion of 1 mg/kg per day from days 1-14, 0.5 mg/kg per day from days 15-21, 0.25 mg/kg per day from days 22-25, and 0.125 mg/kg per day from days 26-28 | Unclear |
| Gordon et al,55 2014 | 4 | 61 | CS: 61; PC: 60 | CS: 42; PC: 40 | Septic shock | Hydrocortisone (50 mg) every 6 h for the first 5 d, 50 mg every 12 h for the next 3 d, 50 mg every 24 h for the last 3 d | Difference in plasma vasopressin concentration between treatment groups |
| Huang et al,56 2014 | 1 | 40 | CS: 53.9; PC: 55.7 | CS: 50.0; PC: 40.0 | Sepsis | Hydrocortisone (300 mg) daily as a continuous infusion for 7 d | 28-d Mortality |
| Torres et al,57 2015 | 3 | 120 | CS: 64.5; PC: 66.1 | CS: 43; PC: 34 | Community-acquired pneumonia and sepsis | Methylprednisolone intravenous bolus of 0.5 mg/kg/12 h for 5 d started within 36 h of hospital admission | Rate of treatment failure |
| Gordon et al,58 2016 | 18 | 421 | 66 | 42 | Septic shock | Hydrocortisone (50 mg) every 6 h for the first 5 d, 50 mg every 12 h for the next 3 d, 50 mg every 24 h for the last 3 d | Kidney failure–free days to 28 d |
| Keh et al,59 2016 | 34 | 380 | CS: 65.5; PC: 64.6 | CS: 33.3; PC: 36.9 | Severe sepsis | Hydrocortisone bolus (50 mg) followed by a continuous infusion of 200 mg daily for 3 d | 14-d Septic shock |
| Tongyoo,60 2016 | 1 | 206 | CS: 64.5; PC: 64.3 | CS: 49.0; PC: 48.5 | Severe sepsis or septic shock and ARDS | Hydrocortisone (50 mg) every 6 h for 7 d | 28-d Mortality |
| Lv et al,61 2017 | 1 | 118 | CS: 68.8; PC: 64.8 | CS: 43.1; PC: 38.3 | Septic shock | Hydrocortisone (200 mg) daily as a continuous infusion for 6 d | 28-d Mortality |
| Annane et al,14 2018 | 34 | 1241 | CS:66; PC: 66 | CS: 34.5; PC: 32.3 | Septic shock | Hydrocortisone bolus (50 mg) every 6 h and oral fludrocortisone (50 μg) every 24 h for 7 d | 90-d Mortality |
| Venkatesh et al,15 2018 | 69 | 3800 | CS: 62.3; PC: 62.7 | CS: 39.6; PC: 38.7 | Septic shock | Hydrocortisone (200 mg) intravenously every day for a maximum of 7 d or until ICU discharge or death | 90-d Mortality |
Abbreviations: ARDS, acute respiratory distress syndrome; CS, corticosteroids; ICU, intensive care unit; NR, not reported; Pao2:FIo2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; PC, placebo or control.
Risk of Bias and Quality of Evidence
Risk-of-bias assessments are reported in eFigures 1 and 2 in the Supplement. Eleven trials had a low risk of bias, 12 trails had an unclear risk, and 14 trials were considered to have a high risk. Key findings of the GRADE assessment of certainty for each outcome are shown in Table 2.
Table 2. Summary of Findings and Strength of Evidence in Studies Comparing Corticosteroids vs Placebo or Standard Supportive Care Among Patients With Sepsis.
