Key Points
Question
Do patients with acute yellow fever infection present with positive ocular findings?
Findings
In this case series, 2 patients with acute yellow fever virus infection presented with bilateral increased choroidal thickness on ocular ultrasonography. One patient, who died after onset of the infection, also presented with bilateral 360° choroidal detachments and yellowish subretinal lesions; the other patient, previously vaccinated for yellow fever virus, presented with bilateral increased retinal venous congestion.
Meaning
Hemodynamic instability or the direct result of viral infection might be associated with the positive ocular findings in these patients with yellow fever virus.
Abstract
Importance
Yellow fever virus (YFV) is a reemerging, potentially lethal arboviral disease that has been occurring recently in Africa and South America. Poor levels of immunization have facilitated the viral spread in southeastern Brazil, leading to an unprecedented outbreak that started in late 2016. Although human cases have been linked to sylvatic mosquitoes, the concern is that YFV may spread to urban centers infested with Aedes aegypti and Aedes albopictus mosquitoes and start a true urban cycle.
Objective
To describe the ocular findings in patients with acute YFV infection.
Design, Setting, Participants
Two adults with an acute YFV infection in southeastern Brazil underwent an ophthalmologic and ocular ultrasonographic examination in early 2018.
Main Outcomes and Measures
Ocular findings in patients with acute YFV infection.
Results
Both patients presented with increased choroidal thickness bilaterally seen on ocular ultrasonography. A man in his late 50s who had not been vaccinated previously also presented with bilateral, midperipheral, 360° choroidal detachment and yellowish subretinal lesions. After clinical deterioration and liver transplant, the man died. A woman in her early 30s who had been vaccinated previously for YFV presented with increased retinal venous congestion bilaterally. She was discharged with mild conjunctival chemosis and icterus.
Conclusions and Relevance
These reports describe different patterns of ocular findings associated with YFV acute infection. However, the exact mechanism involved in the retinal and choroidal findings remains unclear.
This case series describes the ophthalmic manifestations of 2 patients from Brazil with yellow fever virus infection.
Introduction
The yellow fever virus (YFV), an arthropod-borne virus (arbovirus) of the Flavivirus genus in the Flaviviridae family, is a reemerging disease that has caused recent viral outbreaks in central Africa (2015-2016) and Brazil (2016-2018) and become widespread with a large number of cases diagnosed.1,2
In December 2016, Brazil experienced the largest epizootic outbreak of YFV over the past 50 years in nonhuman primates and unvaccinated humans inhabiting the rural areas of 5 states in southeastern Brazil. Thus far, all confirmed YFV cases have been linked to transmission through the jungle mosquito species Haemagogus leucocelaenus and Sabethes cyaneus. However, confirmed cases in humans and nonhuman primates close to large urban areas indicate the potential risk of urbanization, and YFV activity has increased in ecosystems of tropical and subtropical forests close to human populations.2 This report describes the ophthalmologic manifestations of YFV in 2 patients.
Methods
We describe 2 patients with acute YFV infection and positive ocular findings in southeastern Brazil. The Federal University of São Paulo granted permission for the preparation of this report. One patient provided written informed consent. The deceased patient’s family could not be reached for consent; therefore, that patient’s information has been deidentified.
Results
Patient 1
A previously healthy man in his late 50s from southeastern Brazil was hospitalized for evaluation in early 2018 with a 7-day, self-reported history of fever, asthenia, and myalgia. The patient had not been vaccinated previously for YFV. The patient presented with watery diarrhea followed by presyncope, intense myalgia, and orthostatic hypotension; jaundice and tachypnea were observed. The patient was admitted to the intensive care unit with acute respiratory insufficiency and underwent orotracheal intubation, hemodialysis, and treatment with vasoactive drugs. Seven days after hospitalization, worsening of the platelet count, creatinine level, and liver function were observed, as well as anisocoria. A neurologic evaluation suggested metabolic encephalopathy. Anti-YFV IgM and polymerase chain reaction findings were positive for the YFV.