| Outcome | No. of Patients (No. of Studies) | Relative Effect, Risk Ratio, or Mean Difference (95% CI)a | I2, % | Absolute Effect Estimates, No. of Patients per 1000 Population | Quality | ||
|---|---|---|---|---|---|---|---|
| Control | Corticosteroids | Difference (95% CI) | |||||
| Mortality | |||||||
| 28-d | 8729 (34) | 0.89 (0.81 to 0.98) | 27 | 292 | 260 | −32 (−6 to −56) | Moderateb |
| 90-d | 5238 (3) | 0.94 (0.85 to 1.03) | 27 | 329 | 309 | −20 (−49 to 9) | Moderateb |
| In hospital | 3659 (19) | 0.88 (0.79 to 0.99) | 38 | 390 | 343 | −47 (−4 to −82) | High |
| In ICU | 2487 (13) | 0.85 (0.77 to 0.94) | 0 | 369 | 314 | −55 (−22 to −85) | High |
| Length of stay | |||||||
| ICU | 6373 (17) | −1.16 (−2.12 to −0.20) | 30 | NA | NA | −1.16 (−2.12 to −0.2) | High |
| Hospital | 5389 (12) | −0.60 (−2.25 to 1.04) | 48 | NA | NA | −0.6 (−2.25 to 1.04) | Moderated |
| SOFA score at day 7 | 1986 (9) | −1.38 (−1.87 to −0.89) | 50 | NA | NA | −1.38 (−1.87 to −0.89) | Lowc,d |
| Shock reversal at day 7 | 6369 (14) | 1.23 (1.12 to 1.35) | 54 | 370 | 455 | 85 (44 to 129) | Moderatec |
| Time to resolution of shock | 4081 (5) | −1.35 (−1.78 to −0.91) | 68 | NA | NA | −1.35 (−1.78 to −0.91) | Lowc,d |
| Vasopressor-free days to day 28 | 1342 (3) | 1.95 (0.80 to 3.11) | 0 | NA | NA | 1.95 (0.8 to 3.11) | Moderated |
| Ventilation-free days to day 28 | 1630 (5) | 2.03 (−0.38 to 4.44) | 61 | NA | NA | 2.03 (−0.38 to 4.44) | Lowc,d |
| Severe adverse events | |||||||
| Any | 3403 (15) | 1.04 (0.90 to 1.20) | 49 | 428 | 445 | 17 (−43 to 86) | High |
| Gastroduodenal bleeding | 4006 (22) | 1.11 (0.88 to 1.40) | 0 | 30 | 33 | 3 (−4 to 12) | Moderated |
| Superinfection | 7488 (21) | 1.05 (0.93 to 1.19) | 15 | 177 | 186 | 9 (−12 to 34) | High |
| Hyperglycemia | 7332 (17) | 1.19 (1.08 to 1.30) | 41 | 261 | 311 | 50 (21 to 78) | High |
| Hypernatremia | 4844 (5) | 1.57 (1.24 to 1.99) | 0 | 36 | 57 | 21 (9 to 36) | Moderated |
Abbreviations: ICU, intensive care unit; NA, not applicable.
Data on mortality, shock reversal at day 7, and severe adverse events are risk ratios. All other data are mean differences.
The 2 largest trials varied in survival benefit.
Inconsistencies.
Imprecisions.
Primary Outcome: 28-Day Mortality
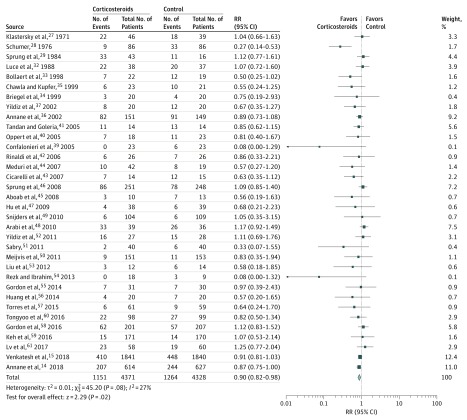
Thirty-four trials with 8699 patients reported 28-day mortality. Overall, 28-day mortality was 26.3% in the patients taking corticosteroids and 29.2% in the patients not taking corticosteroids. The RR (0.90; 95% CI, 0.82-0.98; I2 = 27%) (Figure 2) revealed an association between corticosteroid therapy and improved 28-day mortality. Trial sequential analysis confirmed that the required information size was met for mortality at 28 days (eFigure 3 in the Supplement). Funnel plot analysis suggested some asymmetry (eFigure 5 in the Supplement), and the Egger test (P = .001), Begg test (P = .002), and Harbord test (P = .02) detected significant publication bias.
Figure 2. Mortality at 28 Days in All Trials Evaluating Corticosteroids Among Patients With Sepsis.
The risk ratios (RRs) were determined using the Mantel-Haenszel random-effects model. Square data markers represent RRs, with marker size reflecting the statistical weight of the study using random-effects meta-analysis; horizontal lines, 95% CIs; diamond, the overall RR and 95% CI for the outcome of interest.
Secondary Outcomes
eFigures 13-27 in the Supplement give the forest plots for secondary outcomes. Corticosteroids were associated with significant benefit for in-hospital mortality (RR, 0.88; 95% CI, 0.79-0.99) and ICU mortality (RR, 0.85; 95% CI, 0.77-0.94, I2 = 0%), whereas there was no statistically significant difference in 90-day mortality between groups (3 trials: RR, 0.94; 95% CI, 0.85-1.03, I2 = 27%).
Nine trials provided data on the SOFA score at day 7. The mean difference (MD) in the SOFA score at day 7 was −1.38 (95% CI, −1.87 to −0.89), with patients receiving corticosteroids having lower scores. Fourteen studies reported that shock reversed at day 7 (RR, 1.23; 95% CI, 1.12-1.35), with patients receiving corticosteroids having greater likelihood of reversal of shock. Pooled estimates suggested a marked decrease in ICU length of stay (MD, −1.16; 95% CI, −2.12 to −0.20) and time to resolution of shock (MD, −1.35; 95% CI, −1.78 to −0.91) and a significant increase of vasopressor-free days to day 28 (MD, 1.95; 95% CI, 0.80-3.11) but not ventilation-free days to day 28 (MD, 2.03; 95% CI, −0.38 to 4.44). There was no association between corticosteroids and duration of hospital stay (MD, −0.6; 95% CI, −2.25 to 1.04). To our knowledge, no trial has reported quality of life.
The incidences of hyperglycemia (RR, 1.19; 95% CI, 1.08-1.30) and hypernatremia (RR, 1.57; 95% CI, 1.24-1.99) were higher in the corticosteroid group compared with the control group. Rates of any severe adverse event, gastroduodenal bleeding, and superinfection were not statistically different between treatment groups.
Sensitivity Analysis
Similar results were observed for 28-day mortality in all conducted sensitivity analyses excluding studies only reported as abstracts, published earlier than 2000, that reported ICU mortality or in-hospital mortality, with non-low risk of bias, with fewer than 10 events, with fewer than 200 patients, using fixed-effect models and using odds ratios with the generic inverse variance method (eTable 6 in the Supplement).
Subgroup Analysis and Meta-regression
Subgroup analysis revealed that 28-day mortality was significantly lower in patients taking corticosteroids among the long-course treatment trials, low-dose corticosteroids trials, and trials with low-risk bias (Table 3 and eFigures 6-10 in the Supplement). In the meta-regression analysis exploring the effects of potential sources of heterogeneity (ie, dose of corticosteroids, treatment duration, sepsis population subtype, type of corticosteroids, disease severity, and year of publication), a significant subgroup effect was not found. The meta-regression scatterplots of published year and control groups mortality in the control groups are presented in eFigures 11 and 12 in the Supplement.
Table 3. Subgroup Analysis of the Association of Corticosteroids With 28-Day Mortality Among Patients With Sepsis.
| Subgroup | Studies, No. | Patients, No. | I2, % | Risk Ratio (95% CI) | P Value |
| Dose of corticosteroid, mg/d or equivalent | |||||
| Hydrocortisone <400 | 30 | 8308 | 4 | 0.91 (0.85-0.98) | .70 |
| Hydrocortisone ≥400 | 4 | 389 | 83 | 0.82 (0.47-1.42) | |
| Treatment duration, d | |||||
| <4 (Short course) | 5 | 418 | 79 | 0.78 (0.49-1.24) | .51 |
| ≥4 (Long course) | 29 | 8297 | 2 | 0.92 (0.85-0.98) | |
| Sepsis population subtype | |||||
| Sepsis | 3 | 245 | 0 | 0.89 (0.61-1.31) | .90 |
| Sepsis and ARDS | 4 | 187 | 56 | 0.69 (0.35-1.37) | |
| Sepsis and community-acquired pneumonia | 5 | 840 | 7 | 0.76 (0.50-1.15) | |
| Septic shock | 19 | 7022 | 0 | 0.91 (0.82-1.02) | |
| Severe sepsis | 1 | 52 | 0 | 0.86 (0.33-2.21) | |
| Type of corticosteroids | |||||
| Hydrocortisone | 19 | 5895 | 9 | 0.95 (0.86-1.05) | .31 |
| Hydrocortisone plus fludrocortisone | 3 | 1564 | 0 | 0.87 (0.77-0.98) | |
| Dexamethasone | 3 | 462 | 52 | 0.53 (0.27-1.02) | |
| Betamethasone | 5 | 410 | 70 | 0.56 (0.28-1.13) | |
| Methylprednisolone | 1 | 85 | NA | 1.04 (0.66-1.63) | |
| Prednisolone | 3 | 308 | 0 | 0.94 (0.66-1.35) | |
| Mortality in the control group, % | |||||
| ≥40 (High) | 15 | 998 | 37 | 0.89 (0.75-1.04) | .93 |
| <40 (Low) | 19 | 7699 | 0 | 0.89 (0.79-1.01) |
Abbreviations: ARDS, acute respiratory distress syndrome; NA, not applicable.