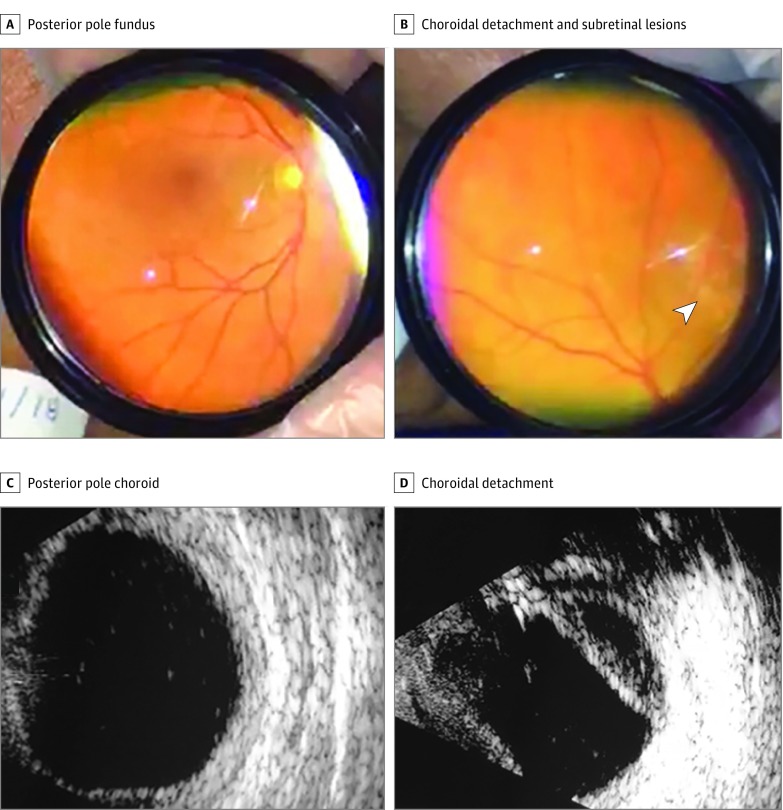
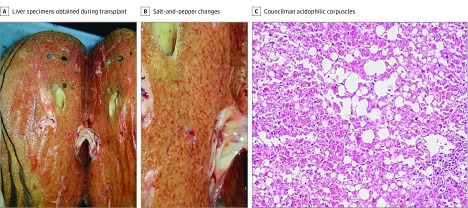
An ophthalmologic examination in the intensive care unit, including Smartphone fundus photographic and ocular ultrasonographic evaluations, revealed mild, bilateral conjunctival chemosis and icterus (eFigure 1A in the Supplement), shallow anterior chambers, and a 360° peripheral choroidal detachment associated with yellowish subretinal lesions (Figure 1 and eFigure 1B-D in the Supplement). Intraocular pressure was 16 mm Hg OU (Perkins tonometry). Ocular ultrasonography showed bilateral, low-intensity echoes suggestive of vitreous cells, choroidal thickening, and 360° midperipheral choroidal detachment (Figure 1C and D). Eight days after onset, the patient underwent liver transplant, but his condition worsened with progressing alveolar hemorrhaging, cardiorespiratory arrest, and death. Macroscopic and microscopic lesions were observed in liver specimens obtained during liver transplant (Figure 2).
Figure 1. Findings in Patient 1.
A, Posterior pole fundus (inverted view). B, Midperipheral 360° choroidal detachment and yellowish subretinal lesions (arrowhead) (inverted view). C, Increased thickness of the posterior pole choroid (1.7 mm). D, Midperipheral choroidal detachment in the right eye and low-reflectivity echoes.
Figure 2. Findings in Liver Specimens from Patient 1.
A, Liver specimens from a patient with liver failure during transplant. B, Salt-and-pepper changes in the macroscopic appearance are seen. C, Hepatic histologic examination shows Councilman acidophilic corpuscles, which represent hepatocytes in apoptosis and indicate extensive cellular death (steatosis or fatty degeneration) (hematoxylin-eosin, original magnification ×40).