Discussion
In this meta-analysis of 37 RCTs (including 9564 patients), corticosteroid treatment was significantly associated with reduced 28-day mortality, ICU mortality, and in-hospital mortality among patients with sepsis. However, this survival benefit was not replicated with 90-day mortality. Subgroup analyses based on treatment modalities demonstrated that the beneficial effect in 28-day mortality was associated with the use of low-dose corticosteroids. The association with 28-day mortality was not observed with high-dose corticosteroids. However, meta-regression did not demonstrate a credible association for any of the subgroup differences.
This meta-analysis showed that the use of corticosteroids in sepsis was associated with a significant increase in shock reversal and vasopressor-free days to day 28 and with a marked decrease in ICU length of stay, SOFA score at 7 days, and time to resolution of shock. However, corticosteroid treatment was not associated with shorter hospital length of stay or fewer ventilation-free days to day 28. To our knowledge, no trial has reported quality of life.
This meta-analysis also showed no association between significant adverse effects and corticosteroid treatment when comparing rates of gastroduodenal bleeding, superinfection, or any severe adverse event. Corticosteroid administration was associated with an increased risk of hypernatremia and hyperglycemia.
Compared With Other Studies
Several meta-analyses have examined the use of corticosteroids in patients with sepsis. However, the results were contradictory and were limited by the small size of the trials. In 2009, Annane et al13 identified 12 eligible trials and found no significant association of corticosteroid treatment with 28-day mortality, hospital mortality, or ICU mortality in severe sepsis or septic shock. In 2015, Annane et al9 published a Cochrane systematic review including a total of 33 trials randomizing 4428 patients. Findings in this review showed that corticosteroid treatment was associated with reduced all-course 28-day mortality (RR, 0.87; 95% CI, 0.76-1.00).9 In parallel, an additional systematic review by Volbeda et al11 included 35 trials and 4682 patients. Conversely, corticosteroids were not statistically significantly associated with mortality (RR, 0.89; 95% CI, 0.74-1.08).11 The results of their meta-analyses were limited owing to imprecision (total information is smaller than the calculated optimal information size), inconsistency (significant heterogeneity across trial results), published bias, and risk of bias.
The findings of this meta-analysis of the association of corticosteroid administration with improved 28-day mortality contrasts with results of previous publications. This difference in part may be explained by additionally including 6 RCTs14,15,58,59,60,61 published after 2015, a feature that accounted for 62.6% (5986 of 9564 patients) of the total number of patients. Moreover, previous meta-analyses pooled only small RCTs, whereas this study included the 2 largest RCTs14,15 available in the literature. The data from these studies helped reinforce the findings, meet the minimum information size required in trial sequential analysis, decrease the heterogeneity, and provide improved precision concerning the treatment effects of corticosteroid therapy.
After this study was submitted for initial review, an additional meta-analysis66 was published. This meta-analysis compared low-dose corticosteroids with placebo in adults with septic shock but found that both short-term and longer-term mortality were unaffected by low-dose corticosteroids. That study differs from the present study in several ways. First, our study included more studies because trials of any dose of corticosteroids for sepsis were reviewed, whereas the other study focused on trials of low-dose corticosteroids for septic shock. Second, the primary outcome of the other study was short-term mortality (defined as death within 90 days), whereas the primary outcome of this study was 28-day mortality. Thus, the studies extracted different data from several included RCTs14,15 that reported 28-day and 90-day mortality at once. In addition, 3 of the trials67,68,69 were excluded from this analysis because 28-day mortality was not reported; however, they were included in the aforementioned report. Third, the authors used a fixed-effects model, whereas the random-effects model was used in this study because of the high level of clinic heterogeneity. The difference in some of the methodologies used in both reports may explain the contrasting results.
Implications for Clinical Practice
Corticosteroids have been used as adjuvant therapy for sepsis for more than 50 years without hard evidence to guide patient selection.7 Physicians have used their clinical judgment to decide how to use corticosteroids. Current Surviving Sepsis Campaign guidelines recommend the use of hydrocortisone in patients with septic shock if adequate fluid resuscitation and treatment with vasopressors have not restored hemodynamic stability (weak recommendation, low quality of evidence).8 This recommendation was based on the absence of convincing evidence of benefit. This analysis of all renewed data from RCTs suggests that corticosteroid treatment is associated with reduced mortality compared with control in patients with sepsis. Furthermore, this study showed that corticosteroid treatment may be associated with increased shock reversal and vasopressor-free days and with decreased ICU length of stay, time to resolution of shock, and SOFA score. These improvements in outcomes are not associated with an increased risk of main complications. These findings appear to indicate that corticosteroids should be prescribed at a low dose and for a long course. However, the optimal strategy for the administration of corticosteroids in patients with sepsis is uncertain. Future studies are needed to associate personalized medicine with clinical phenotyping, genotyping, or metabolomics with the treatment of sepsis for the selection of suitable patients who are more likely to show a benefit.