Patient 2
On January 7, 2018, a previously healthy female nurse in her early 30s from São Paulo City, Brazil, presented with fever (temperature of 38°C), frontal headache, retro-orbital pain, nausea, and myalgia. She had been vaccinated for YFV 18 years previously and returned from a 4-day trip to Brumadinho, Minas Gerais State, where she visited an outdoor art museum surrounded by Atlantic forest 60 km from Belo Horizonte (southeastern Brazil). Three days after onset, she presented with myalgia and worsening nausea, vomiting, adynamia, and asthenia. Two days later, she was admitted to the intensive care unit with hypotension and hepatic insufficiency. Anti-YFV IgM and IgG and polymerase chain reaction findings were positive for YFV.
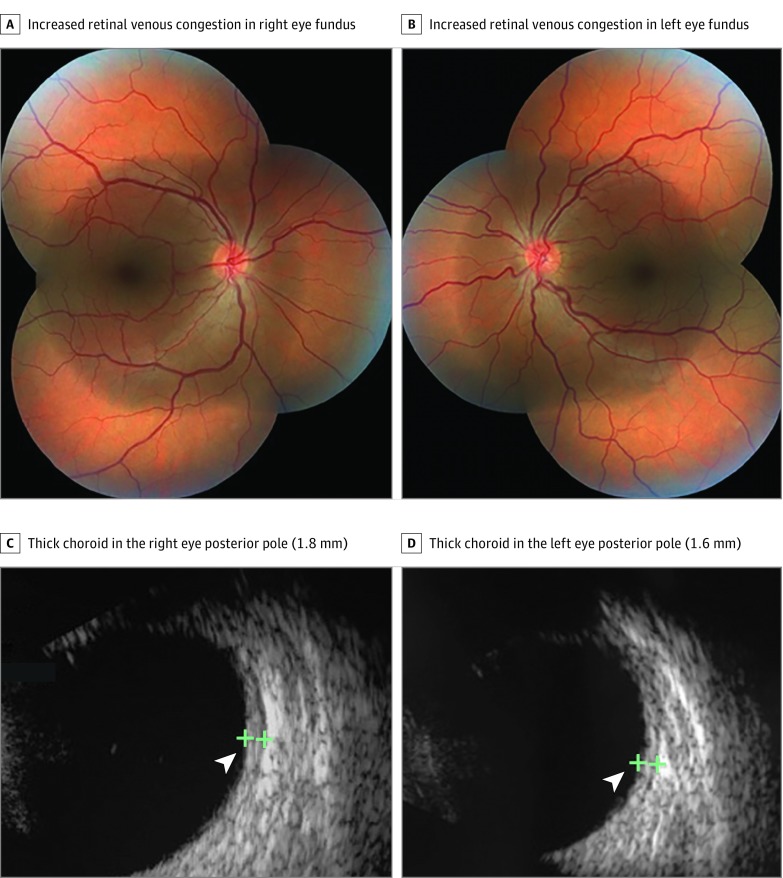
After the clinical symptoms improved, she was discharged and examined in an outpatient setting with ocular ultrasonography and standard fundus photography. Slitlamp biomicroscopy showed mild conjunctival chemosis and icterus. Intraocular pressure was 14 mm Hg OU (Goldmann applanation tonometry). Retinal venous congestion was observed in both fundi (Figure 3A and B). Fundus autofluorescence results were normal bilaterally (eFigure 2 in the Supplement). Ocular ultrasonographic images showed bilateral choroidal thickening (Figure 3C and D).
Figure 3. Findings in Patient 2.
Fundus images show increased retinal venous congestion in the right (A) and left (B) eyes. Ocular ultrasonographic images show a thick choroid in the posterior pole in the right eye (1.8 mm) (C) and left eye (1.6 mm) (D) (arrowheads).