Strengths and Limitations
The strengths of the present review include a comprehensive search strategy, explicit eligibility criteria that enhance generalizability, and rigorous use of the GRADE approach to rate quality of evidence. This meta-analyses of mortality outcomes included more than 8000 patients, which was larger than the minimum information size required in trial sequential analysis and were robust despite multiple subgroup and sensitivity analyses.
This study had limitations. First, the results of this meta-analysis were weakened by significant clinical heterogeneity. The analysis included trials developed almost 5 decades ago; since then, treatments and diagnostic techniques for sepsis have evolved. Therefore, clinical heterogeneity will have inevitably occurred in trials, including type of corticosteroids, dose of drug, timing of administration, and duration of therapy. With regard to statistical heterogeneity, the results of trials included in this study were variable, with a moderate degree of detected heterogeneity for the primary outcome of mortality (I2 = 27%), justifying the use of random-effects models. Heterogeneity was qualitatively and quantitatively investigated and addressed in this analysis. By exclusion of early trials, low-dose trials, or short-course trials, heterogeneity could be resolved without significant change in the primary outcome. These factors were important contributors to heterogeneity in this meta-analysis.
Second, the asymmetry in the funnel plot appeared to be the result of publication bias, mostly secondary to the smaller studies, but a sensitivity analysis that excluded small studies (<200 patients) without evidence of this bias confirmed the findings. Nonetheless, the comprehensive search of the literature and the clinical trial registries may have decreased the risk of missing any study. Beyond small study effect, potential sources of an asymmetrical funnel plot include selective outcome reporting, poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity, artifact, and chance.24
Third, this study might not be powered enough to assess adverse events. The variable definitions of adverse events among trials may have led to inconsistent results. For example, the ADRENAL trial15 and APROCCHSS trial14 reported the ratio of hyperglycemia in the control groups as 0.16% (3 of 1829 patients) and 83.1% (520 of 626 patients), respectively. Moreover, although there was no association of corticosteroid treatment with risk of gastroduodenal bleeding, superinfection, or any severe adverse event, the analysis of rare events in RCTs is associated with its limitations. Observational studies may be more appropriate than RCTs to assess adverse events because these studies may include more patients and follow-up is often longer.
Conclusions
The findings suggest that corticosteroid therapy compared with standard supportive care or placebo is significantly associated with reduced 28-day mortality in patients with sepsis.
eTable 1. Definitions of outcomes
eTable 2. Search strategy
eTable 3. Inclusion criteria and exclusion criteria of including trials
eTable 4. Support for judgement for included trials rated as high risk of bias
eTable 5. Sensitivity analyses
eFigure 1. Risk of bias summary
eFigure 2. Risk of bias graph
eFigure 3. Trial sequential analysis for 28 days mortality
eFigure 4. Trial sequential analysis for 90 days mortality
eFigure 5. Funnel plot for 28 days mortality
eFigure 6. Subgroup analysis for 28 days mortality–based on dose of corticosteroid
eFigure 7. Subgroup analysis for 28 days mortality–based on treatment duration
eFigure 8. Subgroup analysis for 28 days mortality–based on sepsis population subtype
eFigure 9. Subgroup analysis for 28 days mortality–based on type of corticosteroids
eFigure 10. Subgroup analysis for 28 days mortality–based on control group mortality
eFigure 11. Meta-regression for 28-day mortality outcome by control group mortality
eFigure 12. Meta-regression for 28-day mortality outcome by year of study publication
eFigure 13. Forest plot for 90-day mortality
eFigure 14. Forest plot for ICU mortality
eFigure 15. Forest plot for hospital morality
eFigure 16. Forest plot for length of stay in hospital
eFigure 17. Forest plot for length of stay in ICU
eFigure 18. Forest plot for shock reversal at day 7
eFigure 19. Forest plot for SOFA score at day 7
eFigure 20. Forest plot for time to resolution of shock
eFigure 21. Forest plot for vasopressor-free day to day 28
eFigure 22. Forest plot for ventilation-free day to day 28
eFigure 23. Forest plot for any severe adverse event
eFigure 24. Forest plot for gastroduodenal bleeding
eFigure 25. Forest plot for superinfections
eFigure 26. Forest plot for hyperglycemia
eFigure 27. Forest plot for hypernatremia
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar-Hari M, Phillips GS, Levy ML, et al. ; Sepsis Definitions Task Force . Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):775-787. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann C, Scherag A, Adhikari NK, et al. ; International Forum of Acute Care Trialists . Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259-272. doi: 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- 5.Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States: a population-based study. Ann Am Thorac Soc. 2015;12(2):216-220. doi: 10.1513/AnnalsATS.201411-498BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15(5):581-614. doi: 10.1016/S1473-3099(15)70112-X [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Bellissant E, Cavaillon J-M. Septic shock. Lancet. 2005;365(9453):63-78. doi: 10.1016/S0140-6736(04)17667-8 [DOI] [PubMed] [Google Scholar]
- 8.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 9.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for treating sepsis. Cochrane Database Syst Rev. 2015;(12):CD002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004;329(7464):480. doi: 10.1136/bmj.38181.482222.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volbeda M, Wetterslev J, Gluud C, Zijlstra JG, van der Horst IC, Keus F. Glucocorticosteroids for sepsis: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41(7):1220-1234. doi: 10.1007/s00134-015-3899-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sligl WI, Milner DA Jr, Sundar S, Mphatswe W, Majumdar SR. Safety and efficacy of corticosteroids for the treatment of septic shock: a systematic review and meta-analysis. Clin Infect Dis. 2009;49(1):93-101. doi: 10.1086/599343 [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301(22):2362-2375. doi: 10.1001/jama.2009.815 [DOI] [PubMed] [Google Scholar]
- 14.Annane D, Renault A, Brun-Buisson C, et al. ; CRICS-TRIGGERSEP Network . Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378(9):809-818. doi: 10.1056/NEJMoa1705716 [DOI] [PubMed] [Google Scholar]
- 15.Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378(9):797-808. doi: 10.1056/NEJMoa1705835 [DOI] [PubMed] [Google Scholar]
- 16.Bruno JJ, Dee BM, Anderegg BA, Hernandez M, Pravinkumar SE. US practitioner opinions and prescribing practices regarding corticosteroid therapy for severe sepsis and septic shock. J Crit Care. 2012;27(4):351-361. doi: 10.1016/j.jcrc.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 17.Shamseer L, Moher D, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530-538. doi: 10.1007/s00134-003-1662-x [DOI] [PubMed] [Google Scholar]
- 21.Shinichi A. Cochrane Handbook for Systematic Reviews of Interventions. Online Kensaku. 2014;35(3):154-155. https://training.cochrane.org/handbook. Accessed November 13, 2018. [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763-769. doi: 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 26.Singer MRW. Oxford handbook of critical care. 3rd ed. 2009:329. http://oxfordmedicine.com/view/10.1093/med/9780199235339.001.0001/med-9780199235339. Accessed November 9, 2018.
- 27.Klastersky J, Cappel R, Debusscher L. Effectiveness of betamethasone in management of severe infections: a double-blind study. N Engl J Med. 1971;284(22):1248-1250. doi: 10.1056/NEJM197106032842206 [DOI] [PubMed] [Google Scholar]
- 28.Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976;184(3):333-341. doi: 10.1097/00000658-197609000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med. 1984;311(18):1137-1143. doi: 10.1056/NEJM198411013111801 [DOI] [PubMed] [Google Scholar]
- 30.Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317(11):653-658. doi: 10.1056/NEJM198709103171101 [DOI] [PubMed] [Google Scholar]
- 31.Veterans Administration Systemic Sepsis Cooperative Study Group Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317(11):659-665. doi: 10.1056/NEJM198709103171102 [DOI] [PubMed] [Google Scholar]
- 32.Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis. 1988;138(1):62-68. doi: 10.1164/ajrccm/138.1.62 [DOI] [PubMed] [Google Scholar]