Discussion
Yellow fever virus is a potentially lethal arboviral disease characterized by rapid jaundice development in association with liver dysfunction characteristic of clinically apparent human yellow fever.1 Following infection, an acute febrile phase lasts approximately 4 days and is accompanied by myalgia, headache, back pain, nausea, and vomiting, which usually resolve within 1 week.3 Approximately 14% of the patients develop a second, highly morbid phase marked by jaundice, hematemesis, hemorrhage, and renal injury, with mortality rates ranging from 20% to 50%.3 No specific antiviral agent exists to treat acute infections, and treatment remains supportive.
Yellow fever virus has a special affinity for the hepatocyte, resulting in an extensive inflammatory process and destruction of the liver parenchyma (Figure 2). As a result of this aggression, there is extravasation of fluid into the extravascular space. The ocular lesions found in patient 1 might be related to these altered hemodynamic conditions or as a result of inflammatory, metabolic, or hydrostatic ocular conditions.4 The vitreal increased cellularity in both patients might be related to local viral infection or an autoimmune inflammatory mechanism. Ocular histopathologic testing would have documented that the changes seen in patient 1 resulted from YFV. However, the patient’s family did not authorize removal of the eyes for further study.
The less severe form of the disease in patient 2 could have resulted from the partial protection afforded by the previous YFV vaccination. Because the intraocular pressure was within the reference ranges in both patients, we hypothesized that the choroidal findings could have resulted from hemodynamic instability or choroidal direct viral infection/inflammation caused by immunologic systemic processes related to the viral infection.
A 53-year-old Brazilian patient with YFV infection confirmed by polymerase chain reaction presented with unilateral retinal edema, macular exudates, and hemorrhages 19 days after onset. The clinicians hypothesized that the ocular manifestations in the convalescent stage of the infection could have resulted from an immune-mediated mechanism rather than direct viral invasion or infection (Olivia Zin, MD, personal communication, October 9, 2018). Another report described 2 cases of anterior and intermediate uveitis after YFV vaccination with fractional dosing; the authors believed that YFV vaccination could have induced cellular and humoral immune responses, leading to immune cross-reactivity triggering an autoimmune disease, such as anterior or intermediate uveitis.5 The findings seen in these reports differed from those in the patients described herein and resolved after oral corticosteroid therapy.
Yellow fever virus currently resides in the tropical regions of Africa and South America, where it is maintained primarily through a sylvatic cycle between monkeys and arboreal mosquito vectors.6,7 Yellow fever virus is endemic in northern (Amazon) and central-western Brazil and is spreading to southeastern Brazil, from which it has been absent for decades.8 From July 2016 to April 2018, the Brazilian Ministry of Health has reported 1818 confirmed cases that included 548 deaths in 5 states.9 The viral spread occurred in an area where YFV vaccination was not recommended previously and may have been facilitated by the poor vaccination coverage.
Recently, the Evandro Chagas Institute (Belém, Brazil) detected the YFV in Aedes albopictus from rural areas in the state of Minas Gerais, leading to concerns about the possibility of a YFV urban cycle in Brazil.10 The presence of dense populations of A albopictus and Aedes aegypti mosquitoes in numerous urban centers, where most of the inhabitants are not immune, remains a concern in Brazil and other tropical and subtropical countries in South America.11
Although a cost-effective YFV vaccine is available, YFV continues to infect 180 000 people annually and causes 78 000 deaths globally, mostly in Africa.12,13 Over the past 3 years, the global vaccine stockpile has been exhausted and fractional-dosing strategies are recommended to increase the number of vaccinated individuals to maximal achievable levels.14 The concept of the need for reimmunization every 10 years has been challenged because the vaccine might be protective for life. Considering the speed of the spread of the A aegypti–transmitted Zika and chikungunya viruses, the risk cannot be ignored that YFV could become epidemic in densely populated areas of the tropical world other than Latin America and Africa.
Limitations
This report has some limitations. First, only 2 patients were included. Second, patient 1 could not be imaged with standard fundus images and it was not possible to conduct histopathologic tests of his eyes.