- 33.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26(4):645-650. doi: 10.1097/00003246-199804000-00010 [DOI] [PubMed] [Google Scholar]
- 34.Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27(4):723-732. doi: 10.1097/00003246-199904000-00025 [DOI] [PubMed] [Google Scholar]
- 35.Chawla K, Kupfer YST. Hydrocortisone reverses refractory septic shock. Crit Care Med. 1999;27(1):A33. doi: 10.1097/00003246-199901001-00022 [DOI] [Google Scholar]
- 36.Annane D, Sébille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock [correction appears in JAMA. 2008;300(14):1652]. JAMA. 2002;288(7):862-871. doi: 10.1001/jama.288.7.862 [DOI] [PubMed] [Google Scholar]
- 37.Yildiz O, Doğanay M, Aygen B, Güven M, Keleştimur F, Tutuû A. Physiological-dose steroid therapy in sepsis [ISRCTN36253388]. Crit Care. 2002;6(3):251-259. doi: 10.1186/cc1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167(4):512-520. doi: 10.1164/rccm.200205-446OC [DOI] [PubMed] [Google Scholar]
- 39.Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171(3):242-248. doi: 10.1164/rccm.200406-808OC [DOI] [PubMed] [Google Scholar]
- 40.Oppert M, Schindler R, Husung C, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33(11):2457-2464. doi: 10.1097/01.CCM.0000186370.78639.23 [DOI] [PubMed] [Google Scholar]
- 41.Tandan SM, Guleria RNG Low dose steroids and adrenocortical insufficiency in septic shock: a double-blind randomised controlled trial from India. Proceedings of the 2005 American Thoracic Society Meeting; May 20-25, 2005; New York, New York. [Google Scholar]
- 42.Rinaldi S, Adembri C, Grechi S, De Gaudio AR. Low-dose hydrocortisone during severe sepsis: effects on microalbuminuria. Crit Care Med. 2006;34(9):2334-2339. doi: 10.1097/01.CCM.0000233872.04706.BB [DOI] [PubMed] [Google Scholar]
- 43.Cicarelli DD, Vieira JE, Benseñor FE. Early dexamethasone treatment for septic shock patients: a prospective randomized clinical trial. Sao Paulo Med J. 2007;125(4):237-241. doi: 10.1590/S1516-31802007000400009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954-963. doi: 10.1378/chest.06-2100 [DOI] [PubMed] [Google Scholar]
- 45.Aboab J, Polito A, Orlikowski D, Sharshar T, Castel M, Annane D. Hydrocortisone effects on cardiovascular variability in septic shock: a spectral analysis approach. Crit Care Med. 2008;36(5):1481-1486. doi: 10.1097/CCM.0b013e31816f48f2 [DOI] [PubMed] [Google Scholar]
- 46.Sprung CL, Annane D, Keh D, et al. ; CORTICUS Study Group . Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111-124. doi: 10.1056/NEJMoa071366 [DOI] [PubMed] [Google Scholar]
- 47.Hu B, Li JG, Liang H, et al. The effect of low-dose hydrocortisone on requirement of norepinephrine and lactate clearance in patients with refractory septic shock [in Chinese]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21(9):529-531. [PubMed] [Google Scholar]
- 48.Arabi YM, Aljumah A, Dabbagh O, et al. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182(18):1971-1977. doi: 10.1503/cmaj.090707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181(9):975-982. doi: 10.1164/rccm.200905-0808OC [DOI] [PubMed] [Google Scholar]
- 50.Meijvis SC, Hardeman H, Remmelts HH, et al. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9782):2023-2030. doi: 10.1016/S0140-6736(11)60607-7 [DOI] [PubMed] [Google Scholar]
- 51.Sabry NAE-DOE. Corticosteroids and ICU course of community acquired pneumonia in Egyptian settings. Pharmacol Pharm. 2011;2:73-81. doi: 10.4236/pp.2011.22009 [DOI] [Google Scholar]
- 52.Yildiz O, Tanriverdi F, Simsek S, Aygen B, Kelestimur F. The effects of moderate-dose steroid therapy in sepsis: A placebo-controlled, randomized study. J Res Med Sci. 2011;16(11):1410-1421. [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Li J, Huang YZ, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency [in Chinese]. Zhonghua Nei Ke Za Zhi. 2012;51(8):599-603. [PubMed] [Google Scholar]
- 54.Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egypt J Chest Dis Tuberc. 2013;62(1):167-172. doi: 10.1016/j.ejcdt.2013.02.013 [DOI] [Google Scholar]
- 55.Gordon AC, Mason AJ, Perkins GD, et al. The interaction of vasopressin and corticosteroids in septic shock: a pilot randomized controlled trial. Crit Care Med. 2014;42(6):1325-1333. doi: 10.1097/CCM.0000000000000212 [DOI] [PubMed] [Google Scholar]
- 56.Huang R, Zhang Z, Xu M, et al. Effect of Sini decoction on function of hypothalamic-pituitary-adrenal axis in patients with sepsis [in Chinese]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26(3):184-187. [DOI] [PubMed] [Google Scholar]