Conclusions
This report describes different ocular presentations following yellow fever acute infection in 2 patients. The exact mechanism involved in the ocular findings needs further investigation.
eFigure 1. Ocular Findings in Patient 1
eFigure 2. Ocular Findings in Patient 2
References
- 1.Lucey D, Gostin LO. A yellow fever epidemic: a new global health emergency? JAMA. 2016;315(24):2661-2662. doi: 10.1001/jama.2016.6606 [DOI] [PubMed] [Google Scholar]
- 2.Pan American Health Organization Brazil works to control yellow fever outbreak, with PAHO/WHO support. https://www.paho.org/hq/index.php?option=com_content&view=article&id=13098&Itemid=1926&lang=fr. Published March 28, 2017. Accessed November 30, 2018.
- 3.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1(1):11-20. doi: 10.1016/S1473-3099(01)00016-0 [DOI] [PubMed] [Google Scholar]
- 4.Maggio E, Polito A, Prigione G, Pertile G. Uveal effusion syndrome mimicking severe chronic posterior uveitis: a case series of seven eyes of four patients. Graefes Arch Clin Exp Ophthalmol. 2016;254(3):545-552. doi: 10.1007/s00417-015-3176-y [DOI] [PubMed] [Google Scholar]
- 5.Biancardi AL, Moraes HV. Anterior and intermediate uveitis following yellow fever vaccination with fractional dose: case reports [published online August 8, 2018]. Ocul Immunol Inflamm. doi: 10.1080/09273948.2018.1510529 [DOI] [PubMed] [Google Scholar]
- 6.Ross RW, Gillett JD. The cyclical transmission of yellow fever virus through the grivet monkey, Cercopithecus aethiops centralis Neumann, and the mosquito Aëdes (Stegomyia) africanus Theobald. Ann Trop Med Parasitol. 1950;44(4):351-356. doi: 10.1080/00034983.1950.11685460 [DOI] [PubMed] [Google Scholar]
- 7.Cardoso JdaC, de Almeida MA, dos Santos E, et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, southern Brazil, 2008. Emerg Infect Dis. 2010;16(12):1918-1924. doi: 10.3201/eid1612.100608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romano AP, Costa ZG, Ramos DG, et al. Yellow fever outbreaks in unvaccinated populations, Brazil, 2008–2009. PLoS Negl Trop Dis. 2014;8(3):e2740. doi: 10.1371/journal.pntd.0002740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brazilian Ministry of Health Febre amarela: Ministério da Saúde atualiza casos no país. http://portalms.saude.gov.br/noticias/agencia-saude/42940-febre-amarela-ministerio-da-saude-atualiza-casos-no-pais-6. Updated April 4, 2018. Accessed September 7, 2018.
- 10.Evandro Chagas Institute Instituto Evandro Chagas detecta vírus da Febre Amarela em mosquitos Aedes albopictus no Brazil. http://www.iec.gov.br/portal/descoberta/. Published February 5, 2018. Accessed June 24, 2018.
- 11.Possas C, Martins RM, Oliveira RL, Homma A. Urgent call for action: avoiding spread and re-urbanisation of yellow fever in Brazil. Mem Inst Oswaldo Cruz. 2018;113(1):1-2. doi: 10.1590/0074-02760170361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norrby E. Yellow fever and Max Theiler: the only Nobel Prize for a virus vaccine. J Exp Med. 2007;204(12):2779-2784. doi: 10.1084/jem.20072290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett AD. Yellow fever in Angola and beyond—the problem of vaccine supply and demand. N Engl J Med. 2016;375(4):301-303. doi: 10.1056/NEJMp1606997 [DOI] [PubMed] [Google Scholar]
- 14.Klitting R, Gould EA, Paupy C, de Lamballerie X. What does the future hold for yellow fever virus? Genes (Basel). 2018;9(6):E291. doi: 10.3390/genes9060291 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Ocular Findings in Patient 1
eFigure 2. Ocular Findings in Patient 2