- 57.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313(7):677-686. doi: 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 58.Gordon AC, Mason AJ, Thirunavukkarasu N, et al. ; VANISH Investigators . Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA. 2016;316(5):509-518. doi: 10.1001/jama.2016.10485 [DOI] [PubMed] [Google Scholar]
- 59.Keh D, Trips E, Marx G, et al. ; SepNet–Critical Care Trials Group . Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA. 2016;316(17):1775-1785. doi: 10.1001/jama.2016.14799 [DOI] [PubMed] [Google Scholar]
- 60.Tongyoo S, Permpikul C, Mongkolpun W, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care. 2016;20(1):329. doi: 10.1186/s13054-016-1511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv QQ, Gu XH, Chen QH, Yu JQ, Zheng RQ. Early initiation of low-dose hydrocortisone treatment for septic shock in adults: a randomized clinical trial. Am J Emerg Med. 2017;35(12):1810-1814. doi: 10.1016/j.ajem.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 62.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741-1751. doi: 10.1056/NEJMoa040573 [DOI] [PubMed] [Google Scholar]
- 63.CSG The effectiveness of hydrocortisone in the management of severe infections: a double-blind study. JAMA. 1963;183(6):462-465. doi: 10.1001/jama.1963.63700060029012 [DOI] [Google Scholar]
- 64.de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators . Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347(20):1549-1556. doi: 10.1056/NEJMoa021334 [DOI] [PubMed] [Google Scholar]
- 65.Scarborough M, Gordon SB, Whitty CJ, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007;357(24):2441-2450. doi: 10.1056/NEJMoa065711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rygård SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018;44(7):1003-1016. doi: 10.1007/s00134-018-5197-6 [DOI] [PubMed] [Google Scholar]
- 67.Kurugundla N, Irugulapati L, Kilari D, Amchentsev A Effect of steroids in septic shock patients without relative adrenal insufficiency. American Thoracic Society; 2008:116. Presented at the American Thoracic Society International Conference; May 16-21, 2008; Toronto, Ontario, Canada. [Google Scholar]
- 68.Meduri GU, Golden ERU. Prospective double-blind randomized clinical trial on the effects of low-dose hydrocortisone infusion in patients with severe sepsis. Chest. 2009;136:145S. doi: 10.1016/S0012-3692(16)48001-3 [DOI] [Google Scholar]
- 69.Mirea L, Ungureanu R, Pavelescu D, et al. Continuous administration of corticosteroids in septic shock can reduce risk of hypernatremia. Crit Care. 2014;18(1):239. doi: 10.1186/cc13429 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of outcomes
eTable 2. Search strategy
eTable 3. Inclusion criteria and exclusion criteria of including trials
eTable 4. Support for judgement for included trials rated as high risk of bias
eTable 5. Sensitivity analyses
eFigure 1. Risk of bias summary
eFigure 2. Risk of bias graph
eFigure 3. Trial sequential analysis for 28 days mortality
eFigure 4. Trial sequential analysis for 90 days mortality
eFigure 5. Funnel plot for 28 days mortality
eFigure 6. Subgroup analysis for 28 days mortality–based on dose of corticosteroid
eFigure 7. Subgroup analysis for 28 days mortality–based on treatment duration
eFigure 8. Subgroup analysis for 28 days mortality–based on sepsis population subtype
eFigure 9. Subgroup analysis for 28 days mortality–based on type of corticosteroids
eFigure 10. Subgroup analysis for 28 days mortality–based on control group mortality
eFigure 11. Meta-regression for 28-day mortality outcome by control group mortality
eFigure 12. Meta-regression for 28-day mortality outcome by year of study publication
eFigure 13. Forest plot for 90-day mortality
eFigure 14. Forest plot for ICU mortality
eFigure 15. Forest plot for hospital morality
eFigure 16. Forest plot for length of stay in hospital
eFigure 17. Forest plot for length of stay in ICU
eFigure 18. Forest plot for shock reversal at day 7
eFigure 19. Forest plot for SOFA score at day 7
eFigure 20. Forest plot for time to resolution of shock
eFigure 21. Forest plot for vasopressor-free day to day 28
eFigure 22. Forest plot for ventilation-free day to day 28
eFigure 23. Forest plot for any severe adverse event
eFigure 24. Forest plot for gastroduodenal bleeding
eFigure 25. Forest plot for superinfections
eFigure 26. Forest plot for hyperglycemia
eFigure 27. Forest plot for hypernatremia